Highlights
-
•
Wolffia globosa Mankai can serve as a plant substitute for animal protein.
-
•
Genotoxicity and subchronic toxicity studies (GLP) of dry Mankai were conducted.
-
•
Dry Mankai was judged as having no potential to induce gene mutations in Ames test.
-
•
Dry Mankai did not induce chromosomal aberration in in vitro micronucleus test.
-
•
The NOAEL of Dry Mankai in 90-day dietary toxicity study was 20 % (w/w).
Keywords: Wolffia globose, Mankai, Genotoxicity, Subchronic toxicity, Rat, NOAEL
Abstract
Wolffia is a genus of protein-rich aquatic plants. Mankai, a cultivated strain of Wolffia globosa, contains more than 40 % protein based on dry matter evaluation. Furthermore, Mankai is nutritionally excellent as a food material, and is expected to be applicable to various products as a substitute for animal protein. A battery of toxicological studies was conducted on the dried product of Mankai (Dry Mankai), with the expectation to utilize it as a raw material for food applications. Dry Mankai was not genotoxic in a bacterial reverse mutation test and in vitro micronucleus assay. In the subchronic toxicity study, rats were provided Dry Mankai in the diet at levels of 0 %, 5 %, 10 %, or 20 % (w/w), equivalent to 0, 3.18, 6.49, and 13.16 g/kg/day for males and 0, 3.58, 7.42, and 15.03 g/kg/day for females, respectively. No adverse effects that could be attributable to treatment were observed in clinical observations, body weight, food consumption, ophthalmology, hematology and blood chemistry, urinalysis, and macroscopic and microscopic findings. According to the repeated-dose study in rats, the no observed adverse effect level of Dry Mankai was 20 % (w/w) for both sexes (13.16 and 15.03 g/kg/day for males and females, respectively).
1. Introduction
In recent years, plant-based proteins have attracted attention as an alternative to animal proteins. As plant-based proteins have a lower environmental impact and can be produced more efficiently than animal proteins, they can be a means to solve future food insecurity and inadequate protein intake [1]. Furthermore, they are expected to have various health benefits [[2], [3], [4]].
Wolffia spp., from the duckweed family, are protein-rich aquatic plants. Wolffia spp. have been considered vegetables in Southeast Asia for several years [5]. Particularly, Mankai, a cultivated strain of Wolffia globosa (an aquatic plant with a total length of 0.4–0.9 mm), has >40 % protein on a dry matter basis, including all nine essential amino acids and all six conditionally essential amino acids, and its protein digestibility-corrected amino acid score is 89 %. Kaplan et al. [6] reported that the bioavailability of essential amino acids in Wolffia globosa Mankai is comparable to existing animal protein sources such as cheese and existing plant-based protein sources such as peas, and they showed that Mankai is a high-quality protein source for providing essential amino acids in human studies. Moreover, Mankai contains over 60 nutrients and is rich in vitamins (including folic acid), minerals (including iron), polyphenols, and dietary fiber, making it a useful food resource for humans. A recent report showed that the intake of Wolffia globosa Mankai improved iron deficiency anemia in an experimental rat model [7]. Furthermore, in a randomized controlled crossover trial of obese patients, Wolffia globosa Mankai intake showed lower postprandial glucose levels than those of yogurt, and it has been shown to have potential beneficial effects on postprandial glycemic control [8].
As described above, Mankai is nutritionally excellent as a food material and is expected to be applicable in various products as a substitute for animal protein. There is also a plan to use Dry Mankai, a dried product of Mankai, as raw material for food applications. In this study, we conducted genotoxicity and oral subchronic toxicity studies to confirm the safety of Dry Mankai according to the European Food Safety Authority's guidance on novel foods [9]. For genotoxicity studies, a reverse mutation test using bacteria (Ames test) and an in vitro micronucleus assay were conducted. Additionally, a repeated-dose 90-day dietary toxicity study in male and female Sprague-Dawley (SD) rats was performed at high doses (5%–20 % in the diet) as a general toxicity study of oral administration. All studies were performed in accordance with the Organisation for Economic Co-operation and Development (OECD) guidelines for toxicity studies under the Good Laboratory Practice (GLP) Regulations.
2. Materials and methods
2.1. Test material
Dried product of Mankai (Dry Mankai, code name: Mankai D110, Lot No. D20190707) was supplied by Hinoman Ltd. (Rishon LeZion, Israel) and used in the genotoxicity and repeated-dose toxicity studies. Dry Mankai was stored in the refrigerator, protected from light, and was preserved under airtight and moisture-proof conditions until the time of use.
2.2. Genotoxicity studies
2.2.1. Bacterial reverse mutation test (Ames test)
To examine the mutagenic potential of Dry Mankai, a reverse mutation assay was conducted with Salmonella typhimurium TA98, TA100, TA1535, and TA1537, and Escherichia coli WP2 uvrA by the pre-incubation method. This study was conducted in compliance with the GLP standards of OECD and the Ministry of Health, Labour and Welfare of Japan (MHLW) [10,11] and the OECD Guidelines for the Testing of Chemicals 471 [12]. All test strains were obtained from the Division of Genetics and Mutagenesis, National Institute of Health Sciences (Tokyo). Water for injection was used as the vehicle for the test article. Two and three plates were used at each dose level in the dose-finding test and the two main tests, respectively. The S9 microsomal fraction (BoZo Research Center Inc., Tokyo) was freshly prepared on the day of use with liver homogenates from male SD rats induced with phenobarbital and 5,6-benzoflavone.
The dose-finding test was conducted with dose levels between 19.5 and 5000 μg/plate to set the dose levels for the main test. There was no growth inhibition observed in the dose-finding test. Therefore, the main test was conducted with five dose levels (313; 625; 1250; 2500; and 5000 μg/plate) with or without metabolic activation. The main test was conducted twice at the same dose levels and the vehicle, i.e., water for injection, was used as the negative control. As positive controls, 2-(2-furyl)-3-(5-nitro-2-furyl) acrylamide, sodium azide, and 2-methoxy-6-chloro-9-[3-(2-chloroethyl)aminopropylamino]acridineꞏ2HCl were used without metabolic activation, and benzo[a]pyrene and 2-aminoanthracene were used with metabolic activation. After incubation, the number of colonies per plate was counted manually or with a colony counter. The precipitation by test article, growth inhibition, and number of revertant colonies were evaluated for each set of plates.
2.2.2. In vitro micronucleus assay
The potential of Dry Mankai to induce chromosomal aberrations was assessed by in vitro micronucleus test using human lymphoblastoid (TK6) cells with or without metabolic activation by rat liver S9 fraction. This study was conducted in compliance with the GLP standards of OECD and the MHLW [10,11], and the OECD Guidelines for the Testing of Chemicals 487 [13]. TK6 cells were obtained from the National Institute of Health Sciences (Tokyo). A range-finding test was conducted at dose levels from 15.6 to 2000 μg/mL. There was color change of the culture medium to green at dose levels of 2000 μg/mL in the short-term and continuous treatment schedules. This change was considered to be derived from the color of Dry Mankai. Moreover, there were precipitations of the culture medium at all dose levels in all treatment schedules. The IC50 was 1270 and 861 μg/mL in the short-term treatment without metabolic activation and that with metabolic activation, respectively, and it could not be calculated in the continuous treatment owing to no cytotoxicity.
In the main test, based on the results of the range-finding test, the doses showing 40 %–50 % Relative Population Doubling (RPD) were selected as the highest dose in the short-term treatment; 750; 1000; and 1250 μg/mL without metabolic activation and 250, 500, and 750 μg/mL with metabolic activation were selected for observation. In the continuous treatment, all doses (i.e., 250; 500; 1000; and 2000 μg/mL) were observed. The vehicle, i.e., water for injection, was used as the negative control. Cyclophosphamide and colchicine were used as the positive controls for the short-term treatment with metabolic activation and the continuous treatment, respectively. For each treatment schedule, the frequencies of micronucleated cells in the negative control and test article groups, and those of the negative control and positive control group were compared by Fisher's exact test (upper-tailed).
2.3. Ninety-day dietary toxicity study in rats
The ninety-day dietary toxicity study was conducted in accordance with the GLP standards of OECD and MHLW [10,11], and the OECD Guidelines for the Testing of Chemicals 408 [14].
2.3.1. Preparation method of the test substance
Dry Mankai was thoroughly mixed with the powdered basal diet (CR-LPF, Oriental Yeast Co., Ltd., Tokyo) using vinyl bags. To prepare the test article/diet admixture for each concentration, a concentrated admixture was first made by mixing the test article with a small amount of basal diet. Then, the concentrated admixture was mixed with the rest of the basal diet to obtain the prescribed concentration. All test article/diet admixtures were prepared at least once every 7 days and stored in a tightly closed container at room temperature. The test article concentrations were analyzed in the test article/diet admixtures, which were actually used for dosing on Weeks 1 and 13 of the administration period. The proportion of the nominal concentration ranged from 99.3% to 102.1% (acceptable range: 100 % ± 15 %), the coefficient of variation ranged from 0.0% to 1.1% (acceptable range: 10 % or less), and both the concentration and homogeneity met the acceptance criteria. In addition, it was confirmed that the test article/diet admixtures of Dry Mankai at 5% and 20 % (w/w) were stable at room temperature for 18 days (concentrations after 18 days ranged from 99.6% to 100.0%).
2.3.2. Test animals
A total of 46 male and 46 female 4-week-old SD rats [Crl:CD(SD)] were obtained from Charles River Laboratories Japan, Inc. (Atsugi, Kanagawa), and acclimatized for 14 days prior to the start of administration. The animals were distributed randomly to four groups of 10 male and 10 female rats per group according to body weight. Male (179–221 g) and female (141–173 g) animals were 6 weeks of age at the start of the dosing period. Two animals of the same sex were housed in solid-floor plastic cages with bedding. The animal room was controlled to maintain the temperature at 23 ± 3 °C, relative humidity at 50 % ± 20 %, air ventilation at 10–15 times per hour, and 12 -h illumination. The animals were provided free access to powdered diet (CR-LPF) and public tap water. Appropriate environmental enrichment was given to the animals in accordance with the Institutional Animal Care and Use Committee guideline.
2.3.3. Test article administration
Dry Mankai was provided in the diet at 0 %, 5 %, 10 %, or 20 % (w/w) levels for 91 days. Animals in the control group were supplied CR-LPF. The dose levels were selected based on an earlier 14-day repeated dietary toxicity study, showing no abnormality up to 20 % (w/w) Dry Mankai in the diet.
2.3.4. Observation, measurement, and examination
Clinical observations: All animals were observed twice daily for clinical signs. Detailed clinical observations were performed once a week during the administration periods for all animals. The manipulative test and measurements of grip strength and motor activity were conducted on all animals in Week 12 of administration. Detailed clinical observations and manipulative tests were assessed using a scoring system. The animals were placed at random and the information about the treatment was restricted from the analyst except in the case of the measurement of motor activity.
Body weight and food consumption: Individual body weights were measured in the morning on Days 1 and 7 of administration and once per week thereafter at 7-day intervals. Food consumption measurements coincided with all body weight measurements. Body weight gain was calculated for the entire administration period.
Test article intake: Test article intakes (mg/kg/day) each week and those throughout the administration period were calculated from the body weight, food consumption, and nominal concentration of the test article/diet admixture.
Ophthalmology: Ophthalmologic examinations were conducted on all animals in Week 13. The anterior portion (cornea, aqueous chambers, and iris), optic media (lens and vitreous body), and fundus oculi were examined using a binocular indirect ophthalmoscope (Omega 200, HEINE Optotechnik GmbH & Co. KG).
Urinalysis: Urine samples were collected from the animals in Week 13 prior to blood sample collection for clinical pathology evaluation. The animals were placed in a cage with a tray attached for urine collection. Four-hour urine samples were collected under fasting but with free access to drinking water. Then, the following 20 -h urine samples were collected under free access to food and water. With the 4 -h urine samples, pH, protein, ketone body, glucose, occult blood, bilirubin, urobilinogen, and specific gravity using Multistix SG (Siemens Healthcare Diagnostics, Inc., NY, USA), as well as color and urine volume were evaluated. Additionally, microscopic urine sediment examination was conducted. The 20 -h urine samples were analyzed with an Automated Clinical Laboratory System (TBA-120FR, Cannon Medical Systems Corporation, Tochigi) for sodium, potassium, and chloride. Additionally, one-day water intake (during urine sample collection) was measured using the water bottles.
Hematology, blood chemistry, and serum hormone analysis: Blood samples for hematology and blood chemistry analysis were collected from all animals prior to the scheduled necropsy at the end of the dosing period. All animals were fasted overnight prior to blood collection, and blood samples were collected from the abdominal aorta under isoflurane anesthesia. Blood samples containing potassium EDTA as the anticoagulant were analyzed using a total hematology system (ADVIA 2120i, Siemens Healthcare Diagnostics Co. Ltd., Tokyo) for red blood cell count, hemoglobin concentration, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, red blood cell distribution width, reticulocyte count, platelet count, white blood cell count, and differential leukocyte count including lymphocytes, neutrophils, eosinophils, basophils, monocytes, and large unstained cells. Plasma samples anticoagulated with sodium citrate were processed using a coagulometer (ACL Elite Pro, Instrumentation Laboratory Corporation, MA, USA) for prothrombin time, activated partial thromboplastin time, and fibrinogen.
Heparin-anticoagulated plasma samples were evaluated with an automated clinical chemistry analyzer (TBA-120FR, Cannon Medical Systems Corporation, Tochigi) for aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase, alkaline phosphatase, cholinesterase, total bile acid, total cholesterol, high-density lipoprotein, low-density lipoprotein, triglycerides, phospholipids, total bilirubin, glucose, blood urea nitrogen, creatinine, urea, sodium, potassium, chloride, calcium, inorganic phosphorus, total protein, albumin (A), globulin (G), and the A/G ratio. For the analysis of serum hormones, blood samples were collected into a blood coagulation accelerator (Venoject II-Autosep, Terumo Corp.) and centrifuged (3000 rpm, approx. 1600 g, for 10 min) to obtain serum samples. The serum samples were analyzed for triiodothyronine, thyroxine, and thyroid stimulating hormone using RIA-gnost®-T3 kit (Cisbio Bioassays), RIA-gnost®-T4 kit (Cisbio Bioassays), and rTSH(125I) RIA KIT (Institute of Isotopes Co., Ltd., Izotop), respectively.
Necropsy: All animals were euthanized by exsanguination from the abdominal aorta under isoflurane anesthesia at the end of the study period and subjected to a gross necropsy of all internal organs/tissues.
Organ weights: After necropsy, selected organs were weighed, i.e., the brain, pituitary gland, thyroid glands (including parathyroid gland), thymus, heart, liver, spleen, kidneys, adrenal glands, testes, prostate, epididymides, seminal vesicles (including coagulating gland), ovaries, and uterus (cervix and horn). Then, organ-to-body weight percentages (relative organ weights) were calculated.
Histopathology: All the organs/tissues of all animals were fixed and preserved in phosphate-buffered 10 % formalin, while the eyeballs were fixed with a fixation medium containing phosphate-buffered 3% glutaraldehyde and 2.5 % formalin. Testes and epididymides were fixed with Bouin's solution and then preserved in phosphate-buffered 10 % formalin. The organs/tissues listed below from animals in the control and high-dose group, and the adrenal glands from animals in the low- and middle-dose group were embedded in paraffin, sectioned, and stained with hematoxylin and eosin. The collected organs/tissues were the cerebrum, cerebellum (including pons), medullary, spinal cord (cervical, mid-thoracic, and lumbar), sciatic nerve, eyeball, pituitary gland, thyroid gland, parathyroid gland, adrenal gland, thymus, spleen, submandibular lymph node, mesenteric lymph node, heart, thoracic aorta, trachea, lung (including bronchus), tongue, esophagus, stomach, duodenum, jejunum, ileum (including Peyer's patch), cecum, colon, rectum, submandibular gland, sublingual gland, liver, pancreas, kidney, urinary bladder, testis, epididymis, prostate, seminal vesicle (including coagulating gland), ovary, uterus (cervix and horn), vagina, oviduct, mammary gland (inguinal), femur (including knee joint and bone marrow), femoral skeletal muscle, and skin (inguinal).
2.3.5. Statistical analyses
Clinical signs, body weight, food and water consumption, urinalysis, hematology, blood chemistry, and organ weight data, and ophthalmology, necropsy, and histopathology findings were recorded using the MiTOX-BOZO system (Mitsui E & S Systems Research Inc., Chiba) or PATHOS5 system (Pathology Operating Systems Ltd., Harrogate, England). All analyses were performed using an integrated statistical package SAS Release 9.1.3 (SAS Institute Inc., NC, USA). Numerical data were tested by Bartlett's test for homogeneity of variance (level of significance: 0.01). When the variances were homogeneous, Dunnett's test was applied to compare the mean value in the control group with that in each test article group (levels of significance: 0.05 and 0.01, two-tailed). When the variances were heterogeneous, Steel's test was applied to compare the mean rank in the control group with that in each test article group (levels of significance: 0.05 and 0.01, two-tailed).
3. Results
3.1. Genotoxicity studies
3.1.1. Bacterial reverse mutation test (Ames test)
In the main tests, precipitation of Dry Mankai on the plate was observed at ≥313 μg/plate with or without metabolic activation. Observation of the bacterial background lawn using a stereoscopic microscope did not show growth inhibition in any strains irrespective of the presence/absence of metabolic activation. Moreover, there was no dose-dependent increase in the number of revertant colonies of two-fold or more compared with that of the negative control group for any strains irrespective of the presence/absence of metabolic activation (Table 1). As increases in the number of revertant colonies in the positive control group over two-fold were observed compared with that of the negative control group for each tester strain, it was judged that the reactions of bacterial strains to the mutagenic agents were suitable and the study was appropriately conducted. In conclusion, Dry Mankai was judged as having no potential to induce gene mutations under the conditions of this study.
Table 1.
Number of revertant colonies per plate in the reverse mutation test conducted using the pre-incubation method with Dry Mankai.
| Concentration (μg/plate) | TA98 |
TA100 |
TA1535 |
TA1537 |
WP2uvrA |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| -S9 | +S9 | -S9 | +S9 | -S9 | +S9 | -S9 | +S9 | -S9 | +S9 | |
| First test | ||||||||||
| Negative controla | 23 ± 2.1 | 29 ± 3.2 | 121 ± 1.0 | 123 ± 5.1 | 8 ± 1.5 | 8 ± 0.6 | 7 ± 1.0 | 7 ± 1.0 | 24 ± 1.0 | 28 ± 1.5 |
| 313 | 25 ± 1.0 | 26 ± 4.0 | 108 ± 9.7 | 110 ± 11.0 | 10 ± 1.5 | 5 ± 1.0 | 5 ± 2.3 | 5 ± 1.7 | 27 ± 0.6 | 25 ± 3.8 |
| 625 | 27 ± 2.3 | 24 ± 5.2 | 101 ± 10.0 | 106 ± 5.6 | 7 ± 4.0 | 9 ± 1.5 | 4 ± 0.6 | 9 ± 1.0 | 27 ± 1.7 | 22 ± 2.6 |
| 1250 | 21 ± 4.5 | 26 ± 0.6 | 93 ± 9.7 | 111 ± 19.2 | 8 ± 2.5 | 6 ± 3.5 | 6 ± 3.5 | 8 ± 1.0 | 25 ± 4.0 | 28 ± 0.0 |
| 2500 | 27 ± 2.9 | 30 ± 9.7 | 94 ± 9.5 | 106 ± 6.5 | 10 ± 1.2 | 9 ± 2.6 | 7 ± 2.1 | 6 ± 1.2 | 20 ± 3.8 | 22 ± 2.1 |
| 5000 | 21 ± 0.6 | 26 ± 5.1 | 84 ± 2.6 | 98 ± 8.5 | 11 ± 1.0 | 6 ± 1.5 | 6 ± 1.5 | 7 ± 2.3 | 19 ± 1.7 | 18 ± 1.0 |
| Positive control b, c | 336 ± 29.1 | 321 ± 22.2 | 763 ± 45.6 | 1188 ± 60.3 | 301 ± 39.8 | 255 ± 17.8 | 1805 ± 158.5 | 92 ± 4.6 | 149 ± 1.0 | 546 ± 34.3 |
| Second test | ||||||||||
| Negative controla | 22 ± 1.2 | 32 ± 4.6 | 115 ± 16.1 | 134 ± 9.7 | 11 ± 3.2 | 7 ± 0.6 | 8 ± 0.6 | 10 ± 1.5 | 20 ± 1.5 | 28 ± 1.5 |
| 313 | 20 ± 4.2 | 25 ± 1.5 | 112 ± 21.1 | 123 ± 19.1 | 9 ± 3.8 | 7 ± 2.3 | 7 ± 4.0 | 8 ± 2.6 | 19 ± 1.5 | 27 ± 2.3 |
| 625 | 17 ± 5.0 | 26 ± 4.2 | 96 ± 0.6 | 121 ± 5.6 | 6 ± 0.6 | 8 ± 2.6 | 9 ± 2.5 | 7 ± 1.5 | 21 ± 4.4 | 30 ± 4.4 |
| 1250 | 22 ± 2.3 | 28 ± 6.2 | 109 ± 6.7 | 124 ± 15.5 | 10 ± 2.6 | 7 ± 1.7 | 6 ± 2.5 | 8 ± 2.6 | 22 ± 5.0 | 31 ± 4.6 |
| 2500 | 18 ± 5.5 | 30 ± 2.5 | 100 ± 5.5 | 128 ± 7.6 | 10 ± 2.5 | 8 ± 1.0 | 5 ± 2.1 | 6 ± 1.5 | 18 ± 4.0 | 24 ± 1.0 |
| 5000 | 20 ± 3.5 | 21 ± 3.5 | 110 ± 12.9 | 121 ± 5.9 | 11 ± 2.6 | 8 ± 2.3 | 5 ± 1.2 | 8 ± 4.0 | 21 ± 5.7 | 27 ± 3.6 |
| Positive control b, c | 448 ± 31.2 | 284 ± 19.5 | 618 ± 15.6 | 1197 ± 98.2 | 279 ± 6.1 | 229 ± 9.9 | 2135 ± 70.1 | 79 ± 2.3 | 128 ± 7.2 | 589 ± 15.7 |
Values are means ± standard deviation (S.D.).
-S9, without metabolic activation; +S9, with metabolic activation; 2AA, 2-aminoanthracene; AF-2, 2-(2-furyl)-3-(5-nitro-2-furyl)acrylamide; B[a]P, benzo[a]pyrene; ICR-191, 2-methoxy-6-chloro-9-[3-(2-chloroethyl)aminopropylamino]acridine□2HCl ; SAZ, sodium azide.
Water for injection.
Positive controls -S9: TA98, TA100 and WP2uvrA = AF-2; TA1535 = SAZ; TA1537 = ICR-191.
Positive controls + S9: TA98, TA100 and TA1537= B[a]P; TA1535 and WP2uvrA = 2AA.
3.1.2. In vitro micronucleus test
In the main test, there was color change of the culture medium to green at the 2000-μg/mL dose level in continuous treatment, but no color change was observed in the short-term treatment. This change was considered to be derived from the color of Dry Mankai. Precipitation of Dry Mankai, which was observed at all dose levels in all treatment schedules, did not interfere with observations of the micronuclei. The RPD was -13 %–86 %, 38 %–89 %, and 99 %–103 % in the short-term treatment without metabolic activation, the short-term treatment with metabolic activation, and the continuous treatment, respectively (Table 2). For the short-term treatment without metabolic activation, the incidence of cells with a micronucleus was 0.7 %, 0.7 %, and 0.9 % at the dose levels of 750; 1000; and 1250 μg/mL, respectively, while it was 0.9 %, 0.9 %, and 0.8 % at the dose levels of 250, 500, and 750 μg/mL, respectively, for the short-term treatment with metabolic activation. For the continuous treatment, the incidence of cells with a micronucleus was 0.6 %, 0.6 %, 0.6 %, and 0.7 % at the dose levels of 250; 500; 1000; and 2000 μg/mL, respectively. The frequency of cells with a micronucleus did not show a significant increase at any dose levels in any treatment schedules compared with those of the negative control group (Fisher's exact test, p > 0.05). In addition, the incidences of micronucleated cells in each test article group were within the 95 % control limits of distribution of the historical negative control group data. For the positive control group, a statistically significant increase in the frequency of micronucleated cells was recorded compared with those in the concurrent negative control group. Therefore, Dry Mankai did not induce chromosomal aberrations under the conditions of this study.
Table 2.
Results of in vitro micronucleus test with Dry Mankai in TK6 cells.
| Treatment | Short-term treatment |
Continuous treatment |
||||
|---|---|---|---|---|---|---|
| Treatment time (h) | 4 |
4 |
24 |
|||
| Metabolic activation | -S9 |
+S9 |
-S9 |
|||
| Concentration of test article (μg/mL) | RPD (%) | Cells with micronucleus (%) | RPD (%) | Cells with micronucleus (%) | RPD (%) | Cells with micronucleus (%) |
| Negative controla | 100 | 0.6 | 100 | 0.7 | 100 | 0.6 |
| 62.5 | NE | 89 | NO | NE | ||
| 125 | NE | 84 | NO | NE | ||
| 250 | 86 | NO | 72 | 0.9 | 99 | 0.6 |
| 500 | 77 | NO | 68 | 0.9 | 102 | 0.6 |
| 750 | 81 | 0.7 | 41 | 0.8 | NE | |
| 1000 | 59 | 0.7 | 38 | NO | 100 | 0.6 |
| 1250 | 44 | 0.9 | NE | NE | ||
| 1500 | −13 | NO | NE | NE | ||
| 2000 | NE | NE | 103 | 0.7 | ||
| Positive control b, c | NE | 49 | 1.9* | 68 | 4.0* | |
Significantly different from the negative control by Fisher’s exact test: * p < 0.05.
RPD, Relative population doubling; NE, Not examined; NO, Not observed.
Water for injection.
Cyclophosphamide (1.5 μg/mL) in Short-term treatment with S9 mix.
Colchicine (0.006 μg/mL) in Continuous treatment.
3.2. Ninety-day dietary toxicity study
3.2.1. Clinical observations
All control animals and those receiving Dry Mankai survived the 91-day study period. No clinical observations including detailed observations attributable to the administration of Dry Mankai were observed. Although a statistically significant increase in rearing count was recorded in Week 5 in females at 20 % (w/w) Dry Mankai in the diet, this was judged to have no toxicological significance because the change was temporary and minimal.
3.2.2. Body weight and food consumption
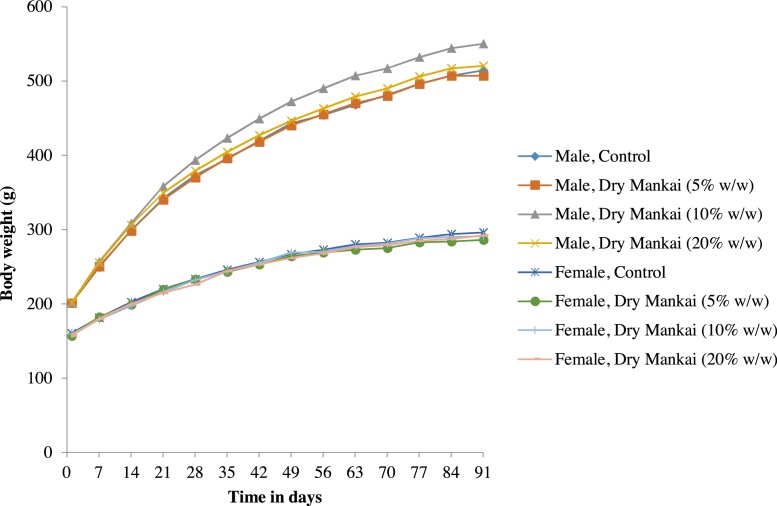
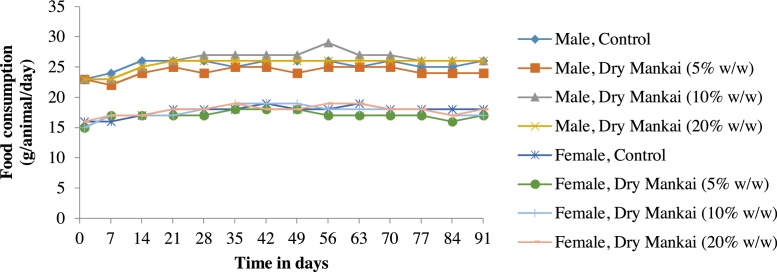
There were no changes in body weights that were attributable to Dry Mankai in either sex (Fig. 1). Furthermore, no significant differences in food consumption were observed between the treatment and control groups (Fig. 2). Although a statistically significant decrease was recorded in females at 5% (w/w) Dry Mankai in the diet on day 91, it was judged to be incidental because it was not dose-related.
Fig. 1.
Changes in body weight in 90-day dietary toxicity study with Dry Mankai in rats.
Fig. 2.
Changes in food consumption in 90-day dietary toxicity study with Dry Mankai in rats.
3.2.3. Test article intake
Overall mean Dry Mankai intakes throughout the administration period were 0, 3.18, 6.49, and 13.16 g/kg/day for males and 0, 3.58, 7.42, and 15.03 g/kg/day for females for the control, 5 %, 10 %, and 20 % (w/w) Dry Mankai diets, respectively (Supplemental Table 1).
3.2.4. Ophthalmology
There were no Dry Mankai-related abnormal ophthalmologic findings on day 90 of the study period.
3.2.5. Urinalysis
Urinalysis (Table 3) showed an increase in water intake and a decrease in one-day excretion of sodium in males at 20 % (w/w) Dry Mankai in the diet, decreases in one-day excretion of sodium and potassium in females at 10 % and 20 % (w/w) Dry Mankai in the diet, and a decrease in one-day excretion of chloride in females in all dose groups.
Table 3.
Summary of urinalysis in 90-day dietary toxicity study with Dry Mankai in rats.
| Male | Female | |||||||
|---|---|---|---|---|---|---|---|---|
| Dose (w/w %) | 0 (Control) | 5 | 10 | 20 | 0 (Control) | 5 | 10 | 20 |
| Number of rats | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| pH | 8.5:3, ≥9:7 | 8.5:1, ≥9:9 | 8.5:3, ≥9:7 | 7:1, 8.5:4, ≥9:5 | 7:2, 8:2, 8.5:3, ≥9:3 | 7.5:2, 8:1, 8.5:3, ≥9:4 | 6.5:1, 7:2, 7.5:1, 8:1, ≥9:5 | 6.5:1, 7:1, 7.5:1, 8.5:2, ≥9:5 |
| Protein | ±:1, +:5, 2+:4 | ±:1, +:5, 2+:3, 3+:1 | +:5, 2+:5 | ±:2, +:4, 2+:4 | ―:5, ±:5 | ―:5, ±:4, +:1 | ―:4, ±:4, +:1, 2+:1 | ―:3, ±:6, +:1 |
| Ketones | ±:5, +:5 | ±:3, +:5, 2+:2 | ―:2, ±:2, +:6 | ―:1, ±:5, +:4 | ―:10 | ―:10 | ―:7, ±:3 | ―:9, ±:1 |
| Glucose | ―:10 | ―:9, +:1 | ―:10 | ―:9, +:1 | ―:10 | ―:10 | ―:10 | ―:10 |
| Occult blood | ―:9, ±:1 | ―:9, ±:1 | ―:8, ±:2 | ―:10 | ―:10 | ―:10 | ―:10 | ―:10 |
| Urobilinogen (EU/dL) | ±:10 | ±:10 | ±:10 | ±:10 | ±:10 | ±:10 | ±:10 | ±:10 |
| Bilirubin | ―:8,+:2 | ―:9,+:1 | ―:9,+:1 | ―:9,+:1 | ―:10 | ―:10 | ―:10 | ―:10 |
| Color | Yellow:10 | Yellow:10 | Yellow:10 | Yellow:10 | Yellow:10 | Yellow:10 | Yellow:10 | Yellow:10 |
| Red blood cells | ―:10 | ―:10 | ―:10 | ―:10 | ―:10 | ―:10 | ―:10 | ―:10 |
| White blood cells | ―:10 | ―:10 | ―:10 | ―:10 | ―:10 | ―:10 | ―:10 | ―:10 |
| Squamous epithelial cells | ±:10 | ±:10 | ±:10 | ±:10 | ±:10 | ±:10 | ±:10 | ±:10 |
| Small round epithelial cells | ―:10 | ―:10 | ―:10 | ―:10 | ―:10 | ―:10 | ―:10 | ―:10 |
| Cast | ―:10 | ―:10 | ―:10 | ―:10 | ―:10 | ―:10 | ―:10 | ―:10 |
| Crystal phosphate salts | ―:9, ±:1 | ―:9, ±:1 | ―:5, ±:5 | ―:6, ±:4 | ―:8, ±:2 | ―:8, ±:2 | ―:8, ±:2 | ―:5, ±:5 |
| Crystal calcium oxalate | ―:10 | ―:10 | ―:10 | ―:10 | ―:10 | ―:10 | ―:9, ±:1 | ―:10 |
| Specific gravity | ≤1.005:3, 1.010:2, 1.015:4, 1.020:1 | ≤1.005:2, 1.010:5, 1.015:2, 1.020:1 | ≤1.005:2, 1.010:4, 1.015:1, 1.020:2 1.025:1 | 1.010:5, 1.015:2, 1.020:3 | ≤1.005:4, 1.010:1, 1.015:4, 1.020:1 | ≤1.005:3, 1.010:4, 1.015:2, 1.020:1 | ≤1.005:3, 1.010:4, 1.015:1, 1.020:1 ≥1.030:1 | ≤1.005:3, 1.010:3, 1.015:2, 1.020:1 1.025:1 |
| Urinary volume (mL/24 h) | 10.8 ± 3.4 | 9.9 ± 3.2 | 11.8 ± 5.5 | 13.5 ± 6.2 | 14.7 ± 8.2 | 8.5 ± 3.3 | 8.0 ± 3.7 | 7.0 ± 2.9 |
| Water consumption (mL/24h) | 36 ± 4 | 37 ± 5 | 35 ± 7 | 45 ± 9** | 39 ± 9 | 31 ± 5 | 32 ± 6 | 37 ± 9 |
| Na (mmol/24 h) | 1.9 ± 0.4 | 1.5 ± 0.4 | 1.7 ± 0.5 | 1.3 ± 0.3* | 1.5 ± 0.5 | 1.3 ± 0.3 | 1.0 ± 0.2** | 0.7 ± 0.3** |
| K (mmol/24 h) | 3.9 ± 0.7 | 3.6 ± 0.8 | 3.8 ± 0.9 | 3.5 ± 0.8 | 3.4 ± 1.0 | 2.7 ± 0.6 | 2.4 ± 0.8* | 2.0 ± 0.7** |
| Cl (mmol/24 h) | 2.8 ± 0.5 | 2.5 ± 0.6 | 2.7 ± 0.6 | 2.3 ± 0.6 | 2.5 ± 0.7 | 1.9 ± 0.4* | 1.5 ± 0.5** | 1.2 ± 0.4** |
Values are means ± S.D. Significantly different from the control using Dunnett’s test (two-tailed): * p ≤ 0.05, ** p ≤ 0.01.
Na, sodium; K, potassium; Cl, chloride.
3.2.6. Hematology, blood chemistry, and serum hormone analysis
There were no Dry Mankai-related changes in hematology in males (Table 4). A statistically significant decrease in fibrinogen was recorded in females at 10 % and 20 % (w/w) Dry Mankai in the diet. Other statistically significant changes were judged to be incidental because they were not dose-related, i.e., decrease in monocyte count in males at 5% (w/w) Dry Mankai in diet, increase in mean corpuscular hemoglobin concentration and in reticulocyte count in females at 5% (w/w) Dry Mankai in the diet. At 20 % (w/w) Dry Mankai in the diet, blood chemistry analyses (Table 5) showed decreases in total cholesterol, phospholipid, calcium, and inorganic phosphorus in males, and increases in glucose, blood urea nitrogen, and urea in females. A statistically significant decrease in calcium recorded in males at 5% (w/w) Dry Mankai in the diet was judged to be incidental because it was not dose-related. There were no Dry Mankai-related changes in serum hormone analysis (Table 5).
Table 4.
Summary of hematology in 90-day dietary toxicity study with Dry Mankai in rats.
| Male | Female | |||||||
|---|---|---|---|---|---|---|---|---|
| Dose (w/w %) | 0 (Control) | 5 | 10 | 20 | 0 (Control) | 5 | 10 | 20 |
| Number of rats | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| RBC (104/μL) | 866 ± 37 | 873 ± 37 | 864 ± 34 | 858 ± 32 | 779 ± 16 | 799 ± 22 | 761 ± 28 | 779 ± 32 |
| HGB (g/dL) | 15.1 ± 0.5 | 15.2 ± 0.7 | 15.2 ± 0.6 | 15.4 ± 0.6 | 14.4 ± 0.4 | 14.7 ± 0.4 | 14.4 ± 0.4 | 14.5 ± 0.5 |
| HCT (%) | 44.0 ± 1.8 | 44.4 ± 2.1 | 44.4 ± 1.9 | 44.5 ± 1.8 | 41.2 ± 1.1 | 41.4 ± 1.0 | 40.7 ± 1.1 | 41.7 ± 1.5 |
| MCV (fL) | 50.8 ± 1.5 | 50.9 ± 2.0 | 51.4 ± 1.8 | 51.8 ± 1.8 | 52.9 ± 1.1 | 51.8 ± 1.2 | 53.6 ± 1.2 | 53.6 ± 1.8 |
| MCH (pg) | 17.5 ± 0.5 | 17.4 ± 0.7 | 17.6 ± 0.6 | 17.9 ± 0.6 | 18.5 ± 0.4 | 18.4 ± 0.5 | 18.9 ± 0.3 | 18.6 ± 0.5 |
| MCHC (g/dL) | 34.4 ± 0.6 | 34.1 ± 0.2 | 34.2 ± 0.3 | 34.6 ± 0.3 | 35.0 ± 0.4 | 35.5 ± 0.4* | 35.3 ± 0.5 | 34.7 ± 0.4 |
| RDW (%) | 13.0 ± 0.3 | 12.9 ± 0.4 | 12.8 ± 0.5 | 12.6 ± 0.7 | 11.8 ± 0.5 | 11.8 ± 0.3 | 11.8 ± 0.5 | 11.7 ± 0.2 |
| RETIC (109/L) | 161.8 ± 25.3 | 150.7 ± 18.7 | 169.9 ± 27.3 | 150.8 ± 26.7 | 157.0 ± 32.7 | 123.7 ± 21.6* | 149.0 ± 33.1 | 148.3 ± 24.0 |
| PLT (104/μL) | 106.5 ± 10.6 | 100.9 ± 11.4 | 112.6 ± 12.7 | 103.9 ± 12.1 | 117.4 ± 18.8 | 113.5 ± 15.9 | 112.5 ± 12.5 | 117.4 ± 13.5 |
| WBC (102/μL) | 91.5 ± 21.3 | 72.9 ± 13.3 | 78.7 ± 23.0 | 78.0 ± 15.3 | 55.3 ± 18.2 | 52.3 ± 13.3 | 50.4 ± 18.7 | 46.8 ± 12.9 |
| LYMP (102/μL) | 71.9 ± 18.7 | 58.3 ± 12.7 | 62.0 ± 20.3 | 60.3 ± 11.9 | 44.4 ± 15.5 | 40.4 ± 12.9 | 39.1 ± 14.6 | 37.0 ± 10.4 |
| NEUT (102/μL) | 15.1 ± 5.4 | 11.6 ± 3.0 | 13.3 ± 5.3 | 13.9 ± 3.5 | 8.4 ± 3.2 | 9.5 ± 4.2 | 8.8 ± 6.2 | 7.9 ± 3.1 |
| EOS (102/μL) | 1.3 ± 0.4 | 1.0 ± 0.2 | 1.0 ± 0.3 | 1.1 ± 0.4 | 0.8 ± 0.3 | 0.7 ± 0.2 | 0.8 ± 0.4 | 0.7 ± 0.3 |
| BASO (102/μL) | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.0 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.1 |
| MONO (102/μL) | 2.2 ± 0.7 | 1.3 ± 0.2* | 1.7 ± 0.5 | 1.8 ± 1.0 | 1.2 ± 0.7 | 1.2 ± 0.5 | 1.2 ± 0.6 | 0.9 ± 0.4 |
| LUC (102/μL) | 0.9 ± 0.6 | 0.5 ± 0.2 | 0.5 ± 0.4 | 0.8 ± 0.4 | 0.4 ± 0.3 | 0.4 ± 0.2 | 0.5 ± 0.4 | 0.3 ± 0.1 |
| PT (sec) | 13.5 ± 1.0 | 13.7 ± 0.7 | 13.4 ± 1.3 | 13.9 ± 1.0 | 12.5 ± 0.6 | 13.1 ± 0.6 | 12.8 ± 1.0 | 13.2 ± 1.2 |
| APTT (sec) | 16.9 ± 1.9 | 16.2 ± 2.0 | 15.4 ± 1.9 | 16.9 ± 1.9 | 14.1 ± 2.0 | 15.4 ± 1.8 | 14.2 ± 1.8 | 15.2 ± 1.6 |
| Fbg (mg/dL) | 290 ± 30 | 295 ± 24 | 300 ± 20 | 289 ± 37 | 221 ± 24 | 206 ± 38 | 183 ± 10** | 180 ± 16** |
Values are means ± S.D. Significantly different from the control using Dunnett’s test (two-tailed) or Steel’s test (two-tailed): * p ≤ 0.05, ** p ≤ 0.01.
RBC, red blood cell count; HGB, hemoglobin; HCT, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; RDW, red blood cell distribution width; RETIC, reticulocyte count; PLT, platelet count; WBC, white blood cell count; LYM, lymphocyte count; NEUT, neutrophil count; EOS, eosinophil count; BASO, basophil count; MONO, monocyte count; LUC, large unstained cell count; PT, prothrombin time; APTT, activated partial thromboplastin time; Fbg, fibrinogen.
Table 5.
Summary of blood chemistry and serum hormone analysis in 90-day dietary toxicity study with Dry Mankai in rats.
| Male | Female | |||||||
|---|---|---|---|---|---|---|---|---|
| Dose (w/w %) | 0 (Control) | 5 | 10 | 20 | 0 (Control) | 5 | 10 | 20 |
| Number of rats | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| AST (IU/L) | 62 ± 7 | 66 ± 9 | 60 ± 8 | 66 ± 12 | 64 ± 8 | 62 ± 12 | 65 ± 16 | 61 ± 6 |
| ALT (IU/L) | 37 ± 4 | 36 ± 8 | 35 ± 7 | 39 ± 6 | 32 ± 5 | 34 ± 7 | 33 ± 5 | 36 ± 6 |
| LDH (IU/L) | 34 ± 9 | 33 ± 8 | 35 ± 12 | 29 ± 6 | 41 ± 12 | 42 ± 13 | 32 ± 11 | 31 ± 5 |
| ALP (IU/L) | 345 ± 47 | 346 ± 87 | 331 ± 87 | 330 ± 47 | 186 ± 49 | 168 ± 41 | 183 ± 54 | 233 ± 80 |
| CHE (IU/L) | 420 ± 52 | 449 ± 73 | 401 ± 74 | 410 ± 93 | 2858 ± 868 | 3005 ± 551 | 3148 ± 911 | 2961 ± 701 |
| TBA (μmol/L) | 35.4 ± 19.8 | 30.5 ± 18.5 | 18.7 ± 14.0 | 19.0 ± 13.3 | 24.2 ± 19.5 | 32.2 ± 12.7 | 23.3 ± 14.4 | 26.1 ± 13.1 |
| T-CHO (mg/dL) | 71 ± 11 | 65 ± 12 | 61 ± 10 | 53 ± 10** | 81 ± 19 | 75 ± 15 | 72 ± 19 | 72 ± 12 |
| HDL (mg/dL) | 22.1 ± 2.4 | 21.2 ± 2.8 | 20.9 ± 2.5 | 19.7 ± 3.0 | 26.3 ± 4.0 | 26.4 ± 3.4 | 25.3 ± 5.3 | 27.6 ± 3.7 |
| LDL (mg/dL) | 4.8 ± 1.2 | 5.8 ± 1.3 | 4.2 ± 0.9 | 6.0 ± 1.5 | 3.7 ± 1.3 | 3.3 ± 1.6 | 3.5 ± 0.7 | 3.4 ± 0.9 |
| TG (mg/dL) | 49 ± 17 | 50 ± 27 | 55 ± 23 | 36 ± 13 | 34 ± 16 | 39 ± 16 | 32 ± 27 | 27 ± 11 |
| PL (mg/dL) | 106 ± 12 | 98 ± 16 | 93 ± 10 | 80 ± 12** | 151 ± 27 | 148 ± 24 | 140 ± 35 | 140 ± 17 |
| T-BIL (mg/dL) | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| GLU (mg/dL) | 151 ± 16 | 169 ± 18 | 166 ± 23 | 157 ± 17 | 112 ± 15 | 113 ± 11 | 124 ± 20 | 131 ± 16* |
| BUN (mg/dL) | 19 ± 2 | 19 ± 3 | 17 ± 3 | 18 ± 3 | 17 ± 2 | 20 ± 3 | 18 ± 2 | 21 ± 3* |
| CRNN (mg/dL) | 0.27 ± 0.03 | 0.30 ± 0.04 | 0.26 ± 0.04 | 0.29 ± 0.04 | 0.34 ± 0.02 | 0.35 ± 0.04 | 0.33 ± 0.04 | 0.32 ± 0.03 |
| Urea (mg/dL) | 40 ± 5 | 42 ± 7 | 36 ± 7 | 39 ± 6 | 37 ± 5 | 44 ± 7 | 39 ± 4 | 44 ± 7* |
| Na (mmol/L) | 144 ± 1 | 144 ± 1 | 144 ± 1 | 144 ± 1 | 143 ± 1 | 143 ± 1 | 142 ± 1 | 143 ± 2 |
| K (mmol/L) | 3.9 ± 0.2 | 3.9 ± 0.3 | 4.0 ± 0.2 | 3.8 ± 0.1 | 3.4 ± 0.1 | 3.5 ± 0.2 | 3.5 ± 0.2 | 3.5 ± 0.2 |
| Cl (mmol/L) | 106 ± 1 | 107 ± 2 | 106 ± 1 | 107 ± 1 | 107 ± 1 | 107 ± 1 | 106 ± 2 | 108 ± 1 |
| Ca (mg/dL) | 10.5 ± 0.3 | 10.1 ± 0.4* | 10.3 ± 0.3 | 10.0 ± 0.4** | 10.6 ± 0.3 | 10.7 ± 0.3 | 10.6 ± 0.5 | 10.4 ± 0.3 |
| IP (mg/dL) | 6.0 ± 0.6 | 5.7 ± 0.7 | 5.7 ± 0.5 | 5.3 ± 0.5* | 5.0 ± 1.0 | 4.5 ± 0.5 | 4.5 ± 0.7 | 4.4 ± 1.2 |
| TP (g/dL) | 6.2 ± 0.1 | 6.3 ± 0.3 | 6.2 ± 0.2 | 6.1 ± 0.2 | 6.7 ± 0.3 | 6.8 ± 0.4 | 6.8 ± 0.6 | 6.6 ± 0.3 |
| ALB (g/dL) | 3.4 ± 0.1 | 3.3 ± 0.2 | 3.3 ± 0.2 | 3.3 ± 0.1 | 3.9 ± 0.3 | 4.0 ± 0.2 | 3.9 ± 0.4 | 3.9 ± 0.2 |
| Globulin (g/dL) | 2.8 ± 0.1 | 2.9 ± 0.2 | 2.9 ± 0.2 | 2.8 ± 0.2 | 2.9 ± 0.1 | 2.9 ± 0.2 | 2.8 ± 0.2 | 2.7 ± 0.1 |
| A/G | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.3 ± 0.1 | 1.4 ± 0.1 | 1.4 ± 0.1 | 1.4 ± 0.1 |
| T3 (ng/mL) | 0.56 ± 0.08 | 0.52 ± 0.09 | 0.50 ± 0.12 | 0.54 ± 0.12 | 0.75 ± 0.11 | 0.78 ± 0.15 | 0.68 ± 0.12 | 0.73 ± 0.08 |
| T4 (ng/mL) | 40.1 ± 4.8 | 39.8 ± 7.3 | 36.0 ± 3.4 | 36.5 ± 7.1 | 28.6 ± 2.7 | 26.4 ± 5.6 | 25.1 ± 7.0 | 25.9 ± 5.9 |
| TSH (ng/mL) | 6.5 ± 4.2 | 5.5 ± 2.2 | 5.9 ± 2.8 | 6.0 ± 2.2 | 3.8 ± 1.3 | 3.2 ± 1.0 | 3.9 ± 1.8 | 3.2 ± 0.9 |
Values are means ± S.D. Significantly different from the control using Dunnett’s test (two-tailed): * p ≤ 0.05, ** p ≤ 0.01.
AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDH, lactate dehydrogenase; ALP, alkaline phosphatase; CHE, cholinesterase; TBA, total bile acid; T−CHO, total cholesterol; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TG, triglycerides; PL, phospholipids; GLU, glucose; T-BIL, total bilirubin; BUN, blood urea nitrogen; CRNN, creatinine; Na, sodium; K, potassium; Cl, chloride; Ca, calcium; IP, inorganic phosphorus; TP, total protein; ALB, albumin; A/G, albumin/globulin ratio; T3, triiodothyronine; T4, thyroxine; TSH, thyroid stimulating hormone.
3.2.7. Necropsy
There were no Dry Mankai-related changes in any organ/tissue in either sex. The macroscopic findings observed in this study were judged to be incidental because they occur spontaneously in this strain of rats at this age and their low incidence.
3.2.8. Organ weights
There were no Dry Mankai-related changes in any organ/tissue in either sex (Supplemental Tables 2 and 3). Although a statistically significant decrease in the relative prostate weight was recorded at 20 % (w/w) Dry Mankai in the diet, it was considered a physiological variation because the individual values in this group were within those in the control group. In addition, a statistically significant decrease was recorded in the relative testis weights at 10 % (w/w) Dry Mankai in the diet and the absolute and relative heart weights in females at 5% (w/w) Dry Mankai in the diet. However, they were judged to be incidental because they were not dose-related.
3.2.9. Histopathology
There were no Dry Mankai-related changes in any organ/tissue in either sex (Supplemental Table 4). In this study, the observed histopathological findings were judged to be incidental because they occur spontaneously in this strain of rats at this age and there were no differences in their incidences between the control and high-dose groups.
4. Discussion
Mankai is nutritionally excellent as a food material, and it is expected to be applicable to various products as a substitute for animal protein. In this study, we performed a battery of toxicity studies on Dry Mankai, which is envisioned to be utilized as raw food material. We confirmed and demonstrated the safety of Dry Mankai by performing a reverse mutation test using bacteria, an in vitro micronucleus test, and a 90-day repeated-dose dietary toxicity study in rats in compliance with GLP standards.
The reverse mutation test using bacteria and in vitro micronucleus test were performed as genotoxicity studies, and Dry Mankai produced negative results. These results indicated that there is very little concern about the inclusion of genotoxic substances in Dry Mankai. Regarding the genotoxicity evaluation of botanical and other related products, several studies have been reported previously. Euglena gracilis is a single-celled microalga, which is cultivated in water with carbon dioxide and sunlight and shares some characteristics with Wolffia globosa Mankai. Dried Euglena gracilis was reported not to be genotoxic in Ames and mammalian micronucleus tests [15]. Although basil-based pesto sauces contain methyleugenol and related alkenylbenzenes known to be genotoxic and carcinogenic compounds, it was judged that there would be no safety concern unless they were consumed on a daily basis for an extended period of time, according to an evaluation using the margin of exposure (MOE) approach [16]. The polyphenolic extract of clove buds was reported to be non-mutagenic and it exhibited antimutagenic potential against known mutagens such as tobacco and sodium azide [17]. Hemp extract was shown to be non-mutagenic in an Ames test conducted in accordance with U.S. FDA Redbook and ICH guidelines [18]. The seed oil of Helianthus annuus Linné (sunflower) was reported to be non-mutagenic in an Ames test performed according to the OECD guideline for testing of chemicals [19].
In the ninety-day dietary toxicity study, the toxicity profile of Dry Mankai was assessed in male and female SD rats by dietary administration for 91 days at dose concentrations of 0 %, 5 %, 10 %, and 20 % (w/w) Dry Mankai in CR-LPF. The control group received CR-LPF in the same manner. In rodent toxicity studies, the addition of a test article to the diet at concentrations greater than 5% (w/w) may raise concerns regarding relative decreases in calories and nutrients such as vitamins and minerals [20]. However, the highest dose was set at 20 % (w/w) in this study considering that Dry Mankai 1) has 400 kcal/100 g which is more calories than those in CR-LPF (347 kcal/100 g), 2) contains vitamins and minerals in a good balance, and 3) is expected to be consumed in large quantities as an alternative protein source in humans in the future.
Overall, the mean test article intake throughout the administration period was 3.18, 6.49, and 13.16 g/kg/day for males and 3.58, 7.42, and 15.03 g/kg/day for females at 5%, 10 %, and 20 % (w/w), respectively.
There were no mortalities during the administration period and no test article-related changes in clinical observations, detailed clinical observations, manipulative tests, measurement of grip strength and motor activity, body weight, food consumption, ophthalmology, plasma hormone levels, organ weight, necropsy, and histopathology. The urinalysis revealed a decrease in the one-day excretion of sodium in males at 20 % (w/w) Dry Mankai in the diet, a decrease in the one-day excretion of sodium and potassium in females at 10 % and 20 % (w/w) Dry Mankai in the diet, and a decrease in the one-day excretion of chlorine in females in all dose groups. However, they were judged to have no toxicological significance because there were no changes in blood electrolytes and no related histopathological changes including injury in the kidney. In the Dry Mankai used in this study, the sodium and potassium contents were 0.054 and 0.65 g/100 g, respectively. Contrastingly, the sodium and potassium contents in CR-LPF were 0.23 and 0.83 g/100 g, respectively. Therefore, it was considered that the above-mentioned findings may be a physiological change owing to the relatively low levels of sodium and potassium in Dry Mankai. Additionally, an increase in water consumption was observed in males at 20 % (w/w) Dry Mankai in the diet, but this was not considered to be toxicologically significant as the degree of change was minimal.
In the hematological study, a decrease in fibrinogen was recorded in females at 10 % and 20 % (w/w) Dry Mankai in the diet. However, this was judged to have no toxicological significance because there were no changes in the coagulation parameters and no related histopathological changes in the liver. In the blood chemistry analysis, decreases in total cholesterol, phospholipid, calcium, and inorganic phosphorus in males at 20 % (w/w) Dry Mankai in the diet and increases in glucose and blood urea nitrogen in females at 20 % (w/w) Dry Mankai in the diet were recorded. However, they were judged to have no toxicological significance because the changes were minimal and individual values were generally within the historical control data in the test facility. In addition, an increase in urea was recorded in females at 20 % (w/w) Mankai in the diet, which was judged to be negligible and have no toxicological significance because it was minimal and the blood urea nitrogen in females at 20 % (w/w) Dry Mankai in the diet was generally within the historical control data in the test facility.
As described above, there were no toxicologically significant findings in any of the parameters. The no observed adverse effect level (NOAEL) of Dry Mankai under these study conditions was judged to be 20 % (w/w) for both males and females, i.e., 13.16 and 15.03 g/kg/day for males and females, respectively. Since no abnormalities were detected up to a very high dose level, i.e., 20 % (w/w), Dry Mankai was considered to be a food with no toxicity concerns and an excellent nutritional balance. In addition, the test results confirmed that Dry Mankai, cultivated and processed by Hinoman Ltd., did not contain any by-products and decomposition components of toxicological concern. Moreover, assuming the body weight of a human adult male to be 70 kg, the NOAEL obtained in this study is equivalent to an intake of approximately 920 g/day in humans. Nine hundred and twenty grams of Dry Mankai contains at least 368 g of protein. The average protein intake of adult males aged 19–30 years was reported to be 104.0 g/day [21]. In addition, the average total protein requirement for healthy adults was estimated as 0.66 g/kg/day (46.2 g/day for persons weighing 70 kg) [22]. Taking into account the above information, it was concluded that there is very little concern of adverse effects even when most of the dietary protein intake is derived from Dry Mankai.
To the best of our knowledge, no toxicity studies have previously been reported on Mankai and Wolffia species. Moreover, there has been no report on the effects of continuous intake of Mankai in dose ranges as high as those used in this study. In animal studies, the repeated oral gavage of Dry Mankai at 1600–3600 mg/(kg·day) for 3 weeks improved anemia in a rat iron-deficiency anemia model [7]. In humans, clinical studies in adults with abdominal obesity showed that cooked Mankai administered at 410 g/day to 13 subjects as a single dose [6], as well as Mankai shakes administered at 75 g/day to 20 subjects for 3 days [8] and 100 g/day for 6 months [7] had no adverse effects. Thus, previous reports are consistent with our current toxicity study results. In addition, Wolffia species have been traditionally eaten under the name "Khai-nam" in Burma, Laos, and northern Thailand [5]. As Wolffia globosa Mankai is taxonomically categorized into Wolffia species and considered to have high similarities with traditionally eaten Wolffia species, Mankai is inferred to be of little safety concern.
In conclusion, results showed that Dry Mankai did not show genotoxicity, and did not show toxicity after subchronic dietary administration at 20 % (w/w) Dry Mankai in the diet to SD rats, supporting the safety of Wolffia globosa Mankai for use in food applications.
Funding
This work was supported by Ajinomoto Co., Inc. and Hinoman Ltd.
CRediT authorship contribution statement
Yasuko Kawamata: Investigation, Validation, Writing - original draft, Visualization. Yusuke Shibui: Conceptualization, Investigation, Writing - original draft, Visualization, Project administration. Asuka Takumi: Investigation, Validation, Writing - review & editing. Takuya Seki: Investigation, Validation, Writing - review & editing. Tomoko Shimada: Investigation, Validation, Writing - review & editing. Masaki Hashimoto: Conceptualization, Resources, Writing - review & editing, Supervision. Naohiko Inoue: Conceptualization, Resources, Writing - review & editing, Project administration. Hisamine Kobayashi: Conceptualization, Resources, Writing - review & editing, Supervision. Takahiro Narita: Conceptualization, Validation, Writing - review & editing.
Declaration of Competing Interest
The authors are planning to develop Dry Mankai as a food product in Japan, the U.S, and Europe.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.toxrep.2020.09.006.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Wu G., Fanzo J., Miller D.D., Pingali P., Post M., Steiner J.L., Thalacker-Mercer A.E. Production and supply of high‐quality food protein for human consumption: sustainability, challenges, and innovations. Ann. N. Y. Acad. Sci. 2014;1321:1–19. doi: 10.1111/nyas.12500. [DOI] [PubMed] [Google Scholar]
- 2.Pan A., Sun Q., Bernstein A.M., Schulze M.B., Manson J.E., Stampfer M.J., Willett W.C., Hu F.B. Red meat consumption and mortality: results from 2 prospective cohort studies. Arch. Intern. Med. 2012;172:555–563. doi: 10.1001/archinternmed.2011.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouvard V., Loomis D., Guyton K.Z., Grosse Y., Ghissassi F.E., Benbrahim-Tallaa L., Guha N., Mattock H., Straif K. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015;16:1599–1600. doi: 10.1016/S1470-2045(15)00444-1. [DOI] [PubMed] [Google Scholar]
- 4.Song M., Fung T.T., Hu F.B., Willett W.C., Longo V.D., Chan A.T., Giovannucci E.L. Association of animal and plant protein intake with all-cause and cause-specific mortality. JAMA Intern. Med. 2016;176:1453–1463. doi: 10.1001/jamainternmed.2016.4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhanthumnavin K., McGarry M.G. Wolffia arrhiza as a possible source of inexpensive protein. Nature. 1971;232:495. doi: 10.1038/232495a0. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan A., Zelicha H., Tsaban G., Yaskolka Meir A., Rinott E., Kovsan J., Novack L., Thiery J., Ceglarek U., Burkhardt R., Willenberg A., Tirosh A., Cabantchik I., Stampfer M.J., Shai I. Protein bioavailability of Wolffia globosa duckweed, a novel aquatic plant–A randomized controlled trial. Clin. Nutr. 2019;38:2576–2582. doi: 10.1016/j.clnu.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Yaskolka Meir A., Tsaban G., Zelicha H., Rinott E., Kaplan A., Youngster I., Rudich A., Shelef I., Tirosh A., Brikner D., Pupkin E., Sarusi B., Blüher M., Stümvoll M., Thiery J., Ceglarek U., Stampfer M.J., Shai I. A green-mediterranean diet, supplemented with Mankai duckweed, preserves iron-homeostasis in humans and is efficient in reversal of anemia in rats. J. Nutr. 2019;149:1004–1011. doi: 10.1093/jn/nxy321. [DOI] [PubMed] [Google Scholar]
- 8.Zelicha H., Kaplan A., Yaskolka Meir A., Tsaban G., Rinott E., Shelef I., Tirosh A., Brikner D., Pupkin E., Qi L., Thiery J., Stumvoll M., Kloting N., von Bergen M., Ceglarek U., Blüher M., Stampfer M.J., Shai I. The effect of Wolffia globosa Mankai, a green aquatic plant, on postprandial glycemic response: a randomized crossover controlled trial. Diabetes Care. 2019;42:1162–1169. doi: 10.2337/dc18-2319. [DOI] [PubMed] [Google Scholar]
- 9.European Food Safety Authority (EFSA) Panel on Dietetic Products, Nutrition and Allergies (NDA) Guidance on the preparation and presentation of an application for authorisation of a novel food in the context of Regulation (EU) 2015/2283. EFSA J. 2016;14:4594. doi: 10.2903/j.efsa.2021.6555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.OECD . 1997. OECD Principles of Good Laboratory Practice. [Google Scholar]
- 11.MHLW . 1997. The Ordinance on Standard for Conduct of Non-Clinical Studies on Safety of Drugs, Ordinance No.21 of the Ministry of Health and Welfare. [Google Scholar]
- 12.OECD . 1997. OECD Guidelines for the Testing of Chemicals 471: Bacterial Reverse Mutation Test. [Google Scholar]
- 13.OECD . 2016. OECD Guidelines for the Testing of Chemicals 487: in Vitro Mammalian Cell Micronucleus Test. [Google Scholar]
- 14.OECD . 2018. OECD Guidelines for the Testing of Chemicals 408: Repeated Dose 90-Day Oral Toxicity Study in Rodents. [Google Scholar]
- 15.Simon R.R., Vo T.D., Levine R. Genotoxicity and subchronic toxicity evaluation of dried Euglena gracilis ATCC PTA-123017. Regul. Toxicol. Pharmacol. 2016;80:71–81. doi: 10.1016/j.yrtph.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Al-Malahmeh A.J., Al-Ajlouni A.M., Wesseling S., Vervoort J., Rietjens I.M.C.M. Determination and risk assessment of naturally occurring genotoxic and carcinogenic alkenylbenzenes in basil-containing sauce of pesto. Toxicol. Rep. 2017;4:1–8. doi: 10.1016/j.toxrep.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vijayasteltar L., Nair G.G., Maliakel B., Kuttan R., Krishnakumar I.M. Safety assessment of a standardized polyphenolic extract of clove buds: subchronic toxicity and mutagenicity studies. Toxicol. Rep. 2016;3:439–449. doi: 10.1016/j.toxrep.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dziwenka M., Coppock R., McCorkle A., Palumbo E., Ramirez C., Lermer S. Safety assessment of a hemp extract using genotoxicity and oral repeated-dose toxicity studies in Sprague-Dawley rats. Toxicol. Rep. 2020;7:376–385. doi: 10.1016/j.toxrep.2020.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oliveira N.M.S., Resende M.R., Morales D.A., Umbuzeiro G.R., Boriollo M.F.G. In vitro mutagenicity assay (Ames test) and phytochemical characterization of seeds oil of Helianthus annuus Linné (sunflower) Toxicol. Rep. 2016;3:733–739. doi: 10.1016/j.toxrep.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Food and Drug Administration (FDA) Subchronic toxicity studies with rodents. In: FDA, editor. Toxicological Principles for the Safety Assessment of Food Ingredients Redbook 2000. 2003. [Google Scholar]
- 21.Institute of Medicine . National Academy Press; Washington, D.C: 2002. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids. Food and Nutrition Board. [DOI] [PubMed] [Google Scholar]
- 22.WHO/FAO/UNU . Protein and Amino Acid Requirements in Human Nutrition. 2007. Report of a joint WHO/FAO/UNU expert consultation. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.