Abstract
LncRNAs play a pivotal role in the regulation of epigenetic modification, cell cycle, differentiation, proliferation, migration and other physiological activities. In particular, considerable studies have shown that the aberrant expression and dysregulation of lncRNAs are widely implicated in cancer initiation and progression by acting as tumour promoters or suppressors. Hippo signalling pathway has attracted researchers’ attention as one of the critical cancer‐related pathways in recent years. Increasing evidences have demonstrated that lncRNAs could interact with Hippo cascade and thereby contribute to acquisition of multiple malignant hallmarks, including proliferation, metastasis, relapse and resistance to anti‐cancer treatment. Specifically, Hippo signalling pathway is reported to modulate or be regulated by widespread lncRNAs. Intriguingly, certain lncRNAs could form a reciprocal feedback loop with Hippo signalling. More speculatively, lncRNAs related to Hippo pathway have been poised to become important putative biomarkers and therapeutic targets in human cancers. Herein, this review focuses on the crosstalk between lncRNAs and Hippo pathway in carcinogenesis, summarizes the comprehensive role of Hippo‐related lncRNAs in tumour progression and depicts their clinical diagnostic, prognostic or therapeutic potentials in tumours.
Keywords: cancer, crosstalk, Hippo, LncRNA, sarcoma, YAP
Increasing evidences have demonstrated that lncRNAs could interact with Hippo signalling cascade and thereby contribute to acquisition of multiple malignant hallmarks, including proliferation, metastasis, relapse and resistance to anti‐cancer treatment. Specifically, Hippo signalling pathway is reported to modulate or be regulated by widespread lncRNAs. Intriguingly, certain lncRNAs could form a reciprocal feedback loop with Hippo signalling. More speculatively, lncRNAs related to Hippo pathway have been poised to become important putative biomarkers and therapeutic targets in human cancers.
1. INTRODUCTION
Cancer is one of the life‐threatening diseases and remains a critical public health issue worldwide. 1 , 2 , 3 Despite the tremendous improvements in cancer therapy in recent decades, there are still many patients who suffer from unsatisfactory outcomes. 4 Currently, the underlying molecular mechanisms in tumour occurrence and progression have not yet been fully elucidated. 5 , 6 Meanwhile, efficient biomarkers for early diagnosis, prognosis prediction and therapeutic targets are still lacking, which may hinder the effective monitoring as well as treatment of cancer. 5 , 7 , 8
Long non‐coding RNAs (lncRNAs) are a large and heterogeneous class of endogenous lncRNA family that are generally greater than 200 nucleotides (nts) in length. 9 Previously, lncRNAs were characterized as transcriptional noise since they exhibit no or limited protein‐coding capacity. 10 , 11 Recently, owing to the advancement of next‐generation sequencing‐based transcriptome profiling, tremendous lncRNAs were identified and functionally annotated. 7 , 12 , 13 LncRNAs are found to execute a wide spectrum of biological processes, 14 such as alternative splicing, chromatin modification, sponging microRNAs (miRNAs) as competing endogenous RNA (ceRNAs), nuclear‐cytoplasmic trafficking or interaction with genes, and thereby involve in crucial regulation of various human diseases including cancer. 15 Compelling experimental evidences indicate an engagement of lncRNAs in pleiotropic pathophysiological functions related to tumorigenesis, like the cell growth, invasion, metastasis, apoptosis and chemo‐resistance, 16 by interaction with other macromolecules. 17 Accumulating studies have shown that lncRNAs could be considered as a promising candidate in cancer prognosis and diagnosis. 1 , 4 , 7 , 18 Accordingly, lncRNAs have attracted great attention due to their multifaceted modulatory functions and the capacity as predictive biomarkers in cancers. 1 , 4 , 7
Hippo signalling pathway consists of a broad range of proteins and controls lots of molecular and cellular processes. 12 It is reported that Hippo pathway could be activated or suppressed by genetic or epigenetic regulation, leading to a plethora of pathological disorders including cancers. 11 Notably, advanced studies have demonstrated that the crosstalk between lncRNAs and Hippo pathway may contribute to cancer occurrence and progression in recent years. For instance, YAP (or YAP1), a major transducer in downstream of Hippo pathway, is amplified and nuclear accumulated in a variety of cancers. 19 LncRNA TNRC6C antisense RNA 1 (TNRC6C‐AS1) was reported to be abundantly expressed in thyroid carcinoma and could regulate the subcellular localization and activation of YAP, leading to the promotion of cell proliferation and tumorigenicity. 20
In this review, we systematically summarize the up‐to‐date insights provided by studies regarding the crosstalk between lncRNAs and Hippo signalling pathways in cancers. In addition, we provide a brief overview of the Hippo‐related lncRNAs as clinicopathological biomarkers and highlight its potential role as therapeutic targets in cancers. The interplay between Hippo and lncRNA may shed light on the role of underlying mechanisms in carcinogenesis.
2. CANONICAL HIPPO SIGNALLING PATHWAY IN TUMORIGENESIS
The Hippo signalling pathway is initially characterized as a critical signalling cascade that regulates organ size in fruit fly (drosophila melanogaster) in 1995. 21 It is an evolutionarily ancient and conserved network among different species, 22 and its homology molecules in mammals have been subsequently identified. A growing number of studies have highlighted a critical role of Hippo pathway in the regulation of organ size, tissue homeostasis, cell proliferation, apoptosis, metastasis, autophagy, angiogenesis and stem cell self‐renewal. 23 , 24 The misregulation of Hippo signalling pathway can cause many disease conditions. 25 In tumorigenesis, Hippo pathway is well‐established as a tumour‐suppressive cascade due to its proliferation restriction and apoptosis induction. 26 , 27
In mammals, the central axis of the Hippo signalling pathway comprises two serine/threonine kinases: mammalian sterile 20‐like kinase 1/2 (MST1/2) and its homolog large tumour suppressor 1 and 2 (LAST1/2); two adaptor/scaffold protein: WW45 for MST1/2 and Mps one binder kinase activator‐like 1 (MOB1) for LAST1/2; downstream transcriptional co‐regulators: yes‐associated protein (YAP) and its paralog transcriptional co‐activator with PDZ‐binding motif (TAZ, also known as WWTR1); and various nuclear transcriptional factors: transcriptional enhancer‐associated domain (TEAD1/2/3/4). 26 , 28 Of them, YAP and TAZ are key intracellular messengers, whose localizations are critical in Hippo pathway. 11 YAP/TAZ could be positively or negatively modulated by phosphorylation at different sites by upstream kinases, elicit target gene expression signature through forming complexes with TEAD family, the major nuclear partner, and thereby play a prominent role in cellular plasticity, lineage differentiation during development, tumour initiation, progression and metastasis. 29 , 30
In canonical Hippo signalling, the cascade is on (‘Hippo On’) when the upstream Hippo pathway is activated by stimuli or regulators, such as mechanical stress, cell polarity determinants and increased cell‐cell contact. 28 , 31 Then, MST1/2 kinase is phosphorylated and subsequently phosphorylates salvador homolog 1 (SAV1) to form a heterotetramer to further promote the LATS1/2 phosphorylation. Activated LATS1/2 could result in inactivation of YAP/TAZ through sequestering its cytoplasmic localization by binding to 14‐3‐3 protein or degradation via ubiquitination, and thereby dampen the transcription of downstream genes. 11 , 31 Conversely, when the Hippo pathway is inactivated (‘Hippo off’), YAP/TAZ translocates to the nucleus and binds primarily to enhancer elements by using TEAD as DNA‐binding sites, 11 , 32 thereby driving target gene (AREG, CTGF, Cyr61, ANKRD1, AXL, etc) transcription and promoting cell tissue growth, survival, proliferation and self‐renewal, 28 , 33 , 34 as presented in Figure 1.
Figure 1.
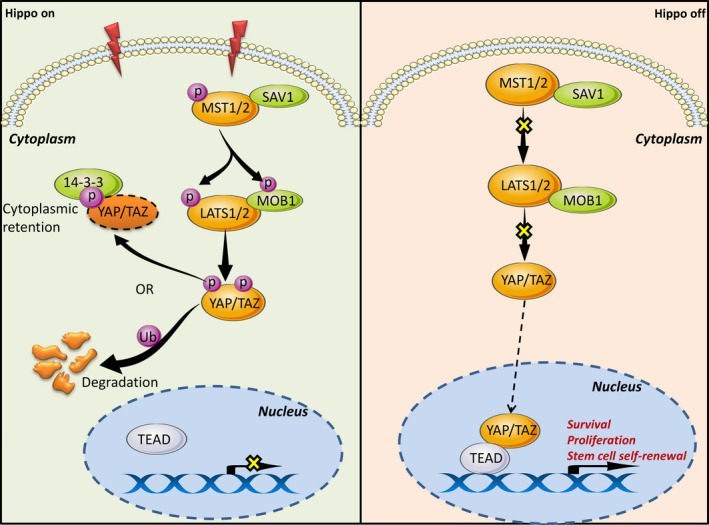
Molecular schematic of canonical Hippo signalling cascade in cancers
3. REGULATORY NETWORK OF LNCRNAS AND HIPPO SIGNALLING PATHWAY IN CANCER
Overall, considerable crosstalk between lncRNAs and Hippo signalling pathway has been revealed in several tumours as demonstrated in Tables 1, 2, 3. A vast majority of lncRNAs were discovered in the regulation of Hippo signalling pathways. Conversely, Hippo pathways were also reported to modulate expression of lncRNAs. 31 These bilateral regulations ultimately impact target gene expressions in cancer progression, indicating a close relationship and complexity between lncRNAs and Hippo signalling cascades.
Table 1.
Overview of lncRNAs that regulate Hippo signalling pathway in cancer development
| LncRNA | Tumour type | Expression | Interaction with Hippo cascade | Biological function in cancers | Ref. |
|---|---|---|---|---|---|
| B4GALT1‐AS1 | Osteosarcoma, colon cancer | ↑ | B4GALT1‐AS1 directly or indirectly binds to YAP to promote its transcription | Proliferation, migration, spheroid formation, stemness, chemo‐resistance | 35, 38 |
| BCYRN1 | Glioma | ↑ | BCYRN1 increases TAZ expression | Proliferation, invasion, migration | 40 |
| BDNF‐AS | Glioblastoma | ↓ | BDNF‐AS increases LATS1 and YAP phosphorylation mediated by RAX2/ DLG5 | Proliferation, apoptosis, migration, invasion | 126 |
| FRMD6‐AS2 | Endometrial cancer | ↓ | FRMD6‐AS2 increases phosphorylation of LATS1 and YAP | Tumour growth, migration and invasion | 127 |
| GHET1 | NSCLC | ↑ | GHET1 enhances YAP expression | Proliferation, invasion, EMT | 36 |
| MAYA | Breast cancer bone metastasis | ↑ | MAYA induces inhibitory methylation of MST1 | Bone metastasis of cancer cells | 128 |
| LEF1‐AS1 | OSCC | ↑ | LEF1‐AS1 inhibits the binding of LATS1 to MOB, and thus suppresses Hippo pathway | Cell survival, proliferation, migration, apoptosis, cell cycle | 129 |
| LINC00174 | CRC | ↑ | LINC00174 sponges to miR‐1910‐3p to activate TAZ | Cell growth | 32 |
| LINC00662 | GC | ↑ | LINC00662 sponges to miR‐497‐5p to promote YAP expression | Proliferation, chemo‐sensitivity | 130 |
| LINC00673 | BC | ↑ | LINC00673 increases MAPK4 and YAP/TAZ expression and reduces YAP phosphorylation | Proliferation, apoptosis, cell cycle | 131 |
| LINC01048 | CSCC | ↑ | LINC01048 interacts with TAF15 to upregulate YAP | Proliferation, apoptosis | 132 |
| LINC01314 | HB | ↓ | LINC01314 inhibits nuclear translocation of YAP | Proliferation, migration, cell cycle | 133 |
| LINC01559 | Pancreatic cancer | ↑ | LINC01559 hinders YAP phosphorylation and enhances its transcription | Proliferation, migration, cell growth | 134 |
| Linc‐OIP5 | BC, glioma | ↑ | Linc‐OIP5 increases YAP expression | Proliferation, migration, invasion, apoptosis, tube formation capacity | 135, 136, 137 |
| LncRNA‐ATB | HCC | ↑ | LncRNA‐ATB activates YAP expression | Cell proliferation, clonogenicity, autophagy | 95 |
| MIR100HG | Osteosarcoma | ↑ | MIR100HG silences LATS1/2 and inactivates Hippo | Proliferation, apoptosis, cell cycle | 42 |
| MRVI1‐AS1 | NPC | ↓ | MRVI1‐AS1 promotes RASSF1 expression to suppress TAZ expression | Paclitaxel‐resistant | 46 |
| Nkx2‐2as | MB | ↓ | Nkx2‐2as upregulates LATS1/2 | Cell division, migration | 138 |
| NSCLCAT1 | NSCLC | ↑ | NSCLCAT1 represses MST1 and LATS1 and increases YAP/TAZ expression | Cell viability, migration, apoptosis, invasion | 139 |
| PCGEM1 | Ovarian carcinoma | ↑ | PCGEM1 upregulates YAP expression | Proliferation, apoptosis, migration, invasion | 122 |
| PLK4 | HCC | ↓ | PLK4 inactivates YAP and induces cell senescence | Cell viability, growth, cellular senescence | 118 |
| SNHG15 | PTC | ↑ | SNHG15 upregulates YAP expression | Proliferation, apoptosis, migration, EMT | 103 |
| THOR | NPC | ↑ | THOR enhances YAP transcriptional activity | Proliferation, migration, invasion, spheres formation, stemness, cisplatin sensitivity | 140 |
| TNRC6C‐AS1 | Thyroid carcinoma | ↑ | TNRC6C‐AS1 regulates MST1 and LATS1/2, and phosphorylation of YAP | Proliferation, apoptosis, autophagy | 20 |
| TUG1 | RCC | ↑ | TUG1 enhances YAP expression | Proliferation, migration | 141 |
| uc.134 | HCC | ↓ | uc.134 inhibits CUL4A‐mediated ubiquitination of LATS1 and increases YAP phosphorylation | Proliferation, invasion, metastasis | 96 |
| XIST | Osteosarcoma | ↑ | XIST increases YAP expression | Proliferation, invasion | 37 |
| ZFAS1 | Prostate cancer | ↑ | ZFAS1 upregulates YAP and TEAD1 expression | Proliferation, invasion, EMT | 91 |
| ZFHX4‐AS1 | BC | ↑ | ZFHX4‐AS1 increases YAP/TAZ expression | Proliferation, migration, apoptosis, invasion, cell cycle | 142 |
Abbreviations: ↑ upregulated; ↓ downregulated; ATF3, activating transcription factor 3; B4GALT1‐AS1, B4GALT1 antisense RNA 1; BC, breast cancer; BCYRN1, brain cytoplasmic RNA 1; BDNF‐AS, BDNF antisense RNA; CRC, colorectal cancer; CSCC, cutaneous squamous cell carcinoma; DLG5, discs large homolog 5; EMT, epithelial‐to‐mesenchymal transition; FRMD6‐AS2, FRMD6 antisense RNA 2; GC, gastric cancer; GHET1, gastric cancer high expressed transcript 1; HB, hepatoblastoma; HCC, hepatocellular carcinoma; LATS1/2, large tumour suppressor homolog 1/2; LEF1‐AS1, LEF1 antisense RNA 1; Linc‐OIP5, linc‐Opa interacting protein 5; LncRNA‐ATB, lncRNA activated by transforming growth factor‐β; LSCC, laryngeal squamous cell carcinoma; MB, medulloblastoma; MIR100HG, mir‐100‐let‐7a‐2‐mir‐125b‐1 cluster host gene; MOB1, Mps one binder kinase activator‐like 1; MRVI1‐AS1, murine retrovirus integration site 1 homolog antisense RNA 1; MST1/2, mammalian sterile twenty‐like 1/2; NPC, nasopharyngeal carcinoma; NSCLC, non‐small‐cell lung cancer; NSCLCAT1, non‐small‐cell lung cancer‐associated transcript‐1; OSCC, oral squamous cell carcinoma; PCGEM1, prostate cancer gene expression marker 1; PDAC, pancreatic ductal adenocarcinoma; PLK4, polo‐like kinase 4; PTC, papillary thyroid carcinoma; RASSF1, ras‐associated domain family member 1; RCC, renal cell carcinoma; SNHG 15, small nucleolar RNA host gene 15; TAZ, transcriptional co‐activator with PDZ‐binding motif; TEAD, transcriptional enhancer‐associated domain; THOR, testis‐associated highly conserved oncogenic long non‐coding RNA; TNRC6C‐AS1, TNRC6C antisense RNA 1; TUG1, taurine upregulated gene 1; XIST, X‐inactive specific transcript; YAP, yes‐associated protein.
Table 2.
Overview of Hippo signalling pathway induced lncRNAs in cancer development
| LncRNA | Tumour type | Expression | Interaction with Hippo cascade | Biological function in cancers | Ref. |
|---|---|---|---|---|---|
| BCAR4 | BC | ↑ | YAP promotes BCAR4 expression | Glycolysis | 26 |
| CYTOR (LINC00152) | CRC | ↑ | YAP increases CYTOR expression, which in turn sponges to miR‐632 and miR‐185‐3p to target FSCN1 | Proliferation, invasion, metastasis | 51 |
| H19 | Osteosarcoma, bladder cancer | ↑ | YAP increases H19 expression | Proliferation, migration | 53, 90 |
| MT1DP | Liver cancer | ↓ | YAP and Runx2 inhibit MT1DP expression dependent on FoxA1 | Proliferation, apoptosis, colony formation | 47 |
| NORAD | Lung and breast cancer metastasis | ↓ | YAP/TAZ‐TEAD and NuRD complex repress NORAD expression | Migration and invasion | 52 |
Abbreviations: ↑ upregulated; ↓ downregulated; BC, breast cancer; BCAR4, breast cancer antiestrogen resistance 4; CRC, colorectal cancer; CYTOR, cytoskeleton regulator RNA; FSCN1, fascin actin‐binding protein 1; MT1DP, metallothionein 1D, pseudogene; NORAD, non‐coding RNA activated by DNA damage; TAZ, transcriptional co‐activator with PDZ‐binding motif; TEAD, transcriptional enhancer‐associated domain; YAP, yes‐associated protein.
Table 3.
Overview of lncRNAs that form reciprocal interactions with Hippo pathway in cancer development
| LncRNA | Tumour type | Expression | Interaction with Hippo cascade | Biological function in cancers | Ref. |
|---|---|---|---|---|---|
| GAS5 | Pancreatic cancer, CRC | ↓ | GAS5 enhances cytoplasm translocation of YAP and promotes phosphorylation and ubiquitin‐mediated YAP degradation. YAP could target YTHDF3, which reversibly bound m6A‐methylated GAS5 to facilitate its decay. | Cell viability, chemo‐resistance | 11, 65 |
| LINC01433 | GC | ↑ | LINC01433 decreases YAP phosphorylation, and YAP activates LINC01433 transcription | Proliferation, migration, invasion, chemo‐resistance | 12 |
| LncARSR | RCC | ↑ | LncARSR inhibits LATS/YAP interaction to facilitate YAP nuclear translocation, which in turn transactivates lnARSR expression | Renal tumour‐initiating cell self‐renewal, tumorigenicity and metastasis | 143 |
| MALAT1 | MM, CRC, pancreatic cancer, liver cancer, Breast cancer, |
↑ ↓ |
MALAT1 directly decreases LATS to increase YAP activity or sponges to miR‐181a‐5p to target YAP. YAP attenuates the nuclear retention of SRSF1 and abrogates its inhibitory effect on MALAT1. Besides, MALAT1 sequesters TEAD and blocks YAP‐TEAD binding | Proliferation, apoptosis, cell adhesion, angiogenesis, migration, invasion, cancer metastasis, EMT | 66, 67, 68, 81, 92 |
| SNHG1 | LSCC | ↑ | SNHG1 sponges to miR‐375 to increase YAP expression, and YAP activates SNHG1 transcription | Proliferation, migration, invasion, apoptosis | 84 |
| THAP9‐AS1 | PDAC | ↑ | THAP9‐AS1 sponges to miR‐484 to indirectly enhance YAP activity, or directly bind to YAP, and in turn inhibit the dephosphorylation of YAP. Besides, YAP/TEAD1 promotes THAP9‐AS1 transcription | Cell growth | 85 |
| UCA1 | Pancreatic cancer, thyroid cancer, ovarian cancer | ↑ | UCA1 enhances AMOT‐YAP interaction to promote YAP nuclear translocation. Increased YAP promotes UCA1 transcription | Proliferation, apoptosis, migration, invasion, EMT | 61, 62, 63, 64, 144 |
Abbreviations: ↑ upregulated; ↓ downregulated; AMOT, angiomotin; CRC, colorectal cancer; GAS5, growth arrest‐specific 5; GC, gastric cancer; LSCC, laryngeal squamous cell carcinoma; MALAT1, metastasis‐associated lung adenocarcinoma transcript 1; MM, multiple myeloma; MOB1, Mps one binder kinase activator‐like 1; PDAC, pancreatic ductal adenocarcinoma; RCC, renal cell carcinoma; SNHG1, small nucleolar RNA host gene 1; SRSF1, serine‐/arginine‐rich splicing factor 1; TAZ, transcriptional co‐activator with PDZ‐binding motif; TEAD, transcriptional enhancer‐associated domain; THAP9‐AS1, THAP9 antisense RNA 1; UCA1, urothelial cancer‐associated 1; YAP, yes‐associated protein.
3.1. LncRNAs regulate members of Hippo pathway
Recently, lncRNAs are emerging as a critical mediator in a wealth of carcinogenic processes by targeting various downstream executors in Hippo signalling pathways (Figure 2). A number of lncRNAs, including B4GALT1 antisense RNA 1 (B4GALT1‐AS1), 35 gastric cancer high expressed transcript 1 (GHET1) 36 and X‐inactive specific transcript (XIST), 37 were tightly associated with YAP to exert their functions in cancers. Zhang et al 35 found that lncRNA B4GALT1‐AS1 was highly expressed in colon cancer cells by RNA‐seq. Depletion of B4GALT1‐AS1 repressed cancer cell colony formation and stemness. Further mechanism assay revealed that B4GalT1‐AS1 could directly bind to YAP. B4GALT1‐AS1 silencing could sequester YAP in cytoplasm and decrease YAP transcriptional activity, while overexpression of YAP attenuated the inhibition effect caused by B4GAlT1‐AS1 knockdown. 35 In similar, another study showed that B4GALT1‐AS1 was expressed in osteosarcoma tissues as well as cell spheres at an enhanced level. 38 Functionally, B4GALT1‐AS1 acted as an oncogene to enhance YAP mRNA stability and transcriptional activity by recruiting HuR, and in turn maintain osteosarcoma cells stemness, and promote migration and chemo‐resistance. 38 Conclusively, these studies clarified an obvious association of B4GALT1‐AS1 and Hippo pathway, which may contribute to the malignant properties of tumour. 35 , 38 GHET1, located in chromosome 7q36.1, was firstly identified as an overexpressed lncRNA in gastric cancer. 39 Guan ZB et al 36 demonstrated an elevated expression of GHET1 in NSCLC and its knockdown could impede YAP expression, and thereby impair tumour cell proliferation, invasion ability and the epithelial‐to‐mesenchymal transition (EMT). XIST is a markedly elevated lncRNA in osteosarcoma tissues and cells. 37 A panel of in vitro and in vivo studies confirmed that XIST knockdown restricted tumour cell growth, invasion and EMT. Interestingly, XIST acted as a decoy for miR‐195‐5p and thereby to alter YAP expression, implicating a regulatory role of XIST/miR‐195‐5p/YAP network in osteosarcoma progression. 37
Figure 2.
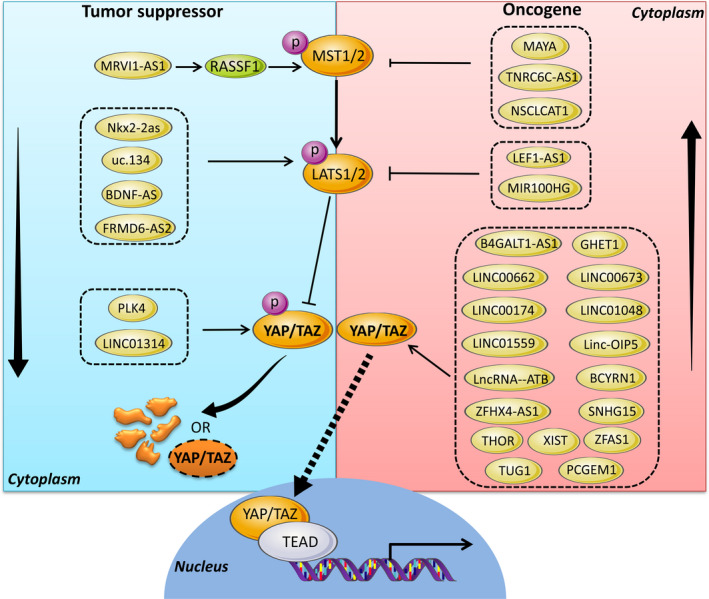
Links between lncRNAs and Hippo signalling cascade. Numerous lncRNAs have been demonstrated to be involved in cancer progression via regulating core components of the Hippo signalling pathway
In addition to YAP, other components of Hippo cascade including TAZ, LATS1/2 and MST1/2 were also found involved in crosstalk with a variety of lncRNAs in carcinogenesis. For example, both LINC00174 and TAZ showed an upregulated expression pattern in human primary colorectal cancer (CRC) tissues as compared to corresponding normal tissues. 32 Overexpression of LINC00174 or TAZ could enhance CRC cell proliferation motility. Bioinformatics and luciferase reporter assays revealed that LINC00174 may competitively bind to miR‐1910‐3p to increase TAZ expression in CRC carcinogenesis. 32 MiR‐125a‐5p, an important endogenous tumour suppressor, 40 was reported to target TAZ and inhibit EGFR pathway to repress retinoblastoma progression. 41 A recent study performed by Yu et al 40 suggested lncRNA BCYRN1 functioned as an oncogene by sponging miR‐125a‐5p to activate TAZ, and then results in cell proliferation, invasion and migration in glioma. In addition, Su et al 42 demonstrated that mir‐100‐let‐7a‐2‐mir‐125b‐1 cluster host gene (MIR100HG), a well‐documented tumour facilitator in breast cancer 43 and acute megakaryoblastic leukaemia, 44 was also highly expressed in osteosarcoma. Functional assay and rescue experiments further confirmed that MIR100HG regulated cell proliferation, apoptosis and cell cycle mediated by epigenetically silencing LATS1/2 and inactivating Hippo pathway. 42 Ras‐associated domain family member 1 (RASSF1) is a scaffold protein and functions as a tumour suppressor through regulation of cell cycle and apoptosis. 45 LncRNA murine retrovirus integration site 1 homolog antisense RNA 1 (MRVI1‐AS1) was reported to be markedly downregulated in paclitaxel‐resistant cells and could promote RASSF1 expression to modulate MST1/2 and suppress downstream TAZ expression, and therefore increase nasopharyngeal cancer (NPC) chemo‐sensivitiy. 46 In summary, these findings help to illuminate the role of lncRNA in the regulation of Hippo signalling to subsequently control cell proliferation and tumorigenesis.
3.2. LncRNAs induced by Hippo pathway
Several studies demonstrated that the core components in Hippo pathways could also exert functions in the regulation of the expression as well as functions of lncRNAs, such as lncRNA breast cancer antiestrogen resistance 4 (BCAR4) 26 and metallothionein 1D, pseudogene (MT1DP) 47 (Figure 3). LncRNA BCAR4 is an upregulated lncRNA in multiple cancers with clinicopathological significance in prognosis. 4 A study showed that BCAR4 and YAP expressions were positively correlated in breast cancer and closely associated with unfavourable recurrence‐free survival. Moreover, YAP could upregulate BCAR4 expression and coordinate the Hedgehog signalling pathway to promote the transcription of glycolysis activators HK2 and PFKFB3, and in turn to reprogramme glucose metabolism in breast cancer. 26 LncRNA MT1DP, a tumour suppressor, could reduce cell proliferation and colony formation, while inducing the apoptosis in liver cancer. 47 Alpha‐fetoprotein (AFP) is a well‐known biomarker in liver cancer progression and recurrence. 48 , 49 Functional assay suggested that MT1DP negatively regulated AFP by suppressing synthesis of Forkhead box A1 (FoxA1). Mechanistically, YAP and Runx2 together displayed an oncogenic activity by hindering lncRNA MT1DP in a FoxA1‐dependent manner in liver cancer. 47 Other lncRNAs that are regulated by Hippo signalling pathway include cytoskeleton regulator RNA (CYTOR), 50 , 51 non‐coding RNA activated by DNA damage (NORAD) 52 and H19. 53 LncRNA CYTOR, also known as long intergenic ncRNA 00 152 (LINC00152), is located on chromosome 2p11.2 with a length of 828 nucleotides. 54 CYTOR was found highly expressed in CRC compared with counterpart controls and proved to sustain proliferation and promote invasion and metastasis of cancer cells. 51 CYTOR could be targeted and transcriptionally regulated by YAP and other Hippo pathway molecules in CRC cells, subsequently regulated fascin actin‐binding protein 1 (FSCN1) expression through sponging to miR‐632 and miR‐185‐3p, and thereby promoted the occurrence and metastasis of CRC. 51 Besides, another study showed that NORAD, a unique kind of lncRNA that responds to DNA damage and maintains genome integrity and stability in cancers, 55 , 56 , 57 was synergistically transcriptionally inhibited by the YAP/TAZ‐TEAD and the NuRD complex, which in turn affected the development and metastasis of lung and breast cancer via sequestration of S100P. 52 Moreover, lncRNA H19, a well‐characterized oncogenic lncRNA in tumour progression, metastasis and chemo‐resistance, 58 , 59 , 60 was also found abnormally expressed in osteosarcoma and could be upregulated by overexpression of YAP. 53 To summarize, it is clear that Hippo pathway could intimately modulate certain lncRNA to engage in multiple processes of cancer development.
Figure 3.
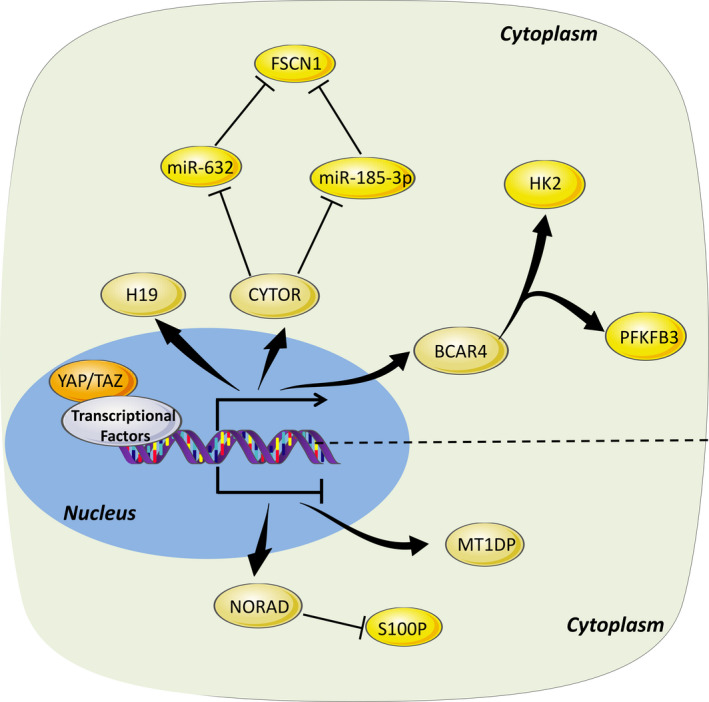
Links between lncRNAs and Hippo signalling cascade. Hippo signalling axis could modulate the transcriptional activity of certain lncRNAs and in turn play a critical role in cancers
3.3. Reciprocal interaction between lncRNAs and Hippo pathway
Of note, there are a number of lncRNAs show reciprocal feedback loop with Hippo signalling pathway, such as urothelial cancer‐associated 1 (UCA1), 61 , 62 , 63 , 64 growth arrest‐specific 5 (GAS5) 11 , 65 and metastasis‐associated lung adenocarcinoma transcript 1 (MALAT1) 66 , 67 , 68 (Figure 4). UCA1 has displayed a trend of significantly increased expression in pancreatic cancer, 64 thyroid cancer 61 and ovarian cancer, 62 when compared to adjacent normal tissue. Loss‐of‐function assay showed that UCA1 knockdown restrained cell proliferation and induced apoptosis, as evidenced by CCK‐8 and flow cytometry. 61 Importantly, UCA1 could interplay with MOB1, LATS1 and YAP to form shielding composites, and thus suppress YAP phosphorylation to upregulate YAP expression. Moreover, UCA1 enhanced YAP nuclear localization and stabilization as well as increase TEAD luciferase activity. Besides, by using reverse‐phase protein array analysis and in vivo RNA antisense purification, Lin X and colleagues 62 further identified that UCA1 could bind to a well‐known YAP regulator, angiomotin (AMOT) in ovarian cancer. Specifically, UCA1 enhanced AMOT‐YAP interaction to enhance YAP dephosphorylation and nuclear translocation. 62 Interestingly, YAP could also promote expression of UCA1, 64 indicating a reciprocal interaction between UCA1 and YAP that maintain the cancerous phenotype.
Figure 4.
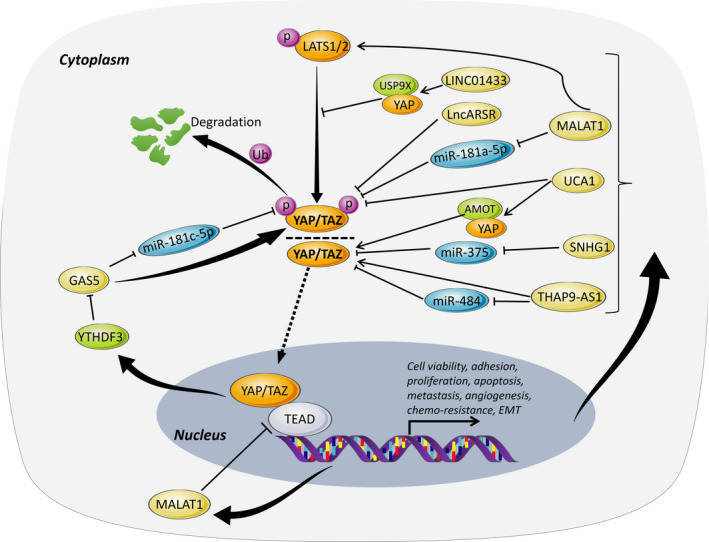
Reciprocal interaction between lncRNAs and Hippo cascade. A number of lncRNAs reciprocally interact with components of the Hippo signalling pathway to complete feedback loop in cancer progression
In addition, GAS5, a well‐acknowledged tumour suppressor, has been shown to exert essential inhibitory roles in cancer development and progression. 69 , 70 Gao et al 65 reported that GAS5 was conspicuously downregulated and inversely correlated with miR‐181c‐5p expression in pancreatic cancer cells. Gain‐of‐function analysis showed that GAS5 dramatically dampened cell viability and antagonized the chemo‐resistance through regulation of miR‐181c‐5p to indirectly activate Hippo signalling. 65 In addition, GAS5 was found to directly interplay with WW domain of YAP to facilitate YAP cytoplasmic localization in CRC. 11 Moreover, GAS5 could trigger YAPSer127 phosphorylation and promote ubiquitin‐mediated YAP degradation as an RNA scaffold. 11 N6‐Methyladenosine (m6A) is the most abundant mRNA modification and plays a critical role in cancer progression. 71 Currently, m6A‐modified lncRNAs in the regulation of YAP activation remain poorly defined. 11 , 72 , 73 By using MeRIP‐seq and lncRNA‐seq, Ni et al 11 further identified YAP could also target m6A reader YTHDF3, which reversibly bound m6A‐methylated GAS5 to facilitate its decay, suggesting a negative functional loop of GAS5‐YAP‐YTHDF3 axis in CRC progression.
Interestingly, lncRNA MALAT1, locating on human chromosome 11q13.1 with a transcript sequence of approximately 8 kb, is a context‐specific lncRNA among mammals that involved in the development of diverse malignancies by crosstalk with Hippo pathway. 74 , 75 , 76 , 77 , 78 , 79 , 80 Early studies consistently showed that MALAT1 is highly expressed in cancerous tissue and facilitates tumour progression and metastasis in various cancers. For instance, a series of in vivo and in vitro experiments showed that MALAT1 knockdown can activate the Hippo cascade by upregulating miR‐181a‐5p, thereby hamper the proliferation and adhesion capacity of tumour cells in myeloma. 66 In pancreatic cancer, MALAT1 showed extremely high expression pattern, leading to increased expression of YAP and decreased LATS1 expression, thus accelerating the tumour growth both in vitro and in vivo. 68 In liver cancer, both YAP and MALAT1 were highly expressed, and YAP could increase MALAT1 expression at both transcriptional and post‐transcriptional levels. 67 Serine‐/arginine‐rich splicing factor 1 (SRSF1) is a negative regulator of MALAT1. Importantly, YAP was reported to attenuate the nuclear retention of SRSF1 via interacting with AMOT and thereby abrogate the inhibitory effect of SRSF1 on MALAT1. 67 Moreover, the combination of YAP overexpression and SRSF1 knockdown led to significantly enhanced tumour growth and migration. 67 In contrast, a recent study by Kim et al demonstrated an opposite phenotype of MALAT1 in breast cancer. 81 MALAT1 was obviously downregulated in breast cancer than parental tissue, and its level was negatively correlated with cancer progression and metastasis potential. MALAT1 acted as a tumour suppressor to impair cancer cell migration, invasion and metastasis by binding to and sequestering TEAD, and thereby blocking its association with co‐activator YAP. 81 , 82 In vivo assay using transgenic, xenograft and syngeneic models consistently showed a metastasis‐inhibitory role of MALAT1 in breast cancer. 81 Hence, collectively, MALAT1 may form a positive bidirectional circuit with oncoprotein YAP in the regulation of cancer development and tumorigenesis in a cancer tissue‐specific manner. More comprehensive studies are therefore required to verify the oncogenic or tumour‐suppressive role in MALAT1 in cancers. 83
Besides, other lncRNAs are also capable of forming feedback loops with Hippo pathway, such as LINC01433, 12 small nucleolar RNA host gene 1 (SNHG1) 84 and THAP9 antisense RNA 1 (THAP9‐AS1). 85 As an oncogenic lncRNA, LINC01433 has been demonstrated to enhance tumour cell aggressiveness, including proliferation, migration, invasion and chemo‐resistance. 12 Intriguingly, Zhang et al 12 reported that LINC01433 stabilized YAP by upregulating the interaction between deubiquitinase USP9X and YAP and reduced YAP phosphorylation through inhibition of YAP‐LATS1 binding. Conversely, YAP could directly bind to LINC01433 promoter region to further activate its transcription. 12 SNHG1 was reported to be remarkably upregulated in several types of human malignancies such as osteosarcoma and laryngeal squamous cell carcinoma (LSCC). 84 , 86 , 87 SNHG1 knockdown obviously impeded tumour cell proliferation, migration and invasion, while induced apoptosis via participating in pleiotropic cancer‐related signalling pathways, such as Notch, 88 Wnt/β‐catenin 87 and Hippo pathway. 84 Specifically, SNHG1 could serve as ceRNA to sponge to miR‐375 and thus promote YAP expression to regulate Hippo pathway in LSCC. Meanwhile, YAP could reversibly occupy promoter of SNHG1 to enhance its transcription, indicating a positive feedback regulation between SNHG1 and YAP. 84 THAP9‐AS1 was found upregulated in pancreatic ductal adenocarcinoma (PDAC) tissues, and its expression was positively associated with YAP levels and remarkably correlated with worse clinical outcomes. 85 THAP9‐AS1 exerted its pro‐carcinogenic role in PDAC both in vitro and in vivo by activating YAP. Notably, ectopic YAP expression could abolish the effects of THAP9‐AS1 knockdown, and vice versa. 85 Mechanistically, THAP9‐AS1 could sponge miR‐484 to indirectly target YAP, or directly bind to YAP to result in upregulation of the expression and activity of YAP. Reciprocally, YAP/TEAD1 complex could enhance THAP9‐AS1 transcription to complete a feed‐forward loop. 85
4. THE CLINICAL SIGNIFICANCE OF LNCRNAS INVOLVED IN HIPPO PATHWAY IN CANCERS
Detection of clinical biomarkers could enable early diagnosis of tumour, which is critical in clinical practice. Several core components of Hippo pathway have been implicated as potential biomarkers for prognosis and chemo‐resistance. For instance, YAP is found consistently elevated expressed in multiple cancers, such as osteosarcoma, 53 breast cancer, 26 liver cancer, 89 bladder cancer, 90 prostate cancer, 91 pancreatic cancer 68 and CRC. 51 , 92 YAP overexpression or increased activity may predict advanced tumour stages and poor clinical outcome in cancer patients. 25 , 85 , 92 A more recent discovery indicated that expression of nuclear YAP (nYAP) was noticeably upregulated in docetaxel‐resistant prostate cancer cell lines than parental cells. 93 Consistently, clinical data also revealed a higher nYAP level in the chemohormonal therapy (CHT) group than other groups, and patients with overexpressed nYAP in residual cancer after CHT predicted higher biochemical recurrence, indicating that nYAP level may be a promising prognostic factor in castration‐resistant prostate cancer patient treated with CHT. 93 Furthermore, in conventional osteosarcoma, YAP/TAZ immune‐reactive score was significantly correlated with the overall survival (OS), and its nuclear expression was associated with progression‐free survival, 94 suggesting a prominent link between YAP/TAZ expression and osteosarcoma prognosis.
Since the lncRNAs interacted with Hippo signalling pathway have a considerable impact on regulation of tumour cell functions, their clinical diagnostic and prognostic significances were also extensively delineated in studies. Some aberrantly expressed lncRNAs involved in Hippo pathway were found overtly correlated with prognosis outcomes and clinicopathological characteristics in cancers. For example, lncRNA‐ATB, a lncRNA activated by TFG‐β, was highly expressed in hepatocellular carcinoma (HCC) tissues compared to corresponding healthy liver samples. 95 In HCC patients, expression of lncRNA‐ATB was positively associated with tumour size, TNM stage and unfavourable survival. 95 A similar conclusion was drawn by Li et al that elevated H19 was associated with poor clinicopathological parameters. 90 Inversely, lncRNA uc.134 was strikingly downregulated in HCC samples than adjacent tissues 96 and its expression was positively associated with LATS1 and pYAPS127 levels in HCC and related to lymphatic metastasis and higher TNM stage. Moreover, HCC patients with lower expression level of uc.134 were apt to worsen OS. 96 Similarly, downregulated expression of NORAD was also associated with lymph node metastasis (LNM) and poor prognosis. 52 By contrast, lncRNA XIST was found markedly increased in osteosarcoma tissues and cell lines as demonstrated by qRT‐PCR, 97 , 98 and its expression was negatively associated with OS, and positively correlated with clinicopathological features, including larger tumour size, advanced Enneking stage, LNM and distant metastasis in osteosarcoma, 99 suggesting XIST may be used as an independent clinical biomarker in osteosarcoma. 100 , 101 , 102 Taken together, Hippo‐related lncRNAs appear to be innovative diagnostic and prognostic biomarkers for multiple cancers. However, there are still numerous challenges for their validation in clinical settings. 80
5. THE THERAPEUTIC POTENTIAL OF LNCRNAS INVOLVED IN HIPPO PATHWAY
As mentioned above, the Hippo pathway comprises multiple downstream signalling proteins, such as YAP/TAZ, whose activation can endow cells with several hallmarks of cancer, 103 , 104 leading to uncontrolled cell growth, malignant transformation, acquisition of EMT and confer tumour cell resistance to chemo‐, radio‐ and even immunotherapy. 19 , 30 , 50 Among them, chemo‐resistance remains a major obstacle to effective cancer treatment, contributing to metastatic progression and tumour relapse. 105 As is shown, Mao et al 106 demonstrated that SIRT1 enhances the interaction between YAP and TEAD4 to maintain cisplatin resistance In HCC. Another recent study confirmed that Hippo cascade also participated in osteosarcoma chemo‐resistance. 107 Upon methotrexate/doxorubicin treatment, MST1 degradation increased, while LATS1/2 expression and YAP phosphorylation decreased in osteosarcoma cells. Further study revealed that activated nYAP subsequently resulted in transcription of downstream target genes, leading to cell proliferation and chemo‐resistance. 107 Autophagy is an essential process implicated in tumour survival and chemo‐resistance. 95 , 108 , 109 Wilkinson et al 110 found that MST1/2 can phosphorylate LC3 and promoted cell autophagy, while decreased MST1 could constrain autophagy and thereby enhance cancer cell chemo‐sensitivity. Besides, EMT is a complicated process which may contribute to cytoskeletal remodelling and tumour cell migration and metastasis. 103 , 111 Shen et al conducted a study to show that TAZ and miR‐135b could form a positive feedback loop to modulate EMT process and metastasis in osteosarcoma. 112 Hereto, researches on Hippo signalling cascade may improve our understanding with regard to a variety of tumour properties including, but not limited to, metastasis, chemo‐resistance and EMT. Therefore, targeting Hippo may be an attractive option for cancer therapy. 30
Given the fact that lncRNAs are involved in cancer‐related signalling pathway to mediate tumorigenic process, it is therefore not surprising that these deregulated lncRNAs in Hippo cascade can also offer with the possibility as the attractive therapeutic candidates. 113 Meanwhile, recent advances in biological drugs, such as antisense oligonucleotides (ASOs), 114 , 115 CRISPR/Cas9 to target lncRNAs, small interfering RNAs (siRNAs) 116 and exosomal vectors, also implicate that lncRNAs could be used as prospective targets in cancer treatments. 111 For instance, Liu et al 50 found that CYTOR was among the most dramatically upregulated lncRNA in tamoxifen‐resistant breast cancer cells and in patient tissues with no response to tamoxifen treatment. CYTOR could activate Hippo and MAPK pathways via regulation of miR‐125a‐5p to enhance breast cancer cell survival upon tamoxifen treatment, indicating that targeting CYTOR may be a possible approach in reversing tamoxifen resistance in breast cancer. 50
Furthermore, glucose metabolism plays a crucial role in promoting and maintaining tumour cell characteristics. 26 , 117 During glucose deprivation, AMPK could phosphorylate and inhibit YAP, and then the activated YAP enhances glucose consumption and lactate production to generate energy to support the tumour cellular activity, 26 suggesting a role of Hippo pathway in promoting Warburg effect during carcinogenesis. A study by Zheng et al 26 showed that BCAR4 acted as a downstream target of YAP‐dependent glycolysis. Of note, BCAR4 antisense‐locked nucleic acid could significantly abolish the YAP‐dependent glycolysis and tumorigenesis. Taken together, targeting YAP‐BCAR4‐glycolysis network may be a putative strategy for breast cancer treatment by reprogramming glucose metabolism. 26 In addition, polo‐like kinase 4‐associated lncRNA (PLK4) is a downregulated lncRNA in HCC tissues and cell lines, and may serve as a tumour suppressor featured with YAP inactivation and subsequent cellular senescence induction. 118 Talazoparib is a potent poly‐ADP‐ribosyl polymerase (PARP) inhibitor that can induce synthetic lethality in cancers with deleterious germline mutations in BRCA. 119 , 120 A very recent study reported that talazoparib could dramatically upregulate expression of PLK4 to show the tumour inhibitory effect in HepG2 tumour cells, which provides us with a novel pathway to target PLK4/YAP axis for the treatment of HCC. 118 Certainly, the modulation of lncRNA/Hippo network may be an interesting and promising avenue for improvement of cancer treatment. However, lncRNA/Hippo‐based targeted therapy is still in its infancy and more experimental strategies as well as clinical trials are required in the near future. 80
6. CONCLUSIONS AND PERSPECTIVES
Hippo pathway is one of the most complicated signalling pathways with multiple downstream effectors that respond to extracellular and intracellular stimuli to coordinately govern cell differentiation, migration and proliferation. 25 Genetic or epigenetically provoked disruption of Hippo pathway leads to imbalanced regulation of these mechanisms, resulting in tumorigenesis. 25 , 121 Targeting Hippo signalling may provide novel approaches in treatment of cancer. However, given the fact that Hippo pathway has striking tumour regulatory activity in various contexts, the factors and concise regulation mechanisms for activation or inactivation of Hippo signalling are still poorly understood. 11
LncRNAs are a subclass of ncRNAs with growing recognition for their role in diverse cellular activities. Altered expression and mutation of lncRNAs are reported to drive multifaceted cancer phenotypes by regulating gene expression and signalling pathways at various levels. 96 Nowadays, a group of lncRNAs have been delineated to directly or indirectly target the core components of Hippo cascade, such as YAP, TAZ, LATS1/2 and MST1. 36 , 58 , 84 , 122 By contrast, Hippo can also modulate certain lncRNAs by affecting their transcriptional activity. 31 The expression of lncRNAs is closely correlated with tumorigenesis and tumour aggressiveness. Importantly, lncRNAs related to Hippo signalling may be useful as predictive indicators for diagnosis and prognosis in cancers. Researches on the interaction between lncRNAs and Hippo signalling pathway may potentially offer us a more comprehensive understanding in cancer occurrence and progression.
However, it should be noticed that the link between lncRNAs and Hippo pathways may be cell type‐, context‐ and even tumour stage‐specific. 31 , 52 Thus, more studies are still warranted to further elucidate their detailed structures and functions for developing biomarker and individualized therapy. 80 Besides the canonical Hippo pathway, there are studies reporting the non‐canonical Hippo signalling axis in the regulation of tumorigenesis. 123 , 124 Currently, the crosstalk between lncRNAs and non‐canonical Hippo pathway has not been elucidated yet, which may merit further exploration. Moreover, despite our understanding of lncRNA has been expanding in past decades, the discovery and functional annotation of lncRNAs still remain just the tip of an iceberg. 125 Furthermore, in order to promote efficient therapeutic interventions in cancers by targeting lncRNAs and Hippo pathway, further in‐depth pre‐clinical and clinical studies are urgently needed.
CONFLICTS OF INTERESTS
The authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
CT, KXY and JYH wrote the manuscript; LW, LQ, WCW, QL and ZHL reviewed and edited the manuscript before submission; CT and ZHL prepared the figures; and all authors read and approved the final version of the manuscript as submitted.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (81902745), Natural Science Foundation of Hunan Province, China (2018JJ3716), and China Scholarship Council (201806375067, 201806375068).
Tu C, Yang K, Wan L, et al. The crosstalk between lncRNAs and the Hippo signalling pathway in cancer progression. Cell Prolif. 2020;53:e12887 10.1111/cpr.12887
Contributor Information
Qiong Lu, Email: christy_luq@csu.edu.cn.
Zhihong Li, Email: lizhihong@csu.edu.cn.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Tu C, Ren X, He J, et al. The predictive value of lncRNA MIR31HG expression on clinical outcomes in patients with solid malignant tumors. Cancer Cell Int. 2020;20:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saad El Din K, Loree JM, Sayre EC, et al. Trends in the epidemiology of young‐onset colorectal cancer: a worldwide systematic review. BMC Cancer. 2020;20:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69:363‐385. [DOI] [PubMed] [Google Scholar]
- 4. Tu C, Ren X, He J, et al. The value of LncRNA BCAR4 as a prognostic biomarker on clinical outcomes in human cancers. J Cancer. 2019;10:5992‐6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang C, Ren X, He J, Wang W, Tu C, Li Z. The prognostic value of long noncoding RNA SNHG16 on clinical outcomes in human cancers: a systematic review and meta‐analysis. Cancer Cell Int. 2019;19:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bin X, Hongjian Y, Xiping Z, Bo C, Shifeng Y, Binbin T. Research progresses in roles of LncRNA and its relationships with breast cancer. Cancer Cell Int. 2018;18:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ren X, He J, Qi L, et al. Prognostic and clinicopathologic significance of long non‐coding RNA opa‐interacting protein 5‐antisense RNA 1 in multiple human cancers. Artif Cells Nanomed Biotechnol. 2020;48:353‐361. [DOI] [PubMed] [Google Scholar]
- 8. Xiao M, Feng Y, Liu C, Zhang Z. Prognostic values of long noncoding RNA PVT1 in various carcinomas: an updated systematic review and meta‐analysis. Cell Prolif. 2018;51:e12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yao F, Wang Q, Wu Q. The prognostic value and mechanisms of lncRNA UCA1 in human cancer. Cancer Manag Res. 2019;11:7685‐7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brannan CI, Dees EC, Ingram RS, Tilghman SM. The product of the H19 gene may function as an RNA. Mol Cell Biol. 1990;10:28‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ni W, Yao S, Zhou Y, et al. Long noncoding RNA GAS5 inhibits progression of colorectal cancer by interacting with and triggering YAP phosphorylation and degradation and is negatively regulated by the m(6)A reader YTHDF3. Mol Cancer. 2019;18:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang C, Qian H, Liu K, Zhao W, Wang L. A feedback loop regulation of LINC01433 and YAP promotes malignant behavior in gastric cancer cells. Onco Targets Ther. 2019;12:7949‐7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tu C, He J, Chen R, Li Z. The emerging role of exosomal non‐coding RNAs in musculoskeletal diseases. Curr Pharm Des. 2019;25:4523‐4535. [DOI] [PubMed] [Google Scholar]
- 14. He J, Tu C, Liu Y. Role of lncRNAs in aging and age‐related diseases. Aging Med (Milton). 2018;1:158‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chi Y, Wang D, Wang J, Yu W, Yang J. Long non‐coding RNA in the pathogenesis of cancers. Cells. 2019;8:1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Qu J, Kamal MA, Yuan C. The regulatory roles of long non‐coding RNA in the chemoresistance process of ovarian cancer. Curr Pharm Des. 2019;25:856‐861. [DOI] [PubMed] [Google Scholar]
- 17. Jiang MC, Ni JJ, Cui WY, Wang BY, Zhuo W. Emerging roles of lncRNA in cancer and therapeutic opportunities. Am J Cancer Res. 2019;9:1354‐1366. [PMC free article] [PubMed] [Google Scholar]
- 18. Fattahi S, Kosari‐Monfared M, Golpour M, et al. LncRNAs as potential diagnostic and prognostic biomarkers in gastric cancer: a novel approach to personalized medicine. J Cell Physiol. 2020;235:3189‐3206. [DOI] [PubMed] [Google Scholar]
- 19. Thompson BJ. YAP/TAZ: drivers of tumor growth, metastasis, and resistance to therapy. BioEssays. 2020;42:e1900162. [DOI] [PubMed] [Google Scholar]
- 20. Yang LX, Wu J, Guo ML, Zhang Y, Ma SG. Suppression of long non‐coding RNA TNRC6C‐AS1 protects against thyroid carcinoma through DNA demethylation of STK4 via the Hippo signalling pathway. Cell Prolif. 2019;52:e12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Justice RW, Zilian O, Woods DF, Noll M, Bryant PJ. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 1995;9:534‐546. [DOI] [PubMed] [Google Scholar]
- 22. Zygulska AL, Krzemieniecki K, Pierzchalski P. Hippo pathway ‐ brief overview of its relevance in cancer. J Physiol Pharmacol. 2017;68:311‐335. [PubMed] [Google Scholar]
- 23. Zeng Q, Hong W. The emerging role of the hippo pathway in cell contact inhibition, organ size control, and cancer development in mammals. Cancer Cell. 2008;13:188‐192. [DOI] [PubMed] [Google Scholar]
- 24. Zhao B, Wei X, Li W, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747‐2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Snigdha K, Gangwani KS, Lapalikar GV, Singh A, Kango‐Singh M. Hippo signaling in cancer: lessons from drosophila models. Front Cell Dev Biol. 2019;7:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zheng X, Han H, Liu GP, et al. LncRNA wires up Hippo and Hedgehog signaling to reprogramme glucose metabolism. EMBO J. 2017;36:3325‐3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fu PF, Zheng X, Fan X, Lin AF. Role of cytoplasmic lncRNAs in regulating cancer signaling pathways. J Zhejiang Univ Sci B. 2019;20:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yu FX, Zhao B, Guan KL. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell. 2015;163:811‐828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kovar H, Bierbaumer L, Radic‐Sarikas B. The YAP/TAZ pathway in osteogenesis and bone sarcoma pathogenesis. Cells. 2020;9:972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pobbati AV, Hong W. A combat with the YAP/TAZ‐TEAD oncoproteins for cancer therapy. Theranostics. 2020;10:3622‐3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shimoda M, Moroishi T. The emerging link between the hippo pathway and non‐coding RNA. Biol Pharm Bull. 2020;43:1‐10. [DOI] [PubMed] [Google Scholar]
- 32. Shen Y, Gao X, Tan W, Xu T. STAT1‐mediated upregulation of lncRNA LINC00174 functions a ceRNA for miR‐1910‐3p to facilitate colorectal carcinoma progression through regulation of TAZ. Gene. 2018;666:64‐71. [DOI] [PubMed] [Google Scholar]
- 33. Zheng Y, Pan D. The Hippo signaling pathway in development and disease. Dev Cell. 2019;50:264‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhao B, Lei QY, Guan KL. The Hippo‐YAP pathway: new connections between regulation of organ size and cancer. Curr Opin Cell Biol. 2008;20:638‐646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang Y, Fang Z, Guo X, et al. lncRNA B4GALT1‐AS1 promotes colon cancer cell stemness and migration by recruiting YAP to the nucleus and enhancing YAP transcriptional activity. J Cell Physiol. 2019;234:18524‐18534. [DOI] [PubMed] [Google Scholar]
- 36. Guan ZB, Cao YS, Li Y, Tong WN, Zhuo AS. Knockdown of lncRNA GHET1 suppresses cell proliferation, invasion and LATS1/YAP pathway in non small cell lung cancer. Cancer Biomark. 2018;21:557‐563. [DOI] [PubMed] [Google Scholar]
- 37. Yang C, Wu K, Wang S, Wei G. Long non‐coding RNA XIST promotes osteosarcoma progression by targeting YAP via miR‐195‐5p. J Cell Biochem. 2018;119:5646‐5656. [DOI] [PubMed] [Google Scholar]
- 38. Li Z, Wang Y, Hu R, Xu R, Xu W. LncRNA B4GALT1‐AS1 recruits HuR to promote osteosarcoma cells stemness and migration via enhancing YAP transcriptional activity. Cell Prolif. 2018;51:e12504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang D, Zhang H, Fang X, Zhang X, Liu H. Prognostic value of long non‐coding RNA GHET1 in cancers: a systematic review and meta‐analysis. Cancer Cell Int. 2020;20:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yu W, Xiang D, Jia H, et al. The lncRNA BCYRN1 functions as an oncogene in human glioma by downregulating miR‐125a‐5p in vitro. Cancer Manag Res. 2020;12:1151‐1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang Y, Xue C, Zhu X, Zhu X, Xian H, Huang Z. Suppression of microRNA‐125a‐5p upregulates the TAZ‐EGFR signaling pathway and promotes retinoblastoma proliferation. Cell Signal. 2016;28:850‐860. [DOI] [PubMed] [Google Scholar]
- 42. Su X, Teng J, Jin G, et al. ELK1‐induced upregulation of long non‐coding RNA MIR100HG predicts poor prognosis and promotes the progression of osteosarcoma by epigenetically silencing LATS1 and LATS2. Biomed Pharmacother. 2019;109:788‐797. [DOI] [PubMed] [Google Scholar]
- 43. Wang S, Ke H, Zhang H, et al. LncRNA MIR100HG promotes cell proliferation in triple‐negative breast cancer through triplex formation with p27 loci. Cell Death Dis. 2018;9:805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Emmrich S, Streltsov A, Schmidt F, Thangapandi VR, Reinhardt D, Klusmann JH. LincRNAs MONC and MIR100HG act as oncogenes in acute megakaryoblastic leukemia. Mol Cancer. 2014;13:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yeung B, Yu J, Yang X. Roles of the Hippo pathway in lung development and tumorigenesis. Int J Cancer. 2016;138:533‐539. [DOI] [PubMed] [Google Scholar]
- 46. Zhu Y, He D, Bo H, et al. The MRVI1‐AS1/ATF3 signaling loop sensitizes nasopharyngeal cancer cells to paclitaxel by regulating the Hippo‐TAZ pathway. Oncogene. 2019;38:6065‐6081. [DOI] [PubMed] [Google Scholar]
- 47. Yu W, Qiao Y, Tang X, et al. Tumor suppressor long non‐coding RNA, MT1DP is negatively regulated by YAP and Runx2 to inhibit FoxA1 in liver cancer cells. Cell Signal. 2014;26:2961‐2968. [DOI] [PubMed] [Google Scholar]
- 48. Huang L, Mo Z, Hu Z, et al. Diagnostic value of fibrinogen to prealbumin ratio and gamma‐glutamyl transpeptidase to platelet ratio in the progression of AFP‐negative hepatocellular carcinoma. Cancer Cell Int. 2020;20:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fan J, He S, Zheng Y. Analyses of clinical efficacy of ultrasound‐guided radiofrequency ablation in liver cancer adjacent to the gallbladder and its prognosis. J BUON. 2019;24:2411‐2417. [PubMed] [Google Scholar]
- 50. Liu Y, Li M, Yu H, Piao H. lncRNA CYTOR promotes tamoxifen resistance in breast cancer cells via sponging miR125a5p. Int J Mol Med. 2020;45:497‐509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ou C, Sun Z, He X, et al. Targeting YAP1/LINC00152/FSCN1 signaling axis prevents the progression of colorectal cancer. Adv Sci (Weinh). 2020;7:1901380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tan BS, Yang MC, Singh S, et al. LncRNA NORAD is repressed by the YAP pathway and suppresses lung and breast cancer metastasis by sequestering S100P. Oncogene. 2019;38:5612‐5626. [DOI] [PubMed] [Google Scholar]
- 53. Chan LH, Wang W, Yeung W, Deng Y, Yuan P, Mak KK. Hedgehog signaling induces osteosarcoma development through Yap1 and H19 overexpression. Oncogene. 2014;33:4857‐4866. [DOI] [PubMed] [Google Scholar]
- 54. Zhang J, Yin M, Huang J, et al. Long noncoding RNA LINC00152 as a novel predictor of lymph node metastasis and survival in human cancer: a systematic review and meta‐analysis. Clin Chim Acta. 2018;483:25‐32. [DOI] [PubMed] [Google Scholar]
- 55. Ventura A. NORAD: defender of the genome. Trends Genet. 2016;32:390‐392. [DOI] [PubMed] [Google Scholar]
- 56. Yang Z, Zhao Y, Lin G, Zhou X, Jiang X, Zhao H. Noncoding RNA activated by DNA damage (NORAD): biologic function and mechanisms in human cancers. Clin Chim Acta. 2019;489:5‐9. [DOI] [PubMed] [Google Scholar]
- 57. Zhou K, Ou Q, Wang G, Zhang W, Hao Y, Li W. High long non‐coding RNA NORAD expression predicts poor prognosis and promotes breast cancer progression by regulating TGF‐beta pathway. Cancer Cell Int. 2019;19:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. An LF, Huang JW, Han X, Wang J. Downregulation of lncRNA H19 sensitizes melanoma cells to cisplatin by regulating the miR‐18b/IGF1 axis. Anticancer Drugs. 2020;31:473‐482. [DOI] [PubMed] [Google Scholar]
- 59. Wang X, Pei X, Guo G, et al. Exosome‐mediated transfer of long noncoding RNA H19 induces doxorubicin resistance in breast cancer. J Cell Physiol. 2020. 10.1002/jcp.29585 [DOI] [PubMed] [Google Scholar]
- 60. Zhang Y, Zhu R, Wang J, Cui Z, Wang Y, Zhao Y. Upregulation of lncRNA H19 promotes nasopharyngeal carcinoma proliferation and metastasis in let‐7 dependent manner. Artif Cells Nanomed Biotechnol. 2019;47:3854‐3861. [DOI] [PubMed] [Google Scholar]
- 61. Li D, Hao S, Zhang J. Long non‐coding RNA UCA1 exerts growth modulation by miR‐15a in human thyroid cancer TPC‐1 cells. Artif Cells Nanomed Biotechnol. 2019;47:1815‐1822. [DOI] [PubMed] [Google Scholar]
- 62. Lin X, Spindler TJ, de Souza Fonseca MA, et al. Super‐enhancer‐associated LncRNA UCA1 interacts directly with AMOT to activate YAP target genes in epithelial ovarian cancer. iScience.. 2019;17:242‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mota MSV, Jackson WP, Bailey SK, et al. Deficiency of tumor suppressor Merlin facilitates metabolic adaptation by co‐operative engagement of SMAD‐Hippo signaling in breast cancer. Carcinogenesis. 2018;39:1165‐1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhang M, Zhao Y, Zhang Y, et al. LncRNA UCA1 promotes migration and invasion in pancreatic cancer cells via the Hippo pathway. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1770‐1782. [DOI] [PubMed] [Google Scholar]
- 65. Gao ZQ, Wang JF, Chen DH, et al. Long non‐coding RNA GAS5 antagonizes the chemoresistance of pancreatic cancer cells through down‐regulation of miR‐181c‐5p. Biomed Pharmacother. 2018;97:809‐817. [DOI] [PubMed] [Google Scholar]
- 66. Sun Y, Jiang T, Jia Y, Zou J, Wang X, Gu W. LncRNA MALAT1/miR‐181a‐5p affects the proliferation and adhesion of myeloma cells via regulation of Hippo‐YAP signaling pathway. Cell Cycle. 2019;18:2509‐2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang J, Wang H, Zhang Y, et al. Mutual inhibition between YAP and SRSF1 maintains long non‐coding RNA, Malat1‐induced tumourigenesis in liver cancer. Cell Signal. 2014;26:1048‐1059. [DOI] [PubMed] [Google Scholar]
- 68. Zhou Y, Shan T, Ding W, et al. Study on mechanism about long noncoding RNA MALAT1 affecting pancreatic cancer by regulating Hippo‐YAP signaling. J Cell Physiol. 2018;233:5805‐5814. [DOI] [PubMed] [Google Scholar]
- 69. Xu W, Yan Z, Hu F, Wei W, Yang C, Sun Z. Long non‐coding RNA GAS5 accelerates oxidative stress in melanoma cells by rescuing EZH2‐mediated CDKN1C downregulation. Cancer Cell Int. 2020;20:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gao C, Ren C, Liu Z, Zhang L, Tang R, Li X. GAS5, a FoxO1‐actived long noncoding RNA, promotes propofol‐induced oral squamous cell carcinoma apoptosis by regulating the miR‐1297‐GSK3beta axis. Artif Cells Nanomed Biotechnol. 2019;47:3985‐3993. [DOI] [PubMed] [Google Scholar]
- 71. He L, Li H, Wu A, Peng Y, Shu G, Yin G. Functions of N6‐methyladenosine and its role in cancer. Mol Cancer. 2019;18:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jin D, Guo J, Wu Y, et al. m(6)A demethylase ALKBH5 inhibits tumor growth and metastasis by reducing YTHDFs‐mediated YAP expression and inhibiting miR‐107/LATS2‐mediated YAP activity in NSCLC. Mol Cancer. 2020;19:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Jin D, Guo J, Wu Y, et al. m(6)A mRNA methylation initiated by METTL3 directly promotes YAP translation and increases YAP activity by regulating the MALAT1‐miR‐1914‐3p‐YAP axis to induce NSCLC drug resistance and metastasis. J Hematol Oncol. 2019;12:135. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 74. Su Y, Wu H, Pavlosky A, et al. Regulatory non‐coding RNA: new instruments in the orchestration of cell death. Cell Death Dis. 2016;7:e2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wen J, Chen L, Tian H, et al. Effect of MALAT1 polymorphisms on papillary thyroid cancer in a chinese population. J Cancer. 2019;10:5714‐5721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Liu S, Qiu J, He G, et al. LncRNA MALAT1 acts as a miR‐125a‐3p sponge to regulate FOXM1 expression and promote hepatocellular carcinoma progression. J Cancer. 2019;10:6649‐6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Tan M, Jiang B, Wang H, et al. Dihydromyricetin induced lncRNA MALAT1‐TFEB‐dependent autophagic cell death in cutaneous squamous cell carcinoma. J Cancer. 2019;10:4245‐4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Qi F, Tan B, Ma F, et al. A Synthetic Light‐switchable System based on CRISPR Cas13a regulates the expression of LncRNA MALAT1 and affects the malignant phenotype of bladder cancer cells. Int J Biol Sci. 2019;15:1630‐1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Li H, He C, Wang X, Wang H, Nan G, Fang L. MicroRNA‐183 affects the development of gastric cancer by regulating autophagy via MALAT1‐miR‐183‐SIRT1 axis and PI3K/AKT/mTOR signals. Artif Cells Nanomed Biotechnol. 2019;47:3163‐3171. [DOI] [PubMed] [Google Scholar]
- 80. Bhan A, Soleimani M, Mandal SS. Long noncoding RNA and cancer: a new paradigm. Cancer Res. 2017;77:3965‐3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kim J, Piao HL, Kim BJ, et al. Long noncoding RNA MALAT1 suppresses breast cancer metastasis. Nat Genet. 2018;50:1705‐1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gomes CP, Nobrega‐Pereira S, Domingues‐Silva B, et al. An antisense transcript mediates MALAT1 response in human breast cancer. BMC Cancer. 2019;19:771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Chen Q, Zhu C, Jin Y. The oncogenic and tumor suppressive functions of the long noncoding RNA MALAT1: an emerging controversy. Front Genet. 2020;11:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Gao L, Cao H, Cheng X. A positive feedback regulation between long noncoding RNA SNHG1 and YAP1 modulates growth and metastasis in laryngeal squamous cell carcinoma. Am J Cancer Res. 2018;8:1712‐1724. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 85. Li N, Yang G, Luo L, et al. lncRNA THAP9‐AS1 promotes pancreatic ductal adenocarcinoma growth and leads to a poor clinical outcome via sponging miR‐484 and interacting with YAP. Clin Cancer Res. 2020;26:1736‐1748. [DOI] [PubMed] [Google Scholar]
- 86. Dong B, Chen X, Zhang Y, Zhu C, Dong Q. The prognostic value of lncRNA SNHG1 in cancer patients: a meta‐analysis. BMC Cancer. 2019;19:780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Jiang Z, Jiang C, Fang J. Up‐regulated lnc‐SNHG1 contributes to osteosarcoma progression through sequestration of miR‐577 and activation of WNT2B/Wnt/beta‐catenin pathway. Biochem Biophys Res Commun. 2018;495:238‐245. [DOI] [PubMed] [Google Scholar]
- 88. Cui L, Dong Y, Wang X, et al. Downregulation of long noncoding RNA SNHG1 inhibits cell proliferation, metastasis, and invasion by suppressing the Notch‐1 signaling pathway in pancreatic cancer. J Cell Biochem. 2019;120:6106‐6112. [DOI] [PubMed] [Google Scholar]
- 89. Zender L, Spector MS, Xue W, et al. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125:1253‐1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Li S, Yu Z, Chen SS, et al. The YAP1 oncogene contributes to bladder cancer cell proliferation and migration by regulating the H19 long noncoding RNA. Urol Oncol. 2015;33(10):427. e421‐410. [DOI] [PubMed] [Google Scholar]
- 91. Cui X, Piao C, Lv C, Lin X, Zhang Z, Liu X. ZNFX1 anti‐sense RNA 1 promotes the tumorigenesis of prostate cancer by regulating c‐Myc expression via a regulatory network of competing endogenous RNAs. Cell Mol Life Sci. 2020;77:1135‐1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sun Z, Ou C, Liu J, et al. YAP1‐induced MALAT1 promotes epithelial‐mesenchymal transition and angiogenesis by sponging miR‐126‐5p in colorectal cancer. Oncogene. 2019;38:2627‐2644. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 93. Matsuda Y, Narita S, Nara T, et al. Impact of nuclear YAP1 expression in residual cancer after neoadjuvant chemohormonal therapy with docetaxel for high‐risk localized prostate cancer. BMC Cancer. 2020;20:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Bouvier C, Macagno N, Nguyen Q, et al. Prognostic value of the Hippo pathway transcriptional coactivators YAP/TAZ and beta1‐integrin in conventional osteosarcoma. Oncotarget. 2016;7:64702‐64710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wang CZ, Yan GX, Dong DS, Xin H, Liu ZY. LncRNA‐ATB promotes autophagy by activating Yes‐associated protein and inducing autophagy‐related protein 5 expression in hepatocellular carcinoma. World J Gastroenterol. 2019;25:5310‐5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ni W, Zhang Y, Zhan Z, et al. A novel lncRNA uc.134 represses hepatocellular carcinoma progression by inhibiting CUL4A‐mediated ubiquitination of LATS1. J Hematol Oncol. 2017;10:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lv GY, Miao J, Zhang XL. Long noncoding RNA XIST promotes osteosarcoma progression by targeting RAS‐related protein RAP2B via miR‐320b. Oncol Res. 2018;26:837‐846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sun X, Wei B, Peng ZH, et al. Knockdown of lncRNA XIST suppresses osteosarcoma progression by inactivating AKT/mTOR signaling pathway by sponging miR‐375‐3p. Int J Clin Exp Pathol. 2019;12:1507‐1517. [PMC free article] [PubMed] [Google Scholar]
- 99. Wang W, Shen H, Cao G, Huang J. Long non‐coding RNA XIST predicts poor prognosis and promotes malignant phenotypes in osteosarcoma. Oncol Lett. 2019;17:256‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Li GL, Wu YX, Li YM, Li J. High expression of long non‐coding RNA XIST in osteosarcoma is associated with cell proliferation and poor prognosis. Eur Rev Med Pharmacol Sci. 2017;21:2829‐2834. [PubMed] [Google Scholar]
- 101. Xu T, Jiang W, Fan L, Gao Q, Li G. Upregulation of long noncoding RNA Xist promotes proliferation of osteosarcoma by epigenetic silencing of P21. Oncotarget. 2017;8:101406‐101417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Deng C, Hu X, Wu K, Tan J, Yang C. Long non‐coding RNA XIST predicting advanced clinical parameters in cancer: A Meta‐Analysis and case series study in a single institution. Oncol Lett. 2019;18:2192‐2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wu DM, Wang S, Wen X, et al. LncRNA SNHG15 acts as a ceRNA to regulate YAP1‐Hippo signaling pathway by sponging miR‐200a‐3p in papillary thyroid carcinoma. Cell Death Dis. 2018;9:947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Su L, Wang S, Yuan T, et al. Anti‐oral squamous cell carcinoma effects of a potent TAZ inhibitor AR‐42. J Cancer. 2020;11:364‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Zhang Z, Qiu N, Yin J, et al. SRGN crosstalks with YAP to maintain chemoresistance and stemness in breast cancer cells by modulating HDAC2 expression. Theranostics. 2020;10:4290‐4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Mao B, Hu F, Cheng J, et al. SIRT1 regulates YAP2‐mediated cell proliferation and chemoresistance in hepatocellular carcinoma. Oncogene. 2014;33:1468‐1474. [DOI] [PubMed] [Google Scholar]
- 107. Wang DY, Wu YN, Huang JQ, et al. Hippo/YAP signaling pathway is involved in osteosarcoma chemoresistance. Chin J Cancer. 2016;35:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Cournoyer S, Addioui A, Belounis A, et al. GX15‐070 (Obatoclax), a Bcl‐2 family proteins inhibitor engenders apoptosis and pro‐survival autophagy and increases Chemosensitivity in neuroblastoma. BMC Cancer. 2019;19:1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Wang X, Lan Z, He J, et al. LncRNA SNHG6 promotes chemoresistance through ULK1‐induced autophagy by sponging miR‐26a‐5p in colorectal cancer cells. Cancer Cell Int. 2019;19:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Wilkinson DS, Hansen M. LC3 is a novel substrate for the mammalian Hippo kinases, STK3/STK4. Autophagy. 2015;11:856‐857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Jin KT, Lu ZB, Lv JQ, Zhang JG. The role of long non‐coding RNAs in mediating chemoresistance by modulating autophagy in cancer. RNA Biol. 2020;1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Shen S, Huang K, Wu Y, et al. A miR‐135b‐TAZ positive feedback loop promotes epithelial‐mesenchymal transition (EMT) and tumorigenesis in osteosarcoma. Cancer Lett. 2017;407:32‐44. [DOI] [PubMed] [Google Scholar]
- 113. Li CH, Chen Y. Targeting long non‐coding RNAs in cancers: progress and prospects. Int J Biochem Cell Biol. 2013;45:1895‐1910. [DOI] [PubMed] [Google Scholar]
- 114. d'Ydewalle C, Ramos DM, Pyles NJ, et al. The antisense transcript SMN‐AS1 regulates SMN expression and is a novel therapeutic target for spinal muscular atrophy. Neuron. 2017;93:66‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Amodio N, Stamato MA, Juli G, et al. Drugging the lncRNA MALAT1 via LNA gapmeR ASO inhibits gene expression of proteasome subunits and triggers anti‐multiple myeloma activity. Leukemia. 2018;32:1948‐1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Jin SJ, Jin MZ, Xia BR, Jin WL. Long non‐coding RNA DANCR as an emerging therapeutic target in human cancers. Front Oncol. 2019;9:1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Liu H, Luo J, Luan S, He C, Li Z. Long non‐coding RNAs involved in cancer metabolic reprogramming. Cell Mol Life Sci. 2019;76:495‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Jia Y, Jin H, Gao L, et al. A novel lncRNA PLK4 up‐regulated by talazoparib represses hepatocellular carcinoma progression by promoting YAP‐mediated cell senescence. J Cell Mol Med. 2020;24(9):5304‐5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Baldwin P, Ohman AW, Medina JE, McCarthy ET, Dinulescu DM, Sridhar S. Nanoformulation of talazoparib delays tumor progression and ascites formation in a late stage cancer model. Front Oncol. 2019;9:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Exman P, Barroso‐Sousa R, Tolaney SM. Evidence to date: talazoparib in the treatment of breast cancer. Onco Targets Ther. 2019;12:5177‐5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Stepan J, Anderzhanova E, Gassen NC. Hippo signaling: emerging pathway in stress‐related psychiatric disorders? Front Psychiatry. 2018;9:715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Chen S, Wang LL, Sun KX, et al. LncRNA PCGEM1 induces ovarian carcinoma tumorigenesis and progression through RhoA pathway. Cell Physiol Biochem. 2018;47:1578‐1588. [DOI] [PubMed] [Google Scholar]
- 123. Du X, Yu A, Tao W. The non‐canonical Hippo/Mst pathway in lymphocyte development and functions. Acta Biochim Biophys Sin (Shanghai). 2015;47:60‐64. [DOI] [PubMed] [Google Scholar]
- 124. An L, Nie P, Chen M, et al. MST4 kinase suppresses gastric tumorigenesis by limiting YAP activation via a non‐canonical pathway. J Exp Med. 2020;217:e20191817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Aboudehen K. Regulation of mTOR signaling by long non‐coding RNA. Biochim Biophys Acta Gene Regul Mech. 2020;1863:194449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Su R, Ma J, Zheng J, et al. PABPC1‐induced stabilization of BDNF‐AS inhibits malignant progression of glioblastoma cells through STAU1‐mediated decay. Cell Death Dis. 2020;11:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Wang J, Li Z, Wang X, Ding Y, Li N. The tumor suppressive effect of long non‐coding RNA FRMD6‐AS2 in uteri corpus endometrial carcinoma. Life Sci. 2020;243:117254. [DOI] [PubMed] [Google Scholar]
- 128. Li C, Wang S, Xing Z, et al. A ROR1‐HER3‐lncRNA signalling axis modulates the Hippo‐YAP pathway to regulate bone metastasis. Nat Cell Biol. 2017;19:106‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Zhang C, Bao C, Zhang X, Lin X, Pan D, Chen Y. Knockdown of lncRNA LEF1‐AS1 inhibited the progression of oral squamous cell carcinoma (OSCC) via Hippo signaling pathway. Cancer Biol Ther. 2019;20:1213‐1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Liu Z, Yao Y, Huang S, et al. LINC00662 promotes gastric cancer cell growth by modulating the Hippo‐YAP1 pathway. Biochem Biophys Res Commun. 2018;505:843‐849. [DOI] [PubMed] [Google Scholar]
- 131. Qiao K, Ning S, Wan L, et al. LINC00673 is activated by YY1 and promotes the proliferation of breast cancer cells via the miR‐515‐5p/MARK4/Hippo signaling pathway. J Exp Clin Cancer Res. 2019;38:418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Chen L, Chen Q, Kuang S, et al. USF1‐induced upregulation of LINC01048 promotes cell proliferation and apoptosis in cutaneous squamous cell carcinoma by binding to TAF15 to transcriptionally activate YAP1. Cell Death Dis. 2019;10:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Lv B, Zhang L, Miao R, et al. Comprehensive analysis and experimental verification of LINC01314 as a tumor suppressor in hepatoblastoma. Biomed Pharmacother. 2018;98:783‐792. [DOI] [PubMed] [Google Scholar]
- 134. Lou C, Zhao J, Gu Y, et al. LINC01559 accelerates pancreatic cancer cell proliferation and migration through YAP‐mediated pathway. J Cell Physiol. 2020;235:3928‐3938. [DOI] [PubMed] [Google Scholar]
- 135. Zhu Q, Li Y, Dong X, Yang Y, Wang H, Guo S. Linc‐OIP5 loss regulates migration and invasion in MDA‐MB‐231 breast cancer cells by inhibiting YAP1/JAG1 signaling. Oncol Lett. 2020;19:103‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Hu GW, Wu L, Kuang W, et al. Knockdown of linc‐OIP5 inhibits proliferation and migration of glioma cells through down‐regulation of YAP‐NOTCH signaling pathway. Gene. 2017;610:24‐31. [DOI] [PubMed] [Google Scholar]
- 137. Zhu Q, Li J, Wu Q, et al. Linc‐OIP5 in the breast cancer cells regulates angiogenesis of human umbilical vein endothelial cells through YAP1/Notch/NRP1 signaling circuit at a tumor microenvironment. Biol Res. 2020;53:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Zhang Y, Wang T, Wang S, et al. Nkx2‐2as suppression contributes to the pathogenesis of sonic hedgehog medulloblastoma. Cancer Res. 2018;78:962‐973. [DOI] [PubMed] [Google Scholar]
- 139. Zhao W, Zhang LN, Wang XL, Zhang J, Yu HX. Long noncoding RNA NSCLCAT1 increases non‐small cell lung cancer cell invasion and migration through the Hippo signaling pathway by interacting with CDH1. FASEB J. 2019;33:1151‐1166. [DOI] [PubMed] [Google Scholar]
- 140. Gao L, Cheng XL, Cao H. LncRNA THOR attenuates cisplatin sensitivity of nasopharyngeal carcinoma cells via enhancing cells stemness. Biochimie. 2018;152:63‐72. [DOI] [PubMed] [Google Scholar]
- 141. Liu S, Yang Y, Wang W, Pan X. Long noncoding RNA TUG1 promotes cell proliferation and migration of renal cell carcinoma via regulation of YAP. J Cell Biochem. 2018;119:9694‐9706. [DOI] [PubMed] [Google Scholar]
- 142. Li SY, Wang H, Mai HF, et al. Down‐regulated long non‐coding RNA RNAZFHX4‐AS1 suppresses invasion and migration of breast cancer cells via FAT4‐dependent Hippo signaling pathway. Cancer Gene Ther. 2019;26:374‐387. [DOI] [PubMed] [Google Scholar]
- 143. Qu L, Wu Z, Li Y, et al. A feed‐forward loop between lncARSR and YAP activity promotes expansion of renal tumour‐initiating cells. Nat Commun. 2016;7:12692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Qin LT, Tang RX, Lin P, et al. Biological function of UCA1 in hepatocellular carcinoma and its clinical significance: Investigation with in vitro and meta‐analysis. Pathol Res Pract. 2018;214:1260‐1272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


