Abstract
Background and purpose
Acute tandem occlusions often require carotid stenting. Combination of mechanical and pharmacologic therapies in addition to antiplatelet drugs administered to prevent acute stent thrombosis might increase the risk of intracerebral hemorrhage. We present a protocol of antiplatelet regimen based on early post-procedural dual-energy CT (DE-CT).
Material and methods
Fifty consecutive stroke patients with tandem occlusions treated with acute carotid stenting after intracranial thrombectomy and TICI 2b/3 were reviewed. All patients received intravenous lysine acetylsalicylate during the procedure. Dual (aspirin+clopidogrel with or without clopidogrel load, groups A and B, respectively) or mono (aspirin) antiplatelet regimen (group C) was administered 12–24 h later according to brain DE-CT findings. Carotid ultrasonography was performed at 24 h and before discharge. We evaluated the rate of subsequent symptomatic intracranial hemorrhage (SICH) and acute stent thrombosis in each group.
Results
Between June 2014 and December 2016, 50 patients were included (mean age 66 years, 76% men, baseline NIHSS 16, median time from symptom onset to recanalization 266 min). According to DE-CT, 24 patients were assigned to group A, 19 to group B and 7 to group C (4 of them had SICH at that time). One patient suffered a subsequent SICH (belonging to group B). There was only one stent thrombosis without clinical repercussions in group B.
Conclusions
DE-CT may contribute to select antiplatelet regimen after acute carotid stenting in tandem occlusions.
Keywords: Carotid stenting, stroke, thrombectomy
Introduction
Tandem lesions are defined as those that combine carotid occlusion with the presence of intracranial thrombus and cause acute ischemic stroke with worse prognosis than strokes caused by isolated intracranial lesions.1,2 These patients have not been included in most large trials of mechanical thrombectomy and therefore highest-level evidence on treatment of this group of patients is lacking.3–8 Nevertheless, there is general agreement that patients with tandem lesions are candidates for endovascular treatment.1,9–12
There are two controversies in the endovascular treatment of tandem lesions: first, the timing of carotid stent implantation before or after extracranial artery recanalization, which can even be delayed for several days after acute treatment1 and second, the antiplatelet regimen to be used in these patients, since carotid stent implantation requires aggressive – usually dual – antiplatelet therapy to achieve stent patency, which can increase bleeding risk due to brain ischemia and hyperperfusion syndrome.13 However, there are few data in the literature on the risk of carotid stent thrombosis in patients not receiving dual antiplatelet therapy.1,12 Moreover, the use of dual antiplatelet therapy in patients receiving intravenous fibrinolysis is controversial, making the decision even more difficult.3–10
Follow-up head CT scans of patients with tandem lesions may show intracranial hemorrhage and sometimes contrast extravasation or retention, which both give rise to the same radiological image, hampering correct diagnosis. Moreover, ischemic lesions can show contrast uptake, hindering even further the identification of intracerebral hemorrhage.14 Dual-energy CT (DE-CT) is a radiological technique that simultaneously obtains two acquisitions with different kilovoltages. Different iodine attenuation at distinct energy levels allows hemorrhage to be differentiated from contrast extravasation in the acute phase of stroke and, in turn, detection of the established ischemic lesion.14,15
Our aim was to report our experience using dual-energy CT in patients who received acute treatment with mechanical thrombectomy and carotid stent implantation for the detection of real intracranial hemorrhage and despite contrast extravasation or retention in the infarct area. DE-CT guided the protocol of antiplatelet therapy (mono or dual, with or without clopidogrel load). We evaluated the rate of subsequent symptomatic intracranial hemorrhage (SICH) and acute stent thrombosis in each group of antiplatelet treatment.
Material and methods
We retrospectively reviewed consecutive patients with acute ischemic stroke due to tandem lesions (carotid extracranial plus intracranial large vessel occlusion) who met the criteria for urgent endovascular treatment in our center from June 2014 to December 2016. The carotid occlusion was diagnosed by CTA and catheter angiography. The inclusion criteria consisted of patients with ischemic stroke and less than 4.5 h from symptom onset to hospital arrival, age between 18 and 80 years, National Institutes of Health Stroke Scale (NIHSS) score > 7, baseline ASPECTS score > 6 and pre-procedural modified Rankin scale (mRS) less than or equal to 1. For the present analysis, we only included those patients that achieved complete recanalization (TICI score 2b-3) after thrombectomy. All patients received multimodal CT-perfusion imaging before endovascular treatment.
Our technical protocol for the treatment carotid tandem occlusion included primary angioplasty of the carotid occlusion through a 5-mm balloon angioplasty, with subsequent advancement of the balloon guide catheter through the carotid stenosis (concentric 8 F) followed by extraction of the intracranial thrombus with stent retriever. A carotid stent (WALLSTENT endoprosthesis, Boston Scientific) was subsequently implanted in all cases, regardless of the result of primary angioplasty, to maintain flow in the occluded artery. During the procedure, 1 g of lysine acetylsalicylate was administered intravenously in all patients, without acute administration of heparin or a second antiplatelet agent. Patients were admitted to the Stroke Unit immediately after endovascular treatment.
DE-CT (Somaton definition Flash CT, Siemens) was performed 12–24 h after the procedure, unless there was clinical deterioration, in which case it was performed urgently. DE-CT allowed the identification of intracranial hemorrhage, contrast retention or extravasation, and the extent of the cerebral infarct. Patients with ischemic lesion were divided using TOAST classification in less of one-third on MCA territory or most of one-third on MCA territory.16
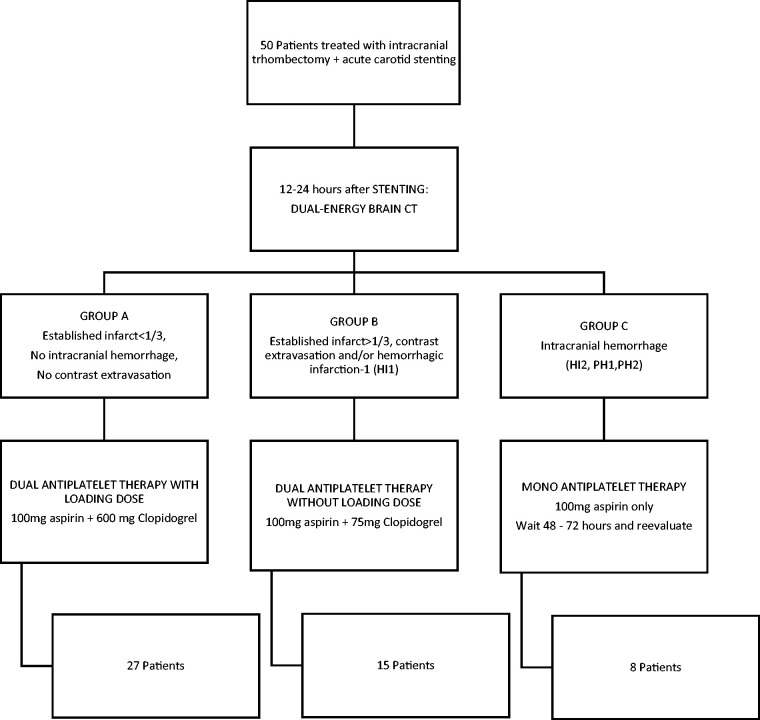
Antiplatelet regimen was then initiated, based on the results of DE-CT, as follows (Figure 1)
Group A: In patients without visible cerebral infarction or established infarction < 1/3 of the ischemic area and with neither intracranial hemorrhage or contrast extravasation, dual antiplatelet treatment was started with a loading dose of 600 mg clopidogrel and 100 mg oral aspirin.
Group B: In patients with petechial hemorrhagic infarction type 1 (HI1), contrast extravasation and those with infarction in > 1/3 of the ischemic area, dual antiplatelet treatment (100 mg aspiring + 75 mg clopidogrel) was used but avoiding clopidogrel loading dose.
Group C: In patients with intracerebral hemorrhage (HI2, PH1, PH2 or SAH), we decided to wait another 24–48 h before introducing the second antiplatelet agent.
Figure 1.
Patient classification with DE-CT in our center and protocol for antiplatelet regimen based on DE-CT.
A second brain CT or MR was performed before discharge, in order to evaluate subsequent intracranial hemorrhage. Intracranial hemorrhage was defined using ECASS2 criteria15
- Hemorrhagic infarction type 1 (HI1)
- ° petechial hemorrhages at the infarct margins
- Hemorrhagic infarction type 2 (HI2)
- ° petechial hemorrhages throughout the infarct
- ° no mass-effect attributable to the hemorrhages
- ° Parenchymal hematoma type 1 (PH1)
- ° ≤30% of the infarcted area
- ° minor mass effect attributable to the hematoma
- Parenchymal hematoma type 2 (PH2)
- ° >30% of infarct zone
- ° substantial mass effect attributable to the hematoma
Symptomatic intracranial hemorrhage (SICH) was defined as any hemorrhagic transformation temporally related to any worsening in neurologic condition, increase by four or more points on the NIHSS.15
Carotid ECO-Doppler ultrasound scan was performed 24 h after the procedure and before discharge to assess the patency of the carotid stent. Clinical exploration was made at the discharge and three months after stroke using NIHSS scale and mRS scale.
We collected clinical (age, sex, vascular risk factors, prior treatments, NIHSS), radiological and procedural-related variables. We evaluated the rate of SICH and stent thrombosis in each group of antiplatelet treatment. We evaluated factors significantly associated with SICH in the entire group using Chi square or Fisher tests for categorical variables and t-Student or U-Mann–Whitney test for continuous variables (normal distribution or not, respectively).
Results
We included 50 consecutive patients with acute tandem occlusions treated with intracranial thrombectomy plus carotid stenting (mean age 66 years, 76% men). Clinical and radiological variables of the sample are represented in Table 1. All patients had a complete occlusion of the cervical carotid artery with an intracranial thrombus (18 TICA, 26 M1 and 6 M2). Seven patients were treated with intravenous thrombolysis prior to endovascular treatment. Complete recanalization (TICI 2b-3) was achieved in all cases (this was a criterion for the selection of patients) with final TICI 3 in 68% of them. The median time from stroke onset to CT-perfusion imaging was 170 min (IQR 40) and until the arterial puncture 217 min (IQR 54). The median time from symptoms onset to vessel recanalization was 266 min (IQR 117). The mean time from door to arterial puncture was 63 min.
Table 1.
Distribution of epidemiological and diagnosis-related variables.
| Characteristics | Global (n = 50) | Correlation with ICHs |
||
|---|---|---|---|---|
| No SICH (n = 45) | SICH (n = 5) | p-value | ||
| Age, mean ± SD, years | 67.62 ± 19 | 67.7 ± 4 | 66.2 ± 10 | 0.712 |
| Male gender, n (%) | 38 (76) | 34 (75) | 4 (80) | 1 |
| Diabetes mellitus, n (%) | 12 (24) | 8 (18) | 4 (80) | 0.009 |
| Hypertension, n (%) | 29 (58) | 24 (53) | 5 (100) | 0.065 |
| Smoker, n (%) | 40 (80) | 18 (40) | 2 (40) | 1 |
| Dyslipidemia, n (%) | 36 (72) | 16 (35) | 2 (40) | 1 |
| Ischemic heart disease, n (%) | 6 (12) | 4 (9) | 2 (40) | 0.103 |
| Anticoagulant therapy, n (%) | 4 (8) | 3 (6) | 1 (20) | 0.353 |
| ASPECT median (IQR) | 9 (2) | 9 (2) | 7 (1) | 0.016 |
| CT Perfusion ≤ 30% volume decrease, n (%) | 46 (92) | 43 (95) | 3 (60) | 0.044 |
| Admission NIHSS, median (IQR) | 16 (2) | 15 (2) | 17 (5) | 0.495 |
| Time to artery recanalization, mean ± SD | 266.4 ± 117 | 271.1 ± 31 | 222.5 ± 150 | 0.306 |
| Intravenous thrombolysis | 7 (14) | 7 (15) | 0 (0) | 1 |
| 3 | 34 (68) | 30 (67) | 4 (80) | 1 |
| 2b | 16 (32) | 15 (33) | 1 (20) | 1 |
Note: Smokers were defined as active smokers at the time of diagnosis. Patients receiving anticoagulants were those receiving this treatment at the time of acute stroke. Assessment with CT perfusion imaging is subjective with color maps and was not used to exclude patients. Time to imaging diagnosis was measured in minutes from symptom onset until identification of the occluded artery in CT angiography postprocessing. Treatment-related variables and postoperative variables included the presence of symptomatic intracranial hemorrhage and outcome measured by the modified Rankin scale at 90 days.
TICA: terminal internal carotid artery; ASPECT: Alberta Stroke Program Early CT Score; NIHSS: National Institute of Health Stroke Scale; IQR: Interquartile range; SD: Standard Deviation; TICI: thrombolysis in cerebral infarction scale; ICHs: intracranial symptomatic scale; mRS: modified Rankin Scale.
Following results of DE-CT performed 12–24 h after endovascular treatment, patients were treated with antiplatelets as follows (Figure 1): 27 with dual antiplatelet using clopidogrel loading dose (group A), 15 with dual antiplatelet without loading dose and 8 patients with aspirin only (4 of them had a SICH at that time).
Regarding outcome variables after initiation of antiplatelet therapy, only one patient had a subsequent hemorrhagic transformation after initiation of the second antiplatelet agent (group B), who had an HI type 1 in first DE-CT. Only one patient had an acute thrombosis of carotid stent, without clinical worsening, also in group B.
Factors significantly associated with SICH in our sample were diabetes, lower ASPECTS score and higher volume lesion in perfusion imaging. Good functional outcome at 90 days (mRS ≤ 2) was achieved in 62% of patients, and there was 8% of mortality at that time.
Discussion
There is no consensus in time and dose of antiplatelet treatment after acute carotid stenting in the acute phase of stroke. Several problems are associated with the endovascular management of tandem lesions. The most widely debated is the timing of carotid stent implantation, before or after intracranial thrombus extraction. However, another issue posing difficulties in clinical practice is the antiplatelet regimen to be used after stent implantation.1,9,12 Although there is no clear evidence in the literature of a greater risk of acute carotid stent thrombosis with antiplatelet monotherapy, the use of dual antiplatelet therapy is practically universal, starting with intravenous administration during acute treatment, normally aspirin, since platelet glycoprotein IIb-IIIA receptor antagonists seem to increase the rate of patients with SICH.13 The administration of a second antiplatelet agent, normally 12–24 h after initial treatment, is also controversial. The second antiplatelet agent increases the risk of hemorrhagic transformation of the cerebral infarction, moreover, hours after the intervention. Routine imaging techniques are unable to distinguish between contrast retention in areas of acute infarction and contrast extravasation during the procedure with intraparenchymal hemorrhage, hyper perfusion syndrome and hemorrhagic conversion of ischemic brain tissue.13,14
DE-CT allows contrast retention to be differentiated from acute hemorrhage by using distinct energy levels in image acquisition, leading to iodine and blood differing in their behavior and density. DE-CT is based on changes in the density of the different substances when examined at different kilovoltages.13,14 This distinct attenuation is especially important with iodine, since its specific value of binding energy of the electrons to the atom makes it susceptible to a greater absorption
DE-CT allows us to use double antiplatelet therapy with load dose of those patients who have a low risk of hemorrhagic transformation. These patients do not have cerebral hemorrhages and only extravasated contrast or those with little ischemic lesion (patients of group A). On the other hand, DE-CT allows us to select patients with an IH1 cerebral hemorrhage or with a high volume of ischemia (group B) to start the double antiplatelet in a more progressive dose, without an antiaggregant loading. This minimizes the risk of acute stent thrombosis without a significant increase in brain hemorrhages (Figures 2 and 3).
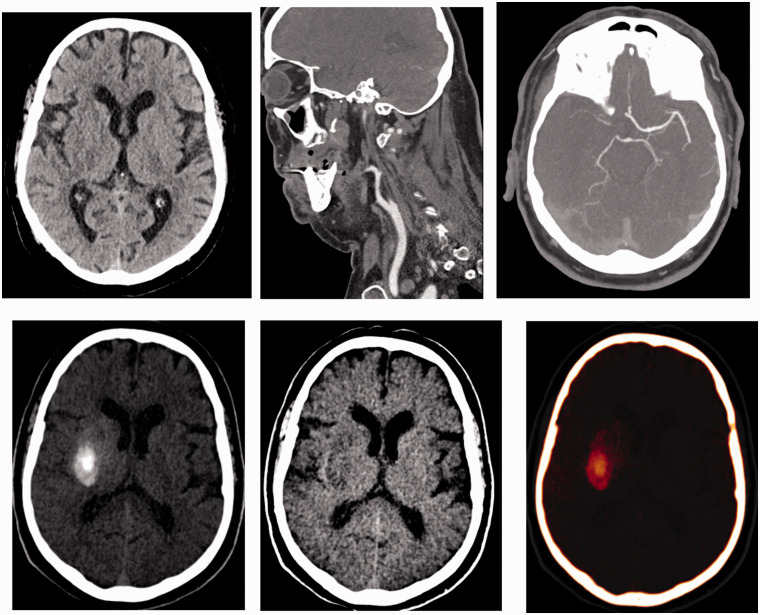
Figure 2.
Patient with occlusion of the right internal carotid artery and intracranial tandem lesion. In the dual energy CT performed after recanalization, the presence of a hyperdense collection is observed. In the study with iodine suppression, there is no cerebral hemorrhage, the collection is extravasation of contrast. This patient is a candidate for dual antiplatelet therapy (group A).
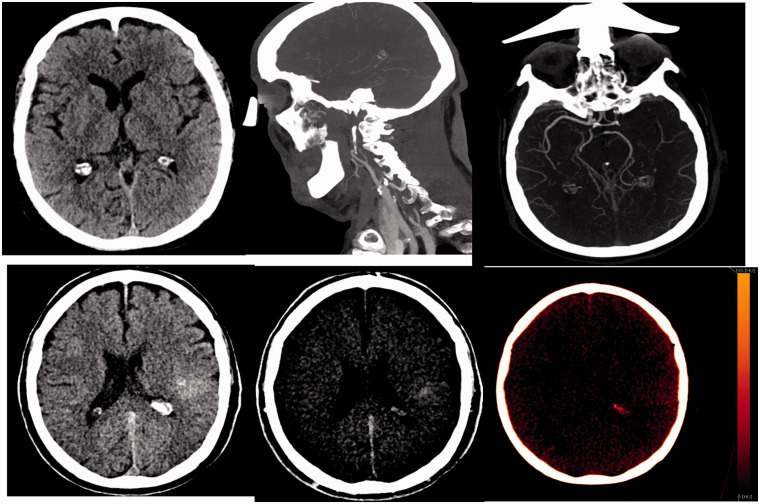
Figure 3.
Patient with occlusion of the left internal carotid artery and intracranial tandem injury. In the dual energy CT performed after recanalization, the presence of a hyperdense collection is observed. In the study with iodine suppression, a double origin is observed. On the one hand, there is extravasation of the contrast and on the other hand there is a hemorrhagic component IH1. It is a candidate to start with double anti-aggregation.
Only one of the patients in our study developed a hemorrhage after administration of the second antiplatelet agent. This was a patient in group B (IH1 petechial hemorrhage after initial treatment starting with 75 mg of oral clopidogrel and 100 mg oral aspirin). The rest of 10 hemorrhages occurred in the first 12 h of treatment with antiplatelet monotherapy. It is reasonable to suppose that they had hyperperfusion syndromes. In series of tandem occlusions, the percentage is usually above 10% and can reach 20% and even 30% with the use of platelet glycoprotein IIb-IIA receptor inhibitors.10,11,17–20,24–26 Probably our bleeding rate is lower due to the adequate control of antiplatelet therapy with the use of DE-CT and avoiding clopidogrel load dose in high-risk patients. Table 2 compares outcomes in our series with those of several recent series of tandem lesions and intra-arterial treatment.18–20,24–26
Table 2.
Dependency at 90 days (mRS < / = 2), SIH (symptomatic intracranial hemorrhage), recanalization (TICI 2b-3) and mortality in retrospective studies with a sample similar to that of the present study.
| Malik et al.27 | Kwak et al.20 | Stampfl et al.24 | Lescher et al.18 | Bheme25 | Heck et al.19 | Cohen et al.26 | Steglich et al.28 | Murias 2019 | |
|---|---|---|---|---|---|---|---|---|---|
| N | 77 | 35 | 24 | 39 | 149 | 23 | 24 | 47 | 50 |
| mRS < / = 2 | 41.6% | 62.9% | 29.2% | 36% | 36% | 39%% | 76% | 68% | 62% |
| SICH | 10.4% | 2.9% | 16.6% | 10% | 9% | 22% | 30%a | 4% | 10% |
| Recanalization (TICI 2b-3) | 75.3% | 74.3% | 62.5% | 64% | 77% | 91% | 79% | 87% | 100%b |
| Mortality | 24.7% | 11.4% | 16.6% | 10% | 19% | 39% | 23% | 9% | 8% |
The study indicates the total number of hemorrhages without indicating how many were symptomatic. bThe current study only included patients with complete recanalization, and consequently the results cannot be compared directly with those of the other series.
We found a statistically significant relationship between epidemiological variables and the presence of SICH. Diabetic patients had a higher incidence of SICH. Likewise, a strong statistical tendency was observed in hypertensive patients and a weaker tendency in patients with ischemic heart disease to develop SICH. A statistically significant association was found between the presence of SICH and the variables that allowed us to evaluate the presence of an established infarction prior to treatment: patients with a low ASPECT score and CT perfusion imaging with more than one-third decrease in cerebral blood volume had a higher proportion of SIHC. No statistically significant relationship was found between the remaining variables and the presence of SICH, including prior oral anticoagulant administration or the use of intravenous fibrinolysis before the endovascular procedure.
We only had one stent thrombosis without clinical repercussions. There are few series that directly report the percentage of thrombosed stents in the acute phase. Malik et al.27 report about 1.3% and Steglich-Arnholm et al.28 reported a higher rate with stent occlusions in 9% of the cases. In a recent study on the type of stent to be used, they conclude that double-layer stents have a significantly higher rate of acute occlusions than single-layer stents (45% vs. 3.7%).29 These single-layer stent occlusions about 3.7% probably shows the real percentage of early occlusions, consequently the real percentage of patients developing this complication is probably low. Moreover, we found no studies in the literature that associate poor functional outcome with stent thrombosis. This is important, since the antiplatelet protocol should be sufficiently conservative to avoid SICH, which seems to be the most important prognostic factor, more so than stent thrombosis.
The dual energy CT allows to avoid second antiplatelet in patients with H1I or with little cerebral infarction (group B) and select patients with intraparenchymal hemorrhage and differentiate it from extravasated iodine to delay the start of the second antiaggregant without a significant increase in acute stent thrombosis (Figure 4).
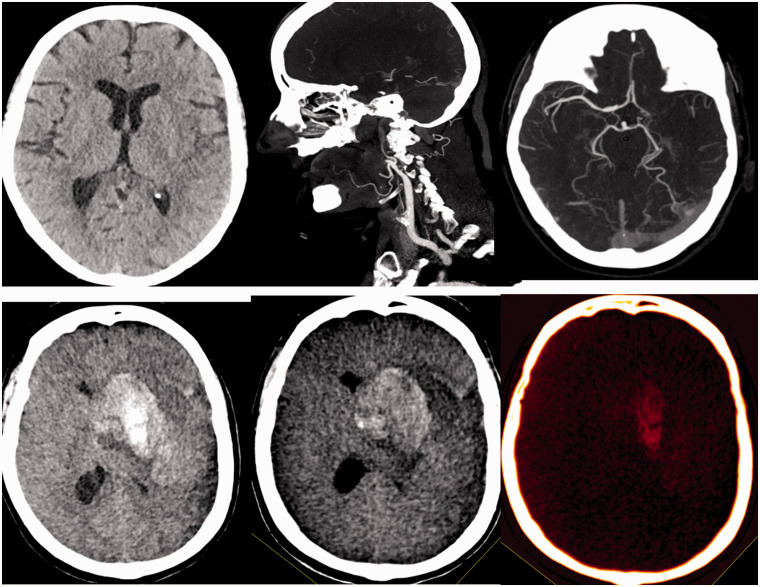
Figure 4.
Patient with occlusion of the left internal carotid artery and intracranial tandem lesion. In the dual energy CT performed after recanalization, the presence of a hyperdense collection is observed. In the study with iodine suppression, a double origin is observed. On the one hand, there is extravasation of the contrast and on the other hand there is a hemorrhagic component PH1. It is a candidate to continue with simple antiaggregation.
DE-CT may guide best antiplatelet regimen in patients with acute stroke due to tandem carotid lesions and treated with acute carotid stenting in our experience. DE-CT can offer new information about what is happening in the cerebral parenchyma that we did not know before. It is maybe useful to include in the imaging protocols. It is safe reducing the rate of SICH and more effective increasing the stent patience. This protocol is effective, since it achieved stent patency in most patients.
The study has some limitations. It has a small sample space, it is a retrospective work and there is some variability in the treatment. Insufficiently controlled hypertension during the intervention is a probably major risk factor for SICH and this analysis was not included into the risk factors in this study. Probably early aggregometry test could be included in the protocol for improving security. Safety and effectiveness of this protocol should be confirmed in future studies.
Authors’ contributions
Eduardo Murias Quintana: Responsible for the integrity of the study; study design; data collection; analysis and interpretation of data; statistical treatment; literature research; text writing; critical revision; approval of the final version.
Pedro Vega Valdés: Responsible for the integrity of the study; analysis and interpretation of data; text writing; critical revision; approval of the final version.
Elena López-Cancio Martínez: Study design; data collection; analysis and interpretation of data; critical revision; approval of the final version.
Jorge Peña Suárez: Analysis and interpretation of data; critical revision; approval of the final version.
Edison Morales Deza: Data collection; text writing; critical revision; approval of the final version.
Lorena Benavente Fernández: Study design; data collection; analysis and interpretation of data; critical revision; approval of the final version.
Montserrat González Delgado: Study design; data collection; critical revision; approval of the final version.
Davinia Larrosa Campo: Analysis and interpretation of data; critical revision; approval of the final version.
Maria Rico Santos: Text writing; critical revision; approval of the final version.
Nuria Riesco: Critical revision; approval of the final version.
María Cadenas Rodríguez: Data collection; analysis and interpretation of data; statistical treatment; literature research; approval of the final version.
Jose María Jimínez: Analysis and interpretation of data; critical revision; approval of the final version.
Juan Chaviano: Analysis and interpretation of data; critical revision; approval of the final version.
Antonio Saiz Ayala: Responsible for the integrity of the study; analysis and interpretation of data; critical revision; approval of the final version.
Sergio Calleja Puerta: Responsible for the integrity of the study; critical revision; approval of the final version.
Faustino Arias: Responsible for the integrity of the study; text writing; critical revision; approval of the final version.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval
The ethics committee of our hospital accepted the study and publication of the text.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Assis Z, Menon BK, Goyal M, et al. Acute ischemic stroke with tandem lesions: technical endovascular management and clinical outcomes from the ESCAPE trial. J Neurointerv Surg 2018; 10: 429–433. [DOI] [PubMed] [Google Scholar]
- 2.Rubiera M, Ribo M, Delgado-Mederos R, et al. Tandem internal carotid artery/middle cerebral artery occlusion: an independent predictor of poor outcome after systemic thrombolysis. Stroke 2006; 37: 2301–2305. [DOI] [PubMed] [Google Scholar]
- 3.ESCAPE Trial Investigators. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015; 372: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 4.REVASCAT Trial Investigators. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015; 372: 2296–2306. [DOI] [PubMed] [Google Scholar]
- 5.EXTEND-IA Trial Investigators. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015; 372: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 6.SWIFT PRIME Investigators. Stent-retriever thrombectomy after intravenous t- PA vs. t-PA alone in stroke. N Engl J Med 2015; 372: 2285–2295. [DOI] [PubMed] [Google Scholar]
- 7.Mr Clean Investigators. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015; 372: 11–20. [DOI] [PubMed] [Google Scholar]
- 8.Powers WJ, Derdeyn CP, Biller J, et al. American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. American Heart Association Stroke Council. Stroke 2015; 46: 3020–3035. [DOI] [PubMed] [Google Scholar]
- 9.Cohen JE, Gomori M, Rajz G, et al. Emergent stent-assisted angioplasty of extracranial internal carotid artery and intracranial stent-based thrombectomy in acute tandem occlusive disease: technical considerations. J Neurointerv Surg 2013; 5: 440–446. [DOI] [PubMed] [Google Scholar]
- 10.Dorado L, Castaño C, Millán M, et al. Hemorrhagic risk of emergent endovascular treatment plus stenting in patients with acute ischemic stroke. J Stroke Cerebrovasc Dis 2013; 22: 1326–1331. [DOI] [PubMed] [Google Scholar]
- 11.Mbabuike N, Gassie K, Brown B, et al. Revascularization of tandem occlusions in acute ischemic stroke: review of the literature and illustrative case. Neurosurg Focus 2017; 42: E15. [DOI] [PubMed] [Google Scholar]
- 12.Kappelhof M, Marquering HA, Berkhemer OA, et al. Intra-arterial treatment of patients with acute ischemic stroke and internal carotid artery occlusion: a literature review. J Neurointerv Surg 2015; 7: 8–15. [DOI] [PubMed] [Google Scholar]
- 13.Heck DV, Brown MD. Carotid stenting and intracranial thrombectomy for treatment of acute stroke due to tandem occlusions with aggressive antiplatelet therapy may be associated with a high incidence of intracranial hemorrhage. J Neurointerv Surg 2015; 7: 170–175. [DOI] [PubMed] [Google Scholar]
- 14.Phan CM, Yoo AJ, Hirsch JA, et al. Differentiation of hemorrhage from iodinated contrast in different intracranial compartments using dual-energy head CT. AJNR Am J Neuroradiol 2012; 33: 1088–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Postma AA, Hofman PA, Stadler AA, et al. Dual-energy CT of the brain and intracranial vessels. AJR Am J Roentgenol 2012; 199(5 Suppl): S26–33. [DOI] [PubMed] [Google Scholar]
- 16.Adams HP,, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. TOAST Stroke 1993; 24: 35–41.. [DOI] [PubMed] [Google Scholar]
- 17.Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet 1998; 352: 1245–1251. [DOI] [PubMed] [Google Scholar]
- 18.Lescher S, Czeppan K, Porto L, et al. Acute stroke and obstruction of the extracranial carotid artery combined with intracranial tandem occlusion: results of interventional revascularization. Cardiovasc Intervent Radiol 2015; 38: 304–313. [DOI] [PubMed] [Google Scholar]
- 19.Heck DV, Brown MD. Carotid stenting and intracranial thrombectomy for treatment of acute stroke due to tandem occlusions with aggressive antiplatelet therapy may be associated with a high incidence of intracranial hemorrhage. J Neurointerv Surg 2015; 7: 170–175. [DOI] [PubMed] [Google Scholar]
- 20.Kwak HS, Hwang SB, Jin GY, et al. Predictors of functional outcome after emergency carotid artery stenting and intra-arterial thrombolysis for treatment of acute stroke associated with obstruction of the proximal internalcarotid artery and tandem downstream occlusion. AJNR Am J Neuroradiol 2013; 34: 841–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delgado C, Martínez C, Trinidad C. Dual-energy computed tomography: what is it useful for? Radiología 2013; 55: 346–352. [DOI] [PubMed] [Google Scholar]
- 22.Kim SJ, Lim HK, Lee HY, et al. Dual-energy CT in the evaluation of intracerebral hemorrhage of unknown origin: differentiation between tumor bleeding and pure hemorrhage. AJNR Am J Neuroradiol 2012; 33: 865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Postma AA, Das M, Stadler AA, et al. Dual-energy CT: what the neuroradiologist should know. Curr Radiol Rep 2015; 3: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stampfl S, Ringleb PA, Moühlenbruch M, et al. Emergency cervical internal carotid artery stenting in combination with intracranial thrombectomy in acute stroke. J Neuroradiol 2014; 35: 741–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Behme D, Mpotsaris A, Zeyen P, et al. Emergency stenting of the extracranial internal carotid artery in combination with anterior circulation thrombectomy in acute ischemic stroke: a retrospective multicenter study. AJNR Am J Neuroradiol 2015; 36: 2340–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen JE, Gomori JM, Rajz G, et al. Extracranial carotid artery stenting followed by intracranial stent-based thrombectomy for acute tandem occlusive disease. J Neurointerv Surg 2015; 7: 412–417. [DOI] [PubMed] [Google Scholar]
- 27.Malik AM, Vora NA, Lin R, et al. Endovascular treatment of tandem extracranial/intracranial anterior circulation occlusions: preliminary single-center experience. Stroke 2011; 42: 1653–1657. [DOI] [PubMed] [Google Scholar]
- 28.Steglich-Arnholm H, Holtmannspötter M, Kondziella D, et al. Thrombectomy assisted by carotid stenting in acute ischemic stroke management: benefits and harms. J Neurol 2015; 262: 2668–2675. [DOI] [PubMed] [Google Scholar]
- 29.Yilmaz U, Körner H, Mühl-Benninghaus R, et al. Acute occlusions of dual-layer carotid stents after endovascular emergency treatment of tandem lesions. Stroke 2017; 48: 2171–2175. [DOI] [PubMed] [Google Scholar]