Abstract
Tuberculosis (TB) is one of the most common infectious diseases globally. The surfactant protein C (SFTPC), which is involved in innate immunity and surfactant function in the lung, may contribute toward the progression of TB. The aim of the present study was to preliminarily investigate the possible association of single nucleotide polymorphisms (SNPs) in the SFTPC gene with TB susceptibility and clinical phenotypes in a Western Chinese Han population. The improved multiplex ligation detection reaction method was used to genotype 6 SNPs in SFTPC, in 900 patients with TB and 1,534 healthy control subjects. It was found that the A allele for rs1124 and the C allele for rs8192313 were associated with increased susceptibility to TB, P=0.024 and P=0.045, respectively. However, these two P-values were not significant following Bonferroni correction. In all samples, the haplotype [CGA], representing three SFTPC variants, was revealed to increase the risk of TB (P=0.001 and P=0.005, following Bonferroni correction). Furthermore, patients with the AA genotype for rs1124 and with the CC genotype for rs8192313 were associated with higher levels of C-reactive protein (P=0.001 and P=0.005, respectively). The results of the present study indicated that the SFTPC SNPs may increase the susceptibility to TB and the immune response of the host to Mycobacterium tuberculosis and may potentially be novel biomarkers for the pathogenesis of TB.
Keywords: genetic, single nucleotide polymorphism, surfactant protein C, tuberculosis, Mycobacterium tuberculosis
Introduction
Tuberculosis (TB), caused by Mycobacterium tuberculosis, is one of the most common infectious diseases worldwide and causes more mortalities than any other pathogen (1). The causative bacteria has developed complex mechanisms to affect the innate and the acquired immune systems of the host (2). It is estimated that more than one third of the world's population is infected with Mycobacterium tuberculosis, but 90% of infected people never develop clinical symptoms, suggesting a natural immunity to TB, due to genetic factors in the host (3). However, the molecular identity and function of these genetic factors remain largely unknown.
Naive immunological responses to Mycobacterium tuberculosis are particularly important in the lung, as inhalation of this harmful bacteria into the alveolar macrophage is a key event in the pathogenesis of the disease (4). Pulmonary surfactant, as a surface-active lipoprotein complex, is composed of 90% lipids and 10% surfactant proteins (SFTPs). Surfactant protein C (SFTPC), a hydrophobic membrane protein, is required for biophysical function, and disruption affects the health of the normal lung (5,6). Therefore, we hypothesized that SFTPC contributes toward the progression of TB. It is well-known that mycobacteria are characterized by a complex cell wall, and various polysaccharides and glycolipids in the cell wall of the mycobacterial species serve significant roles in immune recognition (7-9), including lipoarabinomannan (LAM), which is considered to be a critical virulence factor. LAM from unique strains of mycobacteria have been reported to account for triggering different immune responses (10,11). Furthermore, Sidobre et al (7) reported that the mannosylated LAM was a putative ligand for the attachment of the human pulmonary surfactant protein to the pathogenic Mycobacterium tuberculosis. Mutations in the SFTPC gene have been identified in pulmonary surfactant metabolism dysfunction and were associated with interstitial lung diseases (5,12-17), and multiple genetic variants in the SFTP genes were identified in TB (18,19). However, until now, the association between the SFPTC gene and TB has not been investigated in well-defined populations.
In the present study, in order to provide genetic evidence regarding the effect of SFTPC polymorphisms on TB in the Chinese Han population, a set of single nucleotide polymorphisms (SNPs) within SFTPC were genotyped in 900 patients with TB and 1,534 healthy control subjects, and the association between the risk of TB and the clinical characteristics of active tuberculosis, and selected SFTPC polymorphisms was investigated.
Materials and methods
Study subjects
A total of 900 patients with TB (mean age 42.51 years, range 20-92 years, 542 males) and 1,534 healthy control subjects (mean age 37.96 years, range 17-80 years, 821 males) were included in the present case-controlled study. All samples were obtained from the ‘Tuberculosis Researches’ Bio-Bank located within the West China Hospital between January 2014 and February 2016 in the Department of Laboratory Medicine, West China Hospital, Sichuan University, (China). All the patients with TB, enrolled in the present study, were newly diagnosed by two independent experienced respiratory physicians. The patients with active TB were diagnosed according to their clinical symptoms, sputum smear tests, sputum culture, and radiological and histological pathological examination. Patients with immunodeficiency disease, hepatitis virus infection, human immunodeficiency virus-infection, or other lung diseases were excluded from the study. All the healthy control subjects were recruited from a population of healthy controls, who had not previously suffered from TB and had negative chest radiographs. All participants were of Han ethnicity and were not related to each other.
Demographic data of the enrolled population were reviewed from the medical information system of the West China Hospital of Sichuan University. A total of 2 ml EDTA-anticoagulated blood was collected from each of the subjects for genotyping. Written informed consent was provided by each participant and the study was approved by the Clinical Trials and Biomedical Ethics Committee of West China Hospital, Sichuan University (China) and conducted according to the Declaration of Helsinki.
SNP selection
The human SFTPC gene, which is located on chromosome 8p21.3 is ~7.4 kb nucleotides long, with six exons. The genetic polymorphism data of the whole sequence of SFTPC was obtained from the dbSNP database (http://asia.ensembl.org/Homo_sapiens/Variation/). All SNPs were filtered according to the minor allele frequency (MAF; >0.05) in the Han Chinese population in Beijing. Subsequently, the SNPs were preferentially selected if they were located in potentially functional regions, including an exon, the promoter, an intron and potential regulatory regions (within 2 kb of the genes). Considering the experimental conditions required for genotyping, seven SNPs in SFTPC (rs1124, rs4715, rs8192309, rs4995702, rs8192313, rs13248346 and rs2070686) were eventually included in the present study.
DNA isolation and genotyping
Genomic DNA was extracted from the peripheral blood samples using a QIAamp DNA blood mini kit (Qiagen GmbH), according to the manufacturer's protocol. Candidate SNP genotyping was performed using the improved multiplex ligation detection reaction method (Genesky Biotechnologies, Inc.) as previously described (20). Detailed information regarding the primers is available upon request. For the quality assurance of the genotyping, double distilled water was used as the negative control in each reaction. Furthermore, blinded repeat genotyping of ~10% of all the samples was included in the quality control measures and the concordance rate was 100%.
Statistical analysis
The required sample size was calculated using PASS statistical software version 11 (NCSS LLC), prior to data collection, as previously reported (21). Goodness-of-fit χ2 test was used to evaluate Hardy-Weinberg equilibrium (HWE) for each SNP in the healthy control subjects using PLINK v1.0732 (http://zzz.bwh.harvard.edu/plink/).
Sex and age were compared between patients with TB and healthy control subjects using a χ2 test and an independent t-test, respectively. Dominant and recessive genetic models were used to assess the allele and genotype distribution difference between TB cases and healthy control subjects using multivariate logistic regression, with sex and age as covariates. The Bonferroni method was then used to correct for multiple testing. The associations between the genetic variants and the clinical features of TB were evaluated. The aforementioned statistical methods were performed using SPSS version 19.0 software (IBM Corp.). In addition, the Haploview software package version 4.2 (Broad Institute) was used for analyzing linkage disequilibrium patterns. The haplotype was analyzed using the SHEsis online program (http://analysis.bio-x.cn/) (22). A two-tailed P<0.05 was considered to indicate a statistically significant difference.
Results
Demographic characteristics
As described in Table I, there were significant differences in sex and age between TB cases and healthy control subjects (P<0.001).
Table I.
Demographic characteristics of study participants.
| Characteristic | TB (n=900) | HC (n=1534) | P-valuea |
|---|---|---|---|
| Male, n (%) | 542 (60.22) | 821 (53.52) | <0.001 |
| Age years, mean ± SD | 42.51±18.11 | 37.96±11.07 | <0.001 |
aSex and age were compared between TB patients and healthy controls using a Chi-square test or an independent t-test. TB, tuberculosis; HC, healthy controls; SD, standard deviation.
Genotyping results and single SNP association analysis
The genotype call rates of all the selected SNPs were 99.55%. All healthy control subjects were of Han ethnicity, and genotype distributions of the selected SNPs within the SFTPC gene in the controls did not deviate from HWE (P>0.05), except for rs4995702 (P<0.001). The SNP functional consequence, P-value for the HWE test, the MAF in the healthy controls and the Chinese Han population in Beijing based on the 1,000 Genomes Project database are summarized in Table SI. The majority of MAFs in the healthy controls were similar to that in the Chinese Han Beijing population, but a few were different. However, the MAFs in the healthy controls were consistent with those in the Southern Han Chinese population based on the 1,000 Genomes Project database (data not shown), which indicated that the approach of using the healthy controls was valid in the present study.
There were no data available in the database and reported literature regarding rs4995702 and in association with disease. In addition, rs4995702 was not in HWE in the healthy controls. Numerous factors may contribute toward the inconsistency of the MAF results, including migration, selection and genotyping errors. To produce more reliable conclusions and reduce the chance of error, rs4995702 was removed in the subsequent association analysis. Table II shows the allele frequencies and genotype distributions of the remaining six SNPs in the SFTPC gene between all the TB cases and the healthy control subjects. The minor allele (A) frequency of rs1124 was 35.70 and 32.48% in the TB cases and healthy control subjects, respectively, and was significant after adjusting for sex and age [P=0.024; odds ratio (OR), 1.15; 95% confidence interval (CI), 1.02-1.31)], but was not significant after using Bonferroni correction test (P=0.144). The frequency of the AA genotype for rs1124 was 13.07 and 10.02% in TB cases and in the healthy controls, respectively, with no statistical significance (P=0.097). In addition, the minor allele (C) frequency of rs8192313 was 37.86 and 35.02% in the TB group and in the healthy control subjects, respectively, and was significant after adjusting for sex and age (P=0.045; OR, 1.13; 95% CI, 1.00-1.28); however, it was not significant after Bonferroni correction (P=0.278). The frequency of the CC genotype for rs8192313 was 15.37 and 11.87% in TB cases and in healthy control subjects, respectively. The presence of CC was more common in TB cases, but the difference was borderline significant (P=0.05). The other four loci (rs4715, rs8192309, rs13248346 and rs2070686) were not significantly different between the TB cases and controls in either genotype or allele frequencies (all P>0.05).
Table II.
Association between genetic polymorphisms in SFTPC and tuberculosis risk.
| SNP | Variant | Case, n (%) | Control, n (%) | OR (95% CI) | P-valuea | P-valueb | Variant | Case, n (%) | Control, n (%) | P-valuec |
|---|---|---|---|---|---|---|---|---|---|---|
| rs1124 | A | 639 (35.70) | 992 (32.48) | 1.15 (1.02-1.31) | 0.024 | 0.144 | AA | 117 (13.07) | 153 (10.02) | 0.097 |
| G>A | G | 1,151 (64.30) | 2,062 (67.52) | 1 | - | AG | 405 (45.25) | 686 (44.92) | ||
| GG | 373 (41.68) | 688 (45.06) | ||||||||
| rs4715 | A | 548 (30.44) | 886 (29.03) | 1.07 (0.94-1.21) | 0.318 | AA | 82 (9.11) | 118 (7.73) | 0.100 | |
| C>A | C | 1,252 (69.56) | 2,166 (80.97) | 1 | - | AC | 384 (42.67) | 650 (42.60) | ||
| CC | 434 (48.22) | 758 (49.67) | ||||||||
| rs8192309 | A | 268 (14.92) | 472 (15.48) | 0.95 (0.81-1.13) | 0.577 | AA | 21 (2.34) | 40 (2.62) | 0.578 | |
| G>A | G | 1,528 (85.08) | 2,578 (84.52) | 1 | - | AG | 226 (25.17) | 392 (25.71) | ||
| GG | 651 (72.49) | 1,093 (71.67) | ||||||||
| rs8192313 | C | 680 (37.86) | 1,068 (35.02) | 1.13 (1.00-1.28) | 0.045 | 0.270 | CC | 138 (15.37) | 181 (11.87) | 0.050 |
| A>C | A | 1,116 (62.14) | 1,982 (64.98) | 1 | - | CA | 404 (44.99) | 706 (46.30) | ||
| AA | 356 (39.64) | 638 (41.84) | ||||||||
| rs13248346 | A | 406 (22.61) | 639 (20.92) | 1.10 (0.95-1.26) | 0.211 | AA | 50 (5.57) | 62 (4.06) | 0.265 | |
| G>A | G | 1,390 (77.39) | 2,415 (79.08) | 1 | - | AG | 306 (34.08) | 515 (33.73) | ||
| GG | 542 (60.36) | 950 (62.21) | ||||||||
| rs2070686 | A | 453 (25.25) | 799 (26.23) | 0.95 (0.83-1.08) | 0.427 | AA | 54 (6.02) | 109 (7.16) | 0.452 | |
| G>A | G | 1,341 (74.75) | 2,247 (73.77) | 1 | - | AG | 345 (38.46) | 581 (38.15) | ||
| GG | 498 (55.52) | 833 (54.69) |
aP-value was calculated using logistic regression analysis after adjusting for sex and age;
bP-value after Bonferroni correction.
cP-value was calculated by Chi-square test. SNP, single nucleotide polymorphisms; OR, odds ratio; CI, confidence interval.
The data regarding the dominant and recessive genetic model analysis are shown in Table III. The results indicated that rs1124 was significantly associated with an increased risk of TB in the recessive model (AA vs. AG+GG), with an estimated OR of 1.33 (95% CI, 1.03-1.72; P=0.031 after adjusting for sex and age; P=0.186 after Bonferroni correction). Furthermore, there were similar results for rs8192313 using the recessive model (CC vs. CA+AA), with an estimated OR of 1.36 (95% CI, 1.06-1.73; P=0.014 after adjusting for sex and age; P=0.084 after Bonferroni correction).
Table III.
Association between 6 SNPs of SFTPC and tuberculosis risk in Chinese Han population.
| Dominant model | Recessive model | |||||
|---|---|---|---|---|---|---|
| SNP | OR (95% CI) | P-valuea | P-valueb | OR (95% CI) | P-valuea | P-valueb |
| rs1124 G>A | 1.15 (0.98-1.37) | 0.095 | 1.33 (1.03-1.72) | 0.031 | 0.186 | |
| rs4715 C>A | 1.06 (0.90-1.26) | 0.471 | 1.17 (0.87-1.58) | 0.302 | ||
| rs8192309 G>A | 0.96 (0.19-1.15) | 0.639 | 0.88 (0.51-1.51) | 0.640 | ||
| rs8192313 A>C | 1.10 (0.93-1.30) | 0.286 | 1.36 (1.06-1.73) | 0.014 | 0.084 | |
| rs13248346 G>A | 1.07 (0.90-1.27) | 0.447 | 1.40 (0.95-2.06) | 0.090 | ||
| rs2070686 G>A | 0.97 (0.82-1.14) | 0.684 | 0.82 (0.58-1.15) | 0.248 | ||
aP-value was calculated using logistic regression analysis after adjusting for sex and age;
bP-value after Bonferroni correction. SNP, single nucleotide polymorphisms; OR, odds ratio; CI, confidence interval.
Linage analysis and haplotype construction
The linkage disequilibrium (LD) plots of the genotyped SNPs are displayed in Fig. 1. A total of 3 polymorphisms in SFTPC (rs4715, rs2070686 and rs1124) were revealed to be in high LD with each other. The haplotype frequencies and their associations with TB predisposition are summarized in Table IV. The results indicated that the CGA haplotype was significantly associated with increased susceptibility to TB [P=0.001 with an OR of 1.59 (95% CI, 1.20-2.12)] and remained statistically significant after Bonferroni correction (P=0.005).
Figure 1.
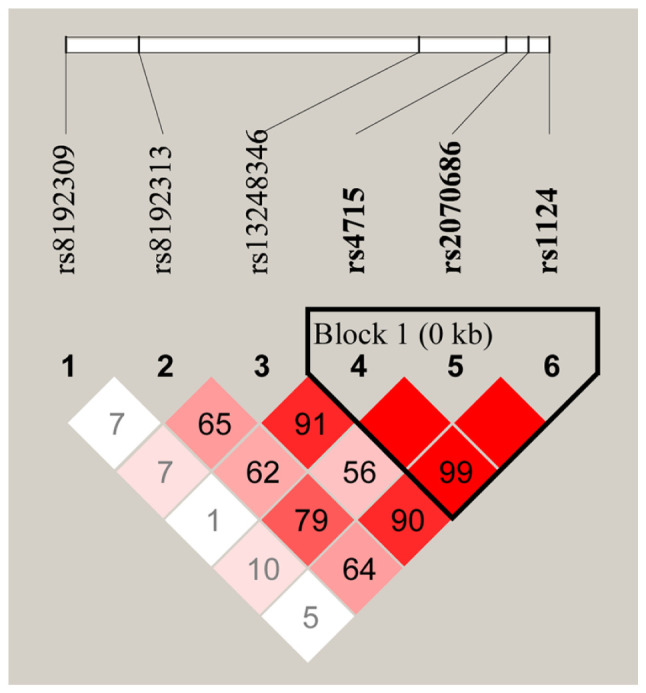
LD of the 6 single nucleotide polymorphisms in surfactant protein C. High LD is represented by a high correlation coefficient and the red color. LD, linkage disequilibrium.
Table IV.
Haplotype constructions of the SFTPC variants associated with the risk of TB.
| Frequency | ||||||
|---|---|---|---|---|---|---|
| Haplotypea | All | TB cases | Healthy controls | OR (95%CI) | P-valueb | P-valuec |
| AGA | 0.295 | 0.303 | 0.290 | 1.06 (0.94-1.21) | 0.340 | |
| CAG | 0.259 | 0.253 | 0.263 | 0.95 (0.93-1.09) | 0.441 | |
| CGA | 0.044 | 0.054 | 0.034 | 1.59 (1.20-2.12) | 0.001 | 0.005 |
| CGG | 0.404 | 0.39 | 0.412 | 0.91 (0.81-1.03) | 0.128 | |
aConsisted of rs4715, rs2070686 and rs1124;
bP-value was calculated using Chi-square test;
cP-value after Bonferroni correction. TB, tuberculosis; OR, odds ratio; CI, confidence interval.
Association between clinical phenotypes and polymorphisms
The clinical symptoms and progression of active TB may be affected by a particular genetic polymorphism as previously reported (23). Therefore, the six candidate SNPs were further analyzed with respect to the clinical symptoms in the patients with TB. The erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), which are considered to be regular inflammatory markers for the host defense (24), were selected and evaluated. As presented in Fig. 2, CRP levels were significantly higher in those who are homozygous for the rs1124 SNP (AA genotype) than in those who had the AG and GG genotypes (P=0.001). A significant association in patients with TB between the CC genotype for rs8192313 and CRP levels was also identified (P=0.005). ESR levels were not associated with anyone with the rs1124 or rs8192313 genotypes; therefore, with respect to the other four SNPs, none of the clinical phenotypes were statistically associated with the genotypes (data not shown).
Figure 2.
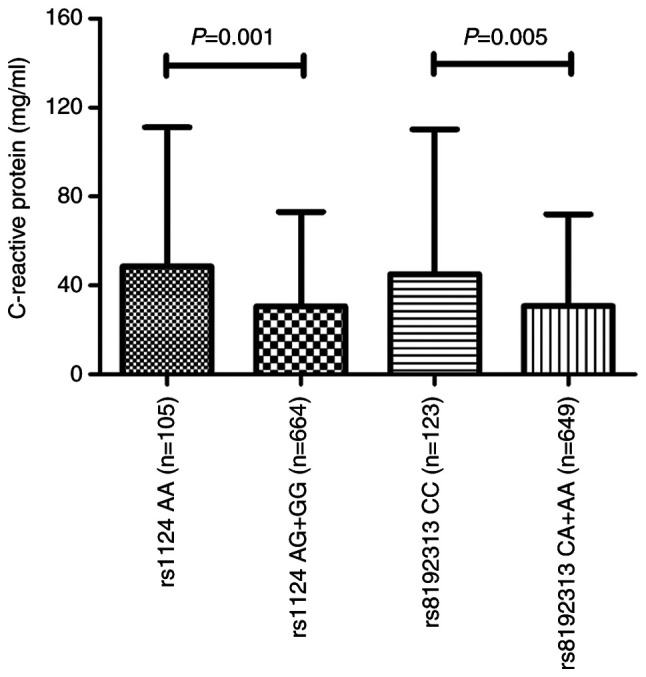
Level of C-reactive protein with respect to rs1124 (n=769) and rs8192313 (n=772) in patients with TB. rs1124 (G>A) and rs8192313 (A>C) genotypes were stratified based on the recessive model, AA vs. (AG + GG) and CC vs. (CA + AA), respectively. TB, tuberculosis.
Discussion
The associations between susceptibility and disease traits in TB were preliminarily investigated in six candidate SNPs in the SFTPC gene within the Western Chinese Han population. The results indicated that the target SFTPC polymorphisms (rs1124 and rs8192313) were not only associated with an increased risk of TB but may also affect the immune response of the host to Mycobacterium tuberculosis. The results of the present study suggested that genetic variants in SFTPC may act as promising novel biomarkers for the pathogenesis of TB.
An improved understanding of the molecular mechanisms underlying host-pathogen interaction would provide a basis for the study of TB pathogenesis. The innate immune response is crucial for the host defense against Mycobacterium tuberculosis (25). During Mycobacterium tuberculosis infection, the pathogen utilizes diverse strategies to circumvent or evade the host innate immunity. By contrast, the host orchestrates multiple signaling cascades to initiate a large variety of innate immune defense functions (26). For example, mannosylated LAM was identified in pathogenic Mycobacterium tuberculosis, which serves as a modulator of the host immune system (27). A study by Zhang et al (11) reported that the pathogens had divergent effects on DC maturation and cytokine responses based on the amount and scaffold of mannose in LAMs with diverse mannosylated structures. By contrast, genetic polymorphisms in host-derived toll-like receptors were associated with susceptibility to TB (23,28). Changes in the alveolar lining fluid, including decreased binding ability of SFTPA and SFTPD to Mycobacterium tuberculosis, may increase TB susceptibility (29). It was reported that SFTPC is a hydrophobic membrane protein and serves a significant role in surfactant function (5), and mutations in SFTPC may modulate recruitment and activation of key myeloid cell populations in interstitial lung disease (30). However, there is very little data supporting the effects of SFTPC genetic polymorphisms on predisposition to and the clinical phenotype for TB, which is highly prevalent in China. Therefore, the present case-control study in the Western Chinese Han population was performed to preliminarily investigate potential TB-associated SNPs within SFTPC and to determine if these loci were associated with clinical manifestations of TB.
There were no significant associations in the risk of TB with rs4715, rs8192309, rs13248346 and rs2070686 within SFTPC between TB cases and healthy control subjects in the Western Chinese Han population. Age did not affect the frequency of genotypes (all P>0.05), and there was no difference in the frequency of genotypes between males and females (all P>0.05) in patients with TB (Table SII). Furthermore, there was no statistical significance in the frequency of genotypes between patients with a positive and negative TB sputum smear test, except for rs8192309 (P=0.025; data not shown). However, the differences in A allele frequencies (P=0.024) and genotype distributions under the recessive model (P=0.031) for the rs1124 SNP were statistically significant. In addition, similar results were observed with the rs8192313 SNP (P=0.045 and P=0.014 for the differences in C allele frequencies and genotype distributions under the recessive model, respectively). The differences reported for the rs1124 and rs8192313 SNPs did not remain statistically significant after Bonferroni correction. However, it was hypothesized that the A allele or the C allele of rs1124 and rs8192313 SNPs, respectively, within the SFTPC gene had a low susceptibility risk for the development of TB based on the results of the present study. The results also indicated that the CGA haplotype, formed by these three SFTPC polymorphisms and possibly due to the A allele for the rs1124 SNP, was significantly associated with increased TB susceptibility (P=0.001) with an OR of 1.59 (95% CI, 1.20-2.12). This finding was consistent with the individual rs1124 SNP analysis. However, the homozygotic genotype CC/GG/AA was not analyzed for TB susceptibility, due to the small homozygous sample size.
In the present study, the rs1124 and rs8192313 SNPs were significantly associated with differences in TB clinical symptoms. The results suggested that carrying the AA genotype for the rs1124 SNP was significantly associated with higher levels of CRP (P=0.001) among patients with active TB, which reflected the host inflammatory response to Mycobacterium tuberculosis infection. This was consistent with the result from the genetic analysis of susceptibility association, and further supported the effect of the A allele in rs1124 on the risk of TB. The effect of the rs8192313 SNP on the clinical features of TB and its effect on the susceptibility to TB were also observed using the same method. The TB susceptibility SNP rs8192313 was associated with CRP levels in patients with active TB within the current dataset. High CRP levels were significantly associated with the CC genotype, compared with the A allele, including heterozygous and homozygous genotypes (P=0.005). The results regarding the susceptibility SNPs to the development of TB and genetic loci associated with TB clinical phenotype indicated that the development of TB, progression and disease manifestation may be affected by diverse genetic loci. The specific molecular mechanisms remain unclear, but the results suggested that SFTPC genetic variants may affect the host defense responses against Mycobacterium tuberculosis and lead to diverse clinical manifestations in patients with active TB.
The SNPs, rs1124 and rs4715, are exonic splicing enhancers, while rs8192309, rs8192313, rs13248346, rs2070686 and rs4995702 are all transcription factor binding sites, as predicted using the SNPinfo method, as previously reported (31) (Table SI). It has also been reported that the rs4715 SNP was associated with respiratory distress syndrome (5,14,16); however, this was not identified in the present study. The results also suggested that the SNP rs1124 may serve an important role in disease development, which was in accordance with the results of previous studies (5,13,14,16). The SNPs, rs4715 and rs1124, are both missense, in which the amino acids 138 (Thr/Asn) and 186 (Ser/Asn) are changed, respectively; however, the potential mechanisms in which they affect function remain unknown. The single SNP statistical analyses of the other four SFTPC variants did not reveal any significant associations, but the investigation of these polymorphisms supplemented the knowledge on the association of SFTPC polymorphisms with the susceptibility to TB. Based on the results of the present study, the association between the SFTPC gene and TB should be interpreted cautiously, as it is hypothesized that this gene was not the primary cause of TB but serves a lesser role in the complex genetic pattern.
To the best of our knowledge, the present study was the first to investigate the association between SFTPC genetic variations and TB in a Western Chinese Han population. Furthermore, to minimize the bias of Mycobacterium tuberculosis complex exposure, all subjects from the same geographical area were recruited over the same study period. However, there are several limitations to the present study. To begin with, only a small number of SNPs within SFTPC and a small sample size were investigated. Additionally, the association between SFTPC SNPs and the risk of TB requires further investigation using subgroup analysis. Furthermore, there were no other clinical characteristics that were matched between the groups for the risk of TB, including smoking and alcohol. Finally, the specific population with latent TB infection among the disease-free controls was not included in the present study. Therefore, further comprehensive, in-depth and multi-center research is required, to reveal the mechanisms of the genetic polymorphisms in SFTPC in TB development and clinical manifestation.
The present study investigated the pathogenesis of TB with respect to surfactant protein-based polymorphisms. A total of 2 SNPs within the SFTPC (rs1124 and rs8192313) were associated with TB susceptibility. Furthermore, the rs1124 AA and rs8192313 CC genotypes were associated with higher CRP levels, in a recessive model. The results indicated that SFTPC polymorphisms may affect TB susceptibility and host immune response to Mycobacterium tuberculosis and may be used as novel biomarkers for the pathogenesis of TB.
Supplementary Material
Acknowledgements
The authors would like to thank Dr Yu Wang from Department of Respiratory and Critical Care Medicine, the First Affiliated Hospital of Zhengzhou University for providing writing assistance.
Funding
The present study was supported by the National Natural Science Foundation of China (grant nos. 81672095 and 81501715) and the Projects of the Health and Family Planning Commission in Sichuan Province (grant no. 16ZD004).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
LM and BWY conceived and designed the present study. JWZ and LJ performed the experiments, analyzed the data and wrote the manuscript. MMG, LZ, XBW and SHG analyzed the data and conceived the study. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Clinical Trials and Biomedical Ethics Committee of West China Hospital, Sichuan University. Written informed consent was obtained from each participant and all experiments were performed according to the Declaration of Helsinki.
Patient consent for publication
All patients provided written informed consent for publication.
Competing interests
The patients declare that they have no competing interests.
References
- 1.McShane H. Insights and challenges in tuberculosis vaccine development. Lancet Respir Med. 2019;7:810–819. doi: 10.1016/S2213-2600(19)30274-7. [DOI] [PubMed] [Google Scholar]
- 2.Orme IM, Robinson RT, Cooper AM. The balance between protective and pathogenic immune responses in the TB-infected lung. Nat Immunol. 2015;16:57–63. doi: 10.1038/ni.3048. [DOI] [PubMed] [Google Scholar]
- 3.Arend SM, Engelhard AC, Groot G, de Boer K, Andersen P, Ottenhoff TH, van Dissel JT. Tuberculin skin testing compared with T-cell responses to Mycobacterium tuberculosis-specific and nonspecific antigens for detection of latent infection in persons with recent tuberculosis contact. Clin Diagn Lab Immunol. 2001;8:1089–1096. doi: 10.1128/CDLI.8.6.1089-1096.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guilliams M, Lambrecht BN, Hammad H. Division of labor between lung dendritic cells and macrophages in the defense against pulmonary infections. Mucosal Immunol. 2013;6:464–473. doi: 10.1038/mi.2013.14. [DOI] [PubMed] [Google Scholar]
- 5.Lin Z, Thorenoor N, Wu R, DiAngelo SL, Ye M, Thomas NJ, Liao X, Lin TR, Warren S, Floros J. Genetic association of pulmonary surfactant protein Genes, SFTPA1, SFTPA2, SFTPB, SFTPC, and SFTPD with cystic fibrosis. Front Immunol. 2018;9(2256) doi: 10.3389/fimmu.2018.02256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li B, Meng YQ, Li Z, Yin C, Lin JP, Zhu DJ, Zhang SB. MiR-629-3p-induced downregulation of SFTPC promotes cell proliferation and predicts poor survival in lung adenocarcinoma. Artif Cells Nanomed Biotechnol. 2019;47:3286–3296. doi: 10.1080/21691401.2019.1648283. [DOI] [PubMed] [Google Scholar]
- 7.Sidobre S, Nigou J, Puzo G, Rivière M. Lipoglycans are putative ligands for the human pulmonary surfactant protein A attachment to mycobacteria. Critical role of the lipids for Lectin-carbohydrate recognition. J Bio Chem. 2000;275:2415–2422. doi: 10.1074/jbc.275.4.2415. [DOI] [PubMed] [Google Scholar]
- 8.Guenin-Macé L, Siméone R, Demangel C. Lipids of pathogenic Mycobacteria: Contributions to virulence and host immune suppression. Transbound Emerg Dis. 2009;56:255–268. doi: 10.1111/j.1865-1682.2009.01072.x. [DOI] [PubMed] [Google Scholar]
- 9.Zheng RB, Jégouzo SAF, Joe M, Bai Y, Tran HA, Shen K, Saupe J, Xia L, Ahmed MF, Liu YH, et al. Insights into interactions of mycobacteria with the host innate immune system from a novel array of synthetic mycobacterial glycans. ACS Chem Biol. 2017;12:2990–3002. doi: 10.1021/acschembio.7b00797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazurek J, Ignatowicz L, Kallenius G, Svenson SB, Pawlowski A, Hamasur B. Divergent effects of mycobacterial cell wall glycolipids on maturation and function of human monocyte-derived dendritic cells. PLoS One. 2012;7(e42515) doi: 10.1371/journal.pone.0042515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang SP, Wu QH, Lei H, Zheng H, Zhou F, Sun ZQ, Zhao JW, Yu XL, Zhang SL. Mannosylated structures of mycobacterial lipoarabinomannans facilitate the maturation and activation of dendritic cells. Cell Immunol. 2019;335:85–92. doi: 10.1016/j.cellimm.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Lahti M, Marttila R, Hallman M. Surfactant protein C gene variation in the Finnish population-association with perinatal respiratory disease. Eur J Hum Genet. 2004;12:312–320. doi: 10.1038/sj.ejhg.5201137. [DOI] [PubMed] [Google Scholar]
- 13.Puthothu B, Krueger M, Heinze J, Forster J, Heinzmann A. Haplotypes of surfactant protein C are associated with common paediatric lung diseases. Pediatr Allergy Immunol. 2006;17:572–577. doi: 10.1111/j.1399-3038.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- 14.Wambach JA, Yang P, Wegner DJ, An P, Hackett BP, Cole FS, Hamvas A. Surfactant protein-C promoter variants associated with neonatal respiratory distress syndrome reduce transcription. Pediatr Res. 2010;68:216–220. doi: 10.1203/PDR.0b013e3181eb5d68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peca D, Boldrini R, Johannson J, Shieh JT, Citti A, Petrini S, Salerno T, Cazzato S, Testa R, Messina F, et al. Clinical and ultrastructural spectrum of diffuse lung disease associated with surfactant protein C mutations. Eur J Hum Genet. 2015;23:1033–1041. doi: 10.1038/ejhg.2015.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fatahi N, Dalili H, Kalani M, Niknafs N, Shariat M, Tavakkoly-Bazzaz J, Amini E, Esmaeilnia Shirvani T, Hardani AK, Taheritafti R, et al. Association of SP-C gene codon 186 polymorphism (rs1124) and risk of RDS. J Matern Fetal Neonatal Med. 2017;30:2585–2589. doi: 10.1080/14767058.2016.1256994. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Chen JH, Wang YQ, Nong GM, Zheng YJ, Hao CL. Genetic variants in the surfactant protein C gene 218 Site are associated with pediatric interstitial lung disease: Seven cases study. Zhonghua Er Ke Za Zhi. 2019;57:21–26. doi: 10.3760/cma.j.issn.0578-1310.2019.01.007. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 18.Hsieh MH, Ou CY, Hsieh WY, Kao HF, Lee SW, Wang JY, Wu LSH. Functional analysis of genetic variations in surfactant protein d in mycobacterial infection and their association with tuberculosis. Front Immunol. 2018;9(1543) doi: 10.3389/fimmu.2018.01543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang HY, Li H, Wang YG, Xu CY, Zhao YL, Ma XG, Li XW, Chen H. Correlation analysis between single nucleotide polymorphisms of pulmonary surfactant protein A gene and pulmonary tuberculosis in the Han population in China. Int J Infect Dis. 2014;26:31–36. doi: 10.1016/j.ijid.2014.03.1395. [DOI] [PubMed] [Google Scholar]
- 20.Bai H, Wu Q, Hu X, Wu T, Song J, Liu T, Meng Z, Lv M, Lu X, Chen X, et al. Clinical significance of lnc-AC145676.2.1-6 and lnc-TGS1-1 and their variants in western Chinese tuberculosis patients. Int J Infect Dis. 2019;84:8–14. doi: 10.1016/j.ijid.2019.04.018. [DOI] [PubMed] [Google Scholar]
- 21.Zhao Z, Zhang M, Ying J, Hu X, Zhang J, Zhou Y, Zhou Y, Song X, Ying B. Significance of genetic polymorphisms in long non-coding RNA AC079767.4 in tuberculosis susceptibility and clinical phenotype in Western Chinese Han population. Sci Rep. 2017;7(965) doi: 10.1038/s41598-017-01163-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Z, Zhang Z, He Z, Tang W, Li T, Zeng Z, He L, Shi Y. A partition-ligation-combination-subdivision EM algorithm for haplotype inference with multiallelic markers: Update of the SHEsis. Cell Res. 2009;19:519–523. doi: 10.1038/cr.2009.33. ( http://analysis.bio-x.cn) [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Zhang MM, Huang WW, Wu SQ, Wang MG, Tang XY, Sandford AJ, He JQ. Polymorphisms in toll-like receptor 10 and tuberculosis susceptibility: Evidence from three independent series. Front Immunol. 2018;9(309) doi: 10.3389/fimmu.2018.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bottazzi B, Doni A, Garlanda C, Mantovani A. An integrated view of humoral innate immunity: Pentraxins as a paradigm. Annu Rev Immunol. 2010;28:157–183. doi: 10.1146/annurev-immunol-030409-101305. [DOI] [PubMed] [Google Scholar]
- 25.Verrall AJ, Schneider M, Alisjahbana B, Apriani L, van Laarhoven A, Koeken VACM, van Dorp S, Diadani E, Utama F, Hannaway RF, et al. Early clearance of Mycobacterium tuberculosis is associated with increased innate immune responses. J Infect Dis. 2019;221:1342–1350. doi: 10.1093/infdis/jiz147. [DOI] [PubMed] [Google Scholar]
- 26.Liu CH, Liu H, Ge B. Innate immunity in tuberculosis: Host defense vs pathogen evasion. Cell Mol Immunol. 2017;14:963–975. doi: 10.1038/cmi.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou KL, Li X, Zhang XL, Pan Q. Mycobacterial Mannose-capped lipoarabinomannan: A modulator bridging innate and adaptive immunity. Emerg Microbes Infect. 2019;8:1168–1177. doi: 10.1080/22221751.2019.1649097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, Zhao Z, Zhong H, Wu L, Zhou W, Peng W, Hu X, Song J, Liu T, Wu Q, et al. Importance of common TLR2 genetic variants on clinical phenotypes and risk in tuberculosis disease in a Western Chinese population. Infect Genet Evol. 2018;60:173–180. doi: 10.1016/j.meegid.2018.02.031. [DOI] [PubMed] [Google Scholar]
- 29.Moliva JI, Duncan MA, Olmo-Fontanez A, Akhter A, Arnett E, Scordo JM, Ault R, Sasindran SJ, Azad AK, Montoya MJ, et al. The lung mucosa environment in the elderly increases host susceptibility to Mycobacterium tuberculosis infection. J Infect Dis. 2019;220:514–523. doi: 10.1093/infdis/jiz138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Venosa A, Katzen J, Tomer Y, Kopp M, Jamil S, Russo S, Mulugeta S, Beers M. Epithelial expression of an interstitial lung disease-associated mutation in surfactant protein-C modulates recruitment and activation of key myeloid cell populations in mice. J Immunol. 2019;202:2760–2771. doi: 10.4049/jimmunol.1900039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu Z, Taylor JA. SNPinfo: integrating GWAS and candidate gene information into functional SNP selection for genetic association studies. Nucleic Acids Res. 2009;37:W600–605. doi: 10.1093/nar/gkp290. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.


