Abstract
Objective
To assess the efficacy of corticosteroids in patients with coronavirus disease 2019 (COVID-19).
Methods
A multicentre observational study was performed from 22 February through 30 June 2020. We included consecutive adult patients with severe COVID-19, defined as respiratory rate ≥30 breath per minute, oxygen saturation ≤93% on ambient air or arterial partial pressure of oxygen to fraction of inspired oxygen ≤300 mm Hg. We excluded patients being treated with other immunomodulant drugs, receiving low-dose corticosteroids and receiving corticosteroids 72 hours after admission. The primary endpoint was 30-day mortality from hospital admission. The main exposure variable was corticosteroid therapy at a dose of ≥0.5 mg/kg of prednisone equivalents. It was introduced as binomial covariate in a logistic regression model for the primary endpoint and inverse probability of treatment weighting using the propensity score.
Results
Of 1717 patients with COVID-19 evaluated, 513 were included in the study, and of these, 170 (33%) were treated with corticosteroids. During hospitalization, 166 patients (34%) met the criteria of the primary outcome (60/170, 35% in the corticosteroid group and 106/343, 31% in the noncorticosteroid group). At multivariable analysis corticosteroid treatment was not associated with lower 30-day mortality rate (adjusted odds ratio, 0.59; 95% confidence interval (CI), 0.20–1.74; p 0.33). After inverse probability of treatment weighting, corticosteroids were not associated with lower 30-day mortality (average treatment effect, 0.05; 95% CI, −0.02 to 0.09; p 0.12). However, subgroup analysis revealed that in patients with PO2/FiO2 < 200 mm Hg at admission (135 patients, 52 (38%) treated with corticosteroids), corticosteroid treatment was associated with a lower risk of 30-day mortality (23/52, 44% vs. 45/83, 54%; adjusted odds ratio, 0.20; 95% CI, 0.04–0.90; p 0.036).
Conclusions
The effect of corticosteroid treatment on mortality might be limited to critically ill COVID-19 patients.
Keywords: ARDS, Corticosteroids, COVID-19, Mortality, SARS-CoV-2
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-associated coronavirus disease 2019 (COVID-19) is characterized by significant morbidity and mortality. The clinical spectrum of COVID-19 is broad, with most infected individuals experiencing only a mild or subclinical illness, especially in the disease's early phase [1]. However, approximately 14% to 30% of hospitalized patients diagnosed with COVID-19 develop severe respiratory failure requiring intensive care [[2], [3], [4], [5]].
It has been hypothesized that the main cause of illness progression is a cytokine storm characterized by dysregulated release of inflammatory products, leading to organ failure and acute respiratory distress syndrome. For this reason, corticosteroids and immunomodulatory drugs have been extensively used during the SARS-CoV-2 pandemic [6,7]. Studies conducted in patients with severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome infections failed to find a benefit of corticosteroids [8]. Among COVID-19 patients, two randomized trials showed conflicting results [9,10].
The objective of this study was therefore to evaluate the efficacy of corticosteroids in a large multicentre observational cohort of patients with SARS-CoV-2 infection.
Methods
Design and setting
We performed a retrospective multicentre cohort study of patients with laboratory-confirmed SARS-CoV-2 virus infection hospitalized from 22 February through 30 June 2020.
Nine hospitals from four Italian regions, including three tertiary-care teaching hospitals, five nonteaching tertiary-care hospitals and one secondary-care hospital, participated in the study.
Diagnostic testing for COVID-19 and hospitalization were dictated by local policies and clinical judgement, and were not encompassed by a general protocol. Local microbiology databases were used to identify patients. Clinical charts and hospital electronic records were used as data sources. Data were collected anonymously and managed using REDCap electronic data capture tools, Alma Mater University of Bologna [11,12].
The study was approved by the ethics committee of the promoting centre (Comitato Etico Indipendente di Area Vasta Emilia Centro, no. 283/2020/Oss/AOUBo).
Participants
All consecutive adult (≥18 years) patients diagnosed with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection were evaluated for study inclusion. Patients were selected if they had severe pneumonia at hospital admission; this was defined as radiologically confirmed pneumonia and respiratory rate ≥30 breaths per minute, oxygen saturation ≤93% on ambient air or partial arterial O2 pressure to fraction of inspired O2 (P/F ratio) ≤300 mm Hg.
Exclusion criteria were: hospital discharge within 24 hours of admission to the emergency department; concomitant treatment with tocilizumab or another immunomodulating drug; immunocompromised condition, defined as neutropenia (neutrophil count <500/mm³), solid organ transplantation, haematopoietic stem-cell transplantation, chronic corticosteroid therapy, uncontrolled HIV infection (<200 CD4/mm³); treatment with low-dose steroids (<0.5 mg/kg prednisone equivalents); death within 48 hours of admission; and steroid treatment initiated >72 hours after admission.
Main exposure variable and endpoints
The exposure variable was corticosteroid treatment, defined as treatment with any corticosteroid drug at dose of ≥0.5 mg/kg of prednisone equivalents initiated within 72 hours of hospital admission; it was treated as a binomial variable in models.
The primary endpoint was 30-day mortality from hospital admission.
Secondary endpoints were time to oxygen discontinuation, defined as definitive discontinuation without any further need for oxygen therapy during the in-hospital stay or return to baseline oxygen support for patients receiving chronic oxygen therapy for other reasons; time to mechanical ventilation; and time to inotropic support. Additionally, we collected the rate of bacterial superinfections in patients treated with or without corticosteroids.
Variables and definitions
Microbiologic diagnosis of SARS-CoV-2 infection was defined as a positive real-time reverse transcriptase PCR (RT-PCR) test of respiratory specimens. These consisted of nasopharyngeal swabs in all cases.
Other exposure variables were assessed at hospital admission and included: age, older age (>70 years), sex and body mass index. Underlying conditions were recorded according to Charlson comorbidity index [13]. Regarding SARS-CoV-2 infection, we collected date and symptoms at onset; date and symptoms at hospitalization; and vital signs and laboratory tests, including arterial blood gas analysis. Clinical severity at hospitalization was recorded according to Sequential Organ Failure Assessment (SOFA) score and PaO2/FiO2 ratio. We also collected treatment received other than steroids and type of oxygen supplementation other than mechanical ventilation. Bacterial superinfections were defined using US Centers for Disease Control and Prevention (CDC) standardized definitions [14]. Endpoint variables were assessed from hospital admission to discharge.
Microbiologic analysis
The presence of SARS-CoV-2 was detected by RT-PCR assay. Briefly, UTM-RT swabs (Copan, Italy) bearing specimens were immediately tested or stored at 4°C until processed, but for no more than 48 hours. Total genomic DNA/RNA was extracted from 280 μL of the clinical swab sample by NucliSENS EasyMag (bioMérieux, Marcy l’Étoile, France) following the manufacturer's instructions. Detection of SARS-CoV-2 virus was performed by RT-PCR following the World Health Organization and/or CDC protocols in a QuantStudio S5 Real-time PCR system (Thermo Fisher Scientific, Waltham, MA, USA).
Power calculation
The power calculation was based on the preliminary analysis of our sample size of 150 patients treated with corticosteroids and 350 patients not receiving corticosteroids. The 30-day mortality in the control group was assumed to be 30% and 11.6% in the treatment group. This sample size would be able to achieve 90% power to detect a difference between the group proportions of −0.1840 with alpha error 0.001, −0.1575 with alpha error 0.01 and −0.1339 with alpha error 0.05, corresponding to a Cohen h (effect size) of 0.196, 0.166 and 0.139 respectively.
Statistical analysis
For descriptive analysis, categorical variables are presented as counts and percentages; continuous variables as mean and standard deviation if normally distributed or as median and interquartile range (IQR) if nonnormally distributed.
For group comparison, Student t test, Mann-Whitney test and ANOVA or Kruskal-Wallis test were used for quantitative variables normally distributed, skewed distributed and for >2 groups, respectively. The Pearson chi-square test (or Fisher exact test where appropriate) was performed for categorical variables. The Shapiro-Wilk and Kolmogorov-Smirnov tests as well as visual methods were applied to test for normality.
The effect of steroid treatment on 30-day mortality was addressed in two ways. Firstly, univariable and multivariable logistic models were fitted. At multivariable models, clinically relevant variables and those with p < 0.10 at univariable analysis were included, with no further selection. To take the time dependency of steroid treatment into the analysis, we expanded our data set with one observation per each day since symptom onset; for each day, a binary indicator for steroid treatment in that day for that patient was created. Finally, time since symptom onset was subsequently included in models as cubic splines interacting with the steroid treatment indicator; to take into account the multiple records per patient, robust variance was estimated clustering by patient.
As a secondary analysis, logistic models with augmented inverse probability weighting (IPW) on propensity score for receiving steroid were also fitted. Risk factors for 30-day mortality, besides corticosteroid treatment, were age, diabetes, hypertension, chronic kidney disease, respiratory rate, SOFA score, creatinine and C-reactive protein (CRP). Variables contributing to the propensity score of receiving steroid in our model were study site, calendar month into the pandemic, age, CRP and days since symptoms onset (as cubic splines). Covariate balance after IPW was evaluated by comparing standardized differences and variance ratios in the crude and weighted analysis and tested by means of the overidentification test; plots the estimated densities of the probability of getting each treatment level were used to check the overlap assumption (Supplementary Methods).
The effect of steroid treatment on time to oxygen discontinuation, mechanical ventilation inotropic support, hospital discharge and occurrence of bacterial superinfection was assessed by competing-risks regression models according to the method of Fine and Gray, with death or discharge as the competing event.
All statistical tests were two sided. Stata 16.1 software (StataCorp, College Station, TX, USA) was used to perform statistical analyses.
Results
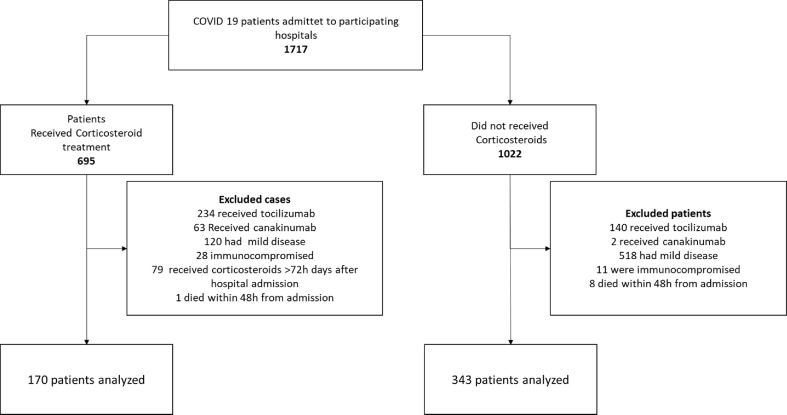
During the study period, 1717 patients with a diagnosis of COVID-19 were evaluated and 513 included in the study cohort (Fig. 1 ); of these, 170 (33%) received corticosteroids. Several important differences in the two groups were detected after comparison (Table 1 ). The percentage of patients receiving the first dose of corticosteroid within 24 and 48 hours after admission was 63% (107/170) and 86% (146/170) respectively. Ninety-eight patients (58%) received dexamethasone at a median (IQR) daily dose of 20 (20–20) mg, whereas the remaining 72 patients (42%) received methylprednisolone at a median (IQR) daily dosage of 80 (60–80) mg. Overall, 166 (98%) of 170 patients received corticosteroids for more than 48 hours. The median (IQR) duration of full dose treatment of corticosteroid was 4 (4–6) days. Thereafter, 95 patients (60%) were managed with steroid tapering of a median (IQR) duration of 9 (4–20) days.
Fig. 1.
Study flowchart.
Table 1.
Characteristics of patients receiving or not receiving corticosteroids
| Characteristic | Overall cohort | Corticosteroid | No treatment | p |
|---|---|---|---|---|
| No. | 513 | 170 | 343 | |
| Age (years), mean ± SD | 71 ± 15 | 74 ± 12 | 69 ± 16 | 0.04 |
| <50 years | 44 (6) | 6 (3) | 38 (11) | 0.001 |
| 50–59 years | 72 (14) | 13 (7) | 59 (17) | |
| 60–69 years | 98 (19) | 38 (22) | 60 (17) | |
| >70 years | 298 (58) | 113 (66) | 185 (54) | |
| Male | 337 (66) | 112 (66) | 225 (66) | 0.98 |
| Underlying diseases | ||||
| Obesity | 95 (18) | 34 (20) | 61 (18) | 0.79 |
| BMI (kg/m2), median (IQR) | 26 (24–30) | 27 (24–30) | 26 (23–30) | 0.23 |
| Hypertension | 303 (59) | 107 (63) | 196 (57) | 0.45 |
| ACE inhibitor treatment | 100 (19) | 37 (22) | 63 (18) | 0.41 |
| Angiotensin receptor blocking treatment | 70 (14) | 22 (13) | 48 (14) | 0.81 |
| Diabetes mellitus | 68 (13) | 22 (13) | 46 (13) | 0.31 |
| Coronary artery disease | 62 (12) | 20 (12) | 42 (12) | 0.87 |
| Congestive heart failure | 46 (9) | 16 (9) | 30 (9) | 0.80 |
| Cerebrovascular disease | 86 (17) | 36 (21) | 50 (15) | 0.06 |
| Peripheral vascular disease | 62 (12) | 29 (17) | 33 (9) | 0.015 |
| Chronic kidney disease | 57 (11) | 21 (12) | 36 (10) | 0.53 |
| COPD | 83 (16) | 33 (19) | 50 (15) | 0.16 |
| ESLD | 14 (3) | 2 (1.5) | 12 (3.0) | 0.12 |
| Malignancy | 49 (10) | 14 (8) | 35 (10) | 0.47 |
| Charlson index, median (IQR) | 4 (2–6) | 5 (3–7) | 4 (2–6) | 0.011 |
| Symptoms at hospitalization | ||||
| Fever (temperature ≥38°C) | 283 (55) | 96 (57) | 187 (54) | 0.30 |
| Cough | 310 (60) | 104 (61) | 206 (60) | 0.13 |
| Dyspnoea | 251 (62) | 95 (55) | 156 (45) | 0.02 |
| Time from symptom onset to hospitalization (days), median (IQR) | 6 (2–10) | 6 (2–10) | 6 (2–10) | 0.27 |
| Vital signs at hospitalization | ||||
| GCS, median (IQR) | 15 (15–15) | 15 (15–15) | 15 (15–15) | 0.21 |
| MAP, median (IQR) | 92 (83–99) | 91 (83–98) | 92 (83–100) | 0.45 |
| PR, median (IQR) | 87 (76–98) | 89 (78–100) | 84 (75–94) | 0.04 |
| RR, median (IQR) | 22 (18–28) | 25 (20–30) | 20 (18–25) | 0.001 |
| Partial arterial O2 pressure to fraction of inspired O2, median (IQR) | 249 (182–276) | 251 (183–276) | 247 (183–276) | 0.98 |
| Laboratory tests at hospitalization | ||||
| Lymphocytes (109/L) median (IQR) | 0.90 (0.64–1.22) | 0.90 (0.61–1.2) | 0.90 (0.67–1.24) | 0.60 |
| CRP (mg/dL), median (IQR) | 8.6 (3.73–14.2) | 11.4 (6.6–17.1) | 6.9 (3.0–12.8) | 0.001 |
| LDH (IU/L), median (IQR) | 335 (257–433) | 373 (289–451) | 309 (243–411) | 0.001 |
| IL-6 (pg/mL), median (IQR) | 43 (22–88) | 43 (30–86) | 41 (17–99) | 0.79 |
| Other treatment during in-hospital stay | ||||
| Hydroxychloroquine | 445 (85.5) | 159 (93) | 286 (84) | 0.003 |
| Lopinavir/ritonavir | 175 (34) | 12 (7) | 163 (48) | <0.001 |
| Darunavir/ritonavir | 77 (15) | 51 (30) | 26 (8) | <0.001 |
| Darunavir/cobicistat | 24 (5) | 14 (8) | 10 (3) | 0.008 |
| Remdesivir | 18 (3) | 2 (1) | 16 (5) | 0.05 |
| LMWH | 292 (57) | 116 (68) | 176 (52) | <0.001 |
| Antibiotic treatment | 312 (61) | 103 (61) | 209 (61) | 0.93 |
| Noninvasive ventilation | 64 (13) | 27 (16) | 37 (10) | 0.21 |
| Continuous positive airway pressure | 106 (21) | 47 (27) | 59 (17) | 0.02 |
| High flow nasal cannula | 12 (5) | 5 (3.5) | 7 (3.7) | >0.99 |
| ICU admission | 116 (23) | 32 (18) | 84 (25) | 0.16 |
| Mechanical ventilation | 103 (20) | 30 (17) | 73 (21) | 0.33 |
| Inotropic support | 66 (13) | 22 (13) | 44 (13) | 0.86 |
| Renal replacement therapy | 17 (3) | 7 (4) | 10 (3) | 0.12 |
| Study centre | <0.001 | |||
| I | 41 (8) | 0 (0) | 41 (12) | |
| II | 47 (9) | 15 (9) | 32 (9) | |
| III | 205 (39) | 48 (28) | 157 (46) | |
| IV | 4 (1) | 2 (1) | 2 (1) | |
| V | 1 (0) | 0 (0) | 1 (0) | |
| VI | 28 (5) | 26 (15) | 2 (1) | |
| VII | 43 (8) | 2 (1) | 41 (12) | |
| VIII | 12 (2) | 0 (0) | 12 (3) | |
| IX | 132 (25) | 77 (45) | 55 (16) | |
Data are presented as n (%) unless otherwise indicated.
ACE, angiotensin-converting enzyme; BMI, body mass index; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; ESLD, end-stage liver disease; GCS, Glasgow coma scale; IL, interleukin; IQR, interquartile range; LDH, lactate dehydrogenase; LMWH, low-molecular-weight heparin; MAP, mean arterial pressure; PR, pulse rate; SD, standard deviation.
Primary endpoint
During hospitalization, 166 patients (34%) died within 30 days of hospital admission (60/170, 35% in the corticosteroid group and 106/343, 31% in the noncorticosteroid group) at a median (IQR) of 12 (4.0–12.5) days after admission. At univariate logistic regression analysis (Table 2 , Supplementary Table S1), several factors were associated with 30-day mortality. At multivariable analysis (Table 2), corticosteroid treatment was not associated with a lower rate of 30-day mortality (adjusted odds ratio (aOR), 0.59; 95% confidence interval (CI), 0.20–1.74; p 0.33), even after adjusting for confounders, study site, month of enrollment and time to corticosteroid treatment. In secondary analyses, after IPW, corticosteroid treatment was not associated with lower 30-day mortality. The average treatment effect for corticosteroids was 0.05 (95% CI, −0.02 to 0.09; p 0.12).
Table 2.
Univariate and multivariate analysis for 30-day mortality
| Characteristic | Univariate |
Multivariate |
||
|---|---|---|---|---|
| Odds ratio (95% CI) | p | Odds ratio (95% CI) | p | |
| Age (years) | 1.07 (1.05–1.09) | <0.001 | 1.06 (1.03–1.09) | <0.001 |
| 50–59 years | 0.80 (0.17–3.77) | 0.78 | ||
| 60–69 years | 3.73 (1.05–13.24) | 0.042 | ||
| >70 years | 11.79 (3.57–38.91) | <0.001 | ||
| Male sex | 1.43 (0.96–2.13) | 0.08 | 1.43 (0.72–2.83) | 0.3 |
| Underlying disease | ||||
| Obesity | 1.40 (0.88–2.23) | 0.16 | ||
| BMI | 1.05 (0.99–1.10) | 0.06 | ||
| Hypertension | 2.89 (1.90–4.39) | 0.001 | 1.66 (0.93–2.96) | 0.09 |
| Diabetes mellitus | 1.7 (1.00–2.87) | 0.04 | 1.20 (0.72–2.83) | 0.43 |
| Coronary artery disease | 2.01 (1.18–3.45) | 0.01 | ||
| Congestive heart failure | 1.25 (0.67–2.35) | 0.48 | ||
| Cerebrovascular disease | 2.78 (1.73–4.45) | <0.001 | ||
| Peripheral vascular disease | 2.74 (1.45–4.26) | <0.001 | ||
| Chronic kidney disease (moderate to severe) | 3.95 (2.23–6.98) | <0.001 | 0.75 (0.28–2.01) | 0.71 |
| COPD | 1.88 (1.16–3.903) | 0.01 | ||
| Charlson index, median (IQR) | 1.32 (1.69–2.23) | <0.001 | ||
| Symptoms at hospitalization | ||||
| Fever (temperature ≥38°C) | 0.92 (0.58–1.48) | 0.59 | ||
| Cough | 0.66 (0.45–0.96) | 0.30 | ||
| Dyspnoea | 1.55 (.91.04–2.31) | 0.03 | ||
| Confusion | 3.11 (1.72–5.60) | 0.03 | ||
| Diarrhoea | 0.34 (0.12–0.94) | 0.03 | ||
| Vital signs at hospitalization | ||||
| GCS, median (IQR) | 0.57 (0.43–0.75) | <0.001 | ||
| MAP, median (IQR) | 0.98 (0.98–1.01) | 0.39 | ||
| Pulse rate | 1.00 (0.98–1.01) | 0.50 | ||
| Respiratory rate | 1.07 (1.04–1.10) | <0.001 | 1.37 (0.76–2.47) | 0.23 |
| Saturated O2 on ambient air, median (IQR) | 0.91 (0.87–0.95) | <0.001 | ||
| Partial arterial O2 pressure to fraction of inspired O2, median (IQR) | 0.99 (0.99–0.99) | <0.001 | ||
| SOFA score | 1.94 (1.69–2.23) | <0.001 | 1.55 (1.23–1.98) | <0.001 |
| Laboratory tests at hospitalization | ||||
| Lymphocytes (109/L) | 0.99 (0.99–1.00) | <0.001 | ||
| CRP (mg/dL) | 1.05 (1.03–1.07) | <0.001 | 0.99 (.097–1.07) | 0.62 |
| LDH (IU/L) | 1.0 (1.00–1.01) | <0.001 | ||
| Glucose (mg/dL) | 1.01 (1.00–1.01) | 0.001 | ||
| Creatinine (mg/dL) | 3.60 (2.38–5.43) | <0.001 | 1.07 (0.51–2.26) | 0.29 |
| Sodium (mmol/L) | 1.01 (1.00–1.09) | 0.003 | ||
| Potassium (mmol/L) | 1.68 (1.14–2.48) | 0.008 | ||
| Bilirubin (mg/dL) | 0.95 (0.59–1.51) | 0.83 | ||
| Aspartate aminotransferase (IU/L) | 1.01 (0.99–1.01 | 0.08 | ||
| Alanine aminotransferase (IU/L) | 1.00 (0.99–1.00) | 0.96 | ||
| Treatment | ||||
| Corticosteroids | 1.10 (0.73–1.65) | 0.62 | 0.59 (0.20–1.74) | 0.33 |
| Additional information | ||||
| Study site | 1.06 (0.97–1.15) | 0.21 | ||
| Period of enrollment (months) | 0.73 (0.43–1.23) | 0.23 | ||
BMI, body mass index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; GCS, Glasgow coma scale; IQR, interquartile range; LDH, lactate dehydrogenase; MAP, mean arterial pressure; PR, pulse rate; SOFA, Sequential Organ Failure Assessment.
Subgroup analysis and secondary endpoints
Subgroup analysis revealed that steroid treatment was not associated with a lower mortality rate in patients aged ≥70 years (aOR, 2.41; 95% CI, 0.47–12.37; p 0.292), in patients with CRP level >10 mg/dL at admission (aOR, 0.70; 95% CI, 0.14–3.36; p 0.65) or in patient with do-not-resuscitate orders in place (aOR, 0.37; 95% CI, 0.06–2.22; p 0.28). However, when selecting only patients with PaO2/FiO2 <200 mm Hg at admission (135 patients, 52 (38%) treated with corticosteroids), a lower mortality was observed among the corticosteroid group compared to no treatment (23/52, 44% vs. 45/83, 54%; aOR, 0.20; 95% CI, 0.04–0.90; p 0.036). At competing-risk analysis using death as the competing event, corticosteroid treatment was associated with earlier discontinuation of oxygen therapy (subdistribution hazard ratio (SHR), 1.189; 95% CI, 1.45–2.46; p < 0.001) without reducing the need for mechanical ventilation (SHR, 0.89; 95% CI, 0.50–1.59; p 0.98) or inotropic support (SHR, 1.27; 95% CI, 0.64–2.51; p 0.50) or reducing the overall length of in-hospital stay (SHR, 0.97; 95% CI, 0.77–1.22; p 0.78).
Incidence of bacterial superinfections
Overall, 89 patients (17%) had at least one episode of bacterial superinfection, mostly consisting of bloodstream infections (35 cases, 39%) and hospital-acquired or ventilator-associated pneumonia (33 cases, 37%). At multivariable competing-risk analysis (Supplementary Table S2), the hazard of bacterial infections was higher in patients receiving steroids than in those not, although this did not reach statistical significance (SHR, 1.55; 95% CI, 0.95–2.55; p 0.08).
Discussion
In this study, we did not find a lower mortality rate among hospitalized patients with COVID-19 treated with corticosteroids after adjusting for confounders and IPW. To date, two large randomized controlled trials on the use of corticosteroids in COVID-19 patients have been published, with conflicting results. The RECOVERY trial showed that patients receiving 6 mg of dexamethasone had a lower mortality rate compared to controls [9], whereas the METACOVID trial found no benefit on 28-day mortality and on several secondary outcomes of treatment with methylprednisolone 0.5 mg/kg twice daily [10]. One possible explanation for these discrepant results is that the dose of corticosteroids in the latter trial was significantly higher than the former. Similarly to the METACOVID trial, in our study, we included only patients receiving dosages of >0.5 mg/kg daily of prednisone equivalents, and we consistently did not find a lower mortality rate.
A potential harm of higher doses of corticosteroids might be hypothesized; this may counterbalance the benefits seen in the RECOVERY trial.
Among patients affected by mild SARS-CoV-1, a randomized controlled trial failed to show a beneficial effect of hydrocortisone administration. Of note, a higher viraemia was observed in the second and third weeks after infection in the hydrocortisone group than in the control group [15]. Similarly, corticosteroids were reported to be associated with delayed SARS-CoV-2 virus shedding, especially when higher doses are administered [16].
The preliminary data of cohort studies has shown a high incidence of bacterial superinfections and pulmonary aspergillosis among COVID-19 patients receiving mechanical ventilation; both these complications might be associated with corticosteroids use [10,[17], [18], [19]].
Another possible explanation of our results is that the subgroup of patients requiring mechanical ventilation represented only a small part of the entire cohort. Previous studies showed higher benefit of corticosteroid therapy in critically ill COVID-19 patients [9]. Consistently, we were able to find a benefit in secondary analysis among patients with severe respiratory failure and PaO2/FiO2 <200.
Our study has a number of limitations. Firstly, being a nonrandomized observational study, bias may had occurred in patients assignment to treatment, and our model might not have taken into account unobserved confounders. To address these limitations, we performed logistic regression and adjusted for IPW for receiving corticosteroids. However, it should be noted that a nonperfect balance was obtained during the IPW model assessment (Supplementary Methods), so we preferred to consider it as a secondary analysis.
Also, we restricted our analysis to patients with severe pneumonia which we assumed would more likely benefit from treatment, but we excluded patients receiving other immunomodulating drugs (i.e. tocilizumab or canakinumab, which were highly prescribed in our centres) in order to obtain estimates of effect of steroids alone; this selected patient group might reduce the generalizability of our results. In addition, patients did not initiate steroid treatment at the same time after admission. We tried to address this issue in two ways: by selecting only those patients administered steroids <72 hours after admission and by adjusting for time from symptom onset as previously described. Finally, the sample size was not based on a priori modelling assumptions, and is likely to be underpowered to detect small effects, as is also indicated by the relatively large CIs of the main effect.
In conclusion, our study showed that use of corticosteroid treatment might not be associated with a lower mortality rate among hospitalized COVID-19 patients. However, in critically ill patients, it could improve outcome.
Acknowledgements
We acknowledge the PREDICO Study Group investigators, who collected data as well as provided and cared for study patients.
Members of the PREDICO Study Group are: University of Bologna, Bologna, Italy—Lorenzo Badia, Filippo Trapani, Luigi Raumer, Luca Guerra, Fabio Tumietto, Alessandra Cascavilla, Eleonora Zamparini, Gabriella Verucchi, Simona Coladonato, Marina Tadolini, Caterina Campoli, Luciano Attard, Stefano Ianniruberto, Eugenia Francalanci, Giulio Virgili, Nicolò Rossi, Elena Rosselli Del Turco, Viola Guardigni, Giovanni Fasulo, Nicola Dentale, Ciro Fulgaro, Giorgio Legnani, Emanuele Campaci, Cristina Basso, Alberto Zuppiroli, Amalia Sanna Passino, Maria Eugenia Giacomini, Davide Maltese, Giulia Tesini, Lucia Angelelli, Adriana Badeanu, Agostino Rossi, Giulia Santangelo, Flovia Dauti, Vidak Koprivika, Nicholas Roncagli, Ioannis Tzimas, Guido Maria Liuzzi, Irid Baxhaku, Letizia Pasinelli, Mattia Neri, Tommaso Zanaboni, Francesco Dell’Omo, Oana Vatamanu, Alice Gori, Idina Zavatta, Stefano Antonini, Chiara Pironi, Elena Piccini, Luca Esposito, Alessandro Zuccotti, Giacomo Urbinati, Agnese Pratelli, Alberto Sarti, Michela Semprini, Enrico Evangelisti, Mara D'Onofrio, Giuseppe Sasdelli; Intensive Care Unit, Department of Medical and Surgical Sciences, Policlinico Sant’Orsola, Bologna, Italy—Giacinto Pizzilli, Elisabetta Pierucci; Centro di riferimento regionale per le emergenze microbiologiche (CRREM), Clinical Microbiology Unit, Department of Experimental, Diagnostic and Specialty Medicine, Policlinico Sant’Orsola, Bologna, Italy—Giada Rossini, Caterina Vocale; Lucia Diella Infectious Disease Unit, Department of Biomedical Sciences and Human Oncology, University of Bari, Policlinico di Bari, Italy—Davide Bavaro, Paola Laghetti.
Editor: L. Leibovici
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2020.09.014.
Transparency declaration
All authors report no conflicts of interest relevant to this article.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Wu Z., McGoogan J.M. JAMA; 2020. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. [DOI] [PubMed] [Google Scholar]
- 2.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W. JAMA; 2020. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grasselli G., Pesenti A., Cecconi M. JAMA; 2020. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. [DOI] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartoletti M., Giannella M., Scudeller L., Tedeschi S., Rinaldi M., Bussini L. Development and validation of a prediction model for severe respiratory failure in hospitalized patients with SARS-CoV-2 infection: a multicenter cohort study (PREDICO study) Clin Microbiol Infect. 2020 doi: 10.1016/j.cmi.2020.08.003. S1198-743X(20)30479-1. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guaraldi G., Meschiari M., Cozzi-Lepri A., Milic J., Tonelli R., Menozzi M. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;2:e474–e484. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li H., Chen C., Hu F., Wang J., Zhao Q., Gale R.P. 2020. Impact of corticosteroid therapy on outcomes of persons with SARS-CoV-2, SARS-CoV, or MERS-CoV infection: a systematic review and metaanalysis. Leukemia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L. Dexamethasone in hospitalized patients with COVID-19—preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. NEJMoa2021436. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeronimo C.M.P., Farias M.E.L., Val F.F.A., Sampaio V.S., Alexandre M.A.A., Melo G.C. Methylprednisolone as adjunctive therapy for patients hospitalized with COVID-19 (Metcovid): a randomised, double-blind, phase IIb, placebo-controlled trial. Clin Infect Dis. 2020:ciaa1177. doi: 10.1093/cid/ciaa1177. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research Electronic Data Capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris P.A., Taylor R., Minor B.L., Elliott V., Fernandez M., O’Neal L. The REDCap Consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 14.Horan T.C., Andrus M., Dudeck M.A. CDC/NHSN surveillance definition of health care–associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Lee N., Allen Chan K.C., Hui D.S., Ng E.K., Wu A., Chiu R.W. Effects of early corticosteroid treatment on plasma SARS-associated coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31:304–309. doi: 10.1016/j.jcv.2004.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li S., Hu Z., Song X. High-dose but not low-dose corticosteroids potentially delay viral shedding of patients with COVID-19. Clin Infect Dis. 2020:ciaa829. doi: 10.1093/cid/ciaa829. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Z., Liu J., Zhou Y., Zhao X., Zhao Q. The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and metaanalysis. J Infect. 2020;81:e13–e20. doi: 10.1016/j.jinf.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartoletti M., Pascale R., Cricca M., Rinaldi M., Maccaro A., Bussini L. Epidemiology of invasive pulmonary aspergillosis among COVID-19 intubated patients: a prospective study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langford B.J., So M., Raybardhan S., Leung V., Westwood D., MacFadden D.R. Bacterial coinfection and secondary infection in patients with COVID-19: a living rapid review and metaanalysis. Clin Microbiol Infect. 2020 doi: 10.1016/j.cmi.2020.07.016. S1198-743X(20)30423-7. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.