Abstract
Imiquimod 3.75% cream is licensed for the treatment of actinic keratosis (AK). Two case reports on the treatment of facial UV‐exposed skin shall open the discussion if subclinical AKs can be detected by the use of imiquimod cream in UV‐exposed areas even if no lesions can be found clinically. A 87‐year old female showing small scaly AK lesions on her right cheek was treated with imiquimod 3.75% cream. A 59‐year old female without obvious clinical signs of UV‐damage on the face experimentally applied imiquimod 3.75% cream twice daily on the entire face for 2 weeks. In the 87‐year old, inflammatory reaction developed from day 3 onward and showed field cancerization, the lesions healed without scarring. In the 59‐year old at the end of the treatment phase, distinct signs of inflammation appeared, then taking 2 weeks for healing without sequalae. These results open the discussion if the use of imiquimod 3.75% cream could be recommended preventively in UV‐exposed skin areas to obviate a later development of AKs/squamous cell carcinoma/nonmelanoma skin cancer.
Keywords: actinic keratosis, cancer prevention, imiquimod, subclinical
1. INTRODUCTION
Depending on age, lifestyle, and skin type of a person, squamous cell carcinoma (SCC)—a nonmelanoma skin cancer (NMSC)—may develop in sun‐exposed skin, predominantly in nasal and frontotemporal areas, and bald male scalps are affected. 1 Actinic keratosis (AK) represents an early or in situ SCC. 1 , 2 , 3 Pathogenesis of AK can be derived from potentially carcinogenic UV light interacting with keratinocyte DNA where DNA repair mechanisms fail. AK evolves slowly in the basal layer until they become thicker and clinically evident as coarse erythematous patches in early stages which may become hyperkeratotic later on (Figure 1). 1 , 4 , 5 , 6 Topical imiquimod has been shown to be useful in clearing AK lesions. 7 , 8 , 9 , 10 , 11 Imiquimod as a toll‐like receptor 7 (TLR‐7) agonist induces cytokines, starting an inflammatory skin reaction directed primarily against malignant or virus‐infected cells, but has virtually no effect on normal skin.
FIGURE 1.
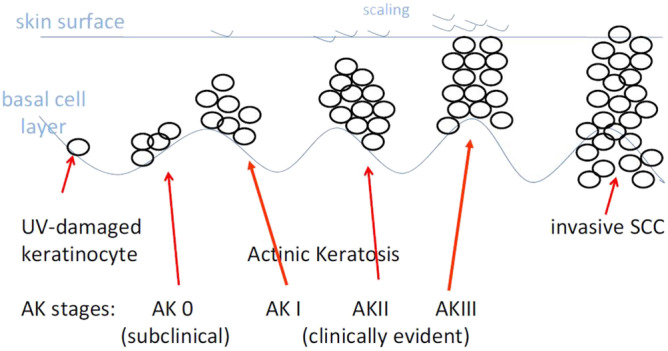
Progression of AK to invasive SCC (NMSC). 1 AK, actinic keratosis; NMSC, nonmelanoma skin cancer; SCC, squamous cell carcinoma
Imiquimod 5% cream is licensed in the United States (FDA) and Europe (EMA) for the treatment of external genital warts, superficial basal cell carcinoma, and AK, and is being experimentally used in various other dermato‐oncological conditions. 12 , 13 , 14 A lesser concentration of imiquimod 3.75% cream is licensed for the treatment of AK on face and scalp. 15 Imiquimod binds to TLR‐7 on monocytes and macrophages indirectly activating intrinsic and acquired immunity due to induction of cytokines with antiviral and antineoplastic abilities. Pro‐inflammatory cytokines subsequently start an inflammatory reaction inducing apoptosis of skin cancer cells. In addition, imiquimod has a direct cytochrome‐mediated proapoptotic effect. 16 , 17
In photodamaged skin featuring AK, these actions of imiquimod are not restricted to visible AK lesions, but often include their vicinity, suggesting that the neoplastic processes are in fact more frequent at cellular level and not confined to clinically evident lesions, supporting the concept of “field cancerization.” 10 Thus, subclinical AKs do exist in an early, macroscopically invisible state and may be rendered visible by imiquimod. At this stage, AKs are being treated before they can be diagnosed by usual clinical means, and well before potential progression to invasive SCC/NMSC. 18
Our objective is demonstrating the effectiveness of topical imiquimod in a case with clinically visible AK on one hand and on the other hand showing the ability of imiquimod in detecting invisible subclinical AK in a case with no obvious signs of UV‐damage. Such early detection and “preventive” treatment might be easier, more effective, and less burdensome than later treatment of clinically evident cancer. 1 , 18
2. MATERIAL AND METHODS
The patients in this article have given written informed consent to publication of their case details.
For demonstration of the interaction of imoquimod and AK, a treatment course in a 87‐year‐old female with AK on her right cheek is shown retrospectively. The treatment was performed according to the recommended regimen of twice daily application for 14 days. For personal documentation, she took selfies with her smartphone (Figure 2).
FIGURE 2.
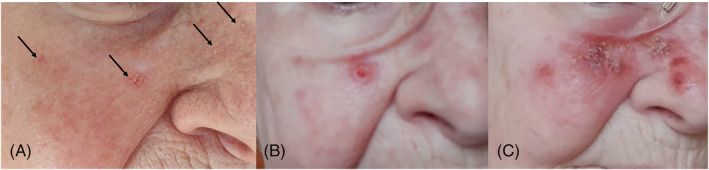
A, Eighty‐seven‐year old female showing small erythematous scaly lesions on her right cheek (arrows). B, Inflammatory reaction starting from day 3 of treatment with topical imiquimod 3.75% cream. C, Day 14, end of treatment phase, showing field cancerization
For demonstration of imiquimosd's ability in detecting clinically invisible, so called “subclinical” AK, a 59‐year‐old female without obvious clinical signs of UV‐damage (Fitzpatrick skin type: 2‐3, photoaging: Glogau score 1) 19 (Figure 3A) was treated according to the same regimen.
FIGURE 3.
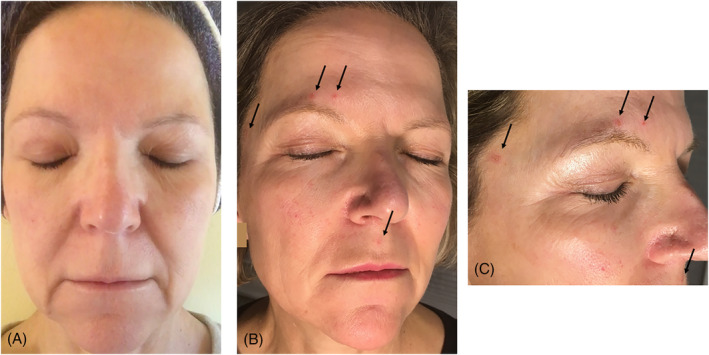
A, UV‐exposed facial skin of a 59‐year old without obvious signs of photodamage. B, Day 13 of imiqimod treatment showing erythematous inflammatory lesions (arrows). C, Close‐up: inflammatory lesions on the upper lip, temporally right and above the right eyebrow (arrows)
3. RESULTS
In the 87‐year‐old, small, coarse erythematous AK on the right cheek could be seen before treatment start (Figure 2A, arrows). On day 3, inflammatory reaction occurred in the lesions (Figure 2B). Ongoing inflammation spread almost over the entire right cheek representing field cancerization on day 14 (Figure 2C). Concurrently she claimed mild to moderate burning sensations and fatigue from day 6 of the treatment until 2 weeks after the end of the treatment phase.
The 59‐year‐old applied imiquimod 3.75% cream twice daily on the entire face for 2 weeks for experimental reasons with the purpose of demonstrating that in UV‐exposed skin even without clinical appearance of lesions UV‐induced photodamage in a subclinical state of AK may be present (Figure 3). There was no skin reaction visible until day 12. On day 13 to 15, small erythematous lesions with a diameter of 5 to 7 mm appeared on the upper lip, temporally right, and above the right eyebrow (Figure 3B,C, arrows). Consequently, there was a faint burning sensation.
Within 2 weeks in the younger patient and 4 weeks in the older, all lesions healed without sequalae.
4. DISCUSSION
UV‐exposed skin may present mottled pigmentation, thinning, dryness, wrinkling, and also AK as signs of photodamage and can be graded according to the Glogau score. Chronic UV exposure leads to cumulative DNA alterations overwhelming physiological DNA repair mechanisms. Consequently, carcinogenic transformation in photodamaged skin occurs sooner or later, its extent depends on the skin type and on the amount of accumulated UV exposure.
Our cases demonstrate on one hand that imiquimod is a potent immunomodulator and is effectively used in the treatment of AK as shown in patient 1. (Figure 3A‐C) On the other hand, the experimental use of imiquimod 3.75% cream in UV‐exposed facial skin led to faint but apparent inflammation as sign of imiquimod binding to TLR‐7 on monocytes and macrophages indirectly activating intrinsic and acquired immunity due to induction of cytokines with antineoplastic abilities. It caused a skin reaction directed against early, clinically invisible, malignant cells (Figure 3A‐C).
The two examples show that earliest treatment detecting very early stages of AK produces only minor inflammatory reaction being tolerated by the patient easily without pain or flu‐like side effects. Even by aesthetic measures, the impact is tolerable. Whereas in later stages of AK, the inflammatory reaction caused by imiquimod provokes a rather incriminating and painful impact on the quality of life of the patient for 3 weeks.
In prior studies, the term “subclinical actinic keratoses” was coined for these clinically, yet nonevident, precursors of AK. 1 In this observation, we found that topical imiquimod applied to apparently clear skin is able to identify submacroscopic actinic damage. In 2015, we posed the question: “At what stage AK progress on to NMSC” (Figure 2). 20 At this stage, the question arises: “When do AK begin to exist.” Obviously they are already there even before they can be clinically diagnosed. Now we have two more challenges, “What may be the most advantageous moment for the treatment of subclinical AK?,” and, “Would it be beneficial to apply imiquimod as a ‘preventive’ avoiding clinically evident AK/SCC/NMSC‐formation and saving the victims from later ailments.”
Thus, opening the discussion whether the use of imiquimod 3.75% cream could be recommended preventively in UV‐exposed skin areas to obviate the presumptive development of AKs.
CONFLICT OF INTEREST
The author declares no potential conflict of interest.
Kopera D. Earliest stage treatment of actinic keratosis with imiquimod 3.75% cream: Two case reports—Perspective for non melanoma skin cancer prevention. Dermatologic Therapy. 2020;33:e13517 10.1111/dth.13517
REFERENCES
- 1. Kopera D, Kerl H. Visualization and treatment of subclinical actinic keratoses with topical imiquimod 5% cream: an observational study. Biomed Res Int. 2014;2014:135916 10.1155/2014/135916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Salasche SJ. Epidemiology of actinic keratoses and squamous cell carcinoma. J Am Acad Dermatol. 2000;42:4‐7. [DOI] [PubMed] [Google Scholar]
- 3. Ackerman AB, Mones JM. Solar (actinic) keratosis is squamous cell carcinoma. Br J Dermatol. 2006;155:9‐22. [DOI] [PubMed] [Google Scholar]
- 4. Cockerell CJ. Pathology and pathobiology of the actinic (solar) keratosis. Br J Dermatol. 2003;149:34‐36. [DOI] [PubMed] [Google Scholar]
- 5. Einspahr JG, Xu MJ, Warneke J, et al. Reproducibility and keratoses. Cancer Epidemiol Biomarkers Prev. 2006;15:1841‐1848. [DOI] [PubMed] [Google Scholar]
- 6. Quatresooz P, Pierard‐Franchimont C, Paquet P, Hubert P, Delvenne P, Piérard GE. Crossroads between actinic keratosis and squamous cell carcinoma, and novel pharmacological issues. Eur J Dermatol. 2008;18:6‐10. [DOI] [PubMed] [Google Scholar]
- 7. Stockfleth E, Meyer T, Benninghoff B, Christophers E. Successful treatment of actinic keratosis with imiquimod cream 5%: a report of six cases. Br J Dermatol. 2001;144:1050‐1053. [DOI] [PubMed] [Google Scholar]
- 8. Hengge UR, Benninghoff B, Ruzicka T, Goos M. Topical immunomodulators—progress towards treating inflammation, infection, and cancer. Lancet Infect Dis. 2001;1:189‐198. [DOI] [PubMed] [Google Scholar]
- 9. Hadley G, Derry S, Moore RA. Imiquimod for actinic keratosis: systematic review and meta‐analysis. J Invest Dermatol. 2006;126:1251‐1255. [DOI] [PubMed] [Google Scholar]
- 10. Stockfleth E, Sterry W, Carey‐Yard M, Bichel J. Multicentre, open‐label study using imiquimod 5% cream in one or two 4‐week courses of treatment for multiple actinic keratoses on the head. Br J Dermatol. 2007;157(suppl 2):41‐46. [DOI] [PubMed] [Google Scholar]
- 11. Berman B, Amini S, Valins W, Block S. Pharmacotherapy of actinic keratosis. Expert Opin Pharmacother. 2009;10:3015‐3031. [DOI] [PubMed] [Google Scholar]
- 12.Aldara® Cream, 5% (Imiquimod). Graceway Pharmaceuticals. U.S. prescribing information. Status October 2010.
- 13.Aldara® 5% Cream. Summary of Product Characteristics (UK Version). MEDA AB. http://emc.medicines.org.uk. Accessed April 2010.
- 14. Schmitt AR, Bordeaux JS. Solar keratoses: photodynamic therapy, cryotherapy, 5‐fluorouracil, imiquimod, diclofenac, or what? Facts and controversies. Clin Dermatol. 2013;31:712‐717. [DOI] [PubMed] [Google Scholar]
- 15. Del Rosso J, Swanson N, Berman B, Martin GM, Lin T, Rosen T. Imiquimod 2.5% and 3.75% cream for the treatment of photodamage: a meta‐analysis of efficacy and tolerability in 969 randomized patients. J Clin Aesthet Dermatol. 2018;11:28‐31. [PMC free article] [PubMed] [Google Scholar]
- 16. Schön MP, Schön M. Imiquimod: mode of action. Br J Dermatol. 2007;157(suppl 2):8‐13. [DOI] [PubMed] [Google Scholar]
- 17. Schön MP, Schön M. TLR7 and TLR8 as targets in cancer therapy. Oncogene. 2008;27:190‐199. [DOI] [PubMed] [Google Scholar]
- 18. Kopera D, Kerl H. Detection and treatment of preclinical actinic keratoses (PAK) with topical imiquimod 5% crème. J Dtsch Dermatol Ges. 2010;8:693‐694. [DOI] [PubMed] [Google Scholar]
- 19. Glogau RG. Physiologic and structural changes associated with aging skin. Dermatol Clin. 1997;15:555‐559. [DOI] [PubMed] [Google Scholar]
- 20. Kopera D, Goswami N, Kerl H. AK progressing to NMSC: at what stage? J Eur Acad Dermatol Venereol. 2016;30:e172‐e173. 10.1111/jdv.13480. [DOI] [PubMed] [Google Scholar]


