Abstract
Problem
Preterm delivery is the leading cause of neonatal mortality and contributes to delayed physical and cognitive development in children. At present, there is no efficient therapy to prevent preterm labor. A large body of evidence suggests that infections might play a significant and potentially preventable cause of premature birth. This work assessed the effects of erythropoietin (EPO) in a murine model of inflammation‐associated preterm delivery, which mimics central features of preterm infections in humans.
Method of study
BALB/c mice were injected i.p. with 20 000 IU/kg EPO or normal saline twice on gestational day (GD) 15, with a 3 hours time interval between injections. An hour after the first EPO or normal saline injection, all mice received two injections of 50 μg/kg LPS, also given 3 hours apart.
Results
EPO significantly prevented preterm labor and increased offspring survival in an LPS induced preterm delivery model. EPO prevented LPS‐induced leukocyte infiltration into the placenta. Moreover, EPO inhibited the expression of pro‐inflammatory cytokines, interleukin‐1β (IL‐1β), interleukin‐6 (IL‐6), and tumour necrosis factor‐α (TNF‐α) in maternal serum and amniotic fluid. EPO also prevented LPS‐induced increase in placental prostaglandin (PG)E2 and uterine inducible nitric oxide synthase (iNOS) production, while decreasing nuclear factor kappa‐B (NF‐κβ) activity in the myometrium. EPO also increased the gene expression of placental programmed cell death ligand 1 (PD‐L1) in LPS‐treated mice.
Conclusions
Our results suggest that EPO could be a potential novel therapeutic strategy to tackle infection‐related preterm labor.
Keywords: erythropoietin, inflammation, offspring survival, PDL1, preterm birth, prostaglandins
1. INTRODUCTION
Preterm delivery (birth before 37 completed weeks of gestation) occurs in 5%‐15% of all pregnancies and remains a significant public health concern worldwide. 1 Premature birth is associated with 75% of infant mortality and 50% of long‐term neurological handicaps. 2 , 3 Despite the advances made in obstetrics and neonatology, the rate of premature delivery has not decreased over the past 20‐30 years. At present, there is no efficient therapy to prevent preterm labor and to increase offspring survival. Although the etiology is multifactorial, intrauterine infections account for 25%‐40% of all premature deliveries. 2 Lipopolysaccharide (LPS) stimulates nuclear factor kappa B (NF‐κβ) 4 , 5 and cytokine responses in the chorioamnion membrane and placenta, leading to an increase in gene expression and protein production levels of proinflammatory cytokines, including tumour necrosis factor‐α (TNF‐α), interleukin‐1β (IL‐1β), interleukin‐6 (IL‐6), prostaglandins (PGs), and nitric oxide (NO), 6 and these factors play a role in preterm labor. 7 , 8 , 9 Moreover, it has been reported previously that a successful pregnancy is closely related to the maintenance of tolerance to the fetus at the feto‐maternal interface (FMI) and a dysregulation in the tolerance to the fetus is associated with adverse gestational outcomes, resulting in preterm labor and fetal loss. 10 Studies have shown that the PD‐1/PD‐L1 (programmed death‐1/programmed death‐ligand 1) pathway is particularly important for the maintenance of tolerance to the fetus. 10 The blockade of the pathway results in a pro‐inflammatory environment and preterm labor in allogeneic mouse models of pregnancy. 10 , 11
Erythropoietin (EPO) is a pleiotropic hormone that regulates the production of red blood cells by binding to the homodimeric EPO receptor and has been widely used to treat anemia. 11 , 12 It has been shown to produce protective effects on kidneys via modulation of the proinflammatory NF‐κB pathway. 5 EPO is also known to reduce proinflammatory cytokines and decrease the levels of serum inflammatory parameters in human brains. 13 , 14 EPO also decreases iNOS/NO and COX‐2/PG expression in the spinal cord ventral horn. 15 , 16 Thus, EPO has been used to treat illnesses such as hepatic ischemia/reperfusion (I/R), preterm brain injury, hypoxic‐ischemic encephalopathy (HIE), and burn‐induced neuromuscular dysfunction and other related illnesses that have inflammatory etiologies. 15 , 17
It was previously detected that, apart from the kidney in adults, EPO is expressed in the uterus 18 , 19 , 20 and placenta throughout the gestation period. 21 , 22 EPO has a protective effect in the placenta by downregulating acute inflammation induced by exposure to LPS. 21 , 23 It also regulates the survival of the first trimester trophoblast cells and decidual stromal cells (DSCs). Deficiency in EPO expression at the FMI could lead to unwanted pregnancy outcomes. 22 , 24 These results suggest that EPO could have an important role in the maintenance of gestation.
EPO binds to two cell surface Epo receptors (EPORs), which form disulfide‐linked homodimers, and selectively activates JAK2 kinase, which in turn phosphorylates Jak2 and EPOR. This process activates a variety of signaling cascades leading to STAT3 and p‐STAT3 activation, which are transcription factors of the anti‐apoptotic gene family consisting of Bcl‐2 and Bcl‐xL proteins. The JAK2/STAT3 signaling pathway is an upstream regulator of PD‐L1. 23 , 24 PD‐L1 has been shown to play an important role in the maintenance of gestation and its blockade results in preterm labor, 10 but whether the effect of EPO on maintenance of gestation is related to PDL1 is unknown. With this background, the aim of this work was to study the effect of EPO on preterm labor and offspring survival in a murine model of LPS induced preterm delivery.
2. MATERIALS AND METHODS
2.1. Animals
Female BALB/c mice from the Institute of Experimental Animals at Guangzhou Medical University were housed in an approved animal room with ad libitum access to food and water. Controlled conditions of humidity (50%‐60%) and temperature (21 ± 2°C) were maintained and the animals were exposed to a 12‐h dark/12‐h light cycle. Virgin female mice were mated with fertile males of the same strain. Pregnancy confirmation was determined by visual inspection of the vaginal plug, which was defined as day 0 of pregnancy. The average gestational length for BALB/c mice is typically 19‐20 days. Animals were killed in a CO2 chamber and all efforts were made to minimize their suffering. All the experimental protocols were approved by Guangzhou Medical University Animal Care and Use Committee and were performed following the guidelines of the National Institutes of Health on the care and ethical treatment of animals.
2.2. Treatments
On gestational day (GD) 15 (day 0 = vaginal plug observed), pregnant mice were assigned to one of four groups: (i) CRL mice (n = 10) were intraperitoneally injected with 0.2ml normal saline twice on GD15, with a 3 hours time interval between injections. One hour after the first normal saline injection, mice then received two injections of normal saline, also given 3 hours apart. (ii) LPS mice (n = 14) were intraperitoneally injected with 0.2 mL normal saline twice on GD15, with a 3 hours time interval between injections. One hour after the first normal saline injection, mice then received two injections of 50 μg/kg LPS (Escherichia coli 0111:B4; Biochemist), also given 3 hours apart. (iii) EPO/LPS mice (n = 11) were intraperitoneally injected with 20 000 IU/kg recombinant erythropoietin (EPO, NeoRecormon®; Boehringer‐La Roche) twice on GD15, with a 3 hours time interval between injections. One hour after the first rhEPO injection, mice then received two injections of 50 μg/kg LPS, also given 3 hours apart. (iv) EPO mice (n = 10) were intraperitoneally injected with 0.2 mL normal saline twice on GD15, with a 3 hours time interval between injections. One hour after the first normal saline injection, mice then received two injections of 20 000 IU/kg EPO, also given 3 hours apart. Animals were observed every hour after the first LPS or vehicle solution injection for any signs of morbidity (decreased movement and/or diarrhea), vaginal bleeding, and preterm delivery. The beginning of preterm delivery was defined by the delivery of the first pup. Viability of the pup was assessed by tactile stimulation.
A second experiment was performed in which the pregnant mice were treated as the first experiment. The CRL, LPS, EPO/LPS, and EPO groups of pregnant mice were deeply anesthetized using sodium pentobarbital (50 mg/kg, i.p.) on GD15 at 12 hours post‐injection of the first vehicle or LPS, (ie, before the onset of preterm labor), and the uterus, placenta, amniotic fluid, and blood samples were collected. To investigate the effects of FA on high‐dose.
2.3. Enzyme‐linked immunosorbent assay
The concentrations of IL‐1β, IL‐6, and TNF‐α in mouse maternal serum and amniotic fluid were determined by enzyme‐linked immunosorbent assay kit (eBioscience) according to the manufacturer's instructions.
2.4. Western blots
For Western blot analysis, five animals were randomly selected from each group. Mice were anesthetized using sodium pentobarbital (50 mg/kg, i.p.) on GD15 at 12 hours after the first vehicle or LPS. Their uteruses were removed and frozen at −80°C until use. The samples were homogenized on ice in 15 mole/L Eris buffer containing a cocktail of proteinase inhibitors and phosphatase inhibitors. The protein samples were separated via gel electroplate (SDS‐PAGE) and transferred onto a polyvinylidene fluoride (PVDF) membrane. The membranes were placed in the blocking buffer for 1 hour at room temperature (RT) and incubated with a primary rabbit antiserum against iNOS (1:1000; CST) or phosphorylated‐NF‐κB‐65 (1:1000; CST) overnight at 4°C. Then, the membranes were incubated in horseradish peroxidase‐conjugated IgG. An enhanced luminescence (ECL) solution (Pierce) was used to detect the immune complexes. Each band was quantified using a computer‐assisted imaging analysis system (NIH ImageJ).
2.5. Immunohistochemistry
Five animals were randomly selected from each group. Mice were deeply anesthetized using sodium pentobarbital (50 mg/kg, i.p.) on GD15 at 12 hours after the first vehicle or LPS treatment. The placentae were removed and fixed in 4% paraformaldehyde solution and embedded in paraffin. Paraffin sections (5 μm) were cut, dehydrated, and microwaved in citrate‐phosphate buffer (pH 6.0) to retrieve antigens. Then, the sections were treated with 3% hydrogen peroxide (H2O2), followed by 10% normal goat serum blocking at RT for 30 minutes. This was followed by incubation with PD‐L1 (1:200; Abcam) or PGE2 antibody (1:200; Abcam) diluted in PBS containing 1% BSA for 24 hours at 4°C. The sections were incubated with mouse anti‐rabbit IgG for 30 minutes at RT after washing with PBS (3 minutes × 3 times). The signals were detected by using a biotin‐streptomycin‐hydroxide system using diamorphine (Charismatic) as the chromosome. Negative controls were performed with primary antibody replaced by PBS.
2.6. Hematoxylin and Eosin (H&E staining)
Five animals were randomly selected from each group. Mice were deeply anesthetized using sodium pentobarbital (50 mg/kg, i.p.) on GD15 at 12 hours after the first vehicle or LPS injection. The placentae were removed and immediately fixed in 10% formalin saline for 24 hours. The placentae were transferred to 70% ethanol and then processed to paraffin‐embedded blocks to generate 5‐µm‐thick sections for hematoxylin and eosin (H&E) staining. The slides were observed under a light microscope for cellular changes of inflammation and photographs of different magnifications were taken digitally with FSX100 (Olympus).
3. DATA PRESENTATION AND STATISTICAL ANALYSIS
Kaplan‐Meier survival curves for preterm labor experiments were compared between different treatment groups using the log‐rank test. Latency intervals (mean ± SD) were analyzed between different experimental groups using a one‐way analysis of variance (ANOVA). Comparisons were made with post hoc Tukey's test. IL‐β, IL‐6, and TNF‐α levels in the mother's serum or amniotic fluid and phosphorylated‐NF‐κB‐65 and iNOS levels in uterine were analyzed between different experimental groups using an ANOVA test. Comparisons were made with post hoc Tukey's test. Statistical analyses were performed using GraphPad Prism 7 (GraphPad) software. The level of significance was set at P < .05 for all analyses.
4. RESULTS
4.1. Effect of EPO on LPS‐induced preterm delivery
Figure 1 illustrates the cumulative percentage of pregnant animals at each time point. In CRL and EPO groups, no pregnant mouse delivered before GD19. LPS resulted in 100% pregnant mice delivering before GD19. Preterm delivery rate dropped to 45.5% when LPS‐treated mice were pretreated with EPO. EPO significantly reduced the preterm delivery rates.
FIGURE 1.
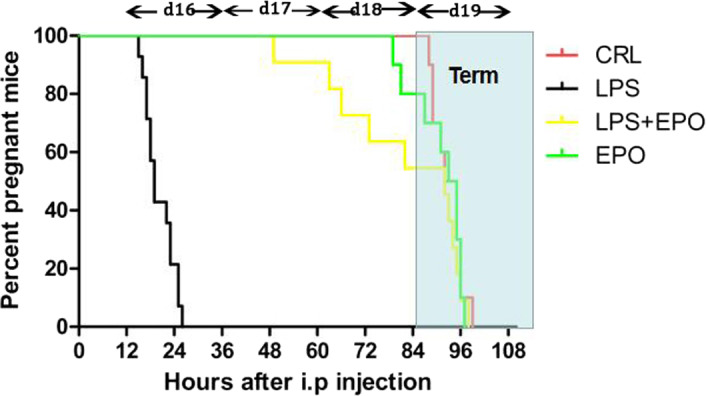
The percentage of mice without delivering after LPS injection. In LPS groups, the pregnant mice were i.p. injected with 0.2 mL normal saline twice on GD15, with a 3 hours time interval between injections. One hour after the first normal saline injection, mice then received two injections of 50 μg/kg LPS, also given 3 hours apart. In EPO/LPS groups, the pregnant mice were i.p. injected with 20 000 IU/kg rhEPO twice on GD15, with a 3 hours time interval between injections. One hour after the first rhEPO injection, mice then received two injections of 50 μg/kg LPS, also given 3 hours apart. All mice were observed for preterm delivery every hour after the first LPS or vehicle solution injection
4.2. Effect of EPO on fetal survivals after LPS injection
Table 1 illustrates EPO reduced fetal mortality. 100% of fetuses were dead in LPS‐treated mice. EPO reduced fetal mortality by 46%, significantly alleviating LPS‐induced fetal death (Table. 1).
TABLE 1.
Effects of EPO on fetal survivals after LPS injection. The percentage of mice after the first LPS or vehicle solution injection. In LPS groups, the pregnant mice were i.p. injected with 0.2 mL normal saline twice on GD15, with a 3 hours time interval between injections. One hour after the first normal saline injection, mice then received two injections of 50 μg/kg LPS, also given 3 hours apart. In EPO/LPS groups, the pregnant mice were i.p. injected with 20 000 IU/kg rhEPO twice on GD15, with a 3 hours time interval between injections. One hour after the first rhEPO injection, mice then received two injections of 50 μg/kg LPS, also given 3 hours apart. Fetal viability was assessed at 36 hours (GD17) after the first LPS or vehicle solution injection. n = 10 for CRL group or EPO group, n = 14 for LPS group and n = 11 for EPO/LPS group
| Viable pup % number/total pup number | Viable pup number/mother | |
|---|---|---|
| CLR | 100 | 8 ± 2 |
| EPO | 100 | 7 ± 2 |
| LPS | 0**** | 0 |
| EPO + LPS | 46**** | 5 ± 1 |
P < 0.0001 vs LPS;
P < 0.0001 vs CRL.
4.3. Effect of EPO on LPS‐induced upregulation of PGE2 in placenta
LPS markedly increased the levels of placental PGE2 which expressed in placental trophoblast cells (Figure 2C),when compared to the negative controls (Figure 2A). EPO itself had no effect on PGE2 production (Figure 2B). EPO pretreatment attenuated LPS‐induced upregulation of PGE2 in placentae (Figure 2D).
FIGURE 2.
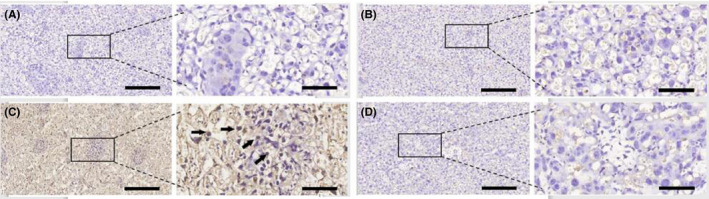
Effect of EPO on LPS‐induced upregulation of PGE2 in placenta. Placentae were collected. PGE2 levels were assessed at 12 hours after the first LPS or vehicle solution injection on GD15.The level of PGE2 was determined by immunohistochemistry. Representative photomicrographs of placental histological specimens from mice treated with normol saline + normol saline (A as control), normal saline + EPO (B), normal saline + LPS (C), and EPO + LPS (D) are shown. PGE2 was observed in placental trophoblast cells (black arrowheads). (C) showed the upregulation of PGE2 in placenta and PGE2 expressed in placental trophoblast cells. The expression of PGE2 in placenta is almost invisible in (A) (B) and (D). Original magnification, ×100 or 400. Scale bars 100 µm for original magnification, ×100. Scale bars 25 µm for original magnification, ×400
4.4. Effect of EPO on LPS‐induced leukocyte infiltration in placenta
Lipopolysaccharide caused an increased leukocyte infiltration in the placenta (Figure 3C), when compared to the negative controls (Figure 3A). EPO itself had no effect on leukocyte infiltration in the placenta (Figure 3B). EPO prevented LPS‐induced leukocyte infiltration in the placenta (Figure 3D).
FIGURE 3.
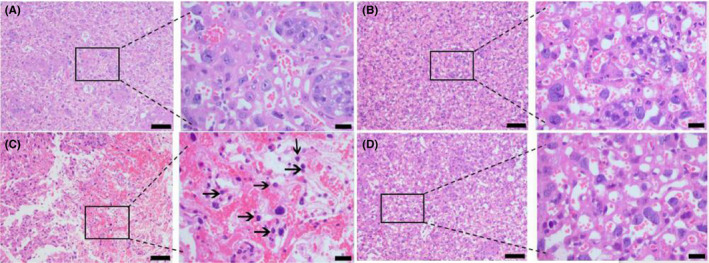
Effect of EPO on LPS‐induced leukocyte infiltration in placenta. Placentae were collected. Total density of leukocytes in placenta was assessed at 12 hours after the first LPS or vehicle solution injection on GD15. Total density of leukocytes in placenta was determined by hematoxylin and eosin. Representative photomicrographs of placental histological specimens from mice treated with normol saline + normol saline (A as control), normal saline + EPO (B), normal saline + LPS (C), and EPO + LPS (D) are shown. (C) shows prominent leukocyte infiltration in placenta (black arrows). In (A), (B) and (D), the infiltration of leukocytes is barely visible. Original magnification, ×100 or 400. Scale bars 100 µm for original magnification, ×100. Scale bars 20 µm for original magnification, ×400
4.5. Effect of EPO on LPS‐induced release of inflammatory cytokines in maternal serum
As shown in Figure 4, maternal serum IL‐1β, IL‐6, and TNF‐α levels significantly increased in LPS‐treated animals, whereas EPO significantly reduced the effect of LPS on this parameter.
FIGURE 4.
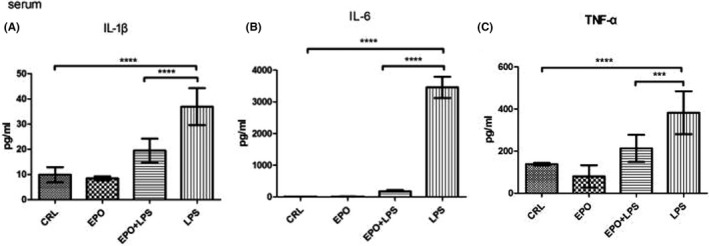
Effect of EPO on LPS‐induced release of IL‐1β, IL‐6, and TNF‐α in maternal serum. Maternal serum was collected. IL‐1β (A), IL‐6 (B), and TNF‐α (C) levels were assessed at 12 hours after the first LPS or vehicle solution injection on GD15. The levels of IL‐1β, IL‐6, and TNF‐α were determined by enzyme‐linked immunosorbent assays. Values are presented as mean ± SD. N = 12. ****P < .0001 vs the control. ***P < .001, ****P < .0001 vs LPS group
4.6. Effects of EPO on LPS‐induced release of inflammatory cytokines in amniotic fluid
As shown in Figure 5, amniotic fluid IL‐1β, IL‐6, and TNF‐α levels significantly increased in LPS‐treated animals, whereas EPO significantly reduced the effect of LPS on this parameter.
FIGURE 5.
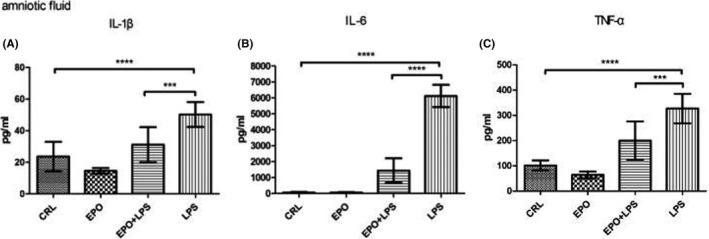
Effects of EPO on LPS‐induced release of IL‐1β, IL‐6, and TNF‐α in amniotic fluid. Maternal amniotic fluid was collected. IL‐1β (A), IL‐6 (B), and TNF‐α (C) levels were assessed at 12 hours after the first LPS or vehicle solution injection on GD15. The levels of IL‐1β, IL‐6, and TNF‐α were determined by enzyme‐linked immunosorbent assay. Values are presented as mean ± SD. N = 12. ****P < .0001 vs the control. ***P < .001, ****P < .0001 vs LPS group
4.7. Effect of EPO on LPS‐induced NF‐κB activation and iNOS protein level in uterus
Uterine phosphorylated‐NF‐κB‐65 and iNOS levels were assessed in control and LPS‐injected mice both in the presence and absence of EPO. As shown in Figure 6, the levels of phosphorylated‐NF‐κB‐65 and iNOS protein were significantly increased in uteruses of mice injected with LPS. EPO significantly prevented increases in phosphorylated‐NF‐κB‐65 and iNOS protein levels induced by LPS (Figure 6A,B, respectively).
FIGURE 6.
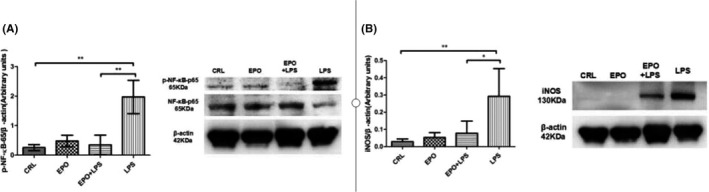
Effect of EPO and LPS on uterine phosphorylated‐NF‐κB‐65 and iNOS protein levels. Uterine strips were collected, phosphorylated‐NF‐κB‐65 and iNOS protein levels were assessed at 12 hours after the first LPS or vehicle solution injection. The levels of phosphorylated‐NF‐κB‐65 and iNOS were determined by Western blots. The levels of phosphorylated‐NF‐κB‐65 (A) and iNOS (B) were determined. Values are presented as mean ± SD. N = 5. * P < .01 vs the control. *P < .05, **P < .01 vs LPS group
4.8. Effects of EPO on the expression of placental PD‐L1 after LPS injection
LPS decreased the expression of placental PD‐L1 (Figure 7C), when compared to the mice receiving saline injection (Figure 7A). EPO, which on its own had no effect on PD‐L1 expression (Figure 7B), increased the expression of placental PD‐L1 after LPS injection (Figure 7D).
FIGURE 7.
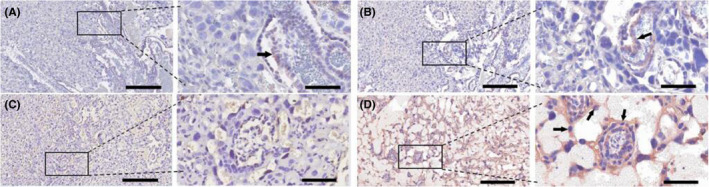
Effects of EPO on the expression of placental PD‐L1 after LPS injection. Placentae were collected. PD‐L1 protein levels were assessed at 12 hours after the first LPS or vehicle solution injection on GD15. The level of PGE2 was determined by immunohistochemistry. Representative photomicrographs of placental histological specimens from mice treated with normol saline + normol saline (A as control), normal saline + EPO alone (B), normal saline + LPS (C), and EPO + LPS (D) are shown. (D) showed the upregulation of PD‐L1 in placenta and PD‐L1 expressed in placental trophoblast cells, including giant trophoblasts (black arrowheads). The expression of PD‐L1 in placenta is almost invisible in (A), (B) and (D). Original magnification, ×100 or 400. Scale bars 100 µm for original magnification, ×100. Scale bars 25 µm for original magnification, ×400
5. DISCUSSION
The current results indicate that EPO prevented preterm labor and increased offspring survival in LPS‐induced preterm delivery. These data indicate that EPO is a potential therapeutic agent for infectious preterm labor.
The administration of LPS to pregnant BALB/c mice on GD15 induced premature parturition, cervical bleeding, and opening. The two doses of 50 μg/kg LPS with 3 hours time difference between injections, resulted in reliable preterm delivery with the least variation in time to delivery between mice and the minimal maternal mortality. In addition, it was considered that prolonged exposure to bacterial products at sub‐clinical doses is a more effective trigger for the onset of preterm labor and delivery. 25
Non‐steroid anti‐inflammatory drugs (NSAIDs) are non‐specific COX inhibitors. At present, NSAIDs are considered the most effective tocolytics. Indomethacin, a nonspecific COX inhibitor, was reported to be an efficient tocolytic drug, significantly delaying preterm delivery. 26 , 27 However, its use should be restricted in duration and limited to pregnancies below 32 weeks because of fetal ductus arteriosus closure risk and decreased urine production, responsible for oligohydramnios. 28 , 29 , 30 , 31 However, the ideal tocolytic agent should be easy to administer, inexpensive, effective in preventing preterm birth and improving neonatal outcomes, with few maternal, fetal, and neonatal side effects, and without long‐term adverse effects. 32 It has been demonstrated that EPO provides a reliable protective strategy with no significant adverse effects in a high preterm and extremely low birth weight infants. 33 , 34 We also demonstrated that EPO prevented preterm labor in LPS‐treated mice and increased the survival of offsprings.
EPO is a broad‐spectrum antioxidant 35 , 36 and a noteworthy anti‐apoptotic agent. 37 Free radical damage 38 and apoptosis 22 are common place during pregnancy and have negative effects on the mother, placenta, and fetus. Thus, the antioxidant and antiapoptotic actions of EPO could also account for the protective effect of EPO on preterm delivery and offspring survival. Although preterm prevalence has decreased in the last decade, there are still high rates of morbidity, even under a tocolytic treatment. Prenatal exposure to inflammation is believed to be an important causal factor in adverse outcomes for children born preterm. It was shown that EPO treatment ameliorates damage to fetal brain and liver, optic nerve injury, and placenta induced by exposure to LPS through anti‐inflammatory, anti‐apoptotic effects and proliferative responses. 21 , 39 It was suggested that due to its multiple organ protection functions and various protection mechanisms, EPO not only behaves as a tocolytic agent, but also might improve fetal development by protecting the fetus. Ongoing studies are in progress to confirm the preventive effects.
Augmentation of PGE2 derived from COX‐2 is a key signal in both the ripening of the cervix and stimulation of uterine contractions. 40 A previous report indicated that LPS increased the levels of PGE2. 41 It was also shown that EPO decreased COX‐2 expression after burn injury. 15 As shown herein, EPO decreased PGE2 levels after LPS injection. EPO has been reported to be an effective inhibitor of iNOS activity. 42 It was shown that EPO attenuates iNOS induction during pentylenetetrazole‐induced generalized seizures. 46 We also demonstrated that EPO prevented the increased production of iNOS protein levels following LPS injection.
Pregnancies affected by LPS are characterized by elevated levels of pro‐inflammatory cytokines, such as TNF‐α, IL‐6, and IL‐1β in the amniotic fluid, myometrium, fetal membranes, and maternal serum. 43 , 44 EPO or EPO analogs prevented the effects of LPS on TNF‐α, IL‐6,, 25 and IL‐1β 45 levels, as previously described in adipose tissues, pancreas, and brain. 25 , 42 , 45 As shown herein, EPO significantly inhibited the production of TNF‐α, IL‐6, and IL‐1β in amniotic fluid and maternal serum.
The release of proinflammatory cytokines plays a critical role in the pathogenesis of inflammation‐associated premature delivery. 46 , 47 NF‐κB is an important factor in the regulation of inflammatory proteins, 48 , 49 such as IL‐6, IL‐1β, iNOS, and TNF‐α, among others. It was reported that N,N‐dimethylacetamide (DMA) significantly suppressed the activation of NF‐κB signaling pathways from LPS‐induced mice 50 and prevents preterm birth. 51 EPO also acts as a potent anti‐inflammatory immune modulator by specifically targeting NF‐κB. 14 , 52 Besides, it was shown that EPO exerts a protective effect in renal IRI via the MAPK/NF‐κB pathway. 5 In accord with the above studies, we also demonstrated that EPO suppressed the action of uterine NF‐κB induced by LPS.
A successful pregnancy is the result of maintenance of tolerance to the fetus by the maternal immune system, as well as, protection from other harmful infections. A dysregulated maternal immunity is related to complications in pregnancy resulting in pathological preterm labor. 57 The maternal immune cells present in the uterus during the period of gestation are tightly regulated, and a very fine balance exists between immunity and tolerance at the FMI. PD1/PD‐L1 pathway plays an important role in fetal protection and the maintenance of tolerance to the fetus. 57 Blockade of this pathway, by anti‐PDL1 or anti‐PD1, abrogates the fine balance of tolerance and immunity at the FMI. 53 Meanwhile, blockade of the PD1/PDL1 pathway results in tolerance to the fetus at the FMI not being maintained resulting in fetal loss in allogeneic mouse model of pregnancy. 54 It has been shown that the JAK2/STAT3 signaling pathway is an upstream regulator of PD‐L1. 23 , 24 EPO activate JAK2/STAT3 signaling pathway, and p‐STAT3 are transcription factors of the anti‐apoptotic gene family consisting of Bcl‐2 and Bcl‐xL proteins. Our study first indicates that EPO increased the expression of placental PD‐L1 in preventing LPS‐induced preterm labor, suggesting that EPO might activate the JAK2/STAT3 signaling pathway and promote the expression of PDL1 protein to prevent preterm labor.
However, the potential therapeutic benefits of EPO therapy are outweighed by its primary erythropoietic activity with a subsequent increase in the risk for thromboembolic complications. 14 , 55 EPO analogs or derivatives, such as EPO‐derived Helix B‐surface peptide (pHBSP), carbamylated EPO (CEPO), asialo‐EPO similar peptides, 13 , 25 which have no erythropoietic activity while retaining the tissue‐protective properties of EPO, might broaden the scope of clinical application.
EPO in human and sheep plasma cannot pass through the placental barrier from mother to fetus. 56 , 57 EPO protected the placenta and fetal liver from damages induced by LPS. 21 Our study has indicated that maternally administered EPO prevented preterm labor induced by LPS and increased offspring survival rates. These findings led us to consider that the placental barrier exposed to inflammation might become more permeable, which meant that EPO could travel across the placenta to the fetus and play a protective role.
Several studies support the inference that increased NO, PG, and cytokine production play significant roles in preterm delivery. 8 , 58 , 59 The administration of indomethacin (a COX‐2 inhibitor), 41 aminoguanidine (an iNOS inhibitor), 41 or etanercept (a competitive inhibitor of TNF‐α) 8 prevents inflammation‐induced preterm delivery. In addition, cytokine suppressive anti‐inflammatory drugs (CSAIDs), 60 which work by specifically targeting the NF‐κB and p38 MAPK inflammatory signaling pathways, could be an available therapeutic option to prevent preterm labor. These and previous studies support the notion that the impairment of PG/COX‐2 system, 61 decrease in NO levels, 41 diminishing cytokine production, 62 inhibiting cytokine signaling pathways, 60 or preferably the combination of these treatments, maybe a therapeutic strategy to prevent preterm delivery. Therefore, EPO could be a promising resource in the management of preterm delivery, as it alone decreased pro‐inflammatory cytokine levels in maternal serum and amniotic fluid, PGE2 contents in placenta, and p‐NF‐κB‐p65 and iNOS levels in mouse uteri from animals treated with LPS. Moreover, EPO promotes expression of placental PD‐L1 in preventing preterm labor induced by LPS. Thus, EPO might be considered as a novel tocolytic agent for infection‐related preterm labor.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ACKNOWLEDGMENTS
There is no funding to report for this submission. We would like to thank Professor RuiPing Pang for technical support.
Zhang J, Luo X, Huang C, et al. Erythropoietin prevents LPS‐induced preterm birth and increases offspring survival. Am J Reprod Immunol. 2020;84:e13283 10.1111/aji.13283
Jie Zhang and Xianqiong Luo contributed equally to this work.
REFERENCES
- 1. Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379(9832):2162‐2172. [DOI] [PubMed] [Google Scholar]
- 2. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McCormick MC. The contribution of low birth weight to infant mortality and childhood morbidity. N Engl J Med. 1985;312:82‐90. [DOI] [PubMed] [Google Scholar]
- 4. Yilun P, Fuai C, Xuejun D, et al. Antioxidative nanofullerol inhibits macrophage activation and development of osteoarthritis in rats. Int J Nanomedicine. 2019;14:4145‐4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang J, Zhao D, Na N, et al. Renoprotective effect of erythropoietin via modulation of the STAT6/MAPK/NF‐κB pathway in ischemia/reperfusion injury after renal transplantation. Int J Mol Med. 201841:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gayle DA, Beloosesky R, Desai M, Amidi F, Nuñez SE, Ross MG. Maternal LPS induces cytokines in the amniotic fluid and corticotropin releasing hormone in the fetal rat brain. Am J Physiol Regul Integr Comp Physiol. 2004;286(6):R1024‐R1029. [DOI] [PubMed] [Google Scholar]
- 7. Brennan KM, Kroener LL, Chazenbalk GD, et al. Polycystic ovary syndrome: impact of lipotoxicity on metabolic and reproductive health. Obstet Gynecol Surv. 2019;74:223‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Burdet J, Zotta E, Cella M, et al. Role of nitric oxide in shiga toxin‐2‐induced premature delivery of dead fetuses in rats. PLoS ONE. 2010;5(12):e15127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vrachnis N, Karavolos S, Iliodromiti Z, et al. Review: impact of mediators present in amniotic fluid on preterm labour. Vivo. 2012;26(5):799‐812. [PubMed] [Google Scholar]
- 10. Tripathi S, Guleria I. Role of PD1/PDL1 pathway, and TH17 and treg cells in maternal tolerance to the fetus. Biomed J. 2015;38(1):25‐31. [DOI] [PubMed] [Google Scholar]
- 11. Guleria I, Khosroshahi A, Ansari MJ, et al. A critical role for the programmed death ligand 1 in fetomaternal tolerance. J Exp Med. 2005;202(2):231‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Krantz SB. Erythropoietin. Blood. 1991;77:419‐434. [PubMed] [Google Scholar]
- 13. Cerami A. TNF and EPO: major players in the innate immune response: their discovery. Ann Rheum Dis. 2012;71(Suppl 2):i55‐i59. [DOI] [PubMed] [Google Scholar]
- 14. Nairz M, Schroll A, Moschen AR, et al. Erythropoietin contrastingly affects bacterial infection and experimental colitis by inhibiting nuclear factor‐κB‐inducible immune pathways. Immunity. 2011;34(1):61‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu S‐H, Lu I‐C, Lee S‐S, et al. Erythropoietin attenuates motor neuron programmed cell death in a burn animal model. PLoS ONE. 2018;13(1):e0190039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Juul SE, Pet GC. Erythropoietin and neonatal neuroprotection. Clin Perinatol. 2015;42(3):469‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aparecida LG, Tartari PPA, Adriana S, et al. Erythropoietin exacerbates inflammation and increases the mortality of histoplasma capsulatum‐infected mice. Mediators Inflamm. 2015;2015:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chikuma M, Masuda S, Kobayashi T, et al. Tissue‐specific regulation of erythropoietin production in the murine kidney, brain, and uterus. Am J Physiol Endocrinol Metab. 2000;279(6):E1242‐E1248. [DOI] [PubMed] [Google Scholar]
- 19. Conrad KP, Benyo DF, Westerhausen‐Larsen A, et al. Expression of erythropoietin by the human placenta. FASEB J. 1996;10(7):760‐768. [DOI] [PubMed] [Google Scholar]
- 20. Fairchild Benyo D, Conrad KP. Expression of the erythropoietin receptor by trophoblast cells in the human placenta. Biol Reprod. 1999;60:861‐870. [DOI] [PubMed] [Google Scholar]
- 21. Dijkstra F, Jozwiak M, De Matteo R, et al. Erythropoietin ameliorates damage to the placenta and fetal liver induced by exposure to lipopolysaccharide. Placenta. 2010;31(4):282‐288. [DOI] [PubMed] [Google Scholar]
- 22. Ji YQ, Zhang YQ, Li MQ, et al. EPO improves the proliferation and inhibits apoptosis of trophoblast and decidual stromal cells through activating STAT‐5 and inactivating p38 signal in human early pregnancy. Int J Clin Exp Pathol. 2011;4(8):765‐774. [PMC free article] [PubMed] [Google Scholar]
- 23. Doi T, Ishikawa T, Okayama T, et al. The JAK/STAT pathway is involved in the upregulation of PD‐L1 expression in pancreatic cancer cell lines. Oncol Rep. 2017;37:1545‐1554 [DOI] [PubMed] [Google Scholar]
- 24. Bellucci R, Martin A, Bommarito D, et al. Interferon‐γ‐induced activation of JAK1 and JAK2 suppresses tumor cell susceptibility to NK cells through upregulation of PD‐L1 expression. Oncoimmunology. 2015;4:e1008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaga N, Katsuki Y, Obata M, et al. Repeated administration of low‐dose lipopolysaccharide induces preterm delivery in mice: a model for human preterm parturition and for assessment of the therapeutic ability of drugs against preterm delivery. Am J Obstet Gynecol. 1996;174:754‐759. [DOI] [PubMed] [Google Scholar]
- 26. Nisell H, Wolff K. Effectiveness and safety of the oxytocin antagonist atosiban versus beta‐adrenergic agonists in the treatment of preterm labour. BJOG. 2003;110:89. [DOI] [PubMed] [Google Scholar]
- 27. Reinebrant HE, Pileggi‐Castro C, Romero CLT, et al. Cyclo‐oxygenase (COX) inhibitors for treating preterm labour. Cochrane Database Syst Rev. 2015;6:CD001992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Smith V, Devane D, Begley CM, et al. A systematic review and quality assessment of systematic reviews of randomised trials of interventions for preventing and treating preterm birth. Eur J Obstet Gynecol Reprod Biol. 2009;142(1):3‐11. [DOI] [PubMed] [Google Scholar]
- 29. Caritis S. Adverse effects of tocolytic therapy. Br J Obstet Gynecol. 2005;112(suppl 1):74‐78. [DOI] [PubMed] [Google Scholar]
- 30. Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. Br J Obstet Gynecol. 2006;113(suppl 3):17‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. King J, Flenady V, Cole S, Thornton S. Cyclo‐oxygenase (COX) inhibitors for treating preterm labour. Cochrane Database Syst Rev. 2005;2:CD001992. [DOI] [PubMed] [Google Scholar]
- 32. Conde‐Agudelo A, Romero R. Transdermal nitroglycerin for the treatment of preterm labor: a systematic review and metaanalysis. Am J Obstet Gynecol. 2013;209(6):551.e1‐551.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. JeanClaude F, Dame C, Vonthein R, et al. An approach to using recombinant erythropoietin for neuroprotection in very preterm infants. Pediatrics. 2015;167(1):52‐57.e3. [DOI] [PubMed] [Google Scholar]
- 34. Juul SE, McPherson RJ, Bauer LA, et al. A phase I/II trial of high‐dose erythropoietin in extremely low birth weight infants: pharmacokinetics and safety. Pediatrics. 2008;122(2):383‐391. [DOI] [PubMed] [Google Scholar]
- 35. Merelli A, Czornyj L, Lazarowski A. Erythropoietin as a new therapeutic opportunity in brain inflammation and neurodegenerative diseases. Int J Neurosci. 2015;125:793‐797. [DOI] [PubMed] [Google Scholar]
- 36. Kumral A, Baskin H, Gokmen N, et al. Selective inhibition of nitric oxide in hypoxic‐ischemic brain model in newborn rats: is it an explanation for the protective role of erythropoietin? Biol Neonate. 2004;85:51‐54. [DOI] [PubMed] [Google Scholar]
- 37. Siren A‐L, Fratelli M, Brines M, et al. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci USA. 2001;98:4044‐4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Al‐Gubory KH, Fowler PA, Garrel C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int J Biochem Cell Biol. 2010;42:1634‐1650. [DOI] [PubMed] [Google Scholar]
- 39. Rees S, Hale N, De Matteo R, et al. Erythropoietin is neuroprotective in a preterm ovine model of endotoxin‐induced brain injury. J Neuropathol Exp Neurol. 2010;69(3):306‐319. [DOI] [PubMed] [Google Scholar]
- 40. Timmons BC, Reese J, Socrate S, et al. Prostaglandins are essential for cervical ripening in LPS‐mediated preterm birth but not term or antiprogestin‐driven preterm ripening. Endocrinology. 2014;155:287‐298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cella M, Farina MG, Dominguez Rubio AP, et al. Dual effect of nitric oxide on uterine prostaglandin synthesis in a murine model of preterm labour. Br J Pharmacol. 2010;161(4):844‐855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bahçekapılı N, Akgün‐Dar K, Albeniz I, et al. Erythropoietin pretreatment suppresses seizures and prevents the increase in inflammatory mediators during pentylenetetrazole‐induced generalized seizures. Int J Neurosci. 2014;124:762‐770. [DOI] [PubMed] [Google Scholar]
- 43. Vyas V, Ashby CR, Olgun NS, et al. Inhibition of sphingosine kinase prevents lipopolysaccharide‐induced preterm birth and suppresses proinflammatory responses in a murine model. Am J Pathol. 2015;185(3):862‐869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen Y‐H, Zhao M, Chen X, et al. Zinc supplementation during pregnancy protects against lipopolysaccharide‐induced fetal growth restriction and demise through its anti‐inflammatory effect. J Immunol. 2012;189(1):454‐463. [DOI] [PubMed] [Google Scholar]
- 45. Jia Z, Xue R, Ma S, et al. Erythropoietin attenuates the memory deficits in aging rats by rescuing the oxidative stress and inflammation and promoting BDNF releasing. Mol Neurobiol. 2016;53(8):5664‐5670. [DOI] [PubMed] [Google Scholar]
- 46. Lappas M, Permezel M, Georgiou HM, Rice GE. Nuclear factor kappa B regulation of proinflammatory cytokines in human gestational tissues in vitro. Biol Reprod. 2002;67:668‐673. [DOI] [PubMed] [Google Scholar]
- 47. Belt AR, Baldassare JJ, Molnár M, Romero R, Hertelendy F. The nuclear transcription factor NF‐kappaB mediates interleukin‐1beta‐induced expression of cyclooxygenase‐2 in human myometrial cells. Am J Obstet Gynecol. 1999;181:359‐366. [DOI] [PubMed] [Google Scholar]
- 48. Xie QW, Kashiwabara Y, Nathan C. Role of transcription factor NF‐kappa B/Rel in induction of nitric oxide synthase. J Biol Chem. 1994;269(7):4705‐4708. [PubMed] [Google Scholar]
- 49. Xiao C, Ghosh S. NF‐κB, an evolutionarily conserved mediator of immune and inflammatory responses. Adv Exp Med Biol. 2005;560:41‐45. [DOI] [PubMed] [Google Scholar]
- 50. Pekson R, Poltoratsky V, Gorasiya S, et al. N, N‐Dimethylacetamide significantly attenuates LPS‐ and TNFα‐induced proinflammatory responses via inhibition of the nuclear factor kappa B pathway. Molec Med. 2016;22:747‐758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sundaram S, Ashby CR, Pekson R, et al. N, N‐Dimethylacetamide regulates the pro‐inflammatory response associated with endotoxin and prevents preterm birth. Am J Path. 2013;183:422‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liu Q‐S, Cheng Z‐W, Xiong J‐G, et al. Erythropoietin pretreatment exerts anti‐inflammatory effects in hepatic ischemia/reperfusion‐injured rats via suppression of the TLR2/NF‐κB pathway. Transpl Proc. 2015;47(2):283‐289. [DOI] [PubMed] [Google Scholar]
- 53. Tripathi S, Guleria I. Role of PD1/PDL1 pathway, and Th17 and treg cells in maternal tolerance to the fetus. Biomed J. 2015;38(1):25‐31. [DOI] [PubMed] [Google Scholar]
- 54. Guleria I. A critical role for the programmed death ligand 1 in fetomaternal toleance. J Exp Med. 2005;202(2):231‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Liu Y, Luo B, Shi R, et al. Nonerythropoietic erythropoietin‐derived peptide suppresses adipogenesis, inflammation, obesity and insulin resistance. Sci Rep. 2015;5:15134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schneider H, Malek A. Lack of permeability of the human placenta for erythropoietin. J Perinat Med. 1995;23:71‐76. [DOI] [PubMed] [Google Scholar]
- 57. Malek A, Sager R, Eckardt K‐U, et al. Lack of transport of erythropoietin across the human placenta as studied by an in vitro perfusion system. Pflügers Archiv. 1994;427(1–2):157‐161. [DOI] [PubMed] [Google Scholar]
- 58. Burdet J, Sacerdoti F, Cella M, et al. Role of TNF‐α in the mechanisms responsible for preterm delivery induced by Stx2 in rats. Br J Pharmacol. 2013;168:946‐953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tiboni GM, Del Corso A, Marotta F. Progestational agents prevent preterm birth induced by a nitric oxide synthesis inhibitor in the mouse. Vivo. 2008;22:447‐450. [PubMed] [Google Scholar]
- 60. Ng PY, Ireland DJ, Keelan JA. Drugs to block cytokine signaling for the prevention and treatment of inflammation‐induced preterm birth. Front Immunol. 2015;6:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Allport VC, Pieber D, Slater DM, et al. Human labour is associated with nuclear factor‐kappaB activity which mediates cyclo‐oxygenase‐2 expression and is involved with the ‘functional progesterone withdrawal’. Mol Hum Reprod. 2001;7:581‐586. [DOI] [PubMed] [Google Scholar]
- 62. Nakajima Y, Masaoka N. Initial experience using Sivelestat to manage preterm labor with a bulging fetal membrane in pregnant women. J Perinatol. 2012;32:466‐468. [DOI] [PubMed] [Google Scholar]


