Abstract
Inflammatory, angiogenic, and immune processes have been associated with uveal melanoma (UM). The aim of the present study was to evaluate the presence of some specific aqueous humor (AH) soluble biomarkers in eyes affected by UM. Thirty-five eyes affected by primary UM and 35 control eyes, scheduled for cataract surgery, underwent full ophthalmic examination and AH sampling at time of surgery (brachytherapy or cataract surgery, respectively). AH samples were analyzed by means of ELISA, to detect the concentration of selected cytokines, chemokines, and growth factors. Compared with the control group, higher levels of IL-6 (P = .049), IL-8 (P = .006), RANTES (P = .008), EGF (P = .032), bFGF (P = .016), MIF (P = .007), and MCP (P = .020) were detected in eyes with UM. VEGF concentration between the two groups was statistically borderline (P = .058). Comparison between clinical characteristics and cytokine concentrations showed a positive correlation between tumor thickness and IL-8 (P = .032), and degree of serous retinal detachment and IL-6 (P = .021). UM is characterized by the presence of retinal neuroinflammatory, angiogenic, and immune biomarkers in AH. The proteomic analysis of AH could characterize UM microenvironment, allowing to better understand its pathophysiology.
Keywords: aqueous humor sample, biomarker, chemokines, cytokines, factors, growth, proteomic analysis, uveal melanoma
1. Introduction
Uveal melanoma (UM) is the most common primary malignant intraocular tumor in adults. Despite improvements of its local treatment, prevention and treatment of the metastatic disease remain still unsolved, and nearly 50% of patients eventually die because of tumor spread. The current model suggests that tumor cells have already diffused outside the eye by the time of diagnosis, remaining dormant until favourable growth conditions develop.[1] This theory is also supported by tumor doubling time calculations, suggesting that the metastatic spread precedes the initial tumor diagnosis.[1,2]
Some clinical and histopathologic parameters have been identified as indicators of UM prognosis, including clinical, histological, and genetic tumor characteristics.[2] However, the mechanisms of UM progression and metastasis are still unknown. Specific angiogenic, immunologic, and inflammatory pathways have been related to UM progression and spreading. An inflammatory phenotype, characterized by an increased number of T-lymphocytes and tumor associated macrophages (TAM), has been associated with the presence of high risk histological and genetic characteristics (epithelioid cells and monosomy 3).[2–4] Furthermore, UM develops in an immunologically privileged environment, where both the adaptive and innate immune systems are suppressed. Therefore, changes in intraocular immune processes (the immunoediting theory) may strongly influence UM progression.[4,5] UM disseminates purely hematogenously. Therefore, the dissemination of the primary tumor through peripheral blood is strictly related to intratumor angiogenesis. Cytokines, chemokines, and growth factors activate and regulate all these processes, and can be produced by both melanoma cells and healthy cells stimulated by the presence of the tumor.[4,5] Notably, UM occurs in a closed microenvironment characterized by a direct contact with ocular fluids. Therefore, the analysis of these fluids may allow to search and identify specific biomarkers providing information about the different pathways affected in an eye with UM.[2] In oncology, biomarkers are used to facilitate diagnosis, establish prognosis and predict the therapeutic response of a tumor. The ideal biomarker should be specific, sensitive, predictive, and easy to be assessed in vivo. Therefore, there is growing interest in the identification of diagnostic and prognostic biomarkers at ocular fluids level. Anterior chamber paracentesis is technically ordinary and safe for the patient, so aqueous humor (AH) evaluation may represent the liquid biopsy approach in UM diagnosis and follow-up. The aim of the present study was to evaluate the presence of AH neuroinflammatory, angiogenic, and immune biomarkers in eyes affected by UM at the time of tumor treatment.
2. Materials and methods
This was a cross-sectional case–control study with prospective enrollment, performed at the Ophthalmology Department of the University of Padova—IRCCS G.B. Bietti Foundation, oncology and toxicology unit. Patients were consecutively recruited from those referred between January and September 2018. Informed consent was obtained from each patient and Institutional Review Board approved the study protocol. The approval for the study was obtained from the local Ethics Committee (Comitato Etico: CESC; study N.:1302P, Prot. N. 65819, November 23, 2006). Patients were diagnosed by a senior ocular oncologist (EM or RP) by ophthalmoscopy and A-B scan ultrasonography. Liver enzymes and liver ultrasonography were used to evaluate the presence of metastatic disease at baseline. Inclusion criteria were: patients affected by UM and planned to be treated with Iodine-125 (I-125) brachytherapy according to the Collaborative Ocular Melanoma Study (COMS) guidelines (85Gy at tumor apex with a dose rate of 0.60–1.05Gy/h). Exclusion criteria were: any history or clinical evidence of ocular and/or systemic diseases (e.g., diabetes), any previous ocular surgery, intravitreal drug injection or laser treatment and any significant refractive error (>6 D). Patients affected by metastatic UM at baseline were also excluded. A control group, composed by age- and gender-matched patients presenting for cataract surgery, without any other ocular or systemic disease, were prospectively recruited during the same period.
2.1. Study procedures
Patients planned for brachytherapy underwent AH sampling during I-125 surgical procedure, immediately before plaque positioning, whereas subjects included in the control group underwent AH sampling at the time of cataract surgery. Each enrolled subject underwent a complete ophthalmologic examination, including slit-lamp biomicroscopy, tonometry, and ophthalmoscopy. The location of the tumor was reported according to the main involved sector (nasal, temporal, superior, inferior) and the tumor origin (choroid, ciliary body). Tumor thickness and largest basal diameter, measured by A- and B-scan ultrasonography (Aviso, Quantel Medical, Clermont-Ferrand, France), were also reported. Tumors were staged using the 8th AJCC classification.[6] The presence of peritumoral serous retinal detachment (SRD) was categorized considering the number of retinal sectors involved (<1 quadrant; 1–2 quadrants; >2 quadrants).
2.2. Sample collection and preparation for analysis
Each enrolled subject underwent standard pre-operative preparation for eye surgery, including disinfection of periocular skin with povidone-iodine 10% (ESO-JOD, ECOLAB, Agrate Brianza, Italia), irrigation of the conjunctival sac with povidone-iodine 5% (Oftasteril, Alphaintes), abundant washing out of the eye with balanced salt solution. AH (150–200 μL) was aspirated from the anterior chamber, using a 30-gauge needle connected to an insulin syringe (1 mL). After aspiration, AH was collected by a second operator in a single microfuge containing 10 mL of a cocktail of protease inhibitors (Pierce Biotechnology, Rockford, IL). Labeled microvials were quickly stored at −70°C. The total protein content was quantified with a digital spectrophotometer (NanoDrop; Thermo Fisher Scientific Inc, Waltham, MA) and protein concentrations were calculated by means of the linearized standard curve (bovine serum albumin) and the A280 software. AH samples where than processed by sonication (VibraCell; Sonics, Newton, CT) and centrifugation to collect the clear supernatant (13,000 rpm/7 min).
2.3. Enzyme-linked immunosorbent assay
Commercial high-sensitive enzyme-linked immunosorbent assays (ELISA, Merck KGaA, Darmstadt, Germany), were used according to the manufacturer's procedures. Briefly, prediluted samples and standard curve were diluted in TBS buffer (20 mM Tris-Cl and 150 mM NaCl; pH 7.5) containing 3% bovine serum albumin, 5 mM EDTA, and 1× protease inhibitor cocktail, and loaded on 96-well precoated plates. Biotin-coupled antibodies and horseradish peroxidase-streptavidin–specific bindings were used, and specific binding was developed with a ready-to-use tetramethylbenzidine substrate (eBioscience, San Diego, CA). The colorimetric signal was quantified using an ELISA reader at 490 to 560 mm (Sunrise; Tecan Group Ltd, Männedorf, Switzerland). The optical density (OD) data were normalized to total protein content (NanoDrop). The specific concentrations of interleukin-6 (IL-6), interleukin-8 (IL-8), regulated upon activation normal T cell expressed, and secreted (RANTES), epidermal growth factor (EGF), basic fibroblast growth factor (bFGF), monocyte chemoattractant protein-1 (MCP-1), macrophage inhibiting factor (MIF) and vascular endothelial growth factor (VEGF) were calculated on the linearized standard curves, as provided at the end of ELISA. Standards and detection limits were 1.5 to 100 ng/mL and 1.5 ng/mL, respectively.
2.4. Statistical analysis
Data are presented as the mean ± standard deviation, and the normality of the distribution was assessed by the Shapiro–Wilk test. The comparison of AH proteins’ concentration in patients with UM and in controls was made, for each protein, by means of Mann–Whitney rank sum test and t test. Linear regression was applied to see whether clinical characteristics correlated with each biomarker level. Data were analyzed using SAS statistical software (SAS 9.2; SAS Institute, Cary, NC). A value of P < .05 was considered statistically significant.
3. Results
Thirty-five consecutive patients (35 eyes) and 35 controls (35 eyes) were consecutively included. Demographic and clinical characteristics of the enrolled population are summarized in Table 1. The mean age at study inclusion was 59.32 ± 11.78 and 61.81 ± 5.42, respectively for cases and controls (P = .3123). There was no significant difference in gender (P = .2169) between the two study groups. Considering the UM group, 29 patients (83%) were affected by choroidal tumor and six patients (17%) by ciliary body tumor. According to the AJCC 8th classification, tumor size categories were T4 in 4 eyes (12%), T3 in 11 eyes (31%), T2 in 12 eyes (34%), and T1 in 8 eyes (23%). The mean tumor largest basal diameter was 13.1 ± 4.5 mm, and the mean tumor thickness 6.2 ± 2.9 mm. All UM were associated with clinically detectable SRD. In 18 tumors (52%) SRD involved <1 quadrant, in 12 (34%) 1 to 2 quadrants and in 5 (14%) >2 quadrants. No complications after AH sampling were documented in both groups. The cytokines, chemokines, and growth factors levels in the AH samples are provided in Table 2 and fold changes are shown in Figure 1. Compared to the control group, significant higher levels of IL-6 (P = .049), IL-8 (P = .006), RANTES (P = .008), EGF (P = .032), bFGF (P = .016), MIF (P = .007), and MCP (P = .020) were detected in eyes with UM.
Table 1.
Clinical and demographic characteristics of enrolled patients.
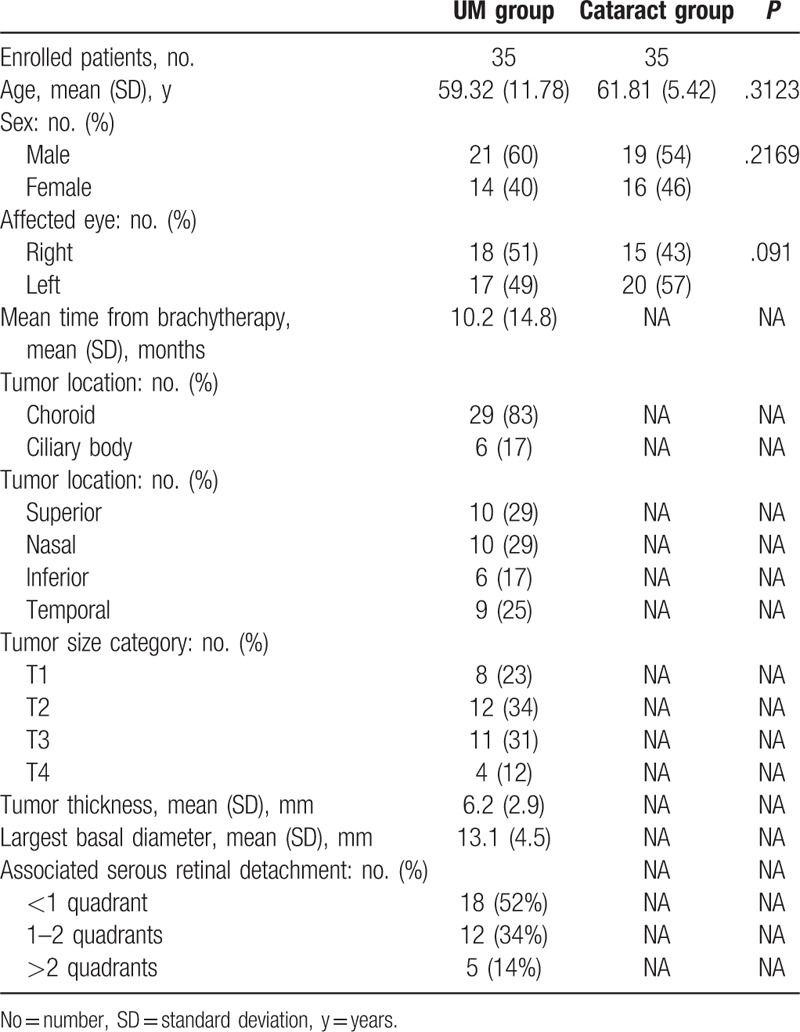
Table 2.
Aqueous humor concentrations (pg/mL) (mean ± SD) of cytokines in uveal melanoma patients (study group) and cataract patients (control group).
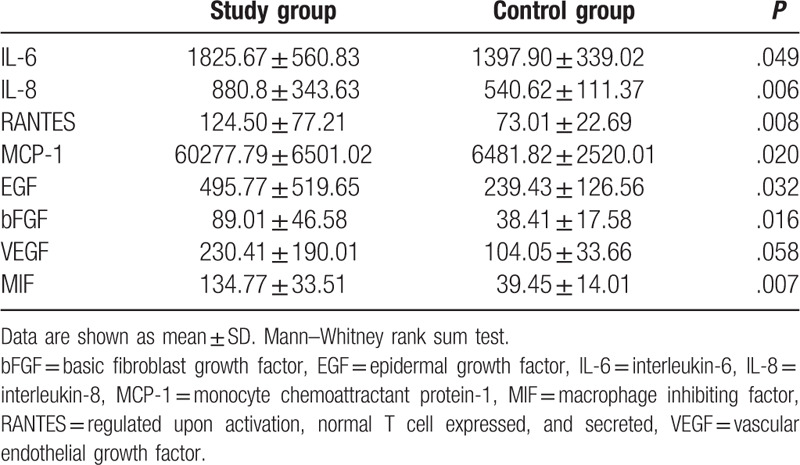
Figure 1.
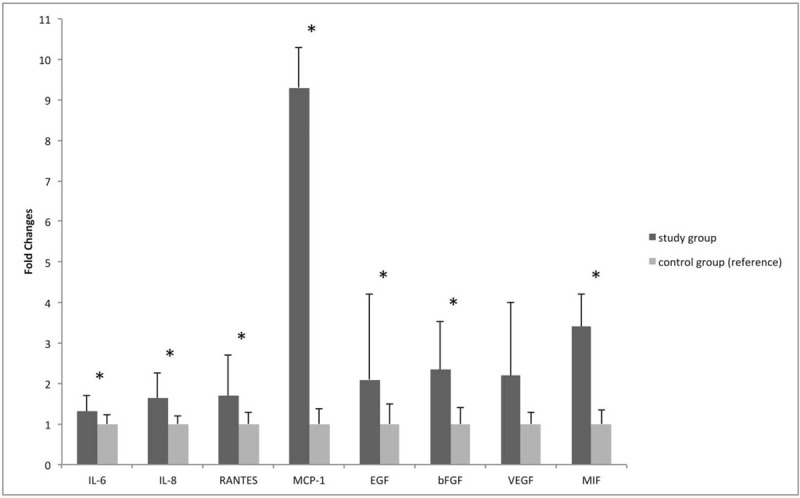
Representation of fold changes ± SD of each AH dosed molecule in uveal melanoma eyes (study group) vs control eyes (control group). Data are shown as mean ± SD. Mann–Whitney rank sum test. ∗ = molecules showing a statistically significant difference (P < .05) between study and control group. VEGF concentration showed a borderline difference (P = .058). AH = aqueous humor, bFGF = basic fibroblast growth factor, EGF = epidermal growth factor, IL-6 = interleukin-6, IL-8 = interleukin-8, MCP-1 = monocyte chemoattractant protein-1, MIF = macrophage inhibiting factor, RANTES = regulated upon activation, normal T cell expressed, and secreted, VEGF = vascular endothelial growth factor.
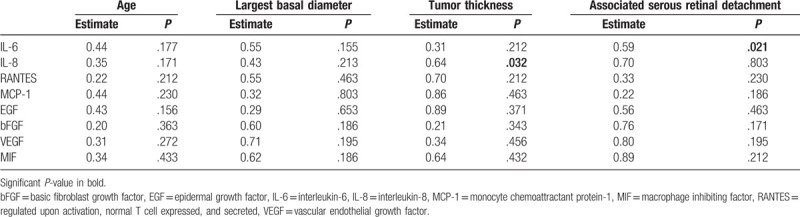
Comparison between clinical characteristics (age, LBD, tumor height, and degree of associated retinal detachment) and cytokine concentration showed a positive correlation between tumor thickness and IL-8 (P = .032), and degree of retinal detachment and IL-6 (P = .021). Table 3 highlights associations between cytokine and chemokine levels in AH and UM clinical characteristics.
Table 3.
Aqueous humor cytokines and chemokines and their correlation to uveal melanoma clinical parameters.
4. Discussion
Angiogenesis, inflammation and immunomodulation are important pathways in the pathogenesis of tumors and in the development and progression of UM. VEGF is the main growth factor in the pathophysiology of a range of retinal and choroidal diseases. And as previously reported, UM cell lines are known to express VEGF.[2,7,8] Other proangiogenic factors, like bFGF and EGF, have been found highly expressed in human eyes with UM.[4,5,7,8] Moreover, some cytokines seem to play a key role in the pathogenesis and/or progression of UM.[2,7,8] Among these, IL-6 is the most thoroughly investigated inflammatory cytokine regarding UM, and it is associated with high levels of IL-8 and MCP-1. These proteins are hypothesized to play a role in the attraction and activation of monocytes and macrophages in UM.[7,8] Regarding MIF and RANTES there is limited knowledge about their role in UM.[4,5,7,8] It has been proven that co-expression of RANTES with others chemokines results in a significantly better T-cell response against tumor cells and that UM derived cells constitutively produce MIF.[4,5,7,8] We focused our study to detect AH concentrations of the cytokines and the chemokines mentioned above.
According to TCGA (The Cancer Genome Atlas) database, high mRNA levels of the overexpressed proteins were found in some histological samples of UM (Fig. 2), ranging from 1.3% of IL-8 to 9% of MIF of the examined cases. Unfortunately, there are not similar public RNA dataset for AH of patients with UM. Intratumoral microenvironment can be considered the main factor influencing the anterior chamber level of tumor related cytokines, sometimes with contrasting data, as reported by Wierenga et al.[9] Thus, we consider that directly comparing intratumoral cytokines expression to AH cytokines level remains controversial and a public RNA dataset for AH is needed to compare any new data.
Figure 2.
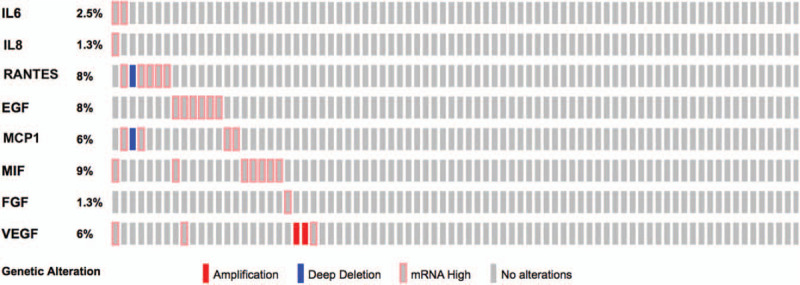
Graphical summary of mRNA expression across the set of the 80 uveal melanoma samples, collected by the TCGA (The Cancer Genome Atlas) project. Rows represent proteins, and columns represent samples. AH = aqueous humour, bFGF = basic fibroblast growth factor, EGF = epidermal growth factor, IL-6 = interleukin-6, IL-8 = interleukin-8, MCP1 = monocyte chemoattractant protein-1, MIF = macrophage inhibiting factor, RANTES = regulated upon activation, normal T cell expressed, and secreted, VEGF = vascular endothelial growth factor.
The identification of soluble molecules in intraocular fluids has recently assumed great relevance in the pathophysiological characterization of different retinal and choroidal diseases.[10,11] The precise correlation between the concentration of molecules present in the vitreous and those in the AH has already been reported, confirming the value of aqueous sampling as a less invasive procedure compared to the vitreous one.[12] Since the identification of aqueous flare in UM eyes, the relevance of soluble factors interactions in UM microenvironment has been suggested.[7–9,13–16] The up-regulation of different growth factors, such as PDGF, VEGF, GM-CSF, EGF, bFGF, has been related to the presence of UM.[2,7,8] Notwithstanding the increase of these factors may be mutually related, as reported by Dunavoelgyi et al[7] and Ly et al.[2] Therefore, we selected to determine the most widely known growth factors. Our study confirmed the higher concentration of EGF, bFGF, and VEGF in eyes affected by UM compared to controls.[8] These findings may have significant implications not only for local progression, but also for the systemic spreading of the disease.
In our cohort, we have found a significantly higher concentration of inflammatory factors such as IL-8, IL-6, RANTES, and MCP in UM eyes compared to controls, even if IL-6 significance was borderline. Probably, our population is still limited in number to reach statistical significance. A significant increase of some pro-inflammatory molecules (mainly IL-8, RANTES, and MCP) has already been reported by Usui et al who compared eyes affected by UM to eyes affected by benign choroidal nevi.[15] Conversely, the same Authors did not find increased IL-6 levels, as we did.[15] IL-6 is a well-known pro-inflammatory cytokine, typically produced by T lymphocytes and macrophages, implicated in the persistence of the inflammatory cycle.[2,14] It has been proposed as stimulator of tumor growth and attractor of tumor associated macrophages (TAM), whose density was correlated with poor survival of UM patients.[2,14] However, conflicting results have been reported considering IL-6 levels and immune cells infiltration.[2,16] Ly et al[2] showed no correlation between the amount of cytokines expressed in the AH and the intratumoral density of M2-type macrophages, suggesting that surroundings tissues are the main source of pro-inflammatory cytokines involved in the inflammatory phenotype.[2] Our results showed a slightly significant increase of IL6 in UM eyes compared to controls, but also a stronger correlation between SRD amount and IL-6, suggesting that IL-6 secretion may be more related to serous retinal detachment than UM growth and spreading. Moreover, previous studies have correlated the intravitreal IL-6 level with the presence of serous retinal detachment in other retinal disorders, such as diabetic macular edema.[17] Furthermore, it is known that the IL-10/IL-6 ratio is usually >1.0 in intraocular lymphoma, whereas is inferior to 1.0 in uveitis, confirming that IL-6 secretion is typically related to inflammation by itself, more than malignancy.[2]
Our data confirmed the relevance of IL-8 and MCP-1 in UM. These molecules are chemotactic factors, involved in the recruitment of TAM into tissues, and facilitating the proliferation and survival of UM.[8,15] Previous studies also reported their ability to promote angiogenesis, suggesting possible alternative pro-angiogenic pathways to VEGF.[8,18] Moreover, we found a significant correlation between IL-8 levels and tumor thickness, suggesting that this soluble factor may be related to an advanced disease stage.
Finally, we found a higher concentration of MIF in UM eyes. MIF is a pleiotropic cytokine normally present in the AH, which inhibits natural killer (NK)-cells, thus protecting malignant cells from their surveillance. NK-cells are bone marrow-derived lymphocytes, which participate in innate immune response, recognizing and eliminating damaged or malignant cells.[4] Several ocular tissues produce MIF, and Repp et al[5] already proved that human UM derived cells constitutively produce MIF. Moreover, they observed that MIF expression was highest in UM metastasis derived cells.[5] NK-cells play a relevant role in controlling UM metastasis. The increased expression of HLA I in UM cells, which reduces the cytolytic activity of NK-cells, has been related to monosomy 3, one of the most relevant prognostic factor for UM.[3] This mechanism assumes specific relevance considering that in the blood and in the liver the NK-cells are extremely active, and the ability to produce factors inhibiting their activity may be essential for the metastatic process.[4,5] Further studies could evaluate whether other immune related factors are involved in UM progression and different AH concentrations may allow an early detection of tumors with greater propensity to metastasize. The main limitation of our study is the small number of patients. Moreover, AH sampling procedure even if easy and safe is still under-utilized, limiting the liquid biopsy approach in ocular oncology. Our results show that the detection of UM-related soluble molecules in AH may be useful to characterize microenvironment and better understanding UM pathogenesis and spreading.
Inter alia, pro-inflammatory cytokines appear to have a primary role in creating an inflammatory environment, which may promote tumor cells survival and metastatization. Moreover, immune related factors, such as MIF, may be significant biomarkers of local immune-editing, strictly related to UM pathophysiology and possible target of new therapies.[4]
Author contributions
Edoardo Midena, Raffaele Parrozzani, Giulia Midena, and Luisa Frizziero had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Sara Trainiti, Giulia Marchione, Eleonora Cosmo, and Davide Londei collected the data. All authors discussed the results and contributed to the final manuscript.
Glossary
Abbreviations: AH= Aqueous Humor, bFGF= basic Fibroblast Growth Factor, EGF= Epidermal Growth Factor, IL-6= Interleukin-6, IL-8= Interleukin-8, MCP= Monocyte Chemo-attractant Protein-1, MIF= Macrophage Inhibiting Factor, RANTES= Regulated upon Activation Normal T cell Expressed and Secreted, UM= Uveal Melanoma, VEGF= Vascular Endothelial Growth Factor.
References
- [1].Horgan N, Shields CL, Mashayekhi A, et al. Early macular morphological changes following plaque radiotherapy for uveal melanoma. Retina 2008;28:263–73.. [DOI] [PubMed] [Google Scholar]
- [2].Ly LV, Bronkhorst IHG, van Beelen E, et al. Inflammatory cytokines in eyes with uveal melanoma and relation with macrophage infiltration. Invest Ophthalmol Vis Sci 2010;51:5445–51.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Maat W, Ly LV, Jordanova ES, et al. Monosomy of chromosome 3 and an inflammatory phenotype occur together in uveal melanoma. Investig Opthalmol Vis Sci 2008;49:505. [DOI] [PubMed] [Google Scholar]
- [4].Oliva M, Rullan AJ, Piulats JM. Uveal melanoma as a target for immune-therapy. Ann Transl Med 2016;4:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Repp AC, Mayhew ES, Apte S, et al. Human uveal melanoma cells produce macrophage migration-inhibitory factor to prevent lysis by NK cells. J Immunol 2000;165:710–5.. [DOI] [PubMed] [Google Scholar]
- [6].Amin MB, Edge S, Greene F, et al. AJCC Cancer Staging Manual. Springer International Publishing: American Joint Commission on Cancer, 8Chicago, IL: 2017. [Google Scholar]
- [7].Dunavoelgyi R, Funk M, Sacu S, et al. Intraocular activation of angiogenic and inflammatory pathways in uveal melanoma. Retina 2012;32:1373–84.. [DOI] [PubMed] [Google Scholar]
- [8].Cheng Y, Feng J, Zhu X, et al. Cytokines concentrations in aqueous humor of eyes with uveal melanoma. Medicine (Baltimore) 2019;98:e14030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wierenga APA, Cao J, Mouthaan H, et al. Aqueous humor biomarkers identify three prognostic groups in uveal melanoma. Invest Ophthalmol Vis Sci 2019;60:4740–7.. [DOI] [PubMed] [Google Scholar]
- [10].Midena E, Bini S, Martini F, et al. Changes of aqueous humor müller cellsʼ biomarkers in human patients affected by diabetic macular edema after subthreshold micropulse laser treatment. Retina 2018;doi:10.1097/IAE.0000000000002356 [DOI] [PubMed] [Google Scholar]
- [11].Pilotto E, Midena E, Longhin E, et al. Müller cells and choriocapillaris in the pathogenesis of geographic atrophy secondary to age-related macular degeneration. Graefe's Arch Clin Exp Ophthalmol 2019;257:1159–67.. [DOI] [PubMed] [Google Scholar]
- [12].Noma H, Funatsu H, Yamasaki M, et al. Aqueous humour levels of cytokines are correlated to vitreous levels and severity of macular oedema in branch retinal vein occlusion. Eye (Lond) 2008;22:42–8.. [DOI] [PubMed] [Google Scholar]
- [13].Küchle M, Nguyen NX, Naumann GO. Quantitative assessment of the blood-aqueous barrier in human eyes with malignant or benign uveal tumors. Am J Ophthalmol 1994;117:521–8.. [DOI] [PubMed] [Google Scholar]
- [14].Lee CS, Jun IH, Kim TI, et al. Expression of 12 cytokines in aqueous humour of uveal melanoma before and after combined Ruthenium-106 brachytherapy and transpupillary thermotherapy. Acta Ophthalmol 2012;90:e314–20.. [DOI] [PubMed] [Google Scholar]
- [15].Usui Y, Tsubota K, Agawa T, et al. Aqueous immune mediators in malignant uveal melanomas in comparison to benign pigmented intraocular tumors. Graefe's Arch Clin Exp Ophthalmol 2017;255:393–9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Nagarkatti-Gude N, Bronkhorst IHG, van Duinen SG, et al. Cytokines and chemokines in the vitreous fluid of eyes with uveal melanoma. Investig Opthalmol Vis Sci 2012;53:6748–55.. [DOI] [PubMed] [Google Scholar]
- [17].Sonoda S, Sakamoto T, Yamashita T, et al. Retinal morphologic changes and concentrations of cytokines in eyes with diabetic macular edema. Retina 2014;34:741–8.. [DOI] [PubMed] [Google Scholar]
- [18].Lattanzio L, Tonissi F, Torta I, et al. Role of IL-8 induced angiogenesis in uveal melanoma. Invest New Drugs 2013;31:1107–14.. [DOI] [PubMed] [Google Scholar]


