Abstract
Outpatient therapeutic feeding protocols for the treatment of uncomplicated severe acute malnutrition in children were initially based on weight gain data from inpatient settings and expert knowledge of the physiological requirements during recovery. However, weight gain and energy requirements from historic inpatient settings may differ from modern outpatient settings and therefore may not be appropriate to guide current therapeutic feeding protocols. We calculated the weight gain and average estimated total daily energy requirement of children successfully treated for uncomplicated severe acute malnutrition as outpatients in Niger (n = 790). Mean energy provided by six therapeutic feeding protocols was calculated and compared with average estimated energy requirements in the study population. Overall weight gain was 5.5 g·kg−1·day−1 among recovered children. Average energy requirements ranged from 92 to 110 kcal·kg−1·day−1 depending on the estimation approach. Two current therapeutic feeding protocols were found to provide an excess of energy after the first week of treatment in our study population, whereas four research protocols tended to provide less energy than the estimated requirement after the first week of treatment. Alternative feeding protocols have the potential to simplify and lead to important savings for programmes but should be evaluated to show adequacy to meet the energy needs of children under treatment, as well as feasibility and cost efficiency. Our findings rely on theoretical calculations based on several assumptions and should be confirmed in field studies.
Keywords: community‐based management of acute malnutrition, energy requirement, Niger, ready‐to‐use therapeutic food, severe acute malnutrition, weight gain
Abbreviations
- mid‐upper arm circumference
MUAC
- ready‐to‐use therapeutic foods
RUTF
- severe acute malnutrition
SAM
Key messages.
Outpatient therapeutic feeding protocols for the treatment of uncomplicated severe acute malnutrition (SAM) in children were initially based on inpatient weight gain and expert knowledge.
Using data from an outpatient clinical trial in Niger, this study provides updated estimates of weight gain and average estimated energy requirements among children treated for uncomplicated SAM as outpatients and compares average estimated energy requirements with current therapeutic feeding protocols.
Two current INGO therapeutic feeding protocols provided an excess of energy and nutrients after the first week of treatment, and four research protocols provided less energy than the estimated requirement after the first week of treatment.
1. INTRODUCTION
The community‐based management of acute malnutrition represents a transformative shift in the clinical management of children with severe acute malnutrition (SAM), where previously standard inpatient treatment is reserved for the stabilization of children with clinical complications and the majority of children are treated on an outpatient basis (Valid International, 2006; World Health Organization [WHO], 2013). This shift to predominantly outpatient care was made possible, in part, with the development of ready‐to‐use therapeutic foods (RUTF). RUTF are energy‐dense, micronutrient‐enriched pastes made of peanuts, oil, sugar, and milk powder. They have a nutritional profile similar to the milk‐based diet (F‐100) previously recommended by the WHO for the catch‐up growth phase of treatment, but unlike F‐100, they do not require on‐site preparation with clean water and can be used safely at home (Briend et al., 1999).
The community‐based management of SAM has been shown to be safe and effective (Ciliberto et al., 2005; Lenters, Wazny, Webb, Ahmed, & Bhutta, 2013; Manary, Ndkeha, Ashorn, Maleta, & Briend, 2004), but at the time of introduction in 2001 and international endorsement in 2007, evidence was limited on how to extend guidance on both nutritional and clinical management from the historical inpatient to the new outpatient setting. With limited experience and no high‐quality evidence, early practice in the outpatient model was guided by inpatient protocols and expert consensus. In particular, the current outpatient therapeutic feeding protocol was based on weight gain data from inpatient settings and extant understanding of physiological requirements during recovery. However, weight gain data from inpatient settings, where children were fed under close supervision by health staff with strict feeding schedules, may not represent weight gain achieved in outpatient settings today with no close supervision of feeding.
An understanding of weight gain and energy requirements in outpatient settings and comparison with current therapeutic feeding protocols are important to assure the optimal use of RUTF and cost‐effectiveness of programmes. Therapeutic feeding protocols that provide an excess of energy and nutrients waste limited resources, whereas providing too little energy and nutrients can jeopardize weight gain and clinical recovery. Using data from an outpatient clinical trial in Niger, this study describes the weight gain and average estimated energy requirements for nutritional recovery among children with uncomplicated SAM. We further compare the coverage of average energy requirements with current feeding protocols in our trial setting to identify possible ways to optimize therapeutic feeding guidelines.
2. METHODS
2.1. Study population
Participants in this study were enrolled in a randomized controlled trial of routine amoxicillin versus placebo in the treatment of uncomplicated SAM (clinicaltrials.gov NCT01613547). The parent study was conducted in four rural health centres in the Madarounfa district of Maradi region, Niger, which is located in the south central part of the country bordering Nigeria. Typical of the rural Sahel, household food production is linked to rain‐fed agriculture, where staple crops such as millet and sorghum are harvested once per year. Each year, the decrease in food quantity and quality experienced in the months preceding the harvest and the concurrent increase in infectious illness, including diarrhoea, pneumonia, and malaria, result in a seasonal increase in acute malnutrition among children <5 years of age. In 2012, the prevalence of acute malnutrition in children under 5 was estimated to be 19% (Institut National de la Statistique/Niger & ICF International, 2013).
The trial design has been described in detail elsewhere (Isanaka et al., 2016). In brief, between October 2012 and November 2013, the trial enrolled 2,412 children aged 6 to 59 months eligible for outpatient treatment of SAM. The trial's primary objective was to determine the effect of routine amoxicillin (80 mg·kg−1·day−1 for 7 days) versus placebo on nutritional recovery by 8 weeks. As per the national protocol, nutritional recovery was defined as weight‐for‐height z‐score ≥ −2 on two consecutive weekly visits and mid‐upper arm circumference (MUAC) ≥ 115 mm; no acute complication or oedema for at least 7 days; and completion of all antibiotic and antimalarial treatments at the time of discharge (Ministre de la Santé Publique (MSP, 2009)). Routine amoxicillin compared with placebo resulted in no significant difference in the risk of nutritional recovery but did improve short‐term weight gain and reduce the time until recovery. As a result of these differences, we restricted this analysis to the 790 children randomly assigned to receive routine amoxicillin (standard of care) and who recovered during the first 8 weeks of follow‐up (Figure S1). The study protocol was approved by the Comité Consultatif National d'Ethique, Niger, and the Comité de Protection des Personnes, Ile‐de‐France XI, Paris. Parents/legal guardians were provided information regarding the study objectives and procedures, and they provided written informed consent for their child's participation before the start of any study activities.
2.2. Data collection and follow‐up
All children received standard nutritional and medical care for outpatient treatment of uncomplicated SAM (excluding the randomized study intervention), described previously (Isanaka et al., 2016) and as per the Médecins Sans Frontières and Government of Niger protocol. Children were followed up on a weekly basis at the health centre until programme discharge (minimum 3 weeks and maximum 8 weeks) and additionally at 4, 8, and 12 weeks from admission according to the study protocol. Each assessment involved the collection of a medical history, physical assessment, and anthropometric assessment. Children who required inpatient care were transferred and censored from the study at the time of transfer.
2.3. Estimation of energy requirements
We calculated the estimated total daily energy requirement of the study population as the sum of energy required for tissue maintenance plus energy required for new tissue deposition (Equation 1) (Food and Agriculture Organization, WHO,, & United Nations University, 2004). Energy required for tissue maintenance was calculated as the energy needed to maintain bodyweight (85.5 kcal·kg−1·day−1) multiplied by the ith individual's average weight (kg) between weeks t and t − 1 (α i,t). Energy required for tissue maintenance was reported by Spady, Payne, Picou, and Waterlow (1976), where energy intake under no growth was estimated by regressing energy intake on weight gain among 11 children recovering from SAM weight gain. Energy required for tissue deposition was calculated as the energy needed to support muscle and fat deposition multiplied by the ith individual's weight change (g·day−1) between week t and t − 1 (δ i,t).
Because of uncertainty regarding the true energetic costs of tissue deposition (i.e., energy needed to support muscle and fat deposition), estimates were calculated using two approaches. The first approach applied an estimate of energetic costs of tissue deposition (4.4 kcal·g−1) published by Spady et al. in 1976, where indirect calorimetry was used among 11 children recovering from SAM in Jamaica (Equation 2). The second approach applied an estimate of energy requirements based on the composition of deposited tissues (93.5% lean tissue and 6.5% fat tissue) reported by Fabiansen et al. in 2017, where the deuterium dilution technique was used among 1,328 children receiving treatment for moderate acute malnutrition in Burkina Faso (Equation 3). Assuming lean tissue is 20% protein and 80% water, and 1 g of protein requires 5.65 kcal and 1 g of fat requires 9.25 kcal (Food and Agriculture Organization et al., 2004), the estimated energy cost of weight gain is 1.66 kcal·g−1. As an illustrative example, consider a child who weighed 6.3 kg at week t − 1 and 6.6 kg at week t, for a gain of 0.3 kg/7 days = 42.9 g·day−1. The child's average weight during this time is therefore (6.3 kg + 6.6 kg)/2 = 6.45 kg. The estimated daily energy needs would be (85.5 kcal·kg−1 * 6.45 kg) + (4.4 kcal·g−1 * 42.9 g) = 740 kcal according to Spady et al. and (85.5 kcal·kg−1 * 6.45 kg) + (1.66 kcal·g−1 * 42.9 g) = 623 kcal according to Fabiensen et al. For comparison, we additionally estimate energy requirements of normal growth assuming the age‐ and sex‐specific energetic requirements of healthy children published by the WHO in 2004 (Food and Agriculture Organization et al., 2004). For example, if the child above was a 12‐month‐old male, his daily caloric needs would be (310.2 kcal + 63.3 kcal·kg−1 * 6.45 kg − 0.263 kcal·kg−2 * 6.45 kg * 6.45 kg) + 13.2 kcal = 721 kcal. All three estimation approaches similarly applied individual weight and weight gain of the analysis population as observed over the course of treatment.
| (1) |
| (2) |
(Spady et al., 1976)
| (3) |
(Fabiansen et al., 2017)
Mean and 95th percentile energy requirements are presented for each estimation approach. Differences in estimated energy requirements by week of treatment (Weeks 1 to 8), weight (<6, 6 to <7, 7 to <8, and ≥8 kg), and MUAC (<115, 115 to <120, 120 to <125, and ≥125 mm) were assessed using linear regression models accounting for repeated measures within individuals (Rogers, 1993). Mean estimated energy requirements in the study population were finally compared with the mean energy provided by two therapeutic feeding protocols currently employed in the field by various international implementing agencies and four research protocols under investigation (Table 1). It is advised to feed children only RUTF during treatment, particularly at the early phase of rehabilitation; therapeutic feeding protocols are thus generally intended to provide sufficient energy and nutrients required for full recovery without use of other foods. The proportion of children receiving more or less energy than estimated to be required on average and the mean difference in energy provided versus required among these children (kcal·day−1) were calculated for each protocol. Analyses were conducted using Stata 13 (College Station, TX, USA).
Table 1.
Energy provided according to six therapeutic feeding protocols
| Protocol | Child characteristic | Energy provided | Source |
|---|---|---|---|
| A | Weight | Action Contre la Faim (2011) | |
| 3 to <3.5 kg | 625 kcal·day−1 | ||
| 3.5 to <5 kg | 750 kcal·day−1 | ||
| 5 to <7 kg | 1,000 kcal·day−1 | ||
| 7 to <10 kg | 1,500 kcal·day−1 | ||
| 10 to <15 kg | 2,000 kcal·day−1 | ||
| B | Weight | Médecins sans Frontières (2015) | |
| < 8 kg | 1,000 kcal·day−1 | ||
| ≥ 8 kg | 1,500 kcal·day−1 | ||
| C | Weight | Action Contre la Faim–Myanmar (James et al., 2015) | |
| If WHZ < −3 or MUAC < 110 mm | |||
| 3 to <3.5 kg | 625 kcal·day−1 | ||
| 3.5 to <5 kg | 750 kcal·day−1 | ||
| 5 to <7 kg | 1,000 kcal·day−1 | ||
| 7 to <10 kg | 1,500 kcal·day−1 | ||
| 10 to <15 kg | 2,000 kcal·day−1 | ||
| If WHZ ≥ 3 and MUAC ≥ 110 mm | 500 kcal·day−1 | ||
| D | MUAC | The Alliance for International Medical Action (Phelan, 2019) | |
| <115 mm or oedema | 175 kcal·kg−1·day−1 × child weight (kg) | ||
| 115 to <120 mm | 125 kcal·kg−1·day−1 × child weight (kg) | ||
| 120 to <125 mm | 75 kcal·kg−1·day−1 × child weight (kg) | ||
| E | MUAC | Combined Protocol for Acute Malnutrition Study (Bailey et al., 2018) | |
| <115 mm or oedema | 1,000 kcal·day−1 | ||
| 115 to <125 mm | 500 kcal·day−1 | ||
| F | MUAC | Randomized Controlled Trial in Sierra Leone (Maust et al., 2015) | |
| <115 mm or oedema | 175 kcal·kg−1·day−1 × child weight (kg) | ||
| 115 to <125 mm | 75 kcal·kg−1·day−1 × child weight (kg) | ||
| ≥125 mm | 200 kcal·day−1 | ||
Note. The World Health Organization recommends between 150 and 220 kcal·kg−1·day−1 to be provided in the inpatient management of severe acute malnutrition (A. Ashworth, Khanum, Jackson, & Schofield, 2003; World Health Organization, 1999). For the integrated management of acute malnutrition adopted in many national protocols, 170 kcal·kg−1·day−1 is currently recommended (Golden & Grellety, 2012).
DATA SHARING AND DATA ACCESSIBILITY
De‐identified individual participant data will be made available upon reasonable request to the corresponding author (sheila.isanaka@epicentre.msf.org).
3. RESULTS
Baseline characteristics of the study population (n = 790) are presented in Table 2. Weight gain and average estimated energy requirements are presented in Table 3 (kcal·kg−1·day−1) and Table S1 (kcal·day−1) according to the three estimation approaches (Spady et al., 1976; Fabiansen et al., 2017; and WHO, 2004) and by week of treatment, weight, and MUAC. Overall, average estimated energy requirements according to Spady et al. (1976) were higher than those according to Fabiansen et al. (2017) and greatest in the first week of treatment. Average proportional estimated energy requirements decreased with both increasing weight and MUAC.
Table 2.
Characteristics of study participants (n = 790)
| Characteristic | Value |
|---|---|
| Sociodemographics | |
| Child age at admission (month) | 17.6 ± 8.3 |
| Female sex | 401 (50.8) |
| Maternal age (year) | 27.1 ± 6.4 |
| Maternal level of education ≥6 years | 21 (2.7) |
| No. of household members | 7.3 ± 3.9 |
| Clinical characteristics at admission | |
| Weight‐for‐height z score (WHZ) | |
| Baseline WHZ (mean) | −3.01 ± 0.59 |
| Baseline WHZ<−3 | 454 (57.5) |
| Mid‐upper arm circumference (MUAC) | |
| MUAC (mean, mm) | 112.8 ± 4.2 |
| MUAC <115 mm | 607 (76.8) |
| Height‐for‐age z score (HAZ) | |
| HAZ (mean) | −3.03 ± 1.19 |
| HAZ<−2 | 633 (80.1) |
| Hemoglobin <11.0 g·dl−1 | 579 (73.3) |
| Rapid diagnostic test positive for malaria | 449 (56.8) |
| Axillary temperature >38.5 °C | 39 (4.9) |
| Signs of infection in previous 24 hr | |
| Diarrhoea | 263 (33.3) |
| Vomiting | 45 (5.7) |
| Cough | 146 (18.5) |
| Treatment outcomes | |
| Duration of treatment (week) | 4.0 ± 1.4 |
| Total weight gain during treatment (g·kg−1) | 168 ± 59 |
| Total MUAC gain during treatment (mm) | 10.9 ± 4.6 |
Note. Values are expressed as mean ± SD or n (%).
Table 3.
Average estimated proportional energy requirement (kcal·kg−1·day−1) among children receiving treatment for uncomplicated severe acute malnutrition in Niger
| Estimated energy requirement | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spady et al. (1976) | Fabiansen et al. (2017) | World Health Organization (2004) | ||||||||||
| N patient visits | Mean weight (kg) | Mean proportional weight gain (g·kg−1·day−1) | Mean | 95th percentile | p trend | Mean | 95th percentile | p trend | Mean | 95th percentile | p trend | |
| Overall | 3,187 | 7.28 | 5.5 | 110 | 162 | — | 95 | 115 | — | 92 | 109 | — |
| Week of treatment | ||||||||||||
| 1 | 790 | 6.95 | 11.8 | 137 | 184 | <.001 | 105 | 123 | <.001 | 94 | 112 | <.001 |
| 2 | 790 | 7.33 | 3.5 | 101 | 136 | 91 | 105 | 93 | 109 | |||
| 3 | 788 | 7.52 | 3.4 | 101 | 136 | 91 | 104 | 92 | 108 | |||
| 4 | 385 | 7.32 | 3.1 | 99 | 132 | 91 | 103 | 91 | 108 | |||
| 5 | 217 | 7.28 | 3.6 | 102 | 133 | 92 | 103 | 91 | 107 | |||
| 6 | 127 | 7.33 | 3.6 | 101 | 137 | 91 | 105 | 91 | 107 | |||
| 7 | 63 | 7.34 | 2.6 | 97 | 122 | 90 | 99 | 91 | 106 | |||
| 8 | 27 | 7.31 | 3.0 | 99 | 127 | 90 | 101 | 89 | 105 | |||
| Weight (kg) | ||||||||||||
| <6 | 540 | 5.64 | 6.6 | 115 | 165 | <.001 | 97 | 116 | <.001 | 80 | 113 | <.001 |
| 6 to <7 | 930 | 6.57 | 5.7 | 111 | 164 | 95 | 115 | 91 | 111 | |||
| 7 to <8 | 889 | 7.59 | 6.2 | 113 | 167 | 96 | 116 | 98 | 106 | |||
| ≥8 | 828 | 8.81 | 3.7 | 102 | 150 | 92 | 110 | 96 | 101 | |||
| MUAC (mm) | ||||||||||||
| <115 | 1,096 | 6.61 | 8.4 | 122 | 174 | <.001 | 99 | 119 | <.001 | 91 | 111 | <.001 |
| 115 to <120 | 924 | 7.17 | 4.7 | 106 | 156 | 93 | 112 | 92 | 108 | |||
| 120 to <125 | 770 | 7.79 | 3.9 | 103 | 152 | 92 | 111 | 94 | 106 | |||
| ≥125 | 397 | 8.38 | 2.2 | 95 | 128 | 89 | 101 | 95 | 106 | |||
Abbreviation: MUAC, mid‐upper arm circumference.
Figure 1 and Table S2 show the difference between the average estimated energy requirement and the energy provided under each of six therapeutic feeding protocols. Current therapeutic Protocols A and B provided sufficient energy to nearly all children (96–100%) after the first week of treatment, with mean excess energy provided in Weeks 2–8 (+386 to +606 kcal·day−1 by Spady et al., 1976, and +449 to +658 kcal·day−1 by Fabiansen et al., 2017).
Figure 1.
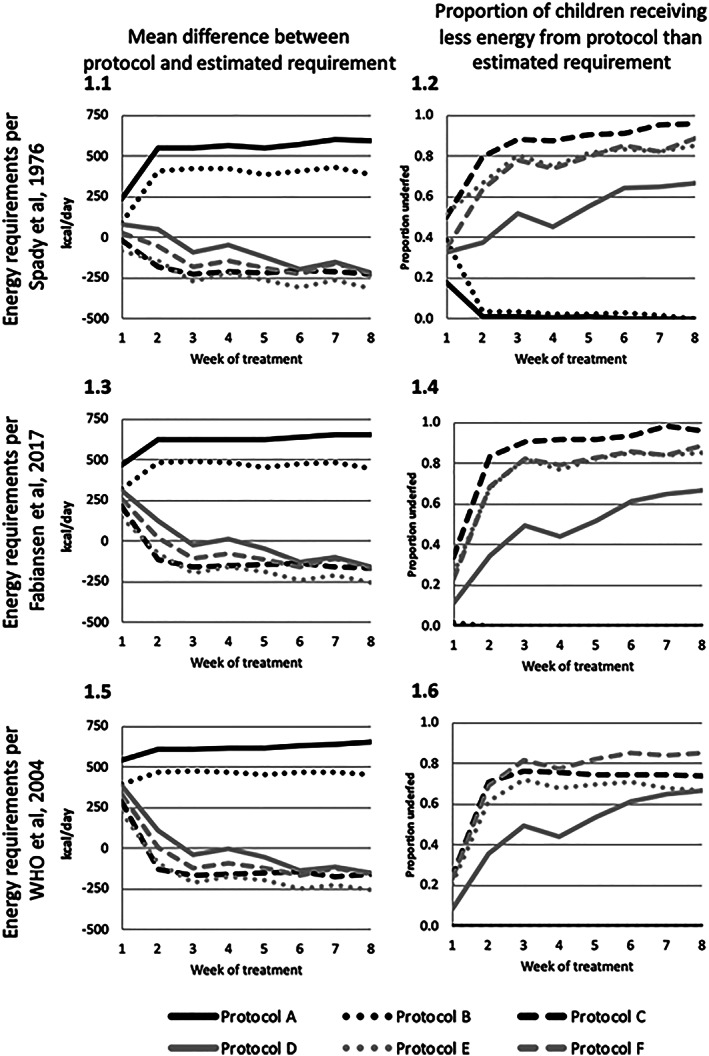
Mean difference in energy provided under six therapeutic feeding protocols and estimated energy requirements, by estimation methods
There was greater variability in performance of research Protocols C–F. Research Protocol C consistently provided less energy than the average estimated energy requirements of the study population, with more than 50% of the study population receiving less energy than the average estimated requirement throughout treatment (Table S2). On average, research Protocol D provided energy above the average estimated energy requirements of the study population in Week 1 (+85 kcal·day−1 by Spady et al., 1976, and +315 kcal·day−1 by Fabiansen et al., 2017) but was closer to the estimated requirement in Weeks 2–8 (−214 to +55 kcal·day−1 by Spady et al., 1976, and −154 to +126 kcal·day−1 by Fabiansen et al., 2017). Approximately one third of children under research Protocol D received less energy than the average estimated requirement in Week 1 by Spady et al. (1976) and 11% by Fabiansen et al. (2017), with the proportion increasing over time by both estimation approaches. In comparison, research Protocols E and F provided less energy than estimated to be required on average in Weeks 2–8 (−53 to −309 kcal·day−1 by Spady et al., 1976, and −255 to +18 kcal·day−1 by Fabiansen et al., 2017), with more than 60% of the study population receiving less than the average estimated requirement. Research Protocols C and E provided less energy than required for normal growth assuming the average energetic requirements of healthy children published by the WHO after the first week of treatment.
4. CONCLUSIONS
The results of this analysis highlight the importance of drawing on the most relevant evidence in the development of clinical guidelines. We provide new data to update our understanding of weight gain and energy requirements in the outpatient treatment of SAM. This analysis is not intended to be prescriptive, as we acknowledge that programmatic and population characteristics vary. However, our findings from Niger suggest current protocols may be providing an excess of energy later in treatment and opportunities may exist to improve program efficiency. An evidence‐based approach similar to that presented here may be used to propose alternative feeding protocols with reduced RUTF dosing. Alternative feeding protocols should be field tested to show adequacy to meet the energy needs of children under treatment, as well as feasibility and cost efficiency.
5. DISCUSSION
Using recent data from Niger, we provide updated estimates of weight gain and energy requirements among children undergoing outpatient treatment for uncomplicated SAM. We found elevated weight gain and energy requirements in the first week of outpatient treatment and decreasing proportional energy requirements with both increasing weight and MUAC. Two current therapeutic feeding protocols were found to largely provide sufficient and on average excess energy to meet the energy needs of the study population, whereas the four research protocols tended to provide less energy than the estimated requirement after the first week of treatment.
At the time of the introduction of community‐based management of SAM in 2001, relatively little experience was available to inform clinical guidelines for outpatient treatment. In the absence of published evidence, expert consensus was applied to allow the early expansion of an innovative model of care at the time. Recent experience with outpatient care, however, suggests that a direct extension of energy needs from historical inpatient to contemporary outpatient settings may not be appropriate. Children under inpatient rehabilitation using a diet of high‐energy density milk‐based formulas (1,350 kcal·L−1 providing 165 kcal·kg−1·day−1 and 3.8 g protein·kg−1·day−1) or three daily meals of RUTF and locally available foods fed ad libitum have achieved very rapid weight gain (20 g·kg−1·day−1, A. Ashworth, 1969; and 15.6 g·kg−1·day−1, Diop el, Dossou, Ndour, Briend, & Wade, 2003). Our outpatient study population achieved an average weight gain of 5.5 g·kg−1·day−1 (ranging from 11.8 in Week 1 to 2.6 in Week 7), whereas other outpatient programmes have achieved weight gains of 3 to 6.8 g·kg−1·day−1 (Collins, 2007). Such differences in weight gain between inpatient and outpatient settings may be expected, as children in inpatient settings were fed under close supervision by health staff with strict feeding schedules, whereas children in outpatient settings may receive no close supervision of feeding and lower quality family foods in addition to RUTF. These differences can influence the estimation of therapeutic energy requirements in each setting. It is clear that clinical guidance cannot always wait for an overwhelming body of evidence, and other fields of public health, such as telemedicine and the decentralization of HIV care and treatment, have advanced in the absence of published evidence. Differences between outpatient and inpatient nutritional treatment outcomes that have become apparent with time, however, highlight the importance of reassessing the adequacy of early guidance with updated evidence whenever possible.
We evaluated the energetic adequacy of six therapeutic feeding protocols using recent data from Niger. The study population benefited from high‐quality clinical management by a dedicated research team and achieved robust rates of weight gain. In this analysis, two therapeutic feeding protocols fared favourably in terms of meeting the energetic needs of children but may provide excess energy after the first week of treatment. Newer research protocols developed to simplify and improve the cost‐effectiveness of treatment did not provide enough RUTF to meet the average energetic needs of a large proportion of children in this analysis. The adequacy of therapeutic feeding protocols may, however, be context and programme specific. For example, Protocol C, used previously in Southeast Asia, reported no major safety issues and acceptable programme outcomes at the time of field implementation but would have provided less energy than estimated to meet the energy needs of this study population in Niger. Protocol F, previously tested in Sierra Leone, provided lower energy from RUTF later in treatment (MUAC > 115 mm) as a supplement to family foods and achieved adequate recovery (Maust et al., 2015). Differences in energy needs across settings can arise for many reasons, including differences in burden of concomitant clinical complications, adequacy of clinical management, individual adherence to treatment with RUTF, intrahousehold sharing, and concurrent provision of family foods.
This analysis highlights the observation of increased weight gain and energy requirements during the first week of treatment. This finding is consistent with the hypothesis that weight gain and recovery is most rapid early in treatment, although no current protocol accounts for the decreased weight gain and average energy requirements that occur later in treatment (Table 1). Our findings suggest that optimal dosing may provide reduced nutritional support later in treatment, providing an opportunity for RUTF savings.
A strength of this analysis is that the data were drawn from a high‐quality treatment programme where children received a high dose of RUTF (approximately 170 kcal·kg−1·day−1), such that weight gain was unlikely to be restricted because of lack of energy. This analysis is, however, limited in its scope as we were not able to consider differences in body composition and only the energetic adequacy of therapeutic feeding. The ideal therapeutic feeding protocol would provide not only adequate energy but also an appropriate amount of protein and micronutrients such as zinc and potassium needed for essential muscle deposition and catch‐up growth. We were further limited in the availability of exact figures with which to calculate energy requirements during treatment. Standard equations to estimate basal energy requirements may not apply to children with SAM who have a body composition different from well‐nourished children and have a reduced metabolism due to adaptation to lower energy intake (Waterlow, 1992). The study by Spady et al. (1976) estimated the energy requirements for maintenance using a regression curve based on 11 children of energy intake in relation to weight gain extrapolated to a zero weight gain. Although the assumption that energy not used for maintenance was used for growth may not be valid in children with uncomplicated malnutrition who may have some physical activity, data applying alternative approaches among malnourished children are limited. Estimates of the energetic costs of tissue deposition during rehabilitation also depend on the proportion of fat versus lean tissue deposition during recovery and therefore on the duration, severity, and nature of the nutritional deficit. The study by Spady et al. estimated the composition of the deposited tissue to be 73.5% lean and 26.5% fat, a proportion that may not be applicable with current diets with a higher mineral content favouring the deposition of lean tissues. Fabiansen et al. (2017) report weight gain following treatment for moderate acute malnutrition and estimate that tissue deposition is 93.5% lean and 6.5% fat. In consideration of this uncertainty, we applied three estimation methods. The Spady et al. method yielded proportional energy requirement estimates that were generally 10–20% higher than those of the Fabiansen et al. method, whereas the Fabiansen et al. and WHO methods produced estimates that were qualitatively similar. Comparison of energy adequacy across the six protocol was, however, largely consistent across estimation approaches.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONTRIBUTIONS
SI and AB designed the research and had primary responsibility for final content. SI and CTA performed statistical analysis and drafted the manuscript. FB collected the data. All authors read and approved the final manuscript. SI and RFG had full access to the data and had final responsibility for the decision to submit for publication.
Supporting information
Figure S1. Study flowchart
Table S1. Average estimated total energy requirement (kcal/day) among children receiving treatment for uncomplicated severe acute malnutrition in Niger
Table S2. Mean difference in energy provided under 6 therapeutic feeding protocols and estimated energy requirements
Isanaka S, Andersen CT, Hanson KE, Berthé F, Grais RF, Briend A. Energy needs in the treatment of uncomplicated severe acute malnutrition: Secondary analysis to optimize delivery of ready‐to‐use therapeutic foods. Matern Child Nutr. 2020;16:e12989 10.1111/mcn.12989
REFERENCES
- Action Contre la Faim . (2011). Guidelines for the integrated management of severe acute malnutrition: In‐ and out‐patient treatment. Retrieved from Paris: https://www.actionagainsthunger.org/sites/default/files/publications/Guidelines_For_the_integrated_management_of_severe_acute_malnutrition_In_and_out_patient_treatment_12.2011.pdf
- Ashworth, A. (1969). Growth rates in children recovering from protein‐calorie malnutrition. The British Journal of Nutrition, 23(4), 835–845. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/5357048. 10.1079/BJN19690094 [DOI] [PubMed] [Google Scholar]
- Ashworth, A. , Khanum, S. , Jackson, A. , & Schofield, C. (2003). Guidelines for the inpatient treatment of severely malnourished children. Retrieved from Geneva:
- Bailey, J. , Lelijveld, N. , Marron, B. , Onyoo, P. , Ho, L. S. , Manary, M. , … Kerac, M. (2018). Combined Protocol for Acute Malnutrition Study (ComPAS) in rural South Sudan and urban Kenya: Study protocol for a randomized controlled trial. Trials, 19(1), 251 10.1186/s13063-018-2643-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briend, A. , Lacsala, R. , Prudhon, C. , Mounier, B. , Grellety, Y. , & Golden, M. H. (1999). Ready‐to‐use therapeutic food for treatment of marasmus. Lancet, 353(9166), 1767–1768. 10.1016/S0140-6736(99)01078-8 [DOI] [PubMed] [Google Scholar]
- Ciliberto, M. A. , Sandige, H. , Ndekha, M. J. , Ashorn, P. , Briend, A. , Ciliberto, H. M. , & Manary, M. J. (2005). Comparison of home‐based therapy with ready‐to‐use therapeutic food with standard therapy in the treatment of malnourished Malawian children: A controlled, clinical effectiveness trial. The American Journal of Clinical Nutrition, 81(4), 864–870. Retrieved from. http://www.ncbi.nlm.nih.gov/pubmed/15817865, 10.1093/ajcn/81.4.864 [DOI] [PubMed] [Google Scholar]
- Collins, S. (2007). Treating severe acute malnutrition seriously. Archives of Disease in Childhood, 92(5), 453–461. 10.1136/adc.2006.098327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diop el, H. I. , Dossou, N. I. , Ndour, M. M. , Briend, A. , & Wade, S. (2003). Comparison of the efficacy of a solid ready‐to‐use food and a liquid, milk‐based diet for the rehabilitation of severely malnourished children: A randomized trial. The American Journal of Clinical Nutrition, 78(2), 302–307. 10.1093/ajcn/78.2.302 [DOI] [PubMed] [Google Scholar]
- Fabiansen, C. , Yameogo, C. W. , Iuel‐Brockdorf, A. S. , Cichon, B. , Rytter, M. J. H. , Kurpad, A. , … Friis, H. (2017). Effectiveness of food supplements in increasing fat‐free tissue accretion in children with moderate acute malnutrition: A randomised 2 × 2 × 3 factorial trial in Burkina Faso. PLoS Medicine, 14(9), e1002387 10.1371/journal.pmed.1002387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Agriculture Organization, World Health Organization, & United Nations University . (2004). Human energy requirements. Retrieved from Rome: http://www.fao.org/3/a-y5686e.pdf
- Golden, M. , & Grellety, Y. (2012). Protocol: Integrated management of acute malnutrition.
- Institut National de la Statistique/Niger, & ICF International (2013). Niger EnquÍte DÈmographique et de SantÈ et ‡ Indicateurs Multiples (EDSN‐MICS IV) 2012. Maryland, USA: Retrieved from Calverton; http://dhsprogram.com/pubs/pdf/FR277/FR277.pdf [Google Scholar]
- Isanaka, S. , Langendorf, C. , Berthe, F. , Gnegne, S. , Li, N. , Ousmane, N. , … Grais, R. F. (2016). Routine amoxicillin for uncomplicated severe acute malnutrition in children. The New England Journal of Medicine, 374(5), 444–453. 10.1056/NEJMoa1507024 [DOI] [PubMed] [Google Scholar]
- James, P. T. , Van den Briel, N. , Rozet, A. , Israel, A. D. , Fenn, B. , & Navarro‐Colorado, C. (2015). Low‐dose RUTF protocol and improved service delivery lead to good programme outcomes in the treatment of uncomplicated SAM: A programme report from Myanmar. Maternal & Child Nutrition, 11(4), 859–869. 10.1111/mcn.12192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenters, L. M. , Wazny, K. , Webb, P. , Ahmed, T. , & Bhutta, Z. A. (2013). Treatment of severe and moderate acute malnutrition in low‐ and middle‐income settings: A systematic review, meta‐analysis and Delphi process. BMC Public Health, 13(Suppl 3), S23 10.1186/1471-2458-13-S3-S23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manary, M. J. , Ndkeha, M. J. , Ashorn, P. , Maleta, K. , & Briend, A. (2004). Home based therapy for severe malnutrition with ready‐to‐use food. Archives of Disease in Childhood, 89(6), 557–561. Retrieved from. http://www.ncbi.nlm.nih.gov/pubmed/15155403, 10.1136/adc.2003.034306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maust, A. , Koroma, A. S. , Abla, C. , Molokwu, N. , Ryan, K. N. , Singh, L. , & Manary, M. J. (2015). Severe and moderate acute malnutrition can be successfully managed with an integrated protocol in Sierra Leone. The Journal of Nutrition, 145(11), 2604–2609. 10.3945/jn.115.214957 [DOI] [PubMed] [Google Scholar]
- Médecins sans Frontières . (2015). Support for severe acute malnutrition in children 6‐59 months.
- MSP, M. D. L. S. P. N (2009). National protocol for the treatment of malnutrition. Niger: Retrieved from Niamey. [Google Scholar]
- Phelan, K. P. (2019). OptiMA study in Burkina Faso: Emerging findings and additional insights. Field Exchange, 60, 40 Retrieved from. www.ennonline.net/fex/60/optimastudyburkinafaso [Google Scholar]
- Rogers, W. (1993). Regression standard errors in clustered samples. Stata Technical Bulletin, 19–23. [Google Scholar]
- Spady, D. W. , Payne, P. R. , Picou, D. , & Waterlow, J. C. (1976). Energy balance during recovery from malnutrition. The American Journal of Clinical Nutrition, 29(10), 1073–1088. 10.1093/ajcn/29.10.1073 [DOI] [PubMed] [Google Scholar]
- Valid International . (2006). Community‐based therapeutic care (CTC): A field manual. Retrieved from Oxford: [Google Scholar]
- Waterlow, J. (1992). In Arnold E. (Ed.), Protein‐energy malnutrition (p. 93). London. [Google Scholar]
- World Health Organization . (1999). Management of severe malnutrition: A manual for physicians and other senior health workers. Retrieved from Geneva:
- World Health Organization . (2013). Guideline updates on the management of severe acute malnutrition in infants and children. Retrieved from 12Geneva: Available at: http://www.who.int/nutrition/publications/guidelines/updates_management_SAM_infantandchildren/en/ [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Study flowchart
Table S1. Average estimated total energy requirement (kcal/day) among children receiving treatment for uncomplicated severe acute malnutrition in Niger
Table S2. Mean difference in energy provided under 6 therapeutic feeding protocols and estimated energy requirements
Data Availability Statement
De‐identified individual participant data will be made available upon reasonable request to the corresponding author (sheila.isanaka@epicentre.msf.org).


