Abstract
Background and aims
The link between diabetes and increased risk of infectious disease has long been recognized, but has re-entered sharp focus following the COVID-19 pandemic.
Methods
A literature search was conducted in PubMed for articles in English on diabetes and infection.
Results
Diabetes predisposes to infections through alterations in innate and acquired immune defenses. Outcomes of infection are worse in people with uncontrolled diabetes, and infection can worsen hyperglycemia in hitherto well controlled diabetes (bidirectional relationship). Diabetes does not increase the risk of infection with COVID-19 per se, but predisposes to severe disease and poor outcomes. COVID-19 has also been linked to deterioration of glycemic control as well as new-onset diabetes.
Conclusions
Clinicians caring for people with diabetes should be aware of the increased risk of infections in this population, as well as the possibility of worsening hyperglycemia. A holistic approach with frequent monitoring of blood glucose levels and appropriate titration of medications, along with close attention to nutritional status, is essential to ensure the best possible outcomes.
Keywords: Diabetes, Hyperglycemia, Infection, COVID-19, Tuberculosis, Vaccination
1. Introduction
Infectious disease is an important, yet oft-neglected corollary of uncontrolled diabetes mellitus. In the pre-insulin era, most deaths among individuals with type 2 diabetes and many among type 1 diabetes occurred as a result of uncontrolled infection. It is a matter of concern that even today, infections continue to cause significant morbidity and mortality in patients with diabetes, notwithstanding the recent advances in antihyperglycemic and antimicrobial therapeutic options.
In this short review, we will review the mechanisms underlying increased susceptibility to infection in diabetes and briefly discuss the clinically relevant infections found in patients with diabetes, with particular reference to the ongoing COVID-19 pandemic. Indian situation has been highlighted whenever data are available.
2. Search strategy
We searched PubMed for original and review articles in English published, using the following keywords: diabetes and infection, hyperglycemia COVID-19, tuberculosis, vaccination, infections, and diabetes in India, from 2000 till September 2020.
3. Why do patients with diabetes have an increased risk of infections?
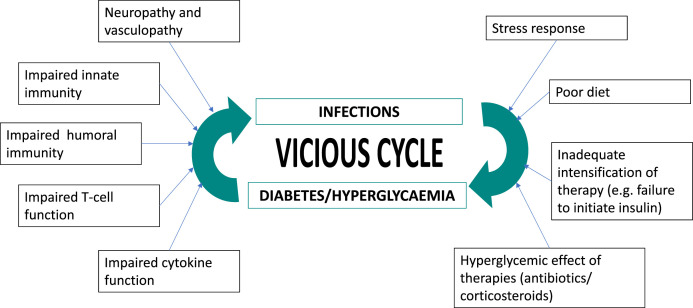
The relationship between diabetes and infection has been known for long and has been traditionally considered as bidirectional (Fig. 1 ) [1]. Uncontrolled diabetes affects almost all components of immunity:
-
1.
Dysregulated innate immunity including defective neutrophil and macrophage function [2].
-
2.
Abnormal complement function, which may be related in part to defects in neutrophil function and cytokine responses [3].
-
3.
Defects in T-cell responses [4].
-
4.
Defective humoral (antibody-mediated) immunity [5].
Fig. 1.
Bidirectional relationship between diabetes/hyperglycemia and infection.
In addition, the widespread vasculopathy typical of longstanding uncontrolled diabetes interferes with the body’s ability to combat infection by limiting the ingress of immune cells as well as antimicrobial factors, and by promoting tissue necrosis and gangrene. Certain features of the hyperglycemic milieu contribute to the growth of specific micro-organisms (e.g. ketosis promoting the growth of fungi causing mucormycosis).
The infections met with in diabetes patients can be broadly classified into two categories.
-
1.
Infections that are common in the non-diabetic population, that also affect people with diabetes, often with more severe clinical presentation and worse outcomes.
-
2.
Infections that are peculiar to individuals with diabetes and virtually unknown in the normal population.
4. Common infections in individuals with diabetes
These include respiratory infections, genitourinary tract infections and skin and soft tissue infections (Table 1 ). In a series of 380 patients with diabetes and infections attending a tertiary care centre in North India, the most commonly encountered infections were those of the soft tissues (42.8%), respiratory tract (30.2%) and genitourinary tract (28.4%). Infection of more than one site was present in 5.3% of patients [6]. Diabetes has been shown to increase the risk of lower, but not upper respiratory tract infections [7].
Table 1.
Infections prevalent in patients with diabetes.
| Common infections |
|
| Uncommon infections peculiar to diabetes |
|
| Diabetic foot infections | Associated with diabetic neuropathy and peripheral vascular disease |
Tuberculosis (TB) is a common comorbidity of diabetes, particularly in developing countries. India faces a double burden with the highest number of TB patients, and the second highest number of individuals with diabetes, living within its borders. The relationship between TB and diabetes is bidirectional; individuals with diabetes are more likely to contract TB, and individuals with diabetes are more likely to have diabetes compared to the general population.
[8]. The greater risk of contracting TB in diabetes, as well as reactivation of latent TB, is postulated to be due to a combination of susceptibility to infection with oxidative stress and increased tissue inflammation [8]. A recent systematic review on the co-prevalence of TB and diabetes in low and middle-income countries found that diabetes was found in 1.8–45% of individuals diagnosed with TB, and that 0.1–6% of individuals with diabetes had TB [9]. TB in diabetes has certain peculiar characteristics that make diagnosis and management difficult (see Box 1 ) [10].
Box 1. Diabetes and tuberculosis (Refs.8–10,39-45).
Magnitude of the problem.
-
•
75 million individuals with diabetes in India
-
•
2.2 million new cases of TB in India every year
Co-prevalence.
-
•
30–60% of individuals with TB have diabetes
-
•
2–5% of individuals with diabetes have TB
-
•
Bidirectional screening is indicated: all patients with TB should be screened for diabetes and vice versa
Peculiarities of TB in diabetes.
-
•
Relative paucity of physical signs
-
•
More extensive caseation and cavitation
-
•
Minimal pleural involvement
-
•
Hemoptysis more common
Issues in management.
-
•
Longer course of treatment may be needed
-
•
Lower likelihood of sputum conversion
-
•
Increased risk of treatment failure, relapse and death
-
•
TB can worsen glycemic control
-
•
Issues with polypharmacy and compliance
-
•
Rifampicin can alter metabolism of oral antidiabetic agents, reducing efficacy
-
•
INH can worsen peripheral neuropathy
Alt-text: Box 1
Genitourinary infections found in patients with diabetes include urethritis, vaginitis, cystitis, and prostatitis. Common causative organisms are Gram negative bacteria such as E. coli and Klebsiella and fungi such as Candida. In Indian patients with diabetes and UTI, the most common organisms isolated were E. coli (64.6%), Klebsiella (12.1%) and Enterococcus (9.9%) [11]. Infection with extended spectrum beta-lactamase producing E. coli was found to be more frequent in individuals with diabetes. Also, nearly 30% of individuals with positive urine culture were found to be asymptomatic. However, current guidelines state that asymptomatic bacteriuria need not be treated, even among patients with diabetes [12].
Use of sodium-glucose cotransporter-2 inhibitors (SGLT2i) for management of hyperglycemia has been associated with increased risk of genital mycotic infections [13]; cystitis and upper urinary tract infections are less common but can occasionally occur [14].
Skin and soft tissue infections found in patients with diabetes include furuncles, carbuncles, and cellulitis. In India, more than 60% of all skin and soft tissue infections have been shown to be associated with Staphylococci [6]. Infection is also an important component of the Diabetic Foot Syndrome. Most cases of diabetic foot infection have been shown to be polymicrobial in nature, with predominance of Gram-negative organisms [[15], [16], [17]]. Prevalence of antimicrobial drug resistance was also found to be higher among patients with diabetes, which could be attributed, at least partially, to the production of biofilms by the causative organisms [18].
The antimicrobial management of these conditions does not differ significantly from that in the population without diabetes. Achievement and maintenance of tight glycemic control, most often requiring the use of intensive insulin therapy, is key to improving outcomes.
5. Diabetes and COVID-19
COVID-19 is an acute, predominantly respiratory viral illness caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV2). From studies conducted in China, Europe, and the U.S., it appears that individuals with diabetes are not at higher risk of infection with SARS-CoV2 compared to the general population [19,20] (Box 2 ). However, it is clear that they do tend to have worse outcomes, with respect to development of more severe illness and mortality risk, than individuals without diabetes [21]. While mortality due to COVID-19 has been lower in south Asian countries such as India, the sheer number of individuals with diabetes in this region represents a huge population at high risk of adverse outcomes due to this infection [22,23]. As individuals with diabetes tend to be older and to have higher burden of cardiometabolic risk factors such as obesity and hypertension (as well as cardiovascular disease per se), it is likely that their increased risk of adverse outcomes is mediated, to a large extent, through these comorbidities than by diabetes per se [24]. Recently, there have been reports of new-onset diabetes following infection with SARS-COV2, mainly from the U.S. [25], but increasingly from elsewhere in the world as well. New-onset hyperglycemia during COVID infection can have multiple causes-weight gain following disordered diet and exercise during lockdown, mental stress, and unwarranted use of dexamethasone for mild to moderate cases of COVID [26]. It is also likely that the novel SARS coronavirus has a direct diabetogenic potential by way of its effects on the pancreas. The angiotensin converting enzyme 2 (ACE2) receptor, by means of which SARS-CoV2 enters target cells, is also present on the pancreatic beta-cell [27]. Infection of the beta cell may lead to acute impairment of insulin secretion or even destruction of the beta cell, as has been reported for human herpesvirus infection in Africa [28].
Box 2. DIABETES AND COVID-19 (Refs.19–26, 37–38, 60).
Magnitude of the problem.
-
•
420 million people with diabetes worldwide (75 million in India)
-
•
28 million infections with COVID worldwide as of September 2020 (4.4 million in India)
Effect of diabetes on COVID.
-
•
Diabetes per se not a risk factor for contracting COVID infection
-
•
Severe disease and adverse outcomes more likely in individuals with diabetes
-
•
Poor outcomes may be linked to other comorbidities such as older age, obesity and hypertension, more frequent among people with diabetes
-
•
Poor long-term (pre-infection) diabetes control, admission hyperglycemia and inpatient hyperglycemia linked with poor outcomes
Effect of COVID and its Treatment on diabetes.
-
•
COVID infection can worsen diabetes control
-
•
New onset diabetes has been reported with COVID
-
•
Some treatments used for COVID treatment (e.g. steroids) can exacerbate hyperglycemia
Social aspects.
-
•
Adverse effect of pandemic and consequent lockdowns on routine diabetes care. This is likely to exacerbate diabetes control and also add new patients of diabetes to already high numbers.
-
•
Patients unable to exercise regularly, access healthy diet and procure medications promptly. This will increase morbidity and even mortality.
Alt-text: Box 2
In individuals with pre-existing diabetes, the current COVID pandemic and the public health/governmental responses to it are also likely to impact glycemic control in significant ways. Lack of accessibility to testing and care during lockdowns, increased snacking and reduced physical activity are likely to worsen diabetes control, and predispose patients to complications [29], although such deterioration has not been found in all studies [30].
6. Rare infections peculiar to patients with diabetes
-
•
Malignant otitis externa refers to Pseudomonal infection of the external acoustic meatus ad middle ear.
-
•
Rhinocerebral mucormycosis is infection of the nasal cavity and orbit by fungi such as Rhizopus, Mucor and Absidia.
-
•
Emphysematous pyelonephritis refers to infection of the renal parenchyma by gas-forming micro-organisms such as E. coli.
-
•
Renal papillary necrosis is a complication of UTI in diabetic patients characterized by necrosis and sloughing off of the renal papillae.
-
•
Emphysematous cholecystitis is inflammation of the gall bladder due to infection by gas-forming micro-organisms.
-
•
Necrotising fasciitis is an uncommon infection of soft tissue caused by various combinations of Streptococci, Staphylococci, and anaerobes. Necrotising fasciitis of the perineum is termed Fournier’s gangrene.
A high index of suspicion is required for the diagnosis of most of these conditions. Treatment involves, in addition to specific antimicrobial agents, early and aggressive surgical intervention wherever indicated. The prognosis for many of these conditions is poor, even with prompt treatment.
7. Effect of hyperglycemia and glycemic control on infections
While the deleterious effects of uncontrolled hyperglycemia on infection have been well characterized, there is less information available on whether controlling hyperglycemia can have beneficial effects on infection prevention and control [31].
7.1. Uncontrolled hyperglycemia increases hospitalization and morbidities of infections
Analysis of patients with type 1 and type 2 diabetes enrolled in primary care in England have shown a clear increase in long-term risk of infection with increasing HbA1c [32]. In a population-based study from Denmark, individuals with HbA1c of 10.5% and above had hazards ratio of 1.64 for infections requiring hospitalization, compared to individuals with HbA1c between 5.50 and 6.49% [33].
7.2. Hyperglycemia and morbidity and mortality due to COVID19
During the ongoing COVID pandemic, attempts have been made to link the severity of disease outcomes in COVID-19 with the levels of background glycemic control, as well as the glucose levels at admission and during the course of hospitalization. Higher HbA1c at hospitalization, indicating poor long-term glycemic control, has been associated with higher risk of in-hospital death due to COVID, although this has not been replicated in all studies [34]. Patients with higher blood glucose levels at admission tended to have the most florid lesions on chest imaging and were more likely to require ICU admission and intubation and to die compared to those who had lower blood glucose levels [35]. In-hospital hyperglycemia was associated with worse clinical outcomes among patients with COVID studied in China and the U.S [36,37]. These findings reinforce the need for ensuring tight glycemic control in patients with diabetes during the current pandemic, and also for the maintenance of euglycemia in patients who are hospitalized for COVID-19. In this context, it should be remembered that some medications used for the treatment of severe COVID (particularly corticosteroids) have the potential to raise blood glucose levels, and that the antidiabetic drug regimen will need to be appropriately titrated in patients receiving these treatments [21]. Even in the absence of these treatments, infection with SARS-COV2 has been associated with extremely high insulin requirements among patients with diabetes, and the development of hyperglycemic crises in some cases [38].
7.3. Hyperglycemia and treatment failure and relapse in TB
The presence of diabetes is associated with increased risk of treatment failure, relapse, and death in patients with TB [39]; similar results have been reported from India as well [40]. The role of tight glycemic control in improving treatment outcomes in TB remains controversial [41]. A study from China showed that treatment outcomes were worse among those with suboptimal glycemic control [42]. Similarly, Mahishale et al. [43] showed that optimal glycemic control resulted in 88% reduction of sputum non-conversion at 2 months of treatment compared to poor glycemic control. However, Nandakumar et al. [44] found no correlation between diabetes control and TB treatment outcomes in their study conducted in Malappuram district of Kerala. In this context, it is interesting to note that recent studies have shown an association of poorly controlled diabetes with better outcomes in individuals with low body-mass index; this needs confirmation in larger studies [45].
7.4. Effect of tight glycemic control on infections
Maintenance of tight glycemic control during the peri- and postoperative period has been found to be associated with a lower incidence of surgical site infections in patients with diabetes [46,47]. Even though maintenance of tight glycemic control has been long considered one of the cornerstones of diabetic foot management, there is little evidence by way of randomized controlled trials to suggest that foot ulcer outcomes are improved by this approach [48]; such trials are urgently needed, considering the global magnitude of the problem of diabetic foot. In a retrospective study of more than 7300 patients with COVID-19 from China, well controlled blood glucose (defined as glycemic variability between 3.9 and 10 mmol/l) was associated with significantly lower mortality compared to higher blood glucose variability (>10 mmol/l) [49].
8. Diet and nutrition in patients with diabetes and infections
The role of diet has often been overlooked while managing infections in patients with diabetes. Some general points can be summarized from available studies:
-
A.
Severe infection is a hypercatabolic state and any diet plan for patients with infection should take this into account, ensuring adequate intake of protein and micronutrients to promote healing. This is particularly relevant in the Indian context, where the baseline protein intake is extremely low. Particularly in patients with diabetes and TB without hepatic or renal insufficiency, it is recommended that proteins should be the major source of energy [50].
-
B.
Supplementation of micronutrients for 6 months has been shown to reduce the incidence of common infections (respiratory, skin, and urogenital) in patients with diabetes [51]. A recent review [52] on the role of micronutrient supplementation in diabetic foot ulcers concluded that while there was some evidence to support the role of Vitamins A, C, D and E, and zinc in ulcer healing, the level of evidence was not strong enough to support any recommendations for routine supplementation with these nutrients. In patients with risk of limb ischemia and/or hypoalbuminemia, supplementation with arginine, glutamine and betahydroxy methyl butyrate has been shown to improve foot ulcer healing [53].
-
C.
Supplementation of vitamin D has been shown to improve the proportion of sputum smear and culture conversion in patients with active pulmonary TB (with or without diabetes) [54].
-
D.
Similarly, vitamin D deficiency was associated with an increased risk of testing positive for COVID-19, raising the possibility that supplementation of this vitamin could reduce the risk of COVID infection; however, it should be noted that this study was not restricted to individuals with diabetes [55].
Recently, consensus guidelines have been published for balanced nutrition in the time of the COVID pandemic [56]. These can be summarized as follows.
-
A.
Individuals not infected with COVID, or those with mild to moderate disease, consume a balanced diet rich in vegetables, fruit, legumes, nuts, and whole grain as well as egg and lean meat wherever applicable.
-
B.
Intake of probiotic-rich food is encouraged, and hydration should be maintained particularly when febrile.
-
C.
Saturated fat and processed food should be avoided, as should extreme “fad” diets.
-
D.
The diet should provide at least 1 g of protein per kg body weight per day in older persons and should contain adequate amounts of micronutrients such as Vitamins A, C, and D, zinc, and selenium.
-
E.
In severely ill patients, these nutritional requirements should be met by way of oral supplementation wherever possible, with enteral and parenteral supplementation being reserved for the most severely ill individuals who cannot tolerate oral intake.
9. Vaccinations in patients with diabetes
As individuals with diabetes represent a vulnerable subgroup of the population with respect to susceptibility to infection, preventing these infections by means of vaccination assumes paramount importance. In addition to all routine immunisations recommended for the general population, the American Diabetes Association provides additional recommendations for the use of pneumococcal, influenza and hepatitis B vaccines in individuals with diabetes [57]. Attempts have been made to formulate similar recommendations for India also [58,59] (Table 2 ).
Table 2.
| Vaccine | Recommendation |
|---|---|
| Pneumococcus |
|
| Influenza |
|
| Hepatitis B |
|
| Other vaccines | Should be administered as appropriate for age |
In this context, it should also be noted that the nationwide lockdowns imposed in India and other countries to combat the spread of the COVID-19 pandemic have had an adverse impact on the coverage of infection control and immunization programs directed against other communicable disease; these countries should gear up to face a recrudescence of many of these hitherto controlled infections in the near future [60].
10. Conclusions
Infectious disease continues to take a heavy toll of the population with diabetes even in the present day. The increased susceptibility of the individual with diabetes to infection has recently returned to sharp focus with the advent of the COVID-19 pandemic, reiterating the need for achieving tight control of hyperglycemia and managing comorbidities appropriately in this population from the time of diagnosis of diabetes. Also, there are certain unusual infections that appear to be exclusively found in patients with diabetes; the clinician dealing with patients with diabetes should be ever alert to the possibility of these infections, as prompt diagnosis may mean the difference between life and death in many cases.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Casquiero J., Casquiero J., Alves M. Infections in patients with diabetes mellitus: a review of pathogenesis. Ind J Endocrinol Metab. 2012;16(Suppl.1):S27–S36. doi: 10.4103/2230-8210.94253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peleg A.Y., Weerarathna T., McCarthy J.S., Davis T.M. Common infections in diabetes: pathogenesis, management and relationship to glycaemic control. Diabet Metab Res Rev. 2007;23:3–13. doi: 10.1002/dmrr.682. [DOI] [PubMed] [Google Scholar]
- 3.Stoeckle M., Kaech C., Trampuz A., Zimmerli W. The role of diabetes mellitus in patients with bloodstream infections. Swiss Med Wkly. 2008;138:512–519. doi: 10.4414/smw.2008.12228. [DOI] [PubMed] [Google Scholar]
- 4.Geerlings S.E., Hoepelman A.I. Immune dysfunction in patients with diabetes mellitus (DM) FEMS Immunol Med Microbiol. 1999;26:259–265. doi: 10.1111/j.1574-695X.1999.tb01397.x. [DOI] [PubMed] [Google Scholar]
- 5.Berbudi A., Rahmadika N., Tjahjadi A.I., Ruslami R. Type 2 diabetes and its impact on the immune system. Curr Diabetes Rev. 2020;16:442–449. doi: 10.2174/1573399815666191024085838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masoodi S.R., Wani A.I., Misgar R.A., Gupta V.K., Bashir M.I., Zargar A.H. Pattern of infections in patients with diabetes mellitus--Data from a tertiary care medical center in Indian Subcontinent. Diabet Metab Syndr Clin Res Rev. 2007;1:91–95. [Google Scholar]
- 7.Muller L.M., Gorter K.J., Hak E., et al. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin Infect Dis. 2005;41:281–288. doi: 10.1086/431587. [DOI] [PubMed] [Google Scholar]
- 8.Bloomgarden Z., Misra A. Diabetes and tuberculosis: an important relationship. J Diabetes. 2017;9:640–643. doi: 10.1111/1753-0407.12547. [DOI] [PubMed] [Google Scholar]
- 9.McMurry H.S., Mendenhall E., Rajendrakumar A., Nambiar L., Satyanarayana S., Shivashankar R. Coprevalence of type 2 diabetes mellitus and tuberculosis in low-income and middle-income countries: a systematic review. Diabet Metab Res Rev. 2019;35(1) doi: 10.1002/dmrr.3066. [DOI] [PubMed] [Google Scholar]
- 10.Guptan A., Shah A. Diabetes and tuberculosis: an appraisal. Indian J Tubercul. 2000;47:2–8. [Google Scholar]
- 11.Aswani S.M., Chandrasekhar U.K., Shivashankara K.N., Pruthvi B.C. Clinical profile of urinary tract infections in diabetics and non-diabetics. Australas Med J. 2014;31:29–34. doi: 10.4066/AMJ.2014.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicolle L.E., Gupta K., Bradley S.F., et al. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America. Clin Infect Dis. 2019;68:e83–e110. doi: 10.1093/cid/ciy1121. [DOI] [PubMed] [Google Scholar]
- 13.Unnikrishnan A.G., Kalra S., Purandare V., Vasnawala H. Genital infections with sodium glucose cotransporter-2 inhibitors: occurrence and management in patients with type 2 diabetes mellitus. Ind J Endocrinol Metab. 2018;22:837–842. doi: 10.4103/ijem.IJEM_159_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta R., Ghosh A., Misra A. Case of acute unilateral emphysematous pyelonephritis and bacteraemia on treatment with canagliflozin. Postgrad Med. 2018;94:714–715. doi: 10.1136/postgradmedj-2018-136109. [DOI] [PubMed] [Google Scholar]
- 15.Saseedharan S., Sahu M., Chaddha R., et al. Epidemiology of diabetic foot infections in a reference tertiary hospital in India. Braz J Microbiol. 2018;49:401–406. doi: 10.1016/j.bjm.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shankar E.M., Mohan V., Premalatha G., Srinivasan R.S., Usha A.R. Bacterial etiology of diabetic foot infections in South India. Eur J Intern Med. 2005;16:567–570. doi: 10.1016/j.ejim.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 17.Tiwari S., Pratyush D.D., Dwivedi A., Gupta S.K., Rai M., Singh S.K. Microbiological and clinical characteristics of diabetic foot infections in northern India. J Infect Dev Ctries. 2012;13:329–332. doi: 10.3855/jidc.1827. [DOI] [PubMed] [Google Scholar]
- 18.Singh S.K., Sridhar G.R. Infections and diabetes. Int J Diabetes Dev Ctries. 2015;35:59–62. [Google Scholar]
- 19.Li B., Yang J., Zhao F., et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109:531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fadini G.P., Morieri M.L., Longato E., Avogaro A. Prevalence and impact of diabetes among people infected with SARS-CoV-2. J Endocrinol Invest. 2020;43:867–869. doi: 10.1007/s40618-020-01236-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Appicella M, Campapiano MC, Mantuano M, Mazoni L, Coppelli A, Del Prato S. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol 20208:782-792.. [DOI] [PMC free article] [PubMed]
- 22.Gupta R., Misra A. COVID19 in South Asians/Asian Indians: heterogeneity of data and implications for pathophysiology and research. Diabetes Res Clin Pract. 2020;165 doi: 10.1016/j.diabres.2020.108267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caballero A.E., Ceriello A., Misra A., et al. COVID-19 in people living with diabetes: an international consensus. J Diabet Complicat. 2020;34 doi: 10.1016/j.jdiacomp.2020.107671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi Qiao, Zhang Xiaoyi, Jiang Fang, et al. Clinical characteristics and risk factors for mortality of COVID-19 patients with diabetes in Wuhan, China: a two-Center, retrospective study. Diabetes Care. 2020;43:1382–1391. doi: 10.2337/dc20-0598. [DOI] [PubMed] [Google Scholar]
- 25.Rubino F., Amiel S.A., Zimmet P., et al. New-onset diabetes in COVID-19. N Engl J Med. 2020;383:789–790. doi: 10.1056/NEJMc2018688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diabetes India National Diabetes Obesity and Cholesterol Foundation (NDOC), and Diabetes Expert Group, India. Strict glycemic control is needed in times of COVID19 epidemic in India: a Call for action for all physicians. Diabet Metab Syndr. 2020;14:1579–1581. doi: 10.1016/j.dsx.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arendse L.B., Jan Danser A.H., Poglitsch M., et al. Novel therapeutic approaches targeting the renin-angiotensin system and associated peptides in hypertension and heart failure. Pharmacol Rev. 2019;71:539–570. doi: 10.1124/pr.118.017129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sobngwi E., Choukem S.P., Agbalika F., et al. Ketosis-prone type 2 diabetes mellitus and human herpesvirus 8 infection in sub-Saharan Africans. J Am Med Assoc. 2008;299:2770–2776. doi: 10.1001/jama.299.23.2770. [DOI] [PubMed] [Google Scholar]
- 29.Ghosal S., Arora B., Dutta K., Ghosh A., Sinha B., Misra A. Increase in the risk of type 2 diabetes during lockdown for the COVID19 pandemic in India: a cohort analysis. Diabet Metab Syndr. 2020;14:949–952. doi: 10.1016/j.dsx.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anjana R.M., Pradeepa R., Deepa M., et al. Acceptability and utilization of newer technologies and effects on glycemic control in type 2 diabetes: lessons learnt from lockdown. Diabetes Technol Therapeut. 2020 doi: 10.1089/dia.2020.0240. [DOI] [PubMed] [Google Scholar]
- 31.Pearson-Stuttard J., Blundell S., Harris T., Cook D.G., Critchley J. Diabetes and infection: assessing the association with glycaemic control in population-based studies. Lancet Diabetes Endocrinol. 2016;4:148–158. doi: 10.1016/S2213-8587(15)00379-4. [DOI] [PubMed] [Google Scholar]
- 32.Critchley J., Carey I.M., Harris T., DeWilde S., Hosking F.J., Cook D.G. Glycemic control and risk of infections among people with type 1 or type 2 diabetes in a large primary care cohort Study. Diabetes Care. 2018;41:2127–2135. doi: 10.2337/dc18-0287. [DOI] [PubMed] [Google Scholar]
- 33.Mor A., Dekkers O.M., Nielsen J.S., Beck-Nielsen H., Sorensen H.T., Thomsen R.W. Impact of glycemic control on risk of infections in patients with type 2 diabetes: a population-based cohort study. Am J Epidemiol. 2017;186:227–236. doi: 10.1093/aje/kwx049. [DOI] [PubMed] [Google Scholar]
- 34.Williamson E., Walker A.J., Bhaskaran K., et al. Factors associated with COVID-19-related hospital death in the linked electronic health records of 17 million adult NHS patients. J Chem Inf Model. 2019;53:1689–1699. [Google Scholar]
- 35.Iacobellis G., Penaherrera C.A., Bermudez L.E., Mizrachi E.B. Admission hyperglycemia and radiological findings of SARS-CoV2 in patients with and without diabetes. Diabetes Res Clin Pract. 2020;164 doi: 10.1016/j.diabres.2020.108185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang F., Yang Y., Dong K., et al. Clinical characteristics of 28 patients with diabetes and COVID-19 in Wuhan, China. Endocr Pract. 2020;26:668–674. doi: 10.4158/EP-2020-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bode B., Garrett V., Messler J., et al. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J Diabet Sci Technol. 2020;14:813–821. doi: 10.1177/1932296820924469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rayman G., Lumb A., Kennon B., et al. Guidance on the management of diabetic ketoacidosis in the exceptional circumstances of the COVID-19 pandemic. Diabet Med. 2020;37:1214–1216. doi: 10.1111/dme.14328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baker M.A., Harries A.D., Jeon C.Y., et al. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med. 2011;9:81. doi: 10.1186/1741-7015-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Viswanathan V., Vigneswari A., Selvan K., Satyavani K., Rajeswari R., Kapur A. Effect of diabetes on treatment outcome of smear-positive pulmonary tuberculosis--a report from South India. J Diabet Complicat. 2014;28:162–165. doi: 10.1016/j.jdiacomp.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 41.Shewade H.D., Jeyashree K., Mahajan P., et al. Effect of glycemic control and type of diabetes treatment on unsuccessful TB treatment outcomes among people with TB-Diabetes: a systematic review. PLoS One. 2017;12(10) doi: 10.1371/journal.pone.0186697. Oct 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiang C.Y., Bai K.J., Lin H.H., et al. The influence of diabetes, glycemic control, and diabetes-related comorbidities on pulmonary tuberculosis. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0121698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahishale V., Avuthu S., Patil B., Lolly M., Eti A., Khan S. Effect of poor glycemic control in newly diagnosed patients with smear-positive pulmonary tuberculosis and type-2 diabetes mellitus. Iran J Med Sci. 2017;42:144–151. [PMC free article] [PubMed] [Google Scholar]
- 44.Nandakumar K.V., Duraisamy K., Balakrishnan S. Outcome of tuberculosis treatment in patients with diabetes mellitus treated in the Revised National Tuberculosis Control Programme in Malappuram district, Kerala, India. PLoS One. 2013;8(10) doi: 10.1371/journal.pone.0076275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kornfeld H., Sahukar S.B., Procter-Gray E., et al. Impact of diabetes and low body mass index on tuberculosis treatment outcomes. Clin Infect Dis. 2020 Jan 19 doi: 10.1093/cid/ciaa054. ciaa054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y.Y., Hu S.F., Ying H.M., et al. Postoperative tight glycemic control significantly reduces postoperative infection rates in patients undergoing surgery: a meta-analysis. BMC Endocr Disord. 2018;18:42. doi: 10.1186/s12902-018-0268-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dronge A.S., Perkal M.F., Kancir S., Concato J., Aslan M., Rosenthal R.A. Long-term glycemic control and postoperative infectious complications. Arch Surg. 2006;141:375–380. doi: 10.1001/archsurg.141.4.375. [DOI] [PubMed] [Google Scholar]
- 48.Patil M.D., Gunasekaran U., La Fontaine J., Meneghini L. Does improving glycemic control accelerate healing of diabetes foot ulcers? Diabetes. 2018;67(Suppl.1) doi: 10.2337/db18-2218-PUB. [DOI] [Google Scholar]
- 49.Zhu L., She Z.G., Cheng X., et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metabol. 2020;31:1068–1077. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Viswanathan V., Krishnan D., Kalra S., et al. Insights on medical nutrition therapy for type 2 diabetes mellitus: an Indian perspective. Adv Ther. 2019;36:520–547. doi: 10.1007/s12325-019-0872-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu Y., Jing H., Wang J., et al. Micronutrients decrease incidence of common infections in type 2 diabetic outpatients. Asia Pac J Clin Nutr. 2011;20:375–382. [PubMed] [Google Scholar]
- 52.Kulprachakarn K., Ounjaijean S., Wungrath J., Mani R., Rerkasem K. Micronutrients and natural compounds status and their effects on wound healing in the diabetic foot ulcer. Int J Low Extrem Wounds. 2017;16:244–250. doi: 10.1177/1534734617737659. [DOI] [PubMed] [Google Scholar]
- 53.Armstrong D.G., Hanft J.R., Driver V.R., et al. Effect of oral nutritional supplementation on wound healing in diabetic foot ulcers: a prospective randomized controlled trial. Diabet Med. 2014;31:1069–1077. doi: 10.1111/dme.12509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu H.X., Xiong X.F., Zhu M., Wei J., Zhuo K.Q., Cheng D.Y. Effects of vitamin D supplementation on the outcomes of patients with pulmonary tuberculosis: a systematic review and meta-analysis. BMC Pulm Med. 2018;18:108. doi: 10.1186/s12890-018-0677-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meltzer D.O., Best T.J., Zhang H., Vokes T., Arora V., Solway J. Association of vitamin d status and other clinical characteristics with COVID-19 test results. JAMA Netw Open. 2020;3(9) doi: 10.1001/jamanetworkopen.2020.19722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Misra A. Balanced nutrition is needed in times of COVID19 epidemic in India: a Call for Action for all nutritionists and physicians. Diabet Metab Syndr. 2020 doi: 10.1016/j.dsx.2020.08.030. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.American Diabetes Association Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes. Diabetes Care. 2020;43(Suppl.1):S37–S47. doi: 10.2337/dc20-S004. [DOI] [PubMed] [Google Scholar]
- 58.Kesavadev J., Misra A., Das A.K., et al. Suggested use of vaccines in diabetes. Indian J Endocrinol Metab. 2012;16:886–893. doi: 10.4103/2230-8210.102982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chawla R., Madhu S.V., Makkar B.M., Ghosh S., Saboo B., Kalra S. RSSDI-ESI clinical practice recommendations for the management of type 2 diabetes mellitus. Indian J Endocrinol Metab. 2020;24:1–122. doi: 10.4103/ijem.IJEM_225_20. RSSDI-ESI Consensus Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gopalan H.S., Misra A. COVID-19 pandemic and challenges for socio-economic issues, healthcare and National Health Programs in India. Diabet Metab Syndr. 2020;14:757–759. doi: 10.1016/j.dsx.2020.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]