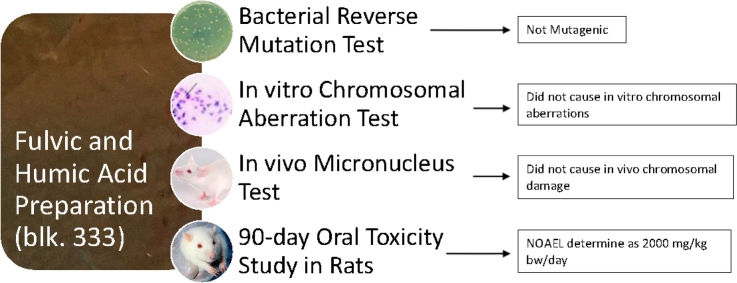
Graphical abstract
Abbreviations: ANOVA, analysis of variance; CDFA, California Department of Food and Agricultural; Cl-HA, chlorinated humic acid; DME, Dulbecco’s modified Eagle’s; EFSA, European Food Safety Authority; FA, fulvic acid; FOB, functional observation battery; fT4, free thyroxine; GLP, good laboratory practice; HA, humic acid; MPCE, micronucleated polychromatic erythrocytes; NOAEL, no observed adverse effect level; O3-HA, ozonated humic acid; O3/Cl2-HA, ozonated and chlorinated humic acid; OECD, Organisation for Economic Co-operation and Development; S9, post mitochondrial supernatant S9-mix Phenobarbital/β-naphthoflavone-induced rat liver S9 metabolic activation system; SCE, sister chromatid exchange; SD, Sprague-Dawley; SOP, standard operating procedure; SPF, specific pathogen-free; TG, test guideline; TSH, thyroid stimulating hormone
Keywords: Fulvic acid, Humic acid, blk. 333, Toxicity, Safety, NOAEL
Highlights
-
•
Toxicological evaluations of blk. 333 according to OECD guidelines were negative.
-
•
Blk. 333 was not mutagenic in vitro and showed no in vivo genotoxic activity.
-
•
The NOAEL of the 90-day study was 2000 mg/kg bw/d blk. 333—the highest dose tested.
-
•
No target organs or treatment-related toxicological effects were identified.
-
•
Our results are relevant to a safety assessment of human ingestion of blk. 333.
Abstract
Humic substances are ubiquitous in soils and waters. These complex superstructures are derived from the decomposition of dead plant and animal matter and are vital to soil health. Their heterogenous composition is specific to their site of origin and is comprised of weakly bound aggregates of small organic compounds that can sequester minerals and make them available to plants. As such, they may possess potential nutritional value for humans, and extractions of fulvic and humic acids can be produced that could be suitable for such purposes. For this reason, we evaluated the toxicological profile of a specific preparation (blk. 333) of fulvic and humic acids derived from a lignite deposit in Alberta, Canada and found it to lack genotoxic potential in a bacterial reverse mutation test, in vitro mammalian chromosomal aberration test, and in vivo mammalian micronucleus test. No general or organ toxicity was observed in Wistar rats following 90 days of continuous exposure, and a no observed adverse effect level (NOEAL) was determined at 2000 mg/kg bw/day, the highest tested dose. Our results suggest the feasibility of further evaluation for development of the preparation as a nutritional supplement in food.
1. Introduction
Humic substances are complex, weakly bound, superstructures of heterogenous, small organic compounds resulting from the decomposition of biological matter (i.e., plants and animals) and are ubiquitously present in soils and waters [1]. These amorphous aggregates cannot be defined by any single molecular structure, or, due to their molecular heterogeneity, even a set of structures; however, they do exhibit considerable uniformity when considered in terms of average properties [2]. The multiple and complex weak forces that stabilize humic substances also give rise to their reactivity and hydrophobic and hydrophilic domains within the small molecules from which the substances derive contribute to their flexible conformational structures [3].
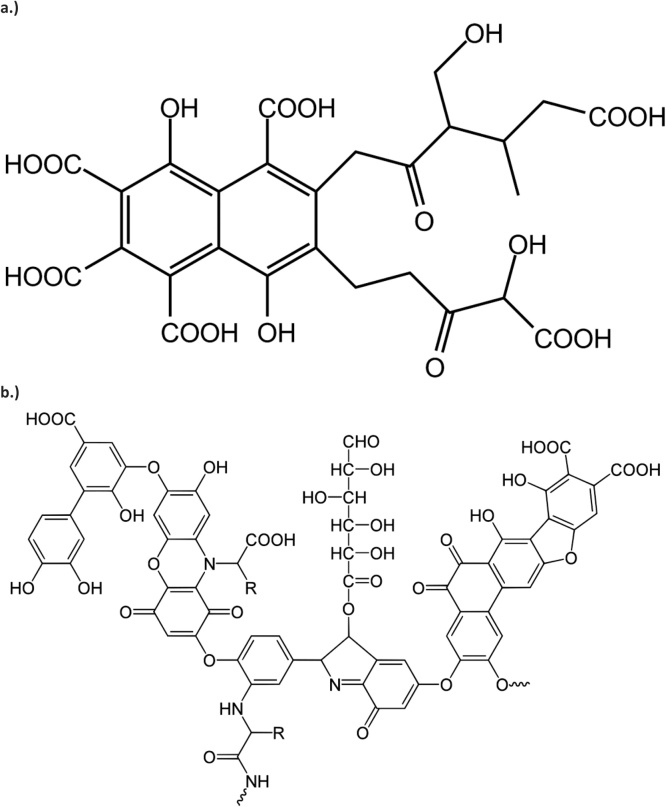
Historically, humic acids (HA; CAS no. 1415-93-6) have been defined as precipitates that form when basic extracts of humic matter are acidified while fulvic acids (FA; CAS no. 479-66-3) are those that remain in solution following this process [1,2]. In other words, HAs are soluble at alkaline pH while FAs exhibit pH independent solubility. The solubility of FAs is imparted by hydrophilicity within the associations of small molecules due to an abundance of acidic functional groups; whereas, the associations within humic acids are hydrophobic resulting in stabilization at neutral pH and clumping at acid pH [3]. However, HA and FA fractions obtained by such extraction methods have not been demonstrated to be present in natural humic matter and certainly contain compounds that are not part of humic matter as well as alteration compounds produced by the extraction technique [2,4]. In fact, it has been argued that defining HA and FA in this way has resulted in the generation of an inaccurate model of humification in an attempt to explain, retrospectively, these operational definitions [4]. Nonetheless, this is a suitable way of thinking about commercial HAs and FAs produced in a similar fashion. Such products are not single definable compounds, but rather, aggregates of multiple compounds [2]. Proposed general pseudostructures of such compounds are shown in Fig. 1.
Fig. 1.
Potential pseudostructures of a.) fulvic acid and b.) humic acid.
General properties of both HAs and FAs relate to their elemental constituents (carbon, hydrogen, nitrogen, and sulfur) and oxygen-containing functional groups (hydroxyl, carboxyl, carbonyl, and phenolic) that give rise to their general chemical properties, such as the ability to react with ionic particles in solution, while the higher molecular weight of HAs relative to FAs affects solubility, carbon and oxygen content, pH, degree of polymerization, and ion exchange capacity [2,5]. Due to their properties, HAs and FAs have been used for various agricultural applications, such as improving nutrient and water utilization and soil quality, including sequestration of carbon [3,[5], [6], [7], [8], [9], [10], [11], [12]]. While a number of mechanisms for the effects on humic substances on plant life have been suggested and/or investigated, much is left to learn as the understanding of these mechanisms is incomplete. One such mechanism is that of ion exchange, by which mineral utilization by plants may be enhanced through prevention of the formation of insoluble mineral complexes in soil; humic substances may then provide absorbable mineral ions to plant roots in exchange for hydrogen and carbonic acid [5,10,11]. The ion exchange mechanism is also active in the sequestration of toxic metals in soil. Because of the role of humic substances in sustaining plant life and their ability to bind and sequester potentially harmful environmental toxicants, it is easy to extrapolate that they might also have an inherent ability to enhance human nourishment and provide some protection against unintentionally ingested dietary toxic elements.
Traditional use, in India, of an FA preparation by humans for antioxidant, adaptogenic, and other effects has been reported, and its antioxidant activity has been assessed in several studies [13,14]; antioxidant properties of HA have also been investigated [15]. One particular preparation of HA and FA derived from Hungarian peat has been the subject of two successful New Dietary Ingredient Notifications (NDIN) to the US Food and Drug Administration for use in a dietary supplement that also contains added minerals in order to enhance mineral and trace element status in the human body [16,17]. The latter NDIN [17] reported 9 unpublished and one published [18] clinical evaluations in which this substance was found to improve mineral status in humans and/or to inhibit absorption and improve excretion of toxic elements. However, due to potential differences between this and other humic preparations, as well as the addition of exogenous minerals, it is unclear whether such results can be extrapolated to humic substances in general. Clinical or mechanistic research, relevant to human ingestion, on other humic preparations is limited, and no other studies related to the above effects have been conducted to the best of our knowledge, although the addition of an extract of humic substances to broiler feed was demonstrated to improve growth of chickens [19].
Due to the heterogeneous nature of humic substances, toxicological evaluation of a single specific substance has been considered inadequate for extrapolation to the group as a whole although efforts have been made to identify a suitable model substance [[20], [21], [22]]. As such, a number of toxicological investigations of humic substances of various origins have been published although, due to the natural occurrence of low levels of humic matter in surface waters, many of these were conducted in order to investigate the mutagenic potential of byproducts formed during disinfection of water supplies. Indeed, chlorination, under conditions of decreasing pH and adequate HA and chlorine concentrations, of organic non-volatile substances contained in HA and FA is known to result in formation of compounds (such as mucochloric acid and 2,3,3-trichloropropenal) that are mutagenic in bacterial reverse mutation tests. However, in general, these studies have been negative with respect to humic substances that have not undergone disinfection, which were often included as control substances or investigated for other reasons [20,[23], [24], [25], [26], [27], [28]].
However, some studies have shown positive or equivocal results in both mutagenicity as well as general toxicity tests [22,[29], [30], [31], [32]]. Additionally, the European Food Safety Authority (EFSA) evaluated an unpublished subchronic toxicity study in rats of a mixture of humic and fulvic acids with added minerals and determined a no observed adverse effect level (NOAEL) of 50 mg/kg bw/day due to body and organ weight decreases at higher doses that could not be laid to rest due to the absence of histological evaluations [21]. A NOEAL of 15 mg/kg bw/day of potassium humate was determined in an unpublished chronic study in dogs due to the occurrence of vomiting and watery feces at 50 mg/kg bw/day and mild heart and liver lesions at 150 mg/kg bw/day. However, EFSA also noted that no behavioral or clinical effects were observed in rats or dogs administered both concentrated HA or its sodium salt at dosages of 100 mg/kg bw/day for 30 days (rats) or 300 mg/kg bw/day for 90-days (dogs).
In light of this conflicting information, in order to evaluate potential concerns, attention should be paid both to harvest site variability in composition and specific extractions methods, both of which may give rise to differences in toxic potential, as well as to robustness of testing protocols. Therefore, with respect to the feasibility for development as a nutritional supplement, we conducted a battery of toxicological studies (as commonly recommended for the evaluation of food ingredients [33,34] and accepted by regulatory agencies as contributing to a weight-of-evidence evaluation in the experience of the authors) in accordance with current Organisation for Economic Co-operation and Development (OECD) standards on a specific preparation (blk. 333) of fulvic and humic acids derived from a lignite deposit in Alberta, Canada.
2. Material and methods
2.1. Test item
The test item was fulvic and humic acid (trade name: blk. 333) powder, a dried aqueous extract of oxidized lignite from a deposit in Alberta, Canada. It is a solid, shiny and/or dull dark and medium brown to black, fine powder that is highly soluble in water. Food grade specifications include >70 % HA content by the California Department of Food and Agricultural (CDFA) method, <5.0 % moisture, and limits on microbial growth and heavy metal content. The humics content (reported as HA content according to the classical definition) assayed by the CDFA method may include some FAs as well other medium molecular weight humic substances. The method employs a base/acid extraction procedure that measures all humics contained in the precipitate obtained at pH 2.0; however, the majority of FAs contained in blk. 333 remain in solution in the filtrate obtained by this procedure. Chemical impurities include silica, aluminosilicates, and other non-humic organic substances. A typical nutritional profile includes approximately 10 % protein, 33 % carbohydrate, 1% dietary fiber, <0.25 % fat, 55 % ash, 0.27 % calcium, 0.12 % iron, 0.48 % sodium, and trace amounts of sugars and vitamins A and C.
Fulvic and humic acid powder is manufactured, packaged, and stored in compliance with current good manufacturing practice for food. Fulvic and humic acid powder lot no. 918-10-11 (HA content 83.50 % by the CDFA method) was utilized for the genetic toxicity studies reported herein and lot no. 17H24-1006-e73c (HA content 103.67 % by the CDFA method) was utilized for the 90-day repeated-dose study.
Test solutions for the performed studies were freshly prepared directly prior to treatment of cells or dosing of animals on each experimental day. Amounts of the test item necessary to achieve the desired test solution concentrations were carefully weighed, suspended in vehicle, and stirred to achieve homogenous solutions; the test solutions were stirred continuously during dosing to maintain homogeneity and dose administration was completed within 2–4 hours of preparation. In all other aspects, the studies herein described were conducted in compliance with OECD Principles of Good Laboratory Practice (GLP) [35].
2.2. Animal husbandry
The animal studies were conducted under the permission of Institutional Animal Care and Use Committee of Toxi-Coop Zrt. The 90-day study was conducted according to the National Research Council Guide for Care and Use of Laboratory Animals [36] and in compliance with the principles of the Hungarian Act 2011 CLVIII (modification of Hungarian Act 1998 XXVIII) and Government Decree 40/2013 regulating animal protection. Animal age and weight ranges, acclimatization, housing, environmental conditions, and food (ssniff® SM R/M-Z+H, ssniff Spezialdiäten GmbH, Soest, Germany) and water (potable tap water) supply were in accordance with the respective OECD test guidelines (TG) [37,38].
2.3. Bacterial reverse mutation test
The experiments were conducted according to OECD TG 471 [39] using bacterial tester strains (Moltox, Inc., Boone, NC, USA) Salmonella typhimurium TA98, TA100, TA1535, and TA1537 and Escherichia coli WP2 uvrA without and with a Phenobarbital/β-naphthoflavone-induced rat liver post mitochondrial supernatant (S9) (Moltox, Inc., Boone, NC, USA) metabolic activation system (S9-mix). Ultrapure water (ASTM Type 1) was chosen as the vehicle, and test item concentrations of 5000, 1600, 500, 160, 50, and 16 μg/plate were chosen for the main tests (initial plate incorporation and confirmatory pre-incubation methods using procedures adapted from Ames et al. [40], Maron and Ames [41], Kier et al. [42], Venitt and Parry [43], and Mortelmans and Zeiger [44]), based on preliminary solubility and concentration range finding tests. Strain specific positive controls for use without (4-Nitro-1,2-phenylenediamine, sodium azide, and 9-aminoacridine obtained from Merck Life Science GmbH (Eppelheim, Germany) and methyl methanesulfonate obtained from Sigma-Aldrich Co. (St. Louis, MO, USA)) and with (2-aminoanthracene, Sigma-Aldrich Co., (St. Louis, MO, USA)) metabolic activation were chosen based on the TG and cited literature. Criteria for evaluation of results based on biological relevance were developed by the laboratory in accordance with the TG and have been described previously [45].
2.4. In vitro mammalian chromosomal aberration test
All experiments were conducted in accordance with OECD TG 473 [46] and the standard operating procedures (SOP) of the laboratory (developed in reference to Preston et al. [47] and Brusick [48]). V79 male Chinese hamster lung cells (European Collection of Authenticated Cell Cultures; Salisbury, England) grown in supplemented Dulbecco’s Modified Eagle’s (DME) medium (Sigma Aldrich, Schnelldorf, Germany) were utilized as the test system. Experimental conditions were short-term (3 h) treatments without and with metabolic activation (i.e., S9-mix) and sampling times of approximately 1.5 (20 h) and 2 (28 h; with S9-mix only) cell cycles and long-term treatments (20 h) without metabolic activation sampled at 20 and 28 h. Based on preliminary solubility and cytotoxicity tests, DME medium was utilized as the vehicle, and test item concentrations of 625, 1250, 2500, and 3000 μg/mL and 39.1, 78.2, 156.3, and 312.5 μg/mL for short- and long-term treatments, respectively, without S9-mix, and 1250, 2500, and 5000 μg/mL for all treatments with S9-mix were chosen for the main test. Positive controls for use without and with S9-mix were ethyl methanesulfonate (a known mutagen and clastogen chosen based on the cited literature and the historical database of the laboratory) and cyclophosphamide, respectively (Sigma Aldrich, Schnelldorf, Germany). The experiments of the main test were conducted in duplicate.
2.5. In vivo mammalian micronucleus test
The micronucleus test was conducted using specific pathogen free (SPF) Crl:NMRI BR mice (Toxi-Coop, Budapest, Hungary) in accordance with OECD TG 474 [37] with reference to the procedures of Salamone and Heddle [49]. Based on a preliminary toxicity test, groups of five male mice each were administered test item doses of 0, 500, 1000, and 2000 mg/kg bw twice, at 24 h intervals, by gavage at a constant volume of 20 mL/kg bw; the vehicle-control was distilled water (Parma Product Kft., Budapest, Hungary). An additional group was administered the positive control, cyclophosphamide (Sigma-Aldrich, Schnelldorf, Germany), once by intraperitoneal injection. Body weight measurements were made prior to the first dose and just before sacrifice, and animals were observed for mortality and signs of toxicity at regular intervals following each dose until sacrifice.
Twenty-four hours following the final treatment, animals were sacrificed by cervical dislocation, and two bone marrow (femur) samples were collected from each animal. From each sample, cell pellets were prepared, and microscope slides were smeared, fixed, and stained for examination. One slide from each animal was coded for blind scoring.
2.6. 90-day repeated-dose oral toxicity study in rats
The study was conducted in accordance with OECD TG 408 [38] in groups of SPF Han:WIST rats (Toxi-Coop, Budapest, Hungary) randomized by weight. Ten rats/sex/group were administered the test item at doses of 0, 500, 1000, and 2000 mg/kg bw/day for 90 (males) or 91 (females) consecutive days (dose and vehicle selection were made on the basis of an unpublished, OECD compliant [50], 14-day repeated-dose range-finding study in which no adverse effects were observed up to the highest dose (2000 mg/kg bw/day) tested). Doses were administered at a constant gavage volume of 10 mL/kg bw, and sunflower oil (Helianthi annui oleum raffinatum; Parma Product Kft., Budapest, Hungary) was chosen as the vehicle because the test solution became too thick/dense for gavage administration at the high concentration of 200 mg/mL when suspended in water or aqueous methylcellulose.
Animals were observed for mortality, clinical signs, behavior, and functional effects, body weight and feeding effects, and ophthalmological changes. The functional observation battery (FOB) was conducted during the last week of treatment according to laboratory SOPs developed as a modification to the of the method of Irwin [51].
Following an overnight fast after the final treatment, a state of deep narcosis was induced in the animals using Isofluran CP® anesthesia (Medicus Partner Kft, Biatorbágy, Hungary), and blood samples for clinical pathology evaluations were collected from the retro orbital venous plexus. Animals were then sacrificed by exsanguination from the abdominal aorta and subject to necropsy. Following gross pathological examinations and determination of organ weights, organ and tissue samples from all animals were preserved for potential future examination. Histopathological examinations of samples from all preserved organs and tissues of control and high-dose animals were performed, and histopathological examinations of all observed gross lesions were also performed.
2.7. Analysis of results
Statistical analyses were performed with SPSS PC + software, version 4 (SPSS, Inc., Chicago, IL, USA), and Microsoft Excel version 2016 (Microsoft, Hungary) was used to check for linear trends. A P-value of <0.05 was considered statistically significant in all tests.
2.7.1. Bacterial reverse mutation test
Mean values, standard deviations, and mutation rates were calculated based on manual counting of colony numbers, and results were evaluated on the basis of biological relevance.
2.7.2. In vitro mammalian chromosomal aberration test
All slides were independently coded and scored blind, and results from the duplicate cultures were pooled for statistical analysis. The number of aberrations and the number of cells with aberrations in the treatment and concurrent positive control groups were compared to the concurrent negative control using Fisher exact and chi-square tests. The concurrent negative and positive controls and the treatment groups were also compared to the laboratory historical controls. The data were evaluated for concentration-related increases in the number of cells with aberrations using the adequate regression analysis.
2.7.3. In vivo mammalian micronucleus test
Differences in frequency of micronucleated polychromatic erythrocytes (MPCE) were assessed using Kruskal-Wallis non-parametric one-way analysis of variance (ANOVA). The data were checked for dose-related increases in MPCE frequency using the adequate regression analysis.
2.7.4. 90-day repeated-dose oral toxicity study in rats
Bartlett’s homogeneity of variance test was used to assess for between group heterogeneity in body weight, body weight gain, food consumption, feed efficiency, clinical pathology parameters, and absolute and relative organ weight data. Heterogeneous data was assessed for normality using the Kolmogorov-Smirnov test. A one-way ANOVA was carried out if data was homogenous or heterogeneous and normally distributed while Kruskal-Wallis non-parametric one-way ANOVA was used in the case of a non-normal distribution. Post hoc analysis to assess the significance of inter-group differences was conducted using Duncan’s Multiple Range test if ANOVA results were statistically significant or using the Mann-Whitney U test if non-parametric ANOVA results were statistically significant. Frequencies of occurrence were calculated to assess the clinical relevance of non-quantitative parameters (clinical and functional observations, ophthalmoscopy, and gross and histopathological findings). Male and female data were evaluated separately.
3. Results
3.1. Bacterial reverse mutation test
The expected increases in revertant colonies were observed with the concurrent positive controls, and all concurrent positive and negative controls were within the corresponding historical control ranges. No biologically relevant (≥2-fold) or concentration-related increases in revertant colonies were observed in the test item-treated tester strains without or with metabolic activation compared to concurrent and historical negative controls (Supplemental Tables S1 and S2).
3.2. In vitro mammalian chromosomal aberration test
Following exposure (short-term without or with metabolic activation or long-term without metabolic activation) to the test item up to the cytotoxic or maximum recommended concentrations, no statistically significant or concentration-related increases in frequency of cells with aberrations compared to concurrent and historical negative controls were observed at sampling times of approximately 1.5 or 2 cell cycles (Supplemental Table S3). Additionally, no polyploidy or endoreduplicated metaphases were observed in the experiments.
3.3. In vivo mammalian micronucleus test
No mortality or abnormal signs or behavior were observed during the period from first dosing until sacrifice. The results of the micronucleus test are summarized in Supplemental Table S4. The ratio of immature to total erythrocytes was similar among treated and negative control samples although a slight, non-statistically significant, decrease was observed. Frequencies of MPCEs observed in bone marrow of treated mice did not differ statistically significantly from those of the concurrent or historical negative controls, and no dose-related increases were observed.
3.4. 90-day repeated-dose oral toxicity study in rats
3.4.1. Mortality, clinical observations, and ophthalmology
No mortality occurred during the study. Dark colored stools were observed in all test item-treated groups throughout the study. No other clinical signs, functional deficits, or abnormal behaviors were observed during the daily cage-side or weekly detailed observations, and physical state, behavior, and reactions to various stimuli were normal in all control and high-dose animals during the FOB (because no functional deficits were observed in the daily and weekly clinical observations, the FOB was not extended to the low- and mid-dose group animals). No eye alterations were observed during the ophthalmologic examinations.
3.4.2. Body weights and food consumption
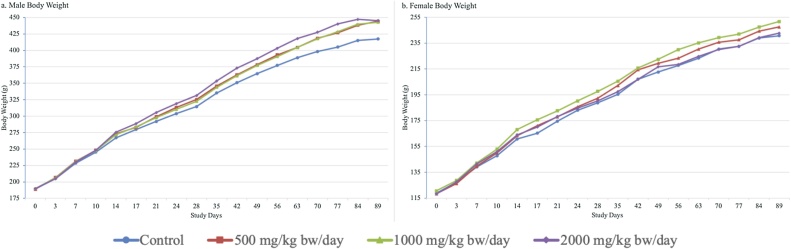
A few transient statistically significant increases in body weight gain of treated males compared to controls were observed over the course of the study but did not affect overall weight gain. Mean food consumption was statistically significantly and dose-dependently increased throughout the study in high-dose male animals compared to controls and correspondingly, overall feed efficiency was slightly worse at the high dose. However, related changes in body weight, body weight gain, clinical chemistry, and organ pathology were not observed. A few statistically significant changes in food consumption and feed efficiency were observed transiently in treated females compared to controls. The mean body weight, body weight gain, food consumption, and feed efficiency data are provided in Supplemental Tables S5–S8. Overall, an adverse test item effect on body weight development of male and female rats was not observed during the study (Fig. 2).
Fig. 2.
Male and Female Body Weight Development. (a) Male body weights. (b) Female body weights.
3.4.3. Clinical pathology
Hematological parameters were comparable in control and treated males while mean percentage of eosinophils and mean activated partial thromboplastin time were statistically significantly decreased and increased, respectively, in mid-dose females compared to controls (Supplemental Table S9). These alterations were within historical control ranges and without relation to dose or correlating histopathology.
Statistically significant, dose-related decreases were observed in alanine aminotransferase in males and calcium in females (Table 1). These changes were well within the historical control ranges and were without correlating histopathology. Statistically significant changes compared to control were also observed in low density lipoprotein and inorganic phosphate in mid-dose males only and were low in magnitude and without correlating findings.
Table 1.
Results of the Clinical Chemistry Evaluation.
| Group | ALT | AST | ALP | TBIL | CREA | UREA | GLUC | CHOL | HDL | LDL | BUN | Pi | Ca++ | Na+ | K+ | Cl− | ALB | TPROT | A/G | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (mg/kg bw/day) | [U/L] | [U/L] | [U/L] | [μmol/L] | [μmol/L] | [mmol/L] | [mmol/L] | [mmol/L] | [mmol/L] | [mmol/L] | [mg/dL] | [mmol/L] | [mmol/L] | [mmol/L] | [mmol/L] | [mmol/L] | [g/L] | [g/L] | ||
| Male | ||||||||||||||||||||
| 0 (Control) | Mean | 56.50 | 79.70 | 104.70 | 2.32 | 30.40 | 4.45 | 5.64 | 1.67 | 1.39 | 0.25 | 12.46 | 2.20 | 2.67 | 146.15 | 4.89 | 101.66 | 47.18 | 63.03 | 2.99 |
| (n = 10) | SD | 7.98 | 11.76 | 25.15 | 0.35 | 5.21 | 0.79 | 0.23 | 0.18 | 0.12 | 0.08 | 2.22 | 0.15 | 0.04 | 1.63 | 0.38 | 2.43 | 1.15 | 2.09 | 0.26 |
| 500 | Mean | 50.70 | 82.20 | 104.50 | 2.28 | 30.50 | 4.51 | 5.94 | 1.76 | 1.46 | 0.27 | 12.63 | 2.19 | 2.65 | 146.65 | 4.80 | 101.81 | 46.90 | 62.06 | 3.11 |
| (n = 10) | SD | 9.87 | 13.53 | 27.50 | 0.83 | 4.50 | 0.39 | 0.55 | 0.22 | 0.17 | 0.09 | 1.08 | 0.21 | 0.07 | 2.32 | 0.17 | 2.40 | 2.46 | 1.97 | 0.34 |
| 1000 | Mean | 46.80 | 82.40 | 103.60 | 2.19 | 28.60 | 4.53 | 5.85 | 1.62 | 1.40 | 0.18 | 12.68 | 2.37 | 2.70 | 146.04 | 5.07 | 100.88 | 47.86 | 65.23 | 2.79 |
| (n = 10) | SD | 4.52 | 7.15 | 26.86 | 0.27 | 3.66 | 0.67 | 0.38 | 0.14 | 0.14 | 0.04 | 1.87 | 0.12 | 0.06 | 1.88 | 0.23 | 1.83 | 1.45 | 2.24 | 0.35 |
| SS | ** | * | * | |||||||||||||||||
| 2000 | Mean | 41.00 | 84.30 | 112.70 | 1.97 | 30.50 | 4.61 | 5.97 | 1.81 | 1.56 | 0.21 | 12.91 | 2.34 | 2.69 | 146.42 | 5.04 | 102.35 | 47.88 | 65.55 | 2.80 |
| (n = 10) | SD | 6.96 | 12.34 | 19.03 | 0.40 | 2.84 | 0.74 | 0.63 | 0.25 | 0.24 | 0.05 | 2.06 | 0.14 | 0.06 | 2.30 | 0.40 | 2.55 | 1.48 | 3.87 | 0.54 |
| SS | ** | |||||||||||||||||||
| Test for Significance | DN | NS | NS | NS | NS | NS | NS | NS | NS | DN | NS | DN | NS | NS | NS | NS | NS | NS | NS | |
| Historical Control Range | 26.0–70.0 | 65.0–131.0 | 62.0–209.0 | 0.4–2.5 | 20.0–35.0 | 3.3–8.9 | 4.7–9.2 | 1.4–3.1 | NE | NE | NE | 1.5–2.3 | 2.4–2.9 | 141.2–148.4 | 4.1–5.2 | 96.8–103.2 | 40.1–47.3 | 59.9–70.1 | 1.5–2.6 | |
| Female | ||||||||||||||||||||
| 0 (Control) | Mean | 42.80 | 80.10 | 57.60 | 2.19 | 31.20 | 5.46 | 5.88 | 1.64 | 1.59 | 0.14 | 15.29 | 1.85 | 2.69 | 143.20 | 4.15 | 100.35 | 53.65 | 67.49 | 3.94 |
| (n = 10) | SD | 8.77 | 17.93 | 12.91 | 0.45 | 2.78 | 0.75 | 0.50 | 0.24 | 0.22 | 0.03 | 2.10 | 0.23 | 0.08 | 1.16 | 0.19 | 2.11 | 2.56 | 3.72 | 0.44 |
| 500 | Mean | 41.90 | 80.00 | 57.20 | 2.28 | 32.30 | 5.22 | 5.55 | 1.52 | 1.52 | 0.11 | 14.62 | 1.75 | 2.67 | 143.65 | 4.21 | 100.78 | 54.39 | 67.09 | 4.29 |
| (n = 10) | SD | 8.27 | 12.88 | 17.48 | 0.34 | 4.06 | 0.80 | 0.62 | 0.16 | 0.14 | 0.03 | 2.23 | 0.25 | 0.08 | 2.68 | 0.31 | 2.54 | 3.16 | 4.13 | 0.24 |
| 1000 | Mean | 34.50 | 78.40 | 52.80 | 1.99 | 31.50 | 4.97 | 5.64 | 1.50 | 1.51 | 0.13 | 13.92 | 1.84 | 2.62 | 142.50 | 4.06 | 99.44 | 53.74 | 67.31 | 4.03 |
| (n = 10) | SD | 7.09 | 18.62 | 17.84 | 0.33 | 4.17 | 0.62 | 0.40 | 0.31 | 0.28 | 0.05 | 1.73 | 0.16 | 0.03 | 1.36 | 0.25 | 1.72 | 2.17 | 2.42 | 0.56 |
| SS | * | |||||||||||||||||||
| 2000 | Mean | 35.90 | 81.20 | 57.00 | 1.91 | 32.80 | 5.02 | 6.32 | 1.59 | 1.61 | 0.11 | 14.06 | 1.71 | 2.60 | 143.03 | 4.10 | 101.76 | 53.59 | 67.79 | 3.86 |
| (n = 10) | SD | 10.02 | 16.68 | 21.16 | 0.37 | 2.62 | 0.80 | 0.42 | 0.27 | 0.26 | 0.04 | 2.25 | 0.20 | 0.05 | 1.96 | 0.17 | 2.37 | 2.23 | 2.30 | 0.65 |
| SS | * | |||||||||||||||||||
| Test for Significance | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | U | NS | NS | NS | NS | NS | NS | |
| Historical Control Range | 28.0–133.0 | 66.0–249.0 | 22.0–162.0 | 0.5–3.6 | 24.0–40.0 | 3.8–9.5 | 4.0–7.3 | 1.1–2.8 | NE | NE | NE | 0.8–2.1 | 2.4–2.9 | 140.9–146.5 | 3.1–4.6 | 97.6–105.0 | 43.8–57.6 | 56.5–78.9 | 1.7–3.7 | |
Abbreviations: A/G, albumin to globulin ratio; ALB, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; Ca++, calcium; CHOL, cholesterol; Cl−, chloride; CREA, creatinine; DN, Duncan's multiple range test; GLUC, glucose; HDL, high-density lipoprotein; K+, potassium; LDL, low-density lipoprotein; Na+, sodium; NE, laboratory historical control data not yet established—new parameter in accordance with OECD 408 (25 June 2018); NS, Not Significant; Pi, inorganic phosphorous; SD, standard deviation; SS, statistically significant compared to control; TBIL, total bilirubin; TPROT, total protein; U, Mann-Whitney U test versus control.
p < 0.05.
p < 0.01.
Free thyroxine (fT4) was statistically significantly increased compared to controls in the mid-dose group males (Supplemental Table S10). While historical control data were not available, the change appeared to be low in magnitude (10 % vs. control) and was without a dose-response or other alterations in thyroid hormones with respect to the control group.
3.4.4. Organ weights
Statistically significant dose-related increases in absolute and relative kidney weights were observed in male animals compared to controls (Table 2, Table 3, Table 4); however, these changes were within the historical control data of the laboratory and were absent of correlating findings. Other statistical significances at the absolute and/or relative weights of some organs (liver, thymus, epididymides, pituitary, and adrenal glands in male animals and brain, heart, ovaries, and pituitary in female animals) with respect to the appropriate control were also not considered to be toxicologically relevant because of their low magnitude and the absence of related histopathological findings at the high dose.
Table 2.
Organ Weights.
| Body weight and Organ weight (g) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Body | Brain | Liver | Kidneys | Heart | Thymus | Spleen | Testes | Epididy- | Seminal | Pituitary | Adrenal | Thyroid | |
| (mg/kg bw/day) | weight | mides | vesiclesa | glands | ||||||||||
| Male | ||||||||||||||
| 0 (Control) | Mean | 411.20 | 2.17 | 9.88 | 2.12 | 1.02 | 0.39 | 0.68 | 3.56 | 1.53 | 2.27 | 0.0078 | 0.071 | 0.017 |
| (n = 10b) | SD | 19.90 | 0.07 | 0.85 | 0.15 | 0.07 | 0.09 | 0.07 | 0.27 | 0.19 | 0.29 | 0.0013 | 0.011 | 0.003 |
| 500 | Mean | 435.50 | 2.20 | 10.71 | 2.36 | 1.08 | 0.40 | 0.71 | 3.68 | 1.65 | 2.45 | 0.0077 | 0.069 | 0.018 |
| (n = 10) | SD | 31.63 | 0.07 | 1.06 | 0.19 | 0.08 | 0.08 | 0.07 | 0.19 | 0.12 | 0.35 | 0.0009 | 0.010 | 0.002 |
| SS | * | |||||||||||||
| 1000 | Mean | 435.90 | 2.18 | 11.19 | 2.40 | 1.10 | 0.40 | 0.71 | 3.73 | 1.69 | 2.44 | 0.0085 | 0.070 | 0.018 |
| (n = 10) | SD | 34.66 | 0.09 | 1.45 | 0.34 | 0.11 | 0.09 | 0.13 | 0.22 | 0.13 | 0.38 | 0.0016 | 0.011 | 0.003 |
| SS | * | * | * | |||||||||||
| 2000 | Mean | 435.90 | 2.18 | 10.87 | 2.46 | 1.08 | 0.29 | 0.69 | 3.63 | 1.55 | 2.42 | 0.0096 | 0.082 | 0.019 |
| (n = 10) | SD | 37.82 | 0.08 | 1.20 | 0.19 | 0.09 | 0.07 | 0.09 | 0.40 | 0.13 | 0.30 | 0.0014 | 0.007 | 0.002 |
| SS | ** | * | ** | * | ||||||||||
| Test for Significance | NS | NS | DN | DN | NS | DN | NS | NS | DN | NS | DN | DN | NS | |
| Historical Control Range | 363.0–548.0 | 2.00–2.35 | 8.20–14.60 | 1.95–3.19 | 0.93–1.37 | 0.25–0.59 | 0.51–0.93 | 2.99–4.43 | 1.20–1.91 | 1.46–3.25 | NE | 0.041–0.091 | NE | |
| Female | Ovaries | Uterus | ||||||||||||
| 0 (Control) | Mean | 236.3 | 1.97 | 7.18 | 1.46 | 0.73 | 0.31 | 0.48 | 0.084 | 0.74 | 0.0097 | 0.078 | 0.021 | |
| (n = 10) | SD | 20.15 | 0.10 | 1.24 | 0.07 | 0.05 | 0.07 | 0.07 | 0.025 | 0.13 | 0.0016 | 0.009 | 0.003 | |
| 500 | Mean | 242.0 | 2.02 | 6.97 | 1.54 | 0.74 | 0.31 | 0.50 | 0.105 | 0.78 | 0.0108 | 0.085 | 0.024 | |
| (n = 10) | SD | 23.08 | 0.09 | 0.92 | 0.19 | 0.06 | 0.06 | 0.09 | 0.025 | 0.13 | 0.0019 | 0.015 | 0.006 | |
| 1000 | Mean | 244.9 | 2.05 | 7.32 | 1.55 | 0.73 | 0.32 | 0.53 | 0.118 | 0.77 | 0.0098 | 0.087 | 0.021 | |
| (n = 10) | SD | 16.58 | 0.06 | 0.91 | 0.14 | 0.06 | 0.05 | 0.08 | 0.031 | 0.13 | 0.0012 | 0.008 | 0.003 | |
| SS | * | ** | ||||||||||||
| 2000 | Mean | 237.5 | 1.95 | 6.51 | 1.47 | 0.68 | 0.29 | 0.46 | 0.105 | 0.75 | 0.0110 | 0.088 | 0.020 | |
| (n = 10) | SD | 21.96 | 0.07 | 0.71 | 0.14 | 0.06 | 0.05 | 0.05 | 0.020 | 0.17 | 0.0013 | 0.011 | 0.002 | |
| Test for Significance | NS | DN | NS | NS | NS | NS | NS | DN | NS | NS | NS | NS | ||
| Historical Control Range | 208–297.0 | 1.83–2.17 | 5.18–8.53 | 1.36–2.34 | 0.63–0.85 | 0.18–0.47 | 0.32–0.56 | 0.07–0.14 | 0.42–1.11 | NE | 0.063–0.104 | NE | ||
Abbreviations: DN, Duncan's multiple range test; NE, laboratory historical control data not yet established—new parameter in accordance with OECD 408 (25 June 2018); NS, Not Significant; SD, standard deviation; SS, statistically significant compared to control.
Remarks: Paired organs were weighed together.
Seminal vesicle coagulating gland and prostate (as a whole).
n = 9 for adrenal glands of control group males (adrenal glands one control male animal were not weighted due to loss at necropsy).
p < 0.05.
p < 0.01.
Table 3.
Organ Weights Relative to Body Weight.
| Organ weight relative to body weight (%) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Brain | Liver | Kidneys | Heart | Thymus | Spleen | Testes | Epididy- | Seminal | Pituitary | Adrenal | Thyroid | |
| (mg/kg bw/day) | mides | vesiclesa | glands | ||||||||||
| Male | |||||||||||||
| 0 (Control) | Mean | 0.528 | 2.403 | 0.515 | 0.248 | 0.094 | 0.166 | 0.866 | 0.374 | 0.551 | 0.0019 | 0.0170 | 0.0041 |
| (n = 10b) | SD | 0.019 | 0.170 | 0.029 | 0.014 | 0.021 | 0.017 | 0.065 | 0.050 | 0.067 | 0.0003 | 0.0028 | 0.0006 |
| 500 | Mean | 0.508 | 2.457 | 0.543 | 0.247 | 0.092 | 0.163 | 0.848 | 0.380 | 0.567 | 0.0018 | 0.0158 | 0.0042 |
| (n = 10) | SD | 0.035 | 0.094 | 0.029 | 0.012 | 0.017 | 0.017 | 0.075 | 0.043 | 0.096 | 0.0002 | 0.0027 | 0.0004 |
| 1000 | Mean | 0.501 | 2.558 | 0.550 | 0.253 | 0.092 | 0.162 | 0.860 | 0.389 | 0.560 | 0.0019 | 0.0162 | 0.0042 |
| (n = 10) | SD | 0.035 | 0.139 | 0.051 | 0.015 | 0.019 | 0.020 | 0.069 | 0.035 | 0.088 | 0.0004 | 0.0027 | 0.0005 |
| 2000 | Mean | 0.504 | 2.493 | 0.565 | 0.249 | 0.066 | 0.158 | 0.837 | 0.357 | 0.556 | 0.0022 | 0.0188 | 0.0042 |
| (n = 10) | SD | 0.056 | 0.149 | 0.043 | 0.009 | 0.012 | 0.017 | 0.103 | 0.039 | 0.063 | 0.0003 | 0.0021 | 0.0003 |
| SS | * | ** | * | ||||||||||
| Test for Significance | NS | NS | DN | NS | DN | NS | NS | NS | NS | DN | NS | NS | |
| Historical Control Range | 0.403–0.606 | 2.055–3.156 | 0.452–0.634 | 0.211–0.284 | 0.063–0.129 | 0.119–0.194 | 0.642–0.963 | 0.279–0.424 | 0.360–0.716 | NE | 0.009–0.020 | NE | |
| Female | Ovaries | Uterus | |||||||||||
| 0 (Control) | Mean | 0.838 | 3.032 | 0.623 | 0.307 | 0.132 | 0.202 | 0.035 | 0.315 | 0.0041 | 0.0333 | 0.0091 | |
| (n = 10) | SD | 0.077 | 0.433 | 0.048 | 0.015 | 0.029 | 0.021 | 0.009 | 0.062 | 0.0006 | 0.0035 | 0.0014 | |
| 500 | Mean | 0.839 | 2.876 | 0.634 | 0.305 | 0.127 | 0.207 | 0.043 | 0.322 | 0.0044 | 0.0350 | 0.0097 | |
| (n = 10) | SD | 0.084 | 0.208 | 0.035 | 0.015 | 0.021 | 0.025 | 0.007 | 0.055 | 0.0005 | 0.0057 | 0.0021 | |
| 1000 | Mean | 0.840 | 2.987 | 0.632 | 0.297 | 0.130 | 0.218 | 0.048 | 0.315 | 0.0040 | 0.0357 | 0.0084 | |
| (n = 10) | SD | 0.050 | 0.280 | 0.028 | 0.019 | 0.014 | 0.032 | 0.011 | 0.056 | 0.0005 | 0.0036 | 0.0009 | |
| SS | ** | ||||||||||||
| 2000 | Mean | 0.828 | 2.743 | 0.621 | 0.285 | 0.122 | 0.196 | 0.044 | 0.318 | 0.0046 | 0.0371 | 0.0086 | |
| (n = 10) | SD | 0.081 | 0.206 | 0.052 | 0.017 | 0.019 | 0.023 | 0.008 | 0.080 | 0.0005 | 0.0045 | 0.0013 | |
| SS | ** | * | * | ||||||||||
| Test for Significance | NS | NS | NS | DN | NS | NS | DN | NS | DN | NS | NS | ||
| Historical Control Range | 0.731–1.000 | 2.183–3.189 | 0.508–0.951 | 0.236–0.333 | 0.078–0.169 | 0.139–0.227 | 0.029–0.054 | 0.161–0.465 | NE | 0.025–0.045 | NE | ||
Abbreviations: DN, Duncan's multiple range test; NE, laboratory historical control data not yet established—new parameter in accordance with OECD 408 (25 June 2018); NS, Not Significant; SD, standard deviation; SS, statistically significant compared to control.
Remarks: Paired organs were weighed together.
Seminal vesicle coagulating gland and prostate (as a whole).
n = 9 for adrenal glands of control group males (adrenal glands one control male animal were not weighted due to loss at necropsy).
p < 0.05.
p < 0.01.
Table 4.
. Organ Weights Relative to Brain Weight.
| Body weight and Organ weight relative to brain weight (%) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Body | Liver | Kidneys | Heart | Thymus | Spleen | Testes | Epididy- | Seminal | Pituitary | Adrenal | Thyroid | |
| (mg/kg bw/day) | weight | mides | vesiclesa | glands | |||||||||
| Male | |||||||||||||
| 0 (Control) | Mean | 18975.6 | 455.67 | 97.78 | 47.01 | 17.86 | 31.55 | 164.10 | 70.69 | 104.36 | 0.36 | 3.23 | 0.78 |
| (n = 10b) | SD | 675.12 | 32.74 | 6.11 | 3.29 | 4.23 | 3.42 | 10.37 | 7.83 | 11.06 | 0.06 | 0.46 | 0.14 |
| 500 | Mean | 19776.2 | 486.45 | 107.30 | 48.82 | 18.10 | 32.15 | 166.85 | 74.66 | 111.27 | 0.35 | 3.12 | 0.84 |
| (n = 10) | SD | 1451.96 | 47.97 | 7.90 | 2.72 | 3.79 | 2.34 | 6.64 | 5.06 | 14.70 | 0.04 | 0.49 | 0.10 |
| SS | * | ||||||||||||
| 1000 | Mean | 20040.8 | 513.91 | 110.33 | 50.59 | 18.49 | 32.57 | 171.80 | 77.87 | 111.86 | 0.39 | 3.22 | 0.84 |
| (n = 10) | SD | 1345.49 | 57.95 | 13.46 | 4.05 | 4.21 | 5.53 | 11.59 | 6.87 | 16.06 | 0.06 | 0.42 | 0.13 |
| SS | * | ** | * | ||||||||||
| 2000 | Mean | 20062.1 | 500.42 | 112.79 | 49.88 | 13.32 | 31.67 | 166.94 | 71.08 | 111.20 | 0.44 | 3.74 | 0.85 |
| (n = 10) | SD | 2257.41 | 65.46 | 8.81 | 5.30 | 2.93 | 4.36 | 19.76 | 6.75 | 14.80 | 0.07 | 0.35 | 0.09 |
| SS | ** | * | ** | * | |||||||||
| Test for Significance | NS | DN | DN | NS | DN | NS | NS | DN | NS | DN | DN | NS | |
| Historical Control Range | 16500.0–24796.4 | 375.45–660.63 | 88.64–144.34 | 42.73–61.99 | 11.31–26.29 | 22.47–41.15 | 146.64–196.02 | 55.16–85.65 | 65.47–154.76 | NE | 1.95–4.27 | NE | |
| Female | Ovaries | Uterus | |||||||||||
| 0 (Control) | Mean | 12016.9 | 364.96 | 74.50 | 36.91 | 15.89 | 24.36 | 4.26 | 37.61 | 0.49 | 3.99 | 1.09 | |
| (n = 10) | SD | 1010.67 | 62.28 | 3.97 | 3.25 | 4.30 | 3.81 | 1.23 | 6.63 | 0.08 | 0.52 | 0.15 | |
| 500 | Mean | 12023.8 | 345.72 | 76.35 | 36.58 | 15.30 | 24.98 | 5.20 | 38.60 | 0.54 | 4.21 | 1.17 | |
| (n = 10) | SD | 1203.76 | 42.81 | 9.88 | 3.33 | 3.26 | 4.08 | 1.21 | 7.10 | 0.09 | 0.83 | 0.32 | |
| 1000 | Mean | 11941.0 | 356.81 | 75.48 | 35.46 | 15.58 | 25.90 | 5.77 | 37.56 | 0.48 | 4.26 | 1.01 | |
| (n = 10) | SD | 743.17 | 40.51 | 5.75 | 2.26 | 2.06 | 3.43 | 1.55 | 6.61 | 0.06 | 0.46 | 0.15 | |
| SS | * | ||||||||||||
| 2000 | Mean | 12180.9 | 333.80 | 75.38 | 34.68 | 14.84 | 23.80 | 5.39 | 38.19 | 0.56 | 4.50 | 1.04 | |
| (n = 10) | SD | 1219.24 | 38.34 | 6.89 | 3.28 | 2.46 | 2.81 | 1.01 | 8.40 | 0.07 | 0.58 | 0.12 | |
| Test for Significance | NS | NS | NS | NS | NS | NS | DN | NS | NS | NS | NS | ||
| Historical Control Range | 10000.0–13686.6 | 263.82–408.74 | 66.67–125.81 | 30.29–44.09 | 8.96–21.86 | 17.11–28.87 | 3.49–7.00 | 20.85–51.63 | NE | 2.99–5.56 | NE | ||
Abbreviations: DN, Duncan's multiple range test; NE, laboratory historical control data not yet established—new parameter in accordance with OECD 408 (25 June 2018); NS, Not Significant; SD, standard deviation; SS, statistically significant compared to control.
Remarks: Paired organs were weighed together.
Seminal vesicle coagulating gland and prostate (as a whole).
n = 9 for adrenal glands of control group males (adrenal glands one control male animal were not weighted due to loss at necropsy).
p < 0.05.
p < 0.01.
3.4.5. Gross and histopathology
Dark colored content was observed in the stomach and small and large intestines of most treated animals, and dark colored content was also observed in the cecum of one low-dose female and two high-dose males and the rectum of one high-dose male (Supplemental Table S11). This finding was correlated to the clinical observation of dark stools in treated animals and was consistent with the color of the test item. No correlated histological lesions were observed in the gastrointestinal tracks of any control or high-dose animals on microscopic examination.
Pyelectasia was observed macroscopically in one or both kidneys in some control, low-dose, and high-dose animals and was correlated to the histological finding (Table 5) of renal pelvic dilatation without pathological changes (e.g., inflammation or necrosis) in the same animals. Frequency of occurrence was low and similar among controls and high-dose animals, and while more low-dose animals were affected, there was no dose-response. Dilatation of the uterine horns was observed macroscopically in some females of the control and all treated groups and microscopically in most of the same control and high-dose animals without associated inflammatory or necrotic changes.
Table 5.
Summary of Histopathology Findings.
| Dose group (mg/kg bw/day) | Control (0) | 500 | 1000 | 2000 | |
|---|---|---|---|---|---|
| Organs | Observations | (n = 10) | N/A | N/A | (n = 10) |
| Male | |||||
| Animals with no microscopic findings | 3/10 | N/A | N/A | 3/10 | |
| Kidneys: | Pelvic dilatation, slight, one or two sides | 1/10 | 5/5 | / | 1/10 |
| Liver: | Cetrilobular vacuolation, mimimal to moderate | 61–3/10 | / | / | 71–3/10 |
| Lungs: | Alveolar emphysema, minimal | 21/10 | / | / | 11/10 |
| Hyperplasia of BALT, minimal to mild | 21–2/10 | / | / | 12/10 | |
| Stomach: | Ulceration, moderate | 0/10 | 13/1 | / | 0/10 |
| Female | |||||
| Animals with no microscopic findings | 7/10 | N/A | N/A | 3/10 | |
| Kidneys: | Pelvic dilatation, slight, both sides | 0/10 | 2/2 | / | 1/10 |
| Lungs: | Alveolar emphysema, minimal | 11/10 | / | / | 0/10 |
| Hyperplasia of BALT, minimal | 11/10 | / | / | 0/10 | |
| Mesenteric lymph nodes: | Hemorrhage, mild | 0/10 | / | / | 12/10 |
| Ovaries: | Lack of corpora lutea | 1/10 | / | / | 0/10 |
| Uterus: | Dilatation | 2/10 | / | / | 6/10 |
| Adenoma | 0/10 | / | / | 1/10 | |
| Abdominal cavity: | Lipoma | 1/1 | / | / | / |
Abbreviations: /, not examined; BALT, bronchus associated lymphoid tissue; N/A, not applicable (only gross lesions were examined).
Data represent incidence of the observation (number of animals with observation per number of animals examined).
Organs without lesions in 10/10 control and high-dose animals or without gross lesions at necropsy not shown.
Superscripts represent grades of lesions: 1 = minimal, 2 = mild, 3 = moderate, 4 = severe.
Minimal, mild, or moderate vacuolation of hepatocytes was observed microscopically, with similar frequency and severity in control and high-dose males, mainly in the centrilobular area of the liver. Minimal alveolar emphysema and minimal or mild hyperplasia of the bronchus associated lymphoid tissue (BALT) were observed with similar low frequencies in the lungs of control and high-dose animals. Other macroscopic and microscope lesions observed, and summarized in Supplemental Table S11 and Table 5, respectively, were considered as individual findings due to their singular occurrences.
4. Discussion
The results of our bacterial reverse mutation test are consistent with those of the majority of previous bacterial reverse mutation experiments on humic test items that had not been subjected to chemical disinfection [20,[23], [24], [25], [26], [27], [28]]; however, Ueno et al. explored the effects of ozonation on three different HA samples (one from peat, one from wastewater, and one from soil) in a bacterial reverse mutation test and observed differences in the mutagenic potential of each [22].
In contrast to our results, the raw (i.e., non-ozonated) sample isolated from wastewater was mutagenic without S9; however, it was not mutagenic with ozonation or with S9. The authors hypothesized that low molecular weight, nitrogen-rich contaminants trapped in the wastewater HA sample, which would have decomposed by ozonation, were likely responsible for the observed mutagenicity. On the other hand, the sample isolated from peat was not mutagenic under any of the tested conditions while the sample isolated from soil was not mutagenic without ozonation with or without S9 but was mutagenic when ozonated without S9. The soil HA had a high content of metal ions known to generate mutagenic reactive oxygen species when ozonated. Thus overall, the results of Ueno et al. support the concept that source specific components of HAs contribute to different toxicological potentials.
Additionally, while we are unaware of any other humic substances having been investigated using an in vitro chromosomal aberration test, mutagenic activity of different HA samples has been observed in sister chromatid exchange (SCE) assays conducted to investigate their potential desmutagenic activities. Cozzi et al. evaluated four different HA samples and found three of them, including one extracted from lignite, to be clearly mutagenic in an in vitro SCE assay in Chinese hamster ovary cells [30]. The sample extracted from volcanic soil was not clearly positive, further suggesting that humic substances from different sources may exhibit different toxic potentials. Ribas et al. also found HA to be mutagenic in an in vitro SCE assay in human lymphocytes [32]. Although the results were statistically significant in both studies, the respective authors considered the observed mutagenicity to be weak when compared to positive controls or other known mutagens. Cozzi et al. seemed surprised by their results as they opined that they were likely due to “some chlorination effect” or contamination with a mutagen during sample preparation, but, while Ribas et al. acknowledged the opinion of Cozzi et al. they conceded that in their experiments, there was “no reliable evidence supporting when and how the humic acid” could have undergone chlorination.
In an in vivo chromosomal aberration study, Bernacchi et al. observed structural aberrations and aneuploidy in intestinal cells and aneuploidy (albeit, without statistical significance) in bone marrow of mice treated with a single dose of HA at 100 mg/kg bw/day [29]. The authors postulated that intestinal chlorination of HA to 3-chloro-4-(dichloromethyl)-5-hydroxy-2(5 H)-furanone may have been responsible for the clastogenic effects on intestinal cells but would not have been expected in bone marrow due to rapid in vivo biotransformation as well as its limited tissue distribution and the low dose of HA that was administered; however, to our knowledge, intestinal chlorination of HA has not been demonstrated.
To the best of our knowledge, ours is the first micronucleus test to have been conducted on a humic substance; nonetheless, in contrast to the in vivo chromosomal aberration test by Bernacchi et al. discussed above, we did not observe a clastogenic or aneugenic effect of our test item in mouse bone marrow based on MPCE frequencies at much higher administered doses nor did we observe structural or numerical chromosomal aberrations in vitro at high concentrations. We note that in both ours and Bernacchi et al.’s in vivo tests, the test solutions were ingested by gavage administration although our test solutions were not exposed to hydrochloric acid during preparation as were those of Bernacchi et al.
Despite the in vitro SCE assays and in vivo chromosomal aberration test demonstrating weak mutagenic activities of some HA samples, a commercial HA preparation (Fluka, Switzerland) was not carcinogenic in mice receiving the test item in drinking water at concentrations corresponding to 0.5 g total organic carbon/L for 24 months [27]. While some of the observed results when the test item was chlorinated may be considered equivocal, no statistically significant increases in incidences of malignant tumors were observed in male or female mice receiving the non-chlorinated HA.
We investigated the repeated-dose toxicity of our test item in rats and observed several statistically significant alterations in various parameters that were of low magnitude and considered to be without toxicological concern. Interestingly, with respect to effects of humic substances on the thyroid, Daniel et al. observed an increased incidence in histological thyroid lesions (colloid depletion, minimal severity and follicular cysts) in male Sprague-Dawley (SD) rats administered a single dose of a commercial HA preparation (Fluka, Switzerland) as a control substance in a subchronic toxicity study of the same HA subjected to ozonation (O3-HA) or subjected to both ozonation and chlorination (O3/Cl2-HA); however, thyroid hormone levels were not measured in the study [31]. Thyroid colloid depletion was also observed in high-dose O3/Cl2-HA group males at lower incidence while thyroid lesions were not observed in the buffered water control or O3-HA high-dose groups. While speculative, it is possible that the HA test item used by Daniel et al. contained a goitrogen, or other thyro-toxicant, that was inactivated by ozonation. As neither alterations in thyroid-stimulating hormone (TSH) levels nor similar lesions were observed in our work, there is no reason to suspect the observed increase in fT4 in mid-dose males was related to administration of the test item as it occurred sporadically without a dose response or related effects on TSH, free triiodothyronine, or thyroid weight, and without any correlating histopathology.
As in the current work, Condie, et al. also reported dose-related increases in absolute and relative kidney weights in SD rats administered chlorinated HA (Cl-HA) for 90-days; however, these changes were accompanied by an increased incidence and severity of crystalline deposits in the renal pelvis and associated hematuria [52]. The authors concluded that the observed renal effects were likely related to a strain-specific predisposition for renal changes in SD rats that may have been accelerated by Cl-HA (although similar effects were not observed by Daniel et al. who also used SD rats and the same commercial HA source). In the current work, the observed alterations in renal weights remained well-within the historical control range of the laboratory and occurred without related changes in clinical chemistry parameters or correlating histopathology and, as such, were considered to be without toxicological relevance.
We also observed several gross and histological lesions in the current work that occurred in both controls and treated animals without dose-responses. Renal pelvic dilatation is a species-specific background lesion that occurs in untreated rats [[53], [54], [55], [56], [57]] and historical control animals of the laboratory, and uterine dilatation (which can be indicative of an estrogenic effect of a test item when a dose-response, degenerative changes, and/or other correlating findings are observed) is a normal occurrence during the proestrus and estrus phases of the sexual cycle due to stimulation by estrogen [[58], [59], [60], [61]].
The vacuolation of hepatocytes observed in the current work was considered an indication of hepatic lipidosis [62], a light reversible liver injury in connection with a disturbance of energy metabolism of affected hepatocytes that can occur in response to dietary fat intake [63,64]. Due to its occurrence with similar frequency and severity in both control and high-dose males, it was considered a response to the sunflower oil vehicle without relevance to the test item.
Alveolar emphysema and hyperplasia of the BALT are both observed with similar frequencies in historical control animals and are known background lesions in rats [[65], [66], [67]]; the former was considered due to the exsanguination procedure while the latter is a antigenic response that is likely due to commensal flora, as it was not associated with inflammatory lesions.
5. Conclusions
All genetic toxicity tests having met their respective acceptance criteria, including validation of the negative and positive controls, the test item was determined to lack genotoxic potential as no frameshift or base pair substitution mutations or in vitro or in vivo chromosomal damage were observed. As discussed above, Bernacchi et al. suggested a potential for gastrointestinal chlorination of ingested HA [29], although, to the best of our knowledge, this has not been demonstrated in any experiments. As such, further investigation into the potential for the current test item to undergo chlorination within the gastrointestinal track could be considered, and if confirmed, an in vivo investigation of genotoxic potential in intestinal cells could be conducted. Nonetheless, in contrast to the test item of Bernacchi, the current test item was not treated with hydrochloric acid during its production, and another HA preparation was not carcinogenic, with or without chlorination, in a 2-year study in mice (although results could be considered equivocal for the chlorinated HA with respect to leukemia incidence in males only) [27]. Thus, it is questionable whether such additional investigations are warranted.
While our 90-day study was not suggestive of any thyrotoxicity of the test item, in the study by Daniel et al. thyroid lesions that could be indicative of the presence of a goitrogen in their test item or iodine sequestration by HA in the gastrointestinal track were observed [31]. Because some known goitrogenic substances have been reported as degradation products of humic substances [68], further investigations into the toxicological potential of blk. 333 could include an assay for known goitrogenic substances under simulated gastrointestinal conditions. With respect to gastrointestinal sequestration of iodine, in addition to a lack of effect on the thyroid, we did not observe any correlating findings, such as estrogenic lesions in breast tissue, suggestive of such an effect. However, in contrast to our study, in which we administered the test item by gavage, Daniel et al. administered their test item in drinking water, and it might be supposed that such effects could be less pronounced or absent when the gavage route is used. Such effects could be further investigated via a binding affinity study and/or a subchronic or chronic repeated-dose study with administration in drinking water. Dark colored stools and intestinal content observed in the 90-day study were considered to be due to the color of the test item and without toxicological relevance. Additionally, statistically significant and dose-related slightly higher food consumption, clinical chemistry alterations, and kidney weights were also considered to have occurred without toxicologic relevance due to their low magnitudes and lack of correlating findings. Histological findings observed were without dose relationships and were generally lesions commonly observed in untreated laboratory rats (including control animals of the current study) without pathological changes while vacuolation of hepatocytes observed in the livers of male control and high-dose animals were considered due to the sunflower oil vehicle; thus, these were considered as unrelated to the test item and without toxicological relevance.
For the reasons stated above, we determined that the test item (blk. 333 fulvic and humic acids preparation derived from a lignite deposit in Alberta, Canada) was not mutagenic or clastogenic under the applied test conditions, and the NOAEL in male and female Han:WIST rats was 2000 mg/kg bw/day, the highest dose tested, following 90 days of continuous exposure by gavage.
Funding
The authors disclose that financial support for the research described herein was provided by the sponsor— BLK International, LLC, 26565 Agoura Road, Suite 205, Calabasas, CA 91302 USA. The sponsor was not involved in study designs; collection, analysis, and interpretation of data; or writing of the formal laboratory reports upon which this article is based. The sponsor did not participate in the writing this article except in providing feedback to the corresponding author regarding the accurate description of the test item in section 2.1. The sponsor was involved in the decision to submit the article for publication in that the sponsor hired AIBMR Life Sciences, Inc. to prepare and submit the article for publication and approved the authors’ choice of ToxicologyReports for submission.
Data availability
The mean data sets generated and utilized for statistical analysis to support the findings of these studies are included within the article or in the supplementary information files. All other raw and processed data used to support the findings of these studies are available from the corresponding author upon request.
CRediT authorship contribution statement
Timothy S. Murbach: Writing - original draft, Visualization. Róbert Glávits: Formal analysis, Data curation, Writing - review & editing. John R. Endres: Conceptualization, Funding acquisition, Writing - review & editing, Funding acquisition. Amy E. Clewell: Writing - review & editing. Gábor Hirka: Resources, Conceptualization, Methodology, Writing - review & editing, Supervision, Project administration. Adél Vértesi: Methodology, Validation, Formal analysis, Investigation, Data curation, Writing - review & editing, Visualization, Supervision, Project administration. Erzsébet Béres: Methodology, Validation, Formal analysis, Investigation, Data curation, Writing - review & editing, Visualization, Supervision, Project administration. Ilona Pasics Szakonyiné: Methodology, Validation, Formal analysis, Investigation, Data curation, Writing - review & editing, Visualization, Supervision, Project administration.
Declaration of Competing Interest
Authors Timothy Murbach, John Endres, and Amy Clewell are salaried employees of AIBMR Life Sciences, Inc. (Seattle, WA, USA). AIBMR was contracted by the study sponsor, as an independent third party, to determine appropriate study protocols and dose selections, place the studies, approve the study plans, and monitor the toxicological studies herein described and to analyze and interpret the resulting data and prepare the manuscript. Author Gábor Hirka is owner and Managing Director at Toxi-Coop Zrt. (with test facilities in Budapest (90-day study) and Balatonfüred (genotoxicity studies), Hungary); authors Adél Vértesi, Erzsébet Béres, and Ilona Pasics Szakonyiné are salaried employees of Toxi-Coop; and author Róbert Glávits is an independent contractor to Toxi-Coop. Toxi-Coop was contracted by AIBMR to develop the study plans and conduct, analyze and interpret, and report the results of the toxicological studies herein described. The authors declare no additional conflicts of interest in regard to the research, authorship, and/or publication of this article.
Acknowledgements
The authors thank the following individuals for their contributions to the work: participating investigators Viktória Polgár-Balogh, Erika Major Biermanné, Ibolya Bogdán, Tamás Buda, Katalin Csendes, Timea Csörge, Kata Eszter Diószegi, Mónika Fekete, Stella Fekete, Zsuzsanna Frank, Irén Somogyi Háriné, Ildikó Hermann, Brigitta Horváth, Istvánné Horváth, Bálint Zsolt Juhari, Kornélia Sereg Jurácsikné, Judit Kálmán, Aranka Kiss, Viktória Koesi, Klára Fritz Kovácsné, Nóra Pongrácz Kurdiné, Marcell Madár, Máté Madár, Viktória Matina, Anikó Légrádi-Maurer, Anita Mayer, Edit Kövári Mesterháziné, Anikó Renkó, Ágota Jó Schüllerné, János Stáhl, Anett Szegner, Ákosné Szabó, Éva Láng-Szabó, Zsuzsanna Szabó, Mariann Lennert Szabóné, Edit Szám, Olga Szász, Márta Tenk, and Erika Misku Vargáné for the performance of experimental tasks, collection of data, statistical analyses, and/or quality assurance; and Jared Brodin for administrative support in preparation of the manuscript.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.toxrep.2020.08.030.
Contributor Information
Timothy S. Murbach, Email: tim@aibmr.com.
Róbert Glávits, Email: glavits.robert.dr@gmail.com.
John R. Endres, Email: john@aibmr.com.
Amy E. Clewell, Email: amy@aibmr.com.
Gábor Hirka, Email: gabor.hirka@toxicoop.com.
Adél Vértesi, Email: adel.vertesi@toxicoop.com.
Erzsébet Béres, Email: erzsebet.beres@toxicoop.com.
Ilona Pasics Szakonyiné, Email: ilona.pasics@toxicoop.com.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Piccolo A. The supramolecular structure of humic substances: a novel understanding of humus chemistry and implications in soil science. Adv. Agronomy. 2002;75:57–134. [Google Scholar]
- 2.MacCarthy P. The principles of humic substances. Soil Sci. 2001;166(11):738–751. [Google Scholar]
- 3.Piccolo A. Carbon Sequestration in Agricultural Soils. Springer-Verlag; 2011. The nature of soil organic matter and innovative soil managements to fight global changes and maintain agricultural productivity; pp. 1–19. [Google Scholar]
- 4.Lehmann J., Kleber M. The contentious nature of soil organic matter. Nature. 2015;528(7580):60–68. doi: 10.1038/nature16069. [DOI] [PubMed] [Google Scholar]
- 5.Malan C. Sustainable Soil Management Symposium. 2015. Review: humic and fulvic acids. A practical approach; p. 21. [Google Scholar]
- 6.O’Donnell R. The auxin-like effects of humic preparations from leonardite. Soil Sci. 1973;116(2):106–112. [Google Scholar]
- 7.Stevenson F. In: Humus Chemistry: Genesis, Composition, Reactions. Wiley; 1994. Organic matter in soils: pools, distribution, transformations, and function; pp. 1–23. [Google Scholar]
- 8.Eyheraguibel B., Silvestre J., Morard P. Effects of humic substances derived from organic waste enhancement on the growth and mineral nutrition of maize. Bioresour. Technol. 2008;99(10):4206–4212. doi: 10.1016/j.biortech.2007.08.082. [DOI] [PubMed] [Google Scholar]
- 9.Mao J.D., Johnson R.L., Lehmann J. Abundant and stable char residues in soils: implications for soil fertility and carbon sequestration. Environ. Sci. Technol. 2012;46(17):9571–9576. doi: 10.1021/es301107c. [DOI] [PubMed] [Google Scholar]
- 10.Rose M., Patti A., Little K. A meta-analysis and review of plant-growth response to humic substances: practical implications for agriculture. Adv. Agronomy. 2014;124:37–89. [Google Scholar]
- 11.Shah Z.H., Rehman H.M., Akhtar T. Humic substances: determining potential molecular regulatory processes in plants. Front. Plant Sci. 2018;9:263. doi: 10.3389/fpls.2018.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin K., Leskovar D. Assessments of humic substances application and deficit irrigation in triploid watermelon. HortScience. 2020;55(5):716–721. [Google Scholar]
- 13.Agarwal S.P., Khanna R., Karmarkar R. Shilajit: a review. Phytother. Res. 2007;21(5):401–405. doi: 10.1002/ptr.2100. [DOI] [PubMed] [Google Scholar]
- 14.Winkler J., Ghosh S. Therapeutic potential of fulvic acid in chronic inflammatory diseases and diabetes. J. Diabetes Res. 2018;2018:5391014. doi: 10.1155/2018/5391014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaskova J., Velika B., Pilatova M. Effects of humic acids in vitro. In Vitro Cell. Dev. Biol. Anim. 2011;47(5–6):376–382. doi: 10.1007/s11626-011-9405-8. [DOI] [PubMed] [Google Scholar]
- 16.Corvina Natural Products Inc., FDA . 2001. NDIN 91. Humifulvate. in. [Google Scholar]
- 17.Humet Plc., FDA . 2003. NDIN 169. Humifulvate. in. [Google Scholar]
- 18.Hudak A., Naray M., Nagy I. Effect of the consumption of humic acid with bound micro elements in cases of occupational cadmium exposure. Central European Journal of Occupational and Environmental Medicine. 1997;3(3):175–186. [Google Scholar]
- 19.Dominguez-Negrete A., Gomez-Rosales S., Angeles M.L. Effect of the addition of humic substances as growth promoter in broiler chickens under two feeding regimens. Animals (Basel) 2019;9(12) doi: 10.3390/ani9121101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bull R.J., Robinson M., Meier J.R. Use of biological assay systems to assess the relative carcinogenic hazards of disinfection by-products. Environ. Health Perspect. 1982;46:215–227. doi: 10.1289/ehp.8246215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) Chromium(III)-, iron(II)- and selenium-humic acid/fulvic acid chelate and supplemented humifulvate added for nutritional purposes to food supplements. Efsa J. 2009;1147:1–12036. [Google Scholar]
- 22.Ueno H., Segawa T., Nakamuro K. Mutagenicity and identification of products formed by aqueous ozonation of humic acids of different origins. Chemosphere. 1989;19(12):1843–1852. [Google Scholar]
- 23.Kringstad K.P., Ljungquist P.O., De Sousa F. On the formation of mutagens in the chlorination of humic acid. Environ. Sci. Technol. 1983;17(9):553–555. doi: 10.1021/es00115a012. [DOI] [PubMed] [Google Scholar]
- 24.LaLonde R.T., Xie S. Glutathione and N-acetylcysteine inactivations of mutagenic 2(5H)-furanones from the chlorination of humics in water. Chem. Res. Toxicol. 1993;6(4):445–451. doi: 10.1021/tx00034a010. [DOI] [PubMed] [Google Scholar]
- 25.Meier J.R., Lingg R.D., Bull R.J. Formation of mutagens following chlorination of humic acid. A model for mutagen formation during drinking water treatment. Mutat. Res. 1983;118(1–2):25–41. doi: 10.1016/0165-1218(83)90113-1. [DOI] [PubMed] [Google Scholar]
- 26.Meier J.R., Ringhand H.P., Coleman W.E. Identification of mutagenic compounds formed during chlorination of humic acid. Mutat. Res. 1985;157(2–3):111–122. doi: 10.1016/0165-1218(85)90105-3. [DOI] [PubMed] [Google Scholar]
- 27.Van Duuren B.L., Melchionne S., Seidman I. Chronic bioassays of chlorinated humic acids in B6C3F1 mice. Environ. Health Perspect. 1986;69:109–117. doi: 10.1289/ehp.8669109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watt M., Malcolm R., Hayes M. Chemistry and potential mutagenicity of humic substances in waters from different watersheds in Britain and Ireland. Water Res. 1996;30(6):1502–1516. [Google Scholar]
- 29.Bernacchi F., Ponzanelli I., Minunni M. In vivo cytogenetic effects of natural humic acid. Mutagenesis. 1996;11(5):467–469. doi: 10.1093/mutage/11.5.467. [DOI] [PubMed] [Google Scholar]
- 30.Cozzi R., Nicolai M., Perticone P. Desmutagenic activity of natural humic acids: inhibition of mitomycin C and maleic hydrazide mutagenicity. Mutat. Res. 1993;299(1):37–44. doi: 10.1016/0165-1218(93)90117-v. [DOI] [PubMed] [Google Scholar]
- 31.Daniel F., Robinson M., Ringhand H. Subchronic toxicity study of ozonated and ozonated/chlorinated humic acids in Sprague-Dawley rats: a model system for drinking water disinfection. Environ. Sci. Technol. 1991;25(1):93–98. [Google Scholar]
- 32.Ribas G., Carbonell E., Creus A. Genotoxicity of humic acid in cultured human lymphocytes and its interaction with the herbicides alachlor and maleic hydrazide. Environ. Mol. Mutagen. 1997;29(3):272–276. doi: 10.1002/(sici)1098-2280(1997)29:3<272::aid-em7>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 33.International Programme on Chemical Safety (IPCS) FAO, WHO; 2009. Principles and Methods for the Risk Assessment of Chemicals in Food. 4. Hazard Identification and Characterization: Toxicological and Human Studies (Environmental Health Criteria 240) p. 187. in. [Google Scholar]
- 34.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH), FDA, Center for Drug Evaluation and Research (CDER) 2012. Guidance for Industry. S2(R1) Genotoxicity Testing and Data Interpretation for Pharmaceuticals Intended for Human Use; p. 31. in. [Google Scholar]
- 35.OECD . OECD Publishing; Paris: 1998. OECD Principles of Good Laboratory Practice. [Google Scholar]
- 36.National Research Council . Committee for the Update of the Guide for the Care and Use of Laboratory Animals, Institute for Laboratory Animal Research, Division on Earth and Life Studies, National Research Council; 2011. Guide for the Care and Use of Laboratory Animals; pp. 1–220. in. [Google Scholar]
- 37.OECD . 2016. Test No. 474: Mammalian Erythrocyte Micronucleus Test, OECD Guidelines for the Testing of Chemicals; p. 21. in. [Google Scholar]
- 38.OECD . 2018. Test No. 408: Repeated Dose 90-Day Oral Toxicity Study in Rodents, OECD Guidelines for the Testing of Chemicals, Section 4; p. 16. in. [Google Scholar]
- 39.OECD . 1997. Test No. 471: Bacterial Reverse Mutation Test, OECD Guidelines for the Testing of Chemicals, Section 4. in. [Google Scholar]
- 40.Ames B.N., McCann J., Yamasaki E. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test. Mutat. Res. 1975;31(6):347–364. doi: 10.1016/0165-1161(75)90046-1. [DOI] [PubMed] [Google Scholar]
- 41.Maron D.M., Ames B.N. Revised methods for the Salmonella mutagenicity test. Mutat. Res. 1983;113(3–4):173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- 42.Kier L.D., Brusick D.J., Auletta A.E. The Salmonella typhimurium/mammalian microsomal assay. A report of the U.S. Environmental Protection Agency gene-tox program. Mutat. Res. 1986;168(2):69–240. doi: 10.1016/0165-1110(86)90002-3. [DOI] [PubMed] [Google Scholar]
- 43.Venitt S., Parry J. IRL Press Limited; Eynsham, Oxford England: 1984. Mutagenicity Testing, a Practical Approach. [Google Scholar]
- 44.Mortelmans K., Zeiger E. The Ames Salmonella/microsome mutagenicity assay. Mutat. Res. 2000;455(1–2):29–60. doi: 10.1016/s0027-5107(00)00064-6. [DOI] [PubMed] [Google Scholar]
- 45.Murbach T., Glavits R., Endres J. A toxicological evaluation of methylliberine (Dynamine®) J. Toxicol. 2019:25. doi: 10.1155/2019/4981420. vol. Article ID 4981420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.OECD . 2016. Test No. 473: In Vitro Mammalian Chromosomal Aberration Test, OECD Guidelines for the Testing of Chemicals; p. 22. in. [Google Scholar]
- 47.Preston R.J., Au W., Bender M.A. Mammalian in vivo and in vitro cytogenetic assays: a report of the U.S. EPA’s gene-tox program. Mutat. Res. 1981;87(2):143–188. doi: 10.1016/0165-1110(81)90030-0. [DOI] [PubMed] [Google Scholar]
- 48.Brusick D. Chapter 14. Genetic toxicology. In: Hayes A., editor. Principles and Methods of Toxicology. second edition. Raven Press; 1989. pp. 407–434. [Google Scholar]
- 49.Salamone M., Heddle J. Chapter 4. The bone marrow micronucleus assay: rationale for a revised protocol. In: de Serres F., editor. Chemical Mutagens. Plenum Press; 1983. pp. 11–149. [Google Scholar]
- 50.OECD . 2008. Test No. 407: Repeated Dose 28-day Oral Toxicity Study in Rodents, OECD Guidelines for the Testing of Chemicals, Section 4. in. [Google Scholar]
- 51.Irwin S. Comprehensive observational assessment: ia. A systematic, quantitative procedure for assessing the behavioral and physiologic state of the mouse. Psychopharmacologia. 1968;13(3):222–257. doi: 10.1007/BF00401402. [DOI] [PubMed] [Google Scholar]
- 52.Condie L.W., Laurie R.D., Bercz J.P. Subchronic toxicology of humic acid following chlorination in the rat. J. Toxicol. Environ. Health. 1985;15(2):305–314. doi: 10.1080/15287398509530656. [DOI] [PubMed] [Google Scholar]
- 53.Hard G., Alden C., Bruner R. Non-proliferative lesions of the kidney and lower urinary tract in rats. Guides Toxicologic Pathol. 1999:1–32. [Google Scholar]
- 54.Johnson M., Gad S. In: Animal Models in Toxicology. CRC Press; 2007. The rat; pp. 147–276. [Google Scholar]
- 55.Frazier K., Seely J. In: Toxicologic Pathology. Nonclinical Safety Assessment. CRC Press; 2013. Chapter 12. Urinary system; pp. 421–484. [Google Scholar]
- 56.Johnson R., Spaet R., Potenta D. In: Toxicologic Pathology. Nonclinical Safety Assessment. CRC Press; 2013. Chapter 8. Spontaneous lesions in control animals used in toxicity studies; pp. 209–254. [Google Scholar]
- 57.NTP, DHHS . 2014. Nonneoplastic Lesion Atlas. Kidney, Pelvis - Dilation. in. [Google Scholar]
- 58.Leininger J., Jokinen M. 27. Oviduct, uterus, and vagina. In: Boorman G., Eustis S., Elwell M., MacKenzie W., editors. In: Pathology of the Fischer Rat: Reference and Atlas. Academic Press; 1990. pp. 443–459. [Google Scholar]
- 59.Vidal J., Mirsky M., Colman K. In: Toxicologic Pathology. Nonclinical Safety Assessment. CRC Press; 2013. Chapter 18. Reproductive system and mammary gland; pp. 717–830. [Google Scholar]
- 60.Dixon D., Alison R., Bach U. Nonproliferative and proliferative lesions of the rat and mouse female reproductive system. J. Toxicol. Pathol. 2014;27(3–4 Suppl):1S–107S. doi: 10.1293/tox.27.1S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.NTP, DHHS . 2014. Nonneoplastic Lesion Atlas. Uterus - Dilation. in. [Google Scholar]
- 62.Foster J. In: Boorman’s Pathology of the Rat. 2nd edition. Elsevier; 2018. Liver; pp. 81–105. [Google Scholar]
- 63.Hammond B.G., Mayhew D.A., Naylor M.W. Safety assessment of DHA-rich microalgae from Schizochytrium sp. Regul. Toxicol. Pharmacol. 2001;33(2):192–204. doi: 10.1006/rtph.2001.1458. [DOI] [PubMed] [Google Scholar]
- 64.Kramer J.K., Hulan H.W., Trenholm H.L. Growth, lipid metabolism and pathology of two strains of rats fed high fat diets. J. Nutr. 1979;109(2):202–213. doi: 10.1093/jn/109.2.202. [DOI] [PubMed] [Google Scholar]
- 65.Vandenberghe J. in, Janssen Research Foundation, Department of Toxicology; Charles River Deutschland: 1990. Life-span Data and Historical Data in Carcinogenicity Testing in Wistar Rats Crl:(WI) BR. Addendum 5.8. [Google Scholar]
- 66.Boorman G., Eustis S. Lung. In: Boorman G., Eustis S., Elwell M., MacKenzie W., editors. In: Pathology of the Fischer Rat: Reference and Atlas. Academic Press; 1990. pp. 339–367. [Google Scholar]
- 67.Haschek W., Rousseaux C., Wallig M. In: Fundamentals of Toxicologic Pathology. Elsevier; 2009. 6. Respiratory system. Structure and cell biology. Physiology and functional considerations - lymphoid tissue; p. 98. [Google Scholar]
- 68.Cooksey R., Gaitan E., Lindsay R. Humic substances, a possible source of environmental goitrogens. Org. Geochem. 1985;8(1):77–80. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The mean data sets generated and utilized for statistical analysis to support the findings of these studies are included within the article or in the supplementary information files. All other raw and processed data used to support the findings of these studies are available from the corresponding author upon request.