Abstract
Clinical manifestations of COVID-19 are known to be variable with growing evidence of nervous system involvement. In this case report, we describe the symptoms of a patient infected with SARS-CoV-2 whose clinical course was complicated with Guillain-Barré syndrome (GBS). We present a case of a 58-year-old woman who was initially diagnosed with COVID-19 pneumonia due to symptoms of fever and cough. Two weeks later, after the resolution of upper respiratory tract symptoms, she developed symmetric ascending quadriparesis and paresthesias. The diagnosis of GBS was made through cerebrospinal fluid analysis and she was successfully treated with intravenous immunoglobulin administration.
Keywords: pneumonia (infectious disease), infection (neurology), peripheral nerve disease
Background
At the time of writing, there exist more than 100 000 new cases, about five million confirmed cases, and over 350 000 deaths from COVID-19 worldwide according to the WHO. The first series of patients were reported in Wuhan, China, in December 2019 and were typically diagnosed in patients with a pneumonia-like presentation.1 There is growing evidence that COVID-19 can also affect the nervous system. To date, there are only 12 published cases of COVID-19-related Guillain-Barré syndrome (GBS).2 We have a limited understanding of how COVID-19 leads to GBS as it needs to be further investigated. We report a case of GBS in a patient with COVID-19 in New Jersey. GBS is an acute, autoimmune, polyradiculoneuropathy characterised by progressive ascending weakness and diminished/absent reflexes. Patients may or may not have additional symptoms involving cranial nerves, sensory symptoms and autonomic dysfunction.2 The abnormal immune response may be triggered by viral/bacterial infections, immunisations or surgery. The diagnosis is made through clinical findings, cerebrospinal fluid analysis (CSF) and nerve conduction studies. Treatment involves the administration of intravenous immunoglobulins, plasma exchange, continuous monitoring and supportive care. Though the syndrome is rare, early diagnosis and treatment can significantly improve outcomes and avoid the need for ventilatory support.3
Case presentation
Our patient was a 58-year-old woman with a medical history significant for cervical spondylosis and disc herniation for which she underwent an anterior cervical discectomy and anterior interbody arthrodesis at C4–C7 3 years ago. Two weeks prior to this hospital admission, she developed a fever, cough and back pain amidst the COVID-19 pandemic. The patient tested positive for COVID-19 and was treated with azithromycin for 5 days at home. Subsequently, over the next 2 weeks, her fever and cough resolved, but the lower back pain persisted. This was characterised by a throbbing, 7/10 intensity (numeric rating scale), radiating to both the lower extremities. She then had a new onset of numbness, pins and needles sensation in the left lower extremity associated with mild weakness. These same symptoms then progressed to the contralateral lower extremity over the next 24 hours with an unstable gait. This progression triggered an emergency room visit. She had no headaches, loss of consciousness, changes in mental status, changes in vision and speech, difficulty swallowing, seizure-like symptoms or urine/bowel incontinence.
She denied any history of tick bites or trauma.
Physical examination findings included stable vital signs and motor strength was 5/5 (Oxford Scale) in all four extremities, normal deep tendon reflexes in both upper and lower extremities and a negative Babinski sign bilaterally. There was no defined spine sensory level and no upper motor neuron weakness findings. She was admitted to the hospital for idiopathic paresthesias with suspicion of lumbosacral radiculopathy.
Investigations
On the day of admission, notable laboratory findings include a white cell count of 13.5×109/L, normal erythrocyte sedimentation rate, C-reactive protein, vitamin B12 level andthyroid stimulating hormone. A CT scan of the head and radiograph of the lumbar spine was performed. The CT scan of the head showed no intraparenchymal mass or lesions, no haemorrhage and no findings of normal pressure hydrocephalus. Radiograph of the spine showed multilevel degenerative changes.
MRI of the lumbar spine without contrast (see figure 1) showed moderate bilateral and moderate left-sided neural foraminal narrowing at L2–L3 and L3–L4, respectively, and unremarkable conus medullaris. Because of persistent headaches, an MRI of the brain was done, which showed normal findings.
Figure 1.
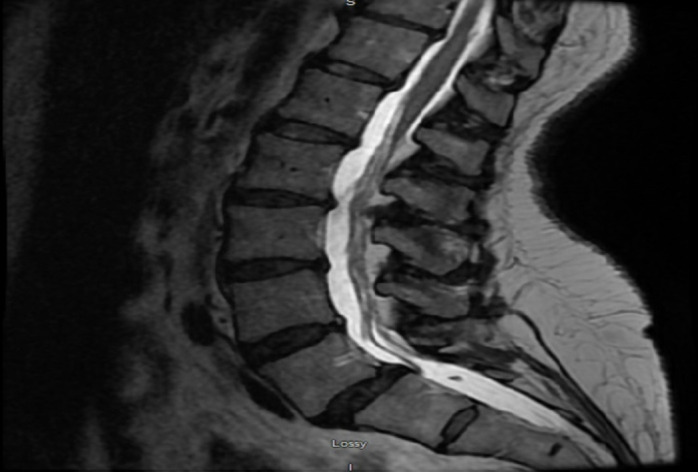
MRI of the lumbar spine without contrast.
Over the next 24 hours, a repeat COVID-19 test sent came back positive. The patient developed worsening weakness with muscle strength 3/5 in both lower extremities and new onset of weakness of her left upper extremity with muscle strength 4/5. Additional symptoms included numbness, tingling and paresthesias of the affected extremities. Now there was a change in her examination with diminished deep tendon reflexes when compared with the previous day. With the patient exhibiting ascending weakness with diminished reflexes, an urgent lumbar puncture was performed to rule out GBS. Initially, a lumbar puncture was deferred as there was a low suspicion for GBS based on the patient’s presentation with normal deep tendon reflexes and an unremarkable conus medullaris. CSF revealed findings suggestive of albumin-cytologic disassociation with a white cell count of 2 cu/mm and protein count of 117 mg/dL. Nerve conduction study was not performed, as this was not available in the inpatient setting.
Differential diagnosis
On the day of hospital presentation, the initial suspicion was lumbar radiculopathy in the setting of back pain with paresthesias. As studies emerged showingpatients with COVID-19 often present with hypercoagulable changes including stroke, imaging of the head was performed to rule out this pathology. Other differentials on admission were transverse myelitis, but the patient did not have a clear sensory level and subsequently did not have inflammatory findings on CSF analysis. Later as the patient exhibited clinical findings of ascending paralysis with diminished deep tendon reflexes, the diagnosis of GBS became more evident.
Treatment
With the initial suspicion of traditional lumbar radiculopathy, physical therapy was initiated. As the diagnosis of GBS became clearer, the patient was given 2 mg/kg intravenous immunoglobulins for 4 days and the respiratory status was monitored by checking vital capacity every 4 hours. She also received gabapentin 300 mg two times per day for neuropathic pain. Fortunately, our patient had stable vital capacity throughout the stay and no signs of respiratory muscle weakness. After receiving this treatment, the patient’s symptoms improved significantly and she was discharged to an acute rehabilitation facility for regular physical therapy.
Outcome and follow-up
Follow-up was established with the patient who mentioned that her symptoms improved. She had complete resolution of paresthesias and about 80% improvement in motor strength of all extremities. Currently, the patient is performing daily physical therapy successfully at her rehab facility.
Discussion
SARS-CoV-2 frequently afflicts the respiratory system and gastrointestinal tracts. It shares its identity with other human coronaviruses including SARS-CoV and Middle East respiratory syndrome coronavirus. In this group of viruses, the respiratory system is commonly affected but they have also shown the involvement of the nervous system.4 Increasing reports of neurologic manifestations of COVID-19 are emerging, but only a few cases of GBS associated with this virus have been established. GBS is an immune-mediated response, likely from a recent infection, where the immune system attacks the peripheral nerves due to a molecular mimicry phenomenon. This has preceded two-thirds of the times by an upper respiratory infection or gastroenteritis.
The case series by Mao et al in Wuhan, China, was one of the first studies that showed neurologic manifestations in patients with COVID-19. They concluded that patients with more severe COVID-19 illness were more likely to have neurologic symptoms.5 In contrast, our patient’s respiratory status was relatively stable.
In Italy, a series of five patients were diagnosed with GBS 5–10 days after a viral illness from COVID-19. Similar to our patient, they did not show typical MRI findings of GBS including surface thickening and contrast enhancement on the conus medullaris and the nerve roots of the cauda equina. Only one of the five patients had a functional recovery to the point of ambulation. We cannot yet conclude the severity of neurologic injury with COVID-19-associated GBS.6
The literature shows there is variability in the presentation of COVID-19 and GBS. Our case had a typical course of viral symptoms preceding GBS findings. However, two other case reports identified concurrent respiratory and neurologic symptoms.7 8 Besides, the duration from onset of viral illness to neurologic manifestations have ranged from 5 to 24 days.9 In all cases reported, treatment with IVIG was administered. However, the recovery varied from full neurologic recovery to no change in extremity function and terminal respiratory failure.7–12
There are several theories on how the virus attacks the nervous system. Studies postulate that the virus can infect a peripheral neuron, use an active retrograde transport mechanism across the synapse onto the cell body and reach the brain.13 Other proposed mechanisms include direct damage through angiotensin converting enzyme-2ACE2 receptors, cytokine-related injury and hypoxia-related sequela.14 It is unclear if the COVID-19 itself triggers the formation of antibodies again any specific forms of glycolipids seen in some forms of GBS.15 There is a need for further investigation into how COVID-19 is related to GBS.
Learning points.
In the spectrum of neurologic manifestations of COVID-19, Guillain-Barré syndrome (GBS) should be considered in patients with peripheral nervous system symptoms.
Early recognition and treatment of GBS can prevent potentially serious morbidity and mortality in patients with COVID-19.
As we evaluate and review more patients with COVID-19, our understanding of neurologic and postviral complications continues to grow.
Footnotes
Contributors: Contributions were collaboratively made by all authors. These contributions include drafting the article, conception or design of the work, critical revision of the article and final approval of the version to be published.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Li Q, Guan X, Wu P, et al. . Early transmission dynamics in Wuhan, China, of novel Coronavirus–Infected pneumonia. N Engl J Med 2020;382:1199–207. 10.1056/NEJMoa2001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asbury AK, Cornblath DR. Assessment of current diagnostic criteria for Guillain-Barr syndrome. Ann Neurol 1990;27:S21–4. 10.1002/ana.410270707 [DOI] [PubMed] [Google Scholar]
- 3.Willison HJ, Jacobs BC, van Doorn PA, et al. . Guillain-Barré syndrome. The Lancet 2016;388:717–27. 10.1016/S0140-6736(16)00339-1 [DOI] [PubMed] [Google Scholar]
- 4.Montalvan V, Lee J, Bueso T, et al. . Neurological manifestations of COVID-19 and other coronavirus infections: a systematic review. Clin Neurol Neurosurg 2020;194:105921–7. 10.1016/j.clineuro.2020.105921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mao L, Jin H, Wang M, et al. . Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurology 2020:E1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toscano G, Palmerini F, Ravaglia S, et al. . Guillain-Barré syndrome associated with SARS-CoV-2. N Engl J Med 2020:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alberti P, Beretta S, Piatti M, et al. . Guillain-Barré syndrome related to COVID-19 infection. Neurol Neuroimmunol Neuroinflamm 2020;7:e741. 10.1212/NXI.0000000000000741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Virani A, Rabold E, Hanson T, et al. . Guillain-Barré syndrome associated with SARS-CoV-2 infection. IDCases 2020;20:e00771. 10.1016/j.idcr.2020.e00771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camdessanche J-P. COVID-19 may induce Guillain–Barré syndrome. RevueNeurologique 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Otmani HEI. Covid-19 and Guillain-Barré syndrome: more than a coincidence! Revue Neurologique 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Padroni M, Mastrangelo V, Asioli GM, et al. . Guillain-Barré syndrome following COVID-19: new infection, old complication? J Neurol 2020:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao H, Shen D, Zhou H, et al. . Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? The Lancet Neurology 2020;19:383–4. 10.1016/S1474-4422(20)30109-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baig AM, Khaleeq A, Ali U, et al. . Evidence of the COVID-19 virus targeting the CNS: tissue distribution, Host–Virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci 2020;11:995–8. 10.1021/acschemneuro.0c00122 [DOI] [PubMed] [Google Scholar]
- 14.Bridwell R, Long B, Gottlieb M. Neurologic complications of COVID-19. Am J Emerg Med 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sedaghat Z, Karimi N. Guillain Barre syndrome associated with COVID-19 infection: a case report. J Clin Neurosci 2020;76:233–5. 10.1016/j.jocn.2020.04.062 [DOI] [PMC free article] [PubMed] [Google Scholar]


