Abstract
The expression of Cystathionine beta-synthase (CBS) and Chemokine ligand 21 (CCL21) is associated with the tumorigenesis and progression of a variety of tumors, but whether alterations in their expression levels correlates with the carcinogenesis and progression of EHCC is still unknown. This study investigated the clinicopathological significance of CBS and CCL21 expression in EHCC.
We investigated the correlations between the expression of CBS and CCL21 and clinicopathological characteristics in EHCC using EnVision immunohistochemistry.
The expression of CBS and CCL21 was significantly higher in EHCC tumors than in nontumor tissues (P < .05 and P < .01). EHCC patients with CBS and CCL21 expression combined with lymph node metastasis, tumor cell invasion, and TNM III/IV stage had more severe conditions than those with no lymph node metastasis, distant invasion and TNM I/II stage (P < .01). Kaplan-Meier survival analysis showed that the overall survival rates for EHCC patients with negative CBS or CCL21 reaction were significantly higher than those for patients with positive CBS or CCL21 reaction((P < .01). CBS or CCL21 expression was revealed as an independent poor prognostic factor for EHCC patients by Cox multivariate analysis.
The present study indicates that CBS and CCL21 expression is closely associated with the pathogenesis of clinical, pathological and biological behaviors and poor prognosis in EHCC.
Keywords: cystathionine beta-synthase, chemokine ligand 21, extrahepatic cholangiocarcinoma, immunohistochemistry, prognosis
1. Introduction
Cholangiocarcinoma (CCA) is a malignant tumor originating from the epithelium of the bile ducts that has a poor prognosis and comprises 3% of all gastrointestinal carcinomas.[1] Globally, CCA is more prevalent in Asia. Compared to Western countries, which have only 2.1 new cases per 100,000, the incidence of CAA in Asia is approximately 113/100,000.[2] The risk factors for CCA are thought to be related to viral hepatitis, cirrhosis, hepatolithiasis, primary sclerosing cholangitis, parasitic infections, and gene mutations.[3] Anatomically, CCA can be distinguished into intrahepatic CCA (IHCC, 8%) and extrahepatic CCA (EHCC, 90%). Based on the cystic duct, EHCC can further be characterized as perihilar (Klatskin) tumors, originating in the bile ducts that are confluent in the liver hilum (50%), or tumors of the distal bile duct (42%).[4] Because of its anatomic location, the diagnosis of EHCC is often incidental, and many patients who receive the first diagnosis are in advanced stages; the clinical manifestations of most patients (>90%) are obstructive jaundice.[5] Unlike superficial tumors, it is always difficult to obtain pathological documentation of CCA. Endoscopic retrograde cholangiopancreatography (ERCP) provides an option for tumor sampling for cytology; however, it should be mentioned that fine-needle aspiration should not be used in order to avoid disseminating the tumor.[6] Thus, in general, only a minority of patients receive a cytologic diagnosis (15%–30%), and the diagnosis of EHCC usually relies on multidisciplinary approaches, including laboratory findings and high-quality abdominal imaging, such as computed tomography (CT) scans or magnetic resonance imaging(MRI).[7] There are four histologic grades of EHCC: well-differentiated, moderately differentiated, poorly differentiated, and undifferentiated. In addition to tumor grade,[8] independent predictors of patient survival and disease recurrence include lymphovascular space invasion, lymph node metastasis, and periductal tumor involvement.[9] Surgical resection is the first choice for EHCC patients, but many patients present with late-stage, unresectable disease. Therefore, further research on new therapeutic targets of EHCC is necessary.
CBS (cystathionine beta-synthase) is one of the heme-containing enzymes and, more importantly, is the first rate-limiting enzyme that catalyzes homocysteine to form the cysteine precursor cystathionine and hydrogen sulfide (H2S) in the transsulfuration pathway.[10] The aberrant expression of CBS was discovered to be strongly involved in various pathological and physiological progressions in various tumors.[11] In a study of colon cancer, researchers found that the expression of CBS in tumor specimens was markedly higher than that in patient-matched normal mucosa tissue; after using short hairpin RNAs (shRNAs) to suppress CBS expression in HCT116 cells, they found that cell proliferation, migration, and invasion were markedly decreased.[12] Evaluated by patient-derived tissue arrays and immunoblot analysis, Sen et al found that significantly increased levels of CBS expression in breast cancer when compared with their normal counterparts.[13] Interestingly, no consensus has been reached on the function of CBS. In colorectal cancer, it was considered a promoter, as the hydrogen sulfide produced by CBS through the transsulfuration pathway could promote cell growth, proliferation, and peritumor angiogenesis. After inhibition or genetic silencing of CBS, Jin found significant inhibitory activity against tumor cells both in vitro and in vivo, which could enhance the antitumor efficacy of anticancer therapeutics.[14] Conversely, Takano et al found that decreased CBS expression could promote glioma tumor formation, as it increases HIF-2α protein levels and HIF-2 target gene expression.[15] Zhao et al also believed CBS to be a tumor suppressor gene since CBS mRNA levels were downregulated through hypermethylation in gastric cancer.[16]
Chemokines are small chemotactic cytokines that attract leukocytes for activation and migration and are currently correlated with the restriction of tumor growth and distant metastasis.[17] Chemokine ligand 21 (CCL21) and chemokine ligand 19 (CCL19) act as ligands interacting with C-C chemokine receptor 7 (CCR7), which plays a role in lymphocyte trafficking as well as an important role in the inflammatory response.[18,19] Regarding malignant diseases, CCR7 is highly expressed in non-small-cell lung cancer (NSCLC)[20]; moreover, once activated by CCL21 and CCL19, it could increase the tumorigenesis, proliferation, and invasion of tumor cells.[21] Accompanied by CCR7, CCL21 expression also correlates with poor prognosis and distant metastasis in breast cancer, thyroid cancer,[22] gastric cancer,[23] and colorectal cancer.[24]
CBS and CCL21 seem to have biological significance in the occurrence and development of many malignant diseases; nonetheless, their roles in EHCC remain to be clarified. Therefore, in our study, the expression of CBS and CCL21 was detected by immunohistochemistry in surgically resected specimens, which contain EHCC, pericancerous tissues, adenoma, and normal biliary tract. Additionally, the clinicopathological significance and prognostic values of CBS and CCL21 expression were analyzed.
2. Material and methods
2.1. Case selection and clinical data
All research protocols were approved by the Ethics Committee for Human Research, Central South University. A total of 100 EHCC tissues, 30 peritumoral tissues, 10 biliary tract adenoma tissues, and 15 normal biliary tract tissues were randomly collected at the Second and Third Xiangya Hospitals, Central South University, from January 2001 to December 2014. All samples were obtained with informed consent. Based on the 7th TNM Classification of Malignant Tumors, the tumors were divided into clear staging classifications. All patients had a defined histological diagnosis of EHCC and were confirmed by two pathologists following the World Health Organization (WHO) tumor classification system. According to the World Health Organization criteria, the tumor differentiation degrees were defined as well, moderately and poorly differentiated. Fifteen normal biliary tract tissues were collected from voluntary organ donors of liver transplantation, and pathological examination revealed normal biliary tract tissues.
All tissues were fixed in neutral buffered formalin for 24 to 48 hours, embedded in paraffin, and cut into sections for micro examination and further processing. Survival data were calculated from the time of diagnosis to 30 months through letters and/or telephone calls, and patients who survived longer than 30 months were included in the analysis as censored cases.
2.2. Immunohistochemistry
Rabbit anti-human CBS and CCL21 polyclonal antibodies were purchased from Dako Corporation (Carpentaria, CA). The EnVisionTM Detection Kit was purchased from Dako Laboratories (CA). Positive controls were provided with the EnVisionTM Detection Kit. Immunohistochemistry (IHC) staining for CBS and CCL21 was performed using the EnVisionTM Detection Kit (Dako Laboratories, CA). In brief, each section was deparaffinized and then incubated with 3% H2O2 in the dark for 15 minutes. Heat-induced epitope retrieval was conducted with sodium citrate buffer (10 mM sodium citrate, 0.05% Tween 20, pH 6.0) at 96°C for 30 minutes. The sections were incubated with rabbit anti-human CBS and CCL21 primary antibodies (1:100 dilution; Dako Corporation, Carpentaria, CA) at 37°C for 2 hours after they were soaked in PBS for 3 × 5 min. The sections were dehydrated, soaked in xylene, and mounted with neutral balsam. Five hundred cells from ten random fields were examined per section by 2 observers independently. An average of the percentages from these two observers was used for final evaluation. Cases with positive cells ≥25% were considered positive, whereas other cases were considered negative.[25–27]
2.3. Statistical analysis
SPSS 21.0 (Statistical Package for the Social Sciences, Version 21.0) was used to analyze the correlations between CBS and CCL21 expression and histological or clinical factors in EHCC by χ2 test or Fisher exact test. Kaplan-Meier univariate survival analysis and the long-rank test were used to assess the overall survival of patients with EHCC. To identify whether the expression of CBS and CCL21 was an independent prognostic factor of OS for EHCC, multivariate analysis was performed with the Cox proportional hazards model, and 95% confidence intervals were calculated. P < 0.05 was considered statistically significant.
3. Results
3.1. The basic characteristics of the patients
As listed in Table 2, there were 100 cases of EHCC (61 males and 39 females, aged from 35 to 80 with a median age of 58.8 years), with 31% well-differentiated, 34% moderately differentiated and 35% poorly differentiated tumors. Surgery included radical resection for 54 patients (54.0%), palliative resection for 36 patients (36.0%) and biopsy for 10 patients (10.0%). The clinical stage of EHCC was partitioned as follows: 35 cases of stage I+II, 38 cases of stage III, and 27 cases of stage IV, which included 67 (67.0%) cases of EHCC with distant metastasis, 38 (38%) cases of EHCC with regional lymph node metastasis, and 31 (31%) EHCC patients with gallstones. Fifty-nine (59%) patients died within 12 months, 24 (24%) patients died within 24 months, 9 (9%) patients died within 30 months, and 8 (8%) patients survived longer than 30 months and were included in the analysis as censored cases.
The pathological examination of 30 pericancerous tissues showed that 12 cases were normal tissues, 8 were mild dysplasia, 6 were moderate dysplasia and 4 were severe dysplasia. The biliary tract adenoma tissues collected from six males and four females showed 6 simple adenoma tissues, 2 mild dysplasia and 2 moderate to severe dysplasia.
3.2. CBS and CCL21 protein expression in EHCC, peritumoral tissues, adenoma, and normal tissues
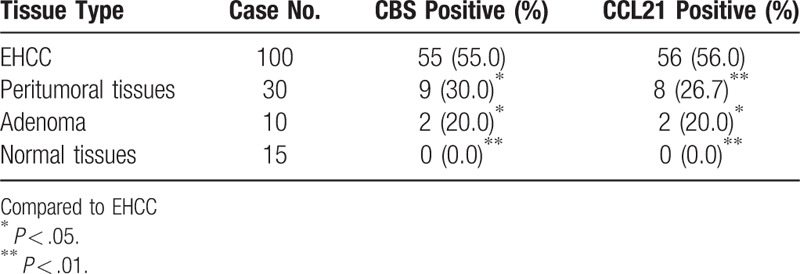
We evaluated the expression and cellular locations of CBS and CCL21 by IHC. As shown in Figures 1 and 2, CBS and CCL21 showed strong positive granule expression in the cytoplasm. In the 100 cases of EHCC, a positive percentage of CBS or CCL21 was 55.0% and 56.0%, respectively, as shown in Figure 1A, B and Figure 2A, B. For the 30 peritumoral tissues, 9 were CBS positive (30.0%) and 8 were CCL21 positive (26.7%), as shown in Figure 1C and Figure 2C. In adenoma, the expression of CBS and CCL21 was found in 2 of 10 cases (20.0%), as shown in Figure 1D and Figure 2D. For normal tissues, CBS and CCL21 expression was negative. Compared with peritumoral, adenoma, and normal tissues, the positive rates of CBS or CCL21 were significantly higher in EHCC (P < .05 and P < .01, respectively) (Table 1). Moreover, peritumoral tissues and adenoma with positive CBS and/or CCL21 expression exhibited moderate to severe dysplasia (Table 1).
Figure 1.
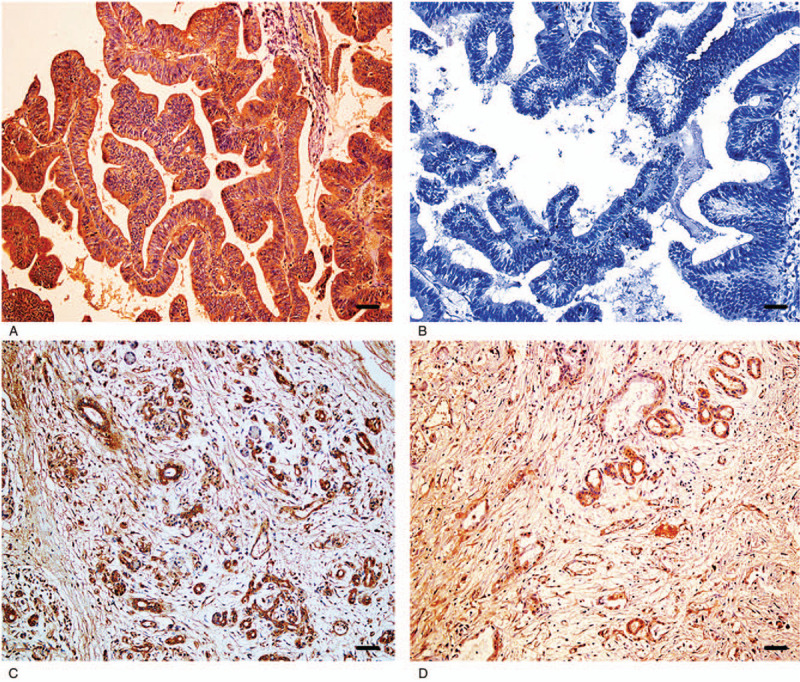
Immunohistochemical staining of CBS, ×200. (A) Positive reaction of CBS in moderately differentiated EHCC. (B) Negative reaction of CBS in well-differentiated EHCC. (C) Positive reaction of CBS in pericancerous tissues. (D) Positive reaction of CBS in adenoma. Scale bars correspond to 50 μm.
Figure 2.
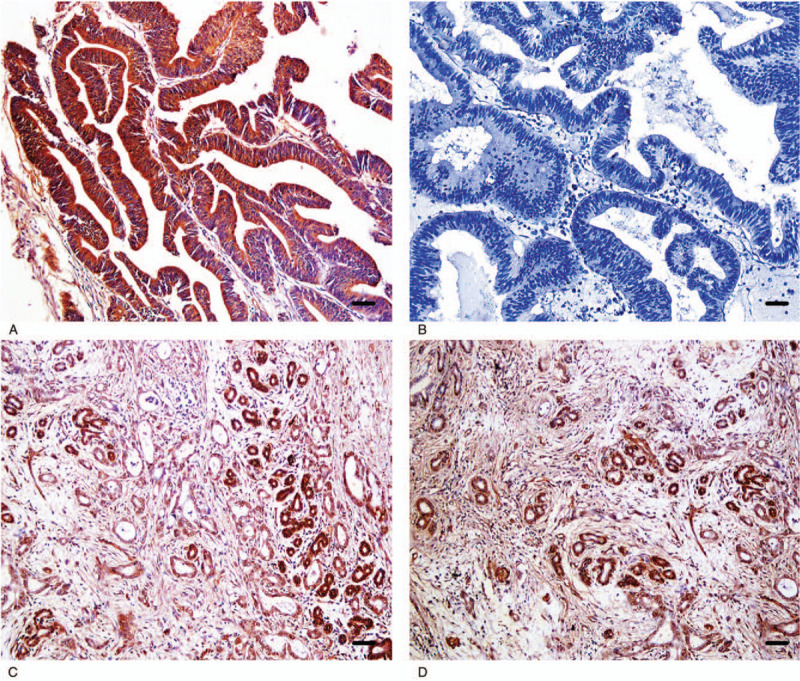
Immunohistochemical staining of CCL21 × 200. (A) Positive reaction of CCL21 in moderately differentiated EHCC. (B) Negative reaction of CCL21 in well-differentiated EHCC. (C) Positive reaction of CCL21 in pericancerous tissues. (D) Positive reaction of CCL21 in adenoma. Scale bars correspond to 50 μm.
Table 1.
Comparison of CBS and CCL21 expression in normal tissue, adenoma, peritumoral tissue and EHCC.
3.3. CBS and CCL21 protein expression was associated with the clinicopathological characteristics of EHCC
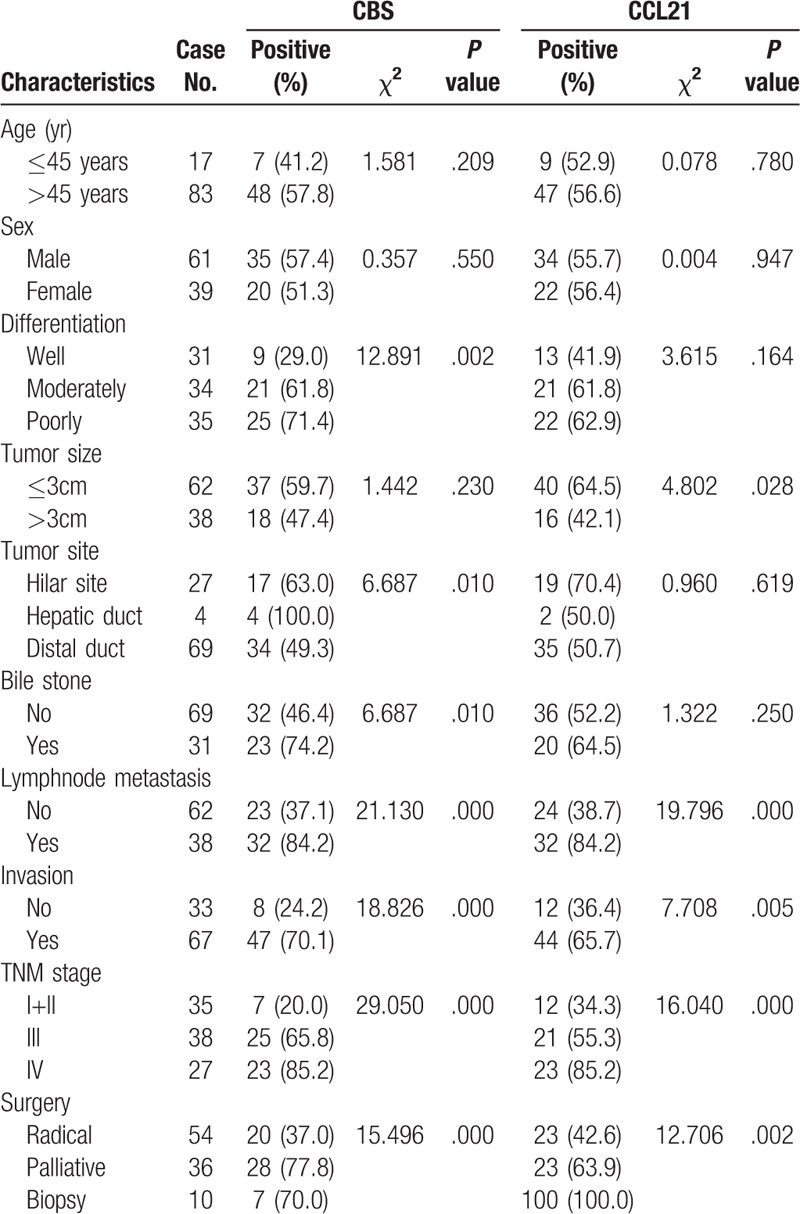
We further investigated the associations between the expression of CBS and CCL21 in EHCC and clinicopathological characteristics, such as age, histological type, and clinical stage, using the chi-square test. As shown in Table 2, the expression of CBS and CCL21 exhibited no significant association with sex, age or tumor site (P > .05). Positive rates of CBS and CCL21 expression in patients with advanced clinical stages (TNM stage III/IV) and no resection (biopsy only) were markedly higher than that of patients in early clinical stages (TNM stage I + II) and in those who underwent radical resection (P < .005). Compared to cases with lymph node metastasis, the expression of CBS and CCL21 was significantly lower in cases with no lymph node metastasis (P < .01). Similarly, distant organ invasion also caused a higher expression of CBS and CCL21 in EHCC. CBS expression was significantly lower in cases with good differentiation than in those with poor differentiation (P < .01). Interestingly, compared with EHCC patients with no bile stones, there was a higher expression of CBS in EHCC patients with bile stones (P = .01). EHCC patients with tumor diameters ≤3 cm showed a significantly higher expression of CCL21 than those with tumor diameters > 3 cm (P < .05).
Table 2.
Correlations of CBS and CCL21 protein expression with the clinicopathological characteristics of EHCC.
We further analyzed the relationship between CBS expression and CCL21 expression in EHCC. Among the 55 cases with positive CBS staining, 41 cases had positive CCL21 staining. Among the 45 cases with negative CBS staining, thirty cases had negative CCL21 staining. The expression of CBS was positively correlated with CCL21 (χ2 = 17.060, P < .001).
3.4. CBS and CCL21 protein expression correlated with the poor overall survival rates of EHCC patients
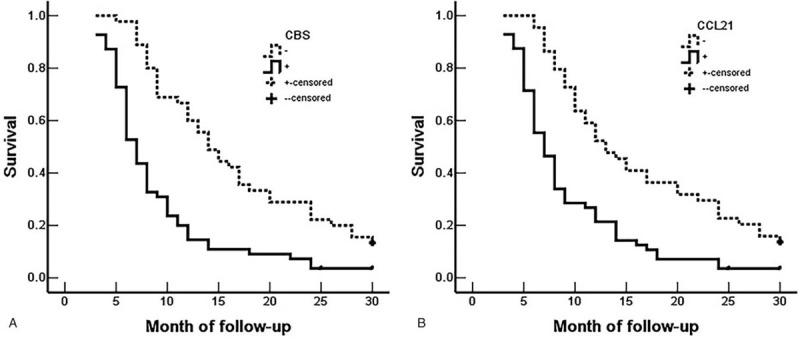
Through careful follow-up, the detailed survival information of all 100 EHCC patients was collected. Fifty-nine patients died within 12 months, 24 patients died within 24 months, 9 patients died within 30 months, and 8 patients who survived longer than 30 months were analyzed as censored cases. Table 3 illustrates the Kaplan-Meier survival analysis for EHCC patients. There was a significant difference in the survival time of EHCC patients between those with negative and reaction of CBS and CCL21 (CBS: negative vs positive, 17.43 months vs 8.27 months; CCL21: negative vs positive, 16.34 months vs 9.30 months, P < .001). Accompanied by the expression of CBS and CCL21, pathological characteristics such as differentiation (P < .001), TNM stage (P < .001), lymph node metastasis (P < .001), invasion (P < .001), and surgical procedure (P < .001) were significantly associated with the average overall survival time of patients with EHCC (Table 3). Figure 3 also illustrates that the overall survival time for CBS- or CCL21-positive reaction EHCC patients was markedly lower than for those with negative reaction. To further investigate whether CBS or CCL21 are independent prognostic factors, we also carried out Cox multivariate analysis. The results showed that along with the expression of CBS and CCL21, poor differentiation, lymph node metastasis, invasion, and high TNM stage (III or IV) were positively correlated with mortality and negatively correlated with overall survival. The results revealed that positive CBS and CCL21 expression can be used as independent prognostic factors for EHCC patients (P = .02 and P = .012, respectively) (Table 4). Finally, we depicted receiver operating characteristic (ROC) curves to evaluate the diagnostic efficacy of CBS expression and CCL21 expression. The AUC for CBS expression was 0.675 (95% CI: 0.588–0.762), and the AUC for CCL21 expression was 0.689 (95% CI: 0.604–0.774) (Fig. 4).
Table 3.
Correlations of clinicopathological characteristics, CBS and CCL21 expression with the mean survival in patients with EHCC.
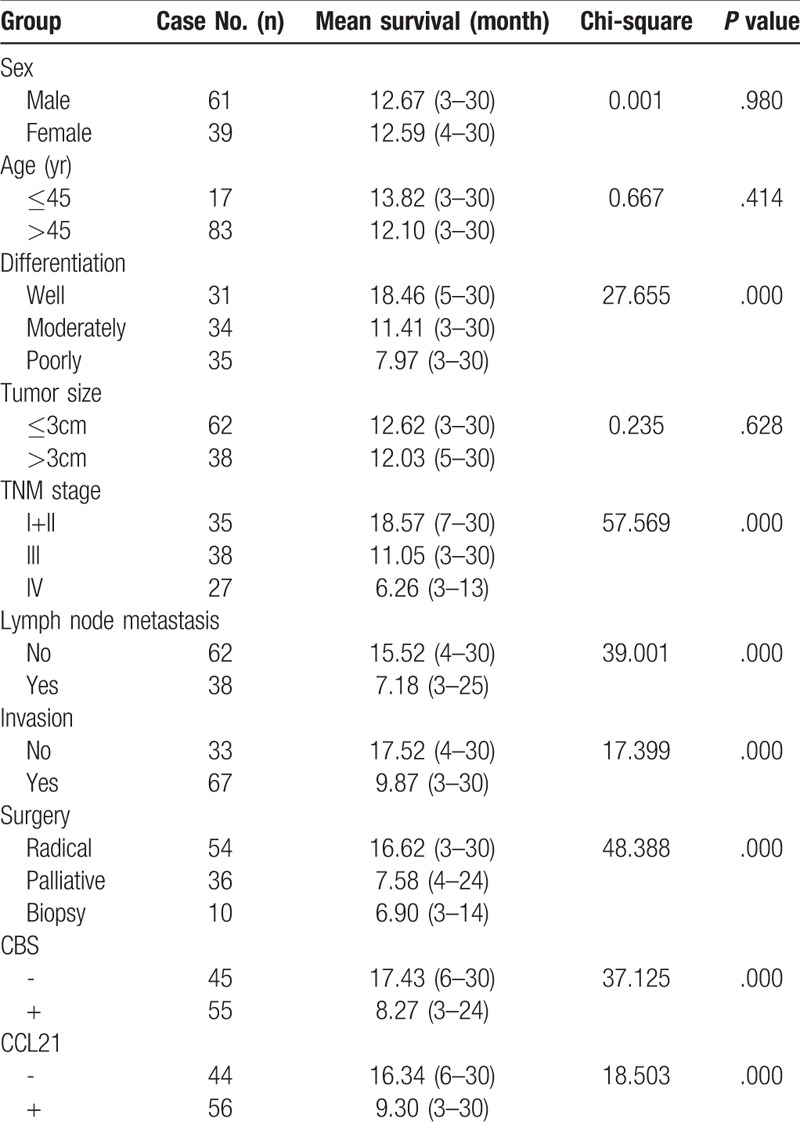
Figure 3.
CBS and CCL21 expression and survival in patients with EHCC. (A) Kaplan–Meier plots of overall survival in patients with CBS-positive and CBS-negative tumors. (B) Kaplan–Meier plots of overall survival in patients with CCL21-positive and CCL21-negative tumors.
Table 4.
Multivariate Cox regression analysis of survival rate in patients with EHCC.
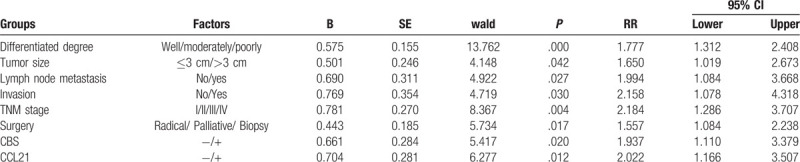
Figure 4.
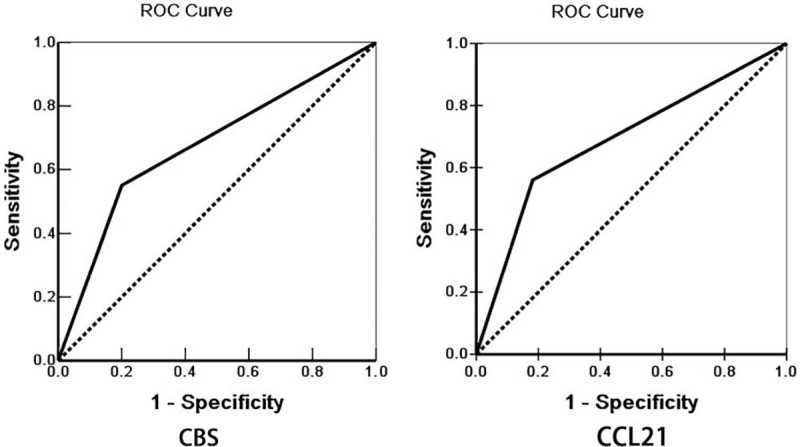
Multivariate analysis. ROC curves of diagonal segments are produced by ties of CBS (left) and CCL21 (right) in EHCC.
4. Discussion
Although the expression of CBS and CCL21 was associated with the progression and prognosis of a variety of tumors, no studies have investigated their expression and biological roles in EHCC. As stated above, EHCC is a highly malignant tumor with poor prognosis. In our study, the mean survival time of patients with well differentiated tumors was much longer than that of patients with poorly differentiated tumors. Additionally, patients in early TNM stages (I/II) had a longer average survival time than those in advanced TNM stages (III or IV). Moreover, the mean survival time of patients who underwent radical surgery was drastically longer than that of patients who underwent biopsy surgery. This result indicates that early diagnosis and treatment can help improve patient outcomes. Therefore, it is urgent to find new specific diagnostic markers for the early diagnosis of EHCC. As the first analysis of the expression of CBS in EHCC, we found a significant increase in CBS and CCL21 expression in EHCC tumors. Moreover, CBS and CCL21 expression are associated with TNM stage, poor differentiation, lymph node metastasis, distant organ invasion and poor prognosis in EHCC.
As one of the “biologic gases”, the function of hydrogen sulfide has attracted much attention. It is mainly catalyzed by cystathionine-β-synthase (CBS) and cystathionine-γ-lyase (CSE), using cystathionine and homocysteine as zymolytes.[10] In a study of ovarian cancer, Bhattacharyya et al found that CBS can serve as a potential therapeutic target for relapsing and drug-resistant tumors.[28] Chen et al also believe that the inhibition of H2S synthesis by reducing the expression of CBS may be a potential target for the treatment of the acquired resistance of colorectal cancer to 5-FU.[29] Gai et al found that in human urothelial cell carcinoma of the bladder (UCB), H2S and its synthase CBS increased as the degree of malignancy increased.[30] Similarly, our study showed that the expression of CBS was significantly increased in EHCC compared with noncancerous tissues and adenoma, which indicates that CBS could serve as a tumor promoter in EHCC. However, some authors found that the high expression of CBS might suppress the progression of some malignant lesions, such as gastrointestinal carcinoma and hepatocellular carcinoma, and lead to a better prognosis.[16,31] Kim et al found that the expression level of CBS in HCC specimens is markedly lower than that in noncancerous liver tissues.[31] Zhao et al also believed CBS to be a tumor suppressor gene since CBS mRNA levels were downregulated through hypermethylation in gastric cancer.[16] Thus, for now, no consensus has been reached on the function of CBS in malignant diseases. CBS may function to either promote or suppress tumor growth, depending on the cancer cell type, and its potential mechanism needs further study.
CCL21 is one of the chemokines secreted by secondary lymphoid organs, lymph nodes, and the spleen, sharing a common receptor, CCR7, with CCL19.[32] The binding of CCL21 and CCR7 mediates T cell recruitment and differentiation. CCL21 is involved in immunosurveillance and plays an important role in antitumor immunity. Previous studies have shown that CCL21/CCR7 is closely related to lymph node metastasis and is involved in many events related to the tumorigenesis of malignant solid tumors, such as angiogenesis, adhesion, and proliferation.[20,33–35] However, no studies have investigated whether it has the same function in EHCC. Our study first demonstrated that the expression of CCL21 was significantly higher in EHCC patients with lymph node metastasis than in patients with no lymph node metastasis. In terms of its mechanism, Tutunea-Fatan et al found that the CCL21/CCR7 chemokine axis could promote the expression of the lymphangiogenic factor VEGF-C. The study suggested that it has direct lymphangiogenic potential by means of the VEGFR-3 signaling pathway, and blocking VEGFR-3 would reduce lymphatic endothelial cell (LEC) proliferation and tube formation.[36] Moreover, it promotes tumor-induced lymph-vascular recruitment. Interestingly, the study found that the expression of CCR7 was significantly upregulated in breast cancer tissues compared to nontumor samples, while no obvious difference in CCL21 expression was observed.[36] Conversely, during tumor development, inhibition of the CCL21/CCR7 axis by a specific antagonist of CCL21 diminishes metastatic tumor formation.[37] The expression of CCL21 is detectable and associated with poor prognosis and distant metastasis in various human cancer types. Shields found a higher expression of CCL21 in human breast invasive ductal carcinoma than in normal tissue.[38] In a study of 48 circulatory inflammation markers, Henrik et al found that CCL21 was closely related to the pathophysiological characteristics of prostate cancer. Additionally, Zhong et al found that CCL21/CCR7 can trigger epithelial-mesenchymal transition (EMT) by activating the ERK1/2 signaling pathway, thereby promoting human lung cancer cell migration and invasion. In agreement with these reports, our study found that CCL21 expression was higher in cases with poor differentiation, lymph node metastasis, invasion, and advanced TNM stages, which means that CCL21 may contribute to the metastasis and development of EHCC.
In our present study, the results showed that the rates of CBS and CCL21 expression were significantly higher in EHCC than in cancerous tissues and adenoma. Adenoma and pericancerous tissues with CBS and/or CCL21 protein expression exhibited atypical hyperplasia. The CBS and CCL21 expression levels in EHCC patients with lymph node metastasis, tumor cell invasion, and TNM stage III/IV were higher than those in patients with no lymph node metastasis and distant invasion and TNM stage I/II. Moreover, patients with positive reaction of CBS and CCL21might have a lower survival probability than patients with negative CBS and CCL21 reaction, and multivariate analysis indicated that CBS and CCL21 expression are independent poor prognostic factors in patients with EHCC.
5. Conclusions
In conclusion, this is the first study to show that high expression levels of CBS and CCL21 are involved in the tumorigenesis and progression of EHCC and are positively related to poor OS rates in EHCC patients. Moreover, CBS and CCL21 expression was associated with poor prognosis in patients with EHCC. Therefore, it could be used as a novel prognostic marker for EHCC.
Author contributions
Lingxiang Wang, Qiong Zou, Zhengchun Wu carried out the study and wrote the paper; Zhulin Yang designed the study and revised the paper; Shu Xu and Daiqiang Li performed the statistical analysis; Yuan Yuan and Jun He collected the specimens and experimental materials. All authors contributed to the data analysis, drafted or revised the article, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Glossary
Abbreviations: CBS = cystathionine beta-synthase, CCL21 = chemokine ligand 21, CI = confidence interval, EHCC = Extrahepatic cholangiocarcinoma, RR = relative risk, TNM = tumor-node-metastasis.
References
- [1].Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology 2013;145:1215–29.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology 2011;54:173–84.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Huang CK, Aihara A, Iwagami Y, et al. Expression of transforming growth factor beta1 promotes cholangiocarcinoma development and progression. Cancer Lett 2016;380:153–62.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Liang Z, Liu X, Zhang Q, et al. Diagnostic value of microRNAs as biomarkers for cholangiocarcinoma. Dig Liver Dis 2016;48:1227–32.. [DOI] [PubMed] [Google Scholar]
- [5].Cho MS, Kim SH, Park SW, et al. Surgical outcomes and predicting factors of curative resection in patients with hilar cholangiocarcinoma: 10-year single-institution experience. J Gastrointest Surg 2012;16:1672–9.. [DOI] [PubMed] [Google Scholar]
- [6].Moreno Luna LE, Kipp B, Halling KC, et al. Advanced cytologic techniques for the detection of malignant pancreatobiliary strictures. Gastroenterology 2006;131:1064–72.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Vasilieva L, Papadhimitriou SI, Alexopoulou A, et al. Clinical presentation, diagnosis, and survival in cholangiocarcinoma: a prospective study. Arab J Gastroenterol 2016;17:181–4.. [DOI] [PubMed] [Google Scholar]
- [8].Nagorney DM, Donohue JH, Farnell MB, et al. Outcomes after curative resections of cholangiocarcinoma. Arch Surg 1993;128:871–7.. discussion 877-879. [DOI] [PubMed] [Google Scholar]
- [9].Oliveira IS, Kilcoyne A, Everett JM, et al. Cholangiocarcinoma: classification, diagnosis, staging, imaging features, and management. Abdom Radiol (NY) 2017;42:1637–49.. [DOI] [PubMed] [Google Scholar]
- [10].Kajimura M, Fukuda R, Bateman RM, et al. Interactions of multiple gas-transducing systems: hallmarks and uncertainties of CO, NO, and H2S gas biology. Antioxid Redox Signal 2010;13:157–92.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Guo H, Gai JW, Wang Y, et al. Characterization of hydrogen sulfide and its synthases, cystathionine beta-synthase and cystathionine gamma-lyase, in human prostatic tissue and cells. Urology 2012;79:483.e481-485. [DOI] [PubMed] [Google Scholar]
- [12].Szabo C, Coletta C, Chao C, et al. Tumor-derived hydrogen sulfide, produced by cystathionine-beta-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc Natl Acad Sci U S A 2013;110:12474–9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sen S, Kawahara B, Gupta D, et al. Role of cystathionine beta-synthase in human breast Cancer. Free Radic Biol Med 2015;86:228–38.. [DOI] [PubMed] [Google Scholar]
- [14].Jin S, Chen Z, Ding X, et al. Cystathionine-beta-synthase inhibition for colon cancer: enhancement of the efficacy of aminooxyacetic acid via the prodrug approach. Mol Med 2016;22:54–63.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Takano N, Sarfraz Y, Gilkes DM, et al. Decreased expression of cystathionine beta-synthase promotes glioma tumorigenesis. Mol Cancer Res 2014;12:1398–406.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhao H, Li Q, Wang J, et al. Frequent epigenetic silencing of the folate-metabolising gene cystathionine-beta-synthase in gastrointestinal cancer. PLoS One 2012;7:e49683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ashour AE, Lin X, Wang X, et al. CCL21 is an effective surgical neoadjuvant for treatment of mammary tumors. Cancer Biol Ther 2007;6:1206–10.. [DOI] [PubMed] [Google Scholar]
- [18].Hirao M, Onai N, Hiroishi K, et al. CC chemokine receptor-7 on dendritic cells is induced after interaction with apoptotic tumor cells: critical role in migration from the tumor site to draining lymph nodes. Cancer Res 2000;60:2209–17.. [PubMed] [Google Scholar]
- [19].Hauser MA, Legler DF. Common and biased signaling pathways of the chemokine receptor CCR7 elicited by its ligands CCL19 and CCL21 in leukocytes. J Leukoc Biol 2016;99:869–82.. [DOI] [PubMed] [Google Scholar]
- [20].Xu Y, Liu L, Qiu X, et al. CCL21/CCR7 promotes G2/M phase progression via the ERK pathway in human non-small cell lung cancer cells. PLoS One 2011;6:e21119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wagner PL, Moo TA, Arora N, et al. The chemokine receptors CXCR4 and CCR7 are associated with tumor size and pathologic indicators of tumor aggressiveness in papillary thyroid carcinoma. Ann Surg Oncol 2008;15:2833–41.. [DOI] [PubMed] [Google Scholar]
- [22].Sancho M, Vieira JM, Casalou C, et al. Expression and function of the chemokine receptor CCR7 in thyroid carcinomas. J Endocrinol 2006;191:229–38.. [DOI] [PubMed] [Google Scholar]
- [23].Hwang TL, Lee LY, Wang CC, et al. CCL7 and CCL21 overexpression in gastric cancer is associated with lymph node metastasis and poor prognosis. World J Gastroenterol 2012;18:1249–56.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zou Y, Chen Y, Wu X, et al. CCL21 as an independent favorable prognostic factor for stage III/IV colorectal cancer. Oncol Rep 2013;30:659–66.. [DOI] [PubMed] [Google Scholar]
- [25].Wang L, Xiong L, Wu Z, et al. Expression of UGP2 and CFL1 expression levels in benign and malignant pancreatic lesions and their clinicopathological significance. World J Surg Oncol 2018;16:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Liu Z, Yang Z, Jiang S, et al. Paxillin and carbonic anhydrase IX are prognostic markers in gallbladder squamous cell/adenosquamous carcinomas and adenocarcinomas. Histopathology 2014;64:921–34.. [DOI] [PubMed] [Google Scholar]
- [27].He J, Yang Z, Wu Z, et al. Expression of FOXP1 and FOXO3a in extrahepatic cholangiocarcinoma and the implications in clinicopathological significance and prognosis. Onco Targets Ther 2019;12:2955–65.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bhattacharyya S, Saha S, Giri K, et al. Cystathionine beta-synthase (CBS) contributes to advanced ovarian cancer progression and drug resistance. PLoS One 2013;8:e79167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chen S, Yue T, Huang Z, et al. Inhibition of hydrogen sulfide synthesis reverses acquired resistance to 5-FU through miR-215-5p-EREG/TYMS axis in colon cancer cells. Cancer Lett 2019;466:49–60.. [DOI] [PubMed] [Google Scholar]
- [30].Gai JW, Qin W, Liu M, et al. Expression profile of hydrogen sulfide and its synthases correlates with tumor stage and grade in urothelial cell carcinoma of bladder. Urol Oncol 2016;34:166.e115-120. [DOI] [PubMed] [Google Scholar]
- [31].Kim J, Hong SJ, Park JH, et al. Expression of cystathionine beta-synthase is downregulated in hepatocellular carcinoma and associated with poor prognosis. Oncol Rep 2009;21:1449–54.. [DOI] [PubMed] [Google Scholar]
- [32].Li F, Zou Z, Suo N, et al. CCL21/CCR7 axis activating chemotaxis accompanied with epithelial-mesenchymal transition in human breast carcinoma. Med Oncol 2014;31:180. [DOI] [PubMed] [Google Scholar]
- [33].Muller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001;410:50–6.. [DOI] [PubMed] [Google Scholar]
- [34].Wang J, Xi L, Hunt JL, et al. Expression pattern of chemokine receptor 6 (CCR6) and CCR7 in squamous cell carcinoma of the head and neck identifies a novel metastatic phenotype. Cancer Res 2004;64:1861–6.. [DOI] [PubMed] [Google Scholar]
- [35].Ishigami S, Natsugoe S, Nakajo A, et al. Prognostic value of CCR7 expression in gastric cancer. Hepatogastroenterology 2007;54:1025–8.. [PubMed] [Google Scholar]
- [36].Tutunea-Fatan E, Majumder M, Xin X, et al. The role of CCL21/CCR7 chemokine axis in breast cancer-induced lymphangiogenesis. Mol Cancer 2015;14:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wang J, Seethala RR, Zhang Q, et al. Autocrine and paracrine chemokine receptor 7 activation in head and neck cancer: implications for therapy. J Natl Cancer Inst 2008;100:502–12.. [DOI] [PubMed] [Google Scholar]
- [38].Shields JD, Fleury ME, Yong C, et al. Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell 2007;11:526–38.. [DOI] [PubMed] [Google Scholar]


