Abstract
Purpose
Long-term non-invasive ventilation (NIV) is recommended for patients with stable chronic obstructive lung disease (COPD) and chronic hypercapnia. High inspiratory pressure NIV (hiNIV) and a significant reduction of arterial pCO2 have been shown to prolong survival. Often, patients on hiNIV describe severe respiratory distress, known as “deventilation syndrome”, after removal of the NIV mask in the morning. Mechanical pursed lips breathing ventilation (PLBV) is a new non-invasive ventilation mode that mimics the pressure-curve of pursed lips breathing during expiration. The clinical impact of switching patients from standard NIV to PLBV has not been studied so far.
Patients and methods
In this hypothesis generating study, we retrospectively analysed the effects of switching COPD patients (stage GOLD III-IV) from conventional NIV to PLBV. Medical records of all patients who had an established NIV and were switched to PLBV between March 2016 and October 2017 were screened. Patients were included if they complained of shortness of breath on mask removal, used their conventional NIV regularly, and had a documented complete diagnostic workup including lung function testing, blood gas analysis and 6-minute walk test (6MWT) before and after 3–7 days of PLBV.
Results
Six male and 10 female patients (median age 65.4 years; IQR 64.0–71.3) with a previous NIV treatment duration of 38 months (median; IQR 20–42) were analysed. After PLVB initiation, the median inspiratory ventilation pressure needed to maintain the capillary pre-switch pCO2 level was reduced from 19.5 mbar (IQR 16.0–26.0) to 13.8 mbar (IQR 12.5–14.9; p<0.001). The median 6MWT distance increased from 200m (IQR 153.8–266.3) to 270m (IQR 211.3–323.8; p<0.001). Median forced vital capacity (FVC) increased from 49.5% to 53.0% of the predicted value (p = 0.04), while changes in FEV1 and residual volume (RV) were non-significant.
Conclusion
Based on this small retrospective analysis, we hypothesise that switching patients with COPD GOLD III-IV and chronic hypercapnia from conventional NIV to PLBV may increase exercise tolerance and FVC in the short term.
Introduction
After non-invasive ventilation (NIV) was established in the 1980s for acute ventilatory failure, it is now also recommended as a long-term treatment for patients with stable chronic obstructive pulmonary diseases (COPD) and concomitant chronic hypercapnia in an outpatient setting [1]. This is based on numerous studies that demonstrated an improvement in the quality of life of patients with advanced COPD and stable hypercapnic respiratory failure [2–6]. In these patients, mortality rates can be reduced when high ventilation pressure is applied and a significant reduction in arterial pCO2 is achieved [7]. A task force of the European Respiratory Society therefore suggests in a recent guideline, that long-term home NIV should be titrated to normalize or reduce pCO2 levels in patients with COPD, but also acknowledges that the certainty of evidence for this recommendation is very low [8]. The term high intensity non-invasive ventilation (hiNIV) has been established for this ventilation method [9]. In patients with advanced COPD treated with NIV, the removal of the ventilation mask after nightly ventilation may result in shortness of breath, which has been described as deventilation syndrome [10]. A possible cause of the deventilation syndrome may be dynamic hyperinflation due to high inspiratory pressure of NIV.
Although high inspiratory pressures are applied in order to reduce hypercapnia, a recent study found no correlation between pCO2 reduction and overall survival in this population [11]. This triggered a debate about the validity of chronic hypercapnia as the sole therapeutic indication for long-term NIV [11].
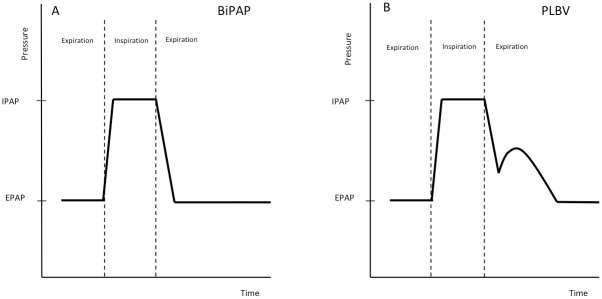
For the treatment of hypercapnia in patients with COPD, a variety of ventilation modes are used but no significant superiority of any mode has been demonstrated so far [12]. Since 2016, another ventilation mode is available through the NIV device "Vigaro" (FLO Medizintechnik GmbH, Melle, Germany). This device works in a time controlled or spontaneously triggered Bilevel Positive Airway Pressure (BiPAP) mode, but also mimics the pressure curve of spontaneous pursed lips breathing during the expiration phase (Fig 1).
Fig 1. Pressure and flow characteristics of BIPAP and PLBV ventilation modes.
Schematic pressure curve of a BiPAP ventilation (A) compared to ventilation in PLBV mode (B). BiPAP: Bilevel positive airway pressure; IPAP: inspiratory positive airway pressure; EPAP: expiratory positive airway pressure; PLBV: pursed lips breathing ventilation.
This pressure curve results from two parameters to be adjusted in the device. The first parameter is called pursed lips breathing delay (PLBD) which determines the point in time at which the pursed lips breathing pressure starts after the onset of the expiration (range of delay: 0.0 to 0.4 sec). The second parameter is called pursed lips breathing pressure (PLBP), which determines the increase in pressure (range of altitude: 0.1 to 4.0 mbar) from the current pressure level. Typically, at low inspiratory positive airway pressure (IPAP) values, short PLBD and low PLBP are set, at higher IPAP values, longer PLBD and higher PLBP are set.
After the PLBP is reached, the pressure decay is adjusted to the respiratory rate and reaches EPAP in the last quarter of the expiration phase.
In this retrospective observational cohort study, we evaluated the effect of switching patients with stable hypercapnic COPD and established NIV to PLBV on exercise tolerance and lung function testing and hypothesized, that the minimization of NIV-induced hyperinflation by PLBV leads to an improvement of the clinical condition.
Material and methods
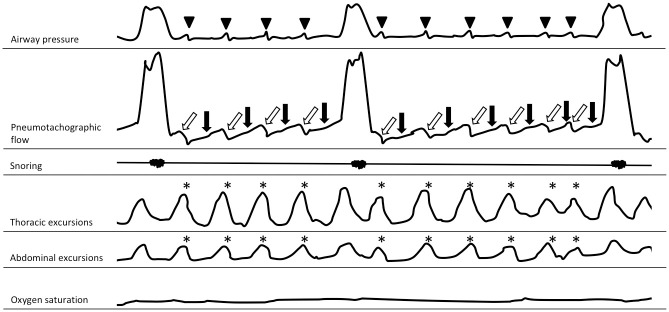
This hypothesis generating, retrospective, monocentric analysis included consecutive patients with hypercapnic COPD on NIV at the Medical Clinic, Research Center Borstel, Germany. All patients with established NIV prior to hospital admission who were switched to PLBV between March 1st, 2016 and October 31st, 2017 were screened for eligibility. Subjects were included in the analysis if they had a diagnosis COPD GOLD stage III or IV without current exacerbation, had a bilevel-NIV therapy that was established and regularly used for at least 4 months, complained of shortness of breath on mask removal after their nightly ventilation, had evidence of hypercapnia (pCO2>55 mbar) in a capillary blood gas analysis obtained recently or prior to NIV initiation, and had a complete diagnostic workup prior to and 3–7 days after PLBV application documented in their medical records. This workup included a 6-minute walk test (6MWT), nocturnal capillary blood gas analysis between 4.00 and 6.00 am under NIV, nocturnal polysomnography (PSG) including pneumotachography (PT) and spirometry with bodyplethysmography. The bodyplethysmograph "Masterscreen" (Jäger/CareFusion, Würzburg, Germany) was used to measure the lung function. Polysomnographic controls were conducted in a sleep laboratory certified according to the guidelines of the German Society for Sleep Research and Sleep Medicine. Respiratory flow was measured with the pneumocontrol sensor of a pneumoflow pressure transducer (ResMed, Martinsried, Germany) that registers flow signals on the principles of a pneumotachograph, but does not require calibration. "Rembrandt" software (Medcare, Iceland) was used to evaluate the polysomnographic information that is routinely used for the therapy adjustment of NIV. PSG allows the detection of AutoPEEP phenomena, as described earlier for invasive ventilation (Fig 2) [13].
Fig 2. AutoPEEP phenomena.
Schematic illustration of a severe AutoPEEP phenomena with repetitive AutoPEEP related frustrating inspirational efforts registered by polysomnography in a patient ventilated in an assisted mode. Inspirational efforts of the thorax and abdomen occur regularly (asterisk). No inspiration signal in the pneumotachographic channel is triggered, but expiratory flow is slowed (black arrows) and then accelerated (white arrows). The pressure channel registers a small peak (arrow head), resulting from a boosted expiratory flow.
An AutoPEEP phenomenon was defined as a pneumotachographic signal due to an inspirational effort of the patient without inspiration but instead reduction of the expirational airflow: In the late phase of expiration, it is often associated with a small short-term peak in the pressure channel and represents a boosted exhalation flow (Fig 2).
The settings of the PLBV were optimized to the lowest possible capillary pCO2 at which no AutoPEEP phenomena occurred. A rising pCO2 triggered an increase of IPAP. AutoPEEP phenomena triggered a decrease of IPAP. After patients were switched to PLBV, ventilation was monitored at night using polysomnography (PSG) including pneumotachography (PT) aiming to detect AutoPEEP (see Fig 2). If AutoPEEP phenomena were still present, the ventilation parameters were further optimized and monitored again by nightly PSG including PT. In most cases, a good NIV adjustment was achieved within 3–4 nights, rarely longer and once only after 7 nights. Follow-up investigations (lung function, blood gas analysis, 6MWT) were only performed after NIV was well adjusted.
COPD assessment test (CAT, GlaxoSmithKline Services Unlimited, Brentford, Middlesex, United Kingdom) as a routinely used surrogate parameter for disease-related quality of life was only available in 4 cases both before and after the NIV switch.
All patients included in this study agreed to the local broad consent of the BioMaterialBank Nord (University of Lübeck, Ethics committee AZ14-225). Use of patient related data for the present study was approved by the University of Lübeck, Ethics committee AZ 16–185).
Statistical analysis
Data were analysed with the statistical program R version 3.5.1, open source software [14]. The Shapiro-Wilk-test was used to determine normal distribution. A paired sample t-test was used to compare variables whose difference between baseline and follow-up showed a normal distribution. The paired Wilcoxon signed-rank test was used as a non-parametric alternative. Effect sizes were calculated using the Cohens d method for normally distributed values and the Vargha & Delaney method for non-normally distributed parameters. For Cohens d, 0.5–0.8 indicates a medium effect size, >0.8 indicates a large effect size. For Vargha & Delaney, 0.64–0.71 indicates a medium effect size, >0.71 indicates a large effect size. Based on the effect sizes and an assumed power of 0.8 at a level of significance at 0.05 the following sample sizes were calculated: IPAP, n = 8 persons; 6MWT, n = 15 persons; FVC, n = 467 persons. Furthermore, ordinary least square regression (OLS) was used to test for correlations between the variables of interest and confounding factors.
Results
Between March 1st, 2016 and October 31st, 2017, 24 patients were identified who received PLBV and had a full diagnostic data set available. From these, eight patients were excluded; two due to confounding pulmonary diagnoses (one with pulmonary fibrosis, one with α1-antitrypsin deficiency), two due to an ongoing acute exacerbation of the COPD and four did not have well established or guideline-based home ventilation before PLBV (Fig 3).
Fig 3. Flowchart of the study.
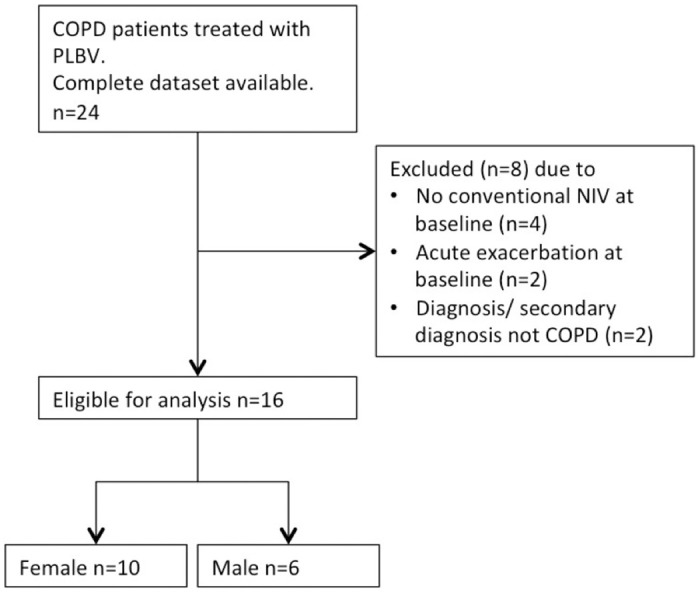
Abbreviations: NIV, non-invasive ventilation.
Data were analysed from 16 patients (Table 1). 6 male (37.5%) 10 female (62.5%) with a median BMI of 25.8 kg/m2 (Interquartile range, IQR 22.7–27.5). All patients lived in their own housing, took inhaled and oral medication in accordance with the current GOLD guidelines and were treated with long-term oxygen therapy, with a median of 2.0 L/min (IQR 1.8–2) that was continued throughout the hospital admission. Patients received NIV therapy for a median of 38 months prior to PLBV initiation (range 4–101 months). For n = 10 patients, categorical data describing exacerbation frequency during the preceding 12 months was available: 2 patients (20%) had no exacerbation, 4 patients (40%) had 1–3 exacerbations and 4 patients (40%) had more than 3 exacerbations.
Table 1. Patient characteristics.
| Parameter | All patients (n = 16) |
|---|---|
| Age [years], median (IQR) | 65.4 (64.0–71.3) |
| Sex, n (%) | Male: 6 (37.5) Female: 10 (62.5) |
| BMI [kg/m2], median (IQR) | 25.8 (22.7–27.5) |
| NT-proBNP [pg/ml], median (IQR) | 65.5 (55.0–160.0) |
| Duration of NIV therapy prior to PLBV [months], median (IQR). | 38 (20–42) |
Abbreviations: BMI, body mass index; NT-proBNP, n-terminal pro brain natriuretic peptide; IQR, interquartile range.
At baseline, the median IPAP was 19.5 mbar (IQR 16.0–26.0), while the EPAP was 6 mbar (IQR 4.8–9.0). As listed in Table 2, the median IPAP was significantly lowered to 13.8 mbar (IQR 12.5–14.9) (p<0.001, Cohens d 1.26), EPAP remained at 6.0 mbar (IQR 5.0–7.0) (p = 0.175). This pressure changes (ΔIPAP) led to various changes in pCO2 (ΔpCO2) in some subjects, but did not result in a significant change of the median nocturnal pCO2, with a median of 46.9 mmHg (IQR 43.8–55.7) at baseline and 50.0 mmHg (45.9–59.5) at follow-up (p = 0.303). ΔpCO2 for each patient is shown in Fig 4A. Fig 4B shows the correlation between ΔpCO2 and ΔIPAP (r = -0.21), which was not significant with β = -0.23 (p = 0.436). The adjusted coefficient of determination of R2 = -0.02 also underlines the lack of impact of ΔIPAP on ΔpCO2.
Table 2. Ventilatory and clinical parameters at baseline and follow-up.
| Parameter | Baseline | Follow up | p-value |
|---|---|---|---|
| Ventilation | |||
| IPAP [mbar], median (IQR) | 19.5 (16.0–26.0) | 13.8 (12.5–14.9) | <0.001* |
| EPAP [mbar], median (IQR) | 6.0 (4.8–9.0) | 6.0 (5.0–7.0) | 0.175 |
| Oxygen supplementation | |||
| oxygen rate on NIV [L/min], median (IQR) | 2.0 (0.8–2.0) | 1.0 (0.9–2.0) | 0.722 |
| oxygen rate off NIV [L/min), median (IQR) | 2.0 (1.8–2) | 2.0 (0.8–2) | 0,371 |
| Capillary blood gas analysis¶ | |||
| pH, median (IQR) | 7.41 (7.40–7.43) | 7.41 (7.39–7.42) | 0.477 |
| pCO2 [mmHg], median (IRQ) | 46.9 (43.8–55.7) | 50.0 (45.9–59.5) | 0,303 |
| pO2 [mmHg], median (IRQ) | 76.5 (73.0–81.4) | 75.2 (70.7–81.0) | 0.713 |
| Base excess [mmol], median (IQR) | 5.2 (3.8–10.7) | 5.4 (3.5–10.2) | 0.900 |
| Exercise tolerance | |||
| 6MWT distance [m], median (IQR) | 200 (153.8–266.3) | 270 (211.3–323.8) | <0.001* |
| BORG scale before 6MWT, median (IQR) | 3 (1.5–3.75) | 3 (2.25–3) | 0.947 |
| BORG scale after 6MWT, median (IQR) | 7.5 (5.625–8.625) | 6 (5–7) | 0.191 |
| Lung function tests | |||
| FEV1 [% predicted], median (IQR) | 24.5 (20.8–32.1) | 27.3 (20.8–31.3) | 0.203 |
| FVC [% predicted], median (IQR) | 49.5 (39.0–57.0) | 53.0 (41.8–60.8) | 0.040* |
| FVC [l], median (IQR) | 1.6 (1.3–2.1) | 1.8 (1.3–2.4) | 0.050* |
| FEV1/FVC [%], median (IQR) | 41.5 (35.1–47.4) | 38.6 (34.0–42.9) | 0.163 |
| VCin[l], median (IQR) | 1.6 (1.3–2.3) | 2.0 (1.3–2.7) | 0.148 |
| TLC [% predicted), median (IQR) | 120.1 (116.8–149.3) | 140.0 (110.3–156) | 0.717 |
| ITGV [l], median (IQR) | 6.7 (5.5–7.3) | 6.6 (5.5–7.4) | 0.774 |
| VCin/TLC, mean (IQR) | 0.3 (0.17–0.27) | 0.3 (0.21–0.32) | 0.177 |
| Reff [kPa×s×l−1], median (IQR) | 1.1 (0.4–1.2) | 0.8 (0.7–1.1) | 0.027* |
| Quality of life | |||
| CAT [score], median (IQR) | 26.5 (24.8–31.0) | 17 (11.3–22.5)§ | n.a. |
§At follow-up, only n = 4 patients competed the CAT questionnaire. Thus, no p-value is given.
¶Blood gas analysis was performed under oxygen supplementation in patients who had an established long-term oxygen therapy.
Abbreviations: IPAP, Inspiratory Positive Airway Pressure; EPAP, Expiratory Positive Airway Pressure; IQR, interquartile range; pCO2, partial pressure of carbon dioxide; pO2, partial pressure of oxygen; BE, Base Excess; 6MWT, 6 minute walk test: FEV1, Forced Expiratory Volume in One Second; FVC, Forced Vital Capacity; VCin, inspiratory vital capacity; TLC, total lung capacity; ITGV, intrathoracic gas volume; Reff, effective respiratory resistance.; CAT, COPD Assessment Test; n.a., not applicable.
Fig 4. pCO2 values at baseline and follow-up and correlation of ΔpCO2 with ΔIPAP.
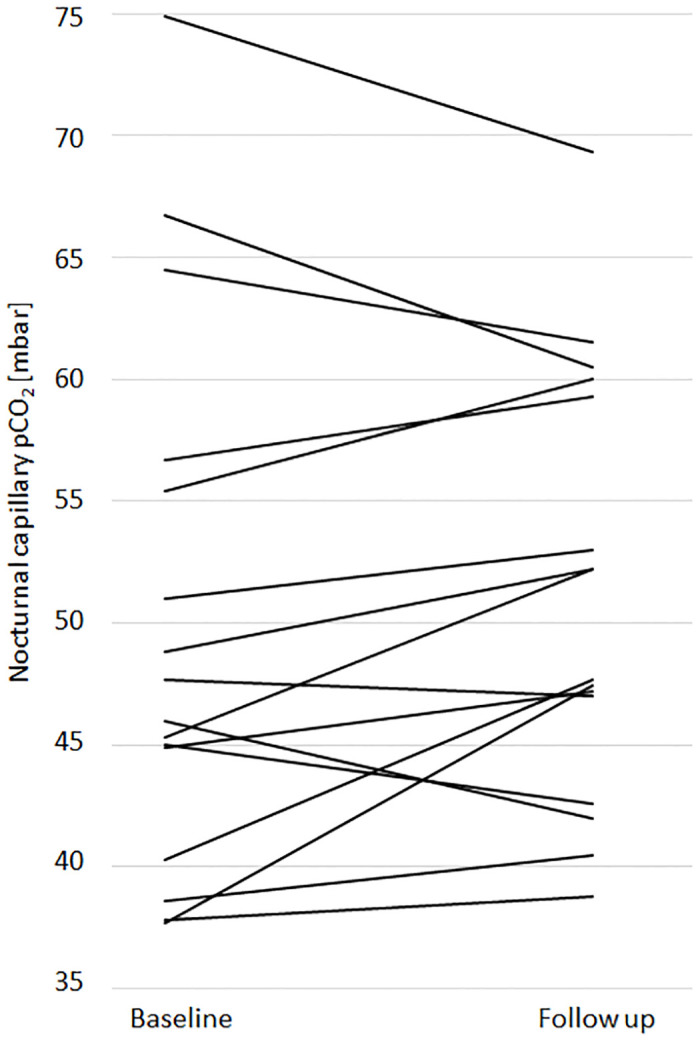
A. Each line represents an early morning pCO2 value for a single patient at baseline and follow up. B. Scatter plot illustrating the correlation of ΔpCO2 (pCO2 at follow-up—pCO2 at baseline) and ΔIPAP (IPAP at follow-up—IPAP at baseline).
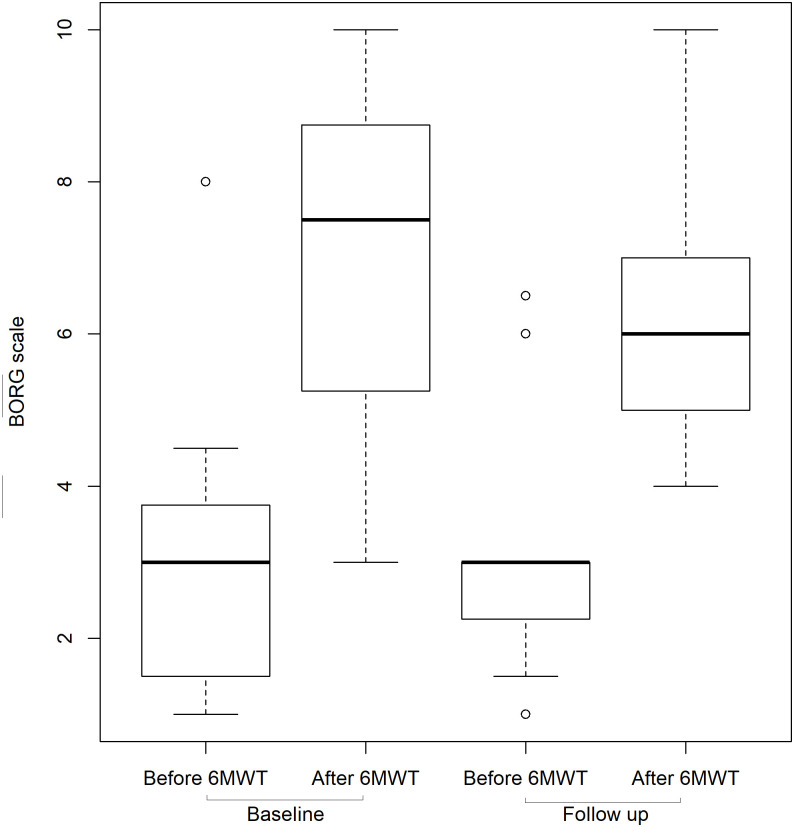
The median walking distance in the 6MWT increased from 200m (IQR 153.8–266.3) to 270m (IQR 211.3–323.8) (Fig 5A). This median improvement of 70 m was highly significant (p<0.001, Cohens d 0.80). Patients reported less dyspnoea on exercise, although the changes in the BORG scale were not statistically significant. During the 6MWT, the median BORG scale increased from 3 to 7.5 prior to PLBV initiation and from 3 to 6 after the switch to PLBV as illustrated in Fig 6 (p = 0.19, Vargha & Delaney 0.64). As shown in Fig 5B, no correlation was found between ΔIPAP and the increase in walking test performance (r = 0.27). Increases in walking test performance of more than 100 meters were found for both small and large changes in IPAP. However, this effect was not significant with β = 3.33 (p = 0.304) and still seems to have a small effect on the variance of walking test performance difference (adjusted R2 = 0.01).
Fig 5. 6MWT distances at baseline and follow-up and correlations of Δ6MWT with ΔIPAP and ΔpCO2.
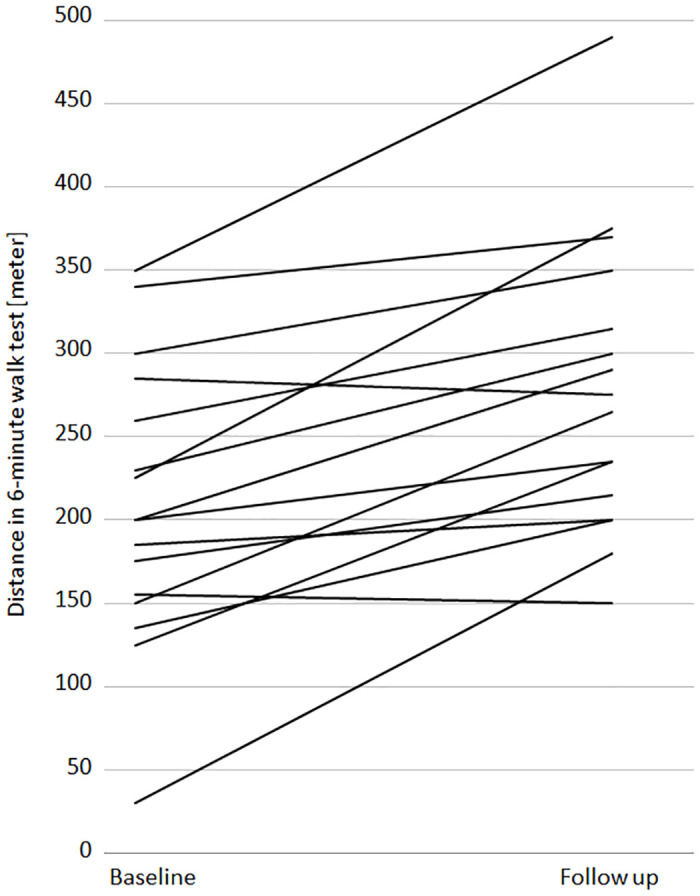
A. Walking distances in the 6MWT at baseline and at follow-up. Each line represents a patient. B. Scatter plot illustrating the correlation of Δ6MWT (6MWT at follow-up– 6MWT at baseline) and ΔIPAP (IPAP at follow-up—IPAP at baseline). C. Scatter plot illustrating the correlation of Δ6MWT (6MWT at follow.up– 6MWT at baseline) and ΔpCO2 (pCO2 at follow-up—pCO2 at baseline).
Fig 6. Exercise induced dyspnoea at baseline and follow up.
Median BORG scale values before and after 6MWT. Baseline values were recorded before PLBV initiation; follow up values 3–7 days after PLBV initiation. Boxplots show median, interquartile range and range of dataset. Dots represent outliers. Abbreviations: 6MWT, 6 minutes walking test.
And as shown in Fig 5C there was also no correlation between the extent of walking distance increase and pCO2 changes (r = 0.04). OLS also confirms this missing correlation with a non-significant b = -0.41 (p = 0.894), and an explanation of variance of ΔpCO2 on the walking test performance is not given with an adjusted R2 of -0.07.
Lung function showed a significant improvement in vital capacity (FVC) from median of 1.6 L (49.5%predicted) to 1.8 L (53.0%predicted) (p = 0.05 and p = 0.04, Cohens d 2.42). The improvement of the median effective airway resistance (Reff) was also significant. It improved from 1.1 kPa*s*L-1 to 0.8 kPa*s*L-1 (p = 0.027, Vargha & Delaney 0.64). Other lung functional parameters showed a tendency but no significant change.
In a few patients (n = 4), data were available from the CAT questionnaires. In these patients the median result showed an improvement of 26.5 (IQR 24.8–31.0) to 17.0 (IQR 11.0–22.5) points (p = n.a.).
Discussion
In this retrospective study, we describe quantifiable and clinically significant effects of switching COPD patients with shortness of breath on discontinuation of their nightly hiNIV to PLBV.
Most striking, the ventilation pressure decreased from a median of 19.5 mbar to 13.8 mbar without a significant increase in the median pCO2. Although individual changes in pCO2 were observed, these changes did neither correlate with the extent of pressure reduction nor with the result of the 6MWT. A plausible explanation for this phenomenon may be that PLBV enables deeper and longer expiration. This may lead to a lower end-expiratory intrathoracic gas volume (ITGV), improved diaphragmatic relaxation, and thus larger tidal volumes. Alternatively, PLBV improves the distribution pattern of ventilated air. Most likely, hiNIV leads to heterogeneous AutoPEEP and local areas of high PEEP associated with inadequate ventilation. Since PLBV is striving to avoid AutoPEEP, these heterogeneously ventilated areas may be minimized. First attempts to support this hypothesis through the use of electrical impedance tomography (EIT) appear to augment this theory (data not published).
The main objective of PLBV was to avoid AutoPEEP phenomena. Nevertheless, the implementation of PLBV led to variable changes in capillary pCO2, with a reduction in some patients, while others showed an increase or no change. Of note, in patients with higher initial pCO2 values, PLBV often led to a pCO2 reduction while an increase was seen in patients with lower initial pCO2 values. One patient had an increase in pCO2 of 7.4 mbar (from 40,3 to 47,7 mbar), another patient an increase of 9.7 mbar (from 37,7 to 47,4 mbar). Despite this laboratory finding, their 6MWT walking distance increased from 230 meters to 300 meters and from 150 meters to 265 meters, respectively. There was no correlation between pCO2 and physical exercise tolerance in our cohort, suggesting an alternative mechanism.
Another striking result was the highly significant increase in 6-minute walking distance by 70 metres within less than a week after switching to PLBV. In two patients only, the 6MWT distance decreased after PLBV initiation. One patient discontinued the test due to knee pain. He walked 285 meters (BORG 7) before the PLBV, and 275 meters (BORG 5) after PLBV initiation. Oxygen therapy during NIV could be reduced from 2 L/min to 1 L/min. The other patient walked 155 meters before and 150 meters after PLBV-initiation. He had a pronounced corticosteroid associated myopathy as a consequence of long-term prednisolone use. In both walking tests, he took five breaks within six minutes. We assume that the myopathy was the performance limiting factor in the walking test.
This overall improved performance was accompanied by concordant spirometric function parameters that are congruent with other published data. Zikyri found a comparable improvement in FVC after initial initiation of outpatient NIV [4]. Nevertheless, the changes in lung function are on average only minor and do not reflect the clear differences in the 6MWT. It should be noted that all data originate from clinical routine and investigations were performed at varying times of the day. It is possible that body plethysmography was to detect more significant changes if performed immediately in the morning after mask removal. Additional bedside spirometry would add useful information and should be used in further studies.
There was also an impressive improvement in quality of life, based on the CAT questionnaire, which was only available in a few patients. However, the results seem plausible in view of the significant improvement in 6MWT.
In several patient files evaluated for this study, a clearly improved quality of sleep and a longer sleeping time were documented: fewer waking-up episodes due to nocturnal breathlessness, refreshing sleep and subjective well-being in the morning.
Before the transition to PLBV, COPD patients had considerable shortness of breath following mask removal in the morning. After conversion to PLBV, a considerable improvement of this deventilation syndrome was documented in patient files frequently. This finding is supported by a preliminary analysis of an ongoing prospective trial (NCT03299764) that showed a reduction of shortness of breath (SOB) from 8.8 (out of 10) points to 4.3 points on a visual analogue scale after 12 weeks PLBV in COPD patients [15].
Our study may have even underestimated the effects on exercise tolerance associated with PLBV. Several patients treated with conventional NIV experienced breathlessness so severe that they were not able to start a 6MWT or a pulmonary function examination because they did not tolerate the transport to the walking parcour or lung function department. Therefore, there was no recorded baseline data for these patients and they were not included in this study. Among them were two patients who were bed-ridden and NIV-dependent for 18 to more than 23 hours per day. After switching them to PLBV, NIV was only required at night and they were able to walk short distances (data not included).
Patients were more likely not to perform the 6MWT prior to the switch to PLBV than after PLBV initiation. Thus, excluding very weak patients from the analysis possibly led to an underestimation of the PLBV effect. To minimize the selection bias, we decided to enrol only patients with a complete dataset (pre- and post-switch) into the study.
Since our results refer to retrospective, uncontrolled, non-blinded and observed data of a small group of patients, the conclusions drawn from the results are methodically limited. Given the small sample size, any additional patient enrolled may have had an impact on the study outcome. In the recruitment period, some patients were considered not to be suitable for PLBV by the treating physician; single patients did not accept the new ventilation method. It is impossible to avoid this subjective selection bias in a routine hospital setting when a new device is introduced since the selection criteria develop iteratively with growing experience. On the other hand, as described above, some patients were excluded due to their frail condition prior to PLBV initiation but would have been able to conduct all investigations some days later.
Furthermore, the algorithm driving the ventilation device was developed at our institution. Physicians who developed the algorithm were directly involved in patient care. This conflict of interest may have impaired an objective view on patient selection and treatment outcome. Potential conflicts of interest are declared in the supplement. Therefore, our observations can be used only to generate the hypothesis rather to confirm any clinical benefit of PLBV.
In order to minimize any potential bias, strict post-hoc inclusion and exclusion criteria were defined and only consecutive patients with complete data sets were included. Furthermore, we only assessed short term effects that were measurable within the clinical routine in the first week of PLBV initiation. A controlled prospective, randomised multicentre study is currently underway [16].
In conclusion, we found short-term improvements in exercise tolerance and FVC when patients with shortness of breath on discontinuation of their nightly hiNIV were switched to PLBV. These results have a limited validity as any retrospective analysis and based on a small number of cases but suggests the hypothesis that the new ventilation algorithm may have beneficial aspects for some COPD patients. Our findings are noteworthy as they suggest symptom improvements in COPD patients when their ventilation mode primarily aimed to avoid AutoPEEP. The findings from this small retrospective analysis warrant further investigations.
Supporting information
(XLSX)
Abbreviations
- 6MWT
6-minute walk test
- AVAPS
average volume assured pressure support
- BE
base excess
- BiPAP
bilevel positive airway pressure
- BMI
body mass index
- CAT
COPD assessment test
- COPD
chronic obstructive pulmonary disease
- DGSM
German society for sleep research and sleep medicine
- EIT
electrical impedance tomography
- EPAP
expiratory positive airway pressure
- FEV1
forced expiratory volume in one second
- FVC
forced vital capacity
- GOLD
Global Initiative for Chronic Obstructive Lung Disease
- hiNIV
high intensity NIV
- IPAP
inspiratory positive airway pressure
- IQR
interquartile range
- ITGV
intrathoracic gas volume
- NIV
non-invasive ventilation
- NT-proBNP
n-terminal pro brain natriuretic peptide
- pCO2
partial pressure of carbon dioxide
- PCV
pressure controlled ventilation
- PLBD
pursed lips breathing delay
- PLBP
pursed lips breathing pressure
- PLBV
pursed lips breathing ventilation
- pO2
partial pressure of oxygen
- PSG
polysomnography
- PT
pneumotachography
- Reff
effective respiratory resistance
- RV
residual volume
- SD
standard deviation
- SOB
shortness of breath
- TLC
total lung capacity
- VCin
inspiratory vital capacity
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
Fees for open access publishing were covered by a grant of the German Leibniz-Gemeinschaft, Open Access Publikation Fonds to Dr Christian Herzmann. The BioMaterialBank Nord is supported by the German Center for Lung Research. The BioMaterialBank Nord is a member of popgen 2.0 network (P2N). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The other authors received no specific funding for this work.
References
- 1.Singh D, Agusti A, Anzueto A, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease: The GOLD Science Committee Report 2019. European Respiratory Journal. 2019:1900164. [DOI] [PubMed]
- 2.Jones DJM, Paul EA, Jones PW, Wedzicha JA. Nasal pressure support ventilation plus oxygen compared with oxygen therapy alone in hypercapnic COPD. American Journal of Respiratory and Critical Care Medicine. 1995;152(2):538–544. 10.1164/ajrccm.152.2.7633704 [DOI] [PubMed] [Google Scholar]
- 3.Clini E, Sturani C, Rossi A, et al. The Italian multicentre study on noninvasive ventilation in chronic obstructive pulmonary disease patients. The European respiratory journal. 2002;20(3):529–538. 10.1183/09031936.02.02162001 [DOI] [PubMed] [Google Scholar]
- 4.Zikyri A, Pastaka C, Gourgoulianis KI. Hypercapnic COPD patients and NIV at home: is there any benefit? Using the CAT and BODE index in an effort to prove benefits of NIV in these patients. International journal of chronic obstructive pulmonary disease. 2018;13:2191 10.2147/COPD.S152574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Budweiser S, Heinemann F, Meyer K, Wild PJ, Pfeifer M. Weight gain in cachectic COPD patients receiving noninvasive positive-pressure ventilation. Respir Care. 2006;51(2):126–132. [PubMed] [Google Scholar]
- 6.Duiverman ML, Wempe JB, Bladder G, et al. Two-year home-based nocturnal noninvasive ventilation added to rehabilitation in chronic obstructive pulmonary disease patients: a randomized controlled trial. Respiratory research. 2011;12(1):112 10.1186/1465-9921-12-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Köhnlein T, Windisch W, Köhler D, et al. Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: a prospective, multicentre, randomised, controlled clinical trial. The Lancet Respiratory Medicine. 2014;2(9):698–705. 10.1016/S2213-2600(14)70153-5 [DOI] [PubMed] [Google Scholar]
- 8.Ergan B, Oczkowski S, Rochwerg B, et al. European Respiratory Society guidelines on long-term home non-invasive ventilation for management of COPD. The European respiratory journal. 2019;54(3). 10.1183/13993003.01003-2019 [DOI] [PubMed] [Google Scholar]
- 9.Dreher M, Storre JH, Schmoor C, Windisch W. High-intensity versus low-intensity non-invasive ventilation in patients with stable hypercapnic COPD: a randomised crossover trial. Thorax. 2010;65(4):303–308. 10.1136/thx.2009.124263 [DOI] [PubMed] [Google Scholar]
- 10.Esquinas AM, Ucar ZZ, Kirakli C. Deventilation syndrome in severe COPD patients during long-term noninvasive mechanical ventilation: poor sleep pattern, hyperinflation, or silent chronic muscular fatigue? Sleep and Breathing. 2014;18(2):225–226. 10.1007/s11325-013-0931-3 [DOI] [PubMed] [Google Scholar]
- 11.Raveling T, Bladder G, Vonk JM, et al. Improvement in hypercapnia does not predict survival in COPD patients on chronic noninvasive ventilation. Int J Chron Obstruct Pulmon Dis. 2018;13:3625–3634. 10.2147/COPD.S169951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Storre J, Schönhofer B. Noninvasive mechanical ventilation in chronic respiratory failure: ventilators and interfaces. J-F Muir, N Ambrosino und AK Simonds (Hg) Noninvasive ventilation, K Larsson (Ed in Chief) European Respiratory Monograph. 2008;2:319–337. [Google Scholar]
- 13.Blanch L, Bernabé F, Lucangelo U. Measurement of air trapping, intrinsic positive end-expiratory pressure, and dynamic hyperinflation in mechanically ventilated patients. Respiratory care. 2005;50(1):110–124. [PubMed] [Google Scholar]
- 14.Team RC. A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2014;https://www.R-project.org.
- 15.Herzmann C, Gaede K, Benz A, et al. NIV in pursed lip breathing mode for severe COPD—a therapeutic option for the deventilation syndrome?. Pneumologie. 2019;73(S 01):P251. [Google Scholar]
- 16.Evaluation of Non-invasive Pursed-lip Breathing Ventilation (PLBV) in Advanced COPD. https://clinicaltrials.gov/ct2/show/study/NCT03299764, 10 Feb 2019.