Abstract
Scope
Akkermansia muciniphila (A. muciniphila) is an intestinal commensal with anti‐inflammatory properties both in the intestine and other organs. The aim is to investigate the effects of oral administration of A. muciniphila on lipid metabolism, immunity, and cuff‐induced neointima formation in hyperlipidemic APOE*3‐Leiden (E3L).CETP mice.
Methods and results
Hyperlipidemic male E3L.CETP mice are daily treated with 2 × 108 CFU A. muciniphila by oral gavage for 4 weeks and the effects are determined on plasma lipid levels, immune parameters, and cuff‐induced neointima formation and composition. A. muciniphila administration lowers body weight and plasma total cholesterol and triglycerides levels. A. muciniphila influences the immune cell composition in mesenteric lymph nodes, as evident from an increased total B cell population, while reducing the total T cell and neutrophil populations. Importantly, A. muciniphila reduces the expression of the activation markers MHCII on dendritic cells and CD86 on B cells. A. muciniphila also increases whole blood ex vivo lipopolysaccharide‐stimulated IL‐10 release. Finally, although treatment with A. muciniphila improves lipid metabolism and immunity, it does not affect neointima formation or composition.
Conclusions
Four weeks of treatment with A. muciniphila exerts lipid‐lowering and immunomodulatory effects, which are insufficient to inhibit neointima formation in hyperlipidemic E3L.CETP mice.
Keywords: Akkermansia muciniphila, atherosclerosis, immunity, lipid metabolism, mesenteric lymph nodes
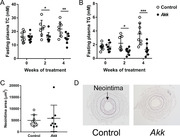
A. muciniphila administration lowers plasma total cholesterol (A) and triglycerides levels (B) and influences the immune cell composition in mesenteric lymph nodes. Importantly, A. muciniphila reduces expression of activation markers MHCII on dendritic cells and CD86 on B cells. Although treatment with A. muciniphila improves lipid metabolism and immunity, it does not affect neointima formation or composition (C and D).
1. Introduction
Our understanding of the role and importance of the intestinal microbiome in health and disease has increased remarkably over the last decades. It is becoming clear that disruption of homeostasis of the intestinal microbiota (i.e., microbial dysbiosis) is involved in the development of various diseases in humans. Dysbiosis does not only play a role in intestinal complications such as inflammatory bowel diseases and ulcerative colitis, but also in rheumatoid arthritis and type‐2 diabetes.[ 1 , 2 , 3 ] The α‐diversity (i.e., the species richness) within the gut microbiota is reduced in these patients, implying that particular bacterial species have disappeared during or preceding the development of these diseases.[ 4 , 5 ]
Akkermansia muciniphila (A. muciniphila) is one of the specific bacterial species whose abundance is inversely correlated with diseases, such as inflammatory bowel diseases, autism, atopy, and metabolic syndrome.[ 6 , 7 , 8 , 9 ] A. muciniphila is a commensal member of human and rodent intestinal microbiota and has been identified as a beneficial bacterium.[ 10 , 11 ] Indeed, daily exposure to A. muciniphila protected mice against high‐fat diet‐induced obesity and improved their metabolic parameters such as obesity, fat mass, glucose tolerance, and hypercholesterolemia.[ 12 , 13 ] In addition, oral supplementation of A. muciniphila reduced experimental alcoholic or immune related liver disease in mice.[ 14 , 15 ]
Although the mechanisms underlying the beneficial effects of A. muciniphila have not been completely resolved, it has been proposed that they involve effects on gut barrier integrity and inflammation. A. muciniphila is thought to counteract metabolic endotoxemia and to improve gut barrier integrity as a consequence of the increased mucin production that is caused by A. muciniphila's mucin degrading capacity.[ 12 , 13 , 16 , 17 ] Oral administration of A. muciniphila to high‐fat diet‐fed mice upregulated colonic expression of the intestinal antimicrobial peptide Regenerating islet‐derived protein 3 gamma (RegIIIγ) and attenuated inflammation in visceral adipose tissue by inducing T‐regulatory cells.[ 12 , 16 ] Moreover, administration of A. muciniphila had anti‐inflammatory effects on splenocytes and macrophages as indicated by an increased ratio of IL‐10/TNF‐α secretion by these cells.[ 18 ] Taken together, these studies suggest that A. muciniphila has beneficial immunomodulating properties both in the intestine and in other tissues.
Atherosclerosis is a chronic, low‐grade inflammatory disease of the vessel wall.[ 19 ] One of the major triggers for vessel wall inflammation is hyperlipidemia. Studies in experimental models support the beneficial effects of inhibiting inflammation to delay atherosclerosis progression.[ 20 , 21 , 22 ] Recently, a clinical study of Ridker et al.[ 23 ] showed anti‐inflammatory therapy by canakinumab lowered the rate of recurrent cardiovascular events, without reducing plasma lipid levels. Therefore, reduction of inflammation as strategy to reduce cardiovascular risk is a viable approach. Given its beneficial immunomodulatory effects, A. muciniphila might be promising in attenuating atherosclerosis. Indeed, in a recent study, A. muciniphila reduced systemic inflammation and atherosclerotic lesion development in apolipoprotein E‐deficient (Apoe −/−) mice, presumably by ameliorating endotoxemia.[ 24 ]
In the present study, we extended these findings and investigated the effect of oral administration of A. muciniphila on lipid metabolism, immunity, and cuff‐induced neointima formation in hypercholesterolemic APOE*3‐Leiden.cholesteryl ester transfer protein (E3L.CETP) mice, an established model for human‐like lipoprotein metabolism.[ 25 , 26 ] In this double transgenic mouse model, both a mutated human APOE*3 gene and the human CETP gene are expressed which leads to diet‐induced hyperlipidemia and atherosclerosis development.[ 27 ] After the placement of a non‐constricting polyethylene cuff around the femoral artery, vascular remodelling with signs of accelerated atherosclerosis takes place in the femoral artery in approximately 2 weeks.[ 25 ] This model of neointima formation resembles both restenosis, as it occurs in patients after balloon angioplasty, as well as accelerated atherosclerotic lesion formation, as the lesions formed after cuff placement contain mainly both smooth muscle cells and foam cells.[ 28 ]
We aimed to investigated the effects of oral administration of A. muciniphila on lipid metabolism, immunity, and cuff‐induced neointima formation in hyperlipidemic APOE*3‐Leiden (E3L).CETP mice. We determined the effect of 4 weeks oral A. muciniphila administration in E3L.CETP mice on 1) plasma lipid levels, 2) the immune response by measuring portal vein lipopolysaccharide (LPS) levels, mesenteric lymph node (mLN) immune cell composition and ex vivo responses of circulating leukocytes to LPS, and 3) neointima formation and composition. We found that 4 weeks of treatment with A. muciniphila beneficially affects hyperlipidemia and had broad immunomodulatory effects in E3L.CETP mice, but that these effects did not result in reduced neointima formation.
2. Experimental Section
2.1. Animals
Male E3L.CETP mice[ 29 ] between 9 and 13 weeks old were fed an atherogenic Western‐type diet containing 1% cholesterol and 0.05% cholate (4021.37 AB Diets, Woerden, The Netherlands) for 7 weeks. All mice received water and food ad libitum. Body weight and food intake per cage were measured weekly. After 3 weeks of run‐in, mice were divided in two groups based on age, body weight, and plasma lipid levels, and thereafter orally gavaged daily for 4 weeks with 100 µL of either 1) anaerobic PBS or 2) 2 × 108 CFU viable A. muciniphila in anaerobic PBS; both containing 2.5% glycerol.
Tail blood samples were obtained in week 0, 2, and 4 after 4‐h fasting for plasma lipid determination. Fresh fecal samples were obtained in week 0 and 4. Neointima formation was induced by placement of a non‐constricted polyethylene cuff (Portex, UK) around the femoral artery of both legs. Mice were anesthetized with a mixture of midazolam/medetomidine/fentanyl. After the surgery, the anesthesia of the mice was antagonized with a mixture of atipamezol/fluminasenil. Buprenorphine was given directly after surgery to relieve pain. The cuffs were placed 2 weeks after starting the A. muciniphila treatment and 2 weeks thereafter the mice were killed. After 4 weeks of treatment with A. muciniphila or vehicle, mice were anesthetized by a mixture of midazolam/fentanyl/dexdomiter/NaCl mix and portal vein samples were obtained in a laminar flow cabinet in pyrogenic‐free tubes. Mice were killed by bleeding via the portal vein and thereafter mesenteric lymph nodes and cecum were dissected. The femoral arteries were harvested, fixed overnight in 4% formaldehyde in PBS, paraffin‐embedded, and sectioned. Animal experiments were performed in compliance with Dutch government guidelines and the Directive 2010/63/EU of the European Parliament and all experiments were approved by the animal ethics committee of the Leiden University Medical Center (protocol no. 14143).
2.2. Culture and Pasteurization of A. muciniphila
Viable A. muciniphila was prepared by the Laboratory of Microbiology, Wageningen University as described previously.[ 13 ] In brief, A. muciniphila MucT (ATTC BAA‐835) was cultured anaerobically in a basal mucin‐based medium as previously described.[ 10 ] Cultures were washed and concentrated in anaerobic PBS with 25% (vol/vol) glycerol under strict anaerobic conditions. Cultures were then immediately frozen and stored at −80 °C. A representative glycerol stock was thawed under anaerobic conditions to determine the CFU mL−1 by plate counting using mucin media containing 1% agarose (agar noble; Difco). Before administration by oral gavage, glycerol stocks were thawed under anaerobic conditions and diluted with anaerobic PBS to an end concentration of 2×108 colony forming unit (CFU) 100 µL and 2.5% glycerol.
2.3. A. muciniphila qPCR
To quantify A. muciniphila in feces or cecum, quantitative PCR was performed. The primers to detect A. muciniphila were based on the 16S ribosomal RNA (16S rRNA) gene sequences: forward CAGCACGTGAAGGTGGGGAC, reverse CCTTGCGGTTGGCTTCAGAT as described previously.[ 12 ] Detection was achieved with CFX96 real‐time PCR system and software (BioRad, Hercules, CA, USA) using EvaGreen (Biotium Inc., Hayward, CA, USA) according to the manufacturer's instructions. Each assay was performed in triplicate in the same run. The cycle threshold of each sample was then compared with a standard curve (performed in triplicate) made by diluting genomic DNA of A. muciniphila (fivefold serial dilution).
2.4. Determination of Plasma Triglycerides and Cholesterol
After 4‐h fasting, tail blood was collected into chilled glass capillaries. Capillaries were placed on ice, centrifuged, and plasma was isolated. Commercially available kits were used to determine plasma total cholesterol (Total Cholesterol Roche diagnostics/Hitachi: 11491458216) and total triglycerides (Total Triglyceride Roche diagnostics/Hitachi: product nr. 11730711216).
2.5. Portal Vein Serum LPS Measurements
LPS concentrations were measured in portal vein serum samples using an Endosafe‐Multi‐Cartridge System (Charles River Laboratories, MA, USA) based on the Limulus Amaebocyte Lysate (LAL) kinetic chromogenic methodology, as described before.[ 12 ] Each sample was diluted when necessary with endotoxin‐free LAL reagent water (Charles River Laboratories, MA, USA) and treated in duplicate. Two spikes for each sample were included in the determination in order to validate the recovery of LPS. All samples were validated for the recovery and the coefficient variation. The lower limit of detection was 0.005 EU mL−1.
2.6. Determination of Immune Composition of Mesenteric Lymph Nodes by Flow Cytometry
Single cell suspensions were prepared from mouse mesenteric lymph nodes (mLNs) for analysis by flow cytometry. Following 20‐min digestion with collagenase II/ DNase in 12 well plates, digested mLNs were dispersed through 100 µm cell strainers. Single cell‐suspensions were plated in 96‐well v‐bottom plates at concentrations of 5 × 105 or 1 × 106 cells per well. Cells were washed, incubated with aqua live‐dead staining (20 min, RT), and stained for respective antibody panels for 20 min (−4 °C). Cells were stained with antibodies against: CD1d, CD3, CD4, CD5, CD11b, CD23, CD44, CTLA‐4, FoxP3 (eBioscience, San Diego, CA, USA) CD11c, CD19, CD21, CD25, CD86, MHC‐II, GR‐1 (BD Biosciences, San Diego, CA, USA), CD24, CD64, GITR, CD103 (BioLegend, San Diego, CA, USA) and F4/80 (SanBio, Uden, The Netherlands). Additionally, FC block (CD16/CD32) was added to the antibody mix to prevent non‐specific binding. For intracellular staining, antibody incubation was preceded by 1 h fixation with eBioscience Fix/Perm buffer and antibody staining mixes were created with Bioscience permeabilization buffer. Cells were acquired with a FACS Canto‐II and analysis was performed with FlowJo (Tree Star, San Carlos, CA, USA) software. For gating strategies see Figure S1, Supporting Information.
2.7. Whole Blood Stimulation with LPS
Portal vein blood samples (16 µL blood in 400 µL RPMI) were stimulated in triplo overnight with 10 ng mL−1 LPS or vehicle and supernatants were collected and stored at −80 °C. IL‐10 and TNF‐α were subsequently measured by ELISA (BD Biosciences, San Diego, CA, USA).
2.8. Neointima Formation Assessment
Serial cross sections 5‐µm thick were made throughout the entire length of the cuffed femoral artery for histological analysis. Weigert's elastin staining was used to visualize elastic laminae. Smooth muscle cells were visualized with smooth muscle cell α‐actin staining (Boehringer Mannheim, Germany), Mac‐3 (Accurate Chemical, Westbury, NY, USA) macrophage staining was used to detect monocytes/macrophages and Sirius red stain (VWR International) was used to detect collagen. Six equally spaced cross sections were used in all mice to quantify intimal lesions. Using image analysis software (Leica, Wetzlar, Germany), total cross‐sectional medial area was measured between the external and internal elastic lamina; total cross‐sectional intimal area was measured between the endothelial cell monolayer and the internal elastic lamina.[ 25 ] For macrophage, smooth muscles cell and collagen composition absolute immuno‐positive area and neointimal area were measured and used to calculate the percentage of immune‐positive area within the neointima area.
2.9. Statistics
Data are presented as mean ± SD. Statistical analyses were performed using Student t‐test analysis. Portal vein serum LPS levels were log normalized before applying Student t‐test analysis. (GraphPad Software Inc., La Jolla, Ca, USA). p < 0.05 was considered statistically significant.
3. Results
3.1. A. muciniphila Decreased Plasma Lipids
To study the effect of administration of A. muciniphila on metabolic and immune parameters as well as on neointima formation, hyperlipidemic male E3L.CETP mice were orally gavaged with 2 × 108 CFU A. muciniphila daily for 4 weeks. As controls, E3L.CETP mice were treated with equivalent volume of vehicle. A. muciniphila abundance, body weight, and food intake were monitored, as well as fasted plasma lipid levels.
Daily gavage with A. muciniphila led to ≈100000‐fold higher CFU levels in feces and ≈1000‐fold higher CFU in cecum at the end of the study (Figure S2A, Supporting Information). A single gavage with A. muciniphila led to a similar increase in CFU levels (Figure S2B, Supporting Information), indicating that A. muciniphila colonized the gastrointestinal tract of the mice. A. muciniphila administration resulted in slightly decreased body weight (Figure 1A), without affecting food intake (data not shown) as compared to control treatment. A. muciniphila did not affect white adipose tissue weight (Figure S3A, Supporting Information); however, there was a trend toward decreased interscapular brown adipose tissue weight (Figure S3B, Supporting Information) as compared to control treatment. A. muciniphila markedly reduced fasting plasma total cholesterol (TC) (Figure 1B) and triglycerides (TG) (Figure 1C) levels after 2 and 4 weeks of treatment as compared to vehicle. Taken together, exposure to A. muciniphila decreased body weight as well as plasma TC and TG levels.
Figure 1.
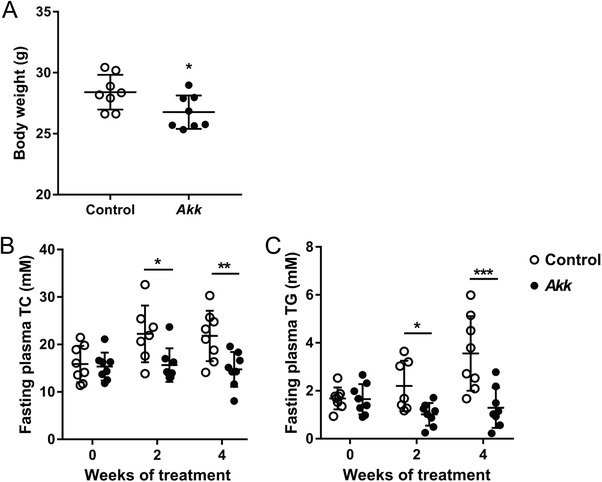
A. muciniphila decreased body weight and plasma lipid levels. A) Body weight (after 4 weeks) as well as fasting plasma B) total cholesterol (TC) and (C) triglyceride (TG) levels were determined at the indicated times after daily oral administration of hyperlipidemic E3L.CETP mice with A. muciniphila (Akk) or vehicle (control). Data are means ± SD; n = 8 per group. * p < 0.05, ** p < 0.01 and *** p < 0.001.
3.2. A. muciniphila Tended to Lower Portal LPS Levels
To assess whether A. muciniphila counteracted endotoxemia, LPS levels were measured in portal vein serum. A. muciniphila administration tended to decrease portal vein serum LPS levels by ≈60% (p = 0.06) as compared to control treatment (Figure S4, Supporting Information).
3.3. A. muciniphila Modulated the Composition and Reduced the Activation Status of Immune Cells in Mesenteric Lymph Nodes
We next assessed the effect of A. muciniphila on the immune system in vivo. The mesenteric lymph node (mLN) is the “first pass” organ for nutrients and microbial substances entering the lymph fluid from the intestinal lamina propria.[ 30 ] As such, it serves as a key site for tolerance induction to food particles and commensals, but at the same time it acts as a firewall to prevent systemic spread of microorganisms.[ 30 , 31 , 32 ] Therefore, we studied the effect of A. muciniphila on immune cells populations locally in the mLNs, using flow cytometry.
A. muciniphila had no effect on the percentage of dendritic cells (DCs) infiltrating the mLNs (Figure 2A), but increased the total B cell population (Figure 2C) and markedly decreased the total neutrophil population (Figure 2B) and the total T cell population (Figure 2D). Subsequently, we studied the composition of various subpopulations of DCs, B cells, and T cells in the mLNs. A. muciniphila had no effect on the percentages of various DC subpopulations (Figure 3A–D). However, A. muciniphila tended to decrease the antigen‐presenting molecule MHCII on CD11b‐CD103+ (tissue resident) DCs (p = 0.07; Figure 3E) and reduced the expression of this marker on the CD11b+CD103‐ DCs subpopulation (Figure 3G). These data indicate reduced activity of these DC subpopulations.
Figure 2.
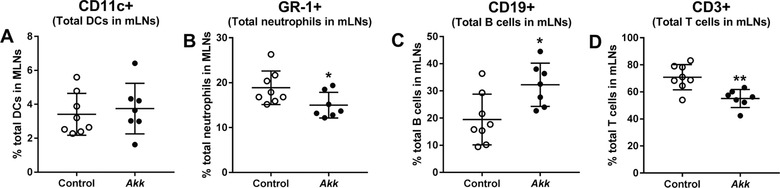
A. muciniphila modulated immune cell populations in mesenteric lymph nodes. Mesenteric lymph nodes (MLNs) were isolated from E3L.CETP mice after 4 weeks of daily oral administration of A. muciniphila (Akk) or vehicle (control), and the percentage of total A) dendritic cells, B) neutrophils, C) B‐cell, and D) T‐cell populations were studied using flow cytometry. Data are means ± SD; n = 7–8 per group. * p < 0.05 and ** p < 0.01.
Figure 3.
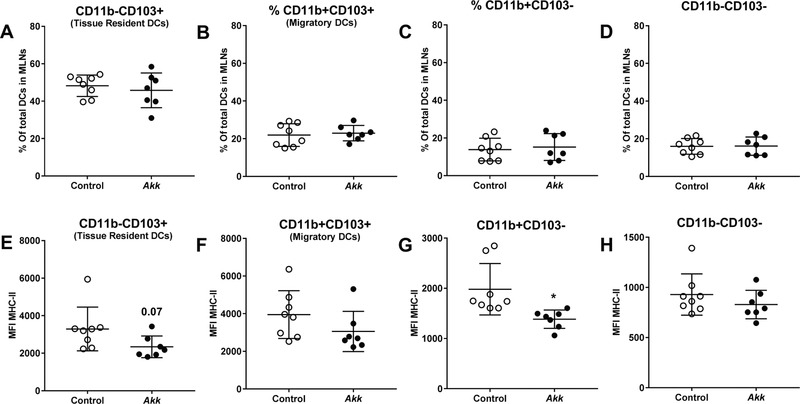
A. muciniphila did not affect percentage of dendritic cell subpopulations but decreased expression of MHC‐II on DCs in mLNs. Mesenteric lymph nodes (MLNs) were isolated from E3L.CETP mice after 4 weeks of daily oral administration of A. muciniphila (Akk) or vehicle (control), and subsets of dendritic cells (DCs) were studied using flow cytometry. A–D) The percentage as well as E–H) the mean fluorescence intensity (MFI) of MHC‐II were determined of A, E) CD11b‐CD103+ (tissue resident DCs), B, F) CD11b+CD103+ (migratory DCs), C, G) CD11b+CD103‐, and D, H) CD11b‐CD103‐ DCs. Data are means ± SD; n = 7–8 per group. * p < 0.05.
A. muciniphila did not affect the percentage of follicular B cells (Figure 4A) or mucosal B cells (Figure 4B) within the total B cell population, indicating that the increase in total B cell population could not be ascribed to changes in these specific B cell subpopulations. A. muciniphila, however, reduced the expression of the T cell co‐stimulatory molecule CD86 on both the follicular (Figure 4C) and mucosal (Figure 4D) B cell population. A. muciniphila did not affect regulatory T cells in the mLNs (Figure S5, Supporting Information). Altogether, A. muciniphila influences the immune cell composition and may reduce the activation status of the immune cells, thereby exerting immunomodulatory properties on mLNs in vivo.
Figure 4.
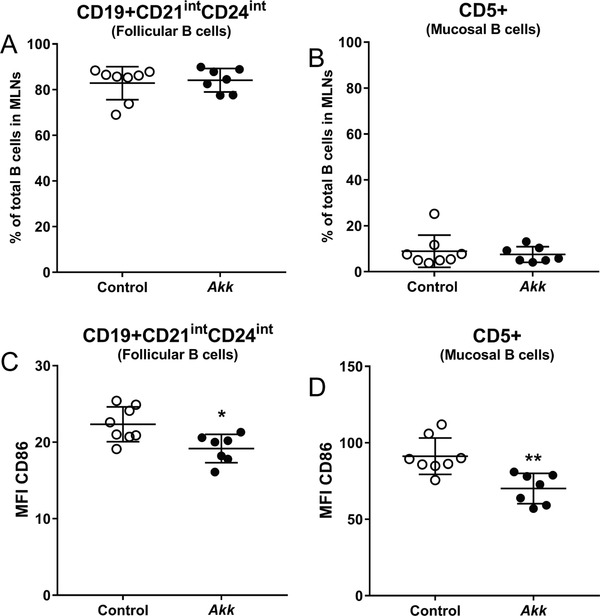
A. muciniphila did not affect abundance of follicular and mucosal B cells in mLNs, but decreased their CD86 expression. Mesenteric lymph nodes (MLNs) were isolated from E3L.CETP mice after 4 weeks of daily oral administration of A. muciniphila (Akk) or vehicle (control), and subsets of B cells were studied using flow cytometry. A,B) The percentage as well as C,D) the mean fluorescence intensity (MFI) of MHC‐II were determined of A, C) CD19+CD21intCD24int (follicular B cells) and B, D) CD5+ (mucosal B cells). Data are means ± SD; n = 7–8 per group. * p < 0.05 and ** p < 0.01.
3.4. A. muciniphila Increased the Anti‐Inflammatory IL‐10 Response after Whole Blood Stimulation
As A. muciniphila modulated the immune system locally in the mLNs, we further studied the effect of A. muciniphila on ex vivo LPS‐stimulated whole blood as a measure for systemic inflammation. Although the overall IL‐10 levels were very low, A. muciniphila tended to increase unstimulated whole blood IL‐10 levels (p = 0.09; Figure 5A) and increased the LPS‐stimulated IL‐10 response (Figure 5B) as compared to whole blood of control mice. Treatment with A. muciniphila did not influence the unstimulated or LPS‐stimulated TNF‐α response (Figure 5C,D). These findings indicate that A. muciniphila increased the whole blood‐(un)stimulated IL‐10 response, and thus modulates systemic cytokine secretion.
Figure 5.
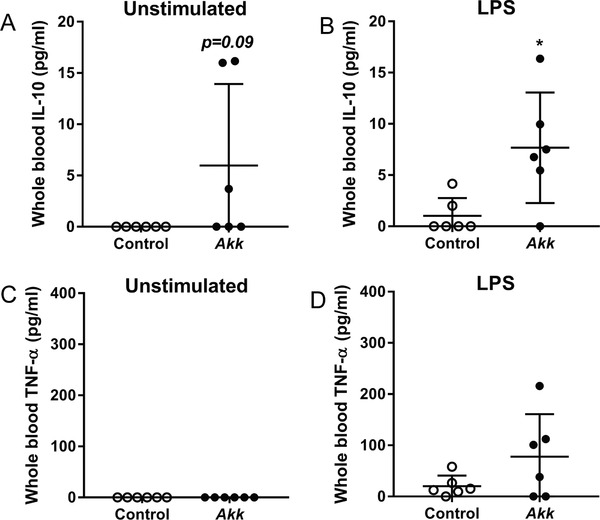
A. muciniphila increased IL‐10 secretion in ex vivo LPS‐stimulated blood. Whole blood was collected from E3L.CETP mice after 4 weeks of daily oral administration of A. muciniphila (Akk) or vehicle (control) and, subsequently, stimulated in vitro A, C) without or B, D) with 10 pg mL−1 LPS for 24 h. A,B) IL‐10 and C,D) TNF‐α secretion was determined by ELISA. Data are means ± SD; n = 6 per group. * p < 0.05.
3.5. A. muciniphila did not Prevent Neointima Formation
We next assessed whether exposure to A. muciniphila prevented neointima formation, a common feature of restenosis and atherosclerosis. Four weeks daily treatment with A. muciniphila did not reduce neointimal area as compared to vehicle (Figure 6A,B). In addition, A. muciniphila did not modulate neointimal content of macrophages (Figure 6C), vascular smooth muscle cells (Figure 6D), and collagen (Figure 6E). These data show that, despite its lipid‐lowering and immunomodulatory properties, A. muciniphila did not reduce neointima formation and vascular lesion composition within this period of time.
Figure 6.
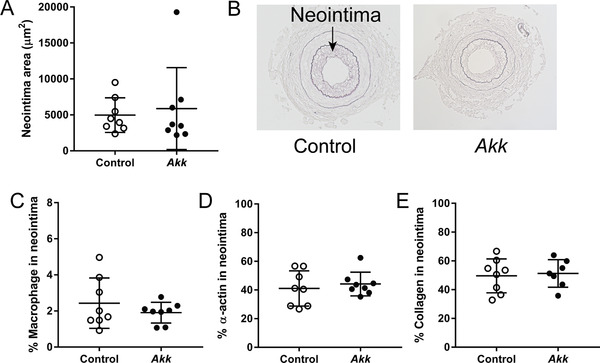
A. muciniphila did not affect neointima formation and composition. A) The neointimal area in the cuffed femoral artery was determined in E3L.CETP mice after 4 weeks of daily oral administration of A. muciniphila (Akk) or vehicle (control) and B) representative pictures are shown. In addition, neointimal content of C) macrophages, D) vascular smooth muscle cells, and E) collagen was determined. Data are means ± SD; n = 7–8 per group.
4. Discussion
We investigated the effect of oral administration of A. muciniphila on body weight, plasma lipid levels, immunity, and cuff‐induced neointima formation in hyperlipidemic E3L.CETP mice, a mouse model for accelerated atherosclerosis driven both by lipids and inflammation.[ 25 , 26 ] Four weeks of treatment with A. muciniphila lightly decreased body weight without affecting food intake, rapidly reduced hyperlipidemia, and exerted immunomodulatory effects. However, within this time‐frame these effects were not accompanied by a reduction in neointima formation or alterations in the composition of the neointima.
Previous studies showed presence or absence of A. muciniphila affects host energy homeostasis parameters. Chevalier et al. showed that oral gavage of cold exposed mice with A. muciniphila reverts cold exposure‐induced intestinal absorptive functions that maximize the caloric uptake during cold.[ 33 ] In line with this finding, Plovier et al. showed that mice treated with pasteurized A. muciniphila had a higher fecal caloric content compared to controls, suggesting a lower energy absorption after treatment with pasteurized A. muciniphila.[ 13 ] Furthermore, recent literature strongly suggests that A. muciniphila promotes browning of adipose tissue,[ 33 , 34 , 35 ] which results in an increased energy expenditure by lipid oxidation. A. muciniphila seems to play a role in reduction of energy absorption and increase in energy expenditure, which is likely to cause a decrease in body weight without an decrease in food intake.
We and others have shown extensively that the E3L.CETP mouse model responds in a human‐like fashion to many of the currently prescribed lipid‐lowering drugs such as atorvastatin and fenofibrate.[ 26 , 29 , 36 ] Since A. muciniphila administration markedly decreased plasma TC and TG, this seems to be a promising lipid‐lowering approach for human interventions that deserves further exploration. Future studies are required to clarify the mechanism of lipid‐lowering by A. muciniphila administration. The main trigger for cuff‐induced neointima formation in E3L.CETP mice is the local injury induced by the cuff, which evolves into a lesion in a two week time span. Hypercholesterolemia is required for this process. Apparently, the reduction in plasma TC and TG levels after A. muciniphila treatment was insufficient to affect the pathological process of neo‐intima formation in the used time‐frame. This finding may not be unexpected based on the correlation that has been described between plasma cholesterol levels and neointimal area formation after cuff placement in E3L.CETP mice.[ 25 ] Applying a linear correlation to the observed reduction in plasma cholesterol after A. muciniphila administration, the expected reduction in neointima formation is well within the standard error of the measurement.
A. muciniphila has been shown to have systemic and organ‐specific immunomodulatory properties,[ 37 , 38 , 39 ] but the underlying mechanisms have not been entirely resolved. Our data clearly showed that A. muciniphila administration modulates the immune cell composition of the mLNs. Importantly, A. muciniphila also reduced the expression of the antigen peptide complex MHCII on DCs as well as the expression of the co‐stimulatory signal CD86 on B cells. Both MHC‐II and CD86 are involved in T‐cell‐mediated immune responses.[ 40 , 41 ] In line with this, A. muciniphila reduced the percentage of total T cells in the mLNs upon administration. Combined, these observations may suggest that A. muciniphila reduces pro‐inflammatory T cell‐mediated immune responses, at least in the mLNs.
Intriguingly, the putative immunomodulatory effects of A. muciniphila were not restricted to the mLNs. A. muciniphila administration also increased IL‐10 secretion from circulating immune cells in whole blood upon ex‐vivo LPS stimulation, although overall IL‐10 levels remained very low. A. muciniphila thus seems to have the capacity to exert complex modulatory effects in vivo, which is in line with a previous study in which daily oral gavage with A. muciniphila diminished Western‐type diet‐induced serum levels of pro‐inflammatory monocyte chemoattractant protein‐1 (MCP‐1) and interleukin‐1β (IL‐1β).[ 24 ] These studies substantiate the findings that intestinal microbiota regulate peripheral immunity, which is an interesting area of further investigation.
The cuff‐induced neointima formation model was shown to be susceptible to immunomodulation in previous studies. For instance, IL‐10 deficiency in hyperlipidemic E3L mice increased cuff‐induced neointima formation, whereas a decrease was observed after IL‐10 overexpression.[ 42 ] Moreover, treatment of hypercholesterolemic E3L mice with abatacept, a protein that prevents CD28–CD80/86 co‐stimulatory T‐cell activation, profoundly inhibited neointima formation.[ 43 ] Although we found that A. muciniphila modulated both the peripheral IL‐10 response and the T cell activation in the mLNs, these effects were apparently insufficient to exert an anti‐atherogenic response in this mouse model, within the period of investigation.
A. muciniphila administration tended to reduce the portal vein LPS levels by ≈60% in our study. The lack of a significant effect may be explained by the low LPS levels observed after the Western‐type diet in our study, which were comparable to previously reported LPS levels in mice fed a chow diet.[ 12 ] Previously, Li et al.[ 24 ] showed that A. muciniphila administration reduced atherosclerotic lesion development in apoe−/‐ mice. In their study, 8 weeks of treatment with 2 × 109 CFU A. muciniphila inhibited atherosclerosis presumably via ameliorating endotoxemia induced by their Western‐type diet. These data thus imply that an increased dose of A. muciniphila, the diet, and/or the duration of treatment might explain the discrepant effects of A. muciniphila on atherosclerosis development between the studies.
Recently, it was observed that pasteurized cells of A. muciniphila were as active as live A. muciniphila cells in protecting mice from diet‐induced obesity.[ 13 ] The outer membrane protein of A. muciniphila, Amuc_1100*, also stimulated TLR2 signaling in vitro, similar to viable A. muciniphila.[ 13 , 44 ] Although our experiments clearly indicate that administration of viable A. muciniphila colonized the mice very well and exerted lipid‐lowering and immunomodulatory effects, it remains to be investigated whether A. muciniphila needs to be viable to retain its efficacy.
In conclusion, administration of A. muciniphila lowered hyperlipidemia in hypercholesterolemic E3L.CETP mice and had immunomodulatory properties. As both hyperlipidemia and immune responses are involved in the pathogenesis of atherosclerosis these observations suggest that A. muciniphila has anti‐atherogenic potential. However, A. muciniphila was unable to ameliorate atherosclerosis in our cuff‐induced neointima formation model, suggesting that the anti‐atherogenic effects of A. muciniphila were not sufficiently strong in this mouse model.
Conflict of Interest
The authors declare no conflict of interest.
Supporting information
Supporting Information
Acknowledgements
J.F.P.B. and V.vH. contributed equally to this work. The authors would like to thank A.C.M. Pronk, S. van Gelderen, T.C.M Streefland, I.M. Mol, H.A.B. Peters, S. Aalvink, and R.C.M de Jong for their excellent technical assistance.
This research was supported by The Netherlands Cardiovascular Research Committee IN‐CONTROL Grant (CVON 2012‐03). Research of WMdV was partly supported by the SIAM Gravitation Grant 024.002.002 of the Netherlands Organization for Scientific Research. PDC is senior research associate at the FRS‐FNRS (Belgium) and recipient of an ERC Starting Grant 2013 (336452‐ENIGMO) and FRFS‐WELBIO under Grant number WELBIO‐CGR‐2017.
S.K., M.R.dV., A.H.C., J.A.vD., H.H.S., W.M.dV., C.B., K.W.vD., J.F.P.B., and V.vH. conceived and designed the experiments. S.K., M.R.dV., J.A.vD., A.H.C., L.R.H., K.T., P.D.C., J.F.P.B., and V.vH. performed the experiments. S.K., A.H.C., K.T., P.D.C., and V.vH. analyzed the data. S.K., K.W.vD., J.F.P.B., and V.vH. wrote the paper. M.R.dV., J.A.vD., A.H.C., L.R.H., K.T., H.H.S., K.E.B., P.C.N.R., P.H.A.Q., M.N., M.G.N., W.M.dV., P.D.C., and C.B. did the critical reading.
Katiraei S., de M. R. Vries, Costain A. H., Thiem K., Hoving L. R., van J. A. Diepen, Smits H. H., Bouter K. E., Rensen P. C. N., Quax P. H. A., Nieuwdorp M., Netea M. G., de W. M. Vos, Cani P. D., Belzer C., van K. W. Dijk, Berbée J. F. P., van V. Harmelen, Akkermansia muciniphila Exerts Lipid‐Lowering and Immunomodulatory Effects without Affecting Neointima Formation in Hyperlipidemic APOE*3‐Leiden.CETP Mice. Mol. Nutr. Food Res. 2020, 64, 1900732 10.1002/mnfr.201900732
The copyright line for this article was changed on 21 May 2020 after original online publication.
References
- 1. Amar J., Chabo C., Waget A., Klopp P., Vachoux C., Bermúdez‐Humarán L. G., Smirnova N., Bergé M., Sulpice T., Lahtinen S., Ouwehand A., Langella P., Rautonen N., Sansonetti P. J., EMBO Mol. Med. 2011, 3, 559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cho I., Blaser M. J., Nat. Rev. Genet. 2012, 13, 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Scher J. U., Abramson S. B., Nat. Rev. Rheumatol. 2011, 135, 612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Karlsson C. L. J., Onnerfält J., Xu J., Molin G., Ahrné S., Thorngren‐Jerneck K., Obesity 2012, 20, 2257. [DOI] [PubMed] [Google Scholar]
- 5. Gevers D., Kugathasan S., Denson L. A., Vázquez‐Baeza Y., Van Treuren W., Ren B., Schwager E., Knights D., Song S. J., Yassour M., Morgan X. C., Kostic A. D., Luo C., González A., McDonald D., Haberman Y., Walters T., Baker S., Rosh J., Stephens M., Heyman M., Markowitz J., Baldassano R., Griffiths A., Sylvester F., Mack D., Kim S., Crandall W., Hyams J., Huttenhower C. et al., Cell Host Microbe 2014, 15, 382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Png C. W., Lindén S. K., Gilshenan K. S., Zoetendal E. G., McSweeney C. S., Sly L. I., McGuckin M. A., Florin T. H. J., Am. J. Gastroenterol. 2010, 105, 2420. [DOI] [PubMed] [Google Scholar]
- 7. Wang L., Christophersen C. T., Sorich M. J., Gerber J. P., Angley M. T., Conlon M. A., Appl. Environ. Microbiol. 2011, 77, 6718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Candela M., Rampelli S., Turroni S., Severgnini M., Consolandi C., De Bellis G., Masetti R., Ricci G., Pession A., Brigidi P., BMC Microbiol. 2012, 12, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dao M. C., Everard A., Aron‐Wisnewsky J., Sokolovska N., Prifti E., Verger E. O., Kayser B. D., Levenez F., Chilloux J., Hoyles L., Dumas M.‐E., Rizkalla S. W., Doré J., Cani P. D., Clément K., Gut 2015, 65, 426. [DOI] [PubMed] [Google Scholar]
- 10. Derrien M., Vaughan E. E., Plugge C. M., de Vos W. M., Int. J. Syst. Evol. Microbiol. 2004, 54, 1469. [DOI] [PubMed] [Google Scholar]
- 11. Belzer C., de Vos W. M., The ISME J. 2012, 6, 1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Everard A., Belzer C., Geurts L., Ouwerkerk J. P., Druart C., Bindels L. B., Guiot Y., Proc. Natl. Acad. Sci. U. S. A. 2013, 110, 9066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Plovier H., Everard A., Druart C., Depommier C., Van Hul M., Geurts L., Chilloux J., Ottman N., Duparc T., Lichtenstein L., Myridakis A., Delzenne N. M., Klievink J., Bhattacharjee A., van der Ark K. C. H., Aalvink S., Martinez L. O., Dumas M.‐E., Maiter D., Loumaye A., Hermans M. P., Thissen J. ‐P., Belzer C., de Vos W. M., Cani P. D., Nat. Med. 2017, 23, 107. [DOI] [PubMed] [Google Scholar]
- 14. Grander C., Adolph T. E., Wieser V., Lowe P., Wrzosek L., Gyongyosi B., Ward D. V, Grabherr F., Gerner R. R., Pfister A., Enrich B., Ciocan D., Macheiner S., Mayr L., Drach M., Moser P., Moschen A. R., Perlemuter G., Szabo G., Cassard A. M., Tilg H., Gut 2017, gutjnl‐2016‐313432. [DOI] [PubMed] [Google Scholar]
- 15. Wu W., Lv L., Shi D., Ye J., Fang D., Guo F., Li Y., He X., Li L., Front. Microbiol. 2017, 8, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shin N.‐R., Lee J.‐C., Lee H.‐Y., Kim M.‐S., Whon T. W., Lee M.‐S., Bae J.‐W., Gut 2014, 63, 727. [DOI] [PubMed] [Google Scholar]
- 17. Lee H., Ko G., Appl. Environ. Microbiol. 2014, 80, 5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Van Beek A. A., Hoogerland J. A., Belzer C., De Vos P., De Vos W. M., Savelkoul H. F. J., Leenen P. J. M., Benef. Microbes 2016, 7, 275. [DOI] [PubMed] [Google Scholar]
- 19. Hansson G. K., Libby P., Tabas I., J. Intern. Med. 2015, 278, 483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mach F., Schönbeck U., Sukhova G. K., Atkinson E., Libby P., Nature 1998, 394, 200. [DOI] [PubMed] [Google Scholar]
- 21. Lutgens E., Lievens D., Beckers L., Wijnands E., Soehnlein O., Zernecke A., Seijkens T., Engel D., Cleutjens J., Keller A. M., Naik S. H., Boon L., Oufella H. A., Mallat Z., Ahonen C. L., Noelle R. J., de Winther M. P., Daemen M. J., Biessen E. A., Weber C., J. Exp. Med. 2010, 207, 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nicoletti A., Kaveri S., Caligiuri G., Bariéty J., Hansson G. K., J. Clin. Invest. 1998, 102, 910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ridker P. M., Everett B. M., Thuren T., MacFadyen J. G., Chang W. H., Ballantyne C., Fonseca F., Nicolau J., Koenig W., Anker S. D., Kastelein J. J. P., Cornel J. H., Pais P., Pella D., Genest J., Cifkova R., Lorenzatti A., Forster T., Kobalava Z., Vida‐Simiti L., Flather M., Shimokawa H., Ogawa H., Dellborg M., Rossi P. R. F., Troquay R. P. T., Libby P., Glynn R. J., CANTOS Trial Group , N. Engl. J. Med. 2017, 377, 1119.28845751 [Google Scholar]
- 24. Li J., Lin S., Vanhoutte P. M., Woo C. W., Xu A., Circulation 2016, 133, 2434. [DOI] [PubMed] [Google Scholar]
- 25. Lardenoye J. H., Delsing D. J., de Vries M. R., Deckers M. M., Princen H. M., Havekes L. M., van Hinsbergh V. W., van Bockel J. H., Quax P. H., Circ. Res. 2000, 87, 248. [DOI] [PubMed] [Google Scholar]
- 26. van den Hoek A. M., van der Hoorn J. W. A., Maas A. C., van den Hoogen R. M., van Nieuwkoop A., Droog S., Offerman E. H., Pieterman E. J., Havekes L. M., Princen H. M. G., Diabetes, Obesity Metabol. 2014, 16, 537. [DOI] [PubMed] [Google Scholar]
- 27. van den Maagdenberg A. M., Hofker M. H., Krimpenfort P. J., de Bruijn I., van Vlijmen B., van der Boom H., Havekes L. M., Frants R. R., J. Biol. Chem. 1993, 268, 10540. [PubMed] [Google Scholar]
- 28. Pires N. M. M., Schepers A., Van Der Hoeven B. L., De Vries M. R., Boesten L. S. M., Jukema J. W., Quax P. H. A., Cardiovasc. Res. 2005, 68, 415. [DOI] [PubMed] [Google Scholar]
- 29. Westerterp M., van der Hoogt C. C., de Haan W., Offerman E. H., Dallinga‐Thie G. M., Jukema J. W., Havekes L. M., Rensen P. C. N., Arterioscler., Thromb., Vasc. Biol. 2006, 26, 2552. [DOI] [PubMed] [Google Scholar]
- 30. Round J. L., Mazmanian S. K., Nat. Rev. Immunol. 2009, 9, 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Scott C. L., Aumeunier A. M., Mowat A. M., Trends Immunol. 2011, 32, 412. [DOI] [PubMed] [Google Scholar]
- 32. Hooper L. V, Macpherson A. J., Nat. Rev. Immunol. 2010, 10, 159. [DOI] [PubMed] [Google Scholar]
- 33. Chevalier C., Stojanović O., Colin D. J., Suarez‐Zamorano N., Tarallo V., Veyrat‐Durebex C., Rigo D., Fabbiano S., Stevanović A., Hagemann S., Montet X., Seimbille Y., Zamboni N., Hapfelmeier S., Trajkovski M., Cell 2015, 163, 1360. [DOI] [PubMed] [Google Scholar]
- 34. Liu J., Li Y., Yang P., Wan J., Chang Q., Wang T. Y., Lu W., Zhang Y., Wang Q., Yu L. L., J. Agric. Food Chem. 2017, 65, 9237. [DOI] [PubMed] [Google Scholar]
- 35. Tea C. P. P., Gao X., Xie Q., Kong P., Liu L., Sun S., Xiong B., Huang B., Yan L., Sheng J., Infect. Immun. 2018, 86, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. de Haan W., van der Hoogt C. C., Westerterp M., Hoekstra M., Dallinga‐Thie G. M., Princen H. M. G., Romijn J. A., Jukema J. W., Havekes L. M., Rensen P. C. N., Atherosclerosis 2008, 197, 57. [DOI] [PubMed] [Google Scholar]
- 37. Ottman N., Reunanen J., Meijerink M., Pietilä T. E., Kainulainen V., Klievink J., Huuskonen L., Aalvink S., Skurnik M., Boeren S., Satokari R., Mercenier A., Palva A., Smidt H., de Vos W. M., Belzer C., PLoS One 2017, 12, e0173004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reunanen J., Kainulainen V., Huuskonen L., Ottman N., Belzer C., Huhtinen H., de Vos W. M., Satokari R., Appl. Environ. Microbiol. 2015, 81, 3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hänninen A., Toivonen R., Pöysti S., Belzer C., Plovier H., Ouwerkerk J. P., Emani R., Cani P. D., De Vos W. M., Gut 2018, 67, 1445. [DOI] [PubMed] [Google Scholar]
- 40. ten Broeke T., Wubbolts R., Stoorvogel W., Cold Spring Harbor Perspect. Biol. 2013, 5, a016873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lim T. S., Goh J. K. H., Mortellaro A., Lim C. T., Hämmerling G. J., Ricciardi‐Castagnoli P., PLoS One 2012, 7, e45185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Eefting D., Schepers A., De Vries M. R., Pires N. M. M., Grimbergen J. M., Lagerweij T., Nagelkerken L. M., Monraats P. S., Jukema J. W., van Bockel J. H., Quax P. H. A., Atherosclerosis 2007, 193, 335. [DOI] [PubMed] [Google Scholar]
- 43. Ewing M. M., Karper J. C., Abdul S., de Jong R. C. M., Peters H. A. B., de Vries M. R., Redeker A., Kuiper J., Toes R. E. M., Arens R., Jukema J. W., Quax P. H. A., Int. J. Cardiol. 2013, 168, 1965. [DOI] [PubMed] [Google Scholar]
- 44. Derrien M., Van Baarlen P., Hooiveld G., Norin E., Müller M., de Vos W. M., Front. Microbiol. 2011, 2, 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


