Abstract
The novel coronavirus disease 2019 (COVID-19) pandemic emerged in Wuhan, China in December 2019 and has subsequently escalated exponentially worldwide. As this virus has never been experienced previously, it poses a significant challenge to healthcare systems who are poorly equipped to handle the large number of gravely ill patients who seek medical attention. Additionally, treating providers are placing their own lives at risk due to the lack of adequate personal protective equipment. We are reporting the proactive measures that were implemented at our healthcare system in a metropolitan community in Kentucky to address COVID-19. The primary goal was to maintain a safe environment for providers, staff, and patients. Three key strategies were incorporated at our healthcare system, including
-
1)
innovative processes/operations;
-
2)
clear and transparent communication; and
-
3)
adaptations in infrastructure. As the COVID-19 pandemic is highly fluid, we continually update our policies according to national, state, and local guidelines and recommendations.
Keywords: coronavirus, coronavirus disease 2019, healthcare system, infectious disease, personal protective equipment
1. Introduction
On December 29, 2019, the first 4 cases of the novel coronavirus disease 2019 (COVID-19) were recognized by hospitals using a surveillance mechanism for “pneumonia of unknown etiology”.[1] All of these patients were linked to the Huanan (Southern China) Seafood Wholesale Market in Wuhan, China where they had been exposed to wildlife animals including snakes and bats.[1,2] This virus was identified as a novel coronavirus or the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).[3,4] Cases detected outside of Wuhan, community infections, and infections among healthcare workers caring for 2019-CoV patients confirmed human to human transmission with the potential of extensive disease dissemination.[5,6] Since its initial recognition, cases of COVID-19 have risen exponentially worldwide, with 216 countries affected and a total of 14,731,563 confirmed cases and 611,284 deaths as of July 21, 2020.[4] The Commonwealth of Kentucky has documented 24,638 positive cases of COVID-19 with 695 virus-related deaths as of July 21, 2020.[7] The first case in the United States was reported on January 20, 2020, involving a 35-year-old man with mild symptoms initially that progressed to pneumonia on day 8.[8] He had returned to his home in Snohomish County, Washington 5 days earlier after visiting family in Wuhan, China.
2019-CoV is the seventh detected coronavirus that is capable of infecting humans, similar to the 2002–2003 SARS-CoV and the 2012 Middle East respiratory syndrome coronavirus (MERS-CoV).[2,3] These 3 infections share numerous common features, including bat origin, respiratory transmission, and the most frequent symptoms of fever, dry cough, and dyspnea. However, COVID-2019 has spread faster than its 2 predecessors.[6] The diagnosis of COVID-2019 may be confirmed by molecular testing on respiratory samples such as throat swabs, nasopharyngeal swabs, sputum, endotracheal aspirates, or bronchoalveolar lavage.[3] On March 29, 2020, the U.S. Food and Drug Administration approved the emergency use of the malarial drugs hydroxychloroquine sulfate and chloroquine phosphate to treat hospitalized patients with COVID-19.[9,10] Remdesivir, an inhibitor of the viral ribonucleic acid-dependent, ribonucleic acid polymerase with inhibitory activity against SARS-CoV and MERS-CoV, was identified as a promising therapy for COVID-19 due to its ability to inhibit SARS-COV-2 in vitro.[11] Remdesivir has been reported as superior to placebo in shortening the time to recovery in adults hospitalized with COVID-19 with evidence of lower respiratory tract infection.[12]
Asymptomatic individuals may easily spread COVID-19, placing countless of older people with pre-existing medical conditions or those who are immunosuppressed at risk.[3,13] The rapidly intensifying pandemic of COVID-19 in the United States has primarily been managed by national, state, and local enforcements of self-quarantine, social distancing, frequent hand washing, and closure of public places that attract large crowds.[14] Certain healthcare systems have been inundated with patients affected by this virus and often have limited personal protective equipment (PPE) for treating providers. Despite gaining experience with emergency preparedness by facing MERS-CoV and SARS-CoV, healthcare systems do not have a playbook for how to handle COVID-19.
We highlight the proactive measures to manage and reduce the spread of COVID-19 that were implemented at our healthcare system in a metropolitan community in Kentucky. The Chair/Vice-Chair of the Institutional Review Board at the University of Louisville determined that this study did not meet the “Common Rule” definition of human subjects’ research.
2. Our healthcare system
Our healthcare system in a metropolitan community in Kentucky consists of 1511 providers (856 physicians, 606 advanced practice providers [nurse practitioners and physician assistants], and 49 other licensed professionals) and more than 16,000 other employees as of July 1, 2020. Our healthcare system includes a Medical Group with more than 250 clinics, 4 adult hospitals, and a pediatric hospital. A multidisciplinary Central Command Center was established at our healthcare system on March 13, 2020, in response to COVID-19 with the primary aim of protecting providers, patients, and staff. This team consisted of infectious disease specialists, other medical providers, nurses, pharmacists, and representatives from clinical administration and Employee Health. The goals of the Central Command Center included maintaining a calm culture and strong leadership while implementing:
-
(1)
innovative processes/operations;
-
(2)
clear communication; and
-
(3)
adaptations in infrastructure (Tables 1 and 2). The Central Command Center continually updated testing and treatment protocols as recommendations changed daily based on guidelines from the Centers for Disease Control and Prevention (CDC) and Kentucky Department for Public Health, as well as other state and local mandates. The Central Command Center was staffed 24 hours a day.
Table 1.
Our Healthcare System's response to coronavirus disease 2019 regarding processes/operations, communication, and infrastructure.

Table 2.
Ambulatory office changes at our Healthcare System in response to coronavirus disease 2019.

3. Processes/operations
A host of processes and operational changes were implemented at our healthcare system to minimize the risks of COVID-19 in the patient population as well as among providers and other employees (Tables 1 and 2). Specific directives focused on patients, providers, staff, and operational leaders of offices and medical groups (Table 2).
3.1. Respiratory illness testing center
On March 11, 2020, the Respiratory Illness Testing Center (RITC) was implemented at our healthcare system to support COVID-19 testing that operated 8 hours per day and 7 days per week. This service was available for employees or patients exposed to COVID-19 or for patients who met screening criteria as determined by the CDC.[15] The CDC reported that the high priority testing included
-
(1)
hospitalized patients with symptoms;
-
(2)
healthcare facility workers, workers in congregate living settings, and first responders with symptoms; and
-
(3)
residents in long-term care facilities or other congregate living settings including prisons and shelters with symptoms.[15] All individuals tested were referred by providers at our healthcare system. Since our healthcare system implemented polymerase chain reaction testing for influenza A and B, group A streptococcus, and respiratory syncytial virus in 2018, we had an adequate supply of viral swab media available. In addition, significant allocations from our suppliers were immediately purchased to initiate the RITC. Influenza A and B testing was performed in conjunction with COVID-19 testing, and the results of the tests were tracked and communicated to the patients and their ordering providers. As of July 21, 2020, our healthcare system had tested 46,225 individuals, 2509 of whom were confirmed COVID-19 positive (Fig. 1). A total of 99 patients who were COVID-19 positive had died. Patients from 13 different states were tested, with the majority being residents of Kentucky and Indiana. On April 8, 2020, the RITC began collecting blood for antibody serum testing upon referral from our Infectious Disease providers and the Central Command Center. The serology testing was utilized in COVID-19 reverse transcription polymerase chain reaction positive patients to identify possible convalescent plasma donors for future patient treatment.
Figure 1.
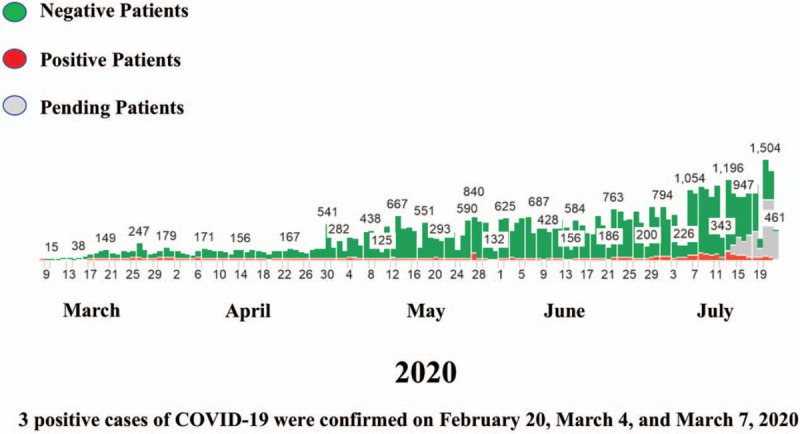
Coronavirus disease 2019 testing and results at our healthcare system.
3.2. Outpatient care
Nonurgent outpatient visits were postponed at our healthcare system to limit the exposure to COVID-19 and to decrease the usage of PPE. Ambulatory telehealth was deployed allowing providers to conduct video visits with their adult and pediatric patients; these visits greatly increased over the first 2 weeks of use (Fig. 2). Patients who required an appointment for necessary care continuity were evaluated in the outpatient office. All patients were called by telephone 1 day before their scheduled routine outpatient appointment at which time COVID-19 screening questions were asked. If a patient was positive for the screening questions but clinically stable, they were instructed on proper self-quarantine procedures, social distancing, and scheduled for a video visit with their provider. High-risk patients who were positive for the screening questions underwent daily telephonic or video visits with their provider as determined by their underlying risk, age, and clinical needs.
Figure 2.
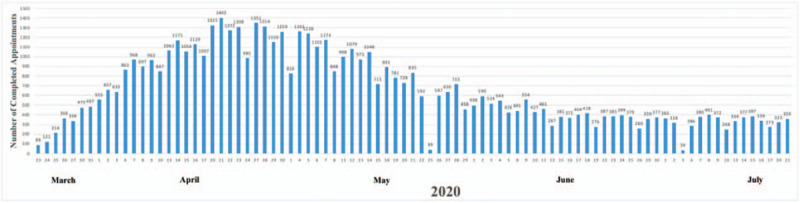
Completed appointments with ambulatory telehealth at our healthcare system in response to coronavirus disease 2019.
For the patients who were evaluated in outpatient clinics, electronic registration was performed during the connection phone call. Patients electronically checked in upon arrival and were advised to remain in their vehicles until text messaged for their appointment to limit the number of patients in waiting rooms. The waiting room structure was also altered to follow social distancing guidelines. Many chairs were removed, and the remaining chairs were no less than 6 feet apart. Patients were required to stay in their car until called by telephone or texted so that the waiting room was not utilized. Patients were instructed to wear masks if they had respiratory symptoms or were positive in the screening questions for COVID-19. Patient check-out was completed electronically or by phone.
After noting a 30% to 50% decrease in outpatient volume and increased employee furloughs secondary to symptoms or exposure, leadership implemented an A/B team strategy for clinical and non-clinical staff on March 30, 2020, to allow more team separation. Team A performed in-person patient visits or assisted providers with setting up daily video visits. Team B worked from home where they called patients, screened the next day's schedule, and completed documentation and medication refills. These teams rotated weekly. This strategy resulted in fewer employees being furloughed and limited the spread of COVID-19 amongst staff.
3.3. Elective procedures
In adherence to our state Governor's request, elective surgical and diagnostic procedures were postponed in our healthcare system on March 16, 2020, except for those that were deemed urgent by the provider, patient, and medical directors.
3.4. No-visitor policy and hospital security
A no-visitor policy was implemented at our healthcare system on March 21, 2020, with a few exceptions. All exempted hospital visitors were screened for COVID-19 symptoms and denied access if present. They were also required to remain in the patient's room for the entire visit. The number of entry points in our hospitals was restricted, with only an entry at the main entrance and Emergency Department (ED). Furthermore, additional security was added to ED entrances, including the National Guard and local police.
3.5. Employee testing
As of July 21, 2020, of the 2561 employees at our healthcare system who had been tested for COVID-19, 267 were positive. The employees in hospitals and outpatient centers at our healthcare system who tested positive for COVID-19 are highlighted in Table 3. Employees were able to complete a self-assessment tool online or by mobile app to determine their likelihood of having COVID-19. If their responses were positive, they received a message to contact Employee Health. All employees underwent temperature testing prior to entering hospitals or outpatient clinics which initiated on March 23, 2020. If an employee's temperature was greater than 100°F, he/she was instructed to contact Employee Health and was furloughed in self-quarantine with pay. The employees were assured that they were not using their paid time off (vacation time). We followed the CDC's guidelines for return to work criteria for healthcare personnel with suspected or confirmed COVID-19.[16] Employees were excluded from work until at least 3 days had passed since recovery, defined as resolution of fever without the use of fever-reducing medication, improvement in respiratory symptoms, and at least 10 days had passed since symptoms first appeared.[16] Of the total 1818 employees who had been furloughed but did not necessarily have COVID-19, 1707 (94%) have already returned to work as of July 21, 2020.
Table 3.
Employees at our Healthcare System who tested positive for COVID-19.

4. Communication
Several opportunities were developed at our healthcare system to enhance communication between leadership, providers, other employees, and patients (Table 1). COVID-19 screening algorithms for employees as well as for adult and pediatric patients treated at outpatient centers were designed, and screening tools for patients, employees, and Access Centers were placed online. Phone tree enhancements and changes to appointment text messages were employed. Conference calls specific to either executive leadership, the Central Command Center, or Operations were incorporated into the daily schedule for operational leadership, clinical leaders, and medical directors. Additionally, the President and Chief Executive Officer of our healthcare system provided daily video updates for all employees, and the Central Command Center sent daily mass e-mails to all employees. Weekly debriefing telephone calls led by Medical Group leadership were also conducted with all providers. An Employee Health Hotline and confidential Employee Emotional Support Line were established to address employees’ questions and offer coping tools, respectively.
5. Infrastructure
Adaptations were created in the infrastructure of our healthcare system in response to COVID-19 that included infectious diseases hospitals/wards (Table 1). The Access centers utilized registered nurse triage support to screen patients regarding COVID-19 risk factors. In the event of a positive screen, patients were referred for eCare, to immediate care centers, or to outpatient primary care offices. Both the registered nurse triage support and customer service for the Access centers were repurposed to serve as back-up for our state's COVID-19 hotline with a real-time dashboard to assess service metrics. Specific scripting was drafted for answering services to educate patients who had questions or concerns. The COVID-19 hotline answered basic COVID-19 questions and assisted with screening for risk factors.
To limit the spread of COVID-19, most vendor representatives were restricted access to our healthcare system, except for those with an approved and specific purpose of directly supporting operations. Large meetings at our healthcare system were cancelled, and meetings of groups larger than 10 individuals occurred virtually or telephonically.
6. Personal protective equipment
The lack of sufficient PPE for healthcare workers initially represented a significant dilemma in the response to COVID-19 which improved on a weekly basis as more supplies were garnered. Several measures were employed at our healthcare system to conserve PPE, while simultaneously protecting the safety of the provider and patient. Tasks were combined that were performed in 1 patient room, and the number of healthcare workers who entered a patient's hospital room in a single day decreased. Providers and other staff who treated patients were encouraged to use innovative technology such as FaceTime or Zoom to communicate with the patient from outside the room.
Each provider in a hospital setting performing aerosolized procedures was issued a N95 mask and told to put his/her name on it. The N95 masks were worn the entire day, exposed to ultraviolet rays for cleansing and sanitizing overnight, and then reused by the same individual the next day. This evidence-based process of utilizing ultraviolet light to clean N95 masks extends the life of these desperately needed items.[17,18] A Universal Mask Protocol was initiated on March 30, 2020, that required all employees who work in medical facilities to wear a surgical mask the entire day and subsequently discard it.
7. Hospital bed and ventilator capacity
With the anticipated surge in positive cases of COVID-19 in our community, predictive analytics were performed to assess the availability of hospital beds and ventilators. Of the total capacity of 1543 available beds, 1121 (73%) were in use as of July 21, 2020. Furthermore, of the total capacity of 312 available intensive care unit beds, 256 (82%) beds were occupied. If needed, medical-surgical beds could be converted to intensive care unit critical care beds.
Of the total capacity of 223 ventilators available at our healthcare system, 86 (38%) were in use as of July 21, 2020. If required, anesthesia machines were capable of being converted to ventilators for a total of 357 ventilators.
8. Key messages
Our healthcare system serves as a pillar of strength in our community which, like many communities, is overwhelmed by anxiety, fear, and uncertainty in the face of COVID-19. Despite lacking a blueprint for direction due to the novel nature of COVID-19, our executive leadership provided clear communication to instill a sense of calmness to manage our culture. The Central Command Center addressed and solved problems in our healthcare system, while maintaining a flexibility to adapt to new regulations proposed daily by the national, state, or local governments.
Employees in our healthcare system responded positively to the assured and empathetic manner of leadership, as exemplified by donating vacation time to those employees who were going through financial hardships. The Chief Executive Officer of our healthcare system commented that employees often stated ‘Yes, we’ll do that. We’re willing to do that. What else can I do?” This statement reflects the communal willingness to share the burden and responsibilities brought on by COVID-19. Furthermore, our healthcare system served as a regional healthcare leader through the COVID-19 hotline, educated individuals to manage COVID-19 symptoms not requiring medical attention by isolating at home and avoid going to the ED, and provided guidance to state and local government officials.
Our healthcare system took a judicious approach to testing for COVID-19 while considering the value of treatment, management, and resources. In the earliest days of the COVID-19 outbreak, testing was performed for surveillance and to better identify individuals who needed to be isolated. As we were concerned about the potential surge of the virus and our limited resources for testing, testing was only conducted for high-risk employees, hospitalized patients, and outpatients who were > 60 years old with co-morbidities. We subsequently expanded testing which included serology and reverse transcription polymerase chain reaction testing at the request of any patient or provider at any of our healthcare settings.
9. Conclusion
The emergence of the COVID-19 pandemic with its shockingly rapid worldwide spread has significant economic, societal, cultural, and financial repercussions. While measures such as hand washing, social distancing, and self-quarantine may curtail the transmission of COVID-19, this coronavirus is anticipated to significantly multiply in the coming months with its associated morbidity and mortality until a vaccine is developed. The strategies implemented at our healthcare system in response to COVID-19 serve as a model that may be replicated at other locations. We describe not only lessons learned but also present a blueprint that may be applied to future pandemics. Continued daily monitoring of the processes, operations, and infrastructure at our healthcare system is warranted to ensure the safest environment possible for providers, patients, and staff and to be prepared for a potential significant influx of patients who are positive for COVID-19.
Acknowledgments
We thank Norton Healthcare for their continued support
Author contributions
LBES made substantial contributions to the conception and design, analyzed and interpreted the data, performed the literature search, and was the major contributor in the writing of the manuscript. STH, PSS, CJ, RH, AMW, and JTH made substantial contributions to the conception and design, analyzed and interpreted the data, and revised the draft critically for important intellectual content. All authors read and approved the final manuscript.
Glossary
Abbreviations: CDC = Centers for Disease Control and Prevention, COVID-19 = coronavirus disease 2019, ED = emergency department, FMLA = Family and Medical Leave Act, MERS-CoV = Middle East respiratory syndrome coronavirus, PPE = personal protective equipment, RITC = Respiratory Illness Testing Center, SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
References
- [1].Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 2020;382:1199–207.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cheng ZJ, Shan J. 2019 novel coronavirus: where we are and what we know. Infection 2020;48:155–63.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Singhal T. A review of coronavirus disease-2019 (COVID-19). Indian J Pediatr 2020;87:281–6.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].World Health Organization. Coronavirus disease (COVID-19) pandemic. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed July 24, 2020. [Google Scholar]
- [5].Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle region – case series. N Eng J Med 2020;382:2012–22.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wang C, Horby PW, Hayden FG, et al. A novel coronavirus outbreak of global health concern. Lancet 2020;395:470–3.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kanik A. Looking for county-level data on the coronavirus? Here's our Kentucky COVID-19 tracker. https://wfpl.org/looking-for-county-level-data-on-the-coronavirus-heres-our-kentucky-covid-19-tracker/. Accessed July 24, 2020. [Google Scholar]
- [8].Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med 2020;382:929–36.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Khemlani A. FDA approves emergency use of malaria pill for COVID-19 treatment. Available at: https://finance.yahoo.com/news/fda-approves-emergency-use-of-malaria-pill-for-covid-19-treatment-133908197.html. Accessed July 22, 2020. [Google Scholar]
- [10].Lurie N, Saville M, Hatchett R, et al. Developing Covid-19 vaccines at pandemic speed. N Eng J Med 2020;382:1969–73.. [DOI] [PubMed] [Google Scholar]
- [11].Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 2020;30:269–71.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19–preliminary report. N Engl J Med 2020;doi:10.1056/NEJMoa2007764. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- [13].Chang, Xu H, Rebaza A, et al. Protecting health-care workers from subclinical coronavirus infection. Lancet Respir Med 2020;8:e13.doi: 10.1016/S2213-2600(20)30066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lipsitch M, Swerdlow DL, Finelli L. Defining the epidemiology of Covid-19–studies needed. N Engl J Med 2020;382:1194–6.. [DOI] [PubMed] [Google Scholar]
- [15].Centers for Disease Control and Prevention. Evaluating and testing persons for coronavirus disease 2019 (COVID-19). Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-criteria.html. Accessed July 24, 2020. [Google Scholar]
- [16].Centers for Disease Control and Prevention. Criteria for return to work ofr healthcare personnel with suspected or confirmed COVID-19 (interim guidance). Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/return-to-work.html. Accessed July 24, 2020. [Google Scholar]
- [17].Centers for Disease Control and Prevention. Decontamination and reuse of filtering facepiece respirators. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/decontamination-reuse-respirators.html. Accessed o July 24, 2020. [Google Scholar]
- [18].Lindsley WG, Martin SB, Jr, Thewlis RE, et al. Effects of ultraviolet germicidal irradiation (UVGI) on N95 respirator filtration performance and structural integrity. J Occup Environ Hyg 2015;12:509–17.. [DOI] [PMC free article] [PubMed] [Google Scholar]


