Abstract
Background.
Two vaccines protect against human papillomaviruses (HPV) 16 and 18, which cause 70% of cervical cancer and 50% of cervical intraepithelial neoplasia 2/3 and adenocarcinoma in situ (CIN2+). Monitoring HPV types in CIN2+ may be used to assess HPV vaccine impact.
Methods.
As part of a multisite vaccine impact monitoring project (HPV-IMPACT), biopsy specimens used to diagnose CIN2+ were obtained for HPV DNA typing for women aged 18–39 years.
Results.
Among 4,121 CIN2+ cases reported during 2008–2009 in 18- to 39-year-old women 3058 (74.2%) were tested; 96% were HPV DNA positive. HPV 16 was most common (49.1%), followed by HPV 31 (10.4%) and HPV 52 (9.7%). HPV 18 prevalence was 5.5% overall. Proportion of CIN2+ cases associated with HPV 16/18 was highest (56.3%) in 25- to 29-year-old women. HPV 16/18-associated lesions were less common in non-Hispanic blacks (41.9%) and Hispanics (46.3%) compared with non-Hispanic whites (59.1%) (P < .0001); the difference remained significant when adjusted for covariates. Compared to non-Hispanic whites, HPV 35 and 58 were significantly more common in non-Hispanic blacks (14.5% vs 4.2%; 12.3% vs 3.4%) and HPV 45 was higher in Hispanics (3.7% vs 1.5%).
Conclusions.
Age and racial/ethnic differences in HPV type distribution may have implications for vaccine impact and should be considered in monitoring trends.
Human papillomavirus (HPV) types 16 and 18 [1–3] are causally related to 70% of cervical cancers and 50% of cervical precancerous lesions worldwide. Since 2006, 2 vaccines directed against these HPV types have become commercially available [1–3]. Cervical cancer develops slowly, over decades, and is often preceded by high-grade cervical lesions that can be detected through routine cervical cancer screening [4]. Histologically confirmed lesions include cervical intraepithelial neoplasia (CIN) grades 2 and 3 and adenocarcinoma in situ (AIS), collectively referred to as CIN2+ for the remainder of this paper. CIN lesions are currently graded from 1 to 3 according to the extent of pathology [5]. CIN3 and AIS are histologic endpoints with good interobserver agreement and are recognized as lesions that are at high enough risk for progression to invasion to warrant treatment. The interobserver agreement on CIN2 is poor; however, because this group of lesions may represent a mix of CIN1 and CIN3 lesions that cannot be readily distinguished by histopathology, CIN2 is the current clinical threshold for treatment [6, 7].
Monitoring HPV type distribution in women diagnosed with CIN2+ noninvasive lesions may provide the earliest evidence of HPV vaccine impact on cervical disease. Baseline data prior to vaccine introduction have been collected internationally [8, 9], but little information exists on HPV type distribution in CIN2+ lesions in the United States. Cervical tissues obtained for CIN2+ diagnosis are archived in the United States, and can be used for HPV DNA testing. Several systems have been established across the country to monitor the impact of HPV vaccine on cervical cancer precursors in different populations [10, 11]. One project, HPV-IMPACT, was established in 2008 to monitor type-specific CIN2/3 and AIS [12]. This paper describes HPV type distribution in US women aged 18–39 years reported to the HPV-IMPACT monitoring system with a diagnosis of CIN2+ during 2008–2009, prior to widescale HPV vaccine introduction.
MATERIALS AND METHODS
Design/Population
The HPV-IMPACT monitoring system is described in detail elsewhere [12]. In brief, HPV-IMPACT is a collaboration between the Centers for Disease Control and Prevention (CDC) and 5 sites in the Emerging Infections Program (EIP) Network [13] to monitor the impact of HPV vaccines on CIN2+ through population-based laboratory surveillance. Catchment areas at 5 participating EIP regions were purposefully selected to include populations that would yield at least 250 CIN2+ cases per site and per year among women 18–39 years of age. The areas are geographically diverse, and include 8 contiguous cities in northwest Alameda County, California; New Haven County, Connecticut; Monroe County, New York; Davidson County, Tennessee; and a contiguous region of Washington and Multnomah counties, Oregon, which includes the city of Portland. The total population of women aged ≥18 years across the 5 participating sites ranges from about 230 000 to 330 000 based on 2010 US Census estimates. Participating sites and the CDC received institutional review board approval or exemption, as appropriate for compliance with local reviews.
Case Ascertainment
Histopathology laboratories serving the HPV-IMPACT catchment areas reported all cases of noninvasive CIN2+ diagnosed in adult (≥18 years) female residents of the catchment area. Because several classification systems and nomenclature for cervical intraepithelial neoplasia are currently in use, a master list of possible CIN2+ codes, terminology, and synonym search terms was provided to the laboratories to achieve complete case ascertainment, and all eligible cases were classified into one of the following categories: CIN2, CIN2/3, CIN3, AIS, or AIS + CIN. The laboratories provided information on each case based on a standard report form that includes date of birth, race/ethnicity, health insurance status, and HPV vaccination history where available. Reports were deduplicated within and between laboratories, either manually or electronically, with an algorithm that utilizes all available identifiers including first name, last name, date of birth, race, and postal code. Unique records for each case were maintained and updated in a central database at the CDC based on ongoing active surveillance and laboratory audits.
Specimen Selection and Processing
Reporting laboratories were asked to provide samples of archived histopathology specimens from CIN2+ cases among women aged 18–39 years. Specimens were available from the majority of the laboratories for approximately 75% of these women. One block representative of the histologic lesion with highest diagnosis was selected for type-specific HPV DNA testing. For each tissue block, serial sections were prepared using precautions to prevent polymerase chain reaction (PCR) contamination. The first and last sections were stained with hematoxylin and eosin (H&E), and 2 intervening 10-μm un-stained sections were placed in sterile microfuge tubes for extraction. Slides and tubes labeled with study ID barcode were shipped to the CDC for HPV testing. Histologic review of the initial and final H&E section performed at the CDC confirmed that diagnostic material was present. HPV typing was performed only on specimens containing diagnostic material.
Laboratory Procedures
DNA was extracted from one tissue section using DNeasy (Qiagen, Valencia, California) with the addition of 1 hour external lysis at 65°C as previously described [14]. If the accessible tissue area was extremely small, 2 sections were combined during the extraction process. For every batch of samples, a water blank was processed through all steps of extraction to serve as a “contamination control.” The final elution volume was 100 μL.
A 10-μL aliquot of the purified DNA was tested with the Linear Array HPV Genotyping Assay (LA; Roche Diagnostics, Indianapolis, Indiana). Hybridization and wash steps of the reverse line blot procedure were performed automatically with Beeblot instruments (Bee Robotics, Caernafon, UK). Other-wise, the manufacturer’s protocol was followed. If HPV 52 status was ambiguous owing to positive results of the XR probe and the cross-hybridizing HPV 33, 35, or 58, a specific quantitative PCR assay was performed to verify the presence of type 52 [15].
Samples with inadequate or HPV negative LA results were retested with the INNO-LiPA HPV Genotyping Extra Assay (Innogenetics, Gent, Belgium) as its short PCR target length might be more suitable for low-quality DNA from formalin-fixed, paraffin-embedded tissues. The manufacturer’s protocol was followed using an AutoBlot 3000H (MedTec, Buffalo, Illinois) for the line blot hybridization. Samples negative for both the genomic control probe and HPV in LA and INNO-LiPA were considered inadequate and omitted from further analysis.
Statistical Methods
Analysis was restricted to participants aged 18–39 years who were diagnosed with noninvasive CIN2+ during 2008–2009, and whose cervical specimens were adequate for DNA typing (n = 3081). Data were analyzed using SAS (version 9.2, SAS Institute, Cary, North Carolina). We calculated the overall and type-specific prevalence of HPV infection for all 37 HPV types detected by the LA genotyping assay. To account for occurrence of multiple HPV types, we further examined HPV type distribution hierarchically based on cervical cancer risk. The HPV risk categories were defined in order as HPV 16 or 18 (with or without other HPV types), else other high risk (HPV 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68), else possible high risk (HPV 26, 53, 67, 69, 70, 73, 82, and IS39), else low risk (HPV 6, 11, 40, 42, 54, 55, 61, 62, 64, 71, 72, 81, 83, 84, and 89). We evaluated the frequency distribution of hierarchical HPV risk categories within selected demographic and clinical strata. Confidence intervals (CIs) were calculated using Wald or exact (Clopper-Pearson) test for the binomial proportion.
We used Pearson χ2 test for independence to evaluate associations between hierarchical HPV risk groups and selected characteristics. Cases were classified into 1 of 5 diagnosis categories: CIN2, CIN3, CIN2/3 (ie, grade not specified), AIS only, and AIS + CIN. We examined age by 5-year age groups, except for those 18–20 years. This group was analyzed separately because new guidelines do not recommend screening before 21 years of age [16]. We distinguished individual race/ ethnicity categories when reported, and combined others with those for whom race/ethnicity information was not available. Two-sided statistical tests were considered significant at the α level of .05. Estimated prevalence and 95% CI are presented.
We examined independent correlates of HPV 16 or 18 infection (vs other HPV type infection) among women aged 21–39 years in a log binomial regression model using back-ward elimination. Covariates included categorical age groups, race/ethnicity, diagnosis, and insurance status. Associations were considered significant if the Pearson χ2 P value was < .05 and those variables were retained in the main effects model. Confounding was assessed to ensure that no parameter estimate of significant variables changed by ≥30%. All pairwise interactions in the final model were examined, and were considered significant if the P value for the likelihood ratio test for the interaction term was < .05.
RESULTS
During 2008–2009, a total of 4121 women aged 18–39 years diagnosed with noninvasive CIN2+ were reported to the HPV-IMPACT monitoring system. Archived specimens were available and retrieved for 3092 of these women for HPV DNA typing. Of these, 3081 (99.6%) specimens had diagnostic material and underwent DNA testing. HPV DNA tests were adequate in 3058 (98.9%) of those tested. This represents specimens from 74.2% of eligible women for that time period. CIN2+ cases with DNA typing results were similar to those for whom specimens were not available with respect to age (median 27 years), race/ethnicity, diagnosis grade/type, and other known characteristics (data not shown).
Overall, 96.0% (2937/3058) of CIN2+ cases had evidence of infection with at least 1 HPV type (Table 1). HPV DNA was detected in 96.8% of cases with CIN3 and 100% of those with AIS. HPV 16 was the most prevalent type detected overall (49.1%) and in all diagnosis categories, ranging from 38.3% in CIN2 to 61.3% in CIN3 lesions. HPV 31 was the second most common (10.4%), followed by HPV 52 (9.7%), HPV 51 (8.3%), and HPV 35 (6.3%) overall. HPV 18 was more common among women with AIS (42.9%) compared to those with CIN2 (4.6%) and CIN3 (5.0%). Among AIS cases (n = 28), the proportion positive for HPV 18 (42.9%) was the same as the percentage positive for HPV 16 (42.9%). HPV 16 was the most common type among cases with both AIS and CIN (n = 33) at 57.6%; HPV 18 was the second highest, followed by high-risk HPV 52, 33, and 31.
Table 1.
Human Papillomavirus Types by Diagnosis and Oncogenic Risk Category Among CIN2+ Cases in Women Aged 18–39 Years, HPV-IMPACT, 2008–2009
| CIN 2 | CIN 2/3 | CIN 3 | AIS | AIS + CIN | Total | |
|---|---|---|---|---|---|---|
| HPV negative | 72 (4.6) | 16 (3.1) | 33 (3.2) | 0 (0) | 0 (0) | 121 (4.0) |
| HPV positive | 1427 (95.4) | 491 (96.9) | 958 (96.8) | 28 (100.0) | 33 (100.0) | 2937 (96.0) |
| High-risk type | ||||||
| 16 | 546 (38.3) | 279 (56.8) | 587 (61.3) | 12 (42.9) | 19 (57.6) | 1443 (49.1) |
| 18 | 65 (4.6) | 27 (5.5) | 48 (5.0) | 12 (42.9) | 9 (27.3) | 161 (5.5) |
| 31 | 158 (10.5) | 46 (9.1) | 112 (11.3) | 0 (0) | 2 (6.1) | 318 (10.4) |
| 33 | 44 (3.1) | 18 (3.7) | 34 (3.6) | 0 (0) | 2 (6.1) | 98 (3.3) |
| 35 | 117 (8.2) | 32 (6.5) | 36 (3.8) | 1 (3.6) | 0 (0) | 186 (6.3) |
| 39 | 66 (4.6) | 15 (3.1) | 27 (2.8) | 0 (0) | 0 (0) | 108 (3.7) |
| 45 | 26 (1.8) | 9 (1.8) | 18 (1.8) | 1 (3.6) | 0 (0) | 54 (1.8) |
| 51 | 154 (10.8) | 35 (7.1) | 53 (5.5) | 0 (0) | 1 (3.0) | 243 (8.3) |
| 52 | 167 (11.7) | 38 (7.7) | 77 (8.0) | 1 (3.6) | 2 (6.1) | 285 (9.7) |
| 56 | 21 (1.5) | 5 (1.0) | 6 (0.6) | 0 (0) | 0 (0) | 32 (1.1) |
| 58 | 90 (6.3) | 33 (6.7) | 34 (3.6) | 0 (0) | 1 (3.0) | 158 (5.4) |
| 59 | 48 (3.4) | 19 (3.9) | 13 (1.4) | 0 (0) | 1 (3.0) | 81 (2.8) |
| 66 | 60 (4.2) | 15 (3.1) | 17 (1.8) | 2 (7.1) | 1 (3.0) | 95 (3.2) |
| 68 | 23 (1.6) | 2 (0.4) | 6 (0.6) | 0 (0) | 1 (3.0) | 32 (1.1) |
| Possible high-risk type | ||||||
| 26 | 8 (0.6) | 2 (0.4) | 2 (0.2) | 0 (0) | 0 (0) | 12 (0.4) |
| 53 | 49 (3.4) | 14 (2.9) | 22 (2.3) | 1 (3.6) | 0 (0) | 86 (2.9) |
| 67 | 16 (1.1) | 4 (0.8) | 3 (0.3) | 0 (0) | 0 (0) | 23 (0.8) |
| 69 | 1 (0.1) | 1 (0.2) | 0 (0) | 0 (0) | 0 (0) | 2 (0.1) |
| 70 | 17 (1.2) | 2 (0.4) | 3 (0.3) | 0 (0) | 1 (3.0) | 23 (0.8) |
| 73 | 24 (1.7) | 5 (1.0) | 3 (0.3) | 0 (0) | 0 (0) | 32 (1.1) |
| 82 | 53 (3.7) | 11 (2.2) | 12 (1.3) | 0 (0) | 1 (3.0) | 77 (2.6) |
| IS39 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Low-risk type | ||||||
| 6 | 25 (1.8) | 10 (2.0) | 14 (1.5) | 1 (3.6) | 0 (0) | 50 (1.7) |
| 11 | 2 (0.1) | 1 (0.2) | 1 (0.1) | 0 (0) | 0 (0) | 4 (0.1) |
| 40 | 3 (0.2) | 1 (0.2) | 1 (0.1) | 0 (0) | 0 (0) | 5 (0.2) |
| 44 | 1 (0.1) | 0 (0) | 1 (0.1) | 0 (0) | 0 (0) | 2 (0.1) |
| 54 | 21 (1.4) | 5 (1.0) | 6 (0.6) | 0 (0) | 0 (0) | 32 (1.1) |
| 55 | 0 (0) | 1 (0.2) | 0 (0) | 1 (3.6) | 0 (0) | 2 (0.1) |
| 61 | 11 (0.8) | 1 (0.2) | 6 (0.6) | 0 (0) | 0 (0) | 18 (0.6) |
| 62 | 10 (0.7) | 6 (1.2) | 3 (0.3) | 0 (0) | 0 (0) | 19 (0.7) |
| 64 | 1 (0.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0.0) |
| 71 | 1 (0.1) | 1 (0.2) | 0 (0) | 0 (0) | 0 (0) | 2 (0.1) |
| 72 | 1 (0.1) | 1 (0.2) | 1 (0.1) | 0 (0) | 0 (0) | 3 (0.1) |
| 81 | 2 (0.1) | 1 (0.2) | 0 (0) | 0 (0) | 0 (0) | 3 (0.1) |
| 83 | 7 (0.5) | 2 (0.4) | 2 (0.2) | 0 (0) | 0 (0) | 11 (0.4) |
| 84 | 8 (0.6) | 5 (1.0) | 2 (0.2) | 1 (3.6) | 0 (0) | 16 (0.5) |
| 89 | 15 (1.1) | 6 (1.2) | 5 (0.5) | 0 (0) | 0 (0) | 26 (0.9) |
All data are presented as no. (%). HPV types were detected by linear array or LiPA assay. Numbers in columns exceed 100% owing to multiple infections. Abbreviations: AIS, adenocarcinoma in situ; CIN, cervical intraepithelial neoplasia; CIN2+, CIN2/3 or AIS; HPV, human papillomavirus.
Table 2 presents HPV prevalence by hierarchical risk group. More than half (53.6%) of CIN2+ cases had HPV 16 or 18 (vaccine-type risk category): 41.8% of CIN2 cases, 65.2% of cases with CIN3, and 82.1% of those with AIS. Most of the remaining CIN2+ cases (41.3%) had at least 1 nonvaccine high-risk HPV type (second risk category). Less than 1% of those with CIN2+ were positive for low-risk types only, and most low-risk types occurred as coinfections with ≥1 high-risk type.
Table 2.
Human Papillomavirus Type Risk Category, CIN2+ Among Women Aged 18–39 Years by Selected Characteristics, HPV-IMPACT, 2008–2009
| Hierarchicala HPV Risk Category | ||||
|---|---|---|---|---|
| HPV 16 or 18 | Other High Risk | Possible High Risk | Low Risk | |
| Overall | 1574 (53.6) | 1214 (41.3) | 124 (4.2) | 25 (0.9) |
| Diagnosisb | ||||
| CIN2 | 597 (41.8) | 721 (50.5) | 91 (6.4) | 18 (1.2) |
| CIN2/3 | 302 (61.5) | 172 (35.0) | 14 (2.9) | 3 (0.6) |
| CIN3 | 625 (65.2) | 311 (32.5) | 18 (1.9) | 4 (0.4) |
| AIS | 23 (82.1) | 5 (17.9) | 0 (0) | 0 (0) |
| AIS + CIN | 27 (81.8) | 5 (15.1) | 1 (3.0) | 0 (0) |
| Age, yb | ||||
| 18–20 | 113 (49.1) | 96 (41.7) | 18 (7.8) | 3 (1.3) |
| 21–24 | 460 (54.9) | 329 (39.3) | 40 (4.8) | 8 (1.0) |
| 25–29 | 510 (56.3) | 358 (39.5) | 30 (3.3) | 8 (0.9) |
| 30–34 | 330 (53.8) | 259 (42.2) | 23 (3.8) | 2 (0.3) |
| 35–39 | 161 (46.0) | 172 (49.1) | 13 (3.7) | 4 (1.1) |
| Race/ethnicityb | ||||
| Non-Hispanic white | 958 (59.1) | 584 (36.0) | 65 (4.0) | 13 (0.8) |
| Non-Hispanic black | 167 (41.9) | 212 (53.3) | 16 (4.0) | 3 (0.7) |
| Hispanic | 151 (46.3) | 155 (47.5) | 15 (4.6) | 5 (1.5) |
| Asian | 42 (43.3) | 47 (48.5) | 7 (7.2) | 1 (1.0) |
| Other/missingc | 256 (51.6) | 216 (43.6) | 21 (4.2) | 3 (0.6) |
| Insurance | ||||
| Private/HMO/MCO | 852 (56.6) | 576 (38.3) | 63 (4.2) | 14 (0.9) |
| Public/state | 291 (50.6) | 259 (45.0) | 22 (3.8) | 3 (0.5) |
| No coverage/self-pay | 60 (60.0) | 36 (36.0) | 3 (3.0) | 1 (1.0) |
| Other/missingd | 371 (49.0) | 343 (45.3) | 36 (4.8) | 7 (0.9) |
| Project siteb | ||||
| California | 241 (52.9) | 193 (42.3) | 18 (4.0) | 4 (0.9) |
| Connecticut | 307 (43.9) | 354 (50.6) | 32 (4.6) | 7 (1.0) |
| New York | 551 (56.3) | 371 (37.9) | 47 (4.8) | 9 (0.9) |
| Oregon | 253 (58.6) | 161 (37.3) | 14 (3.2) | 4 (0.9) |
| Tennessee | 222 (59.8) | 135 (36.4) | 13 (3.5) | 1 (0.3) |
All data are presented as no. (row %).
Abbreviations: AIS, adenocarcinoma in situ; CIN, cervical intraepithelial neoplasia; CIN2+, CIN2/3 or AIS; HMO, health maintenance organization; HPV, human papillomavirus; MCO, managed care organization. .
HPV 16 or 18 > other high-risk HPV types excluding HPV 16 and 18 > possible high-risk HPV > low-risk HPV.
P < .01.
Race/ethnicity was missing for 15.1% of the overall population; 2.3% were classified as “other.”
Insurance status was missing for 22.3% of the overall population; 3.2% were classified as “other.”
HPV 16 or 18 positivity in CIN2+ differed by age group, with the highest proportion (56.3%) among those 25–29 years of age, and decreased with age to 43% among those aged 35–39 years (P < .01). The proportion of women with HPV 16- or 18- related CIN2+ was lowest in Connecticut (43.7%), followed by California (52.9%), New York (56.3%), Oregon (58.6%), and Tennessee (59.8%). Non-Hispanic white women with CIN2+ had a significantly higher prevalence of HPV 16 or 18 types (59.1%) compared with other racial/ethnic groups: 41.9% in non-Hispanic blacks, 46.3% in Hispanics, and 43.3% among Asians (P < .0001) (Figure 1); this trend was similar by diagnosis category. For CIN2, 42.9% of specimens from non-Hispanic white cases had HPV 16 or 18 detected compared with 33.3% in non-Hispanic blacks and 31.9% in Hispanics (P < .01). For CIN3, HPV 16 or 18 was present in 71.4% of specimens from non-Hispanic whites compared with 50.4% in non-Hispanic blacks and 54.6% in Hispanics (P < .0001). HPV 16 or 18 prevalence did not differ significantly by insurance status.
Figure 1.
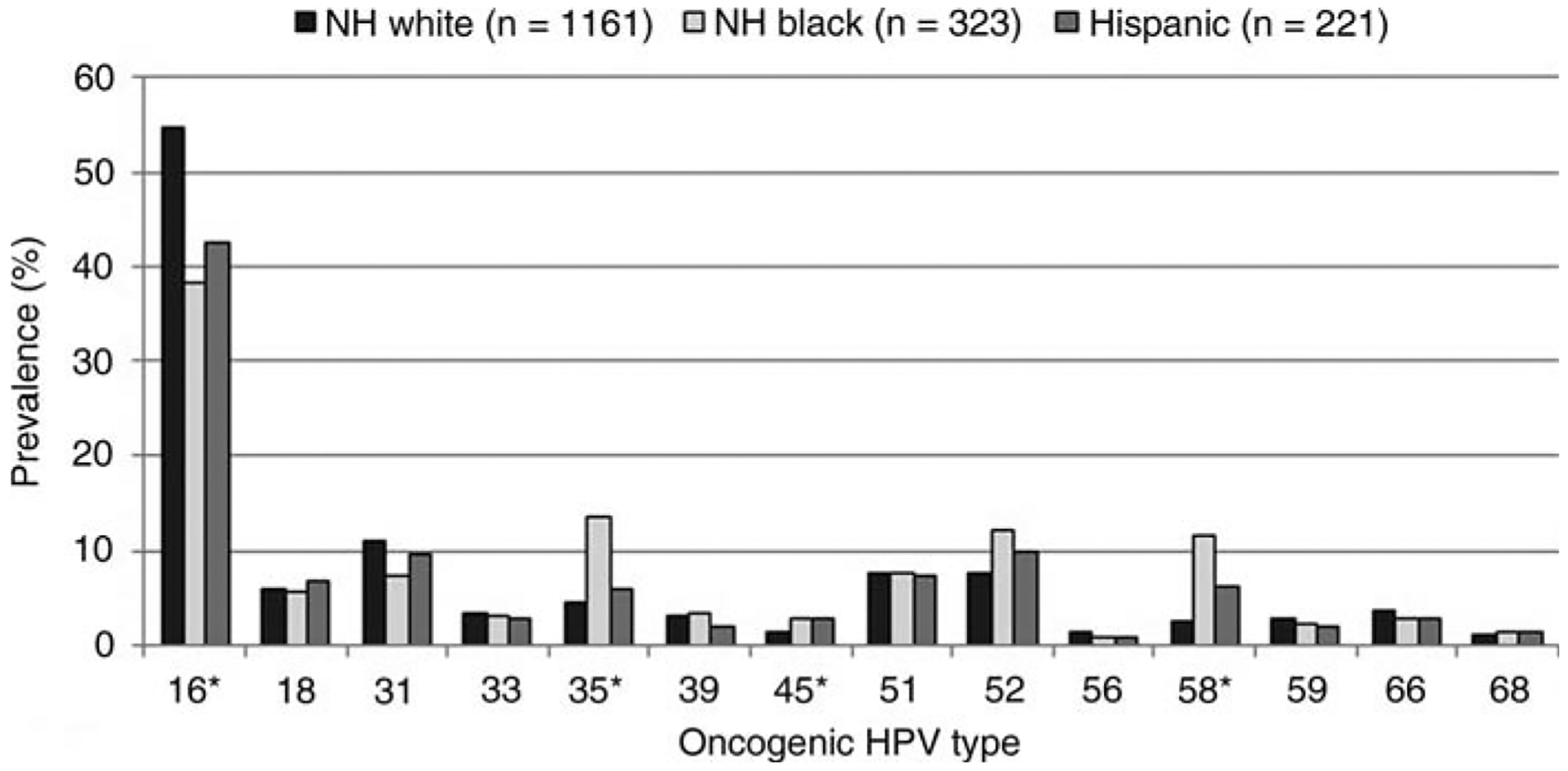
Oncogenic human papillomavirus (HPV) type prevalence by race/ethnicity. *P < .001. NH, non-Hispanic.
Racial/ethnic differences were similar when we excluded women with lesions in which HPV 16 or 18 was detected concurrently with ≥1 nonvaccine oncogenic types. In this subgroup, HPV 16 or 18 positivity was 44.1% in non-Hispanic white compared with 30.2% in non-Hispanic black and 35.2% in Hispanic women with CIN2+ (P < .0001). Among the subset of women with CIN3, the percentage of HPV 16 or 18 was 59.8% in non-Hispanic white compared to 40.7% in non-Hispanic black and 46.2% in Hispanic women (P < .0001) (data not shown). Of note, non-Hispanic black women had significantly higher prevalence of 2 high-risk types compared with non-Hispanic whites: HPV 35 (14.5% vs 4.2%; P < .0001) and HPV 58 (12.3% vs 3.4%; P < .0001); HPV 45 was significantly more common in Hispanic cases compared to non-Hispanic whites (3.7% vs 1.5%; P < .01) (data not shown). In multivariable analysis, the effect of race/ethnicity on HPV 16 or 18 detection was different in the youngest age group of 18- to 20-year-olds compared to all other age groups. However, a stratified analysis was not possible owing to small sample sizes in the youngest age group. Because screening is no longer recommended before age 21, we restricted the multivariable analysis to 21- to 39-year-olds and found no interaction by age. In the final model, the adjusted prevalence ratio (APR) of having HPV 16- or 18-associated CIN2+ was significantly higher in all age categories < 35 years compared to women aged 35–39 years (Table 3). Race was also independently associated with prevalence of HPV 16 or 18 detection. Non-Hispanic blacks (APR = 0.70 [95% CI, .62–.80]) and Hispanics (APR = 0.83 [95% CI, .74–.93]) were less likely to have HPV 16- or 18-associated CIN2+ compared with non-Hispanic whites. As expected, CIN3 cases were more likely to have HPV 16 or 18 compared with CIN2 cases.
Table 3.
Human Papillomavirus (HPV) 16/18 Positivity vs Other High-Risk HPV Positivity in Women Aged 21–39 Years Diagnosed With CIN2+
| Characteristic | PR (95% CI) | APRa (95% CI) |
|---|---|---|
| Diagnosis | ||
| CIN2 | 1.0 | 1.0 |
| CIN2/3 | 1.43 (1.29–1.57) | 1.43 (1.30–1.57) |
| CIN3 | 1.52 (1.41–1.65) | 1.51 (1.40–1.63) |
| AIS | 1.91 (1.58–2.31) | 1.79 (1.49–2.16) |
| AIS/CIN | 1.92 (1.65–2.28) | 1.89 (1.57–2.27) |
| Age group, y | ||
| 21–24 | 1.20 (1.05, 1.36) | 1.28 (1.13–1.44) |
| 25–29 | 1.24 (1.09, 1.41) | 1.26 (1.12–1.42) |
| 30–34 | 1.17 (1.02, 1.34) | 1.16 (1.02–1.31) |
| 35–39 | 1.0 | 1.0 |
| Race/ethnicity | ||
| Non-Hispanic white | 1.0 | 1.0 |
| Non-Hispanic black | .71 (.63, .80) | .70 (.62–.80) |
| Hispanic | .78 (.69, .88) | .83 (.74–.93) |
| Otherb | .85 (.78, .93) | .86 (.79–.94) |
Abbreviations: APR, adjusted prevalence ratio; AIS, adenocarcinoma in situ; CI, confidence interval; CIN, cervical intraepithelial neoplasia; CIN2+, CIN2/3 or adenocarcinoma in situ; HPV, human papillomavirus; PR, prevalence ratio.
Adjusted for diagnosis, age, race, and project site.
Includes Asian, other, and missing race/ethnicity.
Multiple HPV types were detected in 20.6% of CIN2+ cases overall; more among cases with CIN2 (22.4%) compared with 16.4% of cases with CIN3 (data not shown). Among cases with multiple types detected, the majority (77.6%) had only 2 types (n = 488/629). Concurrent HPV types were detected in 14.3% of cases with AIS only, and 21.2% of those with both AIS and CIN had >1 HPV type. The highest number of concurrent HPV types detected was 6 types. Most high-risk HPV types were detected as single types among women with CIN2+ (Figure 2A), as well as among those with CIN3 (Figure 2B). HPV 16 or 18 occurred as a single infection in 39.7% of CIN2+ cases and 51.9% of CIN3 cases.
Figure 2.
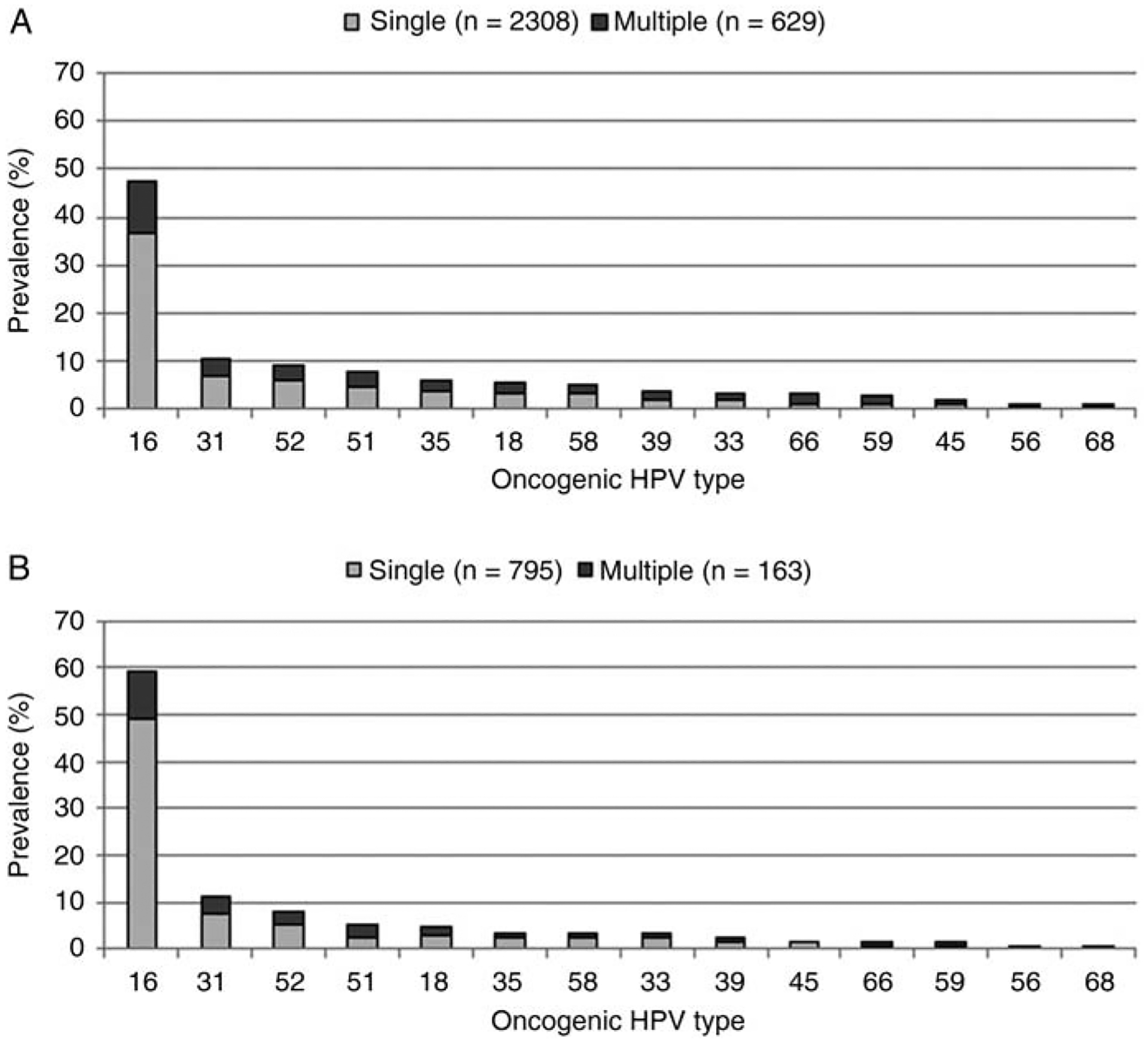
Oncogenic human papillomavirus (HPV) type prevalence in (A) cervical intraepithelial neoplasia 2/3 and adenocarcinoma in situ (CIN2+) and (B) CIN3 lesions by single and multiple infections.
DISCUSSION
In this evaluation of US women diagnosed with noninvasive CIN2 + , almost all cases (95%) were associated with at least 1 high-risk HPV type. As expected, vaccine types HPV 16 and 18 accounted for the majority of CIN2+ diagnoses (53%), and were more prevalent in higher grade CIN3 (65%) and AIS (82%) compared with CIN2. HPV 16 was the most frequent type detected in all diagnosis categories, but HPV 18 was much more common among cases with AIS (43%) compared to those with any grade CIN (5.0%), consistent with other studies [9, 17, 18].
More than 1 HPV type was detected in 20.6% of this population of women with CIN2 + . Similar to our findings, other studies have demonstrated concurrent infection with multiple HPV types in cervical disease [9, 19]. The significance of coinfection in relation to disease risk remains unclear given that each cervical lesion is considered to be independently caused by a single HPV type [20]. Regardless of the association with disease, detection of concurrent HPV types in lesions may complicate evaluations of potential cross-protection against related non-vaccine types because it is difficult to attribute type-specific causality in lesions with multiple types. Likewise, assessment of potential HPV type replacement will be challenging in the presence of HPV coinfections.
We found that vaccine type HPV 16 or 18 prevalence varied by age; prevalence of HPV 16- or 18-associated lesions was highest in the 25- to 29-year age group, and decreased through age 39 years. This observation is consistent with data from other studies [9, 10, 21, 22], and supports the hypothesis that HPV 16 and 18 may require less time to develop into clinically detectable cervical disease [23].
A striking finding in our analysis was differences in HPV type distribution by race/ethnicity. Specifically, HPV 16 or 18 was detected in a significantly lower proportion of CIN2+ lesions among non-Hispanic black and Hispanic women compared with those among non-Hispanic white women, even after adjusting for covariates such as age, diagnosis grade, and geographic location. Conversely, other oncogenic types were more commonly detected in some racial and ethnic subgroups compared to non-Hispanic whites: HPV 35 and 58 in non-Hispanic blacks and HPV 45 in Hispanics. International studies have demonstrated regional and country-to-country variability in the relative prevalence of specific HPV types associated with high-grade and invasive cervical lesions [8, 9, 24]. Interestingly, these studies have consistently shown that the proportion of HPV 16-related lesions is lower in Africa and South America than in Europe and North America. Moreover, a recent meta-analysis by Guan et al found that a much higher proportion of high-grade lesions in Africa were associated with HPV 35 and 58 compared with North America and Europe [24]. The reasons for the reported geographic differences have not been explained, but in this large US study, the distribution of HPV types associated with CIN2+ among non-Hispanic black and Hispanic women in the United States paralleled those found in Africa and South America.
Very few studies have explored racial and ethnic differences in HPV types associated with cervical lesions in the United States. Wheeler et al reported a significantly lower proportion of vaccine type HPV in Hispanic women compared with non-Hispanic whites using data from a state-based monitoring system in New Mexico [10]. However, women with CIN3 and invasive cervical cancer were combined for that analysis and therefore are not comparable to our data. One implication of our results is that current HPV vaccines could have less benefit for prevention of HPV vaccine type–associated CIN2+ lesions in nonwhite racial/ethnic groups of women. However, even if true, these results do not necessarily imply differential effectiveness of vaccine for the prevention of cervical cancer. Among oncogenic HPV types, vaccine type 16 has a higher potential to persist and cause cancer than most other types [25–27], and although not a direct measure of risk, data from the Guan et al study [24] corroborate the relative importance of HPV 16 and 18 for causing cervical cancer, even in regions where lower proportions of these types were found in high-grade lesions [24]. Evaluations are currently under way to investigate race/ethnicity differences in HPV types in invasive cervical cancers in the United States, and examining correlations with HPV types associated with CIN will be critical to interpretation of these findings.
It is important to note that some of the women in this analysis could have received HPV vaccine since the vaccine has been available in the United States since June 2006. However, national data indicate low HPV vaccine coverage in women aged 18 and older in the first few years after vaccine introduction. Estimates from the National Health Interview Survey indicate that HPV vaccine coverage for ≥1 dose was 10.5% in 2008 and 17.1% in 2009 among women aged 19–26 years. Coverage for women >26 years is not provided but is thought to be much lower. [28, 29]. In a separate analysis of our data, Powell et al (manuscript in preparation) present a detailed evaluation of vaccination history, in which they show that few of the women reported to the system were vaccinated, of whom >50% received their first dose after their abnormal pap test that led to the CIN2+ diagnosis. Therefore, women with CIN2+ represented in the current analysis likely did not benefit from receipt of vaccine given that the vaccine has no therapeutic effect.
Our analysis has some limitations. First, the archived histology tissue used for DNA typing may not have been representative of the tissue used for diagnosis. However, all specimens were reviewed by the collaborating pathologist (E.R.U.), and only those with verified cervical lesions were tested for HPV DNA. Second, the inherent heterogeneity in pathologic diagnosis of CIN may have led to misclassification of some cases. However, a master list of codes and terminologies was provided to all reporting laboratories in an effort to standardize case ascertainment. Third, race and ethnicity information was not available for about 15% of the women, which may have biased the observed racial/ethnic differences. Although these data are from a population-based project designed to capture all cases within the designated surveillance areas, we were unable to obtain specimens from some reporting labs, which may have resulted in selection bias. However, in all project areas, HPV typing was performed on >70% of reported cases, and there were no significant differences in demographic or clinical features between cases with and without specimens.
To our knowledge, these results present the most extensive evaluation of HPV types in high-grade cervical disease among subpopulations of women across the United States. Overall HPV type prevalence in women with CIN2+ prior to wide-spread vaccination in the United States provides a baseline for monitoring the population impact of current bivalent and quadrivalent vaccines. Given race/ethnicity and age differences in HPV vaccine type positivity, monitoring HPV types in these subgroups will provide important information for targeting prevention efforts using current vaccines, and also contribute to policy and guidelines for multivalent HPV vaccines that would target more oncogenic HPV types.
Acknowledgments.
HPV-IMPACT Working Group members: Ina Park, MD, MS, Erin Whitney, MPH, Sharon McDonnell, MPH (California Department of Public Health, STD Control Branch); James Hadler, MD, Robert Heimer, PhD, Pamela Julian, MPH, James Meek, MPH (Yale University School of Medicine, Connecticut Emerging Infections Program); Lynn Sosa, MD (Connecticut Department of Public Health); Ghinwa Dumyati, MD, Mary Scahill, Denisse Licon, MPH (University of Rochester-New York Emerging Infections Program); Nasreen Abdullah, MD, MPH, Rob Laing, MPH (Oregon Department of Human Services); Diane Levine, BS, RN, MPH, Manideepthi Pemmaraju, MBBS, MPH, Chasiety Turner, MPH (Vanderbilt University-Tennessee Emerging Infections Program); Jill Sharma (Division of High-Consequence Pathogens and Pathology, National Center for Emerging and Zoonotic Infectious Diseases, CDC).
Financial support. This work was supported by CDC.
Footnotes
The members of the HPV-IMPACT Working Group are listed in the Acknowledgments section.
Publisher's Disclaimer: Disclaimers. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: 2011 International Papillomavirus Conference, Berlin, Germany.
References
- 1.Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56:1–24. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP). MMWR Morbid Mortal Wkly Rep 2010; 59:626–9. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. FDA licensure of quadrivalent human papillomavirus vaccine (HPV4, Gardasil) for use in males and guidance from the Advisory Committee on Immunization Practices (ACIP). MMWR Morbid Mortal Wkly Rep 2010; 59:630–2. [PubMed] [Google Scholar]
- 4.Munoz N, Castellsague X, de Gonzalez AB, Gissmann L. Chapter 1: HPV in the etiology of human cancer. Vaccine 2006; 24(Suppl 3):S3/ 1–10. [DOI] [PubMed] [Google Scholar]
- 5.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet 2007; 370:890–907. [DOI] [PubMed] [Google Scholar]
- 6.Wright TC Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with cervical intraepithelial neoplasia or adenocarcinoma in situ. J Low Genit Tract Dis 2007; 11:223–39. [DOI] [PubMed] [Google Scholar]
- 7.American College of O, Gynecologists. ACOG Committee Opinion No. 463: cervical cancer in adolescents: screening, evaluation, and management. Obstet Gynecol 2010; 116:469–72. [DOI] [PubMed] [Google Scholar]
- 8.Clifford GM, Smith JS, Aguado T, Franceschi S. Comparison of HPV type distribution in high-grade cervical lesions and cervical cancer: a meta-analysis. Br J Cancer 2003; 89:101–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Sanjose S, Quint WG, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 2010; 11:1048–56. [DOI] [PubMed] [Google Scholar]
- 10.Wheeler CM, Hunt WC, Joste NE, Key CR, Quint WG, Castle PE. Human papillomavirus genotype distributions: implications for vaccination and cancer screening in the United States. J Natl Cancer Inst 2009; 101:475–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Markowitz LE, Hariri S, Unger ER, Saraiya M, Datta SD, Dunne EF. Post-licensure monitoring of HPV vaccine in the United States. Vaccine 2010; 28:4731–7. [DOI] [PubMed] [Google Scholar]
- 12.Hariri S, Unger ER, Powell SE, et al. The HPV vaccine impact monitoring project (HPV-IMPACT): assessing early evidence of vaccination impact on HPV-associated cervical cancer precursor lesions. Cancer Causes Control 2012; 23:281–8. [DOI] [PubMed] [Google Scholar]
- 13.Schuchat A, Hilger T, Zell E, et al. Active bacterial core surveillance of the emerging infections program network. Emerg Infect Dis 2001; 7:92–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinau M, Patel SS, Unger ER. Efficient DNA extraction for HPV genotyping in formalin-fixed, paraffin-embedded tissues. J Mol Diagn 2011; 13:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Onyekwuluje JM, Steinau M, Swan DC, Unger ER. A real-time PCR assay for HPV52 detection and viral load quantification. Clin Lab 2012; 58:61–6. [PubMed] [Google Scholar]
- 16.Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol 2012; 137:516–42. [DOI] [PubMed] [Google Scholar]
- 17.Altekruse SF, Lacey JV Jr, Brinton LA, et al. Comparison of human papillomavirus genotypes, sexual, and reproductive risk factors of cervical adenocarcinoma and squamous cell carcinoma: northeastern United States. Am J Obstet Gynecol 2003; 188:657–63. [DOI] [PubMed] [Google Scholar]
- 18.Coutlee F, Ratnam S, Ramanakumar AV, et al. Distribution of human papillomavirus genotypes in cervical intraepithelial neoplasia and invasive cervical cancer in Canada. J Med Virol 2011; 83:1034–41. [DOI] [PubMed] [Google Scholar]
- 19.Li N, Franceschi S, Howell-Jones R, Snijders PJ, Clifford GM. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: variation by geographical region, histological type and year of publication. Int J Cancer 2011; 128:927–35. [DOI] [PubMed] [Google Scholar]
- 20.Quint W, Jenkins D, Molijn A, et al. One virus, one lesion—individual components of CIN lesions contain a specific HPV type. J Pathol 2012; 227:62–71. [DOI] [PubMed] [Google Scholar]
- 21.Munoz N, Bosch FX, Castellsague X, et al. Against which human papillomavirus types shall we vaccinate and screen? The international perspective. Int J Cancer 2004; 111:278–85. [DOI] [PubMed] [Google Scholar]
- 22.Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst 2005; 97:1072–9. [DOI] [PubMed] [Google Scholar]
- 23.Vinokurova S, Wentzensen N, Kraus I, et al. Type-dependent integration frequency of human papillomavirus genomes in cervical lesions. Cancer Res 2008; 68:307–13. [DOI] [PubMed] [Google Scholar]
- 24.Guan P, Howell-Jones R, Li N, et al. Human papillomavirus (HPV) types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer 2012; 131:2349–59. [DOI] [PubMed] [Google Scholar]
- 25.Castle PE, Rodriguez AC, Burk RD, et al. Short term persistence of human papillomavirus and risk of cervical precancer and cancer: population based cohort study. BMJ 2009; 339:b2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schiffman M, Glass AG, Wentzensen N, et al. A long-term prospective study of type-specific human papillomavirus infection and risk of cervical neoplasia among 20,000 women in the Portland Kaiser Cohort Study. Cancer Epidemiol Biomarkers Prev 2011; 20:1398–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen HC, Schiffman M, Lin CY, et al. Persistence of type-specific human papillomavirus infection and increased long-term risk of cervical cancer. J Natl Cancer Inst 2011; 103:1387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jain N, Euler GL, Shefer A, Lu P, Yankey D, Markowitz L. Human papillomavirus (HPV) awareness and vaccination initiation among women in the United States, National Immunization Survey–Adult 2007. Prev Med 2009; 48:426–31. [DOI] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention, National Center for Health Statistics, National Health Interview Survey. http://www.cdc.gov/vaccines/stats-surv/nhis/default.htm. Accessed 25 May 2012.


