Abstract
Introduction
We examined the role of hemodynamic dysfunction in cognition by relating cerebral blood flow (CBF), measured with arterial spin labeling (ASL), to cognitive functioning, in patients with heart failure (HF), carotid occlusive disease (COD), and patients with cognitive complaints and vascular brain injury on magnetic resonance imaging (MRI; ie, possible vascular cognitive impairment [VCI]).
Methods
We included 439 participants (124 HF; 75 COD; 127 possible VCI; 113 reference participants) from the Dutch multi‐center Heart–Brain Study. We used pseudo‐continuous ASL to estimate whole‐brain and regional partial volume‐corrected CBF. Neuropsychological tests covered global cognition and four cognitive domains.
Results
CBF values were lowest in COD, followed by VCI and HF, compared to reference participants. This did not explain cognitive impairment, as we did not find an association between CBF and cognitive functioning.
Discussion
We found that reduced CBF is not the major explanatory factor underlying cognitive impairment in patients with hemodynamic dysfunction along the heart–brain axis.
Keywords: carotid occlusive disease, cognitive impairment, heart failure, perfusion, small vessel disease, vascular cognitive impairment
1. BACKGROUND
Cardiovascular disease and dementia are both common in the aging population and are among the leading causes of death and disability. After Alzheimer's disease (AD), vascular brain injury is the second most common cause of cognitive impairment and dementia. 1 Vascular cognitive impairment (VCI) covers the entire spectrum of cognitive impairment, ranging from mild cognitive impairment (MCI) to fully developed dementia, due to all forms of vascular brain injury. 1 Moreover, research interest is shifting to the earlier stage of subjective cognitive decline (SCD), which refers to patients with cognitive complaints, but without objective impairment on cognitive testing. 2 , 3 Recent studies suggest that cardiovascular disease and dementia are closely related as they share common risk factors such as age, diabetes, smoking, and physical inactivity. 4 In addition, patients with cardiovascular disease are at increased risk for cognitive decline and dementia. 5 This has led to the concept of a “heart–brain axis’’ in cognitive decline and dementia. 6 , 7
Hemodynamic dysfunction or abnormalities of the circulatory system in any component of the heart–brain axis could be a risk factor for the development of vascular brain injury and consequently to the development of cognitive impairment and dementia. 8 In the Heart–Brain study, we investigate if the hemodynamic status of the heart, vessels, and the brain is an important, but underestimated, cause of VCI. We focus on heart failure (HF), symptomatic carotid occlusive disease (COD), and patients with cognitive complaints and vascular brain injury on magnetic resonance imaging (MRI; ie, possible VCI) as three exemplar conditions of hemodynamic dysfunction in different components of the heart–brain axis (ie, heart–carotids–brain). 9
Recently, we found that a substantial number of the patients with HF and COD have cognitive impairment. 10 Reduced cerebral blood flow (CBF) has been associated with cardiovascular disease and is increasingly recognized as an important contributor to cognitive decline. If so, treatment targeting hemodynamic dysfunction in the heart–brain axis might contribute to prevention of cognitive decline. In the Heart–Brain study, we use arterial spin labeling (ASL) MRI for measurement of CBF. ASL is a quantitative MRI technique that enables non‐invasive measurement of CBF at the tissue level. 11 In a previous study in memory‐clinic patients we found associations between reduced CBF and worse cognitive functioning. 12 In the present study, we investigated the cross‐sectional association between CBF, measured with ASL, and cognitive functioning in patients with HF, COD, and possible VCI. We expect this association in all patient groups, but most prominently in VCI.
2. METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request, within the privacy legislation of the Netherlands and after permission of the Heart–Brain study steering committee.
The Heart–Brain study is an ongoing prospective observational cohort study. 9 All patients have been enrolled between September 2014 and September 2017 and here we report on the baseline data (version 2, 1‐1‐2018). We included patients with HF, VCI, and COD from cardiology, memory, and neurology outpatient clinics from four sites in the Netherlands: Leiden University Medical Center (LUMC) in Leiden, Maastricht University Medical Center (MUMC) in Maastricht, University Medical Center Utrecht (UMCU) in Utrecht, and VU University Medical Center (VUMC) in Amsterdam. The study protocol with all in‐ and exclusion criteria per patient group have been described in detail previously. 9 Most important inclusion criteria for all patient groups were a diagnosis of possible VCI, COD, or HF according to current guidelines, age ≥50 years, ability to undergo MRI and cognitive testing, and independence in daily life. Most important exclusion criteria for all patient groups were clinical evidence of a neurodegenerative disease other than VCI or AD, a psychiatric diagnosis that affects cognitive functioning, and atrial fibrillation at the moment of inclusion. For possible VCI, we included patients with cognitive complaints (regardless of the severity of cognitive impairment [ie, subjective cognitive decline to dementia]), combined with moderate to severe vascular brain injury on MRI, or mild vascular brain injury with presence of vascular risk factors, with a Mini‐Mental State Examination (MMSE) 13 of ≥20. Patients with COD had a significant stenosis (>80%) or occlusion of the internal carotid artery as assessed with MR angiography. We included patients with HF irrespective of left ventricular ejection fraction and coronary artery disease according to the European Cardiology Society guidelines with a stable clinical situation. As a reference group, we recruited reference participants via advertisements and among spouses of patients. None of the reference participants had a history of dementia or COD; one reference participant had a prior diagnosis of HF. The study was performed according to the Helsinki Declaration and was approved by the medical ethics committee of LUMC. All participants provided written informed consent prior to research‐related procedures.
2.1. Participants
For the current study, we included all participants with available ASL on MRI and neuropsychological testing at the baseline assessment. Of the total of 559 participants (162 HF, 109 COD, 160 possible VCI, and 128 reference participants), 87 participants were excluded due to missing ASL (n = 83), neuropsychological assessment (n = 2), or both (n = 2). All participants underwent an extensive baseline assessment including medical, neurologic, and cardiovascular history; physical examination including blood pressure measurement and electrocardiography; screening laboratory tests; a neuropsychological assessment; and cardiac and brain MRI. For all participants, history of previous stroke and transient ischemic attack (TIA) and the presence of vascular risk factors (ie, hypertension, hypercholesterolemia, and diabetes mellitus) was determined based on self‐reported medical history and medication use. Smoking status was defined as never, former, or current. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Level of education was classified according to the system of Verhage ranging from 1 to 7 (low to highly educated). 14
2.2. Neuropsychological assessment
Cognitive functioning was assessed using the standardized neuropsychological test battery that has been developed in context of the Dutch Parelsnoer Initiative. 15 As cognitive screening, we used the MMSE. 13 In addition, we assessed four cognitive domains: memory, language, attention‐psychomotor speed, and executive functioning. For memory, we used the Visual Association Test (VAT), part A and the total immediate recall, delayed recall, and recognition score of the Dutch version of the Rey Auditory Verbal Learning Test (RAVLT). 16 , 17 To examine language, we used the VAT naming and the 1‐minute category fluency (animals). 17 , 18 For the domain attention/psychomotor speed, we used the Trail Making Test (TMT) part A, the forward condition of the Digit Span, the Letter Digit Substitution Test, and the Stroop Color Word Test (SCWT) card I and II. 19 , 20 , 21 , 22 To examine executive functioning, we used the index score of TMT part B/part A, the backward condition of the Digit Span and the SCWT interference score, calculated as card III/([card I + card II] / 2). 19 , 20 , 21 The RAVLT recognition score, TMT‐A and TMT‐B and the SCWT scores were inverted, so that higher scores imply a better performance. In participants where the TMT‐B was aborted (n = 14), for example because of lack of time or cognitive impairment, we estimated TMT‐B by multiplying the time needed to complete TMT‐A with the mean B/A index. On the other tests, 0% to 2.7% of the test scores were missing. All neuropsychological test scores were standardized into z‐scores, using the reference participants as reference group. Subsequently, available test scores were averaged to create the four cognitive domains. A score for global cognition was constructed by calculating the mean z‐score across all cognitive domains.
HIGHLIGHTS
We investigated patients with hemodynamic disorders along the heart–brain axis.
We found no association between cerebral blood flow (CBF) and cognitive functioning.
CBF is not the explanatory factor underlying cognitive impairment in these patients.
RESEARCH IN CONTEXT
Systematic review: We reviewed the literature using PubMed regarding cerebral blood flow (CBF) and cognitive impairment in cardiovascular disease and patients with vascular brain injury. We cite several studies that investigate this association in a single group with limited data on cognitive functioning. In our study, we investigated this association in three groups with hemodynamic impairment on different levels along the heart–brain axis using extensive neuropsychological testing.
Interpretation: We found no associations between decreased CBF and cognitive impairment in patients with hemodynamic disorders along the heart–brain axis. This could suggest that the predisposition of cognitive impairment in these patients is likely to be driven by other (hemodynamic) mechanisms than static CBF.
Future directions: Further studies within the Heart–Brain Connection Consortium will focus on other mechanisms, underlying the role of compromised hemodynamics on cognitive impairment, including cardiac output, cerebral autoregulation, and presence of amyloid deposition.
2.3. MRI protocol
All brain MRI scans were acquired on Philips Ingenia, Achieva, and Gemini 3T MRI scanners (Philips, Best, the Netherlands). The standardized MRI protocol included 3D T1‐weighted, T2 fluid‐attenuated inversion recovery (FLAIR), and susceptibility‐weighted imaging (SWI). 9 CBF was measured with pseudo‐continuous ASL (pCASL) (multi‐slice 2D echo planar imaging [EPI] acquisition with background suppression; labeling duration = 1800 milliseconds; post‐labeling delay = 1800 milliseconds; single shot EPI readout; resolution = 3 × 3 × 7 mm). 23 The perfusion measurements were performed in the same scan session as the structural sequences.
2.4. Preprocessing and MRI data analysis
Processing of the brain MRI was performed using two automated pipelines. For each patient, manual segmentation of infarcts and other pathologies that potentially affect automatic tissue segmentation was performed by a neuroradiologist. Subsequently, the annotated infarcts and pathologies were manually segmented by trained students. In addition, an automated pipeline (Quantib Brain, Rotterdam, the Netherlands) was used to segment white matter hyperintensities (WMH) based on FLAIR scans. A brain tissue segmentation method was applied to the 3D T1‐weighted images. From these segmentations, volumes in millilitres (mL) of total brain gray matter (GM), white matter, cerebrospinal fluid, and WMH were computed.
pCASL data were processed using the automated Iris pipeline for CBF quantification. 24 Quantification of ASL data into CBF maps was based on a single‐compartment model after the subtraction of labeled images from control images according to the recommended approach. 23 To scale the signal intensities of the subtracted ASL images to absolute CBF units, a separately acquired proton density‐weighted image (M0) was used. The quantification further included motion‐correction of the raw ASL data 25 and additional partial volume correction (PVC). 26 CBF was quantified in normal‐appearing GM (NAGM) only. To obtain the NAGM mask for each participant, first a binary GM segmentation was obtained using SPM (Statistical Parametric Mapping, London, UK) software. Subsequently, PVC‐uncorrected ASL images of all patients were visually inspected. 23 We excluded nine patients due to suboptimal quality of the ASL images (ie, motion artefacts, incomplete ASL‐sequence, or labeling errors). In addition, 21 patients were excluded due to ASL images with dominant vascular artefacts and little tissue perfusion signal. We excluded three extreme outliers, because their CBF values were more than three standard deviations from the mean. This resulted in a study sample of 439 patients (124 HF, 75 COD, and 127 possible VCI) and 113 reference participants. The regions of interest (ROIs) were defined using a multi‐atlas approach. This involved the registration of 30 manually labeled T1W images, each containing 83 ROIs, 27 , 28 to the participants’ T1 images. In our analyses we combined these ROIs to obtain mean CBF values of the frontal, parietal, temporal, and occipital brain lobes.
2.5. Statistical analysis
PASW Statistics 25.0 for Mac (SPSS Inc., Chicago IL, USA) was used for all statistical analyses. Analyses of variance (ANOVA) and Pearson χ2 tests were performed to compare groups when appropriate.
To investigate the association between CBF and cognitive functioning, we used linear regression analyses with CBF as independent variable and cognitive domains as dependent variables (separate models for each cognitive domain). We adjusted for participant group (using dummy variables), age, sex, education, and center. As the presumed underlying mechanism of reduced CBF differs fundamentally among participant groups (ie, reduced CBF is caused by either an impaired pump function [HF], low blood supply to the brain [COD], or the proposed result of dysfunction of these components [VCI]), we subsequently stratified for participant group to investigate the association between CBF and cognitive functioning per group. For the linear regression analyses, we chose an alpha of 0.01 to adjust for multiple comparisons. A power analysis illustrated that based on two‐tailed testing, an alpha of 0.01, and a power of 80%, our sample size was powered to detect the following effect sizes: 0.11 (reference participants) to 0.10 (HF‐group), 0.16 (COD‐group), and 0.09 (VCI‐group).
3. RESULTS
Compared to participants included in the analysis, excluded participants were older (mean age 70.3 vs 67.2 years, P < 0.001; Table S1 in supporting information).
Demographics are summarized by participant group in Table 1. Patients with COD were less often female than patients in the other participant groups. Patients with HF and possible VCI were older than those with COD and reference participants. Mean MMSE was lowest in patients with possible VCI and COD, compared to patients with HF and reference participants. Prevalence of vascular risk factors (ie, hypertension, hypercholesterolemia, diabetes mellitus, currently smoking, and BMI ≥30) was high among all patient groups. A history of previous stroke or TIA was most frequent in patients with COD and VCI. Compared to the other patient groups and reference participants, total WMH volume was highest and hippocampal volume was lowest in patients with possible VCI.
TABLE 1.
Demographics of the study population
| Demographics | Total (n = 439) | Reference participants (n = 113) | HF (n = 124) | COD (n = 75) | Possible VCI (n = 127) |
|---|---|---|---|---|---|
| Age, years | 67.2 ± 8.6 | 65.6 ± 7.1 | 68.7 ± 9.9 * | 65.1 ± 7.5 † | 68.3 ± 8.7 * , ‡ |
| Women, n (%) | 165 (37.6%) | 55 (48.7%) | 40 (32%) * | 20 (26.7%) † , § | 50 (39.4%) ‡ |
| Education a | 5.2 ± 1.2 | 5.4 ± 1.1 | 5.0 ± 1.3 * | 5.1 ± 1.2 | 5.3 ± 1.2 * |
| MMSE | 28.2 ± 2.1 | 28.8 ± 1.3 | 28.6 ± 1.2 | 27.8 ± 2.3 * , † | 27.4 ± 2.8 § , ¶ |
| CDR, median (IQR) | 0 (0.5) | 0 (0) | 0 (0) * | 0 (0.5) † , § | 0.5 (0.5) § , ¶ . # |
| GDS | 2.1 ± 2.4 | 1.0 ± 1.3 | 2.2 ± 2.7 § | 2.5 ± 2.1 § | 2.9 ± 2.7 † , § |
| Systolic BP, mmHg | 140.3 ± 19.8 | 140.8 ± 18.7 | 133.6 ± 17.2 § | 149.1 ± 20.4 † , ¶ | 141.2 ± 20.5 † , ‡ |
| Diastolic BP, mmHg | 79.9 ± 10.5 | 81.4 ± 9.6 | 76.4 ± 9.9 § | 81.5 ± 11.0 † | 81.0 ± 10.8 † |
| Vascular risk factors b , n (%) | 403 (91.8%) | 88 (77.9%) | 122 (97.6%) § | 74 (98.7%) § | 120 (94.5%) § |
| Hypertension | 279 (63.6%) | 30 (26.5%) | 100 (80%) § | 58 (77.3%) § | 92 (72.4%) § |
| Hypercholesterolemia | 278 (63.6%) | 33 (29.2%) | 80 (64%) § | 69 (92%) § , ¶ | 97 (76.4%) § , # |
| Diabetes mellitus | 61 (13.9%) | 2 (1.8%) | 21 (16.8%) § | 22 (29.3%) † , § | 16 (12.6%) * , # |
| Currently smoking | 72 (16.4%) | 7 (6.2%) | 21 (16.8%) * | 19 (25.3%) † , § | 25 (19.7%) * |
| BMI ≥30 | 90 (20.5%) | 18 (15.9%) | 31 (25%) * | 21 (28%) * | 20 (15.7%) † |
| History of stroke, n (%) | 97 (22.1%) | 0 | 6 (4.8%) * | 38 (50.6%) § , ¶ | 53 (41.7%) § , ¶ |
| History of TIA, n (%) | 102 (23.3%) | 6 (5.3%) | 11 (8.9%) | 56 (74.7%) § , ¶ | 29 (23%) § , ¶ , # |
| Left and right hippocampal volume, mL | 3.8 ± 0.5 | 3.9 ± 0.4 | 3.8 ± 0.5 | 3.9 ± 0.5 | 3.6 ± 0.5 † , § , # |
| Total white matter lesion volume in mL, median (IQR) | 1.6 (5.7) | 0.6 (1.8) | 1.6 (4.0) | 0.9 (1.4) | 7.5 (19.8) § , ¶ , # |
NOTE: Data are presented as mean±SD or number (percentage). One‐way ANOVA or χ2 were performed, respectively.
Abbreviations: BMI, body mass index; BP, blood pressure; CDR, Clinical Dementia Rating; COD, carotid occlusive disease; GDS, Geriatric Depression Scale; HF, heart failure; IQR, interquartile range; LVEF, left ventricular ejection fraction; MMSE, Mini‐Mental State Examination; VCI, vascular cognitive impairment.
Level of education was classified according to the system of Verhage ranging from 1 to 7 (low to highly educated).
Presence of vascular risk factors was determined based on self‐reported medical history and medication use.
P < 0.05 compared to reference participants.
P < 0.05 compared to HF.
P < 0.05 compared to COD.
P < 0.001 compared to reference participants.
P < 0.001 compared to HF.
P < 0.001 compared to COD.
An overview of the CBF values by participant group is shown in Table 2. Whole‐brain and regional CBF values were lower in patients with COD compared to patients with possible VCI, who in turn had lower CBF values than patients with HF and reference participants (whole‐brain PVC CBF in mL/100 g/min: COD: 47.9 ± 10; VCI: 51.2 ± 10.8; HF: 53.6 ± 11.2 vs reference participants: 56.2 ± 11.4). Example CBF maps of three patients and a reference participant are provided in Figure 1. The patient with VCI appears to have slightly reduced CBF compared to the reference participant, mainly in the posterior regions. As clearly seen in the patient with COD, CBF was unilaterally reduced as the patient had a left‐sided unilateral occlusion of the carotid.
TABLE 2.
Values of cerebral blood flow
| CBF values | Total (n = 439) | Reference participants (n = 113) | HF (n = 124) | COD (n = 75) | possible VCI (n = 127) |
|---|---|---|---|---|---|
| Whole brain CBF a | |||||
| Uncorrected CBF | 41.6 ± 8.0 | 42.4 ± 7.8 | 43.1 ± 8.1 | 38.8 ± 7.0 * , † | 40.9 ± 8.4 |
| PVC cortical CBF | 52.6 ± 11.2 | 56.2 ± 11.4 | 53.6 ± 11.2 | 47.9 ± 10.0 † , § | 51.2 ± 10.8 * |
| Regional PVC cortical CBF a | |||||
| Frontal | 54.5 ± 11.3 | 57.9 ± 11.1 | 55.8 ± 11.8 | 49.3 ± 10.5 § , ¶ | 52.9 ± 10.2 * |
| Temporal | 48.7 ± 10.7 | 52.0 ± 11.8 | 50.4 ± 9.9 | 42.9 ± 9.4 § , ¶ | 47.5 ± 9.5 ‡ , § |
| Parietal | 53.9 ± 12.0 | 57.0 ± 12.4 | 55.3 ± 12.4 | 48.9 ± 10.0 † , § | 52.5 ± 11.4 * |
| Occipital | 54.2 ± 13.0 | 56.8 ± 13.1 | 54.6 ± 13.5 | 51.7 ± 12.1 | 52.9 ± 12.5 |
| Central | 51.5 ± 10.3 | 53.3 ± 10.7 | 52.7 ± 10.9 | 48.2 ± 9.7 * , † | 51.0 ± 9.1 |
NOTE: Data are presented as mean ± SD. One‐way ANOVA or χ2 were performed, respectively.
Abbreviations: ANOVA, analysis of variance; CBF, cerebral blood flow; COD, carotid occlusive disease; HF, heart failure; PVC, partial volume corrected; SF, standard deviation; VCI, vascular cognitive impairment.
Cerebral blood flow (CBF) values in mL/100 g/min.
P < 0.05 compared to reference participants.
P < 0.05 compared to HF.
P < 0.05 compared to COD.
P < 0.001 compared to reference participants.
P < 0.001 compared to HF,
P < 0.001 compared to COD.
FIGURE 1.
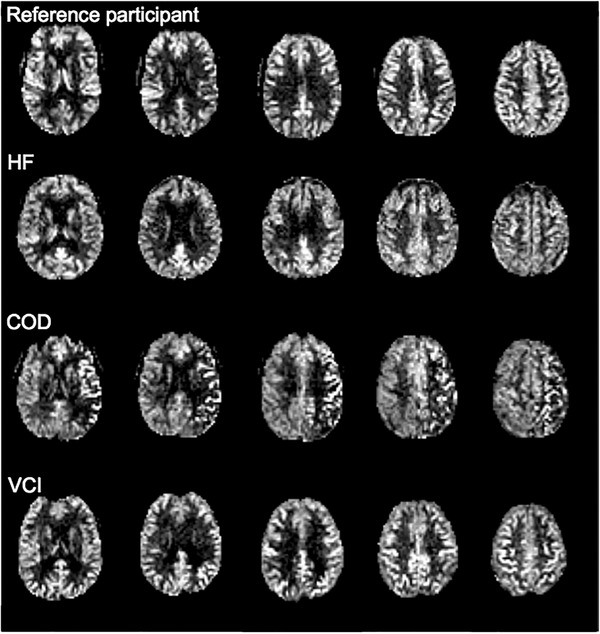
Examples of uncorrected whole‐brain CBF maps in participants of the Heart–Brain study. COD = carotid occlusive disease; HF = heart failure; VCI = vascular cognitive impairment. NOTE: Reference participant: 53‐year‐old woman, Mini‐Mental State Examination [MMSE]: 30, mean uncorrected cerebral blood flow [CBF]: 54 mL/100 g/minute; patient with HF, 85‐year‐old female, MMSE: 27, mean uncorrected CBF: 56 mL/100 g/minute; patient with COD, 66‐year‐old woman, MMSE: 29; mean uncorrected CBF: 42 mL/100 g/minute; patient with possible VCI: 63‐year‐old man, MMSE: 26, mean uncorrected CBF: 48 mL/100 g/min
Table 3 shows the raw neuropsychological data and the z‐scores of all cognitive domains per participant group. As expected, patients with possible VCI had the lowest scores on almost all neuropsychological tests compared to patients with HF and COD and reference participants.
TABLE 3.
Raw neuropsychological test scores per participant group
| Cognitive domains and tests | Total (n = 439) | Reference participants (n = 113) | HF (n = 124) | COD (n = 75) | Possible VCI (n = 127) |
|---|---|---|---|---|---|
| Global cognitive functioning (z‐score) a | −0.4 ± 0.9 | Ref | −0.4 ± 0.6 * | −0.5 ± 0.7 § | −0.9 ± 1.1 ‡ , § , ¶ |
| Memory (z‐score) a | −0.6 ± 1.8 | Ref | −0.4 ± 1.2 | −0.6 ± 1.4 | −1.4 ± 2.6 ‡ , § , ¶ |
| VAT A | 11.1 ± 2.2 | 11.8 ± 0.6 | 11.3 ± 1.6 | 11.3 ± 1.9 | 10.3 ± 3.2 † , ‡ , § |
| RAVLT total immediate | 38.2 ± 11.2 | 41.9 ± 9.4 | 38.5 ± 10.0 | 37.0 ± 11.3 * | 35.1 ± 12.7 § |
| RAVLT delayed | 7.4 ± 3.6 | 8.6 ± 3.1 | 7.7 ± 3.2 | 7.2 ± 3.4 * | 6.2 ± 4.1 † , § |
| RAVLT recognition b | 2.3 ± 2.8 | 1.5 ± 1.6 | 1.9 ± 2.1 | 2.4 ± 2.6 | 3.4 ± 3.8 § , ¶ |
| Attention/psychomotor speed (z‐score) a | −0.5 ± 1.0 | Ref | −0.5 ± 0.9 * | −0.8 ± 1.0 § | −0.8 ± 1.2 † , § |
| TMT part A, seconds b | 46.0 ± 24.4 | 38.1 ± 15.3 | 46.1 ± 17.7 | 49.2 ± 25.7 * | 51.1 ± 32.9 § |
| LDST | 42.1 ± 10.7 | 48.3 ± 8.4 | 42.1 ± 10.0 § | 39.6 ± 10.8 § | 37.9 ± 10.9 † , § |
| Digit span (forward) | 8.4 ± 2.0 | 8.7 ± 2.0 | 8.3 ± 1.9 | 8.3 ± 2.2 | 8.3 ± 2.0 |
| Stroop I, seconds b | 52.9 ± 14.3 | 48.2 ± 9.0 | 52.1 ± 12.1 | 56.4 ± 11.8 * | 55.6 ± 19.4 § |
| Stroop II, seconds b | 70.1 ± 19.9 | 62.7 ± 12.1 | 69.6 ± 16.2 * | 76.2 ± 20.6 § | 73.7 ± 25.7 § |
| Language (z‐score) a | −0.3 ± 0.8 | Ref | −0.4 ± 0.8 * | −0.3 ± 0.6 | −0.6 ± 0.9 ‡ , § |
| VAT naming | 11.9 ± 0.7 | 11.9 ± 0.7 | 11.9 ± 0.8 | 12.0 ± 0.2 | 11.8 ± 0.8 |
| Animal fluency | 22.5 ± 6.4 | 26.0 ± 5.6 | 22.2 ± 6.0 § | 22.8 ± 5.5 * | 19.6 ± 6.4 † , ‡ , § |
| Executive functioning (z‐score) a | −0.3 ± 0.9 | Ref | −0.2 ± 0.8 * | −0.3 ± 0.8 | −0.6 ± 1.1 † , § |
| TMT part B, seconds b | 118.7 ± 77.6 | 88.1 ± 40.6 | 114.1 ± 53.8 * | 129.2 ± 77.7 * | 144.5 ± 107.4 † , § |
| Digit span (backward) | 5.7 ± 1.9 | 5.9 ± 1.9 | 5.8 ± 1.9 | 5.4 ± 1.8 | 5.7 ± 1.9 |
| Stroop III, seconds b | 123.6 ± 52.1 | 103.5 ± 28.5 | 121.0 ± 40.3 * | 131.2 ± 42.5 * | 140.1 ± 74.0 † , § |
NOTE: Raw neuropsychological data are presented as mean ± SD or number (percentage). z‐scores allow comparison of neuropsychological test results within patients and were calculated using the reference participants as reference group. Univariate analyses of variance were performed with diagnosis as between‐subject factor.
Abbreviations: COD, carotid occlusive disease; HF, heart failure; LDST, Letter Digit Substitution Test; RAVLT, Rey Auditory Verbal Learning Test; SD, standard deviation; TMT, Trail Making Test; VAT, Visual Association Test; VCI, vascular cognitive impairment.
Higher z‐scores imply better performance on all tests.
Higher scores imply worse performance.
P < 0.05 compared to reference participants.
P < 0.05 compared to HF.
P < 0.05 compared to COD.
P < 0.001 compared to reference participants.
P < 0.001 compared to HF.
P < 0.001 compared to COD.
3.1. Association between CBF and cognitive functioning
We found hardly any association between whole‐brain and regional PVC CBF values and cognitive functioning (standardized beta [stβ] = ‐0.01 to 0.10, all P > 0.01, Table 4). When we repeated the analysis for whole‐brain uncorrected CBF values, results remained essentially unchanged (data not shown). Subsequent stratification for participant group showed no associations between whole‐brain or regional CBF and cognitive functioning in any of the groups.
TABLE 4.
Linear regression models for the association among PVC, CBF, and cognitive domains
| Region | Cognitive domain | Total (n = 439) | Reference participants (n = 113) | HF (n = 124) | COD (n = 75) | Possible VCI (n = 127) |
|---|---|---|---|---|---|---|
| Whole‐brain | Global cognitive functioning | 0.04 | 0.03 | −0.05 | 0.03 | 0.10 |
| Memory | 0.03 | −0.04 | −0.08 | 0.07 | 0.06 | |
| Attention /psychomotor speed | 0.01 | 0.03 | −0.07 | 0.08 | 0.04 | |
| Language | 0.05 | −0.04 | 0.03 | 0.04 | 0.15 | |
| Executive functioning | 0.04 | 0.14 | −0.01 | −0.17 | 0.10 | |
| Frontal lobe | Global cognitive functioning | 0.05 | 0.08 | −0.05 | 0.07 | 0.08 |
| Memory | 0.01 | 0.06 | −0.11 | 0.09 | 0.00 | |
| Attention /psychomotor speed | 0.03 | −0.01 | −0.05 | 0.12 | 0.07 | |
| Language | 0.09 | 0.10 | 0.03 | 0.07 | 0.12 | |
| Executive functioning | 0.06 | 0.06 | 0.03 | −0.12 | 0.17 | |
| Temporal lobe | Global cognitive functioning | 0.06 | 0.03 | −0.04 | −0.02 | 0.15 |
| Memory | 0.05 | −0.08 | −0.04 | 0.00 | 0.12 | |
| Attention /psychomotor speed | 0.01 | 0.04 | −0.09 | 0.03 | 0.05 | |
| Language | 0.09 | 0.04 | 0.06 | −0.01 | 0.22 | |
| Executive functioning | 0.04 | 0.10 | −0.02 | −0.12 | 0.11 | |
| Parietal lobe | Global cognitive functioning | 0.07 | 0.08 | −0.05 | 0.05 | 0.13 |
| Memory | 0.04 | 0.08 | −0.11 | 0.08 | 0.05 | |
| Attention /psychomotor speed | 0.04 | −0.02 | −0.04 | 0.12 | 0.10 | |
| Language | 0.10 | 0.08 | 0.03 | 0.11 | 0.17 | |
| Executive functioning | 0.05 | 0.07 | 0.01 | −0.19 | 0.15 | |
| Occipital lobe | Global cognitive functioning | 0.04 | 0.02 | −0.12 | 0.06 | 0.12 |
| Memory | 0.00 | −0.05 | −0.18 | 0.07 | 0.04 | |
| Attention /psychomotor speed | 0.07 | 0.04 | −0.04 | 0.13 | 0.13 | |
| Language | 0.06 | 0.02 | −0.02 | 0.06 | 0.16 | |
| Executive functioning | 0.03 | 0.07 | −0.06 | −0.13 | 0.12 | |
| Central lobe | Global cognitive functioning | 0.01 | −0.05 | −0.07 | 0.05 | 0.03 |
| Memory | −0.01 | −0.11 | −0.13 | 0.14 | −0.03 | |
| Attention/psychomotor speed | 0.00 | −0.02 | −0.02 | 0.03 | 0.01 | |
| Language | 0.03 | −0.02 | −0.02 | 0.03 | 0.09 | |
| Executive functioning | 0.03 | 0.02 | 0.03 | −0.12 | 0.09 |
NOTE: Linear regression analyses with data represented as standardized beta (stβ). We performed linear regression analyses with CBF as independent variable and cognitive domains as dependent variable. Cognition is expressed as a (composite) z‐score. We corrected for participant group (using dummy variables), age, sex, education, and center. Subsequently, we stratified for participant group. We used an alpha of 0.01.
Abbreviations: CBF, cerebral blood flow; COD, carotid occlusive disease; HF, heart failure; PVC, partial volume corrected; VCI, vascular cognitive impairment.
4. DISCUSSION
We found reduced whole‐brain and regional CBF values in patients with COD and possible VCI compared to patients with HF and reference participants. However, we found no associations between whole‐brain or regional CBF and cognitive functioning in patients with hemodynamic dysfunctioning along the heart–brain axis.
In the Heart–Brain study, we study the patient groups HF, COD, and possible VCI as exemplar conditions of hemodynamic disorders affecting the heart–brain axis. We investigated the hypothesis that hemodynamic changes, whether brought on by impaired pump function (HF) or low blood supply to the brain (COD) are determinants of impaired cognition. In addition, we included patients with possible VCI to assess if in patients presenting with cognitive complaints and vascular brain injury, compromised hemodynamics contribute to cognitive impairment. Contrary to our expectations, reduced CBF, as measured by ASL, plays a limited role in cognitive functioning and is not the explanatory factor underlying cognitive impairment in patients with hemodynamic disorders along the heart–brain axis.
There are a number of possible explanations for the lack of association between CBF and cognitive functioning in this study. CBF is influenced by several hemodynamic factors, at all levels of the heart–brain axis, ie, the heart, vessels, and the brain. At the level of the heart, suboptimal cardiac function, impaired cardiac output, and myocardial injury could affect endothelial function and decrease perfusion pressure and eventually lead to reduced CBF. 29 These diseases are characteristics of HF, but are also frequently seen in patients with cardio‐ and cerebrovascular disease. In addition, reduced systolic left ventricular functioning could decrease perfusion pressure and thus potentially could lead to reduced CBF. 30 At the level of the vessels, a variability or fluctuations in blood pressure have been associated with cognitive decline and dementia and to structural lesions in the brain. 31 , 32 And finally, at the level of the brain, CBF is controlled by flow‐regulating mechanisms as cerebral autoregulation and cerebrovascular reactivity, which are often impaired in patients with dementia. 8
An alternative explanation for our lack of associations could be the choice of methods to assess CBF in our study. ASL has become an increasingly popular method to measure CBF, because of the non‐invasive nature and the technological benefits over other imaging modalities (eg, single‐photon emission computed tomography). ASL allows visualization and quantification of CBF and the clinical utility of ASL has been demonstrated for several implications, such as cerebrovascular disease (eg, acute and chronic ischemia) and dementia. 33 However, a potential problem with ASL in patients with hemodynamic problems is that the quantification of CBF is hampered due to possible delayed transit time. Transit time is the time it takes for the magnetically labeled arterial blood water to travel from the labeling plane located several centimeters above the carotid bifurcation to the tissue of interest. Transit time is dependent on several factors such as age, arterial size, stiffness, presence of vascular risk factors, and cardiac output. We did not use several delay times to estimate and account for between‐group differences in transit times, as we used the common (single) delay time of 1.8 seconds, as recommended in the consensus paper on ASL. 23 Based on visual inspection, we excluded patients with clearly prolonged transit times, but this might have lowered our sensitivity to detect associations between CBF and cognition.
Finally, our study could have been underpowered, despite our relatively large cohort of patients with HF, COD, and possible VCI. In fact, power analysis suggested sufficient power to demonstrate associations within groups. Furthermore, the observed effect sizes are in line with earlier studies, in which relations between CBF and cognitive functioning are also generally modest. For example, in a previous study of CBF and cognitive functioning in memory‐clinic patients (ie, patients with SCD, mild cognitive impairment, and dementia), we found small effect sizes with standardized betas between 0.05 and 0.18. 12 The Rotterdam Study investigated the association between CBF, using 2D phase‐contrast MRI, and cognitive functioning in cognitively healthy participants and found no association between total CBF and global cognition (difference in z‐score per SD increase [95% confidence interval], reporting small effect sizes: 0.05 [0.01;0.10]). 34
Our study has several strengths, including the achievement of a standardized ASL protocol aligned in a multicenter setting, across three different patient groups. We used an extensive, standardized neuropsychological test battery which allowed us to look at specific cognitive domains. In addition, we included a large cohort of patients with three extreme exemplar conditions of hemodynamic dysfunction of different components of the heart–brain axis. Despite these strengths, several limitations should be considered. First, we used PVC CBF maps, which has as a main drawback that there is currently no consensus on which method is best to correct for partial volume effects. 35 However, the analyses with both uncorrected as PVC CBF yielded comparable results. Second, we included patients with possible VCI, regardless of the severity of cognitive impairment (ie, subjective cognitive decline to dementia). By contrast, most diagnostic criteria on VCI state that this construct only applies to patients with MCI or dementia. 36 , 37 However, the severity of cognitive impairment does not always correspond to the burden of vascular brain injury. In addition, patients with cognitive complaints as a result of vascular brain injury may not always develop cognitive deficits that are severe enough to be classified as MCI. Also, in research on VCI, interest is shifting to the earlier stage of SCD. In the reference group, we included spouses of patients but only on average 20% in all centers. Third, we used large ROIs, which may have obscured subtle regional associations with cognitive functioning. However, the use of large ROIs has the advantage to be more robust. 38 Fourth, we cannot exclude selection bias as we had to exclude participants due to missing ASL or cognitive testing and suboptimal quality or vascular artefacts on ASL as our excluded participants were older compared to our study sample. Given our cross‐sectional method, we cannot rule out the possibility of reverse causality. Finally, we did not adjust for all possible confounders such as cardiac output, caffeine intake, and effects of (vasoactive) medication. A substantial number of the participants in the patients group used antihypertensive medication. It is debated whether angiotensin‐converting enzyme (ACE) inhibitors could maintain or increase CBF as studies in humans are scarce. Studies showed that the use of ACE inhibitors improved CBF and cerebral vasoreactivity in cognitively healthy older individuals with hypertension 39 and in hypertensive patients with stroke. 40
Future research within the Heart–Brain study will include the development of cognitive impairments over time and the longitudinal analyses of the association between CBF and cognitive functioning. In addition, we will address variability and regulation of CBF as well as factors that modulate the impact of hemodynamic changes on the brain (ie, age, sex, and environmental changes).
In conclusion, we have shown that CBF, as measured by ASL, is unlikely to be the explanatory factor underlying cognitive impairment in patients with hemodynamic dysfunction along the heart–brain axis. The predisposition of cognitive impairment in those patients is likely to be driven by other (hemodynamic) mechanisms than CBF. In addition, the interplay with vulnerability factors that modulate the impact of hemodynamics on the brain, such as the presence of atrial fibrillation and the presence of co‐occurring AD pathology, 41 , 42 may play a role in the development of cognitive impairment. Investigating the role of hemodynamic and other factors in cognitive impairment is important to identify potential new treatment targets and the identification of patients that are at risk for cognitive decline.
FUNDING INFORMATION
This work was supported by the Netherlands CardioVascular Research Initiative: the Dutch Heart Foundation (CVON 2018‐28 & 2012‐06 Heart Brain Connection), Dutch Federation of University Medical Centers, the Netherlands Organization for Health Research and Development, and the Royal Netherlands Academy of Sciences.
CONFLICTS OF INTEREST
A.E. Leeuwis, A.M. Hooghiemstra, S. Kuipers, E.A. Oudeman, T. Kalay, H.P. Brunner La Rocca, R.J. van Oostenbrugge, W.J. Niessen, M.A. van Buchem, A.C. van Rossum: report no conflicts.
E.E. Bron and J.P. Greving have been funded by the Dutch Heart Foundation.
N.D. Prins serves on the advisory board of Boehringer Ingelheim and Probiodrug, and on the DSMB of Abbvie's M15‐566 trial. He has provided consultancy services for Sanofi, Takeda, and Kyowa Kirin Pharmaceutical Development. He also receives research support from Alzheimer Nederland (project number WE.03‐2012‐02) and is CEO and co‐owner of Brain Research Center, Amsterdam, the Netherlands.
M.J.P. van Osch has received research funding from Philips, the Netherlands Organisation for Scientific Research (NWO), and European Union Horizon 2020 and serves on the editorial boards of JCBFM and NMR in Biomedicine.
G.J. Biessels has been funded by the Dutch Heart Foundation (grant 2010T073), ZonMW (Vici grant 918.16.616), The Netherlands Organisation for Health Research and Development and European Union Horizon 2020 (grant agreement no. 666881, SVDs@target).
F. Barkhof is supported by the NIHR biomedical research centre at UCLHF. Barkhof serves as a consultant for Biogen‐Idec, Janssen Alzheimer Immunotherapy, Bayer‐Schering, Merck‐Serono, Roche, Novartis, Genzyme, and Sanofi‐aventis.F. Barkhof has received sponsoring from EU‐H2020, NWO, SMSR, TEVA, Novartis, Toshiba, and Imi and serves on the editorial boards of Radiology, Brain, Neuroradiology, MSJ, and Neurology.
Research programs of W.M. van der Flier have been funded by ZonMW, NWO, EU‐FP7, Alzheimer Nederland, Cardiovasculair Onderzoek Nederland, stichting Dioraphte, Gieskes‐Strijbis fonds, Pasman Stichting, Boehringer Ingelheim, Piramal Imaging, Roche BV, Janssen Stellar, Biogen, and Combinostics. All funding is paid to her institution.
Supporting information
Supplementary information
Leeuwis AE, Hooghiemstra AM, Bron EE, et al. Cerebral blood flow and cognitive functioning in patients with disorders along the heart–brain axis. Alzheimer's Dement. 2020;6:e12034 10.1002/trc2.12034
REFERENCES
- 1. van der Flier WM, Skoog I, Schneider JA, et al. Vascular cognitive impairment. Nat Rev Dis Prim. 2018;4:18003. [DOI] [PubMed] [Google Scholar]
- 2. Slot RER, Sikkes SAM, Berkhof J, et al. Subjective cognitive decline and rates of incident Alzheimer's disease and non–Alzheimer's disease dementia. Alzheimers Dement. 2019;15(3):465‐476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benedictus MR, Van Harten AC, Leeuwis AE, et al. White matter hyperintensities relate to clinical progression in subjective cognitive decline. Stroke. 2015;46:2661‐2664. [DOI] [PubMed] [Google Scholar]
- 4. Gottesman RF, Albert MS, Alonso A, et al. Associations between midlife vascular risk factors and 25‐year incident dementia in the Atherosclerosis Risk in Communities (ARIC) Cohort. JAMA Neurol. 2017;388:797‐805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wolters FJ, Segufa RA, Darweesh SKL, et al. Coronary heart disease, heart failure, and the risk of dementia: a systematic review and meta‐analysis. Alzheimers Dementt. 2018;14(11):1493‐1504. [DOI] [PubMed] [Google Scholar]
- 6. Abete P, Della‐Morte D, Gargiulo G, et al. Cognitive impairment and cardiovascular diseases in the elderly. A heart‐brain continuum hypothesis. Ageing Res Rev. 2014;18:41‐52. [DOI] [PubMed] [Google Scholar]
- 7. Van Buchem Ma, Biessels GJ, Brunner La, et al. The heart‐brain connection: a multidisciplinary approach targeting a missing link in the pathophysiology of vascular cognitive impairment. J Alzheimer's Dis. 2014;42:S443‐S451. [DOI] [PubMed] [Google Scholar]
- 8. Kisler K, Nelson AR, Montagne A, Zlokovic BV. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat Rev Neurosci. 2017;18:419‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hooghiemstra AM, Bertens AS, Leeuwis AE, et al. The Missing Link in the Pathophysiology of Vascular Cognitive Impairment: design of the Heart‐Brain Study. Cerebrovasc Dis Extra. 2017;7(3):140‐152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hooghiemstra AM, Leeuwis AE, Bertens AS, Biessels GJ, Bots ML. Frequent Cognitive Impairment in Patients With Disorders Along the Heart‐Brain Axis. Stroke. 2019;50:1‐7. [DOI] [PubMed] [Google Scholar]
- 11. Alsop DC, Detre JA, Golay X, et al. Recommended implementation of arterial spin‐labeled Perfusion mri for clinical applications: a consensus of the ISMRM Perfusion Study group and the European consortium for ASL in dementia. Magn Reson Med. 2015;73:102‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leeuwis AE, Benedictus MR, Kuijer JPA, et al. Lower cerebral blood flow is associated with impairment in multiple cognitive domains in Alzheimer's disease. Alzheimers Dementt. 2017;13(5):531‐540. [DOI] [PubMed] [Google Scholar]
- 13. Folstein MF, Folstein SE, Mchugh PR. Mini‐Mental State ‐ Practical Method for Grading Cognitive State of Patients for Clinician. J Psychiatr Res. 1975;12:189‐198. [DOI] [PubMed] [Google Scholar]
- 14. Verhage F. Intelligentie en leeftijd: onderzoek bij Nederlanders van 12‐77 jaar [in Dutch]. Van Gorcum Assen. 1964. [Google Scholar]
- 15. Aalten P, Ramakers IHGB, Biessels GJ, et al. The Dutch Parelsnoer Institute ‐ Neurodegenerative diseases; methods, design and baseline results. BMC Neurol. 2014;254:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lindeboom J, Schmand B, Tulner L, Walstra G, Jonker C. Visual association test to detect early dementia of the Alzheimer type. J Neurol Neurosurg Psychiatry. 2002;73:126‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saan R, Deelman B. De 15‐woordentest A en B (een voorlopige handleiding) [in Dutch]. Groningen, The Netherlands: AZG: Afdeling Neuropsychologie; 1986. [Google Scholar]
- 18. Van der Elst W, Van Boxtel MPJ, Van Breukelen GJP, Jolles J. Normative data for the Animal, Profession and Letter M Naming verbal fluency tests for Dutch speaking participants and the effects of age, education, and sex. J Int Neuropsychol Soc. 2006;12:80‐89. [DOI] [PubMed] [Google Scholar]
- 19. Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271‐276. [Google Scholar]
- 20. Lindeboom J, Matto D. Digit series and Knox cubes as concentration tests for elderly subjects [in Dutch]. Tijdschr Gerontol Geriatr. 1994;25:63‐68. [PubMed] [Google Scholar]
- 21. Van der Elst W, Van Boxtel MPJ, Van Breukelen GJP, Jolles J. The Stroop color‐word test: influence of age, sex, and education; and normative data for a large sample across the adult age range. Assessment. 2006;13:62‐79. [DOI] [PubMed] [Google Scholar]
- 22. van der Elst W, van Boxtel MPJ, van Breukelen GJP, Jolles J. The Letter Digit Substitution Test: normative data for 1,858 healthy participants aged 24‐81 from the Maastricht Aging Study (MAAS): influence of age, education, and sex. J Clin Exp Neuropsychol. 2006;28:998‐1009. [DOI] [PubMed] [Google Scholar]
- 23. Alsop DC, Ja Detre, Golay X, et al. Recommended implementation of arterial spin‐labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med. 2014;116:102‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bron EE, Steketee RME, Houston GC, et al. Diagnostic classification of arterial spin labeling and structural MRI in presenile early stage dementia. Hum Brain Mapp. 2014;35:4916‐4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huizinga W, Poot D, Guyader J, et al. PCA‐based groupwise image registration for quantitative eMRI. Med Image Anal. 2016;29:65‐78. [DOI] [PubMed] [Google Scholar]
- 26. Asllani I, Borogovac A, Brown TR. Regression algorithm correcting for partial volume effects in arterial spin labeling MRI. Magn Reson Med. 2008;60:1362‐1371. [DOI] [PubMed] [Google Scholar]
- 27. Hammers A, Allom R, Koepp MJ, et al. Three‐dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum Brain Mapp. 2003;19:224‐247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gousias IS, Rueckert D, Heckemann RA, et al. Automatic segmentation of brain MRIs of 2‐year‐olds into 83 regions of interest. Neuroimage. 2008;40:672‐684. [DOI] [PubMed] [Google Scholar]
- 29. Roy B, Woo MA, Wang DJJ, Fonarow GC, Harper RM, Kumar R. Reduced regional cerebral blood flow in patients with heart failure. Eur J Heart Fail. 2017;19:1294‐1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lepic T, Loncar G, Bozic B, et al. Cerebral blood flow in the chronic heart failure patients. Perspect Med. 2012;1:304‐308. [Google Scholar]
- 31. Brickman AM, Reitz C, Luchsinger JA, et al. Long‐term blood pressure fluctuation and cerebrovascular disease in an elderly cohort. Arch Neurol. 2010;67:564‐569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alpérovitch A, Blachier M, Soumaré A, et al. Blood pressure variability and risk of dementia in an elderly cohort, the Three‐City Study. Alzheimers Dementt. 2014;10:330‐337. [DOI] [PubMed] [Google Scholar]
- 33. Watts JM, Whitlow CT, Maldjian JA. Clinical applications of arterial spin labeling. NMR Biomed. 2013;26:892‐900. [DOI] [PubMed] [Google Scholar]
- 34. Poels MMF, Ikram MA, Vernooij MW, et al. Total cerebral blood flow in relation to cognitive function: the Rotterdam Scan study. J Cereb Blood Flow Metab. 2008;28:1652‐1655. [DOI] [PubMed] [Google Scholar]
- 35. Hutton BF, Thomas BA, Erlandsson K, et al. What approach to brain partial volume correction is best for PET / MRI. Nucl Inst Methods Phys Res A. 2013;702:29‐33. [Google Scholar]
- 36. Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2672‐2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sachdev P, Kalaria R, O'Brien J, et al. Diagnostic criteria for vascular cognitive disorders: a VASCOG statement. Alzheimer Dis Assoc Disord. 2014;28:206‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Petersen ET, Zimine I, Ho Y‐CL, Golay X. Non‐invasive measurement of perfusion: a critical review of arterial spin labelling techniques. Br J Radiol. 2006;79:688‐701. [DOI] [PubMed] [Google Scholar]
- 39. Lipsitz LA, Gagnon M, Vyas M, et al. Antihypertensive therapy increases cerebral blood flow and carotid distensibility in hypertensive elderly subjects. Hypertension. 2005;45:216‐221. [DOI] [PubMed] [Google Scholar]
- 40. Moriwaki H, Uno H, Nagakane Y, Hayashida K, Miyashita K, Naritomi H. Losartan, an angiotensin II (AT1) receptor antagonist, preserves cerebral blood flow in hypertensive patients with a history of stroke. J Hum Hypertens. 2004;18:693‐699. [DOI] [PubMed] [Google Scholar]
- 41. De Bruijn RFAG, Heeringa J, Wolters FJ, et al. Association between atrial fibrillation and dementia in the general population. JAMA Neurol. 2015;72:1288‐1294. [DOI] [PubMed] [Google Scholar]
- 42. Vemuri P, Lesnick TG, Przybelski SA, et al. Vascular and amyloid pathologies are independent predictors of cognitive decline in normal elderly. Brain. 2015;138:761‐771. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information


