Abstract
Deficits in social cognition are common in people with psychotic disorders and negatively impact functioning. Social Cognition Training (SCT) has been found to improve social cognition and functioning, but it is unknown which interventions are most effective, how characteristics of treatments and participants moderate efficacy, and whether improvements are durable. This meta-analysis included 46 randomized studies. SCTs were categorized according to their focus (targeted/broad-based) and inclusion of cognitive remediation therapy (CRT). Network meta-analysis was conducted, using both direct (original) and indirect (inferred from the network of comparisons) evidence. All SCT types were compared to treatment as usual (TAU; the chosen reference group). Moderators of outcome were investigated with meta-regression and long-term efficacy with multivariate meta-analysis. Compared to TAU, emotion perception was improved by targeted SCT without CRT (d = 0.68) and broad-based SCT without CRT (d = 0.46). Individual treatments worked better for emotion perception. All treatments significantly improved social perception (active control, d = 0.98, targeted SCT with and without CRT, d = 1.38 and d = 1.36, broad-based SCT with and without CRT, d = 1.45 and d = 1.35). Only broad-based SCT (d = 0.42) improved ToM. Broad-based SCT (d = 0.82 without and d = 0.41 with CRT) improved functioning; group treatments worked significantly better. Male gender was negatively related to effects on social functioning and psychiatric symptoms. At follow-up, a moderate effect on social functioning (d = 0.66) was found. No effect was found on attribution, social cognition (miscellaneous), and psychiatric symptoms. While targeted SCT is the most effective for emotion perception and social perception, broad-based SCT produces the best overall outcomes. CRT did not enhance SCT effects.
Keywords: cognitive remediation, schizophrenia, psychosocial treatment, systematic review, social functioning, social cognition training, social cognition, meta-analysis
Introduction
Psychotic disorders (ie, schizophrenia, schizoaffective disorder, and other diagnoses on the psychotic spectrum) can significantly impair work, relationships, and social functioning.1–3 These functional disabilities are predicted by deficits in social cognition, which are commonly observed in people with psychosis.4
Social cognition refers to the cognitive processes involved in understanding social situations and other people. It is generally divided into different sub-domains: emotion perception and processing (the ability to recognize emotions), social perception and knowledge (understanding social cues and social context), Theory of Mind (ToM; the ability to identify, understand and distinguish other people’s mental state), and attribution (inferences about the causes of events and/or behavior). A large meta-analysis found that people with psychosis have deficits in nearly all aforementioned domains (ie, emotion perception, social perception, and ToM, but no difference in attributional style).5
In the past 2 decades, research efforts have focused on the improvement of social functioning through Social Cognition Training (SCT).6 SCT is an umbrella term of psychosocial interventions focused on the rehabilitation of deficits in social cognition. SCT generally includes a combination of practicing with social stimuli (eg, pictures), and learning strategies to cope with deficits (eg, verbalizing salient emotional features).7 SCT can be divided into 2 categories: targeted interventions, focusing on 1 or 2 specific domains of social cognition (eg, Training of Affect Recognition8) and broad-based SCT, targeting most or all domains of social cognition (eg, Social Cognition & Interaction Training9). Furthermore, some SCTs (eg, Cognitive Enhancement Training10) combine SCT with “Cognitive Remediation Therapy” (CRT), as improvement of neurocognition could provide an important foundation for social cognitive improvement.11
A meta-analysis (k = 19, n = 692) aggregating all forms of SCT,12 found a moderate to large effect on emotion perception (d = 0.71–1.01), ToM (d = 0.46), social functioning (d = 0.78), and psychotic symptoms (d = 0.68), but no effect on social perception and attribution. Other systematic and narrative reviews6,13–17 have also demonstrated the efficacy of SCT, but important questions have remained unanswered.6
First, the efficacy of different SCT types has never been compared meta-analytically, while interventions differ considerably. Grant et al16 systematically reviewed targeted and broad-based SCT, and found an improvement in most outcome domains, irrespective of treatment type. A small (k = 8, n = 300) meta-analysis18 on targeted SCT found large improvements in emotion perception (g = 1.26) and social functioning (k = 3, g = .98). Finally, Kurtz et al19 meta-analyzed broad-based SCT without CRT (k = 16, n = 313) and found moderate to large effects on social cognition (d = 0.40–.1.29). To summarize, while there is evidence for the efficacy of different SCT types, a direct, quantitative comparison has not yet been made.
Second, given the variety in the results of SCT studies, it is likely that treatment outcomes are affected by moderating variables. Only a single study has investigated moderators of treatment outcome in SCT6: Kurtz and Richardson12 found in their meta-analysis that treatment outcomes were moderated by several variables (eg, sample age, education level, illness duration and medication dose, duration of treatment). Since then, several new randomized controlled trials have been published (eg, ref.20–22). Since the optimal parameters of SCT, and whom it benefits, are still largely unknown, it is important to investigate moderators of SCT outcome.
Third, it remains unclear whether gains from SCT are sustained after treatment; reviews indicate mostly positive, but mixed follow-up results.6,14 Long-term effects of SCT have never been meta-analyzed. In sum, there have been several reviews and meta-analyses addressing the effects of SCT, but key questions remain, particularly regarding what is effective, for whom, and for how long.6 From the multitude of existing approaches, we do not know how each one affects different domains of social cognition; previous reviews and meta-analyses have lumped all forms of SCT together,12 investigated only one type of SCT,18,19 or used only qualitative methods.14–17,23 Therefore, this meta-analysis will investigate the following questions:
What effects do different types of SCT have on social cognition and measures of generalization (ie, social functioning and psychiatric symptoms)?
Which characteristics of treatments/studies and participants moderate the effects of SCT?
Are treatment effects of SCT durable (ie, do they persist at follow-up)?
Methods
Systematic Search
In December 2016, PsycInfo and Medline, PubMed, PiCarta, Embase and Web of Science were searched for relevant publications. This search was updated in December 2017 and December 2018. PRISMA guidelines24 were followed. The following string was used: (“social cogn* training” OR “social cogn* rehab*” OR “social cogn* remed*” OR “cognitive remediation” OR “cogn* rehab*”) AND (“social cogn*” OR “social functioning” OR “emotion recognition” OR “theory of mind”) AND (psycho* OR schizophren*). Specific interventions (supplementary materials) were searched for using the string “[intervention name]” AND (psychot* OR psychos* OR schizophren*). Reference lists of relevant publications were checked to identify any missing studies. No specific time range was used. Eligibility was assessed by 2 independent raters (S.A.N. and E.C.D.vdS.) in 3 rounds: titles, abstracts, and full texts. In case of disagreement, publications were reexamined to reach consensus. The following inclusion criteria were applied:
• Randomized Controlled Trial.
• Published in a peer-reviewed journal.
• Conducted in a sample of people with a psychotic disorder.
• Document a form of SCT.
• Report at least one outcome domain of social cognition.
• Report quantitative information (eg, means, SDs) from which effect sizes can be derived. If these were unreported, but measured, authors were contacted to request the missing data.
• Written in English.
Quality Assessment
The methodological quality of the studies was appraised using the Clinical Trials Assessment Measure for Psychological Treatments (CTAM25). This instrument has 15 items grouped in 6 categories: sample size and recruitment method, treatment allocation methods, outcome assessment methods, types of control groups, description of treatment, and statistical methodology. CTAM scores were extracted by 2 raters (S.A.N. and E.C.D.vdS.). In case of discrepancies, publications were reviewed to reach consensus.
Data Extraction
Data on the following characteristics were extracted: (1) Sample characteristics; (2) Intervention type and characteristics; (3) Means and SDs or other statistical parameters (eg, F-statistic) of outcome measures.
Sample characteristics reported separately per group were aggregated using a weighted average and SD. The primary outcome variables were the standardized mean difference in social cognition, social functioning and psychiatric symptoms after SCT; specifically, emotion perception, social perception, ToM, attribution style, miscellaneous measures of social cognition (measures that did not fit one specific social-cognitive domain, were comprised of multiple domains, or were reported as a composite), social functioning (defined as an individual’s ability to fulfill societal roles,26 including functional capacity and functioning in work, interpersonal relationships and self-care26,27), and psychiatric symptoms (ie, total symptom levels or a composite of positive, negative and general symptoms).
If multiple outcome measures were reported for the same domain, the measure with the highest reported psychometric quality26–28 was prioritized for the effect size calculations (supplementary table A1). Outcome measures were assigned to outcome domains following previous reviews and meta-analyses.12,19,26,27
Effect of Different Types of SCT: Network Meta-analysis
To evaluate the effects of different SCT types, network meta-analyses were performed using the “netmeta” package in R,29 because conventional (pairwise) meta-analysis can only compare 2 treatments at the same time: experimental vs control. While calculating the treatment effect, one is tied to the original comparisons in the literature, even if a treatment is an experimental treatment in one study and the control treatment in another. This means that, if 2 treatments were not directly compared by any studies, it is impossible to draw conclusions about their relative efficacy.
Network meta-analysis, however, allows for comparison of any pair, even those that were never compared directly, and all interventions at the same time, because it uses the network of evidence to compare treatments.30 The assumption is as follows: if treatment A is more effective than treatment C, and treatment C is more effective than treatment B, we can deduct that treatment A is more effective than treatment B, even if A and B have never been compared directly—because they were both compared to C.31 Thus, if µ denotes the estimate of the treatment effect, one estimates by inference that µAB = µAC − µBC.
To estimate effect sizes, both direct evidence (original comparisons) and indirect evidence (deducted comparisons) are used. Thus, rather than being forced to classify treatments as “experimental” or “control,” we can choose any pair of interventions (eg, broad-based SCT vs treatment as usual, or TAU) and compare them, using: (1) all studies directly comparing broad-based SCT and TAU: the direct evidence; and (2) the other treatment comparisons in the network (eg, TAU vs targeted SCT, targeted SCT vs broad-based SCT), to deduct the treatment effect: the indirect evidence.
Network meta-analysis, therefore, has the benefit of using available data much more effectively, since all interventions and control conditions can be compared. Different types of treatments do not need to be aggregated or evaluated in subgroup analyses, but can be evaluated simultaneously, as a network. Moreover, all treatments from multi-arm studies can be used, instead of being forced to choose one pair or to combine groups, as one would be in pairwise meta-analysis.32
First, treatments were divided across 6 treatment types, defined in table 1, rated independently by 2 raters (S.A.N. and G.H.M.P.) and if necessary, reexamined to reach consensus. Next, for each treatment arm, a (within-group) pre-post effect size was calculated. Next, pairwise comparisons were calculated for each combination of study arms (direct evidence). Next, direct evidence and indirect evidence were combined in an arm-based random-effects network meta-analysis. We chose TAU as the reference group in the network meta-analysis, as TAU represents the effect of no additional intervention. All other treatments (including active control, reflecting the effect of providing any nonspecific intervention) were compared to TAU; thus, all effect sizes reported represent the effect of that intervention vs TAU. The netmeta package then calculated the indirect treatment comparisons and took these into account, as well as the direct evidence. The outcome statistic used was Cohen’s d on social cognition, social functioning, and psychiatric symptoms for each study arm. We evaluated the consistency assumption of network meta-analysis by examining Q and I2 parameters and their significance.
Table 1.
Treatment Type Definitions
| Type | Social Cognition Trained? | Neurocognition Trained? | Treatments in Category |
|---|---|---|---|
| Treatment as Usual (TAU) | No | No | 18 |
| Active Control Condition (ACC)a | No | In some cases (cf. table 2). | 26 |
| SCT – Targeted (SCTT)b | Yes, 1 or 2 domains | No | 14 |
| SCT – Targeted with CRT (SCTT+)b | Yes, 1 or 2 domains | Yes | 9 |
| SCT – Broad-based (SCTB) | Yes, >2 domains | No | 14 |
| SCT – Broad-based with CRT (SCTB+) | Yes, >2 domains | Yes | 10 |
Note: SCT, Social Cognition Training.
aIn 7 studies, Cognitive Remediation Therapy (CRT) was used as a control group. Since it is an active form of treatment that does not explicitly target social cognition, it was classified as an active control group. To investigate a potential treatment effect of CRT, we added the use of CRT as a variable to the moderator analyses.
bWe defined targeted treatments as those targeting 1 or 2 domains of social cognition, as there were several treatments (eg, training of affect recognition8) that predominantly targeted a single domain, but also included some training of a second domain, and therefore were not as comprehensive as many of the treatments classified as “broad-based.”
Moderators of Treatment Effect
As treatment/study characteristics, we evaluated methodological quality (CTAM score), total time (in hours) of the intervention, use of groups, type of outcome measure (static stimuli, eg, pictures, or dynamic stimuli; eg, videos) and inclusion of CRT. We also examined mean participant characteristics (age, mean illness duration, mean years of education, mean medication dose, and percentage of male participants) as moderators.
Moderators were evaluated for each outcome domain with a random-effects meta-regression model using the “metafor” R package.73 Due to the high likelihood of spurious results in meta-regression, permutation tests were used to correct coefficients and P-values.74,75 Permutation tests work by permuting each row of data to calculate the statistical model, and comparing these random models to the unpermuted, original model.75 Many of the moderator variables had missing data. Since only studies with complete data on each moderator could be used in the models, resulting in a substantial loss of statistical power and data, moderator analyses for participant characteristics were first conducted univariately and corrected with permutation tests. Univariately significant moderators were added to a meta-regression model with multiple predictors and corrected with permutation tests.
Durability/Long-term Efficacy
To analyze the effect of SCT at follow-up, a random-effects multivariate multilevel model was utilized, analyzing all outcome domains simultaneously, using the Metafor R package.73 The outcome statistic was the overall Cohen’s d of the experimental group vs the control group for each study and domain. Long-term outcome was defined as a follow-up period of ≥3 months. Since there was insufficient data to meta-analyze long-term social cognition outcome, we evaluated available effect sizes individually for each study.
Calculations
Effect Sizes
A within-group standardized mean difference between pre- and post-treatment scores was obtained for each outcome by calculating Cohen’s d for each group76,77: (posttreatment analysis), and (follow-up analysis). The overall Cohen’s d was computed by subtracting the effect size of each pair of treatment arms: . For interpretation of effect sizes, we followed convention,78 classifying <|.2| as very small, |.2|–|.5| as small, |.5|–|.8| as moderate, and >|.8| as large.
Standard Errors
A standard error of Cohen’s d was computed for each arm76: , in which n refers to the number of participants in each group, r refers to the correlation between measurements, and d refers to the effect size for that group. Since pre-post treatment correlations were not available for individual study and outcome measures, an r of .7 was assumed, following other meta-analyses.79–81 To ensure this assumption did not have meaningful consequences for our conclusions, we conducted sensitivity analyses with correlations of .3 and .9; results can be found in the supplementary figures A10 and A11.
Variance and Covariance
For the moderator analyses and the follow-up analyses, sampling variance was computed for each overall effect size76: , in which nt and nc refer to the number of participants in the experimental and control groups (respectively), and dj stands for the overall Cohen’s d.
For the follow-up analysis, covariance between outcome variables was calculated76: , in which dj and dj* refer to overall Cohen’s d outcome domains constitutes the estimated correlation between 2 domains. was estimated by using correlations reported in the literature,82,83 extracting recommended measures.27,84
Results
Search Results
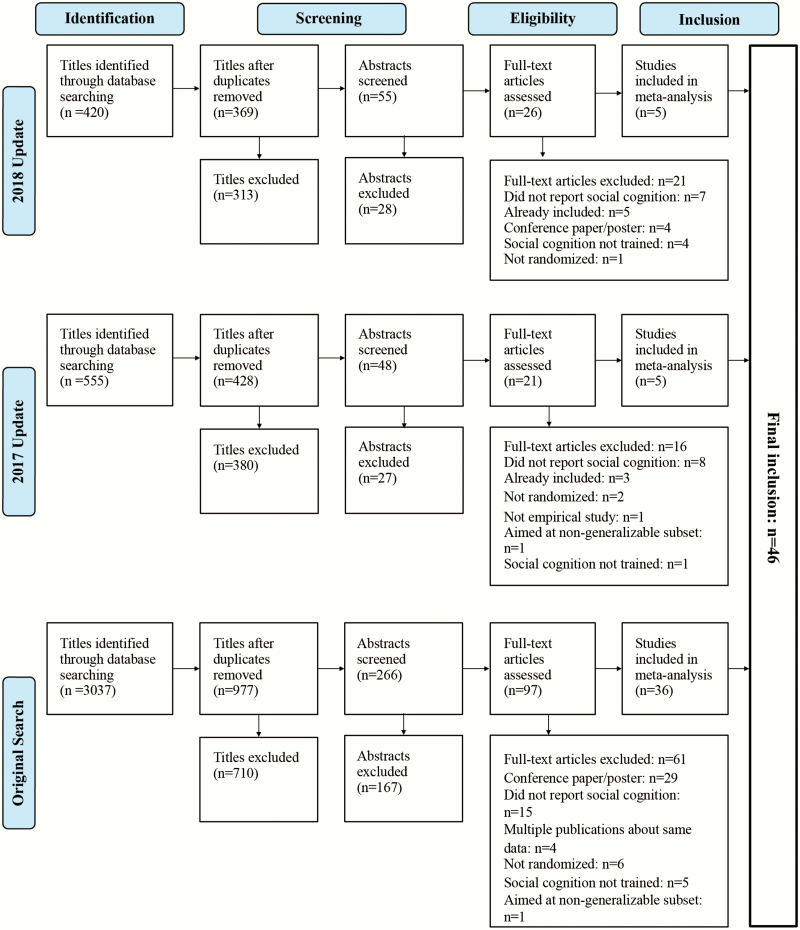
The results of the search are presented in a PRISMA flow chart (figure 1).
Fig. 1.
PRISMA flow chart.
Characteristics of the Sample
The included studies are summarized in table 2. Two48,85 of the 46 studies concerned a follow-up publication to an original study10,38 that was also included. A total of 1979 participants were included in the meta-analytic sample (n = 1290 male, n = 627 female; 6 studies, total n = 164, did not report gender distribution). The weighted average age of the total sample was 37.5 years (SD = 5.3, k = 44, range = 24.6–51.1). The most common diagnosis was schizophrenia (k = 29, n = 1075) and schizophrenia/schizoaffective disorder (k = 11, n = 664), followed by all psychotic disorders (k = 3, n = 182) and early/first-episode psychosis (k = 1, n = 58). Most studies recruited only outpatients (k = 22, n = 1129), 7 studies recruited inpatients (n = 226), and 10 studies (n = 470) recruited both inpatients and outpatients. Five studies (n = 154) did not report hospitalization status. The weighted average illness duration was 13.3 years (SD = 5.8; k = 33, range = 0.5–25.7), and the average medication dose was 488.8 chlorpromazine equivalents (SD = 133.6, k = 20, range = 180.5–654.8). The weighted average number of years of education was 11.5 years (SD = 1.5, k = 29, range = 9.0–14.4).
Table 2.
Included Publications
| Sample Characteristics | Intervention (+type) | Intervention Characteristics | Control (+type) | Follow-up (mo) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | CTAM | Participants | Age (M, SD) | % Male | Years of Education (M, SD) | Medication in CPZ Equivalents (M, SD) | Illness Duration in Years (M, SD) | ||||
| Aloi et al33 | 32 | SZ, inpatients, n = 41 | 51.07 (9.79) | 70.7 | NR | 652.39 (458.51) | 23.14 (2.95) | CRT + Integrated Psychological Therapy (SCTB+) | 46 2h sessions for 12w, 4× per week. Individual. | TAU (TAU) | - |
| Bechi et al34 | 45 | SZ, outpatients, n = 52 | 38.6 (9.60) | 67.3 | 11.4 (3.10) | NR | 15.2 (7.99) | 1. CRT + Video-Based Emotion Processing & ToM Training (SCTT+) 2. CRT+ Integrated Psychological Therapy (SCTB+) | 12 1h sessions for 12w, 1× per week. Group (5pp). | CRT + No Treatment (ACC) | - |
| Bechi et al35 | 52 | SZ, outpatients, n = 75 | 38.8 (10.89) | 57.3 | NR | NR | 15.2 (9.05) | 1. CRT + Video-based SCT (SCTT+); 2. CRT + Theory of mind intervention (SCTT+) | 18 1h sessions for 9w, 2× per week. Group (5pp). | CRT+ Newspaper discussion (ACC) | - |
| Choi and Kwon36 | 35 | SZ and SA, NR, n = 34 | 32.5 (6.96) | 55.9 | 12.4 (2.37) | NR | 11.2 (5.86) | Social Cognition Enhancement Training (SCTB) | 36 1.5h sessions for 18w, 2× per week. Group size NR. | TAU (TAU) | - |
| Combs et al37 | 60 | SZ, inpatients, n = 60 | 38.7 (13.70) | 65 | 12.1 (2.70) | 646.2 (435.00) | 14.6 (12.40) | Attentional Shaping (SCTT) | Single session, duration NR. Individual. | Repeated Practice (ACC) | .23 (1 wk) |
| Eack et al38,39 | 67 | Early psychosis, outpatients, n = 58 | 25.9 (6.31) | 69.0 | NR | NR | 3.2 (2.24) | Cognitive Enhancement Training (SCTB+) | 55 ±1.5h sessions, once per week for ±1 year. Group size NR. | Enriched Supportive Therapy (ACC) | 12 |
| Fernandez-Gonzalo et al40 | 42 | SZ and SA, outpatients, n = 53 | 30.5 (6.60) | 64.2 | 12.1 (3.53) | 257.6 (197.23) | 2.6 (1.47) | Neuro Personal Trainer-Mental Health (SCTB+) | Session number NR, 1-h sessions, 2× per week for 4–5 mo. Group size NR. | Nonspecific computer training (ACC) | - |
| Fisher et al41 | 64 | Psychosis spectrum, outpatients, n = 111 | 43.25 (12.83) | 71.2 | 13.69 (2.24) | NR | NR | Targeted Cognitive Training + SocialVille (SCTT+) | 70 1h sessions, 5× per week, for 14w. Individual. | Targeted Cognitive Training (ACC) | - |
| García et al42 | 30 | Chronic SZ, NR, n = 20 | 38.8 (7.58) | 70 | NR | NR | 18.2 (NR) | Integrated Psychological Therapy (Social Perception) (SCTT) | 21 ± 1h sessions, 2× per week for 9 mo. Group (6pp). | Matched Control (TAU) | - |
| Gaudelus et al43 | 56 | SZ, inpatients and outpatients, n = 33 | 32.67 (SD NR) | 75.8 | NR | 357.7 (SD NR) | NR | GAÏA (SCTT) | 30 60m sessions, 3× per week, for 10w. Individual. | RECOS (ACC) | 6, but follow-up scores NR |
| Gil Sanz et al44 | 24 | SZ, outpatients, n = 14 | 37.4 (9.37) | 50 | 12.9 (2.57) | NR | NR | Social Cognition Training Program (SCTT), uses Social Perception module of IPT | 20 45m sessions, 2× per week, for 10w. Group size NR. | TAU (TAU) | - |
| Gil-Sanz et al45 | 41 | SZ, NR, n = 44 | 40.4 (8.86) | 54.6 | NR | NR | 14.2 (9.21) | Social Cognition Training Program (SCTB) | 28 45m sessions, 1× per week, for 28w. Individual. | Cognitive training (ACC) | - |
| Gohar et al46 | 49 | SZ and SA, outpatients, n = 42 | 31.9 (10.65) | 81.0 | 12.4 (2.09) | NR | 10.2 (9.13) | Social Cognitive Skills Training (SCTB) | 16 1h sessions, 2× per week, for 8w. Group (8pp). | Format- and time-matched illness management (ACC) | - |
| Gordon et al22 | 43 | SZ spectrum, status NR, n = 36 | 35.5 (10.88) | NR | NR | NR | 9.81 (8.64) | Social Cognition & Interaction Training (SCTB) | 20 1h sessions, 2× per week, for 10w. Group size NR. | Treatment as Usual (TAU) | 3–6, only SCIT |
| Habel et al47 | 45 | SZ, NR, n = 20 | 32.6 (9.16) | 100 | NR | NR | NR | Training of Affect Recognition (SCTT) | 12 45m sessions, 2× per week for 6w. Group (2pp). | TAU (TAU) | - |
| Hogarty et al10,48 | 62 | SZ and SA, outpatients, n = 121 | 37.3 (8.90) | 58.7 | NR | NR | 15.7 (9.30) | Cognitive Enhancement Training (SCTB+) | ±56 ±1.5h sessions, once per week for ± 1 year. Group (6pp). | Enriched Supportive Therapy (ACC) | 12 |
| Hooker et al49 | 42 | SZ, outpatients, n = 22 | 46.1 (8.77) | 81.8 | 13.3 (2.34) | 311.8 (396.82) | 24.3 (10.55) | Auditory-based Cognitive Training + Micro-Expression Training Tool (SCTT+) | ±42 ±70m sessions, 5× per week for 10w. Individual. | Computer Games (ACC) | - |
| Horan et al50 | 49 | SZ and SA, outpatients, n = 31 | 48.2 (7.05) | 93.5 | 12.3 (.89) | NR | 19.1 (9.96) | Social Cognitive Skills Training (SCTB) | 12 1h sessions, 2× per week for 12w. Group (6pp). | Illness Management (ACC) | - |
| Horan et al51 | 62 | SZ, SA, delusional disorder, psychosis NOS, outpatients, n = 68 | 47.8 (9.88) | 85.7 | 12.8 (1.77) | NR | 25.7 (9.02) | 1. Social Cognitive Skills Training (SCTB); 2. CRT + Social Cognitive Skills Training (SCTB+); | 24 1h sessions, 2× per week for 12w. Group (8pp). | Ilness Management (ACC) Study also included CRT group (excluded here) | - |
| Kayser et al52 | 27 | SZ, outpatients, n = 14 | 34.9 (9.47) | 78.6 | 10.5 (3.05) | 239.6 (239.93) | NR | ToM Rehabilitation (SCTT) | 2 1h sessions, 2× in 1w. Individual. | TAU (TAU) | - |
| Lindenmayer et al53 | 69 | SZ and SA (duration >5 y), inpatients and outpatients, n = 59 | 43.3 (10.18) | 81.4 | 9.0 (3.68) | NR | 24.7 (10.85) | CRT + MindReading (SCTT+) | 36 1h sessions, 3× per week for 12w. Individual. | CRT (ACC) | - |
| Lindenmayer et al54 | 66 | SZ and SA, inpatients and outpatients, n = 78 | 41.85 (11.61) | 71.79 | 8.99 (2.65) | 491.41 (110.75) | 14.78 (9.06) | CogPack / BrainFitness + MindReading (SCTT+) | 36 50m sessions, 3× per week for 12w. Group (8–10pp). | CogPack / BrainFitness (ACC) | - |
| Maroño Souto et al21 | 50 | SZ, outpatients, n = 60 | 39.17 (7.03) | 78.3 | NR | 599.78 (433.00) | 14.95 (7.18) | e-Motional Training (SCTB) | 12 1h sessions, 1× per week for 12w. Individual. | TAU (TAU) | - |
| Mazza et al55 | 42 | SZ, outpatients, n = 32 | 24.5 (2.12) | 40.6 | 11.4 (2.32) | 654.8 (513.20) | .5 (.28) | Emotion and ToM Imitation Training (SCTT) | 24 50m sessions, 2× per week for 12w. Group size NR. | Problem Solving Group (ACC) | - |
| Mueller et al20 | 84 | SZ and SA, outpatients, n = 156 | 34.2 (8.58) | 69.2 | 11.0 (4.24) | 438.5 (400.92) | 10.1 (7.24) | Integrated Neurocognitive Therapy (SCTB+) | 30 1.5h sessions, 2× per week for 15w. Group (8pp). | TAU (TAU) | 9 |
| Palumbo et al56 | 51 | SZ and SA, outpatients, n = 10 | 36.83 (9.28) | 40 | 13.35 (2.45) | NR | NR | Social Cognition Individualized Activities Lab (SCTB+) | 40 80m sessions (2× per week for 20w). Group size NR. | Social Skills & Neurocognitive Individualized Training (ACC) | - |
| Peña et al57 | 71 | SZ, inpatients and outpatients, n = 101 | 39.03 (9.80) | 72.3 | 10.4 (3.15) | NR | NR | REHACOP (SCTB+) | 39 90m sessions (3× per week for 13w). Group size NR. | Group activities (ACC) | - |
| Penn and Combs58 | 46 | SZ and SA, inpatients, n = 40 | 38.8 (8.06) | 57.5 | 11.5 (2.01) | NR | 17.1 (9.25) | Mimicry (SCTT) | Single session, duration NR. Individual. | Repeated Exposure (ACC) | 0.23 (1 wk) |
| Pino et al59 | 42 | SZ, outpatients, n = 14 | 43.6 (12.85) | 50 | 9.9 (2.76) | 654.8 (513.20) | 15.8 (10.41) | Emotion and ToM Imitation Training (SCTT) | 24 50m sessions, 2× per week for 12w. Group size NR. | Problem Solving Therapy | - |
| Popova et al60 | 45 | Paranoid-hallucinatory SZ, inpatients, n = 38 | 37.8 (8.30) | 60.5 | 11.2 (1.71) | (438.5) | NR | Facial Affect Recognition Training (SCTT+); | 20 1h sessions, 5× per week for 4w. Individual. | TAU (TAU) Study also included CRT group (excluded here) | - |
| Rakitzi et al61 | 52 | SZ, inpatients and outpatients, n = 48 | 32.55 (7) | 66.7 | NR | 527.1 (369.8) | 5.65 (1.22) | Integrated Psychological Therapy (SCTB+) | 20 1h sessions, 2× per week for 10w. Group (8pp) | TAU (TAU) | 3 |
| Roberts et al9 | 72 | SZ and SA, outpatients, | 39.7 (11.44) | 66.7 | NR | 632.6 (534.18) | 16.8 (8.04) | Social Cognition and Interaction Training (SCTB) | 24 1h sessions, 1× per week for 24w. Group (8pp). | TAU (TAU) | 3 |
| Roncone et al62 | 47 | Residual type SZ, inpatients, n = 20 | 33.7 (NR) | 65 | 11.5 (2.65) | 361.2 (203.34) | 14.0 (7.88) | ToM Training (SCTT) | 22 1h sessions, 1× per week for 22w. Group (10pp). | TAU (TAU) | - |
| Russell et al63 | 44 | SZ and SA, outpatients, | 41.4 (9.74) | 67.5 | 14.4 (3.00) | NR | 15.6 (8.19) | Micro-Expression Training Tool (SCTT) | Single 30m session. Individual. | Repeated Exposure (ACC) | - |
| Sachs et al64 | 32 | SZ, inpatients and outpatients, n = 38 | 29.3 (8.47) | 52.6 | 14.2 (5.19) | NR | 5.0 (8.43) | Training of Affect Recognition (SCTT) | 12 45m sessions, 2× per week for 6w. Individual. | TAU (TAU) | - |
| Sevos et al65 | 32 | SZ, inpatients and outpatients, n = 31 | 41.20 (8.36) | NR | 10.80 (2.68) | NR | 15.06 (7.71) | Cinemotion (SCTT) | 10 90m sessions, 1× per week for 10w. Group (3-7pp). | TAU (TAU) | - |
| Tas et al66 | 67 | SZ, outpatients, n = 45 | 34.1 (10.62) | 48.9 | 11.1 (2.92) | 489.6 (340.40) | 12.2 (9.18) | Family Social Cognition and Interaction Training (SCTB) | 14 80m sessions, 1× per week for 14w. Group size NR. | Social Stimulation (ACC) | - |
| Taylor et al67 | 42 | SZ spectrum, inpatients, n = 36 | 40.08 (10.42) | NR | 10.13 (2.34) | NR | NR | Social Cognition & Interaction Training (SCTB) | 16 45m sessions (2× per week, for 8w), group size NR. | Treatment as Usual (TAU) | - |
| Tsotsi et al68 | 42 | SZ, outpatients, n = 27 | 32.54 (6.69) | NR | 11.97 (2.18) | 505.23 (251.20) | 5.24 (3.5) | Facial Affect Recognition Intervention (SCTT) | Single 30m session. Individual. | Attention to Facial Features (ACC) | - |
| van der Gaag et al69 | 48 | SZ, inpatients, n = 42 | 31.1 (7.93) | 64.3 | NR | 627.5 (510.88) | 9.8 (6.96) | CRT + Social Cognitive Remediation (SCTB+) | 22 20m sessions, 3× per week over period of 8w. Group size NR. | TAU (TAU) | - |
| Veltro et al70 | 52 | SZ, outpatients, n = 24 | 38.4 (8.81) | NR | 11.3 (3.29) | 180.5 (148.92) | 13.0 (8.08) | Cognitive-Emotional Rehabilitation (SCTB) | 24 90m sessions, 1× per week for 24w. Group (6pp). | Problem Solving Therapy (ACC) | - |
| Wang et al71 | 58 | SZ, outpatients, n = 39 | 42.6 (10.98) | 51.3 | 10.4 (2.40) | 308.6 (174.12) | NR | Social Cognition and Interaction Training (SCTB) | 20 sessions, 1× per week for 20w. Session duration NR. Group (8pp). | TAU (TAU) | 6, but posttreatment NR |
| Wölwer et al8 | 45 | SZ, outpatients, n = 77 | 34.3 (10.01) | 77.9 | NR | NR | NR | Training of Affect Recognition (SCTT) | 12 45m sessions, 2× per week for 6w. Group (6pp). | TAU (TAU) Study also included CRT group (excluded here) | - |
| Wölwer and Frommann72 | 59 | SZ, inpatients, n = 38 | 37.7 (13.10) | 68.4 | NR | NR | NR | Training of Affect Recognition (SCTT) | 12 45m sessions, 2× per week for 6w. Group (6pp). | CRT (ACC) | - |
Note: [number]h = [number] hours; [number]m = [number] months; NR = Not reported; pp = participants; SA=Schizoaffective, SCT = Social Cognition Training; SZ= schizophrenia; [number]w = [number] weeks. Intervention abbreviations: ACC = Active Control (Group); CRT = Cognitive Remediation Therapy; SCTT = Targeted Social Cognition Training; SCTT+ = Targeted SCT with CRT; SCTB = Broad-based Social Cognition Training; SCTB+ = Broad-based Social Cognition Training with CRT.
Methodological Quality
The median CTAM score was 47.5 (Q1: 42, Q3: 58.25). The intra-class correlation of the ratings was .84, indicating good to excellent reliability. Six9,20,53,54,66,86 of the 44 original publications (13%) were of adequate methodological quality (defined as CTAM ≥65). All studies used a convenience sample (eg, clinic attenders, referred patients). Fifteen studies (34.1%) reported the method of randomization of which 5 (11.4%) reported randomization conducted by someone independent from the research team. Independent assessors were used by 20 (45.5%) studies. Seventeen (38.6%) studies reported using blinded raters, but only one (2.3%) described the blinding process and only 2 studies (4.5%) verified rater blindness. Twenty-seven studies (61.4%) used an active control group, 6 (13.6%) of which also used an additional TAU group. Six studies (13.6%) conducted an intention-to-treat analysis; 5 (11.4%) studies had adequate handling (eg, multiple imputation) of dropout over 15%. Use of a treatment protocol was explicitly reported in 28 studies (63.6%), 9 (20.5%) of which also assessed protocol fidelity.
Effects of SCT (vs TAU): Posttreatment
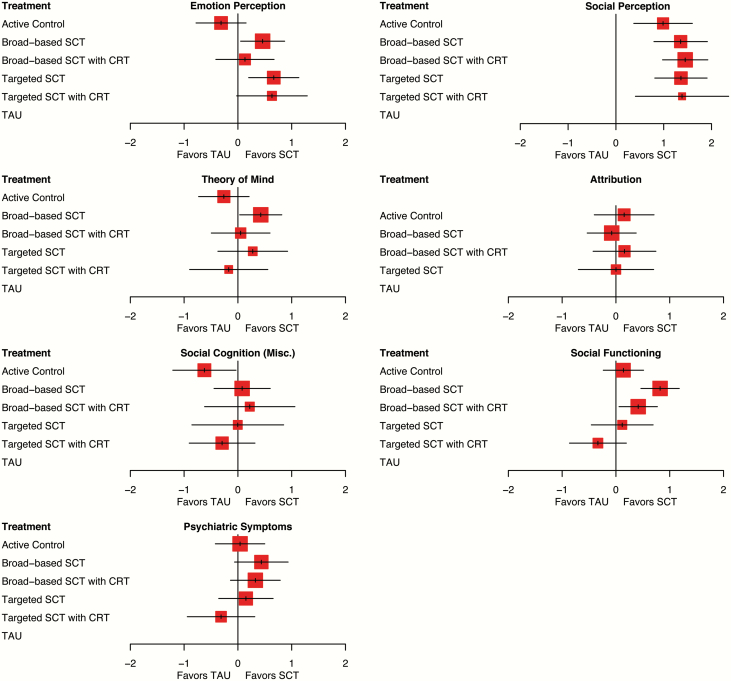
The results of the network meta-analyses are shown in table 3. Forest plots can be found in figure 2. Network graphs and funnel plots are included in the supplementary figures A1–A8. None of the funnel plots showed a statistically significant rank correlation, except for psychiatric symptoms (z = −2.01, P = .045). Heterogeneity was very high in all analyses (I2 ranged between 99.8 and 100%), indicating considerable inconsistency in the network of evidence.
Table 3.
Effect of Different Types of SCT vs Treatment as Usual on Social Cognition, Social Functioning and Psychiatric Symptoms at Posttreatment
| k | m | I 2 (%) | Cohen’s d [95% Confidence Interval] | |||||
|---|---|---|---|---|---|---|---|---|
| Outcome | ACC | SCTT | SCTT+ | SCTB | SCTB+ | |||
| Emotion perception | 31 | 33 | 99.9 | −0.29* [−0.59, −0.00] | 0.68* [0.38, 0.97] | 0.29 [−0.14, 0.72] | 0.46* [0.21, 0.72] | −0.09 [−0.25, 0.45] |
| Social perception | 9 | 11 | 99.8 | 0.98* [0.37, 1.60] | 1.36* [0.81, 1.91] | 1.38* [0.41, 2.36] | 1.35* [0.80, 1.60] | 1.45* [0.98, 1.92] |
| Theory of mind | 22 | 24 | 99.9 | −0.26 [−0.73, 0.21] | 0.28 [−0.38, 0.92] | −0.17 [−0.90, 0.56] | 0.42* [0.03, 0.82] | 0.05 [−0.49, 0.60] |
| Attribution style | 11 | 13 | 100 | 0.15 [−0.40, 0.71] | 0.00 [−0.70, 0.70] | N/A | −0.08 [−0.53, 0.38] | 0.16 [−0.42, 0.74] |
| Social cognition, miscellaneous | 13 | 13 | 100 | −0.62* [−1.21, −0.04] | −0.00 [−0.86, 0.85] | −0.29 [−0.90, 0.32] | 0.08 [−0.44, 0.60] | 0.22 [−0.62, 1.06] |
| Social functioning | 25 | 27 | 100 | 0.14 [−0.24, 0.51] | 0.12 [−0.46, 0.69] | −0.34 [−0.86, 0.19] | 0.82* [0.46, 1.18] | 0.41* [0.06, 0.77] |
| Psychiatric symptoms | 24 | 26 | 100 | 0.04 [−0.42, 0.50] | 0.15 [−0.36, 0.65] | −0.31 [−0.94, 0.31] | 0.44 [−0.06, 0.93] | 0.32 [−0.14, 0.77] |
Note: k, number of studies; m, number of pairwise comparisons; ACC, Active control group; CRT, Cognitive remediation therapy; SCTT, Targeted social cognition training (without CRT); SCTT+, Targeted social cognition training (with CRT); SCTB, Broad-based social cognition training (without CRT); SCTB+, Broad-based social cognition training (with CRT).
*Significant at α = .05.
Fig. 2.
Forest plots of all treatment types, compared using network meta-analysis to TAU, showing effect sizes on social cognition, social functioning and psychiatric symptoms at posttreatment.
Compared to TAU, targeted SCT (without CRT) had a moderate effect on emotion perception (d = 0.68). Broad-based SCT (without CRT) was also significantly more effective than TAU (d = 0.46). Other types of treatment did not have a significant effect on emotion perception in comparison with TAU.
Both targeted (with and without CRT, d = 1.38 and d = 1.36) and broad-based SCT (with and without CRT, d = 1.45 and d = 1.35) had very large effects on social perception, compared to TAU. Interestingly, active control groups were also significantly more effective than TAU (d = 0.98).
For ToM, however, only broad-based SCT without CRT (d = 0.42) had a small to moderate, significant effect, compared to TAU. Other types did not have a significantly larger effect than TAU. No significant effect sizes were found for attribution and miscellaneous measures of social cognition. For social cognition (misc.), active control groups performed significantly worse than TAU (d = −0.62).
Only broad-based SCT with (d = 0.41) and without (d = 0.82) CRT had a significantly larger effect on social functioning than TAU. Finally, none of the treatments had a significantly greater effect on psychiatric symptoms than TAU.
Moderators: Characteristics of Treatments and Study Samples
Full results of the moderator analyses are provided in the supplementary table A3. The majority of studies used static outcome measures; therefore, the effect of type of outcome measure could only be examined for ToM (static k = 18, dynamic k = 4).
Group treatments performed significantly worse for emotion perception (b = −0.74, SE = 0.27, P = .009). Other treatment and participant characteristics did not significantly moderate the effect size. For social perception, no predictors were significant; however, the coefficient of the use of CRT was notably large (b = 2.68, SE = 1.08, P = .140) and trended towards significance.
For ToM, none of variables moderated effect sizes. Treatment characteristics did not significantly moderate the effect on attribution, although higher medication doses trended towards larger effects on attribution (b = 0.02, SE = 0.00, P = .058). For social cognition (miscellaneous), the total time of the intervention was associated with larger effects for longer treatments (b = 0.02, SE = 0.00, P = .005). Participant characteristics did not moderate effect sizes for miscellaneous measures of social cognition.
For functioning, group treatments were significantly more effective (b = 0.53, SE = 0.53, P = .029). The percentage of male participants showed a trend, with higher percentages predicting smaller effects (b = −0.05, SE = 0.02, P = .060). Treatment characteristics did not moderate the effect on psychiatric symptoms, although longer treatments trended towards smaller effects (b = −0.02, SE = 0.01, P = .063). In univariate analyses, effects on symptoms were significantly associated with age (b = −0.06, SE = 0.02, p = .005), illness duration (b = −0.03, SE = 0.01, P = .001) and percentage of male participants (b = −0.03, SE = 0.01, P = .019). In a multivariate model, only the percentage of male participants remained a significant predictor (b = −0.02, SE = 0.01, P = .002).
Effects of SCT (vs Control): Durability
Follow-up data were available from 7 studies.9,20,37,39,48,58,87 Two studies37,58 were excluded because the follow-up period was only 1 week. The length of follow-up of the remaining studies ranged between 3–12 months, with an average of 7.8 months. Social cognition domains (emotion perception, social perception, ToM, attribution, and miscellaneous measures of social cognition) had insufficient follow-up data (k<3) to be analyzed and were therefore reviewed individually.
At follow-up, a statistically significant effect size was found for social functioning (k = 5, d = 0.66, P < .001, 95% CI = [0.27, 1.04]), but not for psychiatric symptoms (k = 4, d = −0.15, P = .587, 95% CI = [−0.71, 0.40]). Residual heterogeneity was high and statistically significant (QE(7) = 39.3, P < .001; social functioning I2 = 73.9%; psychiatric symptoms I2 = 84.8%), indicating inconsistency in treatment effects. There was no evidence of publication bias in the funnel plot (supplementary figure A9) or funnel plot asymmetry (Kendall’s tau = .00, P = 1.00).
For emotion perception, small effects were found at follow-up (Integrative Neurocognitive Therapy or INT,20 SCTB+, d = 0.28 and Social Cognition and Interaction Training or SCIT,9 SCTB, d = 0.22). Both studies on social perception (INT,20 SCTB+, d = 0.02, and integrated psychological therapy,87 SCTB+, d = 4.32) found an effect. A moderate effect on ToM was found (SCIT,9 SCTB, d = 0.50). For attribution style, small (INT,20 SCTB+, d = 0.28) and very small (SCIT,9 SCTB, d = 0.18) improvements were demonstrated. For social cognition (misc.), a very small (SCIT,9 SCTB, d = 0.14) and large (Cognitive Enhancement Therapy,10,48 SCTB+, d = 0.96) effect were found.
In sum, evaluation of individual effect sizes at follow-up suggests that improvements of broad-based SCT with and without CRT are generally maintained at follow-up; however, effect sizes tend to be small.
Discussion
Main Findings
The aim of this meta-analysis was to investigate the efficacy of different types of SCT (research question 1), moderators of treatment outcome (research question 2), and the durability of treatment gains (research question 3). Forty-six RCTs were included.
It was found that broad-based SCT (without CRT) was the most consistently effective form of treatment: it significantly improved emotion perception, social perception, ToM, and social functioning. Targeted SCT had the largest effect on emotion perception and social perception.
The use of groups was the only treatment variable that significantly moderated outcome: individual treatments were more effective for emotion perception, but group treatments worked better to improve social functioning. Gender predicted the effect of SCT on social functioning and psychiatric symptoms: a larger proportion of males was related to poorer generalized outcome.
A durable, moderate effect of SCT was found on social functioning (d = 0.66), but not on psychiatric symptoms. Individual evaluation of effect sizes suggested that improvements in social cognition were maintained at follow-up, but generally smaller than at posttreatment.
Types of SCT: What Works?
For lower-order social cognition (eg, emotion perception), targeted SCT is particularly effective. This makes sense, since the targeted skills and practice stimuli generally resemble the outcome measures. On functioning, a domain further removed from intervention materials and measured in a plethora of ways, however, targeted SCT has no effect. Thus, it appears that targeted SCT works very well, but predominantly for those skills that are explicitly trained. Broad-based SCT appears to be the most consistently effective overall, having moderate to large effects on emotion perception, social perception, ToM, and social functioning. This suggests that, to attain an improvement of social cognition that generalizes to functioning, a broad-based approach is required.
This apparent superiority of broad-based SCT is consistent with Couture et al,1 who hypothesized that the association between social cognition and social functioning is a multi-step process. To respond adequately in a social situation, an emotional cue must be identified (emotion perception); the social context must be evaluated (social perception); inferences must be made about the mental state of the other person (ToM/attribution); and based on these evaluations, an appropriate response must be selected. If only one area of social cognition is targeted, problems might still arise during the other steps of the process, leading to maladaptive social behavior and social dysfunction.
Another notable finding was that adding CRT to SCT did not improve treatment outcomes; with the exception of social perception, SCTs without CRT were consistently more effective than their counterparts with CRT. This is likely because of a larger emphasis on social cognition, rather than neurocognition. Although confidence intervals overlapped, it nevertheless challenges the notion11 that training supportive neurocognitive architecture is important for improvement in social cognition and functioning. This is in line with findings that social cognition and neurocognition are separate domains, and that (higher-order) social cognition more strongly predicts functioning.4,6
The significant effect sizes (positive and negative) for active controls were somewhat puzzling. While we added a comparison of active controls vs TAU to the network analyses to reflect the efficacy of nonspecific treatment characteristics, the significant effect sizes suggest that there is overlap in effective elements that are shared between SCT and active controls, that may be unaccounted for in our comparison of SCT vs TAU. Due to the large variety of active control conditions, it is challenging to identify these characteristics; the use of CRT in the active control category is a potential candidate, since we know from previous studies that CRT alone can improve social cognition.88 It should be noted, however, that the use of CRT was not a significant moderator in our analyses, trending towards significance only for social perception.
None of the treatments significantly improved attribution style (replicating earlier meta-analyses12,19) or symptoms (replicating Kurtz and Richardson12). Some evidence suggests attribution style is a separate construct from social cognition37; it is, therefore, possible that attribution and psychiatric symptoms are too far removed from social cognition, and their improvement requires a specialized approach.
While our results indicate that some types of SCT are more effective than others, they do not tell us why. While the number of domains trained is likely important, there are several characteristics that are associated with SCT type (eg, duration, use of groups) that may be unaccounted for in our moderator analyses. We, therefore, cannot exclude the possibility that our results are caused by other characteristics related to treatment type.
Moderators of SCT Outcome
Given the established efficacy of SCT, it is important to investigate its optimal parameters, and whom it benefits.6 Given the larger efficacy for individual treatments, it is possible that perceptual processes like emotion perception are easier to train individually than in a group. Groups, however, might be helpful for modeling, social support, and interpretation of situations through discussions. These complex skills and processes may be important for the improvement of higher-order social cognition, which is in line with our finding that group interventions had significantly larger effects on social functioning. An alternative explanation might be that targeted SCT was more commonly provided individually than broad-based SCT. Broad-based SCT has a relatively smaller emphasis on emotion perception, and may, therefore, produce smaller effects.
Little is known about gender differences in response to SCT.89 Unlike Kurtz and Richardson,12 we found that a larger proportion of male participants predicted less improvement in functioning and psychiatric symptoms. An opposite pattern was hypothesized by Irani and colleagues,90 who found a stronger association between social cognition and functioning for men. Male gender might predict worse generalized outcome in general: it is associated with lower rates of symptomatic remission, more hospitalizations, lower medication response, and worse psychosocial functioning.91–94
Long-term Effects of SCT
At a mean follow-up length of approximately 8 months, we found a small to moderate effect on social functioning, suggesting that functional improvements from SCT are durable. Individual evaluation of follow-up effect sizes for social cognition suggested that most studies found small effect sizes at follow-up, suggesting that improvements in social cognition may be maintained, but smaller than directly after treatment. However, given the lack of follow-up data, the generalizability and robustness of these findings are unclear; therefore, they should be considered to be preliminary.
Limitations and Strengths
The main limitation of this analysis is the considerable heterogeneity of studies. In a network meta-analysis, this is particularly important since it assumes that estimates of a particular treatment effect are consistent across studies. In our analysis, for the same treatment category, there was a large variety in key characteristics (eg, in terms of methodology, treatment characteristics, and sample), which introduced additional error and has likely affected outcome estimates. While we addressed some heterogeneity by categorizing SCT treatments and conducting moderator analysis, we may not have sufficiently corrected for this inconsistency. Given the variety and inconsistent psychometric quality of outcome measures, it is possible that treatment effects are partly dependent on the outcome measure used, rather than an intervention’s true efficacy.6,12
The heterogeneity in our analyses is likely also a result of our grouping of outcome measures; to maintain an acceptable number of statistical tests, we grouped many different kinds of outcome measures within the same domain (eg, functional capacity and community functioning as “social functioning,” and all symptom domains as “psychiatric symptoms”). It is plausible that SCT affects these sub-domains differentially12,19; further research is necessary to refine these results.
The number of moderators had to be limited to maintain an acceptable ratio of observations to predictors. Therefore, relevant moderators may have been missed. Additionally, the results of the moderator analyses were corrected very conservatively, which may explain why fewer significant moderators were found than by Kurtz and Richardson.12 Finally, patterns of moderation may be more complex than the present design could identify; eg, moderated mediation effects have been found for CRT.95
This meta-analysis is innovative in its use of network meta-analysis (allowing for comparison of SCT types) and multivariate meta-analysis. Furthermore, this meta-analysis is methodologically rigorous (eg, selection of psychometrically high-quality measures, use of sensitivity analyses, the correction of parameters, use of multiple raters for subjective classifications). Finally, it is the first to meta-analytically investigate long-term effects of SCT.
Implications
The results of the current meta-analysis suggest that broad-based SCT without CRT is the best approach to improve social cognition and social functioning. We also found that men achieve poorer generalization of SCT improvements. Gender differences in response to SCT are currently poorly understood and should be studied further.89
We did not find an effect of intervention length, which could imply that longer treatments are not necessarily better. This might be considered in the design of SCT protocols since long programs are more time- and resource-consuming than shorter programs. The lack of outcome moderation of participant characteristics (except gender) implies that SCT is widely applicable.
While the present results indicate that CRT is not necessary to improve social cognition, it is too early to suggest that it has no added value, since functioning is impacted by a number of variables, including neurocognition.96
It is essential to further investigate the working mechanisms of SCT. For this, controlled studies of high methodological quality with long follow-up periods, and well-defined social cognitive target domains are necessary. Mediators and moderators should be investigated to determine the effective ingredients of SCT, and for whom it is most effective. Psychometrically sound outcome measures, such as those recommended by the SCOPE study,97 should be used to improve the quality of evidence.
Ultimately, the skills that participants gain from SCT likely remain close to its content and materials. To improve generalization of social cognition gains to functioning, training procedures and materials with a higher relevance and resemblance to participants’ daily lives (eg, using Virtual Reality6,98) might produce better outcomes.
Supplementary Material
Acknowledgments
We thank Meike Bak, Laura Steenhuis, and Esther Sportel for their aid in the methodological assessment and search update, and Wolfgang Viechtbauer, Leonie Kreuze, and Annelieke Roest for their contributions to the statistical analysis. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Funding
This study was supported by GGZ Drenthe.
References
- 1. Couture SM, Penn DL, Roberts DL. The functional significance of social cognition in schizophrenia: a review. Schizophr Bull. 2006;32(suppl 1):S44–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marwaha S, Johnson S, Bebbington P, et al. . Rates and correlates of employment in people with schizophrenia in the UK, France and Germany. Br J Psychiatry. 2007;191:30–37. [DOI] [PubMed] [Google Scholar]
- 3. Badcock JC, Shah S, Mackinnon A, et al. . Loneliness in psychotic disorders and its association with cognitive function and symptom profile. Schizophr Res. 2015;169(1–3):268–273. [DOI] [PubMed] [Google Scholar]
- 4. Fett AK, Viechtbauer W, Dominguez MD, Penn DL, van Os J, Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci Biobehav Rev. 2011;35(3):573–588. [DOI] [PubMed] [Google Scholar]
- 5. Savla GN, Vella L, Armstrong CC, Penn DL, Twamley EW. Deficits in domains of social cognition in schizophrenia: a meta-analysis of the empirical evidence. Schizophr Bull. 2013;39(5):979–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Horan WP, Green MF. Treatment of social cognition in schizophrenia: current status and future directions. Schizophr Res. 2019;203:3–11. [DOI] [PubMed] [Google Scholar]
- 7. Paquin K, Wilson AL, Cellard C, Lecomte T, Potvin S. A systematic review on improving cognition in schizophrenia: which is the more commonly used type of training, practice or strategy learning? BMC Psychiatry. 2014;14:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wölwer W, Frommann N, Halfmann S, Piaszek A, Streit M, Gaebel W. Remediation of impairments in facial affect recognition in schizophrenia: efficacy and specificity of a new training program. Schizophr Res. 2005;80(2–3):295–303. [DOI] [PubMed] [Google Scholar]
- 9. Roberts DL, Combs DR, Willoughby M, et al. . A randomized, controlled trial of Social Cognition and Interaction Training (SCIT) for outpatients with schizophrenia spectrum disorders. Br J Clin Psychol. 2014;53(3):281–298. [DOI] [PubMed] [Google Scholar]
- 10. Hogarty GE, Flesher S, Ulrich R, et al. . Cognitive enhancement therapy for schizophrenia: effects of a 2-year randomized trial on cognition and behavior. Arch Gen Psychiatry. 2004;61(9):866–876. [DOI] [PubMed] [Google Scholar]
- 11. Hogarty GE, Flesher S. Practice principles of cognitive enhancement therapy for schizophrenia. Schizophr Bull. 1999;25(4):693–708. [DOI] [PubMed] [Google Scholar]
- 12. Kurtz MM, Richardson CL. Social cognitive training for schizophrenia: a meta-analytic investigation of controlled research. Schizophr Bull. 2012;38(5):1092–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Choi JHH, Kim JH, Lee J, Green MF. Social cognition training for individuals with schizophrenia: a review of targeted interventions. Clin Psychopharmacol Neurosci. 2009;7(2):29–38. [Google Scholar]
- 14. Fiszdon JM, Reddy LF. Review of social cognitive treatments for psychosis. Clin Psychol Rev. 2012;32(8):724–740. [DOI] [PubMed] [Google Scholar]
- 15. Horan WP, Kern RS, Green MF, et al. Social Cognition Training for Individuals with Schizophrenia: emerging Evidence. Am J Psychiatr Rehabil. 2008;11(3):205–252. doi: 10.1080/15487760801963652 [DOI] [Google Scholar]
- 16. Grant N, Lawrence M, Preti A, Wykes T, Cella M. Social cognition interventions for people with schizophrenia: a systematic review focussing on methodological quality and intervention modality. Clin Psychol Rev. 2017;56:55–64. [DOI] [PubMed] [Google Scholar]
- 17. Tan BL, Lee SA, Lee J. Social cognitive interventions for people with schizophrenia: a systematic review. Asian J Psychiatr. 2018;35:115–131. [DOI] [PubMed] [Google Scholar]
- 18. Bordon N, O’Rourke S, Hutton P. The feasibility and clinical benefits of improving facial affect recognition impairments in schizophrenia: systematic review and meta-analysis. Schizophr Res. 2017;188:3–12. [DOI] [PubMed] [Google Scholar]
- 19. Kurtz MM, Gagen E, Rocha NB, Machado S, Penn DL. Comprehensive treatments for social cognitive deficits in schizophrenia: a critical review and effect-size analysis of controlled studies. Clin Psychol Rev. 2016;43:80–89. [DOI] [PubMed] [Google Scholar]
- 20. Mueller DR, Schmidt SJ, Roder V. One-year randomized controlled trial and follow-up of integrated neurocognitive therapy for schizophrenia outpatients. Schizophr Bull. 2015;41(3):604–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maroño Souto Y, Vázquez Campo M, Díaz Llenderrozas F, Rodríguez Álvarez M, Mateos R, García Caballero A. Randomized clinical trial with e-Motional Training® 10 for social cognition rehabilitation in schizophrenia. Front Psychiatry. 2018;9:1–9. doi: 10.3389/fpsyt.2018.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gordon A, Davis PJ, Patterson S, et al. . A randomized waitlist control community study of Social Cognition and Interaction Training for people with schizophrenia. Br J Clin Psychol. 2018;57(1):116–130. [DOI] [PubMed] [Google Scholar]
- 23. Choi JH, Kim JH, Lee J, Green MF. Social cognition training for individuals with schizophrenia: a review of targeted interventions. Clin Psychopharmacol Neurosci. 2009;7(2):29–38. [Google Scholar]
- 24. Shamseer L, Moher D, Clarke M, et al. ; PRISMA-P Group Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. [DOI] [PubMed] [Google Scholar]
- 25. Tarrier N, Wykes T. Is there evidence that cognitive behaviour therapy is an effective treatment for schizophrenia? A cautious or cautionary tale? Behav Res Ther. 2004;42(12):1377–1401. [DOI] [PubMed] [Google Scholar]
- 26. Figueira ML, Brissos S. Measuring psychosocial outcomes in schizophrenia patients. Curr Opin Psychiatry. 2011;24(2):91–99. [DOI] [PubMed] [Google Scholar]
- 27. Burns T, Patrick D. Social functioning as an outcome measure in schizophrenia studies. Acta Psychiatr Scand. 2007;116(6):403–418. [DOI] [PubMed] [Google Scholar]
- 28. Pinkham AE, Penn DL, Green MF, Buck B, Healey K, Harvey PD. The social cognition psychometric evaluation study: results of the expert survey and RAND panel. Schizophr Bull. 2014;40(4):813–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rücker G, Schwarzer G, Krahn U, König J. netmeta: Network meta-analysis using frequentist methods. R package version 0.9–8.2016. https://cran.r-project.org/package=netmeta. Accessed August 31, 2016.
- 30. Tonin FS, Rotta I, Mendes AM, Pontarolo R. Network meta-analysis: a technique to gather evidence from direct and indirect comparisons. Pharm Pract (Granada). 2017;15(1):943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Salanti G, Higgins JP, Ades AE, Ioannidis JP. Evaluation of networks of randomized trials. Stat Methods Med Res. 2008;17(3):279–301. [DOI] [PubMed] [Google Scholar]
- 32. The Cochrane Collaboration. 16.5.4 How to include multiple groups from one study. In: Higgins J, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions.2011. www.handbook.cochrane.org. Accessed November 15, 2019.
- 33. Aloi M, de Filippis R, Grosso Lavalle F, et al. . Effectiveness of integrated psychological therapy on clinical, neuropsychological, emotional and functional outcome in schizophrenia: a RCT study. J Ment Health. 2018:1–8. doi: 10.1080/09638237.2018.1521948 [DOI] [PubMed] [Google Scholar]
- 34. Bechi M, Riccaboni R, Ali S, et al. . Theory of mind and emotion processing training for patients with schizophrenia: preliminary findings. Psychiatry Res. 2012;198(3):371–377. [DOI] [PubMed] [Google Scholar]
- 35. Bechi M, Bosia M, Spangaro M, et al. . Combined social cognitive and neurocognitive rehabilitation strategies in schizophrenia: neuropsychological and psychopathological influences on Theory of Mind improvement. Psychol Med. 2015;45(15):3147–3157. [DOI] [PubMed] [Google Scholar]
- 36. Choi K-HH, Kwon J-HH. Social Cognition Enhancement Training for Schizophrenia: a Preliminary Randomized Controlled Trial. Community Ment Health J. 2006;42(2):177–187. doi: 10.1007/s10597-005-9023-6 [DOI] [PubMed] [Google Scholar]
- 37. Combs DR, Tosheva A, Penn DL, Basso MR, Wanner JL, Laib K. Attentional-shaping as a means to improve emotion perception deficits in schizophrenia. Schizophr Res. 2008;105(1–3):68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Eack SM, Greenwald DP, Hogarty SS, et al. . Cognitive enhancement therapy for early-course schizophrenia: effects of a two-year randomized controlled trial. Psychiatr Serv. 2009;60(11):1468–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Eack SM, Greenwald DP, Hogarty SS, Keshavan MS. One-year durability of the effects of cognitive enhancement therapy on functional outcome in early schizophrenia. Schizophr Res. 2010;120(1–3):210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fernandez-Gonzalo S, Turon M, Jodar M, et al. . A new computerized cognitive and social cognition training specifically designed for patients with schizophrenia/schizoaffective disorder in early stages of illness: a pilot study. Psychiatry Res. 2015;228(3):501–509. doi: 10.1016/j.psychres.2015.06.007 [DOI] [PubMed] [Google Scholar]
- 41. Fisher M, Nahum M, Howard E, et al. . Supplementing intensive targeted computerized cognitive training with social cognitive exercises for people with schizophrenia: an interim report. Psychiatr Rehabil J. 2017;40(1):21–32. doi: 10.1037/prj0000244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. García S, Fuentes I, Ruíz JC, Gallach E, Roder V, Volker R. Application of the IPT in a Spanish Sample: evaluation of the “Social Perception Subprogramme.” Int J Psychol Psychol Ther. 2003;3(2):299–310. [Google Scholar]
- 43. Gaudelus B, Virgile J, Geliot S, Franck N; GAÏA/RECOS Study Team Improving facial emotion recognition in schizophrenia: a controlled study comparing specific and attentional focused cognitive remediation. Front Psychiatry. 2016;7:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gil-Sanz DG, Lorenzo MD, Seco RB, et al. . Efficacy of a social cognition training program for schizophrenic patients: a pilot study. Span J Psychol. 2009;12(1):184–191. doi: 10.1017/S1138741600001591 [DOI] [PubMed] [Google Scholar]
- 45. Gil-Sanz D, Fernández-Modamio M, Bengochea-Seco R, Arrieta-Rodríguez M, Pérez-Fuentes G. Efficacy of the Social Cognition Training Program in a sample of outpatients with schizophrenia. Clin Schizophr Relat Psychoses. 2014;4:1–27. doi: 10.1017/CBO9781107415324.004 [DOI] [PubMed] [Google Scholar]
- 46. Gohar SM, Hamdi E, El Ray LA, Horan WP, Green MF. Adapting and evaluating a social cognitive remediation program for schizophrenia in Arabic. Schizophr Res. 2013;148(1–3):12–17. [DOI] [PubMed] [Google Scholar]
- 47. Habel U, Koch K, Kellermann T, et al. . Training of affect recognition in schizophrenia: neurobiological correlates. Soc Neurosci. 2010;5(1):92–104. [DOI] [PubMed] [Google Scholar]
- 48. Hogarty GE, Greenwald DP, Eack SM. Durability and mechanism of effects of cognitive enhancement therapy. Psychiatr Serv. 2006;57(12):1751–1757. [DOI] [PubMed] [Google Scholar]
- 49. Hooker CI, Bruce L, Fisher M, et al. . The influence of combined cognitive plus social-cognitive training on amygdala response during face emotion recognition in schizophrenia. Psychiatry Res. 2013;213(2):99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Horan WP, Kern RS, Shokat-Fadai K, Sergi MJ, Wynn JK, Green MF. Social cognitive skills training in schizophrenia: an initial efficacy study of stabilized outpatients. Schizophr Res. 2009;107(1):47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Horan WP, Kern RS, Tripp C, et al. . Efficacy and specificity of social cognitive skills training for outpatients with psychotic disorders. J Psychiatr Res. 2011;45(8):1113–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kayser N, Sarfati Y, Besche C, Hardy-Baylé MC. Elaboration of a rehabilitation method based on a pathogenetic hypothesis of “theory of mind” impairment in schizophrenia. Neuropsychol Rehabil. 2006;16(1):83–95. [DOI] [PubMed] [Google Scholar]
- 53. Lindenmayer JP, McGurk SR, Khan A, et al. . Improving social cognition in schizophrenia: a pilot intervention combining computerized social cognition training with cognitive remediation. Schizophr Bull. 2013;39(3):507–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lindenmayer JP, Khan A, McGurk SR, et al. . Does social cognition training augment response to computer-assisted cognitive remediation for schizophrenia? Schizophr Res. 2018;201:180–186. [DOI] [PubMed] [Google Scholar]
- 55. Mazza M, Lucci G, Pacitti F, et al. . Could schizophrenic subjects improve their social cognition abilities only with observation and imitation of social situations? Neuropsychol Rehabil. 2010;20(5):675–703. [DOI] [PubMed] [Google Scholar]
- 56. Palumbo D, Mucci A, Piegari G, D’Alise V, Mazza A, Galderisi S. SoCIAL—Training cognition in schizophrenia: a pilot study. Neuropsychiatr Dis Treat. 2017;13. doi: 10.2147/NDT.S136732 [DOI] [PMC free article] [PubMed]
- 57. Peña J, Ibarretxe-Bilbao N, Sánchez P, et al. . Combining social cognitive treatment, cognitive remediation, and functional skills training in schizophrenia: a randomized controlled trial. npj Schizophr. 2016;2:16037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Penn DL, Combs D. Modification of affect perception deficits in schizophrenia. Schizophr Res. 2000;46(2–3):217–229. doi: 10.1016/S0920-9964(00)00005-0 [DOI] [PubMed] [Google Scholar]
- 59. Pino MC, Pettinelli M, Clementi D, Gianfelice C, Mazza M. Improvement in cognitive and affective theory of mind with observation and imitation treatment in subjects with schizophrenia. Clin Neuropsychiatry. 2015;12(3):64–72. [Google Scholar]
- 60. Popova P, Popov TG, Wienbruch C, Carolus AM, Miller GA, Rockstroh BS. Changing facial affect recognition in schizophrenia: effects of training on brain dynamics. Neuroimage Clin. 2014;6:156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rakitzi S, Georgila P, Efthimiou K, Mueller DR. Efficacy and feasibility of the Integrated Psychological Therapy for outpatients with schizophrenia in Greece: final results of a RCT. Psychiatry Res. 2016;242:137–143. [DOI] [PubMed] [Google Scholar]
- 62. Roncone R, Mazza M, Frangou I, et al. . Rehabilitation of theory of mind deficit in schizophrenia: a pilot study of metacognitive strategies in group treatment. Neuropsychol Rehabil. 2004;14(4):421–435. doi: 10.1080/09602010343000291 [DOI] [Google Scholar]
- 63. Russell TA, Green MJ, Simpson I, Coltheart M. Remediation of facial emotion perception in schizophrenia: concomitant changes in visual attention. Schizophr Res. 2008;103(1–3):248–256. [DOI] [PubMed] [Google Scholar]
- 64. Sachs G, Winklbaur B, Jagsch R, et al. . Training of affect recognition (TAR) in schizophrenia–impact on functional outcome. Schizophr Res. 2012;138(2–3):262–267. [DOI] [PubMed] [Google Scholar]
- 65. Sevos J, Grosselin A, Gauthier M, Carmona F, Gay A, Massoubre C. Cinemotion, a program of cognitive remediation to improve the recognition and expression of facial emotions in schizophrenia: a Pilot Study. Front Psychiatry. 2018;9:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tas C, Danaci AE, Cubukcuoglu Z, Brüne M. Impact of family involvement on social cognition training in clinically stable outpatients with schizophrenia – a randomized pilot study. Psychiatry Res. 2012;195(1–2):32–38. [DOI] [PubMed] [Google Scholar]
- 67. Taylor R, Cella M, Csipke E, Heriot-Maitland C, Gibbs C, Wykes T. Tackling Social Cognition in Schizophrenia: a Randomized Feasibility Trial. Behav Cogn Psychother. 2016;44(3):306–317. [DOI] [PubMed] [Google Scholar]
- 68. Tsotsi S, Kosmidis MH, Bozikas VP. Improved facial affect recognition in schizophrenia following an emotion intervention, but not training attention-to-facial-features or treatment-as-usual. Psychiatry Res. 2017;254:135–142. [DOI] [PubMed] [Google Scholar]
- 69. van der Gaag M, Kern RS, van den Bosch RJ, Liberman RP. A controlled trial of cognitive remediation in schizophrenia. Schizophr Bull. 2002;28(1):167–176. [DOI] [PubMed] [Google Scholar]
- 70. Veltro F, Mazza M, Vendittelli N, Alberti M, Casacchia M, Roncone R. A comparison of the effectiveness of problem solving training and of cognitive-emotional rehabilitation on neurocognition, social cognition and social functioning in people with schizophrenia. Clin Pract Epidemiol Ment Health. 2011;7:123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang Y, Roberts DL, Xu B, Cao R, Yan M, Jiang Q. Social cognition and interaction training for patients with stable schizophrenia in Chinese community settings. Psychiatry Res. 2013;210(3):751–755. [DOI] [PubMed] [Google Scholar]
- 72. Wölwer W, Frommann N. Social-cognitive remediation in schizophrenia: generalization of Effects of the Training of Affect Recognition (TAR). Schizophr Bull. 2011;37(Suppl 2):S63–S70. doi: 10.1093/schbul/sbr071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Viechtbauer W. Conducting Meta-Analyses in R with the metafor Package. J Stat Softw. 2010;36(3):1–48. doi: 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 74. Viechtbauer W. Accounting for heterogeneity via random-effects models and moderator analyses in meta-analysis. Z Psychol. 2007;215(2):104–121. [Google Scholar]
- 75. Viechtbauer W, López-López JA, Sánchez-Meca J, Marín-Martínez F. A comparison of procedures to test for moderators in mixed-effects meta-regression models. Psychol Methods. 2015;20(3):360–374. [DOI] [PubMed] [Google Scholar]
- 76. Cooper H, Hedges L V., Valentine JC.. Handbook of Research Synthesis and Meta-Analysis. New York, NY: Russell Sage Foundation; 2009. [Google Scholar]
- 77. Becker BJ. Synthesizing standardized mean-change measures. Br J Math Stat Psychol. 1988;41(2):257–278. doi: 10.1111/j.2044-8317.1988.tb00901.x [DOI] [Google Scholar]
- 78. Cohen J. Statistical power analysis for the behavioral sciences. Stat Power Anal Behav Sci. 1988;2:567. doi: 10.1234/12345678 [DOI] [Google Scholar]
- 79. Hofmann SG, Sawyer AT, Witt AA, Oh D. The effect of mindfulness-based therapy on anxiety and depression: a meta-analytic review. J Consult Clin Psychol. 2010;78(2):169–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Steenhuis LA, Nauta MH, Bocking CLH, Gerdina H. Treating depressive symptoms in psychosis : a network meta-analysis on the effects of non-verbal therapies. PLOS One. 2018;13(12):e0209762. doi: 10.1371/journal.pone.0140637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kreuze LJ, Pijnenborg GHM, de Jonge YB, Nauta MH. Cognitive-behavior therapy for children and adolescents with anxiety disorders: a meta-analysis of secondary outcomes. J Anxiety Disord. 2018;60:43–57. [DOI] [PubMed] [Google Scholar]
- 82. Kalin M, Kaplan S, Gould F, Pinkham AE, Penn DL, Harvey PD. Social cognition, social competence, negative symptoms and social outcomes: inter-relationships in people with schizophrenia. J Psychiatr Res. 2015;68:254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mancuso F, Horan WP, Kern RS, Green MF. Social cognition in psychosis: multidimensional structure, clinical correlates, and relationship with functional outcome. Schizophr Res. 2011;125(2-3):143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Pinkham AE, Penn DL, Green MF, Harvey PD. Social Cognition Psychometric Evaluation: results of the Initial Psychometric Study. Schizophr Bull. 2016;42(2):494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Eack SM, Greenwald DP, Hogarty SS, Keshavan MS. One-year durability of the effects of cognitive enhancement therapy on functional outcome in early schizophrenia. Schizophr Res. 2010;120(1-3):210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Peña J, Ibarretxe-Bilbao N, Sánchez P, et al. . Combining social cognitive treatment, cognitive remediation, and functional skills training in schizophrenia: a randomized controlled trial. npj Schizophr. 2016;2:16037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Rakitzi S, Georgila P, Efthimiou K, Mueller DR. Efficacy and feasibility of the Integrated Psychological Therapy for outpatients with schizophrenia in Greece: final results of a RCT. Psychiatry Res. 2016;242:137–143. [DOI] [PubMed] [Google Scholar]
- 88. Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry. 2011;168(5):472–485. [DOI] [PubMed] [Google Scholar]
- 89. Statucka M, Walder DJ. Efficacy of social cognition remediation programs targeting facial affect recognition deficits in schizophrenia: a review and consideration of high-risk samples and sex differences. Psychiatry Res. 2013;206(2–3):125–139. [DOI] [PubMed] [Google Scholar]
- 90. Irani F, Seligman S, Kamath V, Kohler C, Gur RC. A meta-analysis of emotion perception and functional outcomes in schizophrenia. Schizophr Res. 2012;137(1-3):203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wils RS, Gotfredsen DR, Hjorthøj C, et al. . Antipsychotic medication and remission of psychotic symptoms 10 years after a first-episode psychosis. Schizophr Res. 2017;182:42–48. [DOI] [PubMed] [Google Scholar]
- 92. Ochoa S, Usall J, Cobo J, Labad X, Kulkarni J. Gender differences in schizophrenia and first-episode psychosis: a comprehensive literature review. Schizophr Res Treatment. 2012;2012:916198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Torgalsbøen AK. Full recovery from schizophrenia: the prognostic role of premorbid adjustment, symptoms at first admission, precipitating events and gender. Psychiatry Res. 1999;88(2):143–152. doi: 10.1016/S0165-1781(99)00077-3 [DOI] [PubMed] [Google Scholar]
- 94. Usall J, Haro J, Araya S, et al. . Social functioning in schizophrenia: what is the influence of gender? Eur J Psychiatry. 2007;21(3):199–205. [Google Scholar]
- 95. Wykes T, Reeder C, Huddy V, et al. . Developing models of how cognitive improvements change functioning: mediation, moderation and moderated mediation. Schizophr Res. 2012;138(1):88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Milev P, Ho BC, Arndt S, Andreasen NC. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. Am J Psychiatry. 2005;162(3):495–506. [DOI] [PubMed] [Google Scholar]
- 97. Pinkham AE, Harvey PD, Penn DL. Social Cognition Psychometric Evaluation: results of the Final Validation Study. Schizophr Bull. 2017;44(4):737–748. doi: 10.1093/schbul/sbx117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Nijman SA, Veling W, Greaves-Lord K, et al. . Dynamic Interactive Social Cognition Training in Virtual Reality (DiSCoVR) for social cognition and social functioning in people with a psychotic disorder: study protocol for a multicenter randomized controlled trial. BMC Psychiatry. 2019;19(1):272. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.