Abstract
Background
Immunosuppression induced by anticancer therapy in a COVID-19-positive asymptomatic patient with cancer may have a devastating effect and, eventually, be lethal. To identify asymptomatic cases among patients receiving active cancer treatment, the Federico II University Hospital in Naples performs rapid serological tests in addition to hospital standard clinical triage for COVID-19 infection.
Methods
From 6 to 17 April 2020, all candidates for chemotherapy, radiotherapy or target/immunotherapy, if negative at the standard clinical triage on the day scheduled for anticancer treatment, received a rapid serological test on peripheral blood for COVID-19 IgM and IgG detection. In case of COVID-19 IgM and/or IgG positivity, patients underwent a real-time PCR (RT-PCR) SARS-CoV-2 test to confirm infection, and active cancer treatment was delayed.
Results
Overall 466 patients, negative for COVID-19 symptoms, underwent serological testing in addition to standard clinical triage. The average age was 61 years (range 25–88 years). Most patients (190, 40.8%) had breast cancer, and chemotherapy with or without immunotherapy was administered in 323 (69.3%) patients. Overall 433 (92.9%) patients were IgG-negative and IgM-negative, and 33 (7.1%) were IgM-positive and/or IgG-positive. Among the latter patients, 18 (3.9%), 11 (2.4%) and 4 (0.9%) were IgM-negative/IgG-positive, IgM-positive/IgG-negative and IgM-positive/IgG-positive, respectively. All 33 patients with a positive serological test, tested negative for RT-PCR SARS-CoV-2 test. No patient in our cohort developed symptoms suggestive of active COVID-19 infection.
Conclusion
Rapid serological testing at hospital admission failed to detect active asymptomatic COVID-19 infection. Moreover, it entailed additional economic and human resources, delayed therapy administrationand increased hospital accesses.
Keywords: covid-19, cancer, SARS-CoV-2
Key questions.
What is already known about this subject?
-
•
Immunosuppression induced by anticancer therapy in a COVID-19-positive asymptomatic patient with cancer may have a devastating effect and, eventually, be lethal.
-
•
The incidence of asymptomatic and presymptomatic SARS-COV-2-positive patients ranges from 5% to 80%.
-
•
The role of rapid serological tests in addition to hospital standard clinical triage procedures (patient’s personal and family anamnesis for COVID-19 infection and symptoms, vital signs and temperature check) to identify asymptomatic cases for COVID-19 infection among patients receiving active cancer treatment is currently unknown.
What does this study add?
-
•
Rapid serological testing added to standard clinical triage at hospital admission failed to detect active asymptomatic COVID-19 infection.
-
•
Rapid serological testing entailed additional economic and human resources, a complex rearrangement of day hospital activities, delayed therapy administration by at least 24 hours and increased hospital accesses.
How might this impact on clinical practice?
-
•
More sensitive and specific serological assays are needed.
-
•
All patients who need cancer active immunosuppressive treatment should be screened with real-time PCR SRAS-CoV-2 testing as it has higher sensitivity for COVID-19 detection and currently represent the gold-standard method to diagnose SARS-CoV-2 active infection.
-
•
Implementing different strategies for COVID-19 detection in patients with cancer may be critical to identify asymptomatic cases.
Alt-text: Unlabelled Box
Introduction
WHO declared the coronavirus (COVID-19) outbreak a pandemic in March 11.1 As of mid-July 2020, more than 17 918 582 confirmed cases of COVID-19 disease had been confirmed worldwide and the death toll was 686 703, with USA and Europe accounting for more than 50% of overall cases.2 The incidence and death rate of COVID-19 in the patients with cancer is unknown. However, according to a recent survey, 20% of the Italian COVID-19 patient population who died from the disease had active cancer.3 Data from China3., 4. and, more recently, from Italy and the USA,5 also suggest a higher risk of COVID-related severe events (defined as the percentage of patients admitted to intensive care units and requiring invasive ventilationor death) in patients with cancer versus those without cancer.6
Healthcare systems worldwide have been overwhelmed by COVID-19, and frequently, elective surgery procedures and medical therapies have been suspended, even in patients with cancer in order to concentrate healthcare resources on fighting the COVID-19 pandemic.7., 8., 9., 10., 11. However, many Medical Oncology Societies worldwide recommend that cancer treatment not be delayed especially treatment with curative intent (neoadjuvant or adjuvant curative treatment or treatment for metastatic disease).12., 13., 14., 15., 16., 17.
Current guidelines advise oncologists to monitor fever, coughing, sore throat, breathing difficulty, muscle pain, tiredness, anosmia and dysgeusia, and to implement real-time PCR (RT-PCR) SARS-CoV-2 testing and delay any type of active treatment in case of symptoms. Ideally, RT-PCR SARS-CoV-2 testing should be repeated at each cycle of cancer therapy.16., 18. However, the incidence of asymptomatic and presymptomatic SARS-COV-2-positive patients ranges from 5% to 80%.19., 20. Although the incidence of asymptomatic cases in the population of patient with cancer is unknown, it may not be negligible in areas where COVID-19 infection is endemic and symptom-based screening may not identify completely asymptomatic patients positive for COVID-19 infection. The immunosuppression induced by chemotherapy in a COVID-19-positive asymptomatic patient with cancer may have a devastating effect and maybe lethal. In this report, we describe the proactive approach to identify asymptomatic cases among patients receiving active anticancer treatment by adding a rapid serological test to our hospital’s standard clinical triage procedures.
Methods
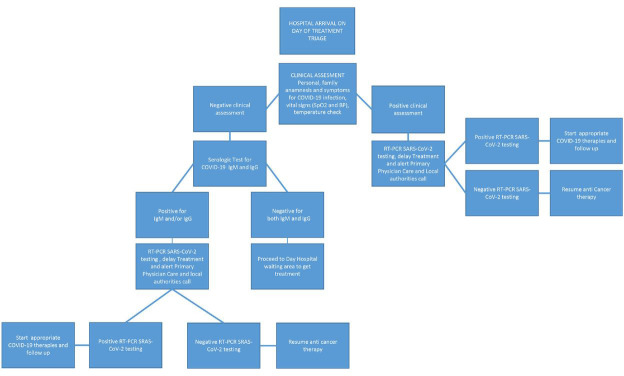
According to the Federico II University Hospital triage procedures, all candidates for active cancer treatment, namely chemotherapy, radiotherapy or target/immunotherapy, are precreened for COVID-19 infection by a phone call the day prior to the planned day hospital admission, and are advised not to go to the hospital if they had symptoms suggestive of COVID-19 infection or had a close contact who was COVID-19-positive and was awaiting RT-PCR SARS-CoV-2 testing. To avoid worthless hospital admissions, the day before the planned hospital access, all patients planned for immunosuppressive anticancer therapy had blood cell count done at a local laboratory nearby their homes, and they were advised by phone not to come to the hospital if blood cell count was too low to be able to receive therapy. In addition, on the day planned for anticancer treatment, patients undergo the standard clinical triage procedures based on their personal and family anamnesis for COVID-19 infection and symptoms, vital signs and temperature check. From 6 to 17 April 2020, if no symptoms of COVID-19 infection were detected by standard clinical triage procedures, patients underwent a rapid serological test on peripheral blood for COVID-19 IgM and IgG detection to identify asymptomatic cases.21 Serological tests were provided by the Local Government of Campania Region and were allocated exclusively for hospital use only. The Institutional Review Board was waived as the delivery of rapid serological test on peripheral blood for COVID-19 IgM and IgG detection to all patients with cancer receiving active anticancer therapy was not conceived as a clinical trial but was an operative procedure taken by the local government to try to trace and contain the infection at the time of its widest outbreak in Italy. The data for the present analysis were retrospectively and anonymously collected from patients’ charts. All patients included in this analyses signed an informed consent to anonymous data treatment for scientific purpose according to the Italian Law (art. 13 del D. Lgs. 196/2003) and to get the rapid serological test.
In case of symptoms suggestive of COVID-19 infection or positivity for IgM or IgG or both, patients underwent RT-PCR SARS-CoV-2 test to eventually confirm the infection. RT-PCR SARS-CoV-2 testing was performed by trained personnel, and samples were analysed at the Infective Disease Laboratory of our University Hospital. The results of the oropharyngeal swab were available within 24 hours. If RT-PCR SARS-CoV-2 testing was positive, active anticancer therapy was halted, the local health authorities and general practitioner were notified by hospital personnel to enable them to trace the case and their contacts and the patient was started on COVID-19 therapy and follow-up according to local procedures.
In case of a negative test and no COVID-19-related symptoms, anticancer treatment was delivered as per schedule (figure 1 ). Triage was mandatory for all patients undergoing active anticancer therapies, and was performed before each hospital access. Procedures were performed in an isolated area in the hospital entrance outside the clinic. All patients with symptoms and/or a positive serological test were isolated from all other patients while awaiting RT-PCR SARS-CoV-2 testing and were confined to an area reserved for suspected positive COVID-19 cases.
Figure 1.
Triage procedures flow chart. BP, blood pressure; SPO2, oxygen saturation.
Importantly, all our patients with cancer were instructed to avoid crowded places; wear personal protective equipment when in the hospital, in crowded placesor indoor public spaces; correctly and frequently wash their hands; not to have contacts with friends and relatives experiencing COVID-19-related symptoms or living in endemic zones; and maintain social distancing in order to reduce the risk of being infected.
Statistical analyses
Descriptive statistics for the categorical data were reported. The χ2 test and the Fisher’s exact tests were used to assess the association between rapid test positivity and clinic-pathological variables. Statistical analyses were performed using GraphPad Prism V.8.01.
Results
A total of 466 patients had a serological test in addition to standard clinical triage procedures between 6 and 17 April 2020. All were negative for symptoms suggestive of COVID-19 infection and denied any contact with COVID-19-nfected friends and relatives. Table 1 shows the patients’ demographics. Average age was 61 years (range 25–88), 146 (31.3%) men and 320 (68.7%) women. Cancer type distribution was the following: 190 (40.8%) breast cancers, 73 (15.7%) gastrointestinal cancers, 56 (12.0%) gynaecological cancers, 18 (3.9%) head-neck cancers, 23 (4.9%) lung cancers, 9 (1.9%) melanoma, 3 (0.6%) neurological cancers 49 (10.5%) pancreatic cancers, 13 (2.8%) rare cancers and 32 (6.9%) urological cancers. Chemotherapy with or without monoclonal antibody therapy (mAb) was the most frequent anticancer treatment and it was administered in 323 (69.3%) patients. Among the 228 mAb-treated patients (with or without chemotherapy), 101 (44%) received trastuzumab-based therapy. Overall, 70% of all patients were resident in the Campania Region and of these, 52% were living in the Naples Metropolitan area.
Table 1.
Patients’ demographics
| Characteristic | n=466 |
|---|---|
| Age (years) | |
| Median | 61 |
| Range | 25–88 |
| Gender | N (%) |
| Male | 146 (31.3) |
| Female | 320 (68.7) |
| Tumour Type | N (%) |
| Breast | 190 (40.8) |
| GI | 73 (15.7) |
| Gynaecological | 56 (12.0) |
| Head-Neck | 18 (3.9) |
| Lung | 23 (4.9) |
| Melanoma | 9 (1.9) |
| Neurological | 3 (0.6) |
| Pancreatic | 49 (10.5) |
| Rare Cancers | 13 (2.8) |
| Urological | 32 (6.9) |
| Treatment | N (%) |
| Chemotherapy | 223 (47.8) |
| Chemotherapy+mAb | 100 (21.5) |
| mAb alone | 128 (27.5) |
| HT+target therapies | 6 (1.3) |
| Small molecules | 4 (0.9) |
| Radiotherapy | 2 (0.4) |
| Other | 3 (0.6) |
GI, gastrointestinal; HT, hormonal therapy; mAb, monoclonal antibody.
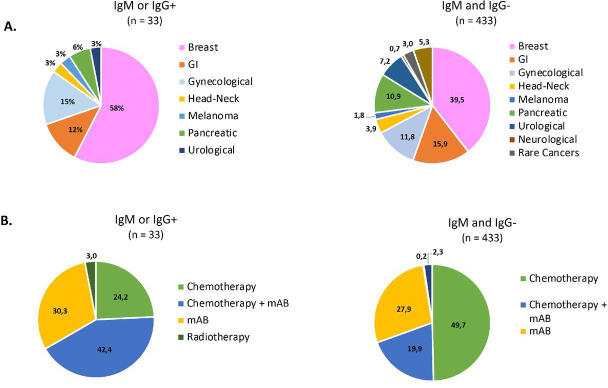
All 466 patients underwent a rapid serological test. In detail, 433 tests (92.9%) were IgG-negative and IgM-negative, and only 33 (7.1%) were IgM-positive and/or IgG-positive (table 2 ). Among the latter, 18 (3.9%) were IgM-negative/IgG-positive, 11 (2.4%) were IgM-positive /IgG- and 4 (0.9%) IgM-positive /IgG-positive. Figure 2 shows the proportions of patients IgM-positive and/or IgG-positive and IgM-negative and IgG-negative at the rapid serological test according to cancer type and anticancer therapy administered. Interestingly, trastuzumab-based anticancer treatment was significantly associated with rapid test positivity for IgM and/or IgG (p=0.0006).
Table 2.
Test results
| Serological rapid test | N (%) |
|---|---|
| n=466 | |
| Negative | |
| IgG-/IgM- | 433 (92.9) |
| Positive | 33 (7.1) |
| IgM-/IgG+ | 18 (3.9) |
| IgM+/IgG- | 11 (2.4) |
| IgM+/IgG+ | 4 (0.9) |
IgG-/IgM-, IgM and IgG-negative; IgM-/IgG+, IgM-negative and IgG- positive; IgM+/IgG+, IgM and IgG-positive; IgM+/IgG-, IgM-positive and IgG-negative.
Figure 2.
Proportions of IgM and/or IgG-positive (IgM and/or IgG+) and IgM and IgG-negative (IgM and IgG-) COVID-19 rapid serological test results according to cancer type (A) and treatment administered (B). mAb, monoclonal antibody; GI, gastrointestinal; HT, hormonotherapy.
All the RT-PCR SARS-CoV-2 tests performed in the 33 patients with a positive IgM and/or IgG serological test were negative. None of the 466 patients developed symptoms suggestive of active COVID-19 infection 14 or 21 days after the serological test and anticancer therapy.
Discussion
Identification of COVID-19 infection in asymptomatic patients with cancer is an urgent medical need as very often cancer treatment cannot be delayed, especially those with curative intent, and because patients receiving active immunosuppressive therapy such as chemotherapy or extensive radiotherapy are at increased risk of developing complicated COVID-19 infection. Most triage procedures have been designed to recognise early COVID-19 infection among patients with respiratory symptoms presenting at the emergency care unit. Very little if anything has been reported about triage for COVID-19 in asymptomatic patients who need such urgent medical procedures as immunosuppressive therapies for cancer. Chest CT scanning or lung ultrasound22 have been proposed to inform decisions on whether to test a patient for COVID-19, admit a patient to hospital or provide other treatment. However, it remains to be established whether to use lung ultrasound or chest CT for the diagnosis and follow-up of COVID-19 infection.23
Serological tests are valuable diagnostic tools especially when combined with RT-PCR SARS-CoV-2 and, because of their scalability, they can be used in large-scale, whole-population testing to assess the overall immune response to the virus and to identify asymptomatic carriers. However, in the present study, adding serological tests to detect anti-OVID-19 IgG and IgM to standard clinical triage procedures, performed at hospital admission before anticancer therapy, did not appear to detect active COVID-19 infection among asymptomatic cancer patients. None of the RT-PCR SARS-CoV-2 tests performed in the 33 patients with a positive serological test revealed COVID-19 virus (100% false positive rate). Interestingly, trastuzumab-based anticancer treatment was significantly associated with rapid test positivity. Further studies are needed to establish whether trastuzumab administration may truly interfere with rapid test outcome. We were unable to calculate the true negative rate of the serological tests we performed because patients negative for COVID-19 IgM and/or IgG did not undergo RT-PCR SARS-CoV-2 testing. However, none of the 433 patients developed COVID-19-related symptoms 14 or 21 days after serological testing and anticancer therapy, which indicates that these patients were free of active infection.
The rapid serological test sensitivity was 85% (95% CI 62.1% to 96.8%) and 100% (95% CI 86% to 100%) for IgM and IgG respectively. The biggest problem for the rapid tests we delivered was their specificity as none of the IgM-positive and/or IgG-positive patients had a positive RT-PCR SARS-CoV-2 test or developed symptoms of COVID-19 infection. Obviously, we cannot exclude that IgM-positive and/or IgG-positive patients had an asymptomatic infection in the weeks preceding serological testing and had developed immunity. However, this is unlikely because all patients ruled out having been in contact with COVID-19-positive subjects.
More sensitive and specific serological assays are currently under evaluation and will be soon available in the clinic, although it is remote that these tests alone will be enough to diagnose SARS-CoV-2 active infection given the slow pace of the human antibody response to SARS-CoV-2.24., 25., 26. Ideally, screening all patients who need cancer active immunosuppressive treatment with RT-PCR SRAS-CoV-2 testing would be preferable as it has higher sensitivity for COVID-19 detection than the available serological tests and because it currently represent the gold standard method to diagnose SARS-CoV-2 active infection. However, employing RT-PCR SRAS-CoV-2 testing for all outpatient admissions may be logistically challenging. This corroborate the importance of implementing new strategies for COVID-19 detection in patients with cancer.
It is feasible that the low incidence of positive RT-PCR SARS-CoV-2 tests in our dataset is due to a low prevalence of COVID-19 infection in the Campania Region, and the relatively low number of cases in the metropolitan area of the city of Naples, which is where the 52% of our patients come from. The prevalence of anti-COVID-19 IgM and IgG detection may be higher in areas where cases are more prevalent. In this regard, we have evidence that no patient included in this analysis developed symptoms at a later follow-up, which means that they were “true negative” cases. It should be noted that the addition of a rapid serological test to standard clinical triage procedures required additional economic and human resources, and entailed a complex rearrangement of day hospital activities and therapy schedules. Moreover, treatment was delayed by at least 24 hours, and led to an increase in hospital accesses.
The goal of hospitals treating patients with cancer in the COVID-19 era should be to ensure continuation of care by reducing the risk of both hospital contamination and of treating asymptomatic or presymptomatic COVID-19-positive patients with immunosuppressive agents. In our experience, rapid serological tests did not help to identify asymptomatic COVID-19-positive patients when added to a very careful clinical triage.
Footnotes
Contributors: All authors of this research paper have directly participated in the planning, execution or analysis of the study. All authors of this paper have read and approved the final version being submitted.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as online supplemental information. Data are not included in a repository and database at Federico II University includes deidentified participant data only.
References
- 1.WHO. Available: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-11-march-2020
- 2.COVID-19 coronavirus pandemic. Available: https://www.worldometers.info/coronavirus/
- 3.Liang W., Guan W., Chen R., et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. doi:10.1016/S1470-2045(20)30096-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. doi:10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onder G., Rezza G., Brusaferro S. JAMA. 2020. Case-Fatality rate and characteristics of patients dying in relation to COVID-19 in Italy.http://www.ncbi.nlm.nih.gov/pubmed/32203977doi:10.1001/jama.2020.4683 doi [Epub ahead of print 23 Mar 2020] [DOI] [PubMed] [Google Scholar]
- 6.Kuderer N.M., Choueiri T.K., Shah D.P., et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. http://www.ncbi.nlm.nih.gov/pubmed/32473681 doi:10.1016/S0140-6736(20)31187-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aeppli S., Eboulet E.I., Eisen T., et al. Impact of COVID-19 pandemic on treatment patterns in metastatic clear cell renal cell carcinoma. ESMO Open. 2020;5:e000852. doi: 10.1136/esmoopen-2020-000852. http://www.ncbi.nlm.nih.gov/pubmed/32669298 doi:10.1136/esmoopen-2020-000852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poggio F., Tagliamento M., Di Maio M., et al. JCO Oncol Pract. 2020. Assessing the impact of the COVID-19 outbreak on the attitudes and practice of Italian oncologists toward breast cancer care and related research activities.http://www.ncbi.nlm.nih.gov/pubmed/32574131doi:10.1200/OP.20.00297 OP.20.00297. [DOI] [PubMed] [Google Scholar]
- 9.Magno S., Linardos M., Carnevale S., et al. The impact of the COVID-19 pandemic on breast cancer patients awaiting surgery: observational survey in an Italian university hospital. Breast J. 2020;26:1597–1602. doi: 10.1111/tbj.13889. http://www.ncbi.nlm.nih.gov/pubmed/32677117 doi:10.1111/tbj.13889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tagliamento M., Bironzo P., Novello S. New emerging targets in cancer immunotherapy: the role of vista. ESMO Open. 2020;4:e000683. doi: 10.1136/esmoopen-2020-000683. http://www.ncbi.nlm.nih.gov/pubmed/32554470 doi:10.1136/esmoopen-2020-000683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marandino L., Di Maio M., Procopio G., et al. The shifting landscape of genitourinary oncology during the COVID-19 pandemic and how Italian oncologists reacted: results from a national survey. Eur Urol. 2020;78:e27–35. doi: 10.1016/j.eururo.2020.04.004. http://www.ncbi.nlm.nih.gov/pubmed/32345523 doi:10.1016/j.eururo.2020.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ueda M., Martins R., Hendrie P.C., et al. Managing cancer care during the COVID-19 pandemic: Agility and collaboration toward a common goal. J Natl Compr Cancer Netw. 2020:1–4. doi: 10.6004/jnccn.2020.7560. doi:10.6004/jnccn.2020.7560 [DOI] [PubMed] [Google Scholar]
- 13.Lambertini M., Toss A., Passaro A., et al. Cancer care during the spread of coronavirus disease 2019 (COVID-19) in Italy: young oncologists’ perspective. ESMO Open. 2020;5:e000759. doi: 10.1136/esmoopen-2020-000759. doi:10.1136/esmoopen-2020-000759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meattini I., Franco P., Belgioia L., et al. Radiation therapy during the coronavirus disease 2019 (covid-19) pandemic in Italy: a view of the nation's young oncologists. ESMO Open. 2020;5:e000779. doi: 10.1136/esmoopen-2020-000779. http://www.ncbi.nlm.nih.gov/pubmed/32295769 doi:10.1136/esmoopen-2020-000779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moujaess E., Kourie H.R., Ghosn M. Cancer patients and research during COVID-19 pandemic: a systematic review of current evidence. Crit Rev Oncol Hematol. 2020;150 doi: 10.1016/j.critrevonc.2020.102972. http://www.ncbi.nlm.nih.gov/pubmed/32344317 doi:10.1016/j.critrevonc.2020.102972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.COVID-19: supporting oncology professionals. Available: https://www.esmo.org/covid-19-and-cancer/supporting-oncology-professionals
- 17.RISCHIO INFETTIVO DA CORONAVIRUS COVID 19: INDICAZIONI PER L’ONCOLOGIA. Available: https://www.aiom.it/wp-content/uploads/2020/03/20200313_COVID-19_indicazioni_AIOM-CIPOMO-COMU.pdf
- 18.Cortiula F., Pettke A., Bartoletti M., et al. Managing COVID-19 in the oncology clinic and avoiding the distraction effect. Ann Oncol. 2020;31:553–555. doi: 10.1016/j.annonc.2020.03.286. http://www.ncbi.nlm.nih.gov/pubmed/32201224 doi:10.1016/j.annonc.2020.03.286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li R., Pei S., Chen B., et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2) Science. 2020;368:489–493. doi: 10.1126/science.abb3221. http://www.ncbi.nlm.nih.gov/pubmed/32179701 doi:10.1126/science.abb3221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Day M. Covid-19: identifying and isolating asymptomatic people helped eliminate virus in Italian village. BMJ. 2020;368 doi: 10.1136/bmj.m1165. http://www.ncbi.nlm.nih.gov/pubmed/32205334 doi:10.1136/bmj.m1165 [DOI] [PubMed] [Google Scholar]
- 21.Screen test COVID-19 IgG/IgM. Available: https://www.screenitalia.it/test-coronavirus-covid-19/
- 22.Soldati G., Smargiassi A., Inchingolo R., et al. Proposal for international standardization of the use of lung ultrasound for patients with COVID-19: a simple, quantitative, reproducible method. J Ultrasound Med. 2020;39:1413–1419. doi: 10.1002/jum.15285. http://www.ncbi.nlm.nih.gov/pubmed/32227492 doi:10.1002/jum.15285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.23. Available: https://www.acr.org/Advocacy-and-Economics/ACR-PositionStatements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection
- 24.Lauer S.A., Grantz K.H., Bi Q., et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172:577–582. doi: 10.7326/M20-0504. http://www.ncbi.nlm.nih.gov/pubmed/32150748 doi:10.7326/M20-0504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.To K.K.-W., Tsang O.T.-Y., Leung W.-S., et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20:565–574. doi: 10.1016/S1473-3099(20)30196-1. http://www.ncbi.nlm.nih.gov/pubmed/32213337 doi:10.1016/S1473-3099(20)30196-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Health Commission of the People’s Republic of China, New Coronavirus Pneumonia Diagnosis and Treatment Program (Trial Version 7)..