Abstract
The correlation between myocardial injury and clinical outcome in COVID-19 patients is gaining attention in the literature. The aim of the present study was to evaluate the role of cardiac involvement and of respiratory failure in a cohort of COVID-19 patients hospitalized in an academic hospital in Lombardy, one of the most affected Italian (and worldwide) regions by the epidemic. The study included 405 consecutive patients with confirmed COVID-19 admitted to a medical ward from February 25th to March 31st, 2020. Follow-up of surviving patients ended either at hospital discharge or by July 30th, 2020. Myocardial injury was defined on the basis of the presence of blood levels of hs-TnI above the 99th percentile upper reference limit. Respiratory function was assessed as PaO2/FiO2 (P/F) ratio. The primary end-point was death for any cause. During hospitalization, 124 patients died. Death rate increased from 7.9% in patients with normal hs-TnI plasma levels and no cardiac comorbidity to 61.5% in patients with elevated hs-TnI and cardiac involvement (p < 0.001). At multivariable analysis, older age, P/F ratio < 200 (both p < 0.001) and hs-TnI plasma levels were independent predictors of death. However, it must be emphasized that the median values of hs-TnI were within normal range in non-survivors. Cardiac involvement at presentation was associated with poor prognosis in COVID-19 patients, but, even in a population of COVID-19 patients who did not require invasive ventilation at hospital admission, mortality was mainly driven by older age and respiratory failure.
Electronic supplementary material
The online version of this article (10.1007/s11739-020-02493-y) contains supplementary material, which is available to authorized users.
Keywords: COVID-19, Cardiac injury, Italy, Prognosis
Introduction
Since the onset of coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a growing body of evidence has shown that patients with confirmed COVID-19 may present elevated blood levels of high-sensitivity TnI (hs-TnI) during hospital stay, which might reflect ischemic cardiovascular complications or acute myocarditis [1–3]. An early experience in Wuhan, China, reported that among 416 hospitalized patients with COVID-19, nearly 20% had elevated levels of TnI at admission and that the overall mortality in this sub-category of patients was higher compared with that of patients with normal TnI levels (52.1 vs 4.5%, respectively, p < 0.001) [4]. Other smaller cohort studies confirmed the poor prognosis of hospitalized COVID-19 patients with concomitant cardiac disease, with or without elevated plasma levels of high-sensitivity troponin [5–7]. On the contrary, in a retrospective, multicenter cohort study including all adult patients with confirmed COVID-19 diagnosis admitted at two Hospitals in Wuhan, hs-TnI values did not correlate with mortality when included in a multivariate analysis [8]. In the same study, the levels of TnI tended to increase during the hospitalization, more specifically around day 15, particularly in the most severe patients [8]. A few studies, including a metanalysis, support the notion that TnI levels are significantly increased when SARS-CoV-2 infection is more severe [9–13]. These data raise the question if cardiac injury is a contributory cause of COVID-19 severity or vice versa if the severity of the infection may lead to cardiac damage [14]. In particular, in most of these previous studies, the degree of respiratory failure at admission was not reported.
Accordingly, we aimed to verify the role of cardiovascular comorbidities and of hs-TnI plasma levels and respiratory failure upon admission during the initial phase of the COVID-19 outbreak in Pavia, Italy. Secondary objective was to verify whether the predictors of outcome differ when evaluating death or the combination of death and/or ICU admission.
Materials and methods
Patients
Four hundred and five consecutive adult (≥ 18 years old) patients admitted with the suspected diagnosis of COVID-19 to the Emergency Department of Fondazione IRCCS Policlinico San Matteo (Pavia, Italy) from February 21st to March 31st 2020 were included in this cohort study. We have also included patients with an initially negative nasopharyngeal swab, but with a clear clinical diagnosis of SARS-CoV-2 infection based on symptoms, radiological and blood test findings, as the diagnosis of COVID-19 remains a hard task to accomplish [15, 16]. In particular, we included only patients coming from high-risk areas, in whom COVID-19 diagnosis was based on the concomitant presence of flu-like symptoms (fever, cough, sore throat), respiratory failure (tachypnoea, hypoxemia), clear radiological signs of interstitial pneumonia, and lymphopenia with increased lactate dehydrogenase. Other potential causes of interstitial pneumonia were ruled out, including atypical bacteria (Mycoplasma or Chlamydia) or other viruses (i.e., influenza A and B, parainfluenza viruses, syncytial respiratory virus, rhinoviruses, adenovirus, other coronaviruses). Patients who were directly hospitalized in the intensive care unit (ICU) were not included in the present study to avoid the confounding factor of extreme hypoxia at admission on the assessment of determinants of in-hospital prognosis. The review board of the Fondazione Policlinico San Matteo approved the publication of anonymized case series of COVID-19 patients using data collected for routine clinical practice and waived the requirement for a specific informed consent.
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Data collection
Demographic characteristics, medical history and previous therapy [ACE inhibitors (ACEi), Angiotensin II receptor blockers (ARB), betablockers, antiarrhythmics, antiplatelets and anticoagulants, clinical data [including PaO2/FiO2 (P/F)] and laboratory findings (including hs-TnI values) of patients upon admission and their subsequent outcome were retrieved from electronic medical records and collected onto a predefined spreadsheet. Determination of hs-TnI blood levels was performed using one validated assay [17].
A 12-lead electrocardiogram (ECG) was collected within the first 24 h of admission. Subsequently, an ECG was performed only in the presence of chest pain or other suspicious symptoms. ECG analysis focused on rhythm, heart rate, conduction disturbances and ST–T pathological abnormalities. QT interval was measured in at least two leads (DII and V2, or V5) and corrected using the Bazett formula.
Definitions of cardiac involvement
Myocardial injury was defined as hs-TnI value above the 99th percentile upper reference limit (URL). History of cardiovascular disease included history of ischemic heart disease, heart failure, valvular heart disease and hypertension. Presence of electrocardiographic abnormality included ST-elevation, ST-depression, negative T waves and atrial fibrillation (AF).
Clinical outcomes
We considered two hard outcome endpoints: death due to any cause during hospitalization and the combination of death and/or transfer to intensive care unit (ICU) due to the necessity of invasive mechanical ventilation. The follow-up of surviving patients ended either at hospital discharge or by July 30th, 2020. By this date, only one patient was still hospitalized in ICU, whilst all the other patients either died during hospitalization or were discharged.
Statistical analysis
All data were entered in anonymous form into a database (Microsoft Excel 2019) and then analyzed with MedCalc version 19.2 (MedCalc Software bvba). Categorical variables were presented as number and percentage and compared with Chi-square test. Continuous variables were tested for normality with the D’Agostino-Pearson’s test. Normally distributed continuous variables were presented as mean ± standard deviation and compared with T Student’s test. Non-normally distributed continuous variables were presented as median and interquartile range [IQR] and compared with Mann–Whitney’s test if independent or with Wilcoxon’s test in case of dependent variables. Logistic regression model was used to identify predictors and we used a stepwise regression to identify the independent predictors. Odds ratio (OR) with their 95% confidence interval (95% CI) are presented. A p value < 0.05 was considered as statistically significant.
Results
Patient characteristics
The main characteristics of the 405 patients admitted in our Emergency Department due to COVID-19 and then hospitalized in an internal ward are as follows. Median age was 69.8 (IQR 58.6–78.3) years and 68.6% of patients were males. Underlying cardiovascular disease (hypertension or heart failure or atrial fibrillation or ischemic or valvular heart disease) was highly prevalent (60%) whereas diabetes was present in 20.2%, pulmonary disease in 14.8%, chronic renal disease in 9.7% and cancer in 12.1% of patients.
A total of 172 patients (44.4%) were treated with ACE inhibitors/ARB/ARNi, and substantial proportion of patients received betablockers, antiarrhythmic drugs (such as amiodarone or flecainide) and antiplatelets or anticoagulants. At hospital arrival, most patients complained of dyspnea and only a small proportion (4%) reported chest pain.
A positive result on polymerase chain reaction testing of a nasopharyngeal swab was obtained in 321 out of 405 patients (79.3%).
Forty (9.9%) patients were transferred to ICU with a median time from hospital admission to ICU of 7 days [IQR 4–13.5] and 124 (30.6%) died during hospital stay in a median of 9 days [IQR 5–15] after hospital admission. The total number of patients who died and/or were transferred to ICU during hospital stay was 145 (35.8%) and this combined outcome occurred in a median of 8 days [IQR 4–11] after hospital admission.
Clinical characteristics according to the hs-TnI plasma levels (Table 1)
Table 1.
Patient characteristics according to hs-TNI plasma levels
| hs-TnI ≤ 47 (n = 266) | hs-TnI > 47 (n = 74) | p | |
|---|---|---|---|
| Age (years), median [IQR] | 68.6 [56.4–76.4] | 76.1 [69.7–82.5] | < 0.001 |
| Males, n (%) | 188 (70.7) | 51 (68.9) | 0.77 |
| Comorbidities | |||
| Cardiovascular disease, n (%) | 165 (63.7) | 59 (80.8) | 0.006 |
| Pulmonary disease, n (%) | 31 (12.1) | 13 (18.1) | 0.19 |
| Chronic renal disease, n (%) | 19 (7.4) | 8 (11.1) | 0.31 |
| Cancer, n (%) | 31 (12.2) | 11 (15.3) | 0.49 |
| Diabetes, n (%) | 51 (20) | 17 (23.6) | 0.51 |
| Theraphy at admission | |||
| ACEi or ARB or ARNi, n (%) | 114 (45.1) | 33 (46.5) | 0.83 |
| Betablockers, n (%) | 88 (34.6) | 23 (31.9) | 0.67 |
| Antiarrhythmics, n (%) | 9 (3.7) | 4 (5.8) | 0.43 |
| Antiplatelets and/or anticoagulants, n (%) | 86 (34) | 33 (45.8) | 0.07 |
| Dyspnea at hospital arrival, n (%) | 170 (65.1) | 50 (68.5) | 0.59 |
| Chest pain at hospital arrival, n (%) | 8 (3.1) | 3 (4.2) | 0.64 |
| Laboratory findings and vital signs at arrival | |||
| Hb (g/dL), median [IQR] | 13.5 [12.3–14.5] | 12.9 [11.1–14.8] | 0.15 |
| WBC (× 103/μL), median [IQR] | 6.42 [5.02–8.34] | 7.45 [5.36–11.42] | 0.01 |
| PLT (× 103/μL), median [IQR] | 180 [145–249] | 202 [138–278] | 0.37 |
| Potassium (mEq/L), median [IQR] | 3.96 [3.88–4.05] | 4.06 [3.8–4.48] | 0.07 |
| Creatinine (mg/dL), median [IQR] | 0.91 [0.73–1.14] | 1.09 [0.82–1.84] | < 0.001 |
| hsTnI (ng/L), median [IQR] | 11 [5–20] | 99.5 [69–186] | < 0.001 |
| P/F (PaO2/FiO2), median [IQR] | 284 [199–336] | 247 [120–324] | 0.017 |
| Systolic blood pressure (mmHg), median [IQR] | 133 [120–145] | 138.5 [123–150] | 0.036 |
| Diastolic blood pressure (mmHg), median [IQR] | 75 [70–85] | 77.5 [68–85] | 0.79 |
| ECG findingsa | |||
| Heart rate (bpm), median [IQR] | 88 [78–99] | 90 [76–106] | 0.41 |
| Atrial fibrillation, n (%) | 19 (11.5) | 6 (10.9) | 0.9 |
| Conduction disturbances, n (%) | 42 (25.5) | 26 (47.3) | 0.002 |
| PR (ms), median [IQR] | 159 [144–180] | 170 [146–191] | 0.14 |
| QTc (ms), median [IQR] | 422 [403–448] | 441 [407–469] | 0.049 |
| Repolarization abnormalities, n (%) | 23 (13.9) | 14 (25.5) | 0.048 |
| Outcomes | |||
| Death | 58 (21.8) | (38 (51.4) | < 0.001 |
| ICU or death | 78 (29.3) | 38 (51.4) | < 0.001 |
| Time for outcome | |||
| Death (days), median [IQR] | 9 [6–18] | 9 [5–15] | 0.76 |
| ICU or death (days), median [IQR] | 8 [5–11] | 7 [5–12] | 0.9 |
ACEi ACE inhibitors, ARB angiotensin II receptor blockers, ARNi angiotensin receptor neprilysin inhibitor, P/F PaO2/FiO2, Hb hemoglobin, WBC white blood cell, ICU intensive care unit
aECG data refer to 220 patients, 165 in the hs-TnI ≤ 47 subgroup and 55 in the hs-TnI > 47 subgroup
A hs-TnI value collected within 1 h after hospital admission was available in 340 patients (84%). The plasma levels of hs-TnI in the entire cohort was within normal range, with a 95th percentile of 189.5 pg/L. Elevated hs-TnI values were present in 74 (21.8%) patients at hospital arrival. The patients with elevated hs-TnI values at hospital arrival were significantly older (p < 0.001) and reported more frequently a history of cardiovascular disease (p = 0.006) compared to patients with normal hs-TnI values. Concerning laboratory findings and vital signs at hospital admission, patients with elevated hs-TnI values showed significantly higher WBC count (p = 0.01), higher creatinine values (p < 0.001) and lower P/F values (p = 0.017) as compared to patients with normal hs-TnI values. Finally, patients with elevated hs-TnI values presented more frequently conduction disturbances (p = 0.002), longer QTc interval (p = 0.049) or any ECG abnormality (p = 0.048) as compared to those with normal hs-TnI values, while atrial fibrillation was similar in the two subgroups.
Death and the composite outcome of death and/or ICU admission occurred in a significantly higher proportion of patients with elevated hs-TnI values at hospital admission as compared to patients with normal hs-TnI values (both p < 0.001). The median time from hospital admission to both the endpoints was similar in the two subgroups.
Clinical characteristics according to positivity to SARS-CoV-2 reverse‐transcriptase polymerase chain reaction (RT‐PCR) test (Supplementary Table 1)
Patients with a positive RT-PCR test for SARS-CoV-2 were more frequently males and more frequently had dyspnea at hospital arrival than patients with a negative test. WBC counts were lower, creatinine values were higher and QTc values were longer in patients with positive RT-PCR test. Both death and the combined outcome occurred more frequently in patients with a positive RT-PCR test for SARS-CoV-2 (p = 0.02 and p = 0.04, respectively).
Clinical characteristics according to outcome (Table 2)
Table 2.
Patient characteristics according to outcome
| Survivors (n = 281) | Non-survivors (n = 124) | p | Event-free survivors (n = 260) | Transferred to ICU or non-survivors (n = 145) | p | |
|---|---|---|---|---|---|---|
| Age (years), median [IQR] | 65 [55–75] | 76 [70–85] | < 0.001 | 66 [55–76] | 74 [69–82] | < 0.001 |
| Males, n (%) | 186 (66.2) | 92 (74.2) | 0.11 | 171 (65.8) | 107 (73.8) | 0.09 |
| Comorbidities | ||||||
| Cardiovascular disease, n (%) | 166 (60.4) | 102 (84.3) | < 0.001 | 151 (59.2) | 117 (83) | < 0.001 |
| Pulmonary disease, n (%) | 39 (14.4) | 19 (15.7) | 0.74 | 38 (15) | 20 (14.5) | 0.9 |
| Chronic renal disease, n (%) | 16 (5.9) | 22 (18.2) | < 0.001 | 15 (5.9) | 23 (16.7) | < 0.001 |
| Cancer, n (%) | 37 (13.8) | 10 (8.3) | 0.12 | 36 (14.2) | 11 (8) | 0.07 |
| Diabetes, n (%) | 43 (15.9) | 36 (29.8) | 0.002 | 41 (16.2) | 38 (27.5) | 0.008 |
| Theraphy at admission | ||||||
| ACEi or ARB or ARNi, n (%) | 114 (42.1) | 58 (50) | 0.15 | 105 (41.3) | 67 (50.4) | 0.09 |
| Betablockers, n (%) | 79 (29) | 56 (48.3) | < 0.001 | 71 (27.8) | 64 (48.1) | < 0.001 |
| Antiarrhythmics, n (%) | 8 (3.1) | 8 (7.1) | 0.08 | 7 (2.9) | 9 (6.9) | 0.07 |
| Antiplatelets and/or anticoagulants, n (%) | 72 (26.6) | 71 (60.7) | < 0.001 | 67 (26.4) | 76 (56.7) | < 0.001 |
| Dyspnea at hospital arrival, n (%) | 171 (62.2) | 93 (75.6) | 0.009 | 157 (61.8) | 107 (74.3) | 0.01 |
| Chest pain at hospital arrival, n (%) | 11 (68.7) | 5 (4.1) | 0.98 | 10 (4) | 6 (4.2) | 0.92 |
| Laboratory findings and vital signs at arrival | ||||||
| Hb (g/dL), median [IQR] | 13.5 [12.3–14.4] | 12.9 [11.8–14.5] | 0.13 | 13.5 [12.3–14.5] | 13 [12–14.3] | 0.19 |
| WBC (× 103/μL), median [IQR] | 6.3 [5–8] | 7.5 [5–10.3] | 0.01 | 6.2 [5–8] | 7.5 [5.2–10.2] | 0.006 |
| PLT (× 103/μL), median [IQR] | 187.5 [149–257] | 174 [129–227] | 0.03 | 188 [150–258] | 174 [130–230] | 0.03 |
| Potassium (mEq/L), median [IQR] | 3.9 [3.6–4.3] | 4.1 [3.8–4.5] | 0.006 | 3.9 [3.6–4.4] | 4.1 [3.8–4.4] | 0.02 |
| Creatinine (mg/dL), median [IQR] | 0.88 [0.72–1.1] | 1.09 [0.88–1.61] | < 0.001 | 0.87 [0.71–1.1] | 1.05 [0.86–1.55] | < 0.001 |
| hsTnI (ng/L), median [IQR] | 11 [5–26] | 31 [15–80] | < 0.001 | 11 [5–27] | 27 [12–62] | < 0.001 |
| P/F (PaO2/FiO2), median [IQR] | 296 [220–351] | 231 [117–316] | < 0.001 | 297 [233–347] | 230 [120–330] | < 0.001 |
| Systolic blood pressure (mmHg), median [IQR] | 135 [120–145] | 130 [120–149] | 0.68 | 134 [120–145] | 130 [120–147] | 0.73 |
| Diastolic blood pressure (mmHg), median [IQR] | 80 [70–85] | 73 [65–80] | 0.003 | 80 [70–86] | 74 [65–80] | 0.002 |
| ECG findings | ||||||
| Heart rate (bpm), median [IQR] | 88 [77–99] | 88 [78–100] | 0.7 | 88 [77–99] | 87 [77–100] | 0.94 |
| Atrial fibrillation, n (%) | 12 (6.9) | 17 (20.2) | 0.002 | 12 (7.1) | 17 (19.3) | 0.003 |
| Conduction disturbances, n (%) | 51 (29) | 31 (36) | 0.25 | 49 (28.5) | 33 (36.7) | 0.18 |
| PR (ms), median [IQR] | 154 [142–172] | 175 [157–190] | < 0.001 | 154 [142–172] | 173 [157–190] | < 0.001 |
| QTc (ms), median [IQR] | 425 [406–450] | 436 [410–460] | 0.12 | 426 [406–450] | 434 [411–460] | 0.12 |
| Repolarization abnormalities, n (%) | 28 (15.9) | 20 (23.3) | 0.15 | 28 (16.3) | 20 (22.2) | 0.24 |
Abbreviations as in Table 1
Patients who died in hospital were significantly older (p < 0.001), had more chronic comorbidities such as cardiovascular disease (p < 0.001), chronic renal disease (p < 0.001) and diabetes (p = 0.002). Patients dying in hospital were more likely to receive drugs such as betablockers (p < 0.001) and antiplatelets and/or anticoagulants (p < 0.001), whilst there were no significant differences in terms of ACEi/ARB/ARNi assumption (p = 0.15). Dyspnea at hospital arrival was more frequently reported by patients who died during the hospitalization (p = 0.009), chest pain was equally present (p = 0.98). WBC count (p = 0.01), platelet count (p = 0.03), creatinine values (p < 0.001) and hsTnI values (p < 0.001) were higher and P/F value was lower (p < 0.001) in patients dying in hospital; in addition, it must be emphasized that the median values of hs-TnI were within normal range in non-survivors. Finally, atrial fibrillation was more frequently present in patients who subsequently died in hospital (p = 0.002).
When considering in-hospital mortality and/or ICU admission (Table 2—right), patients with the worse prognosis were older (p < 0.001) and more frequently had previous history of cardiovascular disease (p < 0.001), chronic renal disease (p < 0.001) and diabetes (p = 0.008). At hospital arrival, dyspnea was highly prevalent (p = 0.01), WBC count (p = 0.006), platelet count (p = 0.03), creatinine values (p < 0.001) and hsTnI values (p < 0.001) were higher in patients who were later transferred to ICU or died during hospital stay, whilst P/F values were lower (p < 0.001); importantly, the median values of hs-TnI were within normal range in patients who died during hospital stay or were transferred to ICU. Atrial fibrillation as presenting rhythm was more frequent in those patients dying or ending up at the ICU (p = 0.003).
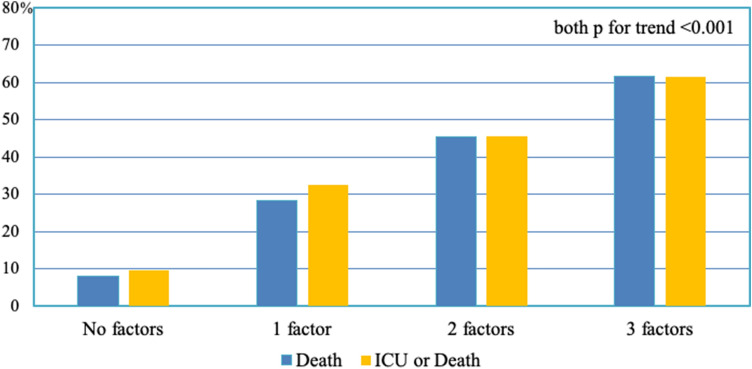
The analysis of patients for whom both hs-TnI and ECG were available showed that the outcome progressively worsened when the number of parameters that we included in the definition of cardiac involvement increased (Fig. 1). This correlation was valid considering both in-hospital death (from 7.9% in patients without cardiac involvement to 61.5% in patients with all three parameters indicative of cardiac involvement) and the composite outcome of death and/or ICU admission (from 9.5% in patients without cardiac involvement to 61.5% in patients with high-grade cardiac involvement).
Fig. 1.
Death rate and ICU or death rate according to the presence of hsTNI plasma levels and/or any electrocardiographic abnormality and/or history of cardiovascular disease
Predictors of death and of the composite outcome
Logistic regression analysis showed that elevated hs-TnI values (p = < 0.001), presence of ECG abnormalities and/or AF at ECG (p = 0.01) and history of cardiovascular disease (p < 0.001) were significant predictors of in-hospital death at univariate analysis, alongside older age (p < 0.001) and P/F less than 200 (p < 0.001). However, only age (p < 0.001), elevated hs-TnI values (p = 0.009) and P/F (p = 0.014) were independent predictors at multivariable analysis (Table 3—upper part). There was no interaction between the result of RT-PCR test for SARS-CoV-2 and death (p = 0.87).
Table 3.
Univariable and multivariable predictors of death or of the combination of death and/or ICU admittance
| Univariable analysis | p | Multivariable analysis | p | |
|---|---|---|---|---|
| Death | ||||
| Age | 1.09 (95% CI 1.07–1.12) | < 0.001 | 1.09 (95% CI 1.06–1.14) | < 0.001 |
| P/F < 200 | 2.89 (95% CI 1.76–4.71) | < 0.001 | 2.53 (95% CI 1.21–5.31) | 0.014 |
| Elevated hs-TnI values | 3.78 (95% CI 2.2–6.5) | < 0.001 | 2.74 (95% CI 1.28–5.84) | 0.009 |
| ECG abnormalities and/or AF at ECG | 2.11 (95% CI 1.19–3.74) | 0.010 | – | |
| History of cardiovascular disease | 3.52 (95% CI 2.04–6.08) | < 0.001 | – | |
| ICU or death | ||||
| Age | 1.06 (95% CI 1.04–1.08) | < 0.001 | 1.09 (95% CI 1.05–1.13) | < 0.001 |
| P/F < 200 | 3.49 (95% CI 2.15–5.65) | < 0.001 | 3.11 (95% CI 1.51–6.4) | 0.002 |
| Elevated hs-TnI values | 2.54 (95% CI 1.5–4.31) | < 0.001 | 2.27 (95% CI 1.07–4.8) | 0.032 |
| ECG abnormalities and/or AF at ECG | 1.92 (95% CI 1.09–3.38) | 0.025 | – | |
| History of cardiovascular disease | 3.36 (95% CI 2.03–5.57) | < 0.001 | – | |
Abbreviations as in Table 1
Considering the combined outcome of death and/or transfer to ICU, elevated hs-TnI values (< 0.001), presence of ECG abnormalities and/or AF at ECG (p = 0.025), history of cardiovascular disease (p < 0.001), older age (p < 0.001) and P/F less than 200 at hospital arrival (p < 0.001) were predictor at univariable analysis but, again, only age (p < 0.001), P/F less than 200 (p = 0.001) and elevated hs-TnI values (p = 0.032), were independent predictors at multivariable analysis (Table 3—lower part). There was no interaction between the result of RT-PCR test for SARS-CoV-2 and the combined outcome (p = 0.94).
Discussion
This study reports the first large case series of consecutively hospitalized patients with COVID-19 in an academic hospital in Lombardy, which is one of the most affected Italian and worldwide regions by the COVID-19 epidemic, both in terms of number of patients needing admission and in terms of fatality rate. The main finding is that, in this cohort, in-hospital mortality was associated with older age, respiratory failure and elevated plasma levels of hs-TnI whereas cardiovascular comorbidities were not an independent risk factor at multivariable analysis. Importantly, plasma levels of hs-TnI were, on average, within normal range in non-survivors.
Cardiac involvement and outcome in COVID-19
Since the onset of the pandemic, a growing body of evidence has shown that a substantial proportion of COVID-19 patients may have cardiovascular complications, up to cardiac arrest [18]. However, it is still a matter of debate whether the presence of elevated hs-TnI plasma levels and/or of cardiovascular comorbid conditions at hospital presentation poses an independent risk in such patients or whether the risk is mediated by other factors. In the present series, cardiac involvement upon admission was associated with a higher risk of events during the subsequent hospital stay at univariable analysis. The mortality rate and the rate of the combined endpoint were more than fivefold greater in patients with elevated hs-TnI plasma levels, electrocardiographic abnormality and history of cardiovascular disease as compared to those without any of these parameters. However, when age and respiratory failure at hospital admission were analyzed together with cardiac involvement in a multivariable analysis, only older age, a P/F ratio < 200 and elevated hs-TnI plasma levels were significant predictor of death and of the combined outcome. Importantly, the hs-TnI plasma levels were generally low and in the normal range even in non-surviving patients. This datum, together with the observation that patients with elevated hs-TnI values at admission had laboratory and respiratory function data indicative of a more severe systemic infection, would support hypothesis that the increase of hs-TnI levels in COVID-19 patients is mainly the consequence of a more severe systemic disease rather being indicative of primary cardiac injury, as already described in the literature [19].
Among the parameters which did not result in statistically significant predictors of events are home therapy with angiotensin converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARB). Concerns about ACEI/ARB have been raised since angiotensin converting enzyme 2 (ACE2) is a potential target for COVID-19 infection. However, although evidence regarding the use of ACEI/ ARB in patients with COVID-19 infection is still emerging, our findings seem to be consistent with the latest studies published on this topic [20, 21].
Mortality rate
It is important to observe that the fatality rate according to cardiovascular involvement in the present cohort is substantially similar to what has been reported in previous studies, ranging from below 10% for subgroups without cardiac injury to greater than 50% for patients with cardiac injury or history of cardiovascular disease. Nonetheless, in the present population, the overall hospital mortality rate was higher compared to previous series [4–7, 22, 23]. The reasons for this may be different. First, the median age in the present cohort of patients is 70 years, greater than in previously reported series, and older age has been shown to be crucial in all studies published so far. Another contributing factor could be the high prevalence of comorbidities in particular when compared to Asian populations. Elderly patients with multimorbidity are usually frail and more likely to have worse outcomes, as already shown in different hospital settings [24]. Moreover, the present cohort was characterized by a substantial pulmonary involvement, in particular considering that the most critical patients, i.e. those who were directly admitted to the ICU, were not included in the present series; this is likely to be a crucial determinant for the dismal prognosis of the present cohort in Pavia. The clinical implication is that COVID-19 patients should possibly be treated earlier, rather than delaying hospital arrival until severe respiratory symptoms have developed. Finally, to the best of our knowledge, the present population is the first one composed almost exclusively of Caucasian patients; it cannot be excluded that ethnicity may play a role in fatality rate and further studies should be focused to address this issue.
Limitations
The main limitation of the present study is its retrospective design; in particular, it lacks echocardiographic imaging of the patients and an in-depth analysis of markers of inflammation such as interleukin-6 which are not routinely performed in the Emergency Department. In addition, how treatment during hospitalization has influenced the outcome has not been analyzed but it was out of the scope of the study. The number of patients enrolled, although quite large for a single center and similar to those included in previous publications, is still too low to allow a differentiation among several types of heart diseases or of ECG abnormalities. This might probably be performed in future metanalysis. We found differences between the baseline characteristics of patients with a positive RT-PCR test for SARS-CoV-2 and those of patients with a negative test. There can be different reasons for these findings, which are out of the scope of our study, but which deserve to be further investigated in future studies.
Finally, considering the retrospective design of the study, unmeasured confounding may be present.
Conclusions
Myocardial injury at presentation was associated with poor prognosis in COVID-19 patients, but, even in a population of patients who did not require invasive ventilation at hospital admission, mortality was mainly driven by older age and respiratory failure.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Stefano Ghio, Luigi Oltrona Visconti, Enrico Baldi, Marco Ferlini, Massimiliano Gnecchi, Alessandra Greco, Giulia Magrini, Laura Scelsi, Rossana Totaro, Alessandro Vicentini, Mauro Acquaro, Michela Coccia, Sabato D'Amore, Simonluca Digiacomo Davide Foglia, Francesco Jeva, Lucrezia Masiello, Claudio Montalto, Martina Moschella, Laura Pezza, Stefano Perlini, Claudia Alfano, Marco Bonzano, Federica Briganti, Giuseppe Crescenzi, Anna Giulia Falchi, Elena Maggi, Roberta Guarnone, Barbara Guglielmana, Ilaria Francesca Martino, Maria Serena Pioli Di Marco, Pietro Pettenazza, Federica Quaglia, Anna Sabena, Francesco Salinaro, Francesco Speciale, Ilaria Zunino, Giulia Sturniolo, Federico Bracchi, Elena Lago, Angelo Corsico, Davide Piloni, Giulia Accordino, Cecilia Burattini, Antonio Di Sabatino, Marco Vincenzo Lenti, Ivan Pellegrino, Simone Soriano, Giovanni Santacroce, Alessandro Parodi, Federica Borrelli de Andreis, Raffaele Bruno, Angela Di Matteo, Elena Maria Seminari, Valentina Zuccaro, Francesco Moioli, Guido Tavazzi, Valentino Dammassi, Riccardo Albertini, and Catherine Klersy.
Investigators and co-authors of the San Matteo COVID Cardiac Injury Task Force are as follows: Division of Cardiology: Stefano Ghio, Luigi Oltrona Visconti, Enrico Baldi, Marco Ferlini, Massimiliano Gnecchi, Alessandra Greco, Giulia Magrini, Laura Scelsi, Rossana Totaro, Alessandro Vicentini, Mauro Acquaro, Michela Coccia, Sabato D'Amore, Simonluca Digiacomo Davide Foglia, Francesco Jeva, Lucrezia Masiello, Claudio Montalto, Martina Moschella, Laura Pezza. Emergency Department: Stefano Perlini, Claudia Alfano, Marco Bonzano, Federica Briganti, Giuseppe Crescenzi, Anna Giulia Falchi, Elena Maggi, Roberta Guarnone, Barbara Guglielmana, Ilaria Francesca Martino, Maria Serena Pioli Di Marco, Pietro Pettenazza, Federica Quaglia, Anna Sabena, Francesco Salinaro, Francesco Speciale, Ilaria Zunino, Giulia Sturniolo, Federico Bracchi, Elena Lago. Division of Respiratory Diseases: Angelo Corsico, Davide Piloni, Giulia Accordino, Cecilia Burattini. Division of Internal Medicine: Antonio Di Sabatino, Marco Vincenzo Lenti, Ivan Pellegrino, Simone Soriano, Giovanni Santacroce, Alessandro Parodi, Federica Borrelli de Andreis. Division of Infectious Disease: Raffaele Bruno, Angela Di Matteo, Elena Maria Seminari, Valentina Zuccaro. Intensive Care Unit: Francesco Moioli, Guido Tavazzi, Valentino Dammassi. Clinical Chemistry Laboratory: Riccardo Albertini. Service of Clinical Epidemiology and Biometry: Catherine Klersy.
Author contributions
SG, EB, LOV and SP planned the study; SG and EB drafted the manuscript; ML, AD, ADM, VZ, DP, AC, MG, FS, SG, EB, LOV, and SP gave substantial contributions to acquisition of data, analysis and interpretation of data, revised the manuscript critically for important intellectual content and provided final approval of the version to be published. All the authors read and approved the final version. SG is responsible for the overall content as guarantor. All above authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Funding
The study was not funded.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights
All procedures performed in studies involving human participants were in according with the ethical standard.
Informed consent
The review board of the Fondazione Policlinico San Matteo approved the publication of anonymized case series of COVID-19 patients using data collected for routine clinical practice and waived the requirement for a specific informed consent.
Footnotes
The original online version of the article was revised due to the incorrect tagging of author name ‘Federica Borrelli de Andreis’ in the xml.
The members of San Matteo COVID Cardiac Injury Task Force are given in Acknowledgements.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Luigi Oltrona Visconti and Stefano Perlini equally contributed as co-last authors.
Change history
1/21/2021
In the original publication of the article, the first name and last name of the author
Contributor Information
Stefano Ghio, Email: s.ghio@smatteo.pv.it.
San Matteo COVID Cardiac Injury Task Force:
Stefano Ghio, Luigi Oltrona Visconti, Enrico Baldi, Marco Ferlini, Massimiliano Gnecchi, Alessandra Greco, Giulia Magrini, Laura Scelsi, Rossana Totaro, Alessandro Vicentini, Mauro Acquaro, Michela Coccia, Sabato D’Amore, Simonluca Digiacomo, Davide Foglia, Francesco Jeva, Lucrezia Masiello, Claudio Montalto, Martina Moschella, Laura Pezza, Stefano Perlini, Claudia Alfano, Marco Bonzano, Federica Briganti, Giuseppe Crescenzi, Anna iulia Falchi, Elena Maggi, Roberta Guarnone, Barbara Guglielmana, Ilaria Francesca Martino, Maria Serena Pioli Di Marco, Pietro Pettenazza, Federica Quaglia, Anna Sabena, Francesco Salinaro, Francesco Speciale, Ilaria Zunino, Giulia Sturniolo, Federico Bracchi, Elena Lago, Angelo Corsico, Davide Piloni, Giulia Accordino, Cecilia Burattini, Antonio Di Sabatino, Marco Vincenzo Lenti, Ivan Pellegrino, Simone Soriano, Giovanni Santacroce, Alessandro Parodi, Federica Borrelli de Andreis, Raffaele Bruno, Angela Di Matteo, Elena Maria Seminari, Valentina Zuccaro, Francesco Moioli, Guido Tavazzi, Valentino Dammassi, Riccardo Albertini, and Catherine Klersy
References
- 1.Zhu N, Zhang D, Wang W, China Novel Coronavirus Investigating and Research Team et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bangalore S, Sharma A, Slotwiner A, et al. ST-segment elevation in patients with COVID-19—a case series. N Engl J Med. 2020;382:2478–2480. doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gnecchi M, Moretti F, Bassi EM, et al. Myocarditis in a 16-year-old boy positive for SARS-CoV-2. Lancet. 2020;395(10242):e116. doi: 10.1016/S0140-6736(20)31307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1–8. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei JF, Huang FY, Xiong TY, et al. Acute myocardial injury is common in patients with COVID-19 and impairs their prognosis. Heart. 2020;106:1154–1159. doi: 10.1136/heartjnl-2020-317007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inciardi RM, Adamo M, Lupi L, Cani DS, Di Pasquale M, Tomasoni D, Italia L, Zaccone G, Tedino C, Fabbricatore D, Curnis A, Faggiano P, Gorga E, Lombardi CM, Milesi G, Vizzardi E, Volpini M, Nodari S, Specchia C, Maroldi R, Bezzi M, Metra M. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur Heart J. 2020;41:1821–1829. doi: 10.1093/eurheartj/ehaa388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis. 2020 doi: 10.1016/j.pcad.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, Yang Y, Zhang C, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng R, Leedy D. COVID-19 and acute myocardial injury: the heart of the matter or an innocent bystander? Heart. 2020;106:1122–1124. doi: 10.1136/heartjnl-2020-317025. [DOI] [PubMed] [Google Scholar]
- 15.Song CY, Yang DG, Lu YQ. A COVID-19 patient with seven consecutive false-negative rRT-PCR results from sputum specimens. Intern Emerg Med. 2020;2:1–4. doi: 10.1007/s11739-020-02423-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sciacqua A, Pujia R, Arturi F, Hribal ML, Montalcini T. COVID-19 and elderly: beyond the respiratory drama. Intern Emerg Med. 2020 doi: 10.1007/s11739-020-02424-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kavsak PA, Worster A, Hill SA, et al. Evaluation of the Siemens ADVIA Centaur high-sensitivity cardiac troponin I assay in serum. Clin Chim Acta. 2018;487:216–221. doi: 10.1016/j.cca.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Baldi E, Sechi GM, Mare C, et al. COVID-19 kills at home: the close relationship between the epidemic and the increase of out-of-hospital cardiac arrests. Eur Heart J. 2020 doi: 10.1093/eurheartj/ehaa508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korff S, Katus HA, Giannitsis E. Differential diagnosis of elevated troponins. Heart. 2006;92:987–993. doi: 10.1136/hrt.2005.071282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mancia G, Rea F, Ludergnani M, et al. Renin-angiotensin-aldosterone system blockers and the risk of Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albini A, Di Guardo G, Noonan DM, Lombardo M. The SARS-CoV-2 receptor, ACE-2, is expressed on many different cell types: implications for ACE-inhibitor- and angiotensin II receptor blocker-based cardiovascular therapies. Intern Emerg Med. 2020 doi: 10.1007/s11739-020-02364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richardson S, Hirsch JS, Narasimhan M, Northwell COVID-19 Research Consortium et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu JT, Leung K, Bushman M, et al. Estimating clinical severity of COVID-19 from the transmission dynamics in Wuhan, China. Nat Med. 2020;26:506–510. doi: 10.1038/s41591-020-0822-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corazza GR, Formagnana P, Lenti MV. Bringing complexity into clinical practice: an internistic approach. Eur J Intern Med. 2019;61:9–14. doi: 10.1016/j.ejim.2018.11.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.