Abstract
Background:
Neurofilament light (NfL) level was obviously increased in traumatic brain injury (TBI) individuals. But, no comprehensive meta-analysis has ever been conducted to assess the diagnostic performance of NfL. This study aims to evaluate the relationship between NfL level and TBI through a meta-analysis.
Methods:
Studies were selected from Pubmed, Web of science, Embase, Google Scholar, PMC and Chinese National Knowledge Infrastructure (CNKI), and the Chinese Biomedical Literature Database (CBM) through inclusion and exclusion criteria. The standard mean difference (SMD) and 95% confidence interval (CI) were calculated using the random-effect model or fixed-effect model to assess the association between NfL level and TBI. Subgroup analysis according to sample collection time, sample type and detection method was performed. The influence analysis and publication bias was also conducted. All analyses were performed using the RevMan 5.3 and Stata 12 software.
Results:
A total of 9 studies were included. Results indicated that TBI individuals had a higher NfL expression level compared with the non-TBI individuals (SMD = 2.48, 95% CI = 1.52–3.43, I2 = 96%, P < .01). Similar NfL increasing was also observed in Caucasian population, 0–48 hour and 6–10 days sample collection time, as well as cerebrospinal fluid (CSF), serum, plasma sample subgroup analysis. Moreover, the NfL increasing still existed no matter the NfL expression level was detected by ELISA or Simoa assay.
Conclusion:
NfL expression level was increased in TBI individuals, which indicated that NfL could be a potential biomarker in the diagnosis of TBI and other neurodegenerative diseases.
Keywords: meta-analysis, neurofilament light, simoa assay, traumatic brain injury
1. Introduction
Traumatic brain injury (TBI), particularly resulting from closed head trauma, mainly led a cause of mortality and morbidity worldwide and significantly increased the risk of developing Alzheimer's disease (AD),[1] Parkinson's disease (PD),[2] and chronic traumatic encephalopathy (CTE).[3] Generally, TBI was determined by the Glascow Coma Scale (GCS) scores, which combined with short or no loss of consciousness and brief periods of post-traumatic amnesia.[4] CT (computed tomography) was generally used to diagnosis more severe intracranial injury. MRI (magnetic resonance imaging) may also be additionally allowed for the detection of more subtle injuries, including diffuse axonal injury, which increased the risks for neurologic symptoms.[5] However, these diagnosis methods are not the standard care for TBI and not high efficiency. Recently, neurofilament light chain (NfL) had been found to be associated with moderate to severe TBI.[5–8] NfL belongs to the intermediate filament protein family, which forming the neuronal cytoskeleton and maintaining neuronal structure.[9] Following CNS (central nervous system) axonal damage, NfL was released into the cerebrospinal fluid (CSF). Hence, it has been considered a potent biomarker of axonal injury in multiple neurological diseases, including TBI, AD, PD.[6,10] Studies found that increased NfL concentration was closely correlated with the progression of TBI pathology, which suggesting that NfL in both CSF and peripheral blood may serve as a marker for the diagnosis of neurodegenerative dementia disease.[3,11]
However, the value of individual study on NfL level varied greatly, and no comprehensive meta-analysis has ever been conducted to assess the association between the change of NfL level and TBI. We examined recent literatures about NfL expression level in CSF, serum, and plasma as a diagnostic measure through evaluating and comparing NfL expression level in subjects with TBI and non-TBI controls. We hope that this meta-analysis could guide the clinical application of NfL level in the early identification, diagnosis, and assessment of the disease progression of TBI.
2. Material and methods
This study extracted the data from previous publication studies, which was not involved in ethics. So it is not necessary approved the study by an ethics committee or institutional review board.
2.1. Search strategy
Two investigators independently conducted an electronic literature (published before Oct 28, 2019) search using PubMed, Web of science, EMbase, Google Scholar, PMC and CNKI, CBM. The publication language was selected as English and Chinese. During our searching process, the following key words were used: “traumatic brain injury”, “brain injury”, “head injury”, “acupuncture”, “electroacupuncture”, and “cerebral injury”, “neurofilament light chain proteins”, “NfL”, “NF-L”. And for further relevant articles, we also checked the reference lists of the included studies. After completing the literature search, the titles and abstracts of the studies were screened, and any disagreement was resolved by discussion.
2.2. Inclusion and exclusion criteria
Two investigators independently evaluated the titles and abstracts of the identified articles to decide whether they met the study criteria. Differences were solved by consensus. Both cross-sectional and longitudinal studies on the relationship between NfL level and TBI were included. Studies were included in this meta-analysis only when they meet the following criteria:
-
1.
study design: published case-control studies,
-
2.
the definitions for TBI and controls were adequate,
-
3.
NfL was detected in CSF, serum or plasma in subjects with TBI and the control group,
-
4.
the investigation of the NfL expression levels was represented in the TBI and control groups.
Studies were excluded if they were
-
1.
no control group,
-
2.
case reports, meta-analysis or review articles,
-
3.
duplicate articles,
-
4.
studies without sufficient data to allow for the extraction of NfL expression levels in TBI patients and controls.
-
5.
animal model or cell line research,
-
6.
two independent reviewers identified the titles and abstracts of literature, and the studies considered irrelevant were excluded.
2.3. Data extraction and quality assessment
Data extraction was performed independently by 2 researchers, which including the following information from each study: basic study information (first author and year of publication), ages of cases, ethnicity, study population characteristics (for example, individual characteristics, sample size), the number of patients and controls, sample type, sample collection time, NfL detection methods, mean NfL and SD or SE of both patients and control individuals. Any inconsistencies were solved by other researchers until a consensus was reached.
The Newcastle-Ottawa Scale (NOS), which was to evaluate the risk of bias of all included case-control studies in a meta-analysis (Stang, 2010), was applied to this study. The NOS is used for quality assessment and intended to assess 3 domains (selection bias, group comparability, and cohort exposure). The total NOS score ranges from 0 to 9 scores, and a higher score stands for better quality. The assessment process was individually performed by 2 researchers, too.
2.4. Statistical analysis
After extracting the mean and SD values of NfL of the TBI and non-TBI groups in included studies, we chose the SMD (standardized mean difference) and 95% confidence interval (CI) as the effect magnitude to calculate the amount of the combined effect (mean and SD of NfL in TBI and non-TBI groups). Subgroup analysis was conducted according to ethnicity, study population, sample collection time, and NfL analysis methods. The heterogeneity of results across trials was assessed using the I2 statistic, which describes the percentage of total variation across studies that was attributable to heterogeneity rather than to chance. I2 values of 25%, 50%, and 75% correspond to cut-off points for low, moderate, and high degrees of heterogeneity. The pooled effect was calculated using the random-effects model when I2 value was >75%. Otherwise, a fixed-effects model was used in the case of significant heterogeneity across studies. A sensitivity analysis was performed to evaluate the influence of each individual study on overall estimates. The Begg and Eggers tests were used to assess potential publication bias. All statistical analyses were conducted by RevMan software (version 5.3, Cochrane Collaboration) and Stata software version 12.0 (Stata Corp LP, TX, USA). A P value <.05 was considered statistically significant.
3. Results
3.1. Characteristics of included studies and subjects
As a result, a total of 287 related studies were obtained. After excluding duplicate literatures, we independently read the article abstracts and their references to assess their eligibility for the meta-analysis. Forty five studies were potentially included in the present analysis for further evaluation. And after read the full text of these articles, thirty six studies were excluded based the exclusion criteria. Finally, 9 articles were included in the quantitative synthesis. A flowchart of the study selection process was shown in Figure 1. The characteristics of the studies included in this meta-analysis were presented in Table 1. The NOS quality score of the included studies ranged from 6 to 9, as illustrated in Table 1. Among these articles, 8 studies were investigated Caucasian ethnicity,[12–19] 1 study was Chinese.[20] Moreover, some included studies contained data on multiple different groups,[12,16,17] so we treated these groups as independent comparisons. The majority of age of case groups was 15 to 85 years. The samples were collected from CSF, serum or plasma at varied time after TBI diagnosis. Six studies of the cases population were patients[12,17–20] and 3 studies of the cases population were athletes.[13,14,16] The ELISA and Simoa assay methods were used to detect NfL concentration.
Figure 1.

Flowchart of studies for selecting process from databases.
Table 1.
Characteristics of the studies included in this meta-analysis.

3.2. Meta-analysis: the association between NfL expression level and TBI
Thirteen comparisons were included to evaluate the NfL expression level in TBI subjects and non-TBI subjects, enrolling a total of 1118 subjects. Among these studies, higher NfL concentration were observed in TBI subjects than non-TBI control subjects (SMD = 2.48, 95% CI = 1.52–3.43, I2 = 96%, P < .01) (Fig. 2A). Subgroup analysis according to ethnicity demonstrated that NfL increasing was significantly associated with TBI in Caucasian population (SMD = 2.23, 95% CI = 1.36–3.09, I2 = 95%, P < .01), shown as Figure 2B. However, there was no significant association between NfL expression level and TBI in athletes subjects (SMD = 1.17, 95% CI = −0.11–2.45, I2 = 86.8%, P = .07) was found when the subgroup analysis according to cases population was performed, shown as Figure 2C. The effect of collection time of each sample on NfL expression level in case and control groups was also explored. As shown in Figure 3A, NfL expression level was significant up-regulated at 0–48 hours (SMD = 2.89, 95% CI = 1.69–4.09, I2 = 97%, P < .01), 610 days (SMD = 2.98, 95% CI = 2.19–3.78, I2 = 0.00%, P < .01), respectively. However, no significance was found when the samples were collected after months of TBI diagnosis (P = .29).
Figure 2.

Forest plots of the association between NfL expression level and TBI individuals. A: overall analysis; B: subgroup analysis according to ethnicity; C: subgroup analysis according to the different included individuals. For each study, the estimate of mean NfL level difference and its 95% confidence interval (95% CI) is plotted with a diamond. Chi2 = Chi-Squared statistic, df = degrees of freedom, I2 = I-squared heterogeneity statistic, IV = inverse variance, SMD = standard mean difference, Z = Z-statistic.
Figure 3.

Forest plots of the association between NfL expression level and TBI individuals. A: subgroup analysis according to sample collection time, B: subgroup analysis according to the detection method of NfL expression level; C: subgroup analysis according to different types of samples. Chi2 = Chi-Squared statistic, df = degrees of freedom, I2 = I-squared heterogeneity statistic, IV = inverse variance, SMD = standard mean difference, Z = Z-statistic.
Recently, Simoa technology, a newly and highly efficiency detection method, had been employed in the detection of NfL expression level. So this study analysized the association between NfL and TBI according to ELISA and Simoa detection method. As shown in Figure 3B, 6 comparisons used the ELISA method (SMD = 4.06, 95% CI = 1.22–6.91, I2 = 98%, P < .01) and 7 comparisons used the Simoa assay (SMD = 1.35, 95% CI = 0.79–1.92, I2 = 87%, P < .01). There was a significant NfL increasing in TBI subjects no matter ELISA or Simoa assay method was used to detect its concentration. However, the heterogeneity of Simoa assay was smaller than the ELISA method. Simoa assay method may be more stability at least. At the end, this meta-analysis also analysised the association between NfL and TBI according to the types of samples. As shown in Figure 3C, 5 comparisons found that higher CSF NfL expression levels in TBI cases than non-TBI subjects (SMD = 4.03, 95% CI = 0.45–7.61, I2 = 98%, P = .03). Five comparisons found that higher serum NfL expression levels in TBI cases than non-TBI subjects (SMD = 2.35, 95% CI = 0.45–4.26, I2 = 97.2%, P = .02). And, 3 comparisons found that higher plasma NfL expression levels in TBI cases than non-TBI subjects (SMD = 1.06, 95% CI = 0.50–1.61, I2 = 82.3%, P < .01).
3.3. Influence analysis and Publication bias
In the present meta-analysis, sensitivity analysis and publication bias were also performed. Shown as Figure 4A, no comparisons were out of the lower limit or the up limit. Eggers test and Beggs shown that there was a low risk of publication bias in this meta-analysis (Fig. 4B).
Figure 4.
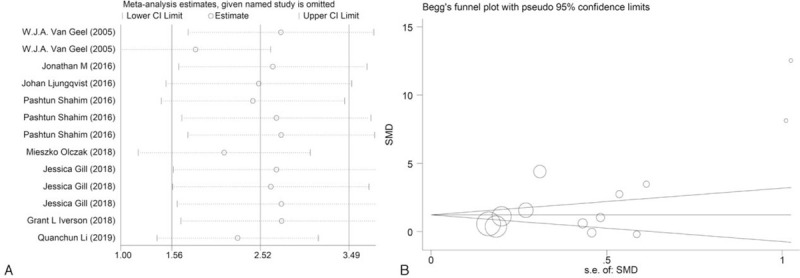
Meta-analysis of influence analysis (A) and publication bias analysis (B).
4. Discussion
In this meta-analysis, we found that NfL level was significant higher in TBI subjects than non-TBI subjects. Compared with non-TBI individuals, TBI was associated with a significant increase of NfL expression level in CSF, serum, plasma sample. Further subgroup analysis showed that TBI significantly increased NfL expression level in Caucasian population but not in athletes subjects. Moreover, TBI significantly increased the NfL expression level only at 0–48 hours, 6–10 days sample collection time, which indicated that the samples should be collected as early as possible after the diagnosis of TBI.
The NfL expression level was detected by ELISA and Simoa assay in the included studies. A commercially available ELISA was used 2 highly specific, non-competing monoclonal antibodies to quantify soluble NfL, but this assay was not recommended for blood measurements by the producer.[21] Recently, single-molecule array (Simoa) technology for digital immunoassays had been confirmed that it was the potential method to significantly improve sensitivity further.[10,22] Actually, the Simoa platform was to be more 100-fold sensitivity (0.62 pg/ml) than ELISA method, and also 25-fold more sensitive than previously described and validated ECL assay (15.6 pg/ml) in NfL level detection.[23] In the subgroup analysis according to the detection method, Simoa assay group indeed obtained a smaller heterogeneity than the ELISA group. However, our results did not confirm that Simoa platform to be more sensitive than ELISA, which needed to be further investigated.
NfL, highly expressed in the large-caliber myelinated subcortical axons of the white matter, released from neurons in Aβ-dependent or Aβ-independent pathological conditions.[24] It is a marker that reflects a downstream response to TBI and other neurodegeneration diseases. However, there is needed more understanding in the diagnostic and prognostic potential of NfL as a biomarker in TBI. Previous study[19] found that age is associated with NfL levels in mild TBI (MTBI) and no pre-injury neurological individuals. For example, the age of MTBI subjects ≥60 years had higher NfL level than individuals under the age of 60 years. Meantime, MTBI and pre-injury neurological disorders individuals had higher NfL level than health individuals. Moreover, this was also found in the orthopedically-injured trauma control sample. Therefore, NfL was not particularly useful for differentiating those with uncomplicated MTBIs from trauma control subjects.
However, the present meta-analysis is also constrained by a number of limitations. First, the sample sizes of the included studies were small, which could cause bias for the results. Second, we could not conduct the subgroup analysis according to severity of TBI because of lack of detailed data for mild TBI or severe TBI. Further studies are needed to determine the relationship between NfL and TBI in different brain injury. Third, the average age of patients is not given in some of included studies, so the subgroup analysis according to individuals age could not be performed, too. However, NfL expression level was varied very large from young adults to older adults. Despite these limitations, this meta-analysis contributed to the current evidence about the relationship of NfL level and TBI disease. NfL might be an important biomarker to help diagnose TBI disease, which can improve the treatment for patients. As for the specific diagnostic threshold, it still needs to be further confirmed through a series of large-scale case-control studies.
In conclusion, TBI individuals had an increasing NfL expression level compared with the non-TBI individuals. In detailed, NfL was also increasing in Caucasian population, 0–48 hours and 6–10 days sample collection time, as well as cerebrospinal fluid (CSF), serum, plasma sample subgroup analysis. Moreover, Simoa assay maybe more precise than ELISA method to detection NfL expression level. Considering the potential limitations in this study, more large scale case-control studies are needed to identify our findings.
Author contributions
Conceptualization: Wenyan Gao, Wenin Xing.
Data curation: Zhongshan Zhang.
Methodology: Xiaoling Lv, Qing Wu.
Validation: Jing Yan, Genxiang Mao.
Writing – original draft: Wenyan Gao, Wenmin Xing.
Writing – review & editing: Wenyan Gao, Wenmin Xing.
Glossary
Abbreviations: AD = Alzheimer's disease, CBM = Chinese Biomedical Literature Database, CI = confidence interval, CNKI = Chinese National Knowledge Infrastructure, CNS = central nervous system, CSF = cerebrospinal fluid, CT = computed tomography, CTE = chronic traumatic encephalopathy, ELISA = enzyme-linked immunosorbent assay, GCS = Glascow Coma Scale, MRI = magnetic resonance imaging, MTBI = mild TBI, NfL = Neurofilament light, NOS = Newcastle-Ottawa Scale, PD = Parkinson's disease, Simoa = single molecule array, SMD = standard mean difference, TBI = traumatic brain injury.
References
- [1].Ramos-Cejudo J, Wisniewski T, Marmar C, et al. Traumatic brain injury and Alzheimer's disease: the cerebrovascular link. EBioMedicine 2018;28:21–30.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Joyce JM, Monchi O, Ismail Z, et al. The impact of traumatic brain injury on cognitive and neuropsychiatric symptoms of Parkinson's disease. Int Rev Psychi 2019;21:1–5.. [DOI] [PubMed] [Google Scholar]
- [3].VanItallie TB. Traumatic brain injury (TBI) in collision sports: possible mechanisms of transformation into chronic traumatic encephalopathy (CTE). Metabolism 2019;100s:153943. [DOI] [PubMed] [Google Scholar]
- [4].Levin HS, Diaz-Arrastia RR. Diagnosis, prognosis, and clinical management of mild traumatic brain injury. Lancet Neurol 2015;14:506–17.. [DOI] [PubMed] [Google Scholar]
- [5].Yuh EL, Mukherjee P, Lingsma HF, et al. Magnetic resonance imaging improves 3-month outcome prediction in mild traumatic brain injury. Ann Neurol 2013;73:224–35.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bagnato S, Grimaldi LME, Di Raimondo G, et al. Prolonged cerebrospinal fluid neurofilament light chain increase in patients with post-traumatic disorders of consciousness. J Neurotrauma 2017;34:2475–9.. [DOI] [PubMed] [Google Scholar]
- [7].Di Pietro V, Yakoub KM, Scarpa U, et al. MicroRNA signature of traumatic brain injury: from the biomarker discovery to the point-of-care. Front Neurol 2018;9:429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Korley FK, Nikolian VC, Williams AM, et al. Valproic Acid treatment decreases serum glial fibrillary acidic protein and neurofilament light chain levels in swine subjected to traumatic brain injury. J Neurotrauma 2018;35:1185–91.. [DOI] [PubMed] [Google Scholar]
- [9].Khalil M, Teunissen CE, Otto M, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol 2018;14:577–89.. [DOI] [PubMed] [Google Scholar]
- [10].Kuhle J, Gaiottino J, Leppert D, et al. Serum neurofilament light chain is a biomarker of human spinal cord injury severity and outcome. J Neurol Neurosurg Psychiatry 2015;86:273–9.. [DOI] [PubMed] [Google Scholar]
- [11].Bacioglu M, Maia LF, Preische O, et al. Neurofilament light chain in blood and CSF as marker of disease progression in mouse models and in neurodegenerative diseases. Neuron 2016;91:56–66.. [DOI] [PubMed] [Google Scholar]
- [12].Van Geel WJ, Rosengren LE, Verbeek MM. An enzyme immunoassay to quantify neurofilament light chain in cerebrospinal fluid. J Immunol Methods 2005;296:179–85.. [DOI] [PubMed] [Google Scholar]
- [13].Oliver JM, Jones MT, Kirk KM, et al. Effect of docosahexaenoic acid on a biomarker of head trauma in american football. Med Sci Sports Exerc 2016;48:974–82.. [DOI] [PubMed] [Google Scholar]
- [14].Shahim P, Tegner Y, Gustafsson B, et al. Neurochemical aftermath of repetitive mild traumatic brain injury. JAMA Neurol 2016;73:1308–15.. [DOI] [PubMed] [Google Scholar]
- [15].Ljungqvist J, Zetterberg H, Mitsis M, et al. Serum neurofilament light protein as a marker for diffuse axonal injury: results from a case series study. J Neurotrauma 2017;34:1124–7.. [DOI] [PubMed] [Google Scholar]
- [16].Shahim P, Zetterberg H, Tegner Y, et al. Serum neurofilament light as a biomarker for mild traumatic brain injury in contact sports. Neurology 2017;88:1788–94.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gill J, Latour L, Diaz-Arrastia R, et al. Glial fibrillary acidic protein elevations relate to neuroimaging abnormalities after mild TBI. Neurology 2018;91:e1385–9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Olczak M, Kwiatkowska M, Niderla-Bielinska J, et al. Brain-originated peptides as possible biochemical markers of traumatic brain injury in cerebrospinal fluid post-mortem examination. Folia Neuropathol 2018;56:97–103.. [DOI] [PubMed] [Google Scholar]
- [19].Iverson GL, Reddi PJ, Posti JP, et al. Serum neurofilament light is elevated differentially in older adults with uncomplicated mild traumatic brain injuries. J Neurotrauma 2019;36:2400–6.. [DOI] [PubMed] [Google Scholar]
- [20].Li Q. Serum neurofilament protein light chain polypeptide level predicts clinical prognosis in patients with acute craniocerebral injury. Neural Injury Function Reconst 2019;14:104–6.. [Google Scholar]
- [21].Norgren N, Karlsson JE, Rosengren L, et al. Monoclonal antibodies selective for low molecular weight neurofilaments. Hybrid Hybridomics 2002;21:53–9.. [DOI] [PubMed] [Google Scholar]
- [22].Rissin DM, Kan CW, Campbell TG, et al. Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat Biotechnol 2010;28:595–9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kuhle J, Barro C, Andreasson U, et al. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin Chem Lab Med 2016;54:1655–61.. [DOI] [PubMed] [Google Scholar]
- [24].Mattsson N, Insel PS, Palmqvist S, et al. Cerebrospinal fluid tau, neurogranin, and neurofilament light in Alzheimer's disease. EMBO Mol Med 2016;8:1184–96.. [DOI] [PMC free article] [PubMed] [Google Scholar]


