Abstract
Background
The unique anthropological coronavirus which has been titled as SARS-CoV-2 was originally arisen in late 2019 in Wuhan, China affecting respiratory infection named as COVID-19. Coronavirus is disturbing human life in an exceptional method and has converted a public health global crisis. Natural products are ahead consideration due to the huge beneficial window and effective anti-inflammatory, immunomodulatory, antioxidant and antiviral possessions. Consequently, the present study was intended to display inhibition ability of natural products coumarins and their analogues against SARS coronavirus.
Methods
The present study, aims to forecast theoretical assembly for the protease of COVID-19 and to discover advance whether this protein may assist as a target for protease inhibitors such as psoralen, bergapten, imperatorin, heraclenin, heraclenol, saxalin, oxepeucedanin, angelicin, toddacoumaquinone, and aesculetin. The docking score of these natural coumarin analogues compared with standard Hydroxychloroquine. Whereas the 3D assembly of main protease of SARS coronavirus was forecast with SWISS MODEL web server, and molecular interaction studies amongst target protein and ligands were done with AutoDock Vina software.
Results
The study more exposed that all the inhibitors acquired with negative dock energy against the target protein. Molecular docking investigation displayed that natural coumarin analogue toddacoumaquinone displayed a remarkable inhibition ability with the binding energy of −7.8 kcal/mol than other compounds against main protease of SARS coronavirus in intricate with α-ketoamide (PDB ID: 5N5O). The synthetic coumarin analogue (1 m) also displayed the comparable inhibition ability with a binding energy of −7.1 kcal/mol against main protease of SARS coronavirus in intricate with α-ketoamide. Keeping the overhead results of ADME and toxicity, all tested compounds were recognized as drug-like nature, passing Lipinski’s “Rule of 5” with 0 violation except α-ketoamide passes Lipinski’s “Rule of 5” with 1 violation MW > 500. The projected constraints are within the assortment of recognized values.
Conclusions
Based upon the results of the manifold sequence alliance, natural and synthetic coumarin binding sites were preserved. The present in silico examination thus, delivers structural awareness about the protease of COVID-19 and molecular relations with some of the recognised protease inhibitors.
Keywords: Coumarin, COVID-19, Molecular docking, Virtual ADME, 5N5O protein
Introduction
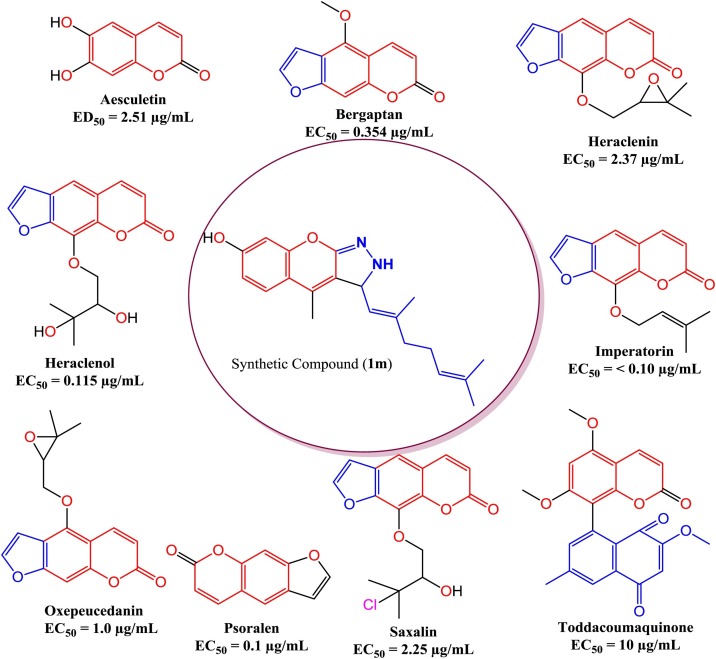
Coronaviruses (CoVs) are an etiologic negotiator of severe infections in both animals and humans, which may origin sickness in respiration territory and also in the digestive area. Earlier inspections of CoVs stated that CoVs can affect assured kinds of animals, as well as reptiles, avian species and mammals [1]. At the end of 2019, the novel species of CoV was recognized and originally termed 2019-nCoV, and occurred through an eruption in Wuhan, China [2]. On January 30, 2020, The Emergency Committee of the WHO acknowledged an eruption in China, which was deliberated to be a Public Health Emergencies of International Concern (PHEIC) [3]. Formally, on February 11, 2020, WHO termed this CoV COVID-19 (coronavirus disease 2019) upon talks and associations with the World Organization for Animal Health and the Food and Agriculture Organization of the United Nations [4]. Now, no precise remedies for COVID-19 are obtainable and research concerning the dealing of COVID-19 is deficient [3]. Yet, the actions that have been instigated persist restricted to protective and compassionate treatments, intended to avoid additional problems and organ injury [3]. As a structural glycoprotein on the virion surface, the main protease of coronavirus is accountable for binding to host cellular receptors and the following fusion between the viral envelope and the cellular membrane [5] and essential for virus replication [6]. Ecological features can significantly stimulus the exudation of secondary metabolites like phytochemicals from tropical plants. Consequently, secondary metabolites concealed by plants in tropical areas prodigious consideration and that may be advanced as remedies [7,8]. Numerous phytochemicals from therapeutic plants, have been testified for antiviral activity [[9], [10], [11]]. Fig. 1 shows that various natural coumarin antiviral agents against viral active Looking for natural anti-AIDS agents several furanocoumarins namely, psoralen (EC50 = 0.1 μg/mL), bergapten (EC50 = 0.354 μg/mL), Imperatorin (EC50 = <0.10 μg/mL), heraclenin (EC50 = 2.37 μg/mL), heraclenol (EC50 = 0.115 μg/mL), saxalin (EC50 = 2.25 μg/mL), and oxepeucedanin (EC50 = 1.0 μg/mL) have been isolated from diverse medicinal plants viz. Prangos tschimganica, and Ferula sumbul and displayed important anti-HIV activity by preventing HIV repetition in H9 lymphocytes [12,13]. An angular furanocoumarin for example angelicin was designate benign natural bio-active element of anti-influenza activity by inhibiting influenza viruses A and B [14]. Ishikawa et al.1995, reported a distinctive dimer of coumarin-naphthoquinone titled toddacoumaquinone isolated from Toddalia asiatica (Rutaceae) and assessed against HIV-1, HSV-1 and HSV-2 for antiviral activity evaluation. The isolated coumarin was originated to be inadequately active against HSV (EC50 = 10 μg/mL) and also ineffectual in contradiction of HIV [15]. A dihydroxy derivative of coumarin termed as aesculetin, acquired from the medicinal plant Artemisia capillaris aerial part and too, described as an anti-HIV agent with ED50 value of 2.51 μg/mL [16]. Keep this in mind, we examined natural coumarin analogues psoralen, bergapten, imperatorin, heraclenin, heraclenol, saxalin, oxapeucedanin, angelicin, toddacoumaquinone, aesculetin as potential inhibitor candidates for protease of SARS coronavirus in intricate with α-ketoamide and compared with hydroxychloroquine and coumarin analogue (1 m). The outcomes of this study will offer other investigators with prospects to find the precise medication to fight COVID-19.
Fig. 1.
Antiviral active various natural coumarin compounds.
Material and methods
The starting material 7-hydroxy-4-methylcoumarin was bought from Sigma-Aldrich and used as received. Chemical characterization was used as following instruments such as Shimadzu 8201 pc for IR spectra on KBr pellets at 4000–400 cm−1, Bruker DRX instrument at 300 MHz was used to inspect 1H & 13C NMR spectra. The elements C, H, N present in synthetic compounds were analyzed with Elemental analyzer (Varian EL III). Thin layer chromatography (TLC) was used to check the purity of the compounds.
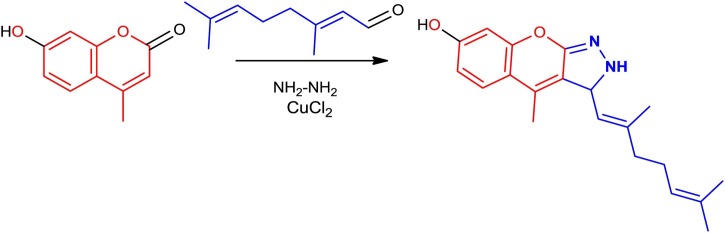
Preparation of synthetic coumarin analogue (1 m)
The synthetic coumarin analogue used in this study was prepared for our previous literature [17]. The mixture of 7-hydroxy-4-methyl-2H-chromen-2-one (0.001 mol, 0.17 g), citral (0.003 mol, 0.5 mL), hydrazine hydrate (0.003 mol, 0.1 mL), potassium phosphate buffer (pH 6.0) and CuCl2 (0.1 mg) is mixed together with continues staring. The final product was washed with ice cold water and purity was confirmed by TLC.
3-(2,6-dimethylhepta-1,5-dien-1-yl)-4-methyl-2,3-dihydrochromeno[2,3-c]pyrazol-7-ol (1 m)
Yield: 86%; mp 168 °C; IR (KBr): 3174.54, 3074.57, 3034, 2596.43 cm−1; 1H NMR (DMSO-d6): δ 10.5 (s, 1 H), 8.70 (s,1 H), 7.04–7.06 (d, J = 6.21 Hz, 1 H), 6.40 (s, 1 H), 6.21−6.18 (d, 1H, J = 6.21 Hz), 5.33−5.30 (d, 1H, J = 6.21 Hz), 5.20−5.17 (d, 1H, J = 6.21 Hz), 3.99−3.96 (d, 1H, J = 6.21 Hz), 2.42−2.39 (s, 3 H), 2.00−1.98 (d, 4H, J = 6.21 Hz), 1.81−1.78 (d, 6H, J = 6.21 Hz), 1.70 (d, 3H, J = 6.21 Hz); 13C NMR (DMSO-d6): 159.5 (1C), 155.7 (1C), 150.6 (1C), 137.2 (1C), 135.5 (1C), 132.0 (1C), 123.5 (1C), 116.7 (1C), 127.2, 108.4, 107.9, 99.5 (4C), 122.3 (1C), 39.8 (1C), 39.3 (1C), 26.4 (1C), 24.6 (1C), 18.6 (1C), 16.5 (1C,), 15.7(1C); EI-MS: m/z 325.19 (M+, 20%).
Molecular docking
In this studies, inspect the binding mode and interaction between compounds psoralen, bergapten, imperatorin, heraclenin, heraclenol, saxalin, oxepeucedanin, angelicin, toddacoumaquinone, aesculetin, hydroxychloroquine, synthetic coumarin analogue (1 m) and co-crystallized ligand α-ketoamide with protein 5N5O were used to analysis via Autodock vina 1.1.2 [18]. The crystal structure of the main protease of SARS coronavirus in complex with α-ketoamide (PDB ID: 5N5O) was downloaded from Protein Data Bank. The 3D structures of the ligands were drawn and energy minimized via ChemDraw Ultra 12.0 software. The binding pocket was identified by using co-crystallized ligand α-ketoamide via discovery studio program and the amino acid residues Thr26, His41, Met49, Phe140, Leu141, Asn142, Gly143, Ser144, Cys145, His163, His164, Met165, Glu166, His172, Asp187, Gln189 and Thr190 were situated in the binding pocket. The search grid of 5N5O protein was recognized as center_x: -23.002, center_y: -3.023, and center_z: 4.681 with size dimensions x: 24, y: 24, and z: 24 with 1.0 Å spacing. The value of exhaustiveness was set to 8. The further constraints were fixed to default for Autodock Vina and not stated. Discovery studio 2019 program was used to investigate visually the best binding affinity of compounds.
ADME and molecular property prediction
In this present study, Lipinski’s “Rule of five” was predicted by theoretical in silico ADME and toxicity of compounds psoralen, bergapten, imperatorin, heraclenin, heraclenol, saxalin, oxepeucedanin, angelicin, toddacoumaquinone, aesculetin, hydroxychloroquine, synthetic coumarin analogue (1 m) and α-ketoamide [19]. A web tool of Swiss ADME was used to predict the Lipinski’s parameters [20]. Topological polar surface area (tPSA) was used to forecast the bioavailability and the transportation of an effective compound via the blood-brain barrier [21]. Bioavailability is extremely multifactorial, then predominantly obsessed through gastrointestinal absorption [22]. The percentage of absorption was calculated from the formula: % ABS = 109 – (0.345 x TPSA). We also projected the water solubility, CYP2D6, CYP2D9, P-glycoprotein inhibition and phospholipidosis (PLD) too.
Result and discussion
Chemistry
The synthesis compounds 1 m was outline in Scheme 1 . The reaction of 7-hydroxy-4-methyl-2H-chromen-2-one was reacted with citral, hydrazine hydrate, and 50 mM potassium phosphate buffer, with using CuCl2 as a catalysis. The compound was recrystallized by suitable alcohol to get pure brown color solid product. The key projections of compound were confirmed by IR, 1 H NMR, and 13C NMR analyses. The FT-IR spectra embrace absorption bands at 2596, 3034 and 3174 cm−1, conforming the -C = N, Ar-H and -NH functional groups. The 1H NMR spectra displays peaks at δ 10.50, 8.70, 5.33−5.30, 5.20−5.17, 3.99−3.96, 2.42, 2.00−1.98 and 1.81−1.78 ppm, conforming the -OH, -NH, -C = C, -CH = C, 4-CH, -CH3, -CH2-CH2-, and -(CH3)2 protons respectively. The 13C NMR spectra displayed signals at δ 159.5, 155.7, 150.6, 137.2, 123.5, 39.3, and 15.7 ppm, conforms -C-OH, -C–O-, -C = N, -C–CH3, -C = C-, -C-NH, and -CH3 carbon atoms. The compound was further confirmed by mass spectroscopy and elemental analysis.
Scheme 1.
Synthesis of coumarine derivative 1m.
Docking studies
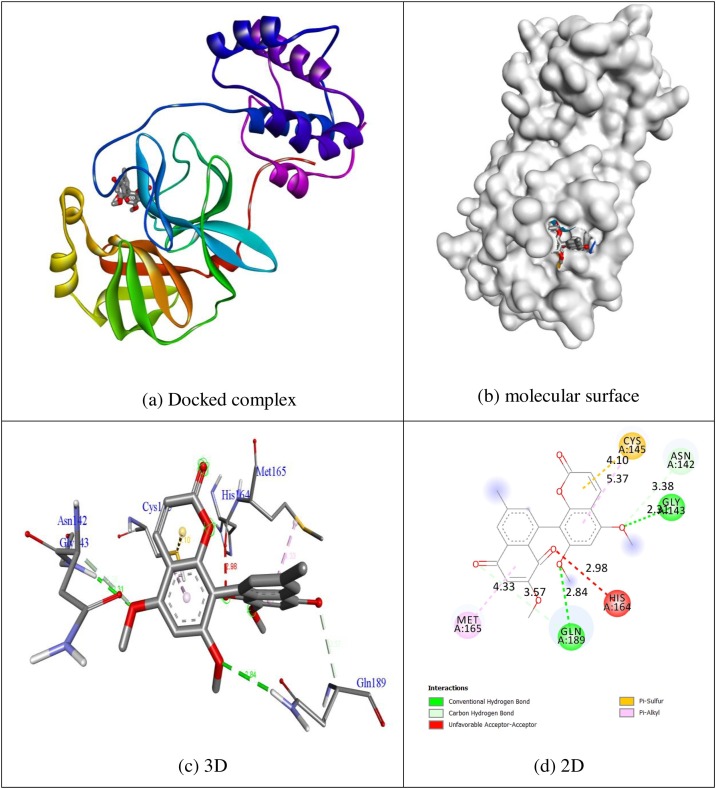
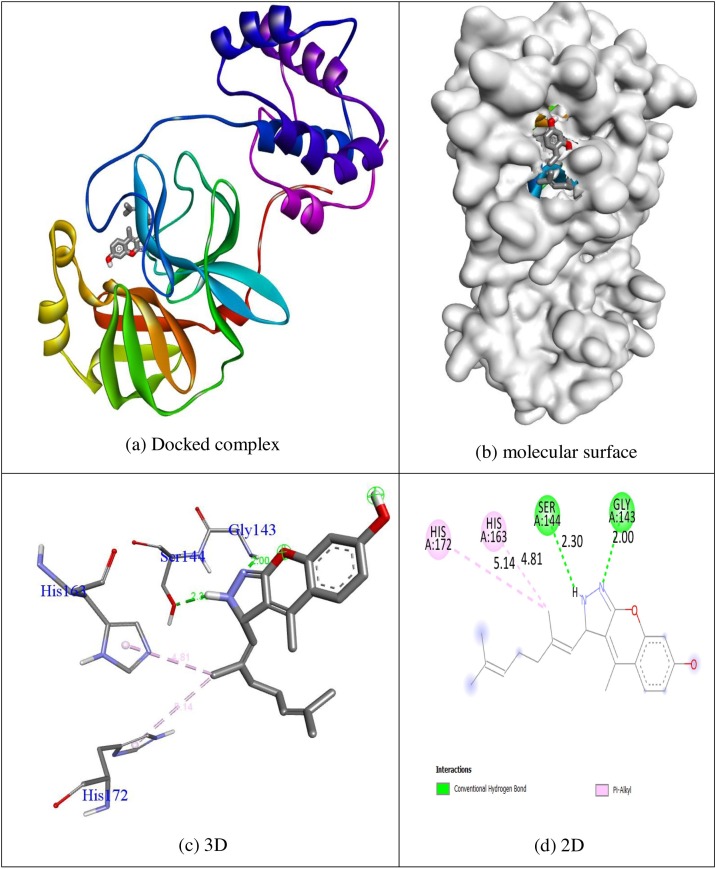
In order to advance perception into the plausible mechanism of biological activities docking, simulations were performed. The compounds psoralen, bergapten, imperatorin, heraclenin, heraclenol, saxalin, oxepeucedanin, angelicin, toddacoumaquinone, aesculetin, hydroxychloroquine, synthetic coumarin analogue (1 m) and co-crystallized ligand α-ketoamide were considered for their docking behaviour of protein 5N5O via Autodock Vina program. All of this tested inhibitors shows negative binding energy. The natural quinone containing coumarin toddacoumaquinone shows remarkable binding affinity (−7.8 kcal/mol) than other compounds psoralen (−5.6 kcal/mol), bergapten (−5.8 kcal/mol), imperatorin (−6.8 kcal/mol), heraclenin (−6.8 kcal/mol) heraclenol (−7.0 kcal/mol), saxalin (−6.4 kcal/mol), oxepeucedanin (−6.8 kcal/mol), angelicin (−5.6 kcal/mol), aesculetin (−6.0 kcal/mol), hydroxychloroquine (−5.8 kcal/mol) and co-crystallized ligand α-ketoamide (−6.6 kcal/mol) in 5N5O protein respectively. The synthesized coumarin analogue (1 m) shows significant inhibition ability with the binding energy of −7.1 kcal/mol. All of the tested ligands shows significant inhibition ability than in-build co-crystallized ligand α-ketoamide with the binding energy of −6.6 kcal/mol except psoralen, bergaptan, saxalin, angelicin, aesculetin and hydroxychloroquine. Hydrogen bonding plays a major role in bonding stability between protein and ligand, and the approving bond distance is less than 3.5 Å amongst H-donor and H-acceptor atoms [23]. The hydrogen bond distances of relevant compounds were less than 3.5 Å in target protein 5N5O indicates the resilient hydrogen bonding between protein and ligands. Compound toddacoumaquinone displays two hydrogen bonding interaction with the receptor 5N5O. The residues of amino acids Gly143 and Gln189 were tangled in hydrogen bonding interaction with the bond lengths of 2.31 and 2.84 Å. The residues of amino acids Asn142, Cys145, His164 and Met165 were tangled in hydrophobic contacts. The hydrogen bonding and hydrophobic contacts of amino acid residues in 5N5O protein with compound toddacoumaquinone was shown in Fig. 2 . The synthesized coumarin analogue (1 m) displays two hydrogen bond interface through the receptor 5N5O. The residues of amino acids Gly143 (bond length: 2.00 Å) and Ser144 (bond length: 2.30 Å) were tangled in hydrogen bonding contacts with the bond lengths of 2.00 and 2.30 Å respectively. The residues amino acids His163 and His172 were tangled in hydrophobic contacts. The hydrogen bonding and hydrophobic contacts of amino acid residues in 3T9T protein with synthesized coumarin analogue (1 m) was shown in Fig. 3 . The fallouts displayed that the compound toddacouma- quinone having the remarkable inhibition ability than other compounds in respective target protein. The outcomes were abridged in Table 1 .
Fig. 2.
Molecular docking interaction of toddacoumaquinone with binding site of 5N5O protein.
Fig. 3.
Molecular docking interaction of 1 m with binding site of 5N5O protein.
Table 1.
Molecular docking interaction of compounds (1a-1 m) and co-crystallized ligand α-ketoamide against 5N5O.
| Compounds | Main protease of SARS coronavirus (PDB ID: 5N5O) |
||
|---|---|---|---|
| Binding affinity (kcal/mol) | No. of H-bonds | H-bonding residues | |
| Psoralen (1a) | −5.6 | 0 | – |
| Bergapten (1b) | −5.8 | 0 | – |
| Imperatorin(1c) | −6.8 | 1 | Cys145 |
| Heraclenin(1d) | −6.8 | 1 | Cys145 |
| Heraclenol(1e) | −7.0 | 5 | Leu141, Gly143, Ser144, Gln189 |
| Saxalin(1f) | −6.4 | 2 | Glu166, Gln189 |
| Oxepeucedanin(1 g) | −6.8 | 0 | – |
| Angelicin(1 h) | −5.6 | 0 | – |
| Toddacoumaquinone(1i) | −7.8 | 2 | Gly143, Gln189 |
| Aesculetin(1 j) | −6.0 | 3 | Ser144, Cys145, Glu166 |
| α-ketoamide(1k) | −6.6 | 0 | – |
| Hydroxychloroquine(1 l) | −5.8 | 2 | Ser144, Cys145 |
| Synthetic compound (1 m) | −7.1 | 2 | Gly143, Ser144 |
ADME property prediction
The bioactive compounds were checked via oral bioavailability, which is plays a key part in compounds for healing agents [24]. The representation such as hydrogen-bounding capacity, low polar surface area, reduced molecular flexibility and intestinal absorption were the main forecasters of this study [25]. All tested compounds passes Lipinski’s “Rule of 5” with 0 violation except α-ketoamide passes Lipinski’s “Rule of 5” with 1 violation MW > 500 (Table 2 ). The conformational changes of molecules were described by the number of rotatable bonds and also, for the binding ability to receptors. The tested compounds except α-ketoamide (17 rotatable bonds) devising under 10 rotatable bonds and without chirality centre, pass one of the oral bioavailability conditions, displaying low conformational flexibility. The passive molecular transport over membranes, as well as the blood–brain barrier was correlated with the property topological polar surface area (tPSA) [21]. The tested compounds having tPSA value of <140 Å2 passes the standards for gastro-intestinal absorption, later oral administration criteria. In contrast, all tested compounds except α-ketoamide (tPSA = 136.63 Å2) and toddacoumaquinone (tPSA = 92.04 Å2) to have a low blood-brain barrier penetration (tPSA >90 Å2), that indicates the side effects of central nervous system (CNS) are compact or inattentive in compounds α-ketoamide and toddacoumaquinone but not in other compounds. The tested compounds displayed absorption percent of (%Abs = >50), which indicates high bioavailability. The acceptable bioavailability through oral route was (>50%). The tested compounds displayed excellent water solubility (-logS value of >−4) except compounds imperaotaarin (-logS value of −4.00) and toddacoumaquinone (-logS value of −4.73) shows moderate water solubility. The liver dysfunction side effect was not anticipated upon the direction of compounds aesculetin, angelicin, bergaptan, heraclenol, imperatorin, α-ketoamide, psoralen and toddacoumaquinone because it predicted as non-inhibitors of CYP2D6 and that was anticipated in heraclenin, hydroxychloroquine, oxepeucedanin and saxalin due to the property of CYP2D6 inhibitor. The member of ATP-binding cassette transporter family P-glycoprotein (P-gp) involves in brain penetration, intestinal absorption, and drug metabolism, and its reticence can extremely modify the drug’s bioavailability and protection [26]. Drug persuaded phospholipidosis is a syndrome regarded by the additional accretion of phospholipids in tissues and that related with drug encouraged toxicity [27]. The outcomes shows that the tested compounds were not a component for P-gp and that were not encourage phospholipidosis. Keeping the overhead results of ADME and toxicity, the compounds synthesized coumarin analogue(1 m), α-ketoamide and toddacoumaquinone shows respectable pharmacokinetic properties, and short gastrointestinal absorption and without blood-brain barrier penetration. All tested compounds were recognized as drug-like nature, passing Lipinski’s “Rule of 5” with 0 violation except α-ketoamide passes Lipinski’s “Rule of 5” with 1 violation MW > 500. The projected constraints are within the assortment of recognized values.
Table 2.
The compounds (1a-1 m) and co-crystallized ligand α-ketoamide prediction of the potent of ADME value.
| Compounds No. | tPSA | %Abs | MW | RoB | HBD | HBA | MR | IlogP (MlogP) |
LogS | CYP2D6 Inhibitor |
|---|---|---|---|---|---|---|---|---|---|---|
| Rule | ≤140 ´Å2 | >50 | ≤500 | ≤10 | ≤5 | ≤10 | 40–130 | <5 | >−4 | – |
| Psoralen (1a) | 43.35 | 94.04 | 186.16 | 0 | 0 | 3 | 52.26 | 2.01 (1.48) |
−2.73 | No |
| Bergapten (1b) | 52.58 | 90.85 | 216.19 | 1 | 0 | 4 | 58.75 | 2.29 (1.18) |
−2.93 | No |
| Imperatorin (1c) | 52.58 | 90.85 | 270.28 | 3 | 0 | 4 | 77.50 | 3.05 (2.14) |
−4.00 | No |
| Heraclenin (1d) | 65.11 | 86.53 | 286.28 | 3 | 0 | 5 | 76.98 | 3.00 (1.39) |
−3.33 | Yes |
| Heraclenol (1e) | 93.04 | 76.90 | 304.29 | 4 | 2 | 6 | 80.34 | 2.57 (0.57) |
−2.73 | No |
| Saxalin (1f) | 72.81 | 83.88 | 322.74 | 4 | 1 | 5 | 83.97 | 2.95 (1.63) |
−3.65 | Yes |
| Oxepeucedanin (1 g) | 65.11 | 86.53 | 286.28 | 3 | 0 | 5 | 76.98 | 3.00 (1.39) |
−3.29 | Yes |
| Angelicin (1 h) | 43.35 | 94.04 | 186.16 | 0 | 0 | 3 | 52.26 | 2.03 (1.48) | −2.99 | No |
| Toddacoumaquinone (1i) | 92.04 | 77.24 | 406.38 | 4 | 0 | 7 | 109.56 | 3.17 (1.08) |
−4.73 | No |
| Aesculetin (1 j) | 70.67 | 84.61 | 178.14 | 0 | 2 | 4 | 46.53 | 1.25 (0.45) | −2.28 | No |
| α-Ketoamide (1k) | 136.63 | 61.86 | 534.65 | 17 | 5 | 5 | 150.47 | 3.58 (1.11) |
−3.72 | No |
| Hydroxychloroquine (1 l) | 48.39 | 92.30 | 335.87 | 9 | 2 | 3 | 98.57 | 3.58 (2.35) |
−3.91 | Yes |
| Synthetic compound (1 m) | 53.85 | 90.42 | 324.42 | 4 | 2 | 3 | 106.48 | 3.42 (3.82) |
−4.92 | No |
Conclusion
At this time, COVID-19 has occurred in the human populace, in China, and is a prospective risk to entire healthiness, worldwide. Still, there is no clinically agreed medicine to cure the illness. The presently accessible medicines for COVID-19 dealing on main protease. The present study, inspect some therapeutic plant-derived coumarin analogues that may be cast-off to combat the COVID-19. Psoralen, bergapten, imperatorin, heraclenin, heraclenol, saxalin, oxepeucedanin, angelicin, toddacoumaquinone, aesculetin were the utmost suggested compounds originate in therapeutic plants that might act as significant inhibitors of COVID-19 main protease (PDB ID: 5N5O). Molecular docking studies showed that natural coumarin analogue toddacoumaquinone displayed a remarkable inhibition ability with the binding energy of −7.8 kcal/mol than other compounds. The synthetic coumarin analogue synthesized coumarin analogue (1 m) also displayed the comparable inhibition ability through a binding energy of −7.1 kcal/mol. ADME properties of the compound synthesized coumarin analogue (1 m), α-ketoamide and toddacoumaquinone shows respectable pharmacokinetic properties, and short gastrointestinal absorption and without blood-brain barrier penetration. All tested compounds were recognized as drug-like nature, passing Lipinski’s “Rule of 5” with 0 violation except α-ketoamide passes Lipinski’s “Rule of 5” with 1 violation MW > 500. The projected constraints are within the assortment of recognized values. However, advance investigation is essential to inspect the probable uses of these coumarin compounds.
Funding
No funding source.
Competing interests
None declared.
Ethical approval
Not required.
Acknowledgments
This work was funded by Researchers Supporting Project number (RSP-2020/27), King Saud University, Riyadh, Saudi Arabia.
References
- 1.Malik W., Sircar Y.S., Bhat S., Sharun S., Dhama K., Dadar K. Emerging novel Coronavirus (2019-nCoV) - current scenario, evolutionary perspective based on genome analysis and recent developments. Vet Q. 2020;40:1–12. doi: 10.1080/01652176.2020.1727993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee P.R., Hsueh P.I. Emerging threats from zoonotic coronaviruses-from SARS and MERS to 2019-nCoV. J Microbiol Immunol Infect. 2020:1–3. doi: 10.1016/j.jmii.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodríguez-Morales A.J., MacGregor K., Kanagarajah S., Patel D., Schlagenhauf P. Going global – travel and the 2019 novel coronavirus. Travel Med Infect Dis. 2020;33:101578. doi: 10.1016/j.tmaid.2020.101578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization (WHO) 2020. Novel coronavirus (2019-nCoV) WHO bull no. JANUARY; pp. 1–7. [Google Scholar]
- 5.Hofmann H., Pohlmann S. Cellular entry of the SARS coronavirus. Trends Microbiol. 2004;12:466–472. doi: 10.1016/j.tim.2004.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hilgenfeld R. From SARS to MERS: crystallographic studies on coronaviral proteases enable antiviral drug design. FEBS J. 2014;281:4085–4096. doi: 10.1111/febs.12936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guerriero G., Berni R., Armando Muñoz-Sanchez J., Apone F., Abdel-Salam E.M., Qahtan A.A. Production of plant secondary metabolites: examples, tips and suggestions for biotechnologists. Genes (Basel) 2018;9:34–46. doi: 10.3390/genes9060309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang L., Wen K.S., Ruan X., Zhao Y.X., Wei F., Wang Q. Response of plant secondary metabolites to environmental factors. Molecules. 2018;23:1–26. doi: 10.3390/molecules23040762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zakaryan H., Arabyan E., Oo A., Zandi K. Flavonoids: promising natural compounds against viral infections. Arch Virol. 2017;162:2539–2551. doi: 10.1007/s00705-017-3417-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thayil Seema M., Thyagarajan S.P. Pa-9: a flavonoid extracted from plectranthus amboinicus inhibits HIV-1 protease. Int J Pharmacogn Phytochem Res. 2016;8:1020–1024. [Google Scholar]
- 11.Jo S., Kim S., Shin D.H., Kim M.S. Inhibition of SARS-CoV 3CL protease by flavonoids. J Enzyme Inhib Med Chem. 2020;35:45–151. doi: 10.1080/14756366.2019.1690480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhoua P., Takaishia Y., Duana H., Chena B., Hondab G., Itohb M. Coumarins and bicoumarin from Ferula sumbul: anti-HIV activity and inhibition of cytokine release. Phytochem. 2000;53:689–697. doi: 10.1016/S0031-9422(99)00554-3. [DOI] [PubMed] [Google Scholar]
- 13.Shikishima Y., Takaishi Y., Honda G., Ito M., Takeda Y., Kodzhimatov O.K. Chemical constituents of Prangos tschimganica structure elucidation and absolute configuration of coumarin and furanocoumarin derivatives with anti-HIV activity. Chem Pharm Bull. 2001;49:877–880. doi: 10.1248/cpb.49.877. [DOI] [PubMed] [Google Scholar]
- 14.Yeh J.Y., Coumar M.S., Horng J.T., Shiao H.Y., Kuo F.M., Lee H.L. Anti-influenza drug discovery: structure-activity relationship and mechanistic insight into novel angelicin derivatives. J Med Chem. 2010;53:1519–1533. doi: 10.1021/jm901570x. [DOI] [PubMed] [Google Scholar]
- 15.Ishikawa T., Kotake K., Ishii H. Synthesis of toddacoumaquinone, a coumarinnaphthoquinone dimer, and its antiviral activities. Chem Pharm Bull. 1995;43:1039–1041. doi: 10.1248/cpb.43.1039. [DOI] [PubMed] [Google Scholar]
- 16.Wu T.S., Tsang Z.J., Wu P.L., Lin F.W., Li C.Y., Teng C.M. New constituents and antiplatelet aggregation and anti-HIV principles of Artemisia capillaris. Bioorg Med Chem. 2001;9:77–83. doi: 10.1016/S09680896(00)00225-X. [DOI] [PubMed] [Google Scholar]
- 17.Mostafa A.A.F., SathishKumar C., Al-Askar A.A., Sayed S.R.M., SurendraKumar R., Idhayadhulla A. Synthesis of novel benzopyran-connected pyrimidine and pyrazole derivatives via a green method using Cu(II)-tyrosinase enzyme catalyst as potential larvicidal, antifeedant activities. RSC Adv. 2019;9:25533–25543. doi: 10.1039/C9RA04496E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trott O., Olson A.J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lipinski C.A., Lombardo F., Dominy B.W., Feeney P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46:3–26. doi: 10.1016/S0169-409X(96)00423-1. [DOI] [PubMed] [Google Scholar]
- 20.Swiss ADME. Available online: http://www.swissadme.ch. (Accessed 10 July 2020).
- 21.Ertl P., Rohde B., Selzer P. Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J Med Chem. 2000;43:3714–3717. doi: 10.1021/jm000942e. [DOI] [PubMed] [Google Scholar]
- 22.Daina A., Zoete V. A BOILED-Egg to predict gastrointestinal absorption and brain penetration of small molecules. Chem Med Chem. 2016;11:1117–1121. doi: 10.1002/cmdc.201600182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taha M., Ismail N.H., Khan A., Shah S.A.A., Anwar A., Halim S.A. Synthesis of novel derivatives of oxindole, their urease inhibition and molecular docking studies. Bioorg Med Chem Lett. 2015;25:3285–3289. doi: 10.1016/j.bmcl.2015.05.069. [DOI] [PubMed] [Google Scholar]
- 24.Newby D., Freitas A.A., Ghafourian T. Decision trees to characterise the roles of permeability and solubility on the prediction of oral absorption. Eur J Med Chem. 2015;90:751–765. doi: 10.1016/j.ejmech.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Azam F., Madi A.M., Ali H.I. Molecular docking and prediction of pharmacokinetic properties of dual mechanism drugs that block MAO-B and Adenosine A2A receptors for the treatment of parkinson’s disease. J Young Pharm. 2012;4:184–192. doi: 10.4103/0975-1483.100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fromm M.F. P-glycoprotein: a defense mechanism limiting oral bioavailability and CNS accumulation of drugs. Int J Clin Pharmacol Ther. 2000;38:69–74. doi: 10.5414/cpp38069. [DOI] [PubMed] [Google Scholar]
- 27.Nonoyama T., Fukuda R. Drug induced phospholipidosis pathological aspects and its prediction. J Toxicol Pathol. 2008;21:9–24. doi: 10.1293/tox.21.9. [DOI] [Google Scholar]