Abstract
Introduction
Several blood‐based biomarkers are associated with neuronal injury, but their utility in interventional clinical trials is unclear. This study retrospectively evaluated the utility of plasma neurofilament light (NfL) and total tau (t‐tau) in an 18‐month trial in mild Alzheimer's disease (AD).
Methods
Correlation and conditional independence analyses and Gaussian graphical models were used to investigate cross‐sectional and longitudinal relations between NfL, t‐tau, and clinical scales.
Results
NfL had a stronger association than t‐tau with clinical scales; t‐tau did not hold additional information to that given by NfL (P > 0.05 at all time points). NfL held independent information about shorter‐term (3‐ to 6‐month) progression beyond patient age and clinical scores. However, no meaningful gain in power was found when adjusting a longitudinal analysis of cognitive scores for baseline NfL.
Discussion
Plasma NfL is superior to t‐tau in mild AD. The ability of NfL to detect changes before clinical manifestations makes it a promising biomarker of drug response in trials of disease‐modifying drugs.
Keywords: Alzheimer's disease, biomarkers, blood biomarkers, clinical trials, dementia, fluid biomarkers, neurofilament light, NfL, plasma biomarkers, total tau, t‐tau
1. INTRODUCTION
Neurofilament light chain (NfL) and total tau (t‐tau) are disease‐unspecific markers of neuronal injury, with increased levels in cerebrospinal fluid (CSF) in many neurodegenerative diseases, including Alzheimer's disease (AD). Ultra‐sensitive assays now enable measurements of both NfL and t‐tau in plasma. Plasma NfL measurements correlate strongly with CSF NfL, with similar capacity for differential diagnosis of dementias and parkinsonian disorders as CSF NfL. 1 This suggests that blood‐based measurements of NfL could be used instead of CSF NfL, which would open for use of NfL on a much larger scale in both clinical practice and drug development. In AD, plasma NfL increases early, 2 , 3 , 4 correlates with other markers of disease intensity, 5 and holds predictive information about future cognitive decline. 6 Together, these findings suggest that plasma NfLs may be predictive of changes in disease stage before they can be detected by clinical evaluation. Plasma or serum levels of t‐tau has also been measured in AD in several studies, with most studies finding slightly increased levels in AD (although with varying effect sizes). 7 , 8 , 9 , 10 However, some studies did not find increased levels in AD. 11 , 12 Potentially, brain injury markers such as plasma NfL and t‐tau may boost enrichment or stratification of clinical trial participants, as well as detection of disease‐modifying effects during follow‐up, and may therefore be considered when designing clinical trials for AD. However, most studies of plasma NfL and t‐tau to date have considered them in isolation, instead of considering their added value to clinical and cognitive information that is already collected in clinical trials. The utility for enrichment in AD clinical trials may therefore be low if basic demographic and cognitive data contain most of the meaningful information. In this study, we critically evaluate plasma NfL and t‐tau in clinical trials of AD. This is done by retrospectively analyzing NfL and t‐tau levels in biobanked plasma samples that were collected longitudinally in an interventional clinical trial in mild AD. In this sample, we analyze how plasma NfL and t‐tau contribute to understanding of clinical presentation and future progression, and we evaluate how to best use these plasma biomarkers in clinical trials.
RESEARCH IN CONTEXT
Systematic review: The authors used PubMed to review literature related to neurofilament light (NfL) and total tau (t‐tau) in Alzheimer's disease (AD). Prior works have shown that both markers are associated with disease stage and progression of AD, but it remains unclear how they contribute additional information beyond demographics and clinical scales.
Interpretation: This study replicated previous results regarding the ability of plasma NfL and t‐tau to predict cognition and function, finding that NfL was generally superior to t‐tau. A novel finding was that when adjusting for patient age and clinical scales, NfL was still predictive of future changes 3 to 6 months before they manifested on clinical scales. Consequently, plasma NfL has promise as a biomarker for detecting disease‐modifying effects in clinical trials in AD.
Future directions: Knowledge of how biomarkers can be used to predict clinical manifestation is key for optimizing clinical trials. Future research is needed to better understand how to use biomarkers in interventional clinical trials in AD.
2. METHODS
2.1. Study population
This study included 500 randomly selected patients with probable mild Alzheimer's dementia (Mini‐Mental Status Examination [MMSE] 20‐26, Clinical Dementia Rating [CDR] 0.5‐1) from an 18‐month phase 3 trial (clinicaltrials.gov Identifier: NCT00105547) of the non‐steroidal anti‐inflammatory and γ‐secretase modulator tarenflurbil. 13 The study was completed in 2008 with the conclusion that 800 mg tarenflurbil given twice a day did not improve cognition or daily function. Included patients were required to have biobanked plasma samples and valid assessments of cognition, function, and neuropsychiatric status available at baseline, and 9 and 18 months.
All participants gave informed consent, and ethics approval for this study was obtained by The Committees on Health Research Ethics in the Capital Region of Denmark (case no. H‐18041352).
2.2. Plasma biomarkers
As part of the study protocol, plasma samples were collected at the baseline visit, and at 9 and 18 months and biobanked for exploratory biomarker analyses.
Plasma NfL concentration was measured at the Clinical Neurochemistry Laboratory, Sahlgrenska University Hospital, Mölndal, Sweden, using an in‐house single molecule array (Simoa) method on an HD‐1 Analyzer (Quanterix, Billerica, MA), as previously described in detail, 14 whereas plasma t‐tau concentration was measured using the commercially available Tau Advantage Kit on an HD‐1 Analyzer, as described by the manufacturer (Quanterix, Billerica, MA). All measurements were performed on one occasion using one batch of reagents by board‐certified laboratory technicians who were blinded to clinical data. Coefficients of variation were below 10%.
2.3. Cognition, function, and neuropsychiatric status
Cognition was assessed using the 12‐item Alzheimer Disease Assessment Scale−Cognitive Subscale 15 (ADAS‐cog; 80‐point version), CDR sum of boxes 16 (CDR‐SB) and MMSE 17 ; function was assessed using the 23‐item ADCS Activities of Daily Living (ADL), 18 and neuropsychiatric status was assessed using the Neuropsychiatric Inventory (NPI). 19
Cognitive, functional, and neuropsychiatric scales were done at baseline, and at 3, 6, 9, 12, 15, and 18 months, except for MMSE, which was done at baseline and 12 and 18 months.
2.4. Statistical analyses
Cross‐sectional correlations between plasma biomarkers and clinical scales were assessed using Spearman rank correlation coefficient. Significance testing of correlation coefficients was done using a t tests.
To assess whether a plasma biomarker contributed additional information when controlling for other information (eg, the other biomarker and/or clinical scale data), conditional independence testing was done using the generalized covariance measure 20 as implemented in the GeneralisedCovarianceMeasure R package. The test used the standard settings of extreme gradient boosting 21 for nonlinear regression and 9999 bootstrap samples to approximate the null distribution of the test statistic.
In cases where a plasma biomarker was found to hold independent information, relations between variables were explored using Gaussian graphical models. For these analyses, each variable in the data was transformed using a rank‐based inverse normal transformation, before a fully connected Gaussian graphical model was fitted. Edges were then iteratively removed using the Bayesian Information Criterion (BIC) 22 to guide backward model selection. The fully connected Gaussian graphical model corresponds to a multivariate Gaussian model with a free covariance structure fitted with maximum likelihood estimation using complete‐case data. The models were fitted using the gRim R package. 23 The edges in a Gaussian graphical model are directly related to the inverse covariance matrix and thus the partial correlations. 24 Testing the individual edges in a graphical model thus corresponds to testing for conditional independence between the two variables given all other variables that are connected to the two variables in the graph.
Whenever relevant, analyses were adjusted for patient age, since plasma NfL has been reported to increase with age independent of other AD‐related variables. 6
Finally, to evaluate the effects of adjusting for biomarkers, analyses of longitudinal ADAS‐cog trajectories were done using mixed models for repeated measures (MMRMs), 25 which are often used to analyze primary end points in clinical trials. The models adjusted for trial site; baseline use of cholinesterase inhibitor, memantine, or both; treatment arm‐by‐visit interaction; and baseline biomarker‐by‐visit interaction. The potential gain in power (t test of primary end point) due to reduced variance was assessed by computing the number of patients per arm (two arms total) needed to detect a 2‐point treatment effect on ADAS‐cog at 18 months.
The statistical analyses were performed using R version 3.6.3 (R Foundation for Statistical Computing).
3. RESULTS
3.1. Study participants and biomarker results
Of the 500 patients, 236 were on active treatment during the study. In line with the negative outcome of the full trial, the treatment arm did not seem to affect plasma NfL and t‐tau (Figure 1A). Therefore, no adjustment for treatment arms were done in the subsequent analyses.
FIGURE 1.
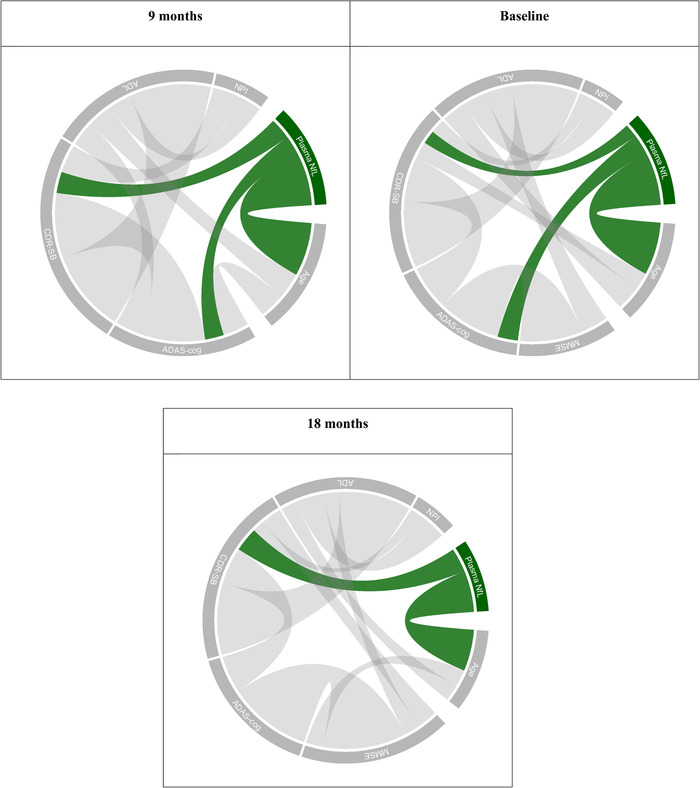
Conditional dependence structure between age, plasma NfL, and clinical scales at baseline and 9 and 18 months. Connection widths are proportional to the size of the estimated conditional correlations between variables. Note that MMSE was not measured at the 9‐month visit. MMSE, Mini‐Mental State Examination; ADAS‐cog, Alzheimer Disease Assessment Scale−Cognitive Subscale; CDR‐SB, Clinical Dementia Rating sum of boxes; ADL,ADCS Activities of Daily Living (ADL); NPI, Neuropsychiatric Inventory
The median age of the 500 patients was 75 years and 51.8% were female. The full demographic characteristic and baseline scores in clinical scales are presented in Table 1.
TABLE 1.
Baseline characteristics
| Characteristic | |
|---|---|
| N | 500 |
| Age, median [IQR] | 75 [68, 79] |
| Female, n (%) | 259 (51.8%) |
| Education, completed secondary school, n (%) | 465 (93.0%) |
| Education, completed college or university, n (%) | 241 (48.2%) |
| MMSE, mean (SD) | 23.4 (1.95) |
| ADAS‐cog, mean (SD) | 25.0 (8.14) |
| CDR‐SB, mean (SD) | 4.60 (1.93) |
| ADL, mean (SD) | 65.7 (9.20) |
| NPI, mean (SD) | 7.75 (8.99) |
IQR, interquartile range; SD, standard deviation; MMSE, Mini‐Mental State Examination; ADAS‐cog, Alzheimer Disease Assessment Scale−Cognitive Subscale; CDR‐SB, Clinical Dementia Rating sum of boxes; ADL,ADCS Activities of Daily Living (ADL); NPI, Neuropsychiatric Inventory (NPI).
At baseline, patients had an average concentration of NfL of 24.6 pg/mL and an average concentration of t‐tau of 2.28 pg/mL in plasma. Plasma NfL increased over the course of the study, whereas t‐tau did not (Table 1A).
3.2. Cross‐sectional biomarker relationships
Cross‐sectional Spearman correlations between plasma biomarkers, clinical scales, and age at baseline and 9 and 18 months are given in Table 2A. Both NfL and t‐tau in plasma had statistically significant correlations with age, MMSE, ADAS‐cog, CDR‐SB, and ADCS‐ADL at all visits except for the correlation between t‐tau and age at 18 months (all other P’s < 0.02). Furthermore, t‐tau in plasma significantly correlated with NPI at baseline (P = 0.0249) and plasma NfL significantly correlated with NPI at 9 and 18 months (P = 0.0210 and P = 0.0017, respectively).
TABLE 2.
Cross‐sectional Spearman correlations between plasma biomarkers, clinical scales, and age at baseline and 9 and 18 months (A), and cross‐sectional test for conditional independence of clinical scales and one biomarker given the other and age (B)
| A. | ||||||
|---|---|---|---|---|---|---|
| Baseline | 9 months | 18 months | ||||
| NfL (n = 500) | t‐tau (n = 368) | NfL (n = 500) | t‐tau (n = 383) | NfL (n = 500) | t‐tau (n = 382) | |
| Age | 0.432 (P < 0.0001) | 0.128 (P = 0.0143) | 0.390 (P < 0.0001) | 0.138 (P = 0.0068) | 0.368 (P < 0.0001) | 0.064 (P = 0.2111) |
| MMSE | −0.127 (P = 0.0043) | −0.130 (P = 0.0123) | — | — | −0.279 (P < 0.0001) | −0.134 (P = 0.0088) |
| ADAS‐cog | 0.285 (P < 0.0001) | 0.144 (P = 0.0057) | 0.269 (P < 0.0001) | 0.167 (P = 0.0011) | 0.296 (P < 0.0001) | 0.134 (P = 0.0086) |
| CDR‐SB | 0.215 (P < 0.0001) | 0.169 (P = 0.0011) | 0.325 (P < 0.0001) | 0.179 (P = 0.0004) | 0.368 (P < 0.0001) | 0.163 (P = 0.0014) |
| ADL | −0.206 (P < 0.0001) | −0.190 (P = 0.0002) | −0.308 (P < 0.0001) | −0.214 (P < 0.0001) | −0.362 (P < 0.0001) | −0.121 (P = 0.0181) |
| NPI | 0.072 (P = 0.1080) | 0.117 (P = 0.0249) | 0.103 (P = 0.0210) | 0.038 (P = 0.4627) | 0.140 (P = 0.0017) | 0.026 (P = 0.6061) |
| B. | |||
|---|---|---|---|
| Baseline (n = 368) | 9 months (n = 383) | 18 months (n = 382) | |
| Test for conditional independence between clinical scales and NfL given t‐tau and age | P < 0.0001 | P = 0.0004 | P < 0.0001 |
| Test for conditional independence between clinical scales and t‐tau given NfL and age | P = 0.4108 | P = 0.0905 | P = 0.4684 |
MMSE, Mini‐Mental State Examination; ADAS‐cog, Alzheimer Disease Assessment Scale‐Cognitive Subscale; CDR‐SB, Clinical Dementia Rating sum of boxes; ADL, ADCS Activities of Daily Living (ADL); NPI, Neuropsychiatric Inventory.
To evaluate if the two plasma biomarkers represented similar or complementary information in terms of clinical status, conditional independence between clinical scales (5‐dimensional outcome of MMSE, ADAS‐cog, CDR‐SB, ADCS‐ADL, and NPI at a visit) and each biomarker given the other biomarker and age was tested at each time point. The hypothesis of conditional independence was rejected for NfL given t‐tau and age (P < 0.0005 at all time points; Table 2B) but not for t‐tau given NfL and age (P > 0.09 at all time points; Table 2B). These results suggest that plasma t‐tau may not hold additional information to plasma NfL and age in terms of predicting clinical status of an individual.
Gaussian graphical models were used to estimate the relations between NfL, age, and clinical scales. The estimated partial correlation networks at baseline and 9 and 18 months are shown in Figure 1.
At baseline, plasma NfL had non‐zero partial correlations with age (partial correlation 0.38, P < 0.0001), ADAS‐cog (partial correlation 0.15, P = 0.0001), and CDR‐SB (partial correlation 0.10, P = 0.0105).
At 9 months, plasma NfL had non‐zero partial correlations with age (partial correlation 0.34, P < 0.0001), ADAS‐cog (partial correlation 0.12, P = 0.0049), and CDR‐SB (partial correlation 0.14, P = 0.0001).
At 18 months, plasma NfL had non‐zero partial correlations with age (partial correlation 0.32, P < 0.0001) and CDR‐SB (partial correlation 0.19, P < 0.0001).
3.3. Baseline biomarker and longitudinal clinical scales
Baseline plasma NfL was significantly correlated with all clinical scales at 18 months (all P’s < 0.002; Table A.2) and change from baseline at 18 months in all scales except NPI (all other P’s < 0.01). Baseline plasma t‐tau was significantly correlated with all clinical scales at 18 months (all P < 0.04), but not with change from baseline at 18 months in any of the clinical scales.
The hypothesis of conditional independence between baseline plasma NfL and 18‐month change from baseline in clinical scales given baseline scores and age was rejected (P = 0.0048, n = 500). The same hypothesis for conditional independence for baseline plasma t‐tau was not rejected (P = 0.5642, n = 368). This suggests that plasma NfL holds additional information about future progression than what is available in the clinical scales and patient age at baseline, but that plasma t‐tau does not. Similarly, it was found that baseline plasma NfL held additional information to baseline clinical scales about 9‐month change from baseline in clinical scales and that 9‐month plasma NfL held additional information to 9‐month clinical scales about 18‐month change from baseline, but that t‐tau did not hold additional information in the same scenarios (Supplementary Material, Table A.3).
A Gaussian graphical model was used to estimate the relations between baseline plasma NfL, age, scores on clinical scales at baseline, and change in scores after 18 months. In the estimated model, plasma NfL had non‐zero partial correlation with age, baseline ADAS‐cog, and baseline CDR‐SB. Furthermore, baseline plasma NfL had a non‐zero partial correlation with ADL change from baseline at 18 months (partial correlation −0.12, P < 0.0001; Figure 2). Note that this suggests that plasma NfL does contain independent predictive information about change in other clinical scales at 18 months, since change in clinical scales at 18 months except NPI were all highly correlated (absolute Spearman correlations between 0.47‐0.68, data not shown).
FIGURE 2.
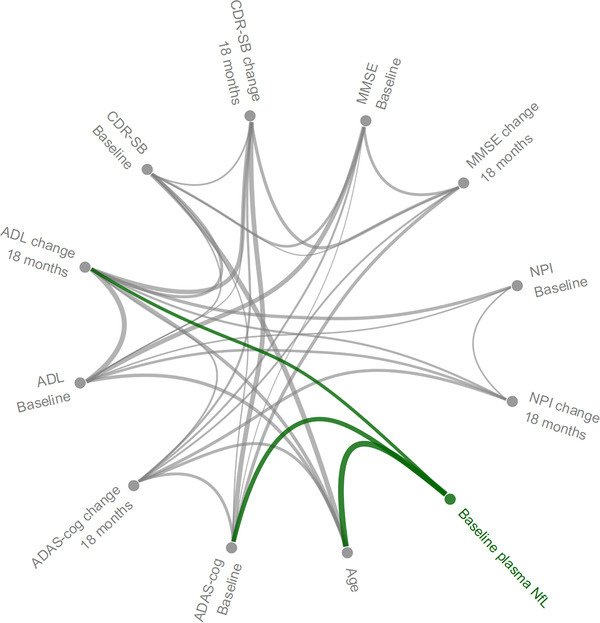
Conditional dependence structure between age, plasma NfL at baseline, clinical scales at baseline, and change in clinical scales at 18 months. Connection widths are proportional to the size of the estimated conditional correlations between variables. MMSE, Mini‐Mental State Examination; ADAS‐cog, Alzheimer Disease Assessment Scale−Cognitive Subscale; CDR‐SB, Clinical Dementia Rating sum of boxes; ADL,ADCS Activities of Daily Living (ADL); NPI, Neuropsychiatric Inventory
3.4. How far into the future is plasma NfL predictive of progression?
To investigate how far into the future plasma NfL was independently predictive of progression on the primary end point, ADAS‐cog, conditional independence tests of plasma NfL, and ADAS‐cog change from baseline at each interval of evaluation time points (3‐18, 6‐18, 9‐18, 12‐18, 15‐18, and 18 months) given the past observations of ADAS‐cog was done (Table 3).
TABLE 3.
Cross‐sectional test for conditional independence of clinical scales and each biomarker given the other and age
| Outcome variable | Conditioned on | NfL at baseline | NfL at 9 months | NfL at 18 months |
|---|---|---|---|---|
| Change from baseline in ADAS‐cog at 3, 6, 9, 12, 15, and 18 months | ADAS‐cog and age at baseline | P = 0.0023 | P = 0.0004 | P = 0.0012 |
| Change from baseline in ADAS‐cog at 6, 9, 12, 15, and 18 months | ADAS‐cog and age at baseline and ADAS‐cog change from baseline at 3 months | P = 0.0294 | P = 0.0044 | P = 0.0075 |
| Change from baseline in ADAS‐cog at 9, 12, 15, and 18 months | ADAS‐cog and age at baseline and ADAS‐cog change from baseline at 3 and 6 months | P = 0.1432 | P = 0.0073 | P = 0.1088 |
| Change from baseline in ADAS‐cog at 12, 15, and 18 months | ADAS‐cog and age at baseline and ADAS‐cog change from baseline at 3, 6, and 9 months | P = 0.0877 | P = 0.0233 | P = 0.0313 |
| Change from baseline in ADAS‐cog at 15 and 18 months given | ADAS‐cog and age at baseline and ADAS‐cog change from baseline at 3, 6, 9, and 12 months | P = 0.2365 | P = 0.5682 | P = 0.0302 |
| Change from baseline in ADAS‐cog at 18 months | ADAS‐cog and age at baseline and ADAS‐cog change from baseline at 3, 6, 9, 12 and 15 months | P = 0.1017 | P = 0.9451 | P = 0.0073 |
MMSE, Mini‐Mental State Examination; ADAS‐cog, Alzheimer Disease Assessment Scale‐Cognitive Subscale; CDR‐SB, Clinical Dementia Rating sum of boxes; ADL, ADCS Activities of Daily Living (ADL); NPI, Neuropsychiatric Inventory (NPI).
It was found that plasma NfL at baseline was independently predictive of future decline up to 6 to 18 months given age, ADAS‐cog at baseline, and change from baseline up to and including 3 months (P = 0.0294), but not beyond that. It was also found that plasma NfL at 9 months was independently predictive of future decline up to 12 to 18 months given age, ADAS‐cog at baseline, and change from baseline up to and including 9 months (P = 0.0233), but not beyond that.
This suggests that in mild AD patients, plasma NfL contains information about progression on ADAS‐cog scores 3 to 6 months into the future that is independent of current and past ADAS‐cog scores.
3.5. Power in clinical trials
Using an MMRM analysis of change from baseline in ADAS‐cog total scores, it was found that there was a significant main effect of baseline plasma NfL (P = 0.0195), but no visit interaction with baseline plasma NfL (P = 0.1917). No significant main or visit‐interaction effects of baseline plasma t‐tau were found (P = 0.7742 and P = 0.1467).
Inclusion of a main effect of baseline plasma NfL decreased the proportion of unexplained variance by less than 1% at all visits (0.5% at 18 months). This resulted in a reduction of one patient per arm (312 vs 313) needed to achieve at least 80% power to detect a 2‐point treatment effect at 18 months.
3.6. Longitudinal biomarkers and longitudinal clinical scales
To explore the full set of relations between biomarkers and clinical scales, a Gaussian graphical model was fitted on complete‐case data of plasma NfL and t‐tau at baseline and 9 and 18 months, as well as all clinical scales at baseline at changes at 9 and 18 months (n = 275). The estimated conditional dependence structure is shown in Figure 3, and suggested that plasma NfL (baseline and 9 and 18 months) contributed independent information about ADAS‐cog at baseline and change from baseline in ADL at 18 months. Furthermore, the model suggested that plasma t‐tau contributed independent information about ADL at baseline.
FIGURE 3.
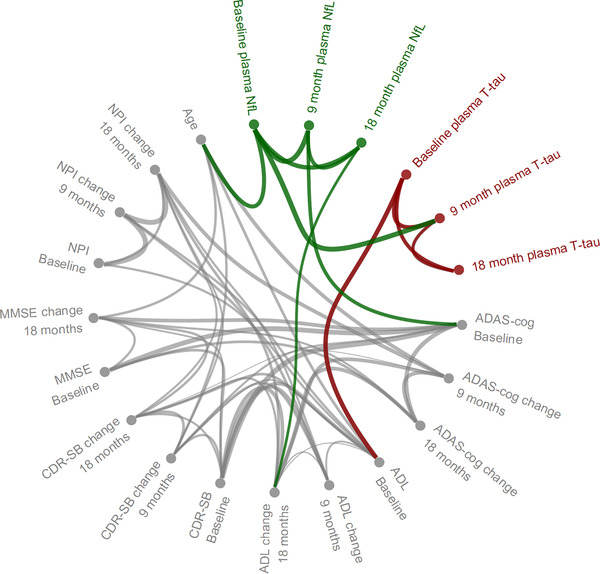
Conditional dependence structure between age, plasma NfL, and t‐tau at baseline and 9 and 18 months, clinical scales at baseline, and changes in clinical scales at 9 and 18 months. Connection widths are proportional to the size of the estimated conditional correlations between variables. MMSE, Mini‐Mental State Examination; ADAS‐cog, Alzheimer Disease Assessment Scale−Cognitive Subscale; CDR‐SB, Clinical Dementia Rating sum of boxes; ADL,ADCS Activities of Daily Living (ADL); NPI, Neuropsychiatric Inventory
4. DISCUSSION
It has been suggested previously that high levels of NfL 5 , 6 and t‐tau 9 , 26 in CSF or plasma are markers of neurodegeneration intensity and hence increased rate of cognitive decline in AD. This motivates detailed analyses of plasma NfL and t‐tau in clinical trial settings, to test if these biomarkers contain information about the disease intensity and prognosis that is independent from demographic information and cognitive data that is already collected in clinical trials. We therefore tested this in a completed 18‐month clinical trial of mild AD, using longitudinal biomarker data. Baseline plasma NfL did predict change in cognition at 18 months, even when adjusting for baseline cognition and demographics; however, baseline plasma NfL was not independently predictive of cognitive changes at 18 months when adjusting for the longitudinal trajectories beyond 6 months into the future. In sum, the current analysis therefore suggested that baseline measures of plasma NfL or t‐tau do not explain variation in observed longitudinal trajectories in longer‐term clinical trials in mild AD beyond what can be predicted by basic demographics and cognitive testing. The results did suggest, however, that plasma NfL may hold independent information about shorter‐term (3‐6 month) progression of disease that is complementary to what is captured in clinical scales (meaning that a measure of plasma NfL may improve the capacity to identify changes in rate of cognitive decline and neurodegeneration). In general, we did not find that plasma t‐tau contained information that was independent from NfL measurements or cognitive data. Taken together, these results are sobering with regard to the ability of these biomarkers to improve power over the full course of a long clinical trial in mild AD. However, the finding that plasma NfL holds independent information about short‐term cognitive decline support its use as a drug‐response biomarker in trials of disease‐modifying drugs, where it may complement the typical clinical scale end point by offering a glimpse 3 to 6 months into the future. Furthermore, it may open a possibility that plasma NfL can be used in shorter trials in early stages of development, by enriching populations for participants more likely to decline. This could be helpful in pilot studies aiming to identify drug candidates that are more likely to ultimately be successful in large phase III trials.
One limitation of this study is that the AD patients were diagnosed according to clinical criteria, without including biomarker information about Aβ pathology. It is therefore likely that some of the individuals did not have AD as the underlying cause of their cognitive decline, but rather other medical conditions. Another limitation is that all participants were symptomatic, precluding us from assessing the value of these biomarkers for clinical trials in the earliest stages of AD, when cognitive tests may be less predictive of future decline. Another limitation is that the samples were stored more than 10 years prior to measurement of NfL and t‐tau. Although repeated freeze‐thaw experiments suggest that the proteins are relatively stable, 27 long‐term stability has not been formally addressed, and degradation may have influenced the results. Finally, we have not measured some of the recently developed very promising blood‐based AD‐specific biomarkers for phosphorylated tau, 28 , 29 , 30 which may be more useful for AD trials than non‐specific markers of neurodegeneration.
5. CONCLUSION
Baseline plasma NfL and t‐tau provide only little information beyond demographics and cognitive data over the full course of an 18‐month clinical trial in mild AD, but plasma NfL may provide unique and complementary information about short‐term (3‐6 month) cognitive decline, which may be useful for assessing treatment response in trials of disease‐modifying drugs and for designing pilot studies for novel AD therapies.
CONFLICT OF INTEREST
Kaj Blennow has served as a consultant, on advisory boards, or on data monitoring committees for Abcam, Axon, Biogen, Julius Clinical, Lilly, MagQu, Novartis, Roche Diagnostics, and Siemens Healthineers; and is a co‐founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. Henrik Zetterberg has served on scientific advisory boards for Denali, Roche Diagnostics, Wave, Samumed, and CogRx; has given lectures in symposia sponsored by Fujirebio, Alzecure and Biogen; and is a co‐founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. Niklas Mattsson‐Carlgren has no disclosures.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
This study was supported by H. Lundbeck A/S. KB is supported by the Swedish Research Council (#2017‐00915), the Alzheimer Drug Discovery Foundation (ADDF) USA (#RDAPB‐201809‐2016615), the Swedish Alzheimer Foundation (#AF‐742881), Hjärnfonden, Sweden (#FO2017‐0243), the Swedish state under the agreement between the Swedish government and the County Councils, the ALF‐agreement (#ALFGBG‐715986), and European Union Joint Program for Neurodegenerative Disorders (JPND2019‐466‐236). Henrik Zetterberg is a Wallenberg Scholar supported by grants from the Swedish Research Council (#2018‐02532), the European Research Council (#681712), the Swedish state under the agreement between the Swedish government and the County Councils, the ALF‐agreement (#ALFGBG‐720931), the Alzheimer Drug Discovery Foundation (ADDF) USA (#201809‐2016862), and the UK Dementia Research Institute at UCL.
Raket LL, Kühnel L, Schmidt E, et al. Utility of plasma neurofilament light and total tau for clinical trials in Alzheimer's disease. Alzheimer's Dement. 2020;12:e12099 10.1002/dad2.12099
Contributor Information
Lars Lau Raket, Email: llra@lundbeck.com.
Niklas Mattsson‐Carlgren, Email: niklas.mattsson-carlgren@med.lu.se.
REFERENCES
- 1. Hansson O, Janelidze S, Hall S, et al. Blood‐based NfL: a biomarker for differential diagnosis of Parkinsonian disorder. Neurology. 2017;88:930‐937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Preische O, Schultz SA, Apel A, et al. Serum neurofilament dynamics predicts neurodegeneration and clinical progression in presymptomatic Alzheimer's disease. Nat Med. 2019;25:277‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Wolf F, Ghanbari M, Licher S, et al. Plasma tau, neurofilament light chain and amyloid‐β levels and risk of dementia; a population‐based cohort study. Brain. 2020;143(4):1220‐1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weston PSJ, Poole T, Ryan NS, et al. Serum neurofilament light in familial Alzheimer disease: a marker of early neurodegeneration. Neurology. 2017;89:2167‐2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mattsson N, Cullen NC, Andreasson U, Zetterberg H, Blennow K. Association between longitudinal plasma neurofilament light and neurodegeneration in patients with Alzheimer disease. JAMA Neurol. 2019;76:791‐799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mattsson N, Andreasson U, Zetterberg H, Blennow K. Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer disease. JAMA Neurol. 2017;74:557‐566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Palmqvist S, Janelidze S, Stomrud E, et al. Performance of fully automated plasma assays as screening tests for Alzheimer disease–related β‐amyloid status. JAMA Neurol. 2019;76:1060‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fossati S, Ramos Cejudo J, Debure L, et al. Plasma tau complements CSF tau and P‐tau in the diagnosis of Alzheimer's disease. Alzheimers Dement (Amst). 2019;11:483‐492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mattsson N, Zetterberg H, Janelidze S, et al. Plasma tau in Alzheimer disease. Neurology. 2016;87:1827‐1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zetterberg H, Wilson D, Andreasson U, et al. Plasma tau levels in Alzheimer's disease. Alzheimers Res Ther. 2013;5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang T, Xiao S, Liu Y, et al. The efficacy of plasma biomarkers in early diagnosis of Alzheimer's disease. Int J Geriatr Psychiatry. 2014;29:713‐719. [DOI] [PubMed] [Google Scholar]
- 12. Sparks DL, Kryscio RJ, Sabbagh MN, et al. Tau is reduced in AD plasma and validation of employed ELISA methods. Am J Neurodegener Dis. 2012;1:99. [PMC free article] [PubMed] [Google Scholar]
- 13. Green RC, Schneider LS, Amato DA, et al. Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: a randomized controlled trial. JAMA. 2009;302:2557‐2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gisslén M, Price RW, Andreasson U, et al. Plasma concentration of the neurofilament light protein (NFL) is a biomarker of CNS injury in HIV infection: a cross‐sectional study. EBioMedicine. 2016;3:135‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984;141(11):1356‐1364. [DOI] [PubMed] [Google Scholar]
- 16. Morris JC. The Clinical Dementia Rating (CDR). Neurology. 1993;43(11):2412‐2412‐a. [DOI] [PubMed] [Google Scholar]
- 17. Folstein MF, Folstein SE, McHugh PR. “Mini‐mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189‐198. [DOI] [PubMed] [Google Scholar]
- 18. Galasko D, Bennett D, Sano M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer's disease. Alzheimer Dis Assoc Disord. 1997;11(Suppl 2):S33‐S39. [PubMed] [Google Scholar]
- 19. Cummings JL, Mega M, Gray K, Rosenberg‐Thompson S, Carusi DA, Gornbein J. The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308‐2308. [DOI] [PubMed] [Google Scholar]
- 20. Shah RD, Peters J. The hardness of conditional independence testing and the generalised covariance measure. Ann Stat. 2020;48(3):1514‐1538. [Google Scholar]
- 21. Chen T, Guestrin C, Xgboost: A scalable tree boosting system. Paper presented at: Proceedings of the 22nd acm sigkdd international conference on knowledge discovery and data mining 2016.
- 22. Schwarz G. Estimating the dimension of a model. Anna Stat. 1978;6:461‐464. [Google Scholar]
- 23. Højsgaard S, Edwards D, Lauritzen S. Graphical Models With R. Springer Science & Business Media; 2012. [Google Scholar]
- 24. Lauritzen SL. Graphical Models. Clarendon Press; 1996. [Google Scholar]
- 25. Laird NM, Ware JH. Random‐effects models for longitudinal data. Biometrics. 1982;38:963‐974. [PubMed] [Google Scholar]
- 26. Buchhave P, Minthon L, Zetterberg H, ÅK Wallin, Blennow K, Hansson O. Cerebrospinal fluid levels ofβ‐amyloid 1‐42, but not of tau, are fully changed already 5 to 10 years before the onset of Alzheimer dementia. Arch Gen Psychiatry. 2012;69:98‐106. [DOI] [PubMed] [Google Scholar]
- 27. Keshavan A, Heslegrave A, Zetterberg H, Schott JM. Stability of blood‐based biomarkers of Alzheimer's disease over multiple freeze‐thaw cycles. Alzheimers Dement (Amst). 2018;10:448‐451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Janelidze S, Mattsson N, Palmqvist S, et al. Plasma P‐tau181 in Alzheimer's disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer's dementia. Nat Med. 2020;26:379‐386. [DOI] [PubMed] [Google Scholar]
- 29. Thijssen EH, La Joie R, Wolf A, et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer's disease and frontotemporal lobar degeneration. Nat Med. 2020;26:387‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Palmqvist S, Janelidze S, Quiroz YT, et al. Discriminative accuracy of plasma Phospho‐tau217 for Alzheimer disease vs other neurodegenerative disorders. JAMA. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.


