Abstract
Background
Hydroxychloroquine is one of several agents being evaluated in the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. We aimed to examine whether patients with rheumatological conditions receiving chronic hydroxychloroquine therapy are at less risk of developing SARS-CoV-2 infection than those not receiving hydroxychloroquine.
Methods
This retrospective cohort study included de-identified information of all veterans in the US Veterans Health Administration clinical administrative database aged 18 years or older with rheumatoid arthritis, systemic lupus erythematosus, or associated rheumatological conditions (based on International Classification of Diseases, 10th edition, diagnostic codes) who were alive on March 1, 2020. A propensity score was calculated for each patient, and each patient who was receiving hydroxychloroquine was matched to two patients who were not receiving hydroxychloroquine (controls). The primary endpoint was the proportion of patients with PCR-confirmed SARS-CoV-2 infection among those receiving chronic hydroxychloroquine versus the propensity-matched patients not receiving chronic hydroxychloroquine between March 1 and June 30, 2020. Secondary outcomes were hospital admission associated with SARS-CoV-2 infection; intensive care requirement associated with SARS-CoV-2 infection; mortality associated with SARS-CoV-2 infection; and overall rates of any hospital admission and mortality (ie, all cause). Multivariate logistic regression analysis was done to determine independent variables for the development of active SARS-CoV-2 infection.
Findings
Between March 1 and June 30, 2020, 10 703 patients receiving hydroxychloroquine and 21 406 patients not receiving hydroxychloroquine were included in the primary analysis. The incidence of active SARS-CoV-2 infections during the study period did not differ between patients receiving hydroxychloroquine and patients not receiving hydroxychloroquine (31 [0·3%] of 10 703 vs 78 [0·4%] of 21 406; odds ratio 0·79, 95% CI 0·52–1·20, p=0·27). There were no significant differences in secondary outcomes between the two groups in patients who developed active SARS-CoV-2 infection. For all patients in the study, overall mortality was lower in the hydroxychloroquine group than in the group of patients who did not receive hydroxychloroquine (odds ratio 0·70, 95% CI 0·55–0·89, p=0·0031). In multivariate logistic regression analysis, receipt of hydroxychloroquine was not associated with the development of active SARS-CoV-2 infection (odds ratio 0·79, 95% CI 0·51–1·42).
Interpretation
Hydroxychloroquine was not associated with a preventive effect against SARS-CoV-2 infection in a large group of patients with rheumatological conditions.
Funding
None.
Introduction
The emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in Wuhan (Hubei province, China) in late 2019 sparked a global pandemic that, towards the end of August, 2020, had resulted in close to 25 million known cases with almost 800 000 attributed deaths.1, 2, 3 The pandemic reached the USA in January, 2020, and by the end of August more than 180 000 deaths had been recorded among 5·8 million cases.4
As the research community launched several efforts to develop vaccine candidates, various potential pharmacotherapy options began to be explored on the basis of previous in-vitro and in-vivo studies of existing coronaviruses and on the few early in-vitro studies on SARS-CoV-2.5, 6 In addition to antiviral agents, some atypical drugs, such as the antimalarial drugs chloroquine and hydroxychloroquine, demonstrated in-vitro activity. In the USA, chloroquine was not on the market, but hydroxychloroquine had been available for several decades, principally used for treatment of patients with rheumatoid arthritis, systemic lupus erythematosus, and other associated autoimmune disorders.7, 8
The US health-care community began to focus on hydroxychloroquine and its potential role in the treatment of SARS-CoV-2 infection, and the federal government and lay media were soon to follow. There was sufficient interest in hydroxychloroquine for health-care institutions to increase procurement of the drug, causing shortages in the supply chain that threatened the continued availability of the drug relied upon by millions of patients with rheumatological conditions.9 Unfortunately, reports began to surface questioning whether patients with active SARS-CoV-2 infection received benefit from hydroxychloroquine, and safety concerns arose that focused on its propensity to induce prolonged QT arrhythmias.10, 11, 12 These data led the US Food and Drug Administration to revoke the emergency use authorisation for hydroxychloroquine and chloroquine to treat active SARS-CoV-2 infection on June 15, 2020, only 11 weeks after initial issuance.13
Research in context.
Evidence before this study
We searched PubMed, up to May 1, 2020, for published clinical trials assessing the effect of hydroxychloroquine to prevent laboratory-confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. The search terms used were “Covid-19”, “SARS-CoV-2”, and “hydroxychloroquine”. We identified a lack of well powered studies to determine the preventive effect of hydroxychloroquine on the development of laboratory-confirmed SARS-CoV-2 infection.
Added value of this study
Our study is the first to examine a large nationwide population of individuals with rheumatological conditions receiving long-term hydroxychloroquine with high adherence rates, comparing the population to a propensity-matched population not receiving hydroxychloroquine. The primary endpoint, the development of laboratory-confirmed SARS-CoV-2 infection, was not significantly different between the two propensity-matched cohorts.
Implications of all the available evidence
The findings of this study expand the knowledge base on the role of hydroxychloroquine in SARS-CoV-2 infection, supporting preliminary data from smaller studies suggesting that hydroxychloroquine might not be an effective agent to prevent SARS-CoV-2 infection.
A small trial reported preliminary evidence of short-term post-exposure prophylaxis of hydroxychloroquine among 821 household and occupational contacts of patients with newly diagnosed SARS-CoV-2 infection.14 The trial failed to demonstrate a difference between hydroxychloroquine and placebo among all participants or among the 20 laboratory-confirmed cases of infection, although the authors noted that a marginal benefit of hydroxychloroquine could not be ruled out. Randomised trials designed to evaluate interventions to prevent active infection have a unique set of challenges, including sparse event rates over time, which results in the need for substantial time and effort to achieve the power necessary to detect an effect. The results of large prevention trials will probably remain unavailable for several months. However, an alternative approach of compiling observational data from large clinical administrative databases might be useful to more rapidly identify preventive effects of an intervention. We aimed to examine whether patients with rheumatological conditions receiving chronic hydroxychloroquine therapy are at less risk of developing SARS-CoV-2 infection compared with a propensity-matched group of patients not receiving hydroxychloroquine.
Methods
Study design and participants
In this retrospective cohort study, de-identified information was obtained from across all US Veterans Affairs Medical Centers (VAMCs) for eligible patients aged 18 years or older throughout the Veterans Health Administration (VHA). The VHA is the largest integrated health-care system in the USA, providing care in 1255 health-care facilities, including 130 health-care centres and 1074 outpatient sites, serving 9 million enrolled veterans each year.15 A central clinical and administrative relational database, the Corporate Data Warehouse, maintains all information from the VHA's comprehensive electronic medical record system and is accessible to VHA clinical researchers following a rigorous approval process.
The patient cohort consisted of all veterans in the VHA system who were alive as of March 1, 2020, who had International Classification of Diseases (10th edition; ICD-10) diagnostic code entries for rheumatoid arthritis, systemic lupus erythematosus, and associated rheumatological conditions recorded from VHA encounters between Oct 1, 2016, and March 1, 2020 (appendix p 1).
Data were collected to determine the following: evidence of receipt of hydroxychloroquine to the equivalent of at least four 90-day supplies since April 1, 2019, and a medication possession ratio calculation of 80% or more from July 1, 2019, to June 30, 2020, with the most recent receipt within a timeframe to include the date of March 1, 2020;16 baseline demographic data as of March 1, 2020, to determine age, race, sex, and any tobacco use; all ICD-10 codes from Oct 1, 2016, to March 1, 2020, to determine the presence of chronic comorbidities; laboratory variables to assess organ dysfunction and to characterise classification and progression of the patient's rheumatological condition from April 1, 2019, to June 30, 2020, including C-reactive protein, erythrocyte sedimentation rate, white blood cell count, haemoglobin, haematocrit, platelet count, blood urea nitrogen, serum creatinine, aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase, and alkaline phosphatase; outpatient prescriptions for methotrexate, leflunomide, sulfasalazine, tofacitinib, prednisone, angiotensin-converting enzyme 2 inhibitors, angiotensin II receptor blockers, and zinc, vitamin D, and vitamin C preparations where availability included the date of March 1, 2020; and outpatient prescriptions or infusion clinic orders for adalimumab, certolizumab, etanercept, golimumab, infliximab, abatacept, rituximab, belimumab, or tocilizumab where last dose administered would remain active (based on frequency given) up to the date of March 1, 2020.
All univariate variables were assessed for their association with the use of hydroxychloroquine. Those univariate variables with a standardised mean difference of more than 0·10 were entered into a nominal multivariate logistic regression model to determine independent variables associated with the use of hydroxychloroquine. This model computed a propensity formula, and a propensity score was calculated for each participant. Each patient who was receiving hydroxychloroquine was matched to two patients who were not receiving hydroxychloroquine (controls) with the next-nearest propensity score to the patient receiving hydroxychloroquine, stratified by the VAMC and rural or urban status, sorted by area zip code.
The resultant propensity population was assessed with the following data collection for data points between March 1 and June 30, 2020: hospital admission dates and discharge dates; ward locations associated with hospital admission for any reason; discharge diagnostic ICD-10 codes associated with each admission; emergency or urgent care clinic encounters; any influenza tests done at the individual facilities; any PCR test results for SARS-CoV-2; and dates of death if applicable.
This study was approved by the University of Oklahoma Health Sciences Center Institutional Review Board and the Oklahoma City VA Healthcare System Research and Development Committee.
Outcomes
The primary endpoint was the proportion of PCR-confirmed SARS-CoV-2 infection among those receiving chronic hydroxychloroquine versus the propensity-matched patients not receiving chronic hydroxychloroquine between March 1 and June 30, 2020.
Secondary endpoints comparing patients receiving hydroxychloroquine with those not receiving hydroxychloroquine within the same time period were: hospital admission associated with SARS-CoV-2 infection; intensive care requirement associated with SARS-CoV-2 infection; mortality associated with SARS-CoV-2 infection; and overall rates of any hospital admission and mortality for both propensity-matched groups.
Statistical analysis
We did a univariate analysis to determine variables associated with the development of SARS-CoV-2 infection, including receipt of chronic hydroxychloroquine. Variables with a p value of 0·05 or less were entered into a multivariate logistic regression model to determine variables independently associated with the development of SARS-CoV-2 infection; receipt of chronic hydroxychloroquine was included in the multivariate model regardless of p value.
For all tests and analyses except where specified, the a-priori level of significance was set at a p value of 0·05 or less. Standardised mean difference measurements were considered well balanced if they were less than 0·25. Categorical variables were assessed using χ2 test and Fisher's exact test where appropriate. Wilcoxon rank sum test was used to assess continuous variables. Univariate and multivariate analyses were done as described. All analyses were done with JMP/SAS statistical software (version 12).
Role of the funding source
There was no funding source for this study. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
An ICD-10 code for any rheumatological-associated condition was found for 194 900 patients. Of these patients, 18 516 were excluded because they died before March 1, 2020. 100 639 patients were excluded because of the presence of only a non-specific ICD-10 diagnostic code indicating arthritis otherwise unspecified, arthropathy, myalgia, myositis, or mono-arthritis. Prescriptions for hydroxychloroquine use that included possession up to March 1, 2020, were identified in 16 178 of the remaining patients. Exclusion due to a medication possession ratio of less than 0·8 was documented in 5475 patients. The final population included in the study was 70 270 patients with a specific rheumatological-associated condition, comprising 10 703 patients with documented adherence with hydroxychloroquine and 59 567 patients not receiving hydroxychloroquine.
Several univariate variables were found to be associated with the selection of hydroxychloroquine at a significant level (appendix pp 2–4). The resultant multivariate logistic regression model derived from these variables resulted in a good fit, and odds ratios (ORs) and 95% CIs for variables found to be independently associated with hydroxychloroquine selection are presented in table 1 .
Table 1.
Baseline variables independently associated with hydroxychloroquine selection by multivariate logistic regression
| Odds ratio | 95% CI | p value | |
|---|---|---|---|
| Rheumatoid arthritis | 1·29 | 1·19–1·40 | <0·0001 |
| Systemic lupus erythematosus | 4·13 | 3·79–4·51 | <0·0001 |
| Polyarthritis | 1·21 | 1·03–1·42 | 0·021 |
| Behcet's syndrome | 0·26 | 0·07–0·67 | 0·0031 |
| Connective tissue disease | 3·58 | 3·11–4·10 | <0·0001 |
| Palindromic rheumatism | 2·74 | 2·07–3·59 | <0·0001 |
| Polyarthralgia rheumatica | 0·81 | 0·69–0·93 | 0·0038 |
| Leflunomide | 1·19 | 1·00–1·40 | 0·049 |
| Sulfasalazine | 2·00 | 1·71–2·29 | <0·0001 |
| Chronic steroids | 1·80 | 1·65–1·96 | <0·0001 |
| Prednisone equivalent >20 mg per day | 0·58 | 0·41–0·81 | 0·0011 |
| Any other csDMARD* | 2·12 | 1·78–2·53 | <0·0001 |
| Other csDMARD* plus biologic | 0·78 | 0·66–0·92 | 0·0024 |
| Methotrexate plus biologic | 0·78 | 0·64–0·96 | 0·018 |
| Vitamin D | 1·48 | 1·40–1·56 | <0·0001 |
| Angiotensin II receptor blocker | 1·43 | 1·32–1·55 | <0·0001 |
| Angiotensin-converting enzyme 2 inhibitor | 1·48 | 1·38–1·57 | <0·0001 |
| Low haemoglobin | 1·44 | 1·37–1·51 | <0·0001 |
| Elevated lactate dehydrogenase | 1·37 | 1·22–1·55 | <0·0001 |
| Thrombocytopenia | 1·12 | 1·02–1·24 | 0·021 |
| Leucopenia | 1·23 | 1·02–1·49 | 0·031 |
| Elevated erythrocyte sedimentation rate | 1·41 | 1·32–1·50 | <0·0001 |
csDMARD=conventional synthetic disease-modifying antirheumatic drug.
csDMARDs include hydroxychloroquine, methotrexate, leflunomide, and sulfasalazine; other csDMARD refers to agents other than hydroxychloroquine.
The baseline characteristics of 10 703 patients who received hydroxychloroquine compared with 21 406 patients who did not receive hydroxychloroquine were largely similar in the propensity-matched analysis, although some small numerical differences were significant, including younger mean age in those receiving hydroxychloroquine (64·8 years [SD 12·9]) compared with those not receiving hydroxychloroquine (65·4 years [13·3], p<0·0001; table 2 ).
Table 2.
Baseline demographic variables for the propensity-matched patients receiving hydroxychloroquine compared with those not receiving hydroxychloroquine
| Patients receiving hydroxychloroquine (n=10 703) | Patients not receiving hydroxychloroquine (n=21 406) | Standardised mean difference in odds ratio (95% CI) | p value | ||
|---|---|---|---|---|---|
| Rheumatological condition | |||||
| Rheumatoid arthritis | 7193 (67·2%) | 15 049 (70·3%) | −0·0805 (−0·107 to 0·520) | <0·0001 | |
| Systemic lupus erythematosus | 2642 (24·7%) | 4475 (20·9%) | 0·119 (0·088 to 0·149) | <0·0001 | |
| Polymyositis | 87 (0·8%) | 317 (1·5%) | −0·334 (−0·466 to −0·203) | <0·0001 | |
| Polyarthritis | 208 (1·9%) | 400 (1·9%) | 0·002 (−0·071 to 0·115) | 0·64 | |
| Sjögren's syndrome | 398 (3·7%) | 406 (1·9%) | 0·382 (0·304 to 0·459) | <0·0001 | |
| Behcet's syndrome | 4 (<0·1%) | 2 (<0·1%) | 0·764 (−0·171 to 1·70) | 0·10 | |
| Jaccoud's syndrome | 1 (<0·1%) | 3 (<0·1%) | −0·224 (−1·470 to 1·020) | 0·72 | |
| Connective tissue disease | 422 (3·9%) | 666 (3·1%) | 0·135 (0·067 to 0·204) | <0·0001 | |
| CREST syndrome | 9 (<0·1%) | 7 (<0·1%) | 0·521 (−0·024 to 1·07) | 0·060 | |
| Felty's syndrome | 12 (0·1%) | 34 (0·2%) | −0·192 (−0·555 to 0·171) | 0·29 | |
| Inflammatory polyarthritis | 346 (3·2%) | 605 (2·8%) | 0·076 (0·002 to 0·150) | 0·044 | |
| Systemic sclerosis | 75 (0·7%) | 148 (0·7%) | 0·008 (−0·146 to 0·161) | 0·92 | |
| Still's disease | 4 (<0·1%) | 11 (<0·1%) | −0·176 (−0·807 to 0·455) | 0·58 | |
| Palindromic rheumatism | 76 (0·7%) | 155 (0·7%) | −0·011 (−0·163 to 0·141) | 0·89 | |
| Polyarthralgia rheumatica | 246 (2·3%) | 574 (2·7%) | −0·087 (−0·170 to −0·004) | 0·038 | |
| Demographics | |||||
| Age, years | 64·8 (12·9) | 65·4 (13·3) | −0·047 (−0·702 to −0·238) | <0·0001 | |
| Sex | .. | .. | −0·063 (−0·093 to 0·033) | <0·0001 | |
| Men | 8029 (75·0%) | 16 502 (77·1%) | .. | .. | |
| Women | 2674 (25·0%) | 4904 (22·9%) | .. | .. | |
| Rural residence | 4126 (38·5%) | 8252 (38·5%) | .. | 1·0 | |
| Race | .. | .. | 0·042 (0·015 to 0·070) | 0·0023 | |
| White | 7292 (68·1%) | 14 221 (66·4%) | .. | .. | |
| Black | 2107 (19·7%) | 4464 (20·9%) | .. | .. | |
| Hispanic | 542 (5·1%) | 1045 (4·9%) | .. | .. | |
| Native American | 148 (1·4%) | 259 (1·2%) | .. | .. | |
| Asian or Pacific Islander | 172 (1·6%) | 343 (1·6%) | .. | .. | |
| Unknown | 442 (4·1%) | 1074 (5·0%) | .. | .. | |
| Body-mass index, kg/m2 | 30·0 (6·2) | 29·7 (6·2) | 0·044 (0·020 to 0·067) | 0·0005 | |
| Any tobacco use | 1175 (11·0%) | 2297 (10·7%) | 0·014 (−0·027 to 0·055) | 0·50 | |
| Rheumatological prescriptions | |||||
| Leflunomide | 654 (6·1%) | 1225 (5·7%) | 0·038 (−0·016 to 0·092) | 0·16 | |
| Methotrexate | 2214 (20·7%) | 4489 (21·0%) | −0·010 (−0·041 to 0·022) | 0·55 | |
| Sulfasalazine | 947 (8·8%) | 1378 (6·4%) | 0·190 (0·142 to 0·237) | <0·0001 | |
| Any other oral csDMARD* | 3388 (31·7%) | 6499 (30·4%) | 0·033 (0·006 to 0·061) | 0·018 | |
| Chronic steroid use | 1001 (9·4%) | 1707 (8·0%) | 0·096 (0·051 to 0·141) | <0·0001 | |
| Prednisone equivalent >20 mg per day | 51 (0·5%) | 89 (0·4%) | 0·076 (−0·115 to 0·266) | 0·44 | |
| Any biological therapy | 1548 (14·5%) | 3228 (15·1%) | −0·027 (−0·063 to 0·009) | 0·14 | |
| Other csDMARD* plus biologic | 760 (7·1%) | 1683 (7·9%) | −0·061 (−0·110 to −0·012) | 0·015 | |
| Methotrexate plus biologic | 473 (4·4%) | 1104 (5·2%) | −0·089 (−0·150 to −0·029) | 0·0036 | |
| Methotrexate plus other csDMARD* plus biologic | 53 (0·5%) | 92 (0·4%) | 0·078 (−0·108 to 0·265) | 0·41 | |
| Other prescriptions of interest | |||||
| Angiotensin II receptor blocker | 943 (8·8%) | 1804 (8·4%) | 0·027 (−0·019 to 0·072) | 0·25 | |
| Angiotensin-converting enzyme 2 inhibitor | 1551 (14·5%) | 2992 (14·0%) | 0·023 (−0·013 to 0·060) | 0·21 | |
| Vitamin D | 2565 (24·0%) | 4679 (21·9%) | 0·066 (0·036 to 0·096) | <0·0001 | |
| Vitamin C | 186 (1·7%) | 326 (1·5%) | 0·074 (−0·026 to 0·174) | 0·15 | |
| Zinc | 14 (0·1%) | 39 (0·2%) | −0·183 (−0·520 to 0·154) | 0·28 | |
| Comorbidities | |||||
| Respiratory | 2342 (21·9%) | 4429 (20·7%) | 0·039 (0·008 to 0·070) | 0·014 | |
| Renal or urinary | 2636 (24·6%) | 4996 (23·3%) | 0·039 (0·009 to 0·069) | 0·011 | |
| Cardiovascular | 4402 (41·1%) | 8895 (41·6%) | −0·010 (−0·036 to 0·016) | 0·47 | |
| Gastrointestinal | 2487 (23·2%) | 4576 (21·4%) | 0·059 (0·029 to 0·090) | 0·0002 | |
| Hepatobiliary | 337 (3·1%) | 651 (3·0%) | 0·020 (−0·054 to 0·093) | 0·60 | |
| Neurological | 3232 (30·2%) | 6046 (28·2%) | 0·052 (0·024 to 0·080) | 0·0003 | |
| Dermatological | 1706 (15·9%) | 3311 (15·5%) | 0·020 (−0·015 to 0·055) | 0·27 | |
| Metabolic or endocrine | 4547 (42·5%) | 9521 (44·5%) | −0·045 (−0·071 to −0·019) | 0·0007 | |
| Haematological | 1423 (13·3%) | 2651 (12·4%) | 0·045 (0·007 to 0·083) | 0·021 | |
| Psychiatric | 3059 (28·6%) | 5994 (28·0%) | 0·016 (−0·013 to 0·044) | 0·28 | |
| Neoplastic | 1379 (12·9%) | 2561 (12·0%) | 0·047 (0·008 to 0·085) | 0·018 | |
| Laboratory abnormalities | |||||
| Elevated alkaline phosphatase | 142 (1·3%) | 336 (1·6%) | −0·094 (−0·203 to 0·015) | 0·087 | |
| Elevated alanine aminotransferase | 199 (1·9%) | 451 (2·1%) | −0·070 (−0·163 to 0·022) | 0·13 | |
| Elevated aspartate aminotransferase | 459 (4·3%) | 978 (4·6%) | −0·036 (−0·099 to 0·026) | 0·25 | |
| Elevated lactate dehydrogenase | 408 (3·8%) | 641 (3·0%) | 0·138 (0·068 to 0·207) | <0·0001 | |
| Low haemoglobin | 4790 (44·8%) | 9290 (43·4%) | 0·030 (0·004 to 0·561) | 0·021 | |
| Thrombocytopenia | 585 (5·5%) | 1218 (5·7%) | −0·024 (−0·079 to 0·325) | 0·41 | |
| Leukocytosis | 558 (5·2%) | 1267 (5·9%) | −0·074 (−0·130 to −0·018) | 0·010 | |
| Leucopenia | 172 (1·6%) | 291 (1·4%) | 0·094 (−0·011 to 0·198) | 0·082 | |
| Elevated urea nitrogen | 1098 (10·3%) | 2353 (11·0%) | −0·043 (−0·084 to −0·001) | 0·045 | |
| Elevated creatinine | 720 (6·7%) | 1497 (7·0%) | −0·023 (−0·074 to 0·028) | 0·37 | |
| Elevated erythrocyte sedimentation rate | 1926 (18·0%) | 3501 (16·4%) | 0·064 (0·030 to 0·097) | 0·0002 | |
| Elevated C-reactive protein >10 μg/mL | 691 (6·5%) | 1303 (6·1%) | 0·035 (−0·018 to 0·087) | 0·20 | |
Data are n (%) or mean (SD) unless otherwise stated. csDMARD=conventional synthetic disease-modifying antirheumatic drug.
csDMARDs include hydroxychloroquine, methotrexate, lefunomide, and sulfasalazine; other csDMARD refers to agents other than hydroxychloroquine.
The incidence of active SARS-CoV-2 infections during the study period did not differ between the two groups (31 [0·3%] of 10 703 vs 78 [0·4%] of 21 406; OR 0·79, 95% CI 0·52–1·20, p=0·27; table 3 ), resulting in an overall rate of infection of 3·39 cases per 1000 patients. The figure shows no difference in time to positive SARS-CoV-2 PCR test between groups (p=0·27), with day 0 indicating March 1, 2020. SARS-CoV-2-related secondary outcomes showed no significant difference between the two groups among patients who developed active SARS-CoV-2 infection. Overall hospital admission did not differ between the groups; however, overall mortality was lower in patients receiving hydroxychloroquine than in those not receiving hydroxychloroquine. A post-hoc analysis demonstrated that a daily dose of hydroxychloroquine of more than 400 mg was not associated with less risk of developing SARS-CoV-2 infection (13 [0·4%] of 2928 for >400 mg daily vs 18 [0·2%] of 7775 for ≤400 mg daily, p=0·081). In a planned analysis, the proportion of patients who tested positive for influenza was low during the study period and did not differ significantly between patients receiving hydroxychloroquine and patients not receiving hydroxychloroquine (13 [0·1%] of 10 703 vs 19 [0·1%] of 21 406, p=0·39).
Table 3.
Primary and secondary outcomes of the propensity-matched comparison of patients treated with hydroxychloroquine versus patients not receiving hydroxychloroquine
| Patients receiving hydroxychloroquine (n=10 703) | Patients not receiving hydroxychloroquine (n=21 406) | Odds ratio (95% CI) | p value | |
|---|---|---|---|---|
| Primary outcome | ||||
| Developed active SARS-CoV-2 infection | 31 (0·3%) | 78 (0·4%) | 0·79 (0·52–1·20) | 0·27 |
| Secondary outcomes | ||||
| Hospital admission associated with SARS-CoV-2 infection | 9/31 (29·0%) | 19/78 (24·4%) | 1·27 (0·50–3·23) | 0·62 |
| Intensive care requirement associated with SARS-CoV-2 infection | 2/9 (22·2%) | 4/19 (21·1%) | 1·07 (0·16–7·31) | 0·99 |
| Mortality associated with SARS-CoV-2 infection | 0 | 7/78 (9·0%) | .. | 0·19 |
| Overall hospital admission | 343 (3·2%) | 733 (3·4%) | 0·93 (0·82–1·06) | 0·30 |
| Overall mortality | 88 (0·8%) | 251 (1·2%) | 0·70 (0·55–0·89) | 0·0031 |
Data are n (%) or n/N (%) unless otherwise stated. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
Figure.
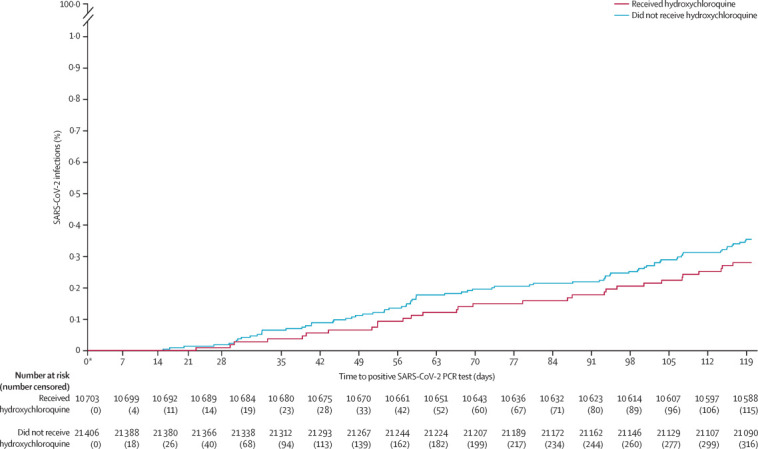
Time to laboratory-confirmed active SARS-CoV-2 infection for the propensity-matched patients receiving hydroxychloroquine and patients not receiving hydroxychloroquine
SARS-CoV-2=severe acute respiratory syndrome coronavirus 2. *Day 0 is March 1, 2020.
Univariate variables associated with the development of SARS-CoV-2 infection are presented in the appendix (pp 5–7). The resultant multivariate logistic regression model showed that the following variables were independently associated with SARS-CoV-2 infection: presence of polyarthritis (OR 4·01, 95% CI 1·76–7·89), non-white race (1·65, 1·08–2·50), urban residence (1·78, 1·14–2·89), receipt of vitamin C (3·31, 1·37–6·83), receipt of an angiotensin-converting enzyme 2 inhibitor (1·74, 1·08–2·72), elevated erythrocyte sedimentation rate (1·69, 1·08–2·59), and baseline C-reactive protein greater than 10 μg/mL (2·14, 1·21–3·63). Receipt of hydroxychloroquine was placed into the model but was not independently associated with SARS-CoV-2 infection (OR 0·79, 95% CI 0·51–1·42).
Discussion
Our study examined a large nationwide population of patients with rheumatological conditions to determine whether chronic hydroxychloroquine might protect against the development of SARS-CoV-2 infection. In this study, the proportion of patients with laboratory-confirmed SARS-CoV-2 infection did not differ between people with rheumatological conditions who received hydroxychloroquine and those who did not, suggesting that chronic hydroxychloroquine might not have a role in the prevention of SARS-CoV-2 infection. The overall rate of infection of 3·39 cases per 1000 patients in this study appears to be relatively close to the rate of infection in the VHA health-care system, as 18 560 infections had been diagnosed—out of a total of close to 9 million enrolled veterans—by June 29, 2020.17 Although there were no differences between groups in infection-related secondary outcomes among patients who developed active SARS-CoV-2 infection, overall mortality was lower in patients receiving hydroxychloroquine. Given that our study's primary purpose was to investigate the association between a drug and prevention of a specific infection, we cannot make conclusions about the observed difference in overall mortality. The high adherence to hydroxychloroquine might result in prolonged survival in patients with systemic lupus erythematosus and rheumatoid arthritis,8 and individuals in the hydroxychloroquine were slightly younger than those in the control group.
Reports of hydroxychloroquine's in-vitro activity against SARS-CoV-2 led to rapid inclusion of the drug in clinical studies and to clinical use in patients with active infection.6, 18 By contrast, other early investigations suggested that hydroxychloroquine might lead to a delay in mounting an initial antiviral response and increase the initial viral load.19, 20, 21 One of the first open-label studies by Gautret and colleagues22 showed that patients infected with SARS-CoV-2 who received hydroxychloroquine and azithromycin had a higher frequency of nasal clearance of the virus compared with patients who did not receive the drug combination. A follow-up study by the same authors that did not include a control group showed rapid transformation to negative PCR test for SARS-CoV-2 in patients receiving hydroxychloroquine and azithromycin.23 Both studies were limited because of the study design, small sample sizes, and questionable exclusion of some patients from data analysis. Despite the scarcity of substantial evidence, use of hydroxychloroquine with and without azithromycin was rapidly and widely incorporated into treatment protocols for SARS-CoV2 infection in the USA and globally in early March, 2020. Rheumatology associations such as the European League Against Rheumatism and the American College of Rheumatology raised concerns over possible hydroxychloroquine shortages, noting its effectiveness to treat joint pain, autoimmune rashes, and autoimmune thrombotic events, to prevent lupus flares, and to potentially prolong survival in patients with systemic lupus erythematosus and rheumatoid arthritis.24 More recently, results of studies evaluating hydroxychloroquine for the treatment of active SARS-CoV-2 infection have been inconclusive. A meta-analysis including three studies did not show earlier or higher frequency of viral clearance in patents receiving hydroxychloroquine, and showed a two times increase in death in these patients.25 In addition, a large observational study of 1376 patients in New York City (NY, USA) reported no significant association between hydroxychloroquine use and a combined endpoint of intubation or death (hazard ratio 1·04, 95% CI 0·82–1·32).10
Effective antiviral pharmacological intervention could fill an important gap to prevent SARS-CoV-2 infection and is likely to play an important part even after effective vaccines become available. So far, there have been no reports of studies evaluating the preventive effects of pharmacological agents other than hydroxychloroquine against SARS-CoV-2 infection. A 5-day high-dose course of hydroxychloroquine given to 821 household and occupational contacts of SARS-CoV-2-infected individuals as post-exposure prophylaxis failed to show a difference between hydroxychloroquine and placebo in the development of compatible symptoms of disease (49 [11·8%] of 414 individuals vs 58 [14·3%] of 407 individuals, p=0·35).14 However, only 20 participants developed laboratory-confirmed SARS-CoV-2 infection in the study (11 [2·7%] of 414 participants in the hydroxychloroquine group vs nine [2·2%] of 407 in the placebo group, p=0·82). The trial was halted prematurely for futility before the a-priori level of power could be reached.14 Another study indirectly investigated usage of hydroxychloroquine and colchicine in 1317 individuals who tested positive for SARS-CoV-2 infection in Israel compared with individuals who tested negative.26 In that study, five of six baseline variables were not balanced between individuals testing positive versus those testing negative, there was no baseline comparison of patients receiving or not receiving hydroxychloroquine, and there was no analysis of adherence among the patients deemed to be receiving hydroxychloroquine (ie, those who had one prescription dispensed between January, 2020, and the patient's first SARS-CoV-2 positive or negative test). The study showed no difference in the proportion of patients receiving hydroxychloroquine who had a positive test versus those who only had a negative test; however, only three (0·23%) patients in the infected group had received a hydroxychloroquine prescription. Studies evaluating prolonged hydroxychloroquine use for prevention of SARS-CoV-2 infection might provide more useful evidence than short post-exposure regimens. Hydroxychloroquine has a long terminal half-life of approximately 45 days and a large volume of distribution.7 These pharmacokinetic characteristics result in substantial drug accumulation in plasma and tissues over time. Our study takes advantage of a setting in which a specific group of patients has been receiving chronic hydroxychloroquine over several months to years as a novel virus emerges among the population, setting up an ideal premise to test the hypothesis that hydroxychloroquine might be effective in preventing SARS-CoV-2 infection.
The standard limitations of a non-randomised, observational retrospective study using a clinical administrative database apply to our study. However, a rigorous multivariate regression-derived, propensity-matching process was used to produce two comparable groups. March 1, 2020, was our baseline event date, just days before the first known entry of SARS-CoV-2 infection into the VHA. To gather baseline data, we collected all chronic comorbidity data for the 4 preceding years, and we collected laboratory data from 1 year previously (using the most recent value for each) for comprehensiveness. Women comprised only 24% of the study population; however, this proportion is much higher than that in most studies done using the VHA, as only approximately 5–10% of the enrolled veterans are female. Adherence to hydroxychloroquine was measured by a 12-month history of prescriptions filled to produce a medication possession ratio. This method does not ensure that patients are taking the medication appropriately, but our strict threshold of including only those with a medication possession ratio of 0·8 or above improves the chances that our population was adherent. No similar measure of adherence was undertaken to evaluate other medications that the patient was receiving, although each medication was documented to have a dispense date with supply that included March 1, 2020. If one extrapolates the high level of adherence of the included patients using hydroxychloroquine to other areas such as overall medication adherence or healthy lifestyle choices (eg, exercise and diet), a claim could be made that this high level of adherence might create an imbalance between the included patients using hydroxychloroquine and those not using hydroxychloroquine. Any perceived imbalance could be corrected with appropriate multivariate analyses. As an alternative, investigators could choose to include patients with poor adherence to hydroxychloroquine (34% of patients receiving hydroxychloroquine were excluded in our study for poor adherence) to assess the endpoint of prevention of SARS-CoV-2 infection; this approach would invite much-deserved criticism, however, of diluting any actual preventive effect of hydroxychloroquine. The primary endpoint was the development of SARS-CoV-2 infection during the initial 4-month period of the pandemic as recorded in the US VHA system. Although we present a time-to-event description in the figure, our study was not designed to provide sophisticated analysis of time-to-event data. Many institutions' policies regarding SARS-CoV-2 PCR testing have depended on test supply availability and prevalence in a particular area; therefore, many people suspected of having SARS-CoV-2 infection might not have been tested, particularly early in March. Thus, given that our outcome measures relied on the objective parameter of a positive SARS-CoV-2 PCR test, some patients with active infection might not have been included. It is also possible that veterans were tested and treated outside the VHA, so we might have not been able to find these patients using the Corporate Data Warehouse database. However, the VHA system is used primarily for the majority of the health-care needs of its enrolled veterans, so its electronic database is quite comprehensive for each veteran's health-care data. To control for the variabilities in testing practices and area prevalence, each propensity-matched patient not on hydroxychloroquine was matched to a patient on hydroxychloroquine enrolled in the same VAMC and by rural or urban residence, sorted by area zip code.
The devastation of the SARS-CoV-2 pandemic and the absence of an available vaccine make it imperative that the research community prioritises pharmacological treatment or prevention strategies effectively and efficiently. The use of observational data drawn rapidly from large clinical administrative databases might be an effective strategy to identify promising agents for further research or to identify agents that might not merit more effort. Our data adds to the expanding knowledge base that suggests that hydroxychloroquine might not be an effective agent in the battle against SARS-CoV-2.
Data sharing
The VHA Corporate Data Warehouse protects information of veterans within the electronic database and does not generally allow sharing of data to individuals or entities outside the VHA.
Acknowledgments
Acknowledgments
No external funding was received for this study. This material is the result of work supported with the resources and use of facilities at the Veterans Affairs Health Care System (Oklahoma City, OK, USA) and the VHA Corporate Data Warehouse.
Contributors
CAG was responsible for the concept of the study. CAG and SKT contributed to drafting of the Article and critical revisions. CAG, SKT, RJW, MBH, GK, and SCH contributed to the study design, data analysis, and data interpretation, and approved the final version of the Article.
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang L, Wang Y, Ye D, Liu Q. Review of the 2019 novel coronavirus (SARS-CoV-2) based on current evidence. Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO Coronavirus disease (Covid-19) weekly epidemiologic update. Aug 30, 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200831-weekly-epi-update-3.pdf?sfvrsn=d7032a2a_4
- 4.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang M, Cao R, Zhang L. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yao X, Ye F, Zhang M. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020;71:732–739. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schrezenmeier E, Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020;16:155–166. doi: 10.1038/s41584-020-0372-x. [DOI] [PubMed] [Google Scholar]
- 8.Singh JA, Saag KG, Bridges SL., Jr 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68:1–26. doi: 10.1002/art.39480. [DOI] [PubMed] [Google Scholar]
- 9.Hernandez AV, Roman YM, Pasupuleti V, Barboza JJ, White CM. Hydroxychloroquine or chloroquine for treatment or prophylaxis of COVID-19: a living systematic review. Ann Intern Med. 2020;173:287–296. doi: 10.7326/M20-2496. [DOI] [PubMed] [Google Scholar]
- 10.Geleris J, Sun Y, Platt J. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;382:2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahévas M, Tran VT, Roumier M. Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data. BMJ. 2020;369 doi: 10.1136/bmj.m1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenberg ES, Dufort EM, Udo T. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State. JAMA. 2020;323 doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.US Food and Drug Administration FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems. 2020. https://www.fda.gov/drugs/drug-safety-and-availability/fda-cautions-against-use-hydroxychloroquine-or-chloroquine-covid-19-outside-hospital-setting-or
- 14.Boulware DR, Pullen MF, Bangdiwala AS. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N Engl J Med. 2020;383:517–525. doi: 10.1056/NEJMoa2016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Center for Veterans Analysis and Statistics Department of Veterans Affairs statistics at a glance. May, 2016. https://www.va.gov/vetdata/docs/Quickfacts/Homepage_slideshow_06_04_16.pdf
- 16.Raebel MA, Schmittdiel J, Karter AJ, Konieczny JL, Steiner JF. Standardizing terminology and definitions of medication adherence and persistence in research employing electronic databases. Med Care. 2013;51(suppl 3):S11–S21. doi: 10.1097/MLR.0b013e31829b1d2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US Department of Veterans Affairs Department of Veterans Affairs COVID-19 national summary. https://www.accesstocare.va.gov/Healthcare/COVID19NationalSummary
- 18.Liu J, Cao R, Xu M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chu CM, Poon LL, Cheng VC. Initial viral load and the outcomes of SARS. CMAJ. 2004;171:1349–1352. doi: 10.1503/cmaj.1040398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paton NI, Goodall RL, Dunn DT. Effects of hydroxychloroquine on immune activation and disease progression among HIV-infected patients not receiving antiretroviral therapy: a randomized controlled trial. JAMA. 2012;308:353–361. doi: 10.1001/jama.2012.6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sperber K, Quraishi H, Kalb TH, Panja A, Stecher V, Mayer L. Selective regulation of cytokine secretion by hydroxychloroquine: inhibition of interleukin 1 alpha (IL-1-alpha) and IL-6 in human monocytes and T cells. J Rheumatol. 1993;20:803–808. [PubMed] [Google Scholar]
- 22.Gautret P, Lagier JC, Parola P. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Gautret P, Lagier JC, Parola P. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: a pilot observational study. Travel Med Infect Dis. 2020;34 doi: 10.1016/j.tmaid.2020.101663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yazdany J, Kim AHJ. Use of hydroxychloroquine and chloroquine during the COVID-19 pandemic: what every clinician should know. Ann Intern Med. 2020;172:754–755. doi: 10.7326/M20-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh AK, Singh A, Singh R, Misra A. Hydroxychloroquine in patients with COVID-19: a systematic review and meta-analysis. Diabetes Metab Syndr. 2020;14:589–596. doi: 10.1016/j.dsx.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gendelman O, Amital H, Bragazzi NL, Watad A, Chodick G. Continuous hydroxychloroquine or colchicine therapy does not prevent infection with SARS-CoV-2: Insights from a large healthcare database analysis. Autoimmun Rev. 2020;19 doi: 10.1016/j.autrev.2020.102566. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The VHA Corporate Data Warehouse protects information of veterans within the electronic database and does not generally allow sharing of data to individuals or entities outside the VHA.


