Abstract
Trichomes are large epidermal cells on the surface of leaves that are thought to deter herbivores, yet the presence of trichomes can also negatively impact plant growth and reproduction. Stomatal guard cells and trichomes have shared developmental origins, and experimental manipulation of trichome formation can lead to changes in stomatal density. The influence of trichome formation upon stomatal development in natural populations of plants is currently unknown. Here, we show that a natural population of Arabidopsis halleri that includes hairy (trichome‐bearing) and glabrous (no trichomes) morphs has differences in stomatal density that are associated with this trichome dimorphism. We found that glabrous morphs had significantly greater stomatal density and stomatal index than hairy morphs. One interpretation is that this arises from a trade‐off between the proportions of cells that have trichome and guard cell fates during leaf development. The differences in stomatal density between the two morphs might have impacts upon environmental adaptation, in addition to herbivory deterrence caused by trichome development.
Keywords: development, environmental adaptation, herbivory, stomata
1. INTRODUCTION
In Arabidopsis, trichomes are large epidermal cells that protrude from the surface of the leaves and petioles. Trichomes play important roles in both biotic defenses and abiotic stress tolerance (Dalin, Agren, Bjorkman, Huttunen, & Karkkainen, 2008; Handley, Ekbom, & Ågren, 2005; Levin, 1973; Mauricio & Rausher, 1997; Sato & Kudoh, 2016; Sletvold & Ågren, 2012; Sletvold, Huttunen, Handley, Kärkkäinen, & Ågren, 2010). However, trichome development appears to impose a fitness cost on growth and reproduction (Kawagoe, Shimizu, Kakutani, & Kudoh, 2011; Mauricio, 1998; Sato & Kudoh, 2016; Sletvold & Ågren, 2012; Sletvold et al., 2010). In addition to trichomes, stomatal guard cells represent another specialized cell type that is present on the leaf surface. Trichome initiation occurs prior to stomatal meristemoid development, and the patterning of trichomes and guard cells appears to be linked (Bean, Marks, Hulskamp, Clayton, & Croxdale, 2002; Bird & Gray, 2003; Galdon‐Armero et al., 2018; Glover, 2000; Larkin, Young, Prigge, & Marks, 1996). Therefore, there might be a trade‐off between trichome and stomatal guard cell development during leaf formation (Glover, Perez‐Rodriguez, & Martin, 1998).
We wished to determine whether trichome formation might be associated with changes in stomatal patterning in natural populations of plants. To achieve this, we investigated stomatal patterning in a naturally occurring population of Arabidopsis halleri subsp. gemmifera that includes trichome‐forming and glabrous morphs (Kawagoe et al., 2011; Sato & Kudoh, 2016). These trichome morph phenotypes are heritable (Sato & Kudoh, 2015, 2017). The glabrous morphs within this population harbor a large transposon‐like insertion within the GLABRA1 (GL1) gene (Kawagoe et al., 2011). GL1 is also required for trichome formation in A. thaliana, with homozygous gl1 mutants being glabrous (Oppenheimer, Herman, Sivakumaran, Esch, & Marks, 1991). Our experiments provide new insights into the relationship between stomatal and trichome patterning under natural conditions.
2. METHODS
2.1. Study site and experimental model
This investigation used a well‐characterized population of Arabidopsis halleri subsp. gemmifera that is located beside a small stream in central Honshu Island, Japan (Figure 1a) (Omoide‐gawa site, 35°06′ N, 134°56′ E; 230 m altitude) (Aikawa, Kobayashi, Satake, Shimizu, & Kudoh, 2010; Kudoh, Honjo, Nishio, & Sugisaka, 2018). A. halleri is metal tolerant and grows essentially as a monoculture at this field site because the water is contaminated by a historical mine (Kudoh et al., 2018). The species was identified by reference to herbarium and museum specimens (Kudoh et al., 2018), and a nearby population that harbors glabrous and hairy morphs supplied material for the genome sequencing and annotation of A. halleri (Briskine et al., 2017; Sato & Kudoh, 2017). The only subspecies of A. halleri present in Japan is A. halleri subsp. gemmifera (Honjo & Kudoh, 2019). Sampling occurred during September 2016 (photoperiod approximately 12 hr, with dawn at 05:40 and dusk at 18:10). During this season, A. halleri bore larger rosette leaves that are well‐suited for quantification of stomatal density (Figure 1b).
FIGURE 1.
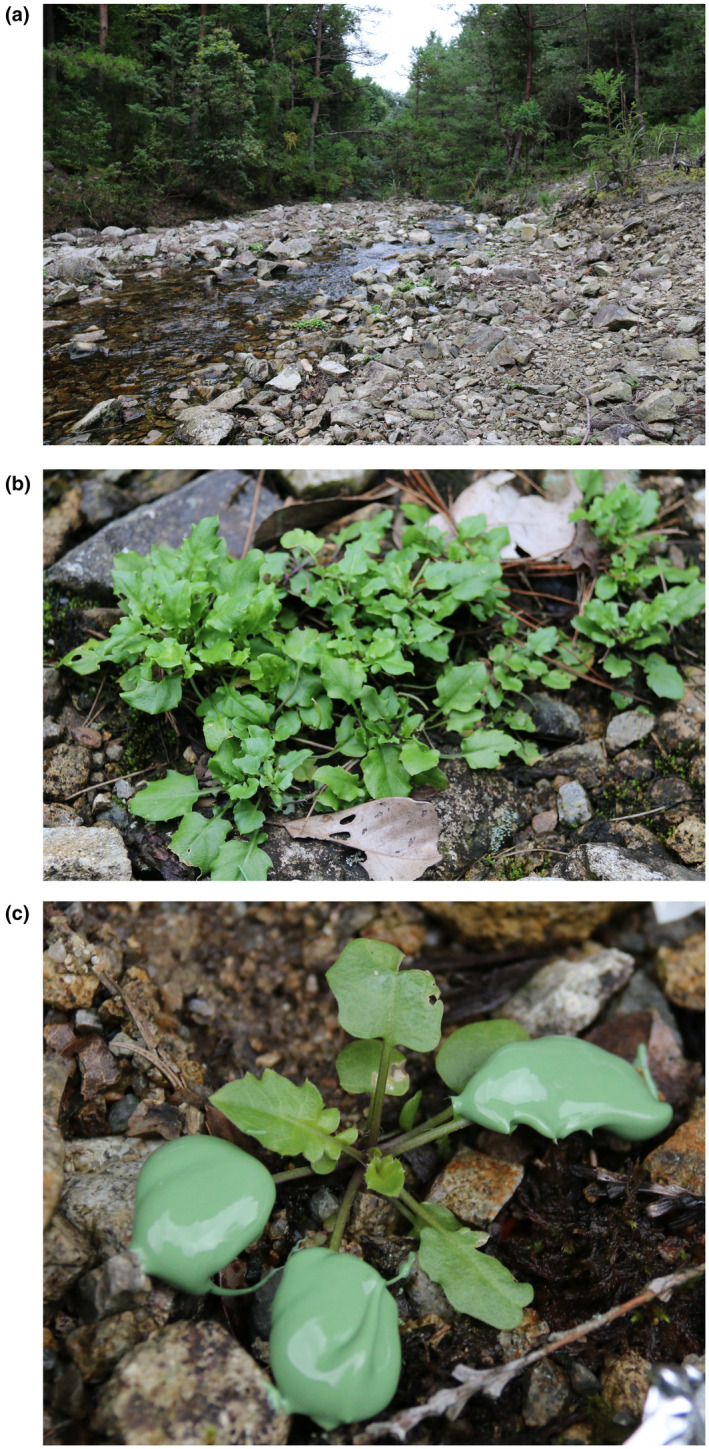
Field sampling of Arabidopsis halleri for stomatal density. (a) Overview of field site; (b) Rosette form of A. halleri plants when sampling during September 2016; (c) Leaf surface impression acquisition using impression paste. The impression paste is green‐colored and occupies the surface of three rosette leaves
2.2. Stomatal density measurement
Eight plants of each trichome morph (hairy or glabrous) were selected at the study site, with individuals chosen such that the replicate plants were distributed evenly across the site. Glabrous and hairy morphs were identified by visual inspection of the leaf surface. It is thought that irradiance and ambient temperature are unlikely to influence the frequency of the morphs (Sato & Kudoh, 2017), but we cannot discount the possibility of microenvironment‐ or field site edge‐effects. Stomatal density was measured by obtaining impressions from the adaxial surfaces of between three and five fully expanded rosette leaves of each plant. We focused on the adaxial surface because this surface also harbors the majority of the trichomes. Between the times of 12:00 and 13:00, President Plus dental impression paste (Coltene) was applied to the adaxial side of each leaf to create a leaf surface impression (Figure 1c). Solidified impression paste was removed from leaves and transported to the laboratory for further processing. First, each impression was assigned a randomly generated number to ensure subsequent steps were performed blind. Each leaf impression was painted with transparent nail varnish (60 s super shine, Rimmel) that, after drying, was peeled away from the dental impression paste using transparent adhesive tape (Scotch Crystal). Next, the adhesive tape was used to attach the nail varnish impression to a 0.8–1 mm thick microscope slide. Leaf impressions were examined using an epifluorescence microscope in white light illumination mode. Images were captured from the center of each leaf half, away from the midrib, using a Hamamatsu camera and Volocity software set to 20x zoom. Two images were captured from each impression, and the number of stomata and pavement cells was counted in an 800 × 800 µm square using the Fiji software to obtain cell density measures. Cell density measures were expressed as per mm2 (multiplication by 1.56).
In total, 29 and 31 leaf impressions were obtained in the field from hairy and glabrous plants, respectively. This produced 58 (hairy) and 62 (glabrous) microscopy images for analysis, because two images were captured from each impression. Stomatal index was calculated according to Equation (1). After all measurements, data were disaggregated according to the blinding/randomization scheme. The differences between hairy and glabrous plants were statistically tested by nested analysis of variance, whereby the mean stomatal density or index per replicate plant was nested within the hairy and glabrous morphs. Tests were conducted using the R 3.6.0 software (R Core Team, 2019) and plots generated with the beeswarm R package (v0.2.3) and Inkscape v0.91. No adjustments are applied to photographs in Figure 1.
| (1) |
Derivation of the stomatal index, where SI is the stomatal index, s is the number of stomata in the field of view, and p is the number of epidermal pavement cells in the field of view.
3. RESULTS
We investigated stomatal patterning in naturally occurring hairy and glabrous morphs of A. halleri (Sato & Kudoh, 2016). Approximately half of the A. halleri population at this study site is glabrous, whilst remaining plants have trichomes (Kawagoe et al., 2011). As trichome initiation occurs prior to stomatal meristemoid formation (Glover, 2000; Larkin et al., 1996), it is likely that trichome and stomatal patterning are linked (Bean et al., 2002), so we hypothesized that this might produce a difference in stomatal density between the two trichome morphs of A. halleri under natural conditions.
We found that the trichome formation dimorphism was accompanied by a difference in stomatal density (Figure 2a; Figure S1; Dataset S1). Fully expanded leaves of glabrous morphs had significantly greater stomatal density on the adaxial surface compared with hairy‐leaved morphs (glabrous: 30.7 ± 2.8 stomata mm−2; hairy: 23.6 ± 2.3 stomata mm−2; mean ± SEM) (Figure 2a; Table S1; Dataset S1). Furthermore, the stomatal index of the adaxial surface was significantly greater in glabrous morphs (18.04 ± 0.92) compared with hairy morphs (16.44 ± 1.03) (Figure 2b; Table S1). The adaxial surface pavement cell density did not differ significantly between the morphs (Table S1). Although leaf widths varied significantly among plants, they did not differ significantly between the morphs (glabrous: 12.4 ± 0.7 mm; hairy: 13.5 ± 1.0 mm; Figure S2; Dataset S1), suggesting that the stomatal density difference between the morphs is not due to differences in leaf expansion between the morphs (Table S1). Mean stomatal density ranged from 17 to 35 stomata mm−2 for hairy morphs and 24–49 stomata mm−2 for glabrous morphs (Figure 2a). This stomatal density was lower than for Arabidopsis thaliana, which has reported stomatal densities of 180–350 stomata mm−2 depending on background accession and growth conditions (Franks, W. Doheny‐Adams, Britton‐Harper, & Gray, 2015; Gray et al., 2000; Zhang, Hu, Cheng, & Huang, 2008). Although lower than in A. thaliana, our measurements of stomatal density in A. halleri are consistent with a previous report of the stomatal density of A. halleri subsp. gemmifera, which measured adaxial stomatal density of 46 stomata mm−2 at 430 m altitude in autumn rosette leaves, with stomatal density progressively increasing with greater altitudes (Aryal, Shinohara, Honjo, & Kudoh, 2018). Our field site was the lower altitude (230 m), so the lower stomatal densities at our study site (Figure 2a) are congruous with this previous study (Aryal et al., 2018).
FIGURE 2.
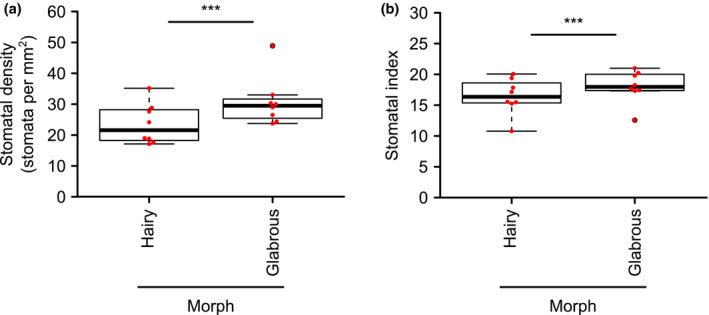
Stomatal density differs between hairy and glabrous morphs within a natural population of Arabidopsis halleri. (a) Stomatal density and (b) stomatal index for fully expanded leaves of hairy and glabrous morphs. Each red point represents the mean stomatal density or stomatal index from one individual plant. The mean stomatal density and stomatal index per plant was derived from two microscopy images analyzed from 3 to 4 leaves of each plant. The center line of the boxplot indicates the median. n = 8 plants from each morph; analyzed by one‐way nested ANOVA. ***Indicates p < .001
4. DISCUSSION
Glabrous plants had significantly greater stomatal density and stomatal index compared with hairy plants (Figure 2a,b). As the density of surrounding pavement cells did not vary between the morphs, these differences in stomatal density and index are due to the greater density of stomata in glabrous morphs compared with hairy morphs (Figure 2b). Our field data are consistent with a laboratory‐based study in which transgenic tobacco plants expressing an Antirrhinum myb‐like transcription factor, which caused an excess of trichomes, also had significantly reduced stomatal density (Glover et al., 1998). In a segregating tomato population, there is a negative correlation between stomatal and trichome density specifically under drought conditions (Galdon‐Armero et al., 2018). Similarly, the trichome‐bearing Col‐0 accession of A. thaliana has lower stomatal density than the glabrous C24 accession (e.g., about 115 mm−2 for Col‐0 and 180 mm−2 for C24) (Lake & Woodward, 2008; Perazza, Vachon, & Herzog, 1998), although factors other than trichome density are likely to influence stomatal density between the accessions. This suggests that in natural populations of A. halleri, there could be a trade‐off between trichome and stomatal development. Since the glabrous gl1 mutant of A. thaliana has a significantly greater density of stomatal units compared with the wild type (Berger, Linstead, Dolan, & Haseloff, 1998) and the glabrous phenotype of A. halleri at this study site is associated with an insertion within GL1 (Kawagoe et al., 2011), it is possible that the GL1 haplotype influences the stomatal density within this population of A. halleri.
In some cases, there does not appear to be a trade‐off between stomatal and trichome density. For example, elevated CO2 decreases stomatal density (Woodward & Kelly, 1995), but might also reduce trichome density (Bidart‐Bouzat, Mithen, & Berenbaum, 2005). Therefore, in the future, it could be informative to examine the relationship between stomatal and trichome density under a range of different experimental conditions that apply different types of selection pressure. Furthermore, we sampled the adaxial leaf surface and it is possible that the presence of trichomes might affect stomatal density differently on the abaxial surface because, depending on environmental conditions, abaxial stomatal density of A. halleri can be 10%–30% greater than the adaxial surface (Aryal et al., 2018).
Interestingly, trichome production appears to impose a fitness cost. For example, glabrous A. halleri plants have 10% greater biomass than hairy plants when grown in the absence of herbivores (Sato & Kudoh, 2016). This cost of herbivore resistance arising from trichome formation also occurs in glabrous and hairy A. lyrata (Løe, Toräng, Gaudeul, & Ågren, 2007; Sletvold et al., 2010) and A. thaliana (Mauricio, 1998; Mauricio & Rausher, 1997) under experimental conditions excluding herbivores. Whilst this fitness advantage of glabrous over hairy leaves in the absence of herbivory might be due to trichome production (Kawagoe & Kudoh, 2010; Kawagoe et al., 2011; Mauricio, 1998; Mauricio & Rausher, 1997; Sletvold & Ågren, 2012; Sletvold et al., 2010), we suggest that glabrous morphs might also gain an advantage by having a greater density or number of stomata. It has been proposed that increasing the number of stomata could increase carbon assimilation (Lawson & Blatt, 2014). For example, Arabidopsis overexpressing STOMAGEN has greater stomatal density and a 30% increase in carbon assimilation compared with the wild type. However, these lines also have higher transpiration rates and consequently lower water use efficiency (Tanaka, Sugano, Shimada, & Hara‐Nishimura, 2013). An alternative interpretation is that differences in the developmental program of the hairy and glabrous morphs might lead to differences in cell or leaf size, which ultimately causes the biomass difference between the morphs. In our samples, the width of fully expanded leaves did not differ significantly between the morphs (Figure S2). Using these leaf width measures as a proxy for leaf size suggests that the biomass difference between the morphs is not due to leaf size differences between the morphs. However, there might be differences in other developmental characteristics that affect biomass, such as leaf thickness, which we did not compare between the morphs.
Optimal stomatal density is important to achieve high photosynthetic rates. A low stomatal density restricts CO2 vertical diffusion through the leaf and reduces photosynthetic rates, whilst high‐density stomatal clustering diminishes CO2 diffusion and causes low carbon assimilation (Lawson & Blatt, 2014). Both A. halleri morphs examined are likely to be within an optimal range of stomatal densities, having evolved and survived under natural conditions. However, the higher stomatal density in the glabrous morph might contribute to its faster growth in the absence of herbivory (Sato & Kudoh, 2016). In the future, it would be interesting to explore this by measuring the CO2 assimilation rate of these trichome morphs under laboratory and/or natural conditions. It would also be informative to determine whether the stomatal density difference between the two trichome morphs confers any advantages within microenvironments characterized by differences in water or light availability. The lower stomatal density of A. halleri compared with A. thaliana (Franks et al., 2015; Gray et al., 2000; Zhang et al., 2008) might reflect differences in growth conditions. An alternative explanation might relate to genome size, because there appears to be a negative correlation between genome size and stomatal density (Beaulieu, Leitch, Patel, Pendharkar, & Knight, 2008), and the genome of A. halleri (250 Mb) is approximately double the size of the A. thaliana genome (125 Mb) (Briskine et al., 2017; The Arabidopsis Genome, 2000).
In summary, we found that a glabrous morph of A. halleri growing under natural conditions had greater stomatal density and stomatal index than a hairy morph. This might contribute to the reported fitness advantage of glabrous plants over hairy plants in the absence of herbivores (Sato & Kudoh, 2017). This differing stomatal density phenotype might derive from the common upstream components in the pathways leading to trichome and guard cell development.
CONFLICT OF INTERESTS
The authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
NMLS, JS, MNH, SAT, GT, HK, and AND performed experimentation and/or analyzed data, and NMLS, MNH, HK, and AND interpreted findings and wrote the paper.
Supporting information
Dataset S1
Table S1‐Fig S1‐S2
ACKNOWLEDGMENTS
We thank Dora Cano‐Ramirez, Haruki Nishio, and Tasuku Ito for experimental assistance. This research was funded by the UK Biotechnology and Biological Sciences Research Council (BBSRC; SWBio DTP award BB/J014400/1 and Institute Strategic Programme GEN BB/P013511/1), the Royal Society (grant IE140501), and the Japan Society for Promotion of Science (JSPS; CREST no. JPMJCR15O1). This research was conducted through the Joint Usage of the Center for Ecological Research, Kyoto University.
Simon NML, Sugisaka J, Honjo MN, et al. Altered stomatal patterning accompanies a trichome dimorphism in a natural population of Arabidopsis . Plant Direct. 2020;4:1–6. 10.1002/pld3.262
DATA AVAILABILITY STATEMENT
All data generated during this study are included in the published article and Supplementary Information files.
REFERENCES
- Aikawa, S. , Kobayashi, M. J. , Satake, A. , Shimizu, K. K. , & Kudoh, H. (2010). Robust control of the seasonal expression of the Arabidopsis FLC gene in a fluctuating environment. Proceedings of the National Academy of Sciences, 107, 11632–11637. 10.1073/pnas.0914293107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aryal, B. , Shinohara, W. , Honjo, M. N. , & Kudoh, H. (2018). Genetic differentiation in cauline‐leaf‐specific wettability of a rosette‐forming perennial Arabidopsis from two contrasting montane habitats. Annals of Botany, 121, 1351–1360. 10.1093/aob/mcy033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean, G. J. , Marks, M. D. , Hulskamp, M. , Clayton, M. , & Croxdale, J. L. (2002). Tissue patterning of Arabidopsis cotyledons. New Phytologist, 153, 461–467. 10.1046/j.0028-646X.2001.00342.x [DOI] [PubMed] [Google Scholar]
- Beaulieu, J. M. , Leitch, I. J. , Patel, S. , Pendharkar, A. , & Knight, C. A. (2008). Genome size is a strong predictor of cell size and stomatal density in angiosperms. New Phytologist, 179, 975–986. 10.1111/j.1469-8137.2008.02528.x [DOI] [PubMed] [Google Scholar]
- Berger, F. , Linstead, P. , Dolan, L. , & Haseloff, J. (1998). Stomata patterning on the hypocotyl of Arabidopsis thaliana is controlled by genes involved in the control of root epidermis patterning. Developmental Biology, 194, 226–234. 10.1006/dbio.1997.8836 [DOI] [PubMed] [Google Scholar]
- Bidart‐Bouzat, M. G. , Mithen, R. , & Berenbaum, M. R. (2005). Elevated CO2 influences herbivory‐induced defense responses of Arabidopsis thaliana . Oecologia, 145, 415–424. 10.1007/s00442-005-0158-5 [DOI] [PubMed] [Google Scholar]
- Bird, S. M. , & Gray, J. E. (2003). Signals from the cuticle affect epidermal cell differentiation. New Phytologist, 157, 9–23. 10.1046/j.1469-8137.2003.00543.x [DOI] [PubMed] [Google Scholar]
- Briskine, R. V. , Paape, T. , Shimizu‐Inatsugi, R. , Nishiyama, T. , Akama, S. , Sese, J. , & Shimizu, K. K. (2017). Genome assembly and annotation of Arabidopsis halleri, a model for heavy metal hyperaccumulation and evolutionary ecology. Molecular Ecology Resources, 17, 1025–1036. [DOI] [PubMed] [Google Scholar]
- Dalin, P. , Agren, J. , Bjorkman, C. , Huttunen, P. , & Karkkainen, K. (2008). Leaf trichome formation and plant resistance to herbivory In Schaller A. (Ed.), Induced plant resistance to herbivory (pp. 89–105). Heidelberg: Springer. [Google Scholar]
- Franks, P. J. , W. Doheny‐Adams, T. , Britton‐Harper, Z. J. , & Gray, J. E. (2015). Increasing water‐use efficiency directly through genetic manipulation of stomatal density. New Phytologist, 207, 188–195. 10.1111/nph.13347 [DOI] [PubMed] [Google Scholar]
- Galdon‐Armero, J. , Fullana‐Pericas, M. , Mulet, P. A. , Conesa, M. A. , Martin, C. , & Galmes, J. (2018). The ratio of trichomes to stomata is associated with water use efficiency in Solanum lycopersicum (tomato). The Plant Journal, 96, 607–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover, B. J. (2000). Differentiation in plant epidermal cells. Journal of Experimental Botany, 51, 497–505. 10.1093/jexbot/51.344.497 [DOI] [PubMed] [Google Scholar]
- Glover, B. J. , Perez‐Rodriguez, M. , & Martin, C. (1998). Development of several epidermal cell types can be specified by the same MYB‐related plant transcription factor. Development, 125, 3497. [DOI] [PubMed] [Google Scholar]
- Gray, J. E. , Holroyd, G. H. , van der Lee, F. M. , Bahrami, A. R. , Sijmons, P. C. , Woodward, F. I. , … Hetherington, A. M. (2000). The HIC signalling pathway links CO2 perception to stomatal development. Nature, 408, 713–716. 10.1038/35047071 [DOI] [PubMed] [Google Scholar]
- Handley, R. , Ekbom, B. , & Ågren, J. (2005). Variation in trichome density and resistance against a specialist insect herbivore in natural populations of Arabidopsis thaliana . Ecological Entomology, 30, 284–292. 10.1111/j.0307-6946.2005.00699.x [DOI] [Google Scholar]
- Honjo, M. N. , & Kudoh, H. (2019). Arabidopsis halleri: A perennial model system for studying population differentiation and local adaptation. AoB Plants, 11, pl2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawagoe, T. , & Kudoh, H. (2010). Escape from floral herbivory by early flowering in Arabidopsis halleri subsp. gemmifera . Oecologia, 164, 713–720. 10.1007/s00442-010-1709-y [DOI] [PubMed] [Google Scholar]
- Kawagoe, T. , Shimizu, K. K. , Kakutani, T. , & Kudoh, H. (2011). Coexistence of trichome variation in a natural plant population: A combined study using ecological and candidate gene approaches. PLoS One, 6, e22184 10.1371/journal.pone.0022184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudoh, H. , Honjo, M. N. , Nishio, H. , & Sugisaka, J. (2018). The long‐term "in natura" study sites of Arabidopsis halleri for plant transcription and epigenetic modification analyses in natural environments In Plant transcription factors (pp. 41–57). Heidelberg: Springer. [DOI] [PubMed] [Google Scholar]
- Lake, J. A. , & Woodward, F. I. (2008). Response of stomatal numbers to CO2 and humidity: Control by transpiration rate and abscisic acid. New Phytologist, 179, 397–404. [DOI] [PubMed] [Google Scholar]
- Larkin, J. C. , Young, N. , Prigge, M. , & Marks, M. D. (1996). The control of trichome spacing and number in Arabidopsis . Development, 122, 997–1005. [DOI] [PubMed] [Google Scholar]
- Lawson, T. , & Blatt, M. R. (2014). Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiology, 164, 1556–1570. 10.1104/pp.114.237107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin, D. A. (1973). The role of trichomes in plant defense. The Quarterly Review of Biology, 48, 3–15. 10.1086/407484 [DOI] [Google Scholar]
- Løe, G. , Toräng, P. , Gaudeul, M. , & Ågren, J. (2007). Trichome production and spatiotemporal variation in herbivory in the perennial herb Arabidopsis lyrata . Oikos, 116, 134–142. [Google Scholar]
- Mauricio, R. (1998). Costs of resistance to natural enemies in field populations of the annual plant Arabidopsis thaliana . The American Naturalist, 151, 20–28. [DOI] [PubMed] [Google Scholar]
- Mauricio, R. , & Rausher, M. D. (1997). Experimental manipulation of putative selective agents provides evidence for the role of natural enemies in the evolution of plant defense. Evolution, 51, 1435–1444. 10.1111/j.1558-5646.1997.tb01467.x [DOI] [PubMed] [Google Scholar]
- Oppenheimer, D. G. , Herman, P. L. , Sivakumaran, S. , Esch, J. , & Marks, M. D. (1991). A myb gene required for leaf trichome differentiation in Arabidopsis is expressed in stipules. Cell, 67, 483–493. 10.1016/0092-8674(91)90523-2 [DOI] [PubMed] [Google Scholar]
- Perazza, D. , Vachon, G. , & Herzog, M. (1998). Gibberellins promote trichome formation by up‐regulating GLABROUS1 in Arabidopsis. Plant Physiology, 117, 375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . (2019). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; Retrieved from https://www.R‐project.org/ [Google Scholar]
- Sato, Y. , & Kudoh, H. (2015). Tests of associational defence provided by hairy plants for glabrous plants of Arabidopsis halleri subsp. gemmifera against insect herbivores. Ecological Entomology, 40, 269–279. [Google Scholar]
- Sato, Y. , & Kudoh, H. (2016). Associational effects against a leaf beetle mediate a minority advantage in defense and growth between hairy and glabrous plants. Evolutionary Ecology, 30, 137–154. 10.1007/s10682-015-9809-0 [DOI] [Google Scholar]
- Sato, Y. , & Kudoh, H. (2017). Fine‐scale frequency differentiation along a herbivory gradient in the trichome dimorphism of a wild Arabidopsis . Ecology and Evolution, 7, 2133–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sletvold, N. , & Ågren, J. (2012). Variation in tolerance to drought among Scandinavian populations of Arabidopsis lyrata . Evolutionary Ecology, 26, 559–577. 10.1007/s10682-011-9502-x [DOI] [Google Scholar]
- Sletvold, N. , Huttunen, P. , Handley, R. , Kärkkäinen, K. , & Ågren, J. (2010). Cost of trichome production and resistance to a specialist insect herbivore in Arabidopsis lyrata . Evolutionary Ecology, 24, 1307–1319. 10.1007/s10682-010-9381-6 [DOI] [Google Scholar]
- Tanaka, Y. , Sugano, S. S. , Shimada, T. , & Hara‐Nishimura, I. (2013). Enhancement of leaf photosynthetic capacity through increased stomatal density in Arabidopsis. New Phytologist, 198, 757–764. 10.1111/nph.12186 [DOI] [PubMed] [Google Scholar]
- The Arabidopsis Genome Initiative . (2000). Analysis of the genome sequence of the flowering plant Arabidopsis thaliana . Nature, 408, 796–815. [DOI] [PubMed] [Google Scholar]
- Woodward, F. I. , & Kelly, C. K. (1995). The influence of CO2 concentration on stomatal density. New Phytologist, 131, 311–327. 10.1111/j.1469-8137.1995.tb03067.x [DOI] [Google Scholar]
- Zhang, L. , Hu, G. , Cheng, Y. , & Huang, J. (2008). Heterotrimeric G protein α and β subunits antagonistically modulate stomatal density in Arabidopsis thaliana . Developmental Biology, 324, 68–75. 10.1016/j.ydbio.2008.09.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dataset S1
Table S1‐Fig S1‐S2
Data Availability Statement
All data generated during this study are included in the published article and Supplementary Information files.


