Abstract
The aim of the present study was to determine the effect of serum deprivation on primary microglia, BV-2 cells and primary astrocytes. Cell morphology combined with the expression of phospho-(p-)38 and p-extracellular signal-regulated kinase (ERK) were assessed. Serum deprivation resulted in various alterations in the three cell cultures. Primary microglia and BV-2 cells exhibited alterations indicative of activation under serum treatment, as well as lipopolysaccharide (LPS) treatment. However, astrocytes did not react as fast. Regarding morphology, the processes present on the primary microglia and BV-2 cells became shorter and the cell bodies became larger, and more transparent vesicles were observed within the cell bodies, which indicated their increased phagocytic ability. At the protein level, p-p38 expression increased quickly within 1 h in the primary microglia culture in response to LPS treatment. Furthermore, the expression levels of p-p38 and p-ERK were elevated in both primary microglia and BV-2 cells under serum deprivation, as well as under LPS treatment, which was not observed in the primary astrocytes. These results suggest that serum deprivation may result in similar changes to cell morphology and the expression levels of p-p38 and p-ERK as LPS treatment in primary microglia and BV-2 cells. These observations suggest that primary microglia and BV-2 cells may become activated under serum deprivation, at least to a certain degree.
Keywords: serum-deprivation, microglia, lipopolysaccharide, mitogen-activated protein kinase, activation-like changes
Introduction
Glia make up the majority of the cells present in the brain. They do not fire action potentials like neurons, but instead surround and ensheath neuronal cell bodies, axons and synapses to protect and maintain the microenvironment throughout the nervous system. Of these glial cells, microglia exhibit unique and specific characteristics in the central nervous system (1). Microglia serve as the resident innate immune cells in the brain and respond quickly to brain injuries and immunological stimuli, changing from resting branched microglia into activated, amoeboid microglia, undergoing dramatic alterations in morphology, which is thought to favor phagocytosis and mobility (2). In order to understand the cell biology of microglia more directly, an increasing number of studies are being performed in vitro using both primary microglial cultures and immortalized cell lines.
Mitogen-activated protein kinase (MAPK)-mediated signaling serves a critical role in the activation of microglia, including modulation of the p38, extracellular signal-regulated kinase (ERK) and JNK cascade (3). The p38 cascade has been implicated in cell death triggered by cytokines, growth factor withdrawal and environmental stressors (4). A previous in vivo study showed that in chronic pain models, p38 phosphorylation in the spinal cord was upregulated (5,6), which has been reported to be exclusively expressed on microglia (7). The ERK pathway, which is activated by growth factors and hormones, is also involved in mediating cellular proliferation, transformation and differentiation (8). Numerous studies have been performed to assess whether certain compounds are able to inhibit microglial activation in vitro, and the effectiveness of these compounds is assessed by measuring the expression of phospho-(p-)p38 and p-ERK as the functional activation markers of microglia (9-11). For in vitro studies, certain studies have cultured microglia in specific media, whereas others have used serum-free media as the control group (12,13), in which case the effect of serum can be disregarded.
In the present study, it was hypothesized that microglial cells could be activated by serum deprivation. The effect of serum deprivation on morphological changes, and on the expression of p-p38 and p-ERK was assessed in primary microglia, BV-2 cell and astrocytes.
Materials and methods
Cell culture
Cell culture preparation experiments were conducted between 8:00 am and 4:00 pm. The experimental protocols were performed in accordance with the Animals in Research: Reporting in vivo Experiments (ARRIVE) guidelines (14), and approved by the Institutional Animal Care and Use Committee of Peking University (approval no. LA2012-34).
Primary cultures of cortical microglia were prepared from newborn (within 24 h after birth) ICR mice (15). Mice were sacrificed by decapitation and the brains were removed, followed by careful elimination of meninges and blood vessels under a dissecting microscope in ice-cold DMEM (Gibco; Thermo Fisher Scientific). After mechanical dissociation, the cell suspension was passed through 70-µm nylon filters (Spectra/Mesh; Spectrum Medical Industries). FBS [10% (v/v); Hyclone; Cytvia] was added to the medium containing filtered cell suspensions. The mixed cell suspensions were first seeded at 1x107 cells/ml in 75-mm2 flasks coated with 0.25% poly-D-lysine. Flasks were then incubated at 37˚C, with 95% air/5% CO2 (v/v) and 95% humidity. Culture medium was changed twice per week with DMEM containing 10% (v/v) FBS. After 12-14 days, when the cells were present as two separate layers, the mixed glial culture was placed on a Forma Orbital Shaker (250 rpm; 37˚C; Thermo Fisher Scientific, Inc.) for 4 h. Following agitation, the suspended cells were collected, pooled, centrifuged (room temperature at 500 x g) and re-suspended. Cells were seeded at 3x105 cells/ml in 35-mm2 tissue culture dishes (Corning, Inc.) for identification and 12-well plates for the other experiments.
BV-2 cells were obtained from the Cell Bank of the Chinese Academy of Medical Sciences and grown in DMEM supplemented with 10% FBS. After 3-5 days of seeding the cells in 60-mm dishes, cells were cultured until they were close to 100% confluent. Cells were split 1:5 when they reached confluence, using trypsin solution in PBS. The cells were then were seeded into 35-mm dishes 1 day prior to subsequent experiments.
Primary cultures of cortical astrocytes were prepared from newborn (within 24 h after birth) ICR mice as previously described (16). Briefly, mice were sacrificed by decapitation and the brain cortexes were removed, followed by careful elimination of meninges and blood vessels under a dissecting microscope in ice-cold modified DMEM. After mechanical dissociation, the cell suspension was passed through a 70-µm nylon and then 10-µm filters (Spectra/Mesh; Spectrum Medical Industries). FBS (10%) was added to the medium containing filtered cell suspensions. These mixed cell suspensions were seeded onto 35-mm dishes at 1x106 cells/ml. Culture medium was changed twice per week with DMEM containing 10% (v/v) FBS. After 4 weeks, these astrocytes became mature and ready for use for the subsequent experiments.
Treatment and cell morphology analysis
The control group were the cells cultured under normal conditions. Cells which were treated with LPS by directly adding LPS (Sigma-Aldrich; Merck KGaA) to the media were considered as the positive control. The remaining two groups were cell cultures in which media was removed and replaced with serum-free medium or serum-free medium containing 100 ng/ml LPS. A light microscope (magnification, x40) was used for observing and capturing images of the morphological changes in cells following treatment. Cell morphology was observed at different time points (within 24 h).
Western blot analysis for p-p38 and p-ERK in cell culture
After washing with ice-cold PBS three times, cell cultures were lysed in ice-cold lysis buffer [150 mM NaCl, 0.1% (w/v) NP-40, 50 mM Tris (pH 8.0), 0.5% (w/v) sodium deoxycholate, 1% (w/v) SDS, 1 mM DTT, 0.1 mM PMSF and protease inhibitor cocktail (Roche Diagnostics)]. Following treatment, the culture plates were briefly centrifuged at room temperature; supernatants were collected and stored for western blot analysis. A Lowry protein assay was used to measure the total protein concentration. The proteins were boiled and analyzed using standard western blotting procedures. Briefly, proteins (20 µg per lane) were loaded on a 12% SDS-gel, resolved using SDS-PAGE and transferred onto PVDF membranes (EMD Millipore). The PVDF membranes were blocked with 5% (w/v) non-fat milk in TBST buffer [0.1 M Tris-HCl (pH 8.0), 0.9% (w/v) NaCl and 0.1% (v/v) Tween-20], and the target proteins were probed with diluted primary antibodies against p-p38 (1:1,000; cat. no. 9211; Cell Signaling Technology, Inc.), p-ERK (1:1,000; cat. no. 9101; Cell Signaling Technology, Inc.) in blocking solution overnight at 4˚C. The membranes were then incubated with a mouse anti-rabbit secondary antibody conjugated to horseradish peroxidase (1:2,000; cat. no. sc-2357; Santa Cruz Biotechnology, Inc.) for 1 h at room temperature. Finally, ECL western blot detection kit (Thermo Fisher Scientific, Inc.) was used to visualize the expression of the target proteins. Densitometry analysis was performed using Quantity One analysis software v4.6.6 (Bio-Rad Laboratories, Inc.), and the density of bands was normalized to the density of internal control and expressed as the fold of change compared with the control group.
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 5 (GraphPad Software, Inc.). All data are presented as the mean ± the standard error of the mean. Differences between groups (quantification of western-blot results) were compared using a one-way ANOVA and corrected using a Bonferroni's correction. P<0.05 was considered to indicate a statistically significant difference.
Results
Morphological changes of microglia, BV-2 cells and astrocytes following serum deprivation and LPS treatment
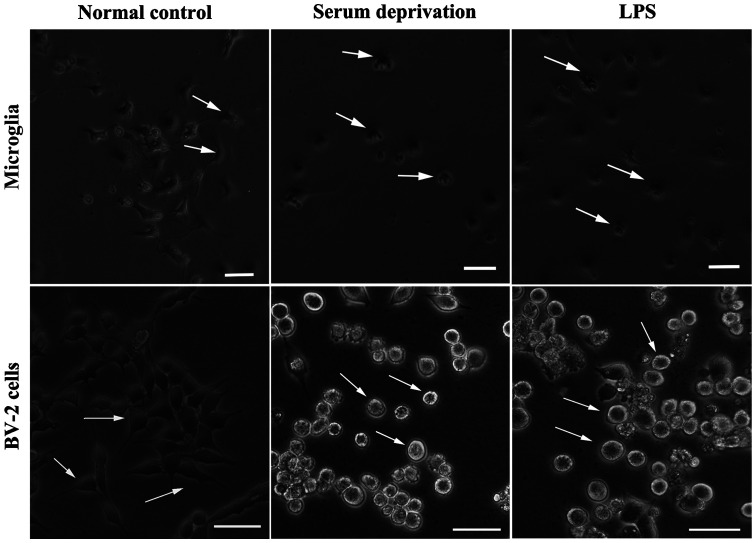
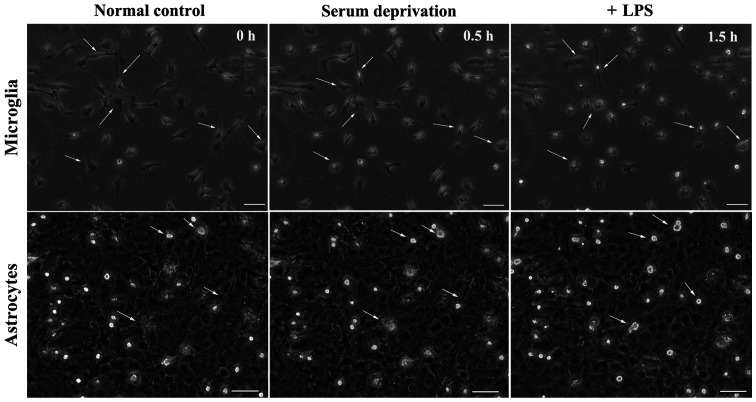
The morphology of microglia change when transitioning from their resting state to an activated state (5,6). In the present study, microglia became rounded and there were several light vesicles present in the body of microglia following LPS treatment (Data S1). Microglia reacted rapidly following LPS treatment; within several minutes. The processes became shorter and the body became notably rounder compared with the resting state. Subsequently, whether serum had any effect on the morphological changes of microglia was assessed (Fig. 1). After 24 h of serum deprivation with or without LPS treatment, the processes of the microglia became shorter and the microglia transformed into an ameboid-like morphology, suggesting that they had become activated. We also detected if this phenomenon appeared in the microglial cell line, BV-2. After 24 h treatment of serum deprivation, the morphology of BV-2 cells looked the same as the LPS-treated group, whose processes disappeared and bodies became fattened and contained vesicles. However, LPS did not result in an immediate effect on the morphology of astrocytes, nor did serum deprivation treatment (Fig. 2). In astrocyte cultures, several microglia were present on the upper layer. Following serum deprivation, the morphology of microglia remained dynamic, but the morphology of astrocytes was stable. Addition of LPS to the cell culture medium showed that the morphology of microglia exhibited a morphology indicative of activation, while the morphology of astrocytes did not change (Fig. 2).
Figure 1.
Effect of serum deprivation and LPS treatment on primary microglia and BV-2 cells. LPS was added to serum deprived cells. Cell morphology was observed after 24 h. Scale bar, 50 µm. LPS, lipopolysaccharide.
Figure 2.
Effect of serum deprivation and LPS treatment on primary astrocytes and microglia between 0-1.5 h. The start of serum deprivation was considered the 0 h time point, and cell morphology was observed every 30 min, for 90 min. LPS was added to the serum-free culture. Scale bar, 50 µm. LPS, lipopolysaccharide.
Temporal profile of p-p38 expression following LPS treatment
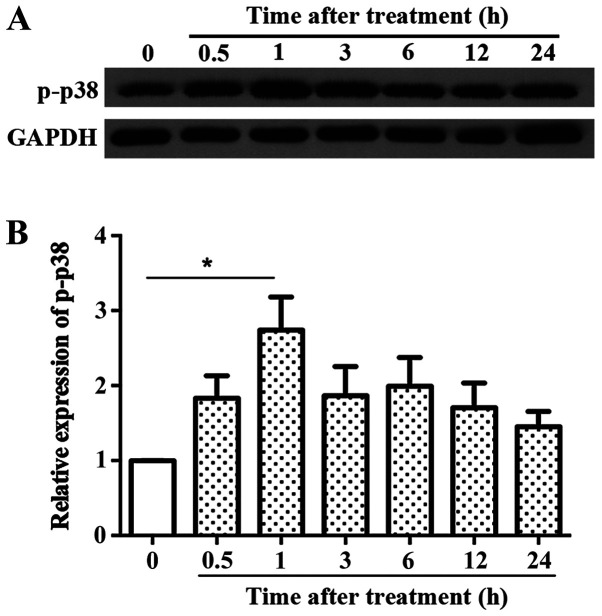
LPS treatment resulted in rapid upregulation of p-p38. The time of peak p-p38 expression was thus determined. Fig. 3 shows that the expression of p-p38 increased within 30 min and peaked at 1 h, which was significantly different from the control group; after which it decreased gradually, but remained higher than the control group. Thus, a time point of 1 h was used for subsequent experiments.
Figure 3.
Effect of LPS treatment on the expression of p-p38 in the primary microglia. LPS was used to treat primary microglia cultures under serum deprived conditions. p-p38 levels were assessed after 0, 0.5, 1, 3, 6, 12 and 24 h. (A) Representative western blots and (B) densitometry analysis showed that the expression levels of p-p38 peaked after 1 h of LPS treatment. n=3. *P<0.05. LPS, lipopolysaccharide; p-, phospho.
Effect of serum deprivation on p-p38 and p-ERK expressions in microglia, BV-2 and astrocytes
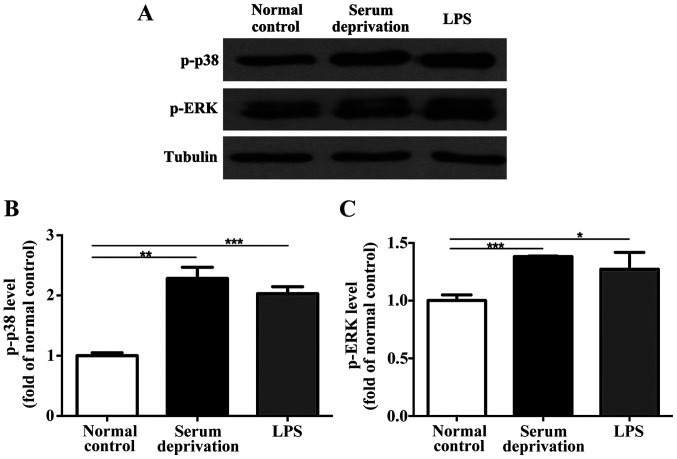
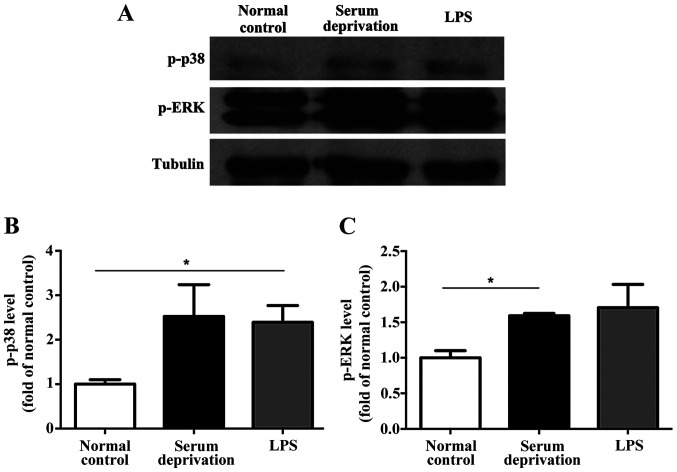
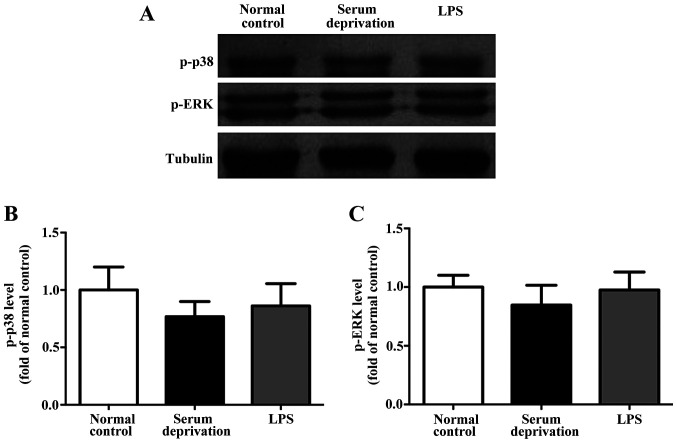
In the primary microglia culture, serum deprivation and LPS treatment both increased the expression levels of p-p38 and p-ERK compared with the normal control (Fig. 4). No significant differences were observed between the serum deprived cultures and those treated with LPS. Additionally, in the BV-2 cells, although the effect of serum deprivation was not as prominent as that in the primary microglia, serum deprivation still resulted in upregulated expression of both p-p38 and p-ERK significantly (Fig. 5). LPS treatment also increased the expression levels of p-p38 and p-ERK compared with the control group. However, no significant differences in the expression of p-p38 and p-ERK were detected in astrocytes following serum deprivation for 1 h. Furthermore, LPS did not increase the expression levels of p-p38 and p-ERK in the astrocytes (Fig. 6). Together, the results suggest that the effect of serum deprivation on the primary microglia and BV-2 cells were specific and not applicable to astrocytes.
Figure 4.
Effect of serum deprivation and LPS treatment on the expression levels of p-p38 and p-ERK in primary microglia. (A) Representative western blots and (B) densitometry analysis showed that serum deprivation significantly upregulated the levels of p-p38 and (C) p-ERK after 1 h. n=3. *P<0.05, **P<0.01, ***P<0.001. LPS, lipopolysaccharide; p-, phospho; ERK, extracellular signal-regulated kinase.
Figure 5.
Effect of serum deprivation and LPS treatment on the expression levels of p-p38 and p-ERK in BV-2 cells. (A) Representative western blots and (B) densitometry analysis showed that serum deprivation upregulated the levels of p-p38 and (C) p-ERK after 1 h. No significant differences were observed between serum deprivation and LPS treated cells. n=3. *P<0.05. LPS, lipopolysaccharide; p-, phospho; ERK, extracellular signal-regulated kinase.
Figure 6.
Effect of serum deprivation and LPS treatment on the expression levels of p-p38 and p-ERK in primary astrocytes. (A) Representative western blots and (B) densitometry analysis showed that the expression levels of p-p38 and (C) p-ERK were not altered following both serum deprivation or LPS treatment after 1 h. n=3. LPS, lipopolysaccharide; p-, phospho; ERK, extracellular signal-regulated kinase.
Discussion
In the present study, it was found that: i) Once serum was depleted, the processes of primary microglia and BV-2 cells became shorter and the body became more rounded in shape, indicative of activation; ii) serum deprivation significantly increased the expression of p-p38 and p-ERK in the primary microglia and BV-2 cell culture, and iii) both morphological changes and upregulation of p-p38 and p-ERK caused by serum deprivation were not observed in the primary astrocytes within the limited time period assessed.
Microglia serve as immune cells monitoring the brain's microenvironment for any damage (17). It has been reported that the rapid and constitutive motion of microglial processes contributes to their function (18). Toll-like receptors (TLRs) are expressed by several cells in the innate immune system, and form part of a larger family of so-called pattern recognition receptors, which sense danger signals in the form of pathogen-associated molecular patterns (19). LPS is a common inflammogen that is used to activate the glia via TLR4 in several animal models of inflammation-mediated neuro-degeneration, including both in vivo and in vitro models (20,21). In the present study, LPS-treated microglia were used as a positive activation control.
It was observed that the processes of microglia became shorter and there was an increase in the presence of phagocytic vesicles in the cell body following LPS treatment. Interestingly, similar morphological changes were detected in both the microglia and BV-2 cells following serum deprivation alone (Fig. 1). These morphological changes observed were consistent with the majority of previous reports on microglia activation (5,6,22). The effects on morphological changes in the cultured microglia in the presence of serum in vitro have been reported previously. Microglia cultured in the presence of serum are considered to be activated microglia, as the shape of the microglia was altered when serum deprived (23,24). Additionally, it was shown that it was difficult to interpret the morphological changes in cultured cells in vitro, due to the possibility of transient changes in cell morphology. Activated macrophages may exhibit increased phagocytic activity compared with ‘resting’ cells (25). Of interest, microglia have been shown to switch to a phenotype contributing to neuronal damage without undergoing notable morphological changes (26). Thus, it is not sufficient to judge the influence of serum deprivation on microglia based only on morphological alterations. The state of microglia and the influence of serum on microglia also depend on the isolation and culture method of microglia (27).
MAPKs are critical regulators of pro-inflammatory cytokines which are involved in the inflammatory process. Of the several MAPKs, p38 has been crucial for anti-inflammatory drug discovery, due to its importance in the production of the pro-inflammatory cytokines and other mediators (28). p-p38 and p-ERK are commonly used in in vitro studies as activation markers of primary microglia, microglial cell lines and astrocytes (29,30). Serum-deprivation has been shown to selectively upregulate the expression of certain genes (31-33). The present study demonstrated that serum deprivation significantly increased the expressions levels of p-p38 and p-ERK in primary microglia and BV-2 cell cultures, and this was not observed in primary astrocytes in the limited time period of assessment. Culture medium supplemented with serum provides a migratory stimulus for BV2 microglia (34). Serum deprivation is not only a simple stressful condition compared with others forms of mimicked stresses. For example, serum depletion but not hypoxia and glucose depletion increased mRNA, protein and enzyme activity of SPHK2, and this was dependent on activation of the JNK/CREB signaling pathway, although the cells grew slowly compared with cells grown in supplemented culture (35). Serum deprivation caused cell death, nuclei condensation and upregulation of Bax expression in cultured microglia. SB203580, a specific inhibitor of p38MAPK, was able to reverse cell death (36). It is also hypothesized that overstimulation or overactivation of microglia results in microglial apoptosis (37,38). Serum exposure influences microglial survival, specification and function (24). MTT analysis (data not shown) was used to assess the cell viability caused by serum deprivation, which did not cause significant levels of cell death. Thus, 24 h was selected as the longest time point for observation of morphological changes. Cell death may have increased if serum deprivation was prolonged. It is speculated that serum deprivation causes the activation of microglia over a short period of time, while microglia may eventually die due to longer periods without an adequate nutrient supply.
In summary, the present study showed that microglia responded quickly to external stimuli both in vivo and in vitro. The biological responses of microglia were different to that of astrocytes to a certain degree. In serum-deprived conditions, the morphology of microglia changed such that the processes became shorter and vesicles were present in the cell body. The expressions levels of p-p38 and p-ERK were also upregulated in both primary microglia culture and BV-2 cell lines. However, all the aforementioned changes were not observed in astrocytes. In addition to the changes in the levels of phosphorylated MAPKs and morphology, other changes may also occur in the cell lines following serum deprivation and/or LPS treatment, and these require further study.
Supplementary Material
Acknowledgements
We would like to thank Professor Albert Cheung Hoi Yu and his lab (Peking University and Department of Neurobiology, Peking University Health Science Center) for their aid with the cell culture.
Funding
The present study was supported by a grant from the Discipline Construction Fund of Beijing Stomatological Hospital (grant no. 17-09-12).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
YY and KYF conceived and designed the experiments. YY performed the experiments, analyzed the data and wrote the manuscript. Both authors have read and approved the final manuscript.
Ethics approval and consent to participate
The experimental protocol was performed in accordance with the Animals in Research: Reporting in vivo Experiments (ARRIVE) guidelines, and approved by the Institutional Animal Care and Use Committee of Peking University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Lund H, Pieber M, Parsa R, Han J, Grommisch D, Ewing E, Kular L, Needhamsen M, Espinosa A, Nilsson E, et al. Competitive repopulation of an empty microglial niche yields functionally distinct subsets of microglia-like cells. Nat Commun. 2018;9(4845) doi: 10.1038/s41467-018-07295-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen SX, Wang SK, Yao PW, Liao GJ, Na XD, Li YY, Zeng WA, Liu XG, Zang Y. Early CALP2 expression and microglial activation are potential inducers of spinal IL-6 up-regulation and bilateral pain following motor nerve injury. J Neurochem. 2018;145:154–169. doi: 10.1111/jnc.14317. [DOI] [PubMed] [Google Scholar]
- 3.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 4.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiological Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 5.Fu KY, Light AR, Matsushima GK, Maixner W. Microglial reactions after subcutaneous formalin injection into the rat hind paw. Brain Res. 1999;825:59–67. doi: 10.1016/s0006-8993(99)01186-5. [DOI] [PubMed] [Google Scholar]
- 6.Tan YH, Li K, Chen XY, Cao Y, Light AR, Fu KY. Activation of Src family kinases in spinal microglia contributes to formalin-induced persistent pain state through p38 pathway. J Pain. 2012;13:1008–1015. doi: 10.1016/j.jpain.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Tsuda M, Mizokoshi A, Shigemoto-Mogami Y, Koizumi S, Inoue K. Activation of p38 mitogen-activated protein kinase in spinal hyperactive microglia contributes to pain hypersensitivity following peripheral nerve injury. Glia. 2004;45:89–95. doi: 10.1002/glia.10308. [DOI] [PubMed] [Google Scholar]
- 8.Grewal SS, York RD, Stork PJ. Extracellular-signal-regulated kinase signalling in neurons. Curr Opin Neurobiol. 1999;9:544–553. doi: 10.1016/S0959-4388(99)00010-0. [DOI] [PubMed] [Google Scholar]
- 9.Chen HL, Jia WJ, Li HE, Han H, Li F, Zhang XL, Li JJ, Yuan Y, Wu CY. Scutellarin exerts anti-inflammatory effects in activated microglia/brain macrophage in cerebral ischemia and in activated BV-2 microglia through regulation of MAPKs signaling pathway. Neuromolecular Med. 2020;22:264–277. doi: 10.1007/s12017-019-08582-2. [DOI] [PubMed] [Google Scholar]
- 10.Chen S, Lyu C, Zhou J, Huang S, Zhang Y, Liu G, Liu K, Chen D, Hu Y, Zhou L, Gu Y. TLR4 signaling pathway mediates the LPS/ischemia-induced expression of monocytechemotactic protein-induced protein 1 in microglia. Neurosci Lett. 2018;686:33–40. doi: 10.1016/j.neulet.2018.08.052. [DOI] [PubMed] [Google Scholar]
- 11.Xu ZZ, Berta T, Ji RR. Resolvin E1 inhibits neuropathic pain and spinal cord microglial activation following peripheral nerve injury. J Neuroimmune Pharmacol. 2013;8:37–41. doi: 10.1007/s11481-012-9394-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kloss CU, Bohatschek M, Kreutzberg GW, Raivich G. Effect of lipopolysaccharide on the morphology and integrin immunoreactivity of ramified microglia in the mouse brain and in cell culture. Exp Neurol. 2001;168:32–46. doi: 10.1006/exnr.2000.7575. [DOI] [PubMed] [Google Scholar]
- 13.Pahan K, Sheikh FG, Namboodiri AM, Singh I. Lovastatin and phenylacetate inhibit the induction of nitric oxide synthase and cytokines in rat primary astrocytes, microglia, and macrophages. J Clin Invest. 1997;100:2671–2679. doi: 10.1172/JCI119812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8(e1000412) doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giulian D, Baker TJ. Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci. 1986;6:2163–2178. doi: 10.1523/JNEUROSCI.06-08-02163.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao K, Wang CR, Jiang F, Wong AY, Su N, Jiang JH, Chai RC, Vatcher G, Teng J, Chen J, et al. Traumatic scratch injury in astrocytes triggers calcium influx to activate the JNK/c-Jun/AP-1 pathway and switch on GFAP expression. Glia. 2013;61:2063–2077. doi: 10.1002/glia.22577. [DOI] [PubMed] [Google Scholar]
- 17.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 18.Parkhurst CN, Gan WB. Microglia dynamics and function in the CNS. Curr Opin Neurobiol. 2010;20:595–600. doi: 10.1016/j.conb.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 20.Carvey PM, Chang Q, Lipton JW, Ling Z. Prenatal exposure to the bacteriotoxin lipopolysaccharide leads to long-term losses of dopamine neurons in offspring: A potential, new model of Parkinson's disease. Front Biosci. 2003;8 (Suppl):S826–S837. doi: 10.2741/1158. [DOI] [PubMed] [Google Scholar]
- 21.Gao JJ, Diesl V, Wittmann T, Morrison DC, Ryan JL, Vogel SN, Follettie MT. Regulation of gene expression in mouse macrophages stimulated with bacterial CpG-DNA and lipopolysaccharide. J Leukoc Biol. 2002;72:1234–1245. [PubMed] [Google Scholar]
- 22.Stence N, Waite M, Dailey ME. Dynamics of microglial activation: A confocal time-lapse analysis in hippocampal slices. Glia. 2001;33:256–266. [PubMed] [Google Scholar]
- 23.Nakamura Y, Si QS, Kataoka K. Lipopolysaccharide-induced microglial activation in culture: Temporal profiles of morphological change and release of cytokines and nitric oxide. Neurosci Res. 1999;35:95–100. doi: 10.1016/s0168-0102(99)00071-1. [DOI] [PubMed] [Google Scholar]
- 24.Bohlen CJ, Bennett FC, Tucker AF, Collins HY, Mulinyawe SB, Barres BA. Diverse requirements for microglial survival, specification, and function revealed by defined-medium cultures. Neuron. 2017;94:759–773.e8. doi: 10.1016/j.neuron.2017.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mosser DM. The many faces of macrophage activation. J Leuko Biol. 2003;73:209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 26.Perry VH. Stress primes microglia to the presence of systemic inflammation: Implications for environmental influences on the brain. Brain Behav Immu. 2007;21:45–46. doi: 10.1016/j.bbi.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Collins HY, Bohlen CJ. doi: 10.3791/57122. Isolation and culture of rodent microglia to promote a dynamic ramified morphology in Serum-free medium. J Vis Exp: 57122, 2018 doi: 10.3791/57122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olajide OA, Bhatia HS, de Oliveira AC, Wright CW, Fiebich BL. Inhibition of neuroinflammation in LPS-activated microglia by cryptolepine. Evid Based Complement Alternat Med. 2013;2013(459723) doi: 10.1155/2013/459723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu LF, Wong PT, Moore PK, Bian JS. Hydrogen sulfide attenuates lipopolysaccharide-induced inflammation by inhibition of p38 mitogen-activated protein kinase in microglia J. Neurochem. 2007;100:1121–1128. doi: 10.1111/j.1471-4159.2006.04283.x. [DOI] [PubMed] [Google Scholar]
- 30.Wang MJ, Jeng KC, Kuo JS, Chen HL, Huang HY, Chen WF, Lin SZ. c-Jun N-terminal kinase and, to a lesser extent, p38 mitogen-activated protein kinase regulate inducible nitric oxide synthase expression in hyaluronan fragments-stimulated BV-2 microglia. J Neuroimmunol. 2004;146:50–62. doi: 10.1016/j.jneuroim.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 31.Huang Z, Huang G, Li Q, Jin J. p38 mitogen-activated protein kinase/activator protein-1 involved in serum deprivation-induced human alkaline ceramidase 2 upregulation. Biom Rep. 2015;3:225–229. doi: 10.3892/br.2014.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Celestino AT, Levy D, Maria Ruiz JL, Bydlowski SP. ABCB1, ABCC1, and LRP gene expressions are altered by LDL, HDL, and serum deprivation in a human doxorubicin-resistant uterine sarcoma cell line. Biochem Biophys Res Commun. 2015;457:664–668. doi: 10.1016/j.bbrc.2015.01.045. [DOI] [PubMed] [Google Scholar]
- 33.Jiang P, Ren YL, Lan Y, Li JL, Luo J, Li J, Cai JP. Phagocytosis of platelets enhances endothelial cell survival under serum deprivation. Exp Biol Med (Maywood) 2015;240:876–883. doi: 10.1177/1535370214565076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Omar Zaki SS, Kanesan L, Leong MYD, Vidyadaran S. The influence of serum-supplemented culture media in a transwell migration assay. Cell Biol Int. 2019;43:1201–1204. doi: 10.1002/cbin.11122. [DOI] [PubMed] [Google Scholar]
- 35.Mizutani N, Omori Y, Tanaka K, Ito H, Takagi A, Kojima T, Nakatochi M, Ogiso H, Kawamoto Y, Nakamura M, et al. Increased SPHK2 transcription of human colon cancer cells in serum-depleted culture: The involvement of CREB transcription factor. J Cell Biochem. 2015;116:2227–2238. doi: 10.1002/jcb.25173. [DOI] [PubMed] [Google Scholar]
- 36.Koyama Y, Kimura Y, Yoshioka Y, Wakamatsu D, Kozaki R, Hashimoto H, Matsuda T, Baba A. Serum-deprivation induces cell death of rat cultured microglia accompanied with expression of Bax protein. Jpn J Pharmacol. 2000;83:351–354. doi: 10.1254/jjp.83.351. [DOI] [PubMed] [Google Scholar]
- 37.Kingham PJ, Cuzner ML, Pocock JM. Apoptotic pathways mobilized in microglia and neurones as a consequence of chromogranin A-induced microglial activation. J Neurochemistry. 1999;73:538–547. doi: 10.1046/j.1471-4159.1999.0730538.x. [DOI] [PubMed] [Google Scholar]
- 38.Liu B, Wang K, Gao HM, Mandavilli B, Wang JY, Hong JS. Molecular consequences of activated microglia in the brain: Overactivation induces apoptosis. J Neurochemistry. 2001;77:182–189. doi: 10.1046/j.1471-4159.2001.t01-1-00216.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.