Abstract
Background:
Atopic dermatitis (AD) is a common eczematous skin disorder that profoundly reduces the quality of life due to intractable pruritus. Excellent therapeutic success of the anti-interleukin 4 receptor-α antibody dupilumab in clinical trials and a real-world clinical context indicates the crucial roles of interleukin (IL)-4 and IL-13 in the pathogenesis of AD. Along with the clinical improvement in skin scores and pruritus, dupilumab significantly and progressively reduces and normalizes the upregulated expression of T helper type 2 signatures such as Chemokine (C-C motif) ligand (CCL)17, CCL18, CCL22, and CCL26 in the lesional skin of AD. However, no blood/serum biomarkers are known to predict good or poor outcome in patients with AD treated with dupilumab.
Methods:
Patients are at least 18 years of age and have moderate-to-severe AD with Eczema Area and Severity Index (EASI) ≥16, Investigator's Global Assessment ≥3, and body surface area ≥10%. We are going to enroll more than 130 subjects from 18 medical facilities. Clinical objective findings will be evaluated by EASI. Subjective symptoms will be assessed by Patient-Oriented Eczema Measure, Numerical Rating Scale for Pruritus (Pruritus-NRS), Skin Comfort-NRS, and Treatment Satisfaction-NRS. We will measure 18 blood/serum biomarkers including % eosinophils in blood cell count, lactate dehydrogenase, total IgE, soluble interleukin 2 receptor, CCL17, CCL18, CCL22, CCL26, CCL27, IL-13, IL-22, IL-24, IL-25, IL-31, IL-33, thymic stromal lymphopoietin, periostin, and squamous cell carcinoma antigen-2. The clinical evaluation and biomarker sampling will be performed at 0, 2, 4, 8, and 16 weeks of dupilumab treatment. We will also perform proteomic analysis (of roughly 300 proteins) of the patients’ sera obtained at 0 and 2 weeks of treatment. The primary endpoint is the association between “baseline levels of 18 biomarkers” and “% change from baseline of EASI at 16 weeks of dupilumab treatment.”
Discussion:
This is the first clinical trial to explore the biomarkers, including potential proteomic markers, most strongly associated with improvement in EASI in patients with moderate-to-severe AD treated with dupilumab for 16 weeks (B-PAD study). A limitation is that we will only enroll Japanese patients.
Keywords: atopic dermatitis, biomarker, chemokines, cytokines, dupilumab, efficacy, treatment
1. Introduction
Atopic dermatitis (AD) is a common eczematous skin disorder, the incidence in the first 5 years of childhood of which is 10% to 16.5%. It is generally considered to have increased in prevalence worldwide, at least from the 1980 s to the early 2000 s.[1] Clinical features of AD include skin inflammation, barrier dysfunction, and chronic pruritus.[2] Its course involves chronic relapse with intense pruritus, which reduces the quality of life and decreases treatment satisfaction among afflicted patients.[3–5] Excellent therapeutic success of the anti-interleukin 4 receptor-α antibody dupilumab in clinical trials and in a real-world clinical context has indicated the crucial roles of T helper type 2 (Th2) cytokines, interleukin (IL)-4 and IL-13, in the pathogenesis of AD.[6–8] Along with the clinical improvement in skin scores and pruritus, dupilumab significantly and progressively reduces and normalizes the elevated expression of Th2 signatures such as Chemokine (C-C motif) ligand (CCL)17, CCL18, CCL22, and CCL26 in the lesional skin of AD.[9,10] Other lesional and blood markers including eosinophils,[11–13] lactate dehydrogenase (LDH),[14] total immunoglobulin E (IgE),[15] soluble IL-2 receptor,[16] CCL27,[14] IL-13,[17] IL-22,[9] IL-24,[18,19] IL-25,[20] IL-31,[21,22] IL-33,[23] thymic stromal lymphopoietin (TSLP),[24] periostin,[9,25] and squamous cell carcinoma antigen-2 (SCCA2)[26,27] are elevated in AD and show substantial correlations with its disease activity.
It is now recognized that AD is not a single or monophenotypic disease, but is composed of heterogenous groups.[11,28–30] In general, we have classified AD patients based on clinical features such as age (pediatric, young adult vs. elderly),[30,31] clinical course (acute vs. chronic),[32] IgE dependence (atopic vs. non-atopic),[33,34] and ethnicity (Caucasian vs. non-Caucasian).[35] In addition, recent approaches based on the molecular mechanisms have subdivided AD into different endoypes, for example, Th2 vs. Th2 + Th17,[36–38] and clinical severity + Th2 / interferon-α/β.[39] The phenotypic and endotypic differences in AD have led to a basis for stratifying patients. Stratifying patients by endotype may be particularly meaningful for the application of molecularly targeted drugs such as dupilumab. Although biomarkers representing the Th2 signature tend to decrease upon dupilumab treatment, the individual degrees of response of biomarkers as well as the rates of clinical improvement vary.[40,41] In addition, it is not fully understood what kinds of biomarkers are responsible for a good/poor clinical outcome of dupilumab treatment.
The purpose of this study is to explore the biomarkers, including potential proteomic markers, that are most strongly associated with clinical improvement in patients with moderate-to-severe AD treated with dupilumab.
2. Methods/Design (Protocol version 1.0, registered on July 8th, 2019)
2.1. Study hypothesis/benefit
Certain biomarkers, including proteomic ones, may be associated with a good/poor clinical response to dupilumab. This information could be very useful for patients for whom the initiation of dupilumab therapy is being considered, given its high cost. Using meaningful stratification of patients, we can expect to increase efficacy of drugs and decrease the economic burden on patients. In addition, new Th2-related serum proteins may be highlighted by proteomic analysis as future target molecules in AD.
2.2. Study design
This is a multi-center, prospective, observational study in which samples/information will be obtained in Japan. This exploratory study will basically be carried out under real-world standard treatment guidelines. We are going to enroll more than 130 subjects from 19 medical facilities joining a consortium. The patients are to cease oral immunosuppressive drugs, oral steroids, or phototherapy at least 1 week before the start of injections of dupilumab. None of the patients is to have any previous experience of dupilumab treatment. They are to be at least 18 years of age, have moderate-to-severe AD with Eczema Area and Severity Index (EASI) ≥16, Investigator's Global Assessment (IGA) ≥3, and body surface area ≥10%, and be individuals for whom topical treatment of steroids provided inadequate control or was medically inadvisable, and had chronic AD for at least 3 years before the start of this study. The use of systemic steroids, systemic calcineurin inhibitors, and phototherapy is not allowed after the initiation of dupilumab.
The continued use of topical steroids, topical calcineurin inhibitors, topical moisturizers, and oral antihistamines used at baseline is allowed. Change of topical drugs to more potent ones is not allowed. The use of ocular, intranasal, or inhalant steroids and calcineurin inhibitors is allowed throughout the study, as is the use of anti-histamine drugs. Subjects are to receive subcutaneous injections of dupilumab (initial dose 600 mg, then 300 mg) biweekly for 16 weeks.
All investigators involved in this study shall carry out this study in accordance with the latest editions of the Declaration of Helsinki and “Ethical Guidelines for Medical and Health Research involving Human Subjects” of the Ministry of Health, Labour and Welfare, Japan. The study protocol has been approved by the Clinical Research Network Fukuoka Certified Review Board (CRB7180004). This study has been registered with the University Hospital Medical Information Network Clinical Trials Registry (UMIN000037307). The enrollment period is set to run from October 10, 2019. Last follow-ep date will be set on September 30, 2021.
2.3. Sample size estimates
The target number of 130 patients aimed to be enrolled was determined based on past experiences and feasibility. From previous phase 3 trials,[7] since it is assumed that approximately 25% of enrolled patients with dupilumab treatment will discontinue the treatment, a plan was set to enroll more than 130 subjects and perform data analysis of at least 100 subjects.
2.4. Eligibility criteria
Inclusion criteria are as follows:
-
(1)
chronic AD that has been present for ≥3 years at enrollment;
-
(2)
moderate-to-severe patients with EASI score of ≥16, IGA score of ≥3, and body surface area ≥10% at enrollment (excluded if inflammation is limited to the head and neck region);
-
(3)
no treatment history of dupilumab;
-
(4)
patients in whom topical steroid treatment provides insufficient inhibition or is medically inadvisable;
-
(5)
patients aged ≥18 years and ≤70 years at enrollment; and
-
(6)
patients who are able to completely understand the study plan and to provide signed informed consent.
Exclusion criteria are as follows:
-
(1)
patients treated with oral immunosuppressive drugs, oral steroid, or phototherapy within 4 weeks before dupilumab administration;
-
(2)
female patients who are breastfeeding, pregnant, or have the possibility of being pregnant; and
-
(3)
any other patients who are regarded as unsuitable for this study by the investigators.
Patient enrollment is performed by a central enrollment method. The investigators confirm that the study subjects meet all of the inclusion criteria and do not meet any of the exclusion criteria, and enter all of the necessary information for patient enrollment in the electronic data capture (EDC) system (Viedoc 4). Data monitoring including adverse events are periodically and independently performed by Clinical Research Support Center Kyushu (CReS Kyushu). Protocol kick-off meeting and amendment committee are also scheduled in the presence of CReS Kyushu.
2.5. Evaluation of clinical findings and biomarkers
Clinical objective findings are evaluated by EASI.[42–44] Subjective symptoms are assessed by Patient-Oriented Eczema Measure (POEM)[44,45] and Numerical Rating Scale for Pruritus (Pruritus-NRS) (Fig. 1).[46,47] Patients are also requested to complete Skin Comfort-NRS (0: no discomfort, 10: worst discomfort imaginable) and Treatment Satisfaction-NRS (0: not satisfied at all, 10: very satisfied) (Fig. 1). We measure 18 biomarkers including % eosinophils in blood cell count, LDH, total IgE, soluble interleukin 2 receptor, CCL17, CCL18, CCL22, CCL26, CCL27, IL-13, IL-22, IL-24, IL-25, IL-31, IL-33, TSLP, periostin, and SCCA2. The clinical evaluation and biomarker sampling are performed on the day that injections of dupilumab start and at 2, 4, 8, and 16 weeks (w) of dupilumab treatment (Fig. 2). We also perform proteomic analysis (of roughly 300 proteins) (Myriad RBM, Austin, TX) of the patients’ sera on the day that injections of dupilumab start and at 2w of treatment.
Figure 1.
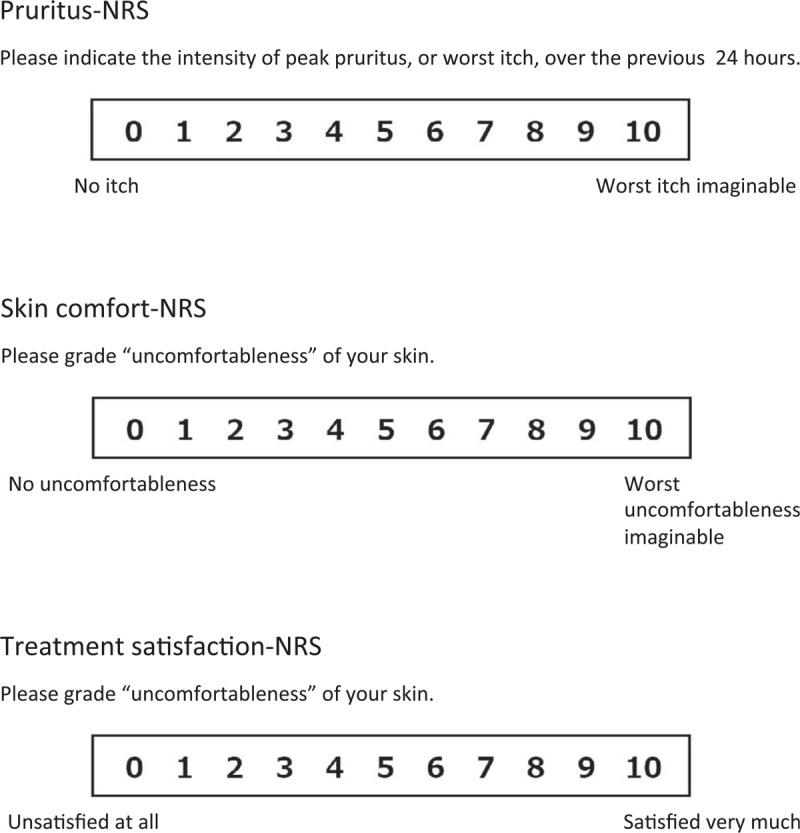
Pruritus-NRS, Skin comfort-NRS and Treatment satisfaction-NRS are used in this study. NRS = numerical rating scale.
Figure 2.
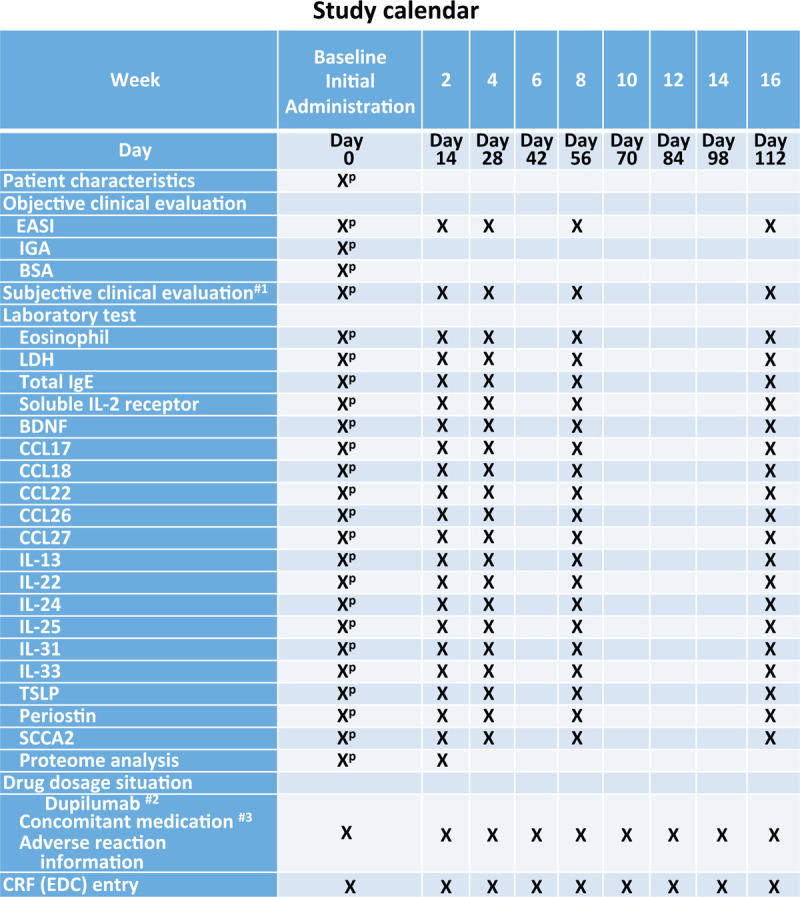
Study calendar is depicted. 1. Subjective clinical evaluation includes POEM, Pruritus-NRS, Uncomfortable skin-NRS, and Treatment satisfaction-NRS. 2. The administration of dupilumab shall be carried out after all assessments and tests are completed. A change of administration day is allowed within the range of +/- 1 week. #3. Use of ocular, intranasal, or inhalant steroids, calcineurin inhibitors, and antihistamines is allowed throughout the study. P = pre-treatment.
2.6. Primary and secondary endpoints
This is an exploratory clinical study to determine which biomarker is most strongly associated with clinical improvement. The primary endpoint is the association between “baseline levels of 18 biomarkers” and “% change from baseline of EASI at 16w of dupilumab treatment.” Secondary endpoints are
-
(1)
the association between “baseline levels of potential proteomic markers” and “% change from baseline of EASI at 16w,”
-
(2)
the association between “baseline levels of 18 biomarkers” and “% change from baseline of POEM at 16w,”
-
(3)
the association between “baseline levels of potential proteomic markers” and “% change from baseline of POEM at 16w,”
-
(4)
the association between “baseline levels of 18 biomarkers” and “% change from baseline of Pruritus-NRS at 16w,”
-
(5)
the association between “baseline levels of potential proteomic markers” and “% change from baseline of Pruritus-NRS at 16w,”
-
(6)
the association between “baseline levels of 18 biomarkers” and “% change from baseline of Skin Comfort-NRS at 16w,”
-
(7)
the association between “baseline levels of potential proteomic markers” and “% change from baseline of Skin Comfort-NRS at 16w,”
-
(8)
the association between “baseline levels of 18 biomarkers” and “% change from baseline of Treatment Satisfaction-NRS at 16w,” and
-
(9)
the association between “baseline levels of potential proteomic markers” and “% change from baseline of Treatment Satisfaction-NRS at 16w.”
3. Statistical analysis
3.1. Relationship between biomarkers and clinical findings
To evaluate the primary and secondary endpoints, we will conduct 2 statistical procedures. First, we will check the distribution of the primary EASI and all secondary clinical findings (POEM, Pruritus-NRS, Skin Comfort-NRS, and Treatment Satisfaction-NRS). The primary EASI and all secondary subjective scores will be logarithmically transformed, and whether the data are normally distributed will be checked. “% change from baseline of EASI and all secondary clinical findings at 16w” will be referred to as the dependent variable, whereas “baseline level of each of the 18 biomarkers” will be referred to as the independent variable.
If we can assume that the data on the log-transformed endpoints at 16w are normally distributed, we will use an analysis of covariance model, adjusting for confounding factors. As potential confounding factors, sex, age, and medical and family history will be included in the model because these are known as important risk factors for AD.
If the data for the log-transformed endpoints at 16w do not fulfil the assumption of normality, we will use generalized linear models, adjusting for confounding factors such as sex, age, and medical and family history.
3.2. Relationship between potential proteomic markers and clinical findings
To evaluate the association between “baseline level of each potential proteomic marker” and “% change from baseline of primary (EASI) and all secondary clinical findings (POEM, Pruritus-NRS, Skin Comfort-NRS, and Treatment Satisfaction-NRS) at 16w,” we will also check the distribution of the primary EASI and all secondary clinical findings as mentioned above. “% change from baseline of the primary endpoint and all secondary clinical findings at 16w” will be referred to as the dependent variable, whereas “baseline level of each potential proteomic marker” will be referred to as the independent variable. Then, we will perform the same statistical analysis as described above.
3.3. Development of a score for evaluating disease activity in AD
Since measuring disease activity is an important component of AD management, biomarkers that capture the complex and heterogeneous biology of AD may have the potential to complement clinical disease activity assessment. We hypothesize that the measurement of multiple biomarkers and potential proteomic markers combined into a more limited score could quantitatively and objectively characterize AD activity and enhance AD activity assessment. Thus, after evaluating the associations of biomarkers and potential proteomic markers with primary and secondary endpoints, we will investigate the possibility of developing a score for evaluating disease activity in AD.
A score for disease activity in AD will be determined using the values of 18 biomarkers (% eosinophils, LDH, total IgE, soluble IL-2 receptor, CCL17, CCL22, CCL27, CCL18, CCL26, IL-13, IL-22, IL-24, IL-25, IL-31, IL-33, TSLP, periostin, and SCCA2) and potential proteomic markers (of roughly 300 molecules) during the 16w period of dupilumab treatment.
To evaluate the internal consistency of biomarkers and potential proteomic markers, Cronbach's α will be calculated. Mutual correlations of biomarkers and potential proteomic markers will be determined using correlation coefficients.
To explore potential groupings of the biomarkers and potential proteomic markers into a more limited number of score components, factorial analysis based on correlation coefficients will be performed. The selection of the number of score components will be based on the eigenvalues. To understand the meaning of the score components, promax rotation will be used. Finally, analysis of covariance or generalized linear models adjusting for confounding factors such as sex, age, and medical and family history will be used to evaluate the associations of combined scores with the primary endpoint and all secondary endpoints.
4. Discussion
The purpose of this study is to explore biomarkers that predict good and poor responders to dupilumab treatment in a real-world setting. As for the biomarkers, we will examine 18 candidates, all of which are known to be associated with disease activity of AD. For example, Guttman-Yassky et al. recently demonstrated that dupilumab treatment does significantly improve type 2 inflammatory signatures (IL-13, IL-31, CCL17, CCL18, and CCL26) in the blood and cutaneous tissues.[9] Our previous studies also demonstrated that periostin and SCCA2 are downstream molecules of IL-4/IL-13 signaling and that these molecules are highly expressed in inflamed sites of AD patients.[48–50] However, none of them has been analyzed as a predictor of response to dupilumab treatment. In addition, no stratification of AD patients to compare the efficacy of dupilumab was performed in 2 phase 3 trials of dupilumab for AD (SOLO1 and SOLO2).[7] In these trials, the improvement as evaluated by IGA score as the primary outcome was 36% to 38%. In addition, the rate of achieving at least 75% improvement from baseline in EASI (EASI-75) as a secondary outcome was 44% to 51%.[7] These results suggest that the efficacy of dupilumab varies among AD patients and that it is important to develop useful biomarkers to predict its efficacy, especially considering the economic burden on patients and the medical insurance cost of such treatment.
In asthma, recent studies have proposed several biomarkers to predict the efficacy of treatments. For example, asthma patients with baseline blood eosinophils of ≥300 cells per μL who are receiving high-dosage inhaled corticosteroids plus long-acting β2-agonists were reported to exhibit a longer exacerbation-free clinical course than those with placebo.[51] Dupilumab was also shown to achieve substantial improvements in asthma patients with a baseline blood eosinophil count of at least 300 eosinophils per μL in terms of patient-reported outcomes such as morning and evening asthma symptom scores.[52] In addition, the eosinophil count is a useful predictor of good treatment response in asthma patients treated with the anti-IL-5 antibody mepolizumab.[53] The anti-IL-13 antibody lebrikizumab is also known to be efficacious for asthma treatment.[54] Patients with high pretreatment levels of serum periostin have greater improvement in lung function upon lebrikizumab treatment than do patients with low periostin levels in asthma.[54] Pretreatment serum levels of dipeptidyl peptidase-4 or periostin are also useful predictors of good therapeutic response in asthma patients administered the anti-IL-13 antibody tralokinumab.[55]
The present clinical trial will be the first to evaluate the pretreatment serum biomarkers that predict a good or poor outcome in patients with AD treated with dupilumab. Eighteen serum biomarkers that are known to reflect disease activity of AD are selected as potential candidates. We will also extend our study to seek new biomarkers using proteomic analysis. However, this study has a limitation that will only enroll Japanese patients. Recent reports suggest that patients of Asian origin with AD have a prominent IL-17 component.[38] Therefore, there is a possibility that the findings of this study cannot be extrapolated to non-Asian AD. However, a biomarker assessment study is now ongoing in the European “BioDay” dupilumab treatment cohort.[40] Although the primary endpoints differ, it will be possible to compare our results with those from “BioDay.”
Author contributions
KI, DO, NK, TN, and MF conceived and designed and drafted the study protocol. All other authors approved the study protocol. TN and MF drafted the manuscript. All other authors critically reviewed the manuscript. All authors approved the final version of the manuscript.
Glossary
Abbreviations: AD = atopic dermatitis, CCL = Chemokine (C-C motif) ligand, EASI = eczema area and severity index, IGA = Investigator's Global Assessment, IgE = immunoglobulin E, IL = interleukin, LDH = lactate dehydrogenase, NRS = numerical rating scale, POEM = patient-oriented eczema measure, SCCA2 = squamous cell carcinoma antigen-2, sIL-2R = soluble interleukin 2 receptor, Th2 = T helper type 2, TSLP = thymic stromal lymphopoietin.
References
- [1].Williams H, Stewart A, von Mutius E, et al. Is eczema really on the increase worldwide? J Allergy Clin Immunol 2008;121:947–54.. [DOI] [PubMed] [Google Scholar]
- [2].Furue M, Ulzii D, Vu YH, et al. Pathogenesis of atopic dermatitis: current paradigm. Iran J Immunol 2019;16:97–107.. [DOI] [PubMed] [Google Scholar]
- [3].Arima K, Gupta S, Gadkari A, et al. Burden of atopic dermatitis in Japanese adults: Analysis of data from the 2013 National Health and Wellness Survey. J Dermatol 2018;45:390–6.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jung HJ, Bae JY, Kim JE, et al. Survey of disease awareness, treatment behavior and treatment satisfaction in patients with atopic dermatitis in Korea: a multicenter study. J Dermatol 2018;45:1172–80.. [DOI] [PubMed] [Google Scholar]
- [5].Komura Y, Kogure T, Kawahara K, et al. Economic assessment of actual prescription of drugs for treatment of atopic dermatitis: Differences between dermatology and pediatrics in large-scale receipt data. J Dermatol 2018;45:165–74.. [DOI] [PubMed] [Google Scholar]
- [6].Fargnoli MC, Esposito M, Ferrucci S, et al. Real-life experience on effectiveness and safety of dupilumab in adult patients with moderate-to-severe atopic dermatitis. J Dermatolog Treat 2019;28:1–7.. [DOI] [PubMed] [Google Scholar]
- [7].Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med 2016;375:2335–48.. [DOI] [PubMed] [Google Scholar]
- [8].Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol 2019;156:44–56.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Guttman-Yassky E, Bissonnette R, Ungar B, et al. Dupilumab progressively improves systemic and cutaneous abnormalities in patients with atopic dermatitis. J Allergy Clin Immunol 2019;143:155–72.. [DOI] [PubMed] [Google Scholar]
- [10].Hamilton JD, Suárez-Fariñas M, Dhingra N, et al. Dupilumab improves the molecular signature in skin of patients with moderate-to-severe atopic dermatitis. J Allergy Clin Immunol 2014;134:1293–300.. [DOI] [PubMed] [Google Scholar]
- [11].Seo E, Yoon J, Jung S, et al. Phenotypes of atopic dermatitis identified by cluster analysis in early childhood. J Dermatol 2019;46:117–23.. [DOI] [PubMed] [Google Scholar]
- [12].Fölster-Holst R, Papakonstantinou E, Rüdrich U, et al. Childhood atopic dermatitis-brain-derived neurotrophic factor correlates with serum eosinophil cationic protein and disease severity. Allergy 2016;71:1062–5.. [DOI] [PubMed] [Google Scholar]
- [13].Tsuda S, Kato K, Miyasato M, et al. Eosinophil involvement in atopic dermatitis as reflected by elevated serum levels of eosinophil cationic protein. J Dermatol 1992;19:208–13.. [DOI] [PubMed] [Google Scholar]
- [14].Thijs J, Krastev T, Weidinger S, et al. Biomarkers for atopic dermatitis: a systematic review and meta-analysis. Curr Opin Allergy Clin Immunol 2015;15:453–60.. [DOI] [PubMed] [Google Scholar]
- [15].Furue M, Chiba T, Tsuji G, et al. Atopic dermatitis: immune deviation, barrier dysfunction, IgE autoreactivity and new therapies. Allergol Int 2017;66:398–403.. [DOI] [PubMed] [Google Scholar]
- [16].Furue M, Sugiyama H, Tsukamoto K, et al. Serum soluble IL-2 receptor (sIL-2R) and eosinophil cationic protein (ECP) levels in atopic dermatitis. J Dermatol Sci 1994;7:89–95.. [DOI] [PubMed] [Google Scholar]
- [17].Tsoi LC, Rodriguez E, Degenhardt F, et al. Atopic dermatitis is an IL-13-dominant disease with greater molecular heterogeneity compared to psoriasis. J Invest Dermatol 2019;139:1480–9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mitamura Y, Nunomura S, Nanri Y, et al. The IL-13/periostin/IL-24 pathway causes epidermal barrier dysfunction in allergic skin inflammation. Allergy 2018;73:1881–91.. [DOI] [PubMed] [Google Scholar]
- [19].Mitamura Y, Nunomura S, Furue M, et al. IL-24: A new player in the pathogenesis of pro-inflammatory and allergic skin diseases. Allergol Int 2020. [DOI] [PubMed] [Google Scholar]
- [20].Aktar MK, Kido-Nakahara M, Furue M, et al. Mutual upregulation of endothelin-1 and IL-25 in atopic dermatitis. Allergy 2015;70:846–54.. [DOI] [PubMed] [Google Scholar]
- [21].Furue M, Yamamura K, Kido-Nakahara M, et al. Emerging role of interleukin-31 and interleukin-31 receptor in pruritus in atopic dermatitis. Allergy 2018;73:29–36.. [DOI] [PubMed] [Google Scholar]
- [22].Ruzicka T, Hanifin JM, Furue M, et al. Anti-interleukin-31 receptor A antibody for atopic dermatitis. N Engl J Med 2017;376:826–35.. [DOI] [PubMed] [Google Scholar]
- [23].Chen YL, Gutowska-Owsiak D, Hardman CS, et al. Proof-of-concept clinical trial of etokimab shows a key role for IL-33 in atopic dermatitis pathogenesis. Sci Transl Med 2019;11:pii:eaax2945. [DOI] [PubMed] [Google Scholar]
- [24].Nygaard U, Hvid M, Johansen C, et al. TSLP, IL-31, IL-33 and sST2 are new biomarkers in endophenotypic profiling of adult and childhood atopic dermatitis. J Eur Acad Dermatol Venereol 2016;30:1930–8.. [DOI] [PubMed] [Google Scholar]
- [25].Kou K, Okawa T, Yamaguchi Y, et al. Periostin levels correlate with disease severity and chronicity in patients with atopic dermatitis. Br J Dermatol 2014;171:283–91.. [DOI] [PubMed] [Google Scholar]
- [26].Nagao M, Inagaki S, Kawano T, et al. SCCA2 is a reliable biomarker for evaluating pediatric atopic dermatitis. J Allergy Clin Immunol 2018;141:1934–6.. [DOI] [PubMed] [Google Scholar]
- [27].Takeuchi S, Furusyo N, Ono J, et al. Serum squamous cell carcinoma antigen (SCCA)-2 correlates with clinical severity of pediatric atopic dermatitis in Ishigaki cohort. J Dermatol Sci 2019;95:70–5.. [DOI] [PubMed] [Google Scholar]
- [28].Bieber T. Atopic dermatitis 2.0: from the clinical phenotype to the molecular taxonomy and stratified medicine. Allergy 2012;67:1475–82.. [DOI] [PubMed] [Google Scholar]
- [29].Leung DY, Guttman-Yassky E. Deciphering the complexities of atopic dermatitis: shifting paradigms in treatment approaches. J Allergy Clin Immunol 2014;134:769–79.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhou L, Leonard A, Pavel AB, et al. Age-specific changes in the molecular phenotype of patients with moderate-to-severe atopic dermatitis. J Allergy Clin Immunol 2019;144:144–56.. [DOI] [PubMed] [Google Scholar]
- [31].Esaki H, Brunner PM, Renert-Yuval Y, et al. Early-onset pediatric atopic dermatitis is T(H)2 but also T(H)17 polarized in skin. J Allergy Clin Immunol 2016;138:1639–51.. [DOI] [PubMed] [Google Scholar]
- [32].Gittler JK, Shemer A, Suárez-Fariñas M, et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol 2012;130:1344–54.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Schmid-Grendelmeier P, Simon D, Simon HU, et al. Epidemiology, clinical features, and immunology of the “intrinsic” (non-IgE-mediated) type of atopic dermatitis (constitutional dermatitis). Allergy 2001;56:841–9.. [DOI] [PubMed] [Google Scholar]
- [34].Tokura Y. Extrinsic and intrinsic types of atopic dermatitis. J Dermatol Sci 2010;58:1–7.. [DOI] [PubMed] [Google Scholar]
- [35].Kaufman BP, Guttman-Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups-Variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol 2018;27:340–57.. [DOI] [PubMed] [Google Scholar]
- [36].Chan TC, Sanyal RD, Pavel AB, et al. Atopic dermatitis in Chinese patients shows T(H)2/T(H)17 skewing with psoriasiform features. J Allergy Clin Immunol 2018;142:1013–7.. [DOI] [PubMed] [Google Scholar]
- [37].Suárez-Fariñas M, Dhingra N, Gittler J, et al. Intrinsic atopic dermatitis shows similar TH2 and higher TH17 immune activation compared with extrinsic atopic dermatitis. J Allergy Clin Immunol 2013;132:361–70.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Guttman-Yassky E, Krueger JG. Atopic dermatitis and psoriasis: two different immune diseases or one spectrum? Curr Opin Immunol 2017;48:68–73.. [DOI] [PubMed] [Google Scholar]
- [39].Thijs JL, Strickland I, Bruijnzeel-Koomen CAFM, et al. Moving toward endotypes in atopic dermatitis: Identification of patient clusters based on serum biomarker analysis. J Allergy Clin Immunol 2017;140:730–7.. [DOI] [PubMed] [Google Scholar]
- [40].Ariëns LFM, van der Schaft J, Bakker DS, et al. Dupilumab is very effective in a large cohort of difficult-to-treat adult atopic dermatitis patients: first clinical and biomarker results from the BioDay registry. Allergy 2020;75:116–26.. [DOI] [PubMed] [Google Scholar]
- [41].Huang TH, Chen YC, Lin SY, et al. Treatment of atopic dermatitis with dupilumab in Taiwan: dynamic changes of IgE levels as a potential response biomarker. Eur J Dermatol 2019;29:658–9.. [DOI] [PubMed] [Google Scholar]
- [42].Hanifin JM, Thurston M, Omoto M, et al. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol 2001;10:11–8.. [DOI] [PubMed] [Google Scholar]
- [43].Schmitt J, Spuls PI, Thomas KS, et al. The Harmonising Outcome Measures for Eczema (HOME) statement to assess clinical signs of atopic eczema in trials. J Allergy Clin Immunol 2014;134:800–7.. [DOI] [PubMed] [Google Scholar]
- [44].Grinich EE, Schmitt J, Küster D, et al. Standardized reporting of the Eczema Area and Severity Index (EASI) and the Patient-Oriented Eczema Measure (POEM): a recommendation by the Harmonising Outcome Measures for Eczema (HOME) Initiative. Br J Dermatol 2018;179:540–1.. [DOI] [PubMed] [Google Scholar]
- [45].Charman CR, Venn AJ, Ravenscroft JC, et al. Translating Patient-Oriented Eczema Measure (POEM) scores into clinical practice by suggesting severity strata derived using anchor-based methods. Br J Dermatol 2013;169:1326–32.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Reich A, Riepe C, Anastasiadou Z, et al. Itch assessment with visual analogue scale and numerical rating scale: determination of minimal clinically important difference in chronic itch. Acta Derm Venereol 2016;96:978–80.. [DOI] [PubMed] [Google Scholar]
- [47].Yosipovitch G, Reaney M, Mastey V, et al. Peak Pruritus Numerical Rating Scale: psychometric validation and responder definition for assessing itch in moderate-to-severe atopic dermatitis. Br J Dermatol 2019;181:761–9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Yuyama N, Davies DE, Akaiwa M, et al. Analysis of novel disease-related genes in bronchial asthma. Cytokine 2002;19:287–96.. [DOI] [PubMed] [Google Scholar]
- [49].Mitsuishi K, Nakamura T, Sakata Y, et al. The squamous cell carcinoma antigens as relevant biomarkers of atopic dermatitis. Clin Exp Allergy 2005;35:1327–33.. [DOI] [PubMed] [Google Scholar]
- [50].Masuoka M, Shiraishi H, Ohta S, et al. Periostin promotes chronic allergic inflammation in response to Th2 cytokines. J Clin Invest 2012;122:2590–600.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor ( monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2016;388:2128–41.. [DOI] [PubMed] [Google Scholar]
- [52].Wenzel S, Castro M, Corren J, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting (2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet 2016;388:31–44.. [DOI] [PubMed] [Google Scholar]
- [53].Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med 2014;371:1198–207.. [DOI] [PubMed] [Google Scholar]
- [54].Corren J, Lemanske RF, Hanania NA, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med 2011;365:1088–98.. [DOI] [PubMed] [Google Scholar]
- [55].Brightling CE, Chanez P, Leigh R, et al. Efficacy and safety of tralokinumab in patients with severe uncontrolled asthma: a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir Med 2015;3:692–701.. [DOI] [PubMed] [Google Scholar]


