Abstract
Unsymmetrically fused porphyrins containing one or two naphthalimide subunits were prepared in modular syntheses relying on electron-rich and electron-poor pyrrole building blocks. These new chromophores show progressive changes in their electron-deficient character, while retaining comparably small optical and electrochemical band gaps. The intrinsic curvature and extended optical absorption of these systems make them of interest as mono- and difunctional components of multichromophoric assemblies.
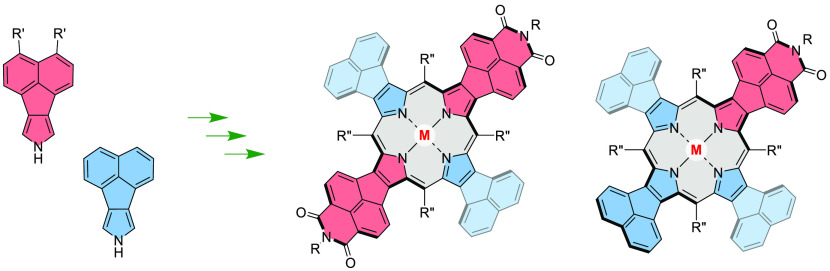
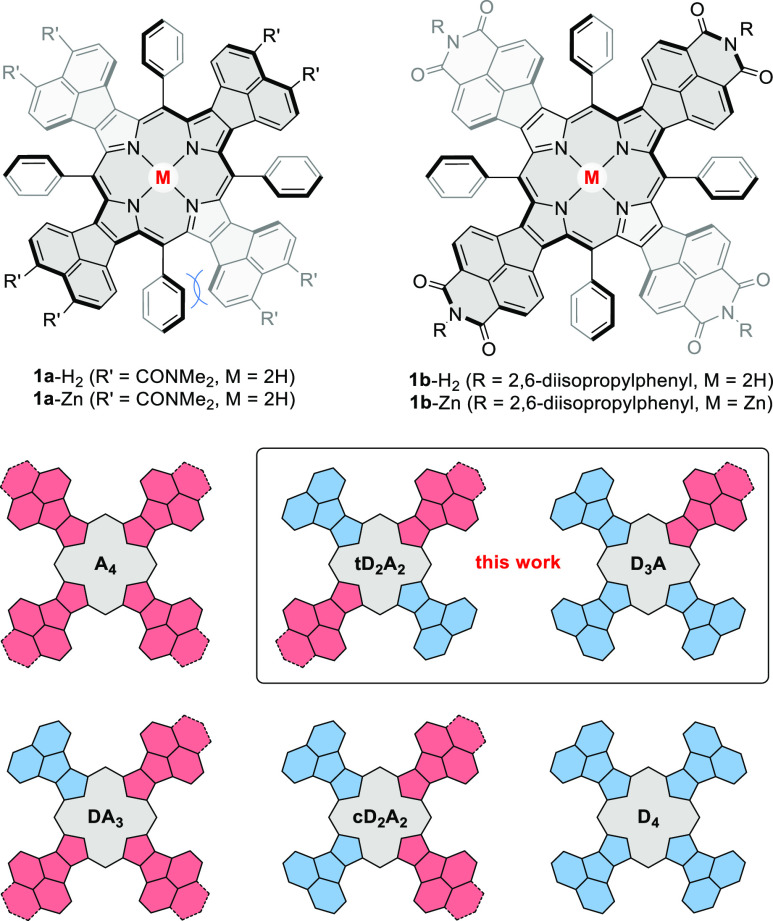
Ryleneimides, most notably perylenediimides (PDIs), are one of the most important classes of organic dyes, with an exceptional scope of industrial and research applications.1−5 Their electronic and self-assembly properties can be tuned by homologation of the rylene backbone,3 modification of imide functionalities,6 peripheral substitution,1 and ring fusion.7−9 Hybridization of ryleneimides with nonbenzenoid and heterocyclic moieties provides a versatile though still relatively unexplored route to structurally unique functional dyes. In one general strategy,9,10 tandem Pd-catalyzed couplings have been used for synthesis of ryleneimide-fused indenes,11 azulenes,12 corannulenes,13,14 and heteroaromatics.15 Our group has employed naphthaleneimide-fused pyrrole building blocks16 for modular synthesis of electron-deficient porphyrins,16 azacoronenes,17−19 bipyrroles,20 and polymers.21 This approach has so far yielded symmetrically fused A4 porphyrins (Scheme 1), containing naphthalenediamide (NDA-fused, 1a-H2) or naphthalenemonoimide acceptor units (NMI-fused, 1b-H2), which have been explored as multielectron acceptors16 and functional dyes.22
Scheme 1. Donor–Acceptor Porphyrins with Mixed Ring Fusion Patterns.
The electronic characteristics of such porphyrins can be tailored by juxtaposition of donor (D) and acceptor (A) units fused to the macrocyclic core (Scheme 1). In particular, we envisaged that the optical band gaps, absorption profiles, and redox properties of such mixed D–A porphyrins might be affected not only by the ratio of D and A subunits but also by the symmetry of the chromophore.23−27 Systems containing imide functionalities are highly attractive as components for multichromophoric systems obtainable by covalent or supramolecular assembly. For instance, donor BODIPY dyes attached radially to A4-type porphyrins via N-imide substituents yield efficient energy transfer according to the Förster mechanism.28 The presence of four functionalization sites in the A4 systems is however unsuitable for construction of unbranched architectures, notably linear and cyclic chromophore arrays. In this regard, D–A porphyrins containing fewer imide functionalities are essential as mono- or bifunctional building blocks, simultaneously analogous to ryleneimides, and other unsymmetrically functionalized porphyrins.29−32
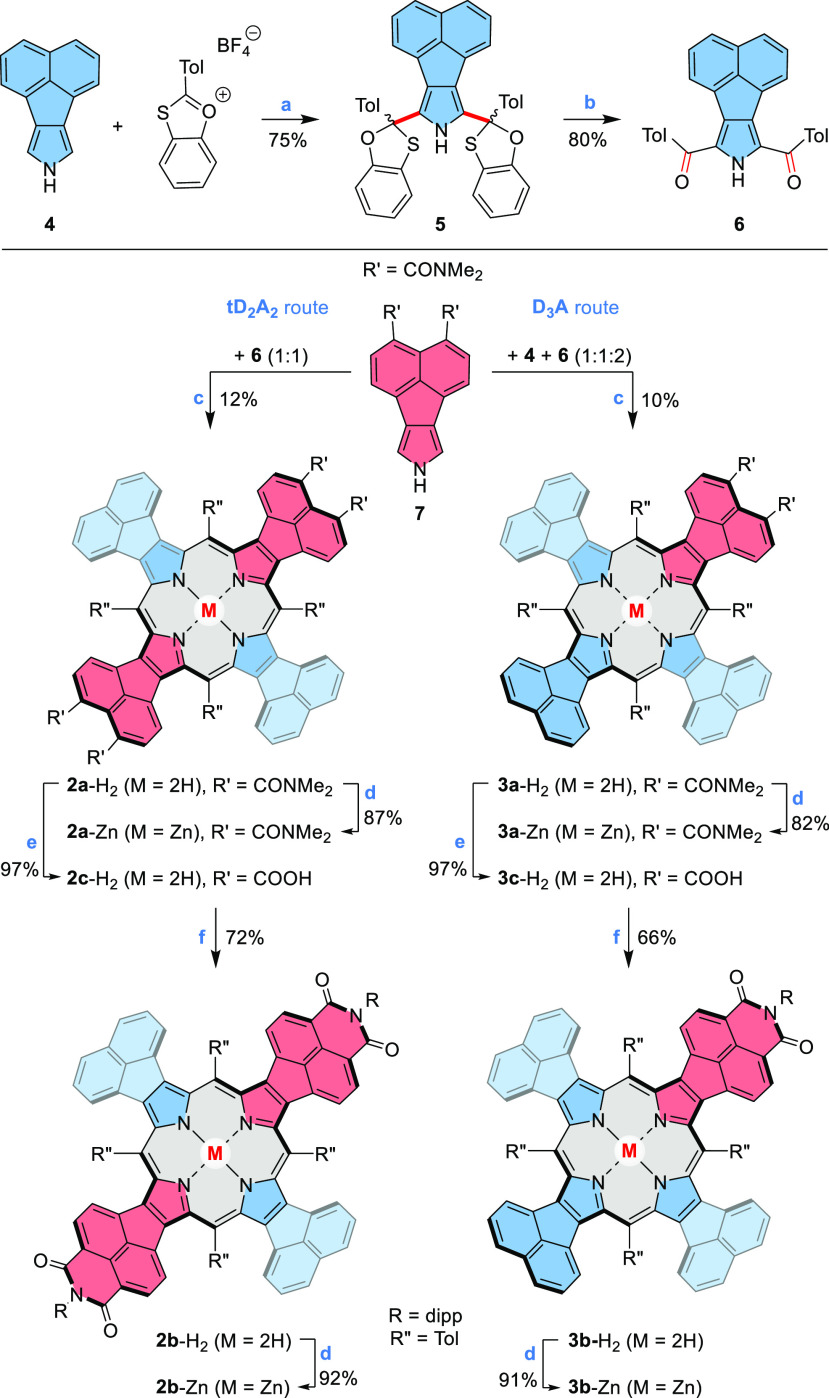
With these possibilities in mind, we now developed synthetic routes to two classes of low-symmetry chromophores: tD2A2 and DA3, which can be viewed as porphyrin-hybridized equivalents of rylene diimides and monoimides, respectively. In principle, mixed donor–acceptor porphyrins, namely tD2A2, D3A, DA3, and cD2A2, are obtainable in a three-component cross-condensation of two different pyrroles and an aldehyde.33 Such a strategy is generally unselective and may fail if the reactivity of the two pyrroles is significantly different. To circumvent these problems, we used a multistep approach shown in Scheme 2. First, the reaction of acenaphthopyrrole 4(34) and 2-tolyl 1,3-benzoxathiolium tetrafluoroborate35,36 afforded the masked diketone 5, which was hydrolyzed to yield the diacyl pyrrole 6. The latter intermediate was reduced with NaBH4 in THF/CH3OH, and the resulting crude acenaphthopyrrole dicarbinol was condensed with 1 equiv of diamide pyrrole 7.16 Following column chromatography, the desired tD2A2 porphyrin 2a-H2 was isolated in a 12% yield. Similarly, a cross-condensation of 7, 4, and reduced 6 in a 1:1:2 molar ratio furnished the D3A-type porphyrin 3a-H2 as the major product. 2a-H2 and 3a-H2 were transformed into the respective imide derivatives 2b-H2 and 3b-H2 using a two-step procedure consisting of acid hydrolysis followed by imidization with 2,6-diisopropylaniline. Finally, the four new porphyrins were metalated with zinc(II) acetate, yielding the corresponding complexes, 2a-Zn, 3a-Zn, 2b-Zn, and 3b-Zn. All the compounds were thoroughly characterized by HR-MS as well as 1D and 2D NMR spectroscopy, and the structures of porphyrins 2b-H2/3b-H2 were confirmed X-ray crystallography.
Scheme 2. Synthesis of Mixed Donor–Acceptor Porphyrins.
Reagents and conditions: (a) pyridine, CH3CN/CHCl3 (1:1 v/v), 1 h; (b) HgO, HBF4, THF, 5 h; (c) (i) 6, NaBH4, THF/MeOH (3:1 v/v), 2 h, (ii) 7 (4, for 3a-H2), p-TSA, CHCl3/MeOH (100:1 v/v), 1 h; (iii) DDQ, 2 h; (d) Zn(OAc)2·2H2O, CHCl3/MeOH (3:1 v/v); (e) HCl, reflux, 24 h; (f) 2,6-diisopropylaniline, acetic acid, 20 h, reflux. Tol = p-tolyl, dipp = 2,6-diisopropylphenyl.
The free bases 2b-H2 and 3b-H2 were characterized crystallographically in the solid state (Figure 1). In each case, a trans tautomer was observed, with the NH protons located on the more electron-rich pair of pyrroles.37 Each macrocycle showed a pronounced saddle-shaped distortion of the aromatic surface. This characteristic feature is caused by the steric congestion between the outer naphthalene moieties and the meso-tolyl substituents, and precludes stacking interactions between the porphyrin π surfaces. The dihedral angles between opposite naphthalene subunits in 2b-H2 are 117.5° (NMI/NMI) and 109.1° (naph/naph, cf. Figure 1), producing a similar level of curvature to that previously observed for 1b-H2. Remarkably, the corresponding angles in 3b-H2 are drastically different (90.2° and 128.8° for naph/NMI and naph/naph, respectively). In DFT-optimized geometries of 2b-H2 and 3b-H2 (Table S14), the dihedrals involving NMI units are very similar (98°–99°), indicating that the geometry difference observed in the solid state is caused by crystal packing and is most likely absent in solution. In the crystal, the surface of 3b-H2 apparently wraps around the unique dipp group of a neighboring molecule, and this interaction is propagated along one direction, to produce densely packed ribbons stabilized by multiple CH···π interactions (Figure 1D). A similar type of interaction between dipp groups and aromatic surfaces is found in the crystal of 2b-H2 (Figure 1C); however, the resulting pattern is less dense and produces no additional distortion of the aromatic core. As a consequence, the latter structure is more highly solvated (2b-H2·1.75C6H14·8.8CHCl3 vs 3b-H2·2.8C6H14·3.1CHCl3), similarly to the previously reported structures of 1b-H2 and 1b-Zn.16
Figure 1.
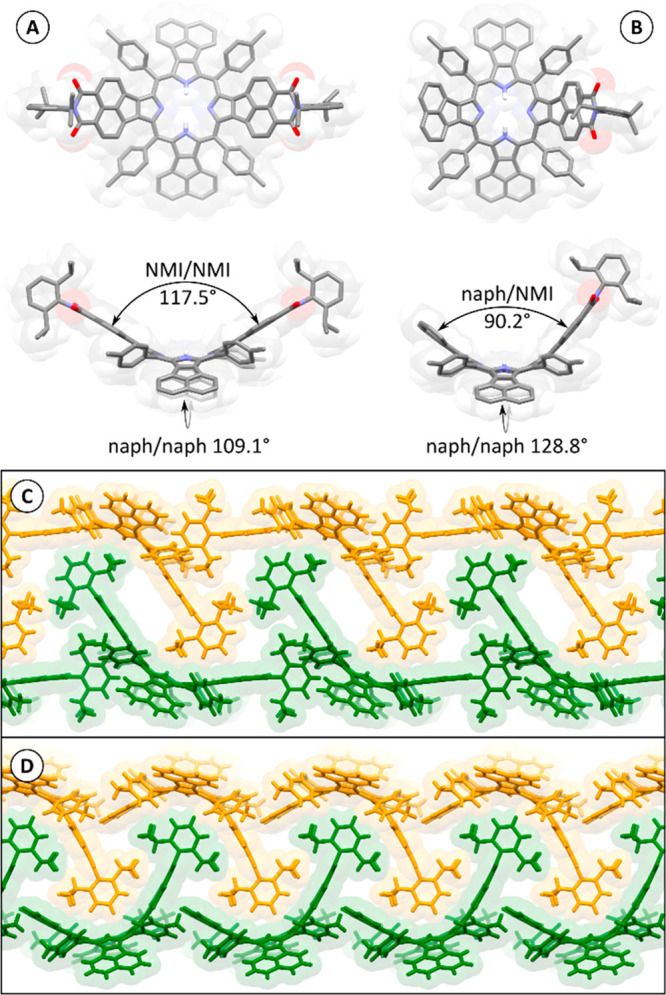
Molecular structures and packing diagrams of 2b-H2 (A and C) and 3b-H2 (B and D) obtained in X-ray crystallographic analyses. C-bound hydrogen atoms (A and B) and solvent molecules (A–D) are omitted for clarity. Interplanar angles (A and B) were calculated between C10 planes of naphthalene (naph) and naphthalenemonoimide (NMI) subunits.
Steady-state absorption spectra of the newly synthesized tD2A2 and D3A porphyrins contain an intense Soret-like band at ca. 600 nm and a series of Q bands extending into the near-infrared (Figure 2 and Tables 1 and S1). Zinc complexes were characterized in the presence of excess pyridine to suppress aggregation previously observed for amide-bearing derivatives.16 For the NDA systems, the difference between the absorption profiles of tD2A2 and D3A-type chromophores (i.e., 2a-M vs 3a-M) is relatively insignificant. In particular, the di-NDA free base 2a-H2 shows a Soret band at 568 nm and three Q bands at 639, 709, 778 nm, whereas, in mono-NDA porphyrin 3a-H2, the Soret band is slightly blue-shifted to 559 nm, whereas the lowest-energy Q-band shows a red shift to 803 nm. In fact these spectra are similar to those of 1a-H216 and the corresponding D4 system,38 implying a relatively weak interaction between the π system and the twisted dimethylaminocarbonyl substituents. In contrast, the optical properties of 2b-M and 3b-M are significantly perturbed by the fusion of NMI subunits. All systems show extended NIR bands, with the absorption onset in each case approaching 1000 nm. Interestingly, in spite of the smaller number of electron-withdrawing NMI moieties, the band gaps of all these systems are apparently reduced relative to that of 1b-M.16 This observation is further confirmed by the positions of emission maxima of the weakly fluorescent complexes 2b-Zn and 3b-Zn (930 and 920 nm, respectively, Figures S7 and S8), which are both red-shifted relative to 1b-Zn16 (893 nm). The mono-NMI systems 3b-M have somewhat larger optical gaps than their di-NMI analogues.
Figure 2.
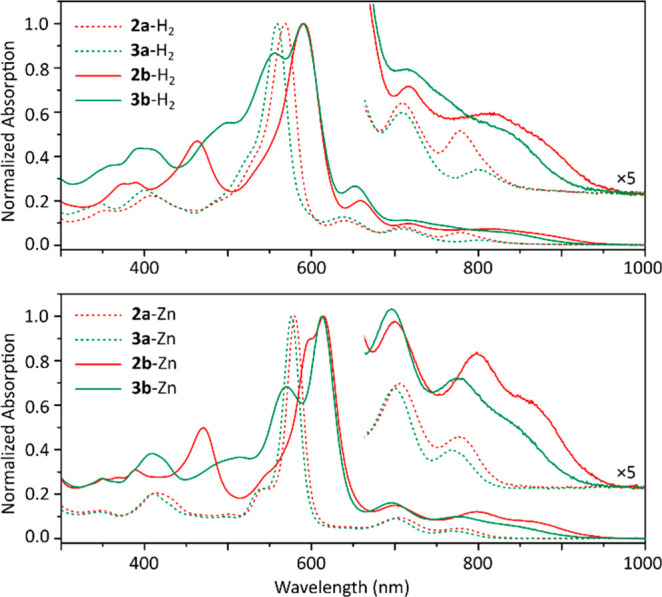
Electronic absorption spectra of 2a-M, 2b-M, 3a-M, and 3b-M (5 μM concentration; M = 2H in dichloromethane, M = Zn in toluene + 1% pyridine).
Table 1. Photophysical and Electrochemical Properties of Compounds 2a-M, 2b-M, 3a-M, and 3b-M (M = 2H, Zn)a,b.
| Species | Q1 (Soret) [nm]b | λmaxem [nm]c | Eox1 [V]d | Ered1 [V]d | ΔE [V]d |
|---|---|---|---|---|---|
| 2a-H2 | 778 (568) | 837 | 0.26 | –1.34 | 1.60 |
| 2b-H2 | 823 (590) | 956 | 0.36 | –1.08 | 1.44 |
| 3a-H2 | 803 (559) | 833 | 0.25 | –1.36 | 1.61 |
| 3b-H2 | 834 (590) | 950 | 0.30 | –1.14 | 1.44 |
| 1a-Zn | 775 (583) | 806 | 0.22 | –1.42 | 1.64 |
| 1b-Zn | 838 (632) | 893 | 0.34 | –1.00 | 1.34 |
| 2a-Zn | 780 (579) | 810 | 0.08 | –1.63 | 1.71 |
| 2b-Zn | 864 (614) | 930 | 0.24 | –1.19 | 1.43 |
| 3a-Zn | 768 (577) | 815 | 0.11 | –1.64 | 1.75 |
| 3b-Zn | 852 (613) | 920 | 0.18 | –1.22 | 1.40 |
Data in dichloromethane. For additional data, see the Supporting Information.
Maximum absorption for the lowest-energy Q-band and for the most intense Soret band. Data for the previously reported161a-Zn and 1b-Zn are provided for comparison.
Fluorescence emission maximum.
First oxidation and reduction potentials (relative to Fc/Fc+) and the electrochemical band gap.
Interestingly, on going from 1b-M, through 2b-M, to 3b-M, gradual reduction of molecular symmetry produces increasingly broadened absorption in the visible (Soret-like) region of the absorption spectrum, which is a beneficial characteristic for potential light-harvesting applications. Moreover, symmetry effects have an influence on excited-state population dynamics of 2b-Zn and 3b-Zn, which were investigated using femtosecond transient absorption spectroscopy. Upon photoexcitation, 2b-Zn and 3b-Zn showed singlet state lifetimes of 460 and 230 ps followed by infinite residues contributed by the intersystem crossing processes to the triplet state (Figures S9 and S10). Thus, the excited singlet states of 2b-Zn and 3b-Zn are quenched 4 to 6 times faster than observed for 1b-Zn,28 indicating a faster deactivation of the excited singlet state in the asymmetrical structures.
In dichloromethane or THF solutions, 2b-M and 3b-M (M = 2H, Zn) showed up to two chemically reversible oxidations, followed by additional nonreversible events at higher potentials (Tables 1 and S2 and Figures S12 and S13). The monoimides (3b-M) are easier to oxidize and more difficult to reduce than the diimides (2b-M), in line with the more electron-deficient character of the latter derivatives. Interestingly, however, electrochemical band gaps of corresponding mono- and diimide species are nearly identical. Similarly to their tetra-NMI congeners,16 the imide-fused systems show multiple reduction events at relatively high potentials. NDA-fused analogues 2a-M and 3a-M showed qualitatively similar electrochemical behavior, with larger band gaps than their NMI analogues.
Kohn–Sham molecular orbitals (MOs), calculated for NMI-fused systems 1b′-M, 2b′-M, and 3b′-M and their imide-free parent 8b′-M (M = 2H, Zn; Figure 3), show progressive lowering of occupied and virtual energy levels when the number of NMI units is increased. Mono-NMI systems 3b′-M have significantly smaller electronic band gaps than their 8b′-M counterparts (by 0.2–0.25 eV). Remarkably, the band gaps of 2b′-M and 1b′-M do not decrease uniformly with the increasing number of NMI groups. Instead, they vary over a relatively small range of energies and, in fact, the smallest gaps are predicted for the di-NMI systems 2b′-M rather than for 1b′-M. meso-Tol substituents are predicted to produce a further small decrease of the optical band gap relative to meso-Ph systems (Table S4), explaining why 2b-M and 3b-M have smaller optical gaps than the Ph-substituted 1b-M. Absorption spectra simulated for the fully substituted 2b-Zn and 3b-Zn using time-dependent DFT are consistent with experimental data, reproducing positions of Q and Soret bands (Figure 3).
Figure 3.
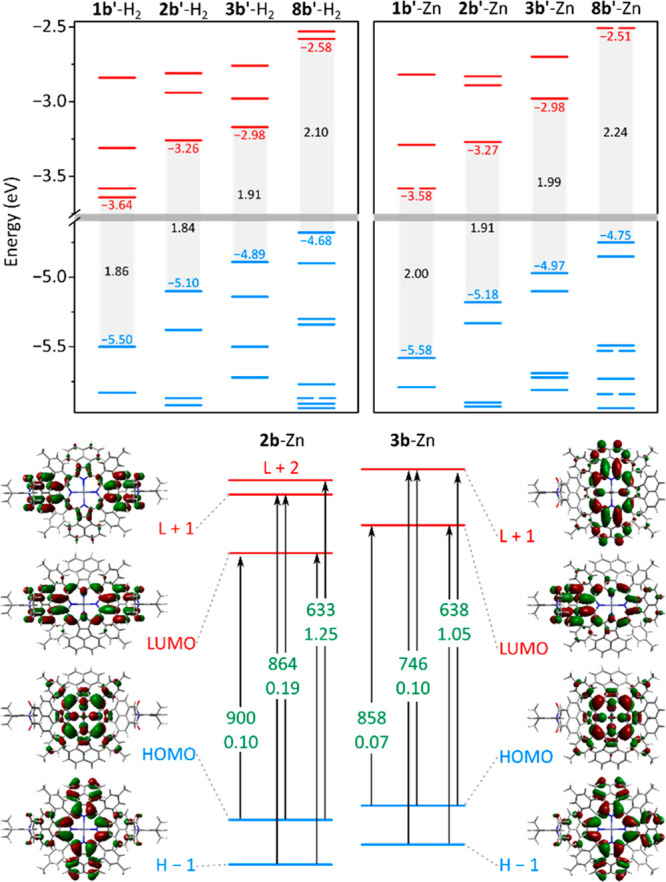
Electronic structure calculations for homologous NMI-fused porphyrins (M = 2H) and zinc porphyrins (M = Zn, B3LYP/6-31G(d,p), DCM solvation included for TD-DFT). Top: Kohn–Sham frontier MO levels for 1b′-M (R = H), 2b′-M and 3b′-M (R = H, R″ = Ph), and 8b′-M (D4-type porphyrin with meso-phenyl substituents). Bottom: MO amplitudes and key TD-DFT transitions for 2b-Zn and 3b-Zn. Transition energies (nm) and oscillator strengths are shown in green.
The porphyrin–ryleneimide hybrids described herein reveal nontrivial relationships between the electronic properties of the π system and the placement of fused acceptor units around the porphyrin core. The electron-deficient character of these molecules is gradually enhanced with the increasing number of acceptors; however, the electronic band gap is significantly reduced only by introduction of the first NMI unit, but it is just moderately affected when further NMI moieties are added. Interestingly, the smallest gap is observed for the tD2A2 porphyrin, which is highly attractive as an NIR-absorbing, intrinsically curved analogue of perylenediimide. In subsequent work, we plan to employ this new chromophore and its monoimide congener as components in supramolecular and covalently linked energy- and charge-transfer systems.
Acknowledgments
Financial support from the Foundation for Polish Science (TEAM POIR.04.04.00-00-5BF1/17-00, M.S.) is gratefully acknowledged. Quantum-chemical calculations were performed in the Wrocław Center for Networking and Supercomputing. The work at Yonsei University was supported the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (NRF-2016R1E1A1A01943379).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.0c02544.
Accession Codes
CCDC 2015790–2015791 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
The authors declare no competing financial interest.
Supplementary Material
References
- Suraru S.-L.; Würthner F. Strategies for the Synthesis of Functional Naphthalene Diimides. Angew. Chem., Int. Ed. 2014, 53 (29), 7428–7448. 10.1002/anie.201309746. [DOI] [PubMed] [Google Scholar]
- Würthner F.; Saha-Möller C. R.; Fimmel B.; Ogi S.; Leowanawat P.; Schmidt D. Perylene Bisimide Dye Assemblies as Archetype Functional Supramolecular Materials. Chem. Rev. 2016, 116 (3), 962–1052. 10.1021/acs.chemrev.5b00188. [DOI] [PubMed] [Google Scholar]
- Chen L.; Li C.; Müllen K. Beyond Perylene Diimides: Synthesis, Assembly and Function of Higher Rylene Chromophores. J. Mater. Chem. C 2014, 2 (11), 1938–1956. 10.1039/C3TC32315C. [DOI] [Google Scholar]
- Al Kobaisi M.; Bhosale S. V.; Latham K.; Raynor A. M.; Bhosale S. V. Functional Naphthalene Diimides: Synthesis, Properties, and Applications. Chem. Rev. 2016, 116 (19), 11685–11796. 10.1021/acs.chemrev.6b00160. [DOI] [PubMed] [Google Scholar]
- Nowak-Król A.; Shoyama K.; Stolte M.; Würthner F. Naphthalene and Perylene Diimides – Better Alternatives to Fullerenes for Organic Electronics?. Chem. Commun. 2018, 54 (98), 13763–13772. 10.1039/C8CC07640E. [DOI] [PubMed] [Google Scholar]
- Jänsch D.; Li C.; Chen L.; Wagner M.; Müllen K. Versatile Colorant Syntheses by Multiple Condensations of Acetyl Anilines with Perylene Anhydrides. Angew. Chem., Int. Ed. 2015, 54 (7), 2285–2289. 10.1002/anie.201409634. [DOI] [PubMed] [Google Scholar]
- Li C.; Lin Z.; Li Y.; Wang Z. Synthesis and Applications of π-Extended Naphthalene Diimides. Chem. Rec. 2016, 16 (2), 873–885. 10.1002/tcr.201500246. [DOI] [PubMed] [Google Scholar]
- Stępień M.; Gońka E.; Żyła M.; Sprutta N. Heterocyclic Nanographenes and Other Polycyclic Heteroaromatic Compounds: Synthetic Routes, Properties, and Applications. Chem. Rev. 2017, 117 (4), 3479–3716. 10.1021/acs.chemrev.6b00076. [DOI] [PubMed] [Google Scholar]
- Zhylitskaya H.; Stępień M. Carbocyclization Approaches to Electron-Deficient Nanographenes and Their Analogues. Org. Chem. Front. 2018, 5 (15), 2395–2414. 10.1039/C8QO00423D. [DOI] [Google Scholar]
- Seifert S.; Shoyama K.; Schmidt D.; Würthner F. An Electron-Poor C 64 Nanographene by Palladium-Catalyzed Cascade C–C Bond Formation: One-Pot Synthesis and Single-Crystal Structure Analysis. Angew. Chem., Int. Ed. 2016, 55 (22), 6390–6395. 10.1002/anie.201601433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert S.; Schmidt D.; Shoyama K.; Würthner F. Base-Selective Five- versus Six-Membered Ring Annulation in Palladium-Catalyzed C–C Coupling Cascade Reactions: New Access to Electron-Poor Polycyclic Aromatic Dicarboximides. Angew. Chem., Int. Ed. 2017, 56 (26), 7595–7600. 10.1002/anie.201702889. [DOI] [PubMed] [Google Scholar]
- Pigulski B.; Shoyama K.; Würthner F.. NIR-Absorbing π-Extended Azulene: Non-Alternant Isomer of Terrylene Bisimide. Angew. Chem., Int. Ed. 2020. 10.1002/anie.202005376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoyama K.; Würthner F. Synthesis of a Carbon Nanocone by Cascade Annulation. J. Am. Chem. Soc. 2019, 141 (33), 13008–13012. 10.1021/jacs.9b06617. [DOI] [PubMed] [Google Scholar]
- Renner R.; Stolte M.; Würthner F. Self-Assembly of Bowl-Shaped Naphthalimide-Annulated Corannulene. ChemistryOpen 2020, 9 (1), 32–39. 10.1002/open.201900291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoyama K.; Mahl M.; Seifert S.; Würthner F. A General Synthetic Route to Polycyclic Aromatic Dicarboximides by Palladium-Catalyzed Annulation Reaction. J. Org. Chem. 2018, 83 (10), 5339–5346. 10.1021/acs.joc.8b00301. [DOI] [PubMed] [Google Scholar]
- Zhylitskaya H.; Cybińska J.; Chmielewski P.; Lis T.; Stępień M. Bandgap Engineering in π-Extended Pyrroles. a Modular Approach to Electron-Deficient Chromophores with Multi-Redox Activity. J. Am. Chem. Soc. 2016, 138 (35), 11390–11398. 10.1021/jacs.6b07826. [DOI] [PubMed] [Google Scholar]
- Żyła-Karwowska M.; Zhylitskaya H.; Cybińska J.; Lis T.; Chmielewski P. J.; Stępień M. An Electron-Deficient Azacoronene Obtained by Radial π Extension. Angew. Chem., Int. Ed. 2016, 55 (47), 14658–14662. 10.1002/anie.201608400. [DOI] [PubMed] [Google Scholar]
- Navakouski M.; Zhylitskaya H.; Chmielewski P. J.; Lis T.; Cybińska J.; Stępień M. Stereocontrolled Synthesis of Chiral Heteroaromatic Propellers with Small Optical Bandgaps. Angew. Chem., Int. Ed. 2019, 58 (15), 4929–4933. 10.1002/anie.201900175. [DOI] [PubMed] [Google Scholar]
- Moshniaha L.; Żyła-Karwowska M.; Chmielewski P. J.; Lis T.; Cybińska J.; Gońka E.; Oschwald J.; Drewello T.; Rivero S. M.; Casado J.; Stępień M. Aromatic Nanosandwich Obtained by σ-Dimerization of a Nanographenoid π-Radical. J. Am. Chem. Soc. 2020, 142 (7), 3626–3635. 10.1021/jacs.9b13942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Żyła-Karwowska M.; Moshniaha L.; Hong Y.; Zhylitskaya H.; Cybińska J.; Chmielewski P. J.; Lis T.; Kim D.; Stępień M. Electron-Deficient Bipyrrole Boomerangs: Bright Fluorophores Obtained via Double C–H Bond Activation. Chem. - Eur. J. 2018, 24 (29), 7525–7530. 10.1002/chem.201801199. [DOI] [PubMed] [Google Scholar]
- Czichy M.; Zhylitskaya H.; Zassowski P.; Navakouski M.; Chulkin P.; Janasik P.; Lapkowski M.; Stępień M. Electrochemical Polymerization of Pyrrole–Perimidine Hybrids: Low-Bandgap Materials with High n-Doping Activity. J. Phys. Chem. C 2020, 124, 14350. 10.1021/acs.jpcc.0c03002. [DOI] [Google Scholar]
- Galstyan A.; Maurya Y. K.; Zhylitskaya H.; Bae Y. J.; Wu Y.-L.; Wasielewski M. R.; Lis T.; Dobrindt U.; Stępień M. π-Extended Donor–Acceptor Porphyrins and Metalloporphyrins for Antimicrobial Photodynamic Inactivation. Chem. - Eur. J. 2020, 26 (37), 8262–8266. 10.1002/chem.201905372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imahori H.; Umeyama T.; Ito S. Large π-Aromatic Molecules as Potential Sensitizers for Highly Efficient Dye-Sensitized Solar Cells. Acc. Chem. Res. 2009, 42 (11), 1809–1818. 10.1021/ar900034t. [DOI] [PubMed] [Google Scholar]
- Kurotobi K.; Toude Y.; Kawamoto K.; Fujimori Y.; Ito S.; Chabera P.; Sundström V.; Imahori H. Highly Asymmetrical Porphyrins with Enhanced Push–Pull Character for Dye-Sensitized Solar Cells. Chem. - Eur. J. 2013, 19 (50), 17075–17081. 10.1002/chem.201303460. [DOI] [PubMed] [Google Scholar]
- Rathi P.; Butcher R.; Sankar M. Unsymmetrical Nonplanar ‘Push–Pull’ β-Octasubstituted Porphyrins: Facile Synthesis, Structural, Photophysical and Electrochemical Redox Properties. Dalton Trans 2019, 48 (40), 15002–15011. 10.1039/C9DT02792K. [DOI] [PubMed] [Google Scholar]
- Sekaran B.; Jang Y.; Misra R.; D’Souza F. Push–Pull Porphyrins via β-Pyrrole Functionalization: Evidence of Excited State Events Leading to High-Potential Charge-Separated States. Chem. - Eur. J. 2019, 25 (56), 12991–13001. 10.1002/chem.201902286. [DOI] [PubMed] [Google Scholar]
- Rathi P.; Ekta; Kumar S.; Banerjee D.; Soma V. R.; Sankar M. Unsymmetrical β-Functionalized ‘Push–Pull’ Porphyrins: Synthesis and Photophysical, Electrochemical and Nonlinear Optical Properties. Dalton Trans 2020, 49 (10), 3198–3208. 10.1039/C9DT04252K. [DOI] [PubMed] [Google Scholar]
- Janiga E.; Kim G.; Chmielewski P. J.; Lis T.; Kim D.; Stępień M.. Porphyrin-Ryleneimide Hybrids: Low-Bandgap Acceptors in Energy-Transfer Cassettes. Chem. - Asian J. 2020. 10.1002/asia.202000762. [DOI] [PubMed] [Google Scholar]
- Lindsey J. S. Synthetic Routes to Meso -Patterned Porphyrins. Acc. Chem. Res. 2010, 43 (2), 300–311. 10.1021/ar900212t. [DOI] [PubMed] [Google Scholar]
- Vicente M.; Smith K. Syntheses and Functionalizations of Porphyrin Macrocycles. Curr. Org. Synth. 2014, 11 (1), 3–28. 10.2174/15701794113106660083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T.; Osuka A. Conjugated Porphyrin Arrays: Synthesis, Properties and Applications for Functional Materials. Chem. Soc. Rev. 2015, 44 (4), 943–969. 10.1039/C3CS60443H. [DOI] [PubMed] [Google Scholar]
- Hiroto S.; Miyake Y.; Shinokubo H. Synthesis and Functionalization of Porphyrins through Organometallic Methodologies. Chem. Rev. 2017, 117 (4), 2910–3043. 10.1021/acs.chemrev.6b00427. [DOI] [PubMed] [Google Scholar]
- Hoshino A.; Ohgo Y.; Nakamura M. Synthesis and Inversion Barriers of Undeca- and Dodeca-Substituted Saddle Shaped Porphyrin Complexes. Tetrahedron Lett. 2005, 46 (30), 4961–4964. 10.1016/j.tetlet.2005.05.100. [DOI] [Google Scholar]
- Spence J. D.; Lash T. D. Porphyrins with Exocyclic Rings. 14. Synthesis of Tetraacenaphthoporphyrins, a New Family of Highly Conjugated Porphyrins with Record-Breaking Long-Wavelength Electronic Absorptions. J. Org. Chem. 2000, 65 (5), 1530–1539. 10.1021/jo991730w. [DOI] [PubMed] [Google Scholar]
- Barbero M.; Cadamuro S.; Degani I.; Fochi R.; Gatti A.; Regondi V. Useful Syntheses of 2-Substituted 1,3-Benzoxathiolium Tetrafluoroborates from Carboxylic Acids, Anhydrides, Esters and Trihalomethyl Compounds. Synthesis 1986, 1986 (12), 1074–1076. 10.1055/s-1986-31882. [DOI] [Google Scholar]
- Barbero M.; Cadamuro S.; Degani I.; Fochi R.; Gatti A.; Regondi V. Pentaatomic Heteroaromatic Cations. 18. Acylation of Pyrrole and N-Methylpyrrole with 1,3-Benzoxathiolium Tetrafluoroborates. A High-Yield Method for the Synthesis of Diacylpyrroles. J. Org. Chem. 1988, 53 (10), 2245–2250. 10.1021/jo00245a022. [DOI] [Google Scholar]
- Crossley M. J.; Harding M. M.; Sternhell S. Tautomerism in 2-Substituted 5,10,15,20-Tetraphenylporphyrins. J. Am. Chem. Soc. 1986, 108 (13), 3608–3613. 10.1021/ja00273a010. [DOI] [Google Scholar]
- Lash T. D.; Chandrasekar P. Synthesis of Tetraphenyltetraacenaphthoporphyrin: A New Highly Conjugated Porphyrin System with Remarkably Red-Shifted Electronic Absorption Spectra. J. Am. Chem. Soc. 1996, 118 (36), 8767–8768. 10.1021/ja961227q. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.