Abstract
Objectives:
To describe patients according to the maximum degree of respiratory support received and report their inpatient mortality due to coronavirus disease 2019.
Design:
Analysis of patients in the Coracle registry from February 22, 2020, to April 1, 2020.
Setting:
Hospitals in the Piedmont, Lombardy, Tuscany, and Lazio regions of Italy.
Patients:
Nine-hundred forty-eight patients hospitalized for coronavirus disease 2019.
Interventions:
None.
Measurements and Main Results:
Among 948 patients, 122 (12.87%) received invasive ventilation, 637 (67.19%) received supplemental oxygen only, and 189 (19.94%) received no respiratory support. The median (quartile 1–quartile 3) age was 65 years (54–76.59 yr), and there was evidence of differential respiratory treatment by decade of life (p = 0.0046); patients greater than 80 years old were generally not intubated. There were 606 men (63.9%) in this study, and they were more likely to receive respiratory support than women (p < 0.0001). The rate of in-hospital death for invasive ventilation recipients was 22.95%, 12.87% for supplemental oxygen recipients, and 7.41% for those who received neither (p = 0.0004). A sensitivity analysis of the 770 patients less than 80 years old revealed a lower, but similar mortality trend (18.02%, 8.10%, 5.23%; p = 0.0008) among the 14.42%, 65.71%, and 19.87% of patients treated with mechanical ventilation, supplemental oxygen only, or neither. Overall, invasive ventilation recipients who died were significantly older than those who survived (median age: 68.5 yr [60–81.36 yr] vs 62.5 yr [55.52–71 yr]; p = 0.0145).
Conclusions:
Among patients hospitalized for coronavirus disease 2019, 13% received mechanical ventilation, which was associated with a mortality rate of 23%.
Keywords: coronavirus disease 2019, inpatient mortality, invasive ventilation, respiratory support, severe acute respiratory syndrome coronavirus 2, supplemental oxygen
Mechanical ventilation may be used to support patients who develop severe symptoms from coronavirus disease 2019 (COVID-19) infection (1–3). However, reported mortality rates for mechanical ventilation recipients vary as a result of a multitude of factors, including the type of patients who receive this therapy, at what point during their illness it is initiated, and at what point during the pandemic the hospitalization occurred. Early mortality rate reports of mechanical ventilation recipients were alarmingly high, suggesting that more patients died than survived (4). However, as the pandemic has progressed, reports indicate that most people who receive this treatment survive (5). Particularly in institutions which may become overwhelmed by large patient volume, providers must consider patients’ chances of survival when evaluating which modality of respiratory support to deliver (6). Hence, we sought to determine the rate at which hospitalized patients received invasive ventilation, their distinguishing characteristics, and their mortality rate compared with those who received other levels of respiratory support using a registry of patients hospitalized in Italian medical centers from February 22, 2020, to April 1, 2020.
MATERIALS AND METHODS
Data
We used the Coracle registry (epidemiology, clinical characteristics, and therapy in real-life patients affected by severe acute respiratory syndrome coronavirus 2), which contains data of COVID-19 patients hospitalized in participating referral centers in the Piedmont, Lombardy, Tuscany, and Lazio regions of Italy, to perform this analysis. All patients in the registry were at least 18 years old and had COVID-19 infection confirmed via positive result of polymerase chain reaction assay of nasal and pharyngeal cultures, on or after February 22, 2020. We limited this analysis to patients whose inpatient mortality status was known (i.e., died in the hospital or discharged alive) as of April 1, 2020. Patients were retrospectively categorized into three mutually exclusive groups according to maximum respiratory support received as follows: invasive ventilation, supplemental oxygen without invasive ventilation, or neither invasive ventilation nor supplemental oxygen. Patients who received both invasive ventilation and supplemental oxygen were analyzed in the invasive ventilation category. Invasive ventilation was initiated for a COVID-19 patient if peripheral oxygen saturation was less than 92% for patients without chronic obstructive pulmonary disease (COPD) or less than 88% for patients with COPD. The deterioration of saturation was evaluated in the presence of noninvasive ventilation with high flow oxygen continuous positive airway pressure with positive end-expiratory pressure of 10–15 cm H2O. The need for supplemental oxygen therapy was evaluated according to peripheral oxygen saturation less than 92–96% in patients without COPD or less than 88–92% in patients with COPD. Generally, patients greater than 80 years old with a high comorbidity burden were maintained noninvasively despite desaturation. As a result, this could have decreased the mortality rate of the mechanical ventilation recipients and increased the mortality rate of those receiving other levels of respiratory support, so we performed a sensitivity analysis in which we considered only patients who were less than 80 years old. This work was approved by the ethical committee of Turin (Comitato Etico Interaziendale A.O.U. Città della Salute e della Scienza di Torino).
Statistical Analysis
Continuous variables were skewed and are presented as median (quartile 1–quartile 3). We categorized age based on decade of life, to be consistent with the COVID-19 literature (7). Categorical variables are presented as frequency (%). Differences in patient characteristics between those who received invasive ventilation, supplemental oxygen (without invasive ventilation), or neither were assessed via the Kruskal-Wallis test and chi-square test, or Fisher exact test, as appropriate. Analyses were performed using SAS Version 9.4 (Cary, NC).
RESULTS
Of the 1,050 patients in the Coracle registry at the time of analysis, 948 (90.3%) had a known mortality status at discharge. Hence, there were 948 patients included in this analysis, 122 (12.87%) of whom received invasive ventilation, 637 (67.19%) received supplemental oxygen without invasive ventilation, and 189 (19.94%) received no respiratory support (Table 1). The median age was 65 years (54–76.59 yr), and although age distribution did not differ significantly according to respiratory support (p = 0.1237), there was evidence of differential treatment by decade of life (Fig. 1). For example, although 60–69 years old constituted approximately 22% of the entire cohort, they represented closer to 32% of invasive ventilation recipients; conversely, although patients 80 years old or more accounted for nearly 19% of the overall cohort, they only made up 9% of invasive ventilation recipients (p = 0.0046). There were 606 men (63.9%) in this study, and they were more likely to receive supplemental oxygen and/or invasive ventilation compared with women (invasive ventilation: 99 [81.15%], supplemental oxygen without invasive ventilation: 407 [63.89%], neither treatment: 100 [52.91%]; p < 0.0001). There were no other significant differences in baseline patient characteristics across treatment groups. Although the overall rate of hypertension was high (51.06%), rates of other comorbidities were fairly low (e.g., diabetes mellitus: 16.16%, chronic heart failure: 7.2%); 59.81% of patients (567/948) had at least one comorbidity.
Figure 1.
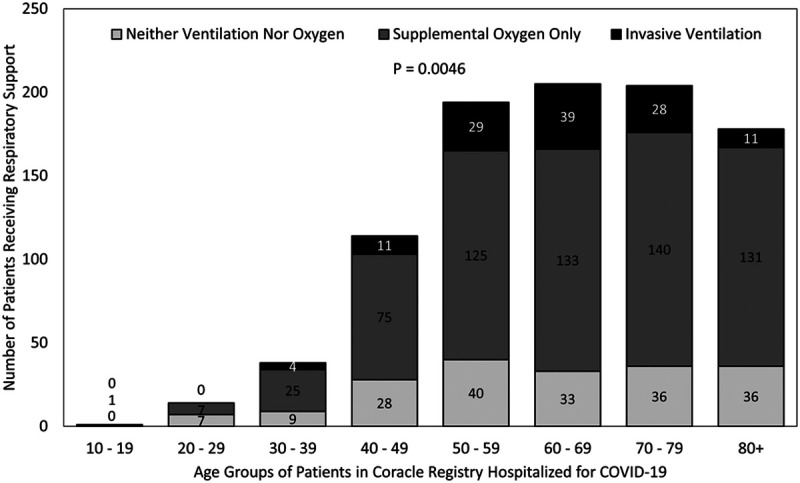
Respiratory support received according to age group of patients in Coracle registry hospitalized in Italy for coronavirus disease 2019 (COVID-19) infection
TABLE 1.
Characteristics of Patients in Coracle Registry Hospitalized for Coronavirus Disease 2019 According to Respiratory Support Received
| Characteristic | Overall (n = 948) | None (n = 189) | Supplemental Oxygen (n = 637) | Invasive Ventilation (n = 122) | p |
|---|---|---|---|---|---|
| Age, yr, median (quartile 1–quartile 3) | 65 (54–76.59) | 63 (50–76) | 66 (54–77) | 63.37 (56–72) | 0.1237 |
| Age category, yr, n (%) | 0.0046 | ||||
| 10–19 | 1 (0.11) | 0 (0) | 1 (0.16) | 0 (0) | |
| 20–29 | 14 (1.48) | 7 (3.7) | 7 (1.1) | 0 (0) | |
| 30–39 | 38 (4.01) | 9 (4.76) | 25 (3.92) | 4 (3.28) | |
| 40–49 | 114 (12.03) | 28 (14.81) | 75 (11.77) | 11 (9.02) | |
| 50–59 | 194 (20.46) | 40 (21.16) | 125 (19.62) | 29 (23.77) | |
| 60–69 | 205 (21.62) | 33 (17.46) | 133 (20.88) | 39 (31.97) | |
| 70–79 | 204 (21.52) | 36 (19.05) | 140 (21.98) | 28 (22.95) | |
| 80+ | 178 (18.78) | 36 (19.05) | 131 (20.57) | 11 (9.02) | |
| Gender (male), n (%) | 606 (63.92) | 100 (52.91) | 407 (63.89) | 99 (81.15) | < 0.0001 |
| Hypertension2, n (%) | 483 (51.06) | 86 (45.74) | 332 (52.12) | 65 (53.72) | 0.2523 |
| Obstructive lung disease1, n (%) | 87 (9.19) | 17 (9.04) | 58 (9.11) | 12 (9.84) | 0.9649 |
| Diabetes mellitus1, n (%) | 153 (16.16) | 28 (14.81) | 110 (17.3) | 15 (12.3) | 0.3323 |
| Smoking status, n (%) | 0.4678 | ||||
| Yes | 82 (8.65) | 13 (6.88) | 54 (8.48) | 15 (12.3) | |
| No | 803 (84.7) | 166 (87.83) | 539 (84.62) | 98 (80.33) | |
| Former | 61 (6.43) | 10 (5.29) | 43 (6.75) | 8 (6.56) | |
| Missing | 2 (0.21) | 0 (0) | 1 (0.16) | 1 (0.82) | |
| Chronic heart failure3, n (%) | 68 (7.2) | 9 (4.84) | 47 (7.38) | 12 (9.84) | 0.2402 |
| Coronary artery disease, n (%) | 106 (11.18) | 14 (7.41) | 79 (12.4) | 13 (10.66) | 0.1572 |
| Beta-blocker1, n (%) | 187 (19.75) | 31 (16.4) | 139 (21.82) | 17 (14.05) | 0.0626 |
| Calcium channel blocker1, n (%) | 162 (17.11) | 28 (14.81) | 120 (18.84) | 14 (11.57) | 0.0972 |
| Thiazide diuretic56, n (%) | 109 (12.22) | 23 (12.17) | 73 (12.27) | 13 (12.04) | 0.9974 |
| Loop diuretic58, n (%) | 103 (11.57) | 16 (8.51) | 78 (13.13) | 9 (8.33) | 0.1200 |
| Renin-angiotensin-aldosterone system inhibition, n (%) | 0.6297 | ||||
| Angiotensin II-converting enzyme inhibitor | 621 (65.51) | 131 (69.31) | 413 (64.84) | 77 (63.11) | |
| Aldosterone receptor blocker | 181 (19.09) | 34 (17.99) | 120 (18.84) | 27 (22.13) | |
| None | 146 (15.4) | 24 (12.7) | 104 (16.33) | 18 (14.75) | |
| ICU11, n (%) | 263 (28.07) | 1 (0.55) | 147 (23.26) | 115 (94.26) | < 0.0001 |
| Hydroxychloroquine162, n (%) | 589 (74.94) | 76 (51.7) | 429 (79.01) | 84 (87.5) | < 0.0001 |
| Anti-interleukin-6 agent132, n (%) | 151 (18.50) | 6 (4.58) | 99 (17.40) | 46 (39.66) | < 0.0001 |
| Length of stay (d)19, median (quartile 1–quartile 3) | 9 (6–12) | 6 (3–10) | 9 (6–12) | 10 (6–15) | < 0.0001 |
Age groups were collapsed into a < 50 yr category for statistical testing due to small counts.
Superscripts indicate missing data.
Overall, 124 patients (13.08%) perished in the hospital. The rates of death differed significantly across respiratory support groups, with 22.95% (28/122) of invasive ventilation recipients, 12.87% (82/637) of supplemental oxygen recipients, and 7.41% (14/189) of those who did not receive invasive ventilation or supplemental oxygen dying (p = 0.0004) (Fig. 2). Of those who received invasive ventilation, the only distinguishing characteristic of those who perished compared with those who survived was older age (68.5 yr [60–81.36 yr] vs 62.5 yr [55.52–71 yr]; p = 0.0145). Among invasive ventilation recipients with hypertension, renin-angiotensin-aldosterone system inhibition use was associated with a significantly lower risk of death (44.44% vs 78.72%; p = 0.0074).
Figure 2.
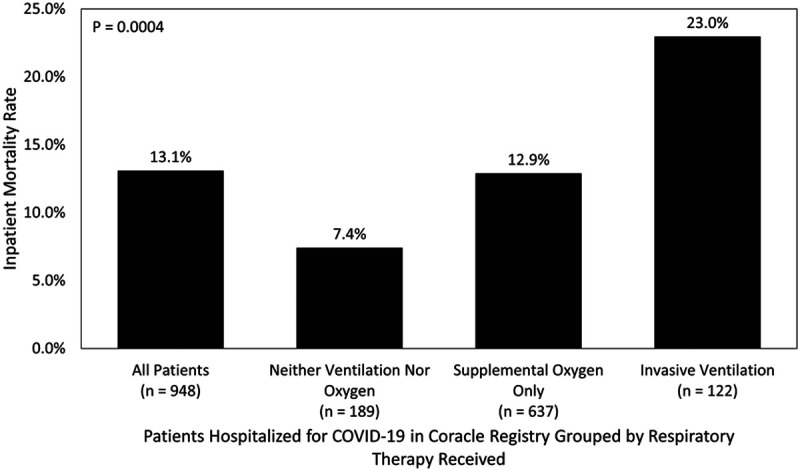
Inpatient survival rates according to age group and respiratory support received of patients in Coracle registry hospitalized in Italy for coronavirus disease 2019 (COVID-19) infection
Additionally, we performed a sensitivity analysis considering only the 770 patients who were younger than 80 years old. In this cohort, 111 patients (14.42%) received mechanical ventilation, 506 (65.71%) received supplemental oxygen only, and 153 (19.87%) received neither. Gender was associated with treatment (men: 81.08%, 66.40%, 53.59%, respectively; p < 0.0001). Age differed significantly across mechanical ventilation recipients, supplemental oxygen recipients, and those who received neither treatment (62 yr [55–70 yr], 61 yr [51–71 yr], and 57 yr [48–68 yr], respectively; p = 0.0360). The rates of congestive heart failure differed significantly across treatment modalities (9.91%, 4.15%, 1.33%; p = 0.0034). These treatments had associated mortality rates of 18.02%, 8.10%, and 5.23%, respectively (p = 0.0008), with an overall in-hospital mortality rate of 8.96%.
DISCUSSION
In this registry study of 948 patients hospitalized for COVID-19, we found that 80.1% received supplemental oxygen and/or invasive ventilation and that 13.1% of patients perished in the hospital, overall. We determined that the rates of death differed significantly across treatment modalities, with those receiving invasive ventilation having the highest rate. Among those who received mechanical ventilation, the mortality rate was 22.95%, much better than early reports from China (8). There were 12.9% of patients in this study who received invasive ventilation, which is less than 20.2% reported out of New York (9). We learned that males in their early-to-mid-60s represented many invasive ventilation recipients and that the recipients who perished were significantly older than their counterparts who survived. Excluding patients 80 years old or more, the mechanical ventilation’s associated mortality rate was 18.02%.
We are not the first to find that patients hospitalized with COVID-19 infection in this timeframe were likely males in their mid-60s. Our 64% male prevalence and overall median age of 65 years is comparable to a study of COVID-19 patients in critical care in the United Kingdom, which revealed male predominance (71%) and a median age of 64 years (10). In Seattle, 63% of such patients were male and had a mean age of 64 years (11). Similar demographic information has been reported in Hong Kong (12). Additionally, Grasselli et al (13) (Lombardy region, Italy) observed an 82% prevalence of males and a median age of 63 in their sample of ICU patients. Our 81% male rate of invasive ventilation recipients is nearly identical (median age = 63 yr). Several hypotheses exist to explain the differential effect of gender on infection severity and outcomes, including sex hormones’ effects on immune and inflammatory responses, stress hormones, and social isolation (14). Comorbidities also play a role in the severity of COVID-19; however, the likelihood of having one or more comorbidities also increases with age.
Death rate reports vary widely. Hong Kong reported a 12% 28-day mortality rate for COVID-19 patients in the ICU (12). Of 66 COVID-19 patients intubated in a Massachusetts study, 16.7% died (15). The rate of inpatient death observed in this study for invasive ventilation recipients (22.95% overall and 18.02% for patients younger than 80 yr) is similar to the 24.5% and 26% reported by Richardson et al (9) in New York and Grasselli et al (13) from the Lombardy region of Italy; however, not all patients had been removed from ventilation at the writing of those articles. Initial reports from Seattle indicate a 50% mortality rate in the ICU (with five-sixths of patients having do-not-resuscitate orders) (11), and critical patients who received invasive ventilation within the first 24 hours of admission in the United Kingdom perished at a rate of 66% (16). Nearly all mortality rates are less than initial reports from (Wuhan) China, in which the 28-day mortality rate among patients who admitted to the ICU and received noninvasive ventilation was 79% (23/29) and 86% (19/22) for those who received invasive mechanical ventilation (4).
This study has limitations inherent to its observational nature, including the inability to fully assess the direct effect of respiratory support on mortality. These data provide information about patients who received respiratory support and do not necessarily inform about patients who may have benefitted from but did not receive it. We recognize that comparing mortality rates between patients receiving different levels of respiratory therapy does not take into account the underlying severity of disease or comorbidity burden of the patients, which influences treatment decisions. However, we presented information indicating whether treatment was or was not provided in the ICU in an effort to describe the severity. Data pertaining to adjunctive therapies of hydroxychloroquine and tocilizumab were missing at high rates. Additionally, we did not have information available pertaining to do-not-resuscitate orders. Other variables of interest, including time on ventilator, were not available for study. Neither race nor ethnicity were available, and data are from the Piedmont, Lombardy, Tuscany, and Lazio regions of Italy, so these results may not be generalizable to other countries. Finally, these data pertain to a time of pandemic “surge” and may not be fully applicable to a nonsurge setting.
CONCLUSIONS
These data reveal that the majority of patients in the Coracle registry hospitalized for COVID-19 infection between February 22, 2020, and April 1, 2020, received some level of respiratory support and that most patients were men, had a median age of 65 years, and had at least one comorbidity. Among such patients, 13% received mechanical ventilation, which had an associated in-hospital mortality rate of 23%.
ACKNOWLEDGMENTS
We are indebted to the following individuals for their contribution to this work: Francesca Montagnani, MD (Infective Diseases Unit, Le Scotte Hospital, University of Siena, Siena, Italy); Bruno Frediani, MD (Rheumatic Diseases, Department of Medical Sciences, University of Siena, Siena, Italy); Marco Schiavone e Gianfranco Mitacchione (Azienda Ospedaliera—Polo Universitario—“Luigi Sacco,” Milano, Italy); Gabriella D’Ettorre, Giancarlo Ceccarelli, Claudio Mastroianni (Department of Public Health and Infectious Diseases, La Sapienza, University of Rome, Rome, Italy); Paolo Severino (Department of Clinical, Internal, Anesthesiological and Cardiovascular Sciences, Sapienza, University of Rome, Rome, Italy); Alessio Mattei (Division of Pneumology, University of Torino, AOU Città della salute e della Scienza, Torino, Italy); and Monica Andriani (Division of Cardiology, University of Torino, AOU Città della salute e della Scienza, Torino, Italy).
Footnotes
Supported, in part, by the Baylor Health Care System Foundation
Dr. Mojoli received funding from GE Healthcare (fee for lectures), Hamilton Medical (fee for lectures), and Seda Spa (fee for lecture) and disclosed a consultancy agreement between University of Pavia and Hamilton Medical. On behalf of the Coracle registry, for the purpose of this study, no ICU data were retrieved by Drs. Corcione or Palazzo. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Ford P, Foale M. Converting gas-driven ventilators from oxygen to air. Anaesthesia. 2020; 75:821. [DOI] [PubMed] [Google Scholar]
- 2.El Majid B, El Hammoumi A, Motahhir S, et al. Preliminary design of an innovative, simple, and easy-to-build portable ventilator for COVID-19 patients. EuroMediterr J Environ Integr. 2020; 5:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herrmann J, Fonseca da Cruz A, Hawley ML, et al. Shared ventilation in the era of COVID-19: A theoretical consideration of the dangers and potential solutions. Respir Care. 2020; 65:932–945 [DOI] [PubMed] [Google Scholar]
- 4.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med. 2020; 8:475–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Auld SC, Caridi-Scheible M, Blum JM, et al. ICU and ventilator mortality among critically ill adults with coronavirus disease 2019. Crit Care Med. 2020; 48:e799–e804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vincent JL, Taccone FS. Understanding pathways to death in patients with COVID-19. Lancet Respir Med. 2020; 8:430–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020; 323:1775–1776 [DOI] [PubMed] [Google Scholar]
- 8.Ñamendys-Silva SA. Respiratory support for patients with COVID-19 infection. Lancet Respir Med. 2020; 8:e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020. Clarification of mortality rate and data in abstract, results, and table 2. JAMA. 2020; 323:2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahase E. Covid-19: Most patients require mechanical ventilation in first 24 hours of critical care. BMJ. 2020; 368:m1201. [DOI] [PubMed] [Google Scholar]
- 11.Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle region - case series. N Engl J Med. 2020; 382:2012–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ling L, So C, Shum HP, et al. Critically ill patients with COVID-19 in Hong Kong: A multicentre retrospective observational cohort study. Crit Care Resusc. 2020; 22:119–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grasselli G, Zangrillo A, Zanella A, et al. COVID-19 Lombardy ICU Network; COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020; 323:1574–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spagnolo PA, Manson JE, Joffe H. Sex and gender differences in health: What the COVID-19 pandemic can teach us. Ann Intern Med. 2020; 173:385–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ziehr DR, Alladina J, Petri CR, et al. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: A cohort study. Am J Respir Crit Care Med. 2020; 201:1560–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Intensive Care National Audit and Research Centre. ICNARC Report on COVID-19 in Critical Care. 2020. Avaiable at: https://www.icnarc.org/Our-Audit/Audits/Cmp/Reports. Accessed April 10, 2020


