Abstract
Background
Cancer is emerging as a major global health-care system challenge with a growing burden worldwide. Due to the inconsistent cancer registry system in Saudi Arabia, the epidemiology of cancer is still dispersed in the country. Consequently, this review aimed to assemble the epidemiological metrics of cancer in Saudi Arabia in light of the available published data during the period from (2010–2019).
Methods
Published literature from Saudi Arabia relating to cancer incidence, prevalence, risk factors, and other epidemiological metrics were accessed through electronic search in Medline/PubMed, Cochrane, Scopus, Web of Knowledge, Google Scholar, and public database that meet the inclusion criteria. Relevant keywords were used during the electronic search about different types of cancers in Saudi Arabia. No filters were used during the electronic searches. Data were pooled and odds ratios (ORs) and 95% confidence interval (95%CI) were calculated. A random-effects meta-analysis was performed to assess the well-determined risk factors associated with different types of cancers.
Results
The most common cancers in Saudi Arabia are breast, colorectal, prostate, brain, lymphoma, kidney and thyroid outnumbering respectively. Their prevalence rates and OR (95%CI) as follow: breast cancer 53% and 0.93 (0.84–1.00); colon-rectal cancer (CRC) 50.9% and 1.2 (0.81–1.77); prostate cancer 42.6% and 3.2 (0.88–31.11); brain/Central Nervous System cancer 9.6% and 2.3 (0.01–4.2); Hodgkin and non-Hodgkin's lymphoma 9.2% and 3.02 (1.48–6.17); kidney cancer 4.6% and 2.05 (1.61–2.61), and thyroid cancer 12.9% and 6.77 (2.34–19.53).
Conclusion
Within the diverse cancers reported from Saudi Arabia, the epidemiology of some cancers magnitude 3-fold in the latest years. This increase might be attributed to the changing in the Saudi population lifestyle (adopting western model), lack of cancer awareness, lack of screening & early detection programs, social barriers toward cancer investigations. Obesity, genetics, sedentary lifestyle, tobacco use, viral infection, and iodine & Vit-D deficiency represent the apparent cancer risk factors in Saudi Arabia.
Keywords: cancer, Saudi Arabia, breast cancer, colon-rectal cancer, risk factors, meta-analysis
1. Introduction
Cancer is responsible for more than 9.6 million deaths in about 185 countries, ranked as the second leading cause of mortality worldwide [1].
Many risk factors have been implicated in the etiology of cancer including; tobacco and alcohol consumption, unhealthy diet, physical inactivity, viral infection, bacterial infection, urban air pollution, ionizing radiation and indoor smoke [1],[2]. It is expected that, due to changes in population demographics in the next decades, cancer will continue rising to 21.4 million deaths worldwide, by 2030 [3]. Overall cancer burden, as well as, increased survival rates can be achieved through cancer prevention, early detection strategies [4].
In 2018, there 10518 cancer deaths with 24,485 new cancer cases in Saudi Arabia (total population = 33,554,333) [5]. The most common cancers include breast cancer, colon-rectum (CRC), and prostate [6]. Frequently reported risk factors associated with breast cancer were hormonal variations, diet, lifestyle, and obesity [7]. Recent researches reported increasing trends of CRC in Saudi Arabia [8],[9]. In 2018, CRC accounts for 14.6% of total cancers in the country [5]. The risk factors for CRC may be genetic, environmental, age, gender and other inflammatory conditions of the digestive tract [10],[11].
Prostate cancer is the other major cause of death in males. Alteration in lipid metabolism, HPV infection and racial difference are some of the risk factors linked with prostate cancer [12].
As per the Saudi cancer registry, the prevalence of brain cancer is comparatively low in Saudi Arabia accounting for only 2.0 to 3.2% in females and males respectively [13]. Like other types of cancers, central nervous system (CNS) tumors are also gaining momentum worldwide and ranks among the top 10 mortalities due to cancers. One of the possible risk factors for CNS tumors is radioactive exposure [14]. This in addition to the metastasis, particularly from breast cancer [15].
Lymphomas Hodgkin's (HL) and non-Hodgkin's (NHL), extranodal non-Hodgkin lymphoma (EN-NHL)) are other common malignancies affecting both young and adult Saudi [16]–[19]. The prevalence of HL (uncommon type) was reported as 3.4% in Saudi Arabia with high clinical variability and frequently observed among the age-range 15–35 years [20]–[22].
Kidney cancer is the other more commonly diagnosed cancer in Saudi Arabia. The tendency is towards the increasing trend. According to the cancer registry in 2013, the age-standardized rate of kidney cancer was 2.3% of all cancers. In recent decades, the incidence is increasing due to various risk factors [23]. The common risk factors associated with kidney cancer are smoking, obesity, diabetes, and hypertension [24]. The common histological type in renal cell carcinoma is clear cell carcinoma [25].
Thyroid carcinoma is one of the frequently diagnosed thyroid disorders [26]. The incidence is more common among females compared to males. The prevalence of thyroid cancer 10.1% [5] and the common malignant type being papillary thyroid carcinoma [27].
Interestingly, in recent years, there were submerging of cancer-related literature (5,000 articles in 2010 to 11,000 in 2017) from Saudi Arabia compared to neighboring Arab countries, as indicated in Figure 1. So this review aimed to assemble the epidemiological metrics of cancer in Saudi Arabia in light of the available published data during the period from (2010–2019).
Figure 1. Cancer-related publications from Saudi Arabia vs. all Arab countries.
2. Methodology
2.1. Data sources and search strategy
Literature from Saudi Arabia related to various cancer types were collected by electronic search in Medline/PubMed, Cochrane Library, Scopus, Web of Knowledge, Google Scholar and public database (GLOBOCAN 2012, IARC) that meet the inclusion criteria. Data from the Saudi cancer registry were also collected. Relevant keywords (breast cancer, colon-rectum (CRC), prostate, Hodgkin's and non-Hodgkin's lymphomas, brain/CNS, kidney, thyroid, etc.) were used in affiliation to Saudi Arabia. No filters were used during the electronic searches.
2.2. Selection of required publications
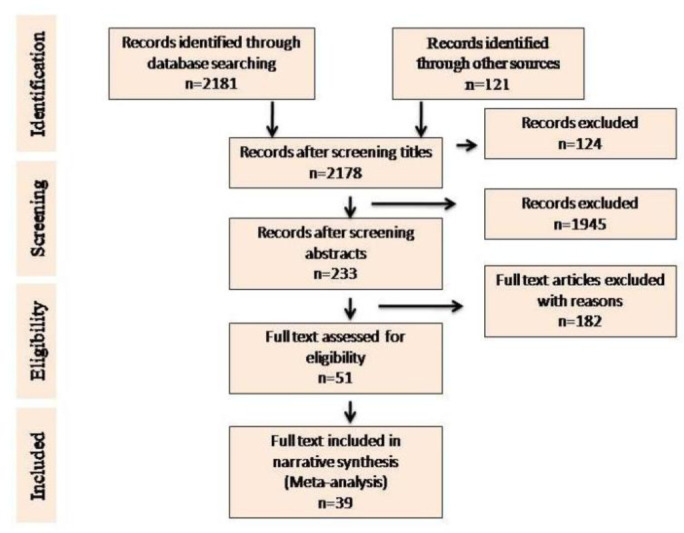
In-depth selections were made through search engines following effective inclusion and exclusion criteria (Figure 2).
Figure 2. Flow chart for the selection process of articles included in the review.
2.2.1. Inclusion criteria
Only literature published during 2010–2019 from Saudi Arabia and related to the epidemiology of cancer and risk factors were included. Data from the Saudi National cancer registry were also considered. All sources which demonstrate the type of malignant neoplasm (C18-21, C-50, C-61, C64-66, C70-72, C73, and C81-85), with possible risk factors were put under inclusion criteria. All relevant articles (including case-control, cohort, cross-sectional, etc.) were included, as they permit the estimation of odds ratios (OR) and 95% confidence intervals (95%CI).
2.2.2. Exclusion criteria
Publications are written in a language other than English and those focussed on survivors of cancer, pharmacological research, qualitative studies, and reviews and meta-analysis were excluded. Publications on laboratory research including animal trials were also excluded.
2.3. Quality appraisal
After scanning the titles of all relevant publications and reading the abstracts of the selected publications full-text papers were appraised by the assigned reviewers using PRISMA guidelines [28].
2.4. Assessment of heterogeneity and statistical parameters
Valid statistical package (Comprehensive Meta-analyses ver. 3) was used to calculate the summary effect estimate and 95% confidence intervals to test for heterogeneity of prevalence and risk factors estimation. Heterogeneity, as determined using the I2 index statistic, was classified into low (scores <25%) and high (scores ≥75%) heterogeneity. DerSimonian-Laird random-effects meta-analysis was used to summarize the prevalence of different cancer types with possible risk factors. Minimum three independent studies were taken into account for summary and subgroup analysis to justify the analysis. Only in one analysis, due to the paucity of data for brain/CNS cancer from Saudi Arabia, two independent studies were used. Statistical package (SPSS ver. 25) were used and p-values <0.05 were considered statistically significant.
3. Results and discussions
3.1. Search results
The systematic database search yielded 2,181 records and 121 additional records from other sources. After excluding 124 records, a total of 2,178 records were further screened. After several selection processes, 39 full-text records were included for meta-analysis (Figure 2).
3.2. Epidemiology
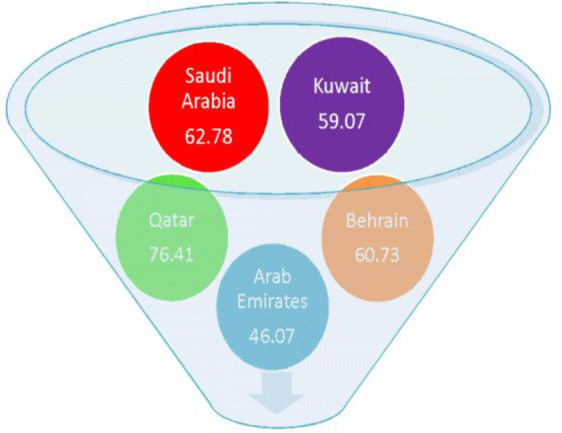
With regard to age-standardized rates (ASR), the incidence of all cancers excluding non-melanoma skin cancer was estimated to be 17,522 cases (8,296 males and 9,226 females) in Saudi Arabia with ASR incidence rates of 0.3–12.6 in males and 0.2–29.5 in females. The estimated mortality was 9,134 cases across all ages and gender [29]. The ASR mortality rates range from 0.1–7.3 in males and 0.1–9.1 in females. Saudi Arabia ranks second in cancer mortality rates amongst all Arabian Gulf countries (Figure 3).
Figure 3. Estimated death rates/100,000 due to cancer in Saudi Arabia in comparison to Arabian Gulf countries (WHO 2017).
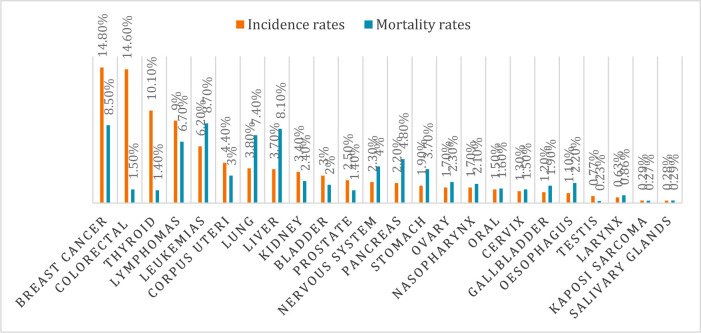
Breast cancer is leading cancer in Saudi Arabia with an incidence and mortality rates of 14.8% (cumulative risk 2.87%) and 8.5%(cumulative risk 0.81%) among both sexes, respectively. In 2018 the incidence of breast cancer among females was 29.7% in Saudi Arabia. Colorectal cancer ranked the third most common cancer in Saudi Arabia with an incidence and mortality rate of 14.6% (cumulative risk 1.47%) and 1.48%(cumulative risk 0.65%) among both sexes, respectively. The incidence among males was 19.6% and females 9.5%. Thyroid cancer ranked the third most common cancer in Saudi Arabia with an incidence and mortality rate of 10.1% (cumulative risk 0.67%) and 1.4% (cumulative risk 0.07%) among both sexes, respectively. The incidence was high among females 14.1% compared to 6% among males [5], as shown in Figure 4 & 5.
Figure 4. Incidence and mortality rates in Saudi Arabia in 2018.
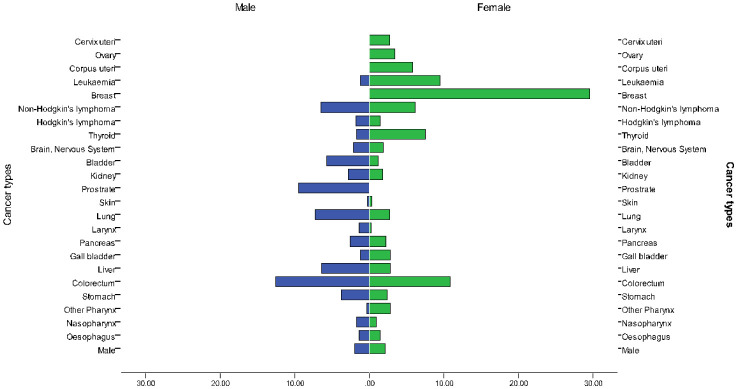
Figure 5. Estimated age-standardized incidence rates of cancers in Saudi Arabia per 100,000 (Source: WHO 2017).
Table 1 provides a summary of the epidemiological indicators as measured in the included studies, viz., incidence, and prevalence, mean age at diagnosis, malignant sites.
Table 1. Incidence, prevalence and possible risk factors of some common and rare cancers in Saudi Arabia.
| Study parameters | Source of data | Period | No. of cases & control/biopsies under study (n) | Age group | Common Malignant sires | Risk factor distribution among patients | Reference |
| Case-control | Hospital-based | 2009 | 200 | 50 ± 5 | Breast | Low intake of folate, MTHFR polymorphism | Alshatwi 2010 |
| Case-control | Hospital-based | 2009–2010 | 200 | 50 ± 5 | Breast | TP 53 & MDM polymorphism | Alshatwi et al. 2011 |
| Case-control | Hospital-based | NS | 208 | 17–80 | Breast | P53 codon 72 polymorphism | Al-Qasem et al. 2012 |
| Case-control | Hospital-based | NS | 189 | NS | Breast | Micro RNA polymorphism | Alshatwi et al. 2012 |
| Retrospective study | Cancer registry | 2001–2008 | 6922 | 30–59 | Breast | NS | Alghamdi et al. 2013 |
| Case-control | Hospital-based | NS | 195 | ~48 | Breast | PARP-1V762A polymorphism | Alanazi et al. 2013 |
| Case-control | Hospital-based | 2009 | 240 | 18–75 | Breast | Vitamin-D deficiency | Yousef et al. 2013 |
| Case-control | Hospital records | NS | 200 | ~48 | Breast | XRCC1 polymorphism | Al-Mutairi et al. 2013 |
| Case-control | Hospital-based | NS | 109 | 9–47 | Breast | Obesity | Alokali et al. 2013 |
| Case-control | Hospital-based | 2010–2011 | 200 | 37–61 | Breast | BRCA1 & BRCA2 gene polymorphism | Hasan et al. 2013 |
| Case-control | Hospital-based | 2007–2012 | 1172 | ~35 | Breast | Obesity | Elkum et al. 2014 |
| Cross-sectional | Hospital-based | 2011–2013 | 801 | 42–57 | Breast | Low vitamin D levels | Abulkhair et al. 2015 |
| Case-control | Hospital-based | 2001–2013 | 192 | 30–65 | Breast | Oral contraceptive, abortion | Karim et al. 2015 |
| Case-control | Hospital-based | NS | 200 | ~40 | Breast | VEGF-gene variation | Al Balawi et al. 2018 |
| Case-control | Hospital-based | NS | 200 | ~40 | Breast | Genetical | Mir et al. 2018 |
| Case-control | Hospital-based | 2009 | 120 | 50–67 | Colon | Serum resistin | Al-Harithy & Al-Ghafari 2010 |
| Case-control | Hospital-based | NS | 120 | 50–65 | Colon | Genetic polymorphism of the ADIPOQ gene | Al-Harithy & Al-Zahrini 2012 |
| Case-control | Hospital-based | 2008–2010 | 130 | 45–80 | Colon | Polymorphism of XRCC1 | Al-Harithy & Al-Ghazzawi 2011 |
| Case-control | Hospital-based | NS | 120 | NS | Colon | Polymorphism of RETN gene | Al-Harithy 2014 |
| Case-control | Hospital-based | 2013–2014 | 200 | 20–80 | Colorectal | Vitamin D receptor (VDR) polymorphism | Alkhayal et al. 2016 |
| Prospective | Hospital-based | 2010–2015 | 280 | ~51 | Colorectal | Family history, ulcerative colitis, fatty diet | Aldiab 2017 |
| Case-control | Hospital-based | 2006–2015 | 297 | 29–60 | Colorectal | Family history, Lynch syndrome, Gene variant | Alqahtani et al. 2017 |
| Retrospective | Hospital records | NS | 572 | <50 to >70 | Prostate | The racial difference, high serum PSA | Al-Abdin 2013 |
| Retrospective | Hospital records | 2006–2013 | 417 | 20–95 | Prostate | NS | Albasri et al. 2014a |
| Retrospective | Hospital records | 2001–2008 | 1739 | 0 to ≥75 | Prostate | NS | Alghamidi et al. 2014 |
| Retrospective | Hospital records | 2010–2015 | 291 | 0–65 | Brain | NS | Taha et al. 2018 |
| Cross-sectional | NS | 2015–2016 | 1500 | <18 to >40 | CNS | Radioactive occupation, genetical, low physical activity | Aljuhani et al. 2018 |
| Retrospective | Hospital records | 1997–2012 | 340 | 25–82 | Hodgkin's lymphoma | EBV virus, genetical, environmental factors | Shafi et al. 2017 |
| Retrospective | Hospital records | 1983–2003 | 83 | <10–80 | Non-Hodgkin's lymphoma (EN-NHL) | NS | Nagi et al. 2010 |
| Retrospective | Hospital records | 1994–1999 | 1209 | 14–60 | Non-Hodgkin's lymphoma (EN-NHL) | Genetical, environmental | Al Diab et al. 2011 |
| Retrospective | Hospital records | 2006–2013 | 346 | 3–96 | Non-Hodgkin's lymphoma | NS | Albasri et al. 2014b |
| Retrospective | Hospital records | 1990–2010 | 382 | ≥18 | Kidney | Smoking, diabetes, obesity, hypertension | Alkhateeb et al. 2015 |
| Retrospective | Hospital records | 1990–2015 | 371 | 45–65 | Kidney | Smoking, diabetes, obesity, hypertension, dyslipidemia, incidental | Alkhateeb et al. 2018 |
| Retrospective | Hospital records | 2003–2013 | 219 | 22–95 | Kidney | NS | Mahasin et al. 2018 |
| Retrospective | Hospital records | 1998–2007 | 668 | 50–60 | Thyroid | NS | Rafeidi et al. 2010 |
| Retrospective | Hospital records | 2000–2010 | 2292 | 39(mean) | Thyroid | Iodine deficiency, family history, high leptin level, radiation exposure | Hussain et al. 2013 |
| Retrospective | Hospital records | 2006–2013 | 292 | <20 to >60 | Thyroid | Iodine deficiency | Albasri et al. 2014 |
| Retrospective | Hospital records | 2008–2010 | 312 | <20 to >60 | Thyroid | NS | Saeed et al. 2018 |
| Retrospective | Hospital-based | 2015 | 168 | 19–23 | Thyroid | NS | Bafaraj et al. 2018 |
Note: NS—Not specified.
3.3. Etiology and meta-analysis
Although the incidence of cancer, particularly breast cancer is high in high-income countries, mortality is high in low or medium-income countries [30]. The polymorphism in BRCA1-3’UTR and VEGF gene are reported to be strong genetic factors linked to breast cancer [31],[32]. Mutation in BRCA1 and BRCA2 genes are reported to increase the development of breast and ovarian cancers [33]. Many factors are associated with the risk of breast cancer including; the use of oral contraceptives, menstrual history, nulliparity obesity, family history of breast cancer, and low vitamin D [34],[35]. Early diagnosis, positive attitude, and awareness are some of the possible measures to mitigate the mortality rate due to breast cancer [36].
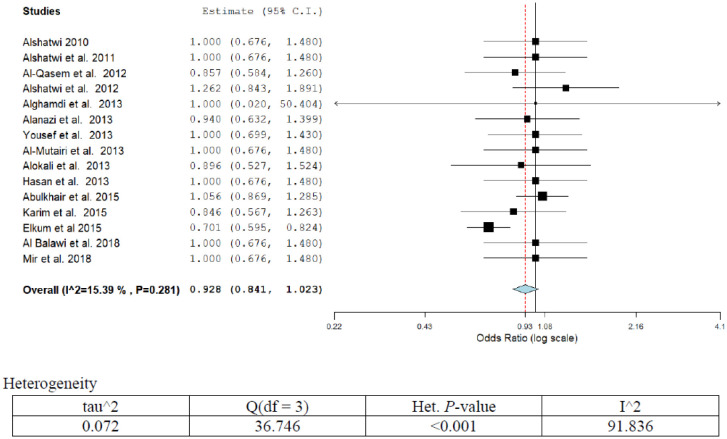
Overall fifteen publications reported different forms of risk factors associated with breast cancer (Table 1; Figure 6). The overall pooled breast cancer prevalence was 53% (95%CI; 42.2–47.5) including 14 studies between 2010 to 2018. The prevalence increased over time significantly in Saudi Arabia (p < 0.002). Nine publications reported OR that has been adjusted for gene variations and other genetic parameters. Three publications have reported on low vitamin D intake and the other three on obesity. The adjusted OR and 95%CI ranged from 0.95 (0.64–1.4) to 1.3 (0.85–2.0) for gene variations or polymorphism [37]–[45],[32],[31]. The OR for low intake of folate or vitamin D ranged from 1.00 (0.87–1.3) to 1.00 (0.70–1.50) [43],[35]. The OR (95%CI) for obesity as a risk factor for breast cancer ranged from 0.70 (0.60–0.83) to 0.90 (0.53–1.53) [44],[46]. Only one publication highlighted the use of oral contraceptives or abortion as possible risk factors with OR (95%CI) of 0.85 (0.57–1.3). The overall pooled estimate was OR (95%CI) = 0.93 (0.84–1.0). A low degree of heterogeneity was observed in the present analysis (I2 = 15.39%, p = 0.29). After eliminating low-quality studies (scoring ≤3 points out of 8), a sensitivity analysis was conducted through repeating meta-analysis, and however, no significant alteration in results was observed.
Figure 6. Meta-analyses (forest plot) of the prevalence of breast cancer with possible risk factors.
A high risk of CRC was observed in the age range of 35–65 years. The common risk factors for CRC are inflammatory bowel disease, smoking, and family history, in addition to environmental and genetic factors. Screening at regular intervals using colonoscopy, sigmoidoscopy and fecal immunochemical tests are recommended for CRC [47]. Some studies suggest that low vitamin D intake is associated with risk of colorectal cancer [48]. The prevalence of colon-rectum cancer was 50.9% (95%CI; 40.22–45.1) as revealed from pooled seven studies of this series. The prevalence significantly increased over time (p < 0.001).
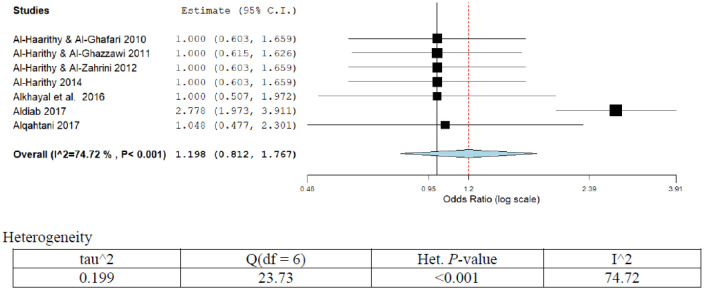
Out of the overall seven publications reporting different forms of risk factors (Table 1; Figure 7), six studies reported genetic polymorphism as the possible risk factors. The adjusted OR (95%CI) ranged from 1.00 (0.50–2.00) to 1.00 (0.50–2.30) [11],[49]–[53]. Two studies have attributed family history as a possible risk for CRC, with OR (95%CI) ranged from 1.00 (0.50–2.3) to 2.8 (2.00–4.1) [8],[11]. Only one study has highlighted ulcerative colitis and fatty diet as possible risk factors for CRC with OR (95%CI) of 2.8 (2.0–2.4) [8]. The pooled OR (95%CI) for the overall risk factors was 1.2 (0.81–1.77). A high degree of heterogeneity was observed in the present analysis (I2 = 75%; p < 0.001). Sensitivity analysis showed a high pooled prevalence of genetic polymorphism positivity as reported by the studies (75%; 95%CI; 15.3–81.1%) and was found statistically significant (p < 0.001).
Figure 7. Meta-analyses (forest plot) of the prevalence of CRC with possible risk factors.
Prostate cancer is one of the common cancers in males with high morbidity and mortality in Saudi Arabia. It is more prevalent in the age group of 50–70 years. The prostatic disease may be benign prostatic hyperplasia or carcinoma prostrates [54]. Several risk factors were implicated in the etiology of prostate cancer [55]–[57].
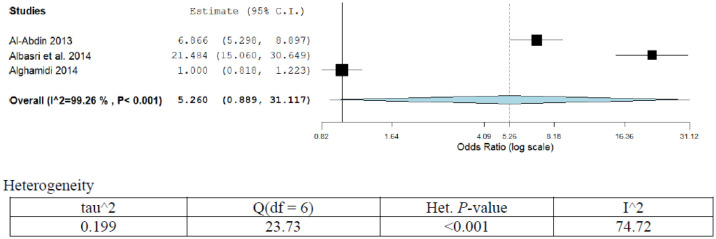
The prevalence of prostate cancer was 42.6% (95%CI; 36.12–41.2) as revealed from pooled three studies in the present series (Table 1; Figure 8). The prevalence was statistically insignificant over time. The OR (95%CI) ranged from 1.00 (0.82–1.22) to 21.5 (15.0–30.6) [54],[57],[58]. Only one study has assigned racial differences and high serum prostate-specific antigen (PSA) as possible risk factors for prostate cancer, OR (95%CI) 6.9 (5.3–8.9). The overall pooled OR was 5.2 (0.89–31). A high degree of heterogeneity was observed in the present analysis (I2 = 99.26%; p < 0.001). Sensitivity analysis showed no significant variation in the results.
Figure 8. Meta-analyses (forest plot) of the prevalence of prostate cancer with possible risk factors.
Tumors of the brain and central nervous system (CNS) differ from other tumors develop in various body tissues. Some of the signs and symptoms associated with CNS tumors are headaches, drowsiness, lack of awareness and increased sleep duration [14]. Some of the risk factors identified are exposure to radioactivity, bisphenol acetate, X-rays, increased body mass index and prolonged use of mobile phones. Vitamin D has been reported to prevent the progression of brain cancer by maintaining the integrity of the adhesion protein.
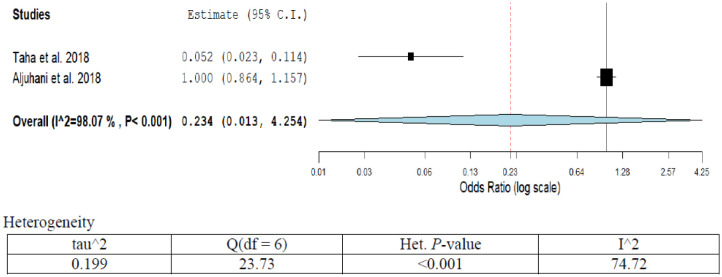
The prevalence of brain/CNS cancer was only 9.6% (95%CI; 11.10–16.2) as revealed from pooled studies this series (Table 1; Figure 9). The OR (95%CI) ranged from 0.05 (0.02–0.11) to 1.0 (0.9–1.2) [13],[14]. The overall pooled OR (95%CI) was 0.23 (0.01–4.2). The attributed risk factors were radioactive exposure, genetic and low physical activity. A high degree of heterogeneity was observed in the present analysis (I2 = 98.0%; p < 0.001). Sensitivity analysis showed no significant variation in the results.
Figure 9. Meta-analyses (forest plot) of the prevalence of brain & CNS cancer with possible risk factors.
The incidence rates of HL are comparatively low than the NHL in Saudi Arabia. The incidence of HL is more prevalent among young people of 15–35 years. However, it may recur after the age of 50 years. In one study from Saudi Arabia, the overall survival rate of patients with HL was found to be 91% [59]. Recent studies showed that infection with hepatitis C&B viruses is a common risk factor [60].
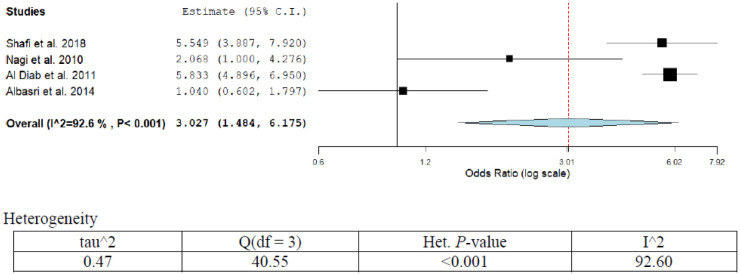
The prevalence of Hodgkin and non-Hodgkin lymphoma was 9.2% (95%CI; 10.1–15.6) as revealed from pooled four studies recruited in this series (Table 1; Figure 10). The pooled OR (95%CI) combining all studies was 3.02 (1.48–6.17). Prevalence was statistically significant over time (p < 0.05). The OR (95%CI) ranged from 1.0 (0.60–1.80) to 5.5 (3.97–7.92). Two studies have assigned genetic and Epstein-Barr viral infection as a possible risk factor for Hodgkin and non-Hodgkin lymphoma, OR (95%CI) 5.5 (3.9–8.0) and 5.8 (4.9–7.0) [19],[59]. A high degree of heterogeneity was observed in the present analysis (I2 = 92.6%; p < 0.001). Sensitivity analysis showed significant variation in the results (p < 0.05). The pooled prevalence was 26.1% (95%CI; 21.0–29.1%).
Figure 10. Meta-analyses (forest plot) of the prevalence of HL & NHL cancers with possible risk factors.
Kidney cancer is the commonest genitourinary tract malignancy [61]. Patients with hypertension, diabetes mellitus, and habitual smoking are more likely to have kidney cancer [24]. Obesity and dyslipidemia are other possible risk factors. Other reported risk factors include gender and age, with the incidence being more prevalent in males and the older age group [23].
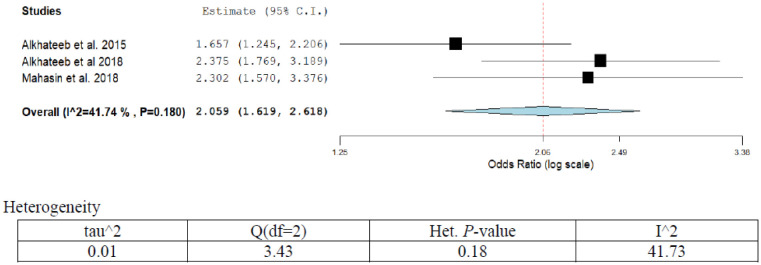
Overall two publications out of total three, reported different forms of risk factors associated with kidney cancer (Table 1; Figure 11). The overall pooled kidney cancer prevalence was 4.6% (95%CI; 2.2–3.7) in the three studies in our series. Prevalence increased over time significantly in Saudi Arabia (p < 0.002). Two publications reported OR that has been adjusted for diabetes and obesity. The adjusted OR (95%CI) ranged from 1.65 (1.24–2.20) to 2.3 (1.57–3.76) for diabetes and obesity [24],[61]. The overall pooled estimate was OR (95%CI) = 2.05 (1.61–2.61). A medium degree of heterogeneity was observed in the present analysis (I2 = 41.74%; p = 0.18). After eliminating low-quality studies (scoring ≤3 points out of 8). Sensitivity analysis was conducted through repeating meta-analysis, however, the results showed no significant alteration.
Figure 11. Meta-analyses (forest plot) of the prevalence of kidney cancer with possible risk factors.
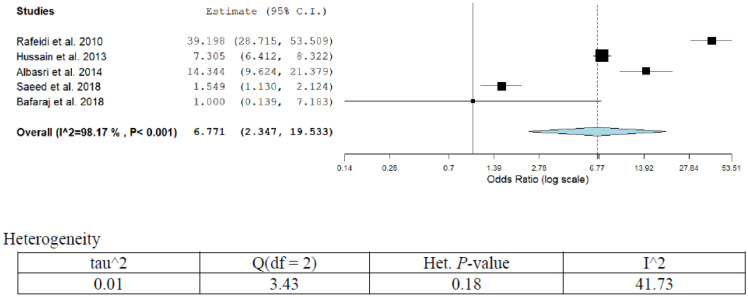
Population-based studies have revealed an increasing trend of thyroid cancer in the past few decades. The disease varies from region to another. The commonest existing histological subtype is papillary thyroid carcinoma [62]. The incidence of thyroid cancer increases with the increase of age. Radiation exposure of the thyroid gland is one of the possible risk factors of thyroid cancer [63]. Thyroid cancer is more prevalent in females compared to males with an incidence rate of 7.5%. Overall five publications reported different forms of risk factors associated with thyroid cancer (Table 1; Figure 12). The overall pooled thyroid cancer prevalence was 12.9% (95%CI; 10.2–11.7) in five studies of our series. Two publications reported OR that has been adjusted for iodine deficiency. The adjusted OR (95%CI) ranged from 1.0 (0.13–7.1) to 39.0 (28.7–53.5) for iodine deficiency [64],[65]. The other risk factors highlighted in the studies are radiation exposure and high leptin level. The overall pooled estimate was 6.77 (2.34–19.53). A high degree of heterogeneity was observed in the present analysis (I2 = 98.17%; p < 0.001). After eliminating low-quality studies (scoring ≤3 points out of 8). Sensitivity analyses were conducted by repeating meta-analysis. Iodine deficiency reported by studies showed medium sensitivity (42.3%; 95%CI; 19.4–55.1%).
Figure 12. Meta-analyses (forest plot) of the prevalence of thyroid cancer with possible risk factors.
The highly recognized risk factors for cancer as revealed by most of the literatures are lack of lifestyle healthy habit, obesity, low vitamin D intake, use of oral contraceptives, abortion, ulcerative colitis, genetic polymorphism, high serum PSA, radioactive exposure, low physical activity, Epstein-Barr viral infection, diabetes, iodine deficiency, smoking, and high leptin levels. Besides these, ambient air pollution is another risk factor for most of the malignancies in Saudi Arabia due to frequent exposure to dust storms containing particulate matters [66]. However, the present study predict that, almost all meta-analyses were highly heterogeneous, which might be attributed to the diverse cancer causes factors, lack of the studies, and intermittence of cancer reporting registries.
3.4. Miscellaneous factors
Within the context of literature pertained to observational studies (assessing perception, attitude, and awareness), many Saudi community-related factors can contribute to the escalating burden of cancer. These increasing trends may be due to changing lifestyles, lack of awareness, embarrassment, fear of testing or non-accessibility to advanced treatment and due to a multitude of factors. Although most of these cancers are preventable, early detection by trained health practitioners is of utmost importance and paramount responsibility. Effective techniques for screening and diagnosis using modern up-graded instruments may minimize the burden of cancer in Saudi Arabia [10],[67]–[69]. The present review has direct implications in improving the health component of cancer patients through the inspiration of health providers towards implementing effective cancer-related health management strategies. These should include; prevention, early detection, proper treatment, and better palliative care. Such strategies will recruit a healthy lifestyle, raised awareness (decreasing cancer morbidity), early detection resulting, appropriate treatment, and better palliative care (decrease mortality).
4. Conclusion
Within the diverse cancers reported from Saudi Arabia, the epidemiology of some cancers magnitude 3-fold in the latest years. This increase might be attributed to the changing in the Saudi population lifestyle (adopting western model), lack of cancer awareness, lack of screening & early detection programs, social barriers toward cancer investigations. Obesity, genetics, sedentary lifestyle, tobacco use, viral infection, and iodine & Vit-D deficiency represent the apparent cancer risk factors in Saudi Arabia.
Footnotes
Conflict of interest: The authors declared no competing interests. The authors had full responsibility for data collection, data interpretation, and writing of the report.
References
- 1.WHO. Cancer, Fact sheets 2018. 2018. Available from: https://www.who.int/news-room/fact-sheets/detail/cancer.
- 2.Ervik M, Lam F, Ferlay J, et al. Cancer Today. Lyon, France: International Agency for Research on Cancer; 2016. Available from: http://gco.iarc.fr/today. [Google Scholar]
- 3.WHO. Global status report on noncommunicable diseases 2010. Available from: https://www.who.int/nmh/publications/ncd_report_full_en.pdf.
- 4.Alharthi H. Healthcare predictive analytics: An overview with a focus on Saudi Arabia. J Infect Public Health. 2018;11:749–756. doi: 10.1016/j.jiph.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 5.WHO, International Agency for Research in Cancer (IARC) Saudi Arabia. Source: Globocan 2018. Available from: https://gco.iarc.fr/today/data/factsheets/populations/682-saudi-arabia-fact-sheets.pdf.
- 6.Bassam AA, Rakan FA, Ahmed AA, et al. Breast cancer in Saudi Arabia and its possible risk factors. J Cancer Policy. 2017;12:83–89. [Google Scholar]
- 7.Agide FD, Sadeghi R, Garmaroudi G, et al. A systematic review of health promotion interventions to increase breast cancer screening uptake: from the last 12 years. Eur J Public Health. 2018;28:1149–1155. doi: 10.1093/eurpub/ckx231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aldiab A, Al Khayal KA, Al Obaid OA, et al. Clinicopathological Features and Predictive Factors for Colorectal Cancer Outcome in the Kingdom of Saudi Arabia. Oncology. 2017;92:75–86. doi: 10.1159/000450857. [DOI] [PubMed] [Google Scholar]
- 9.Alsanea N, Almadi MA, Abduljabbar AS, et al. National Guidelines for Colorectal Cancer Screening in Saudi Arabia with strength of recommendations and quality of evidence. Ann Saudi Med. 2015;35:189–195. doi: 10.5144/0256-4947.2015.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zubaidi AM, AlSubaie NM, AlHumaid AA, et al. Public awareness of colorectal cancer in Saudi Arabia: A survey of 1070 participants in Riyadh. Saudi J Gastroenterol. 2015;21:78–83. doi: 10.4103/1319-3767.153819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alqahtani M, Edwards C, Buzzacott N, et al. Screening for Lynch syndrome in young Saudi colorectal cancer patients using microsatellite instability testing and next generation sequencing. Fam Cancer. 2018;17:197–203. doi: 10.1007/s10689-017-0015-9. [DOI] [PubMed] [Google Scholar]
- 12.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 13.Taha MS, Almsned FM, Hassen MA, et al. Demographic and histopathological patterns of neuro-epithelial brain tumors in Eastern Province of Saudi Arabia. Neurosciences (Riyadh, Saudi Arabia) 2018;23:18–22. doi: 10.17712/nsj.2018.1.20160543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aljuhani SH, Bamaroof SA, Alghamdi TH, et al. Public awareness of central nervous system tumors in the Kingdom of Saudi Arabia. Neurosciences (Riyadh, Saudi Arabia) 2018;23:227–237. doi: 10.17712/nsj.2018.3.20180055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azim HA, Abdel-Malek R, Kassem L. Predicting Brain Metastasis in Breast Cancer Patients: Stage Versus Biology. Clin Breast Cancer. 2018;18:e187–e195. doi: 10.1016/j.clbc.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Rauf MS, Akhtar S, Maghfoor I. Changing trends of adult lymphoma in the Kingdom of Saudi Arabia - comparison of data sources. Asian Pac J Cancer Prev. 2015;16:2069–2072. doi: 10.7314/apjcp.2015.16.5.2069. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed MI, Ahmed MS, Nizamani WM, et al. Beyond PET/CT in lymphoma: Does PET/CT has similar diagnostic accuracy in recurrent lymphoma cases in TB-endemic countries. Pak J Radiol. 2018;28:17–23. [Google Scholar]
- 18.Nagi AH, Minawy LA, Naseem N, et al. A study of the morphological patterns of extranodal non-Hodgkin lymphoma in Pakistani and Saudi populations. Biomedica. 2010;26:118–123. [Google Scholar]
- 19.Al Diab AR, Aleem A, Qayum A, et al. Clinico-pathological pattern of extranodal non-Hodgkin's lymphoma in Saudi Arabia. Asian Pac J Cancer Prev. 2011;12:3277–3282. [PubMed] [Google Scholar]
- 20.Saudi Cancer Registry Annual Report. Riyadh: Ministry of Health; 2014. Available from: https://nhic.gov.sa/eServices/Documents/2014.pdf. [Google Scholar]
- 21.Shamoon RP, Ali MD, Shabila NP. Overview and outcome of Hodgkin's Lymphoma: Experience of a single developing country's oncology centre. Plos One. 2018;13 doi: 10.1371/journal.pone.0195629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toorani ZA, Sridhar S, Roque W. A rare concurrence of Hofgkin's lymphoma, Sickle cell disease and Diabetes mellitus. Bahrain Med Bull. 2018;40:118–120. [Google Scholar]
- 23.Medina-Rico M, Ramos HL, Lobo M, et al. Epidemiology of renal cancer in developing countries: Review of the literature. Can Urol Assoc. 2018;12:154–162. doi: 10.5489/cuaj.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alkhateeb SS, Alothman AS, Addar AM, et al. Kidney cancer in Saudi Arabia. Saudi Med J. 2018;39:459–563. doi: 10.15537/smj.2018.5.22641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahasin SZ, Aloudah N, Al-Surimi K, et al. Epidemiology profile of renal cell carcinoma: A 10-years patients' experience at king Abdulaziz Medical City, National Huard Health Affairs, Saudi Arabia. Urol Ann. 2018;10:59–64. doi: 10.4103/UA.UA_102_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bafaraj S, Awad I, Jastaniah S, et al. Screening for thyroid diseases among students of applied medical sciences at King Abdulaziz University, Saudi Arabia. Saudi Med J. 2018;39:311–314. doi: 10.15537/smj.2018.3.22137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saeed MI, Hassan AA, Butt ME, et al. Pattern of thyroid lesions in western region of Saudi Arabia: A retrospective analysis and literature review. J Clin Med Res. 2018;10:106–116. doi: 10.14740/jocmr3202w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.PRISMA. Statement on transparent reporting of systematic reviews and meta-analysis. 2018. Available from: http://www.prisma-statement.org/.
- 29.GLOBECAN 2012. International Agency for Research on Cancer [online database] Available from: http://www-dep.iarc.fr/
- 30.WHO. Breast cancer burden 2017. Available from: http://www.who.int/cancer/detection/breastcancer/en/index1.html.
- 31.Mir R, Javid J, Al Balawi A, et al. A germline mutation in BRCA1 3’UTR variant predicts susceptibility to breast cancer in a Saudi Arabian population. Asian Pac J Cancer Prev. 2018;19:859–866. doi: 10.22034/APJCP.2018.19.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al Balawi IA, Mir R, Abu-Duhier FM. Potential impact of vascular endothelial growth factor gene variation (−2578C> A) on breast cancer susceptibility in Saudi Arabia: a case-control study. Asian Pac J Cancer Prev. 2018;19:1135–1143. doi: 10.22034/APJCP.2018.19.4.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alhuquail AJ, Alzahrani A, Almubarak H, et al. High prevalence of deliterous BRCA 1 and BRCA 2 germline mutations in arab breast and ovarian cancer patients. Breast Cancer Res Treat. 2018 doi: 10.1007/s10549-017-4635-4. [DOI] [PubMed] [Google Scholar]
- 34.Rahman S, Zayed H. Breast cancer in the GCC countries: A focus on BRCA1/2 and non-BRAC1/2 genes. Gene. 2018 doi: 10.1016/j.gene.2018.05.045. [DOI] [PubMed] [Google Scholar]
- 35.Abulkhair O, Saadeddin A, Makran O, et al. Vitamin D levels and breast cancer characteristics: Findings in patients from Saudi Arabia. J Steroid Biochem Mol Biol. 2015 doi: 10.1016/j.jsbmb.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 36.Ifediora CO. Re-thinking breast and cervical cancer preventive campaigns in developing countries: the case for interventions at high schools. BMC Public Health. 2019;19:503. doi: 10.1186/s12889-019-6890-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alshatwi AA, Hasan TN, Shafi G, et al. A single nucleotide polymorphism in the TP53 and MDM-2 gene modifies breast cancer risk in an ethnic Arab population. Fundam Clin Pharmacol. 2011;26:438–443. doi: 10.1111/j.1472-8206.2011.00939.x. [DOI] [PubMed] [Google Scholar]
- 38.Al-Qasem A, Toulimat M, Tulbah A, et al. The p53 codon 72 polymorphism is associated with risk and early onset of breast cancer among Saudi women. Oncol Lett. 2012;3:875–878. doi: 10.3892/ol.2012.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alshatwi AA, Shafi G, Hasan TN, et al. Differential Expression Profile and Genetic Variants of MicroRNAs Sequences in Breast Cancer Patients. Plos One. 2012;7:e30049. doi: 10.1371/journal.pone.0030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alghamdi IG, Hussain II, Alghamdi MS, et al. The incidence rate of female breast cancer in Saudi Arabia: an observational descriptive epidemiological analysis of data from Saudi Cancer Registry 2001–2008. Breast Cancer: Targets Ther. 2013;5:103–109. doi: 10.2147/BCTT.S50750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alanazi M, Pathan AAK, Arifeen Z, et al. Association between PARP-1 V762A polymorphism and breast cancer susceptibility in Saudi population. Plos One. 2013;8:e85541. doi: 10.1371/journal.pone.0085541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yousef FM, Jacobs ET, Kang PT, et al. Vitamin D status and breast cancer in Saudi Arabian women: case-control study. Am J Clin Nutr. 2013;98:105–110. doi: 10.3945/ajcn.112.054445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Al-Mutairi FM, Alanazi M, Shalaby M, et al. Association of XRCC1 gene polymorphisms with breast cancer susceptibility in Saudi patients. Asian Pac J Cancer Prev. 2013;14:3809–3813. doi: 10.7314/apjcp.2013.14.6.3809. [DOI] [PubMed] [Google Scholar]
- 44.Alokail MS, Al-Daghri N, Abdulkareem A, et al. Metabolic syndrome biomarkers and early breast cancer in Saudi women: evidence for the presence of a systemic stress response and/or a pre existing metabolic syndrome-related neoplasia risk? BMC Cancer. 2013;13:54. doi: 10.1186/1471-2407-13-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hasan TN, Shafi G, Syed NA, et al. Lack of association of BRCA1 and BRCA2 variants with breast cancer in an ethnic population of Saudi Arabia, an emerging high-risk area. Asian Pac J Cancer Prev. 2013;14:5671–5674. doi: 10.7314/apjcp.2013.14.10.5671. [DOI] [PubMed] [Google Scholar]
- 46.Elkum N, Al-Tweigeri T, Ajarim D, et al. Obesity is a significant risk factor for breast cancer. BMC Cancer. 2014;14:788. doi: 10.1186/1471-2407-14-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benard F, Barkun AN, Martel M, et al. Systematic review of colorectal cancer screening guidelines for average-risk adults: Summarising the current global recommendations. World J Gasteroenterol. 2018;24:124–138. doi: 10.3748/wjg.v24.i1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams CE, Williams EA, Corfe BM. Vitamin D status in irritable bowel syndrome and the impact of supplementation on symptoms: what do we know and what do we need to know? Eur J Clin Nutr. 2018;72:1358–1363. doi: 10.1038/s41430-017-0064-z. [DOI] [PubMed] [Google Scholar]
- 49.Al-Harithy RN, Al-Ghafari AB. Risistin in human colon cancer. Saudi Med J. 2010;31:495–500. [PubMed] [Google Scholar]
- 50.Al-Harithy RN, Al-Zahrani MH. The adinponectin gene, ADIPOQ, and genetic susceptibility to colon cancer. Oncol Lett. 2012;3:176–180. doi: 10.3892/ol.2011.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Al-Harithy RN, Al-Ghazzawi Polymorphisms of the deoxyribonucleic acid (DNA) repair gene XRCC1 and risk of colon cancer in Saudi Arabia. Int J Med Med Sci. 2011;3:281–288. [Google Scholar]
- 52.Alharithy RN. Polymorphisms in RETN gene and susceptibility to colon cancer in Saudi patients. Ann Saudi Med. 2014;34:334–339. doi: 10.5144/0256-4947.2014.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alghafari AB, Balamash KS, Doghaither HA, et al. “Relationship between Serum Vitamin D and Calcium Levels and Vitamin D Receptor Gene Polymorphisms in Colorectal Cancer”. BioMed Res Int. 2019 doi: 10.1155/2019/8571541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Albasri A, El-Siddig A, Hussainy A, et al. Histopathologic characterization of prostrate diseases in Madinah, Saudi Arabia. Asian Pac J Cancer Prev. 2014a;15:4175–4179. doi: 10.7314/apjcp.2014.15.10.4175. [DOI] [PubMed] [Google Scholar]
- 55.Ross-Adams H, Lamb AD, Dunning MJ, et al. Integration of copy number and transcriptomics provides risk stratification in prostate cancer: A discovery and validation cohort study. EBioMedicine. 2015;2:1133–1144. doi: 10.1016/j.ebiom.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Travis RC, Appleby PN, Martin RM, et al. A Meta-analysis of Individual Participant Data Reveals an Association between Circulating Levels of IGF-I and Prostate Cancer Risk. Cancer Res. 2016;76:2288–2300. doi: 10.1158/0008-5472.CAN-15-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Al-Abdin OZ, Rabah DM, Badr G, et al. Differences in prostrate cancer detection between Canadian and Saudi populations. Braz J Med Biol Res. 2013;46:539–545. doi: 10.1590/1414-431X20132757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alghamdi IG, Hussain II, Alghamdi MS, et al. The incidence rate of prostate cancer in Saudi Arabia: an observational descriptive epidemiological analysis of data from Saudi Cancer registry 2001–2008. Hematol/Oncol Stem Cell Ther. 2014;7:18–26. doi: 10.1016/j.hemonc.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 59.Shafi RG, Al-Mansour MM, Kanfar SS, et al. Hodgkin Lymphoma Outcome: A Retrospective Study from 3 Tertiary Centers in Saudi Arabia. Oncol Res Treat. 2017;40:288–292. doi: 10.1159/000460819. [DOI] [PubMed] [Google Scholar]
- 60.Dalia S, Chavez J, Castillo JJ, et al. Hepatitis B infection increases the risk of non-Hodkin lymphoma: A meta-analysis of observational studies. Leuk Res. 2013;37:1107–1115. doi: 10.1016/j.leukres.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 61.Alkhateeb SS, Alkhateeb JM, Ahrashidi EA. Increasing trends in kidney cancer over the last 2 decades in Saudi Arabia. Saudi Med J. 2015;36:698–703. doi: 10.15537/smj.2015.6.10841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hajeer MH, Awad HA, Abdullah NI, et al. The rising trend in papillary thyroid carcinoma. Saudi Med J. 2018;39:147–153. doi: 10.15537/smj.2018.2.21211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Y, Chen Y, Huang H, et al. Diagnostic radiography exposure increases the risk for thyroid microcarcinoma: a population-based case-control study. Eur J Cancer Prev. 2015;24:439–446. doi: 10.1097/CEJ.0000000000000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hussain F, Iqbal S, Mehmood A, et al. Incidence of thyroid cancer in kingdom of Saudi Arabia, 2000–2010. Hematol/Oncol Stem Cell Ther. 2013;6:58–64. doi: 10.1016/j.hemonc.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 65.Albasri A, Sawaf Z, Hussainy AS, et al. Histopathological patterns of thyroid disease in Al-Madinah region of Saudi Arabia. Asian Pac J Cancer Prev. 2014c;15:5565–5570. doi: 10.7314/apjcp.2014.15.14.5565. [DOI] [PubMed] [Google Scholar]
- 66.Abdo N, Khader YS, Abdelrahman M, et al. Respiratory health outcomes and air pollution in the Eastern Mediterranean Region: a systematic review. Rev Environ Health. 2016;31:259–280. doi: 10.1515/reveh-2015-0076. [DOI] [PubMed] [Google Scholar]
- 67.Al-Hazzaa HM. Physical inactivity in Saudi Arabia revisited: A systematic review of inactivity prevalence and perceived barriers to active living. Int J Health Sci (Qassim) 2018;12:50–64. [PMC free article] [PubMed] [Google Scholar]
- 68.Al-Musa HM, Awadalla NJ, Mahfouz AA. Male Partners' Knowledge, Attitudes, and Perception of Women's Breast Cancer in Abha, Southwestern Saudi Arabia. Int J Environ Res Public Health. 2019;16:3089. doi: 10.3390/ijerph16173089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aldohaian AI, Alshammari SA, Arafah DM. Using the health belief model to assess beliefs and behaviors regarding cervical cancer screening among Saudi women: a cross-sectional observational study. BMC Womens Health. 2019;19:6. doi: 10.1186/s12905-018-0701-2. [DOI] [PMC free article] [PubMed] [Google Scholar]