Abstract
Background
Persistent poorly-controlled type 2 diabetes mellitus (PPDM), or maintenance of a hemoglobin A1c (HbA1c) ≥8.5% despite receiving clinic-based diabetes care, contributes disproportionately to the national diabetes burden. Comprehensive telehealth interventions may help ameliorate PPDM, but existing approaches have rarely been designed with clinical implementation in mind, limiting use in routine practice. We describe a study testing a novel telehealth intervention that comprehensively targets clinic-refractory PPDM, and was explicitly developed for practical delivery using existing Veterans Health Administration (VHA) clinical infrastructure.
Methods
Practical Telehealth to Improve Control and Engagement for Patients with Clinic-Refractory Diabetes Mellitus (PRACTICE-DM) is an ongoing randomized controlled trial comparing two 12-month interventions: 1) standard VHA Home Telehealth (HT) telemonitoring/care coordination; or 2) the PRACTICE-DM intervention, a comprehensive HT-delivered intervention combining telemonitoring, self-management support, diet/activity support, medication management, and depression management. The primary outcome is HbA1c. Secondary outcomes include diabetes distress, self-care, self-efficacy, weight, depressive symptoms, implementation barriers/facilitators, and costs. We hypothesize that the PRACTICE-DM intervention will reduce HbA1c by >0.6% versus standard HT over 12 months.
Results
Enrollment for this ongoing trial concluded in January 2020; 200 patients were randomized (99 to standard HT and 101 to the PRACTICE-DM intervention). The cohort has a mean age of 58 and is 23% female and 72% African American. Mean baseline HbA1c and BMI were 10.2% and 34.8 kg/m2.
Conclusions
Because it comprehensively targets factors underlying PPDM using existing clinical infrastructure, the PRACTICE-DM intervention may be well suited to lower the complications and costs of PPDM in routine practice.
Keywords: Health services research, Poorly controlled diabetes, Telehealth intervention, Comparative effectiveness
1. Introduction
Type 2 diabetes affects nearly 30 million people in the US [1]. Diabetes is a major cardiovascular risk factor, and is the leading cause of blindness, kidney failure, and non-traumatic lower-limb amputations [2]. Patients with persistent poorly-controlled diabetes mellitus (PPDM), defined as maintenance of an HbA1c ≥8.5 for >1 year despite receiving clinic-based diabetes care, comprise 10–15% of all patients with type 2 diabetes in both Veteran and non-Veteran populations [3,4]. Since the complications and costs of diabetes rise exponentially as hemoglobin A1c (HbA1c) increases [[5], [6], [7]], clinic-refractory individuals with PPDM are likely to be the highest-risk, highest-cost diabetes patients within any healthcare system, making them a compelling population to target for care delivery redesign.
PPDM poses a clinical challenge because factors underlying poor diabetes control, including unreliable/unavailable blood glucose data, medication nonadherence, suboptimal diet, inadequate physical activity, complex treatment regimens, and comorbid depression [4,[8], [9], [10], [11], [12], [13], [14], [15], [16]], are difficult to address with the infrequent patient-provider contact typically attainable with clinic-based care [17,18]. Telehealth, which utilizes information communicated via electronic platforms for medical purposes, may help counter factors that underlie PPDM [19,20]. Telehealth strategies that target individual factors underlying PPDM have been shown to reduce HbA1c by 0.3% to 0.6% compared to clinic-based care. These include telemonitoring (0.48%) [11,21,22], self-management support (0.44%) [14,23], diet/activity support (0.6%) [8,24], medication management (0.51%) [16,25], and depression support (0.33%) [9,26]. While combining these strategies may enhance HbA1c reduction, studies across healthcare settings and patient populations have achieved variable results [[27], [28], [29], [30], [31], [32], [33], [34]]. Additionally, none has specifically targeted the PPDM population in a manner amenable to scaling. Frequently cited barriers to implementation of telehealth interventions broadly include a lack of trained clinical staffing/infrastructure for delivery, insufficient integration of telehealth data into the electronic health record (EHR), and limited reimbursement options [[35], [36], [37], [38]]. In order to address PPDM, there is a need for an intensive, diabetes telehealth intervention that is appropriate for practical delivery.
The Veterans Health Administration (VHA) currently uses telemonitoring and care coordination as part of its Home Telehealth (HT) program, though without integration of additional strategies that target other factors associated with underlying PPDM. VHA's HT program provides an optimal context to evaluate a comprehensive telehealth strategy for PPDM that is amenable to practical translation.
In order to address current gaps in the practical use of comprehensive telehealth for patients with clinic-refractory type 2 diabetes, we are conducting a randomized, controlled trial (RCT) called Practical Telehealth to Improve Control and Engagement for Patients with Clinic-Refractory Diabetes Mellitus (PRACTICE-DM). This ongoing study examines the effectiveness of a comprehensive telehealth intervention for patients with PPDM that combines telemonitoring, self-management support, diet/activity support, medication management, and depression support – 5 evidence-based approaches that target key factors underlying PPDM. The present manuscript details the protocol for PRACTICE-DM, along with baseline population data and lessons learned based on our progress to date.
2. Methods
2.1. Study design
The PRACTICE-DM study (ClinicalTrials.gov NCT03520413) is an ongoing two-site RCT designed to evaluate practical use of a comprehensive telehealth intervention for clinic-refractory patients with PPDM. The study is also exploring intervention acceptability, mechanisms of effect, and intervention and health care costs. By definition, patients with PPDM have proven themselves refractory to clinic-based usual care, so we designed PRACTICE-DM as an active comparator trial with randomization to one of two 12-month interventions: 1) standard VHA Home Telehealth (HT) care coordination and telemonitoring; or 2) the PRACTICE-DM intervention, a comprehensive telehealth intervention that incorporates telemonitoring, self-management support, diet/activity support, medication management (provided in conjunction with a study medication manager), and depression support (provided in conjunction with a study psychiatrist). Both interventions are delivered via existing VHA HT workforce and infrastructure.
Of note, because they receive detail about both study interventions during the consent process, patients are not blinded to their assigned intervention. Research assistants (RAs) participating in study outcome assessment remain blinded to patients' intervention status. The roles and responsibilities for all study staff are outlined in Appendix A.
This study is approved by the Institutional Review Boards at the Durham, North Carolina and Richmond, Virginia VA Medical Centers (VAMCs).
2.2. Study population
Although this study is ongoing, the full study population has been enrolled. Patients were recruited from the Durham and Richmond VAMC catchment areas, which include associated Health Care Centers and geographically distinct Community-Based Outpatient Clinics. Study inclusion criteria include clinic-refractory PPDM, defined as: a diagnosis of type 2 diabetes (based on ICD-10 E11); at least two HbA1c values ≥8.5% during the prior year, with no readings <8.5%; and at least 1 appointment with a VHA Primary Care Provider (PCP) or Endocrinologist during this period (as an indicator of ongoing clinic-based care). Exclusion criteria include: age > 70 years; life expectancy <5 years, or other comorbidities that would offset the benefits of HbA1c <8.5%; inability to communicate by telephone; dementia or psychosis; active alcohol/substance disorder; pregnancy; prior hypoglycemic seizure/coma; explicit refusal to perform self-monitored blood glucose (SMBG) to the degree necessary for VHA HT enrollment (i.e., a “no” response to the question, “Are you willing to check your blood sugar regularly (up to 4 times per day),” with a willingness to try being considered affirmative); use of insulin infusion pumps; hospitalization for stroke, heart attack, or surgery for blocked arteries in the past 12 months; receipt of kidney dialysis; metastatic cancer diagnosis; use of a continuous glucose monitor (due to HT equipment constraints), with refusal to additionally monitor and submit SMBG; or a primary provider request for the patient not to participate. In order to counter the known overrepresentation of men in the VHA population, we oversampled women during recruitment, with an aim to achieve >20% women in the enrolled population. Prior use of standard VHA HT services was not an exclusion criterion.
2.3. Study recruitment
We used a proactive, Institutional Review Board (IRB)-approved process to identify eligible patients through the VHA electronic health record (EHR) at both sites. Research staff mailed invitation letters to eligible patients, which included basic information about the study and instructions to opt out of further contact if desired. For patients not opting out of participation within one week, staff made contact via telephone to gauge interest in participation, explain the study, and briefly screen for eligibility. If appropriate, an in-person appointment for further evaluation of eligibility and possible enrollment was arranged.
2.4. Study enrollment
At each site, and RA obtained written informed consent at an in-person appointment. After consent, participants underwent a baseline assessment including a survey of demographics, clinical history, medications, and measures of relevant psychosocial constructs; measurement of blood pressure and body mass index; and laboratory HbA1c testing. Final study eligibility was determined following this assessment; consented patients with a baseline HbA1c <8.5% were excluded from the study and not randomized.
2.5. Randomization
Eligible patients were randomized in a 1:1 ratio to the two study arms using a stratified, blocked randomization with block size of 2. Randomization strata were study site (Durham or Richmond), pre-enrollment use of standard HT services (yes or no), and pre-enrollment receipt of Endocrinology or Clinical Pharmacy services for diabetes (yes or no). The randomization sequence was generated by a study statistician and accessible only to the statistics team. Patients were informed of their study eligibility and (if eligible) their randomization assignment within 1 week of the consent visit via a telephone call from the project coordinator (PC).
2.6. Study interventions
2.6.1. HT-based delivery
At each site, both study interventions are delivered by VHA HT nurses; in order to minimize bias, a particular HT nurse delivers only one intervention, with no crossover between interventions. At the Durham site, one HT nurse was assigned to cover each intervention arm, while at the Richmond site, two nurses delivered the PRACTICE-DM intervention and five delivered the standard HT intervention.
After randomization, participants in both intervention groups are enrolled into the VHA HT program (unless already enrolled) using standard local processes, and all receive standard HT equipment, which is identical across sites (Medtronic®, Minneapolis, MN). PRACTICE-DM research staff involved with enrollment, randomization, and outcome assessment do not participate in intervention delivery. Of note, all patients in both study groups continue to receive general care from PCPs and other VHA providers throughout the study period. At study completion, all participants may continue to receive standard HT services per local guidelines, and return to diabetes care exclusively with their primary providers.
2.6.2. Standard HT group
Patients randomized to the standard HT group receive HT telemonitoring and care coordination services; because these services are part of routine practice for VHA HT nurses, no specific training is required for HT nurses delivering this intervention. Participants are asked to transmit SMBG data daily using their HT-issued equipment. All HT patients use a connector cable that links the standard blood glucose meter (FreeStyle Freedom Lite, Abbott Diabetes Care Inc., Alameda, CA) and HT device, allowing automatic uploading of data from the meter. Failure to submit data for 3 days triggers a call from HT, followed by a letter after 7 days. After 30 days without a patient response or data transmission, the patient is discharged from HT (but study outcome data is still collected). In addition to telemonitoring, participants in this group receive HT care coordination, including pre-appointment compilation of SMBG data for review by providers and communication for alarm values or other acute issues as needed between appointments. Diabetes management (including selection of HbA1c goals) is otherwise at the discretion of the patient's PCP and any other diabetes providers.
2.6.3. PRACTICE-DM intervention group
2.6.3.1. PRACTICE-DM intervention design
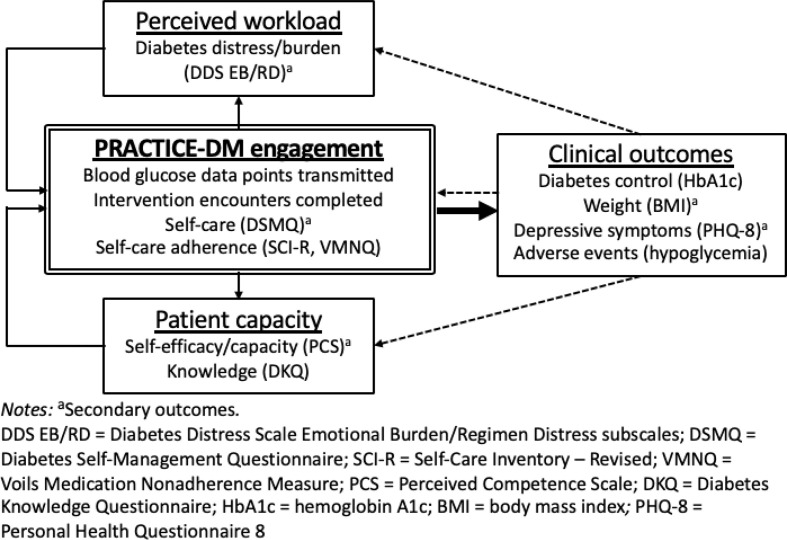
The PRACTICE-DM intervention design was guided by our pilot work and informed by a theoretical framework based on the Cumulative Complexity Model (CCM) [[39], [40], [41], [42], [43], [44]] (Fig. 1 ). Per this model, a balance between patients' workload of demands and capacity to address demands determines intervention engagement and subsequent outcomes. Engagement enhances patient capacity through development of knowledge and self-efficacy, and reduces patients' perceived workload through support from intervention staff. These improvements in patient capacity and perceived workload ultimately sustain further intervention engagement, with additional constructive effects on capacity and workload, and subsequently on clinical outcomes.
Fig. 1.
PRACTICE-DM intervention theoretical framework.
2.6.3.2. PRACTICE-DM intervention overview
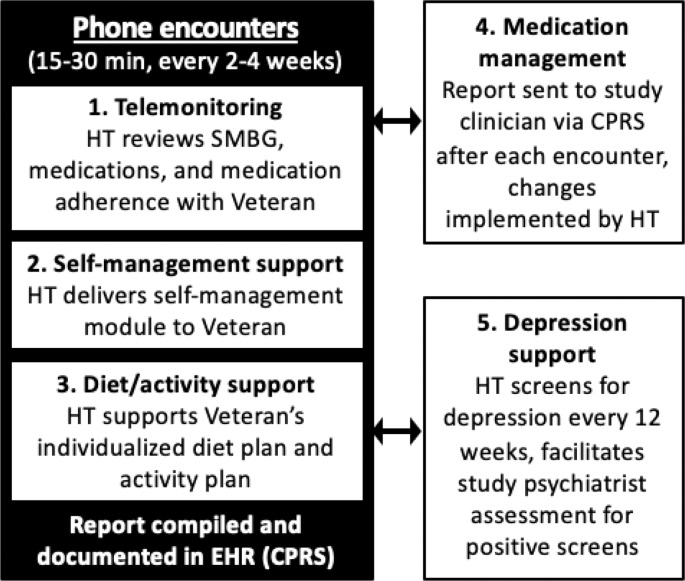
The PRACTICE-DM intervention comprises telemonitoring, self-management support, diet/activity support, medication management (provided in conjunction with a study medication manager), and depression support (provided in conjunction with a study psychiatrist). HT delivers the five intervention components during scheduled telephone encounters (Fig. 2 ) according to a pre-determined encounter schedule (Appendix B). The standard encounter frequency is every two weeks, but may be extended to every four weeks for participants achieving their goal HbA1c. At the beginning of the study, HT nurses delivering the PRACTICE-DM intervention completed a single training session led by the study principal investigator (PI), and received a hardcopy intervention manual containing study materials and procedures. During the training session, the intervention components and encounter schedule were reviewed, the HT nurses received instruction on interaction and communication with the other providers involved with intervention delivery (i.e., the study dietitian, medication managers, and study psychiatrist), and use of the online intervention tracking software (i.e., for attempted/completed phone calls, modules delivered, medication changes) was demonstrated.
Fig. 2.
PRACTICE-DM intervention components.
Per American Diabetes Association (ADA) and VHA guidelines [45,46], patients' HbA1c goals are individualized based on age, comorbidities, and hypoglycemia risk (as should be the case in the standard HT group); most participants target between <7.0% and < 8.0%.
2.6.3.3. Intervention components
Telemonitoring.
As in the standard HT group, participants receive daily prompts to transmit data using their HT-issued equipment. Failure to submit data for 3 days triggers a call from HT, followed by a letter at 7 days. Per ADA guidelines, participants are asked to collect SMBG between one and four times daily, based on their medication regimens [46]; however, patients on stable medication doses and at goal HbA1c may monitor less frequently at the discretion of the HT nurse and study medication manager. For each 2-week telephone encounter, HT reviews SMBG data with the participant, reconciles medications, and assesses self-reported medication adherence. Following each encounter, HT compiles this information in a report documented in the EHR (Appendix C).
Self-management support.
The self-management support component uses a module-based approach appropriate for delivery by HT nurses according to an intervention encounter schedule (Appendix B). The 16 unique modules incorporate patient-centric strategies like tailoring and goal setting to cover topics such as use of SMBG, hypoglycemia self-management, and self-managing insulin. For encounters during which a module is scheduled, the HT nurse delivers the module content using a script (Appendix D). The strategies and content for these modules have been adapted from our team's work across multiple prior trials [33,34,47,48]. Overall, this component builds patients' self-management capacity by focusing on knowledge and self-efficacy, two key determinants of diabetes control [49].
Diet and activity support.
Following the second study encounter, the study dietitian contacts PRACTICE-DM patients by phone to deliver diet and activity support. All patients receive nutrition information and an individualized diet plan. For patients with BMI ≥ 25, this content is specifically designed to target >5% weight loss by targeting a 500–750 cal/day deficit as per ADA guidelines [8]. All PRACTICE-DM patients are also encouraged to maintain ≥150 min of moderate-to-vigorous intensity physical activity per week in accordance with ADA guidelines [50]. HT documents each patient's individualized diet plan, activity recommendations, and weight loss goals, if applicable, in every intervention encounter note (Appendix C). During every study encounter, HT reviews these goals, follows up on progress with regard to diet and physical activity, and records self-reported weight data. This contact frequency is consistent with ADA recommendations for diet and activity interventions. For patients who are not progressing toward their weight loss goal at the 3-, 6-, and 9-month outcome visits, additional phone follow-up may be arranged with the study dietitian.
Medication management.
The medication management component has two key features associated with high impact; it facilitates frequent contact between patients and study staff, and allows modification of diabetes medications without requiring input from primary providers [25]. After each intervention encounter, HT generates an EHR report summarizing participants' SMBG data, reconciled medications, self-reported medication adherence, and the self-management and diet/activity content delivered during the encounter (Appendix C). This report is relayed via the EHR to a study medication manager. Each site has two to three PRACTICE-DM intervention medication managers, all of whom are experienced diabetes providers; the study's group of medication managers includes Clinical Pharmacy Specialists (PharmD), Nurse Practitioners (NP), and physicians. An effort is made to maintain continuity with the same medication manager for a given patient. After receiving the encounter report from the HT nurse, the medication manager considers treatment changes with guidance from a medication protocol (Appendix E). The medication protocol targets fasting blood glucose 90–150 mg/dL and preprandial blood glucose 140–180 mg/dL; these liberal goals may be further tailored to accommodate individualized HbA1c goals or hypoglycemia. The medication manager notifies HT of any recommendations via addendum to the EHR report (Appendix C), and HT contacts the patient by phone to implement the changes. All HT encounter notes are documented in the EHR, including medication changes, and non-study primary care providers or endocrinologists are alerted to notes and changes via the EHR.
Depression management.
All study participants receive baseline screening for depression with the Personal Health Questionnaire-8 (PHQ-8) [51]. For those with PHQ-8 < 10, including those with previously diagnosed depression, the depression protocol is not activated; these patients continue to receive PHQ-8 screening every 12 weeks [51]. Patients with PHQ-8 ≥ 10 enter the depression protocol (Appendix F), which is managed at each site by a study psychiatrist. Consistent with VA/DOD guidelines [52], the depression support protocol offers both pharmacologic and non-pharmacologic options [53,54]. All patients in the depression protocol receive PHQ-8 follow-up and further treatment changes as warranted every 8 weeks. Emergency psychiatric services are available to all throughout the study, and all patients receive the national suicide hotline number at study onset.
2.6.3.4. Fidelity assessment
In order to assure that the PRACTICE-DM intervention is delivered per protocol at both sites, HT nurses deliver each encounter using a shared intervention manual and use an online study database to track all intervention activities (i.e., attempted/completed phone calls, modules delivered, medication changes). The online database is reviewed weekly by the study PC to confirm that all intervention components due at each encounter were delivered. Additionally, for each HT nurse delivering the PRACTICE-DM intervention, several calls are shadowed by the PI and PC to ensure consistency in intervention delivery; any identified problems are addressed through additional training. To assure fidelity to the medication management protocol, the PI and study medication managers at each site conduct periodic case review meetings in-person or via real-time conferencing.
2.7. Outcome measures
All study measurements are performed by trained research staff; as above, RAs involved with outcome assessment are blinded to participants' randomization status. Baseline measures include demographics, clinical history, biomedical data, and measures of theoretical model constructs (Fig. 1, Table 1 ). Follow-up assessments and collection of outcome measures occur at parallel time points in both study arms (3, 6, 9, and 12 months) (Table 1). At 6 months and 12 months, all participants are assessed through an in-person study visit. At 3 months and 9 months, lab-based blood draws for HbA1c assessment are conducted. Participants are compensated a total of $200 for completion of outcome visits throughout the study, including $50 for each of the three in-person visits (baseline, 6, and 12 months), and $25 for each of the two lab-based assessments (3 and 9 months).
Table 1.
Summary and timing of data collection and outcome measures.
| Outcome | Method | Baseline | 3 mo | 6 mo | 9 mo | 12 mo |
|---|---|---|---|---|---|---|
| Primary outcome | ||||||
| Diabetes control | Hemoglobin A1c | * | * | * | * | * |
| Secondary outcomes | ||||||
| Diabetes distress/burden | DDS EB/RD | * | * | * | ||
| Diabetes self-care | DSMQ | * | * | * | ||
| Self-efficacy/capacity | PCS | * | * | * | ||
| Weight (BMI) | Calibrated digital scale | * | * | * | ||
| Depressive symptoms | PHQ-8 | * | * | * | * | * |
| Process evaluation outcomes | ||||||
| Intervention acceptability | Qualitative interview | * | ||||
| Mechanisms of effect | Qualitative interview | * | ||||
| Cost outcomes | ||||||
| Intervention costs | Administrative data, tracking staff data | * | ||||
| Additional outcomes | ||||||
| Social and demographica: Age, sex, race, ethnicity, marital status, education, employment, distance from VA, tobacco use, social support |
Self-reported surveys | * | * | * | ||
| Clinical: Years with diabetes, insulin use, diagnosis of hypertension and high cholesterol |
Self-reported surveys | * | ||||
| Hypoglycemic episodesb | Telemonitoring data | * | * | * | * | * |
| Biomedical: Height, weight |
* | * | * | |||
| Number of encounters completed, number of encounters with data transmission | Home Telehealth Encounter Notes | * | ||||
| Medication changes made during study | Home Telehealth Encounter Survey | * | * | * | * | |
| Psychosocial constructs | ||||||
| Self-care adherence | SCI-R | * | * | * | ||
| Medication adherence | VMNQ | * | * | * | ||
| Diabetes knowledge | DKQ | * | * | * | ||
| Health literacy/numeracy | NVS | * | ||||
| Pain | PROMIS Pain Interference | * | * | * | ||
| Autonomy | Healthcare Climate Questionnaire | * | * | * | ||
| Self-determination | HCCQ Diabetes | * | * | * | ||
DDS EB/RD = Diabetes Distress Scale (Emotional Burden/Regimen Distress subscales only); DSMQ = Diabetes Self-Management Questionnaire; PCS = Perceived Competence Scale; PHQ-8 = Personal Health Questionnaire-8; SCI-R = Self-Care Inventory-Revised; VMNQ = Voils Medication Non-adherence Questionnaire; DKQ = Diabetes Knowledge Questionnaire; NVS = Newest Vital Signs; PROMIS = Patient Reported Outcomes Measurement Information System; HCCQ = Healthcare Climate Questionnaire.
Only a subset of social and demographic outcomes (marital status, employment, tobacco use, social support) are assessed at 6 and 12 months.
Assessed as continuous measure using all submitted self-monitoring blood glucose data.
2.7.1. Primary outcome
The primary outcome is HbA1c. HbA1c is measured at a VA lab at baseline and every 3 months through the 12-month visit.
2.7.2. Secondary outcomes
Secondary outcomes include diabetes distress and burden, diabetes self-care, self-efficacy and capacity, body mass index, and depressive symptoms.
Diabetes distress and burden are measured using the Emotional Burden/Regimen Distress subscales of the Diabetes Distress Scale (DDS) [55,56]. Patient self-care is examined using the Diabetes Self-Management Questionnaire (DSMQ), which assesses self-care activities associated with glycemic control [57,58]. Further, patient self-efficacy and capacity are examined using the Perceived Competence Scale (PCS), which assesses feelings or perceptions of competence with respect to a particular domain such as diabetes [59]. Body weight is examined using body mass index (BMI) at each outcome visit and depressive symptoms are evaluated with the Personal Health Questionnaire-8 (PHQ-8) [51]. These secondary outcomes aim to evaluate the theoretical framework constructs (Fig. 1). In addition, we have included outcomes that will allow us to assess the individual effect of each component of the PRACTICE-DM intervention. Specifically, telemonitoring is assessed with the number of encounters completed and the number of encounters with data transmission, self-management with the Self-Care Inventory-Revised (SCI-R) and Voils Medication Non-adherence Questionnaire (VMNQ), diet/activity support with BMI, medication management with the number of medication changes made during the study, and the depression protocol with the PHQ-8.
2.7.3. Process evaluation outcomes
Acceptability and mechanisms of effect will be evaluated through a mixed-method process evaluation. Using a qualitative approach, phone-based, semi-structured interviews are being conducted with 20 intervention arm subjects, 10 from each site, following the 12-month study visits. Subjects will be selected using purposive sampling, with emphasis on specific factors such as representation of both study sites and different degrees of engagement. Additional interviews may be conducted as needed to achieve thematic saturation. Qualitative interview guide questions probe patients' explanatory models of diabetes, general perception of what did and did not work well with the PRACTICE-DM intervention, and impressions of the 5 intervention components (Appendix G). Further, phone-based, semi-structured qualitative interviews will be conducted with the HT nurses participating in delivery of the PRACTICE-DM intervention, as well as with administrators at each site (Appendix G). Process evaluation metrics align with the Reach, Effectiveness, Adoption, Implementation, and Maintenance (RE-AIM) framework, which seeks to enhance the quality, speed, and impact of efforts to facilitate implementation of research into clinical practice [60].
2.7.4. Cost outcomes
Intervention costs for labor and non-labor inputs associated with delivery of the two interventions are being assessed using a VHA payer perspective. Labor costs include HT nurse and study clinician time, as determined using daily logs documenting engagement in study tasks. Salaries are based on VHA Human Resources salary data. Capital costs include HT telehealth equipment, telephone service costs, overhead costs, and supplies. VHA health care costs include all health care utilization costs that occurs in VHA facilities or facilities reimbursed by VHA during the 12-month study period.
2.8. Data & safety monitoring
2.8.1. Data monitoring committee (DMC)
A DMC has been established to monitor data and oversee participant safety at both sites. The DMC comprises each study site PI, the study statisticians, the overall PC, and two independent experts not affiliated with the study. This committee monitors participant recruitment and retention, randomization, adverse events and safety concerns, data quality and outcomes, and adherence to the proposed timeline. The DMC meets biannually during the study period and as needed to review protocols, procedures, and concerns pertaining to research integrity and safety.
2.8.2. Adverse events (AEs)
AEs from both study arms are assessed by structured self-report [61] and reported per local VAMC requirements. Serious, Unanticipated, and Study-related AEs are reported to the IRB within five business days of noting the event. All other adverse events are reported at continuing review. At study onset, patients were instructed to seek immediate emergency services for any adverse events that require urgent in-person evaluation.
Anticipated adverse events in both arms relate to potential side effects of diabetes treatment, and associated comorbidities in the study cohort. As hypoglycemia is the most common side effect of diabetes therapy, the incidence of blood glucose <70 in both groups will be examined by reviewing SMBG data transmitted to HT during the study.
2.9. Statistical analysis
Our primary analysis and sample size calculations are based on tests of superiority. All hypothesis testing will be conducted with two-sided p-values at the standard 0.05 level. Analyses will be performed according to the intention-to-treat principle using SAS (Version 9.4: SAS Institute, Cary, NC) and R (http://www.R-project.org/).
2.9.1. Primary analysis
A linear mixed model (LMM) will be used to fit a constrained longitudinal data model, in which baseline HbA1c is modeled as a dependent variable in conjunction with the constraint of a common baseline mean across treatment arms, to examine between-arm differences in HbA1c over time [62]. The primary model predictors will include a common intercept, indicator variables for study arm (PRACTICE-DM intervention vs. active control), follow-up times, and the corresponding interactions between study arm and each time point. Random effect(s) or a flexible covariance structure to account for correlation among repeated measures over time will be fit. Our model will also adjust for the baseline stratification variables study site, pre-enrollment use of standard HT services status, and pre-enrollment receipt of Endocrinology or Clinical Pharmacy services [63]. Mixed effects model parameters will be estimated and tested using SAS PROC MIXED (SAS Institute, Cary, NC), with the estimated difference in HbA1c between the PRACTICE-DM intervention and the active comparator at 12 months serving as the primary effectiveness outcome.
2.9.2. Secondary analyses
Since the secondary outcomes are continuous, longitudinally collected measures, we will use similar modeling procedures as those described above for the primary analysis to assess between-group differences. We will also evaluate intervention engagement (SMBG transmission) and adverse events descriptively, examining mean occurrence in each group during the study. Adjusted sensitivity analyses will be conducted to examine how any clinically important between-arm differences at baseline influence intervention effects on the primary outcome.
2.9.3. Process evaluation analyses
All qualitative interviews will be recorded and transcribed verbatim. Transcripts will be analyzed with direct content analysis [64]. A qualitative codebook that includes code definitions, categorizations, and all coding decision rules will be developed as part of a qualitative audit trail. All team members will review and agree upon the coding scheme, including labels and their definitions, by consensus. Qualitative data will be managed in Atlas.ti, which facilitates coding management and analysis of patterns.
2.9.4. Cost analysis
Between-arm differences in annual VHA health care costs over the 12-month intervention period will be analyzed. Outpatient and total VHA costs will be estimated using a quasi-likelihood approach for generalized linear models with the variance and link functions that best fit the data [65]. VHA inpatient costs will be estimated using a marginalized two-part (MTP) model to account for the expected high proportion of zeros associated with lack of admission [66]. All stratification variables and the treatment indicator will be included as covariates in the model.
2.9.5. Missing data
Mechanisms for missing data will be investigated by describing missingness by intervention group, identifying missing data patterns, and understanding which observed covariates predict missingness. The main predictors of interest for the primary analysis are included in our baseline data. Our main analysis technique, LMM, implicitly accommodates missingness when the response is Missing At Random (i.e., due either to treatment, to prior outcome, or to other baseline covariates in the LMM) [67]. Depending on the type and scope of missing data, we will also explore multiple imputation as a sensitivity analysis [68].
2.9.6. Power calculation
Based on previous data [44], we used an alpha of 0.05, 80% power, 20% dropout, a within-patient correlation of HbA1c of 0.55, standard deviation of 1.6, and baseline HbA1c of 10.3% to estimate that n = 200 participants (100 in each arm) would be needed to detect an effect size difference of 0.6% at 12 months. This difference assumes a conservative reduction of 0.5% by 12 months in the standard HT arm, and a conservative reduction of 1.1% by 12 months in the PRACTICE-DM arm [21,23]. Power estimates were derived empirically via simulation (SAS 9.4) by generating 1000 stimulated datasets with these assumptions and then fitting the LMM described previously for our primary and secondary analyses and assessing the effect of interest using two-sided tests with alpha = 0.05. This sample will allow us to detect meaningful between-group differences in our secondary outcomes.
3. Results
3.1. Baseline demographic and clinical characteristics of participants
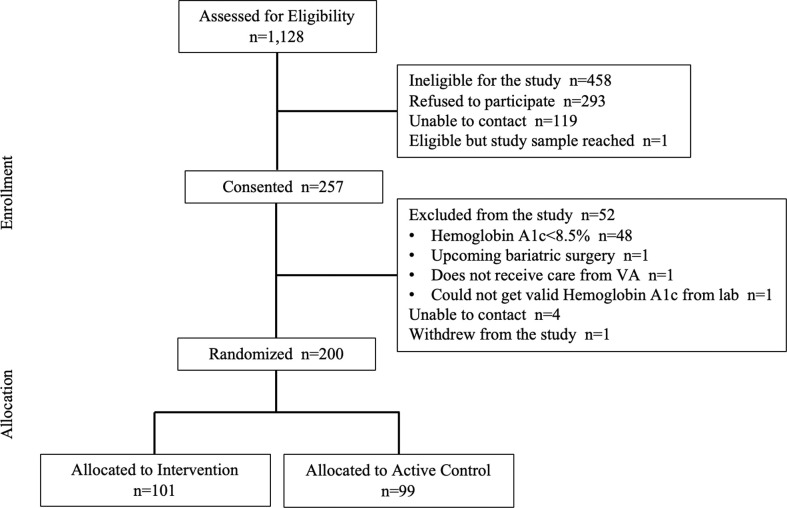
Participant recruitment details are shown in Fig. 3 . Enrollment began in December 2018 and concluded in January 2020. Of the 1128 patients assessed for eligibility, 257 patients were consented and 200 participants were randomized; most of the 57 patients who were consented but not randomized were excluded prior to randomization because their baseline HbA1c did not meet our inclusion criteria. Among randomized patients, 99 were allocated to the standard HT arm and 101 to the PRACTICE-DM arm.
Fig. 3.
PRACTICE-DM participant recruitment and follow-up.
Table 2 presents baseline characteristics of the study population; characteristics were generally well balanced across intervention arms. Participants had a mean (SD) age of 58 years (SD = 8.2), baseline HbA1c 10.2% (SD = 1.3), and baseline BMI 34.8 kg/m2 (SD = 6.7). The study population includes 46 women (23.4%), 144 (72.0%) African American patients, and 11 (5.5%) Hispanic/Latinx patients. Additionally, 12 (6.0%) patients were previously enrolled in Home Telehealth and 136 (68.0%) were receiving Endocrinology care prior to study enrollment.
Table 2.
Baseline Sample Characteristics, Overall and Stratified by Intervention.
| Baseline Characteristics | Overall (n = 200) | Standard Home Telehealth (n = 99) | PRACTICE-DM Intervention (n = 101) |
|---|---|---|---|
| Demographic characteristics | |||
| Age (years), mean (SD) | 57.8 (8.2) | 57.8 (8.0) | 57.7 (8.3) |
| Female, n (%) | 45 (22.5) | 21 (21.2) | 24 (23.8) |
| Race, n (%) | |||
| White or Caucasian | 42 (21.0) | 17 (17.2) | 25 (24.8) |
| Black or African American | 144 (72.0) | 76 (76.8) | 68 (67.3) |
| Other | 14 (7.0) | 6 (6.0) | 8 (7.9) |
| Hispanic/Latino Ethnicity, n (%)a | 11 (5.5) | 5 (5.1) | 6 (5.9) |
| Highest education level n (%) | |||
| ≤ High school graduate | 57 (28.5) | 28 (28.3) | 29 (28.7) |
| Technical school or some college | 80 (40.0) | 43 (43.4) | 37 (36.6) |
| ≥ College graduate | 63 (31.5) | 28 (28.3) | 35 (34.7) |
| Currently married, n (%) | 91 (45.5) | 46 (45.5) | 45 (46.5) |
| Employed (full/part-time/self), n (%) | 90 (45.0) | 42 (42.4) | 48 (47.5) |
| Study site (%) | |||
| Durham | 115 (57.5) | 58 (58.6) | 57 (56.4) |
| Richmond | 85 (42.5) | 41 (41.4) | 44 (43.6) |
| Distance from nearest VA hospital or clinic from home ≤40 miles | 153 (76.5) | 74 (74.7) | 79 (78.2) |
| Tobacco use in past 6 months, n (%) | 30 (25.4) | 14 (21.5) | 16 (30.2) |
| Years with diabetes, mean (SD) | 12.1 (7.7) | 12.0 (7.5) | 12.1 (8.0) |
| Prior Endo care (%) | 136 (68.0) | 67 (67.7) | 69 (68.3) |
| Prior Home Telehealth enrollment (%) | 12 (6.0) | 6 (6.1) | 6 (5.9) |
| Clinical measures | |||
| Baseline HbA1c, mean (SD) | 10.2 (1.3) | 10.2 (1.4) | 10.1 (1.2) |
| Body mass index (kg/m2), mean (SD) | 34.8 (6.7) | 35.2 (7.0) | 34.5 (6.4) |
| Insulin use, n (%) | 143 (71.5) | 64 (64.6) | 79 (78.2) |
| Hypertension, n (%) | 166 (83.0) | 85 (85.9) | 81 (80.2) |
| Hyperlipidemia, n (%)b | 171 (85.5) | 87 (87.9) | 84 (83.2) |
| Social support, n (%)c | 191 (95.5) | 93 (93.9) | 98 (97.0) |
| Psychosocial measures | |||
| NVS score, mean (SD) | 3.5 (2.0) | 3.4 (2.1) | 3.5 (1.8) |
| DKQ score, mean (SD) | 18.2 (2.6) | 17.9 (2.6) | 18.4 (2.6) |
| DSMQ score, mean (SD) | 6.7 (1.6) | 6.5 (1.7) | 6.9 (1.5) |
| VOILS Medication Non-adherence Questionnaire score for Insulin, mean (SD)d |
1.6 (0.8) | 1.8 (0.9) | 1.6 (0.8) |
| VOILS Medication Non-adherence Questionnaire score for Diabetes Pills, mean (SD)e |
1.5 (0.8) | 1.5 (0.8) | 1.4 (0.7) |
| DDS score, mean (SD) | 1.9 (0.8) | 1.9 (0.9) | 1.9 (0.7) |
| PCS score, mean (SD) | 5.2 (1.5) | 5.2 (1.4) | 5.2 (1.5) |
| PROMIS Self-Efficacy score, mean (SD) | 46.4 (7.9) | 45.9 (8.0) | 46.9 (7.9) |
| PROMIS Pain Interference Scale score, mean (SD) |
58.9 (9.8) | 58.6 (10.2) | 59.2 (9.5) |
| HCCQ Diabetes score, mean (SD)f | 5.9 (1.5) | 6.0 (1.3) | 5.8 (1.6) |
| Depression (PHQ-8) score, mean (SD)g | 7.3 (5.7) | 7.6 (6.1) | 7.0 (5.2) |
NVS = Newest Vital Signs; DKQ-24 = Diabetes Knowledge Questionnaire; DSMQ = Diabetes Self-Management Questionnaire; DDS = Diabetes Distress Scale; PCS = Perceived Competence Scale; HCCQ = Health Care Climate Questionnaire; PHQ-8 = Patient Health Questionnaire; BG = blood glucose.
1 patient in the Standard Home Telehealth group responded Don't Know to the Hispanic/Latino Ethnicity question.
1 patient in the Intervention group responded Don't Know to having high cholesterol and to having social support.
Social support was assessed by asking, “Do you have someone you feel close to, someone you can trust and confide in?”
22 patients in the Intervention group and 35 patients in the Standard Telehealth group do not have an insulin adherence score because they reported not taking insulin at baseline.
7 patients in the Intervention group and 11 patients in the Standard Telehealth group do not have a diabetes pill adherence score because they reported not taking diabetes pills at baseline.
1 patient in the Intervention group and 1 patient in the Standard Telehealth group are missing the PHQ score.
1 patient in the Intervention group is missing the HCCQ score.
4. Discussion
4.1. Overview
PRACTICE-DM is an ongoing, active comparator RCT that is examining the effectiveness of a comprehensive telehealth intervention for PPDM. In addition to HbA1c reduction, the impact on several important patient-centered outcomes will be examined, including diabetes distress, medication adherence, and quality of life. We will also evaluate intervention costs and utilization, and perform a process evaluation to help inform future intervention refinement and implementation.
4.2. Importance and relevance of PRACTICE-DM
Several key features contribute to this study's value. The PRACTICE-DM intervention targets patients with PPDM, a group that contributes excessively to the national diabetes burden [7,69]. Our comprehensive focus on this uniquely high-risk and high-cost diabetes population creates substantial potential for prevention of costly short- and long-term diabetes complications. Notably, our PPDM population is 72.0% African American, suggesting that by intervening on PPDM, we may be simultaneously addressing racial disparities in diabetes outcomes.
Critically, the PRACTICE-DM intervention is explicitly designed to use existing VHA clinical staffing, infrastructure, and equipment. As such, the intervention has unique promise for translation and implementation within VHA. Despite its potential, comprehensive telehealth-based diabetes management remains insufficiently utilized in clinical practice, owing mainly to barriers around financial support, availability of staffing and telehealth equipment, and integration with EHR systems. Because the PRACTICE-DM intervention leverages prior VHA investments in telehealth, it could bridge the current gap in utilization of comprehensive telehealth and become a feasible option in practice.
Rather than comparing the PRACTICE-DM intervention to clinic-based diabetes care, we are using standard VHA HT services as an active comparator. Although VHA has successfully implemented its HT network nationwide, this resource remains understudied. Using standard HT as our comparator for the PRACTICE-DM intervention is not only appropriate, given the PPDM population's established resistance to clinic-based diabetes care, but it will also provide valuable data regarding the impact of VHA HT telemonitoring and care coordination.
The PRACTICE-DM intervention exposes patients with PPDM to multiple therapeutic approaches, so it may improve meaningful, patient-centered outcomes beyond glycemic control. We selected outcome measures that will not only facilitate insights regarding the PRACTICE-DM intervention's secondary benefits, but will also allow us to understand the impact of each intervention component individually. Recognizing the relative contributions of each individual component through quantitative measures and qualitative analyses will deepen our understanding of the intervention's mechanisms of effect, and will also suggest opportunities for further modification.
Although the PRACTICE-DM intervention was developed for delivery via VHA infrastructure, the primary concept driving the intervention's design – use of existing staffing and infrastructure – is broadly applicable. In the United States, more than 75% of health systems have some degree of telehealth capacity as of 2017, and this prevalence continues to rise each year [70]. Much of the PRACTICE-DM intervention's approach is amenable to adaptation for other systems. Additionally, while the current study focuses on PPDM, the idea of comprehensive HT-based care for clinic-refractory patients is in essence disease-agnostic. Approximately 60% of all adults have at least one chronic disease, accounting for 3.5 trillion in all healthcare expenditures and 70% of all deaths in the United States [71,72]. The PRACTICE-DM intervention approach could easily be adapted for hypertension, heart failure, and other chronic diseases, thus improving long-term outcomes and costs across a spectrum of conditions. This potential for adaptation to other disease states magnifies the impact of the present study.
4.3. Impact of COVID-19 pandemic
The COVID-19 pandemic has presented substantial challenges for both clinical care and the conduct of clinical research. In spite of the pandemic's widespread impact, delivery of the PRACTICE-DM intervention and active comparator HT intervention has been able to continue uninterrupted at both sites. This robustness against interruption points to a fundamental strength of telehealth – using technology to align care services around patients facilitates patient-provider contact in a manner that circumvents limitations of in-person care. Utilization of telehealth has increased dramatically during the pandemic [73], which accentuates the importance of this study.
While delivery of the study interventions has continued unimpeded during the pandemic, data collection has been affected to a degree. In-person collection of data for research purposes has been temporarily put on hold during the pandemic, which has led to delays in collecting HbA1c, BP, and BMI data for some patients. To mitigate the impact of these delays on our primary outcome, we will conduct analyses allowing for the inclusion of clinical HbA1c data, adapt our modeling approaches to account for HbA1c data collected at varying time points and, and adjust for missing values as necessary. Additionally, patients have continued uploading of SMBG data from their meters during the pandemic; we are able to obtain these data directly from the Medtronic vendor database, and will be able to use them for exploratory assessments of glycemic control. We have also been documenting clinical self-reported home BP and BMI data, which may allow for exploratory sensitivity analyses. Of note, we have been able to continue collecting survey data by phone, so these data are unaffected. Fortunately, collection of lab data has resumed, and we do not anticipate that these challenges will significantly affect our primary analysis plan or the reliability of our findings. Any adaptations to our analysis plan or other impacts from the pandemic will be appropriately described in detail in future manuscripts.
4.4. Limitations
Because the PPDM population comprises individuals with poor glycemic control that persists despite engagement with clinic-based care, our findings may not apply to patients with poorly controlled diabetes who have limited healthcare engagement or newly diagnosed diabetes. However, patients whose diabetes has proven refractory to clinic-based care are likely to reap the greatest benefit from augmenting clinic-based care with approaches like the PRACTICE-DM intervention, making this population a critical target for study. Similarly, because both study arms require willingness to attempt daily blood glucose self-monitoring, our findings may not apply to patients with PPDM who are explicitly unwilling to self-monitor. Our population demographics may likewise limit generalizability; however, the relatively few exclusion criteria and successful recruitment of women (22.5% of overall study population compared to 8.4% of total VHA population) may help mitigate this limitation [74]. Patients with type 1 diabetes were excluded from this study, so our findings may not apply to this population.
As discussed, the PRACTICE-DM intervention is designed for delivery via existing VHA HT services, which may limit generalizability beyond VHA. However, as detailed above, the concept of designing interventions around existing staffing and infrastructure is broadly applicable beyond VHA, and the proposed approach should be adaptable to other systems. Of note, our Durham site used only one HT nurse for each intervention arm (as opposed to our Richmond site, which used 3–5); utilization of fewer nurses at this site was driven by site-specific considerations, and the skills or biases of these individual nurses could in theory confound differences in outcomes. However, the standardized, structured delivery of each PRACTICE-DM intervention component will mitigate this risk, and our ongoing fidelity analysis should identify inconsistent performance between nurses and facilitate rapid intervention as needed.
As above, we selected an active comparator for the PRACTICE-DM intervention because the PPDM population has by definition proven resistant to clinic-based diabetes care. While we view this design as the most ethical option for the PPDM population, it will limit our ability to account for temporal changes in diabetes control within our population. This limitation is mitigated to an extent by our pilot data, which demonstrated that a PPDM cohort exhibited minimal improvement in HbA1c over a 6-month period under usual clinic-based care [44].
Finally, the PRACTICE-DM intervention combines five components, and the study is not designed to determine whether a simpler approach might work equally well. As such, patients in the PRACTICE-DM arm will likely have more intervention contact than in the standard HT arm. Our examinations of each intervention component's impact on secondary outcomes may provide data to help mitigate these limitations. Specifically, we included secondary outcomes that map to each of the five intervention components, which will allow us to examine the components' clinical effectiveness through mediator analyses. This approach will provide evidence as to how each component contributed to any intervention effect, and may permit inference as to which components require refinement. Furthermore, our cost analysis will enable us to quantify added expenses associated with PRACTICE-DM, and whether any clinical benefits offset these costs.
5. Conclusions
The PRACTICE-DM intervention is a novel, comprehensive telehealth approach that seeks to improve glycemic control for patients with PPDM, who represent a uniquely high-risk and high-cost diabetes population. Because the PRACTICE-DM intervention can be delivered using ubiquitous VHA clinical staffing and infrastructure, it may be amenable to broad clinical implementation and dissemination. If successful, the PRACTICE-DM intervention could finally make comprehensive telehealth a viable option for patients whose diabetes is resistant to clinic-based measures.
Acknowledgements
This study is supported by a grant from VA Health Services Research & Development [IIR 16-213, Crowley PI]. The authors also acknowledge support from the Durham Center of Innovation to Accelerate Discovery and Practice Transformation (ADAPT) [CIN 13-410] within the Durham VA Health Care System. EAK is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health [TL1 TR002555]. HBB and MLM are supported by Senior Career Scientist awards from VA Health Services Research & Development (RCS 08-027, RCS 10-391). STS is supported by a Career Development Award from VA Clinical Science Research and Development (IK2CX001397). MJC was supported by a Career Development Award from VA Health Services Research & Development (CDA 13-261) during part of the study. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or VA.
HBB acknowledges receiving grant funding to his institutions from Otsuka, Novo Nordisk, Sanofi, Improved Patient Outcome, Humana, Pharma foundation, and Proteus as well as consulting from Abbott, Preventric Diagnostic, Sanofi. None of these grants or consulting pertained to the current study. Otherwise, the rest of the authors have no conflicts of interest to disclose.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cct.2020.106157.
Supplementary data
Supplementary material
References
- 1.Centers for Disease Control and Prevention Diabetes Report Card 2017. 2018. https://www.cdc.gov/diabetes/pdfs/library/diabetesreportcard2017-508.pdf (Accessed 28 Feb 2020)
- 2.Engelgau M.M., Geiss L.S., Saaddine J.B., Boyle J.P., Benjamin S.M., Gregg E.W., Tierney E.F., Rios-Burrows N., Mokdad A.H., Ford E.S., Imperatore G., Narayan K.M. The evolving diabetes burden in the United States. Ann. Intern. Med. 2004;140(11):945–950. doi: 10.7326/0003-4819-140-11-200406010-00035. [DOI] [PubMed] [Google Scholar]
- 3.Alexopoulos A.S., Jackson G.L., Edelman D., Smith V.A., Berkowitz T.S.Z., Woolson S.L., Bosworth H.B., Crowley M.J. Clinical factors associated with persistently poor diabetes control in the Veterans Health Administration: a nationwide cohort study. PLoS One. 2019;14(3) doi: 10.1371/journal.pone.0214679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crowley M.J., Holleman R., Klamerus M.L., Bosworth H.B., Edelman D., Heisler M. Factors associated with persistent poorly controlled diabetes mellitus: clues to improving management in patients with resistant poor control. Chronic Illn. 2014;10(4):291–302. doi: 10.1177/1742395314523653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilmer T.P., O'Connor P.J., Rush W.A., Crain A.L., Whitebird R.R., Hanson A.M., Solberg L.I. Predictors of health care costs in adults with diabetes. Diabetes Care. 2005;28(1):59–64. doi: 10.2337/diacare.28.1.59. [DOI] [PubMed] [Google Scholar]
- 6.McBrien K.A., Manns B.J., Chui B., Klarenbach S.W., Rabi D., Ravani P., Hemmelgarn B., Wiebe N., Au F., Clement F. Health care costs in people with diabetes and their association with glycemic control and kidney function. Diabetes Care. 2013;36(5):1172–1180. doi: 10.2337/dc12-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stratton I.M., Adler A.I., Neil H.A., Matthews D.R., Manley S.E., Cull C.A., Hadden D., Turner R.C., Holman R.R. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Diabetes Association, 7 Obesity Management for the Treatment of Type 2 Diabetes. Diabetes Care. 2017;40(Suppl. 1):S57–S63. doi: 10.2337/dc17-S010. [DOI] [PubMed] [Google Scholar]
- 9.Anderson R.J., Freedland K.E., Clouse R.E., Lustman P.J. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24(6):1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 10.Chiu C.J., Wray L.A. Factors predicting glycemic control in middle-aged and older adults with type 2 diabetes. Prev. Chronic Dis. 2010;7(1):A08. [PMC free article] [PubMed] [Google Scholar]
- 11.Davidson J. Strategies for improving glycemic control: effective use of glucose monitoring. Am. J. Med. 2005;118(Suppl 9A):27S–32S. doi: 10.1016/j.amjmed.2005.07.054. [DOI] [PubMed] [Google Scholar]
- 12.de Groot M., Anderson R., Freedland K.E., Clouse R.E., Lustman P.J. Association of depression and diabetes complications: a meta-analysis. Psychosom. Med. 2001;63(4):619–630. doi: 10.1097/00006842-200107000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Donnelly L.A., Morris A.D., Evans J.M., D.M. collaboration Adherence to insulin and its association with glycaemic control in patients with type 2 diabetes. QJM. 2007;100(6):345–350. doi: 10.1093/qjmed/hcm031. [DOI] [PubMed] [Google Scholar]
- 14.Hartz A., Kent S., James P., Xu Y., Kelly M., Daly J. Factors that influence improvement for patients with poorly controlled type 2 diabetes. Diabetes Res. Clin. Pract. 2006;74(3):227–232. doi: 10.1016/j.diabres.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 15.Hill-Briggs F., Gary T.L., Bone L.R., Hill M.N., Levine D.M., Brancati F.L. Medication adherence and diabetes control in urban African Americans with type 2 diabetes. Health Psychol. 2005;24(4):349–357. doi: 10.1037/0278-6133.24.4.349. [DOI] [PubMed] [Google Scholar]
- 16.Surwit R.S., van Tilburg M.A., Parekh P.I., Lane J.D., Feinglos M.N. Treatment regimen determines the relationship between depression and glycemic control. Diabetes Res. Clin. Pract. 2005;69(1):78–80. doi: 10.1016/j.diabres.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Morrison F., Shubina M., Turchin A. Encounter frequency and serum glucose level, blood pressure, and cholesterol level control in patients with diabetes mellitus. Arch. Intern. Med. 2011;171(17):1542–1550. doi: 10.1001/archinternmed.2011.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenthal E.S., Bashan E., Herman W.H., Hodish I. The effort required to achieve and maintain optimal glycemic control. J. Diabetes Complicat. 2011;25(5):283–288. doi: 10.1016/j.jdiacomp.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization Telehealth. 2020. https://www.who.int/sustainable-development/health-sector/strategies/telehealth/en/
- 20.U.S. Department of Veterans Affairs, VA Telehealth Services 2020. https://telehealth.va.gov Accessed 29 Feb 2020.
- 21.Secretariat Medical Advisory Home telemonitoring for type 2 diabetes: an evidence-based analysis. Ont Health Technol Assess Ser. 2009;9(24):1–38. [PMC free article] [PubMed] [Google Scholar]
- 22.Kitsiou S., Pare G., Jaana M. Systematic reviews and meta-analyses of home telemonitoring interventions for patients with chronic diseases: a critical assessment of their methodological quality. J. Med. Internet Res. 2013;15(7):e150. doi: 10.2196/jmir.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Secretariat Medical Advisory Behavioural interventions for type 2 diabetes: an evidence-based analysis. Ont Health Technol Assess Ser. 2009;9(21):1–45. [PMC free article] [PubMed] [Google Scholar]
- 24.Kempf K., Altpeter B., Berger J., Reuss O., Fuchs M., Schneider M., Gartner B., Niedermeier K., Martin S. Efficacy of the Telemedical lifestyle intervention program TeLiPro in advanced stages of type 2 diabetes: a randomized controlled trial. Diabetes Care. 2017;40(7):863–871. doi: 10.2337/dc17-0303. [DOI] [PubMed] [Google Scholar]
- 25.Pimouguet C., Le Goff M., Thiebaut R., Dartigues J.F., Helmer C. Effectiveness of disease-management programs for improving diabetes care: a meta-analysis. CMAJ. 2011;183(2):E115–E127. doi: 10.1503/cmaj.091786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atlantis E., Fahey P., Foster J. Collaborative care for comorbid depression and diabetes: a systematic review and meta-analysis. BMJ Open. 2014;4(4) doi: 10.1136/bmjopen-2013-004706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lauffenburger J.C., Ghazinouri R., Jan S., Makanji S., Ferro C.A., Lewey J., Wittbrodt E., Lee J., Haff N., Fontanet C.P., Choudhry N.K. Impact of a novel pharmacist-delivered behavioral intervention for patients with poorly-controlled diabetes: the ENhancing outcomes through goal assessment and generating engagement in diabetes mellitus (ENGAGE-DM) pragmatic randomized trial. PLoS One. 2019;14(4) doi: 10.1371/journal.pone.0214754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nesari M., Zakerimoghadam M., Rajab A., Bassampour S., Faghihzadeh S. Effect of telephone follow-up on adherence to a diabetes therapeutic regimen. Jpn. J. Nurs. Sci. 2010;7(2):121–128. doi: 10.1111/j.1742-7924.2010.00146.x. [DOI] [PubMed] [Google Scholar]
- 29.Sarayani A., Mashayekhi M., Nosrati M., Jahangard-Rafsanjani Z., Javadi M., Saadat N., Najafi S., Gholami K. Efficacy of a telephone-based intervention among patients with type-2 diabetes; a randomized controlled trial in pharmacy practice. Int. J. Clin. Pharm. 2018;40(2):345–353. doi: 10.1007/s11096-018-0593-0. [DOI] [PubMed] [Google Scholar]
- 30.Sherifali D., Bai J.W., Kenny M., Warren R., Ali M.U. Diabetes self-management programmes in older adults: a systematic review and meta-analysis. Diabet. Med. 2015;32(11):1404–1414. doi: 10.1111/dme.12780. [DOI] [PubMed] [Google Scholar]
- 31.von Storch K., Graaf E., Wunderlich M., Rietz C., Polidori M.C., Woopen C. Telemedicine-assisted self-management program for Type 2 diabetes patients. Diabetes Technol. Ther. 2019;21(9):514–521. doi: 10.1089/dia.2019.0056. [DOI] [PubMed] [Google Scholar]
- 32.Wu L., Forbes A., Griffiths P., Milligan P., While A. Telephone follow-up to improve glycaemic control in patients with Type 2 diabetes: systematic review and meta-analysis of controlled trials. Diabet. Med. 2010;27(11):1217–1225. doi: 10.1111/j.1464-5491.2010.03113.x. [DOI] [PubMed] [Google Scholar]
- 33.Crowley M.J., Powers B.J., Olsen M.K., Grubber J.M., Koropchak C., Rose C.M., Gentry P., Bowlby L., Trujillo G., Maciejewski M.L., Bosworth H.B. The Cholesterol, Hypertension, And Glucose Education (CHANGE) study: results from a randomized controlled trial in African Americans with diabetes. Am. Heart J. 2013;166(1):179–186. doi: 10.1016/j.ahj.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Edelman D., Dolor R.J., Coffman C.J., Pereira K.C., Granger B.B., Lindquist J.H., Neary A.M., Harris A.J., Bosworth H.B. Nurse-led behavioral management of diabetes and hypertension in community practices: a randomized trial. J. Gen. Intern. Med. 2015;30(5):626–633. doi: 10.1007/s11606-014-3154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adler-Milstein J., Kvedar J., Bates D.W. Telehealth among US hospitals: several factors, including state reimbursement and licensure policies, influence adoption. Health Aff (Millwood) 2014;33(2):207–215. doi: 10.1377/hlthaff.2013.1054. [DOI] [PubMed] [Google Scholar]
- 36.Glasgow R.E., Lichtenstein E., Marcus A.C. Why don’t we see more translation of health promotion research to practice? Rethinking the efficacy-to-effectiveness transition. Am. J. Public Health. 2003;93(8):1261–1267. doi: 10.2105/ajph.93.8.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kruse C.S., Karem P., Shifflett K., Vegi L., Ravi K., Brooks M. Evaluating barriers to adopting telemedicine worldwide: a systematic review. J. Telemed. Telecare. 2018;24(1):4–12. doi: 10.1177/1357633X16674087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ross J., Stevenson F., Lau R., Murray E. Factors that influence the implementation of e-health: a systematic review of systematic reviews (an update) Implement. Sci. 2016;11 doi: 10.1186/s13012-016-0510-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bodde A.E., Shippee N.D., May C.R., Mair F.S., Erwin P.J., Murad M.H., Montori V.M. Examining health promotion interventions for patients with chronic conditions using a novel patient-centered complexity model: protocol for a systematic review and meta-analysis. Syst Rev. 2013;2:29. doi: 10.1186/2046-4053-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shippee N.D., Allen S.V., Leppin A.L., May C.R., Montori V.M. Attaining minimally disruptive medicine: context, challenges and a roadmap for implementation. J R Coll Physicians Edinb. 2015;45(2):118–122. doi: 10.4997/JRCPE.2015.206. [DOI] [PubMed] [Google Scholar]
- 41.Shippee N.D., Shah N.D., May C.R., Mair F.S., Montori V.M. Cumulative complexity: a functional, patient-centered model of patient complexity can improve research and practice. J. Clin. Epidemiol. 2012;65(10):1041–1051. doi: 10.1016/j.jclinepi.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 42.Zullig L.L., Whitson H.E., Hastings S.N., Beadles C., Kravchenko J., Akushevich I., Maciejewski M.L. A systematic review of conceptual frameworks of medical complexity and new model development. J. Gen. Intern. Med. 2016;31(3):329–337. doi: 10.1007/s11606-015-3512-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andrews S.M., Sperber N.R., Gierisch J.M., Danus S., Macy S.L., Bosworth H.B., Edelman D., Crowley M.J. Patient perceptions of a comprehensive telemedicine intervention to address persistent poorly controlled diabetes. Patient Prefer Adherence. 2017;11:469–478. doi: 10.2147/PPA.S125673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crowley M.J., Edelman D., McAndrew A.T., Kistler S., Danus S., Webb J.A., Zanga J., Sanders L.L., Coffman C.J., Jackson G.L., Bosworth H.B. Practical telemedicine for veterans with persistently poor diabetes control: a randomized pilot trial. Telemed. J. E Health. 2016;22(5):376–384. doi: 10.1089/tmj.2015.0145. [DOI] [PubMed] [Google Scholar]
- 45.U.S. Department of Veterans Affairs VA/DoD Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care. 2017. https://www.healthquality.va.gov/guidelines/CD/diabetes/VADoDDMCPGFinal508.pdf Accessed 29 Feb 2020.
- 46.American Diabetes Association Standards of medical care in diabetes. Diabetes Care. 2017;40:S1–S135. doi: 10.2337/dc17-0299. [DOI] [PubMed] [Google Scholar]
- 47.Crowley M.J., Bosworth H.B., Coffman C.J., Lindquist J.H., Neary A.M., Harris A.C., Datta S.K., Granger B.B., Pereira K., Dolor R.J., Edelman D. Tailored Case Management for Diabetes and Hypertension (TEACH-DM) in a community population: study design and baseline sample characteristics. Contemp Clin Trials. 2013;36(1):298–306. doi: 10.1016/j.cct.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Melnyk S.D., Zullig L.L., McCant F., Danus S., Oddone E., Bastian L., Olsen M., Stechuchak K.M., Edelman D., Rakley S., Morey M., Bosworth H.B. Telemedicine cardiovascular risk reduction in veterans. Am. Heart J. 2013;165(4):501–508. doi: 10.1016/j.ahj.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown S.A., Garcia A.A., Brown A., Becker B.J., Conn V.S., Ramirez G., Winter M.A., Sumlin L.L., Garcia T.J., Cuevas H.E. Biobehavioral determinants of glycemic control in type 2 diabetes: a systematic review and meta-analysis. Patient Educ. Couns. 2016;99(10):1558–1567. doi: 10.1016/j.pec.2016.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.American Diabetes Association, 4 Lifestyle Management. Diabetes Care. 2017;40(Suppl. 1):S33–S43. doi: 10.2337/dc17-S007. [DOI] [PubMed] [Google Scholar]
- 51.Kroenke K., Strine T.W., Spitzer R.L., Williams J.B., Berry J.T., Mokdad A.H. The PHQ-8 as a measure of current depression in the general population. J. Affect. Disord. 2009;114(1–3):163–173. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 52.U.S. Department of Veterans Affairs VA/DoD Clinical Practice Guideline for the Management of Major Depressive Disorder. 2016. https://www.healthquality.va.gov/guidelines/MH/mdd/VADoDMDDCPGFINAL82916.pdf Accessed 29 Feb 2020.
- 53.Lustman P.J., Clouse R.E., Nix B.D., Freedland K.E., Rubin E.H., McGill J.B., Williams M.M., Gelenberg A.J., Ciechanowski P.S., Hirsch I.B. Sertraline for prevention of depression recurrence in diabetes mellitus: a randomized, double-blind, placebo-controlled trial. Arch. Gen. Psychiatry. 2006;63(5):521–529. doi: 10.1001/archpsyc.63.5.521. [DOI] [PubMed] [Google Scholar]
- 54.Lustman P.J., Williams M.M., Sayuk G.S., Nix B.D., Clouse R.E. Factors influencing glycemic control in type 2 diabetes during acute- and maintenance-phase treatment of major depressive disorder with bupropion. Diabetes Care. 2007;30(3):459–466. doi: 10.2337/dc06-1769. [DOI] [PubMed] [Google Scholar]
- 55.Eton D.T., Elraiyah T.A., Yost K.J., Ridgeway J.L., Johnson A., Egginton J.S., Mullan R.J., Murad M.H., Erwin P.J., Montori V.M. A systematic review of patient-reported measures of burden of treatment in three chronic diseases. Patient Relat Outcome Meas. 2013;4:7–20. doi: 10.2147/PROM.S44694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Polonsky W.H., Fisher L., Earles J., Dudl R.J., Lees J., Mullan J., Jackson R.A. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28(3):626–631. doi: 10.2337/diacare.28.3.626. [DOI] [PubMed] [Google Scholar]
- 57.Schmitt A., Gahr A., Hermanns N., Kulzer B., Huber J., Haak T. The Diabetes Self-Management Questionnaire (DSMQ): development and evaluation of an instrument to assess diabetes self-care activities associated with glycaemic control. Health Qual. Life Outcomes. 2013;11:138. doi: 10.1186/1477-7525-11-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmitt A., Reimer A., Hermanns N., Huber J., Ehrmann D., Schall S., Kulzer B. Assessing diabetes self-management with the diabetes self-management questionnaire (DSMQ) can help analyse behavioural problems related to reduced glycaemic control. PLoS One. 2016;11(3) doi: 10.1371/journal.pone.0150774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Williams G.C., Freedman Z.R., Deci E.L. Supporting autonomy to motivate patients with diabetes for glucose control. Diabetes Care. 1998;21(10):1644–1651. doi: 10.2337/diacare.21.10.1644. [DOI] [PubMed] [Google Scholar]
- 60.Glasgow R.E., Vogt T.M., Boles S.M. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am. J. Public Health. 1999;89(9):1322–1327. doi: 10.2105/ajph.89.9.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bent S., Padula A., Avins A.L. Brief communication: better ways to question patients about adverse medical events: a randomized, controlled trial. Ann. Intern. Med. 2006;144(4):257–261. doi: 10.7326/0003-4819-144-4-200602210-00007. [DOI] [PubMed] [Google Scholar]
- 62.Fitzmaurice G.M., Laird N.M., Ware J.H. 2nd ed. Wiley; Hoboken, N.J: 2011. Applied Longitudinal Analysis. [Google Scholar]
- 63.P. Committee for Proprietary Medicinal Committee for Proprietary Medicinal Products (CPMP): points to consider on adjustment for baseline covariates. Stat. Med. 2004;23(5):701–709. doi: 10.1002/sim.1647. [DOI] [PubMed] [Google Scholar]
- 64.Hsieh H.F., Shannon S.E. Three approaches to qualitative content analysis. Qual. Health Res. 2005;15(9):1277–1288. doi: 10.1177/1049732305276687. [DOI] [PubMed] [Google Scholar]
- 65.Manning W.G., Mullahy J. Estimating log models: to transform or not to transform? J. Health Econ. 2001;20(4):461–494. doi: 10.1016/s0167-6296(01)00086-8. [DOI] [PubMed] [Google Scholar]
- 66.Smith V.A., Preisser J.S., Neelon B., Maciejewski M.L. A marginalized two-part model for semicontinuous data. Stat. Med. 2014;33(28):4891–4903. doi: 10.1002/sim.6263. [DOI] [PubMed] [Google Scholar]
- 67.Verbeke G., Molenberghs G. Springer; New York: 1997. Linear Mixed Models in Practice : An SAS-Oriented Approach. [Google Scholar]
- 68.Rubin D.B. Wiley-Interscience; Hoboken, N.J: 2004. Multiple Imputation for Nonresponse in Surveys. [Google Scholar]
- 69.Yoon J., Scott J.Y., Phibbs C.S., Wagner T.H. Recent trends in veterans affairs chronic condition spending. Popul Health Manag. 2011;14(6):293–298. doi: 10.1089/pop.2010.0079. [DOI] [PubMed] [Google Scholar]
- 70.American Hospital Association . 2019. Fact Sheet: Telehealth. [Google Scholar]
- 71.Bashshur R.L., Shannon G.W., Smith B.R., Alverson D.C., Antoniotti N., Barsan W.G., Bashshur N., Brown E.M., Coye M.J., Doarn C.R., Ferguson S., Grigsby J., Krupinski E.A., Kvedar J.C., Linkous J., Merrell R.C., Nesbitt T., Poropatich R., Rheuban K.S., Sanders J.H., Watson A.R., Weinstein R.S., Yellowlees P. The empirical foundations of telemedicine interventions for chronic disease management. Telemed. J. E Health. 2014;20(9):769–800. doi: 10.1089/tmj.2014.9981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Centers for Disease Control and Prevention . 2019. Chronic Diseases in America. [Google Scholar]
- 73.Bashshur R., Doarn C.R., Frenk J.M., Kvedar J.C., Woolliscroft J.O. Telemedicine and the COVID-19 pandemic, lessons for the future. Telemed. J. E Health. 2020;26(5):571–573. doi: 10.1089/tmj.2020.29040.rb. [DOI] [PubMed] [Google Scholar]
- 74.U.S. Census Bureau . 2017. Characteristics of Female Veterans - an Analytic View across Age-Cohorts: 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material