Abstract
The treatment of negative symptoms (NS) in psychosis represents an urgent unmet medical need given the significant functional impairment it contributes to psychosis syndromes. The lack of progress in treating NS is impacted by the lack of known pathophysiology or associated quantitative biomarkers, which could provide tools for research. This current analysis investigated potential associations between NS and an extensive battery of behavioral and brain-based biomarkers in 932 psychosis probands from the B-SNIP database. The current analyses examined associations between PANSS-defined NS and (1) cognition, (2) pro-/anti-saccades, (3) evoked and resting-state electroencephalography (EEG), (4) resting-state fMRI, and (5) tractography. Canonical correlation analyses yielded symptom-biomarker constructs separately for each biomarker modality. Biomarker modalities were integrated using canonical discriminant analysis to summarize the symptom-biomarker relationships into a “biomarker signature” for NS. Finally, distinct biomarker profiles for 2 NS domains (“diminished expression” vs “avolition/apathy”) were computed using step-wise linear regression. NS were associated with cognitive impairment, diminished EEG response amplitudes, deviant resting-state activity, and oculomotor abnormalities. While a connection between NS and poor cognition has been established, association to neurophysiology is novel, suggesting directions for future mechanistic studies. Each biomarker modality was related to NS in distinct and complex ways, giving NS a rich, interconnected fingerprint and suggesting that any one biomarker modality may not adequately capture the full spectrum of symptomology.
Keywords: schizophrenia, bipolar disorder, multivariate statistics/EEG, oculomotor/biotype
Introduction
Negative symptoms (NS) are one of the cardinal manifestations of schizophrenia.1,2 They are considered to be a distinct symptom class, although conceptualizations range from being an entirely independent symptom class to extensively overlapping with positive psychosis and cognitive impairment.3,4 Because these manifestations can be mimicked by other disorders, such as depression or intellectual disability, it is important to elucidate the characteristics of psychosis-related NS whenever possible.5–9 NS are identified in schizophrenia, but the extent to which they appear in other psychotic disorders has been insufficiently studied.10 Finally, the overall neurobiology of NS is not understood, and characteristic phenotypes have not been studied in depth.11 Biological disease features are even more important to identify now that treatments directed toward NS are a focus of development.12–22 With these points in mind, we set out to use proband characteristics from the Bipolar-Schizophrenia Network for Intermediate Phenotypes-1 (B-SNIP1) to identify the neurobiological features of NS in psychotic disorders and to contrast these across conventional psychosis diagnoses and biologically derived psychosis subgroups.23
Features of brain processing which robustly differentiated psychosis from healthy were assessed in B-SNIP1, including cognitive processing, resting and evoked electrophysiology, structural and functional brain imaging, and oculomotor function.24 Several psychosis disorders were included in the B-SNIP deep phenotyping protocol, including schizophrenia, schizoaffective disorder, and psychotic bipolar I disorder.25 Clementz et al used biomarker data to classify psychosis cases into Biotypes (B1-B3), all with similar levels of psychosis. Biotype-1 has severely disordered cognition and deficient electroencephalography (EEG) amplitudes and hyporesponsivity; Biotype-2 has slightly less disordered cognition but neurophysiological hyperactivity; Biotype-3 has almost normal cognition and only slightly deviant EEG responses.23 The broad biomarker profile of predominant NS in such a broad psychosis population, however, has not been examined.
In this article, we (1) identify individual biomarkers which separate B-SNIP1 probands with predominant NS from those without; (2) describe complex relationships between NS and a broad biomarker battery; and (3) demonstrate that 2 domains of NS (“diminished expression” vs “avolition/apathy”) are characterized by distinct biomarker profiles.8,26–31
Methodology
Participants
A total of 932 individuals with psychosis (schizophrenia, N = 397; schizoaffective disorder, N = 223; bipolar I disorder with psychosis, N = 312) were recruited as a part of B-SNIP1, a 5-site deep phenotyping study of the psychosis syndrome (table 1). The SCID Interview was used for clinical diagnosis32; see Tamminga et al25 for methodological details. Clinical assessments included the Global Assessment of Functioning (GAF) scale, Positive and Negative Syndrome Scale (PANSS),33 Young Mania Rating Scale (YMRS), Montgomery-Åsberg Depression Rating Scale (MADRS),34 and Birchwood Social Functioning Scale (BSFS).35 Participants were excluded if they had an active substance use dependence within 3 months or abuse within 1 month, had a known brain disorder, or had a traumatic brain injury resulting in loss of consciousness lasting longer than 30 min. Participants were clinically stable outpatients 15–65 years old (49.7% female), with the majority (92.7%) on at least one psychotropic medication. This study was approved by Institutional Review Boards at all 5 B-SNIP sites, and all participants provided written informed consent.
Table 1.
Clinical Characteristics of Probands With and Without Negative Symptoms
| NS | Non-NS | Statistic | P-value | |||
|---|---|---|---|---|---|---|
| (N = 322) | (N = 515) | |||||
| Sociodemographic Characteristics | ||||||
| Age, years, mean (SD) | 35.6 | (12.4) | 36.7 | (12.6) | F 1,835 = 1.8 | .18 |
| Gender, female, N (%) | 156 | (48.4 %) | 260 | (50.4 %) | χ 2 = 0.25 | .62 |
| Race, N (%) | ||||||
| Caucasian | 169 | (52.5 %) | 326 | (63.3 %) | χ 2 = 9.59 | .002 |
| African American | 137 | (42.5 %) | 183 | (35.5 %) | χ 2 = 3.83 | .050 |
| Education, years, mean (SD) | 13.0 | (2.3) | 13.5 | (2.4) | F 1,833 = 11.1 | .001 |
| Hollingshead score | 51.5 | (14.7) | 56.9 | (15.1) | F 1,779 = 17.6 | < .001 |
| Hollingshead score, maternal | 42.5 | (17.3) | 41.2 | (17.0) | F 1,704 = 0.96 | .33 |
| Hollingshead score, paternal | 39.2 | (18.7) | 37.6 | (17.8) | F 1,612 = 1.10 | .29 |
| Age of illness onset | 20.0 | (8.1) | 20.4 | (8.7) | F 1,804 = 0.30 | .59 |
| Illness duration, years | 15.5 | (12.1) | 16.4 | (12.4) | F 1,804 = 1.09 | .30 |
| Number of hospitalizations | 6.1 | (7.0) | 5.7 | (7.1) | F 1,667 = 0.69 | .41 |
| At least one suicide attempt | 139 | (43.8 %) | 193 | (38.0 %) | χ 2 = 4.27 | .12 |
| Lifetime ECT | 23 | (7.3 %) | 19 | (3.7 %) | χ 2 = 5.09 | .04 |
| Psychosis Subgroups, N (%) | ||||||
| Schizophrenia | 167 | (48.8 %) | 175 | (51.2 %) | χ 2 = 0.19 | .67 |
| Schizoaffective disorder | 93 | (42.1 %) | 118 | (57.9 %) | χ 2 = 2.96 | .09 |
| Bipolar I w/psychosis | 62 | (21.8 %) | 222 | (78.2 %) | χ 2 = 90.14 | < .001 |
| Biotype-1a | 91 | (46.9 %) | 103 | (53.1 %) | χ 2 = 0.74 | .39 |
| Biotype-2 | 92 | (40.7 %) | 134 | (59.3 %) | χ 2 = 7.81 | .005 |
| Biotype-3 | 90 | (32.8 %) | 184 | (47.2 %) | χ 2 = 32.25 | < .001 |
| Clinical Characteristics, Mean (SD) | ||||||
| GAF | 47.7 | (11.7) | 56.6 | (13.4) | F 1,833 = 96.4 | < .001 |
| Birchwood SFS | 114.9 | (25.7) | 130.7 | (23.4) | F 1,665 = 66.8 | < .001 |
| PANSS-Positive | 17.3 | (6.1) | 14.9 | (5.2) | F 1,834 = 36.7 | < .001 |
| PANSS-Negative | 19.5 | (5.2) | 12.0 | (3.4) | F 1,835 = 638.8 | < .001 |
| PANSS-General | 35.8 | (9.2) | 29.4 | (8.0) | F 1,834 = 111.4 | < .001 |
| MADRS | 14.11 | (10.9) | 8.5 | (7.8) | F 1,814 = 74.0 | < .001 |
| Young Mania Rating Scale | 7.0 | (7.0) | 5.9 | (6.1) | F 1,808 = 5.7 | .02 |
| WRAT-4 IQ | 96.0 | (15.6) | 98.2 | (14.8) | F 1,815 = 4.2 | .04 |
| PANSS Negative Symptom Factor items | ||||||
| N1. Blunted affect | 3.3 | (1.5) | 1.7 | (0.8) | F 1,835 = 413.5 | < .001 |
| N2. Emotional withdrawal | 3.0 | (1.2) | 1.7 | (0.8) | F 1,835 = 358.7 | < .001 |
| N3. Poor rapport | 2.5 | (1.3) | 1.4 | (0.6) | F 1,835 = 208.6 | < .001 |
| N4. Passive/apathetic social withdrawal | 3.3 | (1.4) | 1.8 | (0.8) | F 1,835 = 447.1 | < .001 |
| N6. Lack of spontaneity | 2.6 | (1.4) | 1.5 | (0.7) | F 1,835 = 244.8 | < .001 |
| G7. Motor retardation | 2.2 | (1.1) | 1.3 | (0.6) | F 1,835 = 214.9 | < .001 |
| G16. Active social withdrawal | 2.9 | (1.4) | 1.8 | (0.8) | F 1,835 = 242.3 | < .001 |
| Concomitant Medications, N (%) | ||||||
| Any psychotropic medication | 297 | (92.2 %) | 479 | (93.0 %) | χ 2 = 0.18 | .91 |
| Antipsychotics (any) | 272 | (84.4 %) | 411 | (79.8 %) | χ 2 = 3.27 | .20 |
| Antipsychotics, first generation | 41 | (12.7 %) | 39 | (7.6 %) | χ 2 = 6.20 | .05 |
| Antipsychotics, second generation | 230 | (71.4 %) | 372 | (72.2 %) | χ 2 = 0.17 | .97 |
| Mood stabilizers (any) | 117 | (36.3 %) | 256 | (49.7 %) | χ 2 = 14.46 | .001 |
| Antidepressants (any) | 150 | (46.6 %) | 222 | (43.1 %) | χ 2 = 1.03 | .60 |
| Sedatives/anxiolytics | 85 | (26.4 %) | 146 | (28.3 %) | χ 2 = 0.38 | .83 |
| Stimulants | 17 | (5.3 %) | 39 | (7.6 %) | χ 2 = 1.67 | .43 |
| Anticholinergics | 48 | (14.9 %) | 64 | (12.4 %) | χ 2 = 1.08 | .58 |
aOf the 837 probands with complete PANSS Negative data, only 694 had Biotype designations due to biomarker data requirements for the Biotype classification process. These individuals overlapped completely.
The PANSS Negative Symptom Factor (PANSS-NSF) shows superior content validity than the original NS scale, is reliable and sensitive to treatment response, and was used throughout these analyses.36 The PANSS-NSF, which excludes “difficulty in abstract thinking” (N5) and “stereotyped thinking” (N7) and includes “motor retardation” (G7) and “active social avoidance” (G16), has been informative in schizophrenia samples, as well as studies of persistent NS.37–40 For a subset of the current analyses, probands were subdivided into those with (NS) vs without (non-NS) predominant NS based on a previously validated framework, where the threshold for having predominant NS was defined as having at least one of the PANSS-NSF items rated as at least moderate (≥4).37,38 Ninety-six of the 932 B-SNIP probands lacked complete PANSS negative data and were excluded from these analyses. All analyses were performed across the psychosis syndrome, except where specifically noted.
Biomarker Assessment
The extensive B-SNIP phenotyping battery was collected on each participant, including cognitive assessments, electrophysiological measures, and structural and functional brain imaging.24,25,41 This battery included the Brief Assessment of Cognition in Schizophrenia (BACS) battery,42 the Stop Signal Task,43 the Dot Probe Expectancy Task,44,45 the Wechsler Memory Scale spatial span,46 the Penn Conditional Exclusion Test,47 the Penn Emotion Recognition Test,47–49 smooth pursuit eye movements,50–61 pro-/anti-saccades,62–68 electroencephalography (auditory oddball, paired stimuli, resting-state),69–71 resting-state fMRI,72,73 and structural tractography MRI (diffusion tensor imaging; DTI).74 Procedures and findings for each measure from B-SNIP1 are available in previous reports (also see supplemental methods).69–84 Phenotype assessment often included multiple individual variables for each task, paradigm, or imaging modality.23,41,85,86
Statistical Analyses
All biomarker measures were adjusted for age and sex using models constructed from the B-SNIP1 healthy control group (N = 459),24,25 an approach we have taken in previous B-SNIP publications (see supplemental methods).70,75,80,83,85–93 Cognition measures were also adjusted for race in similar manner. To directly examine individual biomarkers differentiating NS and non-NS groups, separate one-way ANOVAs were performed with each individual biomarker as dependent variables and group (NS vs non-NS) as a fixed factor, using SPSS v25 (IBM Corporation). Multiple testing was accounted for using a false discovery rate (FDR) threshold of 5%.94
To examine the structure of associations between NS and biomarkers, we used canonical correlation analysis (CCA), a data-driven, multivariate approach that identifies the bidirectional relationship between 2 sets of variables. This is accomplished by weighting each variable such that the weighted sum of one set of variables (eg, NS) is maximally correlated with a second set (eg, biomarkers). The resulting constructs are interpreted based on the relative strength and polarity of these weights. CCA was performed separately for each biomarker modality, with the biomarker variables on one side of the equation and PANSS-Negative items on the other, using SAS software v9.4 (SAS Institute Inc.). Individual participants with missing data were excluded modality wise. All measures were standardized before the CCA to eliminate differences in scale from contributing to the outcome. The multivariate nature of CCA does not require multiple testing within a CCA, although multiple testing across the separate CCAs was accounted for using an FDR threshold of 5%.94 To evaluate the consistency of the models produced by the CCA solutions and latent variate pairs, we conducted delete-2 jackknife analyses with 1000 replicates constructed using random sampling without replacement, with CCAs conducted on each replicate. No individual measure included zero in the 99% confidence interval across all jackknife outcomes for that behavioral or biological modality.
Psychosis Subgroups.
Subsequent analyses evaluated the similarity of symptom-biomarker associations in NS vs non-NS groups, as well as across conventional clinical diagnoses95 and B-SNIP Biotypes.23 The significant CCA pairs (NS-biomarker/phenotype constructs) were each subjected to a multivariate general linear model with subgroup (either NS/non-NS, DSM diagnosis, or Biotype, respectively) as a fixed factor and the CCA variates as dependent variables. This allowed us to assess group differences across both aspects of the symptom-biomarker construct simultaneously.
Negative Symptom Biosignature in Psychosis.
Finally, we performed a multivariate analysis integrating variables from all biomarker modalities. Canonical discriminant analysis (CDA) with step-wise variable introduction was used to identify those variables that maximally discriminated NS vs non-NS probands. To avoid large decreases in sample size, missing values were imputed using Markov chain Monte Carlo multiple imputation in SPSS v25.
Two-Factor Model of NS.
Confirmatory factor analysis (CFA) was conducted using a 2-factor model of NS identified in previous exploratory and CFA studies, “avolition/apathy” (including anhedonia, avolition, and asociality) and “diminished expression” (including blunted affect and alogia).26,96 The maximum likelihood method was used for estimation as the data did not show a tendency to non-normality (skewness < 2.0, Kurtosis < 3.0).97 CFA was conducted using SAS v9.4 and evaluated using multiple indices of goodness-of-fit: chi-square, comparative fit index (CFI > 0.9), root mean square error of approximation (RMSEA < 0.1), standardized root mean square residual (SRMS < 0.08), and goodness-of-fit index (GFI > 0.9).98–103
The factor loadings from the CFA were used to compute individual subject scores for the 2 NS factors. A stepwise linear regression model (Pin ≤ .05, Pout ≥ .1) was fit for each factor, using all biomarker variables as predictors. This resulted in a biomarker profile for each NS factor.
Results
Clinical Characteristics
Probands without predominant NS were more likely to be Caucasian (P = .002) with more years of education (P = .001) compared to probands with NS, but the groups were otherwise demographically similar (table 1). NS probands exhibited more severe clinical symptoms than non-NS on the PANSS positive and general symptom scores (P < .001), MADRS (P < .001), and YMRS (P = .02), and more severe psychosocial impairments on GAF (P < .001), Birchwood SFS (P < .001), and WRAT-4 (P = .04). There was a large overlap, however, in the distributions of these characteristics between NS and non-NS (supplemental figure 1). To address the question of whether NS were largely secondary to depression in our sample, we examined PANSS Negative scores as a function of MADRS scores (supplemental table 1). Including probands with high MADRS scores showed no appreciable effect on NS scores, so we proceeded by including them in all analyses.
The distribution of NS probands was not equal across DSM diagnoses or Biotypes (supplemental figure 2). Probands diagnosed with schizophrenia (48.8%; χ 2 = 0.19, P = .67) and schizoaffective disorder (42.1%; χ 2 = 42.1, P = .09) were more likely to exhibit NS than those with psychotic bipolar disorder (21.8%). The Biotypes showed a step-wise NS expression, from Biotype-1 (46.9%) to Biotype-2 (40.7%) to Biotype-3 (32.8%) (B1 vs B2, χ 2 = 1.28, P = .26; B2 vs B3, χ 2 = 3.81, P = .05; B1 vs B3, χ 2 = 9.34, P = .002).
Biomarker Characteristics
Individual Biomarkers.
To identify biomarkers specifically associated with NS-psychosis, we examined each biomarker individually. For individual biomarkers/phenotypes, NS cases showed more extensive impairment than non-NS on the BACS (NS = −1.18; non-NS = −0.80; P = 2.3E-4; Cohen’s d = −0.28), the Penn Emotion Recognition Test (NS = 83.7; non-NS = 86.3; P = 2.8E-4; d = −0.28), and the WMS Backward Score (NS = 8.7; non-NS = 9.1; P = .001; d = −0.24). No other individual biomarker differences survived FDR correction.
Modality-Wide Biomarker Associations.
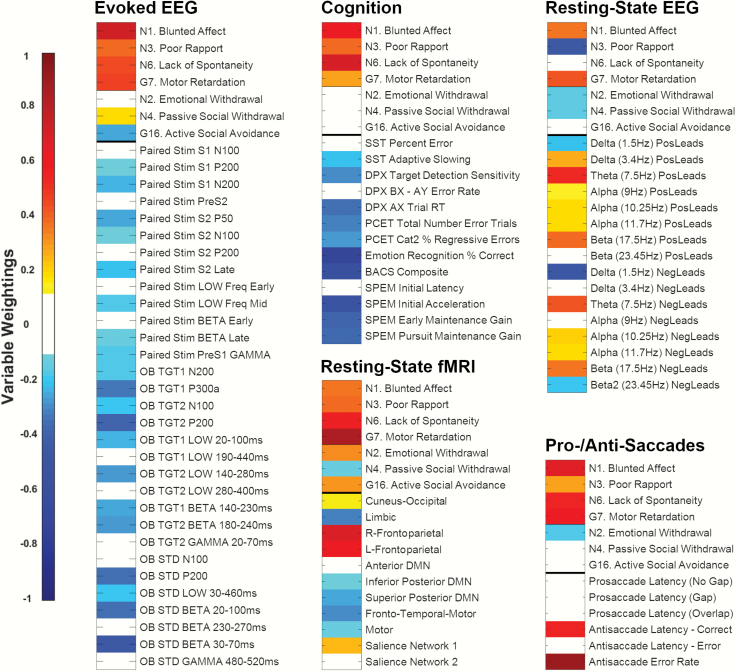
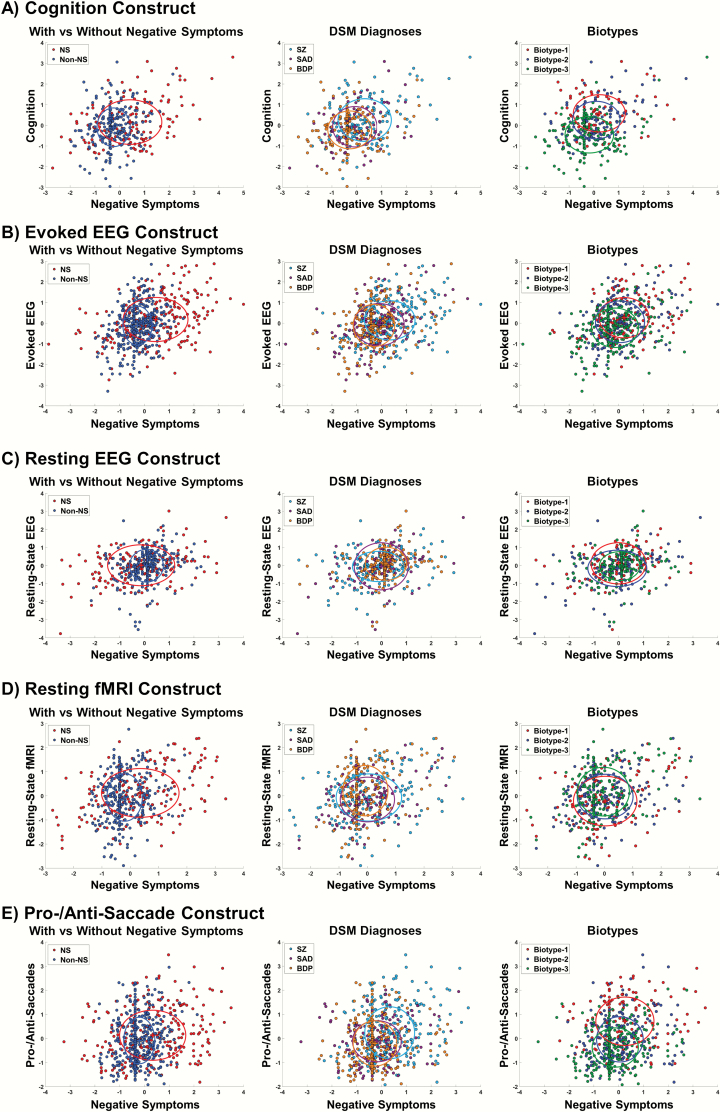
We examined complex relationships between NS and biomarker modalities using CCA. Each biomarker modality had a distinct and unique relationship with NS, giving NS a rich, interconnected fingerprint. For instance, poor performance on several cognitive measures associated with more severe blunted affect (N1), poor rapport (N3), lack of spontaneity (N6), and motor retardation (G7). Evoked-EEG amplitudes were positively correlated with active social avoidance (G16) severity, but negatively correlated with blunted affect (N1), poor rapport (N3), lack of spontaneity (N6), motor retardation (G7), and passive social avoidance (N7) severity. The association patterns for all NS-biomarker constructs are presented in figure 1, with CCA statistics and subgroup comparisons presented in table 2. Although subgroups often differed significantly, scatter and silhouette plots of canonical variate scores indicated large overlaps between subgroups (figure 2 and supplemental figure 3).
Fig. 1.
Each biomarker modality shows a distinct pattern of multivariate associations with negative symptoms. Note: The heat maps show the loading strength of individual negative symptoms and biomarker variables on their respective latent variates for each canonical correlation analysis. Warmer colors indicate stronger positive loadings; cooler colors indicate stronger negative loadings. For clarity, loadings between −.1 and .1 are shown in white. Paired Stim, auditory paired stimulus paradigm; S1/S2, the first or second paired stimulus; OB STD, auditory oddball standard stimuli; TGT1/2, PCA component 1/2 for auditory oddball target stimuli; SST, stop signal task; DPX, dot probe expectancy task; PCET, Penn conditional exclusion task; SPEM, smooth pursuit eye movements; PosLeads, mean of spatial leads that were positively correlated with the frequency component; NegLeads, mean of spatial leads that were negatively correlated with the frequency component; DMN, default mode network.
Table 2.
Modality-Wide Associations and Subgroup Differences
| Canonical Correlation Analysis Results | ||||||||
|---|---|---|---|---|---|---|---|---|
| F-value | P-value | FDR-Adjusted P-value | Canonical Correlation | % Variance Accounted | Wilks’ λ a | N | N–BM b | |
| Cognition | F 91,2159.1 = 1.47 | .003 | .009 | .34 | 34.9 | .69 | 362 | 15 |
| Evoked-EEG | F 217,3838.5 = 1.29 | .003 | .009 | .37 | 30.8 | .62 | 597 | 31 |
| rs-EEG | F 112,2725.1 = 1.31 | .02 | .04 | .34 | 37.7 | .71 | 443 | 16 |
| rs-fMRI | F 77,2620.1 = 1.31 | .04 | .06 | .32 | 47.9 | .80 | 454 | 11 |
| Pro-/anti-saccades | F 42,2944.3 = 1.31 | .09 | .10 | .22 | 61.6 | .93 | 640 | 6 |
| DTIc | F 350,846.05 = 0.92 | .81 | .81 | .38 | 22.2 | .11 | 177 | 50 |
| Probands With vs Without Predominant Negative Symptoms | ||||||||
| ANOVA | NS vs non-NS | |||||||
| F-value | P-value | Cohen’s d | ||||||
| Evoked-EEG | F 1,595 = 60.32 | 3.5E-14 | 0.62 | |||||
| Cognition | F 1,363 = 28.17 | 1.9E-7 | 0.55 | |||||
| rs-EEG | F 1,441 = 1.37 | .24 | — | |||||
| rs-fMRI | F 1,452 = 22.98 | 2.0E-6 | 0.46 | |||||
| Pro-/anti-saccades | F 1,638 = 37.85 | 1.4E-9 | 0.49 | |||||
| Clinical Diagnoses | ||||||||
| ANOVA | SZ vs SAD | SZ vs BDP | SAD vs BDP | |||||
| F-value | P-value | P-value | Cohen’s d | P-value | Cohen’s d | P-value | Cohen’s d | |
| Evoked-EEG | F 2,594 = 18.07 | 2.4E-8 | < .001 | 0.39 | < .001 | 0.57 | ns | — |
| Cognition | F 2,362 = 16.41 | 1.5E-7 | .001 | 0.43 | < .001 | 0.69 | ns | — |
| rs-EEG | F 2,440 = 3.41 | .03 | < .001 | 0.08 | < .001 | 0.30 | ns | — |
| rs-fMRI | F 2,451 = 1.13 | .32 | ns | — | ns | — | ns | — |
| Pro-/anti-saccades | F 2,637 = 33.17 | 2.0E-14 | < .001 | 0.56 | < .001 | 0.69 | ns | — |
| Biotypes | ||||||||
| ANOVA | B1 vs B2 | B1 vs B3 | B2 vs B3 | |||||
| F-value | P-value | P-value | Cohen’s d | P-value | Cohen’s d | P-value | Cohen’s d | |
| Evoked-EEG | F 2,564 = 13.92 | 1.0E-6 | .04 | 0.23 | < .001 | 0.57 | .002 | 0.31 |
| Cognition | F 2,358 = 27.86 | 5.7E-12 | .04 | 0.31 | < .001 | 0.97 | < .001 | 0.68 |
| rs-EEG | F 2,402 = 1.24 | .29 | ns | — | ns | — | ns | — |
| rs-Fmri | F 2,451 = 1.45 | .23 | ns | — | ns | — | ns | — |
| Pro-/anti-saccades | F 2,574 = 44.88 | 7.8E-19 | < .001 | 0.58 | < .001 | 0.89 | .004 | 0.34 |
aWilks’ λ is the product of the values (1—Canonical R2) for the current canonical variate and all variates below it. Therefore, lower values for Wilks’ λ represent a greater proportion of variance shared between the variable sets across all canonical variate functions.
bN-BM, number of biomarker variables included in a given modality.
cAs the negative symptom—DTI construct did not meet even exploratory significance, subgroup comparisons were not performed.
Fig. 2.
Scatterplots of canonical variate scores color coded by probands with vs without predominant negative symptoms (left), clinical diagnosis (middle), and Biotype (right) indicate largely overlapping subgroups. Note: Scores represent the sum of the standardized data weighted by the loading strength of individual negative symptoms and biomarker variables on their respective latent variates for each canonical correlation analysis. Each dot is an individual participant. The color-coded crosses and ellipsoids show the centroids and ±1 SD for each subgroup.
Negative Symptom Biosignature.
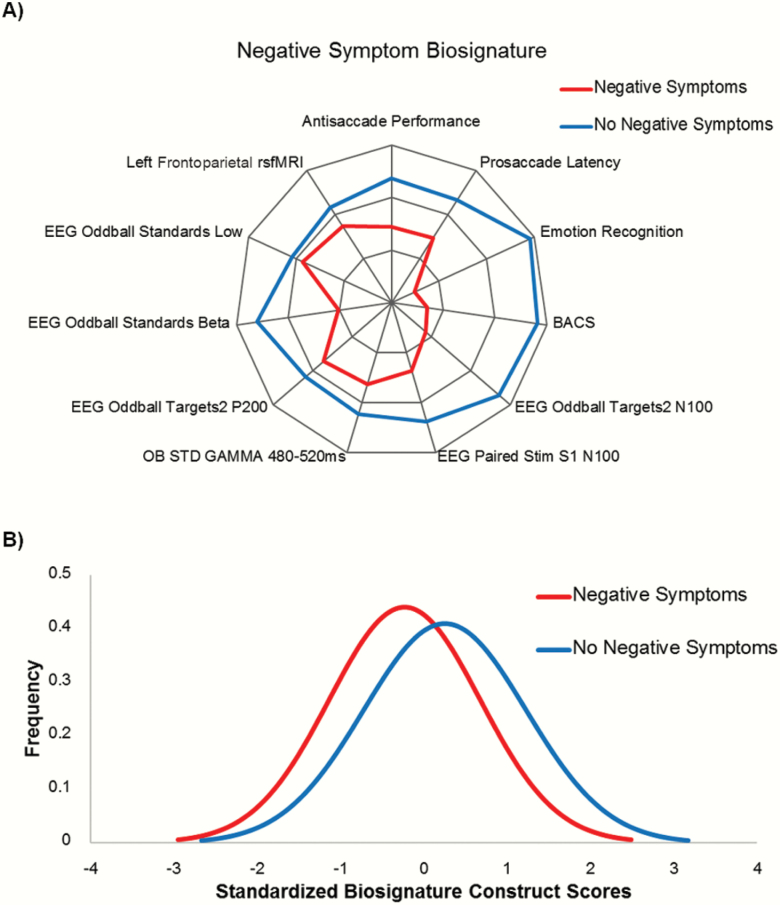
To identify a multimodal biosignature of NS, imputed values for all biomarker variables were used as predictors in a CDA, with NS vs non-NS status as the criterion. The CDA returned a set of 11 biomarkers which together maximally discriminate NS vs non-NS (Wilks’ Δ = .925, F11,781 = 5.77, P = 9.0E-6). The latent biosignature of NS includes poor antisaccade performance, poor emotion recognition, slow prosaccades, low BACS, low evoked-EEG amplitudes, and decreased frontoparietal rs-fMRI activity (figure 3A).
Fig. 3.
Probands with negative symptoms are characterized by slow saccades, low cognitive performance, low EEG amplitudes, and deviant resting-state network activity. Note: The latent biosignature of negative symptoms includes poor antisaccade performance, poor emotion recognition, slow prosaccades, low BACS, low evoked-EEG amplitudes, and decreased frontoparietal rs-fMRI activity. BACS, Brief Assessment of Cognition in Schizophrenia; Paired Stim, auditory paired stimulus paradigm; S1, the first paired stimulus; OB STD, auditory oddball standard stimuli; Targets1/2, PCA component 1/2 for auditory oddball target stimuli.
Two-Factor Model of Negative Symptoms.
Recent factor analyses have identified a 2-factor latent structure for NS, which we examined using CFA.26,96 The 2-factor model yielded the following fit index values: χ 2 = 186.06, df = 11 (P < .0001); CFI = .934; RMSEA = 0.138 [CI: 0.121–0.156, 90%]; SRMR = 0.048; and GFI=0.941, indicating good fit. Therefore, the common practice of considering 2 distinct factors of NS (“diminished expression” vs “avolition/apathy”) is supported by these data.
Standardized factor loadings from the 2-factor model (supplemental table 2) were used to compute individual subject scores for the 2 NS factors. A linear regression model was fit for each factor, using the evoked-EEG, cognition, and saccade measures as predictors. “Diminished expression” was predicted by low BACS (β = −.143, P < .001), low evoked-EEG amplitude on oddball standards beta-band 230–270 ms (β = −.15, P < .001) and 30–70 ms (β = −.08, P = .03), poor antisaccade performance (β = −.092, P = .01), and poor emotion recognition (β = −.072, P = .04; overall model fit: R = .266, F5,787 = 11.95, P = 9.63E-11). “Avolition/apathy” severity was predicted by low evoked-EEG amplitude on oddball standards beta-band 230–270ms (β = −.110, P = .002; overall model fit: R = .11, F1,791 = 9.62, P = .002). These results suggest that the 2 NS domains have distinct underlying biological correlates.
Negative Symptoms and Cognitive Impairment.
The results described above suggest a strong link between NS and cognitive impairment. While multiple individual NS items are significantly correlated with BACS composite scores (N1, P < .001; N2, P = .09; N2, P < .001; N3, P = .002; N4, P = .03; N6, P < .001; G7, P < .001; G16, P = .25), the strength of each correlation is low (all r < .2; supplemental figure 4). Further, NS and non-NS show BACS composite score distributions which are similar in shape but are slightly shifted with respect to one another, with NS showing lower race-adjusted BACS scores (NS: −1.17; non-NS: −0.80; t1,762 = 3.70; P = 2.3E-4; d = −0.28; supplemental figure 1).
Medication Effects.
Of the 837 probands, 776 (92.7%) were actively treated with at least one psychotropic medication, including mood stabilizers and antidepressants in addition to antipsychotics (table 1). NS and non-NS probands had similar medication profiles, with 297 NS (92.2%) and 479 non-NS (93.0%) receiving at least one psychotropic agent. More NS (12.7%) were taking first-generation antipsychotics than non-NS (7.6%) (P = .05). NS probands (36.3%) were less likely than non-NS (49.7%) to be on mood stabilizers of any kind (P = .001). Inclusion of usage (on/off) of first-generation antipsychotics and mood stabilizers as a factor in the individual biomarker ANOVAs and CCA subgroup comparisons did not reveal significant effects related to medication status.
Discussion
We examined the associations between NS and neurobiological characteristics of brain function; identified specific biomarkers, indeed a biomarker fingerprint, differentiating individuals with predominant NS from those without; and demonstrated that two. The first goal was addressed using multivariate approaches across all psychosis probands, which found NS to have strong associations with low cognitive ability and aberrant neurophysiology, highlighting information and sensory processing as potential distinguishing alterations for NS. For the second goal, probands were divided into subgroups, NS and non-NS. The only individual phenotypes which differentiated NS from non-NS related to cognition, suggesting that NS may share a relatively direct (albeit modest) relationship with cognitive impairment in psychosis. In addition to these primary goals, we also assessed a number of outstanding issues from the NS literature, including whether NS items are individually informative or can be meaningfully combined into a NS “total” score, whether NS are secondary to depressive symptoms, and the extent of the relationship between NS and cognitive impairment.
Based on these analyses, the biomarker modalities most strongly associated with NS are cognitive measures and evoked-EEG; secondarily, resting-state EEG and fMRI; and finally, pro-/anti-saccades, emphasizing the importance of both sensory and information processing constructs for understanding negative symptoms. These biomarker associations were largely specific to NS and did not extend to positive symptoms (see supplemental materials). A consensus has formed around the main constructs to be included in the definition of NS–ie, alogia, blunted affect, anhedonia, asociality, and avolition.4 Along with this, NS are commonly reported as an aggregate score, both in treatment trials and in clinical research. In contrast, our data show that individual NS items captured unique biomarker variance (see figure 1). As discussed below, NS are neurobiologically complex, and the individual NS constructs are distinct and informative.104
There is a growing consensus that, in terms of both phenomenology and co-occurrence, NS can be grouped into 2 domains, “avolition/apathy” (including avolition, asociality, and anhedonia) and “diminished expression” (including blunted affect and alogia).8,26,28–30,104 Recent evidence suggests that the “avolition/apathy” domain may be linked specifically to goal-directed behavior and reward system dysfunction.27,28,31 Our biomarker-based testing supports 2 separate NS domains, which were characterized by distinct biomarker profiles. “Avolition/apathy” scores were predicted by decreased delta-band rs-EEG activity, decreased evoked-EEG responses, and decreased ability to identify facial emotion, a critical aspect of social cognition. “Diminished expression” scores were predicted by poor emotion recognition, decreased evoked-EEG responses, and poor antisaccade performance. These observations suggest that battery of biomarkers will be required to fully characterize the biological underpinnings of NS, rather than any particular biomarker in isolation. For example, greater symptom severity in the “diminished expression” domain was associated with lower evoked-EEG amplitude, lower cognitive performance, increased frontoparietal rs-fMRI activity, and poor antisaccade performance in the CCA analysis (figure 1). This was echoed in the follow-up discriminant analysis, which identified low cognitive performance, low evoked-EEG amplitudes, and deviant frontoparietal rs-fMRI as a biosignature of NS (figure 3). Conversely, the relationship between NS in the “avolition/apathy” domain was inconsistent with respect to their association with the various biomarker modalities (see figure 1). These results provide further evidence that neither one biomarker modality nor one biomarker itself adequately captures the full spectrum of symptomology. This also suggests that attempts to determine the latent structure of NS using only symptom scale information may miss important clinically relevant neurobiological information. One caveat is that recent factor analyses of the Scale for the Assessment of Negative Symptoms (SANS),105 Brief Negative Symptom Scale (BNSS),106 and Clinical Assessment Interview for Negative Symptoms (CAINS)28 support a model with separate factors for the 5 domains of the NIMH consensus development conference (blunted affect, alogia, anhedonia, avolition, and asociality).4,104,107 These scales include greater numbers of NS items than the PANSS, perhaps capturing an increased amount of symptom variance.108 To our knowledge, there are no reports of 5-factor models using PANSS Negative data. Accordingly, the extent to which our findings would generalize if another NS scale were used is unclear. While algorithms for converting PANSS scores to SAPS/SANS have been proposed,109 comparisons with newer scales which incorporate a more nuanced understanding of NS psychopathology (eg, CAINS or BNSS) are limited to summary scores in small samples.106,108
NS can be either a primary component of psychosis pathophysiology or secondary to other factors, such as depression, environmental factors, or antipsychotic medication, and they can also be either transient or enduring.1,6,110 It is believed that while no currently available treatments are effective for primary or enduring NS, secondary symptoms, specifically those associated with depression, can be responsive to treatment.1,111,112 Subgrouping individuals with schizophrenia based on this distinction has proven informative in a variety of contexts.6,113–115 Three common approaches to NS study are (1) primary, enduring symptoms or “deficit symptoms”; (2) persistent symptoms, which may include treatment-resistant secondary NS; and (3) NS broadly construed, without differentiating primary from secondary symptoms.6,38,112,113 As the PANSS does not distinguish between primary and secondary NS, the current analyses address NS broadly construed. Surprisingly, in the current study, mean PANSS Negative scores were not impacted by the inclusion of probands with different levels of depression severity (supplemental table 1). From a biological perspective, the body of NS may all converge on the same body of biological functions. Research on NS pathophysiology is surprisingly limited considering the urgent therapeutic need, although work on social cognition, reward processing, reinforcement learning, and oxytocin are promising.116–119
The only individual biomarkers that significantly differentiated NS from non-NS were emotion recognition, BACS, and the working memory span backward score, suggesting that overall NS severity may share a relatively direct relationship with cognitive impairment. While these NS-cognition associations were significant, the correlations were weak, indicating that poor cognition alone does not fully explain NS. The modest association between NS and cognitive impairment found here is consistent with other studies, suggesting that cognitive impairment accounts for only a small amount of the variance in NS. Nonetheless, the potential for medication effects is not easily controlled, and it is of potential relevance that a higher proportion of NS were medicated with first-generation antipsychotics and mood stabilizers, compared to non-NS. This cross-sectional study was not designed to examine medication effects in depth, and the effects of this sort cannot be ruled out completely. Future studies will be needed to examine distinctions in biomarker profiles associated with primary vs antipsychotics-related NS. The relationship between NS and white matter tract integrity also requires further study. Reports of the existence and location of effects are inconsistent, although this could potentially be due to methodological differences.120–123 As a final caveat, the NS-cognition construct correlated with years of education (Pearson r = .23; P < .001). As NS probands had fewer years of education (see table 1), it is possible that the difference between these groups on the cognition construct might be confounded by education levels.
The findings from this investigation highlight the potential value of extensive biomarker batteries and integration across multiple levels of analysis for characterizing a neurobiologically complex clinical construct such as NS. While we observed complex relationships between NS and cognitive and neurophysiological measures, each biomarker modality was related to NS in distinct ways, suggesting that any one biomarker modality may not adequately capture the full spectrum of symptomology and that attempts to determine the latent structure of NS using only symptom scale information may be missing important clinically relevant information. While the multivariate nature of these analyses prohibited splitting our current sample, a larger independent replication sample (B-SNIP2) is in its final year of data collection. Our findings suggest that cognitive impairment, low evoked-EEG amplitude, slow saccades, and deviant resting-state activity may serve as a biosignature for the presence of NS. While the “diminished expression” and “avolition/apathy” domains revealed distinct and condensed biomarker profiles, much more symptom variance was explained in those analyses which included NS as a complex set rather than as a summary score, highlighting the importance of multilevel integration of biomarker and clinical batteries.
Supplementary Material
Acknowledgments
We thank Brad Witte, Gregory Book, and Gaurav Poudyal for their contributions to data management; the small army of students and research assistants required to conduct a study of this scale; and the volunteers who contributed their time and effort to the Bipolar-Schizophrenia Network for Intermediate Phenotypes study. ME Hudgens-Haney and BA Clementz have served as consultants for Astellas, Inc. MS Keshavan is a consultant to Forum Pharmaceuticals and is the editor of Schizophrenia Research. JA Sweeney reports serving on an advisory board for Takeda. CA Tamminga has consulted for Intracellular Therapies (ITI, Inc.), PureTech Ventures, Eli Lilly Pharmaceuticals, Sunovion, Astellas, and Merck. She is also an unpaid volunteer for NAMI, Deputy Editor of the American Psychiatric Association, and an expert witness for Finnegan Henderson Farabow Garrett & Dunner, LLP. F Gaudoux, P Bunouf, B Canolle, F Tonner, and S Gatti-McArthur have been employed by Institut Recherche Pierre-Fabre. The other authors reported no biomedical financial interests or potential conflicts of interest.
Funding
Funding for B-SNIP1 was provided by National Institutes of Health (NIH) grants MH077945, MH077862, MH077851, MH078113, MH085485. Partial funding for an early version of the current analysis was provided by Institut Recherche Pierre-Fabre. ME Hudgens-Haney was partially supported by National Institute of Health grant T32 MH0776690. These funding sources had no influence over the design and execution of the study, the final analyses, or interpretation of the data.
References
- 1. Carpenter WT Jr, Heinrichs DW, Alphs LD. Treatment of negative symptoms. Schizophr Bull. 1985;11(3):440–452. [DOI] [PubMed] [Google Scholar]
- 2. Marder SR, Galderisi S. The current conceptualization of negative symptoms in schizophrenia. World Psychiatry. 2017;16(1):14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grube BS, Bilder RM, Goldman RS. Meta-analysis of symptom factors in schizophrenia. Schizophr Res. 1998;31(2–3):113–120. [DOI] [PubMed] [Google Scholar]
- 4. Kirkpatrick B, Fenton WS, Carpenter WT, Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull. 2006;32(2):214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kirkpatrick B, Mucci A, Galderisi S. Primary, enduring negative symptoms: an update on research. Schizophr Bull. 2017;43(4):730–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kirkpatrick B, Buchanan RW, Ross DE, Carpenter WT Jr. A separate disease within the syndrome of schizophrenia. Arch Gen Psychiatry. 2001;58(2):165–171. [DOI] [PubMed] [Google Scholar]
- 7. Ahmed AO, Strauss GP, Buchanan RW, Kirkpatrick B, Carpenter WT. Schizophrenia heterogeneity revisited: clinical, cognitive, and psychosocial correlates of statistically-derived negative symptoms subgroups. J Psychiatr Res. 2018;97:8–15. [DOI] [PubMed] [Google Scholar]
- 8. Kirkpatrick B. Developing concepts in negative symptoms. J Clin Psychiatry. 2014;75(suppl 1):3–7. [DOI] [PubMed] [Google Scholar]
- 9. Carpenter WT Jr, Heinrichs DW, Wagman AM. Deficit and nondeficit forms of schizophrenia: the concept. Am J Psychiatry. 1988;145(5):578–583. [DOI] [PubMed] [Google Scholar]
- 10. Strauss GP, Esfahlani FZ, Kirkpatrick B, et al. . Network analysis reveals which negative symptom domains are most central in schizophrenia vs bipolar disorder. Schizophr Bull. 2019;45(6):1319–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Strauss GP, Cohen AS. A transdiagnostic review of negative symptom phenomenology and etiology. Schizophr Bull. 2017;43(4):712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Elis O, Caponigro JM, Kring AM. Psychosocial treatments for negative symptoms in schizophrenia: current practices and future directions. Clin Psychol Rev. 2013;33(8):914–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leucht S, Barabássy Á, Laszlovszky I, et al. . Linking PANSS negative symptom scores with the clinical global impressions scale: understanding negative symptom scores in schizophrenia. Neuropsychopharmacology. 2019;44:1589–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Németh G, Laszlovszky I, Czobor P, et al. . Cariprazine versus risperidone monotherapy for treatment of predominant negative symptoms in patients with schizophrenia: a randomised, double-blind, controlled trial. Lancet. 2017;389(10074):1103–1113. [DOI] [PubMed] [Google Scholar]
- 15. Alphs L, Anghelescu I-G, Arango C, et al. . Issues and perspectives in designing clinical trials for negative symptoms in schizophrenia. Schizophr Res. 2013;150(2–3):328–333. [DOI] [PubMed] [Google Scholar]
- 16. Aleman A, Lincoln TM, Bruggeman R, et al. . Treatment of negative symptoms: where do we stand, and where do we go? Schizophr Res. 2017;186:55–62. [DOI] [PubMed] [Google Scholar]
- 17. Bugarski-Kirola D, Blaettler T, Arango C, et al. . Bitopertin in negative symptoms of schizophrenia-results from the phase III flashlyte and daylyte studies. Biol Psychiatry. 2017;82(1):8–16. [DOI] [PubMed] [Google Scholar]
- 18. Galderisi S, Mucci A, Buchanan RW, Arango C. Negative symptoms of schizophrenia: new developments and unanswered research questions. Lancet Psychiatry. 2018;5(8):664–677. [DOI] [PubMed] [Google Scholar]
- 19. Arango C, Garibaldi G, Marder SR. Pharmacological approaches to treating negative symptoms: a review of clinical trials. Schizophr Res. 2013;150(2–3):346–352. [DOI] [PubMed] [Google Scholar]
- 20. Fusar-Poli P, Papanastasiou E, Stahl D, et al. . Treatments of negative symptoms in schizophrenia: meta-analysis of 168 randomized placebo-controlled trials. Schizophr Bull. 2015;41(4):892–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carpenter WT. Evaluating new pharmacotherapies for schizophrenia. Biol Psychiatry. 2016;79(12):948–949. [DOI] [PubMed] [Google Scholar]
- 22. Carpenter WT, Buchanan RW. Negative symptom therapeutics. Schizophr Bull. 2017;43(4):681–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Clementz BA, Sweeney JA, Hamm JP, et al. . Identification of distinct psychosis biotypes using brain-based biomarkers. Am J Psychiatry. 2016;173(4):373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tamminga CA, Pearlson GD, Keshavan MS, Sweeney JA, Clementz BA, Thaker GK. Bipolar and schizophrenia network for intermediate phenotypes: outcomes across the psychosis continuum. Schizophr Bull. 2014;40(suppl. 2):131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tamminga CA, Ivleva EI, Keshavan MS, et al. . Clinical phenotypes of psychosis in the bipolar-schizophrenia network on intermediate phenotypes (B-SNIP). Am J Psychiatry. 2013;170(11):1263–1274. [DOI] [PubMed] [Google Scholar]
- 26. Blanchard JJ, Horan WP, Collins LM. Examining the latent structure of negative symptoms: is there a distinct subtype of negative symptom schizophrenia? Schizophr Res. 2005;77(2–3):151–165. [DOI] [PubMed] [Google Scholar]
- 27. Kaiser S, Lyne J, Agartz I, Clarke M, Mørch-Johnsen L, Faerden A. Individual negative symptoms and domains – relevance for assessment, pathomechanisms and treatment. Schizophr Res. 2017;186:39–45. [DOI] [PubMed] [Google Scholar]
- 28. Kring AM, Gur RE, Blanchard JJ, Horan WP, Reise SP. The Clinical Assessment Interview for Negative Symptoms (CAINS): final development and validation. Am J Psychiatry. 2013;170(2):165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Messinger JW, Trémeau F, Antonius D, et al. . Avolition and expressive deficits capture negative symptom phenomenology: implications for DSM-5 and schizophrenia research. Clin Psychol Rev. 2011;31(1):161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Park SG, Llerena K, McCarthy JM, Couture SM, Bennett ME, Blanchard JJ. Screening for negative symptoms: preliminary results from the self-report version of the clinical assessment interview for negative symptoms. Schizophr Res. 2012;135(1–3):139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Strauss GP, Keller WR, Buchanan RW, et al. . Next-generation negative symptom assessment for clinical trials: validation of the Brief Negative Symptom Scale. Schizophr Res. 2012;142(1–3):88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. First M, Spitzer R, Gibbon M, Williams J.. Structured Clinical Interview for DSM-IV Axis I Disorders. Arlington, VA: American Psychiatric Publishing; 1997. [Google Scholar]
- 33. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. [DOI] [PubMed] [Google Scholar]
- 34. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. [DOI] [PubMed] [Google Scholar]
- 35. Ruhrmann S, Schultze-Lutter F, Salokangas RK, et al. . Prediction of psychosis in adolescents and young adults at high risk: results from the prospective European prediction of psychosis study. Arch Gen Psychiatry. 2010;67(3):241–251. [DOI] [PubMed] [Google Scholar]
- 36. Edgar CJ, Blaettler T, Bugarski-Kirola D, Le Scouiller S, Garibaldi GM, Marder SR. Reliability, validity and ability to detect change of the PANSS negative symptom factor score in outpatients with schizophrenia on select antipsychotics and with prominent negative or disorganized thought symptoms. Psychiatry Res. 2014;218(1–2):219–224. [DOI] [PubMed] [Google Scholar]
- 37. Galderisi S, Mucci A, Bitter I, et al. ; Eufest Study Group Persistent negative symptoms in first episode patients with schizophrenia: results from the European First Episode Schizophrenia Trial. Eur Neuropsychopharmacol. 2013;23(3):196–204. [DOI] [PubMed] [Google Scholar]
- 38. Buchanan RW. Persistent negative symptoms in schizophrenia: an overview. Schizophr Bull. 2007;33(4):1013–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Marder SR, Davis JM, Chouinard G. The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: combined results of the North American trials. J Clin Psychiatry. 1997;58(12):538–546. [DOI] [PubMed] [Google Scholar]
- 40. Wallwork RS, Fortgang R, Hashimoto R, Weinberger DR, Dickinson D. Searching for a consensus five-factor model of the Positive and Negative Syndrome Scale for schizophrenia. Schizophr Res. 2012;137(1–3):246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ivleva EI, Clementz BA, Dutcher AM, et al. . Brain structure biomarkers in the psychosis biotypes: findings from the bipolar-schizophrenia network for intermediate phenotypes. Biol Psychiatry. 2017;82(1):26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 2004;68(2–3):283–297. [DOI] [PubMed] [Google Scholar]
- 43. Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: a model and a method. J Exp Psychol Hum Percept Perform. 1984;10(2):276–291. [DOI] [PubMed] [Google Scholar]
- 44. Henderson D, Poppe AB, Barch DM, et al. . Optimization of a goal maintenance task for use in clinical applications. Schizophr Bull. 2012;38(1):104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jones JA, Sponheim SR, MacDonald AW 3rd. The dot pattern expectancy task: reliability and replication of deficits in schizophrenia. Psychol Assess. 2010;22(1):131–141. [DOI] [PubMed] [Google Scholar]
- 46. Wechsler D. Wechsler Memory Scale (WMS-III). San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 47. Gur RC, Richard J, Hughett P, et al. . A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: standardization and initial construct validation. J Neurosci Methods. 2010;187(2):254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kohler CG, Turner TH, Bilker WB, et al. . Facial emotion recognition in schizophrenia: intensity effects and error pattern. Am J Psychiatry. 2003;160(10):1768–1774. [DOI] [PubMed] [Google Scholar]
- 49. Gur RC, Sara R, Hagendoorn M, et al. . A method for obtaining 3-dimensional facial expressions and its standardization for use in neurocognitive studies. J Neurosci Methods. 2002;115(2):137–143. [DOI] [PubMed] [Google Scholar]
- 50. Thaker GK. Neurophysiological endophenotypes across bipolar and schizophrenia psychosis. Schizophr Bull. 2008;34(4):760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Diefendorf AR, Dodge R. An experimental study of the ocular reactions of the insane from photographic records. Brain. 1908;31(3):451–489. [Google Scholar]
- 52. Lencer R, Sprenger A, Harris MS, Reilly JL, Keshavan MS, Sweeney JA. Effects of second-generation antipsychotic medication on smooth pursuit performance in antipsychotic-naive schizophrenia. Arch Gen Psychiatry. 2008;65(10):1146–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lencer R, Bishop JR, Harris MS, et al. . Association of variants in DRD2 and GRM3 with motor and cognitive function in first-episode psychosis. Eur Arch Psychiatry Clin Neurosci. 2014;264(4):345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Holzman PS. Behavioral markers of schizophrenia useful for genetic studies. J Psychiatr Res. 1992;26(4):427–445. [DOI] [PubMed] [Google Scholar]
- 55. Lencer R, Nagel M, Sprenger A, et al. . Cortical mechanisms of smooth pursuit eye movements with target blanking. An fMRI study. Eur J Neurosci. 2004;19(5):1430–1436. [DOI] [PubMed] [Google Scholar]
- 56. Chen Y, Nakayama K, Levy DL, Matthysse S, Holzman PS. Psychophysical isolation of a motion-processing deficit in schizophrenics and their relatives and its association with impaired smooth pursuit. Proc Natl Acad Sci U S A. 1999;96(8):4724–4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Clementz BA, McDowell JE. Smooth pursuit in schizophrenia: abnormalities of open- and closed-loop responses. Psychophysiology. 1994;31(1):79–86. [DOI] [PubMed] [Google Scholar]
- 58. Lencer R, Reilly JL, Harris MS, Sprenger A, Keshavan MS, Sweeney JA. Sensorimotor transformation deficits for smooth pursuit in first-episode affective psychoses and schizophrenia. Biol Psychiatry. 2010;67(3):217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sweeney JA, Luna B, Haas GL, Keshavan MS, Mann JJ, Thase ME. Pursuit tracking impairments in schizophrenia and mood disorders: step-ramp studies with unmedicated patients. Biol Psychiatry. 1999;46(5):671–680. [DOI] [PubMed] [Google Scholar]
- 60. Thaker GK, Ross DE, Cassady SL, et al. . Smooth pursuit eye movements to extraretinal motion signals: deficits in relatives of patients with schizophrenia. Arch Gen Psychiatry. 1998;55(9):830–836. [DOI] [PubMed] [Google Scholar]
- 61. Lencer R, Keedy SK, Reilly JL, et al. . Altered transfer of visual motion information to parietal association cortex in untreated first-episode psychosis: implications for pursuit eye tracking. Psychiatry Res. 2011;194(1):30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hallett PE. Primary and secondary saccades to goals defined by instructions. Vision Res. 1978;18(10):1279–1296. [DOI] [PubMed] [Google Scholar]
- 63. Reilly JL, Harris MS, Khine TT, Keshavan MS, Sweeney JA. Reduced attentional engagement contributes to deficits in prefrontal inhibitory control in schizophrenia. Biol Psychiatry. 2008;63(8):776–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. McDowell JE, Myles-Worsley M, Coon H, Byerley W, Clementz BA. Measuring liability for schizophrenia using optimized antisaccade stimulus parameters. Psychophysiology. 1999;36(1):138–141. [DOI] [PubMed] [Google Scholar]
- 65. Calkins ME, Iacono WG. Eye movement dysfunction in schizophrenia: a heritable characteristic for enhancing phenotype definition. Am J Med Genet. 2000;97(1):72–76. [DOI] [PubMed] [Google Scholar]
- 66. Calkins ME, Curtis CE, Iacono WG, Grove WM. Antisaccade performance is impaired in medically and psychiatrically healthy biological relatives of schizophrenia patients. Schizophr Res. 2004;71(1):167–178. [DOI] [PubMed] [Google Scholar]
- 67. Pierce JE, Clementz BA, McDowell JE. Saccades: fundamentals and neural mechanisms. In: Klein C, Ettinger U, eds Eye Movement Research Studies in Neuroscience, Psychology and Behavioral Economics; 2000:11–71. doi:10.1007/978-3-030-20085-5_2. [Google Scholar]
- 68. Rodrigue AL, Austin BP, McDowell JE. Plasticity of prefrontal cortex connectivity in schizophrenia in response to antisaccade practice. Psychiatry Res Neuroimaging. 2017;265:77–86. [DOI] [PubMed] [Google Scholar]
- 69. Ethridge LE, Hamm JP, Pearlson GD, et al. . Event-related potential and time-frequency endophenotypes for schizophrenia and psychotic bipolar disorder. Biol Psychiatry. 2015;77(2):127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hamm JP, Ethridge LE, Boutros NN, et al. . Diagnostic specificity and familiality of early versus late evoked potentials to auditory paired stimuli across the schizophrenia-bipolar psychosis spectrum. Psychophysiology. 2014;51(4):348–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Narayanan B, O’Neil K, Berwise C, et al. . Resting state electroencephalogram oscillatory abnormalities in schizophrenia and psychotic bipolar patients and their relatives from the bipolar and schizophrenia network on intermediate phenotypes study. Biol Psychiatry. 2014;76(6):456–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Meda SA, Clementz BA, Sweeney JA, et al. . Examining functional resting-state connectivity in psychosis and its subgroups in the bipolar-schizophrenia network on intermediate phenotypes cohort. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1(6):488–497. [DOI] [PubMed] [Google Scholar]
- 73. Meda SA, Ruaño G, Windemuth A, et al. . Multivariate analysis reveals genetic associations of the resting default mode network in psychotic bipolar disorder and schizophrenia. Proc Natl Acad Sci U S A. 2014;111(19):E2066–E2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Skudlarski P, Schretlen DJ, Thaker GK, et al. . Diffusion tensor imaging white matter endophenotypes in patients with schizophrenia or psychotic bipolar disorder and their relatives. Am J Psychiatry. 2013;170(8):886–898. [DOI] [PubMed] [Google Scholar]
- 75. Kristian Hill S, Buchholz A, Amsbaugh H, et al. . Working memory impairment in probands with schizoaffective disorder and first degree relatives of schizophrenia probands extend beyond deficits predicted by generalized neuropsychological impairment. Schizophr Res. 2015;166(1–3):310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ivleva EI, Moates AF, Hamm JP, et al. . Smooth pursuit eye movement, prepulse inhibition, and auditory paired stimuli processing endophenotypes across the schizophrenia-bipolar disorder psychosis dimension. Schizophr Bull. 2014;40(3):642–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Moates AF, Ivleva EI, O’Neill HB, et al. . Predictive pursuit association with deficits in working memory in psychosis. Biol Psychiatry. 2012;72(9):752–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lencer R, Mills LJ, Alliey-Rodriguez N, et al. . Genome-wide association studies of smooth pursuit and antisaccade eye movements in psychotic disorders: findings from the B-SNIP study. Transl Psychiatry. 2017;7(10):e1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lencer R, Sprenger A, Reilly JL, et al. . Pursuit eye movements as an intermediate phenotype across psychotic disorders: evidence from the B-SNIP study. Schizophr Res. 2015;169(1–3):326–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Reilly JL, Frankovich K, Hill S, et al. . Elevated antisaccade error rate as an intermediate phenotype for psychosis across diagnostic categories. Schizophr Bull. 2014;40(5):1011–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hill SK, Reilly JL, Keefe RS, et al. . Neuropsychological impairments in schizophrenia and psychotic bipolar disorder: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. Am J Psychiatry. 2013;170(11):1275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ethridge LE, Soilleux M, Nakonezny PA, et al. . Behavioral response inhibition in psychotic disorders: diagnostic specificity, familiality and relation to generalized cognitive deficit. Schizophr Res. 2014;159(2–3):491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Reilly JL, Hill SK, Gold JM, et al. . Impaired context processing is attributable to global neuropsychological impairment in schizophrenia and psychotic bipolar disorder. Schizophr Bull. 2017;43(2):397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ruocco AC, Reilly JL, Rubin LH, et al. . Emotion recognition deficits in schizophrenia-spectrum disorders and psychotic bipolar disorder: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. Schizophr Res. 2014;158(1–3):105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hudgens-Haney ME, Ethridge LE, Knight JB, et al. . Intrinsic neural activity differences among psychotic illnesses. Psychophysiology. 2017;54(8):1223–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hudgens-Haney ME, Ethridge LE, McDowell JE, et al. . Psychosis subgroups differ in intrinsic neural activity but not task-specific processing. Schizophr Res. 2018;195:222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Nanda P, Tandon N, Mathew IT, et al. . Local gyrification index in probands with psychotic disorders and their first-degree relatives. Biol Psychiatry. 2014;76(6):447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Padmanabhan JL, Tandon N, Haller CS, et al. . Correlations between brain structure and symptom dimensions of psychosis in schizophrenia, schizoaffective, and psychotic bipolar I disorders. Schizophr Bull. 2015;41(1):154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ethridge LE, Hamm JP, Pearlson GD, et al. . Event-related potential and time-frequency endophenotypes for schizophrenia and psychotic bipolar disorder. Biol Psychiatry. 2015;77(2):127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Rodrigue AL, McDowell JE, Tandon N, et al. . Multivariate relationships between cognition and brain anatomy across the psychosis spectrum. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3(12):992–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Parker DA, Hamm JP, McDowell JE, et al. . Auditory steady-state EEG response across the schizo-bipolar spectrum. Schizophr Res. 2019;209:218–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Trotti RL, Parker DA, Sabatinelli D, et al. . Electrophysiological correlates of emotional scene processing in bipolar disorder. J Psychiatr Res. 2020;120:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Thomas O, Parker DA, Trotti RL, et al. . Intrinsic neural activity differences in psychosis biotypes: findings from the bipolar-schizophrenia network on intermediate phenotypes (b-snip) consortium. Biomarkers in Neuropsychiatry. 2019;1:100002. doi:10.1016/j.bionps.2019.100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Benjamini Y, Hochberg Y. Controlling the false discovery rate : a practical and powerful approach to multiple testing. R Stat Soc. 1995;57(1):289–300. [Google Scholar]
- 95. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition---Text Revision (DSM-IV-TR)). Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 96. Jang SK, Choi HI, Park S, et al. . A two-factor model better explains heterogeneity in negative symptoms: evidence from the positive and negative syndrome scale. Front Psychol. 2016;7:707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. West SG, Finch JF, Curran PJ. Structural equation models with nonnormal variables: problems and remedies. – PsycNET. In: Hoyle RH, ed. Structural Equation Modeling: Concepts, Issues, and Applications. Thousand Oaks, CA: Sage Publications, Inc; 1995:56–75. https://psycnet.apa.org/record/1995-97753-004. Accessed April 8, 2019. [Google Scholar]
- 98. Hayduk LA, Littvay L. Should researchers use single indicators, best indicators, or multiple indicators in structural equation models? BMC Med Res Methodol. 2012;12:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Hu LT, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Model. 1999;6(1):1–55. [Google Scholar]
- 100. Bentler PM. Comparative fit indexes in structural models. Psychol Bull. 1990;107(2):238–246. [DOI] [PubMed] [Google Scholar]
- 101. Steiger JH. Structural model evaluation and modification: an interval estimation approach. Multivariate Behav Res. 1990;25(2):173–180. [DOI] [PubMed] [Google Scholar]
- 102. Akaike H. Factor analysis and AIC. Psychometrika. 1987;52(3):317–332. [Google Scholar]
- 103. Hoyle RH. Confirmatory factor analysis. In: Tinsley HEA, Brown SD, eds Handbook of Applied Multivariate Statistics and Mathematical Modeling London, UK: Academic Press; 2000:465–497. doi:10.1016/B978-012691360-6/50017-3. [Google Scholar]
- 104. Strauss GP, Nuñez A, Ahmed AO, et al. . The latent structure of negative symptoms in schizophrenia. JAMA Psychiatry. 2018;75(12):1271–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS). Iowa City, IA: University of Iowa; 1984. [Google Scholar]
- 106. Kirkpatrick B, Strauss GP, Nguyen L, et al. . The brief negative symptom scale: psychometric properties. Schizophr Bull. 2011;37(2):300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Ahmed AO, Kirkpatrick B, Galderisi S, et al. . Cross-cultural validation of the 5-factor structure of negative symptoms in schizophrenia. Schizophr Bull. 2019;45(2):305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kumari S, Malik M, Florival C, Manalai P, Sonje S. An Assessment of Five (PANSS, SAPS, SANS, NSA-16, CGI-SCH) commonly used Symptoms Rating Scales in Schizophrenia and Comparison to Newer Scales (CAINS, BNSS). J Addict Res Ther. 2017;08(03):324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. van Erp TG, Preda A, Nguyen D, et al. . Converting positive and negative symptom scores between PANSS and SAPS/SANS. Schizophr Res. 2014;152(1):289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Kirkpatrick B, Galderisi S. Deficit schizophrenia: an update. World Psychiatry. 2008;7(3):143–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Kirschner M, Aleman A, Kaiser S. Secondary negative symptoms – a review of mechanisms, assessment and treatment. Schizophr Res. 2017;186:29–38. [DOI] [PubMed] [Google Scholar]
- 112. Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia, “just the facts” 4. Clinical features and conceptualization. Schizophr Res. 2009;110(1–3):1–23. [DOI] [PubMed] [Google Scholar]
- 113. Ahmed AO, Strauss GP, Buchanan RW, Kirkpatrick B, Carpenter WT. Are negative symptoms dimensional or categorical? Detection and validation of deficit schizophrenia with taxometric and latent variable mixture models. Schizophr Bull. 2015;41(4):879–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Cohen AS, Saperstein AM, Gold JM, Kirkpatrick B, Carpenter WT Jr, Buchanan RW. Neuropsychology of the deficit syndrome: new data and meta-analysis of findings to date. Schizophr Bull. 2007;33(5):1201–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Mucci A, Merlotti E, Üçok A, Aleman A, Galderisi S. Primary and persistent negative symptoms: concepts, assessments and neurobiological bases. Schizophr Res. 2017;186:19–28. [DOI] [PubMed] [Google Scholar]
- 116. Strauss GP, Keller WR, Koenig JI, Gold JM, Ossenfort KL, Buchanan RW. Plasma oxytocin levels predict olfactory identification and negative symptoms in individuals with schizophrenia. Schizophr Res. 2015;162(1–3; ):57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Gold JM, Waltz JA, Matveeva TM, et al. . Negative symptoms and the failure to represent the expected reward value of actions: behavioral and computational modeling evidence. Arch Gen Psychiatry. 2012;69(2):129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Allen DN, Strauss GP, Donohue B, van Kammen DP. Factor analytic support for social cognition as a separable cognitive domain in schizophrenia. Schizophr Res. 2007;93(1–3):325–333. [DOI] [PubMed] [Google Scholar]
- 119. Strauss GP, Frank MJ, Waltz JA, Kasanova Z, Herbener ES, Gold JM. Deficits in positive reinforcement learning and uncertainty-driven exploration are associated with distinct aspects of negative symptoms in schizophrenia. Biol Psychiatry. 2011;69(5):424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Skelly LR, Calhoun V, Meda SA, Kim J, Mathalon DH, Pearlson GD. Diffusion tensor imaging in schizophrenia: relationship to symptoms. Schizophr Res. 2008;98(1–3): 157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Foong J, Maier M, Clark CA, Barker GJ, Miller DH, Ron MA. Neuropathological abnormalities of the corpus callosum in schizophrenia: a diffusion tensor imaging study. J Neurol Neurosurg Psychiatry. 2000;68(2):242–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Gu C, Zhang Y, Wei F, Cheng Y, Cao Y, Hou H. Magnetic resonance imaging DTI-FT study on schizophrenic patients with typical negative first symptoms. Exp Ther Med. 2016;12(3):1450–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Brady RO Jr, Gonsalvez I, Lee I, et al. . Cerebellar-prefrontal network connectivity and negative symptoms in schizophrenia. Am J Psychiatry. 2019;176(7):512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.