Radioligand therapy (RLT) using 177Lu-PSMA ligands is highly effective in metastatic castration-resistant prostate cancer (mCRPC); however, failure of 177Lu-PSMA RLT remains challenging as RLT already represents last-line treatment.
The α-emitter 225Ac provides higher biological effectiveness compared with 177Lu [1]. Several centers reported remarkable response after PSMA-targeted alpha therapy (TAT) using 225Ac-PSMA-617 after failure of 177Lu-PSMA RLT [2, 3]. Here we present encouraging response to TAT in a patient with advanced mCRPC showing progression after long-term 177Lu-PSMA RLT (10 cycles). PSA values are provided under the date of each PSMA-PET MIP image (A–B using 68Ga-PSMA-11 and E–H using 18F-PSMA-1007). The patient was referred for RLT after radical prostatectomy and radiotherapy in 2005, and anti-hormonal therapy started in 2013 due to biochemical progression. Further progression was observed in February 2017 (A) after 2nd-line anti-hormonal therapy from 2015 to 2016, 223Ra-Dichloride in 2016, and docetaxel chemotherapy from 2016 to 2017. Two cycles of 177Lu-PSMA-617 were highly effective (B). PSA was still decreasing after two additional 177Lu-PSMA-617 cycles despite increasing PSMA-ligand uptake in PSMA-PET (C). Maintenance therapy using 177Lu-PSMA-617 was continued until January 2019 with further response (D and E); however, disease progression occurred after watchful waiting and two cycles of 177Lu-PSMA-I&T (F and G). The patient then received two cycles of 225Ac-PSMA-I&T and showed encouraging response (H). The main TAT-related side effect was grade 2 xerostomia (grade 2), which was already preexisting after 10 cycles of RLT. No TAT-related grade 3/4 hematological side effects were noted. Further cycles are planned but were suspended due to the COVID-19 crisis upon patient’s request.
Different approaches including tandem therapy with 177Lu or de-escalating doses during consolidation have been proposed for TAT as a trade-off between therapeutic efficacy and tolerable side effects [2, 4], and further studies investigating 225Ac-PSMA remain highly important for prostate cancer theranostics.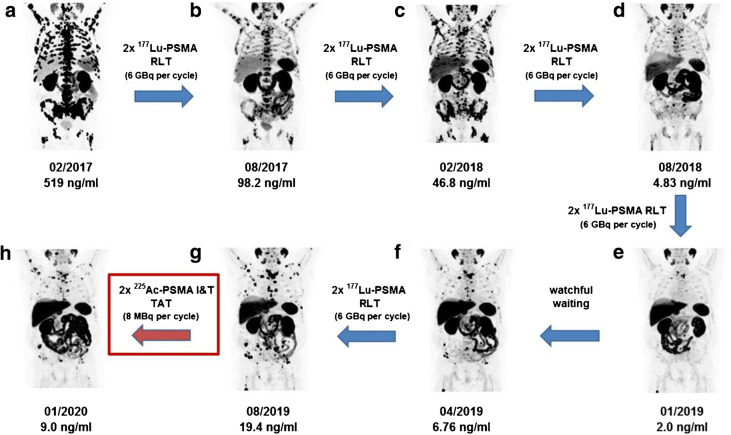
Funding
Open Access funding enabled and organized by Projekt DEAL.
Compliance with ethical standards
All procedures performed in this study involving human participants were in accordance with ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This retrospective evaluation was approved by the local ethic committee (20-178). Written informed consent was obtained prior to the exam.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
This article is part of the Topical Collection on Image of the month.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Czerwinska M, Bilewicz A, Kruszewski M, Wegierek-Ciuk A, Lankoff A. Targeted radionuclide therapy of prostate cancer-from basic research to clinical perspectives. Molecules. 2020;25. 10.3390/molecules25071743. [DOI] [PMC free article] [PubMed]
- 2.Khreish F, Ebert N, Ries M, Maus S, Rosar F, Bohnenberger H, et al. (225)Ac-PSMA-617/(177)Lu-PSMA-617 tandem therapy of metastatic castration-resistant prostate cancer: pilot experience. Eur J Nucl Med Mol Imaging. 2020;47:721–728. doi: 10.1007/s00259-019-04612-0. [DOI] [PubMed] [Google Scholar]
- 3.Sathekge M, Bruchertseifer F, Vorster M, Lawal IO, Knoesen O, Mahapane J, et al. Predictors of overall and disease-free survival in metastatic castration-resistant prostate cancer patients receiving (225)Ac-PSMA-617 radioligand therapy. J Nucl Med. 2020;61:62–69. doi: 10.2967/jnumed.119.229229. [DOI] [PubMed] [Google Scholar]
- 4.Sathekge M, Bruchertseifer F, Knoesen O, Reyneke F, Lawal I, Lengana T, et al. (225)Ac-PSMA-617 in chemotherapy-naive patients with advanced prostate cancer: a pilot study. Eur J Nucl Med Mol Imaging. 2019;46:129–138. doi: 10.1007/s00259-018-4167-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


