Abstract
Ultrasound imaging of the lung (LUS) and associated tissues has demonstrated clinical utility in coronavirus disease 2019 (COVID-19) patients. The aim of the present study was to evaluate the possibilities of a portable pocket-sized ultrasound scanner in the evaluation of lung involvement in patients with COVID-19 pneumonia. We conducted 437 paired readings in 34 LUS evaluations of hospitalized individuals with COVID-19. The LUS scans were performed on the same day with a standard high-end ultrasound scanner (Venue GO, GE Healthcare, Chicago, IL, USA) and a pocket-sized ultrasound scanner (Butterfly iQ, Butterfly Network Inc., Guilford, CT, USA). Fourteen scans were performed on individuals with severe cases, 11 on individuals with moderate cases and nine on individuals with mild cases. No difference was observed between groups in days since onset of symptoms (23.29 ± 10.07, 22.91 ± 8.91 and 28.56 ± 11.13 d, respectively; p = 0.38). No significant differences were found between LUS scores obtained with the high-end and the portable pocket-sized ultrasound scanner. LUS scores in individuals with mild respiratory impairment were significantly lower than in those with moderate and severe cases. Our study confirms the possibilities of portable pocket-sized ultrasound imaging of the lung in COVID-19 patients. Portable pocket-sized ultrasound scanners are cheap, easy to handle and equivalent to standard scanners for non-invasive assessment of severity and dynamic observation of lung lesions in COVID-19 patients.
Key Words: Lung ultrasound, Handheld, COVID-19, SARS-CoV-2, Imaging, Pneumonia, Acute respiratory failure
Introduction
Coronavirus disease 2019 (COVID-19), caused by infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), emerged in China in December 2019 and quickly spread all over the globe. The clinical features are fever, dyspnea, dry cough, fatigue and diarrhea (Wan et al. 2020). Pharyngodynia, nasal congestion, rhinorrhea and anosmia have also been reported (Chen et al. 2020; Gattinoni et al. 2020; Hopkins et al. 2020; Mason 2020). Interstitial pneumonia is very common, and a high percentage of patients (9%–11%) develop severe acute respiratory distress syndrome (ARDS) and require intensive care (Lovato and de Filippis 2020; Remuzzi and Remuzzi 2020; Yuan et al. 2020). The current therapeutic strategy involves agents counteracting viral invasion and replication, and inhibitors of cytokine-sustained inflammatory reactions. No specific antiviral therapy has yet been identified (Capecchi et al. 2020; Conticini et al. 2020).
Ultrasound imaging of the lung (LUS) is a promising technique in many acute and chronic parenchymal conditions that determine interstitial syndrome. These include cardiogenic and non-cardiogenic pulmonary edema, ARDS, interstitial pulmonary fibrosis and a variety of conditions determining lung consolidations, such as pneumonia and lung cancer (Mojoli et al. 2019). In COVID-19 patients, it has demonstrated clinical utility, owing to the typical sonographic characteristics of affected lungs, for providing indications for clinical decisions and the management of associated respiratory failure and lung injury (Smith et al. 2020).
The aim of the present study was to evaluate the possibilities of a portable pocket-sized ultrasound scanner in evaluating lung involvement in individuals with COVID-19 pneumonia.
Materials and Methods
We conducted 34 LUS evaluations on patients admitted to the COVID Unit of Siena University Hospital with symptoms compatible with COVID-19, a positive SARS-CoV-2 nasal-pharyngeal swab and radiologic evidence of interstitial pneumonia.
The participants were divided into three severity categories based on respiratory impairment—mild: PaO2/FiO2 >300 in room air or oxygen flow; moderate: PaO2/FiO2 between 150 and 300 in room air or oxygen therapy, continuous positive airway pressure, non-invasive ventilation or high-flow nasal cannula; and severe: PaO2/FiO2 <150 on oxygen therapy, continuous positive airway pressure, non-invasive ventilation, high-flow nasal cannula or mechanical ventilation.
The LUS scans were performed on the same day with a high-end point-of-care ultrasound scanner (Venue GO, GE Healthcare, Chicago, IL, USA) and a pocket-sized ultrasound scanner (Butterfly iQ, Butterfly Network Inc., Guilford, CT, USA) for clinical purposes; the lung pre-set was used with both scanners. The portable pocket-sized ultrasound scanner we tested has a single silicon chip containing a 2-D array of 9000 capacitive micro-machined ultrasound transducers instead of the standard piezoelectric crystal-based transducers. The chip emulates curved, linear, or phased transducers at any time in M-mode, B-mode or color Doppler with a 2–30 cm scan depth (Liu et al. 2019).
Up to six regions of the chest were identified: anterosuperior (A), anteroinferior (B), lateral superior (C), lateral inferior (D), posterosuperior (E) and posteroinferior (F). One of four different aeration patterns was recorded according to a specific scoring system: A = 0 points (normal aeration, presence of lung sliding with A-lines or no more than two isolated B lines), B1 = 1 point (moderate loss of lung aeration, multiple well-defined B-lines), B2 = 2 points (severe loss of lung aeration, multiple coalescent B-lines) and C = 3 points (lung consolidation and tissue-like pattern). Pleural effusion and pneumothorax were also recorded. A score of 0 was normal, and 36 was the worst. Due to clinical conditions, the posterosuperior region (E) could not be explored in some participants, so the mean of the regions explored was calculated for the purposes of statistical analysis (total sum [0–36] divided by number of regions explored [five or six on each side]). Our step‐by‐step approach to LUS in COVID-19 patients was comparable to the COVID-19 lung ultrasound in emergency department protocol (Manivel et al. 2020). Imaging was obtained by two different operators, both experts in lung ultrasound. The research was approved by the local ethics committee (OSS_REOS number 12908), and informed consent was obtained from each participant.
Statistical analysis
Student's t-test was used to compare pairs of groups, and analysis of variance to compare three or more groups, followed by the Holm–Sidak multiple comparisons test when the former was significant. Normal distribution of data was checked using the D'Agostino–Pearson test (command “sktest” in Stata). The presence and possible sources of systematic bias between the two instruments were investigated in the complete data set of the individual readings at 12 thoracic locations in each participant. We used multilevel mixed-effects linear regression models with the difference in score on the same thoracic location (Butterfly) as the outcome variable, changes in vertical level of the thoracic location (high vs. low), side (right vs. left), horizontal level (anterior, lateral, posterior) and severity as fixed-effect variables and participant as a random-effect variable.
The primary outcome of the study was the assessment of the bias and limits of agreement between the total participant scores obtained with the two instruments, computed with the Bland–Altman method (Bland and Altman 1986). A secondary outcome was the assessment of the concordance between the two instruments. As no single measure of concordance is generally accepted (Bunting et al. 2019), we computed five different parameters: Pearson correlation coefficient, intra-class correlation coefficient, Lin concordance correlation coefficient, Liao improved concordance correlation coefficient and Cohen κ measure of agreement (Liao 2015).
Power calculation
We calculated that 34 participants would be required for comparison of the two methods using the Bland–Altman method (Lu et al. 2016), assuming a mean difference in total score of 1 ± 1, a false positive rate (α) of 0.05 and a false negative rate of 0.1 (β = 0.9). The analyses were performed with Stata for Windows version 16 (Stata Corp., Texas College, TX, USA), except for the Liao improved concordance correlation coefficient (“AgreementInterval” package of R) and power estimation (Medcalc 19.3.1, MedCalc Software Ltd., Ostend, Belgium). A level of p ≤ 0.05 for a two-tailed distribution was considered statistically significant.
Results
The 34 paired LUS scans on 18 COVID-19 patients (14 male and 4 female; age at presentation, 77.6 ± 10.0 y) produced the following results. No difference in age was found between severity groups; 16/34 scans were performed in the severe group, 11 in the moderate group and 7 in the mild group. No difference was observed between groups in days since onset of symptoms (23.29 ± 10.07, 22.91 ± 8.91 and 28.56 ± 11.13 d, respectively; p = 0.38).
When assessed on the full data set of 437 paired readings in 34 LUS scans, no significant differences were found between LUS scores obtained with the high-end and the portable pocket-sized ultrasound, with a mean difference in score of −0.018 ± 0.018 points (not significant). The score difference did not change significantly according to lung side (0.027 ± 0.032 points), vertical level (−0.041 ± 0.033 points) or clinical severity (0.013 ± 0.022 points per each level). A significant difference was found, however, between the two instruments according the horizontal location of the site, with the difference between the two instruments slightly but significantly greater on the posterior compared to the anterior side of the thorax (0.082 ± 0.021 points, p < 0.01).
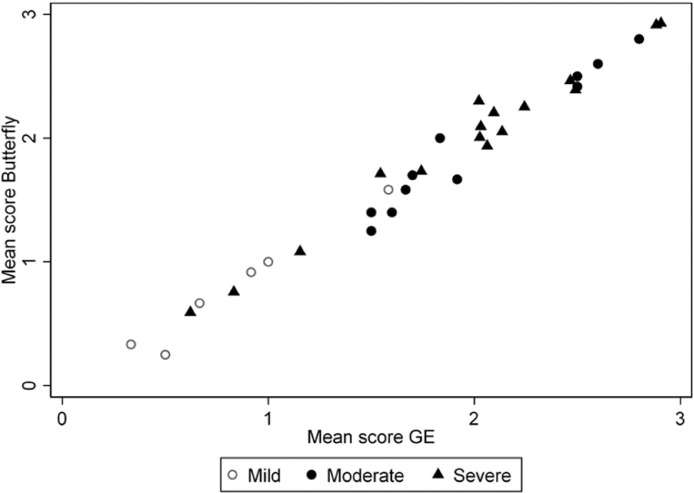
Total average scores obtained with the two instruments were normally distributed, as was their difference. Average participant scores correlated with clinical severity (p < 0.001, Fig. 1 ).
Fig. 1.
Correlation of mean scores obtained with the two instruments, according to disease severity.
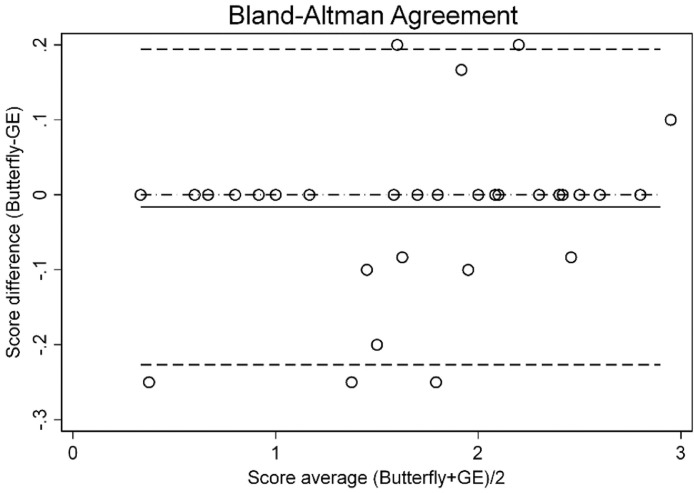
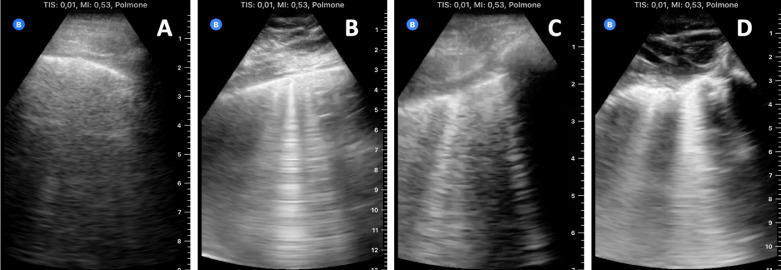
All the computed parameters showed an excellent degree of concordance between the two instruments (Table 1 ). The Bland–Altman plot is shown in Figure 2 . The absolute level of bias computed with the Bland–Altman method was −0.016 (95% confidence interval [CI], −0.054 to 0.021), the lower limit of agreement was −0.227 (95% CI, −0.291 to −0.0162) and the upper limit of agreement was 0.194 (95% CI, 0.129–0.259)—much smaller than the minimum possible change of 1 point. Figure 3 give sample images of different severity grades of lung impairment obtained with the handheld scanner.
Table 1.
Correlation coefficients between total average scores
| Parameter | Coefficient (95% confidence interval) |
|---|---|
| Pearson correlation coefficient | 0.990 (0.980–0.995) |
| Intra-class correlation coefficient | 0.989 (0.980–0.994) |
| Concordance correlation coefficient | 0.989 (0.978–0.994) |
| Improved concordance correlation coefficient | 0.988 (0.927–0.998) |
Fig. 2.
Bland–Altman plot showing the level of bias (solid line) and limits of agreement.
Fig. 3.
Sample images of different grades of severity of lung impairment obtained with the handheld ultrasound scanner. (a) Normal aeration, presence of A-lines or no more than two isolated B-lines; (b) moderate loss of lung aeration, multiple well-defined B-lines; (c) severe loss of lung aeration, multiple coalescent B-lines; (d) lung consolidation and tissue-like pattern.
Discussion
Lung ultrasound imaging is a non-invasive technique that provides useful indications for clinical decisions concerning COVID-19 (Wang et al. 2017; Smith et al. 2020). It is safe, repeatable, radiation-free and economical, and can be used at the point of care. Here we evaluated the possibilities of a portable pocket-sized ultrasound scanner in COVID-19 patients with pneumonia.
We included a cohort of COVID-19 patients who were hospitalized with respiratory failure of different severities. All were scanned with a standard high-end ultrasound scanner and a portable pocket-sized ultrasound scanner.
The results of the portable scanner were practically identical to those of the high-end scanner in assessing lung interstitial syndrome according the bedside lung ultrasound in emergency protocol (Lichtenstein 2015): Bland–Altman bias was found to be close to zero, with very narrow limits of agreement, and all the other parameters of concordance were in the range of substantial or excellent agreement. Furthermore, no systematic bias was observed with disease severity or anatomic site of analysis, except for a statistically significant but practically negligible difference on the posterior side of the thorax, possibly a spurious finding.
Due to its easy handling and dynamic nature, LUS is increasingly used in clinical settings, especially in critical care (Mojoli et al. 2019). In SARS-CoV-2 infection, it is invaluable in clinical management, showing higher accuracy than chest radiography (Smith et al. 2020) and good correlation with computed tomography imaging and pneumonia severity (Nouvenne et al. 2020; Zieleskiewicz et al. 2020). In experimental models of ARDS, it has been found to detect lung lesions before the onset of hypoxemia (Soldati et al. 2020). Point-of-care ultrasound has great possibilities in many branches of medicine, especially emergency and critical care, where it can be invaluable in the safe management of COVID-19, since it allows concomitant clinical examination and lung imaging at the bedside by the same doctor (Smith et al. 2020; Buonsenso et al. 2020). An observational study—the CORonavirus (COVID-19) Diagnostic Lung UltraSound Study (COR-DLUS; ClinicalTrials.gov identifier NCT04351802)—is currently ongoing. The study is designed to assess whether focused LUS examination can improve the diagnosis of COVID-19 lung disease or make an alternative diagnosis at a person's initial hospital presentation.
In our study, we also found a statistically significant correlation between portable-scanner findings and disease severity, confirming previous reports of 68.8%, 77.8% and 100.0% sensitivity, 85.7%, 76.2% and 92.9% specificity and 76.7%, 76.7% and 93.3% diagnostic accuracy in detecting mild, moderate and severe lung lesions, respectively (Lu et al. 2020).
The main limitations of our study were its retrospective nature, preventing analysis of the effect of the order of measurements with the two instruments and the effect of different observers (both can be considered to have been random, but there was no systematic protocol), and the limited number of participants undergoing imaging; however, a considerable number of lung scans were analyzed and clearly demonstrated, for the first time, that the performances of the portable and high-end scanners were interchangeable. The use of portable ultrasound devices has increased in recent years, creating a flourishing market. A big advantage of portable devices is time saved at the bedside and in pre-hospital situations; limits are battery duration, narrow field of vision and low penetration (European Society of Radiology 2019; Stock et al., 2015). In COVID-19 patients, these devices could be of help for triage and in providing instant and objective information on the severity of the disease, and they may avoid the need for further imaging in individuals with mild cases. However, findings are not specific and may not correlate with clinical outcomes, and qualified operators are necessary; combination with clinical and physiologic data is strongly recommended. The utility of portable devices has been argued by several authors (Gibson et al. 2020; Qian et al. 2020), but this is the first study providing a demonstration of their use in daily clinical practice with COVID-19 patients.
In conclusion, our study confirms the possibilities of portable ultrasound imaging of the lung in COVID-19 patients. Portable pocket-sized ultrasound scanners are cheap, easy to handle and equivalent to standard scanners for non-invasive assessment of severity and dynamic observation of lung lesions in COVID-19 patients with pneumonia. These ultrasound scanners can play a decisive role when health care resources are scarce, during pandemics and in emergency situations, such as the present COVID-19 outbreak.
Conflict of interest disclosure
The authors declare no competing interests.
References
- Bland J.M., Altman D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- Bunting K.V., Steeds R.P., Slater L.T., Rogers J.K., Gkoutos G.V., Kotecha D. A practical guide to assess the reproducibility of echocardiographic measurements. J Am Soc Echocardiogr. 2019;32:1505–1515. doi: 10.1016/j.echo.2019.08.015. [DOI] [PubMed] [Google Scholar]
- Buonsenso D., Pata D., Chiaretti A. COVID-19 outbreak: Less stethoscope, more ultrasound. Lancet Respir Med. 2020;8:e27. doi: 10.1016/S2213-2600(20)30120-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capecchi P.L., Lazzerini P.E., Volterrani L., Mazzei M.A., Rossetti B., Zanelli G., Bennett D., Bargagli E., Franchi F., Cameli M., Valente S., Cantarini L., Frediani B. Antirheumatic agents in COVID-19: Is IL-6 the right target? Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-217523. Accessed Apr 16, 2020. [e-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conticini E., Bargagli E., Bardelli M., Rana G.D., Baldi C., Cameli P., Gentileschi S., Bennett D., Falsetti P., Lanzarone N., Bellisai F., Barreca C., D'Alessandro R., Cantarini L., Frediani B. COVID-19 pneumonia in a large cohort of patients treated with biological and targeted synthetic antirheumatic drugs. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-217681. Accessed May 15, 2020. [e-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- European Society of Radiology ESR statement on portable ultrasound devices. Insights Imaging. 2019;10:89. doi: 10.1186/s13244-019-0775-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L., Coppola S., Cressoni M., Busana M., Rossi S., Chiumello D. COVID-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;201:1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson L.E., Bittner E.A., Chang M.G. Handheld ultrasound devices: An emerging technology to reduce viral spread during the Covid-19 pandemic. Am J Infect Control. 2020;48:968–969. doi: 10.1016/j.ajic.2020.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins C., Surda P., Kumar N. Presentation of new onset anosmia during the COVID-19 pandemic. Rhinology. 2020;58:295–298. doi: 10.4193/Rhin20.116. [DOI] [PubMed] [Google Scholar]
- Liao J.J. Quantifying an agreement study. Int J Biostat. 2015;11:125–133. doi: 10.1515/ijb-2014-0030. [DOI] [PubMed] [Google Scholar]
- Lichtenstein D.A. BLUE-protocol and FALLS-protocol: Two applications of lung ultrasound in the critically ill. Chest. 2015;147:1659–1670. doi: 10.1378/chest.14-1313. [DOI] [PubMed] [Google Scholar]
- Liu J.Y., Xu J., Forsberg F., Liu J. CMUT/CMOS-based Butterfly iQ—a portable personal sonoscope. Adv Ultrasound Diagn Ther. 2019;3:115–118. [Google Scholar]
- Lovato A., de Filippis C. Clinical presentation of COVID-19: A systematic review focusing on upper airway symptoms. Ear Nose Throat J. 2020 doi: 10.1177/0145561320920762. Accessed Apr 13, 2020. [e-pub ahead of prints] [DOI] [PubMed] [Google Scholar]
- Lu M.J., Zhong W.H., Liu Y.X., Miao H.Z., Li Y.C., Ji M.H. Sample size for assessing agreement between two methods of measurement by Bland-Altman method. Int J Biostat. 2016;12 doi: 10.1515/ijb-2015-0039. [DOI] [PubMed] [Google Scholar]
- Lu W., Zhang S., Chen B., Chen J., Xian J., Lin Y., Shan H., Su Z.Z. A clinical study of noninvasive assessment of lung lesions in patients with coronavirus disease-19 (COVID-19) by bedside ultrasound. Ultraschall Med. 2020;41:300–307. doi: 10.1055/a-1154-8795. [DOI] [PubMed] [Google Scholar]
- Manivel V., Lesnewski A., Shamim S., Carbonatto G., Govindan T. CLUE: COVID-19 lung ultrasound in emergency department. Emerg Med Australas. 2020;32:694–696. doi: 10.1111/1742-6723.13546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R.J. Pathogenesis of COVID-19 from a cell biology perspective. Eur Respir J. 2020;55 doi: 10.1183/13993003.00607-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojoli F., Bouhemad B., Mongodi S., Lichtenstein D. Lung ultrasound for critically ill patients. Am J Respir Crit Care Med. 2019;199:701–714. doi: 10.1164/rccm.201802-0236CI. [DOI] [PubMed] [Google Scholar]
- Nouvenne A., Zani M.D., Milanese G., Parise A., Baciarello M., Bignami E.G., Odone A., Sverzellati N., Meschi T., Ticinesi A. Lung ultrasound in COVID-19 pneumonia: Correlations with chest CT on hospital admission. Respiration. 2020;99:617–624. doi: 10.1159/000509223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian F., Zhou X., Zhou J., Liu Z., Nie Q. A valuable and affordable handheld ultrasound in combating COVID-19. Crit Care. 2020;24:334. doi: 10.1186/s13054-020-03064-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remuzzi A., Remuzzi G. COVID-19 and Italy: What next? Lancet. 2020;395:1225–1228. doi: 10.1016/S0140-6736(20)30627-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M.J., Hayward S.A., Innes S.M., Miller A.S.C. Point-of-care lung ultrasound in patients with COVID-19—a narrative review. Anaesthesia. 2020;75:1096–1104. doi: 10.1111/anae.15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldati G., Smargiassi A., Inchingolo R., Buonsenso D., Perrone T., Briganti D.F., Perlini S., Torri E., Mariani A., Mossolani E.E., Tursi F., Mento F., Demi L. Is there a role for lung ultrasound during the COVID-19 pandemic? J Ultrasound Med. 2020;39:1459–1462. doi: 10.1002/jum.15284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock K.F., Klein B., Steubl D., Lersch C., Heemann U., Wagenpfeil S., Eyer F., Clevert D.A. Comparison of a pocket-size ultrasound device with a premium ultrasound machine: Diagnostic value and time required in bedside ultrasound examination. Abdom Imaging. 2015;40:2861–2866. doi: 10.1007/s00261-015-0406-z. [DOI] [PubMed] [Google Scholar]
- Wan S., Xiang Y., Fang W., Zheng Y., Li B., Hu Y., Lang C., Huang D., Sun Q., Xiong Y., Huang X., Lv J., Luo Y., Shen L., Yang H., Huang G., Yang R. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. 2020;92:797–806. doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Gargani L., Barskova T., Furst D.E., Cerinic M.M. Usefulness of lung ultrasound B-lines in connective tissue disease-associated interstitial lung disease: A literature review. Arthritis Res Ther. 2017;19:206. doi: 10.1186/s13075-017-1409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J., Li M., Lv G., Lu Z.K. Monitoring transmissibility and mortality of COVID-19 in Europe. Int J Infect Dis. 2020;95:311–315. doi: 10.1016/j.ijid.2020.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieleskiewicz L., Markarian T., Lopez A., Taguet C., Mohammedi N., Boucekine M., Baumstarck K., Besch G., Mathon G., Duclos G., Bouvet L., Michelet P., Allaouchiche B., Chaumoître K., Di Bisceglie M., Leone M., AZUREA Network Comparative study of lung ultrasound and chest computed tomography scan in the assessment of severity of confirmed COVID-19 pneumonia. Intensive Care Med. 2020;46:1707–1713. doi: 10.1007/s00134-020-06186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]