Key Points
Question
Is there an association of gestational age at birth with structural brain morphometry in childhood?
Findings
In this population-based cohort study of 3079 singleton children, gestational duration was associated with global and regional brain volumes 10 years after birth and these linear associations persisted when the sample was restricted to term-born children. Consistent with the rapid expansion of brain volume during the third trimester, gestational age at birth was associated with more gyrification and cortical surface area.
Meaning
Gestational duration may be important for long-term neurodevelopment and should be considered a continuum of development throughout pregnancy.
Abstract
Importance
Preterm and postterm births are associated with adverse neuropsychiatric outcomes. However, it remains unclear whether variation of gestational age within the 37- to 42-week range of term deliveries is associated with neurodevelopment.
Objective
To investigate the association of gestational age at birth (GAB) with structural brain morphometry in children aged 10 years.
Design, Setting, and Participants
This population-based cohort study included pregnant women living in Rotterdam, the Netherlands, with an expected delivery date between April 1, 2002, and January 31, 2006. The study evaluated 3079 singleton children with GAB ranging from 26.3 to 43.3 weeks and structural neuroimaging at 10 years of age from the Generation R Study, a longitudinal, population-based prospective birth cohort from early pregnancy onward in Rotterdam. Data analysis was performed from March 1, 2019, to February 28, 2020, and at the time of the revision based on reviewer suggestions.
Exposures
The GAB was calculated based on ultrasonographic assessment of crown-rump length (<12 weeks 5 days) or biparietal diameter (≥12 weeks 5 days) in dedicated research centers.
Main Outcomes and Measures
Brain structure, including global and regional brain volumes and surface-based cortical measures (thickness, surface area, and gyrification), was quantified by magnetic resonance imaging.
Results
In the 3079 children (1546 [50.2%] female) evaluated at 10 years of age, GAB was linearly associated with global and regional brain volumes. Longer gestational duration was associated with larger brain volumes; for example, every 1-week-longer gestational duration corresponded to an additional 4.5 cm3/wk (95% CI, 2.7-6.3 cm3/wk) larger total brain volume. These associations persisted when the sample was restricted to children born at term (GAB of 37-42 weeks: 4.8 cm3/wk; 95% CI, 1.8-7.7 cm3/wk). No evidence of nonlinear associations between GA and brain morphometry was observed.
Conclusions and Relevance
In this cohort study, gestational duration was linearly associated with brain morphometry during childhood, including within the window of term delivery. These findings may have marked clinical importance, particularly given the prevalence of elective cesarean deliveries.
This cohort study investigates the association of gestational age at birth with structural brain morphometry in children aged 10 years.
Introduction
Gestational age at birth (GAB) is an important determinant of child health and development. Worldwide, approximately 13 million newborns are born preterm (GAB<37 weeks) annually.1 Prematurity is associated with morbidity and mortality,2,3 including neurodevelopmental problems, such as cerebral palsy, intellectual disability, learning disability, and poor motor development.4,5,6 Preterm birth is reportedly associated with increased risks of attention-deficit/hyperactivity disorder (ADHD), autism spectrum disorder,7,8,9 and psychiatric disorders in adulthood.10 In some countries, postterm birth (GAB≥42 weeks) accounts for up to 10% of births11 and is associated with adverse birth outcomes, increased neonatal mortality, cognitive impairment, and increased risk of ADHD.12
Few studies13,14 have investigated the associations of GAB with brain structures despite the dynamic neurodevelopment that occurs during early life. During the third trimester of gestation, there is a 4-fold increase in brain size accompanied by marked growth of brain surface area, resulting in the emergence of sulci and gyri.15,16 Thus, birth before the presumed optimal gestational duration (approximately 40 weeks) may be associated with disruption of neurodevelopmental processes in late pregnancy that persist during postnatal life.
Prior studies17,18,19,20,21,22 often focused on children born extremely preterm (<28 weeks of gestation) or very preterm (<34 weeks of gestation) and found less gray and white matter volume in premature children and adolescents. These studies17,18,19,20,21,22 used categorical indexes for GAB based on somewhat arbitrary cutoffs (eg, extremely, very, or late preterm) rather than gestational duration as a quantitative trait of fetal maturity. Few studies23,24 have examined the associations of gestational duration and developmental outcomes in children born at term. Longer gestation in term-born children has been associated with higher scores of cognitive and motor development early in life. In addition, 2 neuroimaging studies that used a group of approximately 100 term-born children (aged 6-10 years) found that longer gestational duration was associated with larger gray matter volumes, in particular temporal and parietal regions,25 and linear associations of larger GAB with enhanced local and global network efficiency of structural networks.26
Given the novelty of investigating brain morphometric outcomes among children born at term, we examined the prospective association of GAB as a quantitative trait with brain morphometry assessed 10 years later using structural neuroimaging in a large population-based cohort. We used an exploratory approach that involved both global and regional metrics of brain development without defining specific regions of interest. We hypothesized that higher GAB would be associated with larger global and regional brain volumes, even within the term range of gestational duration. Mechanistically, we hypothesized that a higher GAB is positively associated with cortical surface area and gyrification (ie, cortical folding) because of rapid expansion of the cerebral cortex in the third trimester of pregnancy.
Methods
Setting and Design
This cohort study was embedded in the Generation R Study, a population-based cohort in Rotterdam, the Netherlands. Pregnant women living in Rotterdam with an expected delivery date between April 1, 2002, and January 31, 2006, were invited to participate.27 Enrolled children were followed up from fetal life onward. Data analysis was performed from March 1, 2019, to February 28, 2020, and at the time of the revision based on reviewer suggestions. Written informed consent was obtained from all participants, and all data are deidentified. The Generation R Study was approved by the Medical Ethical Committee of the Erasmus Medical Center, Rotterdam. All procedures were conducted in accordance with the World Medical Association Declaration of Helsinki.28
Study Population
The study enrolled 9778 mothers who gave birth to 9749 live-born children (eFigure 1 in the Supplement). Twin pregnancies were excluded because of the increased risk of prematurity and twin-related complications,29 resulting in 9418 singletons with information on GAB. Of these, 8270 children were invited to the research center at 10 years of age. All 5669 children visiting the center were invited to undergo brain magnetic resonance imaging (MRI). In total, 3857 children underwent neuroimaging. The final study population comprised 3079 children after excluding 23 children with major incidental findings, 89 with missing T1-weighted MRIs or artifacts because of braces or retainers, 44 with heterogeneous scanning parameters, and 622 with insufficient image quality (based on visual inspection of T1-weighted MRIs and FreeSurfer reconstructions).30,31
Gestational Age at Birth
Gestational age was determined by ultrasonography during prenatal visits at the research center. Standard methods of fetal ultrasonographic measurements have been previously described.32 Interobserver and intraobserver intraclass correlation coefficients were greater than 0.98.32 Crown-rump length was used for pregnancy dating until a gestational age of 12 weeks 5 days (<65 mm, n = 902). Biparietal diameter was used for pregnancy dating from 12 weeks 5 days onward (>23 mm, n = 1790). For the 387 women who did not attend the prenatal visits, we retrieved gestational age from the Netherlands National Obstetric Register. Preterm birth was defined as delivery occurring at less than 37 weeks of gestation (n = 138), term birth as GAB in the window of 37 weeks 0 days through 41 weeks 6 days (n = 2718), and postterm birth as GAB of 42 weeks or greater (n = 223).33 For illustrative purposes, in eFigure 2 in the Supplement, we subdivided term birth into categories of early, full, and late term.33
Image Acquisition and Processing
Brain images were acquired on a 3.0-T MRI scanner (Discovery MR750, GE Healthcare) using an 8-channel head coil. After a localizer, T1-weighted structural images were acquired with an inversion recovery–prepared fast spoiled gradient recalled sequence. Sequence parameters (option BRAVO) were as follows: repetition time, 8.77 milliseconds; echo time, 3.4 milliseconds; inversion time, 600 milliseconds; flip angle, 10°; field of view, 220 × 220 mm; acquisition matrix, 220 × 220; slice thickness, 1 mm; number of slices, 230; voxel size, 1 × 1 × 1 mm; and arc acceleration, 2.34 Images were processed using FreeSurfer, version 6.0, an open source software for analyzing brain images.35,36 Global and regional volumes, including total brain, cerebral and cerebellar gray, and white matter volumes, as well as subcortical gray matter volumes, including thalamus, amygdala, hippocampus, putamen, pallidum, caudate, and nucleus accumbens, were measured. A surface-based stream quantified cortical thickness, cortical surface area, and gyrification. FreeSurfer morphometry had good test-retest reliability across scanners and field strengths.37,38 FreeSurfer reconstructions were visually inspected,30,31 and images not suitable for analysis were excluded. An automated quality metric39 found no correlation with GAB (r = −0.01, P = .53).
Covariates
Potential confounding variables were selected based on the existing literature.40,41 Self-reported questionnaire data and medical record data measured before child birth provided information on maternal ethnicity,42 age at intake, marital status, educational level, psychopathologic symptoms,43,44 smoking and alcohol use during pregnancy, and family income. Medical registries provided information on obstetric variables, including fetal distress, mode of delivery, 5-minute Apgar score, and calendar year of birth. Child sex and age at neuroimaging were also included.
Statistical Analysis
For descriptive purposes, children were categorized as preterm (<37 weeks), term (≥37 and <42 weeks), and postterm (≥42 weeks) delivery. Linear regression was used to investigate the association of the quantitative trait GAB with global and regional brain volumes (eFigure 3 in the Supplement). Subcortical volumes were standardized to enable comparison of the effect estimates of GAB between subcortical structures. Nonlinear associations of GAB with brain morphometry were examined using quadratic models and models with natural cubic splines with knots at 30, 32, 34, 37, 38, 39, 40, and 42 weeks. We performed additional analyses among children born at term to examine whether associations were also present in this narrow range of gestational duration. To investigate the association between GAB and surface-based cortical measures, we used vertexwise linear regression models with a custom in-house QDECR package. To compare the results with existing studies17,18,19,20,21,22 using clinically defined categories, we also applied linear regression models with the preterm, term, and postterm categories; term-born children served as the reference group.
Model 1 was minimally adjusted for child sex and age at neuroimaging assessment. Model 2 was further adjusted for maternal characteristics, including ethnicity, age at neuroimaging, marital status, educational level, psychopathologic symptoms, smoking and alcohol use during pregnancy, and family income. Calendar year of birth was not included as a covariate because it was not associated with gestational age or brain characteristics. Models with subcortical outcomes were additionally adjusted for intracranial volume.
In addition, we examined whether the association of GAB with brain morphometry was moderated by sex. To ensure that the associations of GAB with brain volumes were not driven by adverse perinatal events, children exposed to perinatal complications, including maternal preeclampsia, diabetes, pregnancy-induced hypertension, urgent cesarean delivery, intrauterine growth restriction, low birth weight (<2500 g), suspected fetal distress, a 5-minute Apgar score below 7, and premature or postterm birth, were excluded in sensitivity analyses.
Missing values of the covariates were imputed using multivariate imputation by chained equations with 10 imputations.45 We report pooled results. Statistical significance was defined as α < .05 (2-sided). In the primary analyses, a false discovery rate correction was applied to minimize false-positive findings attributable to multiple comparisons.46 Surface-based analyses were corrected for multiple testing using built-in gaussian Monte Carlo simulations.47 The cluster-forming threshold was set to P = .001 because this value corresponds closely to a false-positive rate of 0.05,48 with further Bonferroni correction for independent analysis of each hemisphere (P < .025 clusterwise). All statistical analyses were performed using R statistical software, version 3.5.1 (R Foundation for Statistical Computing).
For nonresponse analyses, we used analyses of variance or the Wilcoxon test for continuous variables and the χ2 test for categorical variables to compare maternal and child characteristics between responders and nonresponders at follow-up.
Results
Descriptive Statistics
This study evaluated 3079 children (1546 [50.2%] female) at 10 years of age. Table 1 presents information on the study population. Mothers of preterm-born children less frequently had a partner (109 [79.0%] vs 2405 [88.5%], P = .001), had a lower educational level (53 [38.4%] vs 1411 [51.9%], P = .01), and had a lower income (<€1200 [US $1550] per month: 33 [23.9%] vs 423 [15.6%], P < .001) than did mothers of children born at term. Mothers of children born preterm more often had preeclampsia, diabetes, and pregnancy-related hypertension (22 [15.9%] vs 179 [6.6%], P < .001) and more often underwent cesarean delivery (33 [23.9%] vs 306 [11.3%], P < .001). Maternal sociodemographic or lifestyle factors did not differ between the postterm and the term-born groups. However, children born postterm more often were delivered via cesarean (43 [19.3%] vs 306 [11.3%], P < .001) and more often had signs of fetal distress (27 [12.1%] vs 195 [7.2%], P = .01).
Table 1. Demographic Characteristics of the Study Populationa.
| Characteristic | Term birth (n = 2718) | Preterm birth (n = 138) | Postterm birth (n = 223) |
|---|---|---|---|
| Maternal characteristics | |||
| Age at intake, mean (SD), y | 31.0 (4.9) | 30.7 (5.1) | 31.4 (4.5) |
| Multiparous | 323 (11.9) | 17 (12.3) | 23 (10.3) |
| Pregnancy complicationsb | 179 (6.6) | 22 (15.9) | 11 (4.9) |
| Ethnicity | |||
| Dutch | 1569 (57.7) | 70 (50.7) | 132 (59.2) |
| Non-Dutch, Western | 323 (11.9) | 16 (11.6) | 36 (16.1) |
| Non-Dutch, non-Western | 826 (30.4) | 52 (37.7) | 55 (24.7) |
| Educational level | |||
| Primary or lower | 195 (7.2) | 13 (9.4) | 13 (5.8) |
| Secondary | 1112 (40.9) | 72 (52.2) | 82 (36.8) |
| Higher | 1411 (51.9) | 53 (38.4) | 128 (57.4) |
| Monthly household income, US$ | |||
| <1550 | 423 (15.6) | 33 (23.9) | 34 (15.2) |
| 1550-2580 | 463 (17.0) | 34 (24.6) | 23 (10.3) |
| >2580 | 1832 (67.4) | 71 (51.4) | 166 (74.4) |
| Marital status (married or with partner) | 2405 (88.5) | 109 (79.0) | 202 (90.6) |
| Smoking during pregnancy | |||
| Never in pregnancy | 2101 (77.3) | 104 (75.4) | 176 (78.9) |
| Until pregnancy was known | 231 (8.5) | 10 (7.2) | 23 (10.3) |
| Continued in pregnancy | 386 (14.2) | 24 (17.4) | 24 (10.8) |
| Alcohol use during pregnancy | |||
| Never in pregnancy | 1093 (40.2) | 53 (38.4) | 89 (39.9) |
| Until pregnancy was known | 380 (14.0) | 22 (15.9) | 25 (11.2) |
| Continued in pregnancy, occasionally | 985 (36.2) | 49 (35.5) | 85 (38.1) |
| Continued in pregnancy, frequentlyc | 260 (9.6) | 14 (10.1) | 24 (10.8) |
| Psychopathologic symptoms, mean (SD) | 0.3 (0.4) | 0.4 (0.4) | 0.3 (0.4) |
| Birth and child characteristics | |||
| Cesarean delivery | 306 (11.3) | 33 (23.9) | 43 (19.3) |
| Suspected fetal distress | 195 (7.2) | 15 (10.9) | 27 (12.1) |
| Apgar score at 5 min <7 | 28 (1.0) | 4 (2.9) | 2 (0.9) |
| Gestational age at birth, mean (SD) [range], wk | 40.0 (1.1) [37.0-41.9] | 34.6 (2.7) [26.3-36.9] | 42.3 (0.3) [42.0-43.4] |
| Birth weight, mean (SD), g | 3472.6 (485.9) | 2345.6 (654.7) | 3785.7 (475.6) |
| Male | 1340 (49.3) | 65 (47.1) | 128 (57.4) |
| Age at MRI, mean (SD), y | 10.1 (0.6) | 10.2 (0.6) | 10.1 (0.6) |
Abbreviation: MRI, magnetic resonance imaging.
Data are presented as number (percentage) of study participants unless otherwise indicated. No data were missing for these variables because they were imputed using multiple imputation methods. Categorization was based on gestational age at birth: term birth (gestational age of 37-42 weeks), preterm birth (gestational age <37 weeks), and postterm birth (gestational age of ≥42 weeks).
Pregnancy complications included preeclampsia, diabetes, and/or pregnancy-induced hypertension.
Frequent continued alcohol use is defined as 1 or more glasses of alcohol per week in at least 2 trimesters.
Global and Regional Brain Volumes
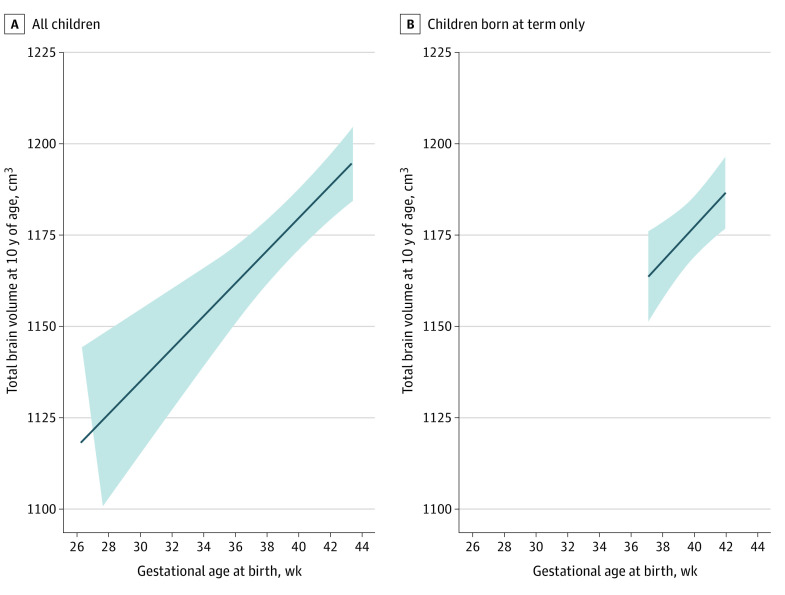
Table 2 and Figure 1 report a positive association of GAB and total brain volume (B = 4.5 cm3/wk; 95% CI, 2.7-6.3 cm3/wk). In addition, GAB was positively associated with cerebral gray matter (2.8 cm3/wk; 95% CI, 1.8-3.7 cm3/wk), cerebral white matter (1.2 cm3/wk; 95% CI, 0.3-2.0 cm3/wk), cerebellar gray matter (0.4 cm3/wk; 95% CI, 0.2-0.5 cm3/wk), cerebellar white matter (0.1 cm3/wk; 95% CI, 0.1-0.2 cm3/wk), and subcortical gray matter (0.2 cm3/wk; 95% CI, 0.1-0.2 cm3/wk) volume. These associations remained after correction for multiple comparisons. Of importance, the associations and effect estimates of GAB with global and regional brain volumes remained intact when restricting the sample to children born at term with the exception of cerebellar gray matter (Table 2 and Figure 1). Furthermore, we did not observe any interaction of sex in the association of GAB with global brain volumes (eTable 1 in the Supplement).
Table 2. Association of Gestational Age at Birth With Global Brain Volumesa.
| Model | Total brain volume | Cerebral gray matter volume | Cerebral white matter volume | Cerebellar gray matter volume | Cerebellar white matter volume | Subcortical gray matter volumeb | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Difference (95% CI) | P value | Difference (95% CI) | P value | Difference (95% CI) | P value | Difference (95% CI) | P value | Difference (95% CI) | P value | Difference (95% CI) | P value | |
| All children | ||||||||||||
| GAB, wk (n = 3079) | ||||||||||||
| Model 1 | 6.1 (4.2 to 8.0) | <.001 | 3.7 (2.7 to 4.7) | <.001 | 1.7 (0.9 to 2.6) | <.001 | 0.5 (0.3 to 0.7) | <.001 | 0.2 (0.1 to 0.2) | <.001 | 0.2 (0.1 to 0.2) | <.001 |
| Model 2 | 4.5 (2.7 to 6.3) | <.001c | 2.8 (1.8 to 3.7) | <.001c | 1.2 (0.3 to 2.0) | .006c | 0.4 (0.2 to 0.5) | <.001c | 0.1 (0.1 to 0.2) | <.001c | 0.2 (0.1 to 0.2) | <.001c |
| Term children | ||||||||||||
| GAB, wk (n = 2718) | ||||||||||||
| Model 1 | 6.7 (3.6 to 9.7) | <.001 | 3.8 (2.2 to 5.4) | <.001 | 2.3 (0.9 to 3.7) | .002 | 0.4 (0.1 to 0.8) | NA | 0.1 (0.05 to 0.2) | .003 | 0.1 (0.01 to 0.2) | .03 |
| Model 2 | 4.8 (1.8 to 7.7) | .002c | 2.8 (1.2 to 4.3) | <.001c | 1.6 (0.3 to 3.0) | .02c | 0.2 (−0.1 to 0.6) | NA | 0.1 (0.03 to 0.2) | .01c | 0.1 (0.01 to 0.2) | .03c |
Abbreviations: GAB, gestational age at birth; NA, not applicable.
In the results of these regression models, the effect estimates represent the difference in cubic centimeters for brain volumes per 1-week-longer gestational duration. Model 1 is a minimally adjusted model corrected for child sex and age at the neuroimaging assessment. Model 2 is a fully adjusted model, corrected for child sex and age at the neuroimaging assessment, maternal ethnicity, age at intake, marital status, educational level, psychopathologic condition, smoking and alcohol use during pregnancy, and family income.
Intracranial volume was additionally adjusted for in both models.
The associations survived a false discovery rate correction for multiple testing (applied model 2 only).
Figure 1. Linear Association Between Gestational Age at Birth and Total Brain Volumes in Children.
Linear association between gestational age at birth and total brain volume in all 3079 children (A) and the 2718 term-birth children (B) at 10 years of age. Models were fully adjusted and corrected for child sex, child age at the neuroimaging assessment, maternal ethnicity, maternal age at intake, marital status, educational level, psychopathologic symptoms during pregnancy, smoking and alcohol use during pregnancy, and family income. Shaded areas indicate the 95% CIs of the predicted values.
Post hoc analyses found that longer gestational duration was associated with larger volumes of the thalamus, caudate, putamen, and pallidum (eFigure 4 in the Supplement). No associations were observed between GAB and amygdala, hippocampus, or nucleus accumbens volume.
Cerebral Cortex Morphometry
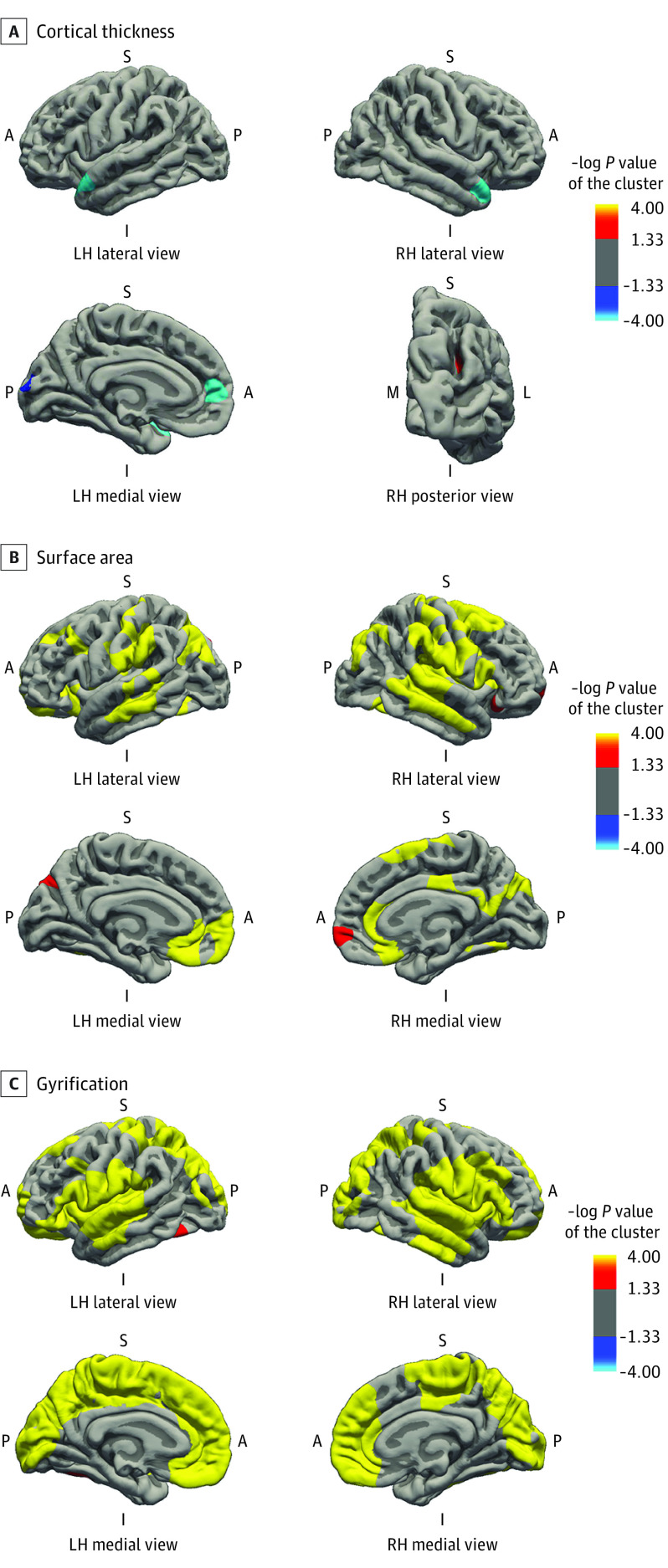
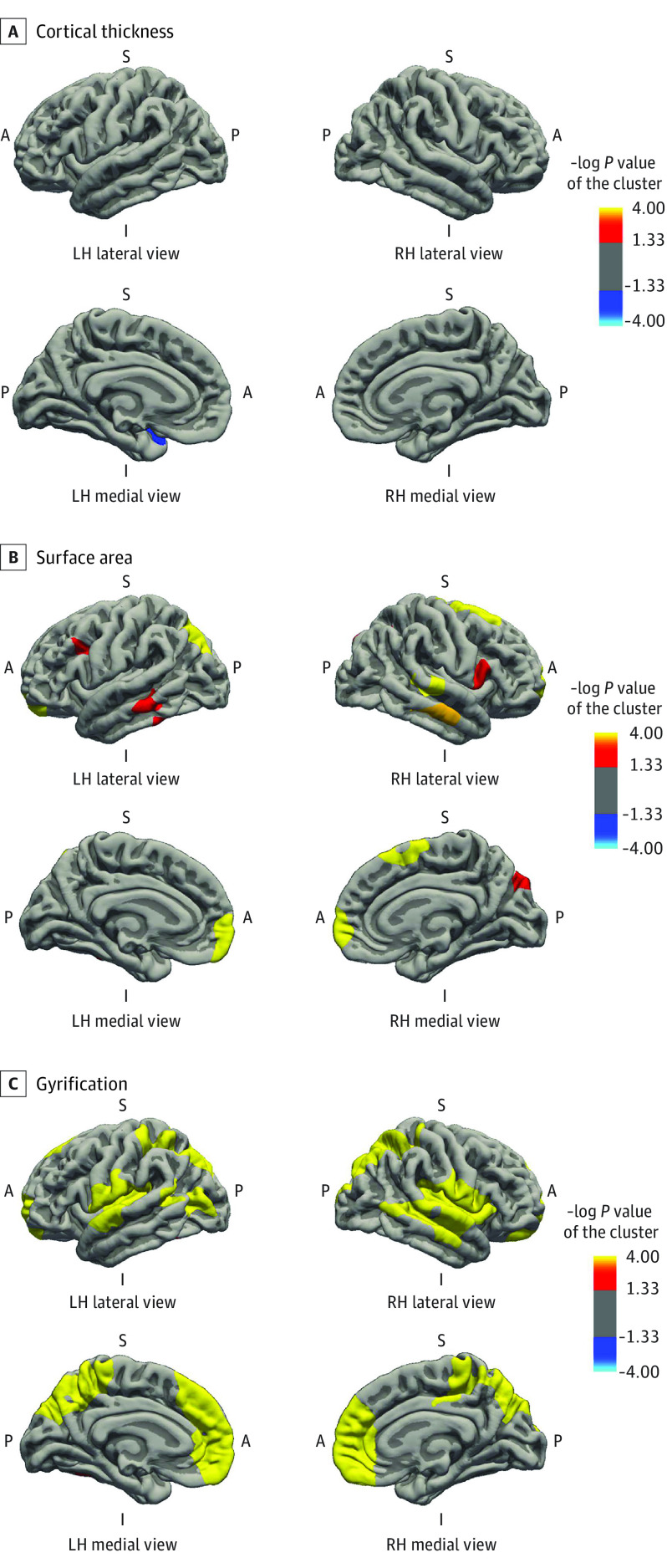
Cortical thickness and GAB were associated in a few small regions of the superior temporal lobe, cuneus, and inferior parietal lobe, exhibiting local reduction of cortical thickness (Figure 2A). In contrast, the association of GAB with cortical surface area was widespread across the neocortex (Figure 2B). Longer gestational duration was associated with larger surface area in the inferior and superior parietal regions, inferior and middle temporal regions, middle frontal and orbitofrontal regions, rostral anterior cingulate, and fusiform gyrus. Likewise, widespread positive associations of GAB and neocortical gyrification, including superior parietal lobe, postcentral region, fusiform gyrus, insular cortex, and anterior cingulate cortex, were observed (Figure 2C). Results were similar in children born at term (Figure 3). Gestational age at birth was associated with a thinner cortex in the superior temporal region (Figure 3A), with larger surface area and gyrification in the frontal, parietal, and temporal regions (Figure 3B and C). Specific information on the associated brain regions (size, Talairach coordinates, and P values) are presented in eTables 2 and 3 in the Supplement.
Figure 2. Gestational Age at Birth and Cortical Thickness, Surface Area, and Gyrification in All Children.
Surface-based analysis was performed for 3065 children with a gestational age at birth ranging from 26.3 to 43.4 weeks. The model was adjusted for child sex, child age at neuroimaging, maternal ethnicity, maternal age at intake, marital status, educational level, psychopathologic conditions during pregnancy, smoking and alcohol use during pregnancy, and family income. Colored clusters represent regions of the brain that were positively (red to yellow) and negatively (dark to light blue) associated with gestational age at birth that survived the clusterwise (Monte Carlo simulation with 5000 iterations) correction for multiple comparisons (P < .001) (eTable 2 in the Supplement). A indicates anterior; I, inferior; L, lateral; LH, left hemisphere; M, medial; P, posterior; RH, right hemisphere; S, superior.
Figure 3. Gestational Age at Birth and Cortical Thickness, Surface Area, and Gyrification in Children Born at Term.
Surface-based analysis was performed for 2706 children with a gestational age at birth ranging from 37.0 weeks to 41.9 weeks. The model was adjusted for child sex, child age at neuroimaging, maternal ethnicity, maternal age at intake, marital status, educational level, psychopathologic conditions during pregnancy, smoking and alcohol use during pregnancy, and family income. Colored clusters represent regions of the brain that were positively (red to yellow) and negatively (dark to light blue) associated with gestational age at birth that survived the clusterwise (Monte Carlo simulation with 5000 iterations) correction for multiple comparisons (P < .001) (eTable 3 in the Supplement). A indicates anterior; I, inferior; L, lateral; LH, left hemisphere; M, medial; P, posterior; RH, right hemisphere; S, superior.
Nonlinear Associations
We found no evidence of nonlinear associations of GAB with brain volume and morphometry. Models with quadratic or natural cubic splines did not improve model fit compared with linear models (eTable 4 in the Supplement).
Categorical Analyses
With the use of a categorical approach, children born preterm had a smaller total brain volume, cerebral gray matter volume, subcortical gray matter volume, cerebellar gray matter volume, and cerebellar white matter volume. Total brain volume of children born preterm had an adjusted difference of 26.5 cm3 compared with children born at term (B = −26.5; 95% CI, −42.1 to −11.0; P < .001). Children born postterm had a larger cerebral gray matter volume and subcortical gray matter volume (eTable 5 in the Supplement).
Sensitivity Analyses
Sensitivity analyses of children born at term without perinatal complications (n = 2264) showed similar associations between GAB and total brain matter (5.5 cm3/wk; 95% CI, 2.2-8.7 cm3/wk), cerebral gray matter (3.1 cm3/wk; 95% CI, 1.4-4.8 cm3/wk), cerebral white matter (2.1 cm3/wk; 95% CI, 0.5-3.6 cm3/wk), cerebellar white matter (0.1 cm3/wk; 95% CI, 0.02-0.2 cm3/wk), and subcortical gray matter (0.1 cm3/wk; 95% CI, 0.01-0.2 cm3/wk) volumes. However, in this subgroup, GAB was not associated with cerebellar gray matter volume (0.1 cm3/wk; 95% CI, −0.2 to 0.5 cm3/wk).
Nonresponse Analyses
Respondents differed from nonrespondents (eTable 6 in the Supplement). Specifically, respondents were older, were more likely of Dutch origin, had a higher educational level, and were less likely to smoke during pregnancy.
Discussion
In this longitudinal, population-based cohort study, we found that GAB was positively associated with global and regional brain volumes in children at 10 years of age. Our findings suggest that the volumetric association with GAB is a consequence of larger cortical surface area and gyrification in the absence of widespread differences in cortical thickness. Our results were robust to confounding by several sociodemographic and lifestyle characteristics. Of importance, we found that these associations remained present when restricting the analyses exclusively to children born at term, which supports a model in which gestational duration should be viewed as a continuum of development throughout pregnancy. Moreover, we observed no evidence of nonlinear associations using spline regression analyses, a flexible and sensitive method for assessing nonlinearity. In addition, child sex did not moderate the association of GAB with brain morphometry. Overall, these findings suggest that a cutoff for the designation of preterm birth as less than 37 weeks of gestation may not be consistent with brain development.
By reporting associations of GAB with global and regional brain volumes, our findings complement previous neuroimaging studies demonstrating that longer gestational duration was associated with larger temporal and parietal gray matter volumes25 and reporting linear associations of higher GA with enhanced local and global network efficiency.26 These results are further supported by our findings that GAB was associated with brain regions that are functionally and structurally integrated (eg, the cerebellum and thalamus) (eFigure 4 in the Supplement). In addition, temporal and parietal regions (Figure 2 and Figure 3) are involved in higher-order cognitive processes, including auditory perception and processing, language production and perception, and declarative memory. Even though larger brain size typically has been associated with enhanced cognitive functioning (including general cognitive ability, working memory, reading, vocabulary, and set-shifting tasks) in children and adolescents,49,50 a previous longitudinal study51 found that neuroanatomical correlates of intelligence, for example, are dynamic and change throughout life. The association between psychiatric disorders and brain size is less evident, but clinical studies52,53,54 in children with ADHD found smaller volumes in frontal, parietal, and cerebellar structures. Furthermore, individual variations in brain size and shape occur.55 Caution is warranted regarding potential functional consequences of the observed association between GAB and brain morphometry.
Several mechanisms are possible. First, the largest absolute volumetric increase in brain volume during fetal development occurs during the third trimester, in particular, the expansion of cortical gray matter.13,56,57,58 The growth rate of the cerebellum also peaks during the third trimester.59 Therefore, on the basis of the observed linear association with global brain volume across the full range of GAB, a parsimonious explanation could be that birth is necessary and sufficient to attenuate the rapid expansion of the neocortex in the late third trimester. In contrast, we did not observe extensive differences by GAB in cortical thickness. Cortical neurogenesis and proliferation are largely complete by the middle of gestation.13,14 In addition, cortical lamination (ie, inside-out layering of the cortex) is already well established by the eighth month of pregnancy, although timing appears to differ slightly among brain regions.14
Second, GAB is often associated with multiple factors,60 including psychological distress, maternal age, substance use, poor nutrition, or fetal growth restriction. Such stress-associated factors may affect the timing of birth and fetal maturation. In line with the developmental origins of health and disease framework, the current study suggests that even small variations in GAB may be associated with fetal programming differences in neurodevelopment.61 Our results suggest that among healthy term-born children, GAB may be linearly and positively associated with brain volume, surface area, and gyrification when assessed in childhood at 10 years of age. Although we considered a variety of confounding factors, we cannot exclude the possibility of residual confounding.
Third, our results could potentially be explained by genetic predisposition. Previous studies30,62,63,64,65 reported genetic variations that are associated with gestational duration. For instance, Adkins et al62 found polymorphisms of insulin and insulinlike growth factor 2 associated with an increased risk of being small for gestational age. Moreover, several maternal and fetal genes have been identified through recent genome-wide association studies63,64 of gestational duration and preterm birth. Genetic factors associated with global and specific brain volumes have also been reported.30,65 Thus, our findings potentially can be explained by a shared genetic susceptibility that is associated with gestational duration and brain morphometry. However, it is likely that both genetic and environmental factors explain the observed associations.
Further research is needed to investigate underlying mechanisms and causal pathways of the association between GAB with childhood brain structure and function. The results would be particularly important for obstetricians, neonatologists, and pediatricians. The findings of the present study have substantial potential clinical importance, considering the discussions regarding expectant management vs labor induction66 and the increasing prevalence of elective cesarean deliveries worldwide, which are typically planned 1 or 2 weeks before the estimated full-term date.67 In line with recommendations of the World Health Organization, our results cautiously support a reduction of elective cesarean deliveries.
Strengths and Limitations
Study strengths were the prospective design, which enabled a temporal association between GA and brain morphometry; measurement of GAB by ultrasonography; adjustment for multiple confounders; and large-scale pediatric neuroimaging (>3000 children), allowing for detection of small effect sizes.
Several limitations should also be mentioned. First, brain morphometry was measured only once, at 10 years of age; thus, whether the observed differences were transient or persistent is unknown. Second, although childhood brain structure has been associated with a diversity of cognitive, emotional, and sensorimotor functions in the general population,68 we cannot yet address the functional implications of the observed morphologic differences. Although the current study was specifically focused on examining brain morphometry, future studies should investigate the associations of GAB with repeated assessments of brain morphometry and multimodal imaging in combination with behavioral and cognitive outcomes in large population-based cohorts. Third, the nonresponse analyses suggested a possible selection bias. Fourth, because this was an observational study, unmeasured residual (genetic and environmental) confounding limited the ability to establish causal inferences.
Conclusions
The findings of this study suggest that GAB is associated with widespread differences in childhood brain morphometry in children born at term. Longer gestational duration was associated with larger brain volume, cortical surface area, and cortical gyrification. Thus, physiologic processes during in utero development may have an enduring influence across the life span. Our findings highlight the importance of the last few weeks of pregnancy in association with neurodevelopment for which additional studies are warranted to evaluate the potential effect on international clinical guidelines for elective cesarean delivery.
eFigure 1. Flow Diagram
eFigure 2. Global Brain Volumes in Children Born Early Term, Full Term, and Late Term
eFigure 3. Linear Relationship Between Gestational Age at Birth and Total Brain Volumes kin Children With Heatmap
eFigure 4. The Association of Gestational Age at Birth With Subcortical Gray Matter Volumes in Children
eTable 1. Gestational Age at Birth and Cortical Morphology at 10 Years: Interaction of GA With Sex
eTable 2. Gestational Age at Birth and Cortical Morphology at 10 Years: Surface-Based Clusters in All Children
eTable 3. Gestational Age at Birth and Cortical Morphology at 10 Years: Surface-Based Clusters in Children Born at Term
eTable 4. Comparison of Linear and Nonlinear (Quadratic and Natural Cubic Spline) Models Using The Likelihood Ratio Test
eTable 5. The Association of Gestational Age at Birth in Categories With Global Brain Volumes
eTable 6. Nonresponse Analysis
References
- 1.Beck S, Wojdyla D, Say L, et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ. 2010;88(1):31-38. doi: 10.2471/BLT.08.062554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of under-5 mortality in 2000-15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet. 2016;388(10063):3027-3035. doi: 10.1016/S0140-6736(16)31593-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Linder N, Hiersch L, Fridman E, et al. Post-term pregnancy is an independent risk factor for neonatal morbidity even in low-risk singleton pregnancies. Arch Dis Child Fetal Neonatal Ed. 2017;102(4):F286-F290. doi: 10.1136/archdischild-2015-308553 [DOI] [PubMed] [Google Scholar]
- 4.Reddihough DS, Collins KJ. The epidemiology and causes of cerebral palsy. Aust J Physiother. 2003;49(1):7-12. doi: 10.1016/S0004-9514(14)60183-5 [DOI] [PubMed] [Google Scholar]
- 5.Brydges CR, Landes JK, Reid CL, Campbell C, French N, Anderson M. Cognitive outcomes in children and adolescents born very preterm: a meta-analysis. Dev Med Child Neurol. 2018;60(5):452-468. doi: 10.1111/dmcn.13685 [DOI] [PubMed] [Google Scholar]
- 6.Lean RE, Paul RA, Smyser TA, Smyser CD, Rogers CE. Social adversity and cognitive, language, and motor development of very preterm children from 2 to 5 years of age. J Pediatr. 2018;203:177-184 e171. [DOI] [PMC free article] [PubMed]
- 7.Ask H, Gustavson K, Ystrom E, et al. Association of gestational age at birth with symptoms of attention-deficit/hyperactivity disorder in children. JAMA Pediatr. 2018;172(8):749-756. doi: 10.1001/jamapediatrics.2018.1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sucksdorff M, Lehtonen L, Chudal R, et al. Preterm birth and poor fetal growth as risk factors of attention-deficit/hyperactivity disorder. Pediatrics. 2015;136(3):e599-e608. doi: 10.1542/peds.2015-1043 [DOI] [PubMed] [Google Scholar]
- 9.Bröring T, Oostrom KJ, van Dijk-Lokkart EM, Lafeber HN, Brugman A, Oosterlaan J. Attention deficit hyperactivity disorder and autism spectrum disorder symptoms in school-age children born very preterm. Res Dev Disabil. 2018;74:103-112. doi: 10.1016/j.ridd.2018.01.001 [DOI] [PubMed] [Google Scholar]
- 10.Nosarti C, Reichenberg A, Murray RM, et al. Preterm birth and psychiatric disorders in young adult life. Arch Gen Psychiatry. 2012;69(6):E1-E8. doi: 10.1001/archgenpsychiatry.2011.1374 [DOI] [PubMed] [Google Scholar]
- 11.Doherty L, Norwitz ER. Prolonged pregnancy: when should we intervene? Curr Opin Obstet Gynecol. 2008;20(6):519-527. doi: 10.1097/GCO.0b013e328314b6f8 [DOI] [PubMed] [Google Scholar]
- 12.Glover Williams A, Odd D. Investigating the association between post-term birth and long term cognitive, developmental and educational impacts: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2020;33(7):1253-1265. doi: 10.1080/14767058.2018.1514379 [DOI] [PubMed] [Google Scholar]
- 13.Keunen K, Counsell SJ, Benders MJNL. The emergence of functional architecture during early brain development. Neuroimage. 2017;160:2-14. doi: 10.1016/j.neuroimage.2017.01.047 [DOI] [PubMed] [Google Scholar]
- 14.Reynolds CR, Fletcher-Janzen E, eds. Handbook of Clinical Child Neuropsychology. 3rd ed. Springer; 2009. doi: 10.1007/978-0-387-78867-8 [DOI] [Google Scholar]
- 15.Clouchoux C, Guizard N, Evans AC, du Plessis AJ, Limperopoulos C. Normative fetal brain growth by quantitative in vivo magnetic resonance imaging. Am J Obstet Gynecol. 2012;206(2):173 e171-178. [DOI] [PubMed]
- 16.Kostovic I, Vasung L. Insights from in vitro fetal magnetic resonance imaging of cerebral development. Semin Perinatol. 2009;33(4):220-233. doi: 10.1053/j.semperi.2009.04.003 [DOI] [PubMed] [Google Scholar]
- 17.Lemola S, Oser N, Urfer-Maurer N, et al. Effects of gestational age on brain volume and cognitive functions in generally healthy very preterm born children during school-age: a voxel-based morphometry study. PLoS One. 2017;12(8):e0183519. doi: 10.1371/journal.pone.0183519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soria-Pastor S, Padilla N, Zubiaurre-Elorza L, et al. Decreased regional brain volume and cognitive impairment in preterm children at low risk. Pediatrics. 2009;124(6):e1161-e1170. doi: 10.1542/peds.2009-0244 [DOI] [PubMed] [Google Scholar]
- 19.Lax ID, Duerden EG, Lin SY, et al. Neuroanatomical consequences of very preterm birth in middle childhood. Brain Struct Funct. 2013;218(2):575-585. doi: 10.1007/s00429-012-0417-2 [DOI] [PubMed] [Google Scholar]
- 20.Nagy Z, Ashburner J, Andersson J, et al. Structural correlates of preterm birth in the adolescent brain. Pediatrics. 2009;124(5):e964-e972. doi: 10.1542/peds.2008-3801 [DOI] [PubMed] [Google Scholar]
- 21.Rogers CE, Barch DM, Sylvester CM, et al. Altered gray matter volume and school age anxiety in children born late preterm. J Pediatr. 2014;165(5):928-935. doi: 10.1016/j.jpeds.2014.06.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson DK, Matthews LG, Alexander B, et al. Tracking regional brain growth up to age 13 in children born term and very preterm. Nat Commun. 2020;11(1):696. doi: 10.1038/s41467-020-14334-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Espel EV, Glynn LM, Sandman CA, Davis EP. Longer gestation among children born full term influences cognitive and motor development. PLoS One. 2014;9(11):e113758. doi: 10.1371/journal.pone.0113758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang S, Platt RW, Kramer MS. Variation in child cognitive ability by week of gestation among healthy term births. Am J Epidemiol. 2010;171(4):399-406. doi: 10.1093/aje/kwp413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis EP, Buss C, Muftuler LT, et al. Children’s brain development benefits from longer gestation. Front Psychol. 2011;2:1. doi: 10.3389/fpsyg.2011.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim DJ, Davis EP, Sandman CA, et al. Longer gestation is associated with more efficient brain networks in preadolescent children. Neuroimage. 2014;100:619-627. doi: 10.1016/j.neuroimage.2014.06.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kooijman MN, Kruithof CJ, van Duijn CM, et al. The Generation R Study: design and cohort update 2017. Eur J Epidemiol. 2016;31(12):1243-1264. doi: 10.1007/s10654-016-0224-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 29.Santolaya J, Faro R. Twins: twice more trouble? Clin Obstet Gynecol. 2012;55(1):296-306. doi: 10.1097/GRF.0b013e3182446f51 [DOI] [PubMed] [Google Scholar]
- 30.Hibar DP, Stein JL, Renteria ME, et al. ; Alzheimer’s Disease Neuroimaging Initiative; CHARGE Consortium; EPIGEN; IMAGEN; SYS . Common genetic variants influence human subcortical brain structures. Nature. 2015;520(7546):224-229. doi: 10.1038/nature14101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muetzel RL, Blanken LME, van der Ende J, et al. Tracking brain development and dimensional psychiatric symptoms in children: a longitudinal population-based neuroimaging study. Am J Psychiatry. 2018;175(1):54-62. doi: 10.1176/appi.ajp.2017.16070813 [DOI] [PubMed] [Google Scholar]
- 32.Verburg BO, Steegers EA, De Ridder M, et al. New charts for ultrasound dating of pregnancy and assessment of fetal growth: longitudinal data from a population-based cohort study. Ultrasound Obstet Gynecol. 2008;31(4):388-396. doi: 10.1002/uog.5225 [DOI] [PubMed] [Google Scholar]
- 33.Spong CY. Defining “term” pregnancy: recommendations from the Defining “Term” Pregnancy Workgroup. JAMA. 2013;309(23):2445-2446. doi: 10.1001/jama.2013.6235 [DOI] [PubMed] [Google Scholar]
- 34.White T, Muetzel RL, El Marroun H, et al. Paediatric population neuroimaging and the Generation R Study: the second wave. Eur J Epidemiol. 2018;33(1):99-125. doi: 10.1007/s10654-017-0319-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fischl B. FreeSurfer. Neuroimage. 2012;62(2):774-781. doi: 10.1016/j.neuroimage.2012.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fischl B, van der Kouwe A, Destrieux C, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14(1):11-22. doi: 10.1093/cercor/bhg087 [DOI] [PubMed] [Google Scholar]
- 37.Han X, Jovicich J, Salat D, et al. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32(1):180-194. doi: 10.1016/j.neuroimage.2006.02.051 [DOI] [PubMed] [Google Scholar]
- 38.Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61(4):1402-1418. doi: 10.1016/j.neuroimage.2012.02.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White T, Jansen PR, Muetzel RL, et al. Automated quality assessment of structural magnetic resonance images in children: comparison with visual inspection and surface-based reconstruction. Hum Brain Mapp. 2018;39(3):1218-1231. doi: 10.1002/hbm.23911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spann MN, Bansal R, Hao X, Rosen TS, Peterson BS. Prenatal socioeconomic status and social support are associated with neonatal brain morphology, toddler language and psychiatric symptoms. Child Neuropsychol. 2020;26(2):170-188. doi: 10.1080/09297049.2019.1648641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El Marroun H, Zeegers M, Steegers EA, et al. Post-term birth and the risk of behavioural and emotional problems in early childhood. Int J Epidemiol. 2012;41(3):773-781. doi: 10.1093/ije/dys043 [DOI] [PubMed] [Google Scholar]
- 42.Centraal Bureau voor de Statistiek . Allochtonen in Nederland 2004. Statistics Netherlands, Voorburg/Heerlen; 2004. [Google Scholar]
- 43.Beurs E. Brief Symptom Inventory Handleiding Addendum. PITS BV; 2009. [Google Scholar]
- 44.Derogatis LR. The Brief Symptom Inventory (BSI): Administration, Scoring and Procedures Manual. National Computer Systems; 1993. [Google Scholar]
- 45.van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate Imputation by Chained Equations in R. J Stat Softw. 2011;45(3):67. doi: 10.18637/jss.v045.i03 [DOI] [Google Scholar]
- 46.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289-300. doi: 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 47.Hagler DJ Jr, Saygin AP, Sereno MI. Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. Neuroimage. 2006;33(4):1093-1103. doi: 10.1016/j.neuroimage.2006.07.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greve DN, Fischl B. False positive rates in surface-based anatomical analysis. Neuroimage. 2018;171:6-14. doi: 10.1016/j.neuroimage.2017.12.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pangelinan MM, Zhang G, VanMeter JW, Clark JE, Hatfield BD, Haufler AJ. Beyond age and gender: relationships between cortical and subcortical brain volume and cognitive-motor abilities in school-age children. Neuroimage. 2011;54(4):3093-3100. doi: 10.1016/j.neuroimage.2010.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moore DM, D’Mello AM, McGrath LM, Stoodley CJ. The developmental relationship between specific cognitive domains and grey matter in the cerebellum. Dev Cogn Neurosci. 2017;24:1-11. doi: 10.1016/j.dcn.2016.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shaw P. Intelligence and the developing human brain. Bioessays. 2007;29(10):962-973. doi: 10.1002/bies.20641 [DOI] [PubMed] [Google Scholar]
- 52.Wyciszkiewicz A, Pawlak MA, Krawiec K. Cerebellar volume in children with attention-deficit hyperactivity disorder (ADHD). J Child Neurol. 2017;32(2):215-221. doi: 10.1177/0883073816678550 [DOI] [PubMed] [Google Scholar]
- 53.Castellanos FX, Lee PP, Sharp W, et al. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA. 2002;288(14):1740-1748. doi: 10.1001/jama.288.14.1740 [DOI] [PubMed] [Google Scholar]
- 54.Mostofsky SH, Cooper KL, Kates WR, Denckla MB, Kaufmann WE. Smaller prefrontal and premotor volumes in boys with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2002;52(8):785-794. doi: 10.1016/S0006-3223(02)01412-9 [DOI] [PubMed] [Google Scholar]
- 55.Reardon PK, Seidlitz J, Vandekar S, et al. Normative brain size variation and brain shape diversity in humans. Science. 2018;360(6394):1222-1227. doi: 10.1126/science.aar2578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoshida R, Ishizu K, Yamada S, et al. Dynamics of gyrification in the human cerebral cortex during development. Congenit Anom (Kyoto). 2017;57(1):8-14. doi: 10.1111/cga.12179 [DOI] [PubMed] [Google Scholar]
- 57.Huang H, Zhang J, Wakana S, et al. White and gray matter development in human fetal, newborn and pediatric brains. Neuroimage. 2006;33(1):27-38. doi: 10.1016/j.neuroimage.2006.06.009 [DOI] [PubMed] [Google Scholar]
- 58.Matthews LG, Walsh BH, Knutsen C, et al. Brain growth in the NICU: critical periods of tissue-specific expansion. Pediatr Res. 2018;83(5):976-981. doi: 10.1038/pr.2018.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koning IV, Dudink J, Groenenberg IAL, Willemsen SP, Reiss IKM, Steegers-Theunissen RPM. Prenatal cerebellar growth trajectories and the impact of periconceptional maternal and fetal factors. Hum Reprod. 2017;32(6):1230-1237. doi: 10.1093/humrep/dex079 [DOI] [PubMed] [Google Scholar]
- 60.Mittendorf R, Williams MA, Berkey CS, Lieberman E, Monson RR. Predictors of human gestational length. Am J Obstet Gynecol. 1993;168(2):480-484. doi: 10.1016/0002-9378(93)90476-Y [DOI] [PubMed] [Google Scholar]
- 61.Baird J, Jacob C, Barker M, et al. Developmental origins of health and disease: a lifecourse approach to the prevention of non-communicable diseases. Healthcare (Basel). 2017;5(1):E14. doi: 10.3390/healthcare5010014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Adkins RM, Krushkal J, Klauser CK, Magann EF, Morrison JC, Somes G. Association between small for gestational age and paternally inherited 5′ insulin haplotypes. Int J Obes (Lond). 2008;32(2):372-380. doi: 10.1038/sj.ijo.0803700 [DOI] [PubMed] [Google Scholar]
- 63.Zhang G, Feenstra B, Bacelis J, et al. Genetic associations with gestational duration and spontaneous preterm birth. N Engl J Med. 2017;377(12):1156-1167. doi: 10.1056/NEJMoa1612665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu X, Helenius D, Skotte L, et al. Variants in the fetal genome near pro-inflammatory cytokine genes on 2q13 associate with gestational duration. Nat Commun. 2019;10(1):3927. doi: 10.1038/s41467-019-11881-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Adams HH, Hibar DP, Chouraki V, et al. Novel genetic loci underlying human intracranial volume identified through genome-wide association. Nat Neurosci. 2016;19(12):1569-1582. doi: 10.1038/nn.4398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grobman WA, Rice MM, Reddy UM, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network . Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379(6):513-523. doi: 10.1056/NEJMoa1800566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boerma T, Ronsmans C, Melesse DY, et al. Global epidemiology of use of and disparities in caesarean sections. Lancet. 2018;392(10155):1341-1348. doi: 10.1016/S0140-6736(18)31928-7 [DOI] [PubMed] [Google Scholar]
- 68.Batista-García-Ramó K, Fernández-Verdecia CI. What we know about the brain structure-function relationship. Behav Sci (Basel). 2018;8(4):E39. doi: 10.3390/bs8040039 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Flow Diagram
eFigure 2. Global Brain Volumes in Children Born Early Term, Full Term, and Late Term
eFigure 3. Linear Relationship Between Gestational Age at Birth and Total Brain Volumes kin Children With Heatmap
eFigure 4. The Association of Gestational Age at Birth With Subcortical Gray Matter Volumes in Children
eTable 1. Gestational Age at Birth and Cortical Morphology at 10 Years: Interaction of GA With Sex
eTable 2. Gestational Age at Birth and Cortical Morphology at 10 Years: Surface-Based Clusters in All Children
eTable 3. Gestational Age at Birth and Cortical Morphology at 10 Years: Surface-Based Clusters in Children Born at Term
eTable 4. Comparison of Linear and Nonlinear (Quadratic and Natural Cubic Spline) Models Using The Likelihood Ratio Test
eTable 5. The Association of Gestational Age at Birth in Categories With Global Brain Volumes
eTable 6. Nonresponse Analysis