Abstract
Most of reported symptoms of SARS-CoV-2 infection are related to the respiratory system. Extra pulmonary manifestations of this novel virus infection are being increasingly reported in the literature, with increased attention on the gastrointestinal symptoms which might be the only presenting symptoms in some patients. These GI symptoms are nonspecific and little reported cases in the literature of confirmed gastrointestinal manifestations of SARS-CoV-2 infection by imaging. Colitis related to SARS-CoV-2 is even less reported in the literature. We present a case of SARS-CoV-2 infection of a 40-year-old lady who presented with GI manifestations and features of colitis of the caecum and ascending colon on CT scan. The patient did not have respiratory symptoms but had incidental lung changes in the visualized lung bases. These features were completely resolved as evident clinically and on follow-up CT scan after only 2 weeks, with only supportive care for SARS-CoV-2 infection. GI symptoms, in general, are very common presenting complain for many patients visiting the emergency department; hence, early recognition and high index of clinical suspicion for SARS-CoV-2 infection with the presence of supporting laboratory and imaging findings are to be considered for early protective measures to be undertaken to help in reducing the spread of this virus; in particular, in the middle of global pandemic of this virus and the fact that GI symptoms could be the only presenting symptoms without any respiratory symptoms. More studies and further invasive investigations in patients with features of colitis in imaging are needed to further understand the pathogenesis and its relation to SARS-CoV-2 infection
Keywords: SARS-CoV-2, COVID 19, Colitis, Gastrointestinal manifestations, CT, Abdomen
Introduction
Our knowledge and understanding of the various manifestations and clinical presentation of the novel SARS-CoV-2 since it was first described in Wuhan, China in late 2019, is being continuously evolving and being reported in the literature.
Most reported symptoms in SARS-CoV-2 infection are related to the respiratory system including in descending frequency, fever, cough, fatigue, and dyspnea [1].
The Centers for Disease Control and Prevention criteria for investigating persons with suspected SARS-CoV-2 infection focus mainly on respiratory symptoms and/or fever [2].
SARS-CoV-2 infection, however, can affect other systems. It is well known that all species of coronaviruses have extra pulmonary presentations; particularly, hematological, neurological, renal, and gastrointestinal symptoms [3].
Recent review articles have demonstrated that SARS-CoV-2 infection can present with variety of gastrointestinal (GI) symptoms including anorexia, diarrhea, vomiting, nausea, abdominal pain, and gastrointestinal bleeding; with these symptoms might be the only presenting findings in some patients [4].
These GI symptoms are nonspecific and little reported cases are there in the literature of confirmed gastrointestinal manifestations of SARS-CoV-2 by imaging.
We present a case of SARS-CoV-2 with GI manifestations and features of colitis of the caecum and ascending colon on CT scan with no respiratory symptoms and incidental lung changes in the visualized lung bases.
Case report
A 40-year-old Nepalese female patient who has no significant previous medical history of note, presented to the ED complaining of increasing abdominal pain in the last 2 days. The patient reported that her symptoms began as nausea and around 7 timed per day, followed shortly by vague diffuse abdominal pain which after a while were localized mainly in the right lower abdomen. Upon her presentation, she denied any other localizing symptoms nor any associated respiratory symptoms, weight loss, night sweats nor fever. She works as housekeeper, with no sick contacts nor recent travel.
At the time of clinical assessment, the patient vital signs were within normal limits as she was afebrile, normotensive and having normal O2 saturation on ambient air. Her physical exam showed signs of dehydration with dry mucous membranes and she was pale, her respiratory examination showed bilateral equal air entry with no added sounds on auscultation. Her abdominal examination revealed soft and lax abdomen per palpation with mild tenderness in the right iliac fossa and right hypochondrium with no rebound tenderness or signs of peritonitis, tip of the spleen was palpated as well but no hepatomegaly. Otherwise, her remaining examination was unremarkable and no skin rash could be appreciated.
Her initial blood investigations showed mild leukopenia with WBC of 3.0 × 103/uL (normal range 4.0-10 × 103/uL) and lymphopenia with 0.8 × 103/uL (normal range 1.0-3.0 × 103/uL). Her hemoglobin level was also slightly low of 9.6 gm/dL (normal range 12-14 gm/dL) with MCV of 63.2 fL (normal range 80-100 fL), the platelet count was normal. Her initial CRP was slightly elevated of 14.4 mg/L (normal <5.0). Otherwise her metabolic profile including kidney function test, serum electrolyte and liver function test was within normal range. Procalcitonin and lactic acid were in normal range. Her pregnancy test was negative.
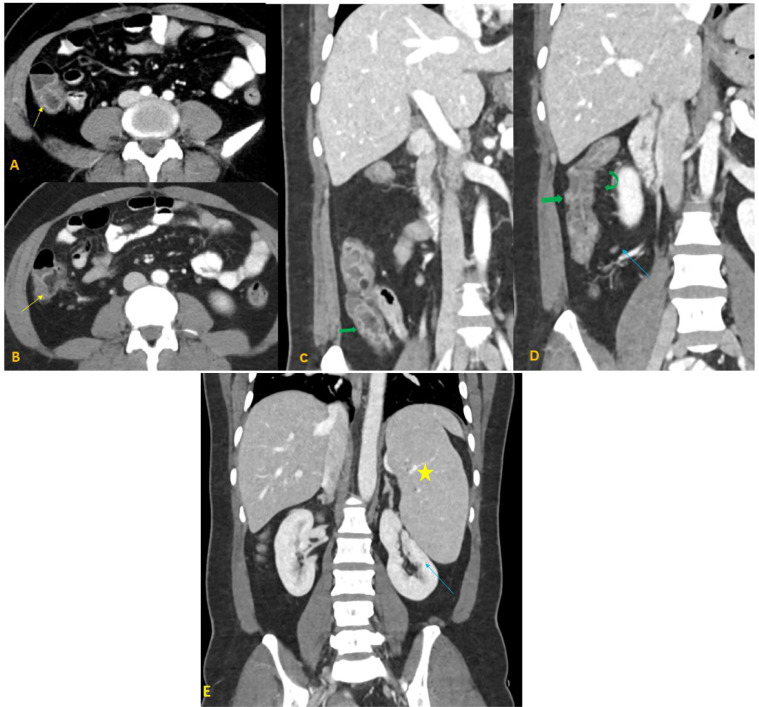
CT scan of the abdomen and pelvis with oral and IV contrast was ordered to rule out the possibility of acute appendicitis. It did not show signs of appendicitis; however, it showed mucosal enhancement and mural thickening of the ascending colon and caecum with adjacent mild mesenteric vascular congestion and small adjacent lymph nodes (Fig. 1).
Fig. 1.
A 40-year-old female with COVID-19 pneumonia presenting with colitis. Axial contrast-enhanced CT abdomen with coronal reformation in venous phase (A-E) demonstrates mucosal enhancement of ascending colon and cecum (yellow arrows in A and B), wall thickening (green arrows in C and D) with adjacent mild mesenteric vascular congestion (curved green arrow in D), and small adjacent lymph nodes (blue arrow in D). Incidental moderate splenomegaly (yellow star in E), compressing the left kidney (blue arrow in E).
Moderate splenomegaly was also evident on the CT scan (Fig. 1).
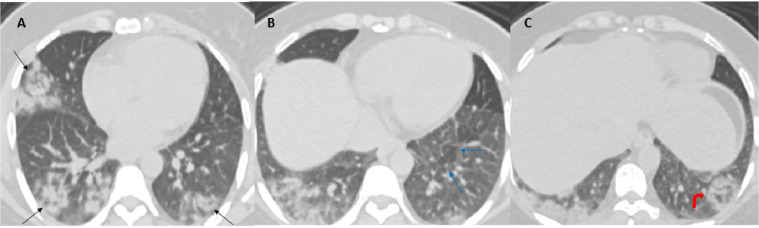
Visualized sections of the lower lungs showed bilateral consolidative changes with a striking peripheral distribution, bilateral ground-glass opacities, focal areas of faint interlobular septal thickening at left lower lobe, and peripheral rim of consolidation with central ground-glass opacities consistent with reverse halo sign (Atoll sign) in left lower lobe basal segment (Fig. 2).
Fig. 2.
Axial contrast-enhanced CT of the abdomen, lower cuts of the chest lung window demonstrate bilateral patchy consolidative changes and ground-glass opacities with a striking peripheral distribution (black arrows in A). Focal areas of faint interlobular septal thickening at left lower lobe (blue arrows in B). Peripheral rim of consolidation with central ground-glass opacities consistent with reverse halo sign (Atoll sign) at the left lower lobe basal segment (curved red arrow in C). Findings are considered typical CT imaging appearance for SARS-CoV-2 pneumonia.
Given the results of CT scan; the patient was admitted in the hospital for further evaluation of the possible causes of colitis and splenomegaly.
The possibility of SARS-CoV-2 infection was also needed to be ruled out; because of the visualized incidental lower lung CT changes which are considered highly suggestive of the virus, even though the patients did not have respiratory symptoms.
During her admission, the patient's clinical status was improving as her pain decreased gradually, her stool studies including stool culture, Clostridium difficile and ova parasite all came back negative apart from raised fecal calprotectin of 264 mg/kg (upper normal limit is 50). The patient during her stay had only 1 spike of fever and her subsequent septic workup was unrevealing.
She continued to improve gradually on supportive measures only with intravenous fluid and antiemetic medications and total resolution of her symptoms by day 4 of admission were evident. As a part of her evaluation, she was planned for colonoscopy; however, as the patient was clinically stable and the results of SARS-CoV-2 PCR were still pending; it was decided for the colonoscopy and follow-up of the patient to be done on outpatient basis.
Given the demographic data as well as multisystem involvement with lung changes, features of colitis, splenomegaly, and anemia, the possibility of underlying TB infection or malignancy was also raised, and hence AFB sputum smears, PCR, and cultures were also obtained which all later came back negative; however, her serum Quantiferon was positive.
On day 5 of admission, the results of SARS-CoV-2 came back positive, and the patient was transferred to communicable disease center for isolation. There the patient was started on ceftriaxone, azithromycin, hydroxychloroquine, oseltamivir and darunavir/cobicistat.
Whether to proceed to bronchoscopy or not given her positive Quantiferon results was discussed and it was decided that the chest CT findings could be explained by SARS-CoV-2 pneumonia and the patient most likely have latent TB. So, the plan was to repeat the CT scan after 2 weeks and proceed according to the results.
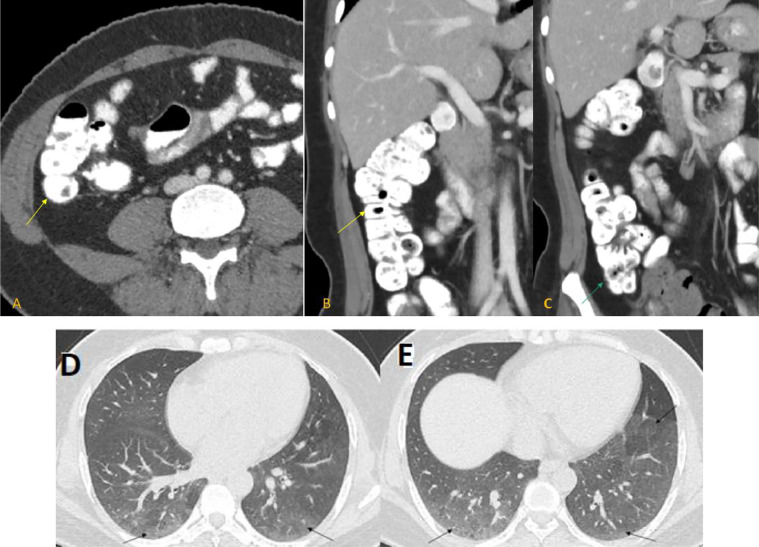
Follow-up CT scan after 2 weeks showed complete resolution of the previously seen mucosal enhancement and mural thickening of the ascending colon and caecum as well as the adjacent mild mesenteric vascular congestion and the small adjacent lymph nodes (Fig. 3).
Fig. 3.
Follow-up CT scan in 3 weeks; axial contrast-enhanced CT abdomen with coronal reformation in venous phase (A-C), showed normal appearance and wall thickness of ascending colon (yellow arrows in A and B) and cecum (green arrow in B) with complete resolution of wall thickening and mucosal enhancement. Axial contrast-enhanced CT chest lung window (D and E) revealed complete resolution of the patchy consolidation with minimal residual ground-glass opacities at both lower lobes (black arrows).
There was also near complete resolution of the previously noted lung changes with only residual minimal ground-glass opacities at both lower lobes (Fig. 3).
For the splenomegaly and the anemia, which were presumed unrelated to the SARS-CoV-2 infection; further workup was obtained including hemoglobin electrophoresis which revealed showed 94.2% of hemoglobin E and 3.8% of hemoglobin F; this pattern together with anemia favor compound heterozygosity for hemoglobin E/beta thalassemia.
On day 12 of admission, the patient remained clinically stable and her CRP was normalized.
The patient was subsequently transferred to quarantine facility with final diagnosis of SARS-CoV-2 infection with GI manifestations, latent tuberculosis infection “given positive Quantiferon results and absence of active MTB infection” and hemoglobin/beta thalassemia.
She completed another 1 week in quarantine facility where she was tested twice negative for SARS-CoV-2 PCR and therefore discharged home.
Discussion
Extrapulmonary manifestations of SARS-CoV-2 infection are being increasingly reported in the literature.
Gastrointestinal symptoms are among the most common of these extrapulmonary manifestations including nausea, vomiting, anorexia, diarrhea, and abdominal pain. They are reported in approximately 3%-50% of patients with SARS-CoV-2 pulmonary infection. It is important to note that these symptoms might be the only presenting findings in some patients [3], [4], [5], [6], [7].
The importance of this increased attention to the GI manifestations of SARS-CoV-2 infection, particularly when they are not accompanied by respiratory symptoms; is that these symptoms are in general a very frequent presenting complain for many patients visiting the emergency departments. Hence, if physicians focus only on respiratory symptoms to identify suspected cases of SARS-CoV-2 infection in the middle of the global pandemic of the virus; many cases with extrapulmonary manifestations may not be diagnosed on time. This will have drastic consequences as there will be delay in the diagnosis and treatment for SARS-CoV-2 infection, and the proper protective measures will not be undertaken early, imposing great risk of widespread of the infection to the medical personnel and other patients. Lower attention of these GI symptoms by the patients them self might also result in the spread of the virus between the family members or in the community [4,7].
SARS-CoV-2 RNA has been identified in many fecal samples from patients with confirmed SARS-CoV-2 pulmonary infection and it can persist after viral clearance from respiratory specimens. It is unclear, however, if the virus is directly responsible for the GI symptoms [6,7].
One theory is that viral entry into a host cell is through ACE-2 protein receptor, which is abundantly expressed in the glandular cells of gastric, duodenal, and rectal epithelia and is proven to be a cell receptor for SARS-CoV-2 [8].
In our patient, initially there was very low index of suspicion for SARS-CoV-2 infection, as the patient had no respiratory symptoms at all and no documented fever (only subjective feverish sensation). The patient had only GI symptoms mimicking appendicitis clinically, hence CT scan of the abdomen and pelvis was performed.
There were no signs of appendicitis on the CT scan; however, it showed features of colitis of the caecum and proximal ascending colon. More striking finding of bilateral lower lung consolidative changes and ground-glass opacities with peripheral distribution, focal areas of faint interlobular septal thickening, and a focal area of reverse halo sign (Atoll sign) in the left lower lobe. These lung changes represent most of the reported typical findings of SARS-CoV-2 infection in the literature [9], [10], [11], [12].
Because of the incidental lower lung findings even without respiratory symptoms, with features of mild leukopenia and lymphopenia in CBC and the fact of the global pandemic of SARS-CoV-2 infection; the suspicious was high and the patient was isolated and tested for the virus, which came back positive later on.
Most hospitalized patients with confirmed SARS-CoV-2 infection and GI symptoms, in general, do not undergo full invasive investigations including endoscopy with or without biopsy unless the patient has severe symptoms, as it is considered probable virus aerosolizing procedure [5,13].
SARS-CoV-2-related colitis is not well reported in the literature. To the best of our knowledge, 2 reported cases of colitis related to SARS-CoV-2 infection.
One reported case of a 31-year-old male who was admitted with abdominal discomfort and absent bowel movement. CT scan of his abdomen showed mild stranding surrounding mildly distended fluid-filled ascending colon and few small surrounding mesenteric lymph nodes were identified, as well as incidental, patchy ground-glass opacities with reverse halo appearance in the visualized lung bases. SARS-CoV-2 PCR was subsequently positive [3].
The other case was reported by Carvalho et al. of confirmed SARS-CoV-2 infection causing hemorrhagic colitis in 71-year-old woman. This patient, however, underwent full invasive investigations as they had low index of suspicion at the time of the patient presentation and they did not take proper precautions and protective measures resulted in subsequent quarantine of 72 healthcare workers [5].
One reported case by Farina et al. confirmed SARS-CoV-2 infection and associated ischemic changes of some of the small bowel loops due to thrombosis of the superior mesenteric artery. Though this case is different from our case, it shows the various possible manifestations of SARS-CoV-2 infection including mesenteric vessel thrombosis and resultant bowel ischemia [14].
Given the demographic data of the patient and multisystem findings, TB needed to be ruled out; however, as the patient was clinically stable and improving on supportive measures only, invasive investigations including possible colonoscopy and bronchoscopy were postponed to be done on outpatient basis after resolution of SARS-CoV-2 infection and according to the result of follow-up CT scan.
We strongly believe that the findings of colitis and lower lung changes are due to SARS-CoV-2 infection; given the fact that these changes were completely resolved in the follow-up CT scan after 2 weeks without any specific treatment other than the supportive treatment of SARS-CoV-2 infection.
The positive serum QuantiFERON test is likely suggestive of latent TB infection, which is an incidental finding; as there were no clinical signs of active TB infection and no abnormal CT scan findings in the follow-up CT scan of the chest, abdomen, and pelvis. The patient is planned to receive treatment for latent TB on outpatient basis.
The other incidental finding of splenomegaly and anemia could be explained by hemoglobin E/beta thalassemia, which was confirmed by peripheral blood smear and hemoglobin electrophoresis.
Author contribution
(1) Mohammed Khader: Contribution: Imaging, literature review, discussion, overall responsibility for the study.
(2) Ahmad Al Bishawi: Contribution: Obtain informed consent, clinical follow up, history & physical examination, laboratory analysis, and prescribing medicine.
(3) Aalaa Kambal: Contribution: Imaging figures and figures legend.
(4) Alaaeldin Abdelmajid: Contribution: Clinical follow up, history & physical examination, laboratory analysis, and prescribing medicine.
Footnotes
Competing interests: Nothing to disclose.
References
- 1.Yang J, Zheng Y, Gou X, Ke P, Zhaofeng C, Qinghong G. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Overview of Testing for SARS-CoV-2 (COVID-19).2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-criteria.html. Accessed date, July 2, 2020.
- 3.Behzad S, Aghaghazvini L, Reza Radmard A, Gholamrezanezhad A. Extrapulmonary manifestations of COVID-19: radiologic and clinical overview. Clin Imaging. 2020;66:35–41. doi: 10.1016/j.clinimag.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tian Y, Rong L, Nian W, He Y. Gastrointestinal features in COVID-19 and the possibility of fecal transmission. Aliment Pharmacol Ther. 2020 doi: 10.1111/apt.15731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carvalho A, Alqusairi R, Adams A, Paul M, Kothari N, Peters S. SARS-CoV-2 gastrointestinal infection causing hemorrhagic colitis: implications for detection and transmission of COVID-19 disease. Am J Gastroenterol. 2020;115(6):942–946. doi: 10.14309/ajg.0000000000000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan L, Mu M, Pengcheng Y, Sun Y, Wang R, Yan J. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol. 2020;115(5):766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao F., Tang M., Zheng X. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158(6):1831–1833.e3. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li M. Chest CT features and their role in COVID-19. Radiol Infect Dis. 2020;7(2):51–54. doi: 10.1016/j.jrid.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanne J.P. Chest CT findings in 2019 novel coronavirus (2019-nCoV) infections from Wuhan, China: key points for the radiologist. Radiology. 2020;295(1):16–17. doi: 10.1148/radiol.2020200241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernheim A., Mei X., Huang M., Yang Y., Fayad Z.A., Zhang N. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020 doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu J., Wu X., Zeng W., Guo D., Fang Z., Chen L. Chest CT findings in patients with corona virus disease 2019 and its relationship with clinical features. Invest Radiol. 2020;55(5):257–261. doi: 10.1097/RLI.0000000000000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corley D.A., Peek R.M. COVID-19: what should clinicians and scientists do and when? Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farina D, Rondi P, Botturi E, Renzulli M, Borghesi A, Guelfi D. Gastrointestinal: bowel ischemia in a suspected coronavirus disease (COVID‐19) patient. J Gastroenterol Hepatol. 2020;10.1111(/jgh.15094) doi: 10.1111/jgh.15094. [DOI] [PMC free article] [PubMed] [Google Scholar]