The increased cardiovascular disease (CVD) risk associated with type 2 diabetes (T2D) is likely due in part to diabetic dyslipidemia—a condition characterized by elevated plasma levels of triglyceride‐rich lipoproteins (TRLs), smaller denser LDL particles, and decreased HDL cholesterol (1,2). Lifestyle modifications and statins are first-line interventions for CVD risk reduction in individuals with T2D, but such individuals remain at considerable risk for cardiovascular events (3). This residual CVD risk appears to be linked to abnormal metabolism of TRLs (3)—chylomicrons, VLDL, and their respective remnant lipoprotein particles (RLPs), many of which are present as intermediate-density lipoproteins (4). Thus, there is a need for developing effective and safe alternative approaches/medications to prevent and reduce diabetic dyslipidemia. In this issue of Diabetes, Roger et al. (5) report a potential new therapeutic strategy to simultaneously reduce LDL and VLDL levels by using a hybrid compound that demonstrates dual inhibition of cannabinoid-1 receptor (CB1R) and inducible nitric oxide synthase (iNOS) in a mouse model.
The elevated TRLs in T2D are generally attributed to hepatic overproduction of apolipoprotein B (APOB)-containing VLDL, increased intestinal secretion of APOB48 (a truncated form of APOB100 in chylomicrons and their remnants), and dysregulated clearance of TRLs (1,2,6,7), as shown by Fig. 1. Insulin resistance and the resulting elevated fatty acids are believed to be responsible for the abnormal TRL metabolism in T2D (1,2,7). For example, inhibition of the transcription factor FoxO1 by insulin-induced Akt activation represses expression of microsomal triglyceride transfer protein (MTP), thereby inhibiting lipidation of APOB and secretion of VLDL (8). In parallel, insulin promotes hepatic clearance of circulating APOB particles via the LDL receptor (LDLR), LDLR-related protein 1 (LRP1), and syndecan, in part by suppressing APOC3, an apolipoprotein that prevents clearance of TRLs (9,10).
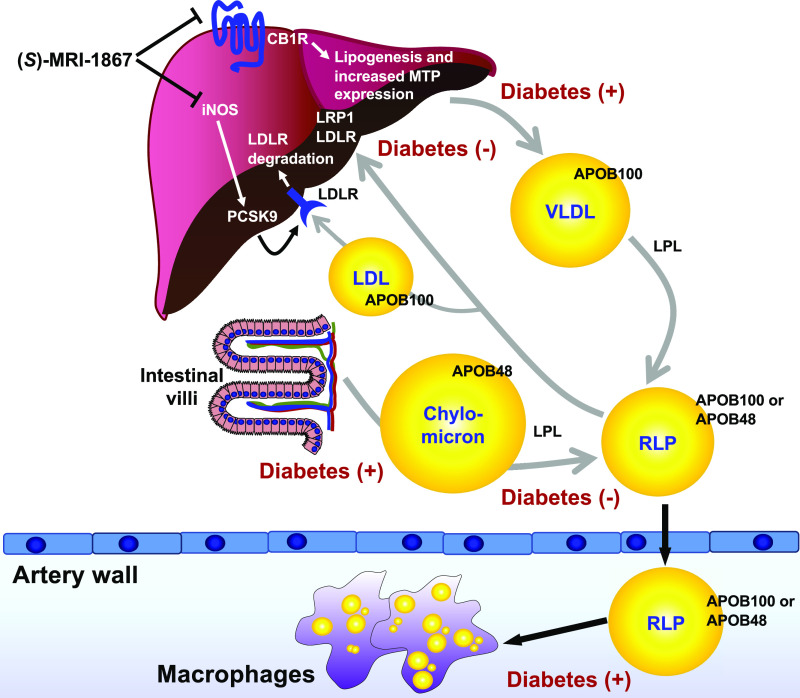
Figure 1.
Diabetes is associated with elevated levels of triglyceride-rich lipoproteins, an effect that might be mitigated by a dual CB1R/iNOS inhibitor. Increased plasma levels of TRLs in T2D can be due to the increased hepatic production of APOB100-containing VLDL, increased intestinal secretion of APOB48, reduced lipolysis of TRLs by LPL, and reduced hepatic clearance of TRLs and their RLPs. A prolonged elevation of RLPs in circulation likely leads to increased trapping of these highly atherogenic particles in the artery wall, where they are taken up by macrophages and are believed to contribute to atherogenesis. The dual CB1R/iNOS inhibitor (S)-MRI-1867 reduces hepatic secretion of VLDL through inhibiting CB1R-mediated MTP expression and reduces PCSK9 expression and subsequently increases LDL receptor expression via iNOS inhibition. The figure was reproduced and modified from Chait et al. (4), with permission of Diabetes.
Cannabinoid signaling through CB1R is involved in the development of obesity, insulin resistance, and elevated plasma lipids (11,12). Thus, CB1R antagonists improve insulin sensitivity and reduce hepatic steatosis and body weight in obese mice (13). However, the CB1R antagonist/reverse agonist rimonabant had unwanted central nervous system–mediated effects in humans, including anxiety and suicidal thoughts, which led to the withdrawal of CB1R antagonists from the market (13). Scientists have continued to explore the beneficial effects of CB1R antagonism by developing second-generation peripherally restricted CB1R antagonists to minimize the neuropsychiatric effects. Moreover, hybrid compounds that are peripheral CB1R antagonists with a secondary target are of recent interest. iNOS has been used as a secondary target for hybrid compounds because it is likely to play a critical role in insulin resistance, nonalcoholic fatty liver disease (NAFLD), and liver fibrosis (14,15).
Roger et al. (5) used elegant pharmacological approaches to characterize the effects of the peripherally restricted hybrid CB1R/iNOS inhibitor (S)-MRI-1867 on dyslipidemia and metabolism in a diet-induced obese mouse model. The authors first showed that (S)-MRI-1867 improves glucose homeostasis and dyslipidemia, associated with a striking reduction in body weight after only 14 days of treatment. (S)-MRI-1867–treated mice exhibited reduced hepatic triglyceride content and an improvement of diet-induced hepatic injury, consistent with a previous report from the same group showing that (S)-MRI-1867 mitigates liver fibrosis (15). Importantly, (S)-MRI-1867 reduced de novo lipogenesis, FoxO1-mediated MTP expression, and VLDL production, likely by increasing hepatic insulin sensitivity. In addition, (S)-MRI-1867–treated mice exhibited increased hepatic expression of LDLRs, likely mediated by suppressed expression of PCSK9 (proprotein convertase subtilisin kexin 9)—a protein that enhances lysosomal degradation of LDLRs (16). PCSK9 inhibitors are now in clinical use for LDL lowering in patients who do not achieve sufficient LDL lowering on statin therapy.
Next, Roger et al. elegantly dissected the relative contribution of CB1R and iNOS inhibition on VLDL secretion and LDLR expression in isolated primary hepatocytes and diet-induced obese mice. A CB1R inhibitor (JD-5037) revealed that CB1R antagonism was responsible for the effect on VLDL secretion, likely through inhibition of the FoxO1-MTP pathway, while iNOS inhibition lacked effect on this pathway. Conversely, inhibition of iNOS reduced PCSK9 expression, an effect not shared by CB1R inhibition. Activation of mTORC1 signaling can lead to reduced PCSK9 and a subsequent increase in LDLR expression (17). Accordingly, the authors suggested that an increase in bioavailability of l-arginine due to inhibition of iNOS (which uses l-arginine as a substrate) explains the activation of mTORC1 and reduction in PCSK9. Together, the findings suggest that (S)-MRI-1867 mitigates dyslipidemia by suppressing CB1R-mediated VLDL production and by increasing LDLR levels by reducing PCSK9 through iNOS inhibition.
This study raises multiple interesting questions and complex challenges. First, to what extent is the improvement in dyslipidemia by (S)-MRI-1867 mediated by weight loss or effects in extrahepatic tissues? This is important because CB1R is widely expressed in peripheral tissues, such as adipose tissue, which can influence systemic lipid metabolism and body weight (18). It is also possible that (S)-MRI-1867 reduces chylomicron secretion from the intestine or improves insulin sensitivity in other tissues. Second, would the improved lipids in (S)-MRI-1867–treated mice translate to reduced atherosclerosis? TRLs and/or their RLPs promote atherosclerosis in diabetic mice with a human-like lipoprotein profile (4,9). However, because the current study was performed in C57BL/6 mice, which have very low LDL levels, lipoprotein profiles were only shown from mice in which lipoprotein lipase (LPL) was inhibited. LPL is the major vascular enzyme responsible for TRL lipolysis (19). Third, the causative roles of the proposed molecular mechanisms need to be validated. For instance, the authors suggest that changes in MTP and PCSK9 expression are responsible for reducing VLDL production and LDL clearance, respectively. It would be important to establish their roles by testing the effect of (S)-MRI-1867 in liver-specific MTP- and PCSK9-deficient mice or hepatocytes, as well as the upstream signaling. Fourth, although hepatic overproduction of VLDL can contribute to diabetic dyslipidemia, defects in clearance of TRLs/RLPs and perhaps reduced HDL cholesterol levels are likely to be important in CVD risk. Fifth, large clinical trials would be needed to establish whether (S)-MRI-1867, or similar hybrid compounds, have beneficial effects on dyslipidemia, NAFLD, and incident CVD in patients with T2D. We look forward to future studies addressing the emerging concept of hybrid inhibition of CB1R/iNOS in mitigating diabetic dyslipidemia and associated complications.
Article Information
Funding. The authors receive research support from the National Institutes of Health (P01HL092969, R01HL127694, R01HL126028, and R35HL150754 to K.E.B.) and a postdoctoral fellowship award from the American Diabetes Association (9-18-CVD1-002 to V.K.).
Duality of Interest. K.E.B. reports receiving research support from Novo Nordisk A/S on an unrelated project. No other potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying article, p. 2120.
References
- 1.Khavandi M, Duarte F, Ginsberg HN, Reyes-Soffer G. Treatment of dyslipidemias to prevent cardiovascular disease in patients with type 2 diabetes. Curr Cardiol Rep 2017;19:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xiao C, Dash S, Morgantini C, Hegele RA, Lewis GF. Pharmacological targeting of the atherogenic dyslipidemia complex: the next frontier in CVD prevention beyond lowering LDL cholesterol. Diabetes 2016;65:1767–1778 [DOI] [PubMed] [Google Scholar]
- 3.Sandesara PB, Virani SS, Fazio S, Shapiro MD. The forgotten lipids: triglycerides, remnant cholesterol, and atherosclerotic cardiovascular disease risk. Endocr Rev 2019;40:537–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chait A, Ginsberg HN, Vaisar T, Heinecke JW, Goldberg IJ, Bornfeldt KE. Remnants of the triglyceride-rich lipoproteins, diabetes, and cardiovascular disease. Diabetes 2020;69:508–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roger C, Buch C, Muller T, et al. . Simultaneous inhibition of peripheral CB1R and iNOS mitigates obesity-related dyslipidemia through distinct mechanisms. Diabetes 2020;69:2120–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gill JM, Sattar N. Hepatic VLDL overproduction: is hyperinsulinemia or insulin resistance the culprit? J Clin Endocrinol Metab 2011;96:2032–2034 [DOI] [PubMed] [Google Scholar]
- 7.Goldberg IJ. Clinical review 124: diabetic dyslipidemia: causes and consequences. J Clin Endocrinol Metab 2001;86:965–971 [DOI] [PubMed] [Google Scholar]
- 8.Haas ME, Attie AD, Biddinger SB. The regulation of ApoB metabolism by insulin. Trends Endocrinol Metab 2013;24:391–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanter JE, Shao B, Kramer F, et al. . Increased apolipoprotein C3 drives cardiovascular risk in type 1 diabetes. J Clin Invest 2019;129:4165–4179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordts PL, Nock R, Son NH, et al. . ApoC-III inhibits clearance of triglyceride-rich lipoproteins through LDL family receptors. J Clin Invest 2016;126:2855–2866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagappan A, Shin J, Jung MH. Role of cannabinoid receptor type 1 in insulin resistance and its biological implications. Int J Mol Sci 2019;20:2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J, Zhou L, Xiong K, et al. Hepatic cannabinoid receptor-1 mediates diet-induced insulin resistance via inhibition of insulin signaling and clearance in mice. Gastroenterology 2012;142:1218–1228.e1 [DOI] [PMC free article] [PubMed]
- 13.Cinar R, Iyer MR, Kunos G. The therapeutic potential of second and third generation CB1R antagonists. Pharmacol Ther 2020;208:107477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujimoto M, Shimizu N, Kunii K, Martyn JA, Ueki K, Kaneki M. A role for iNOS in fasting hyperglycemia and impaired insulin signaling in the liver of obese diabetic mice. Diabetes 2005;54:1340–1348 [DOI] [PubMed] [Google Scholar]
- 15.Cinar R, Iyer MR, Liu Z, et al. . Hybrid inhibitor of peripheral cannabinoid-1 receptors and inducible nitric oxide synthase mitigates liver fibrosis. JCI Insight 2016;1:e87336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peterson AS, Fong LG, Young SG. PCSK9 function and physiology. J Lipid Res 2008;49:1152–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ai D, Chen C, Han S, et al. . Regulation of hepatic LDL receptors by mTORC1 and PCSK9 in mice. J Clin Invest 2012;122:1262–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zou S, Kumar U. Cannabinoid receptors and the endocannabinoid system: signaling and function in the central nervous system. Int J Mol Sci 2018;19:833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldberg IJ, Eckel RH, Abumrad NA. Regulation of fatty acid uptake into tissues: lipoprotein lipase- and CD36-mediated pathways. J Lipid Res 2009;50(Suppl.):S86–S90 [DOI] [PMC free article] [PubMed] [Google Scholar]