Key Points
Question
Is the copy number status of the programmed death ligand 1 (PD-L1) gene in non–small cell lung cancer associated with response to nivolumab monotherapy?
Findings
In this cohort study of 194 patients with non–small cell lung cancer who were treated with nivolumab monotherapy, the proportion of patients with PD-L1 amplification who achieved response was 80.0% vs 18.5% among those with PD-L1 polysomy and 17.9% among those with PD-L1 disomy. Responses among patients with PD-L1 amplification were long lasting, leading to excellent progression-free and overall survival outcomes.
Meaning
The findings of this study suggest that PD-L1 amplification in non–small cell lung cancer is associated with durable benefit from nivolumab treatment.
This cohort study evaluates whether the programmed death ligand 1 (PD-L1) gene copy number gains, comprising amplification and polysomy, in pretreatment specimens are associated with response to nivolumab monotherapy in patients with non–small cell lung cancer (NSCLC).
Abstract
Importance
Robust predictors for response to anti–programmed death 1 and its ligand (PD-1/PD-L1) immunotherapy in non–small cell lung cancer (NSCLC) are not fully characterized.
Objective
To evaluate whether PD-L1 (CD274) copy number gains (CNGs), comprising amplification and polysomy, in pretreatment specimens assessed by fluorescence in situ hybridization are associated with response to nivolumab monotherapy in NSCLC.
Design, Setting, and Participants
This multicenter cohort study enrolled 200 patients, of whom 194 had assessable tumors, with advanced or recurrent NSCLC who were treated with nivolumab after progression following prior treatment at 14 institutions in Japan between July 2016 and December 2018. Median (interquartile range) duration of follow-up was 12.6 (5.6-20.4) months. Data were analyzed from December 2019 to February 2020.
Exposures
Sequential nivolumab was given on day 1 of a 14-day cycle. Response was assessed every 4 cycles using Response Evaluation Criteria in Solid Tumors version 1.1.
Main Outcomes and Measures
Overall response rate (ORR) according to the PD-L1 copy number status. Additional end points were progression-free survival, overall survival, and PD-L1 tumor proportion score (TPS) assessed by immunohistochemistry based on PD-L1 copy number status.
Results
A total of 6 of the 200 patients were excluded because of poor-quality tumor specimens for the biomarker study, resulting in 194 assessable patients. Of these, 155 (79.9%) were men, with a median (range) age of 69 (43-83) years. PD-L1 CNGs were identified in 32 patients (16.5%), including 5 (2.6%) with amplification and 27 (13.9%) with polysomy. The ORR among patients with and without PD-L1 CNGs was 28.1% (95% CI, 13.7%-46.7%) and 17.9% (95% CI, 12.3%-24.7%), respectively. Although patients with PD-L1 polysomy did not demonstrate improved ORR (18.5% [95% CI, 6.3%-38.1%]) compared with those without PD-L1 CNGs, 4 of 5 patients (80.0% [95% CI, 28.4%-99.5%]) with PD-L1 amplification showed response, among whom median duration of response was not reached. Patients with PD-L1 amplification showed excellent survival outcomes for progression-free and overall survival. Overall, 3 PD-L1-amplified tumors (60.0%) showed PD-L1 TPS of at least 80%, but 2 (40.0%) had PD-L1 TPS of 15% or less.
Conclusions and Relevance
In this study, tumor PD-L1 amplification but not polysomy was associated with response to nivolumab monotherapy among patients with NSCLC. External validation with a larger sample size is warranted.
Introduction
Immune checkpoint inhibitors (ICIs) targeting programmed death 1 (PD-1) or its ligand (PD-L1) have offered a subset of cancer patients profound and durable survival benefit and transformed the therapeutic landscape of multiple tumor types, particularly in non–small cell lung cancer (NSCLC).1,2,3,4,5,6 However, the proportion of patients with NSCLC who respond to ICIs is low; response to the anti–PD-1 antibody nivolumab was confirmed in only approximately 20% of patients in the pivotal randomized phase 3 clinical trials.1,2 More troublesome, PD-1/PD-L1 inhibitors can cause immune-related adverse effects7 as well as hyperprogressive disease.8 Therefore, there have been substantial attempts to discover and validate predictive biomarkers to identify patients who may benefit from PD-1/PD-L1 inhibitors by integrating information from tumors, the tumor microenvironment (TME), and the host immune system.9 To date, tumor PD-L1 expression using companion diagnostics is the only approved biomarker to indicate NSCLC patients for PD-1 axis blockade. Several other predictors of responsiveness have also been identified, including mismatch repair deficiency,10,11 tumor mutation burden (TMB),12,13,14 and tumor-infiltrating immune cells.15,16,17 However, none of these factors appear to be satisfactorily sensitive or specific, even when multiple factors are combined,18 in part owing to technical issues, the dynamic nature of the TME, and the complexity and heterogeneity of cancer cells. Therefore, identifying additional factors that are robustly associated with response to anti–PD-1/PD-L1 immunotherapy remains a major clinical need.
PD-L1 is encoded by the PD-L1 gene (CD274; OMIM 605402) located on the chromosome band 9p24.1. Genomic amplification of this locus is associated with distinct features in multiple tumor types.19,20,21 We previously reported that PD-L1 copy number gains (CNGs), including amplification and polysomy, as determined by fluorescence in situ hybridization (FISH), were associated with greater PD-L1 expression in NSCLC,22 suggesting that PD-L1 CNGs are responsible for innate immune resistance through constitutive upregulation of PD-L1. In addition, PD-L1 amplification was shown to enhance PD-L1 induction in response to cytokines, such as interferon-γ and tumor necrosis factor α, as adaptive immune resistance in preclinical models of lung and breast cancer.23,24 Moreover, tumor PD-L1 amplification was associated with a specific type of TME, defined by high PD-L1 and CD8A (OMIM 186910) expression.25 This TME characterized by PD-L1–positive tumors and enriched cytotoxic immune cells appears to be associated with response to PD-1/PD-L1 inhibitors. Based on the encouraging preclinical and clinical studies suggesting the promise of PD-1 axis blockade in PD-L1–amplified tumors, we examined whether increased PD-L1 gene dosage in NSCLC tumors is associated with a greater magnitude of efficacy of nivolumab.
Methods
Study Design and Patients
This prospective, multicenter, investigator-initiated cohort study enrolled patients from 14 hospitals in Japan between July 1, 2016, and December 11, 2018. Data were analyzed from December 2019 to February 2020. This study was conducted in accordance with the Declaration of Helsinki26 and Good Clinical Practice Guidelines, and the protocol was approved by institutional review boards of all participating hospitals. All patients provided written informed consent. This study was registered at the UMIN Clinical Trials Registry as UMIN000022505. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.27
To be eligible for the study, patients for whom nivolumab therapy was planned had to fulfill the following criteria: (1) be aged 18 years or older; (2) have an Eastern Cooperative Oncology Group performance status (PS) of 0 to 2; (3) have histologically proven unresectable stage III or IV or recurrent NSCLC; (4) have progressed following prior treatment; and (5) have available archived formalin-fixed paraffin-embedded tumor for FISH and immunohistochemistry (IHC) analyses of PD-L1. We excluded patients with concomitant autoimmune diseases, interstitial lung diseases, uncontrolled symptomatic brain metastases, or other severe uncontrolled complications.
All patients received nivolumab monotherapy at a dose of 3 mg/kg; the dosage was changed to a flat 240-mg dose in August 2018, according to the renewed approval by the Japanese Ministry of Health, Labor, and Welfare. Nivolumab was repeatedly administered intravenously on day 1 of each 14-day cycle until progressive disease, as defined by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1; discontinuation as a result of unacceptable adverse event; or withdrawal of consent. Response was assessed every 4 cycles (ie, 8 weeks) using RECIST version 1.1 by local investigators. Earlier assessment of progressive disease before 4 cycles was allowed if progression was suspected. Adverse events were graded based on the National Cancer Institute Common Toxicity Criteria version 4.0.
Biomarker Study
Pretreatment tumor samples were collected for biomarker evaluation. PD-L1 copy number was assessed by centrally performed FISH using the Histra PD-L1 FISH kit (Jokoh, Tokyo, Japan) as described elsewhere.22,28 This kit contains the spectrum orange-labeled bacterial artificial chromosome clone RP11-599H20 (9p24.1, PD-L1; Advanced GenoTechs) and the spectrum green-labeled control centromere enumeration probe for chromosome 9 (CEP9; RP11-113O24; Advanced GenoTechs) as PD-L1 locus–specific and referenced chromosome 9 FISH probes, respectively. PD-L1 amplification was defined as a PD-L1 to CEP9 ratio of at least 2.0; polysomy was defined as a mean PD-L1 signal of at least 3.0 and a PD-L1 to CEP9 ratio of less than 2.0; other tumors were defined as disomy.22
Tumor PD-L1 protein expression was assessed in sections adjacent to those used for FISH by IHC using the E1L3N antibody (Cell Signaling Technology) or the 22C3 pharmDX assay (Agilent) before and after the approval of the 22C3 assay in Japan, respectively, followed by calculation of the tumor proportion score (TPS). PD-L1 expression was regarded as positive if membranous expression at any intensity was observed. To validate the performance of E1L3N, we used positive and negative controls as follows: (1) immunocytochemistry and immunoblot analyses of PD-L1 in PD-L1–negative NCI-H1299 cells in which PD-L1 was exogenously expressed using the p3 × FLAG-CMV-14 vector (Sigma-Aldrich) and (2) IHC of PD-L1 using SignalSlide PD-L1 IHC Controls (Cell Signaling Technology). The 28-8 anti–PD-L1 antibody (Abcam) and FLAG-M2 monoclonal antibody (Sigma-Aldrich) were applied for the validation study. Tumor specimens that contained fewer than 100 tumor cells or showed low quality were excluded from FISH and IHC analyses.
Outcomes
The primary end point was the difference in overall response rate (ORR), defined as partial response plus complete response using RECIST version 1.1, according to the PD-L1 copy number status; secondary end points included the differences in progression-free survival (PFS) and overall survival (OS) based on PD-L1 copy number status. PFS was defined as the time between the date of the first administration of nivolumab and the date of progression, defined by RECIST version 1.1, or death due to any cause. OS was defined as the interval from the date of the first administration of nivolumab to the date of death from any cause. Censoring was done at the date of last contact. The associations of tumor PD-L1 protein expression with PD-L1 copy number and outcomes were also included in the secondary end points.
Statistical Analysis
We calculated that a group of 200 individuals would contain approximately 40 patients with tumors carrying PD-L1 CNGs, based on a prevalence of approximately 20%.22 Although no formal hypothesis testing was planned, we assumed that ORR to nivolumab would be approximately 30% in patients with tumors harboring PD-L1 CNGs. With the planned sample size, the ORR would be estimated with the half width of the 95% CI within 15%.
Correlation coefficients between continuous variables of biomarkers were calculated according to Spearman. The Kruskal-Wallis test was used for continuous variables, followed by adjustment using the method of Holm. The Fisher exact test was used for categorical variables. Survival data were estimated using the Kaplan-Meier method, and the log-rank test was used to compare the differences in survival durations. Cox univariable proportional hazards regression model was used to explore the prognostic value of covariables. A 2-tailed P < .05 was considered statistically significant. Statistical analyses were carried out using EZR statistical software29 version 1.35 (Saitama Medical Center, Jichi Medical University) and GraphPad Prism version 8.2.1 (GraphPad Software).
Results
Patient and Tumor Characteristics
Among the 200 patients enrolled in this study, 6 patients were excluded due to poor-quality tumor specimens for FISH (5 [83.3%]) or both FISH and IHC (1 [16.7%]), resulting in 194 assessable patients. At final database lock on December 9, 2019, the median (interquartile range [IQR]) follow-up was 12.6 (5.6-20.4) months, and 127 patients (65.5%) had died, with study treatment ongoing among 16 patients (8.2%). The median (IQR) follow-up period among 67 patients who were censored was 20.5 (15.4-30.4) months.
Baseline patient and tumor characteristics are given in the Table. Median (range) age at enrollment was 69 (43-83) years. Most patients were men (155 [79.9%]) and had a history of smoking (162 [83.5%]), PS 0 or 1 (186 [95.9%]), and stage IV disease (136 [70.6%]). Most patients (184 [94.8%]) received platinum-doublet chemotherapy before nivolumab and received nivolumab as the second line (94 [48.5%]) or third line (55 [28.4%]) of treatment. No patients received ICIs before nivolumab. Nivolumab was discontinued in 178 patients (91.8%), mainly due to disease progression (135 [69.6%]) and adverse events (37 [19.1%]). Biomarker evaluation samples were obtained mainly from primary lesions through several procedures, and the median (IQR) interval between the date of sample collection and nivolumab therapy initiation was 10.7 (5.8-16.8) months, with 111 samples (57.2%) collected within 12 months before treatment.
Table. Patient and Tumor Characteristics at Baseline.
| Characteristic | Patients, No. (%) (N = 194) |
|---|---|
| Age, median (range), y | 69 (43-83) |
| Sex | |
| Men | 155 (79.9) |
| Women | 39 (20.1) |
| ECOG performance status | |
| 0 | 109 (56.2) |
| 1 | 77 (39.7) |
| 2 | 8 (4.1) |
| Smoking status | |
| Never | 32 (16.5) |
| Current or former | 162 (83.5) |
| Histology | |
| Adenocarcinoma | 107 (55.1) |
| Squamous cell carcinoma | 75 (38.7) |
| Othera | 12 (6.2) |
| Stage at treatment | |
| III | 36 (18.6) |
| IV | 137 (70.6) |
| Recurrence | 21 (10.8) |
| Previous systemic therapy, median (range), No.b | 2 (1-8) |
| Nivolumab cycles, median (range), No. | 4 (1-92) |
| Concurrent palliative radiotherapy | |
| Yes | 15 (7.7) |
| No | 179 (92.3) |
| Sample method | |
| TBB | 112 (57.7) |
| Surgery of the lung | 33 (17.0) |
| EBUS-TBNA | 26 (13.4) |
| Thoracoscopic pleural biopsy | 9 (4.7) |
| CT-guided percutaneous transthoracic biopsy | 8 (4.1) |
| Otherc | 6 (3.1) |
| EGFR variant | |
| Presence | 17 (8.8) |
| Absence | 134 (69.1) |
| Unknown | 43 (22.1) |
| ALK rearrangement | |
| Presence | 1 (0.5) |
| Absence | 138 (71.1) |
| Unknown | 55 (28.4) |
Abbreviations: EBUS-TBNA, endobronchial ultrasonograph-guided transbronchial needle aspiration; ECOG, Eastern Cooperative Oncology Group; CT, computed tomography; TBB, transbronchial biopsy.
Other histology includes non–small cell lung cancer not otherwise specified (5 patients [41.7%]), large cell neuroendocrine carcinoma (4 patients [33.3%]), adenosquamous tumors (2 patients [16.7%]), and large cell carcinoma (1 patient [8.3%]).
Includes (neo)adjuvant therapy.
Other includes liver biopsy (2 patients [33.3%]), ultrasonography-guided lymph node biopsy (2 patients [33.3%]), muscle biopsy (1 patient [16.7%]), and colonoscopy (1 patient [16.7%]).
PD-L1 Copy Number and Expression Analysis
In FISH analysis, the median (range) number of tumor PD-L1 signals was 2.3 (1.6-7.8) (eFigure 1A in the Supplement), and the median (range) PD-L1 to CEP9 ratio was 1.1 (0.78-3.5) (eFigure 1B in the Supplement). There was a positive and statistically significant correlation between PD-L1 signals and the PD-L1 to CEP9 ratio (ρ = 0.61; 95% CI, 0.51-0.70; P < .001) (eFigure 1C in the Supplement). Overall, 5 (2.6%) and 27 (13.9%) tumors showed PD-L1 amplification and polysomy, respectively; representative FISH images are shown in Figure 1. All PD-L1–amplified tumors were adenocarcinomas without EGFR and ALK alterations that developed in male smokers, except for 1 with squamous histology (eTable in the Supplement).
Figure 1. Fluorescence In Situ Hybridization Analysis of Programmed Death Ligand 1 (PD-L1).
Representative images of fluorescence in situ hybridization analysis of tumors carrying PD-L1 disomy, polysomy, or amplification obtained from patients enrolled in this study (original magnification ×100). PD-L1 and CEP9 signals are shown in red and green, respectively.
For PD-L1 IHC, 118 (60.8%) and 76 (39.2%) samples were assessed using the E1L3N and 22C3 antibodies, respectively. The number of PD-L1 expression–positive cases at different TPS thresholds were 86 (44.3%) at TPS 1%, 73 (37.6%) at TPS 5%, 61 (31.4%) at TPS 10%, and 24 (12.4%) at TPS 50%. We validated the comparative performance of the E1L3N antibody with the referenced antibodies in immunocytochemistry and IHC (eFigure 2A in the Supplement) and Western blot (eFigure 2B in the Supplement) analyses.
PD-L1 TPS was only weakly correlated with PD-L1 copy number (ρ = 0.24; 95% CI, 0.10 to 0.37; P < .001) (Figure 2A) and was not correlated with the PD-L1 to CEP9 ratio (ρ = 0.12; 95% CI, −0.025 to 0.26; P = .10) (Figure 2B), despite a significant difference in PD-L1 expression levels according to the PD-L1 copy number status (Figure 2C). Of note, the 5 PD-L1–amplified tumors exhibited various PD-L1 TPS values, ranging from 4% to 95% (eTable in the Supplement).
Figure 2. Fluorescence In Situ Hybridization Analysis of Programmed Death Ligand 1 (PD-L1) in Association With PD-L1 Protein Expression.

A, Scatterplot depicting the correlation between PD-L1 tumor proportion score and PD-L1 copy number (Spearman ρ = 0.24; 95% CI, 0.10 to 0.37; P < .001). B, Scatterplot depicting PD-L1 tumor proportion score and PD-L1 to CEP9 ratio (Spearman ρ = 0.12; 95% CI, −0.025 to 0.26; P = .10). C, Violin plot depicting PD-L1 tumor proportion score in association with PD-L1 copy number status.
Correlative Analysis of Efficacy and PD-L1 Copy Number Status
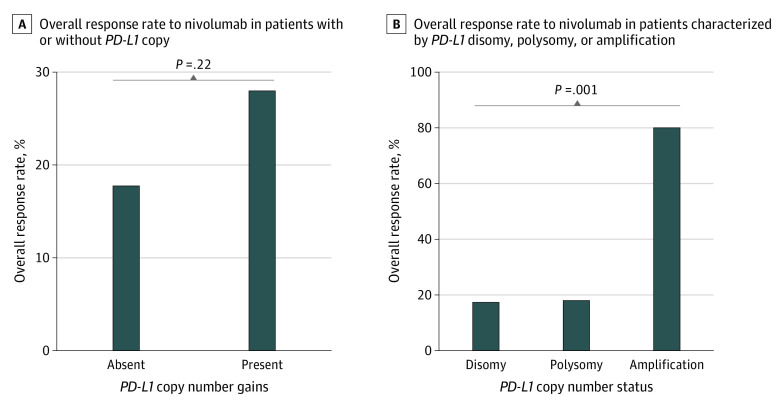
For the overall population, the ORR and disease control rate was 19.6% (95% CI, 14.2%-25.9%) and 50.5% (95% CI, 43.3%-57.8%), respectively. When stratified by the presence of PD-L1 CNGs, there was no significant difference in ORR (with CNGs: 28.1%; 95% CI, 13.7%-46.7%; without CNGs: 17.9%; 95% CI, 12.3%-24.7%; P = .22) (Figure 3A). However, the ORR among patients with PD-L1 amplification was high (80.0%; 95% CI, 28.4%-99.5%), which was a contrast with the low ORR (18.5%; 95% CI, 6.3%-38.1%) among patients with PD-L1 polysomy (Figure 3B). Of the 4 patients with PD-L1 amplification who responded to therapy, 3 patients were still receiving study treatment at the final database lock. For the other patient with PD-L1 amplification who responded, nivolumab was terminated after 5 cycles because of grade 2 colitis as an adverse effect. However, this patient subsequently experienced no disease progression until final database lock, receiving no other systemic antitumor therapy. As a result, median duration of response was not reached (range, 17.7 [ongoing] to 33.7 [ongoing] months) for patients with PD-L1 amplification who responded, which was longer than that among patients with PD-L1 polysomy who responded (14.9 months; 95% CI, 4.6 months to not reached) or those with disomy who responded (16.8 months; 95% CI, 8.1 months to not reached) (eFigure 3A in the Supplement). Representative computed tomography scans demonstrating response in a patient with PD-L1–amplified adenocarcinoma are shown in eFigure 4 in the Supplement. The remaining patient with PD-L1 amplification who did not respond obtained stable disease, demonstrating evidence of antitumor effects, with tumor regression of 20%, as shown in the waterfall plot (eFigure 5 in the Supplement). This patient received 21 cycles of nivolumab with a PFS of 9.7 months (eTable in the Supplement).
Figure 3. Response to Nivolumab According to Programmed Death Ligand 1 (PD-L1) Copy Number Status.
In terms of survival outcome, we observed only 1 event of progression (Figure 4A) and no deaths (Figure 4B) among patients with PD-L1 amplification, with a 1-year PFS rate of 80.0% (95% CI, 20.4%-96.9%) and 1-year OS rate of 100%. In contrast, the 1-year PFS and 1-year OS rates were only 18.5% (95% CI, 6.7%-34.8%) and 46.0% (95% CI, 26.4%-63.6%) for patients with PD-L1 polysomy and 20.8% (95% CI, 14.8%-27.4%) and 57.6% (95% CI, 49.6%-64.8%) for patients with PD-L1 disomy, respectively. When referenced to disomy, PD-L1 amplification was associated with a significantly decreased risk of progression (PFS: hazard ratio [HR], 0.10; 95% CI, 0.01-0.72; P = .02), whereas PD-L1 polysomy did not have an association with risks of PFS (HR, 1.19; 95% CI, 0.78-1.83; P = .42) or OS (HR, 1.30; 95% CI, 0.79-2.12; P = .30).
Figure 4. Survival of Patients.

PD-L1 indicates programmed death ligand 1.
Correlative Analysis of Response and PD-L1 Expression
Patients with high expression of PD-L1 showed significantly higher ORR than those with low expressions of PD-L1 at PD-L1 TPS thresholds of 1%, 10%, and 50% (eFigure 6 in the Supplement). However, survival curves of duration of response overlapped between responders with PD-L1 TPS of at least 50% and those with PD-L1 TPS of less than 50% (eFigure 3B in the Supplement). Patients with a PD-L1 TPS of at least 50% had a superior median PFS of 8.1 (95% CI, 2.1-20.9) months compared with that of 2.2 (95% CI, 1.8-3.4) months in patients with a TPS of less than 50% (HR, 0.54; 95% CI, 0.33-0.90; P = .02) (eFigure 7A in the Supplement). By contrast, there were no significant differences in duration of PFS when stratified by lower PD-L1 TPS thresholds of 10% (eFigure 7B in the Supplement), 5% (eFigure 7C in the Supplement), and 1% (eFigure 7D in the Supplement). An OS benefit for patients with high expression of PD-L1 compared with those with low expression of PD-L1 was observed at the PD-L1 TPS threshold of 50% (HR, 0.44; 95% CI, 0.23-0.84; P = .01) (eFigure 8A in the Supplement), but again, no significant benefit was observed at lower PD-L1 TPS thresholds (eFigures 8B, 8C, and 8D in the Supplement).
Discussion
Although somatic genomic features, such as variations and copy number alterations, have been associated with response and resistance to ICIs,30,31 tumor PD-L1 copy number status has received limited attention in solid tumors. In this prospective study, we demonstrated that FISH-based PD-L1 amplification but not polysomy was associated with durable responses to nivolumab among patients with NSCLC.
The significance of PD-L1 CNGs in the context of ICI therapy was originally highlighted in a previous study showing a high rate (87%) of response to nivolumab, including 17% complete response in heavily pretreated Hodgkin lymphoma32 that usually carries a very low level of TMB,33 given that all tumors analyzed by FISH had an increased PD-L1 gene dosage. However, the reported very low prevalence (0.7%) of PD-L1 amplification, defined as at least 6 copies, assessed by comprehensive genomic profiling across more than 100 types of solid tumors,34 has been considered a major limitation of its widespread use in clinical practice.35 Nevertheless, our previous report22 and the present study showed that PD-L1 amplification was detected in approximately 3% of patients with NSCLC as assessed by FISH. These results justify the clinical application of PD-L1 FISH, considering the strong association of PD-L1 amplification with response to PD-1/PD-L1 blockade.
The mechanisms by which PD-L1–amplified tumors are associated with long-lasting responses to nivolumab remain unclear. Genomic amplification of 9p24.1 targets Janus kinase 2 (JAK2) as well as PD-L1 and PD-L2, resulting in enhanced expression of JAK2 that further augments PD-L1 induction.36 This positive circuit makes the amplification of this locus more impactful in terms of constitutive PD-L1 expression and provides a rationale for response to anti–PD-1/PD-L1 inhibitors. Indeed, previously treated patients with NSCLC who had PD-L1 expression of at least 50% had more response to pembrolizumab compared with those with PD-L1 expression between 1% and 50%.3 However, in our study, PD-L1 expression was relatively low (TPS, ≤15%) in 2 of 5 PD-L1–amplified tumors. This might have been caused by the spatially more heterogeneous nature of PD-L1 expression than copy number,22,37 which was particularly relevant for this study because of the small biopsy specimens used in most cases. In addition, the benefit observed in patients with PD-L1–amplified tumors irrespective of PD-L1 expression levels suggests other mechanisms that render PD-L1–amplified tumors sensitive to ICIs, including the link with known predictive factors such as TMB. However, the association of PD-L1 copy number status with TMB remain contradictory,19,34 although PD-L1 expression does not correlate with TMB.12,13,38 In addition, a TMB-independent association between PD-L1 amplification and inflamed TME was reported.25 Further studies are required to elucidate the mechanistic basis for PD-L1 amplification–associated response to ICIs.
In contrast to our hypothesis that low-grade PD-L1 CNGs represented as polysomy would derive limited benefit from nivolumab therapy, no benefit in response and survival was observed, suggesting distinct roles for PD-L1 amplification and polysomy in tumor immune evasion. This could be partially explained by the finding that group-level and chromosome-level somatic copy number alterations are more negatively associated with cytotoxic immune cell infiltration than the other type of tumor aneuploidy, focal somatic copy number alterations, through a putative mechanism of general gene dosage imbalance rather than the action of specific genes.39 Our definitions of amplification and polysomy are more likely to represent focal and group-level or chromosome-level CNGs, respectively.
Limitations
This study has limitations. First, definite conclusions are still precluded because of the small number of patients with PD-L1 amplification. Second, actual PD-L1 status during nivolumab treatment might not be represented because of the interval between sampling and nivolumab therapy initiation. Future studies will need to investigate the evolving PD-L1 genetic complexity in cancer cells. Third, we could not assess TMB and characteristics of the TME, mainly owing to the limited small biopsy samples.
Conclusions
In this study, a selection of patients with NSCLC based on PD-L1 amplification was associated with greater durable benefit from nivolumab. Despite being hampered by the low prevalence, this association appears to be more clinically meaningful than selection of patients based on PD-L1 expression at any threshold applied. FISH is advantageous for its ability to discriminate PD-L1 amplification from polysomy. External validation with a larger sample size is warranted to facilitate personalization of PD-1/PD-L1 blockade for patients with NSCLC.
eFigure 1. Descriptive Statistics for PD-L1 FISH
eFigure 2. Validation Study of the E1L3N Anti–PD-L1 Antibody
eFigure 3. Duration of Response
eFigure 4. Computed Tomography Scans Before and After Treatment With Nivolumab in a Patient with PD-L1–Amplified Adenocarcinoma
eFigure 5. Waterfall Plot Showing the Best Percentage Change From Baseline
eFigure 6. Response to Nivolumab According to PD-L1 Protein Expression
eFigure 7. Progression-Free Survival of Patients Stratified by PD-L1 Protein Expression
eFigure 8. Overall Survival of Patients Stratified by PD-L1 Protein Expression
eTable. Characteristics of Patients with PD-L1–Amplified Tumors
References
- 1.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small cell lung cancer. N Engl J Med. 2015;373(2):123-135. doi: 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non–small cell lung cancer. N Engl J Med. 2015;373(17):1627-1639. doi: 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540-1550. doi: 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 4.Rittmeyer A, Barlesi F, Waterkamp D, et al. ; OAK Study Group . Atezolizumab versus docetaxel in patients with previously treated non–small cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255-265. doi: 10.1016/S0140-6736(16)32517-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reck M, Rodríguez-Abreu D, Robinson AG, et al. ; KEYNOTE-024 Investigators . Pembrolizumab versus chemotherapy for PD-L1-positive non–small cell lung cancer. N Engl J Med. 2016;375(19):1823-1833. doi: 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 6.Antonia SJ, Villegas A, Daniel D, et al. ; PACIFIC Investigators . Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. N Engl J Med. 2017;377(20):1919-1929. doi: 10.1056/NEJMoa1709937 [DOI] [PubMed] [Google Scholar]
- 7.Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol. 2016;2(10):1346-1353. doi: 10.1001/jamaoncol.2016.1051 [DOI] [PubMed] [Google Scholar]
- 8.Champiat S, Dercle L, Ammari S, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res. 2017;23(8):1920-1928. doi: 10.1158/1078-0432.CCR-16-1741 [DOI] [PubMed] [Google Scholar]
- 9.Prelaj A, Tay R, Ferrara R, Chaput N, Besse B, Califano R. Predictive biomarkers of response for immune checkpoint inhibitors in non-small-cell lung cancer. Eur J Cancer. 2019;106:144-159. doi: 10.1016/j.ejca.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 10.Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409-413. doi: 10.1126/science.aan6733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509-2520. doi: 10.1056/NEJMoa1500596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rizvi H, Sanchez-Vega F, La K, et al. Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non–small cell lung cancer profiled with targeted next-generation sequencing. J Clin Oncol. 2018;36(7):633-641. doi: 10.1200/JCO.2017.75.3384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hellmann MD, Nathanson T, Rizvi H, et al. Genomic features of response to combination immunotherapy in patients with advanced non–small cell lung cancer. Cancer Cell. 2018;33(5):843-852.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology: mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124-128. doi: 10.1126/science.aaa1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568-571. doi: 10.1038/nature13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riaz N, Havel JJ, Makarov V, et al. Tumor and microenvironment evolution during immunotherapy with nivolumab. Cell. 2017;171(4):934-949.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thommen DS, Koelzer VH, Herzig P, et al. A transcriptionally and functionally distinct PD-1+ CD8+ T cell pool with predictive potential in non–small cell lung cancer treated with PD-1 blockade. Nat Med. 2018;24(7):994-1004. doi: 10.1038/s41591-018-0057-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu S, Stein JE, Rimm DL, et al. Comparison of biomarker modalities for predicting response to PD-1/PD-L1 checkpoint blockade: a systematic review and meta-analysis. JAMA Oncol. 2019;5(8):1195-1204. doi: 10.1001/jamaoncol.2019.1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Budczies J, Bockmayr M, Denkert C, et al. Pan-cancer analysis of copy number changes in programmed death-ligand 1 (PD-L1, CD274)—associations with gene expression, mutational load, and survival. Genes Chromosomes Cancer. 2016;55(8):626-639. doi: 10.1002/gcc.22365 [DOI] [PubMed] [Google Scholar]
- 20.Roemer MG, Advani RH, Ligon AH, et al. PD-L1 and PD-L2 genetic alterations define classical Hodgkin lymphoma and predict outcome. J Clin Oncol. 2016;34(23):2690-2697. doi: 10.1200/JCO.2016.66.4482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barrett MT, Anderson KS, Lenkiewicz E, et al. Genomic amplification of 9p24.1 targeting JAK2, PD-L1, and PD-L2 is enriched in high-risk triple negative breast cancer. Oncotarget. 2015;6(28):26483-26493. doi: 10.18632/oncotarget.4494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inoue Y, Yoshimura K, Mori K, et al. Clinical significance of PD-L1 and PD-L2 copy number gains in non–small cell lung cancer. Oncotarget. 2016;7(22):32113-32128. doi: 10.18632/oncotarget.8528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikeda S, Okamoto T, Okano S, et al. PD-L1 Is upregulated by simultaneous amplification of the PD-L1 and JAK2 genes in non–small cell lung cancer. J Thorac Oncol. 2016;11(1):62-71. doi: 10.1016/j.jtho.2015.09.010 [DOI] [PubMed] [Google Scholar]
- 24.Chen M, Pockaj B, Andreozzi M, et al. JAK2 and PD-L1 amplification enhance the dynamic expression of PD-L1 in triple-negative Breast Cancer. Clin Breast Cancer. 2018;18(5):e1205-e1215. doi: 10.1016/j.clbc.2018.05.006 [DOI] [PubMed] [Google Scholar]
- 25.Ock CY, Keam B, Kim S, et al. Pan-cancer immunogenomic perspective on the tumor microenvironment based on PD-L1 and CD8 T-Cell infiltration. Clin Cancer Res. 2016;22(9):2261-2270. doi: 10.1158/1078-0432.CCR-15-2834 [DOI] [PubMed] [Google Scholar]
- 26.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 27.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4(10):e296. doi: 10.1371/journal.pmed.0040296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sugimura H. Detection of chromosome changes in pathology archives: an application of microwave-assisted fluorescence in situ hybridization to human carcinogenesis studies. Carcinogenesis. 2008;29(4):681-687. doi: 10.1093/carcin/bgn046 [DOI] [PubMed] [Google Scholar]
- 29.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452-458. doi: 10.1038/bmt.2012.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skoulidis F, Goldberg ME, Greenawalt DM, et al. STK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS-mutant lung adenocarcinoma. Cancer Discov. 2018;8(7):822-835. doi: 10.1158/2159-8290.CD-18-0099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miao D, Margolis CA, Vokes NI, et al. Genomic correlates of response to immune checkpoint blockade in microsatellite-stable solid tumors. Nat Genet. 2018;50(9):1271-1281. doi: 10.1038/s41588-018-0200-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311-319. doi: 10.1056/NEJMoa1411087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chabanon RM, Pedrero M, Lefebvre C, Marabelle A, Soria JC, Postel-Vinay S. Mutational landscape and sensitivity to immune checkpoint blockers. Clin Cancer Res. 2016;22(17):4309-4321. doi: 10.1158/1078-0432.CCR-16-0903 [DOI] [PubMed] [Google Scholar]
- 34.Goodman AM, Piccioni D, Kato S, et al. Prevalence of PDL1 amplification and preliminary response to immune checkpoint blockade in solid tumors. JAMA Oncol. 2018;4(9):1237-1244. doi: 10.1001/jamaoncol.2018.1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keenan TE, Burke KP, Van Allen EM. Genomic correlates of response to immune checkpoint blockade. Nat Med. 2019;25(3):389-402. doi: 10.1038/s41591-019-0382-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Green MR, Monti S, Rodig SJ, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116(17):3268-3277. doi: 10.1182/blood-2010-05-282780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshimura K, Inoue Y, Karayama M, et al. Heterogeneity analysis of PD-L1 expression and copy number status in EBUS-TBNA biopsy specimens of non–small cell lung cancer: comparative assessment of primary and metastatic sites. Lung Cancer. 2019;134:202-209. doi: 10.1016/j.lungcan.2019.06.002 [DOI] [PubMed] [Google Scholar]
- 38.Ready N, Hellmann MD, Awad MM, et al. First-line nivolumab plus ipilimumab in advanced non–small-cell lung cancer (CheckMate 568): outcomes by programmed death ligand 1 and tumor mutational burden as biomarkers. J Clin Oncol. 2019;37(12):992-1000. doi: 10.1200/JCO.18.01042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davoli T, Uno H, Wooten EC, Elledge SJ. Tumor aneuploidy correlates with markers of immune evasion and with reduced response to immunotherapy. Science. 2017;355(6322):eaaf8399. doi: 10.1126/science.aaf8399 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Descriptive Statistics for PD-L1 FISH
eFigure 2. Validation Study of the E1L3N Anti–PD-L1 Antibody
eFigure 3. Duration of Response
eFigure 4. Computed Tomography Scans Before and After Treatment With Nivolumab in a Patient with PD-L1–Amplified Adenocarcinoma
eFigure 5. Waterfall Plot Showing the Best Percentage Change From Baseline
eFigure 6. Response to Nivolumab According to PD-L1 Protein Expression
eFigure 7. Progression-Free Survival of Patients Stratified by PD-L1 Protein Expression
eFigure 8. Overall Survival of Patients Stratified by PD-L1 Protein Expression
eTable. Characteristics of Patients with PD-L1–Amplified Tumors