Abstract
Introduction:
Pharmacists are poised to be the health care professionals best suited to provide medication-related consults and services based on a patient's genetics. Despite its potential benefits, the implementation of pharmacogenetic (PGx) testing into primary clinical settings has been slow among medically underserved populations. To our knowledge, this is the first time that PGx-driven recommendations have been incorporated into a Comprehensive Medication Management (CMM) service in a Hispanic population.
Objectives:
The aim of this study is to evaluate the clinical utility of adding PGx guidance into pharmacist-driven CMM.
Methods:
This is a pre- and post-interventional design study. Patients were recruited from a psychologist's clinic. A total of 24 patients had a face-to-face interview with a pharmacist to complete a CMM, Personal Medication Record, and Medication-Related Action Plan (MAP) blind to PGx findings. Collected buccal DNA samples were genotyped using drug-metabolizing enzymes and transporters (DMET) Plus Array.
Results:
The pharmacist generated new MAPs for each patient based on PGx results. Genetic variants that could potentially affect the safety and effectiveness of at least one drug in the pharmacotherapy were identified in 96% of patients, for whom the pharmacist changed the initial recommendations. Polymorphisms in genes encoding for isoenzymes CYP2D6, CYP2C19, and CYP2C9 were identified in 83%, 52%, and 41% of patients, respectively. Pharmacists performing CMM identified 22 additional medication problems after PGx determinations. Moreover, they agreed with the clinical utility of PGx in the studied sample based on perceived value of adding PGx to traditional CMM and its utility in the decision-making process of pharmacists.
Conclusions:
The study confirmed the critical role to be played by pharmacists in facilitating the clinical usage of relevant genetic information to optimize drug therapy decisions as well as their involvement on many levels of these multidisciplinary implementation efforts, including championing and leading PGx-guided CMM services.
Keywords: pharmacogenetics, cytochrome P450, clinical decision support
1 ∣. INTRODUCTION
Genetic variants can affect the pharmacokinetics (PK) and pharmacodynamics (PD) of a drug, resulting in variable interpatient response to therapies.1 Pharmacogenetic (PGx) testing identifies clinically actionable genetic variants in genes encoding for relevant drug-metabolizing enzymes, receptors, and transporters, which in turn might impact the individual's metabolic capacity as well as the drug's safety and effectiveness. A retrospective study in 14 578 patients found that 93% presented at least one risk phenotype and 24% of all drug-related interactions were either drug-gene (DGI) or drug-drug-gene interactions (DDGI) of serious consequences that required treatment changes. Notably, such DGIs and DDGIs could not have been identified without PGx information.2
The American Pharmacists Association (APhA) supports the use of genomic data in pharmacy practice.1 Similarly, the American Society of Health-System Pharmacists (ASHP) upheld the pharmacist's role in implementing PGx at clinical settings, which translates into improved outcomes, better drug selection, and increased patient safety.3 Likewise, the American College of Clinical Pharmacy (ACCP) envisions the clinical pharmacogenomic implementation as an essential component of precision pharmacotherapy.4 A feasible approach to integrate PGx into pharmacy practice is through Comprehensive Medication Management (CMM) services.5-7 In a previous report, incorporation of PGx resulted in either elimination or replacement of drug therapies as well as in adequate monitoring of drug response. Interestingly, most referrals for PGx testing came from psychiatry services (77%).8
While PGx variability among patients worldwide is well-documented, studies in Hispanics are scarce. Puerto Ricans are genetically distinct from other Hispanics due to the admixture from several parental groups.9,10 Accordingly, it is a diverse population that could certainly benefit from PGx guidance. However, there is no implementation of preemptive PGx in a CMM service in Puerto Rico. Consequently, we developed this collaborative pilot program aimed at demonstrating the benefit of incorporating PGx information into CMM services.
2 ∣. MATERIALS AND METHODS
2.1 ∣. Study design
This is a pilot study, following a pre- and post-interventional experimental design, to perform an exploratory assessment of PGx testing as add-on information for traditional pharmacist-provided CMM services. Thirty-six patients who routinely receive psychological counseling for their psychiatric and other health conditions at a clinic in Toa Baja, Puerto Rico, were consented to participate in this study. Patients older than 20 years who meet inclusion criteria (ie, with no less than three diagnosed medical conditions and at least one prescribed medication for any of these conditions; or taking six or more prescribed drugs for any condition) were enrolled following a convenience sampling approach. Patients were selected due to a potentially significant benefit, because psychotropic medications are known to be affected by polymorphisms in CYP450 isozymes.11,12 About 32% of all actionable gene-drug pairs included in the Clinical Pharmacogenetics Implementation Consortium (CPIC) evidence-based guidelines relate to psychotropic medications.13 The study was approved by the University of Puerto Rico's institutional review board (approval # A4070117; March 23, 2017).
Participants were scheduled for a one-hour appointment at the clinic facility and were told to bring all of their medications, supplements and any recent laboratory test results. Four buccal swabs were collected from each participant. Clinical nongenetic and demographic information were extracted from medical records.
CMM was offered following the structure recommended by the Patient-Centered Primary Care Collaborative (PCPCC) Resource Guide.5 Clinical nongenetic and demographic information from participants were extracted from medical records. The pharmacist performed a Comprehensive Medication Review (CMR), created a Personal Medication Record (PMR) and identified medication-related problems (MRPs),14 documented therapy goals, and prepared individual Medication-Related Action Plans (MAP). These pre-PGx MAPs contained the initial recommendations for patients, based on the available information collected during a traditional CMM service prior to genotyping results, and served as controls for the post-PGx MAPs.
2.2 ∣. Genotyping
Genotyping was performed by using the Affymetrix DMET-Plus array (ThermoFisher Scientific, CA, USA) and following manufacturer's instructions.15-17
Supporting Information Table 1 depicts all relevant alleles on selected drug-metabolizing enzymes and transporters (DMET) pharmacogenes that were interrogated in our study cohort and their genetically inferred impact on enzyme functionality. Only DMET results for three major CYPs pharmacogenes (ie, CYP2C9, CYP2C19, and CYP2D6) were used.
The xTAG CYP2D6 Kit v3 mutation detection assays (Luminex, TX, USA) and INFINITI CYP2C19/2C9 Multiplex Assays (AutoGenomics, CA, USA) were also utilized at a CLIA-certified laboratory (Genomas, Inc., CT, USA) for interrogating these genes on Luminex xMAP 200 and Auto-Genomics Analyzer instruments, respectively, in order to confirm variant calls used to guide drug therapy recommendations. A custom laboratory information system (LIS) was used to call the result; in the LIS, a combination of automated calling (AC) and expert calling (EC) is implemented.18
2.3 ∣. Clinical decision support algorithm
Individual de-identified genotypes were analyzed in the EC-integrated LIS to report haplotypes and inferred phenotypes. The LIS algorithm integrates data from all three genes and computes Drug Metabolism Reserve indices for each patient from the combinatorial genotyping data.19-21 A higher index indicates a greater innate drug metabolism capacity of the individual. The LIS also provides clinical decision support (CDS) via its MEDtuning module for algorithmic customization of a patient's drug regimen guided by the indices.20,22,23 Gene-specific index values lead to recommendations to avoid those drugs which are substrates of the isoenzymes coded by the altered gene(s). CDS-derived clinically actionable recommendations (eg, best drug options, dose modifications) were then shared with the pharmacist, who completed a new MAP for each patient (post-PGx-MAP) accordingly.
2.4 ∣. Statistical analysis
Descriptive statistics of all demographic and clinical variables were used to characterize the study cohort. The percentage of patients to whom a different recommendation in pharmacotherapy was made based on the PGx results were also reported. Allele and genotype frequencies (mean, 95% confidence interval [CI]) were determined for all identified genetic variants across the three loci of interest. A two-tailed Wilcoxon signed rank test was conducted to examine whether there was a significant difference between the number of pre- and post-PGx MRPs. The same test was performed for each individual item comprising the medication-related problems. Statistical analyses were performed using Intellectus Statistics (Statistics Solutions, 2019).
3 ∣. RESULTS
Figure 1 depicts flowchart of enrollment in this study, between April 22 and November 15, 2017. Twenty-four patients with complete clinical and genomic data were considered for full analysis. The study completion date was April 2018.
FIGURE 1.
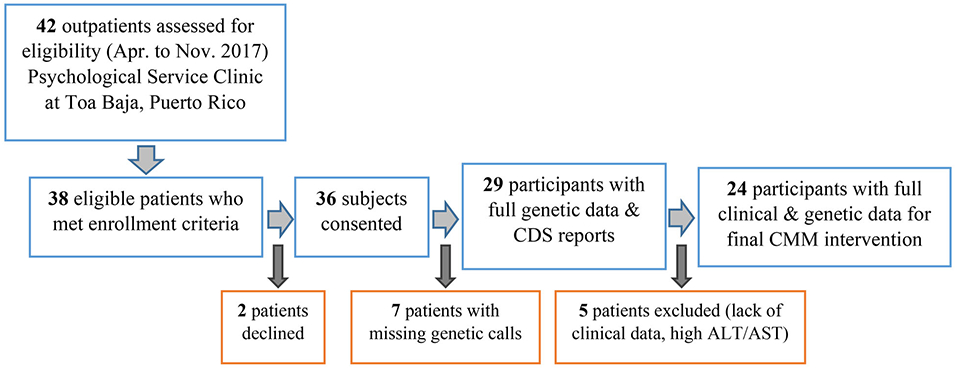
Flow diagram to illustrate the inclusion of participants in this study. A total of 38 eligible patients who met inclusion criteria were approached regarding participation in this study. Two patients declined to participate, resulting in 36 subjects enrolled. Seven patients had incomplete genotypes (eg, poor call rates), leaving only 29 with full data. Another five patients excluded from further analysis due to lack of complete clinical data or increased ALT/AST. ALT, alanine aminotransferase enzyme; AST, aspartate aminotransferase enzyme; CDS, clinical decision support; CMM, comprehensive medication management
Mean age of the patients was 49.9 ± 15 years old. Most were female (26, 89.7%). All patients self-identified as Caribbean Hispanics residing in Puerto Rico. The most prevalent medical conditions in participants were those related to psychiatric disorders (26, 89%), followed by hypertension (14, 48%), and insomnia (9, 31%). Sixteen patients (55%) also suffered from chronic pain. Number of prescriptions and nonprescription medications per patient was on average 8.5 ± 2.8 and 1.0 ± 2.2, respectively. Seventy-nine percent of patients were taking two or more medications to treat psychiatric disorders, and 42% received two or more prescriptions of antidepressants and typical/atypical antipsychotics concomitantly. Notably, all of these individuals were carriers of at least one cytochrome P450 enzymes (CYPs) polymorphism that limited their metabolic capacity. Baseline characteristics are shown in Table 1. Additionally, Supporting Information Table 2 lists all prescription drugs in the pharmacotherapy of patients from the study cohort divided by classes and metabolic pathways; a grand total of 232 drugs were prescribed to the cohort.
TABLE 1.
Baseline clinical and demographic characteristics of patients (N = 29)
| Characteristic | Value |
|---|---|
| Age, years (median ± SD) | 49.9 ± 14.8 |
| Number of prescription drugs per patient (median ± SD) | 8.5 ± 2.8 |
| Number of nonprescription drugs per patient (median ± SD) | 1.0 ± 2.2 |
| Gender, N (%) | |
| Female | 26 (89.7) |
| Male | 3 (10.3) |
| Ethnicity, N (%) | |
| Hispanics | 29 (100) |
| Medical conditions, N (%) | |
| Psychiatric disorder | 26 (89) |
| Pain | 16 (55) |
| Hypertension | 14 (48) |
| Insomnia | 9 (31) |
| Hyperlipidemia | 8 (27) |
| Thyroid disorder | 7 (24) |
| Diabetes | 2 (6.9) |
After conducting the traditional CMM service (ie, before having the PGx results and CDS reports available), the pharmacist identified a median of five MRPs per patient. Accordingly, a total of 129 MRPs were reported. Of the seven predefined MRP categories, the one most frequently identified was “Needs additional drug therapy,” which included both vaccines and medications for diagnosed conditions or patients' symptoms. For the purpose of this study, it was referring to a switch to another drug. The second most frequent MRP was nonadherence. MRPs were translated into pharmacotherapy-related recommendations in the corresponding patients' MAPs. The median number of recommendations per patient was three. Adding a drug therapy was the most frequent recommendation in 75% of patients, followed by the recommendation to discontinue a drug therapy in 58.3% of patients.
PGx testing identified relevant polymorphisms in pharmacogenes encoding for CYP2C19, CYP2C9, and CYP2D6 isozymes in 52%, 41%, and 83% of patients, respectively. The corresponding allele and genotype frequency distributions in the three loci are depicted in Figure 2. As can be seen, the most frequently found genotype was the CYP2C19*1/*2 diplotype that occurred in 38% of patients. Accordingly, the CYP2C19*2 polymorphism was the most prevalent variant across the three loci (minor allele frequency, MAF = 20.7%). As expected, no carrier of the CYP2C19*3 variant was found among participants. It is noteworthy that the no function CYP2C9*3 variant was the minor allele that most frequently occurred at this locus among participants (MAF = 15.5%). The CYP2C9*2 allele showed a MAF of around 7% in our cohort. Three patients harbored the *3/*5 diplotype, two the *2/*3, three the *1/*3, and another one the *3/*9.
FIGURE 2.
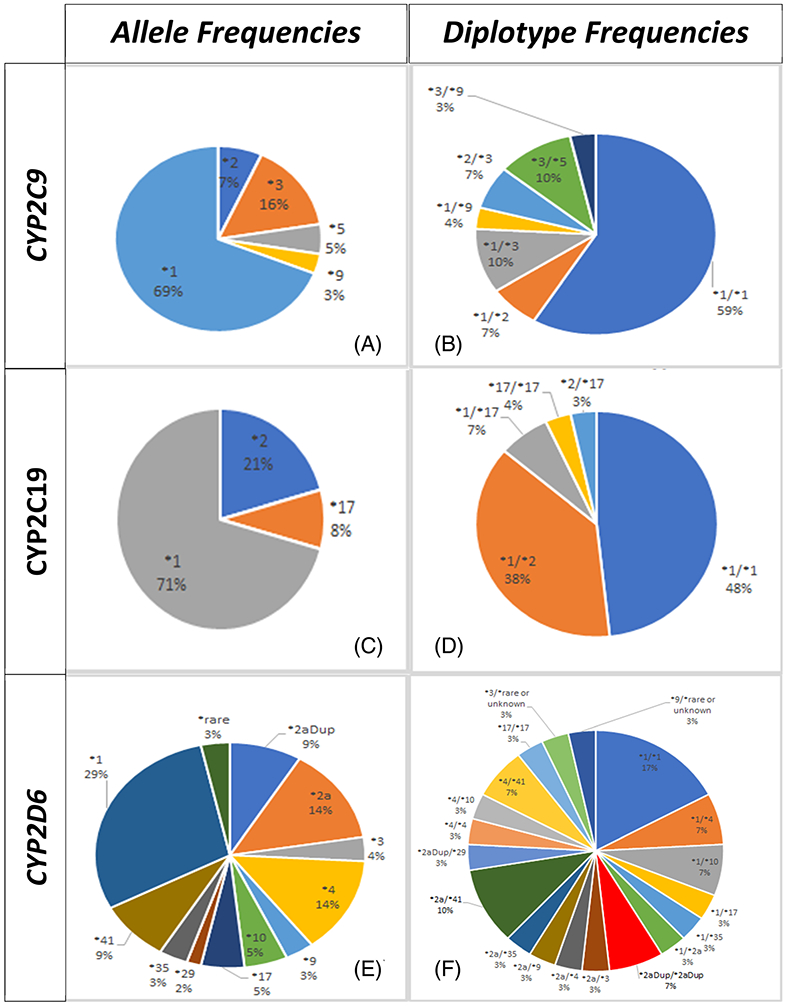
Allele and diplotype frequency distributions across the three genetic loci of interest (ie, CYP2C9, A and B; CYP2C19, C and D; and CYP2D6, E and F, pharmacogenes) in the study cohort
The CYP2D6 gene was more polymorphic than the two other loci among participants, with up to 10 different variant alleles found at this locus (ie, *2a, *2aDup, *3, *4, *9, *10, *17, *29, *35, *41). In addition, two subjects were carriers of a rare or unknown variant in CYP2D6. In this locus, the *2a and *4 alleles were the most frequently found with MAFs of 13.8% at both single nucleotide polymorphisms (SNPs); whereas, the CYP2D6*2a/*41 (n = 3), CYP2D6*1/*4 (n = 2), CYP2D6*1/*10 (n = 2), CYP2D6*2aDup/*2aDup (n = 2), and CYP2D6*4/*41 (n = 2) corresponded to the CYP2D6 diplotypes more commonly observed in participants. Consequently, ~17% of participants were designated as genetically inferred poor metabolizers (PM) for the CYP2D6-mediated metabolic pathway and another 7% were considered as ultrarapid metabolizers (UM) for substrates of this enzyme; whereas, ~17% were also classified as PM for the CYP2C9-mediated metabolism but no genetically inferred PM for the CYP2C19-mediated metabolism were identified among participants. However, about 3% were designated as UM and 38% as intermediate metabolizers (IM) for this pathway. Notably, about one-third (ie, 31%) of participants showed extreme metabolic status (PM or UM) when considering all the three loci combined.
Only one patient had no variants detected in any of the genes encoding for the CYP2C19, CYP2C9, or CYP2D6 drug-metabolizing enzymes, as determined by the PGx results. Because some of these CYP450 genetic variants were associated with an increased risk of adverse drug reactions (ADRs) and intolerance in our patients, an overrepresentation and, therefore, higher prevalence of these polymorphisms in participants might be expected. Accordingly, ~96% of patients harbored at least one genetic variant on any of these three loci that can potentially affect the safety and effectiveness of at least one drug in the current or alternative pharmacotherapy.
On average, the total number of genetic polymorphisms across the three loci combined was approximately three per patient (ie, 2.7 ± 1.14). The distribution of these combinatorial CYP450 polymorphisms is shown in Supporting Information Figure 1. The most abundant combination found in the study cohort was CYP2D6/CYP2C19 (31%) followed by CYP2D6/CYP2C9 (24%). The CDS computed CYP450 combinatorial drug metabolism indexes in the study cohort were the mean values for gene and allele alteration indexes of 3, both of which fell in the upperboundaries (third quartiles) of the distribution curve for these two indexes and hence are greater than their previously reported mean values in a reference population (ie, 1.48 and 1.88, respectively).21 On the other hand, a mean metabolic reserve index of 4.8 ± 1.3 and a corresponding mean metabolic alteration index of 2.0 ± 1.01 were also estimated in our study sample. The metabolic reserve index is a measure of the innate metabolic capacity of the patient, with a mean value of 5.05 in the reference population.21 Accordingly, the estimated mean value of 4.8 in our study cohort was close to the lower tail (first quartile) of the overall distribution curve for this index; whereas, a metabolic alteration index of 2.0 clearly falls in the upper limit of the curve (third quartile) surpassing the mean value of 1.4 for the reference population.21 The drug metabolism alteration index quantifies departure from functional reference alleles on any of the three genes. The greater the allele and drug metabolism alteration indexes, the higher the risk for ADRs. Furthermore, a lower drug metabolism reserve index was associated with subfunctional CYP450 isoenzyme activity and therefore higher risk of toxicity. These indexes also demonstrated that alterations are not concentrated in just one pharmacogene but distributed among the CYP450 gene triad tested.
Patients who were enrolled in this study had, on average, three genetic variants that potentially affected both their current and alternative pharmacotherapy. Figure 3 depicts the most notable drug-gene pairs related to potential DGIs in the study. The total number of drugs with potential DGIs based on PGx-guided CDS reports was 49, which represents approximately 21% of total prescriptions in this cohort. Drugs in current patients' pharmacotherapies that are at risk of potential DGIs, requiring action by the pharmacist, were mostly antidepressants as well as typical and atypical antipsychotics (Figure 3). Other notable drug classes included proton-pump inhibitors (PPIs) and nonsteroidal antiinflammatory drugs (NSAIDs).
FIGURE 3.
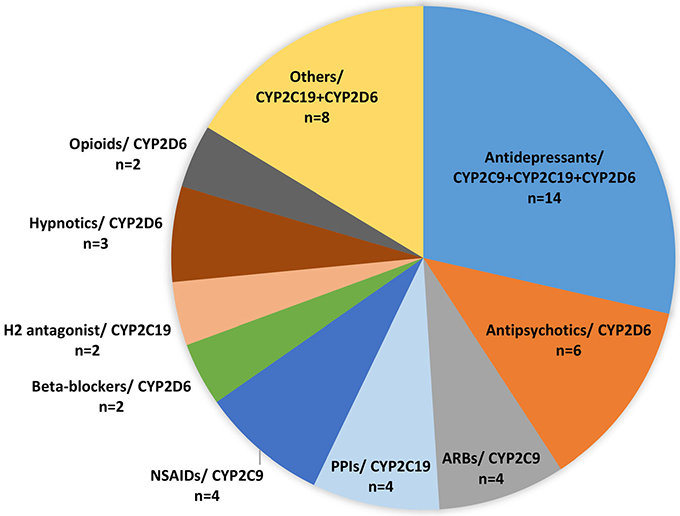
Number of prescription drugs by classes and major metabolic pathways (ie, drug-gene pairs) in the current pharmacotherapy of patients that are prone to DGIs, as predicted by PGx test results plus CDS reports. ARBs, angiotensin II receptor blockers; CDS, clinical decision support; DGI, drug-gene interaction; H2, histamine type2 receptors; NSAIDs, nonsteroidal antiinflammatory drugs; PGx, pharmacogenetic; PPIs, proton-pump inhibitors
Once the PGx test results and the corresponding CDS reports were available, the pharmacist was able to revise all prior CMM interventions and identify new MRPs following the CDS-derived clinically actionable recommendations. Consequently, the median number of MRPs increased from 5 to 6 and the median number of recommendations per patient (not including vaccines) increased from 3 to 5.5 (Figures 4 and 5). The total number of identified problems increased from 129 to 151. Adding a new drug therapy continued to be the most frequent recommendation, which was required in 100% of patients after CDS. An additional recommendation was made for one patient to monitor the response to a drug. The pharmacist also recommended the addition of some drug therapy in all 24 patients, following CDS reports. For the wild-type patient and those having genetically inferred functional metabolic status, the pharmacist did not change any of the prior recommendations made through the traditional CMM. However, a different recommendation in pharmacotherapy was made for the other 21 patients based on CDS reports.
FIGURE 4.
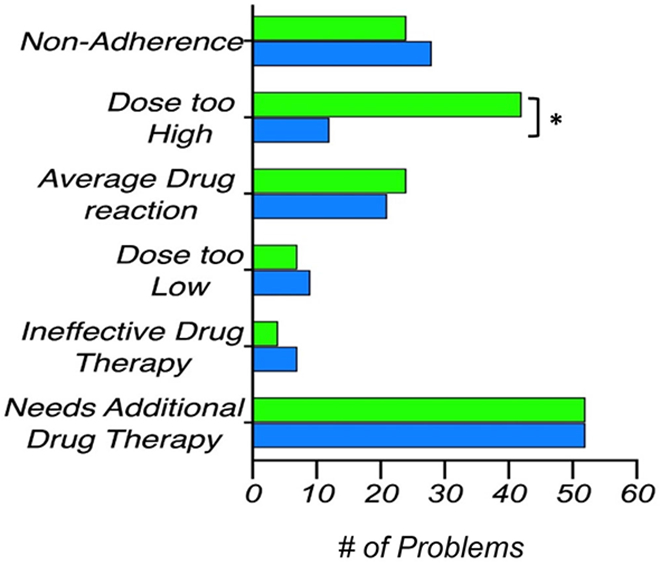
Comparison of the total number of each individual MRP, identified before (blue) and after (green) the PGx test results plus CDS reports became available. Asterisk (*) represents a significant difference (two-tailed Wilcoxon signed rank test). CDS, clinical decision support; MRP, medication-related problem; PGx, pharmacogenetic
FIGURE 5.
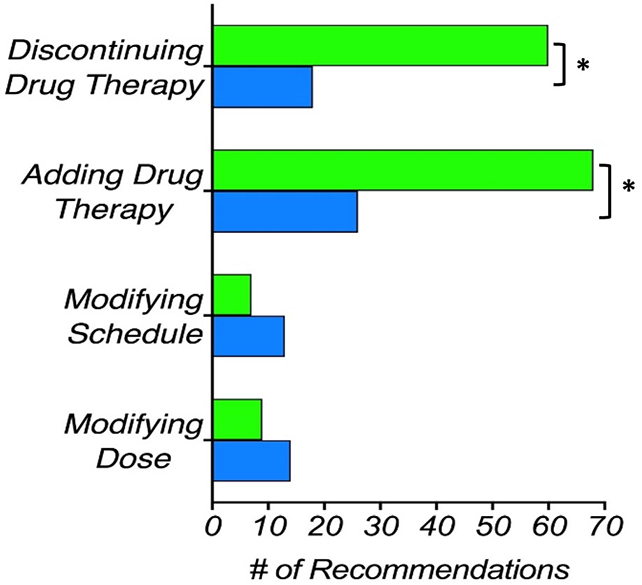
Comparison of total number of pharmacotherapy-related recommendations before (blue) and after (green) the PGx test results plus CDS reports became available. Asterisk (*) represents a significant difference (two-tailed Wilcoxon signed rank test). CDS, clinical decision support; PGx, pharmacogenetic
Figure 6 depicts a boxplot of the ranked values corresponding to the number of pre- and post-PGx MRPs. The median of pre-PGx MRPs was significantly lower than the median of post-PGx MRPs, as suggested by the two-tailed Wilcoxon signed rank test (5.0 vs 6.0; V = 2.50, z = −2.90, P = .004). Likewise, the results of the Wilcoxon's test were also statistically significant for the comparison of the medians corresponding to the following identified problems before and after the PGx testing: dosage too high (P = .039), number of recommendations about pharmacotherapy (P < .001), and discontinuing a drug therapy (P = .002).
FIGURE 6.
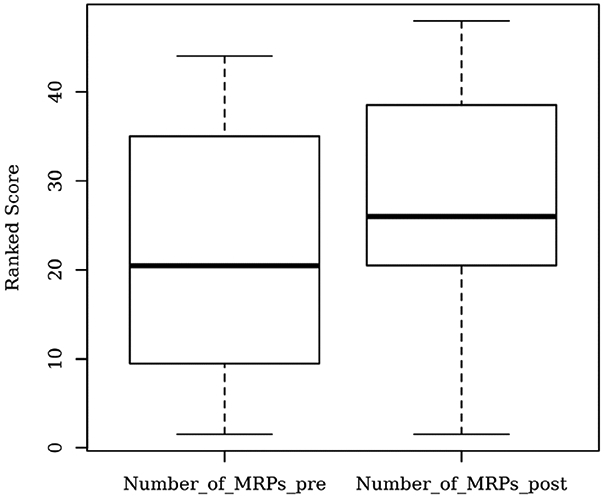
Boxplot of the ranked values corresponding to the total (overall) number of MRPs, identified before and after the PGx test results and subsequent CDS reports became available (ie, pre- and post-PGx). Significant difference found by two-tailed Wilcoxon signed rank test (P = .004). CDS, clinical decision support; MRP, medication-related problem; PGx, pharmacogenetic
4 ∣. DISCUSSION
This paper reports a comparison of the current CMM practice with that supplemented by PGx information. It represents a pioneering example of implementation science in the interface of pharmacy and genetics. CMM encompasses the clinical deployment of pharmacological and medical information in the real world setting of multidrug regimens for comorbid diseases. Pharmacogenetic guidance is evolving and consequently is diverse. Although the CPIC has issued valuable guidance for various drugs in psychiatry, the proportion of drugs with guidelines is still small compared with the range of drugs used in mental health. This void in guidance demonstrates the need for an algorithmic, heuristic logic such as the one provided by CDS based on a quantitative functional categorization and thresholds.24
The CDS tool used in this study has previously proven very useful for supporting clinical decision-making on drug selections as well as in identifying drug-related adverse events and harmful drug interactions in high-risk patients.21,22 To cover the broad array of drugs prescribed to our cohort, and the various drugs prescribed individually, the CDS relied on heuristic inferences from the CYP450 substrate specificity of the drugs. The fact that source data are predominantly biochemical rather than clinical is a limitation of this study. Another limitation of this study is that the actual prescribing by the psychiatrist receiving the CMM report was not ascertained, as it was outside the scope of the protocol. Physician decision-making with CMM supplemented by pharmacogenetic modeling is critical to implementation and will be the subject of future studies.
A strength of this study was the comprehensive panel of variants incorporated in the genotyping assays. It is noteworthy that for CYP2C9, our assays included alleles more prevalent in African descent populations (eg, *5, *6, *11) often missing in other studies. This representation may explain the high proportion of carriers of CYP2C9 variants in our cohort. The prevalence of common CYP2C19 polymorphisms in the Puerto Rican population has previously been reported, with MAFs of 14% and 13% for the CYP2C19*17 and *2, respectively.25 In our study, the MAFs were 8% and 21% for CYP2C19*17 and *2, respectively. As expected, no *3 variant was identified in any participant of the study. This SNP is usually considered rare in non-Asians.26 A prevalence of 25% for combinatorial CYP2C9 polymorphisms was early reported in Puerto Ricans.27 Others have found a prevalence that ranges from 25% to 35% in worldwide populations.28-32 A higher prevalence of 41% was found in this study for CYP2C9 variants combined. Gonzalez-Tejera and colleagues found a 73% prevalence of CYP2D6 variants in 45 psychiatric patients from Puerto Rico, which is comparable to our sample size and the 83% prevalence of CYP2D6 polymorphisms in our study cohort.33
The increased prevalence of polymorphisms in our study cohort may be due to a selection bias, considering that participants came from a clinic where 89% had at least one diagnosed psychiatric condition. Polymorphisms in CYP2C19, CYP2C9, and CYP2D6 have early been studied for their association with psychiatric disorders. The association is believed to result from the metabolism of endogenous neurotransmitters by these enzymes. A study found higher prevalence of CYP2D6 and CYP2C9 polymorphisms in psychiatric patients diagnosed with depression vs a control group.34 CYP2D6 has also been associated with anxiety and socialization disorders.35,36
In our study, polymorphisms affected a median of two medications in the patients' current therapy and 4.5 medications in the alternative pharmacotherapy. Taking altogether, a genetic polymorphism in at least one of the three CYP genes of interest was identified in ~96% of patients. The pharmacist made different pharmacotherapy-related recommendations to all carrier patients after reviewing the corresponding CDS reports. This contrasts with an earlier publication where 93.3% of patients had a genetic variant that affected the drug's metabolism, but a medication change or discontinuation was only done in 20% of patients.37 It reveals differences in criteria and viewpoints of pharmacists providing CMM services with regards to the actions to be taken concerning drugs potentially affected by genetic polymorphisms. In our study, the pharmacist recommended a drug change whenever DGIs were present.
The pharmacist identified five MRPs per patient before receiving the CDS reports. Such identified problems climbed to six after the CDS reports was made available. In an analysis of database from 22 694 ambulatory patients, an average of four MRPs per patient were identified.14 A higher number of MRPs in our study cohort could be due to the fact that psychiatric patients tend to use more medications, which are susceptible to multiple drug interactions. In fact, Cipolle and colleagues14 identified 0.26 adverse drug reaction and 0.15 high dose problem per patient, while we identified 1 adverse drug reaction and 1 high dose problem per patient. Also, nonadherence problems were more common in our study. A median of 1.5 medications per patient with nonadherence issues was reported in our study compared with only 0.55 in Cipolle's study.14 The fact that our MRPs outnumbered the published data supports the idea that the pharmacist did not intentionally underestimate medication problems before the CDS reports to later overestimate the benefit of adding PGx test results.14
With the addition of PGx results in CDS reports, the pharmacist was able to identify a greater number of MRPs. These MRPs were classified under the categories of “dosage too high” and “adverse drug reaction” (Figure 4). Thus, the “dosage too high” category includes drugs for which PGx results would suggest a reduced dose; whereas, the “adverse drug reaction” category includes either drugs to be avoided in the patient or drugs potentially experiencing DGIs due to altered metabolism.
As noted in Figure 4, fewer problems were identified in the categories of “ineffective drug,” “dosage too low,” and “nonadherence.” It is because some drugs with MRPs in these categories also showed DGIs that the pharmacists acted upon by recommending discontinuation, even before the CDS reports were available. The removal of the drug eliminates the problem from such categories. There was a significant increase in recommendations to add and remove drug therapies based on identified DGIs (Figure 5). Fewer recommendations were made to modify either the dose or the proposed schedule, following the CDS reports. It is because the pharmacist mainly chose to substitute drugs susceptible to DGIs instead of modifying their dosing or schedule. PGx-guided CDS reports, along with the previous knowledge of potential drug-drug interactions, helped the pharmacist offering valid recommendations for alternative therapies in order to avoid DGIs and consequently some MRPs. Given the high proportion of drug changes implemented with the CDS, the results in Figure 5 demonstrated the value of PGx guidance in genetically diverse Puerto Ricans.
Pharmacists are very well positioned to make major contributions to the field of Precision Medicine given their knowledge and competencies.4,8,38-49 It is evidenced by their capability to adequately interpret actionable recommendations derived from PGx-guided CDS tools and formulate independent clinical judgments, rendering the pharmacist-provided CMM an ideal niche for PGx-guided CDS. Because pharmacists independently review, evaluate, select, and integrate multiple sources of information for CMM, they are poised to become leaders in the use of CDS for providing PGx-guided CMM services.
Supplementary Material
ACKNOWLEDGMENTS
We want to thank the patients of the clinic for voluntarily participating in this survey. A special acknowledgement to our Pharm.D. students and staff at the Clínica de Servicios Psicológicos in Toa Baja, Puerto Rico, who helped us with data collection as well as the personnel at the Laboratory of Personalized Health, Hartford, Connecticut, for genotyping patients.
This work was supported by CCRHD-RCMI grant #2U54 MD007600-31 from the National Institute on Minority Health and Health Disparities (NIMHD) of the National Institutes of Health.
Funding information
National Institute on Minority Health and Health Disparities (NIMHD) of the National Institutes of Health, Grant/Award Number: #2U54 MD007600-31
Footnotes
CONFLICT OF INTEREST
Gualberto Ruaño is a founder and medical director of the Genomas, Inc. The authors have no further conflict of interest to declare.
DISCLAIMER
The contents of this manuscript do not represent the views of the National Institutes of Health or the United States Government. No funded writing assistance was utilized in the production of this manuscript. The psychologist provided a patient population and clinical histories for the study, but neither the psychologist nor the psychiatrists ordered pharmacogenetic testing. The selection on whom to enroll was made solely by the study personnel. Although the patient's psychiatrists were not involved in the protocol, they received the comprehensive medication management report from the investigators.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.American Pharmacists Association (APhA) Policy. 2018. Annual Meeting & Exposition Leading our Communities in Patient Care: Actions of the 2018 American Pharmacists Association House of Delegates; March 16-19, 2018; Nashville, Tennessee: Available at https://www.pharmacist.com/sites/default/files/files/2018%20Report%20of%20the%20APhA%20House%20of%20Delegates%20-%20FINAL.pdf. [Accessed on March 22, 2020]. [Google Scholar]
- 2.Hocum BT, White JR Jr, Heck JW, et al. Cytochrome P-450 gene and drug interaction analysis in patients referred for pharmacogenetic testing. Am J Health Syst Pharm. 2016;73(2):61–67. [DOI] [PubMed] [Google Scholar]
- 3.American Society of Health-System Pharmacists. ASHP statement on the pharmacist's role in clinical pharmacogenomics. Am J Health Syst Pharm. 2015;72(7):579–581. [DOI] [PubMed] [Google Scholar]
- 4.Hicks KJ, Aquilante CL, Dunnenberger HM, et al. Precision pharmacotherapy: Integrating pharmacogenomics into clinical pharmacy practice. J Am Coll Clin Pharm. 2019;2:303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McInnis T, Webb CE, Strand LM. The patient-centered medical home: Integrating comprehensive medication management to optimize patient outcomes. 2nd ed.Washington, DC: Patient-Centered Primary Care Collaborative, 2012;p. 1–28 Available at https://www.accp.com/docs/positions/misc/CMMResourceGuide.pdf. [Accessed on March 22, 2020]. [Google Scholar]
- 6.Burns A Medication therapy management in pharmacy practice: Core elements of an MTM service model (version 2.0). J Am Pharm Assoc. 2008;48(3):341–353. [DOI] [PubMed] [Google Scholar]
- 7.Viswanathan M, Kahwati LC, Golin CE, et al. Medication therapy management interventions in outpatient settings: A systematic review and meta-analysis. JAMA Intern Med. 2015;175(1):76–87. [DOI] [PubMed] [Google Scholar]
- 8.Dunnenberger HM, Biszewski M, Bell GC, et al. Implementation of a multidisciplinary pharmacogenomics clinic in a community health system. Am J Health Syst Pharm. 2016;73(23):1956–1966. [DOI] [PubMed] [Google Scholar]
- 9.Duconge J, Ruaño G. The emerging role of admixture in the pharmacogenetics of Puerto Rican Hispanics. J Pharmacogenomics Pharmacoproteomics. 2010;1:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Claudio-Campos K, Orengo-Mercado C, Renta JY, et al. Pharmacogenetics of healthy volunteers in Puerto Rico. Drug Metabol Personal Ther. 2015;30(4):239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ventola CL. Role of pharmacogenomic biomarkers in predicting and improving drug response: Part 1: The clinical significance of pharmacogenetic variants. Pharm Ther. 2013;38(9):545–560. [PMC free article] [PubMed] [Google Scholar]
- 12.Belle DJ, Singh H. Genetic factors in drug metabolism. Am Fam Physician. 2008;77(11):1553–1560. [PubMed] [Google Scholar]
- 13.Arwood MJ, Chumnumwat S, Cavallari LH, Nutescu EA, Duarte JD. Implementing pharmacogenomics at your institution: establishment and overcoming implementation challenges. Clin Transl Sci. 2016;9(5): 233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cipolle RJ, Strand LM, Morley PC. Drug therapy problems In: Cipolle RJ, Strand LM, Morley PC, editors. Pharmaceutical care practice: The patient-centered approach to medication management. New York, NY: McGraw Hill Professional, 2012. Available at http://accesspharmacy.mhmedical.com/content.aspx?bookid=491§ionid=39674905 [Accessed on March 22, 2020]. [Google Scholar]
- 15.Guzzi PH, Agapito G, Di Martino MT, et al. DMET-analyzer: Automatic analysis of Affymetrix DMET data. BMC Bioinform. 2012;13 (1):258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sissung TM, English BC, Venzon D, Figg WD, Deeken JF. Clinical pharmacology and pharmacogenetics in a genomics era: The DMET platform. Pharmacogenomics. 2010;11(1):89–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Martino MT, Arbitrio M, Leone E, et al. Single nucleotide polymorphisms of ABCC5 and ABCG1 transporter genes correlate to irinotecan-associated gastrointestinal toxicity in colorectal cancer patients: A DMET microarray profiling study. Cancer Biol Ther. 2011; 12(9):780–787. [DOI] [PubMed] [Google Scholar]
- 18.Ruaño G, Kocherla M, Graydon JS, Holford TR, Makowski GS, Goethe JW. Practical interpretation of CYP2D6 haplotypes: Comparison and integration of automated and expert calling. Clinica Chimica Acta. 2016;456:7–14. [DOI] [PubMed] [Google Scholar]
- 19.Ruaño G, Villagra DV, Kocherla M, Windemuth A, Goethe JW. Physiogenomic method for predicting antidepressant and anxiolytic drug metabolic risk. United States Patent US 9,558,320 B2. January 31, 2017. [Google Scholar]
- 20.Ruaño G, Larsen K, Kocherla M, Graydon JS, Kost J. Complications of psychotropic and pain medications in an ultrarapid metabolizer patient at the upper 1% of cytochrome P450 (CYP450) function quantified by combinatorial CYP450 genotyping. J Pain Palliat Care Pharmacother. 2017;31(2):126–138. [DOI] [PubMed] [Google Scholar]
- 21.Villagra D, Goethe J, Schwartz HI, et al. Novel drug metabolism indices for pharmacogenetic functional status based on combinatory genotyping of CYP2C9, CYP2C19 and CYP2D6 genes. Biomark Med. 2011;5(4):427–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landino J, Buckley J, Roy JM, et al. Guidance of pharmacotherapy in a complex psychiatric case by CYP450 DNA typing. J Am Acad Nurse Pract. 2011;23(9):459–463. [DOI] [PubMed] [Google Scholar]
- 23.Manworren RC, Jeffries L, Pantaleao A, Seip R, Zempsky WT, Ruaño G. Pharmacogenetic testing for analgesic adverse effects. Clin J Pain. 2016;32(2):109–115. [DOI] [PubMed] [Google Scholar]
- 24.Clinical Decision Support Software. Draft guidance for industry and FDA staff. Issued on September 27, 2019. Available at https://www.fda.gov/media/109618/download. [Accessed on October 31, 2019].
- 25.Hernandez-Suarez D, Tomassini-Fernandini J, Cuevas A, et al. Clinical relevant polymorphisms affecting clopidogrel pharmacokinetics and pharmacodynamics: Insights from the Puerto Rico Newborn Screening Program. Int J Environ Res Public Health. 2018;15(6): 1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duconge J, Cadilla CL, Renta JY, et al. Prevalence of CYP2C19 gene polymorphisms in the Puerto Rican population: A preliminary report. PR Health Sci J. 2008;27(4):357. [PMC free article] [PubMed] [Google Scholar]
- 27.Duconge J, Cadilla CL, Windemuth A, et al. Prevalence of combinatorial CYP2C9 and VKORC1 genotypes in Puerto Ricans: Implications for warfarin management in Hispanics. Ethn Dis. 2009;19(4): 390–395. [PMC free article] [PubMed] [Google Scholar]
- 28.Hamdy SI, Hiratsuka M, Narahara K, et al. Allele and genotype frequencies of polymorphic cytochromes P450 (CYP2C9, CYP2C19, CYP2E1) and dihydropyrimidine dehydrogenase (DPYD) in the Egyptian population. Br J Clin Pharmacol. 2002;53(6):596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sipeky C, Lakner L, Szabo M, et al. Interethnic differences of CYP2C9 alleles in healthy Hungarian and Roma population samples: Relationship to worldwide allelic frequencies. Blood Cells Mol Dis. 2009;43(3): 239–242. [DOI] [PubMed] [Google Scholar]
- 30.Arvanitidis K, Ragia G, Iordanidou M, et al. Genetic polymorphisms of drug-metabolizing enzymes CYP2D6, CYP2C9, CYP2C19 and CYP3A5 in the Greek population. Fundam Clin Pharmacol. 2007;21(4):419–426. [DOI] [PubMed] [Google Scholar]
- 31.Allabi AC, Gala JL, Desager JP, Heusterspreute M, Horsmans Y. Genetic polymorphisms of CYP2C9 and CYP2C19 in the Beninese and Belgian populations. Br J Clin Pharmacol. 2003;56(6):653–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bravo-Villalta HV, Yamamoto K, Nakamura K, Bayñ A, Okada Y, Horiuchi R. Genetic polymorphism of CYP2C9 and CYP2C19 in a Bolivian population: An investigative and comparative study. Eur J Clin Pharmacol. 2005;61(3):179–184. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez-Tejera G, Gaedigk A, Corey S. Genetic variants of the drug-metabolizing enzyme CYP2D6 in Puerto Rican psychiatry patients: A preliminary report and potential implications for breast cancer patients. PR Health Sci J. 2010;29(3):299–304. [PubMed] [Google Scholar]
- 34.Ruaño G, Villagra D, Rahim US, et al. Increased carrier prevalence of deficient CYP2C9, CYP2C19 and CYP2D6 alleles in depressed patients referred to a tertiary psychiatric hospital. Per Med. 2008;5(6): 579–587. [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez I, Penas-Lledo EM, Pérez B, Dorado P, Alvarez M, LLerena A. Relation between CYP2D6 phenotype and genotype and personality in healthy volunteers. Pharmacogenomics. 2008;9(7): 833–840. [DOI] [PubMed] [Google Scholar]
- 36.Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabo-lism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther. 2013;138(1):103–141. [DOI] [PubMed] [Google Scholar]
- 37.Haga SB, Moaddeb J, Mills R, Patel M, Kraus W, Allen LaPointe NM. Incorporation of pharmacogenetic testing into medication therapy management. Pharmacogenomics. 2015;16(17):1931–1941. [DOI] [PubMed] [Google Scholar]
- 38.Hoffman JM, Haidar CE, Wilkinson MR, et al. PG4KDS: A model for the clinical implementation of pre-emptive pharmacogenetics. Am J Med Genet C Semin Med Genet. 2014;166C(1):45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson JA, Elsey AR, Clare-Salzler MJ, Nessl D, Conlon M, Nelson DR. Institutional profile: University of Florida and Shands Hospital Personalized Medicine Program: Clinical implementation of pharmacogenetics. Pharmacogenomics. 2013;14(7):723–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Samer CF, Lorenzini KI, Rollason V, Daali Y, Desmeules JA. Applications of CYP450 testing in the clinical setting. Mol Diagn Ther. 2013; 17(3):165–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bielinski SJ, Olson JE, Pathak J, et al. Preemptive genotyping for personalized medicine: Design of the right drug, right dose, right time using genomic data to individualize treatment protocol. Mayo Clin Proc. 2014;89(1):25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gottesman O, Scott SA, Ellis SB, et al. The CLIPMERGE PGx Program: Clinical implementation of personalized medicine through electronic health records and genomics-pharmacogenomics. Clin Pharmacol Ther. 2013;94(2):214–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Driest SL, Shi Y, Bowton EA, et al. Clinically actionable genotypes among 10 000 patients with preemptive pharmacogenomic testing. Clin Pharmacol Ther. 2014;95(4):423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Papastergiou J, Tolios P, Li W, Li J. The Innovative Canadian Pharmacogenomic Screening Initiative in Community Pharmacy (ICANPIC) study. J Am Pharm Assoc. 2017;57(5):624–629. [DOI] [PubMed] [Google Scholar]
- 45.Cavallari LH, Lee CR, Duarte JD, et al. Implementation of inpatient models of pharmacogenetics programs. Am J Health Syst Pharm. 2016;73:1944–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scott SA. Personalizing medicine with clinical pharmacogenetics. Genet Med. 2011;13(12):987–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Owen JA, Reiss SM, American Pharmacists Association. Integrating pharmacogenomics into pharmacy practice via medication therapy management. J Am Pharm Assoc. 2011;51(6):e64–e74. [DOI] [PubMed] [Google Scholar]
- 48.Saldivar JS, Taylor D, Sugarman E, et al. Initial assessment of the benefits of implementing pharmacogenetics into the medical management of patients in a long-term care facility. Pharmacogenom Pers Med. 2016;9:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwartz EJ, Turgeon J, Patel J, et al. Implementation of a standardized medication therapy management plus approach within primary care. J Am Board Fam Med. 2017;30(6):701–714. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


