Abstract
Introduction
Adults with Down syndrome (DS) older than 40 have Alzheimer's disease (AD) neuropathology and high risk for dementia, but little is known about the relationship of sex to AD risk in this population.
Methods
Using nonparametric methods and Cox proportional hazards models we analyzed differences in incidence of dementia, by sex, presence of an apolipoprotein E (APOE) ε4 or ε2 allele, and dementia duration and decline in 246 adults over 40 with DS.
Results
There was no significant sex difference in risk of AD or rate of cognitive decline. APOE ε4 allele significantly increased risk of AD irrespective of sex. No significant interactions were found between sex and APOE status on AD risk. Among those who died, dementia duration was significantly longer in women.
Discussion
This study showed no effect of sex nor interaction between sex and APOE for risk of AD in adults with DS; however, women had longer dementia duration.
Keywords: Alzheimer's disease, apolipoprotein ε2 allele, apolipoprotein ε4 allele, cognitive decline, dementia duration, Down syndrome, sex differences in Alzheimer's disease
1. BACKGROUND
Adults with Down syndrome (DS) are at high risk for Alzheimer's disease (AD) and virtually all adults develop AD‐associated neuropathology by 40 years of age. 1 In less than a century, life expectancy for individuals with DS has increased from an average of 12 years 2 to ≈ 60 years of age 3 and a large increase in AD among adults with DS can be expected. The high risk of AD in those with DS is thought to be due to the early and increased deposition of amyloid beta (Aß) in the brain from overexpression of the gene for the precursor of Aß, which is triplicated on chromosome 21. 4 Although there are neuropathological, neurochemical, and neurophysiological parallels between AD in adults with DS and late‐onset AD (LOAD) in the neurotypical population, 3 the mean age of onset of AD is in the early 50s in adults with DS, 5 , 6 two to three decades earlier than in the general population. In addition, there is considerable variation in age at onset of dementia in adults with DS, ranging from under 40 to over 70 years of age, 3 suggesting that factors other than triplication of the gene for the precursor of Aß may modify risk or affect clinical progression. These factors may provide potential targets for intervention to delay or prevent onset of AD.
Major known risk factors for LOAD are age; the presence of an Apolipoprotein ε4 allele (APOE ε4), which has been associated with an increased risk for LOAD; 7 and sex, where the risk of AD appears to be greater in women compared to men, independent of the longer life expectancy for women. 8 The literature is inconsistent with respect to the differential effects of sex on AD, with several studies finding either no difference between men and women in incidence of AD 9 or a significant effect of age and sex on risk of AD, as the incidence of AD is higher in women in the older age range. 10 , 11 Several lines of investigation have shown a protective role for estrogen in LOAD 12 , 13 and the loss of estrogen has been identified as an important factor in the increased risk of AD in women after menopause. 14 , 15 Sex may also have an effect on rate of cognitive decline after onset of AD 16 and it appears that women with AD have worse cognitive impairment than men at baseline 17 and may decline faster. 18 A recent review on the differential effect of sex on AD highlights knowledge gaps in many aspects of risk factors of progression and biomarkers for AD in the general population and the need for further study. 19
Several risk factors for AD in DS have also been identified, including age and the presence of an APOE ε4; 20 , 21 , 22 however, much less is known about the relationship of sex to risk of AD or to rates of progression in adults with DS, and the few existing reports have been inconsistent. 23 , 24 One community‐based study of ≈ 100 individuals with DS 23 found that men had an earlier onset and an almost three‐fold greater risk for AD than women; however, another similarly powered study 24 found that females had a higher risk for developing AD. Even less is known about the interaction of these risk factors and risk for AD in the DS population.
APOE plays a central role in plasma lipoprotein metabolism and lipid transport within tissues, binds to the Aβ peptide, and plays a role in clearing this peptide from the brain. 7 The APOE ε4 allele has been associated with higher levels of total and low‐density lipoprotein (LDL) cholesterol, and with increased risk for cardiovascular disease and diabetes; the APOE ε2 allele has been associated with decreased risk for AD. 25 The presence of an APOE ε4 allele in the neurotypical population is not only a risk factor for AD but may also confer greater risk of AD to women than men. 26 , 27 , 28 This interaction may be further modified by age, as women in the younger age brackets (65–75 years of age) have greater risk for AD than men. 29 Although the effect of the APOE ε4 haplotype on risk of AD in adults with DS has been confirmed, 20 , 21 , 22 the interaction of sex and APOE ε4 in DS has not been studied. Therefore, a larger study could provide a more reliable determination of sex‐related risk for AD and any sex‐related effect of APOE ε4 in individuals with DS.
In our analysis of a cohort of adults with DS, we address four questions related to effects of sex, and the presence of an APOE ε4 or ε2 allele, on risk for AD: (1) Is there a sex difference in the risk for development of AD? (2) Is there a sex difference in risk for AD in those who carry the ε4 or ε2 alleles? (3) Is there a sex difference in the duration of AD? (4) Is there a sex difference in rate of cognitive decline after the onset of AD?
HIGHLIGHTS
No sex difference in Alzheimer's disease (AD) risk in adults with Down syndrome (DS).
Apolipoprotein ε4 increases risk for AD in DS without sex difference.
Rate of cognitive decline of AD in adults with DS showed no sex difference.
Women with DS have longer duration of AD than men with DS.
RESEARCH IN CONTEXT
Systematic review: Alzheimer's disease (AD) is prevalent in adults with Down syndrome (DS) with many parallels with late onset AD (LOAD) in the neurotypical population. Careful review of the literature using traditional (eg, PubMed) sources indicated that sex differences in the risk for AD in DS are not fully understood. Prior studies had a mix of prevalent and incident cases of AD or had low numbers of participants.
Interpretation: Our study of only incident cases of AD in a large cohort of adults with DS (N = 246) with long follow‐up (≈8.5 years) demonstrates longer duration of AD in women versus men with DS, but no sex differences in risk for AD while accounting for the role of apolipoprotein E (APOE) genotype and other covariates, including presence of an APOE ε4 allele.
Future directions: The role of genetic, hormonal, and environmental factors on the longer duration of AD in women with DS deserves further investigation.
2. METHODS
2.1. Study population
The study sample consisted of 246 adults with DS 40 years of age and older, who were selected from among 758 adults who were consecutively evaluated at a Partners Neurology specialty clinic for adults with DS, where information was collected from both the patients and care providers or family members. Individuals were excluded from the analysis if they had a diagnosis of prevalent dementia, were under 40 years of age at the first visit, had no follow‐up visits after the initial visit, or lacked standardized testing that was used in the determination of dementia.
The study population was composed of 95 (38.6%) females and 151 (61.4%) males, who were 40 to 66 years old, and nondemented at the time of the initial visit (mean 46.7 ± 5.2). The 246 participants were classified into two main groups by level of intellectual disability (LID) before the onset of dementia based on IQ scores, historical designation, or clinical determination to account for the potential influence of LID on cognitive scores: (1) N = 150 mild/moderate (IQ 40–70, at least good activities of daily living [ADL] skills, language with at least short sentences) and (2) N = 96 severe/profound (IQ < 40, at least some help needed for ADL skills, language in short phrases or nonverbal, Table 1). Institutional Review Board approval for a medical records review was obtained from Massachusetts General Hospital.
TABLE 1.
Demographic characteristics of the cohort by dementia status
| Characteristic N (%) | Total N = 246 | Cognitively stable N = 91 (37%) | Demented N = 155 (63%) |
|---|---|---|---|
| Age at first visit: Mean years (S.D.) | 46.7 (5.2) | 45.5 (4.8) | 47.3 (5.3)* |
| Sex: N (%) | |||
| Male | 151 (61.4) | 54 (35.8) | 97 (64.2) |
| Female | 95 (38.6) | 37 (38.9) | 58 (61.1) |
| Level of intellectual disability N (%) | |||
| Mild/moderate | 150 (61.0) | 55 (60.4) | 95 (61.3) |
| Severe/profound | 96 (39.0) | 36 (39.6) | 60 (38.7) |
| Apolipoprotein genotypes: N (%) | N = 184 | N = 58 | N = 126 |
| APOE ε4 allele | 38 (20.7) | 9 (15.5) | 29 (23.0) |
| APOE ε2 allele | 30 (16.3) | 9 (15.5) | 21(16.7) |
| Hypothyroidism: N (%) | 139 (57.2) | 47 (52.8) | 92 (59.7) |
| High cholesterol: N (%) | 75 (30.5) | 29 (31.6) | 46 (29.7) |
| BMI (SD) | 30.2 (6.6) | 30.8 (6.6) | 30.2 (6.3) |
| Diabetes: N (%) | 8 (3.3) | 5 (5.5) | 3 (1.9) |
* P < .05
Abbreviations: APOE, apolipoprotein E; BMI, body mass index; SD, standard deviation.
2.2. Clinical assessments
Regular assessments were conducted every 2 years for those 40 to 50 years old if they did not show any decline from the previous assessment, and yearly for those showing some decline from the previous visit or anyone over 50. The average duration of follow‐up for the study sample was 8.4 ± 4.9 years. Clinical assessments involved evaluation of: (1) medical status (including risk factors for dementia such as body mass index [BMI], LID, hypothyroid status, as well as risk factors for cerebrovascular disease including diabetes or hypercholesterolemia, which could contribute to cognitive dysfunction; (2) educational and vocational history, functional ability; and (3) cognition, including scores on the Test of Severe Impairment (TSI, 30 which includes 24 items assessing language, memory, executive function, and motor performance), scores on the 1‐minute Verbal Fluency Tests (animals and foods) 31 and scoring of cognitive and non‐cognitive/social realms on the Dementia Questionnaire for People with Learning Disabilities (DLD, a 50 item questionnaire covering the areas of short‐term and long‐term memory, spatial and temporal orientation, speech, practical skills, mood, activities and interest, and behavioral disturbance). 32 Higher scores on the TSI and Verbal Fluency Tests were associated with better performance; higher scores on the DLD indicated worsening performance.
2.3. Determination of dementia of the Alzheimer type
Based on information from all available sources, dementia was determined based on a decline in function in two or more areas that was sustained for at least 1 year. Decline in scores on the TSI and on the Verbal Fluency Tests, and worsening function on the DLD were also factored.
2.4. Apolipoprotein E (APOE) genotyping
APOE genotyping was done using standard methods. 33 Of the 246 participants, 186 had genotyping. As those with APOE ε2 and APOE ε4 have opposing effect on risk of AD in the general population, two participants with APOE ε2/ε4 genotype were excluded.
2.5. Statistical analysis
The Kaplan Meier curve is a non‐parametric statistic used to estimate the time to event (or survival time) from a defined starting point and is typically used for exploratory analysis before regression models. 34 The unadjusted Kaplan Meier curve was used prior to conducting the Cox regression models, to assess the effect of sex on age at onset of AD from the age at the initial visit, and the effect of sex on duration from onset of AD to death. We conducted statistical analyses based on data from those individuals who were AD‐free at baseline and used age of AD onset as the outcome variable.
We also conducted a longitudinal data analysis to identify sex differences in cognitive decline after onset of AD for the following five cognitive measures: TSI, Verbal Fluency Test‐Animals (VFTA), Verbal Fluency Test‐Foods (VFTF). Dementia Questionnaire for People with Learning Disabilities (DLD) related to cognitive functions (DLD‐Cog) and non‐cognitive social domains (DLD‐Soc). Using the mixed‐effect model with the cognitive measure as the outcome, we fitted the model with covariates including onset age of AD, sex, time since AD onset, and the interaction term of sex and time since AD onset: Yij = β0+ β1Wi + β2 Xi + β3 tij + β4 Xi tij+ b0i + b1i tij+ εij where Yij is the outcome variable measured at tij for the ith individual; Wi = age at onset of AD; Xi = sex indicator; tij = time since age of AD onset; β0, β1, β2, β3, β4: fixed effects; b0i, b1i:random effects; εij: error term.
The analyses properly accounted for both left truncation and right censoring to ensure unbiased analytical results. 36 The terminology “left truncation” refers to a type of sampling bias which occurs when the data sample includes only individuals who were AD‐free at baseline, as the data sample truncates (excludes) those who were diagnosed with AD at baseline (prevalent cases). “Right censoring” refers to the sampling constraint where age of AD onset cannot be observed due to limited follow‐up time.
3. RESULTS
3.1. Baseline characteristics
The mean age of the cohort at the initial assessment was 46.7 ± 5.2 years with no statistical difference between men and women (mean ages 46.4 ± 4.9 years vs 47.1 ± 5.7 years, P = .26). Among the 246 participants in the study, 91 (37%) remained cognitively stable while 155 (63%) developed dementia over the course of follow‐up: 97 men and 58 women (64.2% vs 61.1%, P = .61). Participants who developed dementia were significantly older at the initial assessment than those who remained cognitively stable (mean age 47.3 ± 5.3 vs 45.5 ± 4.8 years, P = .008; Table 1). Although the mean years of follow‐up was 8.5 years for the whole group, those with incident dementia were followed significantly longer than those who remained dementia‐free (9.5 ± 4.7 vs 6.9 ± 4.8 years, P < .01). When the cohort was divided into a cognitively stable group and a demented group, the following characteristics were not significant between the two groups: (1) mild/moderate LID (60.4% vs 61.3%), severe/profound LID (38.6% vs 38.7%; P = .89); (2) history of hypothyroidism (52.8% vs 59.7%, P = .14); (3) hypercholesterolemia (31.6% vs 29.7%, P = .72); (4) BMI (30.7 vs 30.2, P = .52); and (5) diabetes (5.5% vs 1.9%, P = .13).
3.2. Relation of sex to risk for AD
Among the 155 individuals with dementia in the study, there was no statistical difference in the mean age of AD onset between men and women (53.9 ± 5.7 vs 53.1 ± 5.9, P = .39). The estimated hazard curves for males and females showed that women had higher risk of developing AD, but the difference was not significant (Figure 1). Cox regression analyses adjusted for LID were conducted based on sample size N = 246 to examine the relationship of sex to risk of developing AD. Overall, there was no significant effect of sex on risk for AD (hazard ratio [HR] 1.26, confidence interval [CI]: 0.90 to 1.76, P = .18). Although women had a higher incidence of hypothyroidism than men in the cohort (F 68.1%, M 53.5%, P = .033), there were no significant differences between men and women in the incidence of the other medical comorbidities. Similarly, the effect of sex on risk for AD did not change when the comorbid conditions (hypothyroidism, obesity‐BMI > 30, high cholesterol, and diabetes were added to the analyses (HR = 1.22, CI: 0.85 to 1.75, P = .28).
FIGURE 1.
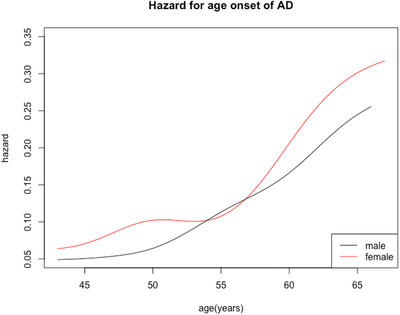
Risk of developing Alzheimer's disease in males (blue) and females (red) with Down syndrome after age 40. Females had higher risk than males, but the difference was not significant
3.3. Relationship of sex to APOE genotype
Among the 246 participants in the study, 184 had APOE genotypes for analysis after excluding two individuals with the APOE‐ε2/ε4 genotype. Of the 184 adults with DS who were genotyped, 115 (62.5%) were men and 69 (37.5%) were women. These percentages were very similar to the sex distribution of the whole cohort (61.4% men and 38.6% women). When further divided for the subanalyses, 40 individuals carried the APOE ε4 allele: 17 (58.6%) men and 12 (41.4%) women, and 30 carried the APOE ε2 allele: 21 (55.3%) men and 17 (44.7%) women. Age at onset of AD was significantly lower in those with the APOE ε4 allele compared to those with the reference APOE‐ε3/ε3 (50.6 ± 5.1 vs 54.6 ± 5.8, P < .001), and there was no difference between men and women who carried the APOE ε4 allele with respect to their mean age of AD onset (M: 50.47 ± 4.9; F: 50.67 ± 5.5).
We used a Cox regression model that included APOE ε3/ε3 as the baseline hazard group and examined the effects of APOE ε4, APOE ε2, and LID on risk of developing AD. Those with APOE ε4 were divided by sex (21 M, 17 F) and further subdivided for “younger” age at first visit (<45) and “older” age at first visit (>46). There were 14 (66.7%) men and 10 women (58.8%) in the “younger” group, of whom 12 (85.7%) men and 7 (70%) women developed AD. In the “older” group at first visit, 7 (33.3%) men and 5 (71%) women developed AD. Analysis of the APOE ε4 group with respect to a younger (<45) versus an older (>46) age at first visit by sex did not show a significant difference in incidence of AD: 12 of 14 (85.7%) younger men versus 7 of 10 (70%) younger women; P = .37. The interaction between sex and APOE ε4 was not significant, so the interaction term was not included in this Cox model. The analysis showed that the presence of APOE ε4 compared to the reference group APOE ε3/ε3 increased the hazard for developing AD (HR = 1.72, CI: 1.09 to 2.70, P = .02). The presence of APOE ε2 did not significantly decrease the hazard for developing AD (HR = 0.88, CI: 0.53 to 1.46, P = .61) and inclusion of LID did not affect the hazard for developing AD (HR = 0.77, CI: 0.53 to 1.12, P = .18).
3.4. Relationship of sex to duration of dementia
We used Kaplan‐Meier curves to estimate the distribution of duration of AD for men and women from onset of AD to death (Figure 2). Among those who died, the mean duration of AD was significantly longer for females than for males (6.7 ± 3.3 vs 5.1 ± 3.1 years, P = .02). Next, a Cox regression analysis was conducted to analyze the sex effect on duration of AD adjusted for onset age of AD. In the Cox model, women with DS had significantly longer duration from onset of dementia to death than men with DS (HR = 0.60, CI: 0.39 to 0.92, P‐value = .02). The result of these two approaches was highly consistent and both approaches concluded that women tended to have longer duration of AD than men from onset to death, even though the mean age of AD onset in those who died showed no significant difference between men and women (53.5 ± 5.5 vs 53.7 ± 5.9 years, P = .89). Although there was a trend for women to die later than men (60.0 ± 5.9 vs 58.6 ± 5.8 years, P = .14), this did not reach statistical significance (Table 2).
FIGURE 2.
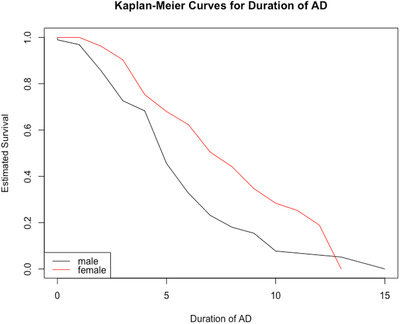
Duration of Alzheimer's disease (AD) in males (blue) and females (red) with Down syndrome from AD onset until death. Mean duration in males 51 ± 3.1 years versus females 6.73 ± 3.3 years (P = .02)
TABLE 2.
Relation of sex to AD onset age, age at death, and dementia duration among those who died
| Characteristic | Males N = 61 | Females N = 33 | P |
|---|---|---|---|
| Age of AD onset: mean years (SD) | 53.5 (5.5) | 53.8 (5.9) | .39 |
| Age of AD death: mean years (SD) | 58.6 (5.8) | 60.0 (5.9) | .14 |
| Dementia duration: mean years (SD) | 5.1 (3.1) | 6.7 (3.2) | .02 * |
Abbreviations: AD, Alzheimer's disease; SD, standard deviation.
3.5. Relationship of sex to cognitive decline
When scores on the TSI, VFTA, or VFTF was the outcome marker, the value of the outcome marker decreased for increasing age of AD onset and the decrease was significant (P = .001, .000, .000, respectively). The value of these outcome markers decreased significantly over time from the onset of AD (P = .003, .014, and .003, respectively). However, both the sex effect at onset age of AD and the sex effect for the rate of change of TSI, VFTA, or VFTF outcome marker values over time were not significant (0.990, 0.700, 0.590; 0.555, 0.991, 0.713). When either DLD‐Cog or DLD‐Soc scores was the outcome marker, the value of the outcome marker increased significantly with increasing age of AD onset (P = .001 and .002, respectively). The value of the DLD‐Cog or DLD‐Soc outcome marker increased with increasing time from onset of AD and this increase was significant (P = .005 and .000, respectively). The sex effect at age of AD onset and the sex effect for rate of change of these marker values of cognitive and social domains were not significant (0.332, 0.977; 0.337, 0.338).
3.6. Potential contribution of comorbid conditions to risk of dementia
We evaluated the potential contribution of medical comorbid conditions to the risk for dementia, including LID, hypothyroid status, BMI as a marker for obesity, hypercholesterolemia, and diabetes. We did not include hypertension, which is a common comorbid condition in the neurotypical population, because hypertension is quite rare in individuals with DS. Although there was a high incidence of hypothyroidism, the presence of hypothyroidism did not have a significant effect on risk for developing AD (HR = 1.26, CI: 0.89 to 1.79, P = .19), even when adjusting for the other covariates (BMI, LID, and age).
4. DISCUSSION
In adults with DS, few studies have examined sex as a risk factor for AD. In our present study of incident AD in adults with DS in which 155 individuals developed dementia, there was no overall sex difference in the risk for the development of AD. These findings are consistent with several large studies in the neurotypical population that showed no difference between men and women in incidence of AD, 9 , 37 , 38 even though two thirds of prevalent cases of AD are women. 39 However, other studies of neurotypical AD indicate that age is a factor in sex differences with females having higher incidence of AD only over age 80 to 90). 10 , 40 , 41 The interaction of sex and incidence of dementia was also analyzed in the Framingham Heart Study, which took selective survival into account; findings indicated similar cumulative incidence of AD in men and women, but lifetime risk accounting for the competing risk of death, was higher in women. 42 These findings contrast with previous smaller studies in DS. Schupf et al., 23 showed a higher risk for AD in men with DS, whereas Lai et al. 24 showed the opposite effect. Only one other study has examined sex differences in adults with DS with respect to AD risk, but this was a small autopsy study (N = 28) 43 in which the onset of AD was earlier in women than men. Nevertheless, despite the lack of an apparent sex effect on AD risk in adults with DS, there was a non‐significant, marginal trend, with women showing higher risk in the estimated hazard curve. In our cohort there were fewer females (N = 95) than males (N = 151); the proportion of incident AD cases is around 60%, so the estimation efficiency might have been affected by the reduced sample size of cases. If it were possible to increase the sample size (particularly for females), there could have been a possibility of significant results. Also, from the hazard plot (Figure 1), the proportionality assumption of the proportional hazard model is not perfectly satisfied, which may also have contributed to the non‐significant results.
In this study, we also found that women with DS had a significantly longer duration of dementia by almost 2 years compared to men with DS from the time of AD onset until death, even though women and men had similar mean ages at onset of AD. These results are most closely aligned with the study by Larson et al. in the neurotypical population of incident AD. 43 They found men had significantly poorer survival across all age groups compared to women. A similar finding of longer duration of AD in women has been reported in another DS study. 44 However, in that study, the age of onset of dementia was older and duration of AD was shorter, likely due to the inclusion of both incident and prevalent cases. We also did not see an effect of sex on the mean age of death, similar to what has been reported previously by Yang et al. in a mortality study in DS spanning 14 years. 45
As expected, we found that the presence of APOE ε4 allele was a genetic risk factor for AD in our overall DS cohort with mean age at onset of AD significantly lower in those with the APOE ε4 allele irrespective of sex, compared to those with the ε3/ε3 genotype. This is consistent with two large meta‐analyses in the general population 29 , 46 and in studies of DS. 47 Other studies in the neurotypical population demonstrated that women with APOE ε4 were at higher risk for AD 26 , 48 , 49 especially those who were younger. 20 We did not find that APOE ε4 women with DS in the younger age bracket had an increased risk for AD compared to APOE ε4 men. However, the proportionately smaller number of women in this study may have contributed to the difference in findings. When the effect of APOE ε2 was examined, there was a nonsignificant protective effect on risk for AD in our cohort. Although this finding differs from the protective APOE ε2 effect on AD risk in the general population 25 and in DS, 24 , 50 this could be due to a relatively small sample size. We also evaluated the effect of sex on rate of decline on scored measures that included memory, word retrieval, temporal and spatial orientation, and behavioral parameters in the 155 adults who developed dementia and found no significant difference between men and women. Although Coppus et al. 51 showed a significant rate of decline on a social competence scale in the 53 persons with DS who developed AD, sex differences were not analyzed.
Comorbid medical conditions such as obesity, diabetes, and hypercholesterolemia are known risk factors for AD in the neurotypical population. 52 In our study, we did not find any contribution from these comorbid conditions to the risk of AD in our cohort. Likewise the presence of hypothyroidism and the difference in LID did not contribute to risk for AD. Similarly, the effect of sex on risk for AD did not change when comorbid conditions were added to the analyses. This suggests that AD in DS may have a somewhat different risk profile compared to the neurotypical population.
In summary, this is one of the largest studies of individuals with DS with incident dementia to evaluate sex differences in onset of AD. The longitudinal nature of our clinically based study with relatively large numbers of patients who were followed by a limited number of the same clinicians are strengths. While we did not find any sex differences in age of AD onset, we did find that women demonstrated longer duration of dementia compared to men; these were not related to any of the comorbid conditions studied or to the presence of an APOE ε4 allele, but does suggest that there may be genetic, environmental, or hormonal explanations that deserve further study.
FUNDING INFORMATION
Support for this work was funded in part through the Alzheimer Disease in Down Syndrome study (NIH U01 AG051412).
ACKNOWLEDGMENTS
We are grateful to the hundreds of patients with Down syndrome who entrusted us to evaluate and follow them over the years. We thank the following individuals who helped with preparing the database: Catherine Walsh, Madison Hebert, Ahalya Parthasarathy, Courtney Jordan, Nusrat Jahan, Giovi Hersch, and Cassandra Wang. The help of librarian Martha Stone and her colleagues at the Massachusetts General Hospital Treadwell Library was invaluable.
Lai F, Mhatre PG, Yang Y, Wang M‐C, Schupf N, Rosas HD. Sex differences in risk of Alzheimer's disease in adults with Down syndrome. Alzheimer's disease. 2020;12:1–8. 10.1002/dad2.12084
REFERENCES
- 1. Wisniewski HM, Silverman W, Wegiel J. Ageing, Alzheimer disease and mental retardation. J Intellect Disabil Res. 1994;38(Pt 3):233‐239. [DOI] [PubMed] [Google Scholar]
- 2. Penrose LS. The incidence of mongolism in the general population. J Ment Sci. 1949;95(400):685‐688. [DOI] [PubMed] [Google Scholar]
- 3. Zigman WB, Lott IT. Alzheimer's disease in Down syndrome: neurobiology and risk. Ment Retard Dev Disabil Res Rev. 2007;13(3):237‐246. [DOI] [PubMed] [Google Scholar]
- 4. Mann DM. The pathological association between Down syndrome and Alzheimer disease. Mech Ageing Dev. 1988;43(2):99‐136. [DOI] [PubMed] [Google Scholar]
- 5. Lai F, Williams RS. A prospective study of Alzheimer disease in Down syndrome. Arch Neurol. 1989;46(8):849‐853. [DOI] [PubMed] [Google Scholar]
- 6. Evenhuis HM. The natural history of dementia in Down's syndrome. Arch Neurol. 1990;47(3):263‐267. [DOI] [PubMed] [Google Scholar]
- 7. Liu CC, Liu CC, kanekiyo T, et al. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9(2):106‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vina J, Lloret A. Why women have more Alzheimer's disease than men: gender and mitochondrial toxicity of amyloid‐beta peptide. J Alzheimers Dis. 2010;20(Suppl 2):S527‐S533. [DOI] [PubMed] [Google Scholar]
- 9. Kawas C, Gray S, Brookmeyer R, et al. Age‐specific incidence rates of Alzheimer's disease: the Baltimore Longitudinal Study of aging. Neurology. 2000;54(11):2072‐2077. [DOI] [PubMed] [Google Scholar]
- 10. Fratiglioni L, Vitanen M, Strauss EV, et al. Very old women at highest risk of dementia and Alzheimer's disease: incidence data from the Kungsholmen Project, Stockholm. Neurology. 1997;48(1):132‐138. [DOI] [PubMed] [Google Scholar]
- 11. Ruitenberg A, Ott A, Swieten JCV, et al. Incidence of dementia: does gender make a difference? Neurobiol Aging. 2001;22(4):575‐580. [DOI] [PubMed] [Google Scholar]
- 12. Pike CJ, Carroll CJ, Rosario RE, et al. Protective actions of sex steroid hormones in Alzheimer's disease. FrontNeuroendocrinol. 2009;30(2):239‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Correia SC, Santos RX, Cardoso S. Effects of estrogen in the brain: is it a neuroprotective agent in Alzheimer's disease?. Curr Aging Sci. 2010;3(2):113‐126. [DOI] [PubMed] [Google Scholar]
- 14. Pike CJ. Sex and the development of Alzheimer's disease. J Neurosci Res. 2017;95(1‐2):671‐680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Snyder HM, Asthana S, Bain L, et al. Sex biology contributions to vulnerability to Alzheimer's disease: a think tank convened by the women's Alzheimer's research initiative. Alzheimers Dement. 2016;12(11):1186‐1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li R, Cui J, Shen Y. Brain sex matters: estrogen in cognition and Alzheimer's disease. Mol Cell Endocrinol. 2014;389(1‐2):13‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tensil M, Hessler JB, Gutsmiedl M, et al. Sex differences in neuropsychological test performance in Alzheimer's disease and the influence of the ApoE genotype. Alzheimer Dis Assoc Disord. 2018;32(2):145‐149. [DOI] [PubMed] [Google Scholar]
- 18. Laws KR, Irvine K, Gale TM. Sex differences in Alzheimer's disease. Curr Opin Psychiatry. 2018;31(2):133‐139. [DOI] [PubMed] [Google Scholar]
- 19. Ferretti MT, Iulita MF, Cavedo E, et al. Sex differences in Alzheimer disease ‐ the gateway to precision medicine. Nat Rev Neurol. 2018;14(8):457‐469. [DOI] [PubMed] [Google Scholar]
- 20. Rubinsztein DC, Hon J, Stevens F, et al. Apo E genotypes and risk of dementia in Down syndrome. Am J Med Genet. 1999;88(4):344‐347. [DOI] [PubMed] [Google Scholar]
- 21. Patel A, Hon F, Stevens F, et al. Association of variants within APOE, SORL1, RUNX1, BACE1 and ALDH18A1 with dementia in Alzheimer's disease in subjects with Down syndrome. Neurosci Lett. 2011;487(2):144‐148. [DOI] [PubMed] [Google Scholar]
- 22. Schupf N, Kapell D, Lee JH, et al. Onset of dementia is associated with apolipoprotein E epsilon4 in Down's syndrome. Ann Neurol. 1996;40(5):799‐801. [DOI] [PubMed] [Google Scholar]
- 23. Schupf N, Kapell D, Nightingale B, et al. Earlier onset of Alzheimer's disease in men with Down syndrome. Neurology. 1998;50(4):991‐995. [DOI] [PubMed] [Google Scholar]
- 24. Lai F, Kammann E, Rebeck GW, et al. APOE genotype and gender effects on Alzheimer disease in 100 adults with Down syndrome. Neurology. 1999;53(2):331‐336. [DOI] [PubMed] [Google Scholar]
- 25. Suri S, Heise V, Trachtenberg AJ, et al. The forgotten APOE allele: a review of the evidence and suggested mechanisms for the protective effect of APOE varepsilon2. Neurosci Biobehav Rev. 2013;37(10 Pt 2):2878‐2886. [DOI] [PubMed] [Google Scholar]
- 26. Altmann A, Tian L, Henderson VW, et al. Sex modifies the APOE‐related risk of developing Alzheimer disease. Ann Neurol. 2014;75(4):563‐573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rasmussen KL, Hnsen AT, Nordestgaard BG, et al. Absolute 10‐year risk of dementia by age, sex and APOE genotype: a population‐based cohort study. CMAJ. 2018;190(35):E1033‐E1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu M, Paranjpe MD, Zhou X, et al. Sex modulates the ApoE epsilon4 effect on brain tau deposition measured by (18)F‐AV‐1451 PET in individuals with mild cognitive impairment. Theranostics. 2019;9(17):4959‐4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Neu SC, Pa J, Kukull W, et al. Apolipoprotein E genotype and sex risk factors for Alzheimer disease: a meta‐analysis. JAMA Neurol. 2017;74(10):1178‐1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Albert M, Cohen C. The test for severe impairment: an instrument for the assessment of patients with severe cognitive dysfunction. J Am Geriatr Soc. 1992;40(5):449‐453. [DOI] [PubMed] [Google Scholar]
- 31. McCarthy D. The McCarthy Scales of Children's Abilities. New York: The Psychological Corporation; 1972. [Google Scholar]
- 32. Evenhuis HM. Further evaluation of the Dementia Questionnaire for Persons with Mental Retardation (DMR). J Intellect Disabil Res. 1996;40 (Pt 4):369‐373. [DOI] [PubMed] [Google Scholar]
- 33. Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31(3):545‐548. [PubMed] [Google Scholar]
- 34. Kaplan EL, Meier M. Nonparametric estimation from incomplete observations. J Amer Statist Assoc. 1958;53(282):457‐481. [Google Scholar]
- 35. Cox D. Regression models and life tables. J R Statist Soc B. 1972;34:187‐220. [Google Scholar]
- 36. Wang M, Brookmeyer R, Jewell N. Statistical models for prevalent cohort data. Biometrics. 1993;49:1‐11. [PubMed] [Google Scholar]
- 37. Knopman DS, Roberts RO, Pankratz VS, et al. Incidence of dementia among participants and nonparticipants in a longitudinal study of cognitive aging. Am J Epidemiol. 2014;180(4):414‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bachman DL, Wolf PA, Linn RT, et al. Incidence of dementia and probable Alzheimer's disease in a general population: the Framingham Study. Neurology. 1993;43(3 Pt 1):515‐519. [DOI] [PubMed] [Google Scholar]
- 39. Hebert LE, Scherr PA, Bieneias JL, et al. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60(8):1119‐1122. [DOI] [PubMed] [Google Scholar]
- 40. Ott A, Breteler MM, Harskamp FV, et al. Incidence and risk of dementia. The Rotterdam Study . Am J Epidemiol. 1998;147(6):574‐580. [DOI] [PubMed] [Google Scholar]
- 41. Brayne C, Gill C, Huppert FA, et al. Incidence of clinically diagnosed subtypes of dementia in an elderly population. Cambridge project for later life. Br J Psychiatry. 1995;167(2):255‐262. [DOI] [PubMed] [Google Scholar]
- 42. Chene G, Beiser A, Au R, et al. Gender and incidence of dementia in the Framingham Heart Study from mid‐adult life. Alzheimers Dement. 2015;11(3):310‐320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Larson EB, Shadlen MF, Wang L, et al. Survival after initial diagnosis of Alzheimer disease. Ann Intern Med. 2004;140(7):501‐509. [DOI] [PubMed] [Google Scholar]
- 44. Sinai A, Mokrysz C, Bernal J, et al. Predictors of age of diagnosis and survival of Alzheimer's disease in Down syndrome. J Alzheimers Dis. 2018;61(2):717‐728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yang Q, Rasmussen SA, Friedman JM. Mortality associated with Down's syndrome in the USA from 1983 to 1997: a population‐based study. Lancet. 2002;359(9311):1019‐1025. [DOI] [PubMed] [Google Scholar]
- 46. Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta‐analysis. APOE and Alzheimer disease meta analysis consortium see comments. JAMA. 1997;278(16):1349‐1356. [PubMed] [Google Scholar]
- 47. Prasher VP, Schupf N, Sajith SG, et al. Significant effect of APOE epsilon 4 genotype on the risk of dementia in Alzheimer's disease and mortality in persons with Down syndrome. Int J Geriatr Psychiatry. 2008;23(11):1134‐1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Payami H, Montee KR, Kaye JA, et al. Alzheimer's disease, apolipoprotein E4, and gender. JAMA. 1994;271(17):1316‐1317. [PubMed] [Google Scholar]
- 49. Riedel BC, Thompson PM, Brinton RD. Age, APOE and sex: triad of risk of Alzheimer's disease. J Steroid Biochem Mol Biol. 2016;160:134‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tyrrell J, Cosgrave M, Hawi Z, et al. A protective effect of apolipoprotein E e2 allele on dementia in Down's syndrome. Biol Psychiatry. 1998;43(6):397‐400. [DOI] [PubMed] [Google Scholar]
- 51. Coppus AMW, Evenhuis HM, Verberne GJ, et al. Survival in elderly persons with Down syndrome. J Am Geriatr Soc. 2008;56(12):2311‐2316. [DOI] [PubMed] [Google Scholar]
- 52. Edwards Iii GA, Gamez N, Escobedo G, Jr . Modifiable risk factors for Alzheimer's disease. Front Aging Neurosci. 2019;11:146. [DOI] [PMC free article] [PubMed] [Google Scholar]


