Abstract
Objective:
To explore the clinical and pathological features of gastric signet ring cell carcinoma, and evaluate the survival impact of preoperative radiotherapy on these patients.
Methods:
The Surveillance, Epidemiology, and End Results database was used to extract eligible patients from 2004 to 2015. The patients were divided into those with and without preoperative radiotherapy. The categorical variables were described by chi-square tests. The patients’ survival was compared between the 2 groups by Kaplan-Meier method with log-rank tests. Cox proportional hazard model was adopted to identify prognostic factors of cancer-specific survival.
Results:
Totally 4771 patients were recruited, of whom 218(4.6%) patients received preoperative radiotherapy, while 4553(95.4%) patients didn’t receive this treatment. Survival analysis of the entire cohort demonstrated that preoperative radiotherapy improved both cancer-specific survival and overall survival (p < 0.001) of the patients. Cox proportional hazard models identified age >60, tumor size >50 mm, TNM stage II-IV as independent risk factors for poor prognosis (HR > 1, p < 0.05). Notably, preoperative radiotherapy was identified as an independent protective factor for favorable prognosis (HR < 1, p < 0.05). Subgroup survival analysis showed that preoperative radiotherapy exerted significant survival benefits for the stages III and IV patients.
Conclusions:
In this population-based study, preoperative radiotherapy is associated with significant survival benefits for the patients with advanced gastric signet ring cell carcinoma. Hence preoperative radiotherapy is feasible for these patients.
Keywords: gastric signet ring cell carcinoma, preoperative radiotherapy, survival
Introduction
Gastric cancer (GC) is the third greatest cause of cancer-related death worldwide.1 In America, about 11,140 deaths were estimated from this disease in 2019.2 The median survival of GC is less than 12 months at advanced stages.3 Adenocarcinoma accounts for the vast majority of GC.4 Gastric signet ring cell carcinoma (SRC) is a unique subtype of gastric adenocarcinoma. In WHO classification, SRC was defined as tumor cell with central optically clear, globoid droplet of cytoplasmic mucin with an eccentrically placed nucleus.5 Recent studies have revealed that the incidence of gastric SRC has been increasing constantly.6 Gastric SRC is also associated with aggressive tumor behavior and early metastasis, posing a major public health problem.7
With respect to the treatment of GC, surgery remains the mainstay for localized GC.8 However, the survival still remains poor for surgery alone, the 5-year survival rate was only 20%-50%, leading to the efforts to improve the prognosis of these patients with adjuvant approaches.9 The INT0116 trial was a milestone study which reported significant survival benefits from adjuvant chemoradiotherapy for the GC patients after gastrectomy.10 Furthermore, the MAGIC trial also demonstrated superior survival of perioperative chemotherapy and surgery as compared to surgery alone for GC.11 On the other hand, the ARTIST trial revealed that adjuvant radiotherapy (RT) combined with chemotherapy did not have a positive impact on the patients’ survival.12 The CRITICS trial also showed that in patients who received preoperative chemotherapy, postoperative chemoradiotherapy did not improve survival as compared to postoperative chemotherapy.13 With the growth of evidence, the benefits of neoadjuvant/adjuvant radiotherapy have become more controversial as therapeutic options. Preoperative chemoradiotherapy has been reported to show significant downstaging, facilitate radical tumor resection, and reduce local relapse for potentially resectable GC.14 However, this therapeutic modality is not the standard of care, with unpredictable outcomes. As a special subtype of GC, the survival impact of preoperative RT on patients with gastric SRC has not been clarified yet. This effect needs to be evaluated, so that clinicians can select more appropriate treatments for these patients.
Patients and Methods
Patient Selection
All the data were extracted from Surveillance, Epidemiology, and End Results (SEER) database (with additional treatment fields). The patients were selected by SEER*Stat version 8.3.5 software directly. The SEER data contain no identifiers and are publicly available, so ethical approval was exempt for our study. We designed the following inclusion criteria: (1) all patients were diagnosed from 2004 to 2015; (2) primary site was stomach; (3) primary gastric cancer was the first or only cancer diagnosis; (4) histological type was confined only to signet ring cell carcinoma (ICD-03, 8490/3); (5) surgery was performed; (6) chemotherapy recode was “yes”. We excluded the patients with unknown information about table variables.
Data Collection
The extracted table variables were: age at diagnosis, gender, race, marital status, tumor size, grade, TNM stage, tumor depth, LN metastasis, radiation, histological type, survival months, SEER cause-specific death classification, and vital status recode. Cancer-specific survival (CSS) was defined as the time from cancer diagnosis to the date of death caused by gastric SRC specifically. Overall survival (OS) was defined as the duration from diagnosis to death from any cause. In this study, the primary endpoint was CSS, and the secondary endpoint was OS.
Statistical Analysis
The eligible patients were divided into those with and without preoperative RT. The categorical variables were compared by chi-square tests. The survival differences between the 2 groups were evaluated by Kaplan-Meier method with log-rank tests. Cox proportional hazard models were utilized to identify prognostic factors associated with CSS. Factors with p < 0.05 in univariate Cox model were further adjusted by multivariate Cox analysis. The statistical analyses were completed by SPSS statistical software, version 25.0 (SPSS, Chicago, IL, USA). A two-tailed p < 0.05 was deemed statistically significant.
Results
Patient Characteristics
We recruited 4771 eligible patients with gastric SRC during the study period. In this cohort, 4553(95.4%) patients didn’t receive preoperative RT, while 218 (4.6%) patients received preoperative RT. There were significant differences between the 2 groups in terms of gender, race, marital status, tumor size, TNM stage, tumor depth, LN metastasis (p < 0.05). Compared with the patients not radiated, those patients who received preoperative RT were more likely to be male (78.4% vs 51.6%), white race (89.0% vs 70.2%). The marital status also displayed significant difference between the 2 comparison groups, with married 68.8% in RT group versus 59.7% in no RT group (p < 0.05). As for tumor characteristics, the RT group showed more tumor size ≤50 mm, more patients with stage II/III, T2/T3, N1. The distributions of age and grade were comparable between the 2 groups (p > 0.05). Patient demographics and clinical features are summarized below (Table 1).
Table 1.
Baseline Characteristics of the Patients Dichotomized by Preoperative Radiotherapy.
| No RT | RT | Total | ||
|---|---|---|---|---|
| Characteristics | n = 4553 (95.4%) | n = 218 (4.6%) | n = 4771 (100%) | p value |
| Age (years) | 0.435 | |||
| ≤60 | 2044(44.9%) | 92(42.2%) | 2136(44.8%) | |
| >60 | 2509(55.1%) | 126(57.8%) | 2635(55.2%) | |
| Gender | <0.001 | |||
| Male | 2351(51.6%) | 171(78.4%) | 2522(52.9%) | |
| Female | 2202(48.4%) | 47(21.6%) | 2249(47.1%) | |
| Race | <0.001 | |||
| White | 3195(70.2%) | 194(89.0%) | 3389(71.0%) | |
| Black | 541(11.9%) | 10(4.6%) | 551(11.5%) | |
| Others | 817(17.9%) | 14(6.4%) | 831(17.4%) | |
| Marital status | 0.007 | |||
| Not married | 1836(40.3%) | 68(31.2%) | 1904(39.9%) | |
| Married | 2717(59.7%) | 150(68.8%) | 2867(60.1%) | |
| Tumor size (mm) | 0.011 | |||
| ≤50 | 1622(35.6%) | 96(44.0%) | 1718(36.0%) | |
| >50 | 2931(64.4%) | 122(56.0%) | 3053(64.0%) | |
| Grade | 0.743 | |||
| I-II | 129(2.8%) | 7(3.2%) | 136(2.9%) | |
| III-IV | 4424(97.2%) | 211(96.8%) | 4635(97.1%) | |
| TNM stage | <0.001 | |||
| I | 1353(29.7%) | 49(22.5%) | 1402(29.4%) | |
| II | 520(11.4%) | 84(38.5%) | 604(12.7%) | |
| III | 619(13.6%) | 58(26.6%) | 677(14.2%) | |
| IV | 2061(45.3%) | 27(12.4%) | 2088(43.8%) | |
| Tumor depth | <0.001 | |||
| T1 | 1284(28.2%) | 23(10.6%) | 1307(27.4%) | |
| T2 | 1494(32.8%) | 120(55.0%) | 1614(33.8%) | |
| T3 | 907(19.9%) | 62(28.4%) | 969(20.3%) | |
| T4 | 868(19.1%) | 13(6.0%) | 881(18.5%) | |
| LN metastasis | <0.001 | |||
| N0 | 2197(48.3%) | 58(26.6%) | 2255(47.3%) | |
| N1 | 1447(31.8%) | 131(60.1%) | 1578(33.1%) | |
| N2 | 561(12.3%) | 21(9.6%) | 582(12.2%) | |
| N3 | 348(7.6%) | 8(3.7%) | 356(7.5%) |
Abbreviations: TNM, tumor-node-metastasis; LN, lymph node; RT, radiotherapy.
Survival Analysis
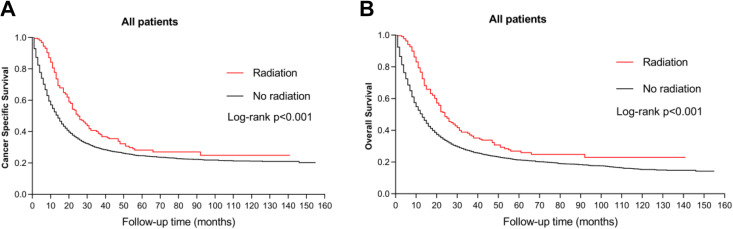
The survival outcomes of RT versus no RT groups were evaluated. The Kaplan-Meier plots demonstrated that the survival of RT group was significantly better than no RT group in both CSS and OS curves (Figure 1, p < 0.001). The median CSS of RT group was 25.0(20.8-29.2) months, while that of no RT group was 12.0(11.3-12.7) months (Table 2, p < 0.001). Likewise, the median OS of RT group was also superior to that of no RT group (Table 2, p < 0.001). These results indicated that preoperative RT exerted notable survival advantages for the patients with gastric SRC.
Figure 1.
Kaplan-Meier survival curves. A, CSS (p < 0.001). B, OS (p < 0.001).
Table 2.
Comparison of Median Survival of the Patients.
| Patients, No. | Median CSS, 95% CI, months | Median OS, 95% CI, months | |
|---|---|---|---|
| No radiation | 4553 | 12.0(11.3-12.7) | 11.0(10.4-11.6) |
| Radiation | 218 | 25.0(20.8-29.2) | 24.0(20.2-27.8) |
| p value | <0.001 | <0.001 |
Abbreviations: No., number; CSS, cancer-specific survival; OS, overall survival.
Identify Prognostic Factors
To identify prognostic factors associated with CSS, we constructed both uni- and multivariate Cox proportional hazard models within the cohort. In univariate analysis, the variables significantly associated with CSS were RT, age, race, marital status, tumor size, TNM stage, tumor depth, LN metastasis (p < 0.05). RT was found to be a significant prognostic factor (HR = 0.641, 95% CI = 0.541-0.759, p < 0.001). All these significant variables in univariate analysis were subsequently recruited into the multivariate Cox regression model. After adjusting for other confounding factors, age >60, tumor size >50 mm, TNM stage II-IV were proved to be independent risk factors for poor prognosis (HR > 1, p < 0.05). Notably, preoperative RT was still significantly associated with the patients’ CSS (HR = 0.714, 95% CI = 0.599-0.850, p < 0.001). Hence preoperative RT was identified as an independent protective factor for favorable prognosis (HR < 1, p < 0.05). The detailed results are shown in Table 3.
Table 3.
Cox Regression Analysis of Cancer-Specific Survival.
| Characteristics | Univariate Cox | Multivariate Cox | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR(95% CI) | p value | |
| RT | ||||
| No | Reference | Reference | ||
| Yes | 0.641(0.541-0.759) | <0.001 | 0.714(0.599-0.850) | <0.001 |
| Age (years) | ||||
| ≤60 | Reference | Reference | ||
| >60 | 1.114(1.041-1.192) | 0.002 | 1.370(1.278-1.468) | <0.001 |
| Gender | ||||
| Male | Reference | NI | ||
| Female | 0.987(0.922-1.056) | 0.694 | ||
| Race | ||||
| White | Reference | Reference | ||
| Black | 0.948(0.852-1.055) | 0.326 | 0.968(0.869-1.079) | 0.560 |
| Others | 0.723(0.657-0.796) | <0.001 | 0.830(0.754-0.914) | <0.001 |
| Marital status | ||||
| Not married | Reference | Reference | ||
| Married | 0.832(0.776-0.891) | <0.001 | 0.810(0.755-0.868) | <0.001 |
| Tumor size (mm) | ||||
| ≤50 | Reference | Reference | ||
| >50 | 2.606(2.410-2.819) | <0.001 | 1.743(1.604-1.895) | <0.001 |
| Grade | ||||
| I-II | Reference | NI | ||
| III-IV | 1.218(0.984-1.509) | 0.071 | ||
| TNM Stage | ||||
| I | Reference | Reference | ||
| II | 1.831(1.610-2.083) | <0.001 | 1.928(1.645-2.260) | <0.001 |
| III | 2.827(2.509-3.186) | <0.001 | 2.973(2.526-3.500) | <0.001 |
| IV | 5.192(4.710-5.724) | <0.001 | 4.996(4.391-5.684) | <0.001 |
| Tumor depth | ||||
| T1 | Reference | Reference | ||
| T2 | 1.485(1.349-1.634) | <0.001 | 0.985(0.882-1.101) | 0.797 |
| T3 | 2.029(1.829-2.251) | <0.001 | 0.932(0.817-1.063) | 0.294 |
| T4 | 3.281(2.953-3.646) | <0.001 | 1.049(0.928-1.187) | 0.444 |
| LN metastasis | ||||
| N0 | Reference | Reference | ||
| N1 | 1.513(1.400-1.635) | <0.001 | 0.881(0.807-0.963) | 0.005 |
| N2 | 1.604(1.443-1.782) | <0.001 | 0.807(0.714-0.911) | 0.001 |
| N3 | 2.006(1.772-2.271) | <0.001 | 0.700(0.610-0.803) | <0.001 |
Abbreviations: RT, radiotherapy; TNM, tumor-node-metastasis; LN, lymph node; HR, hazard ratio; NI, not included.
Subgroup Survival Analysis
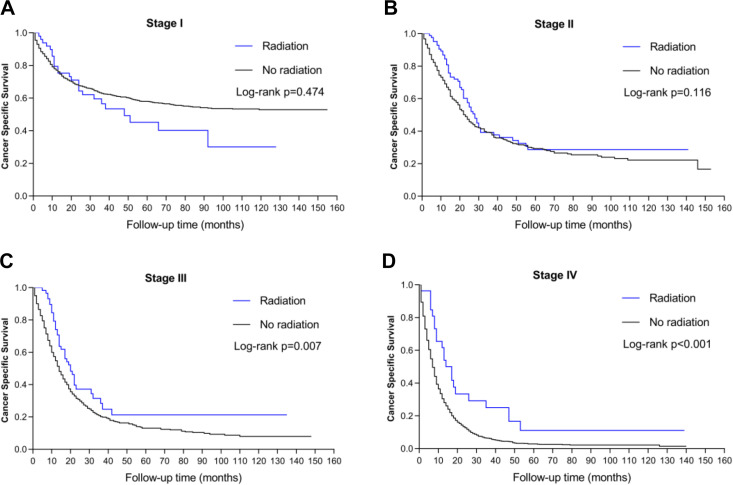
Given that TNM stage is also independently associated with the patients’ CSS, we initiated a subgroup analysis to highlight the impact of preoperative RT on the prognosis of the patients. The Kaplan-Meier plots revealed that preoperative RT showed significant CSS advantages for patients with stages III and IV gastric SRC (p < 0.05). By contrast, no significant survival difference was found between the 2 groups in either stage I or stage II patients (p > 0.05). Thus, preoperative RT showed significant survival benefits for the patients with advanced gastric SRC. The survival curves of CSS stratified by TNM stage can be seen in Figure 2.
Figure 2.
Kaplan-Meier survival curves stratified by TNM stage. A, Stage I (p > 0.05). B, Stage II (p > 0.05). C, Stage III (p < 0.05). D, Stage IV (p < 0.05).
Discussion
Currently, the significance of preoperative RT for patients with gastric SRC has not been widely recognized. Based on a large population from the SEER database, we retrospectively analyzed the clinicopathological features of the patients with gastric SRC, highlighting the effect of preoperative RT on the prognosis of such patients. The overall results indicated that preoperative RT was associated with significant survival benefits for the patients with gastric SRC.
The role of preoperative RT in treating GC patients has been reported by several relevant studies. A recent meta-analysis showed a statistically significant 5-year survival benefit with the addition of RT in patients with resectable GC.15 In another meta-analysis, GC patients could benefit from both preoperative and postoperative RT.16 However, it has not been clarified whether preoperative RT can benefit the survival of patients with gastric SRC likewise. A retrospective study demonstrated that neoadjuvant radiochemotherapy improved the survival of patients with locally advanced SRC in esophagogastric junction.17 Nevertheless, this study has not separately analyzed the survival impact of preoperative RT for gastric SRC. On the other hand, another study has found that gastric SRC was relatively chemoradiation resistant. A higher fraction of SRC was associated with higher resistance.18 Hence the previous studies concerning the prognostic impact of RT on patients with gastric SRC are inconsistent. Comparatively, our study has investigated the influence of preoperative RT on the survival outcomes of patients with gastric SRC based on a large population analysis. Our results indicated the preoperative RT exerted notable survival advantages for these patients. Treatment-associated toxicity is a major contributing factor. A prospective, randomized trial has explored the toxicity and efficacy of surgery and preoperative radiotherapy for treating GC. The hematologic toxicity includes neutropenia, neutropenic fever, and anemia. Common complications were postoperative pancreatitis, anastomotic leakages, intestinal obstruction, and gastrointestinal bleeding. However, the incidence of these complications was relatively low. Preoperative radiotherapy was generally well tolerated, and resulted in a marked survival improvement.19 Therefore, preoperative RT seems applicable for the patients with gastric SRC.
With regard to the prognostic factors for gastric SRC, a recent study has comprehensively analyzed the clinicopathological characteristics and prognosis of such patients. When multivariate analysis with Cox regression was conducted, their results indicated that age, race, histological grade, AJCC stage were independent prognostic factors.20 Based on the analysis of our cohort, we also identified age >60, tumor size >50 mm, TNM stage II-IV as independent risk factors for poor prognosis (HR > 1, p < 0.05). However, preoperative RT was still significantly associated with favorable CSS (HR < 1, p < 0.001). Thus preoperative RT was an independent protective factor for the patients with gastric SRC.
Tumor size is also an important prognostic factor for the GC patients, which is significantly associated with cancer progression, lymph node metastasis, and relapse. The patients with large tumors often indicated more aggressive features and worse prognosis than patients with small tumors. Tumor size could provide vital information for determining the width of surgical margin and the extent of lymph node dissection.21 A recent study also revealed that small GC was appropriate for radical surgery, while large GC with risk factors could not be surgically cured.22 In our study, we have also found that tumor size was an independent prognostic factor for the patients with gastric SRC. As for patients with large advanced tumors, preoperative radiotherapy may offer the potential advantages of reducing tumor size and allowing a R0 resection.23
Based on analysis of the entire cohort, the survival benefits from preoperative RT have been confirmed for the patients with gastric SRC. For solid elucidation, we initiated a subgroup survival analysis by TNM stage. The stratified analysis showed that preoperative RT exerted significant CSS benefits for patients with stages III and IV gastric SRC. Preoperative RT has been reported to downstage the unresectable GC, some advanced patients might be converted into resectable ones.24 The advantages of preoperative RT include intact tumor microenvironment, hence avoiding postoperative hypoxia that may compromise the treatment efficacy. Preoperative RT also has the advantages of clearer target delineation, smaller radiation volumes, and lower doses, which can improve the patients’ survival.24 Thus preoperative RT may be more appropriate for advanced gastric SRC.
There are several limitations of our study. First, this retrospective analysis has an inherent selection bias. The confounders in two cohorts may have an impact on the results. Second, the data are not available in the SEER database regarding radiation techniques and dose, which may have caused potential bias. Third, our findings can only be applied to the America rather than the global population, especially in endemic areas such as China.25 Fourth, the sequences and specific chemotherapy regime are still not available in the SEER database, so the interaction of preoperative RT with distinct chemotherapy can’t be accurately evaluated. To minimize the potential bias from multiple confounding factors, subgroup survival analysis was initiated to compensate the significant differences between the 2 comparison groups.26 Given a large population that represents the real-world patients, our results are still considerably convincing.
Conclusion
Based on a large population from the SEER database, preoperative radiotherapy is associated with significant survival benefits for the patients with advanced gastric SRC. Hence preoperative radiotherapy is feasible for these patients. Our study will hopefully contribute to the future tailored treatment for the patients with gastric SRC.
Acknowledgments
The authors acknowledge the Surveillance, Epidemiology, and End Results database for providing high quality clinical data for our study.
Abbreviations
- SRC
signet ring cell carcinoma
- SEER
Surveillance, Epidemiology, and End Results
- RT
radiotherapy
- CSS
cancer-specific survival
- OS
overall survival
- AJCC
American Joint Committee on Cancer.
Authors’ Note: Our study does not require any ethical approval because we use publicly available SEER database. The SEER data contain no identifiers and are publicly available, so ethical approval was exempt for our study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Natural Science Foundation of China (81670123, 81670144).
ORCID iD: Yuxin Chu  https://orcid.org/0000-0001-5526-997X
https://orcid.org/0000-0001-5526-997X
References
- 1. Corso S, Giordano S. How can gastric cancer molecular profiling guide future therapies? Trends Mol Med. 2016;22(7):534–544. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 3. Zhang XY, Zhang PY. Gastric cancer: somatic genetics as a guide to therapy. J Med Genet. 2017;54(5):305–312. [DOI] [PubMed] [Google Scholar]
- 4. Perrot-Applanat M, Vacher S, Pimpie C, et al. Differential gene expression in growth factors, epithelial mesenchymal transition and chemotaxis in the diffuse type compared with the intestinal type of gastric cancer. Oncol Lett. 2019;18(1):674–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nagtegaal ID, Odze RD, Klimstra D, et al. The 2019 WHO classification of tumors of the digestive system. Histopathology. 2020;76(2):182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Machlowska J, Pucułek M, Sitarz M, Terlecki P, Maciejewski R, Sitarz R. State of the art for gastric signet ring cell carcinoma: from classification, prognosis, and genomic characteristics to specified treatments. Cancer Manag Res. 2019;11:2151–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kazaz İO, Arslan A, Çolak F, Kazaz SN, Mungan S, Karagüzel E. Bladder metastasis of gastric signet-ring cell carcinoma. Urol Case Rep. 2018;22:62–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Song Z, Wu Y, Yang J, Yang D, Fang X. Progress in the treatment of advanced gastric cancer. Tumor Biol. 2017;39(7):1010428317714626. [DOI] [PubMed] [Google Scholar]
- 9. Irino T, Takeuchi H, Terashima M, Wakai T, Kitagawa Y. Gastric cancer in Asia: unique features and management. Am Soc Clin Oncol Educ Book. 2017;37:279–291. [DOI] [PubMed] [Google Scholar]
- 10. Smalley SR, Benedetti JK, Haller DG, et al. Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol. 2012;30(19):2327–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu N, Xu Y, Rahnemai-Azar AA, Abbott DE, Weber SH, Lidor AO. National underutilization of neoadjuvant chemotherapy for gastric cancer. J Gastrointest Surg. 2020;24(4):949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim Y, Kim KM, Choi MG, et al. Adjuvant chemotherapy with or without concurrent radiotherapy for patients with stage IB gastric cancer: a subgroup analysis of the adjuvant chemoradiotherapy in stomach tumors (ARTIST) phase III trial. J Gastric Cancer. 2018;18(4):348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cats A, Jansen EPM, van Grieken NCT, et al. Chemotherapy versus chemoradiotherapy after surgery and preoperative chemotherapy for resectable gastric cancer (CRITICS): an international, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19(5):616–628. [DOI] [PubMed] [Google Scholar]
- 14. Ajani JA, Mansfield PF, Janjan N, et al. Multi-institutional trial of preoperative chemoradiotherapy in patients with potentially resectable gastric carcinoma. J Clin Oncol. 2004;22(14):2774–2780. [DOI] [PubMed] [Google Scholar]
- 15. Valentini V, Cellini F, Minsky BD, et al. Survival after radiotherapy in gastric cancer: systematic review and meta-analysis. Radiother Oncol. 2009;92(2):176–183. [DOI] [PubMed] [Google Scholar]
- 16. Pang X, Wei W, Leng W, et al. Radiotherapy for gastric cancer: a systematic review and meta-analysis. Tumor Biol. 2014;35(1):387–396. [DOI] [PubMed] [Google Scholar]
- 17. Bekkar S, Gronnier C, Messager M, et al. The impact of preoperative radiochemotherapy on survival in advanced esophagogastric junction signet ring cell adenocarcinoma. Ann Thorac Surg. 2014;97(1):303–310. [DOI] [PubMed] [Google Scholar]
- 18. Charalampakis N, Nogueras GM, Elimova E, et al. The proportion of signet ring cell component in patients with localized gastric adenocarcinoma correlates with the degree of response to pre-operative chemoradiation. Oncology. 2016;90(5):239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Skoropad VY, Berdov BA, Mardynski YS, Titova LN. A prospective, randomized trial of pre-operative and intraoperative radiotherapy versus surgery alone in resectable gastric cancer. Eur J Surg Oncol. 2000;26(8):773–779. [DOI] [PubMed] [Google Scholar]
- 20. Liu K, Wan J, Bei Y, Chen X, Lu M. Prognostic impact of different histological types on gastric adenocarcinoma: a surveillance, epidemiology, and end results database analysis. Pathol Oncol Res. 2017;23(4):881–887. [DOI] [PubMed] [Google Scholar]
- 21. Zhao LY, Zhang WH, Chen XZ, et al. Prognostic significance of tumor size in 2405 patients with gastric cancer: a retrospective cohort study. Medicine (Baltimore). 2015;94(50):e2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li C, Oh SJ, Kim S, et al. Risk factors of survival and surgical treatment for advanced gastric cancer with large tumor size. J Gastrointest Surg. 2009;13(5):881–885. [DOI] [PubMed] [Google Scholar]
- 23. Lim L, Michael M, Mann GB, Leong T. Adjuvant therapy in gastric cancer. J Clin Oncol. 2005;23(25):6220–6232. [DOI] [PubMed] [Google Scholar]
- 24. Tian S, Jiang R, Madden NA, et al. Survival outcomes in patients with gastric and gastroesophageal junction adenocarcinomas treated with perioperative chemotherapy with or without preoperative radiotherapy. Cancer. 2020;126(1):37–45. [DOI] [PubMed] [Google Scholar]
- 25. Wu SG, Zhang WW, He ZY, Sun J-Y, Chen Y-X, Guo L. Sites of metastasis and overall survival in esophageal cancer: a population-based study. Cancer Manag Res. 2017;9:781–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heger U, Sisic L, Nienhüser H, et al. Neoadjuvant therapy improves outcomes in locally advanced signet-ring-cell containing esophagogastric adenocarcinomas. Ann Surg Oncol. 2018;25(8):2418–2427. [DOI] [PubMed] [Google Scholar]