To the Editor:
Severe coronavirus disease infection continues to carry a high mortality with no definitive therapy to improve outcomes. Profound inflammation and coagulopathy are often present and predict a poor outcome. Therapeutic plasma exchange has been proposed as a potential therapy in this critically ill subset of coronavirus disease patients through its actions along these pathways. In our series of eight patients receiving adjunct therapeutic plasma exchange for severe coronavirus disease pneumonia complicated by sepsis with multiple organ dysfunction, C-reactive protein and ferritin levels significantly decreased with therapeutic plasma exchange, whereas D-dimer decreased to a lesser degree. Sequential Organ Failure Assessment scores also improved although the clinical impact cannot be assessed due to lack of controls. Our findings offer potentially useful information for the development of prospective trials of therapeutic plasma exchange for severe coronavirus disease infection.
During the severe acute respiratory syndrome (SARS) epidemic of 2012, researchers noted that late-term disease progression was unrelated to the initial viremia, rather to the host’s immunopathologic response (1). This pathologic cascade of cytokine storm, endothelial activation, and microcirculatory thrombosis has been well described in sepsis and appears to be common to coronavirus disease 2019 (COVID-19) (1, 2). Early autopsy reports have demonstrated von Willebrand factor and fibrin clots along with severe endothelial injury and widespread microthrombosis in the lungs of coronavirus disease (COVID) nonsurvivors (3).
Therapeutic plasma exchange (TPE) offers potentially unique therapy by removing excessive, harmful cytokines, stabilizing injured endothelial membranes, and restoring the normal hemostatic milieu. Busundet al (4) showed a trend toward improved mortality is sepsis of any cause with adjunct TPE, whereas Patel et al (5) demonstrated clinical improvement in a case series of pediatric patients with acute respiratory distress syndrome (ARDS) and shock receiving adjunct TPE during the H1N1 influenza pandemic of 2009. These data raise the hypothesis that TPE may be efficacious in critically ill patients with severe COVID infection. We report outcomes of eight critically ill patients with severe COVID complicated by ARDS, sepsis, and multiple organ dysfunction syndrome (MODS) treated with adjunct TPE.
METHODS
We performed a retrospective review of medical records of eight adult patients admitted to Lexington Medical Center (LMC) with laboratory-confirmed SARS coronavirus-2 infection, complicated by ARDS, sepsis, and MODS who received adjunct TPE as part of their management.
Patients were considered for TPE under the 2019 American Society for Apheresis guidelines for sepsis with multiple organ failure (6) if they fulfilled the following criteria: 1) sepsis due to COVID-19 infection, 2) ARDS (as defined by the Berlin criteria), and 3) evidence of greater than or equal to two organ dysfunctions. Patients with poor long-term prognosis not due to COVID-19 were not considered for TPE.
Baseline characteristics of the patients are outlined in Table 1. All patients received standard care for sepsis and ARDS according to the Surviving Sepsis Campaign and ARDS network guidelines. Patients also received specific therapies for COVID-19, as outlined in Table 1.
TABLE 1.
Clinical Characteristics, Treatment, and Outcomes of Eight Patients Treated for Coronavirus Disease 2019 With Therapeutic Plasma Exchange
| Patient Characteristic | Patient Demographics | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Sex | Male | Male | Male | Male | Male | Male | Female | Female |
| Age, yr | 73 | 68 | 67 | 61 | 78 | 41 | 68 | 65 |
| Comorbid conditions | Hyperlipidemia, gastroesophageal reflux disease | Hypertension | Cerebral palsy, diabetes mellitus | Systemic lupus erythematosus, hypertension, benign prostatic hypertrophy | Prostate cancer status post transurethral resection of the prostate | Obesity | Dementia, pseudotumor cerebri, end-stage renal disease, hypertension, stroke | Hypertension, obstructive sleep apnea, chronic kidney disease, obesity, atrial fibrillation, diastolic heart failure |
| Living situation, prior to admission | Home | Home | Extended care | Home | Home | Home | Home | Home |
| COVID 2019 disease presentation | ||||||||
| Admit to ICU transfer, d | 1 | 2 | 5 | 0 | 3 | 0 | 3 | 2 |
| ICU Admission SOFA | 2 | 4 | 7 | 10 | 3 | 15 | 8 | 5 |
| Admit to first TPE, d | 9 | 9 | 11 | 6 | 3 | 0 | 7 | 4 |
| Number of TPE treatments | 2 | 3 | 4 | 7 | 4 | 2 | 1 | 1 |
| Maximum respiratory support | Mechanical ventilation | Mechanical ventilation | Mechanical ventilation | Mechanical ventilation | Mechanical ventilation | Mechanical ventilation | Mechanical ventilation | Bilevel positive airway pressure |
| Proned, yes/no | No | Yes | No | Yes | Yes | Yes | No | No |
| Inhaled nitric oxide, yes/no | No | No | No | Yes | Yes | Yes | No | No |
| Paralytic infusion, yes/no | No | Yes | No | Yes | Yes | Yes | No | No |
| Vasopressor therapy, hr | 50 | 7 | 42 | 219 | 42 | 13 | 2 | 30 |
| Other therapeutic interventions | ||||||||
| Steroids | Methylprednisolone | Methylprednisolone | Methylprednisolone | Methylprednisolone | Methylprednisolone | Methylprednisolone | Methylprednisolone | None |
| COVID-specific medications | Hydroxychloroquine, azithromycin, zinc | Hydroxychloroquine, azithromycin, zinc | Hydroxychloroquine, azithromycin, zinc, tocilizumabe | Hydroxychloroquine, azithromycin, zinc, tocilizumabe | Hydroxychloroquine, azithromycin, zinc | Azithromycin | Azithromycin, Ivermectin | None |
| Convalescent plasma, yes/no | No | No | No | Yes | Yes | Yes | Yes | No |
| Anticoagulation | Enoxaparin prophylaxis | Heparin prophylaxis | Argatroban, apixaban | Enoxaparin full-dose, argatroban | Argatroban, heparin infusion | Argatroban, apixaban | Argatroban, apixaban | Apixaban |
| Clinical outcomes | ||||||||
| Number of ventilator days | 2 | 7 | 6 | 21 | 13 | 9 | 2 | 0 |
| ICU stay, d | 10 | 11 | 18 | 29 | 23 | 11 | 17 | 7 |
| Hospital stay, d | 19 | 17 | 33 | 29 | 26 | 35 | 22 | 14 |
| Hospital discharge SOFA | 2 | 0 | 1 | N/a | N/a | 4 | 4 | 0 |
| Discharge disposition | Acute rehabilitation | Home | Extended care | Deceased | Deceased | Home | Home | Home |
COVID = coronavirus disease, SOFA = Sequential Organ Failure Assessment, TPE = therapeutic plasma exchange.
The primary outcomes were change in Sequential Organ Failure Assessment (SOFA) score, C-reactive protein (CRP), ferritin, and D-dimer levels in relation to TPE. One way repeated measure analysis of variance was used to compare before and after effect of the earliest TPE session on available values. Secondary outcomes included effect on oxygen support, hospital mortality, ICU and hospital lengths of stay, and discharge disposition.
The study was performed in accordance with the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Institutional Review Board at LMC. Consent for treatment was obtained from each patient or his/her surrogate decision-maker at the time of treatment as part of routine care.
TPE TREATMENT
Vascular access was obtained by venous insertion of a 14-French double-lumen temporary hemodialysis catheter. TPE was performed with the Spectra Optia (TerumoBCT, Denver, CO) apheresis system. Unless specified, treatment consisted of three consecutive daily treatments using approximately 100% of the calculated total plasma volume, using fresh frozen plasma as replacement fluid. Patients may not have received all three TPE treatments if their clinical status improved prior to the third treatment. In patients receiving convalescent plasma, no further treatments were planned after convalescent transfusion. Patients with a prolonged course may have received additional treatments based on hemodynamic and/or laboratory values suggesting ongoing organ failure, including inflammation and coagulopathy.
RESULTS
Eight patients were treated with TPE (age range, 41–78 yr; 6 males, 2 females). Six patients were alive at the time of submission, whereas two patients died in the ICU. All six survivors have been discharged from the hospital. Four were discharged home, one discharged to acute rehabilitation, and one returned to extended care (from which he was admitted). ICU lengths of stay were 7–18 days with total hospital stays 14–35 days (Table 1).
SOFA scores were calculated at ICU admission and hospital discharge, as well as prior to, and following, each TPE procedure (Tables 1 and 2). Mean ICU admission SOFA score was 6.8, and mean discharge SOFA was 2.2. A total of 24 TPE procedures were performed: 16 (66.7%) had improved SOFA scores post TPE, six (25%) had no change, whereas two (8.3%) had a worsening SOFA score. SOFA scores significantly decreased with the first TPE treatment (mean ± sd) pre = 9.3 ± 4.5 to post = 6.4 ± 3.5; ratio of variance (F) = 18.6; p = 0.004) (Table 2).
TABLE 2.
Change in Sequential Organ Failure Assessment and Inflammatory Biomarkers Before and After Therapeutic Plasma Exchange Among Eight Patients with Severe Coronavirus Disease 2019 Infection
| Patient | TPE Treatment | Sequential Organ Failure Assessment | C-Reactive Protein | Ferritin | D-Dimer | ||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | ||
| 1 | 1 | 3 | 3 | 147 | 76 | 1,009 | 445 | 2,346 | 1,880 |
| 2 | 3 | 3 | 83 | 35 | 679 | 445 | 2,215 | 1,009 | |
| 2 | 1 | 13 | 7 | — | — | — | — | 4,172 | 6,111 |
| 2 | 7 | 5 | 115 | 94 | 1,324 | 714 | 6,111 | 4,720 | |
| 3 | 4 | 3 | 125 | 24 | 1,397 | 1,980 | 2,854 | 3,323 | |
| 3 | 1 | 12 | 8 | 73 | 23 | 516 | 427 | 25,000 | 9,148 |
| 2 | 8 | 7 | 14 | 10 | 393 | 556 | 7,777 | 3,315 | |
| 3 | 7 | 7 | 10 | 36 | 556 | 904 | 3,315 | 9,553 | |
| 4 | 7 | 7 | 36 | 37 | 904 | 388 | 9,553 | 1,772 | |
| 4 | 1 | 10 | 7 | 588 | 514 | 1,845 | 1,610 | 8,729 | 5,318 |
| 2 | 7 | 4 | 514 | 114 | 1,610 | 847 | 5,318 | 1,542 | |
| 3 | 4 | 3 | 74 | 37 | 841 | 595 | 2,450 | 3,535 | |
| 4 | 6 | 6 | 48 | 28 | 745 | 407 | 6,242 | 4,275 | |
| 5 | 5 | 8 | 105 | 109 | 601 | 495 | 2,853 | 3,278 | |
| 6 | 13 | 9 | 93 | 19 | 1,044 | 365 | 2,811 | 950 | |
| 7 | 18 | 14 | 164 | 74 | 1,600 | 600 | 1,030 | 672 | |
| 5 | 1 | 3 | 2 | 281 | 113 | 1,586 | 990 | 432 | 405 |
| 2 | 2 | 2 | 113 | 26 | 990 | 702 | 405 | 339 | |
| 3 | 2 | 5 | 26 | 11 | 702 | 523 | 339 | 541 | |
| 4 | 6 | 5 | 52 | 121 | 926 | 1,063 | 3,045 | 2,286 | |
| 6 | 1 | 15 | 12 | 311 | 200 | 925 | 494 | 800 | 353 |
| 2 | 12 | 11 | 370 | 80 | 1,721 | 1,025 | 2,811 | 2,911 | |
| 7 | 1 | 11 | 9 | 348 | 168 | 2,629 | 2,211 | 1,832 | 1,907 |
| 8 | 1 | 7 | 3 | — | — | — | — | — | — |
| Means (first TPE Treatment) | 9.3 | 6.4 | 266.1 | 176.5 | 1,404.9 | 984.4 | 6,187.3 | 3,588.8 | |
| Pre-post comparison F, p | 18.6, p < 0.01 | 18.3, p < 0.01 | 32.0, p < 0.01 | 1.3, p = 0.3 | |||||
F = ratio of variance, TPE = therapeutic plasma exchange.
Dashes indicate values are unavailable as they were not measured.
CRP, ferritin, and D-dimer levels in relation to TPE are reported in Table 2. All three typically decreased with each treatment (18/22 CRP; 18/22 ferritin; 15/23 D-dimer). CRP (mean ±SD) pre = 266.1 ± 169.7 to post = 176.5 ± 162.6; F = 18.3; (p = 0.005) and ferritin (mean ± SD) pre = 1404.9 ± 696.3 to post = 984.4 ± 684.5; F = 32.0; (p = 0.001) significantly decreased with the first TPE treatment, whereas D-dimer did not (mean ± SD) pre = 6187.3 ± 8,758.9 to post = 3,588.8 ± 3,332.0; F = 1.3; (p = 0.3).
Daily arterial blood gases were not routinely checked, so Pao2/Fio2 ratios could not be trended to objectively assess changes in respiratory status. Instead, Figure 1 demonstrates changes in the mode of supplemental oxygen support required by each patient. All seven mechanically ventilated patients were initially liberated from the ventilator, although two patients required reintubation and ultimately died from their acute illness. Four survivors were weaned to room air prior to discharge, and two survivors were discharged on low-flow oxygen.
Figure 1.
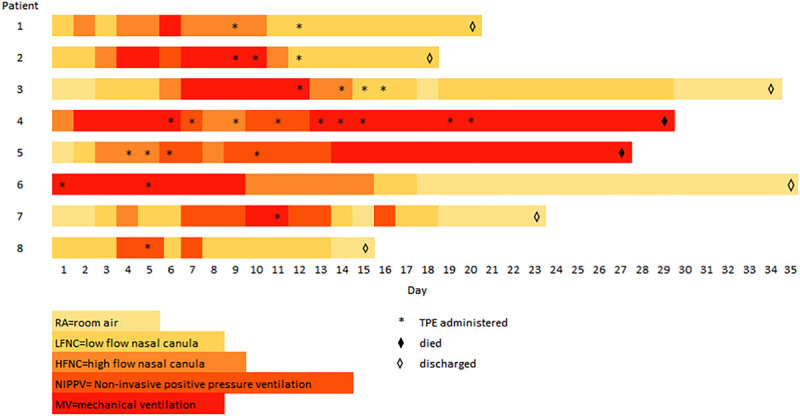
Respiratory support timeline for patients with coronavirus disease 2019 who received therapeutic plasma exchange (TPE) (n = 8).
DISCUSSION
We observed a clinical and laboratory response that may not have been predicted based on early outcome data in severe COVID infection (7), but the relationship of these findings to TPE is uncertain. The temporal relationship of our outcome measures to TPE is undeniable, but the clinical relationship and impact cannot be determined. Without matched controls, it is impossible to determine if these patients would have improved without TPE as part of the natural disease course, or whether other treatments, alone or in combination, are responsible for the outcomes we observed.
Identifying patients with poor prognosis and potential to benefit from adjunct therapy is key in sepsis. Hypercytokinemia is associated with increased mortality in sepsis and may manifest clinically as hypotension and multiple organ failure. CRP, ferritin, and D-dimer may serve as nonspecific markers, and elevated levels have been associated with increased mortality in COVID-19 (8, 9). These levels all generally improved with TPE in our patients. Defining pathologic levels, evaluating their response to TPE, and correlating these values with clinical outlines may prove valuable in future studies of TPE for severe COVID infection.
Although others have reported the feasibility and safety of TPE for sepsis (10), it is important to note that TPE alters the immune system in a nonselective way, and the net effect is not certain. The effect on humoral immunity is a concern, with the potential removal of host-generated antibodies that theoretically may adversely affect the clinical condition. Prospective studies should be performed, not only to evaluate the efficacy of TPE but any potential adverse effects.
As the number of critically ill patients with COVID-19 continues to grow, it is important that we continue to investigate treatment options. TPE offers treatment that targets the pathologic host response on multiple levels and has been effective in patients with a similar presentation of sepsis due to other pathogens. A well-designed prospective trial is desired to investigate this promising therapy for critically ill COVID patients.
CONCLUSIONS
TPE offers a potential therapy in critically ill patients with COVID-19 through its action on the inflammatory and coagulation pathways. Our case series shows favorable decreases in nonspecific markers of these pathways following TPE, but the clinical effect of these changes is uncertain. Prospective trials are needed to investigate the efficacy and safety of TPE in this patient population.
Footnotes
This work was performed at Lexington Medical Center, 2720 Sunset Boulevard, West Columbia, SC.
Dr. Keith had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Drs. Keith, Scott, Day, Goldberg, and Carcillo contributed equally. Drs. Keith, Scott, Day, Guffey, and Carcillo contributed to study concept and design. Drs. Keith, Scott, Weaver, Day, Hewitt, Gravel, Guffey, and Carcillo contributed to acquisition, analysis, or interpretation of data. All authors contributed to drafting of the article. Dr. Carcillo contributed to statistical analysis.
Dr. Carcillo receives grant funding from the National Institute of General Medical Sciences (R01GM108618). The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Gralinski LE, Baric RS. Molecular pathology of emerging coronavirus infections. J Pathol. 2015; 235:185–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang JC. Sepsis and septic shock: Endothelial molecular pathogenesis associated with vascular microthrombotic disease. Thromb J. 2019; 17:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bryce C, Grimes Z, Pujadas E, et al. Pathophysiology of SARS-CoV-2: Targeting of endothelial cells renders a complex disease with thrombotic microangiopathy and aberrant immune response. The Mount Sinai COVID-19 autopsy experience. medRXiv. 2020 [Google Scholar]
- 4.Busund R, Koukline V, Utrobin U, et al. Plasmapheresis in severe sepsis and septic shock: A prospective, randomised, controlled trial. Intensive Care Med. 2002; 28:1434–1439 [DOI] [PubMed] [Google Scholar]
- 5.Patel P, Nandwani V, Vanchiere J, et al. Use of therapeutic plasma exchange as a rescue therapy in 2009 pH1N1 influenza A—an associated respiratory failure and hemodynamic shock. Pediatr Crit Care Med. 2011; 12:e87–e89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Padmanabhan A, Connelly-Smith L, Aqui N, et al. Guidelines on the use of therapeutic apheresis in clinical practice - evidence-based approach from the writing committee of the American Society for Apheresis: The eighth special issue. J Clin Apher. 2019; 34:171–354 [DOI] [PubMed] [Google Scholar]
- 7.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020; 323:2052–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020; 46:846–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020; 395:1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knaup H, Stahl K, Schmidt BMW, et al. Early therapeutic plasma exchange in septic shock: A prospective open-label nonrandomized pilot study focusing on safety, hemodynamics, vascular barrier function, and biologic markers. Crit Care. 2018; 22:285. [DOI] [PMC free article] [PubMed] [Google Scholar]


