Summary
Background/objectives
Several exposures during pregnancy are associated with offspring body mass index (BMI). The objective of this study was to evaluate whether third trimester antibiotic use and vaginal infections are associated with BMI in preschool children.
Subjects/methods
The study population included singletons from the NINFEA birth cohort with available anthropometric measurements at the age of 4 (3151 born with vaginal and 1111 born with caesarean delivery). Self‐reported use of antibiotics and the presence of vaginal infection in the third trimester were analysed in association with the child's BMI, classified into three categories: thinness, normal and overweight/obesity, using both the International Obesity Task Force (IOTF) and the World Health Organization (WHO) recommended cut‐offs.
Results
Maternal vaginal infections in the third trimester of pregnancy were associated with higher relative risk ratios (RRR) for overweight/obesity at age of four in children delivered vaginally: 1.92 (95% confidence interval [CI]: 1.37 to 2.70). This association appeared stronger for children born to women with pre‐pregnancy BMI >25 kg/m2 (RRR: 4.78; 95% CI 2.45 to 9.35), and was robust when different obesity cut‐offs were used. The results regarding third trimester antibiotic use in vaginal deliveries were less conclusive (RRRs for overweight/obesity: 1.43 (0.92 to 2.21) and 1.11 (0.57 to 2.20), for the IOTF and WHO cut‐offs, respectively). Third trimester vaginal infections were not associated with BMI in children delivered by caesarean section.
Conclusions
Maternal third trimester vaginal infections are associated with an increased overweight/obesity risk in children born by vaginal delivery, and especially in children of mothers with pre‐pregnancy overweight/obesity.
Keywords: antibiotics, birth cohort, BMI, prenatal exposures, preschool, vaginal infections
1. INTRODUCTION
Obesity in children is an important public health challenge due to its high prevalence, complex aetiology and long‐term consequences.1, 2 According to the Developmental Origins of Health and Disease hypothesis, the risk for many metabolic diseases, including obesity, can originate during early development.3 Exposures such as high pre‐pregnancy body mass index (BMI), excess gestational weight gain and exposure to tobacco smoke during pregnancy have already been identified as early risk factors for obesity in children.4, 5
Due to the emerging role of altered human gut microbiota in many metabolic and inflammatory diseases, especially obesity, we focused on two common gestational exposures closely linked6 with the maternal and initial neonatal microbiota: antibiotics and vaginal infections in the third trimester of pregnancy. The first major microbial coloniaation of the newborn happens during the birthing process and maternal antibiotic use or vaginal infections in pregnancy may not only disturb the maternal microbiota but also the initial colonising microbiota of the newborn. Antibiotics are among the most commonly prescribed drugs in pregnancy7, 8 and their administration in many circumstances is both effective and life‐saving. However, undesired effects on the long‐term health of the offspring are also linked with their use.9 The association between gestational antibiotic exposure and obesity in children is not well understood, possibly due to the complex interplay between many important factors that exert their effect in this period, with roles difficult to disentangle.10 Some of the studies on this topic reported positive associations between antibiotic exposure in some trimesters, or certain types of antibiotics and overweight or obesity, while others studies reported null associations.11, 12, 13, 14, 15, 16, 17 Most of the studies on antibiotics either used BMI as a continuous variable or included children who are underweight in the reference category, potentially attenuating the association with overweight/obesity. Vaginal dysbiosis in pregnancy is linked with maternal and foetal morbidity, in particular with increased risk for chorionamnionitis and premature rupture of the membranes18 and preterm birth,19, 20 a risk factors for later metabolic diseases.21 To date, there are no studies that explore the possible association between vaginal infections during pregnancy and childhood BMI outcomes.
Since the identification of early modifiable risk factors for metabolic diseases can help promote childhood health as early as from pregnancy, our aim was to evaluate, within the framework of a mother‐child cohort study, whether antibiotic use and vaginal infections in the last trimester of pregnancy are associated with BMI in pre‐school children. To address possible gaps in the literature and explore the association with both sides of the BMI spectrum, we modelled child's BMI as categorical variable with three categories (thinness, normal weight and overweight/obesity). To account for the fact that all mothers undergoing caesarean delivery are pretreated with antibiotics, and therefore, all children could be considered as exposed, our main analyses are focused only on children born by vaginal delivery.
We focused on exposures occurring during the third trimester of pregnancy because, from a theoretical point of view, we hypothesised that exposures that alter the maternal microbiome closer to the time of delivery might be the most relevant ones for obesity risk,22 and from a technical point of view, information on the exposures in the third trimester was the most complete, as detailed in the following section 2.
2. MATERIALS AND METHODS
2.1. Study population
The NINFEA study (Nascita e Infanzia: gli Effetti dell'Ambiente; Birth and Childhood: Effects of the Environment) is an Italian internet‐based mother‐child cohort (https://www.progettoninfea.it/index_en-n1) set up to investigate exposures during prenatal and early postnatal life that may affect health later in life.22, 23 Members of the cohort are children born to women who have access to the Internet and have enough knowledge of the Italian language to complete the online questionnaires. Since 2005, ~7500 pregnant women were recruited and completed the baseline questionnaire at any time during the pregnancy. The follow‐up questionnaires are completed at 6 and 18 months after delivery and when the child turns 4, 7, 10 and 13 years. For this study, we used the 2019.11 version of the NINFEA database, in which 4841 children were followed up at age of 4.
The study population included 4262 singletons with available height and weight measurements at the age of 4. Of them, 3151 were born with vaginal and 1111 with caesarean delivery (Figure S1). The NINFEA study was approved by the Ethical Committee of the San Giovanni Battista Hospital and CTO/CRF/Maria Adelaide Hospital of Turin (approval N.0048362 and following amendments) and all the participants gave written informed consent at enrolment.
2.2. Exposure, outcome and confounding variables
We focused on third trimester exposures (antibiotic use and vaginal infections) due to the two main reasons: proximity of the third trimester exposures to the moment of delivery, and the completeness of information. In particular, the first questionnaire covering first and second trimester exposures can be completed at any point in the pregnancy and investigating exposures during the first two trimesters would imply the exclusion of all participants who completed the questionnaire before the end of the corresponding trimesters, leading to a large reduction in sample size.
We used the data from the second questionnaire completed 6 months after delivery in order to obtain information on antibiotic use and the occurrence of vaginal infections in the third trimester of pregnancy. Mothers were asked to complete two separate pre‐specified checklists, one for medication use and one for pathological conditions. Therefore, antibiotic use was defined as any antibiotic taken by the mother in the last pregnancy trimester, independently of indication, while vaginal infection was defined as any vaginal infection occurring during the third trimester of pregnancy.
Child weight and height measurements at ~4 years of age were reported by mothers at the 4‐year follow‐up questionnaire. Mothers were additionally asked whether the reported anthropometric measures were recalled or taken from the child's health booklets, completed at regular intervals by paediatricians in Italy. Approximately 40% of mothers used health booklets to report their child anthropometric measurements, while the rest of them either recalled the recent measures or measured their child at compilation. The offspring BMI at age of 4 years was calculated using the standard formula, weight in kilogrammes divided by height in metres squared (kg/m2). Since we modelled BMI as a categorical variable with three categories (thinness, normal weight and overweight/obesity) and differences in prevalence are known to exists when different definitions and BMI cut‐offs are used, we decided to use the two most commonly used cut‐offs for child BMI: the ones proposed by the International Obesity Task Force (IOTF)24, 25 and the World Health Organization (WHO).26 The IOTF cut‐offs are based on and linked to the corresponding adult BMI cut‐offs at age 18 of the IOTF reference population, and classify children into six categories: thinness grade 3, 2 and 1, normal BMI, overweight and obesity. The WHO Child Growth Standards (0‐5 years), on the other hand, are based on traditional z‐scores. The z scores recommended to define thinness and overweight/obesity for children under the age of five are −2 and 2. The WHO z score of −2 closely corresponds to the cut‐off for thinness level 2 in IOTF.25 Therefore, for comparability reasons, for the main IOTF analyses we used the age and sex‐specific cut‐off for thinness grade 2 to define thinness and included thinness grade 1 in the normal BMI category. We also conducted sensitivity analyses classifying thinness grade 1 together with thinness grade 2 and 3.
Maternal age (continuous), maternal education (primary school or less, secondary education and university degree or higher), parity (yes, no), maternal pre‐pregnancy BMI (continuous), smoking during pregnancy (ever smoked, never) and gestational diabetes were preselected as potential confounders. We also adjusted for gestational age to account for different pregnancy length and, therefore, the period in which the child could have been exposed prenatally. Although antibiotics can be used to treat vaginal infections, we decided not to adjust for antibiotic use when studying vaginal infections, because in this case the antibiotic would act as a mediator in the association, and not a confounder.27
As children born with caesarean delivery are pretreated with intrapartum antibiotic prophylaxis, they were all exposed to antibiotic use. We therefore restricted the analyses on antibiotics exposure only on children born through vaginal delivery. The analyses on vaginal infections were separately conducted in children born through vaginal delivery and caesarean section.
For completeness and comparison with previous and future studies in the Supporting Information, we additionally reported the findings for antibiotic use and presence of vaginal infections in the first trimester and BMI outcomes in children born with vaginal delivery.
2.3. Statistical analyses
We used a complete case analyses approach, excluding all subjects with missing data in any of the variables of interest. For the main statistical analyses, we used multinomial logistic regression, an extension of logistic regression, used when the dependent variable is nominal with more than two categories. The exponentiated coefficients provide estimate of relative risks and are presented as RRRs and 95% confidence intervals (CIs). RRR represents the relative risk of thinness or overweight/obesity at age 4 (compared to normal BMI) among children of exposed vs unexposed mothers. We further investigated whether the studied associations are modified by maternal pre‐pregnancy BMI (categorised as ≤25 and >25 kg/m2), sex of the child, and parental reporting of the anthropometric measures (whether the height and weight were reported from child's booklets or not). All the analyses were performed using STATA version 15 (STATA Corp., Texas).
3. RESULTS
In 3151 children born with vaginal delivery, the prevalence of antibiotic use in the third trimester was close to 6%, while the prevalence of vaginal infections was slightly higher, around 9%. We observed notable differences in the prevalence of thinness and overweight/obesity when different cut‐offs were used. In particular, according to the WHO z‐score cut‐offs, 4.5% of the NINFEA children were classified in the thinness category, while 7.7% were classified as thinness grade 3 or 2 according to the IOTF cut‐offs. Around 15% of the NINFEA children were classified as IOTF thinness grade 1, but fell within the normal BMI limits according to WHO cut‐offs. (Figure 1) The prevalence of overweight according to WHO and IOTF was 5.3% and 12%, respectively. Other characteristics of the children delivered vaginally are summarised in Table 1.
Figure 1.
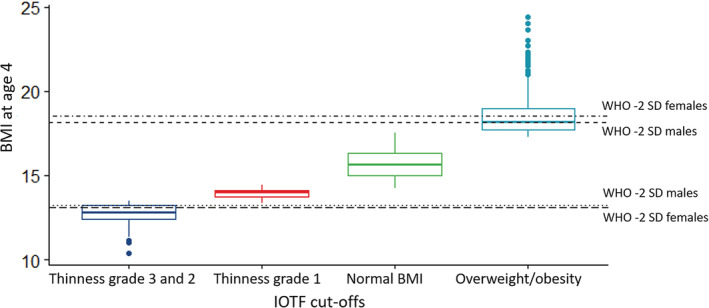
Comparison between the International Obesity Task Force (IOTF) cut‐off and the cut‐offs by the World Health Organization (WHO) growth standards. The figure plots BMI at age of 4 in children born with vaginal delivery
Table 1.
Characteristics of mother‐child dyads born with vaginal delivery and with available height and weight measures at age 4 (N = 3151)
| N | Mean (SD) or % | |
|---|---|---|
| Maternal age at delivery | 3151 | 33.5 (4.1) |
| Maternal pre‐pregnancy BMI | 3100 | 22.2 (3.6) |
| Missing values | 51 | ─ |
| Maternal education | ||
| Primary school or less | 112 | 3.6% |
| Secondary school | 985 | 31.5% |
| University degree or higher | 2035 | 64.8% |
| Missing values | 19 | ─ |
| Gestational diabetes | ||
| No | 2707 | 91.5% |
| Yes | 253 | 8.6% |
| Missing values | 191 | ─ |
| Smoked in pregnancy | ||
| No | 2876 | 92.4% |
| Yes | 237 | 7.6% |
| Missing values | 38 | ─ |
| First pregnancy | ||
| No | 858 | 27.8% |
| Yes | 2227 | 72.8% |
| Missing values | 66 | ─ |
| Antibiotic use in third trimester | ||
| No | 2820 | 94.1% |
| Yes | 177 | 5.9% |
| Missing values | 154 | ─ |
| Vaginal infections in third trimester | ||
| No | 2733 | 91.2% |
| Yes | 264 | 8.8% |
| Missing values | 151 | ─ |
| Child's BMI (IOTF cut‐offs) | ||
| Thinness grade 3 and 2 | 242 | 7.7% |
| Thinness grade 1 | 478 | 15.2% |
| Normal BMI | 2053 | 65.2% |
| Overweight/obesity | 378 | 12% |
| Child's BMI (WHO cut‐offs) | ||
| Thinness | 142 | 4.5% |
| Normal BMI | 2843 | 90.2% |
| Overweight/obesity | 166 | 5.3% |
Abbreviations: BMI, body mass index; IOTF, International Obesity Task Force; WHO, World Health Organization.
The complete case analysis approach led to the exclusion of 10% of the subjects due to missing data, leaving a total of 2837 children for the main analyses. The percentage of missing data was below 6% for all the explanatory variables. There were no differences in the baseline characteristics between included and excluded subjects, with the exception of maternal smoking in pregnancy, which was more common in the excluded group (7.2% vs 12.0%, P‐value .004). The characteristics of the included and excluded subjects are summarised in Table S1.
Using the IOTF cut‐offs, the relative risk of being overweight/obese at 4 years of age in children whose mothers had vaginal infection during the third trimester compared with those without infection was 1.92 (1.37‐2.70; Table 2). The strength of association changed only marginally when the WHO overweight/obesity cut‐off was used. (Table S2). As the prevalence of both thinness and overweight/obesity was lower using WHO standards, these analyses were more limited in power, in comparison to those using IOTF cut‐offs. The association between third trimester antibiotic use and overweight/obesity was less robust. The corresponding RRRs for antibiotic use were 1.43 (0.92 to 2.21) and 1.11 (0.57 to 2.20) for the IOTF and WHO analysis, respectively (Table 2 and Table S2). We did not observe an association between antibiotic use or vaginal infections with child thinness at age 4. These estimates only slightly changed when thinness grade 1 (15% of all children) was included in the thinness category in the IOTF analyses (Table S2).
Table 2.
Associations of antibiotic use and vaginal infections in the third trimester with BMI outcomes at age of 4 in NINFEA children born with vaginal delivery
| Third trimester | Cases/exposed cases (%) | RRRcrude (95% CI) | RRRadja (95% CI) |
|---|---|---|---|
| Antibiotic use | 170/2837 (6.0%) | ||
| Thinness (grades 2‐3) | 14/223 (6.3%) | 1.11 (0.63‐1.96) | 1.10 (0.62‐1.95) |
| Overweight/obesity | 26/328 (7.9%) | 1.43 (0.92‐2.21) | 1.40 (0.90‐2.16) |
| Vaginal infections | 251/2837 (8.8%) | ||
| Thinness (grades 2‐3) | 19/223 (8.5%) | 1.07 (0.65‐1.76) | 1.06 (0.64‐1.74) |
| Overweight/obesity | 49/328 (14.9%) | 2.01 (1.43‐2.85) | 1.92 (1.37‐2.70) |
Notes: International Obesity Task Force cut‐offs were used to define thinness and overweight/obesity. Thinness grade 1 is classified as normal BMI. Data are presented as n, n(%) and relative risk ratios (RRR) and 95% confidence intervals (CI).
Adjusted for: maternal age, education, parity, pre‐pregnancy BMI, smoking during pregnancy, gestational diabetes and gestational age.
We found that maternal pre‐pregnancy BMI modified the association of vaginal infections with overweight/obesity (P‐value for multiplicative interaction .002). The stratified analysis showed that the association was particularly pronounced in the category of mothers with overweight/obesity with RRR of 4.78 (2.45‐9.35; Table 3). There was no evidence of heterogeneity by child sex and reporting modality of anthropometric measures.
Table 3.
Pre‐pregnancy BMI, a potential effect modifier in the association between vaginal infections in the third trimester and overweight/obesity at age 4 in NINFEA children born with vaginal delivery
| RRRs (95% CI) for each stratum of pre‐pregnancy BMI and vaginal infection status with a single reference category | RRRs (95% CI) for vaginal infection in the strata of pre‐pregnancy BMI | ||||
|---|---|---|---|---|---|
| Vaginal infection = 0 | Vaginal infection = 1 | ||||
| Pre‐pregnancy BMI | N | RRR (95% CI) | N | RRR (95% CI) | |
| ≤25 | 208/234 | 1.00 | 26/234 | 1.34 (0.86‐2.08) | 1.33 (0.86‐2.07) |
| >25 | 71/94 | 1.64 (1.22‐2.22) | 23/94 | 7.5 (4.01‐13.97) | 4.78 (2.45‐9.35) |
Note: All RRRs are adjusted for maternal age, education, parity, pre‐pregnancy BMI, smoking during pregnancy, gestational diabetes and gestational age.
Abbreviations: BMI, body mass index; RRR, relative risk ratios.
In our analyses regarding first trimester exposures (Tables S3 and S4), we did not find evidence of association between antibiotic exposure and BMI outcomes, while the association between vaginal infections and overweight/obesity was similar, but lower in magnitude, to the one observed in the third trimester analyses.
The prevalence of vaginal infections in the third trimester and the prevalence of thinness and overweight/obesity in 4‐year‐old children delivered by caesarean section are reported in Table S5. The analyses regarding maternal vaginal infections and BMI outcomes in caesarean deliveries was performed only based on IOTF cut‐offs due to the slightly higher power. We found no evidence of association between vaginal infections during the third trimester and overweight/obesity in children delivered by caesarean section (Table S6). However, these analyses were performed on smaller number of subjects (N = 1111) in comparison with those born with vaginal delivery (N = 3151), resulting in quite wide CIs.
4. DISCUSSION
In the NINFEA cohort, vaginal infections in the third trimester of pregnancy are associated with overweight/obesity at age of 4 in children delivered vaginally, especially if born to mothers with pre‐pregnancy overweight/obesity. We observed no such association in children born by caesarean section. The findings regarding antibiotic use during the third trimester of pregnancy are less conclusive, although there is some indication of a positive association with overweight/obesity when using IOTF cut‐offs.
We did not find a similar study in the literature to compare our findings on vaginal infections. Vaginal infections are one of the most common gynaecological conditions and vaginal dysbiosis is known to be a risk factor for preterm birth, indicating how unbalanced vaginal microbial communities can influence duration of gestation, increasing the risk for some conditions related to metabolic syndrome.21, 28 However, in our cohort, the association between vaginal infections and overweight/obesity is not likely to be explained by prematurity, since the percentage of preterm births is very small (3%).
Taking into account that the first major microbial colonisation of the newborn happens during the birthing process6, 29 and that the vaginal microbiome in pregnant women is characterised by more stable, less rich and diverse communities dominated by Lactobacillus spp that benefit both the mother and the child,30, 31, 32 having a maternal vaginal microbiome enriched with health promoting bacteria, could give competitive advantage to the composition of healthy, non‐obesogenic neonatal intestinal microflora.
Our analyses indicate that maternal pre‐pregnancy BMI modifies the association between vaginal infections in the third trimester and the child's risk for overweight and obesity at age 4. It is known that maternal BMI increases the risk for adverse maternal and birth outcomes,4 as well as childhood obesity risk.5 A recent study33 that analysed the first stool of neonates found that among the neonates born vaginally, those born to mothers with overweight/obesity (BMI ≥25 kg/m2) had different gut microbiota structure compared with children of normal weight mothers that ultimately could lead to metabolic differences later in childhood. As the same associations were not found in children born by caesarean deliveries, the authors argued that the observed differences are likely the result of mother‐to‐offspring transmission of microbiota during childbirth. We also did not observe an association between vaginal infections and overweight/obesity in caesarean deliveries. However, these effect estimates are based on smaller number of children, and additional studies are needed with larger statistical power to confirm the lack of association.
Several studies explored the association between antibiotic use in pregnancy and elevated offspring BMI, and while some found an increased risk of overweight and obesity after prenatal antibiotic exposure, others did not observe any association. Some of the studies modelled BMI z scores and others used percentiles or IOTF cut‐offs, and the inconsistent findings could be, therefore, at least in part explained by different overweight and obesity cut‐offs used. Mueller et al11 reported a higher risk of obesity at age 7 after antibiotic use in the second and third trimester. Mor et al12 reported higher prevalence ratios of overweight and obesity in school‐aged children after antibiotic use in pregnancy (7‐16 years), while Cassidy‐Bushrow et al14 found an increased risk of overweight/obesity and higher BMI z‐score at age 2 after antibiotic use in the first two trimesters. On the contrary, Poulsen et al13 and Sejersen et al34 did not find an association between prenatal antibiotics and BMI z‐scores at age 3 and, 1 and 6 years, respectively. In addition, three recent large studies15, 16, 17 (based on more than 40 000 mother‐child dyads) presented reassuring results with effects going towards the null for most of the associations even when different trimester of exposure, dose‐response, spectrum and type of antibiotic were considered. However, an association with repeated use of antibiotics during pregnancy and exposure to broad spectrum antibiotics and obesity at age 7 was reported by Wang et al15 and Jess et al,17 respectively.
The results from previous studies are not directly comparable to ours, since only some of them performed stratified analyses by delivery mode, and none reported estimates for third trimester antibiotics use and overweight/obesity in vaginal deliveries. The effect estimate and 95% CIs for vaginal deliveries for ever antibiotic use in pregnancy and obesity in children aged 7 to 16 reported by Mor et al was 1.34 (1.08‐1.75). The estimate, however, was not adjusted for maternal BMI. The effect estimates for vaginal deliveries by Wang et al and Jess et al were 1.18 (1.02‐1.38) for repeated use of antibiotics in pregnancy and obesity at age 7 and 1.08 (0.99‐1.17) for ever antibiotic use in pregnancy and overweight/obesity at age of 7, respectively. Despite different exposure definition in our study and a smaller study population with lower prevalence of antibiotic use, the magnitude of the association found for the third trimester antibiotics use is relatively similar to these previous studies.
Several mechanisms may explain the found associations. A recent study by Zhang et al35 found that antibiotic use during mid pregnancy affects infant gut microbiome and subsequent infant adiposity. Vaginal infections could act in much the same way, through alteration of maternal and neonatal microbiota communities. However, other mechanisms such as immunologic, metabolomic or epigenetic pathways could be involved and explored in future studies.
Our study has several limitations. Unfortunately, we were not able to explore several important factors such as the type of vaginal infection, whether it was ever treated and the type of antibiotic used, nor the composition of the maternal vaginal or offspring's gut microbiota that might underlie these associations. We also had limited power to assess second trimester exposures. In the first trimester analysis, although based on smaller number of subjects, the strength of association between first trimester vaginal infections and overweight/obesity seems lower in magnitude than the one in the third trimester, which would be expected if the maternal vaginal microbiota at delivery is the one that underlies the observed associations. (Table S3) It should also be noted that more than 30% of mothers that reported a vaginal infection in the first trimester, also reported one in the third trimester.
We used self‐reported information for both antibiotics and infections, but this is common in large epidemiological studies. The prevalence of antibiotics is only slightly lower in our study population (6% and 7% for vaginal and caesarean deliveries, respectively), compared to the 9% prevalence of antibiotic use in the third trimester for vaginal and caesarean deliveries combined in the study by Jess et al17 based on more than 40 000 subjects and with antibiotic data coming from the Danish National Prescription Registry. Any possible exposure miss‐classification would be however driven by under‐reporting rather than over‐reporting, that would translate into high specificity, such as reporting of well‐known antibiotics and symptomatic vaginal infections, rather that asymptomatic bacterial vaginosis.
Child BMI was measured prospectively, at 4 years of age, and we used parentally reported height and weight measurements to calculate the BMI. As the NINFEA is an internet‐based cohort, only data on parentally reported weight and height is available. However, the NINFEA birth weight data was previously linked with the Piedmont Birth Registry and the comparison showed a very high validity of maternally reported birth weight.36 In addition, the questionnaire at age 4, uses the fact that children in Italy are regularly measured by health professionals and their measures are recorded in the children's personal health booklets. During the compilation of anthropometric measures, mothers were asked how the height and weight measurements were recalled, specifically whether they reported the measurements written in their child's booklets. At age 4, around 40% of the mothers reported written weight and height measures. We did not find evidence of heterogeneity between the children with measures reported from the booklets and the rest of the study population. Parentally reported height and weight compared with height and weight measured in younger children (less than 10 years) has a specificity of ≥95% to classify accurately obesity in several studies, and variable but relatively lower sensitivity,37, 38, 39, 40 that might lead to underestimation of obesity and produce estimates biased towards the null. Such non‐differential misclassification of outcome and/or exposure is of a greater concern in interpreting studies that seem to indicate the absence of an effect, than in studies with relatively strong positive association.41
In the NINFEA cohort, as in many other cohorts, participants mainly originate from a population with high educational attainment. However, it has been extensively shown that baseline selection does not imply biased estimates of the exposure‐disease associations, especially when relevant confounding factors are measured and controlled for.42, 43, 44, 45 In fact, we used the detailed information available in NINFEA to control for a number of carefully chosen covariates, including maternal BMI, maternal education and age, smoking, parity and gestational diabetes, factors that may strongly influence the associations of interest, and we found very little evidence of confounding. We modelled BMI as categorical variable including the thinness as a separate category and we carried out sensitivity analyses that took into account different BMI cut‐offs. Furthermore, we assessed the presence of interaction by sex, reporting modality of anthropometric measures and maternal pre‐pregnancy BMI, and found that the latter modified the effect of vaginal infections on offspring overweight/obesity. We were also able to examine the association between vaginal infections and BMI in children from our cohort born via caesarean section, although with lower power.
In conclusion, we report a novel association of vaginal infections in the third trimester of pregnancy with preschool BMI in children delivered vaginally. Maternal pre‐pregnancy BMI seems to modify this association, which might be an important point for preconception counselling.4 Our results regarding antibiotics were less conclusive.
Obesity has a complex aetiology and is influenced by variety of genetic, dietary and environmental factors. Identifying potential modifiable risk factors for obesity in children as early as in preconception or pregnancy might have long‐term beneficial effect on the child's metabolic health. Future studies should take into careful consideration the complex interplay between maternal pre‐pregnancy BMI, antibiotic use and vaginal infections.
CONFLICT OF INTEREST
The authors declare no competing financial interests.
Supporting information
Figure S1 Flow chart describing the sampling of the study population
Table S1 Characteristics between the included and excluded subjects in the complete case analysis on third trimester exposures in vaginal deliveries
Table S2 Associations of antibiotic use and vaginal infections in the third trimester with BMI outcomes at age of 4 in NINFEA children born with vaginal delivery. The BMI is categorized according to International Obesity Task Force standards (the thinness category includes thinness grades 1‐3) and World Health Organization Growth Standards.
Table S3 Associations of antibiotic use and vaginal infections in the first trimester with BMI outcomes at age of 4 in NINFEA children born with vaginal delivery. The BMI is categorized according to International Obesity Task Force standards.
Table S4 Associations of antibiotic use and vaginal infections in the first trimester with BMI outcomes at age of 4 in NINFEA children born with vaginal delivery. The BMI is categorized according to World Health Organization Growth standards.
Table S5 Characteristics of the study population born with cesarean delivery
Table S6 Associations between vaginal infections and BMI outcomes at age 4 in children born with cesarean delivery. The BMI is categorized according to International Obesity Task Force standards. Thinness grade 1 is included in the reference category.
ACKNOWLEDGEMENTS
M. Maule and L. Richiardi received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No. 774548, (STOP Project Science and Technology in Childhood Obesity Policy). M Maule received funding from the Compagnia di San Paolo.
Isaevska E, Popovic M, Pizzi C, et al. Maternal antibiotic use and vaginal infections in the third trimester of pregnancy and the risk of obesity in preschool children. Pediatric Obesity. 2020;15:e12632 10.1111/ijpo.12632
Funding information STOP Project Science and Technology in Childhood Obesity Policy, Grant/Award Number: 774548; Bando ex‐post di progetti di ricerca di Ateneo ‐ Anno 2018
REFERENCES
- 1. Abarca‐Gómez L, Abdeen ZA, Hamid ZA, et al. Worldwide trends in body‐mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population‐based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390(10113):2627‐2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Commission on Ending Childhood Obesity . Report of the Commission on Ending Childhood Obesity. World Health Organization, Geneva; 2016. Available at: http://www.who.int/end-childhood-obesity/en/. [Google Scholar]
- 3. Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early‐life conditions on adult health and disease. N Engl J Med. 2008;359(1):61‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Voerman E, Santos S, Inskip H, et al. Association of gestational weight gain with adverse maternal and infant outcomes. JAMA. 2019;321(17):1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Woo Baidal JA, Locks LM, Cheng ER, Blake‐Lamb TL, Perkins ME, Taveras EM. Risk factors for childhood obesity in the first 1,000 days: a systematic review. American Journal of Preventive Medicine. 2016;50:761‐779. [DOI] [PubMed] [Google Scholar]
- 6. Stiemsma LT, Michels KB. The role of the microbiome in the developmental origins of health and disease. Pediatrics. 2018;1(4):141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Palmsten K, Hernández‐Díaz S, Chambers CD, et al. The most commonly dispensed prescription medications among pregnant women enrolled in the U.S. Medicaid program. Obstet Gynecol. 2015;126(3):465‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stephansson O, Granath F, Svensson T, Haglund B, Ekbom A, Kieler H. Drug use during pregnancy in Sweden—assessed by the prescribed drug register and the medical birth register. Clin Epidemiol. 2011;3:43‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Popovic M, Rusconi F, Zugna D, et al. Prenatal exposure to antibiotics and wheezing in infancy: a birth cohort study. Eur Respir J. 2016;47(3):810‐817. [DOI] [PubMed] [Google Scholar]
- 10. Cox LM, Blaser MJ. Antibiotics in early life and obesity. Nat Rev Endocrinol. 2015;11:182‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mueller NT, Whyatt R, Hoepner L, et al. Prenatal exposure to antibiotics, cesarean section and risk of childhood obesity. Int J Obes (Lond). 2015;39(4):665‐670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mor A, Antonsen S, Kahlert J, et al. Prenatal exposure to systemic antibacterials and overweight and obesity in Danish schoolchildren: a prevalence study. Int J Obes (Lond). 2015;39(10):1450‐1455. [DOI] [PubMed] [Google Scholar]
- 13. Poulsen MN, Pollak J, Bailey‐Davis L, Hirsch AG, Glass TA, Schwartz BS. Associations of prenatal and childhood antibiotic use with child body mass index at age 3 years. Obesity. 2017;25(2):438‐444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cassidy‐Bushrow AE, Burmeister C, Havstad S, et al. Prenatal antimicrobial use and early‐childhood body mass index. Int J Obes. 2018;42(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang B, Liu J, Zhang Y, et al. Prenatal exposure to antibiotics and risk of childhood obesity in a multicenter cohort study. Am J Epidemiol. 2018;187(10):2159‐2167. [DOI] [PubMed] [Google Scholar]
- 16. Heerman WJ, Daley MF, Boone‐Heinonen J, et al. Maternal antibiotic use during pregnancy and childhood obesity at age 5 years. Int J Obes. 2019;43(6):1202‐1209. 10.1038/s41366-018-0316-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jess T, Morgen CS, Harpsøe MC, et al. Antibiotic use during pregnancy and childhood overweight: a population‐based nationwide cohort study. Sci Rep. 2019. Aug;9:11528 10.1038/s41598-019-48065-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brown RG, Al‐Memar M, Marchesi JR, et al. Establishment of vaginal microbiota composition in early pregnancy and its association with subsequent preterm prelabor rupture of the fetal membranes. Transl Res. 2019;207:30‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fettweis JM, Serrano MG, Brooks JP, et al. The vaginal microbiome and preterm birth. Nat Med. 2019;25(6):1012‐1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hočevar K, Maver A, Vidmar Šimic M, et al. Vaginal microbiome signature is associated with spontaneous preterm delivery. Front Med. 2019;10:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Markopoulou P, Papanikolaou E, Analytis A, Zoumakis E, Siahanidou T. Preterm birth as a risk factor for metabolic syndrome and cardiovascular disease in adult life: a systematic review and meta‐analysis. J Pediatr. 2019;210:69‐80.e5. [DOI] [PubMed] [Google Scholar]
- 22. Firestone R, Cheng S, Pearce N, et al. Internet‐based birth‐cohort studies: is this the future for epidemiology? JMIR Res Protoc. 2015;4(2):e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Blumenberg C, Zugna D, Popovic M, Pizzi C, Barros AJD, Richiardi L. Questionnaire breakoff and item nonresponse in web‐based questionnaires: multilevel analysis of person‐level and item design factors in a birth cohort. J Med Internet Res. 2018;20(12):e11046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cole TJ, Lobstein T. Extended international (IOTF) body mass index cut‐offs for thinness, overweight and obesity. Pediatr Obes. 2012;7(4):284‐294. [DOI] [PubMed] [Google Scholar]
- 25. Cole TJ, Flegal KM, Nicholls D, Jackson AA. Body mass index cut offs to define thinness in children and adolescents: international survey. Br Med J. 2007;335(7612):194‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.WHO Child Growth Standards. Length/height‐for‐age,weight‐for‐age, weight‐for‐length, weight‐for‐height and body massindex‐for‐age: methods and development. Geneva: World Health Organization, 2006. Available at: http://www.who.int/childgrowth/standards/en/.
- 27. Richiardi L, Bellocco R, Zugna D. Mediation analysis in epidemiology: methods, interpretation and bias. Int J Epidemiol. 2013;42(5):1511‐1519. [DOI] [PubMed] [Google Scholar]
- 28. Gritz EC, Bhandari V. The human neonatal gut microbiome: a brief review. Front Pediatr. 2015;5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Robertson RC, Manges AR, Finlay BB, Prendergast AJ. The human microbiome and child growth—first 1000 days and beyond. Trends Microbiol. 2019;27(2):131‐147. [DOI] [PubMed] [Google Scholar]
- 30. Freitas AC, Chaban B, Bocking A, et al. The vaginal microbiome of pregnant women is less rich and diverse, with lower prevalence of Mollicutes, compared to non‐pregnant women. Sci Rep. 2017. Aug 23;(1):9212 10.1038/s41598-017-07790-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. MacIntyre DA, Chandiramani M, Lee YS, et al. The vaginal microbiome during pregnancy and the postpartum period in a European population. Sci Rep. 2015;11:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Romero R, Hassan SS, Gajer P, et al. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non‐pregnant women. Microbiome. 2014;2(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mueller NT, Shin H, Pizoni A, et al. Birth mode‐dependent association between pre‐pregnancy maternal weight status and the neonatal intestinal microbiome. Sci Rep. 2016;6:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sejersen TS, Vinding RK, Stokholm J, et al. Antibiotic Exposure in Infancy and Development of BMI and Body Composition in Childhood. EClinicalMedicine. 2019. Dec; 17:100209 10.1016/j.eclinm.2019.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang M, Differding MK, Benjamin‐Neelon SE, Østbye T, Hoyo C, Mueller NT. Association of prenatal antibiotics with measures of infant adiposity and the gut microbiome. Ann Clin Microbiol Antimicrob. 2019;18(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pizzi C. Validation of the NINFEA birth data. https://www.progettoninfea.it/attachments/27%0D
- 37. Huybrechts I, Himes JH, Ottevaere C, et al. Validity of parent‐reported weight and height of preschool children measured at home or estimated without home measurement: a validation study. BMC Pediatr. 2011;11(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sekine M, Yamagami T, Hamanishi S, Kagamimori S. Accuracy of the estimated prevalence of childhood obesity from height and weight values reported by parents: results of the Toyama birth cohort study. J Epidemiol. 2002;12(1):9‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Garcia‐Marcos L, Valverde‐Molina J, Sanchez‐Solis M, et al. Validity of parent‐reported height and weight for defining obesity among asthmatic and nonasthmatic schoolchildren. Int Arch Allergy Immunol. 2006;139(2):139‐145. [DOI] [PubMed] [Google Scholar]
- 40. O'Connor DP, Gugenheim JJ. Comparison of measured and parents' reported height and weight in children and adolescents. Obesity. 2011;19(5):1040‐1046. [DOI] [PubMed] [Google Scholar]
- 41. Rothman KJ, Greenland S. Validity and Generalizability in Epidemiologic Studies. Wiley: Chichester, UK; 2014. [Google Scholar]
- 42. Pizzi C, De Stavola B, Merletti F, et al. Sample selection and validity of exposure disease association estimates in cohort studies. J Epidemiol Community Health. 2011;65(5):407‐411. [DOI] [PubMed] [Google Scholar]
- 43. Pizzi C, De Stavola BL, Pearce N, et al. Selection bias and patterns of confounding in cohort studies: the case of the NINFEA web‐based birth cohort. J Epidemiol Community Health. 2012;66(11):976‐981. [DOI] [PubMed] [Google Scholar]
- 44. Rothman KJ, Gallacher JE, Hatch EE. Why representativeness should be avoided. Int J Epidemiol. 2013;42(4):1012‐1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Richiardi L, Pizzi C, Pearce N. Commentary: representativeness is usually not necessary and often should be avoided. Int J Epidemiol. 2013;42(4):1018‐1022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Flow chart describing the sampling of the study population
Table S1 Characteristics between the included and excluded subjects in the complete case analysis on third trimester exposures in vaginal deliveries
Table S2 Associations of antibiotic use and vaginal infections in the third trimester with BMI outcomes at age of 4 in NINFEA children born with vaginal delivery. The BMI is categorized according to International Obesity Task Force standards (the thinness category includes thinness grades 1‐3) and World Health Organization Growth Standards.
Table S3 Associations of antibiotic use and vaginal infections in the first trimester with BMI outcomes at age of 4 in NINFEA children born with vaginal delivery. The BMI is categorized according to International Obesity Task Force standards.
Table S4 Associations of antibiotic use and vaginal infections in the first trimester with BMI outcomes at age of 4 in NINFEA children born with vaginal delivery. The BMI is categorized according to World Health Organization Growth standards.
Table S5 Characteristics of the study population born with cesarean delivery
Table S6 Associations between vaginal infections and BMI outcomes at age 4 in children born with cesarean delivery. The BMI is categorized according to International Obesity Task Force standards. Thinness grade 1 is included in the reference category.


