Abstract
Background
For cardiologists, management of acute chest pain continues to be a challenge. Physicians struggle to avoid unnecessary admissions and at the same time not to miss high-risk patients needing urgent intervention. Therefore, diagnostic strategies focus on identifying patients in whom an acute coronary syndrome can be safely ruled out based on findings from history, physical examination, and early cardiac marker measurement. The HEART score, a clinical prediction rule, was developed to provide the clinician with a simple and reliable predictor of cardiac risk.
Aim
This study aimed to investigate the role of neutrophil/lymphocyte ratio (NLR) and platelet/lymphocyte ratio (PLR) as independent laboratory biomarkers when associated with the HEART risk score.
Method
A cross-sectional study of 120 patients who attended the emergency department with acute chest pain. NLR and PLR were both measured. In addition, the HEART score was the valid instrument used in evaluating and risk stratifying patients into low-, intermediate-, and high-risk group.
Results
There was a positive correlation between the HEART score and the mean PLR and NLR (p = 0.000*). PLR and NLR were found to be significantly higher in the high-risk HEART score group (p = 0.05 and 0.0001*, respectively). A PLR of 115.5 and above had a sensitivity of 73% and specificity of 78%, while an NLR of 3.95 and above had a sensitivity of 75% and specificity of 86% to detect high-risk HEART score patients.
Conclusion
PLR and NLR proved to be a useful tool to identify high-risk patients when validated against the HEART score.
Keywords: Platelet/lymphocyte ratio, Neutrophil/lymphocyte ratio, HEART score, Non-ST elevation acute coronary syndrome
Introduction
Acute chest pain is one of the most common reasons for emergency department (ED) attendance and admission to hospitals. Non-ST elevation acute coronary syndrome (NSTE-ACS) is an important cause of chest pain, and accurate diagnosis and risk stratification in the ED must be a clinical priority. Clinical guidelines recommend an early invasive strategy in higher-risk NSTE-ACS [1]. One of the popular risk stratification scores implemented in the ED is the HEART score. It was developed in the Netherlands in 2008 by Six, Backus, and Kelder as a rapid risk stratification tool for patients with chest pain according to their short-term risk MACE (defined as acute myocardial infarction, need for percutaneous coronary intervention or coronary artery bypass graft, and death within 6 weeks) to help identify low-risk patients, suitable for earlier ED discharge within 30 days of index ED visit [2]. This decision tool is considered valuable for several reasons including its ease of application, ready availability of the variables under consideration, the focus on short-term outcome, appropriateness for ED management, and the identification of three discrete subpopulations (low-, moderate-, and high-risk) of ED chest pain patients suspected of ACS.
Today, the neutrophil/lymphocyte count ratio (NLR) is accepted as a parameter that shows the adverse effects of both high neutrophil levels, which reflect acute inflammation, and low lymphocyte levels, which reflect physiological stress [3]. The inflammatory reaction does not only show a response to tissue damage in ACS, but also results in a poor clinical course [4, 5].
Platelets are a source of inflammatory mediators, and they are being influenced by contact with artery surface. The activated platelets release the mediators; then, platelet adhesion and its atherothrombotic potential can lead to the release of mediators, the progression of inflammatory process, and the propagation of intracoronary thrombus predisposing to thrombotic events [4, 6], so the platelet/lymphocyte (PLR) ratio has been shown to be correlated with ACS.
In countries with limited resources, both NLR and PLR are relatively easy, quick, and inexpensive tools that have the potential for both diagnosis and risk stratification of patients admitted with acute chest pain [7].
Patients and Methods
This cross-sectional study included 120 patients who were admitted with chest pain to the ED at Zagazig University Hospitals between July 2019 and December 2019.
Patients were included if they met the following criteria: (a) patients had a diagnosis of non-ST elevation acute myocardial infarction (NSTEMI) or unstable angina (UA) that was objectively confirmed through presence of ischemic symptoms adjudicated with ECG changes consistent with ischemia; (b) elevated cardiac enzyme levels (hsTn and CKMB) were used to differentiate patients with NSTEMI from those with UA; (c) information on neutrophils, lymphocytes and platelets was provided and/or NLR and PLR were provided.
All patients were risk stratified according to the HEART score into low, intermediate, and high-risk group (Table 1) [2].
Table 1.
HEART score for chest pain patients in the emergency department
| Variable | Score of 0 | Score of 1 | Score of 2 |
|---|---|---|---|
| History | Nonspecific history for ACS, a history that is not consistent with chest pain concerning for ACS | Mixed historic elements, a history that contains traditional and non-traditional elements of typical ACS presentation | Specific history for ACS, a history with traditional features of ACS |
| Electrocardiogram | Entirely normal ECG | Abnormal ECG, with repolarization abnormalitiesa yet lacking significant ST depression | Abnormal ECG, with significant ST deviation (depression ± elevation), either new or not known to be old (i.e., no prior ECG available for comparison) |
| Age, years | Age less than 45 years | Age between 45 and 64 years | Age 65 years or older |
| Risk factorsb | No risk factors | 1-2 risk factors | Three or more risk factors OR documented cardiac or systemic atherosclerotic vascular diseasec |
| Troponind | Troponin <discriminative level ± AccuTroponin I <0.04 ng/mL | Troponin elevated 1-3 times discriminative level ± AccuTroponin I 0.04-0.12 ng/mL | Troponin elevated <3 times discriminative level ± AccuTroponin I <0.12 ng/mL |
Total HEART score: risk category and recommended management strategy. 0–3: low risk, potential candidate for early discharge. 4–6: moderate risk, potential candidate for observation and further evaluation. 7–10: high risk, candidate for urgent or emergent intervention.
BBB, LVH, digoxin effect, implanted right-ventricular pacemaker, past Ml, iunchanged repolarization abnormalities.
DM, tobacco smoker, HTN, hypercholesterolemia, obesity, ifamily history of CAD.
Peripheral arterial disease, Ml, past coronary revascularization procedure, istroke.
It is recommended to use the local hospital standards for troponin abnormality determination.
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and has been approved by our institution's ethics committee. A written informed consent was taken from all enrolled patients.
Biochemical Evaluation
Complete blood counts (CBC) with automated differential counts were performed for all patients. CBC analysis was performed in samples anticoagulated with EDTA within 30 min after collection, using an automated cell counter (Sysmex KX21-N, Kobe, Japan). The NLR was calculated by dividing the absolute neutrophil count by the absolute lymphocyte count (ALC); likewise, PLR was calculated by dividing the absolute platelet count by ALC.
Exclusion Criteria
Patients who had a clinically active infection, malignancy, hematological disorders including all types of anemia (hereditary and acquired) and hematological malignancies, patients suffering from intoxication, severe liver disease, active or chronic autoimmune disease, patients on steroid therapy or chemotherapy, and patients with a history of trauma or surgery within 10 days prior to admission were excluded.
Statistical Analysis
All analyses were performed using the SPSS for Windows 20.0 software package. Continuous variables were presented as mean and standard deviation. Categorical variables were presented as percentages. All data were tested for normal distribution with the Kolmogorov-Smirnov test. Differences between frequencies (qualitative variables) and percentages in groups were compared using the χ2 test. Differences between parametric quantitative independent groups were assessed by the t test, and p value was set at <0.05 for significant results and <0.001 for highly significant results. The optimal cut-off values as well as sensitivity and specificity were determined according to the receiver operating characteristic (ROC) analysis. The best cut-off values were expressed using the Youden index. The area under the ROC (AUROC) curve was also calculated.
Results
Baseline characteristics of the study population including CAD risk factors, mean CBC indices, and ECG changes are shown in Table 2; the mean age of our population was 62.7 ± 10.53 years; our study included 86 (71%) males and 34 (28%) females, 76 (63%) patients were diabetic, 64 (53%) were smokers, and 64 (53%) were hypertensive. Of the 120 patients, 54 (45%) were admitted with NSTEMI and 66 (55%) with UA.
Table 2.
Demographic data of the study group
| Age, mean ± SD, years Males, n (%) Females, n (%) DM, n (%) HTN, n (%) Smokers, n (%) |
62.7±10.53 86 (71) 34 (28) 76 (63) 64 (53) 64 (53) |
| Type of ACS NSTEMI, n (%) UA, n (%) |
54 (45) 66 (55) |
| Admission ECG No ECG changes, n (%) Nonspecific repolarization disturbance on ECG, n (%) Significant ST deviation on ECG, n (%) |
67 (56) 6 (5) 46 (38) |
| RBC count, x10<upper>6</upper>/µL Mean ± SD Range Median |
4.6±1.23 3.0–11.4 4.5 |
| RDW, % Mean ± SD Range Median |
14.1±1.5 12.2–19.9 13.8 |
| Platelet count, *103/µL Mean ± SD Range Median |
235.75±72.38 116–521 219.5 |
| MPV, fL Mean ± SD Range Median |
10.42±1.26 7.4–13.7 10.4 |
| WBC count, ×103/µL Mean ± S Range Median |
8.99±3.98 2.7–29 8.5 |
| Neutrophil count, ×103/µL Mean ± SD Range Median |
6.36±2.7 1.6–15 6.36 |
| Lymphocyte count, ×103/µL Mean ± SD Range Median |
2.17±1.13 0.5–5.0 2.0 |
| NLR Mean ± SD Median Range |
4.42±4.64 2.85 0.7–30 |
| PLR Mean ± SD Median Range |
149.45±106.8 104.5 38–534 |
ACS, acute coronary syndrome; NSTEMI, non-ST elevation acute myocardial infarction; UA, unstable angina; RBC, red blood cell; WBC, white blood cell; MPV, mean platelet volume; RDW, red cell distribution width; PLR, platelet/lymphocyte ratio; NLR, neutrophil/lymphocyte ratio.
Significant ECG changes were seen in 46 patients (38%) of our study population, 67 patients (56%) had no ECG changes on admission while 6 patients (5%) had non-specific repolarization abnormality on ECG.
The means of the CBC parameters and deduced indices were compared between low, intermediate, and high-risk HEART score patients as shown in Table 3. The mean PLR, NLR, WBC count, and platelet count were highest in the high-risk group, while the mean lymphocyte count was highest in the low-risk group. The results were statistically significant (p = 0.05, 0.0001, 0.000, 0.005, 0.0001, 0.0001, respectively).
Table 3.
Comparison of the mean CBC values between low-, intermediate-, and high-risk HEART score patients
| Low risk (n = 18) | Intermediate risk (n = 54) | High risk (n = 48) | p value | F value | |
|---|---|---|---|---|---|
| Mean PLR | 77.6667 | 110.2593 | 220.4583 | 0.05 | 25.69 |
| Mean RBC count, 106/pL | 5.0611 | 4.5556 | 4.525 | 0.256 | 1.38 |
| Mean MPV, fL | 9.8 | 10.3 | 10.8 | 0.15 | 4.4 |
| Mean RDW, % | 13.5333 | 13.9 | 14.4583 | 0.06 | 2.9 |
| Mean NLR | 1.9783 | 2.8611 | 7.092 | 0.0001* | 16.9 |
| Mean WBC count, ×103/pL | 7.4 | 7.8 | 10.9 | 0.000* | 10.7 |
| Mean platelet count, ×103/pL | 226 | 216 | 261 | 0.005 | 5.3 |
| Mean neutrophil count, ×l03/pL | 5.6 | 5.2 | 7.8 | 0.0001* | 16 |
| Mean lymphocytes, ×l03/pL | 3 | 2.5 | 1.5 | 0.0001* | 24 |
RBC, red blood cell; WBC, white blood cell; MPV, mean platelet volume; RDW, red cell distribution width; PLR, platelet/lymphocyte ratio; NLR, neutrophil/lymphocyte ratio.
Table 4 shows a significant positive correlation between HEART score and both PLR and NLR.
Table 4.
Correlation between PLR and NLR and the HEART score
| HEART score |
||
|---|---|---|
| r | p value (2-tailed) | |
| PLR | 0.539 | 0.000** |
| NLR | 0.452 | 0.000** |
PLR, platelet/lymphocyte ratio; NLR, neutrophil/lymphocyte ratio.
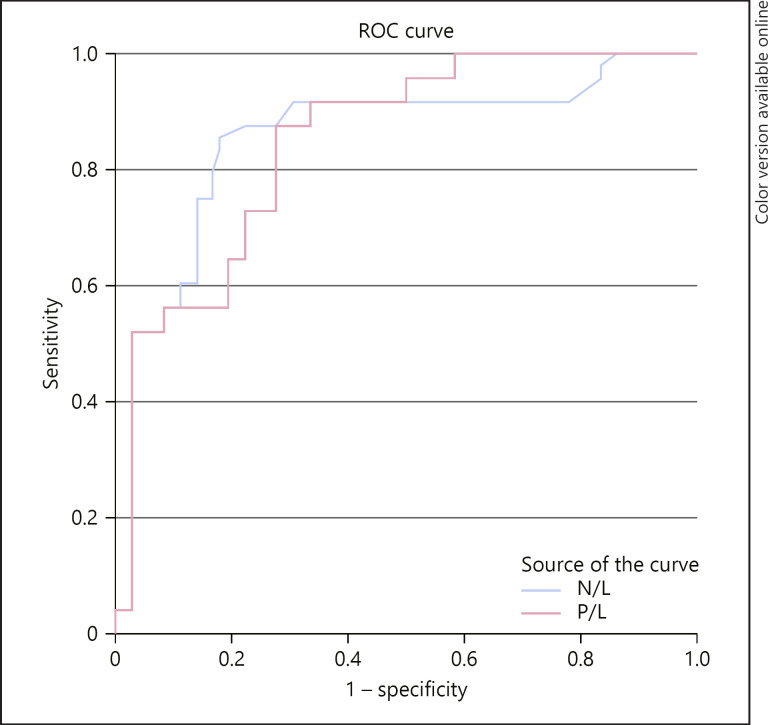
A receiver operating curve shown in Figure 1 and Table 5 was constructed to determine sensitivity and specificity of PLR and NLR in discriminating high-risk HEART score patients. An NLR of 3.95 and above had a sensitivity of 75% and specificity of 86%, while a PLR of 115.5 and above had a sensitivity of 73% and specificity of 78%.
Fig. 1.
Receiver operating curve analysis showing the area under the curve for the PLR and NLR and their sensitivity and specificity in diagnosing high-risk HEART score patients. Diagonal segments are produced by ties.
Table 5.
Receiver operating curve analysis showing the area under the curve for the PLR and NLR and their sensitivity and specificity in diagnosing high-risk HEART score patients
| Cut-off value | AUC | Sensitivity | Specificity | |
|---|---|---|---|---|
| PLR | 115.5 | 0.849 | 73% | 78% |
| NLR | 3.95 | 0.855 | 75% | 86% |
PLR, platelet/lymphocyte ratio; NLR, neutrophil/lymphocyte ratio; AUC, area under the curve.
Discussion
NSTE-ACS is an important cause of chest pain. Risk stratification in the ED must be a clinical priority. Clinical guidelines recommend an early invasive strategy in higher-risk NSTE-ACS [1]. HEART score is a validated rapid risk stratification tool in many countries for patients with chest pain, and thus helps physicians identify high-risk patients who need urgent intervention.
In our study, we found a positive correlation between the HEART score and the mean PLR and NLR of patients admitted with NSTE-ACS. PLR and NLR were both found to be significantly higher in the high-risk HEART score group. A PLR of 115.5 and above had a sensitivity of 73% and specificity of 78%, while an NLR of 3.95 and above had a sensitivity of 75% and specificity of 86% to detect high-risk HEART score patients.
Leukocytes play a key role in the pathophysiology of ACS, given their effect on the instability of atherosclerotic plaques. In the initial stage, leukocytes permeate endothelial cells and become activated when reaching the tunica intima. They induce the formation of microvascularity there and, as a result, make plaques more susceptible to rupture [8].
In a study by Sabatine et al. [9], the elevated WBC count was found to be a relevant death risk factor during the first 30 days and 6 months following the myocardial infarction among patients with ACS (UA, NSTEMI). Furthermore, the elevated level of WBC was also related to a more advanced CAD as well as epicardial and myocardial perfusion disorders.
Several studies have found polymorph nuclear cells (PMN) in coronary thrombi in patients with myocardial infarction who were undergoing primary percutaneous coronary intervention. PMN release neutrophil extracellular traps (NETs) at the culprit lesion site. NETs are highly proinflammatory and prothrombotic fibers which can entrap leucocytes and propagate thrombosis. NETs proved to be correlated negatively with ST-segment resolution and positively with infarct size [10]. By contrast, lymphocytes, especially B2 and T helper, as the elements of the adaptive immune system, could mute and limit inflammation. The lower lymphocyte counts were associated with atherosclerosis progression and adverse clinical outcomes in patients with heart failure and ACS [11].
The true prognostic performance of the HEART score was studied in a retrospective multicenter study published in 2010; the study condensed the findings from many external validation studies. They found a HEART score above the low-risk threshold (≥4) had high sensitivity (95.9%) for short-term MACE (short-term mortality (95.0%) and MI (97.5%), while a high-risk HEART score (≥7) had high specificity (95.0%) for short-term MACE. This work supports the utilization of the HEART for risk stratification of patients presenting with chest pain [12].
In our study, mean PLR and NLR were significantly higher in the high-risk HEART score group compared to the other two groups; furthermore, both were positively correlated with the HEART score.
ROC curve analysis revealed that a PLR of 115.5 and above had a sensitivity of 73% and specificity of 78% to diagnose high-risk patients according to the HEART score. Similarly, an NLR of 3.95 and above had a sensitivity of 75% and specificity of 86% to detect high-risk HEART score patients. However, the sensitivity and specificity of both CBC indices were lower when discriminating between low- and intermediate-risk groups. A PLR of less than 85.5 had a sensitivity of 52% and specificity of 67%, while an NLR below 1.8 had a sensitivity of 52% and specificity of 56% in diagnosing low-risk HEART score patients.
To the best of our knowledge, the relationship between the PLR and NLR with HEART risk score has not been investigated before.
Wikananda et al. [13] studied the relationship between the NLR ratio and GRACE score in patients presenting with acute myocardial infarction. They found that NLR was significantly higher in the high-risk group. Similarly, Acet et al. [14] studied the correlation between NLR and TIMI risk score in a group of STEMI patients; they found that NLR was positively correlated with TIMI risk score.
Azab et al. [15] performed a 4-year follow-up study for NSTEMI patients, and found that PLR was an independent predictor of long-term (4 year) mortality after NSTEMI, and thus they concluded that elevated PLR is a predictor of long-term mortality rather than just a marker of an acute medical condition.
Several mechanisms could explain these findings. Platelets have clear roles in thrombosis and contribute to inflammation. Under stress, activated platelets help neutrophils adhere to the subendothelial matrix. Chirkov et al. [16] have found that there is increased platelet aggregability and resistance to nitric oxide in patients with stable angina pectoris and ACS compared with patients without CHD (coronary heart disease). Platelets synthesize interleukin-1β, an important mediator of platelet-induced activation of the endothelial cells, which, in turn, induces chemokines that upregulate the molecules that promote endothelial adhesion of neutrophils and monocytes.
Activated platelets also have been implicated in the oxidative modification of LDL-C that can contribute to proliferation of smooth muscle cells. Platelets are the source of 90% of the circulating CD40L, which has proatherogenic and prothrombotic functions and is a predictor of incident MI, stroke, and cardiovascular death [17]. In light of our results, we believe that PLR and NLR are easy, inexpensive tools that can be used to identify high-risk HEART score patients in the ED.
Limitations of Our Study
This is a single-center study with a limited number of patients; large, multicenter studies are needed to validate these results. PLR and NLR had a low sensitivity and specificity in discriminating between low- and intermediate-risk HEART score patients.
Conclusion
There is a strong positive correlation between NLR, PLR, and the HEART score. The ease and rapidity of performing these tests make them an independent, simple, inexpensive and accurate early predictor of high-risk HEART score patients in ACS.
Statement of Ethics
The study was approved by Zagazig University Ethical Committee of Zagazig, Egypt. All patients provided written informed consent.
Conflict of Interest Statement
The authors declare that they have no conflicts of interest to disclose.
Funding Sources
The authors are responsible for the study funding without the involvement of grants, scholarship, or any other sources of funding.
Author Contributions
Elshaimaa Seaoud: conceptualization and methodology. Ahmed A.H.A. Mohamed: data curation, writing, and original draft preparation. Moataz A. Elkot and Elshaimaa Seaoud: visualization and investigation. Elshaimaa Seaoud: supervision. Moataz A. Elkot: software, validation, writing, reviewing, and editing.
Acknowledgement
We gratefully acknowledge the invaluable assistance of the physicians of the Department of Cardiology, Zagazig University Hospital, Zagazig, and University School of Medicine. The study would not have been possible without their support.
References
- 1.Corcoran D, Grant P, Berry C. Risk stratification in non-ST elevation acute coronary syndromes: risk scores, biomarkers and clinical judgment. Int J Cardiol Heart Vasc. 2015 Sep;8:131–7. doi: 10.1016/j.ijcha.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Six AJ, Backus BE, Kelder JC. Chest pain in the emergency room: value of the HEART score. Neth Heart J. 2008 Jun;16((6)):191–6. doi: 10.1007/BF03086144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gibson PH, Cuthbertson BH, Croal BL, Rae D, El-Shafei H, Gibson G, et al. Usefulness of neutrophil/lymphocyte ratio as predictor of new-onset atrial fibrillation after coronary artery bypass grafting. Am J Cardiol. 2010 Jan;105((2)):186–91. doi: 10.1016/j.amjcard.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999 Jan;340((2)):115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 5.Palmerini T, Brener SJ, Mehran R, Dangas G, Genereux P, Riva DD, et al. Leukocyte count is a modulating factor for the mortality benefit of bivalirudin in ST-segment-elevation acute myocardial infarction: the HORIZONS-AMI trial. Circ Cardiovasc Interv. 2013 Oct;6((5)):518–26. doi: 10.1161/CIRCINTERVENTIONS.113.000592. [DOI] [PubMed] [Google Scholar]
- 6.Davì G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007 Dec;357((24)):2482–94. doi: 10.1056/NEJMra071014. [DOI] [PubMed] [Google Scholar]
- 7.Zazula AD, Précoma-Neto D, Gomes AM, Kruklis H, Barbieri GF, Forte RY, et al. An assessment of neutrophils/lymphocytes ratio in patients suspected of acute coronary syndrome. Arq Bras Cardiol. 2008 Jan;90((1)):31–6. doi: 10.1590/s0066-782x2008000100006. [DOI] [PubMed] [Google Scholar]
- 8.Madjid M, Awan I, Willerson JT, Casscells SW. Leukocyte count and coronary heart disease: implications for risk assessment. J Am Coll Cardiol. 2004 Nov;44((10)):1945–56. doi: 10.1016/j.jacc.2004.07.056. [DOI] [PubMed] [Google Scholar]
- 9.Sabatine MS, Morrow DA, Cannon CP, Murphy SA, Demopoulos LA, DiBattiste PM, et al. Relationship between baseline white blood cell count and degree of coronary artery disease and mortality in patients with acute coronary syndromes: a TACTICS-TIMI 18 (Treat Angina with Aggrastat and determine Cost of Therapy with an Invasive or Conservative Strategy- Thrombolysis in Myocardial Infarction 18 trial)substudy. J Am Coll Cardiol. 2002 Nov;40((10)):1761–8. doi: 10.1016/s0735-1097(02)02484-1. [DOI] [PubMed] [Google Scholar]
- 10.Mangold A, Alias S, Scherz T, Hofbauer T, Jakowitsch J, Panzenböck A, et al. Coronary neutrophil extracellular trap burden and deoxyribonuclease activity in ST-elevation acute coronary syndrome are predictors of ST-segment resolution and infarct size. Circ Res. 2015 Mar;116((7)):1182–92. doi: 10.1161/CIRCRESAHA.116.304944. [DOI] [PubMed] [Google Scholar]
- 11.Zouridakis EG, Garcia-Moll X, Kaski JC. Usefulness of the blood lymphocyte count in predicting recurrent instability and death in patients with unstable angina pectoris. Am J Cardiol. 2000 Aug;86((4)):449–51. doi: 10.1016/s0002-9149(00)00963-2. [DOI] [PubMed] [Google Scholar]
- 12.Backus BE, Six AJ, Kelder JC, Mast TP, van den Akker F, Mast EG, et al. Chest pain in the emergency room: a multicenter validation of the HEART Score. Crit Pathw Cardiol. 2010 Sep;9((3)):164–9. doi: 10.1097/HPC.0b013e3181ec36d8. [DOI] [PubMed] [Google Scholar]
- 13.Wikananda G, Ariawan E, Husin M. The relationship between neutrophil to lymphocyte ratio (NLR) at admission and GRACE mortality risk score in acute myocardial infarction patient at Tabanan Regency Hospital in IntisariSainsMedis. 2019;10:205–208. [Google Scholar]
- 14.Acet H, Ertaş F, Bilik MZ, Akıl MA, Özyurtlu F, Aydın M, et al. The relationship between neutrophil to lymphocyte ratio, platelet to lymphocyte ratio and thrombolysis in myocardial infarction risk score in patients with ST elevation acute myocardial infarction before primary coronary intervention. Postep Kardiol Inter.201540. :126–135. doi: 10.5114/pwki.2015.52286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azab B, Shah N, Akerman M, McGinn JT., Jr Value of platelet/lymphocyte ratio as a predictor of all-cause mortality after non-ST-elevation myocardial infarction. J Thromb Thrombolysis. 2012 Oct;34((3)):326–34. doi: 10.1007/s11239-012-0718-6. [DOI] [PubMed] [Google Scholar]
- 16.Chirkov YY, Holmes AS, Willoughby SR, Stewart S, Wuttke RD, Sage PR, et al. Stable angina and acute coronary syndromes are associated with nitric oxide resistance in platelets. J Am Coll Cardiol. 2001 Jun;37((7)):1851–7. doi: 10.1016/s0735-1097(01)01238-4. [DOI] [PubMed] [Google Scholar]
- 17.Tousoulis D, Davies G, Stefanadis C, Toutouzas P, Ambrose JA. Inflammatory and thrombotic mechanisms in coronary atherosclerosis. Heart. 2003 Sep;89((9)):993–7. doi: 10.1136/heart.89.9.993. [DOI] [PMC free article] [PubMed] [Google Scholar]