At the beginning of the COVID-19 pandemic, UK national guidance recommended early intubation and ventilation to treat patients with COVID-19. However, multiple Chinese and Italian hospitals were already effectively utilising continuous positive airways pressure (CPAP) and high-flow nasal oxygen (HFNO) to spare ventilator capacity and intensive care resource for the most seriously ill patients, with no obvious risk to their health-care workers from virus aerosolisation. At University College London Hospital (UCLH), London, UK, we thus challenged this guidance in view of the massive anticipated demand that would not be met with existing resources.
A rate-limiting issue was the presence of only 12 stand-alone CPAP devices across the UCLH Trust, and no more were available for purchase. Engineers and intensivists at UCL met on March 17, 2020, to discuss Boris Johnson's Ventilator Challenge—an appeal to British industry to repurpose their engineering know-how and manufacturing capability to build 30 000 ventilators from scratch to augment the 8000 available for British hospitals. The clinicians impressed upon the engineers the low likelihood of timely delivery of suitably sophisticated mechanical ventilators and the severe shortage of trained staff to operate them. Their clinical requisite was for a simple CPAP device that could be designed, tested, mass-manufactured, and deployed at speed.
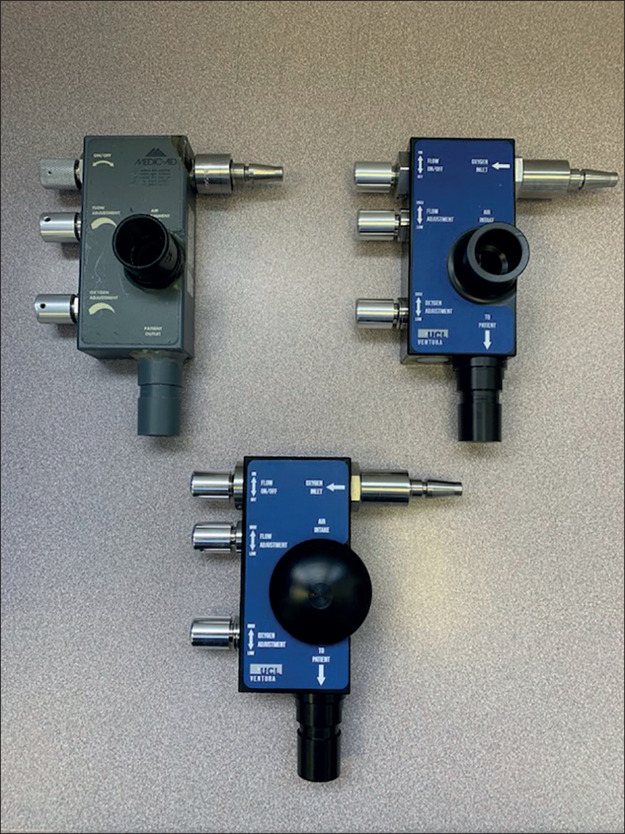
The WhisperFlow (figure 1 ), invented by Medic-Aid (Pagham, Sussex) in 1992, is a purely mechanical CPAP device that plugs directly into the hospital oxygen supply. The only controls are simple rotary valves to alter oxygen concentration and flow rate. The WhisperFlow had been superseded by more sophisticated devices with built-in oxygen analysers, sensors, and alarm systems, and the patent had expired in 2019. Reverse engineering this device would be relatively straightforward, but mass production required an industry partner to provide rapid design and high-precision manufacture. Contact was made with Mercedes AMG High Performance Powertrains (HPP; Brixworth, Northants) who design and build Formula 1 racing car engines. They immediately contributed their considerable expertise and resources into the endeavour.
Figure 1.
CPAP devices
Whisperflow device (top left), and the UCL Ventura CPAP Mark I (top right) and Mark II (bottom) devices. CPAP=continuous positive airway pressure. UCL=University College London.
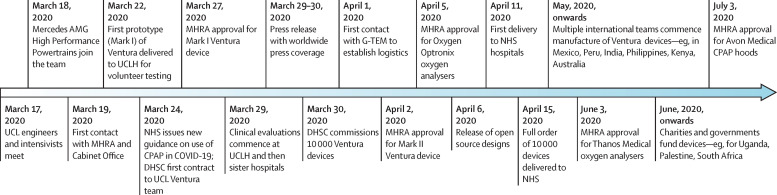
Two WhisperFlow devices were sourced from the UCLH anaesthetic department museum and eBay. Three-dimensional geometries were reconstructed using computer-aided design and verified by CT. Material components were fully characterised. Computational fluid mechanics (CFD) simulators, normally used to inform F1 engine design, characterised air-oxygen flow structures under conditions mimicking clinical use. Exact replicas of the Ventura device were made and bench tested. Just 4 days from starting, the intensivists successfully self-tested the first UCL Ventura device (figure 2 ). For patient use during the pandemic, special authorisation was required from the Medicines and Healthcare products Regulatory Agency (MHRA). Instead of their usual 18–24 month process, they gave approval within 36 h of receipt of a Mark I device and the technical–manufacturing dossier, with confirmatory patient trials performed soon after.
Figure 2.
Development timeline for the UCL Ventura CPAP device
CPAP=continuous positive airway pressure. DHSC=Department of Health and Social Care. MHRA=Medicines and Healthcare products Regulatory Agency. UCLH=University College London Hospital.
The next challenge concerned oxygen. British hospitals had not been traditionally designed, nor upgraded, to cope with a large influx of patients with respiratory failure. Excessive demand for oxygen could potentially collapse the entire hospital supply system, or localised failure to wards or individual bed spaces. High-flow CPAP devices can consume up to 150 L/min of oxygen. To determine whether the UCLH system could cope, and aided by the hospital's Authorised Oxygen Engineer, Ventura devices were sequentially run in an empty ward at maximal oxygen flow. Reassuringly, the oxygen supply pressure only dropped on addition of the tenth Mark I device. The Mercedes HPP team subsequently modelled flows to each floor and bed area.
However, we recognised the imperative of improving oxygen use efficiency as older hospitals could struggle with oxygen supply capability. CFD simulations informed modifications to the Ventura, including alterations to the air-entrainment port geometry, reducing restriction at the air inlet filter, and optimising aerodynamic surfaces. To our surprise, we discovered that disposable tubing, positive end-expiratory pressure valves, masks, and pathogen filters from different commercial suppliers could generate considerable resistance. The resulting pressure drops in the circuit necessitated higher flow rates to avoid increasing the patient's work of breathing. Fortunately, consumables manufactured by Intersurgical (Wokingham, Surrey) met our needs. The device improvements (Mark II Ventura) and optimised circuit reduced total oxygen consumption by up to 70%, both when tested at normal breathing and simulating a tachypnoeic patient receiving high inspired oxygen concentrations. Indeed, the final specification outperformed many commercially available systems. The MHRA authorised use of the Mark II device 6 days after the Mark I. 3 days later, they approved a self-calibrating analyser with alarm functionality (Flo-Ox), developed in just 9 days by Oxford Optronix (Milton Park, UK) to monitor inspired oxygen concentration. The UCL team worked with Thanos Medical (Basildon, UK) to develop a further oxygen analyser, and Avon Medical to develop new CPAP hoods.
While these technical developments were ongoing, there were parallel hurdles to surmount. Our work had commenced when national guidance discouraged use of CPAP. As such, we had to convince clinicians and health-care administrators of the utility and safety of CPAP, reassure about oxygen supplies and staff protection, and encourage the NHS to alter its guidance. Intensive care and respiratory physicians in other British hospitals soon recognised that CPAP could spare critical care and ventilator capacity, and it offered an additional level of support to patients not deemed appropriate for mechanical ventilation. CPAP use was approved in new national guidance on March 24, 2020, with appropriate caveats about patient selection, staff safety, and oxygen supplies.
We also had to secure funding to upscale manufacturing. Mercedes HPP and UCL had covered the initial costs of manufacturing the Ventura, with no guarantee of reimbursement. Government focus and funding were firmly fixed on producing ventilators. There was initial scepticism about CPAP with fears surrounding virus aerosolisation, exacerbated by shortages of personal protective equipment and concerns over hospital oxygen supply. These anxieties were assuaged, enabling an initial commission of 100 Ventura devices from the Cabinet Office on March 27, 2020, in parallel with the first MHRA approval. 2 days later, we went public, receiving widespread coverage across national and international news outlets, followed by a further commission to deliver 10 000 devices within a fortnight. These were delivered by Mercedes HPP by the deadline, with Oxford Optronix delivering 2000 analysers a few days later.
Rapid distribution to hospitals was the next issue because the NHS Supply Chain was under unprecedented strain. Fortunately, G-TEM (Gloucester, UK) offered their services at no cost, taking responsibility for storage and packaging of Ventura devices, oxygen analysers, and tens of thousands of Intersurgical consumable parts, with next-day delivery. More than 60 NHS hospitals were supplied within a few weeks and 110 to date. We also launched a help desk and website providing technical specifications, oxygen use data, guidance documents, training videos, a troubleshooting guide, and an adverse incident and equipment failure reporting process.
We all felt a strong collective responsibility to support other nations, especially low-income and middle-income countries, during the COVID-19 global pandemic. The widespread media coverage generated considerable worldwide interest in the Ventura device. UCL Business and Mercedes HPP legal teams, with support from Dorsey & Whitney LLP, built a dedicated platform and licence agreement within days for release of designs and manufacturing data at no cost to legitimate companies, research institutions, health-care providers, and to the non-profit sector, with the proviso that manufacture was done on a non-profit basis. To date, over 1900 licences have been approved across 105 countries. Countries without the means to manufacture the device have been supported through charities supplying devices and circuits. We encouraged engagement of local health-care teams and provided clear guidance about oxygen supply needs, necessary infrastructure, protective personal equipment, and clinical training. Ongoing support is provided by technical, manufacturing, clinical, and regulatory teams, with assistance from the MHRA. Training materials have been translated into relevant languages, a Facebook group has been established to connect manufacturers in different countries, and webinars are held for manufacturers and clinicians. We have liaised with the WHO, World Bank, overseas governments, and UK governmental departments to encourage a coordinated approach.
The Ventura initiative, translating a brainstorming session into 10 000 devices within 1 month, would not have been possible without the cooperation, dedication, and generosity of individuals, universities, hospitals, companies, governmental bodies, and the media. It shows how usual barriers and procrastinations can be overcome safely and effectively in a time of crisis with a focused, multidisciplinary, agile, and coordinated approach, and a common aim to deliver at pace a device that will hopefully save lives.
The health-care industry can learn some valuable lessons from the motorsports industry in terms of their ability to adapt to ever-evolving situations, their design and manufacturing processes, and their nimble logistic capabilities. Efficient, streamlined, and synergistic partnerships between industry, academia, and health care are needed to break down barriers to innovation and adoption of novel, effective technologies. Worryingly, severe economic recession could further endanger the ability of universities and health care to progress with similar innovative projects. The shrinking UK manufacturing base presents challenges in securing locally sourced parts and product development. Steps should be taken to reduce the heavy reliance on overseas manufacturers who might not be able to deliver during a worldwide crisis. Conversely, developed countries placing trade embargoes on health-care products will help local populations to the detriment of low-income countries. More effort must be made to take collective responsibility for global health.
Acknowledgments
We are hugely indebted to the many individuals, organisations, and companies who provided support during this project. Neil McGuire (MHRA) merits particular mention. MS reports grants from NewB, Defence Science and Technology Laboratory, Critical Pressure, and Apollo Therapeutics, and fees paid to his research fund from Amormed, Baxter, Biotest, General Electric, New B, Roche, Bayer, and Shionogi, outside of the submitted work. RS reports personal fees from the Royal National Orthopaedic Trust, outside of the submitted work. DL reports personal fees, grants, and non-financial support from GlaxoSmithKline, and personal fees from Griffols outside of the submitted work. All other authors declare no competing interests.