Abstract
A randomized complete block design experiment with 30 yearling crossbred steers (initial average body weight [BW] = 297.6 ± 32.8 kg) fed a steam-flaked corn-based diet was used to evaluate finishing performance and carcass characteristics when provided with different concentrations of vitamin A (Rovimix A 1000; DSM Nutritional Products Ltd., Sisseln, Switzerland) subsequent to a depletion phase. Steers were blocked by BW (n = 5 blocks; 6 steers per block), assigned to pens (n = 2 steers per pen), and randomly assigned to one of the following dietary treatments: no added vitamin A (0IU; 0.0 IU/kg dry matter [DM] basis of additional vitamin A), vitamin A supplemented at the estimated National Academies of Sciences, Engineering, and Medicine (NASEM) requirement (2,200IU; 2,200 IU/kg of dietary DM of additional vitamin A), and vitamin A supplemented at 5× the estimated requirement (11,000IU; 11,000 IU/kg of dietary DM of additional vitamin A). The basal diet included minimal vitamin A activity (<200 IU of vitamin A activity/kg of dietary DM) via the provitamin A, beta-carotene. After all animals underwent a 91-d vitamin A depletion period, additional vitamin A was top-dressed at feeding via a ground corn carrier. Liver biopsy samples, BW, and blood were obtained on days −91, −35, 0, 28, 56, 84, and 112. Final BW was collected prior to shipping on day 112. Carcass data were collected by trained personnel upon harvest. Sera and liver samples were used to monitor circulating vitamin A and evaluate true vitamin A status of the cattle. Vitamin A status did not affect interim average daily gain or feed efficiency (G:F; P > 0.05). Throughout the duration of the study, dry matter intake for the 0IU cattle was depressed (P = 0.01). Differences were not observed across treatments for hot carcass weight, rib eye area, back fat thickness, kidney–pelvic–heart fat %, marbling score, or dressing percent (P ≥ 0.10). A treatment × day interaction occurred for both (P < 0.01) sera retinol and liver retinol during phase 2 of the trial. The treatments and sera retinol concentrations were incorporated into a repletion model, resulting in an estimation of liver retinol changes (P < 0.01; R2 = 0.682). However, models used to evaluate depleted animals were less effective. The current NASEM recommended that vitamin A requirement of 2,200 IU/kg is adequate for repletion of vitamin A status of feedlot steers.
Keywords: beef, depletion, liver biopsy, performance, steer, vitamin A
Introduction
The role of vitamin A in fat and muscle development and consequent effects on carcass characteristics is equivocal (Kim et al., 2018). Research has shown that retinoic acid, an active form of vitamin A, inhibits adipogenesis, subsequently reducing marbling (Ohyama et al., 1998; Kawada et al., 2000). Therefore, several countries restrict vitamin A in the finishing diet in attempts to produce highly marbled beef; however, trials evaluating the effects of vitamin A have conflicting results (Oka et al., 1998). Vitamin A deficiency symptoms can include depressed intake and growth, various types of blindness, most recognizably night blindness, reduced reproductive performance, and increased susceptibility to respiratory infections; therefore, vitamin A-depleted diets are scrutinized (NRC, 1996). Steers fed varying doses of vitamin A did not differ in carcass characteristics (Bryant et al., 2010). However, Gorocica-Buenfil et al. (2008) and Pickworth et al. (2012) reported improvements in quality grade for cattle fed decreased concentrations of vitamin A.
The current National Academies of Sciences, Engineering, and Medicine (NASEM, 2016) requirement recommends beef cattle diets should contain 2,200 IU vitamin A/kg of dry matter (DM), which is congruent to the recommendation from the 1996 NRC. However, a review by Vasconcelos and Galyean (2007) stated that nutritionists recommend 5,215 IU of vitamin A/kg of DM on average. As of 2016, consulting nutritionists still recommended the supplementation of vitamin A at more than double the requirement (NASEM, 2016; Samuelson et al., 2016). This is likely due to the historically low cost of vitamin A supplementation. Several studies have suggested that the quality of feed cattle receive during the stocker phase affects their demand for vitamin A (Bryant et al., 2010). Animals entering feedlots from lush pasture likely have increased concentrations of vitamin A in the liver compared with those supplemented with hay (Kohlmeier and Burroughs, 1970). Depletion of vitamin A after the suspension of vitamin A and carotenoid intake varies between classes of cattle, ranging from as little as 40 d in light feeder steers (150 to 300 kg) to as many as 150 d in mature cows (Sewell, 1993). The rate of depletion is likely a product of the animals’ vitamin A status at initiation of restriction (Bryant et al., 2010). The process of repletion of vitamin A in finishing cattle starting with a critically low vitamin A status has not previously been investigated. According to the Michigan State University Veterinary Diagnostic Laboratory, normal ranges for serum and liver retinol for cattle are 225 to 500 ng/mL and 300 to 1,100 µg/g, respectively. It was hypothesized that vitamin A depletion would not affect carcass traits but would reduce performance. Therefore, a study was designed to evaluate liver storage and circulation of vitamin A in cattle throughout finishing and the performance and carcass characteristics of finishing steers provided with different concentrations of vitamin A subsequent to a depletion phase.
Materials and Methods
Animal management and treatments
All procedures were approved by the Texas Tech University Institutional Animal Care and Use Committee (Protocol Number: 18003-01). Bos taurus crossbred (predominantly Angus) steers (n = 30; initial average body weight [BW] = 297.6 ± 32.8 kg) were obtained for a two-phase, 210-d finishing trial from a commercial cattle company in Stratford, TX, after grazing dormant winter forage supplemented with grass hay. They were processed upon arrival at the Burnett Center of Texas Tech University, Idalou, TX after a 3-h transit in late February. Steers received ear tags for identification, were treated with anthelmintics, and vaccinated in accordance with Texas Tech University health protocols. Phase 1 of this trial was a 91-d vitamin A depletion period, during which animals were randomly divided into three groups unrelated to future treatment application, housed on dirt pens (n = 10 per pen), and fed a 65% concentrate growing diet, which contained 11.5% crude protein (CP). Phase 2 was a 119-d finishing period. Between phases, animals were gradually adapted (four steps) to control (CON or basal phase 2 diet) starting on day −21 (Table 1). Steers were fed ad libitum, using clean bunk management. Dietary ingredient selection was consistent between phases. The 90% concentrate finishing diet was designed to deplete vitamin A while meeting the remaining NASEM (2016) requirements for growing-finishing beef cattle, and contained 12.5% CP (DM basis), recommended levels of supplemental vitamins (except vitamin A) and minerals, monensin (Rumensin: Elanco Animal Health, Greenfield, IN; 33 mg/kg of DM), and tylosin phosphate (Tylan: Elanco Animal Health; 11 mg/kg of DM). Depletion diet phase and CON treatment during the finishing phase contained minimal vitamin A activity (<200 IU of vitamin A activity/kg of dietary DM) via the provitamin A, beta-carotene. No beta-adrenergic agonist was fed nor were steers provided with implants during the finishing phase to minimize any factors that could influence the growth performance data beyond the dietary treatments. Animal health and welfare were monitored daily. The cattle were observed for any symptoms relating to night blindness or vitamin A deficiency. Based on previous research, it was estimated that it would require a minimum of 90 d to deplete the liver retinol levels (Bryant et al., 2010). In order to monitor vitamin A status, liver samples were analyzed within 2 wk of collection. Therefore, liver retinol from day −35 was used to estimate the vitamin A status of the animals prior to initiating phase 2, allowing time for sample analysis, treatment allocation, and transfer into concrete-slat treatment pens (two steers per pen). Steers (average BW = 436.3 ± 39.8 kg) were blocked by initial BW and organized in a randomized complete block design (2 steers per pen; 15 pens total; 5 pens per treatment). Animals within a block were randomly assigned to pens administered one of three dietary treatments: no added vitamin A (0IU; 0.0 IU/kg [DM basis] of additional vitamin A), vitamin A supplemented at the estimated requirement (2,200IU; 2,200 IU/kg of dietary DM of additional vitamin A acetate; Rovimix A 1000; DSM Nutritional Products Ltd., Sisseln, SUI), and vitamin A supplemented at 5× the estimated requirement (11,000IU; 11,000 IU/kg of dietary DM of additional vitamin A). Additional vitamin A for phase 2 treatments was top-dressed at feeding time via a ground corn carrier (0.45 kg/[pen · d]) for which the concentration of vitamin A inclusion was adjusted according to the average weekly dry matter intake (DMI). Daily feed deliveries were recorded, and orts were weighed weekly to calculate the feed intake.
Table 1.
Description of experimental diets (DM basis)1
| Ingredients, % DM | Phase 1 | Phase 2 |
|---|---|---|
| Steam-flaked corn | 39.40 | 64.72 |
| Wet corn gluten feed | 17.00 | 19.92 |
| Cottonseed hulls | 10.00 | 4.00 |
| Native grass hay, chopped | 27.00 | 3.94 |
| Fat (yellow grease) | 3.00 | 3.08 |
| Supplement2 | 2.00 | 1.95 |
| Limestone | 1.00 | 1.88 |
| Urea | 0.60 | 0.51 |
| Chemical composition, DM3 | ||
| DM, % | 81.18 | 78.88 |
| CP, % | 11.54 | 12.53 |
| Acid detergent fiber, % | 31.49 | 10.10 |
| Neutral detergent fiber, % | 35.20 | 17.30 |
| Ash, % | 5.68 | 4.63 |
| Total digestible nutrients, % | 72.00 | 84.50 |
| NEM4, Mcal/kg | 1.74 | 2.15 |
| NEG5, Mcal/kg | 1.12 | 1.48 |
| Vitamin A activity, IU/kg6 | 192 | 167 |
1Diets were formulated to meet or exceed NASEM (2016) requirements for growing-finishing beef cattle with the exception of vitamin A requirements.
2Supplement composition (DM basis): 67.755% ground corn, 15.000% NaCl, 10.000% KCl, 3.760% urea, 0.986% zinc sulfate, 0.750% Rumensin-90 (Elanco, Greenfield, IN), 0.506 Tylan-40 (Elanco), 0.500% Endox (Kemin Industries, Des Moines, IA), 0.196% copper sulfate, 0.167% manganese oxide, 0.157% vitamin E (500 IU/g), 0.125% selenium premix (0.2% Se), 0.083% iron sulfate, 0.003% ethylenediamine dihydroiodide, and 0.002% cobalt carbonate.
3Values as measured by proximate analysis of bunk samples on a DM basis except diet DM (Servi Tech Laboratories, Amarillo, TX), and total digestible nutrients (TDN) was determined as in NASEM (2016).
4NEM, net energy for maintenance.5NEG, net energy for gain.
6Vitamin A activity was calculated based on ingredient beta-carotene content, and its conversion in cattle. Treatments were added to basal content: 0IU, 2,200IU, and11,000IU.
Animal removal
One steer (11,000IU) contracted chronic pneumonia resulting in death on day 114. Day-112 liver retinol and blood as well as carcass data were unable to be recorded for this animal. The steer was not an outlier for any data recorded up to the time of death; therefore, data contributions for this steer from day −91 through 84 remained in the analyses.
Sample collection
All data were collected on a designated sampling day before feeding. BWs for all cattle were measured on days −91, −35, 0, 28, 56, 84, 112, and on day 119 relative to treatment initiation. Liver tissue and blood samples were acquired from all cattle on days −91, −35, 0, 28, 56, 84, and 112.
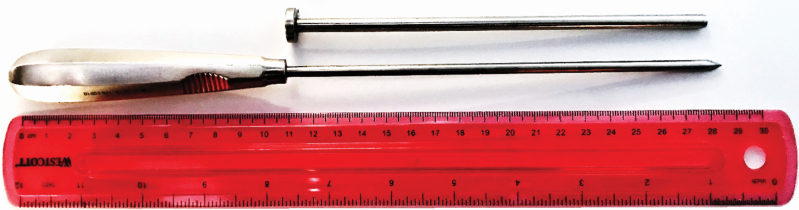
Liver samples were obtained via biopsy to monitor steady-state vitamin A status. During the biopsy procedure, steers were restrained using a hydraulic squeeze chute. The area surrounding the incision site was shaved using a disposable razor, then sanitized using a solution of water, 7.5% povidone–iodine surgical scrub (Betadine, Purdue Products, L.P., Stamford, CT), and 70% ethanol. A local anesthetic (lidocaine HCl, 20 mg/mL, 8 mL per biopsy) was administered subcutaneously in a 6-cm2 rhombus-shaped pattern (four injection sites, 2 mL lidocaine HCl per site) no less than 5 min prior to the biopsy incision. The area was sterilized using 70% ethanol and sterile gauze post-numbing, then a 1-cm incision was made using a sterile scalpel. A sterile 16.5-cm long, 6-mm diameter custom-designed trocar and sharpened cannula biopsy instrument (SurgiPro, Minneapolis, MN) were used to collect approximately 0.4 g of liver tissue using a procedure similar to that used by Chapman et al. (1963; Figure 1). Liver biopsies were collected from an incision made in the 11th intercostal space where it intersects with a line made from the tuber coxae and the point of the shoulder. The incision was closed using veterinary tissue adhesive (VetBond, 3M Animal Care Products, St. Paul, MN) and coated with a protective aerosol bandage (AluShield, Neogen Corp, Lexington, KY) to aid in infection prevention during the healing process. The sample was placed on sterile foil in a covered plastic container to be taken to the sample preparation area, where it was placed in a light-protected microcentrifuge tube and frozen on dry ice.
Figure 1.
The custom biopsy instrument (SurgiPro, Minneapolis, MN) consisted of a sharpened trocar and cannula with a working shaft length of 17.5 cm and a cannula diameter of 6 mm. The combined instrument was inserted through the 11th intercostal space of the animal until the hepatic membrane was pierced, then the trocar was withdrawn, the cannula was inserted into the liver tissue, and the exterior opening of the cannula was covered as the instrument was retrieved from the animal. The negative pressure created by covering the exterior opening aspirated approximately 0.4 g of the liver tissue.
Blood samples were collected via jugular venipuncture using nonadditive silicone-coated glass vacuum blood collection tubes (Vacutainer, BD Diagnostics, Franklin Lakes, NJ). Tubes were stored at 4 °C overnight to allow for clotting and then centrifuged at 1,200 × g for 15 min to separate serum. Serum samples were then stored at −20 °C until laboratory analysis.
Sample analysis
Liver, sera, and feed samples were sent to the Michigan State University Veterinary Diagnostic Laboratory to determine retinol concentrations via saponification, extraction, and quantification as per the AOAC International Official Methods of Analysis (method 2001.13).
Lipids from the liver and sera were saponified with a 40% solution of anhydrous potassium hydroxide in ethanol to reduce vitamin esters to the alcohol form. The vitamins were extracted from the saponification mixture using hexane in liquid–liquid extraction. The hexane was dried under nitrogen, and the vitamins redissolved in the chromatographic mobile phase and placed in autosampler vials. A five-point calibration curve was constructed using the working standards. Stock retinol solution was prepared by adding a retinol standard chromatographic mobile phase. Working solutions were made by diluting the stock in the chromatographic mobile phase to achieve an absorbance of 0.085 to 0.095 at 325 nm. The concentration of the working solution was calculated as retinol (ng/mL) = Abs325/1850 * 10000000 * 0.98. Apocarotenal (R7632; Sigma-Aldrich, St. Louis, MO) was dissolved in methylene chloride to an absorbance of 0.10 to 0.15 at 450 nm to make the working internal standard solution.
Samples were analyzed chromatographically using a Waters Acquity system (Waters Corporation, Milford, MA) and Waters Empower Pro Chromatography Manager software (Waters Corporation, Milford, MA). Elution was isocratic using a mobile phase of acetonitrile:methylene chloride:methanol (70:20:10, v/v/v) and a Symmetry C185, 3.5 µm, 2.1 × 50 mm analytical column (Waters Corporation, Milford, MA). The system also contains a Sentry guard column5, C18, 3.5 µm. The flow rate was 0.5 mL/min and detection was by UV absorption at 325 nm for retinol and 450 nm for apocarotenal. Peak integration was by the ApexTrack method of Empower Pro5. All peaks were reviewed manually after initial autointegration (Waters Corporation, Milford, MA).
Sera retinol concentrations were reported on a ng/mL basis. Liver retinol was reported on a µg/g basis for wet or dry tissue. Values reported on a wet basis were multiplied by 3.33 as a means to convert wet measurements to a dry basis, similar to the comparison discussed in Bryant et al. (2010).
Steers were transported 203 km to a commercial abattoir (Tyson Foods, Amarillo, TX) for harvest on day 119 after undergoing a 7-d observational period to allow for withdrawal from lidocaine. Carcass data were collected by West Texas A&M University Beef Carcass Research Center personnel and included hot carcass weight (HCW), 12th rib fat thickness (FT), longissimus muscle (LM) area, percent kidney–pelvic–heart (KPH) fat, yield grade, and marbling score.
Statistical analyses
Liver retinol and blood parameters were analyzed as a randomized complete block design appropriate for repeated measures using the GLIMMIX procedure of SAS 9.4 (SAS Inst. Inc., Cary, NC), considering pen as the experimental unit. The statistical model for the effect of treatment and days on feed included the fixed effect of treatment, day, and the interaction of treatment × day, while the block was considered a random effect. The model with the lowest Akaike’s information criterion was considered the best fit model for repeated measures analysis.
Carcass traits were also analyzed according to a randomized complete block design using the GLIMMIX procedure of SAS 9.4; the model included the fixed effect of dietary treatment, pen was considered a random effect, and pen served as the experimental unit. Growth performance responses were analyzed according to a randomized complete block design, using the same experiment unit of pen, and fixed and random effects described for carcass data. Least squares means were generated using the LSMEANS statement of SAS. Data were separated and denoted to be different using the pairwise comparisons PDIFF and LINES option of SAS when a significant preliminary F-test was detected. An α level of 0.05 was used to determine the significance, with tendencies discussed at P-values between 0.05 and 0.10.
The REG procedure of SAS was used to evaluate potential relationships between sera and liver concentrations. A simple linear model was initially used to estimate retinol concentration in the liver using sera. There was evidence of nonlinearity detected in the residual analysis of this model; therefore, a logarithmic model was utilized in each of three scenarios: phase 1 (day −91 to 0; short duration depletion), phase 2 (day 0 to 112; repletion), and phases 1 & 2 (day −91 to 112 for the 0IU cattle) as displayed in models 1 through 3:
Results and Discussion
Vitamin A status did not affect the overall average daily gain (ADG) or gain to feed ratio (G:F; P = 0.14 and P = 0.39, respectively); however, DMI for the 0IU cattle was depressed throughout the duration of the study (P = 0.01; Table 2). For the first interim period, ADG had a tendency to differ by treatment (P = 0.07) and did differ during the second interim period (P = 0.02). Additionally, numerical G:F was lower in the 0IU cattle compared with 2,200IU and 11,000IU, but not statistically different (P = 0.16). No differences (P ≥ 0.18) were observed among treatments for HCW, rib eye area (REA), back FT, KPH%, marbling score, or dressing percent (Table 3). However, 2,200IU tended (P = 0.07) to have an increased calculated yield grade compared with 0IU, with 11,000IU being intermediate. Therefore, differences associated with increased vitamin A diets may have been a result of numerical differences in FT. Decreased intake, as well as the minor differences in growth performance, bring doubt to the idea that the cattle were unaffected by vitamin A depletion. No differences between the 2,200IU or 11,000IU treatments were observed. However, this could have been partly due to the inability of the steers to return to or surpass their original vitamin A status, diluting any difference that may have been observed between the treatments with additional dietary vitamin A.
Table 2.
Effects of vitamin A supplementation on feedlot performance
| Treatment1 | ||||||
|---|---|---|---|---|---|---|
| Item2 | 0IU | 2,200IU | 11,000IU | SEM | P-value | |
| Initial BW, kg | 444 | 439 | 444 | 3.4 | 0.80 | |
| Final BW, kg | 557 | 578 | 582 | 7.1 | 0.47 | |
| Day 0 to 112 | ||||||
| ADG, kg | 1.01 | 1.24 | 1.23 | 0.116 | 0.14 | |
| DMI, kg | 8.09b | 9.20a | 9.21a | 0.050 | 0.01 | |
| G:F | 0.125 | 0.135 | 0.134 | 0.0061 | 0.39 | |
| Interim data | ||||||
| Day 0 to 28 | ||||||
| ADG, kg | 1.05 | 1.29 | 1.52 | 0.074 | 0.07 | |
| DMI, kg | 9.00 | 9.67 | 9.70 | 0.058 | 0.12 | |
| G:F | 0.117 | 0.133 | 0.157 | 0.0132 | 0.25 | |
| Day 28 to 56 | ||||||
| ADG, kg | 1.21b | 1.79a | 1.32a | 0.125 | 0.02 | |
| DMI, kg | 8.12b | 9.34a | 9.38a | 0.097 | 0.02 | |
| G:F | 0.149 | 0.192 | 0.141 | 0.0133 | 0.16 | |
| Day 56 to 84 | ||||||
| ADG, kg | 0.92 | 0.75 | 1.01 | 0.086 | 0.30 | |
| DMI, kg | 7.94b | 8.97a | 9.10a | 0.081 | 0.02 | |
| G:F | 0.116 | 0.084 | 0.111 | 0.0095 | 0.17 | |
| Day 84 to 112 | ||||||
| ADG, kg | 0.86 | 1.15 | 1.08 | 0.066 | 0.24 | |
| DMI, kg | 7.29b | 8.82a | 8.66a | 0.111 | <0.01 | |
| G:F | 0.118 | 0.130 | 0.125 | 0.0063 | 0.45 | |
1IU indicates the IU of additional vitamin A per kg diet DM.
2No shrink was applied to interim BW for performance calculations.
a,bMeans within the same row with different superscripts are statistically different (P ≤ 0.05).
Table 3.
Effects of vitamin A supplementation on carcass characteristics
| Treatment1 | |||||
|---|---|---|---|---|---|
| Item2 | 0IU | 2,200IU | 11,000IU | SEM | P-value |
| HCW, kg | 348.3 | 359.2 | 353.6 | 5.28 | 0.79 |
| REA, cm2 | 98.8 | 91.0 | 89.8 | 0.25 | 0.20 |
| FT, cm | 1.07 | 1.54 | 1.30 | 0.040 | 0.18 |
| KPH, % | 1.95 | 1.95 | 2.06 | 0.046 | 0.62 |
| Calculated Yield grade | 2.06 | 2.90 | 2.70 | 0.152 | 0.07 |
| Marbling score3 | 457 | 492 | 469 | 16.2 | 0.79 |
| DP, %4 | 63.77 | 62.32 | 62.80 | 0.004 | 0.36 |
1IU indicates the IU of additional vitamin A per kg diet DM.
2DP, dressing percent.
3400, USDA Small00; 500, USDA Modest00.
4Calculated using HCW/Final BW with a 2% shrink.
In beef cattle, several studies have considered the negative effect of vitamin A on marbling in beef cattle, but the results are inconsistent. Gorocica-Buenfil et al. (2008) demonstrated that low dietary vitamin A (<1,300 compared with 2,700 IU/kg [DM basis]) did not alter ADG, DMI, or G:F. Quality grade tended to increase in the low vitamin A group, but back FT and yield grade were not affected by dietary vitamin A concentration. Bryant et al. (2010) evaluated vitamin A (0; 1,103; 2,205; 4,410; and 8,820 IU/kg [DM basis]), but live growth performance variables were not affected. The vitamin A treatments also had no impact on marbling score, HCW, LM area, back FT, or USDA carcass grades. However, the 2,200IU vitamin A treatment enhanced quality grade and marbling score (Bryant et al., 2010).
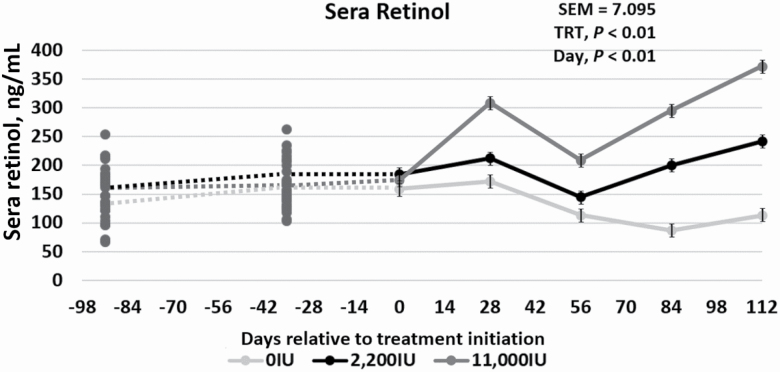
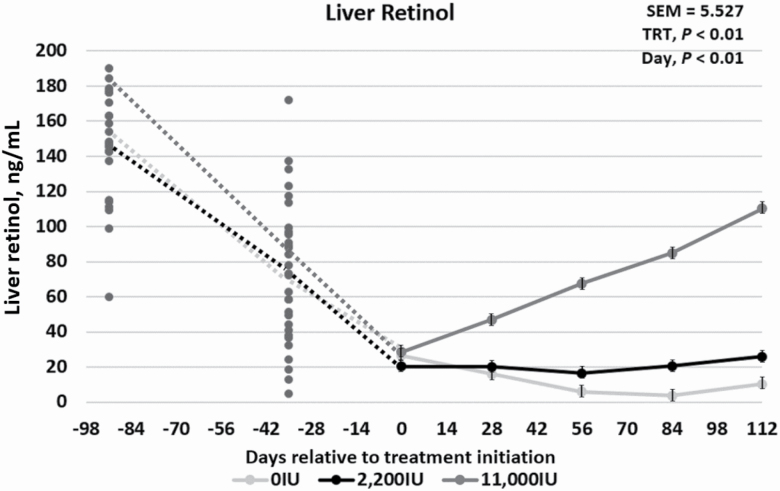
To evaluate the complete vitamin A status of the steers, sera and liver tissue were analyzed for retinol concentrations. A treatment × day interaction occurred for both (P < 0.01) sera retinol and liver retinol during phase 2 of the trial. The sera retinol concentration for the 0IU cattle remained low compared with both the 2,200IU and 11,000IU treatments, which increased over time (Figure 2). In contrast, the liver retinol concentrations for 0IU and 2,200IU remained much lower than the 11,000IU cattle during realimentation (Figure 3). Because the sera concentrations of retinol increased with the 2,200IU inclusion, and liver retinol concentrations did not rebound, it can be hypothesized that dietary vitamin A for those animals was being readily used in the body, rather than being stored. This indicates that 2,200 IU of vitamin A/kg diet DM is a reasonable estimate of animal requirements for vitamin A.
Figure 2.
Effects of vitamin A supplementation on sera retinol concentrations in feedlot steers on days −91, −35, 0, 28, 56, 84, and 112 in relation to treatment initiation (n = 30). Treatments were 0IU, 2,200IU, and 11,000IU. The dotted lines indicate phase 1, a 91-d vitamin A depletion phase prior to treatment allocation or initiation, while the solid lines represent phase 2, where two of the treatments have entered vitamin A repletion. The average sera concentrations were 149.5, 159, and 113 ng/mL for days −91, 0, and 112, respectively.
Figure 3.
Effects of vitamin A supplementation on liver retinol concentrations in feedlot steers on days −91, −35, 0, 28, 56, 84, and 112 in relation to treatment initiation (n = 30). Treatments were 0IU, 2,200IU, and 11,000IU. The dotted lines indicate phase 1, a 91-d vitamin A depletion phase prior to treatment allocation or initiation, while the solid lines represent phase 2, where two of the treatments have entered vitamin A repletion. The average liver concentrations were 145.78, 26.33, and 10.35 µg/g for days −91, 0, and 112, respectively.
Cattle vitamin A sera concentrations normally range between 200 and 300 ng/mL, but vitamin A and carotene can be stored in the fat, liver, and other tissues for later utilization (Bryant et al., 2010). Previous studies have used plasma retinol concentration in several species to indicate vitamin A status, but it only reflects true vitamin A status of the animal when liver stores are almost depleted, and the animal is no longer able to mobilize the various isoforms (Ross et al., 2000; Bryant et al., 2010). The current study supports this conclusion, as the sera levels decreased more steadily as the liver retinol concentration in the 0IU group became more depleted. In another study, plasma vitamin A decreased from initial values of about 30 µg/100 mL to 16 to 18 µg/100 mL in cattle not supplemented with vitamin A (Weichenthal et al., 1963). This decrease occurred to a lesser extent when the cattle were supplemented with about 12,000 IU of vitamin A daily. Liver reserves of vitamin A after the 160-d feeding period were about 1 µg/g in steers not fed vitamin A and about 2 µg/g in steers receiving the additional 12,000 IU vitamin A (Weichenthal et al., 1963). Weichenthal et al. (1963) did not determine initial liver vitamin A content but assumed that there was a gradual depletion in the cattle over time based on the decrease in plasma vitamin A (Weichenthal et al., 1963). Data from the current study, in conjunction with those of Weichenthal et al. (1963), indicate that 2,200 IU/kg of diet DM is the true vitamin A requirement. Both the sera and liver concentrations in the 2,200IU group, which consumed an average of 20,240 IU of vitamin A per animal daily, remained relatively constant throughout phase 2, indicating some instances of metabolic homeostatis regarding vitamin A for cattle fed 2,200 IU of vitamin A/kg diet DM. However, animals fed diets without additional vitamin A were able to somewhat maintain circulating levels of sera retinol comparable to animals fed 2,200IU for approximately 56 d into the feeding period, when the liver retinol concentration truly approached zero. Variation in sera data compared with those for liver retinol values suggests that the prediction of an animal’s actual vitamin A status may not be accurate if estimated solely from circulating blood concentrations.
Between 50% and 80% of body vitamin A is found in the liver, 90% of which is stored within hepatic stellate cells as retinyl esters in lipid droplets, while the remaining percentage is distributed among the kidneys, lungs, adrenals, and blood (Blomhoff et al., 1985). This distribution of vitamin A varies across species, and in some species, the kidneys and adipose tissue are known to store large amounts of vitamin A as well (Blomhoff et al., 1985). Depletion of vitamin A after the cessation of vitamin A and carotenoid intake varies between classes of cattle, ranging from as little as 40 d in light feeder steers to as many as 150 d in mature cows (Sewell, 1993). Knight et al. (1996) noted that the blood carotenoid pool was about twice that of the liver carotenoid concentration for cattle on pasture, but after the cattle consumed rations devoid of carotenoids with or without vitamin A, the carotenoid concentrations in the blood and liver equalized. In a different study, the liver vitamin A content ranged from 1.1 to 6.7 µg retinol equivalents (RE)/g in cattle eating various diets with low-vitamin A contents, with an average of 4.1 ± 1.72 µg RE/g (Majchrzak et al., 2006). Cattle fed commercially formulated diets had the least amount of variation across the group, which indicated that vitamin A supplementation and appropriate incorporation are necessary to maintain vitamin A status in cattle (Majchrzak et al., 2006). Liver biopsy studies have shown that 133 µg of retinol/g of liver tissue on a DM basis can sustain cattle for up to 120 d on a vitamin A-deficient feedlot diet (Bryant et al., 2010). Similarly, the 0IU cattle in the current study had an initial liver concentration of 145.8 µg of retinol/g. After consuming a vitamin A-deficient diet for 147 to 175 d, the liver retinol concentration for 0IU cattle approached zero, and the sera retinol concentration approached 100 ng/mL.
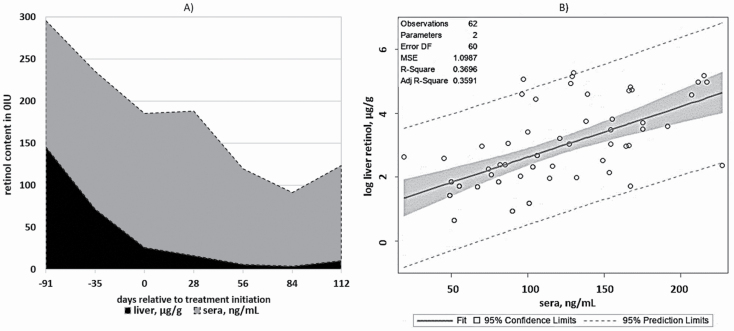
The relationship between liver retinol and sera retinol was evaluated by the phases of the study (Table 4). Phase 1, or the depletion phase, used all the steers (n = 30) to investigate the probability of estimating liver retinol concentrations based on the retinol concentrations of the sera. The model only accounted for 4.4% of the variation in liver retinol concentration (R2 = 0.044; P = 0.06). Liver and sera retinol concentrations from the 0IU treatment were evaluated throughout the duration of phases 1 and 2, allowing the model to account for a greater period of time and vitamin A status fluctuation (Figure 4). This resulted in a more appropriate model (P < 0.01; R2 = 0.370), estimating that a one-unit change of the sera would be associated with a 1.598% change in liver concentrations. The vitamin A feeding period was also used to investigate the sera–liver retinol relationship. In addition to sera, the treatments, 0IU, 2,200IU, and 11,000IU, were incorporated into the repletion model, resulting in the best estimation of changes in the liver retinol (P < 0.01; R2 = 0.682). These data suggested that the detection of an animal with critically low vitamin A concentrations may be difficult using circulating concentrations because for normal functions, animals must maintain their circulating levels of nutrients. In spite of low or no vitamin A in the diet, the 0IU group displayed no symptoms of night blindness or any other clinical symptoms associated with vitamin A deficiency. Contrariwise, sera did respond to supranutritional concentrations of dietary vitamin A, regardless of the fact that they did not return to their initial vitamin A status. The increase in liver concentrations for 11,000IU was reflected in the sera. A study by Montreewasuwat and Olson (1979) discovered no correlation between serum retinol concentrations and liver concentrations unless the value was below 5 µg/g. Additionally, serum retinol concentrations did not serve as an indicator of liver stores of vitamin A. This supports the current conclusion that sera would be the most representative measurement for vitamin A concentration available to tissue, regardless of plummeting values in the liver.
Table 4.
Relationship between liver and sera retinol in various feeding phases
| Phase1 | Day | β 1 value | β 2 value | R 2 value | P-value |
|---|---|---|---|---|---|
| 12 | Day −91 to 0 | −0.00468 | — | 0.0438 | 0.059 |
| 23 | Day 0 to 112 | 0.00220 | 0.0001618 | 0.6821 | <0.001 |
| 1 and 24 | Day −91 to 112 | 0.01586 | — | 0.3696 | <0.001 |
1Feeding phases required different models for the best fit.
2
3
4
Figure 4.
Area plot of liver retinol (µg/g) and sera retinol (ng/mL) concentrations of 0IU vitamin A/kg diet DM (n = 10) to indicate the average change in respective concentrations over time (A). The average liver concentrations were 145.78, 26.33, and 10.35 µg/g for days −91, 0, and 112, respectively. The average sera concentrations were 149.5, 159, and 113 ng/mL for days −91, 0, and 112, respectively. Regression plot for model 3: (B). This plot was developed to investigate the ability to use sera retinol concentrations to predict the liver vitamin A concentration in cattle.
Conclusions
Vitamin A is an essential component of the diet for maintaining healthy feedlot animals. High vitamin A supplementation did not negatively impact the carcass quality of vitamin A-depleted steers, but animals fed low vitamin A diets suffered from depressed intake. However, animals fed diets without additional vitamin A were able to maintain circulating levels of sera retinol until liver retinol concentrations approached 0 µg/g. No differences were observed between steers consuming the recommended dietary inclusion of vitamin A or those consuming a supranutritional quantity. Feeding high levels of vitamin A in feedlot should be further investigated, focusing on maximizing vitamin A storage, rather than depletion. Although not the focus of the current study, the possible role of adipose tissue as a storage site for vitamin A and its effects on circulation should also be evaluated. The sera and liver concentration changes of steers fed moderate and high inclusions of vitamin A reflected the quantity of vitamin A in the diet. A model was developed, based on this small sample of cattle, to estimate a percent change in the liver concentration of retinol based on unit changes in the sera. The relationship between sera and liver vitamin A in steers with depleted vitamin A status fed diets with low vitamin A concentration was more difficult to evaluate, as the sera retinol concentrations changed little in comparison to liver retinol concentration.
Glossary
Abbreviations
- ADG
average daily gain
- BW
body weight
- CON
control
- CP
crude protein
- DM
dry matter
- DMI
dry matter intake
- DP
dressing percent
- G:F
gain to feed ratio
- HCW
hot carcass weight
- KPH
kidney, pelvic, heart fat
- LM
longissimus muscle
- RE
retinol equivalent
- REA
ribeye area
Conflict of interest statement
None of the authors involved in this research have any conflicts of interest to report.
Literature Cited
- Blomhoff R, Rasmussen M, Nilsson A, Norum K R, Berg T, Blaner W S, Kato M, Mertz J R, Goodman D S, and Eriksson U. . 1985. Hepatic retinol metabolism. Distribution of retinoids, enzymes, and binding proteins in isolated rat liver cells. J. Biol. Chem. 260:13560–13565. [PubMed] [Google Scholar]
- Bryant T C, Wagner J J, Tatum J D, Galyean M L, Anthony R V, and Engle T E. . 2010. Effect of dietary supplemental vitamin A concentration on performance, carcass merit, serum metabolites, and lipogenic enzyme activity in yearling beef steers. J. Anim. Sci. 88:1463–1478. doi: 10.2527/jas.2009-2313 [DOI] [PubMed] [Google Scholar]
- Chapman H, Cox D, Haines C, Davis G. . 1963. Evaluation of the liver biopsy technique for mineral nutrition studies with beef cattle. J. Anim. Sci. 22(3):733–737. doi: 10.2527/jas1963.223733x [DOI] [Google Scholar]
- Gorocica-Buenfil M A, Fluharty F L, and Loerch S C. . 2008. Effect of vitamin A restriction on carcass characteristics and immune status of beef steers. J. Anim. Sci. 86:1609–1616. doi: 10.2527/jas.2007-0241 [DOI] [PubMed] [Google Scholar]
- Kawada T, Kamei Y, Fujita A, Hida Y, Takahashi N, Sugimoto E, and Fushiki T. . 2000. Carotenoids and retinoids as suppressors on adipocyte differentiation via nuclear receptors. Biofactors 13:103–109. doi: 10.1002/biof.5520130117 [DOI] [PubMed] [Google Scholar]
- Kim J, Wellmann K B, Smith Z K, and Johnson B J. . 2018. All-trans retinoic acid increases the expression of oxidative myosin heavy chain through the PPARδ pathway in bovine muscle cells derived from satellite cells. J. Anim. Sci. 96:2763–2776. doi: 10.1093/jas/sky155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight T, Death A, Muir P, Ridland M, and Wyeth T. . 1996. Effect of dietary vitamin A on plasma and liver carotenoid concentrations and fat color in Angus and Angus crossbred cattle. New Zeal. J. Agri. Res. 39(2): 281–292. doi: 10.1080/00288233.1996.9513187 [DOI] [Google Scholar]
- Kohlmeier R H, and Burroughs W. . 1970. Estimation of critical plasma and liver vitamin A levels in feedlot cattle with observations upon influences of body stores and daily dietary requirements. J. Anim. Sci. 30:1012–1018. doi: 10.2527/jas1970.3061012x [DOI] [PubMed] [Google Scholar]
- Majchrzak D, Fabian E, and Elmadfa I. . 2006. Vitamin A content (retinol and retinyl esters) in livers of different animals. Food Chem. 98:704–710. doi: 10.1016/j.foodchem.2005.06.035 [DOI] [Google Scholar]
- Montreewasuwat N, and Olson J A. . 1979. Serum and liver concentrations of vitamin A in Thai fetuses as a function of gestational age. Am. J. Clin. Nutr. 32:601–606. doi: 10.1093/ajcn/32.3.601 [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine (NASEM). 2016. Nutrient requirements of beef cattle. Washington, DC: National Academies Press. [Google Scholar]
- National Research Council. NRC 1996. Nutrient requirements of beef cattle. [Google Scholar]
- Ohyama M, Matsuda K, Torii S, Matsui T, Yano H, Kawada T, and Ishihara T. . 1998. The interaction between vitamin A and thiazolidinedione on bovine adipocyte differentiation in primary culture. J. Anim. Sci. 76:61–65. doi: 10.2527/1998.76161x [DOI] [PubMed] [Google Scholar]
- Oka A, Maruo Y, Miki T, Yamasaki T, and Saito T. . 1998. Influence of vitamin A on the quality of beef from the Tajima strain of Japanese Black cattle. Meat Sci. 48:159–167. doi: 10.1016/s0309-1740(97)00086-7 [DOI] [PubMed] [Google Scholar]
- Pickworth C L, Loerch S C, and Fluharty F L. . 2012. Restriction of vitamin A and D in beef cattle finishing diets on feedlot performance and adipose accretion. J. Anim. Sci. 90:1866–1878. doi: 10.2527/jas.2010-3590 [DOI] [PubMed] [Google Scholar]
- Ross S A, McCaffery P J, Drager U C, and De Luca L M. . 2000. Retinoids in embryonal development. Physiol. Rev. 80:1021–1054. doi: 10.1152/physrev.2000.80.3.1021 [DOI] [PubMed] [Google Scholar]
- Samuelson K L, Hubbert M E, Galyean M L, and Löest C A. . 2016. Nutritional recommendations of feedlot consulting nutritionists: the 2015 New Mexico State and Texas Tech University survey. J. Anim. Sci. 94:2648–2663. doi: 10.2527/jas.2016-0282 [DOI] [PubMed] [Google Scholar]
- Sewell H. 1993Vitamins for beef cattle. Extension Publications (MU), Columbia (MO): University of Missouri. [Google Scholar]
- Vasconcelos J T, and Galyean M L. . 2007. Nutritional recommendations of feedlot consulting nutritionists: the 2007 Texas Tech University survey. J. Anim. Sci. 85:2772–2781. doi: 10.2527/jas.2007-0261 [DOI] [PubMed] [Google Scholar]
- Weichenthal B, Embry L, Emerick R, and Whetzal F. . 1963. Influence of sodium nitrate, vitamin A and protein level on feedlot performance and vitamin A status of fattening cattle. J. Anim. Sci. 22(4):979–984. doi: 10.2527/jas1963.224979x [DOI] [Google Scholar]