Abstract
The European Commission asked EFSA for a scientific evaluation on the risks to human health related to the presence of perfluoroalkyl substances (PFASs) in food. Based on several similar effects in animals, toxicokinetics and observed concentrations in human blood, the CONTAM Panel decided to perform the assessment for the sum of four PFASs: PFOA, PFNA, PFHxS and PFOS. These made up half of the lower bound (LB) exposure to those PFASs with available occurrence data, the remaining contribution being primarily from PFASs with short half‐lives. Equal potencies were assumed for the four PFASs included in the assessment. The mean LB exposure in adolescents and adult age groups ranged from 3 to 22, the 95th percentile from 9 to 70 ng/kg body weight (bw) per week. Toddlers and ‘other children’ showed a twofold higher exposure. Upper bound exposure was 4‐ to 49‐fold higher than LB levels, but the latter were considered more reliable. ‘Fish meat’, ‘Fruit and fruit products’ and ‘Eggs and egg products’ contributed most to the exposure. Based on available studies in animals and humans, effects on the immune system were considered the most critical for the risk assessment. From a human study, a lowest BMDL 10 of 17.5 ng/mL for the sum of the four PFASs in serum was identified for 1‐year‐old children. Using PBPK modelling, this serum level of 17.5 ng/mL in children was estimated to correspond to long‐term maternal exposure of 0.63 ng/kg bw per day. Since accumulation over time is important, a tolerable weekly intake (TWI) of 4.4 ng/kg bw per week was established. This TWI also protects against other potential adverse effects observed in humans. Based on the estimated LB exposure, but also reported serum levels, the CONTAM Panel concluded that parts of the European population exceed this TWI, which is of concern.
Keywords: PFAS, food, exposure, mixtures, immune system, PBPK, risk assessment
Short abstract
This publication is linked to the following EFSA Supporting Publications article: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2020.EN-1931/full
Summary
The European Commission asked European Food Safety Authority (EFSA) for a scientific evaluation on the risks to human health related to the presence of 27 per‐ and polyfluoroalkyl substances (PFASs) in food. The Panel developed the draft Scientific Opinion which underwent a public consultation from 24 February 2020 to 20 April 2020. The comments received and how they were taken into account when finalising the Scientific Opinion were published in an EFSA Technical Report (EFSA, 2020).
PFASs evaluated include perfluoroalkyl carboxylates (PFCAs) and sulfonates (PFSAs). These compounds contain a carbon chain with variable chain length (C4–C18), and either a carboxylic acid or sulfonate group. At neutral pH, these compounds have anionic end‐groups, which to some extent explains their properties. In addition, a number of PFASs other than PFCAs and PFSAs are assessed, some of them being precursors of PFCAs and PFSAs.
In 2018, EFSA published an Opinion on two of these PFASs, perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) and derived separate tolerable weekly intakes (TWIs) for these compounds based on effects observed in humans. The current Opinion aimed at assessing the risk for other PFASs, but the EFSA Panel on Contaminants in the Food Chain (CONTAM Panel) was also asked to review the risk assessment in the previous Opinion, including the possibility to apply a mixture approach.
Based on several similar effects in animals, toxicokinetics and observed levels in human blood, the CONTAM Panel performed the current risk assessment for the sum of four PFASs: PFOA, perfluorononanoic acid (PFNA), perfluorohexane sulfonic acid (PFHxS) and PFOS. These four made up approximately half of the lower bound (LB) exposure to those PFASs for which occurrence data were available, the remaining contribution being primarily from PFBA and PFHxA, two PFASs with a short half‐life. Equal, weight‐based, potency was assumed for the four PFASs, since derivation of relative potency factors was considered not possible.
For the exposure assessment, an initial number of 97,434 results for food samples analysed for PFASs, obtained from 16 European countries, were available. Various criteria were used for cleaning the dataset, resulting in 69,433 analytical results for 26 PFASs with 92% of left‐censored (LC) data (results below limit of detection (LOD)/limit of quantification (LOQ)).
The CONTAM Panel decided not to run the exposure assessment for PFASs with 100% of LC data. As a result, the exposure assessment was limited to 17 PFASs (perfluorobutanoic acid (PFBA), perfluoropentanoic acid (PFPeA), perfluorohexanoic acid (PFHxA), perfluoroheptanoic acid (PFHpA), perfluorooctanoic acid (PFOA), perfluorononanoic acid (PFNA), perfluorodecanoic acid (PFDA), perfluoroundecanoic acid (PFUnDA), perfluorododecanoic acid (PFDoDA), perfluorotridecanoic acid (PFTrDA), perfluorotetradecanoic acid (PFTeDA), perfluorobutane sulfonic acid (PFBS), perfluorohexane sulfonic acid (PFHxS), perfluoroheptane sulfonic acid (PFHpS), perfluorooctane sulfonic acid (PFOS), perfluorodecane sulfonic acid (PFDS) and perfluorooctane sulfonamide (FOSA)). For these PFASs, 67,839 analytical data were available. Most data were on PFOS (n = 8,498), PFOA (n = 8,197), PFDA (n = 5,770), PFNA (n = 5,594), PFHxA (n = 5,448) and PFHxS (n = 4,745).
High quantified concentrations (P95 > 10 μg/kg) were reported for some of the PFASs in edible offal from game animals and a number of fish species.
For most food categories and PFASs, calculated mean upper bound (UB) levels (based on using LOQ/LOD in case of non‐detected levels) were much higher than LB levels (using zero for non‐detects). This is due to high proportions of LC results and/or only limited availability of data for many PFASs. Therefore, exposure calculations should be considered only as a rough indication of the range of chronic dietary exposure, and should thus be interpreted with caution. The CONTAM Panel concluded that the calculated LB exposure is likely to be more realistic than the UB exposure.
As mentioned above, the CONTAM Panel decided to limit the risk assessment to the sum of PFOA, PFNA, PFHxS and PFOS. For this sum, the median exposure in adolescents, adults, elderly and very elderly ranged from 0.42 to 3.1 ng/kg body weight (bw) per day at the LB and from 11.4 to 41.5 at the UB. Toddlers and other children had approximately twofold higher mean intake than older age groups (adolescents, adults, elderly, very elderly), ranging from 0.84 to 6.5 ng/kg bw per day at the LB and from 38.5 to 112 ng/kg bw per day at the UB. In infants, the mean exposure ranges were at the LB 2.4–12.2 ng/kg bw per day and at the UB 42.8–115 ng/kg bw per day. The 95th percentile exposures ranged across surveys and age groups at the LB from 1.3 (adults) to 27.9 (infants) ng/kg bw per day and at the UB from 21.9 (very elderly) to 229 (toddlers) ng/kg bw per day.
When focussing on some of the individual PFASs, mean PFOA exposure ranged for the LB from 0.1 to 0.6 ng/kg bw per day and for the UB from 3.0 to 29 ng/kg bw per day across surveys and age groups. At high (95th percentile) exposure, the LB ranged from 0.2 to 2.1 ng/kg bw per day and the UB from 5.6 to 59 ng/kg bw per day. Mean PFNA exposure ranged for the LB from 0.02 to 11.7 ng/kg bw per day and for the UB from 2.7 to 29.4 ng/kg bw per day across surveys and age groups. At high (95th percentile) exposure, the LB ranged from 0.05 to 27.9 ng/kg bw per day and the UB from 4.9 to 57.5 ng/kg bw per day. Mean PFHxS exposure ranged for the LB from 0.04 to 0.36 ng/kg bw per day and for the UB from 2.5 to 29.0 ng/kg bw per day across surveys and age groups. At high (95th percentile) exposure, the LB ranged from 0.09 to 0.86 ng/kg bw per day and the UB from 4.6 to 57.6 ng/kg bw per day. Mean PFOS exposure ranged for the LB from 0.23 to 2.6 ng/kg bw per day and for the UB from 3.3 to 31 ng/kg bw per day across surveys and age groups. At high (95th percentile) exposure, the LB ranged from 1.0 to 8.5 ng/kg bw per day and the UB from 6.25 to 62 ng/kg bw per day.
When comparing exposure of adults to these four PFASs, PFOA contributed 21%, PFNA 4%, PFHxS 10% and PFOS 66% to the sum, based on the median of the mean LB contributions across surveys. The contributions were similar in the age groups ‘Other children’, ‘Adolescents’, ‘Elderly’ and ‘Very elderly’. These four contributed in adults approximately 46% to the sum of all PFASs for which the exposure was calculated, with relative contributions of 9%, 2%, 4% and 30% for, respectively, PFOA, PFNA, PFHxS and PFOS. Other PFASs that contributed more than 5% to this sum were PFBA (16%) and PFHxA (15%) which have short half‐lives in humans.
For PFOS and PFOA, ‘Fish and other seafood’ was the most important contributor to the mean LB exposure, followed by ‘Eggs and egg products’, ‘Meat and meat products’, and ‘Fruit and fruit products’. For PFOA, ‘Vegetables and vegetable products’ and ‘Drinking water’ were also important contributors. For several of the other PFASs, ‘Fish and other seafood’, ‘Fruit and fruit products, ‘Vegetables and vegetable products’, ‘Drinking water’, as well as ‘Starchy roots and tubers’ were the most important food groups. Although for infants and children ‘Food for infants and small children’ was a major contributor, this was highly uncertain since this was based on few samples with detected values. For the combined exposure to PFOA, PFNA, PFHxS and PFOS, the main contributing food categories were ‘Fish meat’, ‘Fruit and fruit products’ and ‘Eggs and egg products’, observed for all population groups.
There were some clear differences with the exposure assessments for PFOS and PFOA in the previous Opinion from 2018, both in terms of the actual exposure and the relative contribution of food groups. These are due to differences in databases, assumptions and treatment of the occurrence data.
The use of PFAS‐containing food contact materials is likely to contribute to human exposure to PFASs, and this may not be included in the reported data. Diet is the major source of PFAS exposure for most of the population, but on an individual basis, other routes such as dust ingestion and indoor air inhalation may also contribute substantially.
Considering toxicokinetics, many of the 27 PFASs considered in this Opinion are shown to be readily absorbed through the gastrointestinal tract in mammals, including humans. They distribute to the plasma and other parts of the body and depending on the specific PFAS, tend to accumulate in the liver. They are excreted in both urine and faeces. Neither PFCAs nor PFSAs are metabolised by animals or humans, whereas precursors such as fluorotelomer alcohols (FTOHs) and polyfluoroalkyl phosphate esters (PAPs) are biotransformed to several metabolites, including PFCAs.
Routes and rates of elimination of PFASs vary according to the chemical end‐group, the chain length and the species. In rats, but not in humans, sex differences were observed in toxicokinetics for a number of PFASs. In rodents, half‐lives vary from few hours to several weeks and are in general much shorter than in humans.
In humans, the estimated half‐lives for short‐chain PFASs (such as PFBA, PFBS and PFHxA) were found to range from a few days to approximately one month, whereas for compounds having a long perfluoroalkyl chain length (such as PFOA, PFNA, PFDA, PFHxS or PFOS), it can be several years. The long elimination half‐lives of these PFASs mainly originate from their interactions with various transporters involved in the reabsorption processes occurring at the hepatic, intestinal and renal level.
The maternal transfer of PFASs to offspring occurs both prenatally (in utero) and postnatally (via breastfeeding).
PFASs are transferred from soil to plants. In general, transfer rates are higher for the short‐chain PFASs. The transfer rates decrease from roots to leaves to fruits, due to natural barriers within the plants. Nevertheless, PFASs have been detected in fruits. This transfer to plant‐derived materials is relevant for both food and feed, including straw. In food producing animals, PFASs transfer from feed to animal derived food like milk, eggs and meat, with clear differences between species and the type of PFAS. This is also relevant for soil ingestion by foraging farm animals.
PFASs have been detected in humans. Blood serum and blood plasma are matrices for biomonitoring of PFASs. Generally, after the year 2000, the concentrations of PFOS, PFOA and in some studies PFHxS have decreased, while the concentrations of PFNA, PFDA and PFUnDA have increased. No clear trends have been reported for other PFASs.
Summary statistics was performed on median levels from the various studies published from 2007 to 2018. Median values for the study medians were determined, referred to as median concentrations. The most prominent PFAS in serum of adults was PFOS (64%), followed by PFOA (16%), PFHxS (5.6%) and PFNA (5.1%). For children, PFOS and PFOA contributed almost the same with 35.0% and 36.6% of the total, followed by PFNA (8.8%) and PFHxS (6.7%). For adults, the median concentrations in serum or plasma were 7.7, 1.9, 0.67, 0.61, 0.30 and 0.28 ng/mL for PFOS, PFOA, PFHxS, PFNA, PFDA and PFUnDA, respectively, while the concentrations of the remaining PFASs were below 0.25 ng/mL. For children, the median concentrations in plasma were 3.2, 3.3, 0.79, 0.60 and 0.30 ng/mL for PFOS, PFOA, PFNA, PFHxS and PFDA, respectively, while the concentrations of the remaining PFASs were below 0.25 ng/mL.
Considerably higher concentrations have been observed for some individuals, including both occupationally exposed adults, and children and adults, which have experienced elevated exposure from e.g. contaminated drinking water. In these cases, the relative abundance of the various PFASs may deviate considerably from what is observed in general populations.
Considering effects in laboratory animals, repeated dose toxicity studies were identified for 11 PFCAs (PFBA, PFHxA, PFHpA, PFOA, PFNA, PFDA, PFUnDA, PFDoDA, PFTeDA, PFHxDA, PFODA), for three PFSAs (PFBS, PFHxS, PFOS) and for two other PFASs (8:2 FtOH, EtFOSE). The most consistent endpoint was increased liver weight, seen for all PFASs studied but with clear differences in relative potencies. Disturbances in lipid metabolism, including hepatocellular steatosis and hepatotoxic effects, were evident mostly at higher dose levels. Many PFASs decreased the levels of thyroid hormones (both T4 and T3). For some PFASs, increased relative kidney weight, and alterations of the mucosa in the nasal cavity and olfactory epithelium were observed.
The most sensitive developmental effect was impaired development of mammary glands in mice, after exposure of the dams late in gestation or the offspring in utero or via lactation. The impairment persisted beyond sexual maturity. Only PFOA has been investigated in relation to this outcome, with a maternal lowest observed adverse effect concentration (LOAEC) around 66 ng/mL serum. A no observed adverse effect concentration (NOAEC) could not be identified from the latter studies.
More general developmental toxicity studies in rodents were identified for 10 PFCAs (PFBA, PFHxA, PFOA, PFNA, PFDA, PFUnDA, PFDoDA, PFTeDA, PFHxDA and PFODA), for three PFSAs (PFBS, PFHxS and PFOS) and for two other PFASs (8:2 FtOH and EtFOSE). The effects most often observed were increased fetal and/or neonatal mortality and reduction in fetal weight and/or postnatal growth. In general, developmental toxicity occurred at similar or slightly lower doses than those exerting maternal toxicity. LOAELs and NOAELs were orders of magnitude higher than those for developmental effects on mammary glands seen with PFOA.
After PFAS exposure, no consistent findings were identified for female reproductive parameters. Effects on male reproductive parameters, including atrophy of the testicular interstitium, accompanied by reduced serum testosterone levels and epidydimal and testicular weights, as well as degenerative changes and spermatid retention in seminiferous tubules, have been reported for PFNA and PFDA.
PFOS and PFOA exert developmental neurotoxic effects in rodents at doses of 0.1–0.3 mg/kg bw per day or higher. PFOS exposure mostly decreased spontaneous activity, while PFOA increased it. Studies with PFDA, PFHXs and PFDoDA indicate developmental neurotoxic effects.
In animal studies, PFOS and PFOA have been shown to cause a reduced response to vaccination (T‐cell‐dependent antibody response) and PFOS also caused a reduced resistance to infection. Effects were noted at doses where no overt toxicity was evident. Whereas effects on the immune system have also been observed for other PFASs, i.e. PFNA and PFDA, the available database for these compounds is more limited and does not include vaccination response. The CONTAM Panel concluded that the immune system is a prime target of PFASs.
For PFOS and PFOA, no evidence for a direct genotoxic mode of action was identified. For PFASs other than PFOS and PFOA, the number of studies and data are limited. However, structural similarity for PFHxS and PFOS, as well as for PFNA and PFOA, indicates that also for these PFASs a direct genotoxic mode of action is unlikely.
Available studies indicate that PFOS and PFOA are tumour promoters in rodent liver and that PFOA also induces Leydig cell tumours in rats. The only long‐term study available for PFHxA provides no evidence for carcinogenicity. PFNA and PFDA promoted liver tumour formation in a trout two‐stage model of hepatocarcinogenesis, while 8:2 FTOH failed to do so.
Many studies have looked into potential effects of PFASs in humans, examining associations between serum levels of PFASs and specific disease incidences or markers of effects. The previous Opinion (EFSA CONTAM Panel, 2018) extensively reviewed the existing data on potential associations, but this was restricted to PFOS and PFOA. For some potential critical effects, new studies appeared since then.
Epidemiological studies published since the publication of the previous EFSA CONTAM Panel Opinion on PFOS and PFOA provide further support for the conclusion that PFOS and PFOA are associated with reduced antibody response to vaccination, observed in several studies. The evidence for other PFASs is weaker, related to the mixture of PFASs in blood, the lower concentrations compared to PFOS and PFOA, and the small number of studies. Some of the studies suggest that serum levels of PFOS and PFOA are associated with increased propensity for infection. As for PFOS and PFOA, epidemiological studies provide insufficient evidence to conclude on associations between exposure to PFASs and asthma and allergies.
Epidemiological studies provide clear evidence for an association between exposure to PFOS, PFOA and PFNA and increased serum levels of cholesterol. As for PFOS and PFOA, epidemiological studies provide insufficient evidence to conclude on associations between exposure to other PFASs and increased risk of cardiovascular disease. Epidemiological studies provide evidence for an association between exposure to PFASs and increased serum levels of the liver enzyme alanine transferase (ALT). The magnitude of the associations was small (~ 3%), however, and few studies found associations with ALT outside the reference range, and there were no associations with liver disease. There is insufficient evidence for associations between exposure to PFASs and diabetes, obesity and metabolic syndrome.
Studies on PFOS and PFOA published since 2018 confirm previous conclusions from the PFOS and PFOA Opinion (EFSA CONTAM Panel, 2018) that ‘there may well be a causal association between PFOS and PFOA and birth weight’. Maternal serum levels in studies reporting results on other PFASs were generally much lower and those studies provide no evidence for an adverse association for other PFASs and birth weight.
As for PFOS and PFOA, epidemiological studies show no associations between other PFASs and fertility and reproductive outcomes in both males and females.
Epidemiological studies provide insufficient evidence for associations between exposure to PFASs and neurodevelopment outcomes, growth in infancy or childhood, neurobehavioural, neuropsychiatric, cognitive outcomes or thyroid function.
Epidemiological studies provide insufficient evidence for associations between exposure to PFASs and changes in kidney function or serum levels of uric acid, as well as low bone mineral density or osteoporosis.
In 2018, the CONTAM Panel concluded that epidemiological studies provide insufficient support for carcinogenicity of PFOS and PFOA in humans. Limited information was identified for other PFASs.
Certain effects seem to be associated with binding of PFASs to the PPARα receptor, but to what extent this mechanism can explain the respective adverse effects is unclear. PPARα knockout mice but also mice expressing human PPARα have been used to study the role of this receptor, including potential differences between rodents and humans. Like peroxisome proliferators, PFASs seem to cause liver hyperplasia via the murine PPARα. However, PFASs induce steatosis in rodent hepatocytes in a PPARα‐independent way. Hepatocellular steatosis appears to be causally related to the occurrence of necrotic hepatocytes and the increased serum transaminase levels, but thorough knowledge of the mode of action underlying this effect is missing.
There is no clear mode of action behind the strong decrease in thyroid hormone levels (T4, T3) observed in rodents exposed to PFASs, which is normally not accompanied by increased thyroid‐stimulating hormone (TSH) levels. PFASs compete with T4 for binding to transthyretin and can induce glucuronosyltransferase activity in the liver, but it is unclear if this is behind the decrease in hormone levels observed in animals.
No mode of action of immunotoxicity by PFASs has been established. Data from in vivo and in vitro studies on PFOS and PFOA suggest that immunotoxic effects may originate from modulation of PPARs, NF‐κB regulated gene transactivation and/or regulation of apoptosis.
The MOA behind the impaired mammary gland development in mice dosed with PFOA during gestation and neonatally is unknown.
Considering critical effects, in human studies, various associations between serum levels and a number of outcomes have been reported. In the previous Opinion (EFSA CONTAM Panel, 2018), four endpoints were selected as potential critical effects for PFOS and/or PFOA. These were (i) increased serum total and LDL cholesterol (risk factor for cardiovascular disease), (ii) increased ALT levels (indicating effects on liver cells), (iii) reduced birth weight and (iv) effects on the immune system as shown by decreased antibody response to vaccines.
In 2018, the CONTAM Panel used the effects on serum cholesterol levels to derive TWIs for both PFOS and PFOA. Those TWIs were also protecting towards the other potential critical endpoints. Although the association with increased cholesterol was observed in a large number of studies, the CONTAM panel now considers the uncertainty regarding causality to be larger. This is primarily due to a postulated biological process around the enterohepatic cycling of both PFASs and bile acids, the latter affecting serum cholesterol levels. This should be further investigated.
For increased ALT, the CONTAM Panel concluded that more studies are needed to support the causality of the effect. Concerning reduced birth weight, a recent study seems to strengthen the causality. However, as concluded in 2018, the decrease in birth weight after adjusting for confounders is not large and the potential longer term consequences of this decrease are unclear.
The CONTAM Panel concluded that effects on the immune system, which were observed at the lowest serum PFAS levels in both animals and humans, are critical for the risk assessment. The findings of a decreased immune response were considered robust since they were consistently observed for the two studied PFASs in rodents (PFOA, PFOS) and in humans. The CONTAM Panel noted that this is not the case for effects on mammary gland development, which are observed at similar low serum levels in mice but have not been studied in other animal models or humans. Therefore, the CONTAM Panel decided to base the present assessment on PFASs on effects on the immune system.
Based on observations in animals and humans, the CONTAM Panel decided to combine its assessment on the sum of four PFASs, i.e. PFOA, PFNA, PFHxS and PFOS. At present, these four PFASs contribute most to the levels observed in human serum. In humans, these four PFASs share toxicokinetic properties and show similar accumulation and long half‐lives. Also, in terms of effects, these compounds in general show the same effects when studied in animals. This also applies to several other PFASs, but the critical studies in humans did not report these in the blood of the participants. Current data do not allow the derivation of potency factors for the critical endpoint. As a pragmatic approach, the CONTAM Panel assumed by default equal potencies for effects of these four PFASs on immune outcomes.
Two critical studies have been considered for the derivation of the Health‐Based Guidance Value. A study with children on the Faroe Islands showed various associations between the serum levels of individual PFASs, but also the sum of PFOA, PFNA, PFHxS and PFOS, and antibody titres against diphtheria and tetanus. An NOAEC of 27.0 ng/mL was identified for the sum of these four PFASs at 5 years of age and the antibody titres against diphtheria at 7 years. In addition, the CONTAM Panel identified a new study with children from Germany showing an inverse association between serum levels of PFOA, but also the sum of PFOA, PFNA, PFHxS and PFOS, and antibody titres against haemophilus influenzae type b (Hib), diphtheria and tetanus in serum sampled from 1‐year‐old children, predominantly breastfed. A lowest BMDL10 of 17.5 ng/mL at the age of 1 year was derived for the sum of PFOA, PFNA, PFHxS and PFOS, based on the inverse association between serum levels of the sum of these four PFASs and antibody titres against diphtheria.
This BMDL10 of 17.5 ng/mL corresponds to a lower intake by the child and thus the mother in her life up to pregnancy, than the NOAEC of 27.0 ng/mL from the study with older children of the Faroe islands. The CONTAM Panel also considered that PFAS serum levels in breastfed children are in general higher at 1 year of age than at 5 years. Therefore, this BMDL10 was used to estimate the daily intake by mothers that would result in this critical serum concentration at 1 year of age in breastfed children. This daily intake was subsequently used to derive an HBGV for the sum of PFOA, PFNA, PFHxS and PFOS.
Using a PBPK model, and assuming 12 months of breastfeeding, it was estimated that the BMDL10 in infants corresponds to an intake by the mother of 0.63 ng/kg bw per day for the sum of the four PFASs. Such intake would result in a serum level in the mother at 35 years of age of 6.9 ng/mL.
The CONTAM Panel decided to use the daily intake of 0.63 ng/kg bw per day as the starting point and established a group tolerable weekly intake (TWI) of 7 × 0.63 = 4.4 ng/kg bw per week for the sum of PFOA, PFNA, PFHxS and PFOS. It was decided that no additional uncertainty factors need to be applied, because the BMDL10 is based on infants which are expected to be a sensitive population group, as is true for many immunotoxic chemicals. In addition, a decreased vaccination response is considered a risk factor for disease rather than a disease.
This TWI should prevent that mothers reach a body burden that results in levels in milk that would lead to serum levels in the infant associated with a decrease in vaccination response. As a result, the higher exposure of breastfed infants is taken into account in the derivation of the TWI and the intake by infants should therefore not be compared with this TWI.
The CONTAM Panel noted that this TWI is protective for the other potential critical endpoints (increase in serum cholesterol, reduced birth weight and high serum levels of ALT) considered in the previous Opinion on PFOS and PFOA (EFSA CONTAM Panel, 2018).
Based on the occurrence data obtained from the Member States, the exposure to individual PFASs was estimated for different age groups across different food consumption surveys. Since it was decided to base the risk assessment on the sum of PFOA, PFNA, PFHxS and PFOS, also the combined exposure to these four PFASs was estimated. The CONTAM Panel noted that several factors contribute to the high uncertainty of the present exposure assessment. Large differences between LB and UB concentrations were observed in foods, as a result of analytical methods being used that are not sufficiently sensitive. This results in a large difference between maximum UB and minimum LB chronic dietary exposure estimates for PFASs. The CONTAM Panel considers that the true exposure level for PFOA, PFNA, PFHxS and PFOS is closer to the LB than the UB values. This assumption is based on two facts. First of all, studies performed using the best analytical methods with high sensitivity and high levels of quality control give results with fewer left censored data and confirm occurrence in foods at levels close to the LB estimates. Secondly, LB exposure estimates in this Opinion are more consistent with reported median blood serum levels.
Regarding the combined exposure to the four PFASs expressed on a weekly basis, for toddlers and other children, mean LB exposure varied between 6 and 46 ng/kg bw per week. The high (P95) LB exposure ranged from 19 to 96 ng/kg bw per week. UB exposure was much higher, for the mean varying between 270 and 785 ng/kg bw per week, with high (P95) UB exposure between 553 and 1603 ng/kg bw per week. For toddlers, the high exposure from food is influenced by a high contribution from PFNA and associated with high uncertainty. For the group of adolescents, adults, elderly and very elderly mean LB exposure varied between 3 and 22 ng/kg bw per week, as compared to high (P95) LB exposure between 9 and 70 ng/kg bw per week. UB exposure for these age groups was higher, for the mean varying between 81 and 290 ng/kg bw per week, with P95 exposure between 153 and 294 ng/kg bw per week. The highest mean LB exposure for adolescents and adults exceeds the TWI by a factor of 5. At the highest LB P95 exposure, this is a factor of 16. At the UB exposure, the exceedance is much larger. These calculated exceedances of the TWI at LB exposure estimates indicate a health concern.
The CONTAM Panel identified several uncertainties and has a number of recommendations to decrease these uncertainties. To improve the exposure assessment, data obtained by more sensitive analytical methods with high levels of quality control (to avoid matrix effects or impact of background contamination) are needed in order to increase the proportion of quantified results and thus reduce uncertainty in the dietary exposure assessment. This is needed for all PFASs and a broad range of widely consumed food products. For the determination of the total amount of PFASs, sensitive and accurate methods, which facilitate determination of the total amount of PFASs in samples of food and drinks are needed. Exposure assessment should be frequently updated, especially when analytical data obtained from more sensitive methods become available. Additional studies on the relative contribution of sources other than food are needed, especially for PFASs which are present in the highest concentrations in indoor air and house dust, such as n:2 FTOHs and PAPs. Also, more studies on the effect of cooking and food processing, in particular in relation to transfer to food from food contact materials that contain PFASs, are needed, given that most food is consumed after cooking/processing, and the fact that data reported in the scientific literature are inconsistent regarding the impact this has on exposure. More information is needed on the transfer of PFASs along the food chain. Furthermore, additional studies on paired human samples are needed to identify the relevant matrices for biomonitoring of various PFASs.
Concerning potential adverse effects, studies on effects of other PFASs, and in particular those of PFNA and PFHxS on the immune system should be conducted. Studies for the potential critical effects that allow for a derivation of potency factors for PFASs should be conducted. In addition, studies to characterise the mode of action of immunotoxicity and mammary gland development of PFASs should be performed. The effects of PFASs on thyroid hormone levels and potential consequences for neurodevelopment should be further investigated.
More longitudinal epidemiological studies are needed on human endpoints, in particular prospective vaccination studies covering more varied types of vaccines, different populations, as well as more studies on other immune outcomes including risk of infections. Most epidemiological studies examine associations between health‐related outcomes and single PFASs separately in spite of co‐exposures. For risk assessment, results for the sum of several PFASs should be reported.
Experimental evidence is needed to understand and quantify the association between PFASs and blood lipids, e.g. the importance of enterohepatic circulation. PBPK models for PFASs should be further optimised.
1. Introduction
1.1. Background and Terms of Reference as provided by the European Commission
Background
Following the Scientific Opinion on Perfluorooctane sulfonate (PFOS) perfluorooctanoic acid (PFOA) and their salts,1 the European Commission recommended an EU‐wide monitoring2 of perfluoroalkylated substances in food. The occurrence data generated by this monitoring have been used in the Scientific Report entitled ‘perfluoroalkylated substances in food: occurrence and dietary exposure’3.
Terms of Reference
In accordance with Art 29 (1) of Regulation (EC) No 178/2002, the European Commission asks the European Food Safety Authority to prepare an Opinion on the risks to human health related to the presence of perfluoroalkylated substances in food, considering existing hazard assessments and available occurrence data
1.2. Interpretation of the Terms of Reference
Following an agreement reached in June 2017 with the European Commission, the CONTAM Panel decided to address the mandate in two separate Opinions, one on perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) (EFSA‐Q‐2015‐00526) and another on other perfluoroalkylated substances (EFSA‐Q‐2017‐00549).
In 2010, EFSA issued a call for data on PFASs in food. Thirteen countries submitted analytical results on 27 PFASs in food covering the sampling period 1998–2012. In addition, data on PFASs in food from a 3‐year EU research project (PERFOOD4) were submitted to EFSA. The selection of compounds in this Opinion is based on these 27 PFASs that were subject of the EFSA 2012 Scientific exposure report (EFSA, 2012).
1.3. Additional information
1.3.1. Chemistry and synthesis
In the 2008 Opinion (EFSA, 2008), it was described that poly‐ and perfluoroalkyl substances (PFASs) are the collective name for a vast group of fluorinated substances, including oligomers and polymers. OECD (2018) has identified 4730 PFAS‐related CAS numbers. In the literature, many individual substances as well as groups of substances are described under more than one acronym and also substances or groups are discussed under identical acronyms.
PFASs (R‐X) are substances consisting of a hydrophobic alkyl chain, R, of varying length (typically C4–C16) and a hydrophilic end group, X. The hydrophobic part may be fully [R=F(CF2)n‐] or partially fluorinated. When fully fluorinated, the molecules are also called perfluoroalkyl substances. Their general structure is given in Figure 1 and some chemical characteristics of individual PFASs considered in this Opinion are given in Table 1.
Figure 1.
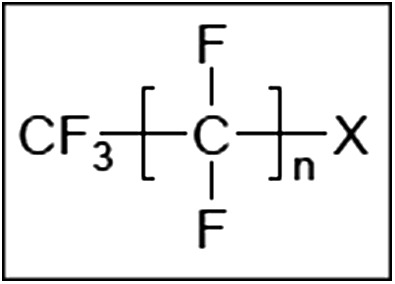
General structure of PFASs
Table 1.
Chemical characteristics of individual perfluoroalkyl substances considered within this Opinion and their uses as derived from Buck et al. (2011)
| Acronym | Chemical name | CAS number | Structural formula | Molecular weight | Uses |
|---|---|---|---|---|---|
| Perfluoroalkyl carboxylic acids (PFCAs) | |||||
| PFBA | Perfluorobutanoic acid | 375‐22‐4 |
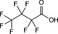
|
214.04 | Surfactant |
| PFPeA | Perfluoropentanoic acid | 2706‐90‐3 |
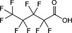
|
264.05 | Surfactant |
| PFHxA | Perfluorohexanoic acid | 307‐24‐4 |
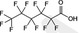
|
314.05 | Surfactant |
| PFHpA | Perfluoroheptanoic acid | 375‐85‐9 |

|
364.06 | Surfactant |
| PFOA | Perfluorooctanoic acid | 335‐67‐1 |
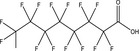
|
414.07 | Surfactant |
| PFNA | Perfluorononanoic acid | 375‐95‐1 |

|
464.08 | Surfactant |
| PFDA | Perfluorodecanoic acid | 335‐76‐2 |
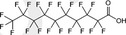
|
514.08 | Surfactant |
| PFUnDA | Perfluoroundecanoic acid | 2058‐94‐8 |

|
564.09 | Surfactant |
| PFDoDA | Perfluorododecanoic acid | 307‐55‐1 |
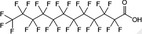
|
614.10 | Surfactant |
| PFTrDA | Perfluorotridecanoic acid | 72629‐94‐8 |

|
664.11 | Surfactant |
| PFTeDA | Perfluorotetradecanoic acid | 376‐06‐7 |
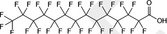
|
714.11 | Surfactant |
| PFPeDA | Perfluoropentadecanoic acid | 18024‐09‐4 |

|
764.12 | Surfactant |
| PFHxDA | Perfluorohexadecanoic acid | 67905‐19‐5 |

|
814.13 | Surfactant |
| PFODA | Perfluorooctadecanoic acid | 16517‐11‐6 |
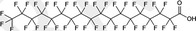
|
914.15 | Surfactant |
| Perfluoroalkane sulfonic acids (PFSAs) | |||||
| PFBS | Perfluorobutane sulfonic acid |
375‐73‐5/45187‐15‐3 [59933‐66‐3 as hydrate] |
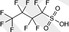
|
300.10/299.09 [318.11] |
Surfactant |
| PFHxS | Perfluorohexane sulfonic acid | 355‐46‐4/ 108427‐53‐8 |
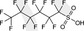
|
400.11/399.10 | Surfactant |
| PFHpS | Perfluoroheptane sulfonic acid | 375‐92‐8 |

|
450.12 | Surfactant |
| PFOS | Perfluorooctane sulfonic acid | 2795‐39‐3 (Potassium salt; 1763‐23‐1 (Acid) |
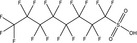
|
538.22 (Potassium salt) 500.13 (Acid) |
Surfactant |
| PFDS | Perfluorodecane sulfonic acid | 335‐77‐3 |
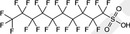
|
600.14 | Surfactant |
| Perfluoroalkane sulfinic acids (PFSIAs) | |||||
| PFOSI | Perfluorooctane sulfinic acid | 647‐29‐0 |

|
484.13 | Intermediate environmental transformation product |
| n:2 fluorotelomer alcohols (n:2 FTOHs) | |||||
| 8:2 FTOH | 8:2 Fluorotelomer alcohol | 678‐39‐7 |
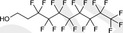
|
464.12 | Major raw material for surfactant and surface production products |
| n:2 polyfluoroalkyl phosphoric acid esters (PAPs) | |||||
| 8:2 monoPAP | 8:2 Fluorotelomer phosphate monoester | 57678‐03‐2 |

|
544.10 | Surfactant and surface protection products |
| 8:2 diPAP | 8:2 Fluorotelomer phosphate diester | 678‐41‐1 |
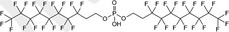
|
990.20 | Surfactant and surface protection products |
| Perfluoroalkane sulfonamides (FASAs) | |||||
| FOSA | Perfluorooctane sulfonamide | 754‐91‐6 |
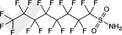
|
499.14 | Major raw material for surfactant and surface production products |
| N‐ethyl perfluoroalkane sulfonamides (EtFASAs) | |||||
| EtFOSA | N‐ethyl perfluorooctane sulfonamide | 4151‐50‐2 |
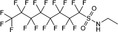
|
527.20 | Major raw material for surfactant and surface production products |
| N‐ethylperfluoroalkane sulfonamidoethanols (EtFASEs) | |||||
| EtFOSE | N‐ethyl perfluorooctane sulfonamidoethanol | 1691‐99‐2 |
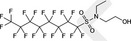
|
571.25 | Major raw material for surfactant and surface production products |
| Perfluoroalkyl phosphate | |||||
| FC‐807 | Ammonium bis[2‐[N‐ethyl(heptadecafluorooctane)sulfonylamino]ethyl]phosphate | 30381‐98‐7 |
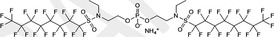
|
1,221.49 | Surfactant |
The hydrophilic end group can be neutral, or positively or negatively charged. The resulting substances are non‐ionic, cationic or anionic surface‐active agents due to their amphiphilic character. Examples of substances with anionic end groups are the perfluoroalkane sulfonic acids (PFSAs), which include PFOS, and the perfluoroalkyl carboxylic acids (PFCAs), which include PFOA. In cationic PFASs, the fluorinated hydrophobic part is attached to e.g. a quaternary ammonium group. Examples of neutral end groups X are: ‐OH, ‐SO3NH2 and include among others the fluoroteleomer alcohols (FTOHs) and perfluoroalkane sulfonamides (FASAs). Due to the strong covalent C‐F bond, perfluoroalkyl substances are highly persistent.
Many PFASs are considered to be potential precursors of other PFASs, which has been described in more detail in EFSA CONTAM Panel (2018). These precursors are usually not environmentally persistent but may be transformed in the environment among others through biodegradation. For instance, N‐ethylperfluoroalkane sulfonamidoethanols (EtFASEs) and N‐ethyl perfluoroalkane sulfonamides (EtFASAs) can biodegrade to FASAs and further to PFSAs. Because the precursors include products that are not fully fluorinated, some of the partially fluorinated substances are also discussed in this Opinion. For the partially fluorinated compounds, the position and number of fluorines determine their characteristics. Considering the entire family of PFASs, it should be noted that there are interrelationships between individual substances as industrial, environmental or metabolic precursors or transformation products of one another. This is described in more detail by e.g. Buck et al. (2011) and the Swedish Chemical Agency (2015).
This Opinion only considers those partially fluorinated substances that contain a ‐ CH2CH2‐ moiety between the hydrophilic part and the remaining fully fluorinated carbon chain: F(CF2)n‐CH2CH2‐X. These partially fluorinated compounds are called telomer substances and derive their name from the telomerisation production process (see Section 1.3.3). The telomerisation process results only in substances consisting of a linear alkyl chain with an even number of carbon atoms’, whereas the electrochemical process produces a mixture of branched (br‐PFAS) and linear (n‐PFAS) isomers (EFSA, 2008; Buck et al., 2011; Löfstedt Gilliam et al., 2016). In environmental and human samples, PFASs are found as a mixture of the linear and the branched isomers (De Silva and Mabury, 2006; Riddell et al., 2009; EFSA CONTAM Panel, 2018).
1.3.2. Methods of analysis
1.3.2.1. Analytical methods for PFASs
Analytical methods for PFASs were described in the Opinion on PFOS and PFOA in Food (EFSA CONTAM Panel, 2018). PFASs are normally measured alongside PFOS and PFOA as part of multi‐analyte methods. These do not always measure the same PFASs and some methods measure more compounds than others. Methods with best sensitivity and quality control usually involve stable isotope dilution (internal standards) and consist of extraction and clean‐up of food samples followed by determination using liquid chromatography (LC) coupled to quadrupole tandem mass spectrometry (LC‐MS/MS) with electrospray ionisation (ESI).
1.3.2.2. Analytical methods for determination of PFASs in biological samples
The instrumental detection of PFASs in biological samples is usually done in the same way as for food samples (EFSA CONTAM Panel, 2018). However, the sample preparation methods may differ substantially. It is common to make use of liquid–liquid extraction or off‐line solid‐phase extraction, but also quite a few laboratories apply direct analysis or on‐line column switching technology after protein precipitation (Jahnke and Berger, 2009; Salihovic et al., 2013).
1.3.3. Production and use of the substances
PFASs are reported to be, or have been, used in e.g. oil‐, water‐ and stain‐resistant coatings for clothing, personal protective equipment and workwear as well as leather and carpets; oil‐resistant coatings for food contact paper; aviation hydraulic fluids; fire‐fighting foams; paints, adhesives, waxes, polishes; in industrial applications as surfactants, emulsifiers and coatings and personal care products including cosmetics (see Section 3.2.4.2) (Kissa, 2001; Prevedouros et al., 2006; Lindstrom et al., 2011; Fujii et al., 2013; Wang et al., 2014a,b). Specific information relating to the volumes of production for each of these uses is not publically available.
Wang et al. (2014a) have further estimated the global cumulative emissions of C4–C14 PFCA homologues during 1951–2030. The estimations are presented as the outcome from two scenarios, low and high. For perfluorobutanoic acid (PFBA), global cumulative emissions (low and high) are estimated to 15 and 915 tonnes, whereof 47% and 26% are estimated to come from direct sources. Direct sources are here defined as all sources of individual PFASs through their whole product cycles (production to disposal), whereas indirect sources are those where the same compounds appear as a result of degradation, or other conversion process in the environment, of precursor compounds as described in Section 1.3.1. The corresponding figures for PFHpA, PFOA and PFTeDA are (low and high) 59 and 3,264 tonnes, 26% and 26%; 2,078 and 18,366 tonnes, 100% and 98%; and 0 and 19 tonnes, 0% and 1%, respectively. Comprehensive overviews of historic and current synthesis scheme of PFOA‐, PFOS‐ and common fluorotelomer‐based products applied by different companies are described by Wang et al. (2014a,b). Furthermore, estimated use of ammonium perfluorooctanoate/sodium perfluooctanoate (APFO/NaPFO) for fluorotelomer production is also given for different country groups. The estimates of consumption of APFO/NaPFO volumes for fluorotelomer production are decreasing in one group of countries: Western Europe, Japan and the US whereas it is indicated to increase in another group of countries: China, India, Poland and Russia. However, the total consumption during 2011–2015 is indicated to be in the same range, around 350 tonnes in the two country groups. In Wang et al. (2017a), an overview of the production, use and emissions of PFSAs from perfluorooctanesulfonyl fluoride (POSF)‐based products is given along with a description of the production pattern of POSF‐based substances.
FTOH‐based products have been produced since the 1970s and the global production in the early 2000s has been estimated to be between 5,000 and 6,500 tonnes per year (US EPA, 2002) but already in 2004, it was estimated to be around 12,000 tonnes per year. No more recent production volumes have been identified, but it is likely that the production has continued to increase.
According to the Swedish Chemical Agency (2015), there were at least 3,000 PFASs on the global market, and recently, OECD (2018) has identified 4,730 PFAS‐related CAS numbers. There is however just minor or no information on the production and use for most PFASs on the market, resulting in little or no understanding on how much has been, and will be, released, transformed and accumulated in the environment and biota over time.
1.3.4. Environmental fate
A wide range of PFASs have been used in numerous industrial applications and consumer products due to their unique properties such as the ability to create stable foams, chemical resistance and surface tension lowering properties (Buck et al., 2011; Kissa, 2001). Among the various PFASs, some groups of compounds are extremely persistent such as PFCAs and PFSAs (Kissa, 2001) while others are easily degraded in the environment and in humans (Ellis et al., 2004; Martin et al., 2006). Some of the non‐persistent compounds, e.g. PAPs, FTOHs and EtFASEs, are degraded to highly persistent PFASs such as PFCAs and PFSAs and are thus contributing to the exposure to these groups of persistent compounds (D'Eon and Mabury, 2007; Lee et al., 2010; Nabb et al., 2007; Peng et al., 2014). Due to their extensive use and high persistency in the environment, a wide range of PFASs have frequently been detected in the environment, as well as in wildlife and humans (Giesy and Kannan, 2001).
1.3.4.1. Release and distribution in the environment
PFASs can be released to the environment during production, use and disposal (Ahrens and Bundschuh, 2014). Municipal wastewater treatment plants and landfill waste sites represent important direct sources of PFASs in aquatic ecosystems, while atmospheric deposition is also a major contributor (Ahrens and Bundschuh, 2014). As many of these compounds are persistent and relatively water soluble, they may be transported long distances in water and also in aerosols (Ahrens et al., 2011a; Prevedouros et al., 2006). In addition, volatile precursors may undergo long‐range transport in the atmosphere (Benskin et al., 2011; Young and Mabury, 2010). One or more PFASs have been detected in more than 90% of all European rivers investigated and several PFASs have been detected in drinking water (Ahrens and Bundschuh, 2014).
1.3.4.2. Bioaccumulation in aquatic and terrestrial food chains
The octanol–water equilibrium coefficient is usually reflecting the bioaccumulation potential for fat soluble compounds, while for PFASs, the partitioning to serum proteins is likely to be one of the driving mechanisms (Kelly et al., 2009; Martin et al., 2003a,b). Gebbink et al. (2016) reported on field‐based sediment‐water distribution coefficients (K D) for PFSAs and PFCAs. The K D for PFSAs was found to increase from around 0.3 to 2.5 with increasing chain length between four to eight carbon atoms. For PFCAs, the corresponding increase was reported to be from around 2.5 to 4.7 with increasing chain length between 7 and 10 carbon atoms. The same authors also reported on bioaccumulation factors (BAFs) from water to Baltic Herring (Clupea harengus). For PFSAs, the BAF increased from 3.3 to 4.1 with increasing chain length from six to eight carbon atoms, whereas the corresponding increase for PFCAs was reported to be from around 2.0 to 5.3 with increasing chain length between 6 and 10 carbon atoms.
The bioaccumulation processes is more complex for terrestrial food chains. In a study by Stahl et al. (2009), it was demonstrated that PFASs are taken up by the roots from the soil, and Felizeter et al. (2012) reported that PFASs with longer chain lengths have a greater potential to bioaccumulate in hydroponically grown plants. Sorption to organic matter is also known to increase with chain length (Higgins and Luthy, 2006) and so the uptake of long‐chain PFASs may become limited by the fraction available in the pore water. Based on the strong bioaccumulation of several PFASs in humans (Olsen et al., 2007) and other mammals (Houde et al., 2006, 2011), it is no surprise that bioaccumulation of PFSAs and PFCAs is also important in farm animals. Biomagnification of PFNA, PFDA, PFUnDA and PFDoDA from lichen to caribou to wolf has been reported (Müeller et al., 2011), and it has been concluded that the trophic magnification factors are higher in the aquatic food webs compared to terrestrial food webs (Müeller et al., 2011).
Biotransformation and fate of PFASs are also described in Sections 1.3.1 ‘Chemistry’ and 3.3.1 ‘Toxicokinetics’.
1.3.4.3. Time trends
Concentrations of PFASs in environmental samples vary among others depending on compounds and sampling period. As an example, Table 2 summarises temporal variation of many PFASs from the Baltic Sea area and the Swedish mainland. A comprehensive review published in 2018 (Land et al., 2018) of the effects of phasing out long‐chain PFASs on their (and ‘possible precursors’) occurrence in the environment and how temporal variation of concentrations in abiotic and biological samples could be retrieved and analysed in a systematic way.
Table 2.
Temporal trends for a range of perfluoroalkyl substances in Grey seal (Halichoerus grypus) and Peregrine falcon (Falco peregrinus) (from Holmström et al., 2010 and Kratzer et al., 2011)
| Compound | Species/organ, period | |||
|---|---|---|---|---|
| Peregrine falcon/egg | Grey seal/liver | |||
| 1974–2007 | 2000–2007 | 1974–2008 | 1998–2008 | |
| FOSA | N.A. | N.A. | −−− | −−−a |
| PFNA | +++ | 0 | +++ | −− |
| PFDA | +++ | 0 | +++ | 0 |
| PFUnDA | +++ | 0 | +++ | 0 |
| PFDoDA | +++ | 0 | +++ | 0 |
| PFTrDA | +++ | − | +++ | 0 |
| PFTeDA | +++ | −− | +++ | +++ |
| PFPeDA | +++ | −− | N.A. | N.A. |
| PFHxS | +++ | 0 | −−− | −− |
| PFHpS | N.A. | N.A. | +++ | −− |
| PFDS | +++ | 0 | N.A. | N.A. |
| PFOSI | N.A. | N.A. | 0 | −−− |
1987–2008.
N.A. = Not available, +++ = increasing trend (p < 0.02), −−− = decreasing trend (p < 0.02), −− = decreasing trend (p > 0.02 but < 0.05), − = decreasing trend (p = 0.09), 0 = no significant trend. For Peregrine falcon log‐linear regression; for Grey seal two‐way ANOVA.
Note: These studies are also discussed with respect to temporal variation in Swedish Environmental Agency (2012).
1.3.4.4. Contamination of food
Two main processes are thought to lead to contamination of food with PFASs, namely bioaccumulation in aquatic and terrestrial food chains, and transfer from contact materials used in food processing and packaging (for further information on migration from food contact materials, including non‐stick coatings used on cookware see Section 3.1.4.1). Terrestrial food ingredients could of course also be contaminated directly via atmospheric deposition, but it is likely that this route could only be of importance close to substantial sources. It can be assumed that contamination from packaging and processing reflects current production and use of PFASs, while bioaccumulation is regarded to reflect long‐term use. Knowledge on the relative contribution from bioaccumulation and food processing is useful for i. a. prediction of possible future dietary exposure.
1.3.4.5. Levels of PFASs in environmental samples useful for human exposure assessment
Information on the occurrence of PFASs in the environment could also be considered in the human exposure assessment, especially when data on occurrence in food are scarce or lacking. Occurrence of individual PFASs in ground and surface water, plants and wildlife such as mammals, birds, fish and shellfish, could raise concern as such data indicate that these compounds are also likely to be found in food originating from similar environmental habitats. This concern could be strengthened when environmental monitoring indicates increasing temporal trends of individual PFASs.
In Section 3.2.1 (Current exposure assessment), it is described that in addition to PFOA and PFOS, a dietary chronic exposure assessment has been performed for PFBA, PFPeA, PFHxA, PFHpA, PFNA, PFDA, PFUnDA, PFDoDA, PFTrDA, PFTeDA, PFBS, PFHxS, PFHpS, PFDS and FOSA. Due to a very high proportion of results below the limit of detection/quantification (LOD/LOQ) (around 99%) and/or only limited availability of data, exposure calculations for PFBA, PFHpA, PFTeDA and PFBS, PFHpS, PFDS should be considered only as a rough indication of the range of chronic dietary exposure. For the remaining PFASs; PFPeDA, PFHxDA, PFODA, PFOSI, 8:2 FTOH, 8:2 monoPAP, 8:2 diPAP, EtFOSA, EtFOSE and FC‐807 no exposure assessment could be carried out.
Considering this categorisation, information on the occurrence of PFBA, PFHpA, PFTeDA, PFPeDA, PFHxDA, PFODA, PFBS, PFHpS, PFDS, PFOSI, 8:2 FTOH, 8:2 monoPAP, 8:2 diPAP, EtFOSA, EtFOSE and FC‐807 in the environment could be of importance for the recommendations on future actions. The compilation below is therefore restricted to these compounds. Occurrence of PFOS and PFOA in the environment was described in the previous Opinion (EFSA CONTAM Panel, 2018).
1.3.4.5.1. Levels and temporal trends of PFASs for which none or only rough exposure assessment was performed
PFBA
PFBA has been found in the environment in Europe and the USA in an array of matrices, such as groundwater, wastewater, plants, limnic biota and birds’ liver (Campo et al., 2015; Chu et al., 2015; Eschauzier et al., 2013; Gobelius et al., 2017). In a study by Campo et al. (2014), PFBA was found in wastewater in concentrations up to 20.5 ng/L with an increasing temporal trend. Eschauzier et al. (2013) reported extremely high concentrations in groundwater, up to 1,208 ng/L from the Netherlands. Potential point sources in the area are a former landfill and a military camp with an airbase. Möller et al. (2010) reported on PFASs in the Rhine/Meuse delta but also River Rhine from Constance to its estuary. Downstream from Leverkusen, the authors reported a profound increase of the concentration of many PFASs but particularly PFBS and PFBA. This increase became even more obvious upstream from Rees. These increases are probably due to impact from local sources. The maximum concentration of PFBA recorded in the Rhine/Meuse delta was 330 ng/L as estimated from the author's figure.
Zhang et al. (2017) reported on PFBA in food from a contaminated site and a site without known contamination. The concentration in legumes and leafy vegetables from the contaminated site was up to 200 and 1,000 ng/g ww, respectively, whereas the concentrations in the same vegetables from the non‐contaminated site were 1 ng/g ww or lower. The relative contribution from different food categories was estimated to be almost 100%, as attributed to leafy vegetables in the contaminated area whereas some 85% was attributed to legumes in the non‐contaminated area. Gobelius et al. (2017) analysed sap from birch (Betula pendula) sampled in the vicinity of fire training facilities. Concentrations up to 0.17 ng/L, corresponding to bioconcentration factors (related to concentration in ground water) up to 0.03 were reported. In a study by García‐Valcárcel et al. (2014) on Great Brome (Anisantha diandra), the ratio between the concentration in the plant and that in the nutrient solution (0.5 μg/mL) was reported to be in the order of 0.258–5.65 depending on exposure time (1–20 days, respectively). In a study by Blaine et al. (2014), celery was grown in biosolid‐amended soil. Mean concentration in shoots was reported to be 232 ng/g. Different species of fish from Spain, prepared as total homogenates, showed a mean concentration of 0.6 ng/g ww and the maximum concentration was 4.92 ng/g ww (Lorenzo et al., 2016). In liver from Baltic herring (Clupea harengus) and flounder (Platichthus flesus), PFBA has been reported at concentrations up to 0.23 and 0.45 ng/g ww, respectively (Lilja et al., 2009). Mink (Neovison vison), which to a great extent is a fish‐eating mammal, sampled in Sweden had mean and maximum concentrations in the liver of 0.6 ng/g ww and 4.7 ng/g ww, respectively (Persson et al., 2013). Plasma samples from another fish‐eating species, Bald eagle (Haliaeetus leucocephalus), collected in the USA, were analysed to determine PFBA, and mean and maximum concentrations of 0.55 and 78 ng/mL, respectively, were reported (Route et al., 2014).
PFHpA
More than 50 reports on PFHpA levels in the environment have been found in the scientific literature and these reports demonstrate its worldwide occurrence in the environment, e.g. groundwater, surface water, seawater, sediment but also wastewater. Eschauzier et al. (2013) reported up to 320 ng/L in groundwater from the Netherlands. Potential point sources in the area are a former landfill and a military camp with an airbase.
In a study by Blaine et al. (2014), celery (Apium graveolens) was grown in biosolid‐amended soil. The mean concentration in shoots was reported to be 20 ng/g ww. Gobelius et al. (2017) analysed sap from birch (Betula pendula) sampled in the vicinity of fire training facilities. Concentrations up to 0.80 ng/L, corresponding to a bioconcentration factor (related to concentration in ground water) of 0.072, were reported. Zhang et al. (2017) report on PFHpA in food from a contaminated site and a site without known contamination. The authors calculated that the total daily intake by adults (fish liver not considered due to lack of data) from the contaminated site was 2.94 ng/kg bw per day and 0.003 ng/kg bw per day at the non‐contaminated site. The relative contribution from different food categories was estimated, with almost 100% being attributed to leafy vegetables in the contaminated area whereas some 50% was attributed to each leafy vegetables and offal in the non‐contaminated area.
PFHpA has also been detected in biological organisms such as decapods, mussels, fish, birds and mammals (Butt et al., 2008; Furdui et al., 2007; Bytingsvik et al., 2013; Baduel et al., 2014; Blaine et al., 2014; Fang et al., 2014; Arvaniti and Stasinakis., 2015; Campo et al., 2015; Armstrong et al., 2016; Cerveny et al., 2016; Gebbink et al., 2016). Mean concentrations of PFHpA in Baltic Herring (Clupea harengus) and Sprat (Sprattus sprattus) were reported to be around 0.025 ng/g ww, whereas maximum concentrations in Lake trout (Salvelinus namaycush) whole body homogenate from the US and European Chub (Squalius cephalus) liver sampled in Poland were reported to be 1.43 and 27.2 pg/g, respectively. There is no obvious temporal trend for the occurrence of PFHpA in the environment, but the concentrations in biosolids in the US and Spain have been reported to decrease during 2005–2013 and 2010–2011 by Armstrong et al. (2016) and Campo et al. (2014), respectively. In a systematic review and compilation of data (including recalculations) performed by Land et al. (2018), most studies of biota and indoor air and dust show decreasing or non‐significant temporal trends. However, two out of three Chinese lake sediments show significant increasing temporal trends.
PFTeDA
Around 25 scientific reports have reported on levels of PFTeDA in the environment. These studies contain results from a worldwide study of wastewater and biosolids (maximum concentration 20 ng/L and 50 ng/g, respectively (Arvaniti and Stasinakis, 2015)), as well as a French study (Munoz et al., 2015) of river sediments showing a maximum concentration of 1.3 ng/g dry weight (dw). Occurrence in biota is reported from Europe including Greenland, North America, Oceania and Asia. In Europe, mean concentrations of PFTeDA in Baltic Herring (Clupea harengus) and Sprat (Sprattus sprattus), analysed by Gebbink et al. (2016), were 0.0087 and 0.0027 ng/g ww, respectively. Roos et al. (2013) studied Swedish Otters (Lutra lutra) and found a maximum concentration in liver of 11 ng/g ww. The same authors reported on a rapid increase in the concentrations of PFTeDA in Otter liver from 1974 onwards. This increase is supported by results from a study by Kratzer et al. (2011) where increasing concentrations were observed between 1974 and 2008 in Grey Seal (Halichoerus grypus) from the Baltic Sea area. The maximum concentration in liver in this study was 0.7 ng/g ww. Eggs (yolk only) from Swedish Cormorants (Phalacrocorax carbo), a fish‐eating species, were shown to contain, on average, 10.4 ng/g ww with a maximum of 32.9 ng/g ww (Nordén et al., 2013). Holmström et al. (2010) reported on the occurrence of PFTeDA in Peregrine falcon (Falco peregrinus) eggs from 1974 to 2007 and identified an increasing trend up to 2004. The increase during this period was from 0.2 to 3.2 ng/g ww, as read from the author's figure.
PFPeDA
PFPeDA is mainly identified in biological samples from Europe, including Greenland, and the US. Furdui et al. (2007) analysed whole fish homogenates of Lake Trout from Lake Huron and found a mean concentration of 1.10 ng/g ww. In Baltic Herring (Clupea harengus) and Sprat (Sprattus sprattus), analysed by Gebbink et al. (2016), the concentrations were almost three orders of magnitude lower, while egg yolk from Swedish Cormorants (Phalacrocorax carbo), a fish‐eating species, were shown to contain on average 3.23 ng/g ww with a maximum of 24.9 ng/g ww (Nordén et al., 2013). There was no evident temporal trend in the occurrence of PFPeDA in the environment.
PFHxDA
Campo et al. (2014) has presented results from analyses of wastewater and WWTP sludge in Spain. The concentrations in 2011 were 0.04 ng/L and 0.13 ng/g dw, respectively. In another study, Campo et al. (2015) reported a much higher maximum concentration in the Llobregat River system (Spain), being 4.25 ng/L.
There are also a few reports on PFHxDA in biota. Chu et al. (2015) studied PFHxDA in liver, muscle and adipose tissue of Black‐footed Albatross (Phoebastria nigripes) at Midway Atoll. The authors reported concentrations in liver up to 0.14 ng/g ww. Muscle and adipose tissue samples were all below LOQ (0.08 ng/g). Letcher et al. (2015) assessed PFASs in Herring Gull (Larus argentatus) eggs at 19 locations around the Great Lakes and found mean concentrations of PFHxDA up to 0.15 ng/g ww (maximum concentration 0.24 ng/g ww). Rubarth et al. (2011) found PFHxDA in Red‐throated Divers (Gavia stellata), a mainly fish‐eating bird, but no numbers were presented due to sparse and irregular detection.
PFODA
Campo et al. (2014) identified PFODA in influent and effluent wastewater at Spanish Sewage treatment plants (maximum concentrations 300 and 160 ng/L, respectively) in 2011. In 2010, PFODA was not detected at the same plants, which could indicate an increasing temporal trend in this matrix.
Letcher et al. (2015) studied PFASs in Herring Gull (Larus argentatus) eggs at 19 locations at the Great Lakes and found PFODA in only two out of 114 samples. The concentrations were above LOD (0.012 ng/g ww) but below LOQ (0.030 ng/g ww).
PFBS
PFBS has been identified in the environment in more than 30 studies from Europe, North America, Asia and Africa. In a worldwide study on PFASs in wastewater treatment plants, Arvaniti and Stasinakis (2015) reported concentrations as high as 100 ng/L in wastewater and 80 ng/g ww in biosolids. Möller et al. (2010) assessed PFASs in water from the Rhine/Meuse delta but also River Rhine from Constance to its estuary. Downstream Leverkusen, the authors report a profound increase of the concentration of many PFASs but particularly PFBS and PFBA. This increase became even more obvious upstream Rees. These increases are probably due to the impact from local sources. The maximum concentration of PFBS recorded in the Rhine/Meuse delta was 115 ng/L, as estimated from a figure. Even higher concentrations of PFBS, up to 2,900 ng/L, were reported from the Rhine tributary Aare (Lange et al., 2007 as cited by Möller et al., 2010). Möller et al. (2010) estimated a yearly mass flow of around 17 tonnes PFASs from the River Rhine into the delta and further into the North Sea, whereof PFBS represented 5.1 tonnes. In a study from the Netherlands, Eschauzier et al. (2013) reported concentrations in groundwater up to 104 ng/L.
PFBS has also been shown to be available to plants via the root system. Blaine et al. (2014) performed a study on the uptake into pea shoots grown in soil amended with biosolids, and the mean concentration in the shoots was 150 ng/g ww. Gobelius et al. (2017) analysed sap from birch (Betula pendula) sampled in the vicinity of fire training facilities. Concentrations up to 6.2 ng/L, corresponding to bioconcentration factors (related to concentration in ground water) up to 0.027 were reported.
Different species of fish from Spain had a mean and maximum concentration of 4.9 ng/g ww and 7.3 ng/g ww, respectively (Lorenzo et al., 2016). In Germany, PFBS has been detected in Harbour Seal (Phoca vitulina) liver (maximum concentration 3.1 ng/g ww) (Ahrens et al., 2009). In the same study, a decreasing temporal trend has been observed since 2002. In a systematic review and compilation of data (including recalculations) performed by Land et al. (2018), studies on biota sediments, as well as fresh‐ and seawater samples showed non‐significant temporal trends.
PFHpS
There are around 10 studies reporting on the environmental occurrence of PFHpS. These studies contain results from worldwide studies of wastewater and biosolids (maximum concentrations 10 ng/L and 100 ng/g, respectively (Arvaniti and Stasinakis, 2015)), as well as a French study of river water and sediments showing maximum concentrations of 17 ng/L and 0.15 ng/g dw, respectively.
There are also a few studies on the occurrence of PFHpS in biota. Ahrens et al. (2011b) reported decreasing trends in the concentrations of PFHpS in Tawny Owl (Strix aluco) eggs sampled between 1986 and 2009. The mean concentration in eggs was 0.08 ng/g ww. A decreasing temporal trend was also reported by Ahrens et al. (2009) in a study from Germany on Harbor Seal (Phoca vitulina) liver, but here the maximum concentration was as high as 53 ng/g ww. The reported decreasing temporal trend as described above, is to some extent supported by observations by Kratzer et al. (2011) studying Grey Seal (Halichoerus grypus) in the Baltic Sea area during 1974–2008, where a maximum concentration in liver of 1.3 ng/g ww was recorded in 2000.
PFDS
Occurrence of PFDS in the environment has been reported from Europe including Greenland, North America and one worldwide study. The worldwide study by Arvaniti and Stasinakis (2015) covers PFASs in wastewater treatment plants, and they report concentrations up to 150 ng/L in wastewater and 40 ng/g ww in biosolids.
In biota, PFDS has been found in German Harbor seal (Phoca vitulina) liver (maximum concentration 4.1 ng/g ww), in a study by Ahrens et al. (2009). In the same study, an increasing trend was reported from 2002 onwards. In the US, Lake trout (Salvelinus namaycush) from Lake Erie showed a mean concentration of 9.8 ng/g ww (Furdui et al., 2007). Bald eagle plasma samples (Haliaeetus leucocephalus) from the USA were analysed for PFDS by Route et al. (2014) and mean and maximum concentrations of 265 and 1,400 ng/mL were found, respectively. In Sweden, Roos et al. (2013) reported on increasing concentrations in Otter (Lutra lutra) liver between 1972 and 2011. On the other hand, Ahrens et al. (2011b) and Ullah et al. (2014) observed decreasing trends in Tawny Owl (Strix aluco) eggs and Herring (Clupea harengus) liver during 1986–2009 and 1991–2011, respectively. The inconsistent outcome of studies of environmental temporal trends is confirmed by a systematic review and compilation of data (including recalculations) performed by Land et al. (2018).
PFOSI
Perfluorooctane sulfinic acid (and perfluorooctane sulfinate) was considered in a small number of studies. Liver from Harbour seals sampled in Germany was reported to contain up to 1.7 ng/g ww (Ahrens et al., 2009). Kratzer et al. (2011) assessed PFOSI concentrations in Grey Seal (Halichoerus grypus) from the Baltic Sea area during 1974–2008 and reported a maximum concentration in liver of 0.5 ng/g ww in the sample from 1998. Statistically significantly increasing concentrations were observed until 1997, while a statistically significant decrease was seen during 1997–2008.
8:2 FTOH
Environmental occurrence of 8:2 FTOH was studied by a number of research groups. Gewurtz et al. (2013) reported concentrations in air sampled in Arctic Canada (Alert, Nunavit) between 1 and 10 pg/m3, as estimated from the author's figure. Del Vento et al. (2012) analysed a number of PFASs in eight atmospheric samples from western Antarctic Peninsula and found 8:2 FTOH in all samples ranging from 3.9 to 15.4 pg/m3, with a mean of 9.9 pg/m3. Dreyer et al. (2009) collected high volume air samples on‐board research vessels in the Atlantic Ocean and the Southern Ocean in 2007 and 2008. The samples were analysed for a set of PFASs. The concentrations of 8:2 FTOH were between 1.8 and 130 pg/m3, and among the compounds studied, 8:2 FTOH was the analyte that usually was observed at the highest concentrations. Furthermore, the highest concentrations were recorded close to European source regions, whereas the lowest concentrations were found in samples from the Southern Ocean.
These concentrations could be compared with concentrations reported from indoor air, e.g. in Shoeib et al. (2011) in Vancouver, where the geometrical mean was 2,900 pg/m3.
It should also be noted that despite the presence of 8:2 FTOH in many environmental matrices, it may also undergo degradation in the environment. For instance, Wang et al. (2009) reported that 8:2 FTOH could be biodegraded in different aerobic soils, with a half‐life generally less than 7 days. Depending on soil characteristics, around 10–30% was shown to be degraded into PFOA.
8:2 monoPaP
No relevant information on the occurrence in the environment was identified.
8:2 diPaP
Very few results above LOD/LOQ have been identified in the literature. Eriksson et al. (2016) reported the maximum concentration of 8:2 diPAP in eggs of Swedish Common kestrels (Falco tinnunculus) to be 0.16 ng/g ww, and Gebbink et al. (2016) reported the mean concentration in zooplankton in the Baltic Sea to be 0.057 ng/g ww. No temporal trend was identified.
EtFOSA
EtFOSA has been identified in a number of biological matrices. Taniyasu et al. (2013) studied its occurrence in blood samples from wild rats and mice in Japan (Rattus rattus, R. norvegicus and Apodemus specious). EtFOSA (reported as its acetate, N‐EtFOSAA) was identified in most samples and the maximum concentration in rats was 102 ng/mL. Swedish Guillemot (Uria aalge) eggs and Grey seal (Halichoerus grypus) livers were analysed by Löfstrand et al. (2008) and Kratzer et al. (2011), respectively. The highest concentration in Guillemot eggs was 1.1 ng/g ww, while the geometric mean concentration in Grey seal was 0.03 ng/g ww.
EtFOSE
EtFOSE has often been identified in atmospheric samples. One study in Canada by Gewurtz et al. (2013) reported a mean concentration in ambient air to around 3 pg/m3 (as estimated from an author's figure) and another Canadian study by Ahrens et al. (2011c) reported a mean concentration of 20 pg/m3 in air inside a wastewater treatment plant. Shoeib et al. (2006) collected in 2005 20 high‐volume air samples during a crossing of the North Atlantic and Canadian Archipelago. The concentrations ranged from < 1 to 8.9 pg/m3. Dreyer et al. (2009) collected high volume air samples on‐board research vessels in the Atlantic Ocean and the Southern Ocean in 2007 and 2008. The samples were analysed for a set of PFASs. The average concentrations of EtFOSE in the gas phase and the particle phase from the Northern hemisphere were 0.7 and 0.5 pg/m3, respectively. The corresponding figures for the Southern hemisphere were 0.6 and 0.3 pg/m3, respectively.
FC‐807
No relevant information on the occurrence in the environment was identified.
1.3.4.5.2. Temporal environmental trends of PFASs for which exposure assessment was performed
In a recent review by Land et al. (2018), environmental trends have been reviewed for nine PFASs, as follows:
PFPeA and PFHxA; no temporal trends for PFPeA or PFHxA were reported. However, Wang et al. (2013a) reported that it is likely that emissions of PFHxA could occur as a result of increasing use of polymers based on 6:2 FTOHs in surface treatment products, as this compound could undergo environmental degradation to PFHxA but also to PFBA and PFBS.
PFNA; concentrations in biota generally show an increasing trend (21 studies) or no significant trend (19 studies), whereas one study on Beluga Whales (Delphinapterus leucas) showed a decreasing trend.
PFDA; concentrations in biota generally show an increasing trend (21 studies) or no significant trend (20 studies), whereas one study on Beluga whales (Delphinapterus leucas) showed a decreasing trend.
PFUnDA; concentrations in biota generally show an increasing trend (22 studies) or no significant trend (nine studies). Just one study Bald eagle nestlings (Haliaeetus leucocephalus) showed a decreasing trend but this study had a short duration.
PFDoDA; concentrations in biota generally show an increasing trend (13 studies) or no significant trend (13 studies), whereas one study on Beluga Whales (Delphinapterus leucas) showed a decreasing trend since 2000.
PFTrDA; concentrations in biota generally show an increasing trend (14 studies) or no significant trend (13 studies), whereas one study on Beluga Whales (Delphinapterus leucas) shows a decreasing trend since 2000 and another study on Harbor seals (Phoca vitulina) since 2004.
PFHxS; concentrations in biota show an increasing trend in five studies whereas no significant trends were observed in 23 studies. In one study on Loggerhead sea turtle (Caretta caretta), a previously decreasing temporal trend was in 2000 replaced by an increasing temporal trend as high as 40% per year. On the other hand, significant decreasing temporal trends were reported in one study of fish (Swedish west coast) and two studies of seals (German Bight and Greenland) and one study of Harbor porpoise (North Sea). These results illustrate that the temporal trends could vary in a considerable way between different geographical areas.
FOSA; two studies showed increasing temporal trends, whereas eight showed decreasing and 14 showed no significant temporal trends. This is similar to what was observed in corresponding analyses for PFOS.
1.3.4.6. Summary
PFASs constitute a vast and complex group with respect to differences in i.a. chain‐length, molecular weight, degree and pattern of fluorination and polar functional groups. Therefore, it is probably not meaningful to generalise their properties or environmental fate. From the compilations presented above, it is clear that most of the substances selected can be found in environmental samples. These matrices are more or less closely related to food. Matrices as fish and fish‐eating birds or mammals could to a large extent serve as indicators of food contamination. Reliable information on the occurrence in such matrices are found for PFBA, PFHpA, PFTeDA, PFPeDA, PFBS, PFHpS, PFDS, PFOSI and FOSA and possibly also for EtFOSA, PFHxDA and 8:2 diPaP. There are consequently good arguments for further investigations of the occurrence of these compounds in food. The presence of PFASs in groundwater and other freshwater bodies could be an indicator for possible occurrence in drinking water. Reliable information on the occurrence in these matrices is found for PFBA, PFHpA, PFBS and PFHpS. Likewise, these findings support further investigations of the occurrence of these compounds in drinking water.
From the compilation of observations of compounds in this Opinion, categorised to have sufficient data for conducting a dietary exposure assessment, it can be concluded that the concentrations of PFASs in the environment show a generally increasing temporal trend. This should be considered when estimating human dietary exposure to these compounds.
Regarding the 16 compounds presented in more detail in this section, an important observation to be further considered is the increasing temporal trends of some compounds in relevant environmental matrices. Matrices considered relevant are those that, with respect to their content and spatial and/or temporal concentration trends of contaminants, could be associated with the occurrence of the same contaminants in food. Such matrices are e.g. biota inhabiting the same or similar environmental matrices as biota used as food. This could be the case for fish, marine vertebrates and wild mammals. Regarding drinking water, surface‐ and groundwater, and to some extent also freshwater sediments could be relevant matrices.
Unfortunately, very few studies are performed in such a way that reliable temporal trends could be identified. However, increasing temporal trends in relevant matrices have been identified for PFTeDA and EtFOSA. An increasing trend was also identified for PFODA in wastewater.
One further factor that needs attention is the long‐range transport properties of the compounds (usually based on their detection in remote areas). This is the case for PFBA, PFHpA, PFTeDA, PFPeDA, PFBS and PFDS. It is therefore likely that these compounds are able to stay for a long time in the environment, which could increase the probability for finding these compounds in food.
Finally, substances that have been regulated (banned or with reductions of their production volumes and/or restrictions to certain applications only) generally show a reduction in their occurrence in environmental matrices. As mentioned above and seen from Table 2, different environmental matrices show different temporal trends, indicating possible differences in environmental transport but also in kinetics of the compounds in animal species studied that could influence the outcome.
1.3.5. Previous risk assessments
This chapter reviews risk assessments for PFOS and PFOA that have been published after the 2018 EFSA Contam Panel Opinion, and previous risk assessments for PFASs other than PFOS and PFOA.
In 2018, the EFSA CONTAM Panel established a tolerable weekly intake (TWI) of 13 ng/kg bw per week for PFOS and 6 ng/kg bw per week for PFOA. The derivation of TWIs was based on human epidemiological studies. For PFOS, the increase in serum total cholesterol in adults and the decrease in antibody response at vaccination in children were identified as the critical effects. For PFOA, the increase in serum total cholesterol was the critical effect. Also reduced birth weight (for both compounds) and increased prevalence of high serum levels of the liver enzyme alanine aminotransferase (ALT) (for PFOA) were considered. Due to the nature of the scientific uncertainties described in the EFSA CONTAM Panel (2018) Opinion and the possible application of the forthcoming Scientific Committee guidance on combined exposure to multiple chemicals, it was stated in a explanatory note that the conclusions of this assessment will be reviewed, also taking into account PFASs other than PFOS and PFOA. Until such time, the derived TWIs shall be considered provisional.
In 2012, the Swedish Environmental Protection Agency published an Environmental and Health Risk Assessment of 23 PFASs in Sweden. Hepatotoxicity and reproductive toxicity were identified and selected as toxicological endpoints common for PFASs. For PFASs lacking internal dose measurements or toxicity data (PFHxA, PFHpA, PFDA, PFUnDA, PFDoDA, PFTrDA, PFTeDA, 6:2 FTSA, PFBS, PFDS and FOSA), a read‐across was carried out to the closest most potent congener for the respective endpoint. For the general population, the risk characterisation did not indicate any cause of concern for reproductive toxicity or hepatotoxicity, neither for most assessed individually nor in combination. In the general population, for hepatotoxicity, RCRs (risk characterisation ratios) were highest for PFOS (0.17) and PFOA (0.04) contributing in total with 77% to the cumulative RCRs. For PFNA and PFHxS, RCRs of 0.014 and 0.0048 were calculated. For reproductive toxicity, the highest RCR was identified for PFOS (0.14), contributing with 76% to the cumulative RCR. RCRs for PFOA, PFNA and PFHxS were, respectively, 0.008, 0.0073 and < 0.0035 (Swedish Environmental Protection Agency, 2012).
In 2015, the Danish Environmental Protection Agency stated that for FOSA, no sufficient data were available for derivation of a specific tolerable daily intake (TDI). However, because FOSA is the amide derivate of PFOS and is used as a precursor for PFOS formation, the Danish Environmental Protection Agency concluded that it seems justifiable to apply the TDI for PFOS (0.03 μg/kg bw per day) on FOSA as well (Danish EPA, 2015).
The French Agency for Food, Environmental and Occupational Health & Safety (ANSES) published an Opinion on PFBA, PFHxA, PFBS and PFHxS (ANSES, 2017). Chronic indicative toxicity values (iTV) were established for PFBA (0.024 mg/kg bw per day) and PFHxS (0.004 mg/kg bw per day) based on liver effects (increase in absolute and relative liver weight, hepatocellular hypertrophy) in rodents. Chronic toxicity reference values (TRV) were established for PFHxA (0.32 mg/kg bw per day) and PFBS (0.08 mg/kg bw per day) based on kidney effects in rodents.
In 2017, the German Environment Agency, following consultation with the German Drinking Water Commission, recommended the following water guide values, (TWLW, Trinkwasser‐Leitwerte) for PFBA (TWLW 10 μg/L), PFHxA (TWLW 6 μg/L), PFHpA (TWLW 10 μg/L), PFOA (TWLW 0.1 μg/L), PFNA (TWLW 0.06 μg/L), PFBS (TWLW 6 μg/L), PFHxS (TWLW 0.1 μg/L), PFOS (TWLW 0.1 μg/L) or health‐based orientation values (GOWs, gesundheitliche Orientierungswerte) for PFPeA (GOW 3.0 μg/L), PFHpA (GOW 0.3 μg/L), PFDA (GOW 0.1 μg/L), PFHPs (GOW 0.3 μg/L) and FOSA (GOW 0.1 μg/L) (Bundesgesundheitsblatt 2017, 60:350–352).
In 2017, FSANZ (Food Standards Australia New Zealand) published a hazard assessment report for PFOS, PFOA and PFHxS. With respect to PFHxS, FSANZ stated that there was insufficient toxicological and epidemiological information to justify establishing a TDI. They concluded that the Environmental Health Standing Committee (enHealth) 2016 approach of using the TDI for PFOS (0.02 μg/kg bw per day based on decreased parental and offspring body weight gains in a multi‐generation reproductive toxicity study in rats) is likely to be conservative and protective of public health as an interim measure. For PFOA, a TDI 0.16 μg/kg bw per day based on an NOAEL for fetal toxicity in a developmental and reproductive study in mice was recommended (FSANZ, 2017).
In 2017, the Department of Environmental Protection (New Jersey, US) developed a health‐based maximum contaminant level (MCL) for PFOA of 0.014 μg/L using a risk assessment approach intended to protect for chronic (lifetime) drinking water exposure (DEP, 02/2017). For PFOS, an MCL of 0.013 μg/L has been proposed (DEP, 11/2017) and for PFNA an MCL of 0.013 μg/L. The PFNA MCL is based on increased liver weight (BMD10) in pregnant mice exposed to PFNA for 16 days (DEP 10/2017).
In 2018, ATSDR has prepared a draft for public comment on the Toxicological Profile for 14 PFASs (PFOS, PFOA, PFBA, PFHxA, PFHpA, PFNA, PFDA, PFUnDA, PFDoDA, PFBS, PFHxS, FOSA, 2‐(N‐methyl‐perfluorooctanesulfonamido)acetic acid, 2‐(N‐ethyl‐perfluorooctane‐sulfon‐amido)acetic acid. The document summarises that sufficient data are available for derivation of provisional intermediate duration oral maximum residue levels (MRLs) for PFOA, PFOS, PFHxS and PFNA based on laboratory animal data. The databases were not considered adequate for derivation of MRLs for the other PFASs. Hepatic, immune and developmental endpoints were the most sensitive targets for PFOA (MRL 0.003 μg/kg per day) and PFOS (MRL 0.002 μg/kg per day), hepatic (increased liver weight and centrilobular hepatocellular hypertrophy) and thyroid endpoints (thyroid follicular cell damage, hypertrophy and hyperplasia) for PFHxS (MRL 0.002 μg/kg per day) and decreased body weight gain and developmental delays in the offspring for PFNA (MRL 0.003 μg/kg per day).
In 2018, RIVM published a Relative Potency Factor (RPF) approach for 19 PFASs and selected PFOA as the Index Compound (Zeilmaker et al., 2018). Liver toxicity in rodents, as revealed by liver hypertrophy (hepatocellular, centrilobular) and accompanying liver enlargement, i.e. absolute and relative liver weight, was selected as the critical effect and the basis to derive RPFs for all 19 PFASs. For PFASs with no experimental animal data available for the selected endpoint a read across approach was applied. The assessed PFAS (with the respective RPF reported in bracket) were: PFBA (0.05), PFPeA (0.01–0.05), PFHxA (0.01), PFHpA (0.01–1), PFOA (1), PFNA (10), PFDA (4–10), PFunDA (4), PFDoDA (3), PFTrDA (0.3–3), PFTeDA (0.3), PFHxDA (0.02), PFODA (0.02), PFBS (0.001), PFPeS (0.001–0.6), PFHxS (0.6), PFHpS (0.6–2), PFOS (2).
The German Human Biomonitoring (HBM) Commission at the German Environment Agency has derived HBM‐I and ‐II values for the health‐related assessment of human biomonitoring data for PFOA and PFOS. The HBM‐I value corresponds to the concentration of a substance in human biological material below which, according to the current status of assessment, no adverse health effects are to be expected. It is set at 2 ng PFOA/mL and 5 ng PFOS/mL in blood serum or plasma, respectively (HBM, 2018). The HBM‐II value corresponds to the concentration of a substance in human biological material which, when exceeded, may lead to health impairment which is considered as relevant to affected individuals. The HBM Commission established HBM‐II values for women at child‐bearing age of 5 ng PFOA/mL blood plasma and 10 ng PFOS/mL blood plasma such as for all other population groups of 10 ng PFOA/mL blood plasma and 20 ng PFOS/mL blood plasma. Both the HBM‐I and the HBM‐II values for PFOA and PFOS are based on the assessment of the population‐related risk of changes in selected effect indicators (HBM, 2020).
Michigan Science Advisory Workgroup recommended health‐based drinking water values (HBV) for six PFASs (Michigan Science Advisory Workgroup 71, 2019). The recommended HBV for PFHxA (400,000 ng/L), PFOA (8 ng/L), PFNA (6 ng/L), PFBS (420 ng/L), PFHxS (51 ng/L) and PFOS (16 ng/L) were based on experimental animal studies. It is also stated in the document that based on the similarity in toxicity for the long‐chain PFASs, the Workgroup recommends the use of the established HBV for PFNA as a screening value for other long‐chain PFASs, which are included in the US EPA method 537.1 but for which so far not enough information is available to support HBVs.
1.3.6. Legislation
The legal status for PFOS and PFOA was summarised in the Scientific Opinion on the risk to human health related to the presence of perfluorooctanesulfonic acid and perfluorooctanoic acid in food (EFSA CONTAM Panel, 2018). PFOS, including its salts and perfluorooctane sulfonyl fluoride (PFOSF), is now listed in Annex I of the POP Regulation (Regulation (EU) 2019/1021) and added to Annex B (Restriction) to the Stockholm Convention. These documents are relevant for considerations of whether other PFAS are to be considered as PFOS‐related substances e.g. as precursors. The definition of PFOS‐related substances is described in ‘Guidance on PFOS, its salts and PFOSF.5’ According to Commission Regulation (EU) No 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food,6 PFOS is not allowed for use in the production of plastics food contact materials (FCM).
Regarding PFOA, in FCM made of plastic, regulation (EU) No 10/2011 is applicable. According to it, the use of PFOA (ammonium salt) as an additive or as polymer production aid is allowed with the specification to only be used in repeated use articles sintered at high temperatures. Furthermore, in 2013, the Member State Committee, referred to in Article 76(1)(e) of Regulation (EC) No 1907/2006, identified PFOA as a persistent, bioaccumulative and toxic (PBT) substance, in accordance with Article 57(d) of that Regulation. PFOA was also in 2013 included in the Candidate List of Substances of Very High Concern (SVHC), for possible inclusion into Annex XIV to Regulation (EC) No 1907/2006. By means of Commission Regulation (EU) 2017/1000, PFOA was included in Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as regards PFOA, its salts and PFOA‐related substances. In May 2019, the COP (Conference of Parties) of the Stockholm Convention adopted a corresponding, but slightly more comprehensive, regulation of PFOA. In November 2019, the Commission presented a proposal on how to amend Annex I to Regulation (EU) 2019/1021 of the European Parliament and of the Council as regards the listing of perfluorooctanoic acid (PFOA), its salts and PFOA‐related compounds. Deadline for responses from member states was 5 December 2019 and the regulation will come into force on 4 July 20207 . This regulation would open the field for classifying certain PFAS, e.g. as precursors, as PFOA‐related based on their structural properties. The term PFOA‐related compounds have been defined by the Persistent Organic Pollutants Review Committee (POPRC)8 as:
‘(a) PFOA (pentadecafluorooctanoic acid, CAS No: 335‐67-1, EC No: 206‐397-9) including any of its branched isomers;
(b) Its salts; and
(c) PFOA‐related compounds which, for the purposes of this risk management evaluation, are any substances that degrade to PFOA, including any substances (including salts and polymers) having a linear or branched perfluoroheptyl group with the moiety (C7F15)C as one of the structural elements, for example:
(i) Polymers with ≥ C8 based perfluoroalkyl side chains;
(ii) 8:2 fluorotelomer compounds;
(iii) 10:2 fluorotelomer compounds.’
In practice, this makes it possible to classify a number of PFASs as PFOA‐ or PFOS‐related. This is described in more detail below under each substance.
In contrast to PFOS and PFOA, no other PFASs addressed in this Opinion have so far been legally restricted in Europe with respect to production, marketing or use. There are, however, a number of initiated or ongoing activities with the aim to reduce human and environmental risk connected to a number of PFASs discussed in this Opinion. C9–C14 PFCA are included in the SVHC candidate list which implies certain terms of their use.
PFBA and PFPeA
No activities identified.
PFHxA
A risk management option analysis (RMOA) performed by Germany and SVHC (substance of very high concern) classification was proposed but later withdrawn9. A restriction proposal was submitted in December 2019.10
PFHpA
Substance evaluation is performed by Belgium. A proposal for harmonised classification as Repr. 1B11 , H360D, and STOT RE 1,12 H372, is under development.13
PFNA, PFDA, PFUnDA, PFDoDA, PFTrDA and PFTeDA
Proposals on restriction of PFNA, PFDA, PFUnDA, PFDoDA, PFTrDA and PFTeDA have passed the ECHA RAC (Risk Assessment Committee) and SEAC (Socioeconomic Assessment Committee) and the proposal has been submitted to the European Commission.14 According to EU No 10/2011, PFNA is not allowed for use in the production of plastics in FCM.
PFPeDA, PFHxDA and PFODA
Under the scope of the PFOA restriction in the Stockholm Convention (includes related substances in polymers up to C18).8
PFBS
An RMOA was developed by Norway.15 Proposal on SVHC was submitted in August 2019.16 At ECHA's 67th Member States Committee Meeting, in December 2019, PFBS was classified as SVHC with reference to article 57 f.17
PFHxS
According to EU No 10/2011, PFHxS is not allowed for use in the production of plastics in FCM. An SVHC under REACH based on its vPvB properties (very Persistent and very Bioaccumulative),18 passed screening criteria for inclusion into Annex A of the Stockholm Convention.19 Proposal on EU restriction was submitted by Norway in April 2019.15 A risk profile was adopted by POPRC.20 Risk Management Evaluation to be discussed at nCOP21.21
PFHpS and PFDS
No activities identified.
8:2 FTOH, 8:2 monoPAP and 8:2 diPAP
All three substances are PFOA related.
PFOSI, FOSA, EtFOSA, EtFOSE and FC‐807
All five substances are PFOS‐related and should therefore fall under the current PFOS regulation.
2. Data and methodologies
2.1. Data
2.1.1. Occurrence in food data
2.1.1.1. Data collection and validation
Following an European Commission mandate to EFSA, a call for annual collection of chemical contaminant occurrence data in food, including PFASs, was issued by the former EFSA Dietary and Chemical Monitoring Unit (now DATA Unit)22 in December 2010 with a closing date of 01 October of each year.23 European national authorities and similar bodies, research institutions, academia, food business operators and other stakeholders were invited to submit analytical data on PFASs in food. The data for PFASs were provided by national authorities from Austria, Belgium, Cyprus, the Czech Republic, Denmark, Finland, France, Germany, Greece, Ireland, Italy, Malta, Norway, Slovenia, Spain and the United Kingdom.
The data submission to EFSA followed the requirements of the EFSA Guidance on Standard Sample Description for Food and Feed (EFSA, 2010a); occurrence data were managed following the EFSA standard operational procedures (SOPs) on ‘Data collection and validation’ and on ‘Data analysis of food consumption and occurrence data’.
By the 16th of May 2018, a total of 97,448 results (representing 11,528 samples) of food and beverages with analytical data on PFASs were available in the EFSA database.
Data received after that date were not included in the dataset used for further evaluation for this Opinion.
2.1.1.2. Data analysis
Following the EFSA SOP on ‘Data analysis of food consumption and occurrence data’ to guarantee an appropriate quality of the data used in the exposure assessment, the initial dataset was carefully evaluated applying several data cleaning and validation steps. Special attention was paid to different parameters such as ‘Sampling strategy’, ‘Sampling method’, ‘Sampling year’, ‘Sampling country’, ‘Analytical methods’, ‘Reporting unit’, ‘Limit of detection’ and the codification of the different samples under FoodEx classification. The outcome of the data analysis is presented in Section 3.1.1.
In the analysis of PFAS occurrence data, the left‐censored data (results below LOD or below LOQ) were treated by the substitution method as recommended in the ‘Principles and Methods for the Risk Assessment of Chemicals in Food’ (WHO/IPCS, 2009). The same method is indicated in the EFSA scientific report ‘Management of left‐censored data in dietary exposure assessment of chemical substances’ (EFSA, 2010b) as an option in the treatment of left‐censored data. The guidance suggests that the lower bound (LB) and upper bound (UB) approach should be used for chemicals likely to be present in the food (e.g. naturally occurring contaminants, nutrients and mycotoxins). The LB is obtained by assigning a value of zero (minimum possible value) to all samples reported as lower than the LOD (< LOD) or LOQ (< LOQ). The UB is obtained by assigning the numerical value of LOD to values reported as < LOD and LOQ to values reported as < LOQ (maximum possible value), depending on whether LOD or LOQ is reported by the laboratory.
2.1.2. Consumption data
2.1.2.1. Food consumption data
The EFSA Comprehensive European Food Consumption Database (Comprehensive Database) provides a compilation of existing national information on food consumption at individual level. Details on how the Comprehensive Database is used are published in the Guidance of EFSA (EFSA, 2011a). The food consumption data gathered by EFSA in the Comprehensive Database are the most complete and detailed data currently available in the EU. The latest version of the Comprehensive Database updated in 2018 contains results from a total of 60 different dietary surveys carried out in 25 different Member States covering 119,458 individuals. The age classes considered are the following:
Infants: < 12 months old
Toddlers: ≥ 12 months to < 36 months old
Other children: ≥ 36 months to < 10 years old
Adolescents: ≥ 10 years to < 18 years old
Adults: ≥ 18 years to < 65 years old
Elderly: ≥ 65 years to < 75 years old
Very elderly: ≥ 75 years old
Four additional surveys provided information on specific population groups: ‘Pregnant women’ (≥ 15 to ≤ 45 years old for Latvia; 17–46 years for Portugal) and ‘Lactating women’ (≥ 28 to ≤ 39 years old for Greece; 18–45 years for Estonia). When for one country and age class two different dietary surveys were available, only the most recent one was used. Additionally, for chronic exposure assessment, surveys that lasted only 1 day were excluded. This resulted in a total of 38 different dietary surveys carried out in 23 different European countries used for the chronic dietary exposure assessment. In Annex A, Table A.1, these dietary surveys and the number of subjects available for the chronic exposure assessment are described.
Overall, the food consumption data gathered by EFSA in the Comprehensive Database are the most complete and detailed data currently available in the EU. Consumption data were collected using single or repeated 24‐ or 48‐h dietary recalls or dietary records covering from 3 to 7 days per subject. Owing to the differences in the methods used for data collection, direct country‐to‐country comparisons can be misleading.
2.1.2.2. Food classification
Consumption data were classified according to the FoodEx classification system (EFSA, 2011b). FoodEx is a food classification system developed by EFSA in 2009 with the objective of simplifying the linkage between occurrence and food consumption data when assessing the exposure to hazardous substances. It contains 20 main food categories (first level), which are further divided into subgroups having 140 items at the second level, 1,261 items at the third level and reaching about 1,800 end‐points (food names or generic food names) at the fourth level.
In 2011, a new version of FoodEx, named FoodEx2 has been developed and is described in the scientific document ‘Report on the development of a Food Classification and Description System for exposure assessment and guidance on its implementation and use’ (EFSA, 2011c). The last release of FoodEx2 complements the previous hierarchical classification system of basic codes with more detailed food levels and gives the possibility of reporting additional information through the use of facets and facet descriptors (EFSA, 2015).
2.1.3. Toxicokinetic and toxicological data
Data were obtained from the scientific literature as described in Section 2.2.2.
2.2. Methodologies
2.2.1. Dietary Exposure assessment
The CONTAM Panel considered it appropriate to estimate only chronic exposure to PFASs (see Section 3.4). As suggested by the EFSA Working Group on Food Consumption and Exposure (EFSA, 2011a), dietary surveys with only 1 day per subject were not considered as they are not adequate to assess repeated exposure. Similarly, subjects who participated only 1 day in the dietary studies, when the protocol prescribed more reporting days per individual, were also excluded for the chronic exposure assessment. Thus, for chronic exposure assessment, food consumption data were used from 35 different and most recent dietary surveys carried out in 19 different European countries present in the latest version of the Comprehensive Database (Annex A, Table A.1). Not all countries provided consumption information for all age groups, and in some cases the same country provided more than one consumption survey.
For calculating chronic dietary exposure to PFASs, food consumption and body weight data at the individual level were accessed in the Comprehensive Database.
Occurrence concentrations were averaged at FoodEx level 1, 2 or 3 depending on the food category. For Meat and meat products, and Milk and dairy products, occurrence was averaged at FoodEx level 2 to allow for a more detailed assessment and for Fish and other seafood at FoodEx level 3 to account for different fish species. When the available number of samples for a certain food category was less than 6 or zero, the mean occurrence value of the parent level was used in the analysis if available. FoodEx level 1 categories with less than six samples were retained in the analysis and the mean occurrence value calculated on the available samples.
Also, food categories that have so far not been analysed are expected to contain PFASs. Thus, it was decided to include all food categories in the exposure assessment and assume that measured levels in a subgroup are representative for the whole food group (e.g. data in apples used for all fruit). This led to including 1,491 different food categories in the exposure assessment.
In cases where the food consumption data are only specified at a higher FoodEx level, like fish meat, the mean occurrence value of certain FoodEx level 1 categories could be strongly affected by high occurrence values in FoodEx level 2 categories that are commonly analysed, but that are rarely consumed (e.g. Meat and Meat products mean affected by occurrence results in Edible offal from game mammals).
To mitigate this effect, all FoodEx level 2 occurrence values for all food categories were weighted against their consumption amount when calculating the mean for FoodEx level 1. The same was done with FoodEx level 3 categories when calculating the mean occurrence value of FoodEx level 2 categories related to Fish and Fish products.
To assess the combined exposure to PFOA, PFNA, PFHxS and PFOS, the mean occurrence value of each of the four PFASs for each food category was summed and considered to be the combined occurrence of these PFASs within the food category.
Occurrence data and consumption data were then linked at the appropriate FoodEx level.
The mean and the high (95th percentile) chronic dietary exposures were calculated by combining mean PFAS occurrence values for food samples collected in different countries (pooled European occurrence data) with the average daily consumption for each food at individual level in each dietary survey and age class. Consequently, individual average exposures per day and body weight were obtained for all individuals. On the basis of distributions of individual exposures, the mean and 95th percentile exposure were calculated per survey and per age class. Dietary exposure was assessed using overall European LB and UB mean occurrence values of PFASs.
All analyses were run using the SAS Statistical Software (SAS enterprise guide 5.1).
2.2.2. Literature search and appraisal of studies
EFSA outsourced an extensive literature search related to the oral toxicity of PFASs, their precursors and potential replacements, in experimental animals and humans (contract: RC/EFSA/BIOCONTAM/2012/02). The aim of the assignment was to identify and collect all relevant literature regarding PFASs. The search was performed in March 2013. The methodology and the results are detailed in Bull et al. (2014).
The following areas were covered:
Area 1: Data on toxicokinetics (absorption, distribution, metabolism and excretion) in in vitro studies, experimental animals and humans.
Area 2: Data on toxicity in experimental animals (i.e. acute and repeat dose toxicity, immunotoxicity, developmental and reproductive toxicity, neurotoxicity, carcinogenicity and other effects).
Area 3: Data on observations in humans, including epidemiology, case reports and biomarkers of exposure and effects.
In addition to the literature search outsourced by EFSA, further literature searches were performed covering up to 16 August 2019, for the above three areas, in order to cover peer‐reviewed literature published between the beginning of 2013 and August 2019. Further search strategies were designed to identify literature published after 2007, which covered additional areas, including, chemistry, analysis, synthesis, production, use, environmental fate, food occurrence and human exposure. An overview of the search terms and dates are given in Appendix N (Table N.1).
Table N.1.
Search terms
| Human observations | |
|---|---|
|
Search terms Dates |
perfluoro* OR pfos OR pfoa OR pfas OR ‘fluorotelomer alcohol’ AND ‘human health’ OR ‘adverse effect*’ OR ‘occupational case*’ OR occupational OR epidemiol* OR biomarker OR ‘biological marker’ OR poison* OR ‘incidental poison*’ OR ‘case stud*’ OR adverse OR ‘case control*’ OR ‘case report*’ OR human OR adult OR man OR woman OR men OR women OR female OR male OR child OR children OR infant OR neonate OR maternal OR cohort OR prenatal 01 January 2013–31 Dec 2017. Extended to 01 August 2019 for studies on blood lipids, liver/ALT, birthweight, immune outcomes, carcinogenicity and endocrine effects (other than those under thyroid function & disease) |
| Toxicity | |
| Search terms Dates | perfluoro* OR pfos OR pfoa OR pfas OR ‘fluorotelomer alcohol’ AND toxicity OR toxi* OR acute OR subacute OR subchronic OR chronic OR mutagen* OR carcino* OR cardiotox* OR genotox* OR reprotox* OR nephrotox* OR neurotox* OR hepatotox* OR immune OR immuno* OR hematotox* OR haematotox* OR cytotox* OR ‘developmental tox*’ OR thyroid OR endocri* OR endocrine OR estrogen OR oestrogen OR fertility OR tumour OR tumor OR gestat* OR lactat* OR ‘DNA damage’ OR mortality OR adverse OR ‘adverse effect’ OR ‘blood lipid*’ OR ‘serum lipid*’ OR PPAR OR ‘ex vivo’ OR ‘in vitro’ OR ‘in vivo’ OR exvivo OR invitro OR invivo OR cell* OR tissue* OR rodent* OR mouse OR animal* OR rat* OR mice OR rabbit* OR dog* OR monkey* OR ‘experimental animal*’ OR ‘lab* animal*’ 01 January 2013–16 August 2019 |
| Toxicokinetics | |
| Search terms Dates | perfluoro* OR pfos OR pfoa OR pfas OR ‘fluorotelomer alcohol’ AND toxicokinetic* OR absorption OR distribution OR metabolism OR excretion OR ADME OR biotransformation OR pharmacokinetic* OR disposition OR fate OR transfer OR conjugat* OR hydroxylation OR ‘half‐life’ OR ‘half life’ OR PBPK OR ‘physiologically based pharmacokinetic modelling*’ OR uptake OR elimination OR urine OR bile OR faeces OR feces OR milk 01 January 2013–16 August 2019 |
| Biomonitoring | |
| Search terms Dates |
perfluoro* OR pfos OR pfoa OR pfas OR ‘fluorotelomer alcohol’ AND teeth OR tooth OR skin OR bone OR sperm or semen OR tissue OR level* OR concentration* OR ‘time trend’ OR milk OR blood OR ‘whole blood’ OR serum OR plasma OR ‘breast milk’ OR biomarker OR ‘human milk’ OR ‘cord blood’ OR urine OR ‘amniotic fluid’ OR faeces OR placenta OR meconium OR hair OR nail* OR sweat OR saliva OR level* OR concentration* 01 January 2007–31 August 2018 for PFASs other than PFOS and PFOA Data from EFSA CONTAM Panel et al. (2018) Opinion for PFOS and PFOA |
| Occurrence in Food | |
| Search terms Dates | perfluoro* OR pfos OR pfoa OR pfas OR ‘fluorotelomer alcohol’ AND occurrence* OR level* OR concentration* OR amount*OR food OR beverage OR ‘drinking water’ OR ‘bottled water’ OR vegetable* OR legume* OR fruit* OR grain* OR cereal* OR poultry OR chicken OR beef OR turkey OR meat OR egg* OR milk OR seafood OR fish OR shrimp OR prawn* OR mollusc* OR feed OR feedstuff OR beef OR pork OR livestock OR bivalve* 01 January 2007–01 September 2018 |
| Food Processing | |
| Search terms Dates | perfluoro* OR pfos OR pfoa OR pfas OR ‘fluorotelomer alcohol’ AND process* OR cook* OR roast* OR fry* OR boil* OR bak* OR ‘thermal processing’ OR sterilisation OR sterilization OR sterilise OR sterilize OR freez* OR heat* 01 January 2007–01 September 2018 |
| (Dietary) Exposure | |
| Search terms Dates | perfluoro* OR pfos OR pfoa OR pfas OR ‘fluorotelomer alcohol’ AND ‘exposure assessment*’ OR ‘dietary exposure assessment*’ OR ‘human dietary exposure assessment*’ 01 January 2007–01 September 2018 |
| (Non‐dietary) Exposure | |
| Search terms Dates | perfluoro* OR pfos OR pfoa OR pfas OR ‘fluorotelomer alcohol’ AND ‘non‐dietary exposure assessment*’ OR ‘human non‐dietary exposure assessment*’ OR ‘exposure pathway*’ OR ‘indoor exposure’ OR ‘dermal exposure’ OR occupational OR dust OR air OR ‘in‐utero’ OR inutero OR ‘in utero’ OR skin 01 January 2007–31 December 2017 |
| Chemistry and analysis | |
| Search terms Dates | perfluoro* OR pfos OR pfoa OR pfas OR ‘fluorotelomer alcohol’ AND chemistry OR analysis OR determination OR detection OR spectroscopy OR chromatography OR TLC OR GC OR GC‐MS OR HPLC OR LC‐MS OR ICP‐MS 01 January 2007 – 29 June 2016 |
| Production/Use | |
| Search terms Dates | perfluoro* OR pfos OR pfoa OR pfas OR ‘fluorotelomer alcohol’ AND source* OR application OR use* OR production OR ‘production volume’ OR application 01 January 2007–29 June 2016 |
| Environmental fate | |
| Search terms Dates | perfluoro* OR pfos OR pfoa OR pfas OR ‘fluorotelomer alcohol’ AND ‘environmental fate’ OR ‘environmental monitoring’ OR soil OR biosolid OR manure OR sediment OR sewage OR sludge OR water OR ‘waste water*’ OR ‘ground water*’ OR wastewater* OR groundwater* OR river OR land OR lake OR grass OR vegetation 01 January 2007 – 29 June 2016 |
Web of Science24 and PubMed25 were identified as databases appropriate for retrieving literature for the present evaluation. The references resulting from the literature search were imported and saved using a software package (EndNote26), which allows effective management of references and citations.
Reviews, relevant scientific evaluations and assessments by national or international bodies were also considered for the current risk assessment. When relevant papers were identified during the risk assessment process (e.g. from other studies or reviews), they were also considered.
The references obtained were screened using title and abstract to identify relevant literature.
The information retrieved was subsequently reviewed by the CONTAM working group (WG) on PFASs in food and has been used for the present assessment based on expert judgement. Selection of the scientific papers for inclusion or exclusion was based on consideration of the extent to which the study was relevant to the assessment and general study quality considerations.
2.2.3. Benchmark dose analysis
Benchmark dose analysis was done according to EFSA guidance (EFSA Scientific Committee, 2017) and using the R package PROAST, version 69.0. The BMD modelling of the individual data from the study by Abraham et al. (2020) was performed by the authors since these could not be provided to EFSA. However, the CONTAM Panel was able to reproduce the results using data extracted from the graphs provided by the authors (Appendix K).
2.2.4. Methodology applied for risk assessment
The CONTAM Panel applied the general principles of the risk assessment process for chemicals in food as described by WHO/IPCS (2009), which include hazard identification and characterisation, exposure assessment and risk characterisation. In addition to the principles described by WHO/IPCS (2009), any EFSA guidance pertaining to risk assessment and relevant for the present assessment has been duly considered (see Appendix O).
The Panel developed the draft Scientific Opinion which underwent a public consultation from 24 February 2020 to 20 April 2020. The comments received and how they were taken into account when finalising the Scientific Opinion were published in an EFSA Technical Report (EFSA, 2020).
3. Assessment
3.1. Occurrence data
3.1.1. Current occurrence data in food
An initial number of 97,434 results for food samples analysed on PFASs from 16 European countries were available for the assessment. The major contributor of data on PFASs in terms of number of results was Norway which reported 38% of the data, followed by Germany (33%) and France (15%). In terms of number of samples, Germany provided 58% of all data.
The current data were not systematically checked for possible duplicate occurrence with the data reported in Section 3.1.3. This might have resulted in a partial overlap between the data reported in the scientific literature and the data reported to EFSA and used in the current exposure assessment.
Data were reported on samples collected between the years 2000 and 2016 with 74% of the samples collected after 2007.
Approximately 79% of the data were obtained from samples collected within official monitoring programmes, 7% from dietary exposure studies, 11% from surveys and 3% from a combination of programmes.
Most results were obtained by LC‐MS/MS based methods (86%). Very few (< 1%) results were reported as obtained using GC‐based methods. For the remaining data, no information on analytical methods was reported.
All analytical results were expressed on whole weight basis; thus, no conversion had to be applied.
A proportion of analytical results (9%) was obtained from pooled samples, meaning that the result represented an average of a number of samples taken in equal parts from different consignments/batches and pooled together for the laboratory analysis. Since the level of aggregation for pooled samples matched the level of classification of the individual samples (only similar food matrices were pooled together), results from pooled samples were retained for further evaluation. To ensure a proportionate representation of the individual samples and thus an accurate use of occurrence data in assessing the dietary exposure, the mean concentrations per food category were calculated by weighting the reported analytical results for the number of samples pooled.
Quantitative data were available for only 17 PFASs out of the 28 for which results were available. Overall, 93.5% of the results were left‐censored (below LOQ or LOD).
The number of reported analytical results for 28 different PFASs and the % of left‐censored results for each PFAS are presented in Table 3 (see also Annex B, Table B.1).
Table 3.
List of perfluoroalkyl substances (PFASs) for which data were reported (before applying exclusion criteria)
| PFASs(a) | Acronyms | No of results | % LC(c) |
|---|---|---|---|
| Perfluoroalkyl carboxylic acids (PFCAs) | |||
| Perfluorobutanoic acid | PFBA | 3,096 | 98.6 |
| Perfluoropentanoic acid | PFPeA | 4,882 | 95.7 |
| Perfluorohexanoic acid | PFHxA | 7,019 | 96.4 |
| Perfluoroheptanoic acid | PFHpA | 4,882 | 98.4 |
| Perfluorooctanoic acid | PFOA | 11,506 | 91.3 |
| Perfluorononanoic acid | PFNA | 6,970 | 95.4 |
| Perfluorodecanoic acid | PFDA | 6,979 | 94.2 |
| Perfluoroundecanoic acid | PFUnDA | 4,885 | 93.8 |
| Perfluorododecanoic acid | PFDoDA | 5,234 | 95.0 |
| Perfluorotridecanoic acid | PFTrDA | 2,673 | 95.1 |
| Perfluorotetradecanoic acid | PFTeDA | 2,992 | 98.9 |
| Perfluoropentadecanoic acid | PFPeDA | 14 | 100 |
| Perfluorohexadecanoic acid | PFHxDA | 1,886 | 100 |
| Perfluorooctodecanoic acid | PFODA | 1,865 | 100 |
| Perfluoroalkane sulfonic acids (PFSAs) | |||
| Perfluorobutane sulfonate | PFBS | 6,370 | 98.4 |
| Perfluorohexane sulfonate | PFHxS | 6,010 | 98.2 |
| Perfluoroheptane sulfonate | PFHpS | 1,387 | 99.9 |
| Perfluorooctane sulfonic acid | PFOS | 11,476 | 75.2 |
| Perfluorodecane sulfonate | PFDS | 3,418 | 99.5 |
| Perfluoroalkane sulfinic acids | |||
| Perfluorooctane sulfinic acid | PFOSi | 320 | 100 |
| n:2 Fluorotelomer alcohols (n:2 FTOHs) | |||
| 8:2 fluorotelomer alcohol | 8:2 FTOH | 36 | 100 |
| n:2 polyfluoroalkyl phosphoric acid esters (PAPs) | |||
| 8:2 fluorotelomer alcohol (8:2 FTOH) mono‐phosphate | 8:2 monoPAP | 22 | 100 |
| 8:2 fluorotelomer alcohol (8:2 FTOH) di‐phosphate | 8:2 diPAP | 12 | 100 |
| Perfluoroalkane sulfonamides (FASAs) | |||
| Perfluorooctane sulfonamide | FOSA | 3,361 | 93.3 |
| Perfluorooctane sulfonylamide(b) | 36 | 100 | |
| N‐ethyl perfluoroalkane sulfonamides (EtFASAs) | |||
| N‐ethyl perfluorooctane sulfonamide | EtFOSA | 46 | 100 |
| N‐ethyl perfluoroalkane sulfonamidoethanol (EtFASEs) | |||
| N‐ethyl perfluorooctane sulfonamidoethanol | EtFOSE | 45 | 100 |
| Perfluoroalkyl phosphate | |||
| Perfluoroalkyl phosphate | FC‐807 | 12 | 100 |
| Total | 97,434 | 93.5 | |
N: number; LC: left‐censored.
PFAS names as reported by data providers. They may differ from those reported in Table 1.
There is no acronym available, and it was not clear to which PFAS the compound refers to, and therefore, it was not considered for further evaluation. These data were not used for the assessment.
The % LC was calculated over the number of analytical results and not weighted for the number of samples included in pooled samples.
As stated in Section 3.1.1, the occurrence data were carefully evaluated and a list of validation steps was applied before being used to estimate dietary exposure.
Outdated results may not reflect current levels of contamination. Therefore, only results from samples collected after the beginning of 2007 were retained for the further assessment and samples collected before then (n = 2,587) were excluded.
Particular attention was paid to data reported as suspect samples. Suspect samples are samples taken repeatedly from the same site as a consequence of evidence or suspicion of contamination, and are often taken as a follow‐up of demonstrated non‐compliance. Because inclusion of these data may result in an overestimation of the contamination levels, the CONTAM Panel decided to exclude them (n = 486) from further analysis. However, it cannot be excluded that some of the remaining samples included in the analysis may also have been collected in a targeted way, including suspect samples not reported as such or samples sampled under other targeted criteria.27
In addition, 16 samples of cow's milk from Germany (144 results) were discarded based on the indication of the data provider that concentrations were determined with a non‐validated method. These samples showed unusually high PFOA levels (> 1 ng/mL), whereas a study with dairy cows showed poor transfer of PFOA into milk (Kowalczyk et al., 2013). For EFSA, this was a reason to start an inquiry.
The analytical results (n = 36) with unclear identification of the PFAS compound, reported as perfluorooctane sulfonylamide, were excluded from further evaluation.
The Commission Recommendation 2010/161/EC28 recommends an LOQ of 1 μg/kg for the monitoring of PFASs in food. The LODs/LOQs of the PFAS data reported to EFSA varied between laboratories, food matrices and substances. A high percentage of results below LOD/LOQ in combination with high LODs/LOQs increases the uncertainty associated with the dietary exposure estimations. In order to reduce the impact of this, the recommended LOQ cut‐off of 1 μg/kg was applied to all food categories with two exceptions. One was ‘Drinking water’ for which a cut‐off of 0.01 μg/kg was applied, because of the high intake of water and reported low LOQs/LODs. The other one was the food categories of ‘Edible offal, game animals’ and ‘Fish offal’ for which no cut‐off was applied to avoid the loss of too many quantitative samples. For those results for which only the LOD was available, the LOQ was calculated by multiplying the LOD by a factor of 3. Quantified results for which neither the LOD nor the LOQ was available, with an occurrence value greater than 1 μg/kg and not belonging to the food categories ‘Edible offal, game animals’ and ‘Fish offal’, were excluded from the final dataset (n = 217). Quantified results for which neither the LOD nor the LOQ was available, with an occurrence value below 1 μg/kg (implying a low LOQ), were retained in the final dataset (n = 1,271).
For FTOH, all results (n = 36) were generated using a method with an LOQ > 1 μg/kg; thus, all data for this substance were discarded. As all results were left‐censored, the substance would not have been included anyway in the exposure assessment.
An additional 24,531 results were excluded following the application of the 1 μg/kg cut‐off. Out of these, 99% were left‐censored. After cut‐off application and removal of quantified results with missing LOQ information, 92% of the results were left‐censored.
Annex A Table A.2 shows the effect of the LOQ cut‐off application on data availability and mean occurrence values for each food category.
Figure 2 shows the distribution of the reported LOQs before and after applying cut‐offs.
Figure 2.
Distribution of the limits of quantification (LOQs) of PFASs for reported results before (upper figure) and after (lower figure) applying the LOQ cut‐off
- Box plot showing whiskers at minimum and maximum, box at P25 and P75 with line at P50, outliers shown as dots. The vertical green line shows the 1 μg/kg LOQ cut‐off, applied to most foods categories except drinking water (0.01 μg/kg) and ‘Edible offal, game animals’ and ‘Fish offal’ (no cut‐off).
As a consequence of the cleaning, a total of 28,001 analytical results were excluded (Annex A Table A.3).
The final validated dataset contained 69,433 analytical results for food samples analysed for 26 PFASs.
The analytical results included in the final dataset were as in the original dataset, mostly from Norway (24,869 analytical results), Germany (19,980 analytical results) and France (13,594 analytical results) (Annex B, Table B.2). It should be noted that the origin of the data was not always the European country reporting the data, i.e. the dataset also contained samples originating from North and South America, Africa, Asia and Australia, but available on the European market. The distribution of analytical results per sampling country and sampling year are summarised in Figures 3 and 4 and Annex B, Tables B.2–B.4.
Figure 3.
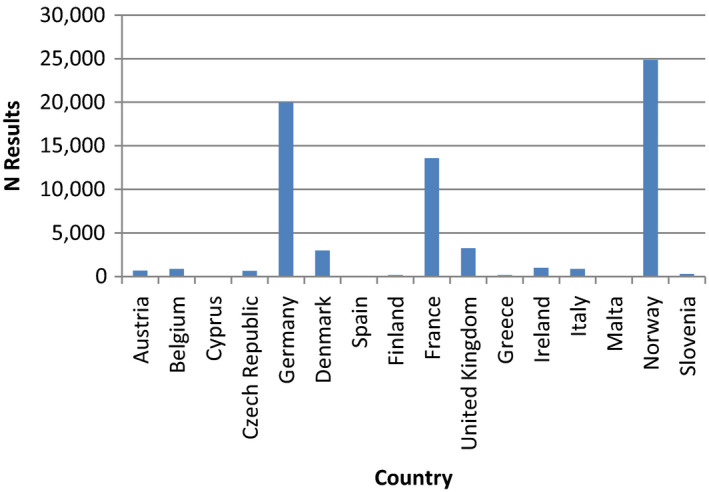
Distribution of analytical results for PFASs divided by sampling country
Figure 4.
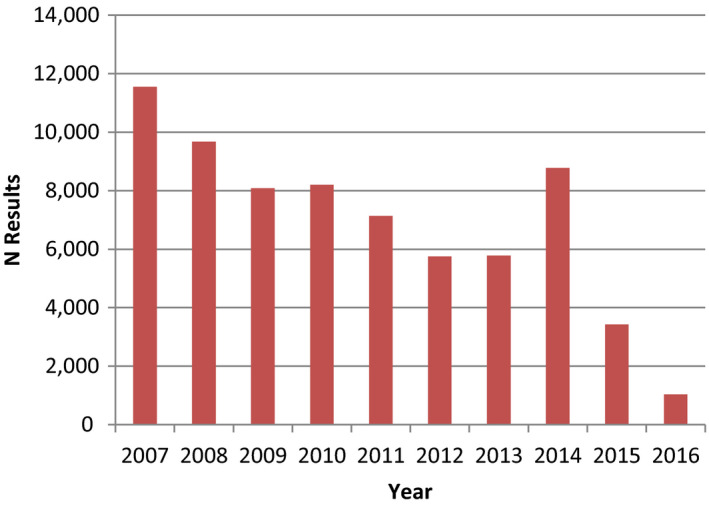
Distribution of analytical results for PFASs divided by sampling year
The analytical results in the final dataset were reported as corrected for recovery, or not in need of correction in approximately 69% of the cases. For results which were reported as not corrected for recovery and for which the recovery rates were available (19%), the results were corrected prior to being used for exposure assessment. For results for which the information about the need for correction or the recovery value was not available were used as provided (12%).
The list of food categories and the number of results available for each of them and for each PFAS is detailed in Annex B, Table B.5. The five most frequently analysed food categories were ‘Fish meat’ (n = 23,572), ‘Fish offal’ (n = 9,745), ‘Livestock meat’ (n = 4,475) and ‘Drinking water’ (n = 3,803) and ‘Vegetables and vegetable products’ (n = 3,697). The number of data available per food category was not evenly distributed across the 26 PFASs. Most data were on levels of PFOS (n = 8,498) followed by PFOA (n = 8,197), PFDA (n = 5,770), PFNA (n = 5,594), PFHxA (n = 5,448) and PFHxS (n = 4,745).
The CONTAM Panel decided not to run the exposure assessment for PFASs for which the fraction of left‐censored data was 100%. As a result, the exposure assessment was limited to 17 PFASs. In total, 67,839 analytical results were used for calculating the mean level in food and used in the exposure assessment.
3.1.2. Analytical results
The mean occurrence values calculated on the final dataset for the selected 17 PFASs in each food category and used in the exposure assessment are displayed in Annex A, Table A.4. The mean levels for each PFAS in each of these food categories were used for the exposure assessment. Table 4 shows the mean levels in a number of food categories for the four PFASs selected for the risk assessment, being PFOA, PFNA, PFHxS and PFOS (see critical effects, Section 3.4). Table 5 shows the mean concentrations of these PFASs in a number of fish species (see Appendix A and Annex A, Table A.4 for more fish species).
Table 4.
Mean levels (μg/kg) of PFOS, PFOA, PFNA and PFHxS in selected food categories (except fish)
| Food category | PFOS | PFOA | PFNA | PFHxS | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | %LC | LB | UB | N | %LC | LB | UB | N | %LC | LB | UB | N | %LC | LB | UB | |
| Vegetables and vegetable products* | 477 | 95% | 0.003 | 0.15 | 489 | 86% | 0.006 | 0.16 | 275 | 96% | 0.001 | 0.12 | 274 | 98% | 0.000 | 0.10 |
| Fruit and fruit products | 143 | 77% | 0.027 | 0.25 | 144 | 63% | 0.009 | 0.26 | 98 | 73% | 0.011 | 0.17 | 94 | 84% | 0.022 | 0.16 |
| Livestock meat | 461 | 93% | 0.028 | 0.17 | 459 | 96% | 0.028 | 0.17 | 348 | 99% | 0.000 | 0.14 | 222 | 100% | 0.000 | 0.09 |
| Poultry | 169 | 99% | 0.009 | 0.13 | 185 | 98% | 0.002 | 0.15 | 170 | 100% | 0.000 | 0.14 | 130 | 100% | 0.000 | 0.11 |
| Game mammals (meat) | 574 | 71% | 0.94 | 1.59 | 572 | 91% | 0.38 | 1.23 | 33 | 100% | 0.000 | 0.67 | 28 | 96% | 0.015 | 0.68 |
| Milk and dairy products | 13 | 85% | 0.001 | 0.12 | 13 | 85% | 0.001 | 0.13 | 13 | 92% | 0.000 | 0.10 | 13 | 92% | 0.000 | 0.08 |
| Liquid milk | 235 | 96% | 0.001 | 0.14 | 236 | 100% | 0.000 | 0.15 | 111 | 100% | 0.000 | 0.11 | 126 | 100% | 0.000 | 0.10 |
| Eggs and egg products | 174 | 92% | 0.27 | 0.35 | 177 | 92% | 0.106 | 0.21 | 124 | 100% | 0.000 | 0.098 | 107 | 97% | 0.000 | 0.06 |
| Animal and vegetable fats and oils | 38 | 90% | 0.004 | 0.11 | 38 | 90% | 0.002 | 0.11 | 36 | 100% | 0.000 | 0.12 | 53 | 97% | 0.000 | 0.102 |
| Alcoholic beverages | 6 | 100% | 0.000 | 0.002 | 6 | 84% | 0.010 | 0.014 | 6 | 100% | 0.000 | 0.005 | 6 | 84% | 0.006 | 0.007 |
| Drinking water** | 451 | 88% | 0.0001 | 0.003 | 452 | 78% | 0.001 | 0.003 | 449 | 99% | 0.000 | 0.002 | 449 | 85% | 0.002 | 0.004 |
| Food for infants and small children | 11 | 100% | 0.000 | 0.24 | 11 | 100% | 0.000 | 0.15 | 10 | 90% | 0.126 | 0.24 | 10 | 100% | 0.000 | 0.24 |
| Edible offal, farmed animals | 495 | 80% | 0.87 | 1.18 | 542 | 94% | 0.092 | 0.36 | 285 | 84% | 0.087 | 0.32 | 170 | 99% | 0.014 | 0.52 |
| Edible offal, game animals | 903 | 4% | 214 | 215 | 898 | 58% | 5.48 | 8.18 | 105 | 10% | 9.77 | 9.87 | 105 | 99% | 0.010 | 2.52 |
*: Includes fungi; **: without additives except carbon dioxide; includes water ice for consumption.
N: number; %LC: Percentage left‐censored; LB: Lower bound; UB: upper bound; PFOS: perfluoroheptane sulfonate; PFOA: perfluorooctanoic acid; PFNA: perfluorononanoic acid; PFHxS: perfluorohexane sulfonic acid.
Table 5.
Mean levels (μg/kg) of PFOS, PFOA, PFNA and PFHxS in selected fish species and fish offal
| Fish species | PFOS | PFOA | PFNA | PFHxS | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | %LC | LB | UB | N | %LC | LB | UB | N | %LC | LB | UB | N | %LC | LB | UB | |
| Herring (Clupea) | 288 | 74% | 0.32 | 0.62 | 290 | 96% | 0.016 | 0.38 | 243 | 90% | 0.023 | 0.38 | 237 | 99% | 0.000 | 0.38 |
| Sardine and pilchard (Sardina) | 14 | 0% | 4.73 | 4.73 | 28 | 64% | 0.101 | 0.37 | 14 | 57% | 0.084 | 0.53 | 14 | 64% | 0.014 | 0.45 |
| Anchovy (Engraulis) | 5 | 0% | 0.58 | 0.98 | 13 | 62% | 0.044 | 0.12 | – | – | – | – | – | – | – | – |
| Salmon and trout (Salmo spp.) | 574 | 88% | 0.31 | 0.83 | 521 | 95% | 0.13 | 0.63 | 522 | 100% | 0.003 | 0.70 | 365 | 100% | 0.000 | 0.63 |
| Mackerel (Scomber) | 125 | 79% | 0.36 | 0.93 | 136 | 81% | 0.31 | 0.88 | 129 | 96% | 0.004 | 0.74 | 122 | 99% | 0.001 | 0.74 |
| Tuna (Thunnus) | 21 | 39% | 0.16 | 0.26 | 34 | 100% | 0.000 | 0.12 | 17 | 100% | 0.000 | 0.13 | 17 | 100% | 0.000 | 0.11 |
| Cod and whiting (Gadus spp.) | 174 | 67% | 0.47 | 1.05 | 145 | 93% | 0.012 | 0.74 | 130 | 92% | 0.016 | 0.78 | 27 | 100% | 0.000 | 0.53 |
| Halibut (Hippoglossus spp.) | 487 | 71% | 0.26 | 0.81 | 106 | 99% | 0.003 | 0.30 | 487 | 100% | 0.000 | 0.77 | 487 | 100% | 0.002 | 0.69 |
| Carp (Cyprinus) | 145 | 14% | 14.12 | 14.21 | 149 | 32% | 4.10 | 4.33 | 125 | 65% | 0.84 | 1.47 | 126 | 97% | 0.066 | 1.01 |
| Eels (Apodes) | 164 | 35% | 9.23 | 9.44 | 177 | 96% | 0.071 | 0.68 | 54 | 91% | 0.98 | 1.66 | 58 | 98% | 0.017 | 0.73 |
| Fish offal | 208 | 83% | 3.38 | 4.99 | 208 | 100% | 0.010 | 3.51 | 204 | 99% | 0.011 | 2.41 | 202 | 100% | 0.031 | 1.65 |
N: number; %LC: Percentage left‐censored; LB: Lower bound; UB: upper bound; –: no data provided to EFSA; PFOS: perfluoroheptane sulfonate; PFOA: perfluorooctanoic acid; PFNA: perfluorononanoic acid; PFHxS: perfluorohexane sulfonic acid.
High quantified concentrations (P95 > 10 μg/kg) were reported for PFOS, PFOA, PFDA and PFNA in edible offal from game animals, PFOS in a number of fish species, like sardine and pilchard, sea catfish and wolf‐fish (Anarhichas), eel, perch, barbel, roach and bream, PFOA in carp and PFDA in perch. The highest mean and P95 LB concentrations were for PFOS in edible offal from game animals, being, respectively, 214 and 753 μg/kg.
It is relevant to focus on regularly consumed food categories, which contribute most to the exposure to each of the PFASs. For PFOS, highest mean LB levels were observed for various fish species, followed by eggs and egg products, livestock meat and fruit and fruit products. For PFOA, in addition to fish, eggs and egg products and livestock meat, also drinking water, fruit and fruit products and vegetables and vegetable products showed relatively high levels, considering the large consumption of these products. In addition, a relatively high mean level was calculated for alcoholic beverages, but this was based on one out of six samples with a level above the LOQ. For PFNA, relatively high levels were observed for fruit and fruit products, and for food for infants and small children, the latter due to one sample out of 10 with a detectable level. For PFHxS, highest mean levels were reported for fruit and fruit products, but also the level in alcoholic beverages (one sample > LOQ) caused a significant contribution to the exposure to this PFAS. For most food categories and PFASs, UB levels were much higher than LB levels.
In fish, highest mean PFOS levels were reported for carp, eel, roach, perch, bream, barbel and sardine, in addition to fish offal. In general, mean LB levels of PFOA, PFNA and PFHxS in fish are much lower, with the exception of carp.
Concerning drinking water, there were a considerable number of analytical results (206–452) for nine PFASs, varying between 78% and 100% left‐censored. To calculate the mean occurrence for drinking water, occurrence values for FoodEx level 2 categories of water (tap, well and bottled) were weighted according to the consumption of these categories. The highest mean LB level was for PFHxA, followed by PFHxS, PFBS and PFOA, being, respectively, 0.0022, 0.0018, 0.0015, and 0.0013 μg/L. Despite the low LOQ cut‐off applied (0.010 μg/L), mean UB levels were a factor of two higher.
For fruit and fruit products, PFOS and PFOA were detected in, respectively, 23% and 37% of the samples with mean LB levels of 0.0267 and 0.0086 μg/kg. Mean UB levels were 10‐ and 31‐fold higher. Rather high mean LB levels were also detected for PFHxA and PFHxS, being 0.0529 and 0.0224 μg/kg, followed by PFNA with a mean LB level of 0.0111 μg/kg. Detectable levels for PFOS were reported for some samples of apple, pear, peaches, table grapes and strawberries, and for PFOA in some samples of apple, pear and strawberries. These results were obtained from the EU‐project Perfood4 that applied a very sensitive method and investigated pooled samples from a number of European countries. PFOS was detected in 11 out of 24 samples, PFOA in 16 out of 25 samples, PFNA in 9 out of 25 samples and PFHxS in 6 out of 25 samples.
For pooled samples of vegetables from the Perfood project, the detection rates were lower, PFOS, PFOA, PFNA and PFHxS being detected in 9, 28, 7 and 3 out of 62 samples, respectively. The detected levels were in general below LOQs reported by other data providers. Regarding the large consumption of fruit and vegetables, these low levels may still be relevant for exposure. The high detection frequency for fruit and, to a lesser extent, vegetables in the Perfood project was a major reason to assume that all kinds of fruits and vegetables may contain PFASs.
3.1.3. Comparison of previous and current occurrence
Occurrence of PFASs in foods were summarised in the Opinion on PFOS and PFOA in food (EFSA CONTAM Panel, 2018). A direct comparison of reported data is difficult due to a variety of reasons. For example, different laboratories and methodologies lead to different sets of PFASs that are measured; data can be reported with very different LODs/LOQs; much of the literature data did not provide the mean concentration (LB and UB) required for an accurate comparison.
It was concluded that fish was the best studied of all food types in terms of this contaminant group. This is because several PFASs are present in fish at higher concentrations than in other food groups and because they are also useful as a marker of environmental quality resulting in their use as environmental indicators. PFOS is usually the PFAS that is present at the highest concentration in fish and shellfish, and lean predatory fish usually have the highest concentrations.
Many studies that had included a wide range of food types suffered from inadequate sensitivity resulting in left‐censored data. The more recent studies that have been conducted with more sensitive methods (Haug et al., 2010a,b; Lacina et al., 2011; Noorlander et al., 2011; Vestergren et al., 2012) reported concentrations for PFASs with a similar profile to those reported to EFSA and used for the current exposure assessment in this Opinion. For these studies, fish had the highest concentrations of PFOS (0.013–5.4 μg/kg), followed by meat, meat products and chicken eggs which had concentrations of PFOS in the range 0.013–1.281 μg/kg—although there were some anomalies, such as a pooled Swedish egg sample that contained an unusually large amount of PFOS. PFOA has similar concentrations in food samples of both vegetable and animal origin (< 0.003–0.102 μg/kg), whereas PFOS is generally higher in foods of animal origin. These differences in homologue patterns may result from different pollution sources, differences in bioaccumulation or could derive from different uptake and elimination pathways in terrestrial and aquatic food webs.
The concentration ranges reported in the literature for fish were in line with the mean concentrations reported to EFSA. PFOS levels for milk and eggs submitted to EFSA were also consistent to those reported in the literature, while for other foods of animal origin, an accurate comparison was not possible due to limited information (e.g. information on meat type missing). For most foods of plant origin (e.g. olive oil, apple, potatoes etc.), PFAS levels reported to EFSA were usually a little lower than those reported in the literature. Johansson et al. (2014) reported temporal trends in dietary exposure to PFASs. Archived samples of eggs, milk and farmed rainbow trout collected between 1999 and 2010, which covered a period when major production changes occurred, were assessed. The results showed significantly decreasing concentrations of PFOS in fish (p < 0.002) and eggs (p < 0.001). Concentrations of PFOS in fish and eggs decreased by a factor of 10 and 40, respectively. In eggs there was also a statistically significant decreasing trend for PFOA.
There have been several reports in the literature of PFASs in foods published since the Opinion on PFOS and PFOA (EFSA CONTAM Panel, 2018). Most of these have focused on PFOS and PFOA, although some do include other PFASs. In general, these data support the findings described in the current occurrence section above (Section 3.1.1).
In addition to the individual studies, a recent review collates data published in the period 2011–2016 for all PFASs (Domingo and Nadal, 2017). This review updated their previous report (Domingo et al., 2012) that covered the concentrations of PFASs in foodstuffs, human dietary exposure and health risks. Although it is apparent that most of the data published are for PFOS and PFOA, there are also data for other PFASs included in the review. The limited amount of data for other PFASs supports the current occurrence data of PFASs as submitted to EFSA (Section 3.1.1).
A study by Scher et al. (2018) investigated PFASs in garden produce from homes that have elevated levels of PFASs in their municipal water supply. PFBA was the main PFAS present in the water supply, followed by PFPeA. Although PFBA, PFOA and PFOS were present in all soil samples analysed and were found at higher concentrations than the other PFASs, only PFBA was found to be translocated to plants. PFBA in the garden produce was found to be related to water that was applied to the garden via watering and the type of produce grown. The study confirmed that short‐chain PFASs have the highest potential to translocate to and bioaccumulate in edible plants. PFBA was frequently detected across all plant types and parts examined, but differed widely in concentration, with median concentrations ranging from 0.11 mg/kg in stems to 15 mg/kg in florets. PFHxA, PFHxS and PFPeA also had the highest concentrations in florets.
In a review on the accumulation of PFASs in agricultural plants, Ghisi et al. (2019) indicated a direct correlation between PFAS concentrations in soil and bioaccumulation in plants. It was established that plants could uptake PFASs from water, and that the amount of uptake was associated with chain length, functional group, plant species and organ. Low accumulation of PFOA and PFOS was found in peeled potatoes and cereal seeds, while short‐chain compounds were found to be present in substantial amounts in leafy vegetables and fruits.
Short‐chain PFASs are increasing in terms of production volume as they are being used as substitutes for longer chain compounds and are found in water due to their relatively high solubility and mobility (Blum et al., 2015).
In summary, although it is not possible to make a direct comparison with data in the scientific literature due to differences in sampling and reporting, there is broad agreement with the data reported to EFSA that have been used for the exposure assessment. Most data reported in the literature are for fish. There is evidence that PFOS and PFOA concentrations in food are decreasing. Production and use of short‐chain PFASs are increasing, and there is evidence that these compounds may be preferentially taken up by leafy vegetables and fruits.
3.1.4. Food processing
Most studies on food processing focus on PFOS and PFOA with only very limited information in the literature about other PFASs, partly due to the low levels found and insufficiently sensitive analytical methods. Where other PFASs have been measured, these have been discussed in the Opinion on PFOS and PFOA (EFSA CONTAM Panel, 2018).
3.1.4.1. Migration from food contact materials, including non‐stick coatings used on cookware
As described in the Opinion on PFOS and PFOA (EFSA CONTAM Panel, 2018), there are several studies that found polytetrafluoroethylene (PTFE) cookware to contain residual PFOA in the low μg/kg range. These studies concluded that fluoropolymer food contact materials were not likely to be a major source of PFASs. PFCAs, particularly PFOA, and fluorotelomer alcohols (FTOHs) have been shown to be released from coated cookware at normal cooking temperatures (179–233°C surface temperature). Therefore, they have the potential to migrate into food during the cooking process, but studies are inconclusive and show that only relatively small amounts are released into foods, when compared to concentrations that are found in the raw food.
Since the literature was searched for the previous Opinion (EFSA CONTAM Panel, 2018), a comprehensive study of per‐ and polyfluoroalkyl substances in microwave popcorn bags has been published by Zabaleta et al. (2017) where the bags were extracted with methanol using ultrasonification to establish a potential for migration. Different global regions were found to use different PFASs in their products. In bags from European and American countries, short‐chain PFASs were detected, whereas long‐chain PFASs were found to still be used in Asian countries. Whilst the study identified the PFAS compounds in the bags, the potential of PFASs to migrate into food was not fully established.
A study by Schaider et al. (2017) reported the presence of PFASs in grease‐resistant packaging used for fast food. It was found that 46% of food contact papers and 20% of paperboard samples contained detectable fluorine (> 16 nmol/cm2). A subset of 20 samples found PFOA (in six samples) and unidentified PFCAs, PFSAs and other PFASs that were found using a non‐targeted analysis approach with LC/MS. Concentrations in foods as a result of using these packaging materials were not measured.
Jogsten et al. (2009) investigated sources of PFASs in a variety of foodstuffs as a result of food processing and packaging. PFHxS, PFOS, PFHxA and PFOA were detected in at least one sample, whereas PFBS, PFHpA, PFNA, PFDA, PFUnDA and PFDoDA were found to be < LOD in all samples. PFOS was most commonly found (8 out of the 20 composites), and PFHxA was detected in samples of raw veal, chicken nuggets, frankfurter sausages and packaged lettuce. It was not sufficiently clear whether non‐stick cookware, or packaging used for some foods, was a significant source of exposure.
Choi et al. (2018) investigated PFASs in food simulants after migration from fluorocarbon resin‐coated frying pans, baking utensils and non‐stick baking papers on the Korean market. Estimated daily intakes as a result of migration for PFOA, PFNA, PFDoDA, PFTrDA, PFTeDA, PFHxDA and PFODA were 0.35, 0.09, 0.35, 0.2, 0.1, 0.3 and 0.4 ng/kg bw per day, respectively. In this study, repeated use articles were tested in more than one consecutive use, Regulation (EU) No 10/2011, requires that the third consecutive test is used for the evaluation of migration. No PFASs were found to migrate after first use and their exposure assessment reflects a worst‐case scenario.
Hu et al. (2018) investigated whether profiles of PFASs in human serum were able to provide information on major exposure sources. Several associations of potential predictors with serum PFAS concentrations were established, including positive associations (p‐value < 0.05) between serum PFHxS and increased use of Teflon cookware and preheated packaged foods. PFOS was also associated with increased use of packaged foods. Results were adjusted for PFAS exposure sources from other consumer goods, seafood consumption and demographic factors that may influence toxicokinetics, such as race, ethnicity, previous pregnancy and breastfeeding history.
Susmann et al. (2019) investigated associations between serum PFASs and consumption of restaurant food and popcorn in a representative sample of the US population. Associations between serum PFAS and popcorn consumption were found to be a potential consequence of PFAS migration from microwave popcorn bags. Inverse associations between serum PFAS and food eaten at home was consistent with less contact between home‐prepared food and food contact materials, some of which contain PFASs.
In summary, PTFE cookware may contain residual PFOA in the low μg/kg range, and food packaging may contain PFASs where they are used because of their grease‐resistant properties. Studies conducted to date continue to support the conclusions reported in the previous Opinion (EFSA CONTAM Panel, 2018) that the use of this type of material is likely to contribute to human exposure to PFASs, but that the contribution is small compared with other sources of exposure.
3.1.4.2. Effect of cooking not related to coatings on cookware and food contact materials
The 2018 Opinion on PFOS and PFOA (EFSA CONTAM Panel, 2018) summarised the impact of cooking on concentrations of PFASs in foods. The limited number of studies gave an inconsistent view about whether or not losses or increases occur. Domingo (2011) found that it was the cooking process that had the greatest impact rather than the food type that was being cooked, but that different cooking methods could either reduce or increase the levels of chemical contaminants in food. Cooking procedures that released or removed fat from the product generally reduce the total concentrations of organic contaminants, but this was not necessarily the case for PFASs since they are not associated with the fat component of the food in the same way as for other organic contaminants.
Since the literature was searched for EFSA CONTAM Panel (2018), Alves et al. (2017) reported on changes in the bioaccessibility of several contaminants including PFASs, i.e. PFOS and PFUnDA, as a result of cooking of seafood. A range of contaminant classes was studied including brominated flame retardants (i.e. brominated diphenylether 47, brominated diphenylether 100, alpha‐hexabromocyclododecane), pharmaceuticals and personal care products (i.e. venlafaxine, methylparaben and UV‐filter octocrylene). While there was some variation between contaminant class and seafood species, PFASs were found to be generally more available, i.e. to have higher bioaccessibility percentages as a result of cooking when compared to some of the other contaminant classes (between 71% and 95%).
Binnington et al. (2017) studied the effects of preparation on nutrient and environmental contaminant levels in traditional foods derived from the Arctic beluga whale (Delphinapterus leucas), and found that the process of roasting blubber increased concentrations of hydrophilic substances including certain PFASs (PFNA, PFDA, PFUnDA; based on measured concentrations of PFOS and the fact that all of these compounds were not present in oil from the blubber suggesting that concentrations in the remaining blubber after cooking must have increased).
Barbosa et al. (2018) examined the effect of steaming to cook seafood on a range of contaminants of emerging concern, including some PFASs. Out of all the PFASs measured, only PFUnDA, PFDoDA, PFTrDA, PFTeDA and PFOS were detected in raw and steamed samples of Katsuwonus pelamis and Pleuronectes platessa. Other PFASs, like PFPeA, PFHxA, PFHpA, PFOA, PFNA, PFBS, PFHxS, PFHpS and PFDS were not detected (i.e. < LOD) in the analysed species (i.e. P. platessa, Merluccius australis, Merluccius capensis, K. pelamis and Mytilus edulis. In raw samples of M. edulis, only PFDA was detected but not after steaming (< LOD). However, in this species, a large increase for PFBA (> 100%) was detected after steaming. In K. pelamis, cooking by steaming resulted in a significant decrease of PFUnDA (68%), PFDoDA (10%) and PFOS (53%), but a significant increase of PFTrDA (50%) and PFDA (> 100%).
In summary, the literature above covers the few papers describing the impact of cooking on PFASs in foods. This limited number of studies gives an inconsistent view about whether or not losses or increases occur as a result of cooking and processing.
3.2. Dietary exposure assessment
3.2.1. Current exposure assessment
Among the 26 PFASs for which data were available after validation, quantified results were reported for 17 PFASs (Section 3.1.1). The proportion of left‐censored data across food categories was above 90% for all PFASs with the exception of PFOS for which it was 80%. The chronic dietary exposure assessment cannot be performed accurately if a large proportion of left‐censored data is included (EFSA, 2010b; WHO/IPCS, 2009). The exposure is likely to be underestimated with the LB approach whereas it may be overestimated with the UB approach, even to a high extent. For compounds with very high proportions of results below LOD/LOQ and/or only limited availability of data, the exposure calculations should therefore be considered only as a rough indication of the range of chronic dietary exposure. Since this was the case for all the PFASs, results of the present assessment should be interpreted with caution. The CONTAM Panel concluded that the calculated LB exposure is likely to be more realistic than the UB exposure.
The CONTAM Panel assessed the refined dietary chronic exposure (following the methodology described in Section 2.2.1) to the following 17 PFASs: PFBA, PFPeA, PFHxA, PFHpA, PFOA, PFNA, PFDA, PFUnDA, PFDoDA, PFTrDA, PFTeDA, PFBS, PFHxS, PFHpS, PFOS, PFDS and FOSA.
Based on the available data with the limitations described, the chronic dietary exposure to the 17 individual PFASs was estimated to be at the level of a few ng/kg bw per day considering all population groups. By analogy, for the PFASs for which no exposure assessment could be carried out, the chronic dietary exposure is likely to be lower.
Tables 6–12 show summary statistics of the estimated chronic dietary exposure to 17 individual PFASs for each age group using the available occurrence data. Table 13 shows summary statistics of the estimated chronic dietary exposure to the sum of PFOA, PFNA, PFHxS, PFOS for each age group using the available occurrence data. Detailed mean and 95th percentile dietary exposure estimates calculated for all population groups and each of the 35 dietary surveys are presented in Annex A, Table A.5.
Table 6.
Summary statistics of the mean and 95th percentile LB and UB chronic dietary exposure to 17 PFASs (ng/kg bw per day) for infants in 11 surveys across European countries
| PFAS | Range of mean dietary exposure in infants (LB–UB) (ng/kg bw per day) | |||||
|---|---|---|---|---|---|---|
| Mean LB dietary exposure | Mean UB dietary exposure | |||||
| Minimum | Median | Maximum | Minimum | Median | Maximum | |
| PFBA | 0.15 | 0.68 | 2.37 | 2.49 | 8.99 | 19.89 |
| PFPeA | 0.06 | 0.38 | 0.78 | 6.32 | 13.20 | 35.72 |
| PFHxA | 0.29 | 0.63 | 1.85 | 8.76 | 17.55 | 28.74 |
| PFHpA | 0.84 | 2.49 | 6.92 | 8.75 | 15.59 | 24.87 |
| PFOA | 0.11 | 0.19 | 0.39 | 8.88 | 17.33 | 27.76 |
| PFNA | 1.14 | 4.13 | 11.73 | 11.11 | 17.42 | 29.43 |
| PFDA | 0.99 | 3.73 | 10.48 | 10.55 | 16.49 | 27.85 |
| PFUnDA | < 0.01 | 0.01 | 0.22 | 10.34 | 18.83 | 29.37 |
| PFDoDA | 1.85 | 7.40 | 21.28 | 13.72 | 21.44 | 43.41 |
| PFTrDA | < 0.01 | 0.01 | 0.13 | 3.85 | 11.50 | 22.24 |
| PFTeDA | < 0.01 | < 0.01 | 0.01 | 2.37 | 6.60 | 18.35 |
| PFBS | 0.03 | 0.10 | 0.12 | 11.37 | 18.73 | 30.47 |
| PFHxS | 0.09 | 0.19 | 0.36 | 11.00 | 16.74 | 29.03 |
| PFHpS | < 0.01 | < 0.01 | < 0.01 | 5.48 | 11.13 | 26.67 |
| PFOS | 0.23 | 0.36 | 1.26 | 11.78 | 20.10 | 31.44 |
| PFDS | < 0.01 | < 0.01 | < 0.01 | 5.05 | 10.81 | 26.47 |
| FOSA | < 0.01 | 0.01 | 0.14 | 15.78 | 30.52 | 47.63 |
| PFAS | Range of 95th percentile dietary exposure in infants (LB–UB) (ng/kg bw per day) | |||||
| 95th percentile LB dietary exposure | 95th percentile UB dietary exposure | |||||
| Minimum | Median | Maximum | Minimum | Median | Maximum | |
| PFBA | 0.63 | 2.22 | 4.86 | 8.37 | 21.21 | 49.68 |
| PFPeA | 0.15 | 1.15 | 2.87 | 22.17 | 36.53 | 70.87 |
| PFHxA | 1.47 | 1.81 | 3.86 | 21.49 | 27.74 | 58.17 |
| PFHpA | 1.97 | 7.68 | 16.05 | 17.32 | 25.76 | 45.44 |
| PFOA | 0.37 | 0.58 | 1.02 | 21.83 | 26.88 | 54.70 |
| PFNA | 2.77 | 13.16 | 27.88 | 21.38 | 31.40 | 57.54 |
| PFDA | 2.63 | 11.70 | 24.93 | 20.76 | 29.94 | 54.52 |
| PFUnDA | 0.01 | 0.02 | 1.41 | 21.08 | 30.39 | 54.10 |
| PFDoDA | 4.92 | 23.74 | 50.70 | 23.47 | 45.99 | 84.67 |
| PFTrDA | < 0.01 | 0.02 | 0.85 | 10.04 | 22.02 | 42.56 |
| PFTeDA | 0.01 | 0.01 | 0.06 | 5.09 | 20.05 | 41.99 |
| PFBS | 0.13 | 0.18 | 0.42 | 22.28 | 32.31 | 59.77 |
| PFHxS | 0.38 | 0.51 | 0.86 | 20.11 | 31.25 | 57.63 |
| PFHpS | < 0.01 | < 0.01 | 0.01 | 10.60 | 27.84 | 54.19 |
| PFOS | 0.74 | 1.59 | 4.15 | 26.36 | 32.56 | 61.95 |
| PFDS | < 0.01 | < 0.01 | 0.01 | 10.04 | 28.02 | 54.19 |
| FOSA | 0.02 | 0.03 | 0.65 | 37.83 | 48.84 | 90.22 |
Table 12.
Summary statistics of the mean and 95th percentile LB and UB chronic dietary exposure to 17 PFASs (ng/kg bw per day) for the very elderly in 14 surveys across European countries
| PFAS | Range of mean dietary exposure in the very elderly (LB–UB) (ng/kg bw per day) | |||||
|---|---|---|---|---|---|---|
| Mean LB dietary exposure | Mean UB dietary exposure | |||||
| Minimum | Median | Maximum | Minimum | Median | Maximum | |
| PFBA | 0.12 | 0.38 | 0.50 | 2.29 | 2.75 | 3.54 |
| PFPeA | 0.02 | 0.09 | 0.25 | 4.23 | 5.56 | 6.75 |
| PFHxA | 0.23 | 0.33 | 0.42 | 3.20 | 3.97 | 4.73 |
| PFHpA | 0.06 | 0.11 | 0.15 | 2.16 | 2.69 | 3.52 |
| PFOA | 0.08 | 0.15 | 0.37 | 3.29 | 4.08 | 4.82 |
| PFNA | 0.02 | 0.04 | 0.15 | 2.85 | 3.63 | 4.25 |
| PFDA | 0.04 | 0.05 | 0.14 | 2.72 | 3.50 | 4.11 |
| PFUnDA | 0.01 | 0.02 | 0.05 | 2.83 | 3.68 | 4.38 |
| PFDoDA | 0.01 | 0.02 | 0.07 | 2.56 | 3.07 | 3.94 |
| PFTrDA | < 0.01 | 0.01 | 0.03 | 0.48 | 0.68 | 0.88 |
| PFTeDA | < 0.01 | < 0.01 | 0.01 | 0.42 | 0.51 | 0.73 |
| PFBS | 0.02 | 0.03 | 0.04 | 3.17 | 4.07 | 4.72 |
| PFHxS | 0.04 | 0.08 | 0.11 | 2.69 | 3.41 | 4.04 |
| PFHpS | < 0.01 | < 0.01 | < 0.01 | 0.65 | 0.80 | 0.93 |
| PFOS | 0.28 | 0.57 | 2.62 | 3.61 | 4.42 | 7.03 |
| PFDS | < < 0.01 | < 0.01 | < 0.01 | 0.51 | 0.66 | 1.06 |
| FOSA | 0.01 | 0.03 | 0.21 | 7.21 | 9.54 | 12.10 |
| PFAS | Range of 95th percentile dietary exposure in the very elderly (LB–UB) (ng/kg bw per day) | |||||
| 95th percentile LB dietary exposure | 95th percentile UB dietary exposure | |||||
| Minimum | Median | Maximum | Minimum | Median | Maximum | |
| PFBA | 0.39 | 0.87 | 1.47 | 3.22 | 4.47 | 5.19 |
| PFPeA | 0.05 | 0.41 | 1.56 | 7.96 | 9.22 | 12.72 |
| PFHxA | 0.47 | 0.65 | 0.95 | 5.41 | 7.27 | 10.51 |
| PFHpA | 0.12 | 0.21 | 0.30 | 3.93 | 5.39 | 8.99 |
| PFOA | 0.21 | 0.32 | 2.06 | 5.56 | 7.23 | 10.81 |
| PFNA | 0.05 | 0.11 | 0.39 | 5.10 | 6.82 | 10.27 |
| PFDA | 0.08 | 0.13 | 0.88 | 4.97 | 6.69 | 10.17 |
| PFUnDA | 0.04 | 0.06 | 0.26 | 5.18 | 6.61 | 10.06 |
| PFDoDA | 0.03 | 0.05 | 0.39 | 4.65 | 5.72 | 9.80 |
| PFTrDA | 0.01 | 0.04 | 0.14 | 0.91 | 1.23 | 1.74 |
| PFTeDA | < 0.01 | 0.01 | 0.08 | 0.75 | 0.87 | 1.26 |
| PFBS | 0.05 | 0.06 | 0.21 | 5.53 | 7.40 | 10.58 |
| PFHxS | 0.09 | 0.15 | 0.23 | 4.86 | 6.55 | 9.88 |
| PFHpS | < 0.01 | < 0.01 | < 0.01 | 1.06 | 1.35 | 1.62 |
| PFOS | 1.03 | 1.83 | 7.40 | 6.25 | 8.37 | 12.27 |
| PFDS | < 0.01 | < 0.01 | < 0.01 | 1.10 | 1.27 | 1.79 |
| FOSA | 0.01 | 0.13 | 1.28 | 11.67 | 15.85 | 20.45 |
Table 13.
Summary statistics of the mean and 95th percentile for the combined LB and UB chronic dietary exposure to PFOA, PFNA, PFHxS and PFOS (ng/kg bw per day) for each age group across surveys
| Age group | Range of mean dietary exposure (LB–UB) (ng/kg bw per day) | |||||
|---|---|---|---|---|---|---|
| Mean LB dietary exposure | Mean UB dietary exposure | |||||
| Minimum | Median | Maximum | Minimum | Median | Maximum | |
| Infants | 2.39 | 4.87 | 12.19 | 42.77 | 71.44 | 114.62 |
| Toddlers | 1.47 | 2.94 | 6.51 | 61.20 | 74.17 | 112.09 |
| Other children | 0.84 | 1.54 | 3.07 | 38.50 | 53.23 | 81.78 |
| Adolescents | 0.42 | 0.84 | 1.52 | 20.59 | 26.48 | 41.45 |
| Adults | 0.55 | 0.92 | 1.34 | 13.54 | 15.94 | 21.97 |
| Elderly | 0.71 | 0.89 | 2.08 | 11.51 | 15.07 | 18.77 |
| Very elderly | 0.42 | 0.86 | 3.10 | 12.56 | 15.41 | 19.85 |
| Age group | Range of 95th percentile dietary exposure (LB–UB) (ng/kg bw per day) | |||||
| 95th percentile LB dietary exposure | 95th percentile UB dietary exposure | |||||
| Minimum | Median | Maximum | Minimum | Median | Maximum | |
| Infants | 4.50 | 13.65 | 27.88 | 92.77 | 122.25 | 224.84 |
| Toddlers | 3.35 | 7.55 | 13.69 | 100.65 | 134.01 | 229.04 |
| Children | 2.66 | 4.21 | 9.69 | 78.97 | 109.03 | 165.31 |
| Adolescents | 1.27 | 2.13 | 5.22 | 44.17 | 57.04 | 89.40 |
| Adults | 1.30 | 2.29 | 5.04 | 26.29 | 32.78 | 62.70 |
| Elderly | 1.76 | 2.37 | 5.58 | 22.96 | 28.77 | 46.73 |
| Very elderly | 1.32 | 2.23 | 9.93 | 21.90 | 28.32 | 42.03 |
LB: lower bound; PFOA: perfluorooctanoic acid; PFNA: perfluorononanoic acid; PFHxS: perfluorohexane sulfonic acid; PFOS: perfluoroheptane sulfonate.
Table 7.
Summary statistics of the mean and 95th percentile LB and UB chronic dietary exposure to 17 PFASs (ng/kg bw per day) for toddlers in 14 surveys across European countries
| PFAS | Range of mean dietary exposure in toddlers (LB–UB) (ng/kg bw per day) | |||||
|---|---|---|---|---|---|---|
| Mean LB dietary exposure | Mean UB dietary exposure | |||||
| Minimum | Median | Maximum | Minimum | Median | Maximum | |
| PFBA | 0.53 | 0.96 | 1.82 | 7.96 | 10.50 | 11.37 |
| PFPeA | 0.18 | 0.40 | 1.34 | 17.45 | 20.76 | 23.96 |
| PFHxA | 0.80 | 0.89 | 1.37 | 16.79 | 18.87 | 29.37 |
| PFHpA | 0.36 | 0.73 | 2.20 | 11.69 | 14.69 | 23.27 |
| PFOA | 0.25 | 0.41 | 0.59 | 16.58 | 18.87 | 29.03 |
| PFNA | 0.25 | 0.81 | 3.42 | 14.13 | 17.71 | 27.35 |
| PFDA | 0.21 | 0.80 | 3.08 | 13.38 | 17.02 | 26.51 |
| PFUnDA | 0.01 | 0.04 | 0.46 | 14.94 | 17.92 | 27.47 |
| PFDoDA | 0.28 | 1.29 | 6.05 | 14.29 | 17.81 | 25.37 |
| PFTrDA | 0.01 | 0.03 | 0.29 | 2.36 | 4.48 | 7.78 |
| PFTeDA | < 0.01 | 0.01 | 0.07 | 1.38 | 2.46 | 6.26 |
| PFBS | 0.07 | 0.09 | 0.14 | 15.62 | 18.92 | 28.96 |
| PFHxS | 0.19 | 0.23 | 0.32 | 13.03 | 16.86 | 26.33 |
| PFHpS | < 0.01 | < 0.01 | < 0.01 | 3.61 | 4.69 | 9.28 |
| PFOS | 0.70 | 1.34 | 2.58 | 17.41 | 20.43 | 29.38 |
| PFDS | < 0.01 | < 0.01 | < 0.01 | 3.01 | 4.28 | 9.25 |
| FOSA | 0.02 | 0.08 | 0.23 | 31.26 | 35.68 | 48.62 |
| PFAS | Range of 95th percentile dietary exposure in toddlers (LB–UB) (ng/kg bw per day) | |||||
| 95th percentile LB dietary exposure | 95th percentile UB dietary exposure | |||||
| Minimum | Median | Maximum | Minimum | Median | Maximum | |
| PFBA | 1.54 | 2.57 | 4.03 | 13.82 | 17.40 | 21.60 |
| PFPeA | 0.46 | 1.04 | 5.18 | 30.73 | 34.02 | 44.63 |
| PFHxA | 1.46 | 1.98 | 2.67 | 26.61 | 33.06 | 60.85 |
| PFHpA | 0.81 | 2.39 | 6.63 | 21.46 | 28.62 | 53.73 |
| PFOA | 0.59 | 0.88 | 1.23 | 26.63 | 33.44 | 59.09 |
| PFNA | 0.64 | 3.58 | 11.32 | 24.18 | 32.89 | 56.46 |
| PFDA | 0.61 | 3.53 | 10.09 | 23.25 | 32.54 | 55.01 |
| PFUnDA | 0.06 | 0.14 | 1.79 | 25.17 | 32.63 | 60.16 |
| PFDoDA | 0.97 | 6.24 | 20.55 | 24.01 | 36.70 | 56.35 |
| PFTrDA | 0.04 | 0.10 | 1.17 | 4.96 | 9.26 | 17.71 |
| PFTeDA | 0.01 | 0.04 | 0.31 | 2.68 | 6.90 | 17.70 |
| PFBS | 0.14 | 0.17 | 0.23 | 25.98 | 33.26 | 58.83 |
| PFHxS | 0.43 | 0.48 | 0.80 | 22.99 | 31.70 | 54.90 |
| PFHpS | < 0.01 | < 0.01 | 0.01 | 6.09 | 10.83 | 22.45 |
| PFOS | 2.05 | 3.77 | 6.89 | 27.59 | 36.48 | 58.85 |
| PFDS | < 0.01 | < 0.01 | 0.01 | 5.90 | 10.44 | 22.44 |
| FOSA | 0.06 | 0.31 | 3.32 | 47.33 | 55.86 | 92.17 |
Table 8.
Summary statistics of the mean and 95th percentile LB and UB chronic dietary exposure to 17 PFASs (ng/kg bw per day) for other children in 19 surveys across European countries
| PFAS | Range of mean dietary exposure in other children (LB–UB) (ng/kg bw per day) | |||||
|---|---|---|---|---|---|---|
| Mean LB dietary exposure | Mean UB dietary exposure | |||||
| Minimum | Median | Maximum | Minimum | Median | Maximum | |
| PFBA | 0.40 | 0.77 | 1.50 | 5.48 | 7.32 | 10.07 |
| PFPeA | 0.09 | 0.28 | 0.50 | 9.47 | 14.49 | 19.32 |
| PFHxA | 0.42 | 0.65 | 1.17 | 10.25 | 13.88 | 21.51 |
| PFHpA | 0.10 | 0.26 | 0.33 | 6.80 | 10.15 | 17.13 |
| PFOA | 0.16 | 0.30 | 0.63 | 10.06 | 13.82 | 21.23 |
| PFNA | 0.05 | 0.12 | 0.24 | 9.29 | 12.51 | 19.86 |
| PFDA | 0.07 | 0.14 | 0.26 | 8.86 | 12.05 | 19.18 |
| PFUnDA | 0.01 | 0.02 | 0.24 | 9.13 | 13.04 | 19.84 |
| PFDoDA | 0.02 | 0.08 | 0.33 | 7.50 | 11.50 | 18.34 |
| PFTrDA | 0.01 | 0.02 | 0.16 | 1.29 | 2.06 | 3.56 |
| PFTeDA | < 0.01 | 0.01 | 0.03 | 0.85 | 1.23 | 1.52 |
| PFBS | 0.03 | 0.07 | 0.10 | 10.33 | 13.50 | 21.18 |
| PFHxS | 0.07 | 0.16 | 0.25 | 8.78 | 11.73 | 19.02 |
| PFHpS | < 0.01 | < 0.01 | < 0.01 | 1.73 | 2.53 | 3.70 |
| PFOS | 0.52 | 1.02 | 2.28 | 10.37 | 14.95 | 21.66 |
| PFDS | < 0.01 | < 0.01 | < 0.01 | 1.42 | 2.11 | 3.38 |
| FOSA | 0.02 | 0.06 | 0.54 | 20.21 | 26.36 | 36.69 |
| PFAS | Range of 95th percentile dietary exposure in other children (LB–UB) (ng/kg bw per day) | |||||
| 95th percentile LB dietary exposure | 95th percentile UB dietary exposure | |||||
| Minimum | Median | Maximum | Minimum | Median | Maximum | |
| PFBA | 1.23 | 2.02 | 3.27 | 8.72 | 12.39 | 15.19 |
| PFPeA | 0.26 | 0.75 | 2.48 | 15.89 | 25.05 | 35.27 |
| PFHxA | 0.90 | 1.36 | 2.32 | 20.24 | 26.93 | 41.79 |
| PFHpA | 0.24 | 0.50 | 0.74 | 16.13 | 21.48 | 36.34 |
| PFOA | 0.40 | 0.65 | 1.03 | 19.97 | 27.67 | 41.74 |
| PFNA | 0.14 | 0.28 | 0.95 | 19.18 | 25.90 | 40.70 |
| PFDA | 0.18 | 0.31 | 1.49 | 18.80 | 25.31 | 40.42 |
| PFUnDA | 0.05 | 0.09 | 1.11 | 18.64 | 25.31 | 39.13 |
| PFDoDA | 0.05 | 0.14 | 1.50 | 17.22 | 23.14 | 37.11 |
| PFTrDA | 0.04 | 0.07 | 0.66 | 2.68 | 4.14 | 5.64 |
| PFTeDA | 0.01 | 0.03 | 0.15 | 1.40 | 2.05 | 3.09 |
| PFBS | 0.06 | 0.12 | 0.19 | 20.05 | 26.10 | 41.69 |
| PFHxS | 0.21 | 0.35 | 0.58 | 18.79 | 24.54 | 40.54 |
| PFHpS | < 0.01 | < 0.01 | < 0.01 | 2.97 | 4.58 | 5.66 |
| PFOS | 1.92 | 3.12 | 8.51 | 20.27 | 29.48 | 42.05 |
| PFDS | < 0.01 | < 0.01 | 0.01 | 2.79 | 4.15 | 5.61 |
| FOSA | 0.09 | 0.23 | 2.93 | 34.22 | 45.12 | 67.20 |
Table 9.
Summary statistics of the mean and 95th percentile LB and UB chronic dietary exposure to 17 PFASs (ng/kg bw per day) for adolescents in 18 surveys across European countries
| PFAS | Range of mean dietary exposure in adolescents (LB–UB) (ng/kg bw per day) | |||||
|---|---|---|---|---|---|---|
| Mean LB dietary exposure | Mean UB dietary exposure | |||||
| Minimum | Median | Maximum | Minimum | Median | Maximum | |
| PFBA | 0.22 | 0.43 | 0.93 | 2.43 | 3.95 | 5.26 |
| PFPeA | 0.05 | 0.11 | 0.21 | 4.34 | 6.96 | 11.83 |
| PFHxA | 0.23 | 0.36 | 0.65 | 5.36 | 6.99 | 10.67 |
| PFHpA | 0.05 | 0.15 | 0.21 | 4.09 | 4.96 | 8.68 |
| PFOA | 0.09 | 0.17 | 0.31 | 5.36 | 7.00 | 10.78 |
| PFNA | 0.02 | 0.04 | 0.08 | 4.93 | 6.25 | 9.92 |
| PFDA | 0.02 | 0.06 | 0.10 | 4.78 | 6.01 | 9.66 |
| PFUnDA | < 0.01 | 0.01 | 0.12 | 5.28 | 6.49 | 10.27 |
| PFDoDA | 0.01 | 0.02 | 0.09 | 4.37 | 5.57 | 9.35 |
| PFTrDA | < 0.01 | 0.01 | 0.08 | 0.58 | 1.07 | 1.53 |
| PFTeDA | < 0.01 | < 0.01 | 0.02 | 0.45 | 0.68 | 0.87 |
| PFBS | 0.02 | 0.04 | 0.06 | 5.56 | 7.00 | 10.78 |
| PFHxS | 0.04 | 0.07 | 0.16 | 4.72 | 5.90 | 9.54 |
| PFHpS | < 0.01 | < 0.01 | < 0.01 | 0.74 | 1.23 | 1.69 |
| PFOS | 0.23 | 0.53 | 1.10 | 5.57 | 7.30 | 11.20 |
| PFDS | < 0.01 | < 0.01 | < 0.01 | 0.53 | 1.00 | 1.42 |
| FOSA | 0.01 | 0.04 | 0.52 | 11.07 | 13.17 | 18.42 |
| PFAS | Range of 95th percentile dietary exposure in adolescents (LB–UB) (ng/kg bw per day) | |||||
| 95th percentile LB dietary exposure | 95th percentile UB dietary exposure | |||||
| Minimum | Median | Maximum | Minimum | Median | Maximum | |
| PFBA | 0.76 | 1.03 | 2.04 | 4.56 | 6.90 | 9.40 |
| PFPeA | 0.12 | 0.26 | 1.18 | 9.44 | 13.62 | 19.78 |
| PFHxA | 0.51 | 0.80 | 1.29 | 11.33 | 14.62 | 22.57 |
| PFHpA | 0.11 | 0.29 | 0.38 | 9.54 | 11.56 | 20.65 |
| PFOA | 0.23 | 0.40 | 0.59 | 11.33 | 14.59 | 22.64 |
| PFNA | 0.06 | 0.10 | 0.29 | 10.85 | 13.56 | 22.03 |
| PFDA | 0.08 | 0.16 | 0.36 | 10.59 | 13.28 | 21.83 |
| PFUnDA | 0.03 | 0.06 | 0.53 | 11.06 | 13.32 | 22.15 |
| PFDoDA | 0.03 | 0.05 | 0.47 | 10.38 | 12.55 | 21.44 |
| PFTrDA | 0.02 | 0.05 | 0.32 | 1.28 | 2.12 | 3.36 |
| PFTeDA | 0.00 | 0.01 | 0.08 | 0.85 | 1.19 | 1.54 |
| PFBS | 0.03 | 0.07 | 0.12 | 11.63 | 14.69 | 22.72 |
| PFHxS | 0.13 | 0.19 | 0.29 | 10.55 | 13.12 | 21.66 |
| PFHpS | 0.00 | 0.00 | 0.00 | 1.45 | 2.27 | 3.50 |
| PFOS | 0.85 | 1.59 | 4.95 | 11.84 | 15.78 | 23.43 |
| PFDS | 0.00 | 0.00 | 0.00 | 1.17 | 2.06 | 3.16 |
| FOSA | 0.04 | 0.15 | 2.86 | 20.35 | 24.69 | 31.70 |
Table 11.
Summary statistics of the mean and 95th percentile LB and UB chronic dietary exposure to 17 PFASs (ng/kg bw per day) for the elderly in 18 surveys across European countries
| PFAS | Range of mean dietary exposure in the elderly (LB–UB) (ng/kg bw per day) | |||||
|---|---|---|---|---|---|---|
| Mean LB dietary exposure | Mean UB dietary exposure | |||||
| Minimum | Median | Maximum | Minimum | Median | Maximum | |
| PFBA | 0.12 | 0.36 | 0.47 | 2.07 | 2.60 | 3.29 |
| PFPeA | 0.05 | 0.08 | 0.23 | 3.81 | 5.45 | 6.31 |
| PFHxA | 0.22 | 0.33 | 0.40 | 2.96 | 3.78 | 4.68 |
| PFHpA | 0.06 | 0.10 | 0.14 | 2.06 | 2.58 | 3.53 |
| PFOA | 0.15 | 0.17 | 0.29 | 3.04 | 4.01 | 4.88 |
| PFNA | 0.03 | 0.04 | 0.09 | 2.66 | 3.46 | 4.36 |
| PFDA | 0.03 | 0.06 | 0.09 | 2.51 | 3.35 | 4.25 |
| PFUnDA | 0.01 | 0.02 | 0.08 | 2.75 | 3.49 | 4.50 |
| PFDoDA | 0.01 | 0.02 | 0.05 | 2.38 | 3.01 | 4.07 |
| PFTrDA | < 0.01 | 0.01 | 0.05 | 0.45 | 0.66 | 0.82 |
| PFTeDA | < 0.01 | < 0.01 | 0.01 | 0.42 | 0.48 | 0.70 |
| PFBS | 0.03 | 0.03 | 0.04 | 3.07 | 3.84 | 4.76 |
| PFHxS | 0.06 | 0.08 | 0.11 | 2.49 | 3.26 | 4.17 |
| PFHpS | < 0.01 | < 0.01 | < 0.01 | 0.56 | 0.76 | 0.91 |
| PFOS | 0.45 | 0.59 | 1.66 | 3.33 | 4.41 | 5.96 |
| PFDS | < 0.01 | < 0.01 | < 0.01 | 0.51 | 0.65 | 0.99 |
| FOSA | 0.01 | 0.03 | 0.30 | 7.20 | 9.18 | 11.44 |
| PFAS | Range of 95th percentile dietary exposure in the elderly (LB–UB) (ng/kg bw per day) | |||||
| 95th percentile LB dietary exposure | 95th percentile UB dietary exposure | |||||
| Minimum | Median | Maximum | Minimum | Median | Maximum | |
| PFBA | 0.49 | 0.82 | 1.21 | 3.32 | 4.20 | 5.23 |
| PFPeA | 0.12 | 0.31 | 1.13 | 6.96 | 10.21 | 11.09 |
| PFHxA | 0.46 | 0.64 | 0.81 | 5.52 | 7.14 | 11.65 |
| PFHpA | 0.13 | 0.22 | 0.26 | 3.49 | 5.44 | 10.21 |
| PFOA | 0.27 | 0.36 | 1.13 | 6.10 | 7.33 | 12.03 |
| PFNA | 0.08 | 0.10 | 0.19 | 4.88 | 6.67 | 11.39 |
| PFDA | 0.09 | 0.13 | 0.29 | 5.07 | 6.49 | 11.25 |
| PFUnDA | 0.04 | 0.07 | 0.32 | 4.71 | 6.69 | 11.15 |
| PFDoDA | 0.03 | 0.05 | 0.21 | 4.37 | 6.12 | 10.88 |
| PFTrDA | 0.01 | 0.05 | 0.19 | 0.88 | 1.17 | 1.67 |
| PFTeDA | < 0.01 | 0.01 | 0.07 | 0.75 | 0.88 | 1.32 |
| PFBS | 0.05 | 0.06 | 0.09 | 5.32 | 7.25 | 11.64 |
| PFHxS | 0.14 | 0.18 | 0.22 | 4.58 | 6.42 | 11.02 |
| PFHpS | < 0.01 | < 0.01 | < 0.01 | 1.07 | 1.29 | 1.63 |
| PFOS | 1.32 | 1.87 | 4.30 | 6.54 | 8.27 | 13.49 |
| PFDS | < 0.01 | < 0.01 | < 0.01 | 1.03 | 1.35 | 1.87 |
| FOSA | 0.02 | 0.10 | 1.98 | 12.35 | 15.43 | 20.39 |
Based on the medians across food surveys for the mean exposure, highest exposure for adults, adolescents, elderly and other children was for PFOS, followed by PFBA, PFHxA, PFOA and either PFHpA or PFPeA. For infants, the highest median mean LB exposure was to PFDoDA, followed by PFNA, PFDA, PFHpA, PFBA and PFHxA, so only a minor contribution for PFOS and PFOA. For toddlers, PFOS was most important, as for older age groups, but this was followed by PFDoDA and then PFBA, PFHxA, PFNA and PFDA. This implies that the median mean LB exposure to PFNA and PFHxS was relatively small for most age groups
In adults, the median of mean LB contribution to the sum of the four PFASs was for PFOA 21%, PFNA 4%, PFHxS 10% and PFOS 66%. The contributions were similar in other children, adolescents, elderly and very elderly. Overall, across surveys in adults, the median contribution of these four was 46% (range 33–56%) to the sum of all PFASs for which the exposure was calculated, with contributions of 9%, 2%, 4% and 30% for, respectively, PFOA, PFNA, PFHxS and PFOS. Other PFASs that contributed more than 5% to this sum were PFBA (16%) and PFHxA (15%). This is illustrated in Figure 5.
Figure 5.
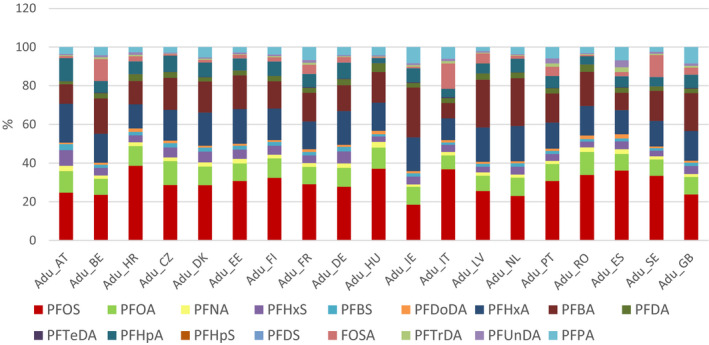
Percent contribution of each perfluoroalkyl substance (PFAS) to total exposure to 17 PFASs in adults
For infants and toddlers, the median of mean contribution of PFNA to the sum of the four PFASs was, respectively, 79% and 26% (Table 14). This was highly influenced by one detected sample out of 10 samples in the food category ‘Food for infants and small children’, and this sample had relatively high PFNA concentration. Since the proportions of contributions across the other age groups are similar as in adults, this high contribution from PFNA is not expected to be representative for the usual exposure in infants and toddlers. This is further elaborated in Section 3.2.1.1. Also the LB exposure of infants to PFDoDA (Table 6) was affected by the single sample with a quantified level, (details are provided in Annex A, Table A.7).
Table 14.
Mean contribution (%) of PFOA, PFNA, PFHxS and PFOS to the dietary exposure to the sum of the four PFASs (minimum, medium, maximum across surveys)
| PFOA | PFNA | PFHxS | PFOS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Med | Max | Min | Med | Max | Min | Med | Max | Min | Med | Max | |
| Infants | 1 | 4 | 12 | 48 | 79 | 96 | 1 | 4 | 13 | 2 | 9 | 28 |
| Toddlers | 7 | 14 | 22 | 10 | 26 | 63 | 4 | 8 | 15 | 21 | 47 | 66 |
| Other children | 14 | 18 | 24 | 4 | 6 | 14 | 5 | 10 | 14 | 57 | 64 | 74 |
| Adolescents | 14 | 21 | 24 | 3 | 5 | 7 | 6 | 10 | 16 | 55 | 64 | 72 |
| Adults | 14 | 21 | 28 | 3 | 4 | 6 | 5 | 10 | 17 | 53 | 66 | 72 |
| Elderly | 12 | 20 | 23 | 4 | 5 | 6 | 4 | 9 | 12 | 63 | 66 | 80 |
| Very elderly | 8 | 19 | 23 | 3 | 5 | 7 | 3 | 9 | 12 | 60 | 68 | 84 |
PFAS: perfluoroalkyl substance; PFOA: perfluorooctanoic acid; PFNA: perfluorononanoic acid; PFHxS: perfluorohexane sulfonic acid; PFOS: perfluoroheptane sulfonate.
3.2.1.1. Relative contribution from different food groups
The contribution (%) of each food category to the overall mean LB exposure for each PFAS, included in the exposure assessment, was calculated for each age group and dietary survey. It was decided to do this only for the mean LB exposure. The relative contribution of the different food categories is detailed in Annex A, Tables A.6 and A.8, and shown for adults and toddlers for PFOA, PFNA, PFOS and PFHxS in Figures 6, 7, 8–9, respectively. Similar FoodEx 1 food groups are shown for these PFASs, and no other food groups contributed to the LB exposure. Results should be interpreted with caution as neither all nor the same food categories were analysed for the various PFASs and the proportion of left‐censored data across food categories was above 89% for all PFASs. In principle, the relative contribution is determined by both occurrence and the amounts consumed, implying that food categories with high levels but rarely consumed may not be very relevant for mean exposure (like offal of wild game). It should also be pointed out that in some cases the mean level for a certain food category was only based on very few quantified results.
Figure 6.
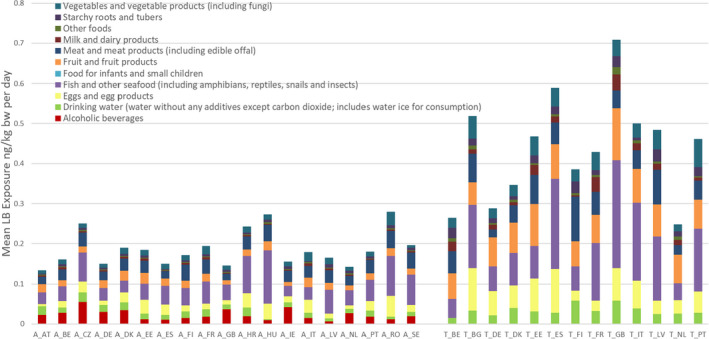
- LB: lower bound; PFOA: perfluorooctanoic acid.
Figure 7.
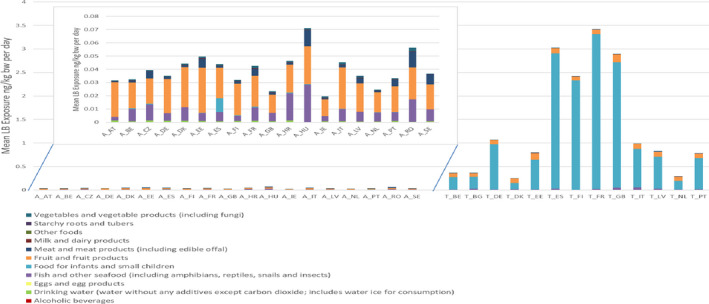
Mean LB exposure from different food categories of adults (A, left, inserted with different scale as toddlers) and toddlers (T, right) to PFNA, for various surveys
- LB: lower bound; PFOA: perfluorooctanoic acid; PFNA: perfluorononanoic acid. Note: It should be stressed that the exposure from food for infants and small children was based on only one quantified level in 10 samples. This sample had a quantified PFNA level above the mean in liver from farmed animals, and PFOS or PFOA were not detected in the same sample, which is unexpected.
Figure 8.
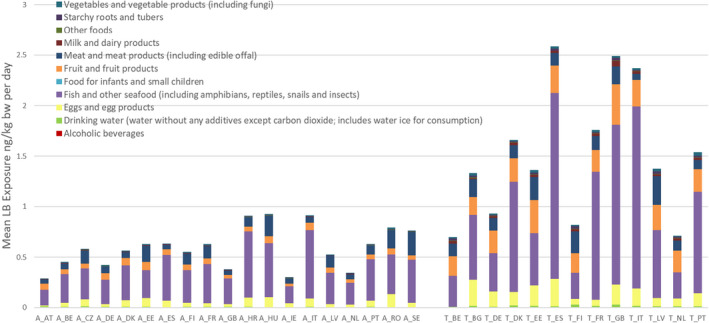
- LB: lower bound; PFOS: perfluoroheptane sulfonate.
Figure 9.
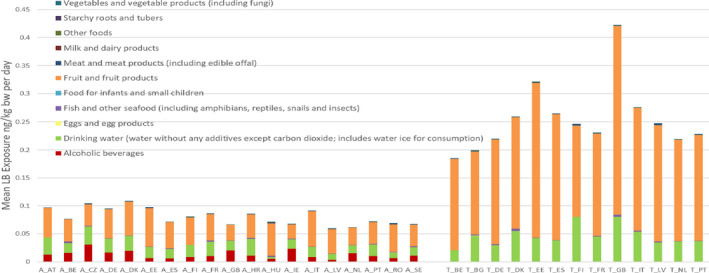
- LB: lower bound; PFHxS: perfluorohexane sulfonic acid.
As shown in Figure 8, for PFOS, ‘Fish and other seafood’ was the most important contributor to the mean LB exposure, followed by ‘Eggs and egg products’, ‘Meat and meat products’, and especially for children also ‘Fruit and fruit products’. Although to a lesser extent, ‘Fish and other seafood’ was also an important contributor for PFOA (see Figure 6), again followed by ‘Eggs and egg products’, ‘Meat and meat products’, but also ‘Fruit and fruit products’, ‘Vegetables and vegetable products’ and ‘Drinking water’.
‘Fish and other seafood’ was also among the main contributing food categories for most age groups for FOSA, PFPeA, PFNA, PFDA, PFUnDA, PFDoDA, PFTrDA and PFTeDA. ‘Fruit and fruit products’ was also among the main contributing food categories for most age groups for PFHxA, PFNA, PFTeDA, PFBS and PFHxS. ‘Vegetables and vegetable products’ was also among the main contributing food categories for most age groups for PFDoDA and PFDS. In addition to PFOA, ‘Drinking water’ was among the main contributing food categories for most age groups for PFHxA, PFHpA, PFBS and PFHxS.
‘Grains and grain‐based products’ was among the main contributing food categories for most age groups for PFDS. ‘Starchy roots and tubers’ was among the main contributing food categories for most age groups for PFBA, PFHxA, PFHpA, PFDA and PFBS. For PFBA, this is based on just one quantified result out of three samples, with a relatively high level. ‘Liquid milk’ was a main contributing food category for all age groups for PFPeA, but based on 1 quantified result out of 13 samples. For children, infants and toddlers, ‘Food for infants and small children’ was a main contributing category for PFDA, PFHpA, PFNA and PFDoDA. It should be stressed that this was based on 10 samples with only one quantified level for each of these PFASs. For PFNA, this sample had a quantified level above the mean in liver from farmed animals, and PFOS or PFOA were not detected in the same sample. Similarly, ‘Alcoholic beverages’ was a main contributing category for PFHxS and PFOA in adults, elderly and very elderly, but again based on only one quantified result out of six samples.
For the combined exposure to PFOA, PFOS, PFHxS and PFNA, the main contributing categories were ‘Fish meat’ and ‘Fruit and fruit products’ for all population groups and ‘Food for infants and small children’ was a main contributor for children, toddlers and infants, but only based on one quantified level for PFNA. The category ‘Eggs and egg products’ was also a main contributor for all population groups except infants, based on detected levels for PFOS and PFOA in 15 and 14 samples out of 174 and 177, respectively.
3.2.1.2. Exposure of breastfed infants
To assess the exposure of breastfed infants below 6 months of age, a median age of 3 months was selected, equivalent to a body weight of about 6.1 kg, with an estimated average daily milk consumption of about 800 mL and a high consumption of 1,200 mL. Levels in milk have been investigated but were often below the LOQs of the method. However, some studies determined the ratio between milk levels and maternal serum levels, being on average 0.015 for PFOS and 0.03 for PFOA. Based on the mean serum levels in adults of 7.5 ng/mL for PFOS and 2.1 ng/mL for PFOA (see Section 3.3.2), this would result in milk levels of 0.113 and 0.063 ng/mL, respectively.
For PFOS, a daily average and high consumption of 800 or 1,200 mL milk (EFSA NDA Panel, 2013) would result in an intake of 90 and 135 ng PFOS per day or, respectively, 15 and 22 ng/kg bw per day (103 and 155 ng/kg bw per week). For PFOA, the daily intake for average and high consumers would be 50 and 76 ng, or, respectively, 8 and 12 ng/kg bw per day (58 and 87 ng/kg bw per week).
For compounds that accumulate in the body over time and transfer to milk during breastfeeding, high levels in mothers will result in a prolonged high exposure of the infant. Using only the mean serum levels results in underestimating the exposure of these children. However, the CONTAM Panel decided that the available data on P95 serum levels are insufficient to estimate the exposure for higher milk levels.
3.2.2. Comparison with the exposure in the CONTAM Panel 2018 Opinion
In the previous Opinion, the CONTAM Panel estimated the exposure to PFOS and PFOA for the European population across the same age groups. To a large extent, similar consumption surveys and occurrence data were used as those used in the present Opinion. There are, however, some differences in the surveys, occurrence data and especially the way the data were treated in the present Opinion, as shown in detail in Annex C:
Updated and additional food consumption surveys and occurrence data were used
Changes in cut‐offs applied for LOQs
Replacing missing occurrence data with values in similar food categories
Mean occurrence levels changed, in particular in drinking water, fish and meat, because occurrence was weighted for consumption
Mean PFOA levels in milk were reduced due to withdrawal of data by the data provider.
These changes led to differences in the LB and UB exposure assessments (see Annex C).
For exposure of adults to PFOA, when comparing the newly estimated mean LB exposure, they are on average 45% lower than the ones from 2018, and at the higher end (P95) 40% lower. Similar lower exposures are observed for most other age classes. For infants, the difference is even larger, the exposure estimate being 73% and 68% lower at the mean and P95, respectively. A major explanation for this large decrease lies in the fact that the LB level for milk in the present assessment was reduced to 0, as compared to 0.067 ng/g in the previous assessment. This was due to the withdrawal of all the samples with detected levels by the data provider (see Section 3.1.1). Milk was one of the major contributors in the previous Opinion. Also the mean LB level of another important contributor, drinking water, was much lower in the present Opinion, being 0.001 instead of 0.009 ng/g, due to weighing of the levels reported for bottled, drinking and tap water according to their relative consumption reported in the consumption surveys.
A factor that should have resulted in an increase in the exposure was the decision to include all food categories, also those without reported data but considered analogous to those for which occurrence data were available. This led to including 1491 different food categories in the exposure assessment of PFOA and PFOS against the 184 and 406, respectively, in the previous Opinion. It is unclear to what extent this affected the intake. As a result of the various changes, the relative contribution of food products to the LB exposure to PFOA also changed, with fish meat, eggs and egg products, fruit and fruit products and livestock meat being the most important sources, and for adults also alcoholic beverages (but based on one measured level).
With respect to UB exposure, in order to reduce the uncertainty due to large numbers of LC data, it was decided to apply more cut‐offs for LOQs in different food products and drinking water, resulting in the loss of some data points. This should have resulted in lower UB exposure estimates, but in practice, the UB exposure for adults increased by a factor of 2.6 and 2.4 for the medians of, respectively, the mean and P95 exposure. A major reason is the decision to include more food categories based on occurrence in similar food category (see above). Since e.g. fruit and vegetables are highly consumed food categories, this has a major impact on the UB exposure.
In the case of PFOS, the medians for the mean and P95 LB exposure of adults estimated in the current Opinion were similar as in the previous Opinion (EFSA CONTAM Panel, 2018), being 4% and 13% lower. This was also the case for most other age classes, whereas for toddlers, it was 79% higher. For the median P95 intakes by infants and toddlers, they were both higher, respectively, 34 and 81%. Overall, it is difficult to point out a specific reason for the observed changes. The UB exposure estimates for PFOS were at least twofold higher than in the previous Opinion (EFSA CONTAM Panel, 2018), but in some cases even up to ninefold (infants, median of the means). This may be explained by the assumption that in principle all vegetables and fruits could be contaminated with PFOS, as explained above. As in the previous Opinion (EFSA CONTAM Panel, 2018), fish meat was the most important source for the LB exposure, followed by eggs and egg products, but fruit and fruit products now became more important than meat and meat products.
3.2.3. Comparison with previously reported exposure assessments
EFSA reported details of occurrence and dietary exposure to PFASs in food in 2012 (EFSA, 2012). The report summarised occurrence data for PFASs collected in 13 European countries during the period 2006–2012. The report considered 54,195 analytical results covering the same list of 27 substances included in this Opinion. The overall proportion of quantified results was very low due to the analytical LODs of the methods used resulting in a large amount of left‐censored data. PFASs were found more frequently in fish and other seafood and in meat and meat products (liver in particular) than in other food groups. Based on the available data with a low proportion of quantified results, the chronic dietary exposure to PFASs other than PFOS and PFOA was not quantified, but was expected to be in the low ng/kg bw per day range or even lower. Since this period, new data have been provided, some of which has been generated using methods with improved LODs, and hence an exposure estimate has been possible (see Section 3.2.1).
The 2018 Opinion on PFOS and PFOA in food (EFSA CONTAM Panel, 2018) included details reported in the scientific literature for dietary exposure to PFASs. It was still difficult to make reliable dietary exposure estimates to compare with those reported in Section 3.2.1 due to uncertainties arising from the use of left‐censored data, the fact that many reports were not clear whether UB or LB data had been used, and sampling strategies were not always reported. There are still insufficient data reported in the literature to make reliable dietary exposure estimates for most PFASs. There have been many studies that link data from blood monitoring studies to overall exposure and these show that blood can be a useful biomarker. For many individuals and locations, drinking water was shown to be a significant contributor to overall exposure to PFASs, either as a direct result of consumption or because of the use of water during cooking (EFSA CONTAM Panel, 2018).
Exposure to PFASs other that PFOS and PFOA have been reported by Haug et al. (2010a). Associations were found between estimated individual total dietary intakes of PFASs and serum. PFAS concentrations in serum were associated with lean fish, fish liver, shrimp and meat consumption. The estimated dietary intakes of PFOA, PFUnDA and PFOS were 0.60, 0.34 and 1.5 ng/kg bw per day, respectively. The major dietary source was seafood (fish and shellfish) contributing 38% of the estimated dietary intakes of PFOA, 93% of PFUnDA and 81% of PFOS. Haug et al. (2010b) reported that in the general Norwegian adult population, the highest dietary intake was estimated for PFOA (31 ng/day) followed by PFOS (18 ng/day), PFDA (13 ng/day) and PFNA (9.5 ng/day) using a value of 1/2 LOD for left‐censored data. For PFHxS, PFOS, PFHxA, PFHpA, PFOA, PFNA and PFDA, more than 85% of the estimated intake was based on measurements above LOD. The estimated total dietary intake of PFASs for the general Norwegian adult population (all age groups and both genders) was 103 ng/day.
Papadopoulou et al. (2017) estimated dietary exposure to PFASs in 61 Norwegian adults (74% women, average age: 42 years) using three methods: (i) a 1‐day duplicate diet study; (ii) by estimating intake after combining food contamination with food consumption data and iii) by a Food Frequency Questionnaire (FFQ). The PFASs found at highest concentrations in the duplicate diet samples were PFOA, PFOS and PFHxS where median total intakes were calculated to be 5.6 ng/day, 11 ng/day and 0.78 ng/day, respectively.
Johansson et al. (2014) investigated temporal trends of several different PFASs in commonly consumed food items over the period 1999–2010. Statistically significant decreasing trends were observed for concentrations of PFOS and PFHxS in fish and eggs. Concentrations of PFOS in fish and eggs decreased by a factor of 10 and 40, respectively. In eggs, concentrations of PFOA were also shown to decrease. While the concentrations of both linear and branch chained PFOS decreased in eggs, especially after 2004, the concentration of the branched PFOS isomers dropped more quickly than the linear isomer, leading to an average increase of the ratio between the linear and sum of branched PFOS by around 12% per year. A reduction in concentrations of PFHxS and PFOS was found in rainbow trout, whereas PFUnDA concentrations did not change significantly over the time period. The PFOS levels decreased by about 18% per year and PFHxS levels by about 4% per year between 1999 and 2010.
Several papers have been published since the 2018 Opinion (EFSA CONTAM Panel, 2018).
Arrebola et al. (2018) reported on the differential contribution of animal and vegetable food items to persistent organic pollutant serum concentrations in Spanish adults, using data generated from the BIOAMBIENT.ES project. PFOS and PFDA serum concentrations were primarily influenced by fish consumption.
Dietary exposure to PFASs for the general population and pregnant women in Taiwan was reported by Chen et al. (2018). Compared to Western populations, the Taiwanese were found to be exposed to higher levels of PFOA, PFDA and PFUnDA (11.2, 85.1, 44.2, and 4.45 ng/kg bw per day, respectively), mainly due to the higher contaminations in food. The exposure of 8.0 mg PFOA/person per day in the 95 percentile of pregnant women was largely due to frequent consumption of pork liver.
Exposure to PFHxS and other PFASs in Baiyangdian Lake (China) associated with industrial contamination including from a photographic film production plant, were reported by Cui et al. (2018). Estimated daily intake for the highest PFASs was for PFOS where exposure was 16.56 ng/kg bw per day and for PFHxS where it was 16.11 ng/kg bw per day, via aquatic food and drinking water. Fish consumption was the most important exposure pathway for residents to PFOA, PFHxS, PFOS and 6:2 chlorinated polyfluorinated ether sulfonate (6:2 Cl‐PFESA). The authors stated that this was the highest PFHxS levels ever recorded in surface water.
Sznajder‐Katarzyńska et al. (2018) examined exposure to PFASs as a result of contamination of fruits and vegetables. PFBA, PFOA and PFOS were the only 3 of 10 PFAS compounds that were quantitatively determined. The detection frequency for PFASs for the 55 samples analysed was < 10%. The major contributor to exposure was PFBA for which the concentration, reported only for banana, apple and orange samples, was 50.740 ng/g ww. PFOA was the most commonly detected PFAS. The origin and growing region were said to be possible factors that may influence the distribution profile of PFASs and their levels in food.
Contaminated drinking water in New Hampshire was a source of exposure to PFOS, PFOA and PFHxS (Daly et al., 2018), and PFOA and PFHxA were the dominant compounds in drinking water in central eastern China (Lu et al., 2018).
As with the findings reported in the current exposure section above, fish and meat products such as offal are generally the highest reported sources of dietary exposure to most PFASs, except where contaminated drinking water has been used to prepare dishes with a high water content (Saito et al., 2004; Quiñones and Snyder, 2009; Rayne and Forest, 2009).
Exposures in different parts of the world can vary according to usage of PFASs and sources of water contamination. Water is used extensively in the home for cooking and for food preparation, and therefore, contaminated water can have a major influence in dietary exposure even if concentrations of contaminants in foods are low.
In summary, it is difficult to compare exposure estimates with literature sources due to several factors. These include uncertainties arising from the use of left‐censored data, the fact that many reports are not clear whether UB or LB data had been used, and sampling strategies are not always reported. It is nevertheless clear that drinking water can be an important factor, whether consumed directly or used as part of the cooking process. PFASs are found more frequently in fish and other seafood, and in meat and meat products (liver in particular) than in other food groups, although local sources can have an impact in certain geographical regions. There is evidence that dietary exposure to some PFASs has decreased over recent decades.
3.2.4. Non‐dietary exposure
The large historical production volumes and widespread applications of PFASs in consumer products represent a potential for contamination of the indoor environment. Recently, OECD (2018) has identified 4,730 PFAS‐related CAS numbers. Non‐dietary exposure to PFOS and PFOA has been described in the previous Opinion (EFSA CONTAM Panel, 2018). PFASs might be leaching from consumer products into house dust as well as to both indoor and outdoor air. Thus, ingestion of house dust and inhalation of air (in both gas and particulate phase) are potential exposure routes for PFASs. Exposure can also occur through direct contact with consumer products such as all‐weather clothing and other textiles. In addition, PFAS manufacturing and downstream production may lead to occupational non‐dietary exposure to these compounds (Olsen, 2015).
3.2.4.1. Exposure through air and dust
Vapour pressures of PFCAs and PFSAs in dissociated forms are expected to be low; thus, exposure from air primarily occurs through inhalation of neutral PFASs (Stock et al., 2010). Concentrations of PFASs in indoor air usually exceed those in outdoor air considerably and thus exposure through inhalation of air is mainly through indoor air (Harrad et al., 2010). Concentrations of PFASs in indoor air have been found to vary a lot between homes (Ericson Jogsten et al., 2012; Fromme et al., 2015; Haug et al., 2011a). FTOHs have in general been found in higher concentrations than perfluoroalkane sulfonamido substances such as EtFASAs and EtFASEs. In a recent study from Germany with samples collected in 2014, the median concentration of 8:2 FTOH was 8,679 pg/m3 (range 4,702–21,698 pg/m3), while median concentrations of 69 pg/m3 (range 27–155 pg/m3) and < 54 pg/m3 (< 54–119 pg/m3) were reported for EtFOSE and EtFOSA, respectively (Fromme et al., 2015).
Ingestion of house dust is also a potential exposure route for PFASs. As for indoor air, the concentrations in house dust are quite variable. The distribution pattern is often following a lognormal distribution, with some samples having concentrations far exceeding the mean and median values of the dataset (Harrad et al., 2010). In a review by Eriksson and Kärrman (2015), concentrations of PFCAs, PFSAs, PAPs as well as perfluoroalkane sulfonamido substances in dust from various countries are described. The highest median sum concentrations were observed for n:2 diPAPs, followed by PFCAs, PFSAs and perfluoroalkane sulfonamido substances. For example, the median sum concentrations of n:2 diPAPs, PFCAs, PFSAs and perfluoroalkane sulfonamido substances in Sweden were 62 ng/g, 44 ng/g, 4.6 ng/g and < 0.1 ng/g (Eriksson and Kärrman, 2015).
3.2.4.2. Dermal exposure
Dermal exposure to PFASs can occur through direct contact with consumer products, but so far data are very limited. Both ionic and neutral PFASs have been detected in a wide range of consumer products (Kotthoff et al., 2015; Liu et al., 2014a) including carpets and textiles such as technical clothing (Gremmel et al., 2016; Washburn et al., 2005), waxes and paints (Washburn et al., 2005), food contact materials (Begley et al., 2005), non‐stick cookware (Sinclair et al., 2007) and personal care products including cosmetics (Fujii et al., 2013; Framtiden i våre hender, online) (see Section 1.3.3). So far very little is known on the dermal absorption of PFASs. In a study by Fasano et al. (2005), the dermal absorption of ammonium perfluorooctanoate was only 0.048% (Fasano et al., 2005), and for PFOS and PFOA, this pathway is thought to give a minor contribution to the intake both for infants, toddlers, children and adults (Trudel et al., 2008). A more recent study indicates that the dermal absorption is dependent on the ionisation state, and that there is a potential for a significant dermal absorption of PFOA through human skin. However, this is likely not the case when exposed through the environment, as PFOA then exists in its ionised form resulting in a low dermal absorption (Franko et al., 2012). Based on similarities in the kinetics of other PFCAs and PFSAs compared to PFOA and PFOS, it can be expected that the dermal exposure to these compounds is also low. No information on dermal exposure is available for the other PFASs covered in this Opinion.
3.2.4.3. Relative contribution to overall exposure
In a very recent study (published after the literature deadline) from Norway, the relative exposure from ingestion of food and drinks, inhalation of air, ingestion of dust and dermal absorption was assessed in a study group from the general population (Poothong et al., 2020). For most of the participants, diet was the predominant exposure pathway, but for 7, 12, 14 and 18 of the 61 participants, more than 20% of the exposure was attributed to other exposure pathways than diet for PFHxS, PFOS, PFOA and PFNA, respectively. For some individuals less than 50% of the exposure to one or more of the compounds studied came from ingestion of food and drinks. Also in a study from China, diet was identified as the major exposure pathway for ordinary residents while in occupational workers ingestion of dust contributed most to the total exposure (Fu et al., 2015). A study from Sweden demonstrated that, similar to PFOS and PFOA, contaminated drinking water may in particular cases be the major exposure pathway to PFHxS (Li et al., 2018a). For infants and toddlers, the relative exposure from ingestion of house dust may be larger when compared to adults, as infants may ingest considerable amounts of dust by crawling on the floor and by putting toys and other objects in their mouth. In a study on PFOS from the US, Egeghy and Lorber (2011) found that in a typical exposure scenario for 2‐year‐old children, the relative contribution from dust ingestion (36%) was about the same as from food ingestion (42%) followed by ingestion from drinking water (20%), dermal absorption (2%), inhalation of indoor air (< 1%) and inhalation of outdoor air (< 1%).
3.3. Hazard identification and characterisation
3.3.1. Toxicokinetics
This chapter reviews data on absorption, distribution, metabolism and elimination of PFASs in animals and humans. PFOS and PFOA toxicokinetics studies which have been published before 2017 are documented in previous EFSA Opinions (EFSA, 2008; EFSA CONTAM Panel, 2018). Additional studies published since 2017 have been analysed and reported in the current Opinion.
3.3.1.1. Experimental animals
Most of the information on the fate of PFSAs and PFCAs is based on PFOS and PFOA, respectively (EFSA, 2008; EFSA CONTAM Panel, 2018). These compounds have been shown to be readily absorbed in the gastrointestinal tract in mammals, including humans, and to distribute to the plasma and other parts of the body and depending on the specific PFAS, tend to accumulate in the liver. PFOS and PFOA are not metabolised and are excreted in both urine and faeces. They may be subject to extensive enterohepatic recirculation. For PFOS, the serum elimination half‐lives in rats and mice were slightly higher than one month, whereas in rabbits and monkeys, they were 3–4 months. Significant sex differences in the elimination of PFOA are observed in some species such as rats, for which half‐lives may vary from few hours (in females) to several days (in males). Differences in biological half‐lives between species for both PFOS and PFOA and between sexes for PFOA are mainly due to differences in renal clearance. For both PFOS and PFOA, maternal transfer occurs prenatally to the fetus through placental transfer and postnatally through the consumption of maternal milk. A detailed description of the individual studies summarised here below is provided in Appendix C.
PFCAs
In the past decades, a limited set of data was published on the toxicokinetics of PFCAs other than PFOA, such as PFBA, PFHxA, PFHpA, PFNA, PFDA, PFUnDA, PFDoDA, PFTrDA and PFTeDA. The most extensive studies have been carried out in rodents. Oral exposure, mainly by gavage, of experimental animals to PFCAs having a perfluorinated carbon chain length of 3–11 was shown to result in an estimated absorption fraction greater than 95% of the administered dose (ATSDR, 2018). None of the experimental studies observed the formation of metabolites, suggesting, as previously reported for PFOA (EFSA CONTAM Panel, 2018), that the biotransformation of PFCAs is unlikely in mammals, irrespective of their chain length.
Although distribution of PFCAs shows species and sex differences, which are attributed, at least in part, to differences in elimination kinetics, blood, liver and kidney are the tissues exhibiting the highest concentrations of absorbed PFCAs. In blood, PFCAs were found to bind to serum albumin, the affinity generally increasing with PFCAs hydrophobicity, but decreasing for perfluorinated carbon chain length beyond eight carbons.
The primary route of elimination of PFCAs having a carbon chain length below 10 is via urine, whereas for PFDA, PFUnDA, PFDoDA, PFTrDA and PFTeDA, faecal excretion is predominant. In rodents, half‐lives may vary from few hours (PFBA, PFHxA) to more than 1 month (PFNA, PFDA). Elimination of PFCAs exhibits pronounced sex differences in rats, with faster elimination in females than in males.
It was shown that transport proteins such as serum albumin, liver fatty acid‐binding proteins (L‐FABP) and organic anion transporters play a key role in PFCA excretion and/or reabsorption (Appendix C).
PFSAs
In addition to PFOS, toxicokinetics data in experimental animals were identified for PFBS and PFHxS, showing that the gastrointestinal absorption of these compounds was almost complete. They distributed mainly to blood, liver, and at a lesser extent to kidney. Neither in vitro nor in vivo biotransformation was reported for PFSAs. As for PFCAs, a slower elimination rate in males, compared to females was also observed for PFSAs in rats.
PFSAs are primarily eliminated in urine, and to a lesser extent in faeces. They are excreted into the bile but undergo extensive intestinal reabsorption, explaining the limited faecal excretion. Half‐lives of PFSAs are usually longer than for PFCAs with identical fluorocarbon chain length.
PFSAs are able to bind to serum albumin and with organic anion transporters, which are expressed in hepatocytes and enterocytes (Appendix C).
Other PFASs
Most studies on fluorotelomer‐based polyfluoroalkyl substances have used 8:2 FTOH as the parent compound, showing the formation of PFOA and, to a smaller extent, PFNA and lower chain length PFCAs. In general, the yield is low, probably because of the multiple biotransformation pathways, including conjugation reactions in animals. The PAPs were shown to be direct precursors to FTOHs and thus follow similar degradation pathways (Appendix C).
3.3.1.2. Humans
In the previous Opinion dealing with PFOS and PFOA (EFSA CONTAM Panel, 2018), it was reported that both compounds were extensively absorbed in humans and mainly distributed in plasma (in which they are predominantly bound to albumin), liver and kidney. PFOS and PFOA do not undergo metabolism and are eliminated in urine and bile. Biliary excretion of PFOS and PFOA is significantly higher than elimination via the urine, but does not predominantly contribute to overall elimination, due to high biliary reabsorption. Humans have a high percentage of PFOA renal tubular reabsorption due to the high affinity of PFOA for human uptake transport proteins such as OAT1, OAT3 and URAT1. Although a limited number of papers have been published on PFOS transporting proteins, compared to PFOA, some authors showed that these proteins are involved in the transport of PFSAs, including PFOS (Zhao et al., 2015, 2017). These studies are described in detail in this Opinion. Several studies estimated the half‐lives of PFOS and PFOA in humans, most of them suggesting values between 2 and 6 years (Table 15). PFOS and PFOA have been detected in umbilical cord blood, breast milk and plasma samples of breastfed toddlers indicating that maternal transfer occurs pre‐ and postnatally.
Table 15.
Toxicokinetics parameters for PFASs in humans
| PFAS | Gender | Half‐life | Clearancetot (mL/kg per day) | Reference |
|---|---|---|---|---|
| PFBA | M (n = 7), F (n = 2) | 2.5 d (1.8–6.3) | NR | Chang et al. (2008) |
| PFHxA | M (n = 7) | 32 d(a) (14–49) | 4.33 | Russell et al. (2013) |
| PFHpA |
M (n = 5), F (n = 5) F(b) (n = 12) M(c) + F(d) (n = 31) |
NR 1.0 y(a) (0.11–3.3) 0.82 y(a) (0.12–5.1) |
0.674 0.61(k) (95% CI 0.022–1.2) 0.61(k) (95% CI 0.38–0.83) |
Fujii et al. (2015) Zhang et al. (2013a) Zhang et al. (2013a) |
| PFOA |
M (n = 20) M + F (n = 4) M (n = 24), F (n = 2) M + F (n = 200) M + F (n = 643)(i) M + F (n = 1,029)(j) M (n = 5), F (n = 5) F(b) (n = 20) M(c) + F(d) (n = 66) M + F (n = 207) M (n = 22), F (n = 23) |
NR NR 3.8 y (95% CI 3.1–4.4) 2.3 y (95% CI 2.1–2.4) 2.9 y (95% CI 2.3–3.8) 8.5 y (95% CI 7.1–10.1) NR 1.5 y(a) (0.19–5.2) 1.2 y(a) (0.04–14) 1.7 y(a) 3.9 y |
0.132 0.150 0.150 NR NR NR 0.096 0.30(k) (95% CI 0.11−0.49) 0.77(k) (95% CI 0.47−1.1) NR NR |
Harada et al. (2005) Harada et al. (2007) Olsen et al. (2007) Bartell et al. (2010) Seals et al. (2011) Seals et al. (2011) Fujii et al. (2015) Zhang et al. (2013a) Zhang et al. (2013a) Fu et al. (2016) Worley et al. (2017) |
| PFNA |
M (n = 5), F (n = 5) F(b) (n = 16) M(c) + F(d) (n = 50) |
NR 1.7 y(a) (0.38–7.7) 3.2 y(a) (0.34–20) |
0.062 0.25(k) (95% CI 0.13–0.37) 0.15(k) (95% CI 0.099–0.20) |
Fujii et al. (2015) Zhang et al. (2013a) Zhang et al. (2013a) |
| PFDA |
M (n = 5), F (n = 5) F(b) (n = 19) M(c) + F(d) (n = 60) |
NR 4.0 y(a) (1.2–7.7) 7.1 y(a) (0.58–60) |
0.066 0.066(k) (95% CI 0.035–0.097) 0.096(k) (95% CI 0.050–0.13) |
Fujii et al. (2015) Zhang et al. (2013a) Zhang et al. (2013a) |
| PFUnDA |
M (n = 5), F (n = 5) F(b) (n = 19) M(c) + F(d) (n = 63) |
NR 4.0 y(a) (1.1–7.8) 7.4 y(a) (1.1–104) |
0.065 0.064(k) (95% CI 0.033–0.095) 0.065(k) (95% CI 0.050–0.080) |
Fujii et al. (2015) Zhang et al. (2013a) Zhang et al. (2013a) |
| PFDoDA | M (n = 5), F (n = 5) | NR | 0.070 | Fujii et al. (2015) |
| PFTrDA | M (n = 5), F (n = 5) | NR | 0.077 | Fujii et al. (2015) |
| PFTeDA | M (n = 5), F (n = 5) | NR | 0.224 | Fujii et al. (2015) |
| PFBS | M (n = 5), F (n = 1) | 27.7 d (95% CI 16.1–39.3) | NR | Olsen et al. (2009) |
| PFHxS |
M (n = 24), F (n = 2) F(b) (n = 19) M(c) + F(d) (n = 64) M (n = 22), F (n = 23) M (n = 20) F (n = 30) |
8.5 y (95% CI 6.4–10.6) 7.1 y(a) (2.3–13) 25 y(a) (1.6–182) 15.5 y 7.4 y (95% CI 6.0–9.7) 4.7 y (95% CI 4.6–6.0) |
NR 0.039(k) (95% CI 0.020–0.057) 0.027(k) (95% CI 0.018–0.037) NR NR NR |
Olsen et al. (2007) Zhang et al. (2013a) Zhang et al. (2013a) Worley et al. (2017) Li et al. (2018a) Li et al. (2018a) |
| PFOS |
M (n = 20) M+F (n = 4) M (n = 24), F (n = 2) F(b) (n = 19) M(c) + F(d) (n = 64) M (n = ca 1,000) F (n = ca 1,000) M + F (n = 207) M (n = 22), F (n = 23) M (n = 20) F (n = 30) |
NR NR 5.4 y (95% CI 3.9–6.9) 5.8 y(a) (3.2–10) 18 y(a) (1.6–121) 4.7 y (95% CI 4.2–5.3) 4.3 y (95% CI 4.1–4.5) 1.9 y(a) 3.3 y 4.6 y (95% CI 3.7–6.1) 3.1 y (95% CI 2.7–3.7) |
0.066 0.106 NR 0.05(e) (95% CI 0.037–0.064) 0.037(e) (95% CI 0.026–0.049) NR NR NR NR NR NR |
Harada et al. (2005) Harada et al. (2007) Olsen et al. (2007) Zhang et al. (2013a) Zhang et al. (2013a) Wong et al. (2014) Wong et al. (2015) Fu et al. (2016) Worley et al. (2017) Li et al. (2018a) Li et al. (2018a) |
Values are means and 95% confidence interval (95% CI) or means and range.
NR: Not Reported; M: male; F: female; y: years; d: days.
Geometric mean.
Age ≤ 50 years.
20 y < age < 88 y.
Age > 50 years.
Age 22 ± 0.9.
Age 68 ± 5.
Age 23 ± 3.
Age 69 ± 5.
< 4 years elapsed in a water district with high exposure levels.
< 9 years elapsed in a water district with low exposure levels.
Renal clearance (mean).
Most of the human data published on the toxicokinetics of PFASs other than PFOS and PFOA are related to their distribution and elimination. Because humans are exposed to PFAS mixtures, these compounds will not be considered individually in this section, as it was the case for experimental animal data. Quantitative oral absorption data in humans were not identified, but reports of high levels of PFASs in blood of individuals exposed to contaminated water (see Section 3.3.2.5) indicate that gastrointestinal absorption of these compounds had occurred. Although studies of metabolism of PFASs in humans were not identified, it is unlikely that these compounds could be biotransformed in human tissues. However, similar to experimental animals, humans are able to transform precursors to PFCAs and PFSAs. The FTOH metabolites FTCAs and FTUCAs were detected in the blood from ski wax technicians exposed through inhalation to high levels of 8:2 FTOH, suggesting metabolism of FTOH to PFOA and PFNA (Nilsson et al., 2013). Using human hepatocytes, Nabb et al. (2007) demonstrated the biotransformation of 8:2 FTOH to PFPeA, PFHxA, PFHpA, PFOA and PFNA (Appendix C, Table C.4). After having detected and quantified, FTOH−sulfate conjugate in serum and/or urine of rats dosed with 8:2 FTOH and 8:2 diPAP (see Appendix C, Section C.4), Dagnino et al. (2016) found the presence of measurable levels of FTOH−sulfate in the serum of more than 5% of the general population and suggested that this metabolite can be considered as a biomarker of exposure to FTOHs and PAPs in humans.
Table C.4.
Summary of perfluorinated biotransformation products observed in metabolic studies of fluorotelomer compounds (FTOHs), polyfluoroalkyl phosphoric acid esters (PAPs) and other PFAS precursors
| Systems | Precursors | Metabolites | References | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PFBA | PFPA | PFHxA | PFHpA | PFOA | PFNA | PFDA | PFOS | |||
| Male rats (gavage, single dose) | 8:2 FTOH | NE | NE | NE | NE | Plasma | ND | NS | Hagen et al. (1981) | |
| Male rats (single ip injection) | 8:2 FTOH | NE | NE | NE | NE | Plasma, Liver Kidney | Plasma, Liver Kidney | NE | Martin et al. (2005) | |
| Rats (gavage, single dose) | 14C‐8:2 FTOH | ND | ND |
Plasma Urine Faeces |
Plasma |
Plasma Urine Faeces |
Plasma | ND | Fasano et al. (2006) | |
| Rats (gavage, repeated doses) | 8:2 FTOH + 14C‐8:2 FTOH | ND |
Plasma Tissues(a) |
Plasma Tissues(b) Urine |
Plasma Tissues(c) Urine |
Plasma Tissues(c) Urine |
Plasma Tissues(c) Urine |
ND | Fasano et al. (2009) | |
| Male and female rats (inhalation) | 8:2 FTOH | NE | ND | Plasma | Plasma | Plasma | Plasma | NE | Himmelstein et al. (2012) | |
| Male rats (gavage, single dose) | 8:2 FTOH | NE | NE | NE | NE |
Serum Urine Faeces |
Serum Urine Faeces |
NE | Dagnino et al. (2016) | |
| Male and female rats (single i.v. dose) | 8:2 FTOH | NE | NE | NE | NE | Serum | NE | NE | Huang et al. (2019b) | |
| Male and female rats (gavage, single dose) | 8:2 FTOH | NE | NE | NE | NE | Serum, liver, kidney | Huang et al. (2019b) | |||
| Male rats (gavage, single dose or two doses) |
8:2 monoPAPs 8:2 diPAPs |
NE | NE | ND | Blood(d) |
Blood Liver Kidney |
ND | NE | D'Eon and Mabury (2007) | |
| Male rats (single iv injection or gavage, single dose) | Mixtures of 4:2, 6:2, 8:2, 10:2 monoPAPs or diPAPs |
Blood Urine |
Blood(e) Urine |
Blood Urine |
Blood Urine |
Blood Urine(f) |
Blood | Blood | D'Eon and Mabury (2011) | |
| Male rats (gavage, single dose) | 8:2 diPAP | NE | NE | NE | NE |
Serum Urine Faeces |
Serum Urine Faeces |
NE | Dagnino et al. (2016) | |
| Female rats (gavage, repeated doses) | EtFOSE | NE | NE | NE | NE | NE | NE | NE |
Liver Serum |
Xie et al. (2009) |
| Male rat hepatocytes | 8:2 FTOH | NE | NE | NE | NE | Major PFCA(g) | Traces | NE | Martin et al. (2005) | |
| Rat hepatocytes(h) | 14C‐8:2 FTOH | NE | 0.15% | 0.27% | 0.08% | 0.24% | 0.06% | NE | Nabb et al. (2007) | |
| Rat microsomesa) | 14C‐8:2 FTOH | NE | < LOQ | 0.06% | 0.15% | 1.33% | 0.02% | NE | Nabb et al. (2007) | |
| Male mice (dietary exposure) | 8:2 FTOH | NE | NE | NE | NE | Liver | Liver | NE | Kudo et al. (2005) | |
| Pregnant mice (gavage, single dose at GD8) | 8:2 FTOH | NE | NE | NE | NE |
Liver, serum (dams) Liver, serum (pups) Placenta |
Liver, serum (dams) Liver, serum (pups) Placenta |
NE | Henderson and Smith (2007) | |
| Mouse hepatocytes(h) | 14C‐8:2 FTOH | NE | 0.21% | 0.21% | 0.20% | 0.47% | 0.08% | NE | Nabb et al. (2007) | |
| Mouse microsomesa) | 14C‐8:2 FTOH | NE | < LOQ | 0.04% | 0.05% | 0.48% | < LOQ | NE | Nabb et al. (2007) | |
| Human hepatocytes(h) | 14C‐8:2 FTOH | NE | 0.08% | 0.04% | 0.03% | 0.02% | 0.02% | NE | Nabb et al. (2007) | |
| Human microsomesa) | 14C‐8:2 FTOH | NE | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | NE | Nabb et al. (2007) | |
Only end‐metabolites are mentioned.
Present in liver, kidney and thyroid.
Present in liver > kidney > thyroid > adipose tissue ≈ skin.
Present in liver > kidney > thyroid > adipose tissue ≈ bone marrow ≈ skin ≈ thymus.
Detected in animals exposed to 8:2 monoPAPS.
not detected in blood from animals exposed via oral gavage.
not detected in urine from animals injected intravenously.
After 4 h incubation with 18μM 8:2 FTOH, 78% of the parent material had been biotransformed, PFOA representing 1.4% and PFNA < 0.2% of the formed products.
Values are reported as percent of incubated dose following 120 min incubations.
Values are reported as percent of incubated dose following 30 min incubations
ND: Not detected.
NE: Not evaluated.
NS: Not specified.
Compared to PFOA and PFOS, a limited number of studies investigated the interaction of other PFASs with human serum albumin. However, evidence exists that PFBS, PFHxS, PFHpA, PFNA, PFDA, PFUnDA PFDoDA can bind to human serum albumin (see Liu et al., 2019 for review). Among PFCA, the binding strength follows the order PFHpA < PFOA = PFNA < PFDA > PFUnDA; whereas for PFSAs, the order is PFOS < PFHxS (Hebert and MacManus‐Spencer, 2010).
The concentrations of 21 PFASs in 99 samples of autopsy tissues (liver, lung, kidney, brain and bone) from subjects who had been living in Tarragona (Catalonia, Spain) were measured by Pérez et al. (2013). Unexpectedly, in the liver and brain, the PFAS found at the highest median concentration was PFHxA (68.3 and 141 ng/g, respectively), whereas in kidney and lung, PFBA was predominant (263 and 807 ng/g, respectively). In bone, PFHpA was the main PFAS when the mean was considered (77.1 ng/g), but based on the median, PFOA was predominant.
Partitioning behaviours of PFASs between human plasma and blood cells were investigated by several authors (Ehresman et al., 2007; Hanssen et al., 2013; Jin et al., 2016; Kärrman et al., 2006; Poothong et al., 2017). Median serum (or plasma) to whole blood ratio of PFOS, PFOA, PFHxS, PFNA and PFUnDA was approximately 2. However, for other PFASs, the ratio may be different, suggesting that blood can be a preferable matrix compared to serum or plasma for monitoring these compounds (see Section 3.3.2.1). The toxicokinetics of PFCAs with 6–14 carbon atoms (C6–C14) was investigated in humans by Fujii et al. (2015). Urine from healthy volunteers, bile from patients who underwent biliary drainage, and cerebral spinal fluid (CSF) from brain drainage were collected for analysis. Urinary and biliary clearance of each PFCA was determined by dividing the cumulative urine or bile excretion in a 24‐h period by the serum concentration of each PFAS. Urinary clearance decreased as a function of chain length, from 0.67 mL/kg per day for PFHpA to 0.005 mL/kg per day for PFUnDA. No differences were observed between PFUnDA, PFDoDA and PFTrDA. For comparison, PFOA and PFOS renal clearance values were 0.03–0.04 and 0.015 mL/kg per day (Harada et al., 2005; Fujii et al., 2015), respectively. Biliary clearance varied from 1.2 mL/kg per day (PFNA) to 11.22 mL/kg per day (PFTeDA). For PFOA and PFOS biliary excretion rates, reported mean values were 1.06–2.62 and 2.98 mL/kg per day, respectively (Harada et al., 2007, Fujii et al., 2015). These data suggest that shorter chain PFCAs, which are less bound to serum protein, are preferentially excreted in urine, whereas PFNA and longer chain PFASs are preferentially eliminated through the bile. This is in accordance with the conclusion reached by Zhang et al. (2013a) on the preferential excretion of short chain PFCAs in urine, whereas other excretion routes contributed to the elimination of longer PFASs. Levels of PFCAs in the human CSF ranged from 1.0 to 2.5% of the concentrations in serum, confirming that, irrespective of the chain length, crossing the blood–brain barrier occurred at a very limited extent (Fujii et al., 2015).
Available total clearance values (sum of urinary and biliary clearance) are presented in Table 15 and are generally 50–100 times smaller than in rodents (Appendix C, Tables C.2 and C.3). Values for PFNA, PFDA, PFUnDA, PFDoDA and PFTrDA estimated by Fujii et al. (2015) are approximately 0.07 mL/kg per day. Parts of these values are in accordance with the data reported by Zhang et al. (2013a). Higher values were determined for PFHpA and PFTeDA (Fujii et al., 2015) and by Russell et al. (2013) for PFHxA.
Table C.2.
Selected TK parameters for PFOS and PFOA in animals
| PFAS | Species (sex) | Route(c) | Dose (μmol/kg) | Half‐life | Clearancetot (mL/kg per day) | Volume of distribution (mL/kg) | Reference |
|---|---|---|---|---|---|---|---|
| PFOS |
Rat (F) Rat (M) Rat (F) Rat (M) Rat (F) Rat (M) Rat (F) Rat (M) Rat (F) Rat (M) Rat (F) Rat (M) Rat (F) Rat (M) Mice (F) Mice (M) Mice (F) Mice (M) Monkey (F) Monkey (M) |
G G G G i.v. i.v. G G i.v. i.v. G G G G G G G G i.v. i.v. |
3.72 3.72 27.84 27.84 4 4 4 4 4 4 4 4 20 20 1.86 1.86 37.2 37.2 3.72 3.72 |
62.30 ± 2.09 d 38.31 ± 2.32 d 71.13 ± 11.25d 41.19 ± 2.01 d 24.80 ± 1.52 d 28.70 ± 1.85 d 23.50 ± 1.75 d 28.70 ± 1.85 d 23.0 ± 3.7 d(a) 22.0 ± 2.1 d(a) 28.4 ± 11.0 d(a) 19.9 ± 3.8 d(a) 18.0 ± 3.1 d(a) 14.5 ± 2.1 d(a) 37.80 d 42.81 d 30.45 d 36.42 d 110 ± 15 d 132 ± 7 d |
5.4 ± 0.2 22.2 ± 0.3 4.9 ± 0.5 11.3 ± 0.6 9.8 ± 0.2 9.2 ± 0.4 8.5 ± 0.4 7.3 ± 0.6 9.0 ± 0.6 13.1 ± 0.7 5.4 ± 0.3 9.7 ± 0.7 4.5 ± 0.3 6.4 ± 0.5 4.7 4.7 6.0 5.0 1.65 ± 0.04 1.10 ± 0.06 |
484 ± 24 1228 ± 97 468 ± 25 666 ± 21 352 ± 19 383 ± 18 289 ± 16 280 ± 17 297 ± 43(b) 417 ± 31(b) 222 ± 84(b) 280 ± 48(b) 417 ± 31(b) 417 ± 31(b) 258 290 261 263 274 ± 28 202 ± 13 |
Chang et al. (2012) Chang et al. (2012) Chang et al. (2012) Chang et al. (2012) Kim et al. (2016a) Kim et al. (2016a) Kim et al. (2016a) Kim et al. (2016a) Huang et al. (2019a) Huang et al. (2019a) Huang et al (2019a) Huang et al (2019a) Huang et al (2019a) Huang et al (2019a) Chang et al. (2012) Chang et al. (2012) Chang et al. (2012) Chang et al. (2012) Chang et al. (2012) Chang et al. (2012) |
| n‐PFOS |
Rat (M) Rat (M) Rat (F) |
G Oral, 3 m Oral, 3 m |
5.4 0.04 0.04 |
33.7 d 82 (66–107) d 83 (73–90) d |
NR NR NR |
NR NR NR |
Benskin et al. (2009) De Silva et al. (2009) De Silva et al. (2009) |
| iso‐PFOS |
Rat (M) Rat (M) Rat (F) |
G Oral, 3 m Oral, 3 m |
0.8 0.004 0.004 |
23.4 d 65 (47–107) d 38 (25‐81) d |
NR NR NR |
NR NR NR |
Benskin et al. (2009) De Silva et al. (2009) De Silva et al. (2009) |
| 1m‐PFOS |
Rat (M) Rat (M) |
G Oral, 3 m |
< 1.5 < 0.006 |
102 d 103 (63–288) d |
NR NR |
NR NR |
Benskin et al. (2009) De Silva et al. (2009) |
| PFOA |
Rat (F) Rat (M) Rat (F) Rat (M) Rat (F) Rat (M) Rat (F) Rat (M) Rat (M) Rat (M) Rat (M) Rat (M) Mice (F) Mice (M) Mice (F) Mice (M) Monkey (F) Monkey (M) Monkey (M) |
i.v. i.v. i.v. i.v. i.v. i.v. G G G G Oral, 3 m Oral, 3 m i.v. i.v. G G i.v. i.v. Oral, 6 m |
48.6 48.6 48.6 48.6 2.42 2.42 2.42 2.42 1 (n‐PFOA) 0.1 (iso‐PFOA) 0.05 (n‐PFOA) 0.005 (iso‐PFOA) 0.31 0.31 3.13 3.13 24.15 24.15 24.15 |
0.08 ± 0.03 d 5.68 ± 0.99 d 0.08 ± 0.03 d 5.63 ± 1.20 d 0.19 ± 0.01 d 1.64 ± 0.44 d 0.15 ± 0.01 d 1.83 ± 0.47 d 13.4 d 8.1 d 9.1 (5.3–33) d 6.3 (4.3–12) d NR NR NR NR 32.6 ± 8.0 d 20.9 ± 12.5 d 19.5 d |
2233 ± 805 50.4 ± 14.4 2233 ± 805 50 ± 17 612.8 ± 32.5 47.4 ± 3.4 645.1 ± 43.4 40.3 ± 3.4 NR NR NR NR 11.8 ± 6.1 14.2 ± 8.4 9.0 ± 1.5 13.1 ± 7.4 NR NR NR |
211 ± 28 346 ± 57 211 ± 28 339 ± 68 171 ± 11 112 ± 29 154 ± 9 106 ± 9 NR NR NR NR 150 ± 40 180 ± 40 NR NR 198 ± 69 181 ± 12 NR |
Kudo et al. (2002) Kudo et al. (2002) Ohmori et al. (2003) Ohmori et al. (2003) Kim et al. (2018) Kim et al. (2018) Kim et al. (2018) Kim et al. (2018) Benskin et al. (2009) Benskin et al. (2009) De Silva et al. (2009) De Silva et al. (2009) Fujii et al. (2015) Fujii et al. (2015) Fujii et al. (2015) Fujii et al. (2015) Butenhoff et al. (2004) Butenhoff et al. (2004) Butenhoff et al. (2004) |
NR: Not Reported; d: day; F: female; M: male; G: gavage; i.v.: intravenous; m: month; PFAS: perfluoroalkyl substance; PFOS: perfluoroheptane sulfonate; PFOA: perfluorooctanoic acid; TK toxicokinetic.
Elimination half‐life based on k10 calculation.
Volume of distribution for the central compartment.
Unless otherwise stated, single dose exposure is given.
Table C.3.
Selected TK parameters for PFASs other than PFOS and PFOA in animals
| PFAS | Species (sex) | Route(j) | Dose (μmol/kg) | Half‐life | Clearancetot (mL/kg per day) | Volume of distribution (mL/kg) | Reference |
|---|---|---|---|---|---|---|---|
| PFBA |
Rat (F) Rat (M) Rat (F) Rat (M) Mice (F) Mice (M) Mice (F) Mice (M) Monkey (F) Monkey (M) |
i.v. i.v. G G G G G G i.v. i.v. |
140 140 140 140 140 140 47 47 47 47 |
1.03 ± 0.03 h 6.4 ± 0.54 h 1.8 ± 0.3 h 9.2 ± 0.8 h 3.1 ± 0.3 h 16.3 ± 7.2 h 2.9 ± 0.3 h 13.3 ± 4.6 h 41.0 ± 4.7 h 40.3 ± 2.4 h |
3,318 ± 66(a) 766 ± 55(b) 1,718 ± 163(a) 444 ± 27(b) 835 ± 38(c) 254 ± 549(d) 730 ± 29(c) 240 ± 63(d) 1,792 ± 152(e) 1,694 ± 209(f) |
187 ± 3 253 ± 6 173 ± 21 209 ± 10 134 296 107 152 443 ± 59 526 ± 68 |
Chang et al. (2008) Chang et al. (2008) Chang et al. (2008) Chang et al. (2008) Chang et al. (2008) Chang et al. (2008) Chang et al. (2008) Chang et al. (2008) Chang et al. (2008) Chang et al. (2008) |
| PFHxA |
Rat (F) Rat (M) Rat (F) Rat (M) Rat (F) Rat (M) Rat (M) Monkey (F) Monkey (M) |
i.v. i.v. G G G (25 d) G (25 d) G i.v. i.v. |
31.8 31.8 159.2 159.2 159.2 159.2 0.32 31.8 31.8 |
0.4 h 1.0 h 2.6 h 2.2 h 2.7 h 2.2 h 2.9 (2.1‐4.3) h 2.4 ± 1.7 h 5.3 ± 2.5 h |
18,600 2,784 NR NR – – NR 3,264 ± 528 2,928 ± 576 |
466 175 NR NR – – 760 474 ± 349 989 ± 579 |
Chengelis et al. (2009) Chengelis et al. (2009) Chengelis et al. (2009) Chengelis et al. (2009) Chengelis et al. (2009) Chengelis et al. (2009) Iwabuchi et al. (2017) Chengelis et al. (2009) Chengelis et al. (2009) |
| PFHpA |
Rat (F) Rat (M) Mice (F) Mice (M) Mice (F) Mice (M) |
i.v. i.v. i.v. i.v. G G |
48.6 48.6 0.31 0.31 3.13 3.13 |
0.05 ± 0.01 d 0.10 ± 0.05 d NR NR NR NR |
3,070 ± 781 1,604 ± 558 257 ± 124 347 ± 86 190 ± 22 293 ± 154 |
201 ± 29 196 ± 19 80 ± 20 70 ± 10 NR NR |
Ohmori et al. (2003) Ohmori et al. (2003) Fujii et al. (2015) Fujii et al. (2015) Fujii et al. (2015) Fujii et al. (2015) |
| PFNA |
Rat (F) Rat (M) Rat (F) Rat (M) Rat (F) Rat (M) Rat (F) Rat (M) Rat (M) Rat (F) Rat (M) Rat (F) Rat (M) Mice (F) Mice (M) Mice (F) Mice (M) Mice (F) Mice (M) Mice (F) Mice (M) |
i.v. i.v. G G G G G G G i.v. i.v. G G G G G G i.v. i.v. i.v. G |
48.6 48.6 2.16 2.16 6.47 6.47 21.6 21.6 0.11 6.47 6.47 6.47 6.47 2.16 2.16 21.6 21.6 0.31 0.31 3.13 3.13 |
2.44 ± 0.41 d 29.6 ± 2.3 d NR 42.1(33‐56) d 32.0(3‐119) d 23.6(20‐28) d NR 28.0(25‐32) d 29(18‐64) d 4.4 ± 0.2 d 40.2 ± 18.7 d 6.4 ± 1.1 d 54.6 ± 2.5 25.7(23‐29) d 34.4(29‐41) d 68.8(42‐120) d 228(70‐796) d NR NR NR NR |
106 ± 31 6.9 ± 0.6 NR NR NR NR NR NR NR 16.6 ± 1.3 7.4 ± 1.4 NR NR NR NR NR NR 5.1 ± 2.3 3.9 ± 1.9 2.4 ± 1.0 4.0 ± 1.7 |
243 ± 49 287 ± 13 125(86‐164) 113(67‐158) 171(104‐238) 139(82‐196) 146(90‐201) 110(65‐154) 880 45.9 ± 3.7 363 ± 183 NR NR 192(165‐220) 328(0‐1060) 192(165‐220) 328(0‐1060) 150 ± 40 220 ± 60 NR NR |
Ohmori et al. (2003) Ohmori et al. (2003) Tatum‐Gibbs et al. (2011) Tatum‐Gibbs et al. (2011) Tatum‐Gibbs et al. (2011) Tatum‐Gibbs et al. (2011) Tatum‐Gibbs et al. (2011) Tatum‐Gibbs et al. (2011) Iwabuchi et al. (2017) Kim et al. (2019) Kim et al. (2019) Kim et al. (2019) Kim et al. (2019) Tatum‐Gibbs et al. (2011) Tatum‐Gibbs et al. (2011) Tatum‐Gibbs et al. (2011) Tatum‐Gibbs et al. (2011) Fujii et al. (2015) Fujii et al. (2015) Fujii et al. (2015) Fujii et al. (2015) |
| PFDA |
Rat (F) Rat (M) Rat (F) Rat (M) Rat (F) Rat (M) Mice (F) Mice (M) Mice (F) Mice (M) |
i.v. i.v. i.v. i.v. G G i.v. i.v. G G |
48.6 48.6 1.95 1.95 1.95 1.95 0.31 0.31 3.13 3.13 |
58.6 ± 5.8 d 39.9 ± 8.6 d 50.0 ± 2.3 d 109.4 ± 18.7 d 74.6 ± 10.2 d 80.0 ± 5.5 d NR NR NR NR |
5.3 ± 0.2 5.2 ± 1.3 0.8 ± 0.1 0.8 ± 0.1 NR NR 2.8 ± 1.2 2.2 ± 0.9 2.2 ± 1.1 3.9 ± 1.8 |
441 ± 55 348 ± 15 58.4 ± 4.5 118.2 ± 9.3 NR NR 200 ± 50 250 ± 60 NR NR |
Ohmori et al. (2003) Ohmori et al. (2003) Kim et al. (2019) Kim et al. (2019) Kim et al. (2019) Kim et al. (2019) Fujii et al. (2015) Fujii et al. (2015) Fujii et al. (2015) Fujii et al. (2015) |
| PFUnDA |
Mice (F) Mice (M) Mice (F) Mice (M) |
i.v. i.v. G G |
0.31 0.31 3.13 3.13 |
NR NR NR NR |
3.4 ± 1.5 2.8 ± 1.0 3.1C1.7 5.7 ± 2.6 |
280 ± 80 33 ± 60 NR NR |
Fujii et al. (2015) Fujii et al. (2015) Fujii et al. (2015) Fujii et al. (2015) |
| PFDoDA |
Mice (F) Mice (M) Mice (F) Mice (M) |
i.v. i.v. G G |
0.31 0.31 3.13 3.13 |
NR NR NR NR |
4.8 ± 2.4 4.4 ± 1.6 5.2 ± 3.2 9.4 ± 4.1 |
350 ± 100 570 ± 210 NR NR |
Fujii et al. (2015) Fujii et al. (2015) Fujii et al. (2015) Fujii et al. (2015) |
| PFTrDA |
Mice (F) Mice (M) Mice (F) Mice (M) |
i.v. i.v. G G |
0.31 0.31 3.13 3.13 |
NR NR NR NR |
7.2 ± 3.2 6.8 ± 2.5 17.1 ± 12.0 34.2 ± 16.6 |
430 ± 140 580 ± 200 NR NR |
Fujii et al. (2015) Fujii et al. (2015) Fujii et al. (2015) Fujii et al. (2015) |
| PFTeDA |
Mice (F) Mice (M) Mice (F) Mice (M) |
i.v. i.v. G G |
0.31 0.31 3.13 3.13 |
NR NR NR NR |
10.4 ± 6.0 10.4 ± 4.6 48.7 ± 381 106.3 ± 46.6 |
430 ± 130 550 ± 180 NR NR |
Fujii et al. (2015) Fujii et al. (2015) Fujii et al. (2015) Fujii et al. (2015) |
| PFBS |
Rat (F) Rat (M) Rat (F) Rat (M) Rat (F) Rat (M) Rat (F) Rat (M) Rat (F) Rat (M) Rat (F) Rat (M) Rat (F) Rat (M) Monkey (F) Monkey (M) Monkey (F) Monkey (M) |
i.v. i.v. i.v. i.v. G G i.v. i.v. G G G G G G i.v. i.v. i.v. i.v. |
33.3 33.3 100 100 100 100 11.8 11.8 11.8 11.8 59.1 59.1 295.7 295.7 33.3 33.3 33.3 33.3 |
0.6 h 2.1 h 4.0 ± 0.2 h 4.5 ± 2.2 h 7.4 ± 0.8 h 4.7 ± 0.4 h 0.36 ± 0.03 h(k) 2.26 ± 0.3 h(k) 1.5 ± 0.1 h(k) 4.4 ± 18.1 h(k) 1.2 ± 0.1 h(k) 2.7 ± 0.8 h(k) 1.1 ± 0.1 h(k) 2.86 ± 0.4 h(k) 11.3 ± 2.5 h 83.2 ± 41.9 h(g) 13.2 ± 2.9 h 95.2 ± 27.1 h(g) 8.1 ± 2.0 h 15 ± 9.4 h |
7,464 946 56,280 ± 4,800(a) 11,424 ± 3,264(b) NR NR 6,048 ± 432 8,028 ± 48 3,648 ± 480 624 ± 60 4,392 ± 936 902 ± 74 6,216 ± 792 1,812 ± 139 8,832 ± 2,880(h) 12,264 ± 3,384 552 ± 240 298 ± 158 |
288 118 351 ± 34 330 ± 32 391 ± 105 676 ± 55 123 ± 12(l) 113 ± 16(l) 328 ± 57(l) 164 ± 677(l) 326 ± 95(l) 148 ± 52(l) 415 ± 83(l) 311 ± 55(l) 255 ± 17 254 ± 31 248 ± 45 209 ± 29 |
Chengelis et al. (2009) Chengelis et al. (2009) Olsen et al. (2009) Olsen et al. (2009) Olsen et al. (2009) Olsen et al. (2009) Huang et al. (2019a) Huang et al. (2019a) Huang et al (2019a) Huang et al (2019a) Huang et al (2019a) Huang et al (2019a) Huang et al (2019a) Huang et al (2019a) Olsen et al. (2009) Olsen et al. (2009) Chengelis et al. (2009) Chengelis et al. (2009) |
| PFHxS |
Rat (F) Rat (M) Rat (F) Rat (M) Rat (F) Rat (M) Rat (F) Rat (M) Rat (F) Rat (M) Rat (F) Rat (F) Rat (M) Rat (F) Rat (M) Rat (F) Rat (M) Rat (F) Rat (M) Rat (F) Rat (M) Rat (F) Rat (M) Mice (F) Mice (M) Mice (F) Mice (M) Monkey (F) Monkey (M) |
i.v. i.v. G G i.v. i.v. i.v. i.v. G G i.v. i.v. i.v. G G i.v i.v. G G G G G G G G G G i.v. i.v. |
22.8 22.8 22.8 22.8 22.8 22.8 10 10 10 10 10 25 25 10 25 9.1 9.1 9.1 9.1 36.5 36.5 73 73 2.3 2.3 45.7 45.7 22.8 22.8 |
1.83 ± 0.26 d 6.83 d 0.83 ± 0.53 d NR 1.64 ± 0.08 d 29.1 ± 0.6 d 0.88 ± 0.07 d(i) 20.7 ± 4.0 d(i) 1.72 ± 0.11 d(i) 26.9 ± 0.4 d(i) 1.61 ± 0.06 d 2.03 ± 0.09 d 34.1 ± 0.88 d 1.69 ± 0.06 d 34.1 ± 4.85 d 0.7 ± 0.08 d(k) 13.0 ± 1.5 d(k) 2.33 ± 0.07 d(k) 17.6 ± 1.8 d(k) 2.19 ± 0.06 d(k) 16.5 ± 1.1 d(k) 1.98 ± 0.05 d(k) 14.8 ± 1.2 d(k) 24.8 d 30.5 d 26.8 d 28.0 d 87 ± 27 d 141 ± 30 d |
119 ± 47 40.32 NR NR 53.35 ± 4.38 6.75 ± 0.06 227.9 ± 6.7(i) 9.0 ± 0.05(i) 124.8 ± 3.4(i) 7.2 ± 0.06(i) 56.2 ± 6.2 52.6 ± 8.6 6.4 ± 0.06 65.3 ± 8.2 6.6 ± 1.2 65.5 ± 3.1 4.7 ± 0.5 46.1 ± 2.2 4.8 ± 0.4 59.0 ± 3.6 5.7 ± 0.3 96.2 ± 5.8 9.0 ± 0.6 2.7 2.9 3.8 4.8 1.9 ± 0.4 1.3 ± 0.1 |
278 ± 66 NR NR NR 126 ± 14 275 ± 5 289.3 ± 23.8(i) 268.9 ± 52.1(i) 255.9 ± 18.2(i) 277.6 ± 3.9(i) 130.4 ± 5.5 153.9 ± 20.2 314.8 ± 23.2 158.6 ± 7.8 326.7 ± 20.0 66.3 ± 7.6(l) 123 ± 11(l) 155 ± 9(l) 123 ± 11(l) 186 ± 14(l) 137 ± 9(l) 264 ± 20(l) 192 ± 17(l) 96 129 147 195 213 ± 28 287 ± 52 |
Sundström et al. (2012) Sundström et al. (2012) Sundström et al. (2012) Sundström et al. (2012) Sundström et al. (2012) Sundström et al. (2012) Kim et al. (2016a) Kim et al. (2016a) Kim et al. (2016a) Kim et al. (2016a) Kim et al. (2018) Kim et al. (2018) Kim et al. (2018) Kim et al. (2018) Kim et al. (2018) Huang et al. (2019a) Huang et al. (2019) Huang et al. (2019) Huang et al. (2019a) Huang et al. (2019a) Huang et al. (2019a) Huang et al. (2019a) Huang et al. (2019a) Sundström et al. (2012) Sundström et al. (2012) Sundström et al. (2012) Sundström et al. (2012) Sundström et al. (2012) Sundström et al. (2012) |
d: day; F: female; M: male; G: gavage; i.v.: intravenous; m: month; PFAS: perfluoroalkyl substance; PFOS: perfluoroheptane sulfonate; PFOA: perfluorooctanoic acid; TK toxicokinetic; y: years; NR: Not Reported.
Unless otherwise specified, values are means ± SD or means and range.
(a): Based on an average weight of 200 g.
(b): Based on an average weight of 250 g.
(c): Based on an average weight of 25 g.
(d): Based on an average weight of 35 g.
(e): Based on an average weight of 3 kg.
(f): Based on an average weight of 7 kg.
(g): Calculated from γ phase of elimination profile.
Expressed as ml/d (weight of the animals not reported).
Values reported as mean ± SEM.
Unless otherwise stated, single dose exposure is given.
Elimination half‐life based on k10 calculation.
Volume of distribution for the central compartment.
Zhang et al. (2013b) investigated the binding affinity of 12 PFCAs with L‐FABP. They found that binding affinity increased significantly with their perfluorinated carbon number from 4 to 11, and decreased slightly when the number was over 11, suggesting that PFNA, PFDA and PFUnDA may accumulate preferentially in the liver. PFSAs have stronger L‐FABP interactions than PFCAs with the same perfluorinated chain length (Ng and Hungerbühler, 2014; Zhang et al., 2013b).
Zhao et al. (2015) showed that human Na+/taurocholate co‐transporting polypeptide (NTCP), which is highly expressed at the basolateral membrane of hepatocytes and mediates the uptake of bile acids into hepatocytes, can transport PFBS, PFHxS and PFOS (Km values = 39.6, 112 and 130 μM, respectively), whereas the human sodium‐dependent bile salt transporter (ASBT), which is mainly localised to the brush border membrane in the terminal ileum and is involved in the enterohepatic circulation of bile acids, only transports PFOS (see Appendix C, Table C.1). Human organic solute transporter (OST) α/β, which is expressed at the basolateral membrane of enterocytes to mediate the efflux of bile acids, can also transport the 3 PFSAs tested (Zhao et al., 2015).
Table C.1.
Uptake transporters that have been reported to mediate PFAS membrane transport in renal tubular cells, hepatocytes and enterocytes (extracted from Weaver et al., 2010; Han et al., 2012; Zhao et al., 2015, 2017)
| Cell types | Excretion | (re‐)absorption | ||
|---|---|---|---|---|
| Human | Rat | Human | Rat | |
| Renal proximal tubular cells |
OAT1[Link] (SLC22A6) OAT2[Link] (SLC22A7) OAT3[Link] (SLC22A8) |
Oat1[Link] (Slc22a6) Oat3[Link] (Slc22a8) |
OAT4[Link] (SLC22A11) URAT1[Link] (SLC22A12) |
Oat2[Link] (Slc22a7) Urat1[Link] (Slc22a12) Oatp1a1[Link] (Slco1a1) |
| Hepatocyteb |
OATP1B1[Link] (SLCO1B1) OATP1B3[Link] (SLCO1B3) OATP2B1[Link] (SLCO2B1) NTCP[Link] (SLC10A1) |
Oatp1a1[Link] (Slco1a1) Oatp1a5[Link] (Slco1a5) Oatp1b2[Link] (Slco1b2) Oatp2b1[Link] (Slco2b1) NTCP[Link] (Slc10a1) |
||
| Enterocytes |
OATP2B1[Link] (SLCO2B1) ASBT[Link] (SLC10A2) OSTα/β[Link] (SLC51A/SLC51B) |
Oatp1a5[Link] (Slco1a5) Oatp2b1[Link] (Slco2b1) |
||
(a) Expressed at the basolateral membrane (exchanges with blood).
(b) Expressed at the apical membrane (exchanges with urine or bile or intestinal lumen).
Note that uptake transporters may be capable of being bi‐directional (uptake and efflux).
OAT = organic anion transporter,
SLC = solute carrier family gene,
URAT = uric acid transporter, a member of the OAT family,
OATP = organic anion transporting polypeptide,
NTCP = Na + /taurocholate cotransporting polypeptide,
ASBT = apical sodium‐dependent bile salt transporter,
OST = organic solute transporter.
The role of human renal organic anion transporters (OAT), organic anion‐transporting polypeptide (OATP) and urate transporter (URAT) in transporting PFCAs was investigated by Yang et al. (2010). The inhibition of OATP1A2‐ or OAT4‐mediated estrone‐3‐sulfate uptake or URAT1‐mediated urate uptake has been compared for PFCAs with carbon chain lengths from 4 (PFBA) to 12 (PFDoDA). A chain length–dependent inhibition was observed, suggesting that PFCAs in general are substrates of OAT4 and URAT1, and to a lesser extent for OATP1A2, but with different levels of affinities to the transporters depending on their chain length. Regarding OATP1A2 and OAT4, the maximum inhibition was observed for PFNA and PFDA, whereas URAT1 was inhibited the most and roughly at the same levels by PFHpA, PFOA, PFNA and PFDA, PFCAs with shorter or longer chain lengths showed relatively less inhibition. These data suggest that OAT4 and URAT1 are key transporters in renal reabsorption of PFCAs in humans and, as a result, may contribute to their long half‐life.
Zhao et al. (2017) demonstrated that organic anion‐transporting polypeptides OATP1B1, OATP1B3, OATP2B1, which are expressed in human hepatocytes and/or enterocytes, can transport PFBS, PFHxS, PFOS, PFOA and PFNA, facilitating the enterohepatic recycling process. Furthermore, they showed that the organic solute transporter OSTα/β was also able to transport PFBS, PFHxS and PFOS.
All these transporters can potentially play a role in the accumulation of PFASs in the liver and contribute to their long elimination half‐life in humans.
The half‐lives of various PFASs have been estimated in humans (Table 15). Short‐chain PFASs were found to have half‐lives ranging from few days (PFBA) to approximately 1 month (PFBS, PFHxA). For comparison, estimated half‐lives for PFOA and PFOS (expressed as arithmetic means) vary from 2.3 to 8.5 years and from 3.1 to 7.4 years, respectively (EFSA, 2008; ATSDR, 2018; EFSA CONTAM Panel, 2018). Values for PFHxS were between approximately 4.7 and 8.5 years according to Olsen et al. (2007) (based on data from retired fluorochemical production workers) and Li et al. (2018a) (from the general population mainly exposed to PFASs through drinking water). Higher values were reported by Zhang et al. (2013a) (general population biomonitoring) and Worley et al. (2017) (drinking water exposure to PFASs). According to these authors, the half‐life estimated by Zhang et al. (2013a) on the basis of renal clearance should be viewed as upper limits due to the possibility that there might be other elimination routes than via the urine. Other uncertainties in the results provided by Zhang et al. (2013a) include the estimation of half‐lives from single time‐point serum measurements and based on volume of distribution values reported by Thompson et al. (2010) for PFOA. The half‐life calculation published by Worley et al. (2017) is based on serum concentrations measured at two time points and on intake rates estimated from PFHxS concentrations in drinking water supplying a residentially exposed population in Alabama (USA). The values, which are reported as means but without indications of range or inter‐individual variability, may be overestimated, due to other sources of ongoing exposure. Bearing in mind these limitations in kinetic calculation or reporting, the CONTAM Panel considered that Olsen et al. (2007) and Li et al. (2018a) (see Table 15) provided the best estimate.
Gao et al. (2015) measured the levels of linear and branched PFOS, PFOA and PFHxS isomers in serum and urine samples collected from 36 occupational workers in a fluorochemical manufacturing plant in China from 2008 to 2012 and they evaluated the daily clearance of these compounds. The median concentration of PFHxS isomers in the serum was 995 ng/mL (min–max = 12.8–10,546 ng/mL), with a mean linear isomer proportion of 93%. In urine, concentrations varied from LOQ (see supporting information) to a maximum of 40 ng/mL, with a mean proportion of linear isomers of 74%. The renal clearance was estimated on the basis of paired serum and urine concentrations and was found to be 0.04 mL/kg bw per day (0.03 for linear isomer and 0.19 for branched isomers).
In the study published by Zhang et al. (2013a), estimated geometric mean elimination half‐lives for PFNA in young females and in a group of males and older females were 1.7 and 3.2 years, respectively (Table 15). Corresponding values for PFHpA and PFDA were 1.0 years/0.82 years and 4.0 years/7.1 years. The values for PFUnDA (4.0 years/7.4 years) were similar to those of PFDA (Table 14). Some uncertainties regarding these estimated values were already mentioned in this section (see above).
For PFHxS, ATSDR reported fetal/maternal serum ratios ranging from 0.23 to 1.25. For PFNA, PFDA, PFUnDA and PFDoDA, the ratios were 0.4–1.18, 0.03–0.41, 0.29–0.53 and 0.61–0.68, respectively (ATSDR, 2018). The median, calculated from the ratios reported by Inoue et al. (2004), Fromme et al. (2010), Kim et al. (2011a), Gützkow et al. (2012), Hanssen et al. (2013), Lee et al. (2013), Ode et al. (2013), Porpora et al. (2013), Zhang et al. (2013c), Cariou et al. (2015), Fisher et al. (2016), Eryasa et al. (2019) for PFHxS, PFOS, PFOA and PFNA were 0.56, 0.36, 0.74 and 0.48, respectively. These values have been used for the calculation of the serum level at birth (see Section 3.4 on PBPK/TWI calculation).
The maternal‐infant transfer via breast milk was estimated using the ratio of the concentrations in milk vs. plasma. The ratio is in the range of 0.01–0.07 for PFHxS, PFNA, PFDA PFUnDA and FOSA (ATSDR, 2018), indicating that the transfer occurs at similar levels as for PFOS (ratio of 0.01–0.02), but at a lesser extent than for PFOA (ratio of 0.03–0.12) (EFSA CONTAM Panel, 2018). Mondal et al. (2014) calculated that each month, breastfeeding was associated with 1% and 2% decrease in maternal serum concentration of PFHxS and PFNA, respectively, compared to 3% for both PFOS and PFOA. Several authors estimated the decline in PFAS concentrations in breast milk, due to breastfeeding. The calculation of the decline was based 1) on repeated analyses of breast milk samples collected at different time points during lactation (Thomsen et al., 2010), 2) on measured concentrations in maternal serum at different time points during lactation (Fromme et al., 2010), 3) on measured concentrations in maternal serum of mothers that had breastfed their children during variable time (Bjermo et al., 2013; Brantsæter et al., 2013; Lauritzen et al., 2016; Mondal et al., 2014). In addition, calculations can be based on intakes, distribution volumes, breastfeeding amount and ratios between breast milk and serum concentrations. All these methods show that monthly decrease in breast milk concentrations ranged from 1.2% to 3.1% for PFOS and from 1.3% to the 7.7% for PFOA. Percentages reported for PFHxS and PFNA varied between 1% and 6.7% and between 1.1% and 2.8%, respectively.
To summarise, based on the high levels of PFASs observed in the blood of individuals exposed to contaminated water and by analogy to what is known for PFOS and PFOA, it can be assumed that the gastrointestinal absorption of most of the PFASs occurs to a significant extent in humans. PFASs are widely distributed in the body, with the highest concentrations generally found in blood, liver and kidney. In blood PFASs bind to albumin. Metabolism of PFSAs and PFCAs has never been observed; however, precursor compounds such as FTOHs and PAPs can be biotransformed in the human body to PFCAs and other metabolites. PFASs are eliminated in urine and in faeces, but breast milk was also found to be a substantial route of excretion. Shorter chain PFCAs are preferentially excreted in urine, whereas PFNA and longer chain PFASs are preferentially eliminated through the bile and subsequently the faeces. Extensive uptake from enterohepatic circulation and reabsorption by OATs in the kidneys are believed to be more active processes in humans compared to rodents, slowing down the excretion of these substances. Short‐chain PFASs were found to have half‐lives ranging from few days (PFBA) to approximately 1 month (PFBS, PFHxA), whereas for PFHxS, PFOS, PFOA, PFNA, PFDA and PFUnDA, estimated half‐lives can exceed 3 years.
Based on levels in cord blood, PFASs can be transferred to the fetus during pregnancy with varying transfer rates depending on the structure of the compound. Generally, longer fluoroalkyl chain length and a terminal sulfonate group are associated with lower fetal/maternal ratios.
Limited data were identified on the toxicokinetics of FTOHs and other precursors in humans.
3.3.1.3. PBPK modelling
Published PBPK models for PFOS and PFOA were already summarised in the previous Opinion (EFSA CONTAM Panel, 2018). Since then, other models have been developed, such as the PFOS PBPK model published by Chou and Lin (2019) in which a Bayesian statistical analysis using Markov chain Monte Carlo simulation was performed to optimise the model and to characterise the uncertainty and interspecies (rat, monkey and humans) variability of chemical‐specific parameters. Although this type of model can be applied to other PFASs, there are some limitations in this extrapolation. A limited number of validated PBPK models specifically based on PFASs other than PFOS and PFOA has been published.
Kim et al. (2018) developed a model for simulating the kinetics of PFHxS in male and female rats. The model includes compartments representing plasma (including a bound and free fraction), kidney and renal glomerular filtrate, gastrointestinal tract as absorption site, liver (taking into consideration enterohepatic circulation), brain, heart, lung and the rest of the body. A storage compartment was included in the model, receiving PFHxS from the glomerular filtrate. Absorption from the gastrointestinal tract is simulated as the balance between first‐order absorption and faecal excretion of unabsorbed chemical. A PBPK model for PFOA and PFOS in monkeys was referred to describe the renal tubular reabsorption (Loccisano et al., 2011). The tissue to plasma partition coefficients used in the model were calculated from data obtained in rats 14 days after iv injection of PFHxS at a dose of 0.5–10 mg/kg per bw. The transporter maximum, the transporter affinity constant, the rate constant to storage compartment and the free fraction of PFHxS in plasma were estimated by fitting the plasma and urine concentration data in rats.
To validate the PBPK model, male and female rats were both orally administrated 1 and 4 mg/kg bw doses and the model was evaluated according to the values observed for the PFHxS concentrations in plasma, tissues and urine, compared to the predicted concentrations at different time points. The authors concluded that the experimental results fitted well with the model by visual inspection.
The developed PBPK model was extrapolated to humans, considering the interspecies differences of physiological and chemical‐specific parameters using the following allometric equation:
where khuman and krat are the rate constants in human and rats, respectively, and BWhuman/BWrat refers to the body weight of humans and rats, respectively.
Kim et al. (2019) explored gender differences in humans for PFNA and PFDA using a PBPK model based on an in vivo study in male and female rats. The PBPK model was validated by comparing visually the data obtained from the analysis of the biological samples collected during the in vivo experiment with the predicted concentrations by model simulation. In addition, a normalised sensitivity analysis was carried out comparing the area under the curve (AUC) obtained from the original parameter and the changed parameter after increasing by 1%. The rat PBPK model was extrapolated to humans, considering the interspecies physiological parameters, on the basis of the same allometric equation described by Kim et al. (2018), as reported above.
3.3.1.4. Transfer from feed to food
Numata et al. (2014) estimated the bioaccumulation factors (ratio of the concentration in the tissue vs. concentration in feed) of several PFSAs and PFCAs in growing pigs fed for 21 days a diet containing PFOS, PFBS, PFHxS, PFHxA, PFOA, PFHpA, PFHpS at concentrations of 137, 132, 91.3, 47.8, 22.4, 10.2 and 4 μg/kg dry feed, respectively. Although the steady state was not reached at the end of the experiment for most of the investigated substances, bioaccumulation factors were reported for the whole body as well as for some tissues. For PFBS, the bioaccumulation factors calculated for the whole animals, meat and liver were 1.2, 0.8 and 6.0, respectively, whereas it was 17.9, 9.7 and 503, respectively, for PFOS. The corresponding values for PFHxS were 20.1, 13.1 and 48, whereas for PFHpS, the values were 12.7, 8.3 and 81, respectively. The bioconcentration factors for PFHpA were 2.7, 1.8 and 7.0 for the whole animal, meat and liver, whereas for PFOA corresponding values were 7.9, 5.3 and 32.8. For PFHxA, the bioconcentration factor was < 1 in each case.
Kowalczyk et al. (2013) investigated the transfer of PFBS, PFHxS, PFOS and PFOA into milk in dairy cows fed a PFAS‐contaminated feed for 28 days. On the basis of the daily intake of PFAS‐contaminated grass silage and hay, the total intake of PFBS and PFHxS was 3.4 ± 0.7 and 4.6 ± 1.0 μg/kg bw per day, respectively, compared to 7.6 ± 3.7 and 2.0 ± 1.2 μg/kg bw per day for PFOS and PFOA, respectively. The estimated transfers of PFOS, PFBS, PFHxS and PFOA into milk were found to be approximately 14, 0.01, 2.5 and 0.1%, respectively. The accumulated amount of PFASs in tissue samples of dairy cows showed the highest accumulation potential for PFOS (approximately 18% and 43% of ingested dose in liver and muscle, respectively), less for PFHxS (0.6 and 9%, respectively) as well as negligible potential for PFBS and PFOA accumulation.
Martin et al. (2003a) exposed juvenile rainbow trout (Oncorhynchus mykiss) to a mixture of PFASs for 34 days in the diet. The concentrations of PFCAs in feed were 0.50, 0.52, 0.46, 0.42, 0.39, 0.57, 1.1 and 1.2 mg/kg, for PFPeA, PFHxA, PFHpA, PFOA, PFDA, PFUnDA, PFDoDA and PFTeDA, respectively, whereas concentrations of PFSAs were 0.32, 0.51, 0.54 mg/kg, for PFBS, PFHxS and PFOS, respectively. PFBS, PFHxA and PFHpA were not detected or quantified in fish carcasses (LOQ not specified), suggesting that their bioaccumulation potential was negligible. For other PFASs, the bioaccumulation factors increased with increasing perfluoroalkyl chain length, varying from 0.04 for PFOA to 1 for PFTeDA. Overall, these values indicate that dietary exposure does not result in bioaccumulation of PFASs in juvenile trout.
This result was confirmed by Goeritz et al. (2013) in adult rainbow trout dietary exposed for 28 days to a mixture of PFASs (PFBS, PFHxS, PFOS, PFOA and PFNA incorporated into the feed at 0.50 mg/kg each). In these experimental conditions, bioaccumulation factors were calculated as 0.42 for PFOS, > 0.23 for PFNA, > 0.18 for PFHxS, > 0.04 for PFOA, and > 0.02 for PFBS.
Gaillard et al. (2017) investigated the fate of EtFOSE in juvenile perch (Perca fluviatilis) dietary exposed for 45 days to EtFOSE‐based phosphate diester incorporated into feed at a concentration of 1.6 mg/kg feed. EtFOSE and metabolites, including PFOS, were monitored in fish tissues (muscle, liver, serum) to study the bioaccumulation. Although a bioaccumulation factor cannot be calculated from the data provided by the authors, the concentrations found in muscle, liver and serum for EtFOSE and the sum of corresponding metabolites (< 0.04 μg/g) suggest that bioaccumulation does not occur.
3.3.2. Biomonitoring
3.3.2.1. Selection of biomarker and appropriate matrix for assessing internal dose
Due to their chemical structure, perfluoroalkyl sulfonates (PFSAs) and perfluoroalkyl carboxylates (PFCAs) are highly persistent and are not metabolised in the human body. Thus, when biomonitoring studies are conducted, PFSAs and PFCAs can be measured unchanged in biological matrices. The most frequently assessed PFSAs and PFCAs are PFOS and PFOA, followed by PFHxS and PFNA (see Appendix B, Table B.1). Quite a few studies have also reported levels of PFDA and PFUnDA, while data on other compounds are scarce. Biomonitoring of PFOS and PFOA has been described in detail in the previous Opinion (EFSA CONTAM Panel, 2018).
Table B.1.
Time trends for PFASs in blood and breast milk
| Period | Study population | Country | PFASs | Trend | Reference |
|---|---|---|---|---|---|
| European time trend studies | |||||
| 1987–2007 |
n = 80 plasma samples women, sampled in connection with breast reduction surgery (1987–1991), or as wives of men with cancer (2006–2007) mean age (range): 48 (36–56) years Cross sectional |
Sweden | PFHxS, PFOS, PFOA, PFNA, PFDA, PFUnDA | PFHxS, PFOS and PFOA peaked during the period 1990–2000. PFHxS increased during the whole period. PFOS and PFOA decreased from around 2000, even though only PFOS was significant. PFNA, PFDA and PFUnDA increased during the whole period, largest increase after the year 2000 | Axmon et al. (2014) |
| 1972–2008 | n = 20 pools of breast milk healthy native Swedish mothers at the Mothers’ milk center in Stockholm cross sectional | Sweden | PFOS, PFOA, PFHxS | Concentrations of PFOS, PFOA and PFHxS increased from 1972 to the 1990s. From around 2001 to 2008 the concentrations of all three PFASs decreased, while the trend for PFHxS was not significant | Sundström et al. (2011) |
| 1996–2010 | n = 36 pools of serum primiparous women living in Uppsala County, donated serum samples within the third week after delivery age: 19–41 years cross sectional | Sweden | PFHxA, PFHpA, PFOA, PFNA, PFDA, PFUnDA, PFDoDA, PFTrDA, PFTeDA, PFBS, PFHxS, PFOS, PFDS, FOSA | PFBS, PFHxS, PFNA and PFDA increased during the period 1996–2010, while PFOS, PFDS, FOSA and PFOA decreased in the same period. For PFHpA and PFUnDA, no significant trend was observed. PFHxA, PFDoDA, PFTrDA and PFTeDA were not detected in any sample above LOQ. The increasing trends of PFBS and PFHxS have later been shown to be due to contamination of drinking water with these PFASs (Gyllenhammar et al., 2013) | Glynn et al. (2012) |
| 1997–2012 | n = 27 pools of serum (three pools at each time point) primiparous women living in Uppsala County, donated serum samples within the fourth week after delivery cross sectional | Sweden | PFBS, PFHxS (branched and linear), PFOS (branched and linear), FOSA (branched and linear), EtFOSA, PFHpA, PFOA (branched and linear), PFNA, PFDA, PFUnDA, PFDoDA, PFTrDA,, 8:2/8:2 diPAP | Significant decreasing trends were observed for branched and linear PFOS, PFDS, branched and linear FOSA and for PFOA. Significant increasing trends were observed for branched and linear PFHxS, PFNA, PFDA, PFUnDA, PFDoDA and PFTrDA. No significant trends were observed for PFBS, and 8:2/8:2 diPAP. While EtFOSA, was detected in less than 60% of the samples and thus no statistical calculations were made | Gebbink et al. (2015) |
| 1974–2010 |
Archived serum samples from Swedish primiparous women living in the Uppsala county (n = 36 pools between 1996 and 2010) Archived American serum or plasma samples from 1974, 1989, 2000/2001, 2006 and 2010 were collected from individuals residing in the Hagerstown, Maryland (n = 60) cross sectional |
Sweden and USA | PFOS, PFOS isomers and 1‐m PFOS enantiomers |
Swedish population: For ΣPFOS; No significant trend was seen up to 2000, but between 2000 and 2010 a downward trend was observed. For % branched PFOS; no significant trend was found between 1996 and 2000, but a significant upward trend was seen between 2000 and 2010. For the enantiomeric fraction of 1‐m PFOS; a significant decreasing temporal trend was observed between 1996 and 2000, but no significant trend was seen between 2000 and 2010 American population: For ΣPFOS; No significant temporal trend was found for from 1974 to 2000/2001, but in the 2000/2001–2010 period, the downward trend was seen. For % branched PFOS; no significant trend was found for the period 1974–2010. For the enantiomeric fraction of 1‐m PFOS; no significant temporal trend was observed |
Liu et al. (2015) |
| 1996–2004 | n = 9 pools from Swedish primiparous women with a median age ranging from 27 to 30 years for the various pools cross sectional | Sweden | PFHxS, PFOS, FOSA, PFOA and PFNA | PFOA and FOSA were not observed above LOQ in any samples while PFNA was only found in three samples in similar concentrations. Quite stable concentrations were observed for both PFHxS and PFOS between 1996 and 2000, while a slight decrease was observed in 2003–2004 for PFHxS and in 2002–2004 for PFOS | Kärrman et al. (2007) |
| 2001–2014 | n = 579, men and women, 70 years (2001–2004), 75 years (2006–2009) and 80 years (2011–2014) at the three sampling time points, serum longitudinal sampling | Sweden | Fourteen PFASs, whereof trends were evaluated for the following: PFHpA, PFOA, PFNA, PFDA, PFUnDA, PFHxS, PFOS, FOSA | The concentrations of all PFASs except FOSA and PFOS increased from the first to the second sampling. Between the two last samplings, the concentrations decreased for all PFASs | Stubleski et al. (2016) |
| 1972–2016 | n = 20 pools from Stockholm (9–116 individuals per pool), women, breast milk | Sweden | PFPeA, PFHxA, PFHpA, PFOA, PFNA, PFDA, PFUnDA, PFDoDA, PFTrDA, PFTeDA, PFPeDA, PFBS, PFHxS, PFOS, PFDS, FOSA, MeFOSAA, EtFOSAA | PFOS, PFOA, PFHxS and PFNA increased from 1972 but decreased from 1988, 2000, 2004 and 2010, respectively. PFUnDA increased throughout the whole period, while PFTrDA, PFBS and PFHxA decreased from 1972 to 2004, 2011 and 2011, respectively, before increasing until 2016. No trends were observed for PFHpA and PFDoDA | Nyberg et al. (2018) |
| 2007–2015 | n = 11 pools from Gothenburg (5–11 individuals per pool), women, breast milk | Sweden | PFPeA, PFHxA, PFHpA, PFOA, PFNA, PFDA, PFUnDA, PFDoDA, PFTrDA, PFTeDA, PFPeDA, PFBS, PFHxS, PFOS, PFDS, FOSA, MeFOSAA, EtFOSAA | PFHxS, PFOS and PFDoDA decreased significantly from 2007 to 2015. None of the other PFASs showed any statistically significant trends | Nyberg et al. (2018) |
| 1977–2006 | n = 24 pools of serum males 40–50 years cross sectional | Norway | Nineteen PFASs, whereof trends were evaluated for the following 13: PFPeA, PFHpA, PFOA, PFNA, PFDA, PFUnDA, PFDoDA, PFTrDA, PFBS, PFHxS, PFHpS, PFOS, FOSA | PFOA, PFNA, PFDA, PFUnDA, PFHxS, PFHpS and PFOS all increased from the mid‐1970s up to the mid‐1990s, while FOSA reached a plateau between 1985 and 1993. The concentrations of PFOS, PFOA and PFHpS decreased from around 2,000, while for FOSA the decrease started somewhat earlier. No particular trends were observed for PFNA, PFDA and PFUnDA after around 2,000. The concentrations of PFPeA, PFHpA, PFDoDA and PFTrDA were quite stable during the whole study period | Haug et al. (2009) |
| 1979–2007 | n = 254 serum samples from 53 males from Northern Norway median age at first and last sampling was 43 and 71 years, respectively repeated sampling and measurements at up to five time points (1979, 1986, 1994, 2001 and 2007) longitudinal sampling | Norway | Ten PFASs were quantified, from which trends were assessed for eight: PFOA, PFNA, PFDA, PFUnDA, PFHxS, PFHpS, PFOS, FOSA | Increasing trends were observed for all the eight PFAS from 1979 and onwards. For PFOS, PFOA and FOSA, significant decreasing concentrations were observed from 2001 to 2007. In contrast, increasing concentrations were observed throughout the whole study period for PFNA, PFDA and PFUnDA, while only the trends for PFNA and PFDA were statistically significant for all time periods. Similar concentrations of PFHxS were observed in 2001 and 2007. Somewhat lower concentrations of PFHpS were observed in 2007 when compared to 2001, but this trend was not significant | Nøst et al. (2014) |
| 2008–2013 | n = 1,533 serum samples pregnant nulliparous women from the Aarhus Birth Cohort Biobank most participants gave a blood sample between 11 weeks and 14 weeks of gestation median age 29 years cross sectional | Denmark | Sixteen PFASs; whereof trends were assessed for PFASs detected in > 50% of the samples, i.e. PFHxS, PFHpS, PFOS, PFOA, PFNA, PFDA, PFUnDA | All seven PFASs decreased in the period 2008–2013 | Bjerregaard‐Olesen et al. (2016) |
| 1982–2010 | n = 258 plasma samples from the German Environmental Specimen Bank males and females, approximately 50% of each age range 20–29 years cross sectional | Germany | PFBA, PFPeA, PFHxA, PFHpA, PFOA, PFNA, PFDA, PFUnDA, PFDoDA, PFTrDA, PFTeDA, PFBS, PFHxS, PFHpS, PFOS, PFDS |
PFTrDA, PFTeDA, PFBS, PFDS not found in any samples. For PFBA, PFPeA, PFHxA, PFUnDA and PFDoDA, only very few samples had concentrations above LOQ. PFHpA, PFDA and PFHpS were quantifiable in 20–30% of the samples PFOS increased from 1982 to 1986, and remained quite stable up to 2001. From 2001 to 2010, a steadily decrease has been observed. PFOA concentrations also increased from 1982 to 1986 were quite stable up to 2008 and decreased from 2008 to 2010. PFHxS increased from 1982 to 2001, was stable up to 2005 and decreased after that. For PFNA, no trend was observed |
Schröter‐Kermani et al. (2013) |
| 1982–2009 | n = 420, students, male and female 20–29 years from Halle and Munster cross sectional | Germany | PFHxA, PFHpA, PFOA, PFNA, PFDA, PFUnDA, PFDoDA, PFTrDA, PFTeDA, 8:2 diPAP | From 2000 to 2009, the concentrations of PFOA decreased, while the concentrations of PFNA, PFDA and PFUnDA increased in the same period. No significant trend was observed for 8:2 di‐PAPs throughout the same period | Yeung et al. (2013a) |
| 1982–2009 | n = 420, students, male and female 20–29 years from Halle and Münster cross sectional | Germany | PFOS, PFHxS | For some of the compounds increasing concentrations were observed during the first years of the study. From around 1995 to 2009, decreasing concentrations were observed for PFOS, while no clear trend was seen for PFHxS | Yeung et al. (2013b) |
| 1977–2004 | n = 30, students, 19 males and 11 female, 20–29 years from Arnsberg cross sectional | Germany | PFBS, PFHxA, PFPeA, PFOA, PFOS, PFHxS | PFOS and PFOA levels remained fairly stable throughout the period, while PFHxS concentrations increased during the whole study period | Wilhelm et al. (2009) |
| Examples of time trend studies in other countries | |||||
| 2003–2013 | n = 71 persons. 81% of the participants were female Gullah African Americans participating in the SLEIGH study serum The age ranged from 6.1 to 77.6 years at the first visit longitudinal study; two time points | USA | Trends reported for PFHxS, PFOS, PFOA, PFNA, PFDA, PFUnDA |
At a population level, an overall decrease in the concentration of PFOA, PFOS, PFHxS and PFUnDA was seen. No significant trend was observed for PFDA Some individuals had a considerable increase of PFHxS concentration from the first to the second blood sample, but for the overall population, the PFHxS concentration decreased |
Gribble et al. (2015) |
| 2003–2011 | 30 random samples from 2003, 2005, 2007, 2009 and 2011 (n = 150) pregnant women, between 28 and 32 weeks of gestation, who were enrolled in a prospective birth cohort study in Hokkaido (the Hokkaido Study on Environment and Children's Health) mean age: 30.3 years cross sectional | Japan | PFHxS, PFOS PFHxA, PFHpA, PFOA, PFNA, PFDA, PFUnDA, PFDoDA, PFTrDA and PFTeDA | Concentrations of PFOS and PFOA decreased during the period 2003–2011, while the levels of PFNA and PFDA increased in the same period. The trends for the remaining compounds were not significant | Okada et al. (2013) |
| 2002–2011 | n = 158 pools of serum males and females, aged from 0 to > 60 years four time points 2002/2003, 2006/2007, 2008/2009 and 2010/2011 cross sectional | Australia | PFDA, PFHxS, PFNA, PFOA, PFOS, FOSA | Decreasing trends were observed for PFOS and PFOA, while more variable trends were observed for the other PFASs and the various age groups | Toms et al. (2014) |
| 2002–2013 | n = 54 pools from 4,920 individuals, men and women, 0–> 60 years, serum cross sectional | Australia | 8:2 diPAP, EtFOSA, EtFOSE, PFBA, PFPeA, PFHxA, PFHpA, PFOA, PFNA, PFDA, PFUnDA, PFDoDA, PFTrDA, PFTeDA, PFHxDA, PFODA, PFHxS, PFOS, PFDS | Decreasing trends were observed throughout the entire period for PFHxS, PFHpS, PFOS and PFOA, while PFNA, PFDA and PFUnDA concentrations started to decrease from 2006. PFDoDA increased from 2006 and onwards | Eriksson et al. (2017) |
| 1999–2008 | n = 7,876 samples from the NHANES study age: ≥ 12 years, males and females cross sectional | USA | PFOS, PFHxS, PFOA, PFNA | The PFOS concentrations decreased significantly during the period, while the PFNA concentrations had a significant upward trend. The highest concentrations of PFOA were observed in 1999–2000, while the concentrations were quite stable from 2003 to 2008. Decreasing concentrations were observed for PFHxS in the period 1999–2006, but an increase has been observed in 2007–2008 | Kato et al. (2011) |
Blood, specifically plasma or serum, is generally the preferred matrix to assess internal doses of PFSAs and PFCAs in humans (Butenhoff et al., 2006). As described in detail in Section 3.3.2.3 below, PFHxS, PFOS, PFOA and PFNA are in general the most abundant PFSAs and PFCAs. Studies on whole blood have also been conducted, with the advantage that whole blood represents the entire circulating fluid. However, due to practical reasons, serum and plasma are more frequently used. When comparing levels in whole blood, serum and plasma, it has been common to multiply the whole blood concentration with a factor of 2 to be comparable with concentrations in serum and plasma (Ehresman et al., 2007). In a study on Norwegian adults (n = 61), a range of PFCAs and PFSAs were determined in plasma, serum and whole blood. For PFHxS, PFNA and PFUnDA, as well as for PFOS and PFOA, the ratio between serum/plasma and whole blood was close to 2, while for PFBS, PFHpS, PFDS, PFDA, PFDoDA and PFTrDA, the results were more variable (Poothong et al., 2017). This could be due to differences in the distribution between blood compartments, but the very low concentrations (median < 0.4 ng/mL) of these PFASs in all three matrices may also have had an influence on this, as the measurement of uncertainty is considerably higher for such low concentrations. Furthermore, PFHxA was detected in all samples of whole blood but none of the serum/plasma samples from the same individuals (Poothong et al., 2017). Based on this study, whole blood is the preferred blood matrix for PFHxA.
For PFASs other than PFCAs and PFSAs, much less is known. Methods for determination of most of these compounds in blood have been established, but the data are very limited (see below). Similar to PFHxA, a study on Norwegian adults (n = 61) found considerably higher concentrations of FOSA in whole blood compared to serum/plasma samples from the same individuals, and thus, whole blood is also the preferred blood matrix for FOSA (Poothong et al., 2017).
As described in Section 1.3.1, FTOHs and PAPs can be transformed in the human body to PFCAs, and EtFASEs and EtFASAs to FASAs and further to PFSAs, and thus contribute to the internal dose of PFCAs and PFSAs. As EtFOSA is transformed to EtFOSAA in the body, EtFOSAA would be the preferred biomarkers of exposure to EtFOSA.
Some studies on breast milk have been conducted, and a limited number of studies have assessed the levels of blood and breast milk from the same individuals. The levels in breast milk are substantially lower than in serum. In a study including 61 paired samples of breast milk and serum from France, only PFHxS in addition to PFOS and PFOA was observed above the LOQ in some of the milk samples (15% > LOQ), with a mean concentration of 0.026 ng/mL (Cariou et al., 2015). The ratio between the mean concentrations of PFHxS in breast milk and serum was 0.01. In a Swedish study on matched samples of breast milk and serum, mean ratios (breast milk:serum) of 0.02, 0.07 and 0.01 were reported for PFHxS, FOSA and PFNA (Kärrman et al., 2007). Distribution of PFASs between serum and breast milk was also explored in a Korean study, and a ratio (breast milk:serum) of 0.008 was reported for PFHxS (Kim et al., 2011b). In a study on matched serum and breast milk samples from China, ratios (breast milk:serum) of 0.04, 0.03 and 0.04 were reported for PFNA, PFDA and PFUnDA, respectively (Liu et al., 2011).
Also, some few studies have reported concentrations of PFSAs and PFCAs in urine, and the concentrations are in general low. In a recent study by Kato et al. (2018), a range of PFASs were measured in 50 commercially available paired urine and serum samples. The concentrations observed in the serum samples were similar to concentrations reported in samples from NHANES collected between 2013 and 2014, while in urine only, PFBA was observed above the LOD (0.1 ng/mL) with a median concentration of 0.2 ng/mL (56% detection frequency). In a study on matched urine and serum samples from children in South Korea, short‐chain PFCAs were the compounds most frequently detected in urine, while none of the PFSAs were observed above the LOD in any of the urine samples (Kim et al., 2014). In general, the detection frequency in urine was very low except for PFPeA which was found above the LOQ in 70% of the samples with a mean concentration of 2.34 ng/mL (range: not detected–11.6 ng/mL). In comparison, the mean PFPeA concentration in serum (detection frequency of 37%) was 0.497 ng/mL (range: not detected–0.942 ng/mL). In a study from China with samples collected in the Hebei province, 86 paired samples of urine and blood were collected (Zhang et al., 2013a). The median concentrations in urine were 0.0011, 0.025, 0.00082, 0.019, 0.0017, 0.00022, 0.00030 and 0.00044 ng/mL for PFHxS, PFOS, PFHpA, PFOA, PFNA, PFDA, PFUnDA and FOSA, respectively. The ratios between the mean serum and urine concentrations were 30, 33 (whole blood:urine), 163, 218, 600, 682, 760 and 1,320 for PFHpA, FOSA, PFOA, PFNA, PFUnDA, PFDA, PFOS and PFHxS. In another study from China, 64 paired samples of urine and serum were analysed (Li et al., 2013). The median concentrations of PFOA and PFOS in urine were approximately 125 and 175 times lower than the corresponding median serum concentrations, respectively. In a study of 55 highly exposed fishery employees from Wuhan, China, with paired samples of urine and serum, high correlations (Spearman Rank Correlation > 0.65) between concentrations in urine and serum were observed for PFBA, PFOA, PFBS, PFHxS and PFOS, and the corresponding median ratios were 0.139, 0.003, 0.0001, 0,0003 and 0,0006, respectively (Wang et al., 2018a).
Other non‐invasive matrices which have been assessed are hair and nails, but it is still not clear how to interpret these results (Alves et al., 2014, 2015; Li et al., 2013).
3.3.2.2. Time trends
The production and use of PFSAs and PFCAs have changed dramatically during the last decades (see Section 1.3.3). To understand how concentrations of various PFCAs and PFSAs have changed over time, several time‐trend studies have been conducted (see detailed information in Appendix B, Table B.1). The time frames of the studies vary, and so do the observed trends.
To illustrate this, results from a time‐trend study conducted in Germany based on samples from the German Environmental Specimen bank (Yeung et al., 2013a,b) are described in detail below. This study covers many sampling years and many different PFASs, including both males and females, and the LOQs are satisfactory low to be able to determine a wide range of PFASs in the samples.
In this particular study, 420 blood samples were collected in Münster (n = 270) and Halle (n = 150) from students in the age group 20–29. The samples from Münster (mean age 24 years) were collected between 1982 and 2009, and PFASs concentrations were determined in 10 samples per year except in 2006 (n = 9) and 2004 (n = 0). Samples were collected in Halle during the period 1995–2009, and 10 samples per year were analysed to determine PFAS concentrations except in 2006 (n = 11). The mean age of the individuals that donated samples in Halle was 23 years. The gender distribution was approximately even in the samples from both Münster and Halle.
Information on the trends of PFOS and PFOA in this study is given in the EFSA CONTAM Panel (2018) Opinion.
PFHxS: no particular trend was observed for either the Münster or Halle samples. In the samples from Münster, the mean concentration in males varied between 1.28 ng/mL in 1985 and 2.49 ng/mL in 2002. The lowest observed mean concentration in males from Halle was 1.21 ng/mL in 1995, while it was 1.91 ng/mL in 2003.
PFNA: in the Münster samples, a steep increase was observed from 1982, with a mean concentration in males of 0.13 ng/mL, to 1989 where the mean concentration in males was 1.58 ng/mL. Following this, a decreasing trend was observed up to the year 2000. The mean concentration in males was then 0.34 ng/mL. From 2000 to 2006, the concentration increased again, with a mean concentration in males of 0.95 ng/mL in 2006. In the Halle samples, the concentration of PFNA was quite stable from 1995 to the end of the century, with a mean concentration in males of 0.30 ng/mL in 1995. From around the year 2000, an increasing trend was observed with a mean concentration in males of 0.86 ng/mL in 2008.
PFDA: an increasing concentration was observed in the Münster samples from 1982 up to 2006 after which it seemed to stabilise. The mean concentration in males was 0.061 ng/mL in 1982 while it was 0.42 ng/mL in 2006. A comparable trend and similar concentrations were observed for the Halle samples.
PFUnDA: the trend in the Münster samples was similar to the trend for PFOS and PFOA with an increasing trend from 1982 to around 1990, followed by decreasing concentrations between 1990 and 1995 and a new increasing trend from 1995 to 2009. The mean concentration in males in 1982 was 0.034 ng/mL while it was 0.218 ng/mL in 1989 and 0.127 ng/mL in 2009.
No particular trends were observed for the remaining detected substances (PFHxA, PFHpA, PFDoDA, PFTrDA, PFTeDA, 8:2 diPAP, PFDS, EtFOSAA).
The general trends observed in the time‐trend studies (see Appendix B, Table B.1) can be summarised as follows: (i) the concentrations of most PFSAs and PFCAs increased from the early 1970s up to around the year 2000, (ii) the concentrations of PFOS and PFOA have in most studies been observed to decrease from approximately the year 2000, while in many studies, PFNA, PFDA and PFUnDA have increased or at least remained stable during the same time period, (iii) variable trends have been reported for PFHxS, while no particular change has been reported for some of the PFASs that are present in low concentrations in humans. This is in line with the conclusions in a recent review on time trends of PFASs (Land et al., 2018).
3.3.2.3. Levels in general European populations with serum or plasma samples collected from 2007–2008 and onwards
In Appendix B, Tables B.2 and B.4, concentrations of all PFASs included in this Opinion in plasma and serum from adults and children are reported, respectively. Furthermore, in Appendix B (Table B.3), concentrations of PFHxA and FOSA in whole blood are presented. European studies with samples collected from general populations in 2007–2008 and onwards are included. For the time‐trend studies, only results from the most recent years have been included. Descriptive statistics summarising the studies in Appendix B, Tables B.2–B.4, are presented in Tables 16 and 17 for adults and Tables 18 and 19 for children, respectively. The summary statistics on PFOS and PFOA are given in the EFSA CONTAM Panel et al. (2018) Opinion but are also included in Tables 16–19 for comparison.
Table B.2.
Concentrations of PFASs in general European adult populations with serum or plasma samples collected from 2007 to 2008 and onwards
| Year | Country | Study population | Median (ng/mL) | Geometric mean (ng/mL) | Aritmetric mean (ng/mL) | Min (ng/mL) | Max (ng/mL) | Reference |
|---|---|---|---|---|---|---|---|---|
| PFBA | ||||||||
| 2012 | Sweden | n = 3 pools primiparous women living in Uppsala County, donated serum samples within the fourth week after delivery. Serum | NR | NR | NR | < LOQ (0.3)a | < LOQ (0.3)a | Gebbink et al. (2015) |
| 2015 | Czech Republic | n = 300, men and women, mean age: 40.8 years. Serum | < LOQ (0.013) | < LOQ (0.013) | NR | < LOQ (0.013) | < LOQ (0.013) | Sochorová et al. (2017) |
| 2015–2016 | Italy | n = 250, men and women, age: 20–51 years. Background exposed population. Serum | < LOQ (NR) | NR | NR | < LOQ (NR) | 0.35 | Ingelido et al. (2018) |
| 2015–2016 | Italy | n = 257, men and women, age: 20–51 years. Exposed to elevated PFAS concentrations through drinking water. Serum | < LOQ (NR) | NR | NR | < LOQ (NR) | 3.59 | Ingelido et al. (2018) |
| 2015 | UK | n = 29, women, mean age: 32.9 years, Controls. Serum. n = 30, women, mean age 32.9 years, Cases with polycystic ovary syndrome. Serum | NR | < LOQ (0.5) | NR | < LOQ (0.5) | < LOQ (0.5) | Heffernan et al. (2018) |
| PFPeA | ||||||||
| 2012 | Sweden | n = 3 pools primiparous women living in Uppsala County, donated serum samples within the fourth week after delivery. Serum | NR | NR | NR | < LOQ (0.1)a | < LOQ (0.1)a | Gebbink et al. (2015) |
| 2013–2014 | Norway | n = 61, men and women, median (range) age: 41 (20–66) years. Serum | < LOQ (0.09) | NR | < LOQ (0.09) | < LOQ (0.09) | < LOQ (0.09) | Poothong et al. (2017) |
| 2008–2013 | Denmark |
n =1,533 samples, pregnant nulliparous women from the Aarhus Birth Cohort Biobank Most participants gave a blood sample between 11 weeks and 14 weeks of gestation. Median age 29 years. Serum |
NR | NR | NR | < LOQ (0.19) | < 20% of samples above LOQ | Bjerregaard‐Olesen et al. (2016) |
| 2015 | Czech Republic | n = 300, men and women, mean age 40.8 years. Serum | < LOQ (0.013) | < LOQ (0.013) | NR | < LOQ (0.013) | < LOQ (0.013) | Sochorová et al. (2017) |
| 2015–2016 | Italy | n = 250, men and women, 20–51 years. Background exposed population. Serum | < LOQ (NR) | NR | NR | < LOQ (NR) | 0.22 | Ingelido et al. (2018) |
| 2015–2016 | Italy | n = 257, men and women, 20–51 years. Exposed to elevated PFAS concentrations through drinking water. Serum | < LOQ (NR) | NR | NR | < LOQ (NR) | 0.46 | Ingelido et al. (2018) |
| 2015 | UK | n = 29, women, mean age 32.9 years, Controls. Serum. n = 30, women, mean age 32.9 years, Cases with polycystic ovary syndrome. Serum | NR | < LOQ (0.5) | NR | < LOQ (0.5) | 2.02 | Heffernan et al. (2018) |
| PFHxA | ||||||||
| 2012 | Sweden | n = 3 pools primiparous women living in Uppsala County, donated serum samples within the fourth week after delivery. Serum | NR | NR | NR | < LOQ (0.05)a | < LOQ (0.05)a | Gebbink et al. (2015) |
| 2013–2014 | Norway | n = 61, men and women, median age 41 years (range: 20–66 years). Serum | < LOQ (0.045) | NR | < LOQ (0.045) | < LOQ (0.045) | < LOQ (0.045) | Poothong et al. (2017) |
| 2008–2013 | Denmark |
n= 1,533, samples pregnant nulliparous women from the Aarhus Birth Cohort Biobank Most participants gave a blood sample between 11 weeks and 14 weeks of gestation. Median age 29 years. Serum |
NR | NR | NR | < LOQ (0.19) | < LOQ (0.19) | Bjerregaard‐Olesen et al. (2016) |
| 2007–2009 | Germany | n = 30, students, male and female, age: 20–29 years from Münster. Plasma | NR | NR | NR | < LOQ (0.0003) | 0.0617 | Yeung et al. (2013a) |
| 2007–2009 | Germany | n = 30, students, male and female, age: 20–29 years from Halle. Plasma | NR | NR | NR | < LOQ (0.0014‐0.005) | 1.58 | Yeung et al. (2013a) |
| 2015 | Czech Republic | n = 300, men and women, mean age: 40.8 years. Serum | < LOQ (0.013) | < LOQ (0.013) | NR | < LOQ (0.013) | < LOQ (0.013) | Sochorová et al. (2017) |
| 2015–2016 | Italy | n = 250, men and women, age: 20–51 years. Background exposed population. Serum | < LOQ (NR) | NR | NR | < LOQ (NR) | 0.26 | Ingelido et al. (2018) |
| 2015–2016 | Italy | n = 257, men and women, 20–51 years. Exposed to elevated PFAS concentrations through drinking water. Serum | < LOQ (NR) | NR | NR | < LOQ (NR) | 0.68 | Ingelido et al. (2018) |
| 2015 | UK | n = 29, women, mean age 32.9 years, Controls. Serum. n = 30, women, mean age 32.9 years, Cases with polycystic ovary syndrome. Serum | NR | < LOQ (0.5) | NR | < LOQ (0.5) | < LOQ (0.5) | Heffernan et al. (2018) |
| PFHpA | ||||||||
| 2008–2011 | Sweden | n = 150, primiparous women, within the third week after delivery. The County has a known contamination of the drinking water with PFAS. Mean (range) age: 30.2 (21–40 years). Serum | 0.045a | NR | 0.059a | < LOQ (0.04)a | 0.296a | Gyllenhammar et al. (2015) |
| 2010 | Sweden | n = 36 pools of serum. Primiparous women living in Uppsala county, donated serum samples within the third week after delivery. Age 19–41 years | NR | NR | NR | 0.073 | 0.11 | Glynn et al. (2012) |
| 2012 | Sweden | n = 3 pools primiparous women living in Uppsala County, donated serum samples within the fourth week after delivery. Serum | NR | NR | NR | 0.022a | 0.030a | Gebbink et al. (2015) |
| 2011–2014 | Sweden | n = 579, men and women, 80 years | 0.03 | 0.02 | 0.04 | 0.01 | 0.70 | Stubleski et al. (2016) |
| 2007–2008 | Norway | n = 41, women, mean age: 36.7 years (range 25–45 years). Serum | < LOQ (0.05) | NR | < LOQ (0.05) | < LOQ (0.05) | 0.10 | Haug et al. (2011b) |
| 2007–2009 | Norway | n = 391, pregnant women, mean (range) age: 31 (18–43) years. Serum | NR | NR | NR | < LOQ (0.03) | 0.45 | Berg et al. (2014) |
| 2013–2014 | Norway | n = 61, men and women, median (range) age: 41 (20–66) years. Serum | < LOQ (0.045) | NR | 0.06 | < LOQ (0.045) | 0.34 | Poothong et al. (2017) |
| 2010–2013 | Greenland | n = 207, pregnant women age > 18 years, Inuits. Serum | 0.03 | 0.03 | 0.04 | 0.03 | 0.26 | Long et al. (2015) |
| 2011–2014 | Greenland | n = 128, women, median age around 50 years, breast cancer cases and controls. Serum | 0.14 | NR | NR | NR | NR | Wielsøe et al. (2017) |
| 2015 | Czech Republic | n = 300, men and women, mean age: 40.8 years. Serum | NR | NR | NR | < LOQ (0.013) | 0.52 | Sochorová et al. (2017) |
| 2015–2016 | Italy | n = 250, men and women, age: 20–51 years. Background exposed population. Serum | < LOQ (NR) | NR | NR | < LOQ (NR) | 0.26 | Ingelido et al. (2018) |
| 2015–2016 | Italy | n = 257, men and women, age: 20–51 years. Exposed to elevated PFAS concentrations through drinking water. Serum | < LOQ (NR) | NR | NR | < LOQ (NR) | 0.42 | Ingelido et al. (2018) |
| 2015 | UK | n = 29, women, mean age 32.9 years, Controls. Serum. n = 30, women, mean age 32.9 years, Cases with polycystic ovary syndrome. Serum | NR | < LOQ (0.1) | NR | < LOQ (0.1) | 0.70 | Heffernan et al. (2018) |
| PFNA | ||||||||
| 2007 | Sweden | n = 10, women, mean (range) age: 48 (36–56 years). Serum | 0.87 | NR | NR | NR | NR | Axmon et al. (2014) |
| 2008–2010 | Sweden | n = 153, males, mean (range) age: 67 (53–79) years. Whole blood | 0.58 | 0.56 | 0.64 | 0.09 | 1.6 | Bao et al. (2014) |
| 2010–2011 | Sweden | n = 270, women, median (range) age: 50 (22–75 years). Serum | 0.80 | NR | NR | 0.35 (P5) | 1.66 (P95) | Bjermo et al. (2013) |
| 2007–2011 | Sweden | n = 201, men with prostate cancer. Median (range) age: 67 (49–79) years. Serum | 0.612 | NR | 0.679 | 0.05 | 4.6 | Hardell et al. (2014) |
| 2007–2011 | Sweden | n = 186, men without prostate cancer. Median (range) age: 67 (50–79) years. Serum | 0.572 | NR | 0.631 | 0.0850 | 2.1 | Hardell et al. (2014) |
| 2008–2011 | Sweden | n = 150, primiparous women, within the third week after delivery. The County has a known contamination of the drinking water with PFAS. Mean (range) age: 30.2 (21–40) years. Serum | 0.46a | NR | 0.52a | 0.064a | 2.2a | Gyllenhammar et al. (2015) |
| 2010 | Sweden | n = 36 pools of serum. Primiparous women living in Uppsala county, donated serum samples within the third week after delivery. Age: 19–41 years | NR | NR | NR | 0.59 | 0.86 | Glynn et al. (2012) |
| 2012 | Sweden | n = 3 pools primiparous women living in Uppsala County, donated serum samples within the fourth week after delivery. Serum | NR | NR | NR | 0.48a | 0.56a | Gebbink et al. (2015) |
| 2011–2014 | Sweden | n = 579, men and women, 80 years | 0.87 | 0.89 | 1.1 | 0.07 | 6.9 | Stubleski et al. (2016) |
| 2007–2008 | Norway | n = 41, women, mean (range) age: 36.7 (25–45) years. Serum | 0.63 | NR | 0.64 | 0.28 | 1.3 | Haug et al. (2011b) |
| 2007–2008 | Norway | n = 123, pregnant women. Plasma | 0.34 | NR | 0.4 | < LOQ (0.05) | 2.18 | Gützkow et al. (2012) |
| 2007–2008 | Norway | n = 99, pregnant women. Plasma | 0.3 | NR | 0.3 | < LOQ (0.05) | 0.9 | Granum et al. (2013) |
| 2007–2009 | Norway | n = 391, pregnant women, mean (range) age: 31 (18–43) years. Serum | 0.56 | NR | 0.67 | 0.15 | 4.36 | Berg et al. (2014) |
| 2012–2014 | Norway | n = 74, 59 consumers (HC) and 15 non‐consumers (NC) of fish from AFFF‐affected waters. Median (range) age: 58.5 (32–79) years. Serum | 1.72 (HC) | 1.57 (HC) | 2.18 (HC) | 0.58 (HC, P10) | 5.22 (HC, P90) | Hansen et al. (2016) |
| 2012–2014 | Norway | n = 74, 59 consumers (HC) and 15 non‐consumers (NC) of fish from AFFF‐affected waters. Median (range) age: 58.5 (32–79) years. Serum | 0.80 (NC) | 0.80 (NC) | 0.95 (NC) | 0.30 (NC, P10) | 1.88 (NC, P10) | Hansen et al. (2016) |
| 2013–2014 | Norway | n = 61, men and women, median (range) age: 41 (20–66) years. Serum | 0.94 | NR | 1.06 | 0.19 | 2.73 | Poothong et al. (2017) |
| 2008–2009 | Denmark | n = 247, adult men. Mean age: 19.6 years. Serum | 1.07 | NR | 1.23 | 0,64 (P5) | 2.41 (P95) | Joensen et al. (2013) |
| 2011 | Denmark | n = 200 samples of serum from pregnant women. Serum | 0.61 | NR | 0.69 | 0.18 | 4.4 | Vorkamp et al. (2014) |
| 2010–2012 | Denmark | n = 392, newly pregnant women. Serum | 0.72 | NR | NR | 0.18 (P5) | 4.40 (P95) | Jensen et al. (2015) |
| 2011 | Denmark | n = 145, women, mean (range) age: 41 (31–52) years. Plasma | 0.64 | NR | 0.75 | 0.26 | 2.55 | Mørck et al. (2015) |
| 2008–2013 | Denmark | n = 1,507 pregnant women, primiparous, median age between 29 and 31 years for the four quartiles of participants. Serum | 0.8 | NR | NR | 0.6 (IQR) | 1.0 (IQR) | Bach et al. (2016) |
| 2010–2012 | Denmark | n = 649 pregnant women, mean age: 30.7 years. Serum | NR | NR | 0.7 | 0.5 (IQR) | 0.9 (IQR) | Lind et al. (2017a) |
| 2007–2009 | Faroe Islands | n = 487, pregnant women, mean age: 30.6 years. Serum | 0.66 | NR | NR | 0.52 (IQR) | 0.86 (IQR) | Timmermann et al. (2017a) |
| 2010–2013 | Greenland | n = 207, pregnant women age: > 18 years, Inuits. Serum | 1.30 | 1.29 | 1.49 | 0.41 | 7.71 | Long et al. (2015) |
| 2011–2014 | Greenland | n = 128, women, median age around 50 years, breast cancer cases and controls. Serum | 2.64 | NR | NR | NR | NR | Wielsøe et al. (2017) |
| 2008 | France | n = 478, age: 18 and 75 years, males and females, had current residence in targeted areas and had a fishing license. Serum | 1.3 | 1.4 | 1.6 | 0.2 | 8 | Denys et al. (2014) |
| 2010–2013 | France | n = 100, pregnant women, median (range) age: 32 (20–46) years. Serum | 0.430 | NR | 0.519 | < LOQ (0.3) | 3.29 | Cariou et al. (2015) |
| 2007–2009 | Germany | n = 44, pregnant women, mean (range) age: 33 (21–43 years), samples collected during pregnancy. Plasma | 0.6 | NR | 0.8 | NR | NR | Fromme et al. (2010) |
| 2009 | Germany | n = 60, men and women, age: 18–67 years. Plasma (rural town, near fluoropolymer production plant) | 0.8 | NR | 1.1 | NR | 8.6 | Fromme et al. (2017) |
| 2015 | Germany | n = 26, men and women, age: 18–67 years. Plasma (rural town, near fluoropolymer production plant) | 0.5 | NR | 0.7 | NR | 3.8 | Fromme et al. (2017) |
| 2009 | Germany | n = 60, men and women, age: 18–67 years. Plasma (potential contamination of drinking water) | 0.8 | NR | 0.8 | NR | 2.9 | Fromme et al. (2017) |
| 2015 | Germany | n = 50, men and women, age: 18–67 years. Plasma (potential contamination of drinking water) | 0.5 | NR | 0.5 | NR | 2.6 | Fromme et al. (2017) |
| 2014 | Germany | n = 42, men and women, age: 18–67 years. Plasma | 0.4 | NR | 0.4 | NR | 0.8 | Fromme et al. (2017) |
| 2016 | Germany | n = 158, men and women, age: 18–67 years. Plasma | 0.4 | NR | 0.4 | NR | 1.5 | Fromme et al (2017) |
| 2009–2010 | Spain | n = 46, males and females, age: 19–53 years. Whole blood | NR | NR | 0.12 | < LOQ (0.05) | 2.94 | Gómez‐Canela et al. (2015) |
| 2009–2010 | Spain | n = 755, men and women, age: 18–65 years. Serum | 0.92 | 0.96 | 1.11 | 0.51 (P10) | 2.55 (P95) | Bartolome et al. (2017) |
| 2010–2012 | Slovakia | n = 120, pregnant women. Age range: 18–45 years. Plasma | 0.44 | 0.44 | 0.54 | < LOQ (NR) | 1.7 | Uhl (2016) |
| 2010–2012 | Austria | n = 114, pregnant women. Age range: 18–45 years. Plasma | 0.38 | 0.41 | 0.45 | < LOQ (NR) | 1.1 | Uhl (2016) |
| 2015 | Czech Republic | n = 300, men and women, mean age: 40.8 years. Serum | 0.325 | 0.300 | NR | < LOQ (0.013) | 6.55 | Sochorová et al. (2017) |
| 2015–2016 | Italy | n = 250, men and women, age: 20–51 years. Background exposed population. Serum | 0.58 | NR | NR | 0.03 | 7.72 | Ingelido et al. (2018) |
| 2015–2016 | Italy | n = 257, men and women, age: 20–51 years. Exposed to elevated PFAS concentrations through drinking water. Serum | 0.61 | NR | NR | < LOQ (NR) | 2.46 | Ingelido et al. (2018) |
| 2015 | UK | n = 29, women, mean age: 32.9 years, Controls. Serum. n = 30, women, mean age: 32.9 years, Cases with polycystic ovary syndrome. Serum | NR | 0.57 | NR | 0.2 | 1.79 | Heffernan et al. (2018) |
| PFDA | ||||||||
| 2007 | Sweden | n = 10, women, mean (range) age: 48 (36–56) years. Serum | 0.35 | NR | NR | NR | NR | Axmon et al. (2014) |
| 2010–2011 | Sweden | n = 270, women, median (range) age: 50 (22–75) years. Serum | 0.39 | NR | NR | 0.19 (P5) | 0.84 (P95) | Bjermo et al. (2013) |
| 2008–2010 | Sweden | n = 153 males, mean (range) age: 67 (53–79) years. Whole blood | 0.27 | 0.23 | 0.29 | < LOQ (0.2) | 1.0 | Bao et al. (2014) |
| 2007–2011 | Sweden | n = 201, men with prostate cancer. Median (range) age: 67 (49–79) years. Serum | 0.301 | NR | 0.338 | 0.0300 | 1.2 | Hardell et al. (2014) |
| 2007–2011 | Sweden | n = 186, men without prostate cancer. Median (range) age: 67 (50–79) years. Serum | 0.269 | NR | 0.291 | 0.0244 | 1.0 | Hardell et al. (2014) |
| 2008–2011 | Sweden | n = 150, primiparous women, within the third week after delivery. The County has a known contamination of the drinking water with PFAS. Mean (range) age: 30.2 (21–40) years. Serum | 0.26a | NR | 0.28a | < LOQ (0.05) a | 1.1a | Gyllenhammar et al. (2015) |
| 2010 | Sweden | n = 36 pools of serum. Primiparous women living in Uppsala county, donated serum samples within the third week after delivery. Age: 19–41 years | NR | NR | NR | 0.28 | 0.42 | Glynn et al. (2012) |
| 2012 | Sweden | n = 3 pools primiparous women living in Uppsala County, donated serum samples within the fourth week after delivery. Serum | NR | NR | NR | 0.27a | 0.29a | Gebbink et al. (2015) |
| 2011–2014 | Sweden | n = 579, men and women, age: 80 years | 0.34 | 0.34 | 0.40 | 0.03 | 2.0 | Stubleski et al. (2016) |
| 2007–2008 | Norway | n=41, women, mean (range) age: 36.7 (25–45) years. Serum | 0.23 | NR | 0.25 | 0.10 | 0.59 | Haug et al. (2011b) |
| 2007–2008 | Norway | n = 123, pregnant women. Plasma | 0.07 | NR | 0.10 | < LOQ (0.05) | 1.14 | Gützkow et al. (2012) |
| 2007–2009 | Norway | n = 391, pregnant women, mean (range) age: 31 (18–43) years. Serum | 0.23 | NR | 0.26 | 0.05 | 2.34 | Berg et al. (2014) |
| 2012–2014 | Norway | n = 74, 59 consumers (HC) and 15 non‐consumers (NC) of fish from AFFF‐affected waters. Median (range) age: 58.5 (32–79) years. Serum | 0.47 (HC) | 0.43 (HC) | 0.59 (HC) | 0.11 (HC, P10) | 1.40 (HC, P90) | Hansen et al. (2016) |
| 2012–2014 | Norway | n = 74, 59 consumers (HC) and 15 non‐consumers (NC) of fish from AFFF‐affected waters. Median (range) age: 58.5 (32–79) years. Serum | 0.37 (NC) | 0.22 (NC) | 0.31 (NC) | 0.04 (NC, P10) | 0.66 (NC, P90) | Hansen et al. (2016) |
| 2013–2014 | Norway | n = 61, men and women, median (range) age: 41 (20–66 years). Serum | 0.37 | NR | 0.40 | 0.15 | 1.07 | Poothong et al. (2017) |
| 2008–2009 | Denmark | n = 247, adult men. Mean age: 19.6 years. Serum | 0.35 | NR | 0.38 | 0.22 (P5) | 0.61 (P95) | Joensen et al. (2013) |
| 2011 | Denmark | n = 200 samples of pregnant women. Serum | 0.27 | NR | 0.31 | 0.088 | 1.7 | Vorkamp et al. (2014) |
| 2010–2012 | Denmark | n = 392, newly pregnant women. Serum | 0.27 | NR | NR | 0.07 (P5) | 1.75 (P95) | Jensen et al. (2015) |
| 2011 | Denmark | n = 145, women, mean (range) age: 41 (31–52) years. Plasma | 0.28 | NR | 0.33 | 0.08 | 1.14 | Mørck et al. (2015) |
| 2008–2013 | Denmark | n = 1,507, pregnant women, primiparous, median age between 29 and 31 for the four quartiles of participants. Serum | 0.3 | NR | NR | 0.2 (IQR) | 0.4 (IQR) | Bach et al. (2016) |
| 2010–2012 | Denmark | n = 649 pregnant women, mean age: 30.7 years. Serum | NR | NR | 0.3 | 0.2 (IQR) | 0.3 (IQR) | Lind et al. (2017a) |
| 2007–2009 | Faroe Islands | n = 487, pregnant women, mean age: 30.6 years. Serum | 0.26 | NR | NR | 0.19 (IQR) | 0.35 (IQR) | Timmermann et al. (2017a) |
| 2010–2013 | Greenland | n = 207, pregnant women age: > 18 years, Inuits. Serum | 0.72 | 0.78 | 0.99 | 0.12 | 7.84 | Long et al. (2015) |
| 2011–2014 | Greenland | n = 128, women, median age around 50 years, breast cancer cases and controls. Serum | 1.34 | NR | NR | NR | NR | Wielsøe et al. (2017) |
| 2008 | France | n = 478, age: 18 and 75 years, males and females, had current residence in targeted areas and had a fishing license. Serum | 0.5 | 0.6 | 0.7 | < LOQ (NR) | 11.2 | Denys et al. (2014) |
| 2010–2013 | France | n = 100, pregnant women, median (range) age: 32 (20–46) years. Serum | < LOQ (0.4) | NR | 0.277 | < LOQ (0.4) | 1.99 | Cariou et al. (2015) |
| 2007–2009 | Germany | n = 44, pregnant women, mean (range) age: 33 (21–43) years, samples collected during pregnancy. Plasma | < LOQ (0.4) | NR | < LOQ (0.4) | NR | NR | Fromme et al. (2010) |
| 2009 | Germany | n = 60, men and women, age: 18–67 years. Plasma (rural town, near fluoropolymer production plant) | 0.5 | NR | 1.1 | NR | 19.2 | Fromme et al. (2017) |
| 2015 | Germany | n = 26, men and women, age: 18–67 years. Plasma (rural town, near fluoropolymer production plant) | NR | NR | < LOQ (0.4) | NR | 3.3 | Fromme et al. (2017) |
| 2009 | Germany | n = 60, men and women, age: 18–67 years. Plasma (potential contamination of drinking water) | 0.3 | NR | 0.4 | NR | 1.3 | Fromme et al. (2017) |
| 2015 | Germany | n = 50, men and women, age: 18–67 years. Plasma (potential contamination of drinking water) | 0.2 | NR | 0.3 | NR | 2.6 | Fromme et al. (2017) |
| 2014 | Germany | n = 42, men and women, age: 18–67 years. Plasma | NR | NR | NR | NR | 0.5 | Fromme et al. (2017) |
| 2016 | Germany | n = 158, men and women, age: 18–67 years. Plasma | NR | NR | NR | NR | 1.0 | Fromme et al. (2017) |
| 2009–2010 | Spain | n = 755, men and women, age: 18–65 years. Serum | 0.36 | 0.42 | 0.49 | < LOQ (0.20, P10) | 0.99 (P95) | Bartolome et al. (2017) |
| 2010–2012 | Slovakia | n = 120, pregnant women. Age range: 18–45 years. Plasma | 0.27 | 0.29 | 0.36 | < LOQ (NR) | 1.1 | Uhl (2016) |
| 2010–2012 | Austria | n = 114, pregnant women. Age range: 18–45 years. Plasma | 0.18 | 0.18 | 0.20 | < LOQ (NR) | 0.5 | Uhl (2016) |
| 2015 | Czech Republic | n = 300, men and women, mean age: 40.8 years. Serum | 0.145 | 0.141 | NR | 0.013 | 1.81 | Sochorová et al. (2017) |
| 2015–2016 | Italy | n = 250, men and women, age: 20–51 years. Background exposed population. Serum | 0.32 | NR | NR | < LOQ (NR) | 3.07 | Ingelido et al. (2018) |
| 2015–2016 | Italy | n = 257, men and women, age: 20–51 years. Exposed to elevated PFAS concentrations through drinking water. Serum | 0.33 | NR | NR | < LOQ (NR) | 1.96 | Ingelido et al. (2018) |
| 2015 | UK | n = 29, women, mean age: 32.9 years, Controls. Serum. n = 30, women, mean age: 32.9 years, Cases with polycystic ovary syndrome. Serum | NR | 0.31 | NR | < LOQ (0.2) | 1.17 | Heffernan et al. (2018) |
| PFUnDA | ||||||||
| 2007 | Sweden | n = 10, women, mean (range) age: 48 (36–56) years. Serum | 0.24 | NR | NR | NR | NR | Axmon et al. (2014) |
| 2010–2011 | Sweden | n = 270, women, median (range) age: 50 (22–75 years). Serum | 0.33 | NR | NR | 0.11 (P5) | 0.86 (P95) | Bjermo et al. (2013) |
| 2008–2010 | Sweden | n = 153 males, mean (range) age: 67 (53–79) years. Whole blood | 0.25 | 0.20 | 0.28 | < LOQ (0.2) | 1.5 | Bao et al. (2014) |
| 2007–2011 | Sweden | n = 201, men with prostate cancer. Median (range) age: 67 (49–79 years). Serum | 0.264 | NR | 0.308 | 0.0150 | 1.3 | Hardell et al. (2014) |
| 2007–2011 | Sweden | n = 186, men without prostate cancer. Median (range) age: 67 (50–79) years. Serum | 0.254 | NR | 0.285 | 0.0250 | 1.5 | Hardell et al. (2014) |
| 2008–2011 | Sweden | n = 150, primiparous women, within the third week after delivery. The County has a known contamination of the drinking water with PFAS. Mean (range) age: 30.2 (21–40 years). Serum | 0.23a | NR | 0.26a | < LOQ (0.05) a | 0.91a | Gyllenhammar et al. (2015) |
| 2010 | Sweden | n = 36 pools of serum. Primiparous women living in Uppsala county, donated serum samples within the third week after delivery. Age: 19–41 years | NR | NR | NR | 0.19 | 0.31 | Glynn et al. (2012) |
| 2012 | Sweden | n = 3 pools primiparous women living in Uppsala County, donated serum samples within the fourth week after delivery. Serum | NR | NR | NR | 0.23a | 0.27a | Gebbink et al. (2015) |
| 2011–2014 | Sweden | n = 579, men and women, 80 years | 0.36 | 0.36 | 0.42 | 0.12 | 1.8 | Stubleski et al. (2016) |
| 2007–2008 | Norway | n = 41, women, mean (range) age: 36.7 (25–45 years). Serum | 0.42 | NR | 0.44 | 0.080 | 1.1 | Haug et al. (2011b) |
| 2007–2008 | Norway | n = 123, pregnant women. Plasma | 0.16 | NR | 0.19 | < LOQ (0.05) | 0.54 | Gützkow et al. (2012) |
| 2007–2009 | Norway | n = 391, pregnant women, mean (range) age: 31 (18–43) years. Serum | 0.26 | NR | 0.30 | 0.03 | 1.46 | Berg et al. (2014) |
| 2012–2014 | Norway | n = 74, 59 consumers (HC) and 15 non‐consumers (NC) of fish from AFFF‐affected waters. Median (range) age: 58.5 (32–79) years. Serum | 0.71 (HC) | 0.66 (HC) | 1.10 (HC) | 0.12 (HC, P10) | 3.01 (HC, P90) | Hansen et al. (2016) |
| 2012–2014 | Norway | n = 74, 59 consumers (HC) and 15 non‐consumers (NC) of fish from AFFF‐affected waters. Median (range) age: 58.5 (32–79) years. Serum | 0.21 (NC) | 0.25 (NC) | 0.45 (NC) | 0.03 (NC, P10) | 1.05 (NC, P90) | Hansen et al. (2016) |
| 2013–2014 | Norway | n = 61, men and women, median (range) age: 41 (20–66) years. Serum | 0.37 | NR | 0.43 | 0.05 | 1.67 | Poothong et al. (2017) |
| 2008–2013 | Denmark | n = 1,507 pregnant women, primiparous, median age between 29 and 31 for the four quartiles of participants. Serum | 0.3 | NR | NR | 0.2 (IQR) | 0.4 (IQR) | Bach et al. (2016) |
| 2010–2013 | Greenland | n = 207, pregnant women age > 18 years, Inuits. Serum | 1.60 | 1.68 | 2.58 | 0.18 | 18.2 | Long et al. (2015) |
| 2011–2014 | Greenland | n = 128, women, median age around 50 years, breast cancer cases and controls. Serum | 2.49 | NR | NR | NR | NR | Wielsøe et al. (2017) |
| 2010–2013 | France | n = 100, pregnant women, median (range) age: 32 (20–46) years. Serum | < LOQ (0.35) | NR | 0.21 | < LOQ (0.35) | 2.60 | Cariou et al. (2015) |
| 2010–2012 | Slovakia | n = 120, pregnant women. Age range: 18–45 years. Plasma | 0.61 | 0.58 | 0.62 | < LOQ (NR) | 0.9 | Uhl (2016) |
| 2010–2012 | Austria | n = 114, pregnant women. Age range: 18–45 years. Plasma | 0.40 | 0.37 | 0.37 | < LOQ (NR) | 0.4 | Uhl (2016) |
| 2015 | Czech Republic | n = 300, men and women, mean age: 40.8 years. Serum | 0.058 | 0.055 | NR | < LOQ (0.013) | 0.417 | Sochorová et al. (2017) |
| 2015–2016 | Italy | n = 250, men and women, age: 20–51 years. Background exposed population. Serum | 0.18 | NR | NR | < LOQ (NR) | 1.35 | Ingelido et al. (2018) |
| 2015–2016 | Italy | n = 257, men and women, age: 20–51 years. Exposed to elevated PFAS concentrations through drinking water. Serum | 0.16 | NR | NR | < LOQ (NR) | 1.02 | Ingelido et al. (2018) |
| 2015 | UK | n = 29, women, mean age 32.9 years, Controls. Serum. N = 30, women, mean age 32.9 years, Cases with polycystic ovary syndrome. Serum | NR | < LOQ (0.2) | NR | < LOQ (0.2) | 0.47 | Heffernan et al. (2018) |
| PFDoDA | ||||||||
| 2008–2011 | Sweden | n = 150, primiparous women, within the third week after delivery. The County has a known contamination of the drinking water with PFAS. Mean (range) age: 30.2 (21–40) years. Serum | < LOQ (0.05)a | NR | NR | < LOQ (0.05)a | 0.25a | Gyllenhammar et al. (2015) |
| 2012 | Sweden | n = 3 pools primiparous women living in Uppsala County, donated serum samples within the fourth week after delivery. Serum | NR | NR | NR | 0.027a | 0.033a | Gebbink et al. (2015) |
| 2007–2008 | Norway | n = 41, women, mean (range) age: 36.7 (25–45 years). Serum | < LOQ (0.05) | NR | 0.050 | < LOQ (0.05) | 0.14 | Haug et al. (2011b) |
| 2007–2009 | Norway | n = 391, pregnant women, mean (range) age: 31 (18–43) years. Serum | 0.03 | NR | 0.04 | < LOQ (0.03) | 0.20 | Berg et al. (2014) |
| 2013–2014 | Norway | n = 61, men and women, median (range) age: 41 (20–66) years. Serum | 0.07 | NR | 0.07 | < LOQ (0.0036) | 0.26 | Poothong et al. (2017) |
| 2010–2013 | Greenland | n = 207, pregnant women age > 18 years, Inuits. Serum | 0.21 | 0.31 | 0.39 | 0.20 | 1.85 | Long et al. (2015) |
| 2011–2014 | Greenland | n = 128, women, median age around 50 years, breast cancer cases and controls. Serum | 0.54 | NR | NR | NR | NR | Wielsøe et al. (2017) |
| 2009 | Germany | n = 60, men and women, age: 18–67 years. Plasma (rural town, near fluoropolymer production plant) | NR | NR | < LOQ (0.4) | NR | 3.9 | Fromme et al. (2017) |
| 2015 | Germany | n = 26, men and women, age: 18–67 years. Plasma (rural town, near fluoropolymer production plant) | NR | NR | < LOQ (0.4) | NR | 0.8 | Fromme et al. (2017) |
| 2009 | Germany | n = 60, men and women, age: 18–67 years. Plasma (potential contamination of drinking water) | NR | NR | NR | NR | < LOQ (0.4) | Fromme et al. (2017) |
| 2015 | Germany | n = 50, men and women, age: 18–67 years. Plasma (potential contamination of drinking water) | NR | NR | NR | NR | < LOQ (0.4) | Fromme et al. (2017) |
| 2014 | Germany | n = 42, men and women, age: 18–67 years. Plasma | NR | NR | NR | NR | < LOQ (0.4) | Fromme et al. (2017) |
| 2016 | Germany | n = 158, men and women, age: 18–67 years. Plasma | NR | NR | NR | NR | < LOQ (0.4) | Fromme et al. (2017) |
| 2015 | Czech Republic | n = 300, men and women, mean age: 40.8 years. Serum | NR | NR | NR | < LOQ (0.013) | 0.196 | Sochorová et al. (2017) |
| 2015–2016 | Italy | n = 250, men and women, age: 20–51 years. Background exposed population. Serum | 0.04 | NR | NR | < LOQ (NR) | 1.67 | Ingelido et al. (2018) |
| 2015–2016 | Italy | n = 257, men and women, age: 20–51 years. Exposed to elevated PFAS concentrations through drinking water. Serum | < LOQ (NR) | NR | NR | < LOQ (NR) | 1.33 | Ingelido et al. (2018) |
| 2015 | UK | n = 29, women, mean age 32.9 years, Controls. Serum. n = 30, women, mean age 32.9 years, Cases with polycystic ovary syndrome. Serum | NR | < LOQ (0.5) | NR | < LOQ (0.5) | < LOQ (0.5) | Heffernan et al. (2018) |
| PFTrDA | ||||||||
| 2012 | Sweden | n = 3 pools primiparous women living in Uppsala County, donated serum samples within the fourth week after delivery. Serum | NR | NR | NR | 0.030a | 0.038a | Gebbink et al. (2015) |
| 2007–2008 | Norway | n = 41, women, mean (range) age: 36.7 (25–45 years). Serum | < LOQ (0.05) | NR | 0.056 | < LOQ (0.05) | 0.22 | Haug et al. (2011b) |
| 2007–2008 | Norway | n = 123, pregnant women. Plasma | < LOQ (0.05) | NR | 0.06 | < LOQ (0.05) | 0.23 | Gützkow et al. (2012) |
| 2013–2014 | Norway | n = 61, men and women, median (range) age: 41 (20–66) years. Serum | < LOQ (0.009) | NR | < LOQ (0.009) | < LOQ (0.009) | 0.05 | Poothong et al. (2017) |
| 2010–2013 | Greenland | n = 207, pregnant women age > 18 years, Inuits. Serum | 0.21 | 0.21 | 0.21 | 0.21 | 0.90 | Long et al. (2015) |
| 2015 | Czech Republic | n = 300, men and women, mean age: 40.8 years. Serum | NR | NR | NR | < LOQ (0.013) | 0.094 | Sochorová et al. (2017) |
| PFTeDA | ||||||||
| 2012 | Sweden | n = 3 pools of serum primiparous women living in Uppsala County, donated serum samples within the fourth week after delivery. Serum | NR | NR | NR | < LOQ (0.002)a | < LOQ (0.002)a | Gebbink et al. (2015) |
| 2007–2009 | Germany | n = 30, students, male and female, age: 20–29 years from Münster. Plasma | NR | NR | NR | < LOQ (0.004) | 0.0169 | Yeung et al. (2013a) |
| 2007–2009 | Germany | n = 30, students, male and female, age: 20–29 years from Halle. Serum | NR | NR | NR | < LOQ (0.0103‐0.0304) | 0.0077 | Yeung et al. (2013a) |
| 2015 | Czech Republic | n = 300, men and women, mean age: 40.8 years. Serum | NR | NR | NR | < LOQ (0.013) | 0.029 | Sochorová et al. (2017) |
| PFBS (total) | ||||||||
| 2008–2011 | Sweden | n = 150, primiparous women, within the third week after delivery. The County has a known contamination of the drinking water with PFAS. Mean (range) age: 30.2 (21–40 years). Serum | 0.027a | NR | 0.055a | < LOQ (0.01)a | 0.80a | Gyllenhammar et al. (2015) |
| 2010 | Sweden | n = 36 pools of serum. Primiparous women living in Uppsala county, donated serum samples within the third week after delivery. Age: 19–41 years | NR | NR | NR | 0.070 | 0.10 | Glynn et al. (2012) |
| 2012 | Sweden | n = 3 pools primiparous women living in Uppsala County, donated serum samples within the fourth week after delivery. Serum | NR | NR | NR | < LOQ (< 0.009) a | 0.02a | Gebbink et al. (2015) |
| 2013–2014 | Norway | n = 61, men and women, median (range) age: 41 (20–66 years). Serum | 0.04 | NR | 0.05 | < LOQ (0.009) | 0.20 | Poothong et al. (2017) |
| 2009–2010 | Spain | n = 46, males and females, age: 19–53 years. Whole blood | NR | NR | 0.20 | < LOQ (0.04) | 0.43 | Gómez‐Canela et al. (2015) |
| 2009 | Germany | n = 60, men and women, age: 18–67 years. Plasma (rural town, near fluoropolymer production plant) | NR | NR | NR | NR | < LOQ (0.4) | Fromme et al. (2017) |
| 2015 | Germany | n = 26, men and women, age: 18–67 years. Plasma (rural town, near fluoropolymer production plant) | NR | NR | NR | NR | < LOQ (0.4) | Fromme et al. (2017) |
| 2009 | Germany | n = 60, men and women, age: 18–67 years. Plasma (potential contamination of drinking water) | NR | NR | NR | NR | < LOQ (0.4) | Fromme et al. (2017) |
| 2015 | Germany | n = 50, men and women, age: 18–67 years. Plasma (potential contamination of drinking water) | NR | NR | NR | NR | < LOQ (0.4) | Fromme et al. (2017) |
| 2014 | Germany | n = 42, men and women, age: 18–67 years. Plasma | NR | NR | NR | NR | < LOQ (0.4) | Fromme et al. (2017) |
| 2016 | Germany | n = 158, men and women, age: 18–67 years. Plasma | NR | NR | NR | NR | < LOQ (0.4) | Fromme et al. (2017) |
| 2015 | Czech Republic | n = 300, men and women, mean age: 40.8 years. Serum | NR | NR | NR | < LOQ (0.006) | 0.057 | Sochorová et al. (2017) |
| 2015–2016 | Italy | n = 250, men and women, age: 20–51 years. Background exposed population. Serum | < LOQ (NR) | NR | NR | < LOQ (NR) | 0.36 | Ingelido et al. (2018) |
| 2015–2016 | Italy | n = 257, men and women, age: 20–51 years. Exposed to elevated PFAS concentrations through drinking water. Serum | < LOQ (NR) | NR | NR | < LOQ (NR) | 4.26 | Ingelido et al. (2018) |
| 2015 | UK | n = 29, women, mean age 32.9 years, Controls. Serum. n = 30, women, mean age 32.9 years, Cases with polycystic ovary syndrome. Serum | NR | < LOQ (0.2) | NR | < LOQ (0.2) | 0.46 | Heffernan et al. (2018) |
| PFHxS (total) | ||||||||
| 2007 | Sweden | n = 10, women, mean (range) age: 48 (36–56) years. Serum | 0.93 | NR | NR | NR | NR | Axmon et al. (2014) |
| 2010–2011 | Sweden | n = 270, women, median (range) age: 50 (22–75) years, serum | 1.95 | NR | NR | 0.73 (P5) | 10.29 P95) | Bjermo et al. (2013) |
| 2008–2010 | Sweden | n = 153, males, mean (range) age: 67 (53–79) years. Whole blood | 0.88 | 0.86 | 0.96 | 0.19 | 2.8 | Bao et al. (2014) |
| 2007–2011 | Sweden | n = 201, men with prostate cancer. Median (range) age: 67 (49–79) years. Serum | 0.909 | NR | 1.1 | 0.0876 | 16 | Hardell et al. (2014) |
| 2007–2011 | Sweden | n = 186, men without prostate cancer. Median (range) age: 67 (50–79) years. Serum | 0.865 | NR | 0.940 | 0.154 | 3.0 | Hardell et al. (2014) |
| 2008‐2011 | Sweden | n = 150, primiparous women, within the third week after delivery. The County has a known contamination of the drinking water with PFAS. Mean (range) age: 30.2 (21–40 years). Serum | 3.7a | NR | 5.4a | 0.32a | 34a | Gyllenhammar et al. (2015) |
| 2010 | Sweden | n = 36 pools of serum. Primiparous women living in Uppsala county, donated serum samples within the third week after delivery. Age 19–41 years | NR | NR | NR | 5.6 | 8.0 | Glynn et al. (2012) |
| 2012 | Sweden | n = 3 pools primiparous women living in Uppsala County, donated serum samples within the fourth week after delivery. Serum | NR | NR | NR | 2.0a | 5.0a | Gebbink et al. (2015) |
| 2014–2016 | Sweden | n = 3,418, men and women from Ronneby municipality where drinking water was highly contaminated for many years, wide age range from children to elderly, plasma | 152 | NR | 228 | < LOQ (0.5) | 1790 | Li et al. (2018a) |
| 2014–2016 | Sweden | n = 242, men and women in a wide age range from children to elderly, plasma | 0.84 | NR | 1.91 | < LOQ (0.5) | 60.1 | Li et al. (2018a) |
| 2011–2014 | Sweden | n = 579, men and women, age: 80 years | 2.9 | 3.9 | 7.5 | 0.14 | 77 | Stubleski et al. (2016) |
| 2007–2008 | Norway | n = 41, women, mean (range) age: 36.7 (25–45 years). Serum | 0.39 | NR | 0.57 | 0.16 | 4.1 | Haug et al. (2011b) |
| 2007–2008 | Norway | n = 123, pregnant women. Plasma | 0.28 | NR | 0.34 | 0.04 | 1.64 | Gützkow et al. (2012) |
| 2007–2008 | Norway | n = 99, pregnant women. Plasma | 0.3 | NR | 0.3 | < LOQ (0.05) | 2.8 | Granum et al. (2013) |
| 2007–2009 | Norway | n = 391 pregnant women, mean (range) age: 31 (18–43) years. Serum | 0.44 | NR | 0.61 | < LOQ (0.06) | 14.8 | Berg et al. (2014) |
| 2012‐2014 | Norway | n = 74, 59 consumers (HC) and 15 non‐consumers (NC) of fish from AFFF‐affected waters. Median age 58.5 years (range 32–79 years). Serum | 2.15 (HC) | 2.66 (HC) | 4.07 (HC) | 0.86 (HC, P10) | 14.0 (HC, P90) | Hansen et al. 2016 |
| 2012–2014 | Norway | n = 74, 59 consumers (HC) and 15 non‐consumers (NC) of fish from AFFF‐affected waters. Median age 58.5 years (range 32–79 years). Serum | 1.06 (NC) | 0.89 (NC) | 1.29 (NC) | 0.15 (NC, P10) | 3.03 (NC, P90) | Hansen et al. (2016) |
| 2013–2014 | Norway | n = 61, men and women, median (range) age: 41 (20–66 years). Serum | 0.78 | NR | 0.95 | 0.23 | 2.34 | Poothong et al. (2017) |
| 2008–2009 | Denmark | n = 247, adult men. Mean age: 19.6 years. Serum | 0.67 | NR | 0.81 | 0.37 (P5) | 1.58 (P95) | Joensen et al. (2013) |
| 2011 | Denmark | n = 200 samples from pregnant women. Serum | 0.22 | NR | 0.25 | < LOQ (0.03) | 0.75 | Vorkamp et al. (2014) |
| 2010–2012 | Denmark | n = 392, newly pregnant women. Serum | 0.29 | NR | NR | 0.02 (P5) | 7.28 (P95) | Jensen et al. (2015) |
| 2011 | Denmark | n = 145, women, mean (range) age: 41 (31–52 years). Plasma | 0.32 | NR | 0.39 | 0.08 | 1.74 | Mørck et al. (2015) |
| 2008–2013 | Denmark | n = 1,507, pregnant women, primiparous, median age between 29 and 31 for the four quartiles of participants. Serum | 0.5 | NR | NR | 0.4 (IQR) | 0.6 (IQR) | Bach et al. (2016) |
| 2010–2012 | Denmark | n = 649, pregnant women, mean age 30.7 years. Serum | NR | NR | 0.3 | 0.2 (IQR) | 0.4 (IQR) | Lind et al. (2017a) |
| 2007–2009 | Faroe Islands | n = 487, pregnant women, mean age: 30.6 years. Serum | 0.20 | NR | NR | 0.13 (IQR) | 0.31 (IQR) | Timmermann et al. (2017a) |
| 2010–2013 | Greenland | n = 207, pregnant women age > 18 years, Inuits. Serum | 0.70 | 0.69 | 0.81 | 0.13 | 4.48 | Long et al. (2015) |
| 2011–2014 | Greenland | n = 128, women, median age around 50 years, breast cancer cases and controls. Serum | 1.11 | NR | NR | NR | NR | Wielsøe et al. (2017) |
| 2008 | France | n = 478, age: 18 and 75 years, males and females, had current residence in targeted areas and had a fishing license. Serum | 2.3 | 2.3 | 2.8 | 0.1 | 14.5 | Denys et al. (2014) |
| 2010–2013 | France | n = 100, pregnant women, median (range) age: 32 (20–46) years. Serum | 0.619 | NR | 2.28 | < LOQ (0.3) | 31 | Cariou et al. (2015) |
| 2007–2009 | Germany | n = 44 pregnant women, mean (range) age: 33 (21–43) years, samples collected during pregnancy. Plasma | 0.5 | NR | 0.6 | NR | NR | Fromme et al. (2010) |
| 2010 | Germany | n = 18, male and female, age: 20–29 years. Plasma | 0.86 | 0.82 | NR | 0.25 | 1.39 | Schrôter‐Kermani et al. (2013) |
| 2009 | Germany | n = 60, men and women, age: 18–67 years. Plasma (rural town, near fluoropolymer production plant) | 0.4 | NR | 0.5 | NR | 2.3 | Fromme et al. (2017) |
| 2015 | Germany | n = 26, men and women, age: 18–67 years. Plasma (rural town, near fluoropolymer production plant) | 0.4 | NR | 0.4 | NR | 1.1 | Fromme et al. (2017) |
| 2009 | Germany | n = 60, men and women, age: 18–67 years. Plasma (potential contamination of drinking water) | 0.7 | NR | 0.8 | NR | 5.7 | Fromme et al. (2017) |
| 2015 | Germany | n = 50, men and women, age: 18–67 years. Plasma (potential contamination of drinking water) | 0.3 | NR | 0.4 | NR | 1.0 | Fromme et al. (2017) |
| 2014 | Germany | n = 42, men and women, age: 18–67 years. Plasma | 0.2 | NR | 0.2 | NR | 0.6 | Fromme et al. (2017) |
| 2016 | Germany | n = 158, men and women, age: 18–67 years. Plasma | 0.5 | NR | 0.7 | NR | 11.6 | Fromme et al. (2017) |
| 2009–2010 | Spain | n = 46, males and females, age: 19–53 years. Whole blood | NR | NR | 0.26 | < LOQ (0.07) | 0.87 | Gómez‐Canela et al. (2015) |
| 2009–2010 | Spain | n = 755, men and women, age: 18–65 years. Serum | 0.82 | 0.91 | 1.18 | < LOQ (0.34. P10) | 2.85 (P95) | Bartolome et al. (2017) |
| 2010–2012 | Slovakia | n = 120, pregnant women. Age range: 18–45 years. Plasma | 0.35 | 0.37 | 0.38 | < LOQ (NR) | 0.7 | Uhl (2016) |
| 2010–2012 | Austria | n = 114, pregnant women. Age range: 18–45 years. Plasma | 0.36 | 0.38 | 0.43 | < LOQ (NR) | 1.3 | Uhl (2016) |
| 2015 | Czech Republic | n = 300, men and women, mean age: 40.8 years. Serum | 0.184 | 0.171 | NR | 0.008 | 1.39 | Sochorová et al. (2017) |
| 2015–2016 | Italy | n = 250, men and women, age: 20–51 years. Background exposed population. Serum | 2.49 | NR | NR | < LOQ (NR) | 9.14 | Ingelido et al. (2018) |
| 2015–2016 | Italy | n = 257, men and women, age: 20–51 years. Exposed to elevated PFAS concentrations through drinking water. Serum | 2.98 | NR | NR | < LOQ (NR) | 43.43 | Ingelido et al. (2018) |
| 2015 | UK | n = 29, women, mean age 32.9 years, Controls. Serum. n = 30, women, mean age 32.9 years, Cases with polycystic ovary syndrome. Serum | NR | 1.04 | NR | 0.2 | 10.2 | Heffernan et al. (2018) |
| PFHpS (total) | ||||||||
| 2007–2008 | Norway | n = 41, women, mean age: 36.7 years (range 25–45 years). Serum | 0.079 | NR | 0.083 | < LOQ (0.05) | 0.19 | Haug et al. (2011b) |
| 2007–2009 | Norway | n = 391, pregnant women, age: mean 31 (18–43 years). Serum | 0.10 | NR | 0.12 | < LOQ (0.06) | 1.10 | Berg et al. (2014) |
| 2013–2014 | Norway | n = 61, men and women, median (range) age: 41 (20–66 years). Serum | 0.20 | NR | 0.25 | 0.06 | 0.63 | Poothong et al (2017) |
| 2008‐2009 | Denmark | n = 247, adult men. Mean age: 19.6 years. Serum | 0.26 | NR | 0.29 | 0.15 (P5) | 0.52 (P95) | Joensen et al. (2013) |
| 2008–2013 | Denmark | n = 1,507, pregnant women, primiparous, median age between 29 and 31 for the four quartiles of participants. Serum | 0.2 | NR | NR | 0.1 (IQR) | 0.2 (IQR) | Bach et al. (2016) |
| 2010–2013 | Greenland | n = 207, pregnant women age: > 18 years, Inuits. Serum | 0.19 | 0.19 | 0.23 | 0.06 | 1.44 | Long et al. (2015) |
| 2008 | France | n = 478, age 18 and 75 years, males and females, had current residence in targeted areas and had a fishing license. Serum | 0.6 | 0.6 | 0.8 | < LOQ (NR) | 13.3 | Denys et al. (2014) |
| 2010–2013 | France | n = 100, pregnant women, median (range) age: 32 (20–46) years. Serum | < LOQ (0.4) | NR | 0.182 | < LOQ (0.4) | 0.808 | Cariou et al. (2015) |
| PFDS (total) | ||||||||
| 2008–2011 | Sweden | n = 150, primiparous women, within the third week after delivery. The County has a known contamination of the drinking water with PFAS. Mean (range) age: 30.2 (21–40 years). Serum | < LOQ (0.005)a | NR | < LOQ (0.005) a | 0.13(a) , a | Gyllenhammar et al. (2015) | |
| 2010 | Sweden | n = 36 pools of serum. Primiparous women living in Uppsala county, donated serum samples within the third week after delivery. Age: 19–41 years | NR | NR | NR | 0.011 | 0.025 | Glynn et al. (2012) |
| 2012 | Sweden | n = 3 pools primiparous women living in Uppsala County, donated serum samples within the fourth week after delivery. Serum | NR | NR | NR | < LOQ (0.005)a | < LOQ (0.005)a | Gebbink et al. (2015) |
| 2013–2014 | Norway | n = 61, men and women, median (range) age: 41 (20–66 years). Serum | 0.06 | NR | 0.06 | < LOQ (0.0018) | 0.17 | Poothong et al. (2017) |
| 2015 | Czech Republic | n = 300, men and women, mean age: 40.8 years. Serum | NR | NR | NR | < LOQ (0.006) | 0.245 | Sochorová et al. (2017) |
| 2015 | UK | n = 29, women, mean age: 32.9 years, Controls. Serum. n = 30, women, mean age: 32.9 years, Cases with polycystic ovary syndrome. Serum | NR | < LOQ (0.5) | NR | < LOQ (0.5) | < LOQ (0.5) | Heffernan et al. (2018) |
| PFOSI | ||||||||
| 2008–2009 | Denmark | n = 247, adult men. Mean age: 19.6 years. Serum | NR | NR | NR | < LOQ (0.15) | NR | Joensen et al. (2013) |
| FOSA | ||||||||
| 2008–2010 | Sweden |
n = 9 pools primiparous women living in Uppsala County, donated serum samples within the third week after delivery Age: 19–41 years. Serum |
NR | NR | NR | < LOQ (0.040)a | 0.049a | Glynn et al. (2012) |
| 2008–2012 | Sweden | n = 3 pools primiparous women living in Uppsala County, donated serum samples within the fourth week after delivery. Serum | NR | NR | NR | 0.002a | 0.004a | Gebbink et al. (2015) |
| 2010 | Sweden | n = 36 pools of serum. Primiparous women living in Uppsala county, donated serum samples within the third week after delivery. Age: 19–41 years | NR | NR | NR | < LOQ (0.040) | < LOQ (0.040) | Glynn et al. (2012) |
| 2011–2014 | Sweden | n = 579, men and women, age: 80 years | 0.02 | 0.03 | 0.04 | 0.01 | 0.52 | Stubleski et al. (2016) |
| 2007–2008 | Norway | n = 41, women, mean (range) age: 36.7 (25–45) years. Serum | < LOQ (0.035) | NR | < LOQ (0.035) | < LOQ (0.035) | 0.10 | Haug et al. (2011b) |
| 2007–2009 | Norway | n = 391 pregnant women, mean (range) age: 31 (18–43) years. Serum | NR | NR | NR | < LOQ (0.01) | 0.38 | Berg et al. (2014) |
| 2013–2014 | Norway | n = 61, men and women, median (range) age: 41 (20–66) years. Serum | 0.03 | NR | 0.03 | < LOQ (0.0018) | 0.05 | Poothong et al. (2017) |
| 2008–2013 | Denmark |
n = 1,533 serum samples pregnant nulliparous women from the Aarhus Birth Cohort Biobank Most participants gave a blood sample between 11 weeks and 14 weeks of gestation. Median age: 29 years |
NR | NR | NR | < LOQ (1.19) | < LOQ (1.19) | Bjerregaard‐Olesen et al. (2016) |
| 2015 | Czech Republic | n = 300, men and women, mean age: 40.8 years. Serum | < LOQ (0.007) | < LOQ (0.007) | NR | < LOQ (0.007) | < LOQ (0.007) | Sochorová et al. (2017) |
| 8:2 monoPAP | ||||||||
| 2013–2014 | Norway | n = 61, men and women, median (range) age: 41 (20–66 years). Serum | < LOQ (0.045) | NR | < LOQ (0.045) | < LOQ (0.045) | < LOQ (0.045) | Poothong et al. (2017) |
| 8:2 diPAP | ||||||||
| 2008–2012 | Sweden | n = 9 pools (three pools at each time point) primiparous women living in Uppsala County, donated serum samples within the fourth week after delivery. Serum | NR | NR | NR | < LOQ (0.0005)a | 0.0183a | Gebbink et al. (2015) |
| 2013–2014 | Norway | n = 61, men and women, median (range) age: 41 (20–66) years. Serum | < LOQ (0.009) | NR | 0.03 | < LOQ (0.009) | 0.11 | Poothong et al. (2017) |
| 2007–2009 | Germany | n = 30, students, male and female, age: 20–29 years from Münster. Serum | NR | NR | NR | < LOQ (0.001) | 0.0721 | Yeung et al. (2013a) |
| 2007–2009 | Germany | n = 30, students, male and female, age: 20–29 years from Halle. Serum | NR | NR | NR | < LOQ (0.0008‐0.002) | 0.0131 | Yeung et al. (2013a) |
| EtFOSA | ||||||||
| 2007–2008 | Norway | n = 41, women, mean (range) age: 36.7 (25–45) years. Serum | NR | NR | NR | < LOQ (0.05) | < LOQ (0.05) | Haug et al. (2011b) |
| 2013–2014 | Norway | n = 61, men and women, median (range) age: 41 (20–66) years. Serum | < LOQ (0.045) | NR | < LOQ (0.045) | < LOQ (0.045) | < LOQ (0.045) | Poothong et al. (2017) |
| 2015 | Czech Republic | n = 300, men and women, mean age: 40.8 years old. Serum | < LOQ (0.004) | < LOQ (0.004) | NR | < LOQ (0.004) | < LOQ (0.004) | Sochorová et al. (2017) |
| EtFOSAA | ||||||||
| 2009–2010 | Spain | n = 755, men and women, age: 18–65 years old. Serum | NR | NR | NR | < LOQ (0.27, P10) | < LOQ (0.27, P95) | Bartolome et al. (2017) |
| FC‐807 | ||||||||
| 2007–2009 | Germany | n = 30, students, male and female, age: 20–29 years from Münster. Serum | NR | NR | NR | < LOQ (0.0004–0.0009) | < LOQ (0.0004–0.0009) | Yeung et al. (2013b) |
| 2007–2009 | Germany | n = 30, students, male and female, age: 20–29 years from Halle. Serum | NR | NR | NR | < LOQ (0.0004–0.0007) | < LOQ (0.0004–0.0007) | Yeung et al. (2013b) |
The term LOQ has been used for both limit of quantification, limit of detection and method detection limit.
P5: 5th percentile; P10: 10th percentile; P90: 90th percentile; P95: 95th percentile; HC: high consumers; NC: non‐consumers; IQR: Interquartile range; NR: not reported; LOQ: limit of quantification.
Linear PFDS.
Reported in ng/g.
Table B.4.
Concentrations of PFASs in general European children populations with serum or plasma samples collected from 2007 to 2008 and onwards
| Year | Country | Study population | Median (ng/mL) | Geometric mean (ng/mL) | Arithmetric mean (ng/mL) | Min (ng/mL) | Max (ng/mL) | Reference |
|---|---|---|---|---|---|---|---|---|
| PFBA | ||||||||
| 2011 | Faroe Islands | n = 51, boys and girls, age: 13 years. Serum | < LOQ (0.1) | NR | NR | < LOQ (0.1) (IQR) | < LOQ (0.1) (IQR) | Dassuncao et al. (2018) |
| 2012 | Faroe Islands | n = 51, boys and girls, age: 5 years. Serum | < LOQ (0.1) | NR | NR | < LOQ (0.1) (IQR) | < LOQ (0.1) (IQR) | Dassuncao et al. (2018) |
| PFPeA | ||||||||
| 2011 | Faroe Islands | n = 51, boys and girls, age: 13 years. Serum | < LOQ (0.05) | NR | NR | < LOQ (0.05) (IQR) | < LOQ (0.05) (IQR) | Dassuncao et al. (2018) |
| 2012 | Faroe Islands | n = 51, boys and girls, age: 5 years. Serum | < LOQ (0.05) | NR | NR | < LOQ (0.05) (IQR) | < LOQ (0.05) (IQR) | Dassuncao et al. (2018) |
| PFHxA | ||||||||
| 2010–2011 | Norway | n = 940, boys and girls, age: 15–19 years. Serum | 0.08 | NR | 0.11 | 0.03 | 1.34 | Averina et al. (2018) |
| 2011 | Faroe Islands | n = 51, boys and girls, age: 13 years. Serum | < LOQ (0.05) | NR | NR | < LOQ (0.05) (IQR) | < LOQ (0.05) (IQR) | Dassuncao et al. (2018) |
| 2012 | Faroe Islands | n = 51, boys and girls, age: 5 years. Serum | < LOQ (0.05) | NR | NR | < LOQ (0.05) (IQR) | < LOQ (0.05) (IQR) | Dassuncao et al. (2018) |
| PFHpA | ||||||||
| 2010–2011 | Norway | n = 112, toddlers, age: 3 years. Serum | 0.15 | NR | NR | 0.05 | 0.82 | Papadopoulou et al. (2016) |
| 2010–2011 | Norway | n = 940, boys and girls, age: 15–19 years. Serum | 0.11 | NR | 0.14 | 0.07 | 1.47 | Averina et al. (2018) |
| 2011 | Faroe Islands | n = 51, boys and girls, age 13 years. Serum | 0.06 | NR | NR | 0.05 (IQR) | 0.08 (IQR) | Dassuncao et al. (2018) |
| 2012 | Faroe Islands | n = 51, boys and girls, age 5 years. Serum | < LOQ (0.03) | NR | NR | < LOQ (0.03) (IQR) | < LOQ (0.03) (IQR) | Dassuncao et al. (2018) |
| PFNA | ||||||||
| 2011 | Denmark | n = 145, children, mean (range) age: 8.7 (6–11) years. Plasma | 0.82 | NR | 0.88 | 0.28 | 2.16 | Mørck et al. (2015) |
| 2007–2009 | Germany | n = 44, infants, age: 6 months. Plasma | 1.0 | NR | 1.1 | NR | 2.3 (P95) | Fromme et al. (2010) |
| 2007–2009 | Germany | n = 24, infants, age: 19 months. Plasma | 0.6 | NR | 0.7 | NR | 1.4 (P95) | Fromme et al. (2010) |
| 2007–2008 | Germany | n = 112, children, mean age: 6.6 years. Plasma | 0.79 | NR | 0.84 | 0.42 | 2.38 | Wilhelm et al. (2015) |
| 2009–2010 | Germany | n = 101, children, mean age: 8.5 years. Plasma. | 0.68 | NR | 0.70 | < LOQ (0.4) | 1.59 | Wilhelm et al. (2015) |
| 2010–2011 | Norway | n = 112, toddlers, age: 3 years. Serum | 2.13 | 1.31 | NR | 0.17 | 23.96 | Papadopoulou et al. (2016) |
| 2010–2011 | Norway | n = 940, boys and girls, age: 15–19 years. Serum | 0.50 | NR | 0.60 | 0.12 | 5.35 | Averina et al. (2018) |
| 2012–2014 | Faroe Island | n = 349, children, age: 5 years. Serum | 1.1 | NR | NR | 0.8 (IQR) | 1.6 (IQR) | Grandjean et al. (2017a) |
| 2010–2013 | Faroe Island | n = 587, children, age: 13 years. Serum | 0.7 | NR | NR | 0.6 (IQR) | 0.9 (IQR) | Grandjean et al. (2017b) |
| PFDA | ||||||||
| 2011 | Denmark | n = 145, children, mean (range) age: 8.7 (6–11) years. Plasma | 0.32 | NR | 0.34 | 0.11 | 0.75 | Mørck et al. (2015) |
| 2007–2009 | Germany | n = 44, infants, age: 6 months. Plasma | < LOQ (0.4) | NR | < LOQ (0.4) | NR | 0.7 (P95) | Fromme et al. (2010) |
| 2007–2009 | Germany | n = 24, infants, age: 19 months. Plasma | < LOQ (0.4) | NR | < LOQ (0.4) | NR | < LOQ (P95) | Fromme et al. (2010) |
| 2010–2011 | Norway | n = 112, toddlers, age: 3 years. Serum | 0.13 | NR | NR | 0.05 | 0.55 | Papadopoulou et al. (2016) |
| 2010–2011 | Norway | n = 940, boys and girls, age: 15–19 years. Serum | 0.21 | NR | 0.26 | 0.05 | 1.89 | Averina et al. (2018) |
| 2012–2014 | Faroe Island | n = 349, children, age: 5 years. Serum | 0.3 | NR | NR | 0.2 (IQR) | 0.5 (IQR) | Grandjean et al. (2017a) |
| 2010–2013 | Faroe Island | n = 587, children, age: 13 years. Serum | 0.3 | NR | NR | 0.2 (IQR) | 0.4 (IQR) | Grandjean et al. (2017b) |
| PFUnDA | ||||||||
| 2010–2011 | Norway | n = 112, toddlers, age: 3 years. Serum | 0.21 | 0.14 | NR | < LOQ (0.05) | 1.08 | Papadopoulou et al. (2016) |
| 2010–2011 | Norway | n = 940, boys and girls, age: 15–19 years. Serum | 0.16 | NR | 0.18 | 0.03 | 0.85 | Averina et al. (2018) |
| 2011 | Faroe Islands | n = 51, boys and girls, age: 13 years. Serum | 0.29 | NR | NR | 0.16 (IQR) | 0.45 (IQR) | Dassuncao et al. (2018) |
| 2012 | Faroe Islands | n = 51, boys and girls, age: 5 years. Serum | 0.15 | NR | NR | 0.1 (IQR) | 0.26 (IQR) | Dassuncao et al. (2018) |
| PFDoDA | ||||||||
| 2010–2011 | Norway | n = 112, toddlers, age: 3 years. Serum | 0.08 | NR | NR | 0.07 | 0.11 | Papadopoulou et al. (2016) |
| 2010–2011 | Norway | n = 940, boys and girls, age: 15–19 years. Serum | 0.04 | NR | 0.06 | 0.01 | 0.24 | Averina et al. (2018) |
| 2011 | Faroe Islands | n = 51, boys and girls, age: 13 years. Serum | < LOQ (0.05) | NR | NR | < LOQ (0.05) (IQR) | < LOQ (0.05) (IQR) | Dassuncao et al. (2018) |
| 2012 | Faroe Islands | n = 51, boys and girls, age: 5 years. Serum | < LOQ (0.05) | NR | NR | < LOQ (0.05) (IQR) | < LOQ (0.05) (IQR) | Dassuncao et al. (2018) |
| PFTrDA | ||||||||
| 2010–2011 | Norway | n = 112, toddlers, age: 3 years. Serum | 0.10 | NR | NR | 0.05 | 0.17 | Papadopoulou et al. 2016) |
| PFBS | ||||||||
| 2011 | Faroe Islands | n = 51, boys and girls, age: 13 years. Serum | < LOQ (0.1) | NR | NR | < LOQ (0.1) (IQR) | < LOQ (0.1) (IQR) | Dassuncao et al. 2018) |
| 2012 | Faroe Islands | n = 51, boys and girls, age: 5 years. Serum | < LOQ (0.1) | NR | NR | < LOQ (0.1) (IQR) | < LOQ (0.1) (IQR) | Dassuncao et al. 2018) |
| PFHxS | ||||||||
| 2011 | Denmark | n = 145, children, mean age: 8.7 years (range 6–11 years). Plasma | 0.34 | NR | 0.44 | < LOQ (0.03) | 3.68 | Mørck et al. (2015) |
| 2007–2009 | Germany | n = 44, infants, age: 6 months. Plasma | 0.6 | NR | 0.7 | NR | 1.6 (P95) | Fromme et al. (2010) |
| 2007–2009 | Germany | n = 24, infants, age: 19 months. Plasma | 0.6 | NR | 0.7 | NR | 1.2 (P95) | Fromme et al. (2010) |
| 2007–2008 | Germany | n = 112, children, mean age: 6.6 years. Plasma | 0.81 | NR | 0.91 | 0.27 | 4.73 | Wilhelm et al. (2015) |
| 2009–2010 | Germany | n = 101, children, mean age: 8.5 years. Plasma | 0.75 | NR | 0.83 | 0.32 | 2.92 | Wilhelm et al. (2015) |
| 2010–2011 | Norway | n = 112, toddlers, age: 3 years. Serum | 0.55 | 0.60 | NR | 0.16 | 6.73 | Papadopoulou et al. (2016) |
| 2010–2011 | Norway | n = 940, boys and girls, age: 15–19 years. Serum | 0.71 | NR | 1.53 | 0.18 | 84.7 | Averina et al. (2018) |
| 2012–2014 | Faroe Island | n = 349, children, age: 5 years. Serum | 0.3 | NR | NR | 0.2 (IQR) | 0.4 (IQR) | Grandjean et al. (2017a) |
| 2010–2013 | Faroe Island | n = 587, children, age: 13 years. Serum | 0.4 | NR | NR | 0.3 (IQR) | 0.5 (IQR) | Grandjean et al. (2017b) |
| PFHpS | ||||||||
| 2010–2011 | Norway | n = 112, toddlers, age: 3 years. Serum | 0.12 | 0.11 | NR | < LOQ (0.05) | 0.48 | Papadopoulou et al. (2016) |
| 2010–2011 | Norway | n = 940, boys and girls, age: 15–19 years. Serum | 0.15 | NR | 0.17 | 0.03 | 7.62 | Averina et al. (2018) |
| 2011 | Faroe Islands | n = 51, boys and girls, age: 13 years. Serum | 0.19 | NR | NR | 0.14 (IQR) | 0.24 (IQR) | Dassuncao et al. (2018) |
| 2012 | Faroe Islands | n = 51, boys and girls, age: 5 years. Serum | 0.07 | NR | NR | 0.04 (IQR) | 0.11 (IQR) | Dassuncao et al. (2018) |
| PFDS | ||||||||
| 2011 | Faroe Islands | n = 51, boys and girls, age: 13 years. Serum | < LOQ (0.03) | NR | NR | < LOQ (0.03) (IQR) | < LOQ (0.03) (IQR) | Dassuncao et al. (2018) |
| 2012 | Faroe Islands | n = 51, boys and girls, age: 5 years. Serum | < LOQ (0.03) | NR | NR | < LOQ (0.03) (IQR) | 0.04 (IQR) | Dassuncao et al. (2018) |
| FOSA | ||||||||
| 2010–2011 | Norway | n = 112, toddlers, age: 3 years. Serum | 0.11 | NR | NR | < LOQ (0.05) | 0.16 | Papadopoulou et al. 2016 |
| 2011 | Faroe Islands | n = 51, boys and girls, age: 13 years. Serum | < LOQ (0.03) | NR | NR | < LOQ (0.03) (IQR) | 0.05 (IQR) | Dassuncao et al. (2018) |
| 2012 | Faroe Islands | n = 51, boys and girls, age: 5 years. Serum | < LOQ (0.03) | NR | NR | < LOQ (0.03) (IQR) | < LOQ (0.03) (IQR) | Dassuncao et al. (2018) |
| ETFOSAA | ||||||||
| 2011 | Faroe Islands | n = 51, boys and girls, age: 13 years. Serum | < LOQ (0.03) | NR | NR | < LOQ (0.03) (IQR) | 0.03 (IQR) | Dassuncao et al. (2018) |
| 2012 | Faroe Islands | n = 51, boys and girls, age: 5 years. Serum | < LOQ (0.03) | NR | NR | < LOQ (0.03) (IQR) | 0.02 (IQR) | Dassuncao et al. (2018) |
| MeFOSAA | ||||||||
| 2011 | Faroe Islands | n = 51, boys and girls, age: 13 years. Serum | 0.05 | NR | NR | 0.03 (IQR) | 0.08 (IQR) | Dassuncao et al. (2018) |
| 2012 | Faroe Islands | n = 51, boys and girls, age: 5 years. Serum | < LOQ (0.03) | NR | NR | < LOQ (0.03) (IQR) | 0.02 (IQR) | Dassuncao et al. (2018) |
The term LOQ was used for both limit of quantification, limit of detection and method detection limit.
P5: 5th percentile. P95: 95th percentile. NR: not reported. LOQ: limit of quantification. IQR: interquartile range.
Table B.3.
Concentrations of PFHxA and FOSA in general European adult populations with whole blood samples collected from 2007 to 2008 and onwards
| Year | Country | Study population | Median, ng/mL | Geometric mean (ng/mL) | Arithmetic mean (ng/mL) | Min (ng/mL) | Max (ng/mL) | Reference |
|---|---|---|---|---|---|---|---|---|
| PFHxA | ||||||||
| 2013–2014 | Norway | n = 61, men and women, median (range) age: 41 (20–66) years. Whole blood | 0.62 | NR | 0.68 | 0.14 | 1.65 | Poothong et al. (2017) |
| FOSA | ||||||||
| 2013–2014 | Norway | N = 61, men and women, median (range) age: 41 (20–66) years. Whole blood | 0.14 | NR | 0.22 | 0.05 | 2.35 | Poothong et al. (2017) |
NR: not reported.
Table 16.
Summarising statistics describing serum concentrations of perfluoroalkyl carboxylic acids (PFCAs) in the European adult population based on studies included in Tables B.2 and B.3 in Appendix B (from 2007–2008 and onwards)
| Concentration (ng/mL) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PFBA | PFPeA | PFHxA | PFHxA whole blood | PFHpA | PFOAa | PFNA | PFDA | PFUnDA | PFDoDA | PFTrDA | PFTeDA | ||
| Statistics based on medians reported in studies included in Tables B.2 and B.3 in Appendix B, b | Median | 0.01 | 0.05 | 0.03 | 0.620 | 0.05 | 1.9 | 0.61 | 0.30 | 0.28 | 0.05 | 0.05 | – |
| Mean | 0.01 | 0.05 | 0.03 | 0.620 | 0.06 | 2.1 | 0.74 | 0.35 | 0.46 | 0.14 | 0.08 | – | |
| Minimum | 0.01 | 0.01 | 0.01 | 0.620 | 0.03 | 0.76 | 0.30 | 0.07 | 0.06 | 0.03 | 0.01 | – | |
| Maximum | 0.013 | 0.09 | 0.045 | 0.62 | 0.14 | 4.9 | 2.64 | 1.34 | 2.49 | 0.54 | 0.21 | – | |
| Number of studies c | 1 | 2 | 2 | 1 | 6 | 32 | 37 | 33 | 22 | 7 | 4 | 0 | |
| Information based on individual samples reported in studies included in Tables B.2 and B.3 in Appendix B | Minimum individual samples | < LOQ (0.013) | < LOQ (0.013) | < LOQ (0.0003) | 0.14 | < LOQ (0.01) | 0.03 | < LOQ (0.013) | 0.013 | < LOQ (0.013) | < LOQ (0.0036) | < LOQ (0.009) | < LOQ (0.002) |
| Maximum individual samples | 3.59 | 2.02 | 1.58 | 1.65 | 0.70 | 80.8 | 8.6 | 19.2 | 18.2 | 3.9 | 0.9 | 0.029 | |
| Country reporting maximum individual samples | Italy | UK | Germany | Norway | Sweden | France | Germany | Germany | Greenland | Germany | Greenland | Czech Republic | |
| Reference for maximum | Ingelido et al. (2018) | Heffernan et al. (2018) | Yeung et al. (2013a) | Poothong et al. (2017) | Stubleski et al. (2016) | Denys et al. (2014) | Fromme et al. (2017) | Fromme et al. (2017) | Long et al. (2015) | Fromme et al. (2017) | Long et al. (2015) | Sochorová et al. (2017) | |
NR: not reported; LOQ: limit of quantification.
For studies with median concentration lower than LOQ, the LOQ was used for calculations. For studies reporting the concentrations in ng/g it was assumed that 1 g = 1 mL of serum/plasma.
Data from EFSA CONTAM Panel (2018) Opinion.
Based only on studies where median levels were reported.
Only studies where median is > LOQ are included in this number.
Table 17.
Summarising statistics describing serum concentrations of perfluoroalkyl sulfonic acids (PFSAs) and other selected PFASs in the European adult population based on studies included in Table B.2 and B.3 in Appendix B (from 2007–2008 and onwards)
| Concentration (ng/mL) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PFBS | PFHxS | PFHpS | PFOSa | PFDS | FOSA | FOSA whole blood | 8:2 monoPAP | 8:2 diPAP | EtFOSA | EtFOSAA | FC‐807 | PFOSI | ||
| Statistics based on medians reported in studies included in Tables B.2 and B.3 in Appendix B, b | Median | 0.03 | 0.67 | 0.20 | 7.7 | 0.03 | 0.025 | 0.140 | 0.045 | 0.009 | 0.025 | – | – | – |
| Mean | 0.03 | 4.94 | 0.25 | 7.5 | 0.03 | 0.023 | 0.140 | 0.045 | 0.009 | 0.025 | – | – | – | |
| Minimum | 0.03 | 0.20 | 0.08 | 1.7 | 0.01 | 0.007 | 0.140 | 0.045 | 0.009 | 0.004 | – | – | – | |
| Maximum | 0.04 | 152 | 0.6 | 27.4 | 0.06 | 0.035 | 0.14 | 0.045 | 0.009 | 0.045 | – | – | – | |
| Number of studies c | 2 | 37 | 8 | 32 | 2 | 4 | 1 | 1 | 1 | 2 | 0 | 0 | 0 | |
| Information based on individual samples reported in studies included in Tables B.2 and B.3 in Appendix B | Minimum individual samples | 0.006 | 0.008 | 0.05 | 0.06 | 0.005 | 0.0018 | 0.05 | 0.045 | 0.0005 | 0.004 | 0.27 | 0.0004 | 0.15 |
| Maximum individual samples | 4.6 | 1790 | 13.3 | 392.3 | 0.245 | 0.52 | 2.35 | 0.045 | 0.11 | 0.05 | 0.27 | 0.0007 | NR | |
| Country reporting maximum individual samples | Italy | Sweden | France | France | Czech Republic | Sweden | Norway | Norway | Norway | Norway | Spain | Germany | Denmark | |
| Reference for maximum | Ingelido et al. (2018) | Li et al. (2018a) | Denys et al. (2014) | Denys et al. (2014) | Sochorová et al. (2017) | Stubleski et al. (2016) | Poothong et al. (2017) | Poothong et al. (2017) | Poothong et al. (2017) | Haug et al. (2011b) | Bartolome et al. (2017) | Yeung et al. (2013b) | Joensen et al. (2013) | |
NR: not reported; LOQ: limit of quantification.
For studies with median concentration lower than LOQ, the LOQ was used for calculations. For studies reporting the concentrations in ng/g, it was assumed that 1 g = 1 mL of serum/plasma.
Data from EFSA CONTAM Panel (2018) Opinion.
Based only on studies where median levels were reported.
Only studies where median is > LOQ are included in this number.
Table 18.
Summarising statistics describing serum concentrations of perfluoroalkyl carboxylic acids (PFCAs) in the European children population based on studies included in Table B.4 in Appendix B (from 2007 to 2008 and onwards)
| Concentration (ng/mL) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PFBA | PFPeA | PFHxA | PFHpA | PFOAa | PFNA | PFDA | PFUnDA | PFDoDA | PFTrDA | ||
| Statistics based on medians reported in studies included in Table B.4 in Appendix B, b | Median | 0.08 | 0.09 | 3.3 | 0.79 | 0.30 | 0.19 | 0.05 | 0.10 | ||
| Mean | 0.08 | 0.09 | 3.3 | 0.92 | 0.29 | 0.20 | 0.06 | 0.10 | |||
| Minimum | 0.08 | 0.03 | 0.49 | 0.5 | 0.13 | 0.15 | 0.04 | 0.1 | |||
| Maximum | 0.08 | 0.15 | 6.9 | 2.13 | 0.4 | 0.29 | 0.08 | 0.1 | |||
| Number of studies c | 0 | 0 | 1 | 4 | 8 | 9 | 7 | 4 | 4 | 1 | |
| Information based on individual samples reported n studies included in Table B.4 in Appendix B | Minimum individual samples | < 0.1 (IQR) | < 0.05 (IQR) | 0.03 | < 0.03 (IQR) | 0.45 | 0.12 | < 0.05 | 0.03 | 0.01 | 0.05 |
| Maximum individual samples | < 0.1 (IQR) | < 0.05 (IQR) | 1.34 | 1.47 | 19.5 (P95) | 23.96 | 1.89 | 1.08 | 0.24 | 0.17 | |
| Country reporting maximum individual samples | Faroe Islands | Faroe Islands | Norway | Norway | Germany | Norway | Norway | Norway | Norway | Norway | |
| Reference for maximum | Dassuncao et al. (2018) | Dassuncao et al. (2018) | Averina et al. (2018) | Averina et al. (2018) | Fromme et al. (2010) | Papadopoulou et al. (2016) | Averina et al. (2018) | Papadopoulou et al. (2016) | Averina et al. (2018) | Papadopoulou et al. (2016) | |
P95: 95th percentile; IQR: interquartile range; LOQ: limit of quantification.
For studies with median concentration lower than LOQ, the LOQ was used for calculations. For studies reporting the concentrations in ng/g, it was assumed that 1 g = 1 mL of serum/plasma.
Data from EFSA CONTAM Panel (2018) Opinion.
Based only on studies where median levels were reported.
Only studies where median is > LOQ are included in this number.
Table 19.
Summarising statistics describing serum concentrations of perfluoroalkyl sulfonic acids (PFSAs) in the European children population based on studies included in Table B.4 in Appendix B (from 2007–2008 and onwards)
| Concentration (ng/mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| PFBS | PFHxS | PFHpS | PFOSa | PFDS | FOSA | MeFOSAA | EtFOSAA | ||
| Statistics based on medians reported in studies included in Table B.4 in Appendix B, b | Median | 0.10 | 0.60 | 0.14 | 3.2 | 0.03 | 0.03 | 0.04 | 0.03 |
| Mean | 0.10 | 0.56 | 0.13 | 3.3 | 0.03 | 0.06 | 0.04 | 0.03 | |
| Minimum | 0.1 | 0.3 | 0.07 | 0.49 | 0.03 | 0.03 | 0.03 | 0.03 | |
| Maximum | 0.1 | 0.81 | 0.19 | 8.6 | 0.03 | 0.11 | 0.05 | 0.03 | |
| Number of studies c | 2 | 9 | 4 | 8 | 2 | 3 | 2 | 2 | |
| Information based on individual samples reported in studies included in Table B.4 in Appendix B | Minimum individual samples | < 0.1 (IQR) | < 0.03 | 0.03 | 0.47 | < 0.03 (IQR) | < 0.03 | < 0.03 (IQR) | < 0.03 (IQR) |
| Maximum individual samples | < 0.1 (IQR) | 84.7 | 7.62 | 23.0 | 0.04 (IQR) | 0.16 | 0.08 (IQR) | < 0.03 (IQR) | |
| Country reporting maximum individual samples | Faroe Islands | Norway | Norway | Denmark | Faroe Islands | Norway | Faroe Islands | Faroe Islands | |
| Reference for maximum | Dassuncao et al. (2018) | Averina et al. (2018) | Averina et al. (2018) | Mørck et al. (2015) | Dassuncao et al. (2018) | Papadopoulou et al. (2016) | Dassuncao et al. (2018) | Dassuncao et al. (2018) | |
IQR: interquartile range; LOQ: limit of quantification.
For studies with median concentration lower than LOQ, the LOQ was used for calculations. For studies reporting the concentrations in ng/g, it was assumed that 1 g = 1 mL of serum/plasma.
Data from EFSA CONTAM Panel (2018) Opinion.
Based only on studies where median levels were reported.
Only studies where median is > LOQ are included in this number.
Despite variations in design, populations (e.g. sex, age, race), analytical methods and geographic location the median concentrations in the different European studies are similar, with ratios between the highest and lowest median concentration usually being less than 10. The European levels are also comparable to levels observed in general populations world‐wide (Haines and Murray, 2012; Khalil et al., 2016; Wan et al., 2013; Zhang et al., 2014a).
No European studies reporting concentrations of PFHxDA, PFODA and EtFOSE were identified. Two studies from Australia and China have reported that PFHxDA and PFODA were determined, but not observed above LOD (< 0.2 ng/mL) in any of the samples (Eriksson et al., 2017; Gao et al., 2016).
3.3.2.4. Levels in blood from occupationally exposed adults
Fluorochemical production workers have in several studies been reported to have elevated exposure to PFASs. Most frequently, levels of PFOS and PFOA are presented, with mean concentrations typically in the range 500–7,000 ng/mL (Fromme et al., 2009), but also studies on PFHxS have been described with geometric mean concentrations up to 700 ng/mL (Olsen, 2015). In Olsen (2015), levels in fluorochemical production workers have been discussed in further detail.
Elevated concentrations of a range of PFCAs in serum from professional ski waxers have been reported in two Nordic studies (Freberg et al., 2010; Nilsson et al., 2010). As an example, in a Swedish study, the whole blood concentrations of PFNA were in the range 0.8–163 ng/mL, while the range of concentrations for PFDA and PFUnDA were 0.9–24 ng/mL and 0.1–2.8 ng/mL, respectively.
Several studies have found increased concentrations of different PFSAs and PFCAs in blood from firefighters compared to the general population. In a study from Australia, elevated serum levels of PFOS and PFHxS were observed (Rotander et al., 2015). Median concentrations of 66 and 25 ng/mL were reported for PFOS and PFHxS, respectively. The PFOS and PFHxS concentrations were around 6–15 times higher than the corresponding concentrations in the general population. Elevated concentrations of PFOA, PFNA and PFDA compared to the general US population were observed in two studies on Californian firefighters (Dobraca et al., 2015; Shaw et al., 2013). Further, Tao et al. (2008) concluded that New York State employees and National Guard personnel were exposed to PFSAs and PFCAs, especially PFOA, PFNA and PFHxS, through inhalation of dust and smoke released during and after the collapse of the World Trade Center. In a study on Finnish firefighters, PFNA and PFHxS concentrations in serum increased during three consecutive training sessions, although this was not possible to test statistically due to few included individuals (Laitinen et al., 2014).
3.3.2.5. Levels in blood from populations with elevated drinking water exposure
Drinking water contaminated with PFASs has been identified in many countries, and several biomonitoring studies have observed elevated concentrations of PFASs in blood from general populations drinking the contaminated water (ATSDR, 2013; Frisbee et al., 2009; Gyllenhammar et al., 2015; Hölzer et al., 2008; Ingelido et al., 2018; Jakobsson et al., 2014; MDH, 2012). Most of the studies have focused on PFOA and PFOS, but also increased concentrations of other PFASs such as PFHxS and PFNA are described. For example, in a study from Sweden, a maximum concentration of PFHxS of 1,790 ng/mL was reported (Li et al., 2018a). These findings emphasise that, even though most general populations worldwide are similarly exposed to PFSAs and PFCAs, elevated exposure may be experienced by large populations (Post et al., 2012).
3.3.2.6. Factors that may have an impact on the internal dose of PFASs
Exposure to PFASs can occur throughout our entire lives. Trans‐placental transfer has been confirmed for many PFASs (Apelberg et al., 2007a; Fisher et al., 2016; Gützkow et al., 2012; Kim et al., 2011a; Ode et al., 2013; Porpora et al., 2013; Zhang and Qin, 2014; Zhang et al., 2013c), and the exposure continues after birth among others through breastfeeding (Fromme et al., 2010; Haug et al., 2011b; Papadopoulou et al., 2016).
In contrast to some other environmental contaminants, no clear regional differences in PFAS concentrations have been reported for general populations, but there are indications that low income countries may have somewhat lower prevalence of these compounds (Fromme et al., 2009; Kannan et al., 2004).
The body burden of PFASs may be influenced by several biological factors. In general, lower concentrations have been observed in women compared to men (Bjermo et al., 2013; Fromme et al., 2009; Haines and Murray, 2012; Ingelido et al., 2010). Factors that may explain this are differences in exposure (Kato et al., 2015a), physiological differences including urinary elimination (Han et al., 2012), menses (Wong et al., 2014), use of oral contraceptives (Rush et al., 2018), pregnancy and lactation (Brantsæter et al., 2013). The relation between PFAS concentrations and age is more questionable (Fromme et al., 2009), and due to temporal changes in exposure, it is hard to distinguish between the effect of age and the effect of changes in exposure (Nøst et al., 2014). However, due to long elimination half‐lives for several PFASs (see Section 3.3.1), bioaccumulation and thereby increasing concentrations with increasing age, is anticipated.
With respect to ethnicity, lower PFSA and PFCA concentrations have been reported in Mexican Americans compared to non‐hispanic whites (Calafat et al., 2007; Khalil et al., 2016). The effect of body weight/body mass index (BMI) on PFAS concentrations in blood has been evaluated in many studies, and both inverse associations (Eriksen et al., 2011; Kato et al., 2014; Lauritzen et al., 2016), positive associations (Halldorsson et al., 2008) and no associations (Rylander et al., 2009) have been reported. Also, the impact of socio‐economical status on the concentrations of PFASs in blood has been assessed in a range of studies. Increasing PFAS concentrations with increasing income has frequently been reported (Calafat et al., 2007; Nelson et al., 2012; Brantsæter et al., 2013; Kato et al., 2014; Jain, 2014; Sagiv et al., 2015), while the impact of education is not as clear (Apelberg et al., 2007b; Calafat et al., 2007; Halldorsson et al., 2008; Nelson et al., 2012; Brantsæter et al., 2013; Kato et al., 2014; Jain, 2014; Sagiv et al., 2015).
3.3.2.7. Relative contribution and correlations between PFASs
The relative contribution of different PFASs in blood varies between individuals. In Figure 10, the relative contribution based on medians of medians presented in Tables 16 and 17 (adults) and Tables 18 and 19 (children) are shown. The seven most prominent PFASs (PFOS, PFOA, PFHxS, PFNA, PFDA, PFUnDA, PFHpS) contributed with 96.6% and 93.4% of the total for adults and children, respectively. As can be seen from Figure 10, for adults the most prominent PFAS was PFOS (64%) followed by PFOA (16%), PFHxS (5.6%) and PFNA (5.1%). For children, PFOS and PFOA contributed almost the same with 35.0% and 36.6% of the total, followed by PFNA (8.8%) and PFHxS (6.7%). Also in most of the individual studies contributing to calculations of medians of medians, both for adults and children, these four PFASs were predominant (e.g. Fromme et al., 2010; Haug et al., 2011b; Bjermo et al., 2013; Bao et al., 2014; Denys et al., 2014; Mørck et al., 2015; Bach et al., 2016; Hansen et al., 2016; Papadopoulou et al., 2016; Stubleski et al., 2016; Bartolome et al., 2017; Grandjean et al., 2017a; Sochorová et al., 2017; Timmermann et al., 2017a; Averina et al., 2018; Heffernan et al., 2018; Ingelido et al., 2018). As can be noted from Figure 10, the relative contribution of the different PFASs differs between children and adults. Several factors may partly explain this, such as differences in the transplacental transfer of various PFASs, differences in the relative transfer of PFASs during breastfeeding, and differences in the relative contribution of various exposure pathways for children and adults.
Figure 10.
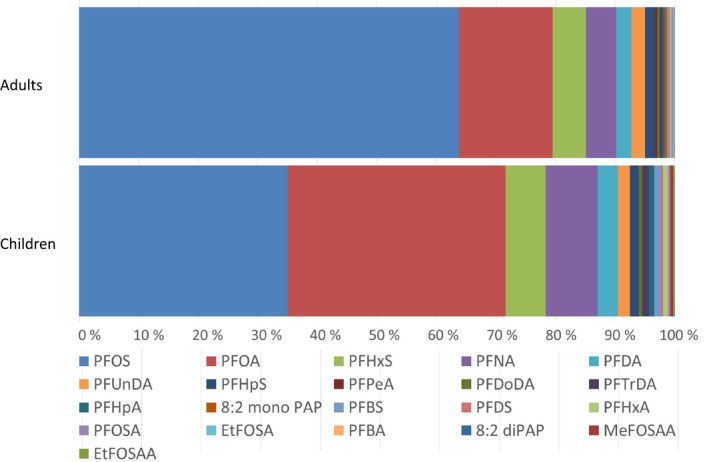
The relative contribution of various perfluoroalkyl substances (PFASs) based on medians of median serum concentrations in biomonitoring studies in Europe for adults presented in Tables 16 and 17 and for children presented in Tables 18 and 19
Pair‐wise correlations between PFAS concentrations have been assessed in several studies. Pair‐wise correlations for the four most prominent PFASs in general European populations (PFOA, PFNA, PFHxS and PFOS) were summarised based on various studies (without known pronounced contamination of drinking water) from Table B.2 in Appendix B (Bao et al., 2014; Cariou et al., 2015; Hansen et al., 2016; Bach et al., 2016; Sochorová et al., 2017; Ingelido et al., 2018 (non‐exposed subjects)). High correlations indicate similar exposure pathways among PFASs, while low correlations indicate distinct exposure pathways for specific compounds. The correlations varied to a large extent for all compounds (PFOS vs. PFOA: 0.40–0.71; PFOS vs. PFHxS: 0.38–0.83; PFOS vs. PFNA: 0.46–0.89; PFOA vs. PFHxS: 0.23–0.87; PFOA vs. PFNA: 0.58–0.82; PFNA vs. PFHxS: non‐significant–0.74). This shows that it is difficult to predict concentrations of one PFAS based on another PFAS.
3.3.2.8. Linear and branched isomers
PFCAs and PFSAs may exist as both linear and branched isomers (see Section 1.3.1). So far most studies have been focussing on isomers of PFOS and PFOA (see Section 1.3.1 of EFSA CONTAM Panel, 2018), but isomer patterns may be of particular interest for exploring sources of exposure also for other PFASs (Martin et al., 2010; Miralles‐Marco and Harrad, 2015).
3.3.2.9. Summary
Taken together, blood serum and plasma have been the most commonly used matrices for biomonitoring of PFASs, even though for some specific compounds, whole blood may be more appropriate. Different time trends have been observed for the various PFASs the last 50 years. Generally, decreasing concentrations have been observed for PFOS, PFOA and in some studies PFHxS after the year 2000, while the concentrations of PFNA, PFDA and PFUnDA have increased in the same time span. In samples collected from general populations in 2007/2008 and onwards, the median of the median concentrations of all PFASs (except for PFOA and PFOS) in all included studies were below 1 ng/mL for both adults and children. However, considerably higher concentrations have been observed for some individuals, and for occupationally exposed adults with for example mean PFHxS concentrations up to 700 ng/mL. Further, higher levels have also been reported following elevated exposure from for instance contaminated drinking water. Age, gender and socio‐economic status may also have an impact on the determined PFAS concentrations.
3.3.3. Toxicity in experimental animals
With a few exceptions, information is missing as to whether or not linear or branched forms of PFASs were applied. The NOEL/LOEL and NOAEL/LOAEL values indicated have been derived by the authors and agreed by the CONTAM Panel, unless otherwise stated.
3.3.3.1. Effects following acute exposure
Considering the limited number of published data on acute exposure effects, studies on oral as well as non‐oral exposure were considered. Reports on effects following acute exposure to PFOS and PFOA, which have been published between 2008 and 2016, are documented in the previous Opinion (EFSA CONTAM Panel, 2018).
For the group of PFCAs, studies on PFHxA and PFDA were identified. The LD50 for PFHxA ranged between 1,750 and 5,000 mg/kg bw in female rats and for PFDA between 120 and 129 mg/kg bw in female mice. In male mice, PFDA lowered major transporters for bile acids into the liver; as a result, 80 mg/kg bw increased serum bile acid concentrations. The same dose elevated the hepatic expression of Mrp3 and Mrp4 interfering with the efflux of bilirubin and bile acids to serum. Hepatocellular injury and inflammation at 50–80 mg of PFDA/kg bw were reported as well.
With regard to other PFASs, EtFOSE did not alter peroxisomal ß‐oxidation or relative liver weights, when applied i.p. to male rats at 100 mg/kg bw. Cynomolgus monkeys, treated with a single dose of 9 mg PFOS/kg bw by gavage, showed no significant alterations. A single gavage of 8:2 FTOH at 500 and 2,000 mg/kg bw exerted no effects in male and female rats. For details, see Appendix D.
3.3.3.2. Effects following repeated exposure
Studies on effects following repeated exposure to PFOS and PFOA, which have been published between 2008 and 2016, are documented in the previous Opinion (EFSA CONTAM Panel, 2018).
3.3.3.2.1. PFCAs
The available repeated dose studies for PFBA, PFHxA, PFHpA, PFNA, PFDA, PFUnDA, PFDoDA, PFTeDA, PFHxDA and PFODA are listed in Appendix E, Table E.1. Reports on effects following repeated exposure to PFOA, which have been published between 2008 and 2016, are documented in the EFSA Opinion of 2018. For PFTrDa, no studies have been identified.
Table E.1.
Repeated dose toxicity studies for PFCAs
| Substance (purity) | Species/Experimental design and doses | Observed effect | Highest dose with no effects (mg/kg bw per day) | Lowest dose with effect (mg/kg bw per day) | Serum/tissue level of compound | Reference | |
|---|---|---|---|---|---|---|---|
| Perfluorobutanoic acid (PFBA) | |||||||
| PFBA (purity not specified) |
SV/129 wt mice (m) No/sex/group: 10 Duration: 28 days Gavage: 0, 35, 175 or 350 mg/kg bw per day |
Incr rel liver weight Incr hepatic replicative DNA synthesis Incr mRNA of Cyp4A10/ACO Hepatocellular necrosis |
35 |
35 35 35 175 |
At 35 mg/kg bw per day Serum: ~ 80 μg/mL Liver: ~ 27 μg/g |
Foreman et al. (2009) | |
| PFBA (ammonium salt, 3M, purity not specified) |
SD rats (m/f) No/sex/group: 10 Duration: 28 days Gavage: 0, 6, 30 or 150 mg/kg bw per day |
Incr abs liver weight (m) Decr serum cholesterol (m) Decr serum total T4 (m) Decr free T4 in serum (m) Incr mRNA of Acox, Cyp4A1 (m) |
6 6 6 |
30 30 6(a) 6(a) 30 |
At 6 mg/kg bw per day in males(f) Serum: 24.7 +/– 17.6 μg/mL Liver: 7.5 +/– 4.5 μg/g At 30 mg/kg bw per day in males(f) Serum: 38.04 +/– 23.2 μg/mL Liver: 17.4 +/– 8.2 μg/g |
Butenhoff et al. (2012) | |
| PFBA (ammonium salt, 3M, purity not specified) |
SD rats (m/f) No/sex/group: 10 Duration: 90 days Gavage: 0, 1.2, 6, or 30 mg/kg bw per day |
Incr abs liver weight (m) Incr mRNA of Cyp4A1 (m) Decr serum total T4 (m) |
6 6 6 |
30 30 30 |
At 6 mg/kg bw per day in males(f) Serum: 13.6 +/– 9.1 μg/mL Liver: 3.1 +/– 2 μg/g At 30 mg/kg bw per day in males(f) Serum: 52.2 +/– 25 μg/mL Liver: 16.1 +/– 9.1 μg/g |
Butenhoff et al. (2012) | |
| Perfluorohexanoic acid (PFHxA) | |||||||
| PFHxA (Sigma‐Aldrich) |
ddY mice (m/f) No/sex/group: 3–5 Duration: 5 days i.p.: 50, 100, 150 mg/kg bw per day |
Incr abs & rel liver weight a | 50 | 100 | Kudo et al. (2006) | ||
| PFHxA (98.5% purity) |
SD rats (m) No/sex/group: 10–15 Duration: 28 days Gavage: 0, 50, 150, 450/300* mg/kg bw per day (*reduced on day 4 from 450 to 300 mg/kg bw per day due to lethality in 5/15 males) |
Lethality, reduced body weight Decr mean corpuscular haemoglobin Incr abs and rel liver weight Decr serum cholesterol |
150 150 50 50 |
450/300 450/300 150 150 |
WIL Research Laboratories (2005) | ||
| PFHxA (98.5% purity) |
SD rats (m, f) no/sex/group: 10 Duration: 90 days Gavage: 0, 10, 50 or 200 mg/kg bw per day |
Incr rel liver weight, (m) Incr rel kidney weight, (m) Decr serum cholesterol, (m) Incr serum ALT and ALP, (m) |
50 10 50 |
200 10 50 200 |
Chengelis et al. (2009) | ||
| PFHxA (sodium salt, 100% purity) |
Crl:CD(SD) rats (m/f) No/sex/group: 10 Duration: 92 days Gavage: 0, 20, 100, or 500 mg/kg bw per day |
Decr body weight (m) Incr perox ß‐oxidation (m/f) Incr rel & abs liver weight (m/f) Incr ALT (m) Incr rel kidney weight (m/f) Incr urine volume (m/f) Degeneration/atrophy in nasal cavity (m,f) |
100 20/100 m/f: 100/100 100 100 20 |
500 100/500 m/f: 500/500 20 500 500 100 |
Loveless et al. (2009) | ||
| PFHxA (sodium salt, 100% purity) |
Crl:CD(SD) rats (m) No/sex/group: 10 Duration: ~ 110 days Gavage: 0, 20, 100 or 500 mg/kg bw per day |
Decr body weight (m, day 105) | 20 | 100 | Loveless et al. (2009) | ||
| PFHxA (> 99% purity) |
SD rats (m, f) No/sex/group: 10 Duration: 28 days Gavage: 0, 62.6, 125, 250, 500 or 1,000 mg/kg per day |
Incr rel liver weight (m) Incr abs liver weight (m) Incr rel+abs liver weight a Incr. acyl‐CoA oxidase activity (m) Decr haematocrit, haemoglobin, erys (m) Decr blood cholesterol (m) Decr T3 and free+total T4 (m) Incr ALT and AST (m+f) and ALP (m) Degeneration and hyperplasia of olfactory epithelium (m+f) |
125 250 250 125 250 125 |
250 500 500 250 62.6 62.6 62.6 500 250 |
Plasma conc. (ng/ml) at 62.6 mg/kg bw per day: 378 ± 178 (m) 129 ± 16 a at 250 mg/kg bw per day: 1,297± 265 (m) Liver conc (ng/g) at 250 mg/kg bw per day 655 ± 148 (m) |
NTP (2019a) | |
| Perfluoroheptanoic acid (PFHpA) | |||||||
| PFHpA (analytical grade) |
Wistar rats (m/f) No/sex/group: 4 Duration: 5 days i.p.: 0–160 mg/kg bw per day |
Incr hep peroxisomal ß‐oxidation (m) Incr hep peroxisomal ß‐oxidation a |
30(b) 160(b) |
160(b) | Kudo et al. (2000) | ||
| PFHpA (Sigma‐Aldrich) |
ddY mice (m/f) No/sex/group: 3–5 Duration: 5 days i.p.: 0, 20, 50, 100 mg/kg bw per day |
Incr abs & rel liver weight (m) Incr abs & rel liver weight a Incr hep peroxisomal ß‐oxidation (m) Incr hep peroxisomal ß‐oxidation a |
20 50 20 |
50 100 20 50 |
Kudo et al. (2006) | ||
| PFHpA (Sigma, purity not specified) |
C57BL/6 mice (sex) No/sex/group: 4 Duration: 3 days i.p. 0 or 20 mg/kg bw per day |
Incr rel liver weight | 20 | Abe et al. (2017) | |||
| Perfluoroctanoic acid PFOA | |||||||
| PFOA (> 96% purity) |
Balb/c mice (m) No/sex/group: not reported Duration: 7 days Via distilled water: 0, 1 or 5 mg/kg bw per day |
Incr ALT Necrosis and vacuolation of hepatocytes Incr abs liver weight Incr triglycerides in liver Decr body weight Decr free fatty acids in serum Decr triglycerides in serum |
1 1 |
1 1 5 1 1 5 1 |
Hui et al. (2017) | ||
| PFOA (96% purity) |
Balb/c mice (m) No/sex/group: 3–10 Duration: 28 days Orally: 0 or 1.25 mg/kg bw per day |
Incr rel liver weight Incr fasting blood glucose levels Decr glycogen and glucose content in the liver Incr blood glucagon |
1.25 1.25 1.25 1.25 |
Zheng et al. (2017) | |||
| PFOA |
SD rats (m) No/sex/group: 7 Duration: 14 days Gavage: 0, 1, 5, 25 mg/kg bw per day |
Incr abs & rel liver weight Incr activity of superoxide dismutase and glutathione peroxidase in the liver Incr MDA content in liver |
1 1 |
5 1 5 |
Wang et al. (2017b) | ||
| PFOA (commercial source, purity not specified) |
C57BL/6 mice (sex) No/sex/group: 4 Duration: 3 days i.p. 0 or 20 mg/kg bw per day |
Incr rel liver weight | 20 | Abe et al (2017) | |||
| PFOA (98% purity) |
Kunming mice (m) No/sex/group: 8 Duration: 21 days Gavage: 0, 1 or 5 mg/kg bw per day |
Incr abs & rel liver weight Incr ALT and AST Decr serum triglycerides Incr hepatic triglycerides Decr hepatic FGF21 protein |
1 1 1 1 1 |
5 5 5 5 5 |
Wu et al. (2018) | ||
| PFOA (ammonium salt, > 98% purity) |
C57BlL/6 mice (m) No/sex/group: 5 Duration: 2, 8 or 16 weeks Gavage: 1 mg/kg bw per day |
Decr body weight (week 8+16) Incr liver weight (week 8) Incr rel liver weight (week 2–16) Incr replication of hepatocytes (week 2+8) Incr hepatic peroxisomal ß‐oxidation activity (week 2–16) |
1 1 1 1 1 1 |
Li et al. (2019a) | |||
| PFOA (> 98% purity) |
SD rats (m, f) No/sex/group: 10 Duration: 28 days Gavage: males: 0, 0.625, 1.25, 2.5, 5, or 10 mg/kg bw per day; females: 0, 6.25, 12.5, 25, 50, or 100 mg/kg bw per day |
Incr rel+abs liver weight (m) Incr acyl‐CoA‐oxidase activity (m) Incr rel kidney weight (m) Incr rel+abs liver weight a Incr rel kidney weight a Incr rel thyroid weight (m) Decr serum cholesterin & triglyceride (m) Incr ALT, ALP, albumin/globulin ratio (m) Decr T3, free+total T4 (m) Decr haematocrit a Incr TSH, ALP a Incr serum cholesterin+triglycerides a Degeneration and inflammation of olfactory epithelium (m) |
25 25 0.625 25 |
0.625 0.625 0.625 50 50 1.25 0.625 0.625 0.625 6.25 6.25 50 0.625 |
Plasma conc. (ug/ml) at 0.625 mg/kg bw per day: 50.7 ± 2.2 (m) at 5 mg/kg bw per day: 110.7 ± 3.8 (m) at 6.25 mg/kg bw per day: 491 ± 72.1a Liver conc (ug/g) at 0.625 mg/kg bw per day 54.6 ± 2.2 (m) |
NTP (2019a) | |
| Perfluorononanoic acid (PFNA) | |||||||
| PFNA (analytical grade) |
Wistar rats (m/f) No/sex/group: 4 Duration: 5 days i.p.: 0, 2.5, 5, 10,15,20 mg/kg bw per day |
Incr hep peroxisomal ß‐oxidation (m) Incr hep peroxisomal ß‐oxidation a |
5(b) |
2.5(b) 10(b) |
Liver conc (ug/g) 20 mg/kg bw: 358 +/−19 (m) 102 +/−11 a |
Kudo et al. (2000) | |
| PFNA (commercial source; purity not specified) |
ddY mice(m/f) No/sex/group: 3–5 Duration: 5 days i.p.: 0, 2.5, 5, 10, 20 mg/kg bw per day |
Incr abs & rel liver weight a Incr abs liver weight (m) Incr rel liver weight (m) Incr perox ß‐oxidation (m/f) |
2.5 |
2.5 5 2.5 2.5 |
Kudo et al. (2006) | ||
| PFNA (97% purity) |
SD rats (m) No/sex/group: 6 Duration: 14 days Gavage: 0, 0.2, 1 or 5 mg/kg bw per day |
Incr abs & rel liver weight Decr total serum cholesterol Incr mRNA of SREBP‐1c, ACOT1/2 Incr hepatic levels of Il1ß,Il10,TNFa Incr serum levels of ALT, AST, ALP, LDH |
0.2 0.2 1 |
1 0.2 1 0.2 5 |
Liver conc. (ug/g) 0.2 mg/kg bw per day: 12.2 5 mg/kg bw per day: 135 |
Fang et al. (2012a) | |
| PFNA (97% purity) |
SD rats (m) No/sex/group: 6 Duration: 14 days Gavage: 0, 0.2, 1 or 5 mg/kg bw per day |
Incr serum glucose Decr serum HDL Incr liver glycogen Incr liver MDA Incr mRNA of G6PC/GLUT2 |
0.2 1 1 1 |
1 0.2 5 5 5 |
Fang et al. (2012b) | ||
| PFNA (97% purity) |
Balb/c mice (m) No/sex/group: 8 Duration: 14 days Gavage: 0, 0.2, 1 or 5 mg/kg bw per day |
Incr rel liver weight Incr total hepatic cholesterol/triglycer. Incr mRNA of Cyp4A1/ACOX1 Incr serum levels of AST, ALT |
1 |
0.2 0.2 0.2 5 |
Wang et al. (2015a) | ||
| PFNA (97% purity) |
SD rats (m)(c) No/sex/group: 10 Duration: 7 days Gavage: 0, 0.2, 1, 5 mg/kg bw per day |
Incr hepatic cholesterol Incr activity of glucose‐6‐P‐dehydrogenase Incr serum ALT |
0.2 0.2 0.2 |
1 1 1 |
Fang et al. (2015) | ||
| PFNA (purity not specified) |
Wistar rat (m) No/sex/group: 10 Duration: 14 days Gavage: 0, 0.0125, 0.25 or 5 mg/kg bw per day |
Incr plasma corticosterone Decr hepatic OATP4C1 protein |
0.0125(b) 0.0125(b) |
Serum levels (ug/ml): at 0.0125 mg/kg bw per day: 0.396 at 0.25 mg/kg bw per day: 30 at 5 mg/kg bw per day: 602 |
Hadrup et al. (2016) | ||
| PFNA (Sigma purity not specified) |
SV129 mice (m) No/sex/group: 4 Duration: 7 days Gavage: 0, 1 or 3 mg/kg bw per day |
Incr abs & rel liver weight | 1 | Rosen et al. (2017) | |||
| PFNA (97% purity) |
SV129 mice (m) No/sex/group: 4 Duration: 7 days Gavage: 0 or 10 mg/kg bw per day |
Incr abs & rel liver weight Incr hepatic lipid and triglyceride content |
10 10 |
Das et al. (2017) | |||
| PFNA (Sigma, purity not specified) |
C57BL/6 mice (sex) No/sex/group: 4 Duration: 3 days i.p. 0 or 20 mg/kg bw per day |
Incr rel liver weight | 20 | Abe et al. (2017) | |||
| PFNA (> 98% purity) |
SD rats (m, f) No/sex/group: 10 Duration: 28 days Gavage: males: 0, 0.625, 1.25, 2.5, 5, or 10 mg/kg bw per day; females: 0, 1.56, 3.12, 6.25, 12.5, or 25 mg/kg bw per day |
Decr body weight (m) Decr body weight a Incr rel + abs liver weight (m) Incr acyl‐CoA oxidase activity (m) Incr rel kidney weight (m) Incr rel + abs liver weight a Incr rel kidney weight a Decr serum cholesterol+triglyceride (m) Decr free + total T4 (m) Incr serum bile salts (m) Incr urea, albumin/globulin ratio (m) Incr total+direct bilirubin, ALP/ALT/AST (m) Incr albumin/globulin ratio a Incr urea and serum bile salts a Decr total+free T4 a |
0.625 0.156 0.625 0.625 1.56 1.56 |
1.25 3.12 0.625 0.625 0.625 1.56 1.56 0.625 0.625 0.625 1.25 1.25 1.56 3.12 3.12 |
Plasma conc. (ug/ml) at 0.625 mg/kg bw per day: 56.7 ± 1.9 (m) at 1.56 mg/kg bw per day: 26.4 ± 1.1 a Liver conc (u g/g) at 0.625 mg/kg bw per day 145.5 ± 2.7 (m) |
NTP (2019a) | |
| Perfluorodecanoic acid (PFDA) | |||||||
| PFDA (Aldrich) |
Wistar rats (m) No/sex/group: 4 Duration: 1 week Diet: 0, 0.00125, 0.0025, 0.005 or 0.01%; equivalent to 0, 1.5, 3, 6, or 12 mg/kg bw per day(e) |
Incr rel liver weight Incr abs liver weight Incr peroxisomal ß‐oxidation Incr acyltransferase activity Incr intrahepatic triacylglycerol |
1.5 1.5 |
1.5 3 3 1.5 1.5 |
Kawashima et al. (1995) | ||
| PFDA (analytical grade) |
Wistar rats (m/f) No/sex/group: 4 Duration: 5 days. i.p.: 0, 2.5, 5, 10, 15, 20 mg/kg bw per day |
Incr hep peroxisomal ß‐oxidation (m) Incr hep peroxisomal ß‐oxidation a |
2.5(d) 2.5(d) |
5(d) 5(d) |
Liver conc (ug/g) 20 mg/kg bw: 453 +/−19 (m) 412 +/−33 a |
Kudo et al. (2000) | |
| PFDA (Sigma, purity not specified) |
C57BL/6 mice (sex not reported) No/sex/group: 4 Duration: 3 days i.p. 0 or 20 mg/kg bw per day |
Incr rel liver weight | 20 | Abe et al. (2017) | |||
| PFDA (97.8% purity) |
SD rats a No/sex/group: 8 Gavage at 0, 0.125, 0.25, 0.5, 1, or 2 mg/kg bw per day Duration: 28 days |
Incr rel liver weight Incr abs liver and rel kidney weight Incr abs kidney weight |
0.125 0.25 |
0.125 0.25 0.5 |
Frawley et al. (2018) | ||
| PFDA (97.8% purity) |
B6C3F1 mice a No/sex/group: 8 Gavage at 0, 0.31, 0.625, 1.125, 2.5, or 5 mg/ kg bw per day Duration: 28 days |
Incr abs & rel liver weight Incr rel spleen weight |
0.31 0.625 |
0.625 1.125 |
Frawley et al. (2018) | ||
| PFDA (> 97% purity) |
SD rats (m, f) No/sex/group: 10 Duration: 28 days Gavage: 0, 0.156, 0.312, 0.625, 1.25, or 2.5 mg/kg bw per day |
Incr abs+rel liver weight (m, f) Incr acyl‐CoA‐oxidase activity (m) Decr abs weight of adrenal gland (m) Incr abs+rel weight of thyroid a Incr albumin/globulin ratio (m, f) Decr cholesterol in blood (m) Incr AST (m/f) Incr ALT (m/f) Incr ALP (m, f) |
0.156 −/0.625 0.156/0.625 0.156 |
0.156 0.156 0.156 0.312 0.156 0.156 0.156/1.25 0.312/1.25 0.312 |
Plasma conc. (ug/ml) at 0.156 mg/kg bw/day: 8.5 ± .6 (m) 11.2 ± 0.4 a Liver conc (ug/g) at 0.156 mg/kg bw/day 44.7 ± 1.5 (m) |
NTP (2019a) | |
| Perfluoroundecanoic acid (PFUnDA) | |||||||
| PFUnDA (98.5% purity) |
Crl:CD (SD) rats (m/f) No/sex/group: 12 Duration: 42 days Gavage: 0, 0.1, 0.3, 1 mg/kg bw per day |
Incr abs liver weight (m, f) Incr rel liver weight (m/f) Decr abs & rel spleen weight (m) Incr serum ALP, AST (m) Incr serum BUN (m/f) Decr serum albumin (m) |
0.3 0.1/0.3 0.3 0.3 0.3 0.3 |
1 0.3/1 1 1 1 1 |
Takahashi et al. (2014) | ||
| Perfluorododecanoic acid (PFDoDA) | |||||||
| PFDoDA (> 99% purity) |
SD Rats (m) No/sex/group: 6 Duration: 14 days Gavage: 0, 1, 5, or 10 mg/kg bw per day |
Incr abs & rel liver weight Incr serum triglyceride Incr hepatic triglyceride Incr hepatic SOD activity Incr hepatic mRNA of PPARα/g, ACOX, CypA4 Incr hepatic content of cholesterol |
1 5 5 5 |
5 10 10 1 1 10 |
Zhang et al. (2008) | ||
| PFDoDA (95% purity) |
SD Rats (m) No/sex/group: 10 Duration: 110 days Gavage: 0, 0.02, 0.05, 0.2, or 0.5 mg/kg bw per day |
Incr serum glucose Incr serum albumin Incr hepatic mRNA of PPARα, Cyp4A1, ACOX, cd36 |
0.05 |
0.02 0.02 0.2 |
Ding et al. (2009) | ||
| PFDoDA (95% purity) |
SD Rats (m) No/sex/group: 6 Duration: 110 days Gavage: 0, 0.05, 0.2, or 0.5 mg/kg bw per day |
Incr protein level of pyruvate carboxylase in kidney Incr protein level of isovaleryl coenzyme A dehydrogenase, malate dehydrogenase 1 and dihydrolipoamide S‐acetyltransferase in kidney |
0.05 |
0.05 0.2 |
Zhang et al. (2011) | ||
| PFDoDA (Sigma, purity not specified) |
SD rats (m) No/sex/group: 6 Duration: 110 days Gavage: 0, 0.2, or 0.5 mg/kg bw per day |
Incr hepatic cholesterol Incr hepatic triglycerides Altered hepatic levels of signal transduction proteins (e.g. glycogen synthase kinase, insulin receptor substrate) |
0.2 0.2 0.2 |
Zhang et al. (2013e) | |||
| PFDoDA (97% purity) |
SD Rats (m/f) No/sex/group: 7 Duration: 42 days Gavage: 0, 0.1, 0.5, or 2.5 mg/kg bw per day |
Incr rel liver weight (m/f) Decr weight of spleen/heart a Decr in reticulocytes (m) Incr serum ALP (m) Decr serum total cholesterol (m) Liver hypertrophy (m) Hepatic necrosis a Pancreas: decr zymogen granules (m) Decr serum glucose (m) |
0.1 0.1 0.5 0.1 0.5 0.5 0.5 0.5 |
0.5 0.5 2.5 0.5 0.1 2.5 2.5 2.5 2.5 |
Kato et al. (2015b) | ||
| PFDoDA (> 95% purity) |
SD Rats (m) No/sex/group: 4‐10 Duration: 110 days Gavage: 0, 0.05, 0.2, or 0.5 mg/kg bw per day |
Incr hepatic SOD activity Incr TBARS in liver Decr hepatic GPX activity Incr mRNA of PPARα/Cyp4A1 Incr mRNA of mitochondrial acyl‐CoA‐thioesterase 1 and hydroxyacyl‐CoA‐dehydrogenase |
0.2 0.2 0.2 0.05 |
0.5 0.5 0.5 0.2 0.05 |
Liu et al. (2016) | ||
| Perfluorotetradecanoic acid (PFTeDA) | |||||||
| PFTeDA (96.5% purity) |
Crl:CD (SD) rats (m) No/sex/group: 12 Duration: 42 days Gavage: 0, 1, 3, or 10 mg/kg bw per day |
Decr body weight Decr hindlimb strength Incr serum ALP and BUN Incr abs & rel liver weight Centrolob. liver hypertrophy & steatosis Decr abs & rel pituitary gland weight Decr abs weight of semin. vesicles Hypertrophy of thyroid follicular cells |
3 1 3 1 1 1 0 1 |
10 3 10 3 3 3 1 3 |
Hirata‐Koizumi et al. (2015) | ||
| Perfluorohexadecanoic acid (PFHxDA) | |||||||
| PFHxDA (95.3% purity) |
Crl:CD (SD) rats (m) No/sex/group: 12 Duration: 42 days Gavage: 0, 4, 20, or 100 mg/kg bw per day |
Decr body weight Incr abs & rel liver weight Centrolob. liver hypertrophy & steatosis Incr rel thyroid weight |
20 20 20 20 |
100 100 100 100 |
Hirata‐Koizumi et al. (2015) | ||
| Perfluorooctadecanoic acid (PFODA) | |||||||
| PFODA (98.9% purity) |
Crl:CD (SD) rats (m) No/sex/group: 12 Duration: 42 days Gavage: 0, 40, 200, or 1,000 mg/kg bw per day |
Decr body weight/food consumption Decr red blood cells/haemoglobin/haematocrit Decr serum gamma‐GTP Incr abs & rel liver weight Liver hypertrophy Incr serum ALP, ALT |
200 40 40 40 40 200 |
1,000 200 200 200 200 1,000 |
Hirata‐Koizumi et al. (2012) | ||
ALP: alanine phosphatase; ALT: alanine aminotransferase; AST: aspartate aminotransferase; BUN: blood urea nitrogen; bw: body weight; f: female; m: male; i.p.: intraperitoneal; SOD: superoxide dismutase; T3: triiodothyronine; T4: thyroxine; TBARS: Thiobarbituric acid reactive substances.
Please note that the effect was not evident at the 90‐day time point of investigation.
Adequate statistical evaluation is missing.
Rats were treated with streptozotocin to induce diabetes.
Derived from figure 2D of Kudo et al. (2000). No statistical evaluation is given and significance of data is unclear.
Applying default values, provided by EFSA.
More data on serum and tissue concentrations are given in Butenhoff et al. (2012). The table is confined to serum/tissue concentration at doses with sensitive endpoints in males of the highest dose group.
In two independent studies, PFBA increased the liver weight in male mice (28 days) and male rats (28 or 90 days) at oral doses of 35 (Foreman et al., 2009) and 30 mg/kg bw per day (Butenhoff et al., 2012), respectively. Butenhoff et al. (2012) treated male and female SD rats (10/sex per group) by gavage with ammonium PFBA at doses of 0, 6, 30 and 150 mg/kg bw per day for 28 days and at doses of 0, 1.2, 6 and 30 mg/kg bw per day for 90 days. At the lowest dose, there were no treatment‐related alterations in females. In males, PFBA induced dose‐dependently an increased liver weight, accompanied by hepatocellular hypertrophy and coagulative necrosis. In females, the relative liver weight remained unchanged. In males, total serum cholesterol was lowered. Thyroid follicular hypertrophy and hyperplasia were also noted. In the 28‐day study, a reduced total and free serum thyroxin level was observed in males already at 6 mg/kg bw per day but no changes occurred in female animals in any group. In the 90‐day study, the serum thyroxin levels were decreased again in males only at 30 mg/kg bw per day. The considerably higher NOAEL in females was attributed to significantly lower levels of PFBA in serum and the liver, e.g. after 28 days of 150 mg PFBA/kg bw per day levels reached 82.2 μg/mL serum and 37.4 ug/g in males, while females showed levels of 10.2 μg/mL serum and 2.7 μg/g in liver tissue only. The same trend could be seen also after 90 days of 30 mg PFBA/kg bw per day. Females exhibited 5.2 μg/mL serum and 0.9 ug/g liver tissue and males 52.2 μg/mL serum and 16.1 μg/g liver.
PFHxA, increased absolute and relative liver weights after oral application in male and female rats (Chengelis et al., 2009; Loveless et al., 2009; NTP, 2019a; WIL Research Laboratories, 2005) and after i.p. application in female mice (Kudo et al., 2006) occurring at doses of 100 mg/kg bw per day and above. In male rats, liver enzymes in serum were increased at 20 mg/kg bw per day (Loveless et al., 2009). The most sensitive parameter was increased relative kidney weight in male animals, treated with the lowest dose of 10 mg/kg bw per day for 90 days (Chengelis et al., 2009). This finding could not be reproduced by another study, applying the same rat strain and treating the animals for an almost identical period of time (Loveless et al., 2009). Here, an increased relative kidney weight occurred in both sexes only at the highest dose of 500 mg/kg bw per day. Further sensitive endpoints were degenerations and atrophy in the nasal cavity occurring in male and female rats at 100 mg/kg bw per day (Loveless et al., 2009). Loveless et al. (2009) performed also a one‐generation reproduction study in rats (see Section 3.3.3.3.3). In this part of the study, body weight of parental males was reduced at 500 mg/kg bw per day after exposure for approximately 105 days.
In the NTP (2019a) study, male SD rats exhibited 1.6‐ to 2.9‐fold higher plasma concentration compared to females, which was consistently seen among all treatment groups. Increased relative liver weight occurred at doses of 250 (males) and 500 mg/kg bw per day (females) and above. Serum cholesterol concentrations were significantly decreased in all male dose groups. Lowered haematocrit, haemoglobin and RBC were evident in males from 62.6 and in females from 250 mg/kg bw per day upwards. ALT, AST were elevated in both sexes at 500 mg/kg bw per day. Total thyroxine (T4), free T4 and triiodothyronine (T3) concentrations were significantly decreased in all male dose groups, while females exhibited no alterations. In the histopathological evaluations, the most prominent findings were degeneration and hyperplasia of the olfactory epithelium occurring in both sexes at doses of 250 mg/kg bw per day and above.
A few studies on the subacute effects of i.p. administration of PFHpA indicate that PFHpA induces peroxisomal ß oxidation enzymes in rats at a dose of 160 mg/kg bw per day (sex not specified) and in male mice at 20 mg/kg bw per day. PFHpA elevates the relative and absolute liver weight in male mice (Abe et al., 2017; Kudo et al., 2000, 2006). Female mice required higher doses to show liver enlargement and induction of peroxisomal ß‐oxidation (Kudo et al., 2006).
Reports on PFOA, being published between 2008 and 2016 are documented in the previous Opinion (EFSA CONTAM Panel, 2018). In the NTP (2019a) study on PFOA male SD rats exhibited increased absolute and relative liver weights as well as increased relative kidney weights at 0.625 mg/kg bw per day, when given for 28 days. Relative and absolute liver weights were elevated also in other studies applying doses above 1 mg/kg bw per day to mice or rats (Hui et al., 2017; Wang et al., 2017b; Wu et al., 2018; Zheng et al., 2017). Li et al.. (2019a) showed that the PFOA‐induced liver enlargement was due to elevated hepatocellular DNA replication, as demonstrated in mice receiving 1 mg/kg bw per day for up to 8 weeks.
With regard to hepatotoxic effects of PFOA, male rats exhibited increased ALT and ALP serum levels at already 0.625 mg/kg bw per day (NTP 2019a). PFOA elevated transaminases and induced hepatocellular necrosis at 1 mg/kg bw per day and above also in mice (Hui et al., 2017; Wu et al., 2018) and increased the malondialdehyde concentration in rat liver at 5 mg/kg bw per day.
Most of the recent studies on PFOA focused on metabolic effects of this compound. PFOA‐exposed mice showed elevated hepatic peroxisomal ß‐oxidation, reduced triglyceride and free fatty acid contents in serum, as well as elevated levels of hepatic triglycerides (Hui et al., 2017). Liver sections displayed visible vacuolation of hepatocytes. This was associated with upregulated mRNA as well as protein of hepatic lipoprotein lipase (LPL) and fatty acid translocase (CD36) and downregulated mRNA and protein of apolipoprotein‐B100 (APOB). These findings suggest dysregulations of fatty acid trafficking. Also, Wang et al. (2017b) observed that the frequency of CD36‐positive cells was elevated and of ApoB‐positive cells reduced. Li et al. (2019a) reported that in mice, kept on a high‐fat diet, 1 mg PFOA/kg bw per day decreased the severity of hepatic steatosis within a treatment period of 2–16 weeks.
Zheng et al. (2017) studied the impact of PFOA on liver glucose homoeostasis. In male mice 4 weeks of 1.25 mg/kg of PFOA per day increased fasting blood glucose levels and decreased glycogen and glucose content in the liver. In blood insulin levels remained unchanged while those of glucagon were elevated, possibly contributing to the lowered protein levels of glycogen synthase and elevated levels of glucokinase in the liver and to the hyperglycaemia observed in the treated animals. PFOA exposure did not affect muscle glucose or glycogen levels. Indirect calorimetry revealed elevated energy consumption in PFOA‐treated animals. The findings give indication that PFOA may induce glycogenolysis and gluconeogenesis in murine livers. In contrast to observations of Zheng et al. (2017), Wu et al. (2018) found dose‐dependent but insiginificant increases in insulin and decreases in glucose in blood of fasted mice, starting at 1 mg PFOA/kg bw per day. This was associated with increased insulin‐positive cells in the pancreas of the animals. Furthermore, PFOA downregulated the protein amount of hepatocellular fibroblast growth factor 21 (FGF21) dose‐dependently. In the liver, FGF21 expression is regulated by PPARα and the effect of this hepatokine is additive to the activity of insulin with regard to glucose uptake in the peripheral tissues. The observations of Zheng et al. (2017) and Wu et al. (2018) indicate complex alterations in the regulation of carbohydrate metabolism.
In the NTP study (NTP, 2019a), many haematological and clinical chemistry parameters were altered in male rats at already 0.625 mg/kg bw per day, such as decreased serum cholesterol and triglycerides, increased albumin/globulin ratios and lowered levels in T3, free and total T4. In females, some of these alterations occurred at much higher doses, such as elevated albumin/globulin ratios at 12.5 mg/kg bw per day or lowered total and free T4 at 100 mg/kg/bw per day. At the pathohistological examination, cytoplasmic changes of hepatocytes and degeneration and inflammation in the olfactory epithelium were observed at 0.625 mg/kg bw per day in the males. Similar changes were seen in females at 12.5 or 25 mg/kg bw per day and above. This indicates a marked sex difference in the sensitivity towards PFOA, related to the much lower serum levels in female rats.
For PFNA, most repeated‐dose toxicity studies focused on hepatic effects. Sensitive endpoints were increased relative liver weight and increased intrahepatic levels of total cholesterol and triglycerides in male mice at 0.2 mg/kg bw per day (Wang et al., 2015a), and decreased serum HDL and cholesterol at 0.2 mg/kg bw per day in male rats (Fang et al., 2012a,b). Hepatotoxic effects, as indicated by elevated serum transaminases, became evident at 1 mg/kg bw per day and more (Fang et al., 2012b, 2015; Wang et al., 2015a). Subsequent studies (Abe et al., 2017; Das et al., 2017; Rosen et al., 2017) focused on the MoA of PFNA and applied doses of 1 mg/kg bw per day and above (see also Section 3.3.5). Again, increased relative and absolute liver weights as well as intrahepatic lipid storage were reported. Das et al. (2017) showed a decrease in DNA content per mg liver but an overall increase in the amount of total DNA per liver, suggesting both hypertrophy and hyperplasia, as also shown for PFOA in this study.
In the NTP study (NTP, 2019a) on PFNA, most male and female animals died at the higher dose groups (5 and 10 mg/kg bw per day for males, 12.5 and 25 mg/kg bw per day for females). Body weights decreased at lower dose levels. The absolute and relative liver weights and the relative kidney weights were increased, starting at 0.625 mg/kg bw per day in male and 1.56 mg/kg bw per day in female SD rats. Blood cholesterol was consistently lowered in males exposed to 0.625 and 1.25 mg/kg bw per day but not in those exposed to 2.5 mg/kg bw per day, while in females, no changes were found. Urea concentrations and the albumin/globulin ratio were elevated in almost all dose groups in males and in females at 3.12 and 1.56 mg/kg bw per day, respectively. In male rats, increased bile acid concentrations were evident at 0.625 mg/kg bw per day, followed by elevated bilirubin, AST, ALT and ALP at 1.25 mg/kg bw per day. In females, similar alterations were observed at higher PFNA doses, possibly related to the lower serum levels in females at similar doses. In almost all PFNA‐treated groups of males, free and total T4 concentrations were decreased. In the histopathological evaluations, alteration in the cytoplasm and hypertrophy of hepatocytes was the most sensitive endpoint occurring already at the lowest doses in both sexes.
Kawashima et al. (1995) treated male rats with a diet containing PFDA at concentrations ranging from 1.5 to 12 mg/kg bw per day for 1 week. PFDA treatment induced absolute and relative liver weights, peroxisomal beta‐oxidation, microsomal 1‐acylglycerophosphocholine acyltransferase and cytosolic long‐chain acyl‐CoA hydrolase in a dose‐dependent manner. In male and female rats, PFDA was a potent inducer of peroxisomal ß‐oxidation in the liver; hepatic concentrations of PFDA were similar in both sexes (Kudo et al., 2000). Abe et al. (2017) observed an increased relative liver weight in mice at an i.p. dose of 20 mg/kg bw per day.
Frawley et al. (2018) reported on the effects of PFDA on the immune system, when administered by gavage to female rats and mice for a period of 28 days (for further details, see Section 3.3.3.5). The authors provide some additional information on the changes in the organ weights. In female SD rats, the relative liver weight was increased from 0.125 mg/kg bw per day onwards, the absolute liver weight and relative kidney weight at 0.25 mg/kg bw per day and above and the absolute kidney weight at the highest dose of 0.5 mg/kg bw per day. At this dose single necrotic hepatocytes occurred in the centrilobular region in 3/8 rats. Mice were less sensitive than rats and showed an increased relative and absolute liver weight from 0.625 mg/kg bw per day and elevated relative spleen weight from 1.125 mg/kg bw per day onwards.
In the NTP study (NTP, 2019a) on PFDA absolute and relative liver weight were increased in both, male and female SD rats, from the lowest dose onwards. The relative and absolute weight of the thyroid gland was elevated in females only at 0.312 mg/kg bw per day and above, whereas males showed decreased absolute weights of adrenal glands in all dose groups. The albumin/globulin ratio significantly increased with 0.156 mg/kg bw per day or greater in both sexes. A decrease in blood cholesterol levels was evident in males at the three lower dose groups only and in females only at the high dose. ALT, AST and ALP were significantly elevated at lower doses in both, male and female rats. Free and total T4 concentrations were lowered in males at the higher PFDA dose groups, while females showed only a decreased fT4 level at the highest dose level. In the histopathological evaluations, alteration in the hepatocellular cytoplasm and hypertrophy was the most sensitive endpoint occurring already at the lowest doses in both sexes.
For PFUnDA, decreases in body weight gain in male and female rats were noted, which were most pronounced at the highest dose (Takahashi et al., 2014). Further effects were increases in relative liver weight, blood urea nitrogen, centrilobular hypertrophy of hepatocytes and focal hepatocellular necrosis. A decrease in serum cholesterol observed at 0.3 mg/kg bw per day was not evident at the highest dose of 1 mg/kg bw per day. The most sensitive parameter was an increase in the relative liver weight in the males at the dose of 0.3 mg/kg bw per day.
When PFDoDA was applied to male and female rats, the most consistent treatment‐related effects were described for the liver. The majority of alterations occurred at gavage doses of 0.5 mg/kg bw per day and above, and comprised increases in relative liver weight and serum ALP (Kato et al., 2015b; Liu et al., 2016). Several studies provided direct or indirect evidence for intrahepatic lipid peroxidation (Liu et al., 2016; Zhang et al., 2008). Lowered serum cholesterol, altered blood levels of albumin and glucose, as well as renal alterations occurred in an inconsistent way.
PFTeDA was administered to male rats by daily gavage at 1, 3 or 10 mg/kg bw per day for 42 days. One of the most sensitive endpoints was the lowered absolute weight of the seminal vesicles occurring at 1 mg/kg bw per day. The middle and the high dose caused increases in relative and absolute liver weight, hepatocyte hypertrophy and fatty changes in the centre of liver lobules, reduced absolute and relative weight of the pituitary gland, reduced hindlimb strength, as well as follicular cell hypertrophy in the thyroid gland (Hirata‐Koizumi et al., 2015).
PFHxDA was applied at 4, 20 or 100 mg/kg bw by daily gavage to male rats for 6 weeks. Liver enlargement, hepatocellular hypertrophy and steatosis and an elevated relative weight of the thyroid gland were observed mainly at the dose of 100 mg/kg bw per day (Hirata‐Koizumi et al., 2015).
Hirata‐Koizumi et al. (2012) treated male rats with PFODA at 0, 40, 200 or 1,000 mg/kg bw per day by gavage for 42 days. Rats were mated after about 2 weeks of treatment. Alterations of the liver (increased absolute and relative liver weight, centrilobular hepatocyte hypertrophy, necrosis) and of haematological parameters (decreased red blood cells count, lowered haemoglobin/haematocrit) appeared to be the most sensitive endpoints and occurred at a dose of 200 mg/kg bw per day.
In summary, the most consistent and most sensitive endpoint was the increased relative liver weight, especially in male rodents, seen for all PFCAs studied. The disturbances in lipid metabolism, hepatotoxic effects and signs of cholestasis were mostly evident at higher dose levels. For some PFCAs increased relative kidney weight, alterations of the mucosa in the nasal cavity and olfactory epithelium and disturbed thyroid hormone levels were among the most sensitive endpoints. For details, see Appendix E.
3.3.3.2.2. PFSAs
The available repeated dose studies for PFBS, PFHxS and PFOS (appearing after literature deadline of the previous Opinion (EFSA CONTAM Panel, 2018)) are listed in Appendix E, Table E.2. For PFHpS and PFDS, no studies have been identified.
Table E.2.
Repeated dose toxicity studies for PFSAs
| Substance (Purity) | Species/Experimental design and doses | Observed effect | Highest dose with no effects (mg/kg bw per day) | Lowest dose with effect (mg/kg bw per day) | Serum/tissue level of compound | Reference |
|---|---|---|---|---|---|---|
| Perfluorobutane sulfonic acid (PFBS) | ||||||
| PFBS, potassium salt (98.2% purity) |
Sprague‐Dawley rats (m/f) No/sex/group: 10 Duration: 28 days Gavage: 0, 100, 300, or 900 mg/kg bw per day |
Decr serum phosphorus and potassium (m) Incr rel and absolute liver weight (m) |
100 300 |
300 900 |
N/A | NICNAS (2005) |
| PFBS, potassium salt (98.2% purity) |
Crl:CD(SD)IGS BR VAF/PlusTM rats (m/f) No/sex/group: 10 Duration: 90 days Gavage: 0, 60, 200, or 600 mg/kg bw per day |
Decr abs &rel spleen weight(m) Decr red blood cells (m) Decr haematocrit (m) Decr haemoglobin (m) Incr serum chloride (m) Decr serum albumin and total protein (f) |
200 60 60 200 200 |
60 600 200 200 600 600 |
Lieder et al. (2009a) | |
| PFBS, potassium salt (97.9% purity) |
Crl:CD(SD)IGS BR VAF/PlusTM rats (m) No/sex/group: 30 Duration: 10 weeks Gavage: 0, 30, 100, 300 or 1,000 mg/kg bw per day |
Incr abs & rel liver weight | 100 | 300 | Lieder et al. (2009b) | |
| PFBS (> 97% purity) |
Sprague‐Dawley rats (m, f) No/sex/group: 10 Duration: 28 days Gavage: 0, 62.6, 125, 250, 500, or 1,000 mg/kg bw per day |
Incr rel liver weight (m,f) Incr abs liver weight (m/f) Incr acyl‐CoA‐oxidase activity (m) Incr rel kidney weight (f) Decr haematocrit, RBC, cholesterol, T3, total+free T4 (m,f) Incr albumin/globulin ratio (m,f) |
−/62.6 125 62.6 |
62.6/125 125/250 250 62.6 62.6 125 |
Plasma conc. (ug/mL at 62.6 mg/kg bw per day: 2.2 ± 4.8 (m) 0.2 ± 0.05 (f) Liver conc (ug/g) at 62.6 mg/kg bw per day 1.3 ± 0.2 (m) |
NTP, 2019b) |
| Perfluorohexane sulfonic acid (PFHxS) | ||||||
| PFHxS (potassium salt; 99.98% purity) |
Sprague‐Dawley rats (m) No/sex/group: 10 Duration: 42 days Gavage: 0, 0.3, 1, 3 or 10 mg/kg bw per day |
Incr rel liver weight Decr prothrombin time Decr serum cholesterol Decr haemoglobin Decr haematocrit Decr RBC Thyroid hypertrophy/hyperplasia |
1 0.3 1 1 1 |
3 0.3 0.3 1 3 3 3 |
Concentration in μg/g at 0.3 mg/kg bw per day in liver 43.8 +/− 8.1 in serum 44.2 +/− 12.7 3 mg/kg bw per day in liver 339 +/− 128 in serum 128 +/− 10 10 mg/kg bw per day in liver 593 +/− 81.4 in serum 201.5 +/− 20 | Butenhoff et al. (2009) |
| PFHxS (3M, salt, purity not specified*) |
SV129 mice (m) No/sex/group: 4 Duration: 7 days Gavage: 0, 3 or 10 mg/kg bw per day |
Incr rel liver weight Incr abs liver weight |
3 |
3 10 |
Rosen et al. (2017) | |
| PFHxS (potassium salt, 97% purity) |
SV129 mice (m) No/sex/group: 4 Duration: 7 days Gavage: 0 or 10 mg/kg bw per day |
Incr abs and rel liver weight Incr hepatic lipid and triglyceride content |
10 10 |
Das et al. (2017) | ||
| PFHxS (potassium salt; 98.9% purity) |
Crl:CD1 (IRC) mice (m) No/sex/group: 30 Duration: 42 days Gavage: 0, 0.3, 1, 3 mg/kg bw per day |
Incr abs & rel liver weight Centrilobular hypertrophy Incr ALP Decr serum cholesterol Necrotic hepatocytes and lipid vesicles in hepatocytes |
0.3 1 1 1 |
1 0.3 3 3 3 |
Liver conc. (μg/g): 0.3 mg/kg bw per day: 25.9 ± 3.5 1 mg/kg bw per day: 98.5 ± 22.7 3 mg/kg bw per day: 281.1 ± 45.4 |
Chang et al. (2018) |
| PFHxS (potassium salt; > 98% purity) |
Sprague‐Dawley rats (m, f) No/sex/group: 10 Duration: 28 days Gavage: 0, 0.625, 1.25, 2.5, 5, or 10 mg/kg bw per day (males) 0, 3.12, 6.25, 12.5, 25 or 50 mg/kg bw per day (females) |
Incr rel and abs liver weight (m/f) Decr T3 and cholesterol (m) Decr total T4 (m/f) Decr free T4 (m/f) Incr acyl‐CoA‐oxidase activity (m) |
0.625/− −/3.12 −/6.25 2.5 |
1.25/3.12 0.625 0.625/6.25 0.625/12.5 5 |
Plasma conc. (ug/mL) 0.625 mg/kg bw per day: 66.8 ± 3.5 (m) 3.12 mg/kg bw per day: 37.0 ± 1.7 (f) Liver conc (ug/g) 0.625 mg/kg bw per day 39.9 ± 1.3 (m) |
|
| Perfluorooctane sulfonic acid (PFOS) | ||||||
| PFOS (commercial source, Sigma) |
Swiss albino rats (m) No/sex/group: 6 Duration: 30 days Gavage every second day: 0, 0.6, 1.25 or 2.5 mg/kg bw |
DNA fragmentation in liver (Comet Assay), indication of apoptosis/necrosis? |
~ 0.6 | Eke et al. (2017) | ||
| PFOS (Potassium salt from 3M, approx. 88.9% purity, containing PFHxS at 3.2%, PFHpS at 1.2%, PFPeS at 1.1%, PFBS at 0.97%, PFPS at 0.74%) |
Cynomolgus monkey (m/f) No/sex/group: 4–6 Gavage: day 43: 14 mg/kg (m, f) day 288: 14.8 (m) and 17.2 mg/kg (f) day 358: 11 mg/kg (m, f) End of observation: day 422 |
Insignificant reduction of serum cholesterol post treatments (m) |
Serum conc (ug/mL): 11 mg/kg bw per day at day 365: 160.8 +/− 14.2 (m) 165.0 +/−6.7 (f) |
Chang et al. (2017) | ||
| PFOS (commercial source, Sigma) |
C57BL/6 J mice (m) No/sex/group: 5 Duration: 28 days Diet: 0 or 0.2 mg/kg bw per day |
Incr epididymal adipose tissue Incr rel liver weight Incr hepatic triglycerides Incr hepatocellular lipid storage Incr blood glucose |
0.2 0.2 0.2 0.2 0.2 |
Huck et al. (2018) | ||
| PFOS (> 98% purity) |
Mice (m) No/sex/group: 4 Duration: 21 days Gavage: 0 or 10 mg/kg bw per day |
Incr AST, ALT, LDH Incr rel liver weight Incr content of hepatic MDA and H2O2 Incr hepatic levels of TNFa and IL6 Incr hepatic caspase 3 activity |
10 10 10 10 10 |
Lv et al. (2018) | ||
| PFOS (potassium salt, 98% purity) |
SD rats (m) No/sex/group: 6 Duration: 28 days Gavage: 0, 1 or 10 mg/kg bw per day |
Incr ALT Incr AST Incr TNFa in serum Incr hepatic MDA content Decr hepatic catalase activity Decr hepatic content of GSH and GSH/GSSG Incr cleavage of caspase 3 in liver |
1 |
1 10 1 1 1 1 1 |
Han et al. (2018a) | |
| PFOS (potassium salt, 98% purity) |
SD rats (m) No/sex/group: 6 Duration: 28 days Gavage: 0, 1 or 10 mg/kg bw per day |
Incr ALT Incr AST Incr TNFa in serum Incr IL6 in serum Incr PCNA positive nuclei |
1 |
1 10 1 1 1 |
Han et al. (2018b) | |
| PFOS (commercial source, no further details indicated purity and type of salt not specified) |
C47Bl6/J mice (m) No/sex/group: 5 Duration: 6 weeks Diet: 0.089 mg/kg bw per day |
Incr rel liver weight Incr hepatic triglyceride conc. Incr blood glucose Incr. serum triglyceride & cholesterol |
0.089 0.089 0.089 0.089 |
Huck et al. (2018) | ||
| PFOS (type of salt not specific, 9(8% purity) |
CD mice (f) No/sex/group: 4 Duration: 7 weeks Diet: 0, 0.3 or 3 mg/kg bw per day |
Incr abs & rel liver weight Incr liver triglyceride Decr serum triglyceride Altered pyruvate tolerance test Altered gut microbiome |
0.3 0.3 |
3 0.3 3 0.3 0.3 |
Plasma conc (ug/g) at 0.3 mg/kg bw per day: 33.8 ± 4.4 at 3 mg/kg bw per day: 109.5 ± 19.4 Liver conc (ug/g) at 0.3 mg/kg bw per day: 32.9 ± 13.5 at 3 mg/kg bw per day: 503.8 ± 326 |
Lai et al. (2018) |
| PFOS (98% purity, salt not specified) |
ICR mice (m) No/sex/group: 10 Duration: 21 days Treatment: 10 mg/kg bw per day |
Incr. ALT & AST Incr total cholesterol & triglycerides in serum Incr TNFa & IL6 in serum |
10 10 10 |
Su et al. (2019) | ||
| PFOS (> 96% purity) |
SD rats (m, f) No/sex/group: 10 Duration: 28 days Gavage: 0, 0.312, 0.625, 1.25, 2.5, or 5 mg/kg bw per day |
Incr rel and abs liver weight (m/f) Incr acyl‐CoA‐oxidase activity (m) Decr blood cholesterol (m) Decr total and free T4 (m, f) |
1.25 |
0.312 2.5 0.312 0.312 |
Plasma conc. (ug/ml) at 0.312 mg/kg bw per day: 23.7 ± 1.1 (m) 30.5 ± 0.9 (f) Liver conc (ug/g) at 0.312 mg/kg bw per day 87.2 ± 3.04 (m) |
NTP (2019b) |
ALP: alanine phosphatase; ALT: alanine aminotransferase; AST: aspartate aminotransferase; bw: body weight; f: female; m: male; GSH: glutathione; GSSG: oxidized glutathione; i.p.: intraperitoneal; IL: interleukin; LDH: lactate dehydrogenase; MDA: malondialdehyde; PCNA: proliferating cell nuclear antigen; T3: triiodothyronine; T4: thyroxine; TNF: tumour necrosis factor.
Purity assumed to be 99.98%.
In a 4‐week study, PFBS lowered phosphorus and potassium in the serum and increased relative and absolute liver weights in male rats at 300 and 900 mg/kg bw per day (NICNAS, 2005). No alterations were noted for female rats. Lieder et al. (2009a) treated male and female rats with PFBS at 0, 60, 200 and 600 mg/kg bw per day by gavage for 90 days. The most sensitive endpoints were decreased absolute and relative spleen weights. Further treatment‐related effects comprised haematological parameters, such as decreased haemoglobin concentration and haematocrit. Histological alterations in the kidneys were found at the two highest doses (modest hyperplasia of the epithelial cells of the medullary and papillary tubules and the ducts in the inner medullary region) but were not considered to be adverse due to an absence of any changes in clinical chemistry or urinary parameters. In the liver signs of inflammation became evident at the highest dose in 3 of 10 males and in 4 of 10 female rats. In a further study, Lieder et al. (2009b) noted increased absolute and relative liver weight in male rats at a dose of 300 mg/kg bw per day, applied over a period of 10 weeks.
In the NTP study (NTP, 2019b) on the effects of PFBS in SD rats, increases in the relative right kidney weights (females) and in relative liver weights (males and females) occurred in most dose groups. Decreases in T3 and free and total T4 occurred in both sexes at all doses applied. Haematocrit, red blood cell counts and blood cholesterol were decreased at most doses in males, but in females, only a decrease in cholesterol was observed and only at the highest dose. Also for this PFAS, serum levels were lower in females than males. An increased albumin/globulin ratio was evident in males at females at 125 mg/kg bw per day and above. In the histopathological evaluation, hepatocellular cytoplasmic alteration and hypertrophy became evident at 125 mg/kg bw per day and above. Animals exhibited degeneration, necrosis, inflammation and/or hyperplasia of the olfactory epithelium, being significant at 125 mg/kg bw per day in females and at 250 mg/kg bw per day in males.
Butenhoff et al. (2009) treated male SD rats with PFHxS at 0, 0.3, 1, 3 or 10 mg/kg bw per day by gavage, during cohabitation (see Section 3.3.3.3). At day 42, low doses of PFHxS reduced serum cholesterol and prothrombin time (0.3 mg/kg bw per day corresponding to 44.2 μg/mL of PFHxS in serum). The prolonged prothrombin time at 0.3 mg/kg bw per day was not evident at the next higher dose. Increased liver‐to‐body weight ratio, centrilobular hepatocellular hypertrophy, hyperplasia of thyroid follicular cells and decreased haematocrit occurred at 3 mg/kg bw per day (corresponding to 128.7 μg/mL serum or 338.7 μg/g liver tissue). Decreased body weight gain and elevations in serum albumin, BUN, ALP, Ca2+ and albumin/globulin ratio were observed at the highest dose only. Similar findings were reported in an industry study, reviewed by the Swedish EPA (2012).
Das et al. (2017) studied the impact of PFHxS on hepatic gene expression patterns and lipid metabolism in male mice (see section on mode of action). An elevated relative liver weight was observed at a dose of 3 mg/kg bw per day and above; the increases in organ weight appeared to be due to hepatocellular hypertrophy, as reflected by decreases in DNA content per mg of liver (Das et al., 2017). Oil Red O stained sections revealed lipid accumulation in PFHxS groups, accompanied by elevated hepatic triglyceride levels and indicating the development of liver steatosis.
Chang et al. (2018) studied PFHxS for reproductive/developmental toxicity in CD‐1 mice. In F0 males, 0.3–3 mg/kg bw per day was administered before mating, which induced centrilobular hepatocellular hypertrophy at 0.3 mg/kg bw per day and increased relative liver weight at 1 mg/kg bw per day. Decreased serum cholesterol and increased alkaline phosphatase were observed as well. Hepatotoxicity became evident at 3 mg/kg bw per day, as indicated by hepatocellular lipid vesicles and necrotic hepatocytes (see Section 3.3.3.3.14 for further study details in relation to reproductive/developmental toxicity).
In the NTP study (NTP, 2019b) on the potassium salt of PFHxS, absolute and relative liver weights were elevated at 1.25 mg/kg bw per day in males and 3.12 mg/kg bw per day (lowest dose) in females, and above. In males, serum levels of T3, total and free T4 were reduced already at the lowest dose (0.625 mg/kg bw per day). Blood cholesterol levels were lowered from 1.25 mg/kg bw per day upwards. Females showed reductions in total T4 at 6.25 mg/kg bw per day and free T4 at 12.5 mg/kg bw per day, but no significant decrease for T3, and also not for cholesterol. Serum PFHxS levels in females were lower than in males. In the histopathological evaluation, hepatocellular hypertrophy became evident at 2.5 mg/kg bw per day in males only. Female rats exhibited degeneration, necrosis, inflammation and/or hyperplasia of the olfactory epithelium, but in the highest dose group only.
PFOS elevated the relative liver weight in mice at already 0.2 mg/kg bw per day, when applied over a period of 4 weeks (Huck et al., 2018). Elevated levels of ALT were reported for rats after treatment with 1 mg/kg bw per day for 4 weeks (Han et al., 2018a,b). This was associated with increased serum levels of the pro‐inflammatory cytokines TNFα and IL6, elevated content of malondialdehyde (a lipid peroxidation product), a decrease in glutathione and increased occurrence of macrophages in the liver. This may indicate hepatocellular lipid peroxidation and damage, followed by a pro‐inflammatory response in the affected organ. Similar alterations were observed by Lv et al. (2018) and Su et al. (2019), after application of 10 mg/kg bw per day for 3 weeks to mice. At doses of approximately 0.6 mg/kg bw, applied every second day over a period of a month, DNA fragmentation became obvious, which may be a primary effect of PFOS or secondary due to induction of apoptosis or necrosis of hepatocytes (Eke et al., 2017). This is substantiated by the observation that PFOS induces caspase‐3 cleavage in the liver (Lv et al., 2018). Huck et al. (2018) reported on hepatic steatosis and elevated levels of blood glucose and serum cholesterol and triglycerides in mice, exposed to 1 mg PFOS/kg bw per day for 6 weeks. Similar alterations were seen when animals were kept on a high‐fat diet. Mechanistic studies revealed that PFOS affected the expression of lipid trafficking genes, such as CD36, the major lipid transporter of cells. The combination of high‐fat diet with PFOS, however, reduced steatosis and hepatomegaly probably by leaving the expression of genes of the lipid‐transport machinery more or less unchanged.
In the NTP study (NTP, 2019b), increased absolute and relative liver weights were observed in both male and female SD rats at 0.312 mg/kg bw per day. At 0.312 mg PFOS/kg bw per day, serum levels of free and bound T4 were reduced in both sexes, and T3 levels at 0.625 mg/kg bw per day and higher. In males, cholesterol blood levels were lowered from the lowest dose upwards (0.312 mg/kg bw per day), in females only significantly at the highest dose of 5 mg/kg bw per day. Serum levels of PFOS were similar in males and females. In the histopathological evaluation in both sexes, decreased extramedullary haematopoiesis and hypocellularity became evident at 1.25 mg/kg bw per day and hepatocellular alteration (hypertrophy and/or cytoplasmic alterations) at 2.5 mg/kg bw per day.
Lai et al. (2018) studied the impact of PFOS on the energy metabolism and the gut microbiome by feeding PFOS at 0.3 or 3 mg/kg bw per day to female CD mice over a period of 7 weeks. The authors noted that PFOS treatment elicited disturbances in the lipid and glucose metabolism and changes in the abundance of metabolism‐associated bacteria belonging to the phyla Firmicutes, Bacteroidetes, Proteobacteria and Cyanobacteria in the gut. This was associated with alterations in the gut metabolism of amino acids, methane and butanoate.
In summary, an elevated absolute and relative liver weight was the most sensitive endpoint for PFBS, PFHxS and PFOS. No repeated dose toxicity studies were available for PFHpS and PFDS. Disturbed lipid metabolism, necrosis and inflammation in the liver were mostly seen at higher dose levels. Also disturbed thyroid hormones and alterations in the kidney (PFBS only) were documented. For details, see Appendix E.
3.3.3.2.3. Other PFASs
The available repeated dose studies for 8:2 FTOH and EtFOSE are listed in Appendix E, Table E.3. For FOSA and EtFOSA, no studies have been identified.
Table E.3.
Repeated dose toxicity studies for further PFASs
| Substance (Purity) | Species/Experimental design and doses | Observed effect | Highest dose with no effects (mg/kg bw per day) | Lowest dose with effect (mg/kg bw per day) | Serum/tissue level of compound | Reference |
|---|---|---|---|---|---|---|
| N:2 fluorotelomer alcohols (n:2 FTOHs) | ||||||
| 8:2 FTOH (commercial source, purity not specified) |
Wistar rat (m) No/sex/group: 4 Duration: 14 days Diet: 0, 0.2, 0.4, or 0.8% corresponding to 0, 240, 480, or 960 mg/kg bw per day(a) |
Incr rel liver weight Decr of 16:0 fatty acid in liver |
240 240 |
Iwase et al. (2006) | ||
| 8:2 FTOH (99.2% purity) |
Crl:CD(SD)IGS BR rat (m/f) No/sex/group: 10 Duration: 90 days Gavage: 0, 1, 5, 25, or 125 mg/kg bw per day |
Focal hepatic necrosis (m) Incr rel liver weight (m/f) Incr abs liver weight (f) Decr RBC, m Incr serum cholesterol, (f) Incr hepatic perox ß‐oxidation (f) Incr urinary fluorides (m) Incr urinary fluorides (f) |
5 5 5 5 25 5 1 5 |
25 25 25 25 125 25 5 25 |
Ladics et al. (2008) | |
| 8:2 FTOH (> 97% purity) |
C57Bl/6 mice (m) No/sex/group: 6 Duration: 28 days Gavage: 0, 10, 30, or 100 mg/kg bw per day |
Incr abs& rel liver weight Incr abs thymus weight Serum SOD activity Serum GSH content |
10 10 10 10 |
Wang et al. (2018b) | ||
| N‐ethyl perfluorooctanesulfonamidoethanol (EtFOSE) | ||||||
| EtFOSE (> 99% purity) |
SD rat (f) No/sex/group: 9 Duration: 5 days/week for 3 weeks Gavage: 0 or 5 mg/kg bw/day |
Decr body weight gain Incr rel liver weight Incr rel spleen weight Incr catalase activity (uterus) Decr total GPx activity (uterus) Incr CuZn‐SOD activity (uterus) Incr CuZn‐SOD and MnZn‐SOD activity (liver) |
5 5 5 5 5 5 5 |
Serum concentr. (ng/mL) 177+/−86 |
Xie et al. (2009) | |
bw: body weight; f: female; m: male; GSH: glutathione; GPx: glutathione peroxidase; RBC: red blood cells; SOD: superoxide dismutase.
Applying default values, provided by EFSA.
Iwase et al. (2006) applied 8:2 FTOH at 240, 480 or 960 mg/kg bw per day via diet to male rats for a period of 2 weeks. The treatment elevated dose‐dependently the relative liver weight and the proportion and content of 18:1 (n‐9) fatty acid due to the induction of palmitoyl‐CoA chain elongase and stearoyl‐CoA desaturase. As a result, there was a relative decrease in 16:0 fatty acid in the liver starting at a dose of 240 mg/kg bw per day. The body weight remained unaffected. Ladics et al. (2008) treated rats with 0, 1, 5, 25 or 125 mg/kg bw per day for 90 days. There were no treatment‐related alterations in body weight, food consumption or neurobehavioural parameters. The liver was the main target organ, as indicated by increased organ weight and focal necrosis, peroxisome proliferation and induction of hepatic β‐oxidation at 25 mg/kg bw per day and above. Chronic progressive nephrotoxicity was observed in females at 125 mg/kg bw per day. The authors derived an NOAEL of 5 mg/kg bw per day. Wang et al. (2018b) applied 8:2 FTOH to adult male C57BL/6 mice at 10, 30 and 100 mg/kg bw per day by gavage for 28 days. Liver toxicity became evident by increased absolute and relative liver weights and histological changes comprising vacuolation, cell swelling, immune cell infiltration, karyopyknosis and nuclear swelling. Thymus and spleen were hardy affected. 8:2 FTOH reduced the concentration of glutathione and the activity of superoxide dismutase (SOD) in the serum.
Xie et al. (2009) treated female rats with EtFOSE at 5.0 mg/kg bw per day for a period of 21 days (once daily by gavage for 5 days per week, no administration on days 6 and 7). The EtFOSE metabolite levels in liver and serum decreased in the order PFOS > FOSA and N‐ethyl perfluorooctanesulfonamidoacetate > perfluorooctanesulfonamidoethanol and EtFOSE. EtFOSE treatment lowered the body growth rate, increased relative liver weight and activity of catalase (uterus), superoxide dismutases (CuZnSOD and MnSOD; liver and uterus) and lowered the activity of glutathione peroxidase (GPX, uterus). Peroxisomal ß‐oxidation activity was elevated on average by 16‐fold but did not reach significance. Absolute weights of liver and absolute and relative weights of uterus, kidney and spleen remained unaffected.
In summary, repeated dose studies were available for 8:2 FTOH and EtFOSE, while for FOSA and EtFOSA, no studies have been identified. 8:2 FTOH treatment increased dose‐dependently the relative liver weight and hepatic β‐oxidation. Liver toxicity became evident by histological changes, comprising vacuolation, cell swelling, immune cell infiltration, karyopyknosis and nuclear swelling. Several EtFOSE metabolites occurred in liver and serum, with PFOS and FOSA being predominant. EtFOSE treatment lowered the body growth rate and increased the relative liver weight. Peroxisomal ß‐oxidation activity was elevated insignificantly. For details, see Appendix E.
3.3.3.3. Developmental and reproductive toxicity
The previous Opinion (EFSA CONTAM Panel, 2018) documented reproductive and developmental toxicity studies for PFOS and PFOA, which have been published between 2008 and 2016. These studies are included in Appendix F (Tables F.6–F.8), in which also some key studies evaluated by EFSA in the PFOS and PFOA Opinion in 2008 (EFSA, 2008) are included. Additional studies (Abbott et al., 2009; Keil et al., 2008; Suh et al., 2011), not reviewed in EFSA CONTAM Panel (2018), were identified based on the literature list in the previously reviewed studies and these are also included in Appendix F, Tables F.6 and F.7.
Table F.6.
Perfluorooctanesulfonic acid (PFOS) reproductive and developmental toxicity studies
| Substance/(Purity) | Species/Experimental design and doses | Most sensitive endpoints | Highest dose with no effect (mg/kg bw per day) | Significant effect level (mg/kg bw per day) | Serum/tissue levels of compound | Reference | ||
|---|---|---|---|---|---|---|---|---|
| Mother | Offspring | Mother | offspring | |||||
|
PFOS (91% K+‐Salt) ‘Our analysis indicated that approximately 71% of the chemical was straight‐chain, and the remaining 29% was branched. Additional analysis indicated that the chemical obtained from Fluka appeared to be identical to that produced by 3M.’ |
Sprague‐Dawley rats Exposure: 0, 1, 2, 3, 5, 10 mg/kg per day per gavage GD2–GD21 |
Decreased postnatal survival Decreased growth in surviving pups Delay in eye opening Decreased thyroxin No consistent change in liver weight or relative liver weight in surviving pups PND 0–35 Maternal effects not assessed |
1 (BMDL05 0.58) 1 1 1 |
2 2 2 2 |
Read from figures. Serum in pups at PND 1: Control: 0 1 mg/kg: 36 μg/mL 2 mg/kg: 71 μg/mL 3 mg/kg: 85 μg/mL 5 mg/kg: 108 μg/mL Slightly lower at PND 5 Liver in pups at pnd 1: Control: 0 1 mg/kg: 45 μg/g 2 mg/kg: 68 μg/g 3 mg/kg: 100 μg/g 5 mg/kg: 160 μg/g |
Lau et al. (2003) | ||
|
PFOS (86.9%, K+‐Salt. Lesser homologues (C4–C7) at 8.4%; impurities (by quantitative 19F Nuclear Magnetic Resonance) at 1.9%; metals (calcium, magnesium, sodium, nickel, and iron) at 1.5%; inorganic fluoride at 0.6%; perfluorooctanoic acid at 0.3%; nonofluoropentanoic acid at 0.3%; heptafluorobutyric acid at 0.1% |
Sprague‐Dawley rats, 20 dams per group. Exposure: 0, 0.1, 0.4, 1.6, and 3.2 mg/kg bw per day by gavage for 6 weeks prior to mating, during mating and through gestation and lactation, across two generations for females (only 0, 0.1, 0.4 mg/kg bw per day continued to F2). Cross fostering (0 and 1.6 mg/kg bw per day) as follow‐up study |
F0 males and females: reduced bw gain. In F0 shorter gestation, lower n implantation sites, increase in stillborn pups or early neonatal death of litter. F1 reduced survival and bw gain. Delayed eye opening F1 Pre‐ and postnatal exposure additive to pup toxicity |
0.4 1.6 0.4 0.1 |
1.6 3.2 1.6 0.4 |
Serum levels (μg/mL) from cross foster study at end of lactation. Control litter nursed by control dams:< 0.05. Control litter nursed by treated dam: 22.4 Treated litter nursed by control dam: 53.9 Treated litter nursed by treated dam: 89.7 Control dams nursing control litter: < 0.05 Treated dams nursing control litter: 83.0 Control dams nursing treated litter: 2.02 Treated dams nursing treated litter: 89.0 |
Luebker et al. (2005a) | ||
|
PFOS (86.9%, K+‐Salt. Impurities as in Luebker 2005a |
Sprague‐Dawley rats, 35 dams per group. Exposure: 0, 0.4, 0.8, 1.0, 1.2, 1.6, 2,0 mg/kg bw per day by gavage from 6 weeks prior to mating to day four of lactation |
Decrease in gestation length Decrease in postnatal survival day 5 Mean pup weight at birth Increase in serum T4 on lactation day 5 |
BMDL05 0.31 |
BMDL05 0.89 BMDL05 0.39 |
0.4 | 0.4 |
Measured in dams GD 1, 7, 15 and 21. Constant GD 1–15, drop at GD 21 Serum levels GD 1–15 (μg/mL): 0.1 mg/kg: 7.8–8.9 0.4 mg/kg: 40.7–41.4 1.6 mg/kg: 154–160 3.2 mg/kg: 275–318 |
Luebker et al (2005b) |
| PFOS (purity 98%, salt not specified) |
Sprague‐Dawley rats Exposure: 0, 0.1, 0.6, 2 mg/kg per day per gavage GD2–GD21 Dams: 10 per group (not studied) Follow up offspring: Neonatal survival PND 4 (all), survivors until PND 21: 6 per group |
Increased mortality of offspring at 2 mg/kg per day Reduced body weight offspring Increased heart to body weight ratio at PND 21 Cardiac mitochondrial injury |
0.6 0.6 0.6 0.6 |
2 2 2 2 |
Serum PND21, dose dependent increase: 4.26 μg/mL at 2 mg/kg per day Heart, dose‐dependent increase: 9.59 μg/g at 2 mg/kg per day |
Xia et al. (2011) | ||
| PFOS, purity not specified |
Sprague‐Dawley rats Exposure: 0, 5, 20, mg/kg per day by oral gavage, GD 11‐19 n = 4 per group |
At GD 20: Decreased body weight Decreased testis weight and liver HDL‐cholesterol Decreased body weight Decreased testis weight Change anogenital distance Increased apoptosis rate in testicular cells Decreased number of Leydig cells Decreased testis testosterone Decreased testis progesterone |
5 |
5 n.d. 5 5 5 5 5 5 |
20 |
20 5 20 20 20 20 20 20 |
Not reported | Zhao et al. (2014) |
| PFOS (98% K+‐Salt) |
ICR mice 15 dams/group (5 each selected for specific endpoints Exposure: 0, 1, 10, 20 mg/kg per day, GD 1–17/18 |
Reduced weight gain Increased liver weight Liver hypertrophy Decreased neonatal survival Developmental/teratological alterations Sternal defects |
10 1 10 |
1 1 n.d |
20 10 20 |
10 10 1 |
Not reported | Yahia et al. (2008) |
| PFOS, 91%, K+‐Salt |
B6C3F1 mice Exposure: GD 1–17, 0, 0.1, 1.0, 5.0 mg/kg bw per day by gavage. |
Decrease in NK cell activity at 8 weeks Decrease in IgM production assessed by PFC assay at 8 weeks (spleen) |
0.1 (males) 1 (females) 1 (males) 5 (females) |
1 (males) 5 (females) 5 (males) |
Not reported | Keil et al. (2008) | ||
| PFOS (purity 98%, K+‐salt) |
ICR mice Exposure 0, 9, 13, 20, 30 mg/kg bw per day by gavage GD 1–17 (3 dams/group). 20 mg/kg bw per day GD 1–17 and 50 mg/kg per day GD11–15. 5–8 dams per group 67–103 fetuses: (examined animals, total number higher) 20 mg/kg per day GD 1–15/18 for histology. |
Sharp increase in cleft palate between 13 (7.3%) and 20 (78.35) mg/kg per day |
13 50% effective dose expected 17.7 mg/kg per day |
20 |
Serum level on GD17 (μg/mL) reported for dams at 30 mg/kg per day and other values estimated from figure: 9 mg/kg bw per day: 58 in dam, 62 in fetus. 13 mg/kg bw per day: 105 in dam and fetus. 20 mg/kg bw per day: 135 in dam and fetus. 30 mg/kg bw per day: 162.3 in dams and 130 in fetus. 50% effective fetal serum concentration for cleft palate: 121 μg/mL |
Era et al. (2009) | ||
| PFOS (K+‐salt, > 91% pure) |
129S1/Svlm WT and PPARαa KO. Exposure: Gavage, (WT) 0, 4.5, 6.5, 8.5, 10.5 mg/kg bw per day, (KO) 0, 8.5 10.5 mg/kg per day GD 15–18. Number of dams varied from 8 (WT. 4.4 mg) to 20 (WT control) |
Postnatal death Delayed eye opening (Litter loss not affected) |
4.5 |
4.5 6.5 |
Measured at PND15 in adult females with and without pups and in pups | Abbott et al. (2009) | ||
| PFOS (98% K+‐Salt) |
C57BL/6J‐Apc+/+ female mated with C67BL6J‐Min/+ males (n=20/21) Exposure: 0, 0.1, 3 mg/kg per day experimental block 1 per drinking water 0, 0.01, 0.1, 3 mg/kg per day experimental block 2 per drinking water GD 1–17 ApcMin/+ genotype mice were terminated at 11 weeks of age for tumourigenesis. Wild‐type mice were kept until week 20 for obesogenic effects |
No obesogenic effects, no intestinal tumourigenesis Comparative study approach revealed that mild toxicity effects seen for PFOA did not occur in response to PFOS |
3 |
0.1 mg/kg PFOS: 2.2/2.7 μg/mL in GD 18 Dams, 0.48/0.54 μg/mL dams after weaning and 0.38/0.3 μg/mL pups after weaning 3.0 mg/kg PFOS: 36.6/44.6 μg/mL in GD 18 dams; 17.2/22.2 μg/mL Dams after weaning and n.d in pups after weaning |
Ngo et al. (2014) | |||
| PFOS (purity 98%, salt not specified) |
CD1 mice Exposure: 0, 0.3, 3 mg/kg per day GD1–PND21 by gavage. Then no dosing in offspring until sacrifice at PND 63. Dams: 6 per group (sacrifice after weaning (PND 21)) Offspring: animals per treatment equally distributed in a low and a high fat feeding group. Termination on PND 63 |
Increased liver weight Relative liver weight increase Increased HOMA‐IR Elevated fasting glucose Additional effects related to high fat diet, but only results from normal diet included here |
3 0.3 |
0.3 (male) 0.3 3 3 (PND 21) |
3 0.3 |
3 (male) 3 0.3 (PND 63) |
Serum levels in dams at 0, 0.3 and 3 mg/kg per day: 0.25, 15.3 and 131.7 μg/mL. Liver levels in dams at 0, 0.3 and 3 mg/kg per day: 0.15, 49.1 and 338.9 μg/g. Serum levels in pups PND21 at 0.3 mg/kg per day: 12.7 μg/mL in males and 11.4 μg/mL in females Serum levels at 3 mg/kg per day: 98.7 μg/mL in males and 87.2 μg/mL in females Liver levels in pups PND21 at 0.3 mg/kg per day: 20.1 μg/g in males and 18.0 μg/g in females Liver levels at 3 mg/kg per day: 243 μg/g in males and 178 μg/g in females |
Wan et al. (2014) |
| PFOS (source, purity and salt not specified) |
Sprague‐Dawley rats Exposure: 18.75 mg/kg per day GD 2–6 by gavage 10–12 Offspring animals per group |
Delayed weight gain Reduced birth weight both sexes Increase blood pressure in male offspring from PND7 to PND52 Increase blood pressure in female offspring from PND37 to PND65 |
n.d. n.d. n.d. n.d. |
18.75 18.75 18.75 18.75 |
Rogers et al. (2014) | |||
| PFOS (purity not specified, K+‐salt) |
CD1 mice Exposure: Gavage, 0, 0.5, 2, 8 mg/kg per day, GD11–GD16 10 dams per group |
Body weight decrease Dose‐dependent decrease of placental weight and capacity Dose‐dependent increase of number of resorptions and dead fetuses Decrease in the numbers of glycogen trophoblast cells in the junctional zone and the number of sinusoidal trophoblast giant cells in the labyrinth zone Decrease of mPL‐II, mPLP‐Cα and mPLP‐K expression levels and serum concentrations |
2 |
n.d n.d. n.d. n.d. |
8 |
0.5 0.5 0.5 0.5 |
Not reported | Lee et al. (2015) |
| PFOS, purity not given |
Kunming mice. Exposure: 0, 0.5, 5 mg/bw per day, intragastric, from GD1 to parturition, 5 dams per group |
Increased liver weight and triglycerides Changes in liver lipid homeostasis |
0.5 0.5 |
5 5 |
Not given | Liang et al. (2019) | ||
n.d: not determined; BMDL: benchmark dose limit; bw: body weight; GD: gestation day; HDL: high‐density lipoprotein; HOMA‐IR: Homeostatic Model Assessment of Insulin Resistance; IgM: immunoglobulin M; NK: natural killer (cell); PFC: perfluorinated compound; PFOA: perfluorooctanoic acid; PND: postnatal day.
Note: Studies are in chronological order with studies in rats listed before studies in mice.
Table F.8.
Perfluorooctanoic acid (PFOA) developmental toxicity studies with exposure in pubertal or adult animals
| Substance/ (Purity) | Species/Experimental design and doses | Most sensitive endpoints | Highest dose with no effect (mg/kg bw per day) | Significant effect level (mg/kg bw per day) | Serum/tissue levels of compound | Reference |
|---|---|---|---|---|---|---|
| PFOA ammonium salt, 98% pure |
BALB/c and C57BL/6 mice (comparative approach); n = 20 0, 1, 5, 10 mg/kg bw per day, 5 days per week Exposure: PND 21 for 28 days (pubertal period) |
Increase of relative liver weight in both strains Delayed vaginal opening Balb/c mice Delayed vaginal opening C57BL/6 mice Dose‐dependent decrease uterine wet weight in Balb/c mice Increase of uterine wet weight for C57/BL6 mice Reduction of ductal length, number of terminal end buds and number of terminal ducts in Balb/c mice Increase of mammary gland parameters in C57/BL6 mice |
n.d. n.d. 1 n.d. n.d. 1 n.d. |
1 1 5 1 1 5 1 |
BALB/c at 0, 1, 5 ng/kg bw per day: < 10, 29,500, 109,000 ng/mL. C57BL/6 at 0, 1, 5, 10 ng/kg bw per day: < 10, 26,000, 68,200, 96,600 ng/mL |
Yang et al. (2009) |
| PFOA ammonium salt, 98% pure |
129/Sv wild‐type, PPARα knockout mice, humanised PPARα mice, adult 0, 1, 5 mg/kg per day, n = 8–10, 42 days |
Sperm abnormalities, decreased testosterone level | n.d. | 1 | Li et al. (2011) | |
| PFOA ammonium salt, 98% pure |
Exposure: PND 21 for 28 days (pubertal period). 5‐10 mice per group. Dosing 5 days a week Balb/c: 0 and 2.5 mg/kg bw per day C57Bl/6: 0 and 7.5 mg/kg bw per day |
Reduction in mammary gland development (reduced ductal length, number of terminal end buds, stimulated terminal ducts) |
2.5 7.5 |
Balb/c < 10, 51,100 C57Bl/6 < 10, 93,400 |
Zhao et al. (2012)1 | |
| PFOA ammonium salt, 98% pure |
CD1 mice PND18, three day uterotrophic assay 0, 0.01, 0.1, 1 mg/kg bw per day (n = 8) |
1.46‐fold increase of uterine wet weight. Further supported by histopathological examination | n.d. | 0.01 | Dixon et al. (2012) | |
| PFOA ammonium salt, 98% pure |
BALB/c mice, adult 0, 0.31, 1.25, 5, 20 mg/kg bw per day, n = 16, 28 days |
Sperm count reduced, sperm motility, sperm progression increased, percentage of teratosperm increased Testosterone and progesterone levels decreased Mild phenotype in seminiferous tubules |
1.25 0.31 0.31 |
5 1.25 1.25 |
Zhang et al. (2014b) | |
| PFOA, > 98% pure |
Sprague‐Dawley rats, 10–11 weeks old at start. 0, 0.625, 1.25, 2.5, 5, 10 mg/kg bw per day, N = 10 per sex per group 28 days |
Reduced epidydimal weight Reduced epidydimal sperm count |
5 5 |
10 10 |
Plasma conc. (ug/mL) at 0.625 mg/kg bw per day: 50.7 + 2.2 (m) at 5 mg/kg bw per day: 110.7 + 3.8 (m) at 6.25 mg/kg bw per day: 491 + 72.1 (f) Liver conc (ug/g) at 0.625 mg/kg bw per day 54.6 + 2.2 (m) |
NTP (2019a) |
n.d.: not determined from the study; bw: body weight; f: female; m: male; PND: postnatal day.
1Concentration in serum extracted from figure in Zhao et al. (2012).
Table F.7.
Perfluorooctanoic acid (PFOA) reproductive and developmental toxicity studies with pre‐ and perinatal exposure
| Substance/ (Purity) | Species/Experimental design and doses | Most sensitive endpoints | Highest dose with no effect (mg/kg bw per day) | Significant effect level (mg/kg bw per day) | Serum/tissue levels of compound | Reference | ||
|---|---|---|---|---|---|---|---|---|
| Mother | offspring | Mother | offspring | |||||
| PFOA ammonium salt, 98% pure | CD1 mice, GD1‐birth, 0, 1, 3, 5, 10, 20, 40 mg/kg per day |
Liver weight resorption of litters, reduced percentage of live fetuses, reduced weight of fetuses, reduced postnatal survival and growth deficits (ossification) |
n.d. | 1 | 1 | 3 |
Maternal serum levels (ng/mL) at term (estimated from figure). 0: n.d. 1: 20,000 3: 40,000 5: 70,000 10: 110,000 20: 170,000 40: 260,000 |
Lau et al. (2006) |
| PFOA ammonium salt, 97,99% pure | 129S1/SvlmJ wild‐type and PPARα knockout mice, 0.1, 0.3, 0.6, 1, 3, 5, 10, 20 mg/kg per day, GD 1–17. Results for WT |
Relative liver weight Relative liver weight Litter loss Postnatal survival |
0.6 |
n.d. 0.3 0.3 |
1 |
0.1 0.6 0.3 |
P0 with no pups, PND22 serum at 0, 0.1, 0.3, 0.6 and 1 mg/kg per day: 131, 4,400, 10,400, 17400 and 26,300 ng/mL P0 with pups, PND22 serum at 0, 0.1, 0.3, 0.6 and 1 mg/kg per day: 33.2, 1,600, 2,840, 5,170, 9,290 ng/mL Pups at PND22 serum at 0, 0.1, 0.3, 0.6 and 1 mg/kg per day: 17.3, 798, 2,150, 3,810, 9,860 ng/mL |
Abbott et al. (2007) |
| PFOA ammonium salt, 98% pure |
CD1 mice GD1–17, GD8–17, GD12–17: 0 and 5 ng/kg bw/day |
Reduced bw Decreased weaning‐induced mammary involution (P0) Mammary gland development retardation irrespective of the timing of exposure |
5 |
5 5 |
White et al. (2007) | |||
| PFOA ammonium salt, 98% pure |
CD1 mice Full gestational study (GD 1–17): 0 (n = 48), 3 (n = 28), 5 (n = 36) mg/kg bw (pregnant dams) cross‐fostering during lactational exposure leading to 7 groups. GD 8–17: 0 (n = 56), 5 (n = 56) ng/kg bw (pregnant dams). Cross‐fostering during lactational exposure. Early life effects restricted gestational (GD 7,10,13,15–17) exposure study at 5 mg/kg per day |
Mammary gland development retardation irrespective of the timing of exposure | n.d. |
3 5 5 |
Serum levels (ng/mL). Exposure GD 8‐17, dams on lactation day 1,3,5,10: Control dams nursing control pups: 11, 6, 4, 0. Control dams nursing treated pups: 26, 1,300, 6,510, 5,000. Treated dam nursing control pups: 47,900, 39,300, 34,000, 16,400. Treated dam nursing treated pups: 42,200, 43,000, 38,800, 24,400. Pups on PND 1, 3, 5, 10 (exposure GD 8–17) and PND 22, 42, 63 (exposure GD 1–17). Control pup nursed by control dam: 23, 16, 10, 8, nd, nd, nd. Treated pup nursed by control dam: 66,200, 43,900, 33,100, 20,500, 8,300, 646, nd. Control pups nursed by treated dam: 1,560, 10,500, 15,000, 15,700, 12,100, 894, nd. Treated pups nursed by treated dam: 70,000, 54,400, 52,200, 31,300, 21,900, 4,050, 500. |
White et al. (2009)1 | ||
| PFOA, ammonium salt > 98% pure |
CD‐1 mice Exp. 1: 0,1, 3, or 5 mg/kg; n = 5, 5, 8, 7 dams Exp. 2: 0, 0.01, 0.1, 0.3, 1 or 5 mg/kg; n = 14 dams except for 5 mg/kg n = 10 Exposure GD1–17 |
Transient body weight gain at mid age observation group of the pups (21–33 weeks) Increase of insulin and leptin 0.01–0.1 mg/kg. |
n.d. n.d. |
0.01– 0.3 0.01 |
Hines et al. (2009) | |||
| PFOA, purity 90%, salt unknown | ICR mice, 15–19 dams/group, 1, 5, 10 mg/kg per day, exposure GD1–17/18 |
Dose dependent liver weight gain, Significant change of 12 out of 20 metabolic parameters with phosphorus and urea levels being most sensitive Decreased neonatal survival |
1 n.d. |
1 |
5 1 |
5 | Yahia et al. (2010) | |
| PFOA, ammonium salt > 98% pure |
CD1 mice Full gestational study (GD 1–17): 0, 0.3, 1, 3 mg/kg per day, dams (n = 13) Late gestational study (GD 10–17): 0, 0.01, 0.1, 1.0 mg/kg per day, dams (n = 7–13) Offspring: 7–9 animals per litter |
Full gestational exposure: Transient (until PND7) increase in liver weight Decreased mammary gland developmental score Late gestational exposure: decreased mammary gland developmental score number of terminal end buds |
n.d n.d. n.d. 0.01 |
0.3 0.3 0.01 0.1 |
Full gest. exposure, female serum at 0.3 mg/kg per day: 4,980 (PND7)–16 ng/mL (PND84) Liver at 0.3 mg/kg per day: 2,078 (PND7)–43 ng/g (PND84) Late gest. exposure: Control 22.6 (PND1)–4.1 ng/mL (PND21) Female serum at 0.01 mg/kg per day: 284.5 (PND1)–16.5 ng/mL (PND21) Serum at 0.1 mg/kg per day: 2,303.5 ng/mL (PND1)–131.7 ng/mL (PND21) More concentration data in supplementary |
Macon et al. (2011) | ||
| PFOA, ammonium salt, purity not stated |
CD‐1 mice, 10 dams per group, 2, 10, 25 mg/kg bw/day, GD 11–16. Sacrificed on GD 16 |
No of resorptions and dead fetuses Decrease of placental weight Decrease in trophoblast cells in the placenta Decrease of mPL‐II, mPLP‐Cα and mPLP‐K expression levels and serum concentrations |
10 |
2 2 25 2 |
Suh et al. (2011) | |||
| PFOA, ammonium salt 98% pure |
Pregnant CD‐1 mice receiving 0, 1, 5 mg/kg bw per day PFOA by oral gavage from GD1–17. Pregnant CD‐1 mice receiving 0 and 1 mg/kg bw per day PFOA by gavage from GD1–17 and additional drinking water containing 5 μg/L (0.00045 mg/kg bw per day) of PFOA from GD7 until termination of the experiment for P0, F1 and F2 generations Dams: n = 5–12 Litter size neonates F1: 12–13 pups Litter size neonates F2: 10 pups |
Prenatal loss (P0) Decreased weaning‐induced mammary involution (P0) Postnatal survival (F1) |
1 | 1 |
5 0.00045 (5 μg/L in water) |
5 |
P0 dams at weaning (PND 22) Control: 4.0 ng/mL Control + 5 μg/L in water: 74.8 ng/mL 1 mg/kg bw per day: 6,658 ng/mL 1 mg/kg bw per day + 5 μg/L in water: 4,772 ng/mL 5 mg/kg/ bw per day: 26,980 ng/mL |
White et al. (2011) |
| F1 developmental indices mammary gland without PFOA in drinking water (PND 22, 42, 63) | n.d. | 1 |
F1 pups PND 22 Control 0.6 ng/mL Control + 5 μg/L in water: 21.3 ng/mL 1 mg/kg bw per day: 2,444 ng/mL 1 mg/kg bw per day + 5 μg/L in water: 2,744 ng/mL 5 mg/kg bw per day: 10,045 ng/mL |
|||||
| F1 developmental indices mammary gland with PFOA in drinking water (PND 22, 42, 63) | n.d. | 0.00045 5 μg/L in water |
F1 pups PND 42 Control: 1.4 ng/mL Control + 5 μg/L in water: 48.9 ng/mL 1 mg/kg bw per day: 610 ng/mL 1 mg/kg bw per day + 5 μg/L in water: 558 ng/mL 5 mg/kg bw per day: 1,581 ng/mL |
|||||
| F1 maternal indices mammary gland without PFOA in drinking water (PND 10) | n.d. | 1 |
F1 pups PND 63 Control 3.1 ng/mL Control + 5 μg/L in water: 66.2 ng/mL 1 mg/kg bw per day: 211 ng/mL 1 mg/kg bw per day + 5 μg/L in water: 187 ng/mL 5 mg/kg bw per day: 760 ng/mL |
|||||
| F1 maternal indices mammary gland with PFOA in drinking water (PND 10) | n.d. | 0.00045 5 μg/L in water |
F1 dams at weaning (PND 22) Control 2.0 ng/mL Control + 5 μg/L in water: 86.9 ng/mL 1 mg/kg bw per day: 9.3 ng/mL 1 mg/kg bw per day + 5 μg/L in water: 173 ng/mL 5 mg/kg bw per day: 18.7 ng/mL |
|||||
| F2 developmental indices mammary gland without PFOA in drinking water (PND 63) | n.d. | 1 |
F2 pups PND 22 Control 0.4 ng/mL Control + 5 μg/L in water: 26.6 ng/mL 1 mg/kg bw per day: 4.6 ng/mL 1 mg/kg bw per day + 5 μg/L in water: 28.5 ng/mL 5 mg/kg bw per day: 7.8 ng/mL |
|||||
| F1 developmental indices mammary gland with PFOA in drinking water (PND 42) | n.d. | 0.00045 5 μg/L in water |
F2 pups PND 42 Control 0.7 ng/mL Control + 5 μg/L in water: 57.4 ng/mL 1 mg/kg bw per day: 0.4 ng/mL 1 mg/kg bw per day + 5 μg/L in water: 72.8 ng/mL 5 mg/kg bw per day: 0.4 ng/mL |
|||||
|
F1 pups PND 63 Control: 1.1 ng/mL Control + 5 μg/L in water: 68.5 ng/mL 1 mg/kg bw per day: 1.1 ng/mL 1 mg/kg bw per day + 5 μg/L in water: 69.2 ng/mL 5 mg/kg bw per day: 1.2 ng/mL |
||||||||
| PFOA ammonium salt, 98% pure | CD‐1 mice, 5 dams/group, 5 mg/kg bw per day, exposure GD 1–17 | Decreased neonatal survival | n.d. | 5 | Abbott et al. (2012) | |||
| PFOA, purity not stated |
Sv/129 Mice WT, mPPARα KO and expressing hPPARα GD 1–17 3 mg/kg bw per day. Results for WT (5–6 dams per group) |
Decrease postnatal survival Decrease mammary development |
3 | 3 | 19,000 ng/mL | Albrecht et al. (2013) | ||
| PFOA ammonium salt, 98% pure |
C57BL/6J‐Apc+/+ female mated with C67BL6J‐Min/+ males 10–24 dams/group 0.1 and 3 mg/kg bw per day (study 1) and 0.01 and 0.1 mg/kg bw per day (study 2) Exposure: GD 1 – 14–18 |
Decreased neonatal survival (not detectable for PFOS) Small increase of liver weight at 0.01 mg/kg per day, but not at 0.1 mg/kg per day |
0.1 n.d. |
3 0.01 (not detectable at 0.1) |
Serum levels in pups at 0, 0.01/0.1/3 mg/kg bw per day: < 0.05, 12–26, 213–216/n.d. ng/mL | Ngo et al. (2014) | ||
| PFOA ammonium salt, 98% pure |
CD‐1 mice, 12, 12, 14, 13, 12, and 6 pregnant dams resulting in 29, 29, 37, 26, 31, and 21 female offspring, exposure 0.01, 0.1, 0.3, 1, 5 mg/kg bw per day, GD 1 –17 129/Sv WT mice, 7, 7, 5, 3, and 5 pregnant dams resulting in 10, 10, 8, 6, and 8 female offspring, exposure 0, 0.1, 0.3, 0.6, 1 mg/kg bw per day, GD 1–17 129/Sv PPARα ko mice, 5, 9, 8, 7, and 9 pregnant dams resulting in 6, 10, 10,9 and 9 female offspring, exposure 0.1, 0.3, 1, 3 mg/kg bw per day, GD1–17 Investigation at 18 months |
CD1 mice: Several non‐neoplastic alterations in livers 129/Sv mice: bile duct hyperplasia in 129/Sv PPARα knockout, but not wild‐type mice liver lesions can occur independent of PPARα |
1 1 |
5 5 |
Filgo et al. (2014) | |||
| PFOA, ammonium salt 98% pure |
CD‐1 mice, 163 dams equally distributed to treatment groups, 6–7 females and 3–4 males per litter after birth C57Bl/6 mice, 41 dams divided to 5 groups, litter sizes > 5 were maintained Exposure 0, 0.01, 0.1, 0.3 1.0 mg/kg bw per day, GD1–17 |
CD‐1 mice: Decrease of mammary gland development score C57Bl/6 mice: Decrease of mammary gland development score |
0.01 (PND21) n.d. (PND 35, PND 56) 0.1 (all time points) |
0.1 (PND21) 0.01 (PND 35, PND 56) 0.3 (all time points) |
CD‐1: Female pups, PND 21 serum at 0, 0.01, 0.1, 0.3 and 1 mg/kg bw per day: < 5, 74.8, 457.3, 904.8, 3,119 ng/mL. C57Bl/6: Female pups, PND 21 serum at 0, 0.01, 0.1, 0.3 and 1 mg/kg bw per day: < 10, 26.1, 247.1, 891.3, 2,141.7 Concentrations in CD‐1 at PND 35 and 56 and in B6 at PND 61 available |
Tucker et al. (2015) | ||
| PFOA Na+ Salt > 99% |
Female C57BL/6J mice mated with male FVB mice. Supplemented through feed: 0, 0.017, 0.056, 0.17, 0.56, 1.7, 5.6 and 17 mg/kg bw per day. Corresponding exposure: 0, 3, 10, 30, 100, 300, 1,000 and 3,000 μg/kg bw per day. Feeding to females was started 2 weeks prior to mating and maintained through mating, gestation and lactation (6 F0 females) Follow up of 9 offspring animals per sex into juvenile and adult stages Switch to high fat diet at 21 weeks |
Decreased litter size Decreased Body weight Decreased perirenal fat pads weight Decreased cholesterol (female) Triglycerides (female offspring) |
BMDL 0.299 |
BMDL5 (week 25) 0.85 BMDL5 0.65 BMDL5 0.40 BMDL5 0.006 (BMDU/BMDL = 100) |
1 | Van Esterik et al. (2016) | ||
| PFOA salt not specified, 96% pure |
C57BL/6/Bkl female mice Exposure 0, 0.3 mg/kg per day, during gestation, n = 6 dams per group |
Periostal areas and medullary areas of the femur were increased at 17 months of age, the bone mineral density of the femur unaffected. Tibial bone mass was decreased both at 13 and 17 months. Biomechanical properties unaffected | n.d. | 0.3 |
Bone levels: 13 months: control 0.73 ng/g, PFOA 3 ng/g 17 months: control 0.64 ng/g, PFOA 3.7 ng/g |
Koskela et al. (2016) | ||
| PFOA, 99.2% pure |
Kunming mice Gavage, 0, 1, 5, 10, 20, 40 mg/kg bw per day, GD 1–17, 10 dams per group. Terminated on GD 18 |
Decreased bw gain Increased relative liver weight Decreased relative uterus weight Decreased embryo weight Decreased embryo survival |
10 n.d. 1 1 5 |
5 1 5 5 10 |
Li et al. (2018b) | |||
| PFOA > 98% pure |
Kunming mice, Gavage, 1, 2.5, 5 mg/kg bw per day from GD 1 to 17, 10 dams per group. Follow up on male offspring PND 21 and 70 |
Decreased postnatal survival Decreased bw PND 21 Decreased testosterone Decreased number of Leydig cells |
2.5 1 n.d. 1 |
5 2.5 1 2.5 |
Mice serum 0.17 μg/mL. At which dose or age of mice was not explained, and method not given | Song et al. (2018a) | ||
| PFOA, 99.2% pure |
Kunming mice Gavage 0, 1, 2.5, 5, 10 mg/kg bw per day, GD 1 to 17, 10 dams per group. Follow up on female offspring on PND21 |
Decreased postnatal survival Decreased weight gain Increased liver weight |
2.5 1 n.d. |
5 2.5 1 |
Li et al. (2019b) | |||
n.d.: not determined from the study; bw: body weight; BMDL: benchmark dose limit; BMDU: benchmark dose upper confidence limit; GD: gestation day; PND: postnatal day.
1Concentrations in serum quantified based on figure.
3.3.3.3.1. PFOS
Developmental studies on PFOS show effects in offspring at doses similar to, or below, those showing maternal toxicity (EFSA, 2008; EFSA CONTAM Panel, 2018). Among effects observed in rats and/or mice are high mortality early after birth, reduced fetal weight, reduced postnatal growth, increased liver weight, anasarca, impaired immune effects, cardiac abnormalities, cleft palate, delayed ossification of bones and a decrease in placental weight and capacity. Increase in liver weight (NOAEL 0.3 mg/kg bw per day) effects on placental physiology (LOAEL 0.5 mg/kg bw per day, NOAEL not determined) and aspects of glucose homoeostasis (LOAEL 0.3 mg/kg bw per day, NOAEL not determined) were the most sensitive endpoints (EFSA CONTAM Panel, 2018). One study (Liang et al., 2019), that did not change this conclusion, was identified by the updated literature search and included in Appendix F, Table F.6.
3.3.3.3.2. PFOA
Regarding reproductive effects, in adult male mice reproductive organs and male sex hormone levels were affected by PFOA (NOAEL 0.3 mg/kg bw per day). Among developmental effects in rodents were increased resorption of litters, dead foetuses, reduced postnatal survival (NOAEL 0.3 mg/kg bw per day), impaired development of mammary gland (LOAEL 0.00045 mg/kg bw per day, NOAEL not determined)), reduced postnatal weight, pathological alterations in liver and increased liver weight in pups (LOAEL 0.1 mg/kg bw per day, NOAEL not determined) (EFSA CONTAM Panel, 2018). Relevant studies identified by the updated literature search are included in the Appendix F, Table F.7 (Li et al., 2018b, 2019b; NTP 2019a; Song et al., 2018a), but they did not change these previous conclusions.
The most sensitive developmental effect observed is delayed and impaired development of the mammary gland upon prenatal and early postnatal exposure. The effect is also observed after higher exposure from puberty onwards. Delay in mammary gland development can be documented by decreased scores in whole mounts of mammary glands in early neonatal life or in puberty and later ages, including pregnancy and the first day after birth, or by increased scores in dams at the time of weaning, when the mammary gland is reducing and stopping the milk production in a process called involution (see Section 3.3.5.4). Delays were reported in all these stages. The eight studies on the developmental effects on mammary gland were performed in four different mouse strains. They are described in more detail below and summarised in Appendix F, Table F.1. The table also includes a selection of the PFOA concentrations measured in serum or tissues at different time points, and the NOAEL/LOAEL in each study is indicated. First the studies with exposure in utero and/or via lactation are listed chronologically, followed by studies with pubertal exposure.
Table F.1.
Studies on mammary gland development in mice with PFOA exposure in utero, postnatally or during puberty
| Mouse strain | Study design, exposure duration | Dosage (mg/kg bw per day) | NOAEL (mg/kg bw per day) | LOAEL (mg/kg bw per day) | Serum or tissue levels (ng/mL) | NOAEC (ng/mL) | LOAEC (ng/mL) | Reference |
|---|---|---|---|---|---|---|---|---|
| Prenatal and lactational exposure | ||||||||
| CD‐1 | GD 1‐17, GD 8‐17, GD 12‐17 | 0, 5 | 5 | Semi‐quantitative in blood of dams and pups at PND 10 and 20; quantitatively in livers of pups at PND 1, 10, 20 (data presented but not shown here) | White et al. (2007) | |||
| CD‐1 |
GD 1‐17 and GD 8‐17, + cross‐fostering (lactation) GD 7/10/13/15‐17 |
0, 3, 5 0, 5 |
3 5 |
Serum11 levels in GD 8–17 dams, 5 mg/kg bw per day: 42,200 or 47,900 at lactation day (LD) 1, decreasing to 16,400 or 24,400 at LD 10, depending on lactating control or treated pups. In pups exposed in utero GD 8–17, 66,200 or 70,000 at PND 1, decreasing to 20,500 or 31,300 at PND 10, when nursed by control or treated dams, respectively. In pups from control dams, maximum 15,700 at PND 10 when nursed by treated dams. Below 1,000 in all pups at PND 63 (weaning from PND 22) |
White et al. (2009) | |||
| CD‐1 |
GD 1‐17 GD 10‐17 |
0, 0.3, 1, 3 0, 0.01, 0.1, 1 |
0.3 0.01 |
Pup PND 7: < 20, 4,980, 11,026, 20,700 Pup PND 1: 22.6, 285, 2304, 16,306 Pup PND 21: 4.1, 16.5, 132, 2,025 |
4,980 285 16.5 |
Macon et al. (2011) | ||
| CD‐1 |
3‐generations, P0 GD 1‐17, +/− 5 μg/L in drinking water (0.00045 mg/kg bw per day) continuously from P0 GD 7 |
0, 0+5 μg/L, 1, 1+5 μg/L, 5 |
0+5 μg/L |
F1 PND 22: 0.6, 21.3, 2,444, 2,744, 10,045 F1 PND 63: 3.1, 66.2, 210.7, 187, 760 |
21.3 66.2 |
White et al. (2011) | ||
| Sv/129 | GD 1‐17 | 0, 3 | 3 | GD 18 dam: 19,000 | 19,000 | Albrecht et al. (2013) | ||
| CD‐1 | GD 1‐17 | 0, 0.01, 0.1, 0.3, 1 | 0.01 | Pup PND 21: < 5, 74.8, 457, 905, 3,119 | 74.8 | Tucker et al. (2015) | ||
| C57Bl/6 | 0, 0.01, 0.1, 0.3, 1 | 0.1 | 0.3 | Pup PND 21: < 10, 26.1, 247, 891, 2,142 | 247 | 891 | ||
| Pubertal exposure | ||||||||
| Balb/c | From PND 21 for 28 days, 5 days/week22 | 0, 0.7, 3.6, 7.1 | 0.722 | 3.622 | < 10, 29,500, 109,000, NR | 29,500 | 109,000 | Yang et al. (2009)11, 33 |
| C57Bl/6 | 0, 0.7, 3.6, 7.1 | For ↓ 3.622 |
For ↓ 7.122 For ↑ 0.722 |
< 10, 26,000, 68,200, 96,600 |
For ↓ 96,600 For ↑ 26,000 |
|||
| Balb/c | From PND 21 for 28 days, 5 days/week22 | 0, 1.8 | 1.822 | Serum at termination: < 10, 51,100 | 51,100 | Zhao et al. (2012)11 | ||
| C57Bl/6 | 0, 5.4 | 5.422 | Serum at termination: < 10, 93,400 | 93,400 | ||||
NR: Not reported; bw: body weight; GD: gestation day; LD: lactation day; LOAEC: lowest‐observed‐adverse‐effect concentration; LOAEL: lowest‐observed‐adverse‐effect level; NOAEC: no‐observed‐adverse‐effect concentration; NOAEL: no‐observed‐adverse‐effect level; PND: postnatal day.
Concentration in serum extracted from figure.
Values adjusted for dosing 5 days per week.
Serum levels reported in Zhao et al. (2012).
Note: Results concern decreased scores unless otherwise indicated.
Prenatal and early postnatal exposure via milk
White et al. (2007) studied the consequences of gestational exposure on mammary gland development, since prenatal PFOA exposure in previous studies led to postnatal death and a dose‐related decrease in neonatal body weight, which could be indicative of lower milk production by the dam. Pregnant CD‐1 mice were exposed to 0 and 5 mg/kg bw per day from either GD 1–17, GD 8–17 or GD 12–17. The body weight of offspring at PND 1–20 was reduced in all treated groups. Mammary gland differentiation was significantly reduced in the dams at PND 10 (time of maximal milk production), as assessed by the amount of epithelial tissue filling of the gland and the presence of well‐formed, productive alveoli (presence of milk), after treatment during GD 1–17 and 8–17 (not significant for GD 12–17). At PND 20, the normal presence of apoptotic bodies in the mammary gland during involution at weaning was observed in controls but to a lesser extent in the treated dams. Development of the mammary gland in dams and female pups was scored on a 1 (poor) to 4 (normal) age‐adjusted developmental scale by two independent scorers, blinded to the treatment. The scoring included assessment of the number of primary ducts, number of large secondary ducts, lateral side‐branching, appearance of budding from the ductal tree and longitudinal outgrowth of the epithelia. In the pups, the mammary gland development was significantly impaired at both PND 10 and 20 in all three treatment groups, showing stunted epithelial branching and longitudinal growth. Differences in blood PFOA levels between the groups were small at PND 10, but larger at PND 20, dams and pups treated during GD 1–17 showing the highest levels. Liver PFOA levels in pups also increased with treatment duration at all three time points (PNDs 1, 10 and 20) but were significantly different only at PND 20. The study showed that the susceptible period for mammary gland development in offspring is in late gestation or postnatal.
In a follow‐up study, White et al. (2009) confirmed that the effects in mammary gland development in pups (examined on PND 29 and 32) were similar when the dams were only treated during GD 15 to 17, as compared to much longer treatments starting at GD 7, 10 or 13 (daily dose 5 mg/kg bw per day). Furthermore, the effects of in utero vs. lactational exposure were investigated by cross‐fostering pups of untreated and treated dams (3 and 5 mg/kg bw per day, GD 1–17 and 5 mg/kg bw per day, GD 8–17). Pups from untreated dams receiving milk from treated dams (5 mg/kg bw per day) had mammary glands with impaired development at all investigated time points (PND 1, 3, 5, 10, 22, 42 and 63). Similar effects were seen in pups exposed in utero and cross‐fostered by untreated dams. The PFOA concentration in serum from treated pups was highest on PND 1 in both the groups nursed by treated and untreated dams. In pups from untreated dams, but nursed by treated dams, levels increased up to PND 10 and to a level similar to that in pups only exposed in utero. At PND 63, the serum levels in all groups of pups were close to that in controls. The study showed that both late gestational or only lactational exposure to PFOA can result in impaired development of mammary glands, and that this can still be observed in adult animals.
Macon et al. (2011) studied the effect of lower doses during gestation in CD1 mice treated during GD 1–17 (0, 0.3, 1.0, 3 mg/kg bw per day) or during GD 10–17 (0, 0.01, 0.1, 1.0 mg/kg bw per day). Exposure during GD 1–17 led to a decrease in mammary gland development scores in pups at all doses at PND 14, 21, 42 and 84. After exposure during GD 10–17, the overall mammary gland developmental score in pups at PND 21 was dose‐dependently and significantly decreased already at 0.01 mg/kg bw per day, and no NOAEL could be derived. The overall developmental score was composed of, but not restricted to, longitudinal and lateral growth, change in longitudinal and lateral growth, the number of terminal end buds (TEBs) and the number of terminal ends. The number of TEBs was the most sensitive individual parameter and showed a dose‐related reduction at all doses, which was significant at 0.1 and 1.0 mg/kg bw per day. This study confirmed that the impairment of mammary gland development was permanent and that the sensitive period is late gestation or the first weeks postnatally. The LOAEL for exposure during GD 10–17 was 0.01 mg/kg bw per day. This treatment resulted in serum PFOA concentrations in pups of 285, 184, 151, 80 and 17 ng/mL at PNDs 1, 4, 7, 14 and 21, respectively.
White et al. (2011) performed a three‐generation study, exposing CD‐1 dams (P0) to 0, 1 or 5 mg/kg bw per day from GD 1 to GD 17. In addition, groups of dams dosed with 0 or 1 mg/kg bw per day during GD 1–17 received a continued lifelong exposure to 5 μg/L of PFOA through the drinking water. This started on GD 8 in the dams and continued also in pups throughout F1 and F2 (estimated to correspond to a dose of 0.00045 mg/kg bw per day29). At weaning (PND 22), maternal mammary gland indices were increased in all dosed P0 dams, indicating a delay in involution. The female F1 generation was used for breeding (F1 dams) or assessment of developmental scores (F1 females). Mammary gland development scores were significantly decreased in all treated F1 pup groups on PND 22, 42 and 63, indicating that at the time when F1 females became pregnant, the mammary glands were not fully developed. This effect was still observed in the F1 dams on PND 10, but such changes were not consistently seen on PND 22. Effects of treatment on mammary gland development were also less evident in F2 pups with significant decreases only observed at PND 42 and only in the groups receiving contaminated drinking water. A lactational challenge in F1 dams on PND 10 did not indicate lower milk production or change in time to initiate milk production in any of the dosed groups. Furthermore, growth of the F2 offspring was not altered. Nursing behaviour, such as number of nursing events or duration of nursing per event was not investigated. No other impact such as on the number of live fetuses, prenatal loss, postnatal survival, body and liver weight were reported following chronic exposure to 5 μg/L of PFOA through the drinking water. The serum PFOA concentration increased with age at different developmental time points after chronic exposure to 5 μg/L PFOA in the drinking water and corresponded to a lifetime average serum concentration in F1 females of 60 ng/mL and in F2 females of 51 ng/mL. At PND 22, the serum concentrations were 21 and 27 ng/mL in F1 and F2 pups, respectively.
In Sv/129 mice, a PFOA dose of 3 mg/kg bw per day during GD 1–17 induced postnatal lethality in offspring but no changes in mammary gland parameters on PND 20 in offspring (Albrecht et al., 2013). The CONTAM Panel noted limitations in the study in that only one dose and one time point was investigated, the number of animals was low and that the mean number of TEBs per gland was reported to be only 2.1.
The effects of PFOA in CD‐1 mice were confirmed in a comparative study with CD‐1 and C57Bl/6 mice (Tucker et al., 2015). In this study, timed pregnant dams received oral daily doses of 0, 0.01, 0.1, 0.3 or 1.0 mg/kg bw per day of PFOA from GD 1 to 17. No treatment group showed an effect on absolute and relative body or liver weight, or on puberty onset measured by vaginal opening. Mammary gland development scores in CD‐1 mice were significantly decreased in all dose groups at PND 35 and PND 56 and from 0.1 mg/kg bw per day on PND 21. C57Bl/6 mice appeared to be less sensitive since the decrease in the scores at PND 21 and 61 were only significant for the treatment with 0.3 and 1.0 mg/kg bw per day. However, the CONTAM Panel noted high uncertainty regarding differences in strain sensitivity since the treatment groups for C57Bl/6 were smaller than for CD‐1. The concentration of PFOA in serum in general also appeared to be lower in C57Bl/6 than in CD‐1 mice.
Pubertal exposure
Balb/c and C57BL/6 mice were orally treated with PFOA at doses of 0, 1, 5 and 10 mg/kg bw per day during 5 days a week for 4 weeks starting at PND 21 (Yang et al., 2009). An increase in absolute (at 5 and 10 mg/kg bw per day) and relative (at 1, 5 and 10 mg/kg bw per day) liver weight was observed in both strains. In Balb/c mice, a significant delay in vaginal opening was observed for 1 mg/kg bw per day and no vaginal opening at all for 5 and 10 mg/kg bw per day. In C57BL/6 mice, a significant delay of vaginal opening was seen at 5 mg/kg bw per day and no vaginal opening at 10 mg/kg bw per day. Uteri of Balb/c mice showed a dose‐dependent decrease of absolute and relative weight, whereas in C57BL/6 mice a dose of 1 mg/kg bw per day resulted in increased absolute and relative uterine weight and only 10 mg/kg bw per day led to a decrease in uterine weight. The ductal length, the number of mammary gland TEBs and the number of terminal ducts of the developing mammary gland were also investigated. In Balb/c mice, a dose‐related decrease in all three parameters was observed and reached statistical significance at 5 mg/kg bw. In C57BL/6 mice, a decrease of all three parameters was only observed at 10 mg/kg bw per day, whereas at 1 and 5 mg/kg bw per day, the number of terminal end buds and stimulated terminal ducts was increased, being significant at 5 mg/kg bw per day. Since the two mouse strains responded with different sensitivity, which might be indicative for strain specificity, Balb/c mice were also treated with 0.1 mg/kg bw per day with no findings in comparison to controls (data not shown).
The decrease in mammary gland development in Balb/c and C57BL/6 mice was confirmed by Zhao et al. (2012). The effect was seen at a dose level of 2.5 mg/kg per day for Balb/c and a dose level of 7.5 mg/kg bw per day in C57BL/6 mice treated from PND 28 for 4 weeks with dosing at 5 days a week. No other dose levels were applied.
The mechanistic results from the studies on pubertal exposure are described further in Section 3.3.5.4.
In summary, PFOA exposure was shown to impair normal development of the mammary gland in CD‐1 and C57Bl/6 mice exposed late in gestation or via lactation. This impairment in development of the gland was still observed at PND 84 (12 weeks) in CD‐1 mice and is thus present beyond sexual maturity. Normal involution in CD‐1 dams at weaning was also disturbed (as indicated by increased scores). Exposure starting at puberty also caused delay or impairment of mammary gland development in Balb/c and C57Bl/6 mice. However, this was observed at substantially higher doses and serum levels than after exposure during gestation and lactation. In C57Bl/6 mice, an inhibitory effect on mammary gland development was seen at 7.1 mg/kg bw, but a stimulatory effect at dose levels 0.7 and 3.6 mg/kg bw, all three levels being inhibitory in Balb/c mice.
At present, the impaired development of mammary glands, as shown in two mouse strains (CD‐1 and C57Bl/6), is the most sensitive developmental outcome. No NOAEL has been identified in CD‐1 mice based on the three studies using low doses during gestation (Macon et al., 2011; Tucker et al., 2015; White et al., 2011). The LOAEL of 0.01 mg/kg bw per day in CD‐1 mice dosed during GD 10–17 resulted in a serum concentration in pups on PND 1 of 285 ng/mL, which decreased to 16.5 ng/mL on PND 21 (Macon et al., 2011). A same LOAEL of 0.01 mg/kg bw per day was observed in another study with dosing during GD 1–17, leading to a serum concentration of 74.8 ng/mL in CD‐1 pups on PND 21 (Tucker et al., 2015). In the three‐generation study by White et al. (2011), a chronic dose of 5 μg/L in drinking water (0.00045 mg/kg bw per day) from GD 7 in P0 dams and continued for the F1 pups led to a lowest observed adverse effect concentration (LOAEC) serum concentration of 21.3 ng/mL in the pups on PND 22. These LOAEC serum concentrations in pups on PND 21/22 are quite similar in the three studies. In the mice in the three‐generation study by White et al. (2011) under constant exposure via drinking water, the LOAEC of 21.3 ng/mL increased to 66.2 ng/mL on PND 63, at an age the mice get pregnant. Thus, the maternal LOAEC in the experiment was around 66 ng/mL and the average lifetime serum concentrations in F1 and F2 females were 60 and 51 ng/mL, respectively.
3.3.3.3.3. PFCAs
Reproductive and developmental toxicity studies were identified for PFBA, PFHxA, PFNA, PFDA, PFUnDA, PFDoDA, PFTeDA, PFHxDA and PFODA. No studies were identified for PFPeA, PFHpA, PFTrDA, PFPeDA and PFHpDA. Appendix F Table F.1 contains reproductive and developmental studies with exposure during pregnancy and Appendix F Table F.2 studies with exposure in puberty or adulthood. In the text below, all developmental and reproductive studies are described together for each PFAS.
Table F.2.
Perfluoroalkyl carboxylic acids (PFCAs): reproductive and developmental toxicity
| Substance/(Purity) | Species/Experimental design and doses | Observed effects | Highest dose with no effect (mg/kg bw per day) | Lowest dose with effects (mg/kg bw per day) | Serum/tissue levels of compound | Reference | ||
|---|---|---|---|---|---|---|---|---|
| Mother | Offspring | Mother | Offspring | |||||
| Perfluorobutanoic acid (PFBA) | ||||||||
| PFBA (98% pure) |
CD‐1 mice 0, 35, 175, 350 mg/kg per day Exposure GD 1 – GD 17 30 mice per dose group, in three blocks |
Increased liver weight Increased liver weight (PND 1) Delayed eye opening Delayed vaginal opening Delayed preputial separation |
35 |
35 n.d. 35 175 |
175 |
175 35 175 350 |
Serum in μg/mL, liver in μg/g. Serum in non‐pregnant female: < LOD (0.01), 1.96, 2.41, 2.67. Serum in pregnant dam: < LOD (0.01), 3.78, 4.44, 2.49 Liver in non‐pregnant female: 0.038, 0.51, 0.86, 0.89. Liver in pregnant female: < LOD (0.01), 1.41, 1.60, 0.96. Serum pups PND 1: ND, 0.56, 0.61, 0.37. Serum pups PND 10: < LOD (0.01), 0.11, 0.14, 0.12 |
Das et al. (2008) |
| (Perfluorohexanoic acid (PFHxA) | ||||||||
| PFHxA, 93.4% pure (ammonium salt) |
CD‐1 mice Exposure GD 6 – GD 18 20 mice per dose group Phase 1: 0, 100, 350, 500 mg/kg per day, gavage. Phase 2: 0, 7, 35, 175 mg/kg per day, gavage. |
Maternal mortality Increased % pups found dead day 1–4 Increase in stillborn pups and pups dying on PND1 |
100 35 |
100 |
350 175 |
350 | Concentrations in serum and liver from dams at weaning and in F1 at day 41 after birth were generally below the LOQ (LOQ 0.02 μg/mL) | Iwai and Hoberman (2014) |
| As above | As Phase 2 above | Reassessment of Phase 1 in Iwai and Hoberman (2014), combining Phase 1 and Phase 2 controls in statistical analysis. | 175 | Iwai et al. (2019) | ||||
| PFHxA, 100% pure (sodium salt) |
Crl:CD(SD) rats One generation reproduction study: 0, 20, 100, 500 mg/kg bw per day, gavage. Females and males: 70 days prior to cohabitation until weaning, approximately 126 day totally for females and 110 days totally for males |
Reduced body weight gain (females) during first week of exposure and during lactation Reduced mean F1 pup weight |
100 | 100 | 500 | 500 | Not reported | Loveless et al. (2009) |
| PFHxA, 100% pure (sodium salt) |
Crl:CD(SD) rats Developmental toxicity study: 0, 20, 100, 500 mg/kg bw per day, gavage. 22 female rats/group. Exposure from GD 6 to GD 20 according to OECD Guideline 414, sacrifice on GD 21. |
Reduced maternal body weight 10% decrease of fetal weight |
100 | 100 | 500 | 500 | Not reported | Loveless et al. (2009) |
| Perfluorononanoic acid (PFNA) | ||||||||
| PFNA 97% pure, (linear isomer according to supplier) free acid |
CD‐1 mice 0, 1, 3, 5, 10 mg/kg bw per day Exposure GD 1–GD 17 n = 11–27 animal per group, depending on outcome (details not specified) Experiment performed in three blocks |
Dams at 10 mg/kg failed to carry pregnancy (no follow up in this dose group) Increase in absolute and relative liver weight Dose dependent increase in relative liver weight in pups Neonatal mortality within the first 10 days Postnatal reduction of body weight gain Delay in eye opening, preputial separation and vaginal opening |
5 n.d. |
n.d. 3 n.d. 1 |
10 1 |
1 5 1 3 |
Read from graphs at 1, 3, 5 mg/kg bw per day: Serum, non‐pregnant: 30, 45, 210 μg/mL Serum, dam at term: 20, 25, 75 μg/mL Serum dam after weaning: 10, 25, 85 μg/mL Liver, non‐pregnant: 170, 320, 470 μg/g Liver, dam at term: 100, 270, 320 μg/g Dam, post weaning: 35, 125, 210 μg/g Fetal liver at term: 10, 35, 70 μg/g Serum levels in pups at PND 1: 25, 50 and 75 μg/mL Liver from pups at PND 1 and PND 10: 60, 150, 200 μg/g, thereafter decreasing, but still elevated at PND 40 and 70 |
Das et al. 2015 |
| PFNA source, purity and salt not specified |
Sprague‐Dawley rats 5 mg/kg bw per day, gavage. Exposure GD 1–20 10–12 Offspring animals per group |
Delayed weight gain Lower birth weight in females Increased blood pressure in males and females at 10 weeks of age Reduced nephron endowment (nephrons per kidney) in males at week 22 |
n.d. |
n.d. n.d. n.d. |
5 |
5 5 5 |
Not reported | Rogers et al. 2014 |
| PFNA 97% pure |
Parkes mice, 0, 2, 5 mg/kg bw per day, by oral feeding needle, from GD 12 to parturition. 10 dams per group, 2 male offspring per dam investigated on PND 3 |
Decreased testicular testosterone PND3 | 2 | 5 | Not reported | Singh and Singh, 2019a | ||
| Perfluorodecanoic acid (PFDA) | ||||||||
| PFDA, 96% pure salt not specified |
C57BL/6N Mice, gavage, GD10‐13 at 0, 0.25, 0.5, 1, 2, 4, 8, 16 and 32 mg/kg bw per day. GD 5‐15 at 0.03, 0.1, 0.3, 1, 3, 6.4, 12.8 mg/kg bw per day. |
Increased liver weight Reduced bw Increased liver weight Reduced body weight |
0.25 8 0.3 3 |
0.25 0.1 |
0.5 16 1.0 6.4 |
0.5 0.3 |
Not reported | Harris and Birnbaum 1989a |
| Perfluoroundecanoic acid (PFUnDA) | ||||||||
| PFUnDA, 98.5% pure, salt not specified |
Crl:CD Sprague‐Dawley rats, 0, 0.1, 0.3, 1 mg/kg per day by gavage. Males: 12 per group for 42 days beginning 14 days before mating Females (12 per group): 14 days prior to mating, through gestation until 4 days of lactation. Recovery groups: 5 of 12 males were allowed a 14 days recovery period. Females in recovery group were treated like males in this group |
Lowered body weights in male and female pups at PNDs 0 and 4 | 0.3 | 1 | Not reported | Takahashi et al. 2014 | ||
| Perfluorododecanoic acid (PFDoDA) | ||||||||
| PFDoDA (97% pure, salt not specified |
Crl:CD Sprague‐Dawley rats. 0, 0.1, 0.5, 2.5 mg/kg per day by gavage. Males: 12 per group for 42 days beginning 14 days before mating Females: 14 days prior to mating, through gestation until 6 days of nursing |
Reproductive endpoints only Decreased spermatid and spermatozoa count at 2.5 mg/kg per day |
0.5 | 2.5 | Not reported | Kato et al.2015b | ||
| Continuous dioestrus | 0.5 | 2.5 | ||||||
| Death in late pregnancy (7 out of 12 animals) | 0.5 | 2.5 | ||||||
| Failure to deliver live pups (4 out of 12 animals) | 0.5 | 2.5 | ||||||
| Perfluorotetradecanoic acid (PFTeDA) | ||||||||
| PFTeDA, (salt not specified), 96.5% pure |
Crl:CD(SD) rats, males and females, from 14 days before mating (12 per group) were dosed for 42 days (males), or until PND5 (females), Dosage 0, 1, 3 and 10 mg/kg bw per day, gavage. |
Females: centrilobular hepatocyte hypertrophy. Decreased body weight. |
3 | 3 | 10 | 10 | Not reported | Hirata‐Koizumi et al. 2015 |
| Perfluorohexadecanoic acid (PFHxDA) | ||||||||
| PFHxDA (salt not specified), 95.3% pure |
Crl:CD(SD) rats males and females, from 14 days before mating (12 per group) were dosed for 42 days (males), or until PND5 (females). Dosage 0, 4, 20 and 100 mg/kg bw per day, gavage |
Centrilobular hepatocellular hypertrophy of hepatocytes Reproductive/ developmental parameters not affected |
20 100 |
100 | 100 | Not reported | Hirata‐Koizumi et al. 2015 | |
| Perfluorooctadecanoic acid (PFODA) | ||||||||
| PFODA, 98.9% pure | Crl:CD(SD) rats, males were dosed for 42 days, females until PND 5, dosage 0, 40, 200 and 1,000 mg/kg per day, gavage |
Hepatic changes (centrilobular hepatocellular hypertrophy) Reduced numbers of implantation, total number of born pups and number of live pups on PND 0 and 4, decreased birth weight and postnatal weight gain |
200 | 200 | 1,000 | 1,000 | Not reported | Hirata‐Koizumi et al. 2012 |
n.d: not determined; bw: body weight; LOD: limit of detection; LOQ: limit of quantification; PND: postnatal day.
PFBA was tested at doses of 0, 35, 175 and 350 mg/kg bw per day following oral gavage from GD 1–17, to 10 mice per dose group (Das et al., 2008). Serum levels in dams at the end of dosing were < 0.01, 3.8, 4.4 and 2.5 μg/mL and liver levels were < 0.01, 1.41, 1.6 and 0.96 μg/g. On the maternal side, a modest (30%) increase in absolute and relative liver weight became apparent at 175 and 350 mg/kg bw per day, and there was an increase in full litter resorption at the highest dose. There were no changes in maternal weight gain, and survival in newborns and postnatal weight gain were not affected. Liver weight at 175 and 350 mg/kg bw per day was also increased in the offspring at PND 1, but not on PND 10. Eye opening of pups was delayed by between 1 and 1.5 days at all doses. A dose‐related 2–3 days delay in vaginal opening became significant at 175 mg/kg bw per day and a delay in preputial separation was seen at 350 mg/kg bw per day. Expression of genes regulated by PPARα and PXR was unaltered in liver from pups at PND1. Overall, gestational exposure to PFBA induced a delay in developmental markers with an LOAEL of 35 mg/kg bw per day for delayed eye opening, and a modest increase in liver weight in dams and in pups on PND 1 with an NOAEL of 35 mg/kg bw per day. The early neonatal mortality and decrease in postnatal growth that were seen with PFOS and PFOA were not observed for PFBA.
A study with PFHxA (NH4 +‐PFHxA) on developmental and perinatal/postnatal reproduction toxicity was performed in CD‐1 mice (Iwai and Hoberman, 2014). In the first phase of the experiment, mice (20 per dose group) were orally treated with 0, 100, 350 and 500 mg/kg bw per day on GD 6–18. In the second phase of the experiment, the dosage was modified to 0, 7, 35 and 175 mg/kg bw per day. Increased neonatal mortality, reduced viability index, reduced weight gain during lactation and an increased liver to body weight ratio in the offspring were detected at doses of 350 mg/kg bw per day and higher in the first phase experiment. In the second phase, there was an increased number of stillborn pups and pups dying on PND 1 and decreased pup weight on PND 1 at 175 mg/kg bw per day. No developmental changes were reported in F1. The overall NOAEL for maternal and developmental effects in the two studies was 100 mg/kg bw per day. Iwai et al. (2019) undertook a reassessment of the two studies, combining the two experimental phases in statistical analyses, and concluded that there was no significant decrease in viability at 175 mg/kg bw per day.
Loveless et al. (2009) performed a comprehensive toxicological evaluation of Na+‐PFHxA, which included a 90‐day subchronic, a one‐generation reproduction and a developmental toxicity study in rats (see also Section 3.3.3.2). In the one‐generation reproduction studies, parental animals were exposed from 70 days prior to cohabitation until weaning with 0, 20, 100 and 500 mg/kg bw per day by gavage. There were no detectable effects related to reproduction. In the developmental study, females were exposed from GD 6–20 to 0, 20, 100 and 500 mg/kg bw per day by gavage, according to OECD guideline 414. No effects linked to developmental processes could be identified. An NOAEL of 100 mg/kg bw day was delineated by the authors based on reduced maternal and fetal body weight in the 500 mg/kg bw per day exposure group.
In a 28‐day study in male rats orally treated with PFHxA at doses from 62.6 to 1,000 mg/kg bw per day did not show significant effects on sperm count per g cauda epididymis or serum testosterone, but a slight (5%) decrease in epidydimal weight was reported for the highest dose. Oestrus cyclicity was normal in dosed females (highest dose tested 500 mg/kg bw per day) (NTP, 2019a).
The effects of the free acid of PFNA (97% pure) were assessed in a study design that was delineated from PFOS and PFOA studies. PFNA was tested in CD‐1 mice (n = 11–27 dams per dose group) with exposure during GD 1–17 at doses of 1, 3, 5 or 10 mg/kg bw per day (Das et al., 2015). Dams in the dose group of 10 mg/kg bw per day could not carry their pregnancies and were not subjected to follow‐up studies. Serum concentrations in pregnant dams at term at 1, 3 and 5 mg/kg bw were approximately 20, 25 and 75 μg/mL and the corresponding liver levels were 100, 270 and 320 μg/g. Liver weight was dose dependently increased at 1, 3 and 5 mg/kg bw per day in both non‐pregnant animals and pregnant animals at term. Neonatal survival was around 90% for the 0, 1 and 3 mg/kg per day dose groups, but decreased during the first 10 days in the 5 mg/kg bw per day group and was at weaning only 20%. Serum levels in pups in the 1, 3 and 5 mg/kg per day dose groups were around 25, 50 and 75 μg/mL at PND 1 and decreased to control levels at weaning for the two lower dose groups. In pup livers, the PFNA levels were similar at PND 1 and PND 10 (60, 150 and 200 μg/g in the three dose groups) and thereafter decreased, but were still elevated at PND 40 and 70. The liver to serum ratio in pups was approximately 3:1 the first 10 weeks after birth. In surviving pups, a dose‐dependent reduction of postnatal body weight gain reached statistical significance at 3 and 5 mg/kg bw per day. The lower body weight persisted up to PND 49 in females and up to PND 297 in males. Relative liver weight was dose‐relatedly increased in pups in all dose groups at PND 1, 10 and 24, but was not anymore significantly increased in the lower dose at PND 42, and no significant effects were seen at PND 60 in any of the groups. Together with decreased postnatal growth, the surviving pups also showed a dose‐dependent delay in developmental landmarks (eye opening, preputial separation and vaginal opening). The authors also provided benchmark dose estimates. The most sensitive ones were BMD5/BMDL5 values of 0.43/0.27 mg/kg bw per day for increased relative liver weights in mothers and BMD5/BMDL5 of 0.24/0.19 mg/kg bw per day for increased relative liver weight of pups at PND1.
Rogers et al. (2014) comparatively assessed the impact of several compounds on parameters related to cardiovascular functions in Sprague‐Dawley rats. Animals were exposed from GD 1 to 20 to PFNA at 5 mg/kg bw per day, and 10–12 offspring animals per group were followed up for testing cardiovascular functions. On the maternal side, a delayed weight gain was observed. For the offspring, a lower birth weight was recorded in females. Blood pressure was found to be increased but only at the time point of 10 weeks of age of the offspring. A reduced nephron endowment (nephrons per kidney) was found in males at 22 weeks of age.
PFNA (males dosed at 0, 0.625, 1.25 and 2.5 mg/kg bw per day, females at 0, 1.56, 3.12, 6.25 mg/kg bw per day) showed effects on reproductive parameters in the 28‐day study in rats conducted by NTP (2019a). Epidydimal weight decreased dose relatedly (33% reduction at the highest dose) and was significantly reduced from the lowest dose. Cauda epidydimal sperm count was significantly decreased, but was similar to the control per g tissue. There were also histopathological findings in epidydimis at the highest dose (hypospermia, exfoliated germ cells, epithelial apoptosis, granuloma sperm). Testis weight was reduced in the two highest dose groups, and was accompanied by histopathological changes (degeneration, spermatid retention, interstitial cell atrophy). Testosterone was also significantly decreased in the two higher dose groups (28% and 81%, respectively). In females, testosterone was dose‐relatedly and significantly increased (66% increase at highest dose) but vaginal cytological data indicated cyclicity similar to controls.
Singh and Singh (2019a) exposed Parkes mice orally to PFNA (0, 2 and 5 mg/kg bw per day, 10 dams per group) from GD 12 to parturition, and testes of two male pups per dam were investigated on PND 3. In dams, the body weight was not affected, and there were no changes in birth rate, number of pups per dam and weight of male pups. Testicular testosterone was decreased in the highest dose group, together with reduced levels of proteins involved in testosterone synthesis (StAR, CYP11A1, 3β and 17β‐HSD), gonad development (WT1 and SF1) and cell proliferation (PCNA). However, testis weight and testis histology were not affected.
Singh and Singh (2019b) investigated the consequences of PFNA exposure on parameters of male fertility (Appendix F Table F.3). After a dose range finding study, 14 male Parkes mice were orally treated at 0, 0.2 and 0.5 mg/kg bw per day for 90 days using a feeding needle, starting at prepubertal age (PND 25) and ending on PND 114. At 24 h after the last dosing, the authors observed a dose‐related reduction of sperm number in cauda epidydimis (significant at the highest dose, 74% of control), motility and viability. This was associated with a decreased proliferation (number of PCNA labelled cells) and an increase in apoptosis (caspase 3 staining) in the testicular tissue. Testis weight was not affected. In a reproduction assay, exposure of male mice led to a dose‐related decrease in litter size (significant at the highest dose, 50% of control). The authors also showed a dose‐dependent decrease of total serum cholesterol (67% of control) and of testosterone (69% of control), also being significant at 0.5 mg/kg bw per day. On a molecular level, decreased levels of proteins and mRNA involved in the steroidogenic process (StAR, Cyp11A1, 3β‐HSD and 17β‐HSD [isoform not specified]) were observed at the highest dose. Finally, the dose of 0.5 mg/kg bw per day of PFNA increased lipid peroxidation. This effect was accompanied by a decrease of SOD and catalase activity which was significant at 0.2 mg/kg bw per day.
Table F.3.
PFCA reproductive toxicity studies with exposure in pubertal or adult animals
| Substance/ (Purity) | Species/Experimental design and doses | Most sensitive endpoints | Highest dose with no effect (mg/kg bw per day) | Significant effect level (mg/kg bw per day) | Serum/tissue levels of compound | Reference |
|---|---|---|---|---|---|---|
| Perfluorohexanoic acid (PFHxA) | ||||||
| PFHxA (> 99% purity) |
Sprague‐Dawley rats Gavage 0, 62.6, 125, 250, 500, or 1,000 mg/kg per day |
Cauda epidydimis sperm count Serum testosterone Oestrus cyclicity |
1,000 1,000 1,000 |
Plasma conc. (ng/mL) at 62.6 mg/kg bw per day: 378 ± 178 (m) 129 ± 16 (f) at 250 mg/kg bw per day: 1297± 265 (m) Liver conc (ng/g) at 250 mg/kg bw/day 655 ± 148 (m) |
NTP (2019a) | |
| Perfluorononanoic acid (PFNA) | ||||||
| PFNA (> 98% purity) |
Sprague‐Dawley rats Gavage Male: 0, 0.625, 1.25, 2.5 mg/kg bw per day Female: 0, 1.56, 3.12, 6.25 mg/kg bw per day |
Decreased epidydimal weight with histopathological findings. Reduced testis weight with histopathological changes Decrease serum testosterone |
0.625 0.625 |
0.625 1.25 1.25 |
Plasma conc. (ug/mL) at 0.625 mg/kg bw per day: 56.7 ± 1.9 (m) at 1.56 mg/kg bw per day: 26.4 ± 1.1 (f) Liver conc (u g/g) at 0.625 mg/kg bw per day 145.5 ± 2.7 (m) |
NTP (2019a) |
| PFNA 97% pure |
Male Parkes mice, 0, 0.2 and 0.5 mg/kg bw per day by oral feeding needle. 90 days exposure (PND 25 to PND 114) n = 14 per group 7 animals were selected for fertility test, the remaining 7 for assessment of toxicological parameters |
Reduced male fertility (reduced sperm number, viability and motility) Decreased cholesterol Decreased testosterone Reduced litter size Reduced expression of steroidogenic enzymes in testes Reduced PCNA (proliferation marker) and increased caspase3 (apoptosis marker) expression in testis Decreased SOD and catalase activity in testes |
0.2 0.2 0.2 0.2 0.2 0.2 n.d. |
0.5 0.5 0.5 0.5 0.5 0.5 0.2 |
Not reported | Singh and Singh (2019b) |
| PFNA 97% pure | Male Parkes mice, 0, 2, 5 mg/kg bw per day, by oral feeding needle for 14 days, from PND 25 to PND 38. 10 mice per dose group. 5 mice per dose group for some outcomes. |
Reduced body weight gain Reduced serum and testicular testosterone Degenerative changes in seminiferous tubules |
2 n.d. n.d |
5 2 2 |
Not reported | Sing and Singh (2019c) |
| PFNA 97% pure | Male Parkes mice, 0, 2, 5 mg/kg bw per day, by oral feeding needle for 14 days, from PND 25 to PND 38. 10 mice per dose group treated, but generally 5 mice per dose group for outcomes. |
Increased liver weight and hepatocellular hypertrophy. Altered proportions of 4C and 2C cells in testis |
n.d. n.d. |
2 2 |
Not reported | Singh and Singh (2019d) |
| Perfluorodecanoic acid (PFDA) | ||||||
| PFDA (> 97% purity) |
Sprague‐Dawley rats Gavage 0, 0.156, 0.312, 0.625, 1.25 and 2.5 mg/kg bw per day |
Reduced epidydimal weight and cauda epidydimis sperm count Reduced testis weight Reduced testosterone |
0.625 1.25 1.25 |
1.25 2.5 2.5 |
Plasma conc. (ug/mL) at 0.156 mg/kg bw per day: 8.5 ± .6 (m) 11.2 ± 0.4 (f) Liver conc (ug/g) at 0.156 mg/kg bw per day 44.7 ± 1.5 (m) |
NTP (2019a) |
| Perfluorododecanoic acid (PFDoDA) | ||||||
| PFDoDA (95% pure, salt not specified) |
Female Sprague‐Dawley rats, weaned (PND 21; 8 per group) 0, 0.5, 1.5, 3 mg/kg bw per day orally 28 days (PND 24 – PND 52) |
Body weight decreased Absolute and relative weight of uterus and ovary Age, weight at vaginal opening Oestrus cyclicity Increased cholesterol Decreased estradiol |
1.5 3 3 3 1.5 1.5 |
3 3 3 |
Not given | Shi et al. (2009a) |
| PFDoDA (95% pure, salt not specified) |
Male Sprague‐Dawley rats, weaned (PND21; 6 per group) 0, 0.02, 0.05, 0.2, 0.5 mg/kg bw per day orally 110 days exposure |
Body weight decreased Absolute and relative weight of testis, prostate, seminal vesicle, vas deferens Cholesterol Decreased testosterone |
0.2 0.5 0.5 0.2 |
0.5 0.5 |
Not given | Shi et al. (2009b) |
| PFDoDA (purity and salt not specified) |
Male Sprague‐Dawley rats 21 days old (8 per group) 0, 5, 10 mg/kg per day gavage 14 days exposure (sacrifice PND 35) |
Body weight decrease Decreased testis weight Decreased testosterone, LH, FSH Leydig and Sertoli cell number |
5 5 |
10 10 5 |
Not given | Chen et al. (2019) |
| Perfluorotetradecanoic acid (PFTeDA) | ||||||
| PFTeDA, (salt not specified), 96.5% pure |
Crl:CD(SD) rats, 12 males per group dosed for 42 days. Dosage 0, 1, 3 and 10 mg/kg bw per day, gavage. |
Decreased weight of seminal vesicles | 1 | Not reported | Hirata‐Koizumi et al. (2015) | |
n.d: not determined.
The same authors also investigated parameters related to male reproduction and steroidogenesis in Parkes mice (n = 10 per group) orally exposed to PFNA (97% purity) at 0, 2 and 5 mg/kg bw for 14 days, from PND 25 to PND 38 (Singh and Singh, 2019c,d). Mice were sacrificed 24 h after the last treatment. A dose‐related decrease in body weight gain became statistically significant at 5 mg/kg bw per day. Serum and intratesticular testosterone levels (five mice per group) were decreased in both dose groups, and the percent of seminiferous tubules showing degenerative changes were increased. A dose‐related decrease of levels of markers involved in steroidogenesis (SF‐1, StAR, CYP11A1 and 3β and 17β‐HSD) in testis was reported. Lipid peroxidation was increased, whereas SOD, catalase and glutathione S‐transferase were decreased in testis, indicating oxidative stress. Proliferation index measured by % PCNA positive cells per tubule was decreased, whereas the apoptotic index was increased (Singh and Singh, 2019c). Increased liver weight as well as hepatocellular hypertrophy was reported for both dose groups, and hepatic lipid peroxidation was increased. Serum cholesterol was decreased at the highest dose. Blood glucose was increased, whereas testicular glucose, lactate and lactate dehydrogenase were decreased (Singh and Singh, 2019d).
In summary, the most sensitive endpoint after gestational exposure to PFNA was increased liver weight in both maternal and offspring CD‐1 mice, and a reduction in postnatal weight gain in F1, with an LOAEL of 1 mg/kg bw per day, with corresponding concentration in serum from the dam at term of 20 μg/mL. Delay in development was seen at 3 mg/kg bw per day, and at 5 mg/kg bw per day, there was an increase in neonatal mortality. A 90‐day male reproductive study reported decreased sperm production, decrease in cholesterol, steroidogenic enzymes and testosterone, as well as decreased number of pups in the next generation, with an NOAEL and LOAEL of 0.2 and 0.5 mg/kg bw per day, respectively. Effects on male reproduction parameters were also reported by NTP in rats at somewhat higher exposure levels, and it was noted that 28 days is shorter than one spermatogenic cycle and too short to fully assess male reproductive parameters.
Harris and Birnbaum (1989a) dosed C57BL/6N mice with PFDA during GD 10–13 with 0, 0.25, 0.5, 1.0, 2.0, 4.0, 8.0, 16.0 or 32.0 mg/kg bw per day and during GD 5–15 with 0, 0.03, 0.1, 0.3, 1.0, 3.0, 6.4 or 12.8 mg/kg bw per day. Toxicity was assessed in dams and fetuses on GD 18. Reduced maternal body weight gain and reduced fetal viability was observed in the two highest dose groups in both dosing regimes. After exposure during GD 5–15, a statistically significant increase in maternal liver weight could be observed in response to doses of 1 mg/kg per day and higher. No effects were observed at 0.3 mg/kg bw per day and below. There was a dose‐related reduction in live fetal body weight, being statistically significant at 0.1 mg/kg bw per day, not significant at 0.3 mg/kg per day, but again significant at 1 mg/kg bw per day. In conclusion, effects in offspring were seen at doses that also indicated maternal toxicity, with the exception of reduction of live fetal body weight, being reduced already at 0.1 mg/kg bw per day.
In the 28‐day study in rats conducted by NTP (NTP 2019a), PFDA was dosed at 0, 0.156, 0.312, 0.625, 1.25 and 2.5 mg/kg bw per day. Reproductive tissue parameters were reported in animals in the three higher dose groups and controls, and showed lower number of sperms in cauda epidydimis in the two highest dose groups (significant in the highest dose group, 30% decrease). Also the body weight was significantly decreased at these doses. This was accompanied by significantly lower epidydimal weights (10% and 23%), and the number of sperm per g epidydimis was unaltered. Serum testosterone decreased with dose, becoming statistically significant (75% reduction) at the highest dose, at which also testis weight was reduced (11%), but with unaltered spermatid counts. There were also multiple histopathological findings in testis at this dose. In females, cyclicity was disrupted at the highest dose, with prolonged time in dioestrus. Serum testosterone in females increased with dose (41–141%), becoming significant from 0.312 mg/kg bw.
In a study on PFUnDA, a combined repeated dose (see Section 3.3.3.2) and reproductive/developmental toxicity approach according to OECD guideline 422 were used (Takahashi et al., 2014). Here, only the effects on reproductive and developmental endpoints are reported. Male and female Crl:CD(SD) rats (12 per group) were dosed for 42 days with 0, 0.1, 0.3 and 1 mg/kg bw per day per gavage from 14 days prior to mating for 42 days in males and until PND 5 in females. The following reproductive and developmental parameters were assessed: copulation index, fertility index, gestation index, number of pregnant animals, gestation length, numbers of corpora lutea and implantation sites, implantation index, number of litters, number of live pups at PND 0 and 4, live birth index, sex ratio, viability index, as well as body weights of male and female pups at PND 0 and PND 4. The only statistically significant change observed in response to the treatment was a reduction in body weight of pups of both sexes on PND 0 and PND 4 at 1 mg/kg bw per day. Increased liver weight was observed at 1 mg/kg bw per day in the repeated dose part of the study (see Section 3.3.3.2.1).
Kato et al. (2015b) performed a combined repeated dose (see Section 3.3.3.2) and reproductive toxicity study in rats with PFDoDA at 0, 0.1, 0.5, 2.5 mg/kg bw per day, according to OECD guideline 422. Here, only the effects on reproductive and developmental endpoints are reported. Twelve males per dose were orally treated for 42 days starting 14 days prior to mating. Twelve females per dose were treated starting 14 days before mating, throughout gestation until 6 days of nursing. Reproductive effects were only detected at the highest dose. Among them were decreased spermatid and spermatozoa counts in males, as well as a continuous dioestrus in females. At this dose, 7 out of 12 animals died in late pregnancy, 4 did not deliver live pups. In summary, gestational exposure to PFDoDA induces maternal and reproductive effects with an NOAEL of 0.5 mg/kg bw per days, which is higher than the NOAEL observed for repeated dose toxicity of 0.1 mg/kg bw per day (see Section 3.3.3.2).
Shi et al. (2009a) investigated the effect of PFDoDA exposure on pubertal transition in female Sprague‐Dawley rats orally treated from PND 24 to PND 52 with 0, 0.5, 1.5 and 3 mg/kg bw per day. Body weight was decreased at 3 mg/kg bw per day. No effects were observed for absolute and relative weights of uteri and ovaries, age and weight at vaginal opening and on oestrus cyclicity. At 3 mg/kg bw per day, cholesterol levels were increased and estradiol levels were decreased, while LH and FSH levels were unchanged. Authors in addition performed an extensive gene expression analysis of molecules involved in steroid metabolism and regulation of steroid metabolism. The most sensitive response was observed for 17β‐HSD mRNA expression, which was found to be upregulated at the lowest dose tested.
Shi et al. (2009b) investigated the effect of PFDoDA exposure on pubertal transition in male Sprague‐Dawley rats, by treating 21‐day‐old animals orally for 110 days with 0, 0.02, 0.05, 0.2 and 0.5 mg/kg bw per day. Body weight was decreased at 0.5 mg/kg bw per day. No effects were observed for absolute and relative weights of testis, prostate, seminal vesicle and vas deferens. At 0.5 mg/kg bw per day, testosterone levels were decreased, while LH and FSH and total cholesterol levels were unaffected. Authors in addition performed an extensive gene expression analysis of molecules involved in steroid metabolism and regulation of steroid metabolism. The most sensitive response was observed for StAR, which was downregulated at 0.02 mg/kg bw per day at the mRNA level and at the protein level at 0.05 mg/kg bw per day.
Chen et al. (2019) investigated the effect of PFDoDA on rat Leydig cell development in puberty of 21‐day‐old male Sprague‐Dawley rats orally treated for 14 days with 0, 5, 10 mg/kg bw per day. Body weight and absolute testis weight were decreased at 10 mg/kg bw per day. No effects were observed for Leydig and Sertoli cell number. Levels of serum testosterone, LH and FSH were decreased at 5 mg/kg bw per day. In addition to the described changes in hormone levels, authors base this suggestion on the downregulation of Leydig cell protein levels of LHCGR, SCARB1, STAR, CYP11A1, SIRT1 (5 mg/kg bw per day) and CYP17A1, HSD11B1, PGC1‐α (10 mg/kg bw per day). In addition, a reduced viability and reduced mitochondrial membrane potential of isolated Leydig progenitor cells in culture is described in response to 10 μM of PFDoDA.
In summary, exposure of rats to PFDoDA prior to and during gestation induced maternal and reproductive effects (continuous dioestrus and fetal loss) with an NOAEL of 0.5 mg/kg bw per day. Male reproductive effects (decreased spermatid and spermatozoa counts) were seen at a similar NOAEL of 0.5 mg/kg bw per day, which is higher than the NOAEL of 0.1 mg/kg per day observed for repeated dose toxicity in the same experiment.
Hirata‐Koizumi et al. (2015) also assessed PFTeDA in a combined repeated dose and reproductive/developmental toxicity study according to OECD guideline 422 (see Section 3.3.3.2). Here only the effects on reproductive and developmental endpoints are reported. Male and female Crl:CD(SD) rats (12 per group) were dosed from 14 days prior to mating with 0, 1, 3 and 10 mg/kg bw per day by gavage for 42 days in males and until PND 5 in females. In females, centrilobular hepatocyte hypertrophy was observed at 10 mg/kg bw per day. In males, a decreased absolute weight of the seminal vesicles was observed at 1 mg/kg bw per day. No significant changes regarding reproductive parameters were observed. Investigations comprised oestrus cyclicity, copulation index, fertility index, gestation index or length, number of corpora lutea, number of implantation sites, number of delivered pups as well as number of live pups at PND 1 and PND 4. However, on PND 1 and 4, the body weights of both male and female pups were significantly lower in the group with highest exposure.
In the paper described above (Hirata‐Koizumi et al., 2015), the authors also report on a combined repeated dose and reproductive/developmental toxicity study with PFHxDA following the same experimental design, with dose levels of 0, 4, 20 and 100 mg/kg bw per day per gavage. The authors investigated the same reproductive parameters as specified for PFTeDA (see above) and observed no effects on reproductive/developmental parameters. Hepatic changes (centrilobular hepatocellular hypertrophy) were observed in the dams in the highest dose group. The NOAEL for developmental and reproductive effects was 100 mg/kg bw per day, the highest dose tested.
Hirata‐Koizumi et al. (2012) performed a combined repeated dose (see Section 3.3.3.2) and reproductive toxicity screening study with PFODA according to OECD guideline 422. Here only the effects on reproductive and developmental endpoints are reported. Twelve male and female Crl:CD(SD) rats were dosed by gavage starting 14 days prior to mating at 0, 40, 200 and 1,000 mg/kg bw per day. Males were dosed for 42 days including the mating period and females until lactation day 5. In dams, hepatic changes (centrilobular hepatocellular hypertrophy and necrosis), reduced feed consumption and reduced body weight gain were seen at 1000 mg/kg bw per day. For offspring, reduced numbers of implantation, reduced total number of born pups and number of live pups occurred at 1,000 mg/kg bw per day. The NOAEL was 200 mg/kg bw per day for both maternal and developmental toxicity.
3.3.3.3.4. PFSAs
Appendix F Table F.4, 30 lists the identified reproductive and developmental toxicity studies for PFBS and PFHxS. No studies were identified for PFHpS and PFDS.
Table F.4.
Perfluoroalkane sulfonic acids (PFSAs): reproductive and developmental toxicity
| Substance/ (Purity) | Species/Experimental design and doses | Observed effects | Highest dose with no effect (mg/kg bw per day) | Lowest dose with effect (mg/kg bw per day) | Serum/tissue levels of compound | Reference | ||
|---|---|---|---|---|---|---|---|---|
| Mother | offspring | Mother | offspring | |||||
| Perfluorobutane sulfonic acid (PFBS) | ||||||||
| PFBS (97.9% pure, K+‐salt) |
Sprague Dawley rats 2‐generation reproduction study according to OECD guideline 416 Exposure: 0, 30, 100, 300, 1,000 mg/kg bw per day, gavage. Parental (F0) animals (males and females, n = 29–30 per sex per group) dosed from 70 days prior to mating, females were continued through gestation and lactation. F1 offspring (n = 29‐30) was dosed from weaning (lactational day 22) onwards. F2 generation was exposed through placenta and lactation. Experiment was terminated at lactational day 22 of F2 generation animals |
Increased liver weight Increased hepatocellular hypertrophy (P and F1 adult males) Increased incidence of mild microscopic findings in kidney No reproductive and developmental toxicity findings |
100 100 100 |
1,000 |
300 300 300 |
Not determined | Lieder et al. (2009b) | |
| PFBS (97% pure, K+‐salt) |
ICR mice Dosing: 0, 50, 200 and 500 mg/kg bw per day from GD 1–20, orally. Only female offspring follow‐up reported. 30 dams per dose group, randomly allocated to three experimental subgroups per dose: group 1: perinatal survival and growth, pubertal onset, and ovarian and uterine development (10 dams, 50 female offspring per dose), group 2: hypothalamic–pituitary–gonadal hormone and hypothalamic– pituitary–thyroid hormone levels (10 dams, 30 PND 1 female offspring, 10 female PND 30 offspring, and 10 PND 60 female offspring), group 3: levels of serum PFBS (10 dams) |
Decreased body weight from PND 1 to adulthood Delayed eye opening Delayed vaginal opening Impaired ovarian and uterine development Delayed oestrus cyclicity and reduced E2/increased LH Decreased T3/T4, increased TSH Decreased T3/T4, increased TSH at GD 20 |
50 |
50 50 50 50 50 50 |
200 |
200 200 200 200 200 200 |
Mean (10 dams) serum concentration (ng/mL) on GD 20, 12 h after last dosing, at 0, 50, 200 and 500 mg/kg bw per day: 1.73, 74, 332, 721 |
Feng et al. (2017) |
| Perfluorohexane sulfonic acid (PFHxS) | ||||||||
| PFHxS (99.98% pure, K+‐salt) |
Sprague‐Dawley rats, OECD guideline 422‐based design Exposure: 0, 0.3, 1, 3, 10 mg/kg bw per day by oral gavage to F0 (n = 15 + 3; main study + serum sampling) starting 14 days prior to mating until PND 22 or presumed gestation day 25 for rats without litter |
No reproductive or developmental effect No treatment‐related effects in dams |
10 | 10 |
Elaborated serum and liver values in dams and fetuses. Serum levels at study day 14 and GD 21 in dams were similar. For GD 21 females in serum and liver: 0 mg/kg per day: < LOQ of 0.1 μg/mL and 0.1 μg/g. 0.3 mg/kg per day: 3.32 μg/mL and 0.79 μg/g 1 mg/kg per day: 10.65 μg/mL and 2.61 μg/g 3 mg/kg per day: 32.75 μg/mL and 7.80 μg/g 10 mg/kg per day: 59.80 μg/mL and 16.53 μg/g |
Butenhoff et al. (2009) | ||
| PFHxS, K+‐salt, purity > 98% |
Wistar rats. Oral gavage from GD 7 until PND 22 (except for day of delivery). Range finding study (8 time mated rats per group): 0, 25, 46 mg/kg bw per day. Main study (16–20 time mated rats per group): 0, 0.05, 5, 25 mg/kg bw per day. |
Increased liver weight. ‐ Males ‐ Females Pronounced reduction of T4 levels, detectable at different time points. Dams: Offspring: Mildly decreased body weight. Male pups Female pups |
0.05 |
5 0.05 0.05 5 0.05 |
5 |
25 5 5 25 5 |
Serum levels in dam at PND 22 in the range finding study. 139 and 174 μg/mL in 25 and 45 mg/kg bw per day groups, respectively | Ramhøj et al. (2018) |
| K+PFHxS (98.9% pure) |
Crl:CD1 (IRC) mice Study design according to OECD guideline 422, modified). Exposure: gavage at 0, 0.3, 1, 3 mg/kg bw per day (n=30+12 per treatment; main study + toxicokinetic arm). Main experiment: For F0 males treatment from 14 days prior to cohabitation for to at least 42 days total (one day post‐last dosing). Treatment of F0 females started 14 days prior to cohabitation with continuation through mating, gestation and lactation. F0 dams were sacrificed on lactation day 22 (one day after last dosing). F1 offspring, first exposure in utero and via lactation. After weaning (PND 22), F1 direct dosing for 14 days at maternal dose. Toxicokinetic experiment: 12 animals per sex and dose. Subset 1 (5/sex/dose group) daily oral gavage for 14 days prior to sacrifice. Subset 2 (7/sex/dose group) dosing for 14 days prior to cohabitation. Serum and liver samples were collected at study day 14 for both sexes, at study day 28 for males and GD 18 (for females). On GD 18, pooled fetal blood and fetal liver samples were collected |
Reduced litter size (without impact on born pup to implant ratio) F0 animals: Increased mean and relative liver weight. F1 animals: Increased relative liver weight ♀ and ♂ Increased thyroid weight ♂ |
0.3 1 1 |
0.3 |
1 3 3 |
1 |
Serum and liver were measured at study day 14, GD 18 (toxicokinetic study arm) and lactational day 22 (main experiment) in dams and in F1 at GD 18 (pooled serum fetuses), PND 4 (pooled liver litter) and PND 21 and 36 (males and females). Serum and liver in dams, and serum from pooled fetus on GD18: 0 mg/kg per day: < 0.001 μg/mL, < 0.005 μg/g and < 0.001 μg/mL. 0.3 mg/kg per day: 16.8 μg/mL, 5.3 μg/g, and 20.8 μg/mL 1 mg/kg per day: 51.5 μg/mL, 15.1 μg/g and 62.3 μg/mL 3 mg/kg per day: 111.3 μg/mL, 88.4 μg/g and 137.7 μg/mL. |
Chang et al. (2018) |
bw: body weight; FSH: follicle stimulating hormone; GD: gestation day; LH: luteinizing hormone; LOQ: limit of quantification; PCNA: proliferating cell nuclear antigen; PND: postnatal day; T3: triiodothyronine; T4: thyroxine; TSH: thyroid stimulating hormone.
A two‐generation reproduction study with PFBS according to OECD guideline 416 was conducted in Sprague‐Dawley rats (Lieder et al., 2009b). Parental males and females (n = 29–30 per sex per group) were exposed to 0, 30, 100, 300, 1,000 mg/kg bw per day for 7 weeks prior to mating. Exposure of parental females was continued through gestation and lactation. F1 offspring (n = 29–30) was dosed from weaning at PND 22 onwards. F2 generation was only exposed in utero and during lactation. The experiment was terminated at PND 21 of the F2 generation animals. There were no treatment‐related findings on reproductive toxicity endpoints. In parental animals and in adult F1 offspring animals, increased liver weight, increased hepatocellular hypertrophy in males and increased incidence of mild microscopic findings in the medulla and papilla of the kidney were detected from 300 mg/kg bw per day. Delayed preputial separation was observed in F1 males in the lowest and highest dose group, but this was not significant after adjustment for body weight. No relevant treatment‐related developmental changes were found in the study. The NOAEL for reproductive and developmental effects was the highest dose tested (1,000 mg/kg bw per day).
In the 28‐day study conducted by NTP (NTP 2019b) PFBS at doses up to 1,000 mg/kg bw per day had no effect on sperm parameters, testosterone levels or oestrus cyclicity in rats.
Feng et al. (2017) orally exposed 30 pregnant female ICR mice per dose group to PFBS at 0, 50, 200 and 500 mg/kg bw per day from GD 1 (as assessed by the formation of a vaginal plug) until GD 20. Mean PFBS levels on GD 20 in serum sampled 12 h after the last dosing in 10 dams per dose group were 1.73, 74, 332 and 721 ng/mL. On PND 21, all offspring were weaned. Only female offspring animals were followed up. Offspring from 10 dams (50 offspring) per dose group were assessed for perinatal survival and growth, pubertal onset and ovarian and uterine development. Another group of offspring was assessed for the hormones representing the hypothalamic, pituitary gland and thyroid, as well as gonadal axis (n = 30 PND 1 offspring, n = 30 PND 30 offspring and n = 10 PND 60 offspring from 10 dams). In the two higher dose groups, PFBS exposure resulted in a dose‐related decrease in body weight from PND 1 to adulthood, delayed eye opening, delayed vaginal opening and occurrence of the first oestrus and prolongation of dioestrus phase, which was matched by decreased serum levels of estradiol and progesterone, as well as elevated levels of LH. The absolute and relative weight of the ovaries and the number of ovarian follicles, as well as the absolute and relative uterus weight were reduced. Regarding thyroid hormones in offspring, decreased T3 and T4 levels were observed throughout the observation period (PND 1, PND 30 and PND 60), whereas increases in TSH were seen only on PND 30. In dams on GD 20, there was a decrease in T3 and T4 and an increase in TSH in the two higher dose groups, while serum levels of estradiol and progesterone remained unchanged. Authors suggest that the observed hypothyroxinaemia may relate to general growth deficits observed. The NOAEL for developmental effects was 50 mg/kg bw per day, resulting in a serum level of 74 ng/mL in the dam at GD 20.
In summary, reproductive toxicity was not reported in rats exposed to PFBS up to 1,000 mg/kg bw per day. Delay in development and decrease in body weight gain were seen in mice exposed during gestation, with an NOAEL of 50 mg/kg bw per day (74 ng/mL serum in the dam at GD 20).
PFHxS was investigated in rats in a combined repeated dose toxicity study with the reproduction/developmental toxicity screening setting adapted from OECD guideline 422 (Butenhoff et al., 2009). Doses of exposure were 0, 0.3, 1, 3 and 10 mg/kg bw per day for n = 15 animals per sex and dose group in the main study, as well as three animals per group for determination of levels of PFHxS in blood and liver. Exposure per gavage was initiated 14 days prior to mating until termination at PND 22 or presumed GD 25 for females and study day 42 for males. No reproductive or developmental effects became apparent and no treatment‐related effects were observed in dams. For effects and serum concentrations in males, see Section 3.3.3.2.
Ramhøj et al. (2018) performed reproductive toxicity studies with PFHxS alone and in combination with an endocrine disruptor mix in Wistar rats. Only results with PFHxS alone are described here. Pregnant rats (16–20 per dose group) were exposed to PFHxS at doses of 0, 0.05, 5, 25 mg/kg bw per day by gavage from GD 7 to PND 22, except for the day of delivering offspring. In F1 females and males, an increased liver weight was observable at 5 and 25 mg/kg bw per day, respectively. A pronounced statistically significant reduction of T4 levels occurred from 5 mg/kg bw per day and was detectable at different investigated time points for both dams and offspring. PFHxS exposure mildly decreased the body weight of female (5 mg/kg bw per day) and male (25 mg/kg bw per day) pups. No other relevant effects on anogenital distance, nipple retention or organ weights were identified.
Potential reproductive and/or developmental toxicity of PFHxS was also evaluated in CD1 mice (Chang et al., 2018), using oral gavage at 0, 0.3, 1 and 3 mg/kg bw per day (n = 30 animals per sex, and a toxicokinetic experiment with 12 mice per group). F0 males and females were treated from 14 days prior to cohabitation for at least 42 days (males) and throughout mating, gestation, until PND 21 (dams). F0 dams were sacrificed on PND 22. After weaning (PND 22), F1 animals were directly dosed for 14 days. Evaluated parameters included clinical observations, mating and oestrus cyclicity, functional observational batteries, related to general animal health and behaviour, motor activity, in response to noise, blood parameters, organ weights, organ histology by microscopy, reproductive parameters including sperm parameters, TSH levels and gene expression. Results for other endpoints than reproductive ones are for males reported in Section 3.3.3.2. In F0, there was an increased mean and relative liver weight at 1 mg/kg bw per day. Regarding reproductive parameters, a reduced litter size (without impact on the born pup to implant ratio) was observed at 1 and 3 mg/kg bw per day, but without clear dose‐response. In F1 animals, an increased relative liver weight in females and males, as well as an increased thyroid weight in males was observed at 3 mg/kg bw per day.
In the 28‐day study conducted by NTP (NTP 2019b), PFHxS at doses up to 10 mg/kg bw per day in males and up to 50 mg/kg bw in females had no effect on sperm parameters, testosterone levels or oestrus cyclicity in rats.
In summary, the most sensitive reproductive endpoint for PFHxS exposure was reduced litter size at 1 mg/kg bw per day in mice (51.5 μg/mL serum on GD 18 in dams) with an NOAEL of 0.3 mg/kg bw per day (16.8 μg/mL serum on GD 18 in dams). At 1 mg/kg bw per day, increased liver weight was seen in the dams. In general, gestational exposure to PFHxS produces effects in offspring animals at doses which were equal to or higher than those inducing responses in parental animals (see also Section 3.3.3.2).
3.3.3.3.5. Other PFASs
Appendix F Table F.5 lists the available reproductive and developmental studies for 8:2 FTOH and EtFOSE. No studies were identified for PFOSI, 8:2 monoPAP, 8:2 diPAP and FOSA.
Table F.5.
8:2 FTOH and EtFOSE reproductive and developmental toxicity
| Substance/ (Purity) | Species/Experimental design and doses | Observed effects | Highest dose with no effect (mg/kg bw per day) | Lowest dose with effect (mg/kg bw per day) | Serum/tissue levels of compound | Reference | ||
|---|---|---|---|---|---|---|---|---|
| Mother | offspring | Mother | offspring | |||||
| 8:2 FTOH (99.2% pure) |
Crl:CD (SD)IGS BR rats (time mated) 0, 50, 200, 500 mg/kg bw per day, oral gavage Exposure GD 6–GD 20 22 rats per dose group |
Mortality Decreased body weight and body weight gain (Slight) reduction in food consumption Slight increases in delayed skull ossification Increased skeletal variations (due to delayed ossification) |
200 200 100 |
100 200 |
500 500 200 |
200 500 |
Mylchreest et al. (2005) | |
| 8:2 FTOH (purity not specified) |
CD1 mice Experiment 1: 30 mg/kg per day on GD 8 (n = 15 control, n = 26 treatment group). Follow‐up by serial sacrifice at GD 9, GD 10, GD 13, GD 15, and GD 18 Experiment 2 (cross fostering): 30 mg/kg per day on GD 8 (n = 34 control, n = 36 treatment group). From PND 0 cross‐fostering of half of the animals Follow‐up on PND 1, 5, 15 |
Pre‐ and postnatal exposure to PFOA and PFNA following maternal treatment at GD 8 with 8:2 FTOH |
No 8:2 FTOH detectable after 24 h of treatment of dams. Only quantifiable compounds PFOA and PFNA. 8:2 FTOH not detectable in fetal or neonatal tissue. Placental PFOA 49 ± 13 ng/g. Fetal period: Maternal serum: Decrease 789 to 668 ng/mL (GD 9–18) Maternal liver: Decrease 673 to 587 ng/g (GD 9–18). Fetuses (whole body burden): Increase PFOA 49 ng/g to 140 ng/g (GD 9–18), PFNA only quantifiable at GD 18 at 31 ng/g Postpartum period: Maternal serum: Decrease PFOA 451–52 ng/mL (PND 1–PND 15) Neonates (treated mothers, whole body burden): 200 to 149 ng/g (PND 1 – PND 3) Neonates (untreated mothers, cross fostering, whole body burden): 57 to 58 ng/g (PND 3–PND 15) Levels of PFOA were higher than levels of PFNA in serum and liver of dams, fetuses and neonates Cross fostering experiments provide evidence for lactational exposure |
Henderson and Smith (2007) | ||||
| EtFOSE (98.2% pure |
Female Crl:CD BR rats Dose range finder study: Dosage: 0, 1, 5, 10, 20, 25, 35 mg/kg bw per day, 8 pregnant animals/group, oral administration from GD 6–17. Necroscopy on GD20. Developmental toxicity (teratology) study: Dosage 0, 1, 5, 10, 20 mg/kg bw per day, 25 pregnant animals/group, oral administration GD 6–17. Necroscopy at GD 20 |
Results from dose range finding study Emaciation Reduced maternal body weight Reduced fetal body weight Results from developmental toxicology study Reduced body weight gain during pregnancy Reduction of fetal weight |
25 5 10 5 |
5 |
35 10 20 10 |
10 | No information given | Case et al. (2001) |
|
Rabbits Dose range finder study: Dosage: 0, 1, 5, 10, 25, 50, 75 mg/kg bw per day, 5 mated rabbits, oral administration GD 6–20. Sacrifice at GD 29. Developmental toxicity (teratology) study: Dosage 0, 0.1, 1, 2.5, 3.75 mg/kg bw per day, 22 pregnant does/group, oral administration GD 7–20. Sacrifice at GD 29. |
Results from dose range finding study Weight loss Severe toxicity and death Abortion Results from developmental toxicology study Abortion Permanent reduced body weight gain Transient reduction of body weight (GD 7–13) |
1 10 1 1 0.1 |
1 |
5 25 2.5 2.5 1 |
5 | No information given | Case et al. (2001) | |
EtFOSE: N‐ethyl perfluorooctane sulfonamido ethanol; FTOH: fluorotelomer alcohol; GD: gestation day; PFOA: perfluorooctanoic acid; PFNA: perfluorononanoic acid; PND: postnatal day.
The potential maternal and developmental toxicity of 8:2 FTOH was assessed in time‐mated female Crl:CD (SD)IGS BR rats (Mylchreest et al., 2005). 22 animals per group were orally gavaged with 0, 50, 200, 500 mg/kg bw per day from GD 6 to 20. In dams, exposure to 500 mg/kg bw per day resulted in mortality, a decreased body weight and body weight gain, which were not observed at 200 mg/kg bw per day. A slight reduction in food consumption was in some time periods detectable at 200 mg/kg bw per day. In offspring animals, slight increases in delayed skull ossification became apparent at 200 mg/kg bw per day, but was not considered a reliable indicator of developmental toxicity by the authors, since a common observation and within historical control values. Increased skeletal variations, due to delayed ossification, became apparent at 500 mg/kg bw per day, and the NOAEL for developmental and maternal toxicity was 200 mg/kg bw per day.
Toxicokinetic experiments in rodents have indicated that 8:2 FTOH is rapidly metabolised into PFOA and PFNA and any toxicity may not be attributable to the parent compound (see Appendix C, Section C.4).
A developmental toxicity study with EtFOSE, which was designed as a teratology study with termination of the experiment prior to the end of gestation, was performed with pregnant Crl:CD BR rats and with pregnant rabbits (Case et al., 2001). For both animal species, a dose range finding was performed prior to the teratology study. In the dose finding studies, eight pregnant rats per group were orally dosed with 0, 1, 5, 10, 20, 25 and 35 mg/kg bw per day of EtFOSE from GD 6 to GD 17. Developmental toxicity studies were performed with 25 pregnant rats in a dose range of 0, 1, 5, 10 and 20 mg/kg bw per day from GD 6 to GD 17. Rats were sacrificed at GD 20. With rats, a maternal and a developmental NOAEL for EtFOSE of 5 mg/kg bw per day was determined. This was based on reduced maternal weight and weight gain during pregnancy and reduced fetal weight, which occurred at doses of 10 mg/kg bw per day and higher and which was most probably caused by reduced food consumption. Fetal effects did not occur at doses below those of maternal toxicity. No indications for selective developmental toxicity was found in rat fetuses.
In the dose range finding study with rabbits, five pregnant rabbits were orally dosed with EtFOSE at 0, 1, 5, 10, 25, 50 and 75 mg/kg bw per day from GD 7 to 20. In developmental toxicity studies (22 pregnant does per dose), animals were orally dosed with 0, 0.1, 1, 2.5 and 3.75 mg/kg bw per day during GD 7 to 20. Rabbits were sacrificed at GD 29. The authors established a maternal NOEL of 0.1 mg/kg bw per day based on a transient reduction in maternal body weight during GD 7–13 in response to 1 mg/kg bw per day. An NOAEL of 1 mg/kg bw per day was derived based on spontaneous abortions at doses of 2.5 mg/kg bw per day and higher. Like for the rats, no selective developmental toxicity could be observed in rabbits.
3.3.3.3.6. Summary
The most sensitive developmental effect observed was impaired development of the mammary gland in mice, after exposure of the dams late in gestation or the offspring in utero or via lactation. The impairment persisted beyond sexual maturity. Only PFOA has been investigated in relation to this outcome, with LOAELs of 0.01–0.00045 mg/kg bw per day, depending on the dosing period. These NOAELs resulted in a similar serum concentration in pups on PND 21, which corresponded to a maternal LOAEC around 66 ng/mL serum.
In addition, developmental toxicity studies in rodents were identified for the 10 PFCAs (PFBA, PFHxA, PFOA, PFNA, PFDA, PFUnDA, PFDoDA, PFTeDA, PFHxDA and PFODA), for three PFSAs (PFBS, PFHxS and PFOS) and for the two other PFASs (8:2 FtOH and EtFOSE). The effects most often observed were increased fetal and/or neonatal mortality and reduction in fetal weight and/or postnatal growth. In general, developmental toxicity occurred at similar or slightly lower doses than those exerting maternal toxicity. The LOAELs and NOAELs were generally orders of magnitude higher than for the developmental effects on mammary gland seen in response to PFOA.
Reproductive parameters were investigated in a comparative way in rat for PFHxA, PFOA, PFNA, PFDA, PFBS, PFHXsS and PFOS in the 28‐day studies by NTP (2019a,b), although noting that 28 days is shorter than one spermatogenic cycle, and too short to fully assess male reproductive parameters. There were no consistent findings for female reproductive parameters. Effects on male reproductive parameters, including atrophy of the testicular interstitium, accompanied by reduced serum testosterone levels and epidydimal and testicular weights, as well as degenerative changes and spermatid retention in seminiferous tubules, have been reported for PFNA and PFDA.
3.3.3.4. Neurotoxicity
3.3.3.4.1. PFOS and PFOA
In 2018, the EFSA CONTAM Panel concluded that both PFOS and PFOA exert developmental neurotoxic effects in rodents at doses of 0.1–0.3 mg/kg bw per day or higher. The analysis of behaviour showed that the most frequent alterations observed are related to locomotor activity. PFOS exposure mostly decreased spontaneous activity, while PFOA increased it. In several neurodevelopmental exposure studies, a sex‐related difference has been observed with males being more sensitive than females. The CONTAM Panel has not identified relevant new neurotoxicity studies in experimental animals.
3.3.3.4.2. PFASs other than PFOS and PFOA
The literature search from 2000 onwards identified the following studies.
In vivo neurotoxicity studies were identified for PFDA, PFHxS and PFDoDA and are listed in Appendix G Table G.1. All studies were single exposure studies.
Table G.1.
Developmental neurotoxicity studies
| Substance (Purity) | Species/Experimental design and doses | Observed effects | Highest dose with no effect (mg/kg bw per day | Lowest dose with effect (mg/kg bw per day) | Serum/tissue concentration | Reference |
|---|---|---|---|---|---|---|
| Perfluorodecanoic acid (PFDA) | ||||||
| PFDA; purity 98% |
Mouse, NMRI developmental exposure, single exposure at PND10 1.4, 21 μmol/kg bw (0.72; 10.8 mg/kg bw per day) oral (n = 4 to 7 per group) |
Decreased locomotor activity | N/A | 10.8 at 2 months; 0.72 at 4 months | N/A | Johansson et al. (2008) |
| Perfluorohexane sulfonic acid (PFHxS) | ||||||
| PFHxS; purity > 98% |
Mouse, NMRI developmental exposure, single exposure at PND10 1.4; 14; 21 μmol/kg bw (0.61; 6.1; 9.2 mg/kg bw per day) oral (n = 12 per group) |
Decreased locomotor activity at 2 months | N/A | 0.61 | N/A | Viberg et al. (2013) |
| PFHxS; purity > 98% |
Mouse, NMRI developmental exposure, single exposure at PND10 14, 21 μmol/kg bw (6.1; 9.2 mg/kg bw) oral (n = not reported) |
Increased expression of CaMKII and Tau in hippocampus at PND11; increased Tau level in cortex at 4 months | N/A | 6.1 | N/A | Lee and Viberg (2013) |
PND=postnatal day. Oral defines exposure via ingestion of drinking water, food or via gavage; N/A: Not applicable.
In the paper by Johansson et al. (2008), NMRI mice were exposed at PND10 by gavage to 1.4 or 21 μmol/kg bw (0.72 or 10.8 mg/kg bw) PFDA, PFOS or PFOA. The effects on behaviour were investigated at the age of 2 and 4 months by monitoring spontaneous activity in the open field, and by recording the activity in the elevated plus maze in order to assess anxiety‐like behaviour. With the exception of a decrease in spontaneous activity in the first 20 min of testing in the lower dose group at 2 months of age, the authors did not find significant differences between PFDA‐exposed mice and controls.
Viberg et al. (2013) exposed NMRI mice to 1.4, 14 or 21 μmol/kg bw (0.61, 6.1 or 9.2 mg/kg bw) PFHxS at PND10 via gavage. Spontaneous locomotor activity was assessed in 2‐ and 4‐month‐old mice, both males and females. In addition, the increase in locomotor activity stimulated by nicotine (80 μg/kg bw, s.c.) was assessed at the age of 4 months. At the age of 2 months, the mice exposed to the highest dose (both males and females) displayed globally lower levels of activity, and decreased habituation. Male mice exposed to the lowest dose displayed hyperactivity in the beginning of the testing period. When the animals were tested at the age of 4 months, the authors describe a similar pattern of alterations as at 2 months. Upon stimulation by subcutaneous administration of nicotine, the normal increase in activity was blunted in the animals exposed to the highest dose. In a study by Lee and Viberg (2013), NMRI mice were exposed to 14 or 21 μmol/kg bw (6.1 or 9.2 mg/kg bw) PFHxS at PND10 via gavage. The mice were killed at 24 h and 4 months after exposure, and the levels of CAMK‐II, GAP‐43, synaptophysin, Tau and BDNF were measured in the frontal cortex and in the hippocampus. In the hippocampus, the lower dose increased the expression levels of CAMK‐II and Tau, while the higher dose increased the expression of CAMK‐II, synaptophysin and Tau in both male and female mice. No alterations in protein expression were detected in the hippocampus 4 months after exposure. In the cerebral cortex, the expression of GAP‐43 was decreased 24 h after exposure in both males and females. Four months after exposure, only male mice displayed an increase in Tau expression, while females showed no change in protein expression.
Kawabata et al. (2017) compared the level of PFDoDA with those of PFOA and PFDA in rats 9 days after a single oral dose (50 mg/kg bw). The PFDoDA level in the brain was 44.4 ± 2.0 μg/g, higher than that in the serum (24.4 ± 1.0 μg/mL). Conversely, the brain concentrations of PFOA and PFDA were low (< 0.8 and 4.7 ± 0.4 μg/g), one‐tenth of those in the serum. Behavioural analyses were performed to assess cognitive function alterations induced by PFOA, PFDA and PFDoDA. The novel object recognition test performed 5–6 days after exposure revealed a significant decrease in the discrimination index in PFDoDA‐exposed rats, whereas there were no significant alterations in PFDA‐ and PFOA‐treated rats. The effects of PFDoDA were also assessed by the elevated‐plus maze test, the Y‐maze test, open‐field test and forced swim test. PFDoDA induced alterations in the elevated‐plus maze test, but not in the Y‐maze test, open‐field test and forced swim test. The results show that PFDoDA easily distributes in the brain and causes cognitive behavioural alterations.
3.3.3.4.3. In vitro studies
Three in vitro studies have been identified for PFHxS, PFNA, PFUnDA.
Berntsen et al. (2017) compared the cytotoxicity of PFASs with different length of the perfluorinated carbon chain and functional group attached. Two PFSAs, chain length C6 and C8, and four PFCAs, chain length C8–C11, were studied using primary cultures of rat cerebellar granule neurons (CGNs). Toxicity potencies were determined by evaluating cell viability by trypan blue and MTT assays after exposure to PFUnDA, PFDA, PFOS, PFNA, PFOA or PFHxS at concentrations determined by preliminary range‐finding experiments. The following NOEC–LOEC values were identified: PFHxS 300–400 μM; PFOS 10–20 μM; PFOA 200–300 μM; PFNA 20–40 μM; PFDA 10–20 μM; PFUnDA 10–15 μM. Different PFAS doses, producing equipotent effects after 24 h exposure, were applied to study the dynamics of viability changes after 10, 30, 60, 90, 120 and 180 min, as well as 6, 12, 18 and 24 h. The following concentrations were used: 550 μM PFHxS, 60 μM PFOS, 500 μM PFOA, 100 μM PFNA, 40 μM PFDA and 30 μM PFUnDA. The onset of reduction in viability occurred relatively quickly (30–60 min) for PFOS, PFDA and PFUnDA as compared to PFHxS, PFOA and PFNA (12–24 h). Vitamin E exerted a slight protective effect against PFOA, PFNA and PFOS‐induced reduction in viability, suggesting the occurrence of oxidative stress. At cell membrane level, PFHxS, PFOS and PFUnDA aggregated in large hotspots, whereas PFOA, PFNA and PFDA had a scattered distribution pattern. In summary, PFAS toxicity increased with increasing length of carbon chain, and a sulfonate functional group induced greater toxicity than a carboxyl group.
In a follow‐up study, Berntsen et al. (2018) showed that excitotoxicity induction by PFOS in CGNs is likely due to over‐activation of the N‐methyl‐D‐aspartate receptor (NMDA‐R) and excess influx of Ca2+. Channel blockers (memantine and MK‐801) also showed some effects against PFHxS‐induced reductions in viability. No substantial involvement of the NMDA‐R in PFOA‐, PFNA‐ and PFDA‐induced effects on viability were observed.
Fang et al. (2018) showed that in PC12 cells PFNA (0, 10 20, 50, 100 μM, 24 h) dose‐dependently decreased the viability (≥ 20 μM PFNA), increased the malondialdehyde content (100 μM PFNA) and the intracellular calcium level (≥ 50 μM PFNA), and decreased the total antioxidant capacity (≥ 50 μM PFNA). Additionally, an upregulation of the Ca2+calmodulin‐dependent protein kinase II (CaMKII) expression and decrease in Bcl‐2 expression and increased Bax were observed.
3.3.3.4.4. Summary
In summary, as reported before for PFOS (EFSA CONTAM Panel, 2018), PFHxS and PFDA have been shown to decrease locomotor activity in rodents. Data from one study indicate that PFDoDA, in contrast to PFDA and PFOA, can efficiently transfer into rat brain and causes cognitive behavioural changes.
3.3.3.5. Immunotoxicity
In general, effects on the immune system include among others, changes in weights of lymphoid organs (thymus, spleen), cellularity of these organs, expression of interleukins and total immunoglobulin levels. Such parameters may indicate effects on the immune system. There are also more direct parameters that gauge the functionality of the immune system, such as antibody responses to T‐cell‐dependent antigens. The antibody response to a T‐cell‐dependent antigen in experimental animals is a prime parameter to indicate immunotoxic effects in experimental animals (Luster et al., 1993). A model that has mostly been used is to sensitise rodents by i.v. or i.p. injection of sheep erythrocytes (SRBCs), followed by enumerating specific antibody producing cells in a blood‐agar plate, by counting the plaques formed. These plaques are formed by lysis of erythrocytes by IgM produced by plasma cells, in conjunction with complement that is also in the system. This so‐called plaque forming assay (PFC) is a model for vaccination to a T‐cell‐dependent antigen in humans. An alternative way to measure the antibody response to the PFC assay is to measure the antibodies in serum by Enzyme‐Linked Immunosorbent Assay (ELISA). Other antigens, than SRBCs used in experimental studies include ovalbumin, keyhole limpet haemocyanin (KLH) or TNP‐LPS (trinitrophenol coupled to lipopolysaccharide). In order to achieve antibody production specific for a particular antigen, different components of the immune system are required, i.e. antigen processing by phagocytes, antigen presentation by antigen presenting cells, induction of T‐helper lymphocytes, maturation of B cells into plasma cells and eventually production of different isotypes of specific antibodies. Different antigens depend, to a different extent, on the various components of the immune system. For instance, particulate antigens such as sheep erythrocytes require more processing than soluble antigens. KLH is more T‐cell dependent than TNP‐LPS. Also, the duration of the sensitisation schedule is a determinant of the eventual outcome. Hence, effects of an exogenous factor on antibody production may depend on the antigen and immunisation schedule used. A decrement in antibody titres may not only signify decreased protection to the specific pathogen, but also signifies a decrement in functionality of the immune system, potentially resulting in reduced resistance to infectious diseases as well as resistance to tumours (van Loveren et al., 2001; WHO/IPCS, 2012). An approach that directly probes the efficacy of the immune system is the use of experimental infections. A reduced resistance to an infectious agent may represent the ultimate consequence of a suppressed immune functionality.
3.3.3.5.1. PFOS
Studies already assessed in 2018
In the previous Opinion (EFSA CONTAM Panel, 2018), several animal studies that have investigated effects of PFOS exposure on the immune system were reviewed. The studies on PFOS are briefly summarised below (Table 20, see also Appendix H). The studies had different study design, used different strains of mice or rats, applied different doses of PFOS and investigated different parameters that may shed light on effects on the immune system. Also duration was different and studies are presented according to short‐term or longer term exposure.
Table 20.
Studies on immunological effects of PFASs in rodents
| Species | Strain | Sex | Route | Duration | PFAS | NOAEL | LOAEL | NOAEC | LOAEC | Immune treatment | Days before sacrifice | Immune endpoint (*) | Effect | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (days) | (mg/kg per day) | (ng/mL) | ||||||||||||
| Mouse | B6C3F1 | Male | Gavage | 28 | PFOS | 0.000166 | 0.00166 | 18 | 92 | SRBC | 5 days | PFCs | ↓ | Peden‐Adams et al. (2008) |
| Mouse | B6C3F1 | Female | Gavage | 28 | PFOS | 0.00331 | 0.0166 | 123 | 666 | SRBC | 5 days | PFCs | ↓ | |
| Mouse | B6C3F1 | Female | Gavage | 21 | PFOS | 0.005 | 0.025 | 189 | 670 | Influenza | None | Survival | ↓ | Guruge et al. (2009) |
| Mouse | B6C3F1 | Male | Diet | 28 | PFOS | 0.25 | – | 11,600 | – | SRBC | 5 days | Serum IgM, PFCs | – | Qazi et al. (2010) |
| Mouse | B6C3F1 | Male | via dams, gavage | GD 1–17 | PFOS | 1 | 5 | NR | NR | SRBC | 4 days | PFCs | ↓ | Keil et al. (2008) |
| Mouse | B6C3F1 | Female | PFOS | 5 | – | NR | – | SRBC | 4 days | PFCs | – | |||
| Mouse | C57BL/6 | Male | Gavage | 60 | PFOS | 0.00833 | 0.0833 | 674 | 7,132 | SRBC | 4 days | PFCs | ↓ | Dong et al. (2009) |
| Mouse | C57BL/6 | Male | Gavage | 60 | PFOS | 0.0167 | 0.0833 | 2,360 | 10,750 | SRBC | 7 days | Serum IgM | ↓ | Dong et al. (2011) |
| Mouse | C57BL/6 | Male | Gavage | 60 | PFOS | 0.0833 | 0.4167 | 8,210 | 24,530 | None | TNF‐a, IL‐6 | ↑ | Dong et al. (2012) | |
| Mouse | C57BL/6 | Male | Gavage | 7 | PFOS | – | 5 | – | 110,460 | SRBC | 5 days | PFCs | ↓ | Zheng et al. (2009) |
| Mouse | C57BL/6 | Male | Gavage | 7 | PFOS | – | 5 | – | 97,250 | None | Non‐specific IgM | ↓ | Zheng et al. (2011) | |
| Mouse | BALB/c | Female | Gavage | 21 | PFOS | – | 20 | – | NR | Ovalbumin | 14 and 7 days (two injections) | Serum IgM | ↓ | Vetvicka and Vetvickova, 2013) |
| PFOA | – | 20 | – | NR | ↓ | |||||||||
| Mouse | CD‐1(ICR)BR | Male | Gavage | 29 | APFO | 1 | 10 | 32,000 | 225,000 | SRBC | 5 days | Serum IgM | ↓ | Loveless et al. (2008) |
| Mouse | C57BL/6 | Female | Gavage | 15 | PFOA | 1.88 | 3.75 | NR | 74,913 | SRBC | 5 days | Serum IgM | ↓ | DeWitt et al. (2008) |
| Mouse | C57BL/6 | Female | Water | 15 | PFOA | 7.5 | 30 | NR | NR | SRBC | 5 days | Serum IgM | ↓ | DeWitt et al. (2016) |
| Rat | SD | Male | Diet | 28 | PFOS | – | 0.14 | 470 | 950 | None | Serum IgG1 | ↓ | Lefebvre et al. (2008) | |
| Rat | SD | Female | Diet | 28 | PFOS | 7.58 | – | 43,200 | – | None | – | – | Lefebvre et al. (2008) | |
| Rat | CD(SD)IGS BR | Male | Gavage | 29 | APFO | 30 | – | 223,000 | – | SRBC | 5 days | Serum IgM | – | Loveless et al. (2008) |
APFO: ammonium perfluorooctanoate; LOAEL: lowest‐observed‐no‐adverse‐effect level; LOAEC: lowest‐observed‐no‐adverse‐effect concentration; NOAEL: no‐observed‐no‐adverse‐effect level; NOAEC: no‐observed‐no‐adverse‐effect concentration; NR: not reported; PFAS: perfluoroalkyl substance; PFOS: perfluoroheptane sulfonate; PFCs: plaque‐forming colonies in spleen cells producing anti‐SRBC antibodies.
In case serum IgM is mentioned as well as the time between injection of antigen and sacrifice, authors looked for antigen‐specific IgMs.
Qazi et al. (2009a,b, 2012) carried out a number of short‐term exposure studies and observed that after PFOS administration, body weight, thymus, spleen and epididymal fat weights were reduced compared to controls. A significant reduction in the number of total white blood cells and lymphocytes, but not neutrophils, was observed in the circulation, in addition to reduced numbers of macrophages in the bone marrow, but not the spleen or peritoneal cavity. Production of TNF‐α and IL‐6 by macrophages isolated from animals treated with PFOS was modestly increased, while ex vivo stimulation with LPS enhanced production of TNF‐α and IL‐6 by peritoneal and bone marrow (but not splenic) macrophages taken from PFOS exposed animals. These results may indicate immunomodulatory activity but cannot rule out that the effects were secondary to a general toxic effect as also effects on body weights were noted. It was noted that after 10 days of cessation of exposure, body weight had recovered, whereas relative weights of liver, thymus and spleen remained largely unchanged, while numbers of lymphocytes of the B‐cell lineage did not recover, hence were still reduced.
Wang et al. (2011a) reported the outcome of short‐term exposure studies that are in line with those of Qazi et al. (2009a,b, 2012), i.e. significantly decreased thymus and spleen weights, compared with controls. These effects were associated with increased thymus and spleen atrophy. Immune effects were noted at doses that also affected body weights. In line with the latter were results from a study by Zhang et al. (2013d), who also reported induction of apoptosis in splenocytes and thymocytes isolated from mice exposed to PFOS.
Also, Vetvicka and Vetvickova (2013) found effects on the immune system after short‐term exposure to PFOS. As in the studies by Qazi et al. (2009a,b, 2012) and Wang et al. (2011a), a significant decrease in cellularity of both the spleen and thymus was observed. In addition, significantly inhibited phagocytosis (by peripheral blood neutrophils) and NK splenic activity was observed, as well as suppressed T‐lymphocyte proliferation but not B‐cell proliferation. Also in this study, effects on immune parameters were noted while also body weights were depressed.
Whereas these studies have indicated some effects of short‐term exposure to PFOS on general parameters of the immune system, another approach is to investigate the functionality of the immune system, as indicated above. Zheng et al. (2009, 2011) achieved this by sensitising animals to sheep erythrocytes. While effects on the cellularity in spleen and thymus were noted as in other studies, as well as effects on cytokine expression, the antibody response to the sheep erythrocytes as measured by plaque‐forming activity, was reduced at lower exposure doses that did not affect body weights or other parameters of general toxicity. It is noteworthy that in contrast to IgM, IgG was increased rather than decreased. In addition to effects on antibody production, also lymphocyte proliferation and NK‐cell activity, both parameters of the functionality of the immune system, were reduced at the lowest dose tested.
In addition to short‐term studies, also studies with a longer exposure time were performed, i.e. 21, 28 or 60 days, as well as during development.
Fair et al. (2011) observed in a 28‐day exposure study with adult female B6C3F1 mice variable effects on several non‐functional parameters of the immune system, with increased IL‐6 production by B cells as the most sensitive parameter, observed at 0.1 mg/kg (TAD). Mollenhauer et al. (2011), observed in a 28‐day exposure study variable effects on various non‐functional parameters of the immune system, but only at the higher dose at which also effects on body weights were noted.
In a 28‐day exposure study by Qazi et al. (2010), applying only one dose of PFOS, no effects on circulating lymphocytes, the number of cells in the thymus or spleen, the number of plasma cells secreting anti‐SRBC IgM, the levels of IgM, or of IgG directed to SRBCs were noted, nor were effects on IgM antibody response to TNP recorded.
In contrast, Vetvicka and Vetvickova (2013) who administered PFOS to mice also for 21 days and sensitised the animals to ovalbumin, found significantly reduced NK splenic activity and antibody responses.
Lefebvre et al. (2008) performed a 28‐day exposure study with Sprague‐Dawley rats and applied two concurrent study protocols: one in which animals were only exposed to PFOS, the other where animals were also sensitised to keyhole limpet haemocyanin (KLH), to investigate antibody production and delayed‐type hypersensitivity to KLH. In the study performed according to the first protocol, significant trends in the elevation of levels of total IgG, IgG2a and IgG2c were reported in males (and IgG2c also in females), whereas serum IgG1 levels were significantly reduced in the male dose groups that did not show effects on body weight. In the study performed according to the second protocol, there was a trend towards increasing concentrations of IgG specific for KLH in males, but none of the doses showing a significant difference.
Dong et al. (2009) investigated the effects of PFOS on immunotoxicity in male C57BL/6 mice after longer term exposure. PFOS (potassium salt, purity 98%) was administered via oral gavage (in deionised water in 2% Tween 80) to groups of 10 mice at dose levels of 0, 8.3, 83.33, 416.67, 833.33 or 2,083.33 μg/kg bw per day for 60 days (to achieve total added doses (TAD) of 0.5, 5, 25, 50 or 125 mg/kg bw). A dose‐related decrease in specific IgM antibody production as measured by the PFC assay was observed at doses of 5 mg/kg bw TAD and higher. The level of PFOS in serum at the 5 mg/kg bw TAD dose was determined to be 7,132 ng/mL. These functional effects on the immune system were noted at doses not causing other toxicity. It should be noted that, in contrast to the other studies reported here that used the antibody response to SRBC as a read out of functionality of the immune system, Dong et al. (2009) applied a shorter sensitisation schedule for the PFC assay (4 days instead of 5 or 6), which may result in a lower PFC response at the time of harvesting, as demonstrated by the lower numbers of plaques counted as compared for instance with the study by Peden‐Adams et al. (2008), presented below.
In a follow‐up study by Dong et al. (2011), with doses of (TAD) 0.5, 1, 5, 25 and 50 mg/kg bw for 60 days, antibody responses to SRBC were measured using another approach, i.e. an ELISA to specific antigens of SRBC. SRBC‐specific serum IgM levels were decreased at doses of 5 mg/kg bw (TAD) and higher. Specific IgG, IgG1 and IgE levels were significantly elevated at the highest dose of 50 mg/kg bw (TAD). The serum level at the 5 mg/kg bw dose was 10,750 ng/mL.
In a study by Peden‐Adams et al. (2008), PFOS (potassium salt, purity > 98%, doses of 0, 0.166, 1.66, 3.31, 16.6, 33.1 or 166 μg/kg bw per day) were administered to B6C3F1 mice (groups of 5 males or females) via oral gavage (0.5% Tween 20 in Milli‐Q water) for 28 days. The TAD over 28 days was 0, 0.005, 0.05, 0.1, 0.5, 1 or 5 mg PFOS/kg bw (reported as free ion doses). There were no signs of overt toxic effects on body weight or organ weight of spleen, thymus, liver, kidney or gonads following PFOS exposure, nor were the cellularity and viability of spleen and thymus affected. NK‐cell activity was significantly increased by 2‐ to 2.5‐fold in males exposed to ≥ 0.5 mg/kg bw TAD but not in females. The specific IgM SRBC response, as measured by plaque forming colonies, was suppressed in males (52–78%) and females (50–74%) at ≥ 0.05 and ≥ 0.5 mg/kg bw TAD, respectively. In males, T‐cell CD4/CD8 subpopulations in the thymus were not affected by PFOS whereas the numbers of all T‐cell subpopulations were altered in the spleen at ≥ 0.1 mg/kg bw TAD (CD4‐/CD8+ and CD4‐/CD8‐ were increased while CD4+/CD8 and CD4+/CD8+ were decreased). In females, the numbers of T‐cell populations were minimally affected by PFOS. Splenic CD4–/CD8+ cells were decreased at ≥ 0.1 mg/kg TAD, whereas CD4+/CD8 were decreased at 0.1 and 0.5 mg/kg bw TAD only. The effects on sensitisation to SRBC was corroborated in a separate experiment, where female mice were dosed with 0.344 mg PFOS/kg bw per day for 21 days (TAD, 7 mg/kg bw) and, 7 days before termination, animals were injected iv with TNP. Serum levels of TNP‐specific IgM were significantly suppressed (62%) compared with controls. The authors determined an LOAEL of 0.05 mg/kg bw TAD for males and 0.5 mg/kg bw TAD for females, i.e. a LOAEL in males of 1.66 μg/kg bw per day, corresponding to a measured serum level of 91.5 ng/g. The NOAEL of 0.166 μg/kg bw per day resulted in a no observed adverse effect concentration (NOAEC) serum level of 18 ng/mL. Peden‐Adams et al. (2008) mention that the study was repeated with similar results. Upon request from EFSA, data on both studies were provided to EFSA. It was stressed that the results were obtained by a standardised protocol (Peden‐Adams, 2020, personal communication) and that for the results in the second study, shown in the paper, samples were blinded for the person scoring the PFCs. In addition, data were provided on an unpublished study with C57BL/6 mice (WT and PPARα mutant) where a similar protocol was applied. The results suggest that the B6C3F1 strain is more sensitive than the C57Bl/6 strain used by Dong et al. (2009), which may be another reason for the lower doses of PFOS causing the effect on the antibody titres in the study by Peden‐Adams et al. (2008) compared to Dong et al. (2009) (see Appendix H, Table H.3 and Figures H.1–H.3).
Table H.3.
Data provided by Peden‐Adams on request of EFSA, showing the results from two independent studies, each with both female and male B6C3F1 mice treated with perfluorooctanoic acid (PFOA) via gavage (n = 5 per dose per sex)
| first experiment | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| females | males | |||||||||||
| TAD | serum (ng/g) | PFCs/million cells | % of | serum (ng/g) | PFCs/million cells | % of | ||||||
| (mg/kg bw) | n | mean | SD | mean | SD | control | n | mean | SD | mean | SD | control |
| 0 | 5 | ND | 2,631 | 814 | 100% | 5 | 4,641 | 879 | 100% | |||
| 0.005 | 4 | ND | 1,539 | 563 | 58% | 5 | 4,019 | 2,026 | 87% | |||
| 0.05 | 5 | ND | 1,472 | 306 | 56% | 5 | 1,853 | 579 | 40% | |||
| 0.1 | 5 | ND | 1,422 | 550 | 54% | 5 | 2,225 | 567 | 48% | |||
| 0.5 | 5 | ND | 766 | 267 | 29% | 5 | 2,006 | 296 | 43% | |||
| 1 | 5 | ND | 891 | 548 | 34% | 5 | 1,016 | 437 | 22% | |||
| 5 | 5 | ND | 750 | 166 | 29% | 5 | 1,416 | 618 | 31% | |||
| second experiment (published data) | ||||||||||||
| females | males | |||||||||||
| TAD | serum (ng/g) | PFCs/million cells | % of | serum (ng/g) | PFCs/million cells | % of | ||||||
| (mg/kg bw) | n | mean | SD | mean | SD | control | n | mean | SD | mean | SD | control |
| 0 | 5 | 17 | 4 | 3,013 | 694 | 100% | 5 | 12 | 5 | 3,519 | 1,848 | 100% |
| 0.005 | 5 | ND | 2,706 | 733 | 90% | 5 | 18 | 4 | 2,747 | 1,211 | 78% | |
| 0.05 | 5 | 88 | 11 | 3,250 | 514 | 108% | 5 | 92(a) | 22 | 1,328 | 471 | 38% |
| 0.1 | 5 | 123 | 19 | 2,509 | 816 | 83% | 5 | 131 | 15 | 1,094 | 270 | 31% |
| 0.5 | 5 | 666 | 108 | 1,494 | 997 | 50% | 5 | ND | 1,413 | 467 | 40% | |
| 1 | 5 | ND | 1,019 | 362 | 34% | 5 | ND | 1,488 | 323 | 42% | ||
| 5 | 5 | NR | 791 | 364 | 26% | 5 | NR | 1,353 | 472 | 38% | ||
ND: not done; NR: not reported, outside calibration curve; PFC: perfluorinated compound; SD: standard deviation; TAD: total applied dose.
(a): a: n = 4.
Note: Studies were carried out in 2 subsequent years. The data show that the results were reproducible. Data on serum levels were only provided for the second study. TAD is the total applied dose over the 28‐day study.
Figure H.1.
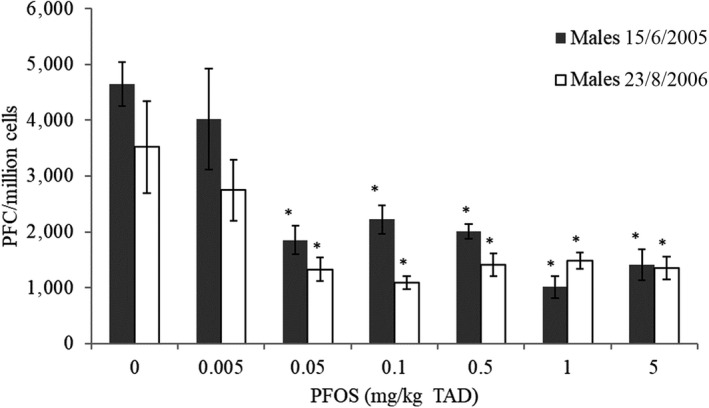
PFC response in male B6C3F1 mice treated with 0, 0.005, 0.05, 0.1, 0.5, 1 or 5 mg PFOS/kg bw (TAD) for 28 days by oral gavage (n = 5)
-
* Significantly different from control (p < 0.05). (Mean and SEM). Two independent experiments.PFC: perfluorinated compound; PFOS: Perfluorooctanesulfonic acid; SEM: standard error of mean; TAD: total applied dose.
Figure H.3.
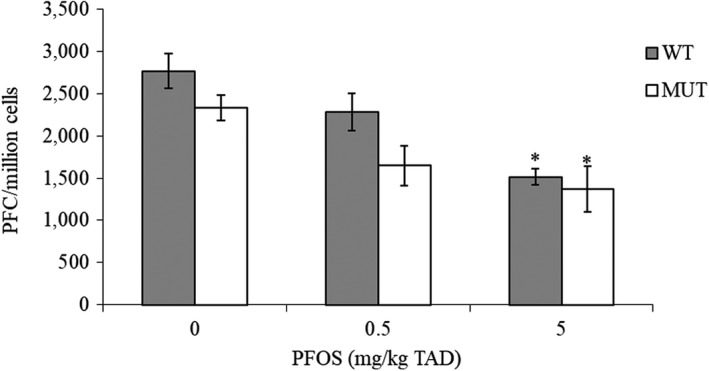
PFC response in female C57Bl/6 mice (WT) and PPARα targeted mutation mouse model (MUT; Taconic), treated with PFOS for 28 d by gavage, with N = 5 (mean, SEM)
-
* Significantly different from control (p < 0.05). Samples were blinded to person reading slides. Doses of 0, 0.5 and 5 mg/kg bw (TAD).PFC: perfluorinated compound; PFOS: Perfluorooctanesulfonic acid; SEM: standard error of mean; TAD: total applied dose.
Keil et al. (2008) measured antibody responses to SRBCs using the PFC in the offspring following maternal exposure to PFOS, thereby establishing an NOAEL of 0.1 mg/kg per day for decreased NK activity and 1.0 mg/kg per day for the antibody response to SRBCs. Also, these authors used a shorter immunisation protocol of 4 days. It is noteworthy that in the study by Keil et al. (2008), there was a considerable sex difference, in that an effect was found in males, but not in females. This was found also by the publication of the same group presented above (Peden‐Adams et al., 2008), be it to a lesser extent.
Guruge et al. (2009) administered PFOS to B6C3F1 mice for 21 days prior to inoculation with mouse‐adapted influenza virus A/PR/8/34 (H1N1) and examined the animals for signs of morbidity and mortality for a further 20 days. The survival rates on day 20 after virus infection were dose‐dependently reduced, being significant at 25 μg/kg bw per day.
New studies on PFOS
Since the deadline for inclusion of studies in the previous EFSA Opinion (EFSA CONTAM Panel, 2018), two additional studies have been published.
For PFOS, Suo et al. (2017) used a mouse infection model using Citrobacter rodentium. Wild‐type mice on a C57Bl background (4 per group), 6–8 weeks old, were exposed to 2 mg PFOS/kg bw per day for a period of 7 days, followed by exposure by gavage of 1010 C. rodentium in 200 μL phosphate‐buffered saline (PBS). PFOS exposure was continued until the end of the experiment, i.e. day 18. Intestinal lamina propria lymphocytes were harvested in the course of the exposure and infection. Whereas early on, PFOS exposure inhibited the outgrowth of the pathogen, which coincided with enhanced production of IL22. In the later phase, enhanced bacterial counts were noted, accompanied by increased display of inflammatory cytokines, reduced mucus production, dysbiosis and increased levels of E. coli.
Lee et al. (2018) investigated the effect of PFOS exposure on allergic inflammation in ICR mice in an ovalbumin model. Sensitised mice, 6 weeks of age, were intraperitoneally sensitised to ovalbumin with adjuvant on day 0 and 7. On days 9, 11 and 13, they were orally exposed to relatively high doses of 50–150 mg PFOS/kg bw, and subsequently challenged i.p. with ovalbumin on day 14. PFOS aggravated the allergic symptoms, i.e. induced hypothermia, and increased serum histamine, TNFα, IgG1 and IgE. The authors did not evaluate other toxic effects of PFOS exposure in these animals, which reduces its validity.
3.3.3.5.2. PFOA
In the previous Opinion (EFSA CONTAM Panel, 2018), also several studies on effects of PFOA on the immune system were reviewed.
In two oral exposure studies, DeWitt et al. (2008, 2009) performed in female C57BL/J mice, in which PFOA (98% purity) were administered by oral gavage for 10 days, IgM synthesis after immunisation to sheep red blood cells (SRBCs) was significantly decreased, with an NOAEL for this parameter at 1.88 mg/kg per day. Mice were also immunised to ovalbumin, and also IgM responses to this antigen were suppressed with an NOAEL at 1.88 mg/kg bw per day. The same group repeated studies in 2016 (DeWitt et al., 2016), studies were repeated using a T‐cell independent antibody response (to i.v. DNP sensitisation) and also using this endpoint, an NOAEL of 1.88 mg/kg bw per day was found. Similar studies were performed by Vetvicka and Vetvickova (2013), who administered PFOA female BALB/c mice, for either 10 or 21 days, and significantly reduced antibody responses to ovalbumin and the formation of IgM directed against tri‐nitrophenyl (TNP) were observed at doses of 20 mg/kg bw per day (lowest dose tested).
Another important study was performed by Loveless et al. (2008). These authors administered PFOA (100% pure, 0, 0.3, 1, 10, 30 mg/kg bw per day) by oral gavage to groups of male CD‐1 mice and 10 male CD rats for 29 days. Rats and mice were given an i.v. dose of SRBCs on day 23 or 24, respectively, and sacrificed on day 28. Immune effects included reduced IgM titres to SRBCs (at 10 and 30 mg/kg bw per day), decreased spleen and thymus weights and numbers of cells in these. In rats, unlike mice, no effects on SRBC‐IgM titres were reported. The NOAEL for immunotoxicity in this study was 1 mg/kg bw per day based on suppression of the anti‐SRBC IgM response.
3.3.3.5.3. PFASs other than PFOS and PFOA
In two studies by Fang et al. (2008, 2009), indications of immunotoxic activity were noted. In the first study, PFNA was administered via oral gavage (vehicle not stated) to groups of 6 male BALB/c mice at 0, 1, 3 or 5 mg/kg bw per day for 14 days. Levels of serum adrenocorticotropic hormone and cortisol were increased at 5 mg/kg bw per day and at 3 and 5 mg/kg bw per day, respectively. Body weight was reduced from 3 mg/kg bw per day. Absolute and relative thymus and spleen weights, as well as cell cycle parameters in thymus and spleen were significantly influenced from 3 mg/kg bw, while in the thymus, T‐cell subpopulations were altered at only the highest dose. At the higher doses also markers for apoptosis were noted in the spleen. Whereas these effects might have been secondary to effects on body weights and hormonal alterations, in the spleen, a significant decrease in the percentage of F4/80+, CD49+ immature T cells was seen at all dose levels and a significant decrease in CD11c+CD8+ at 1 mg/kg bw per day only. In spleen cells, IL‐4 was reduced at all dose levels in a dose‐related manner. Whereas in this study, no functional parameters of the immune system were included, the data suggest that effects on the immune system are evident at the lowest dose tested, i.e. 1 mg/kg bw per day.
In a similar study, PFNA (0, 1, 3 or 5 mg/kg bw per day) was administered via oral gavage (vehicle 0.5% Tween 20) to male Sprague‐Dawley rats for 14 days; 6 rats/dose level were analysed. The results largely confirmed the mouse study, i.e. decreased body weights were noted from 3 mg/kg bw per day, while immune effects were noted at 1 mg/kg bw per day, albeit in a bit less pronounced fashion than in mice. The effects at the lower dose pertained to thymus weight that was increased at 1 mg/kg bw per day and decreased at 3 and 5 mg/kg bw per day, while an increase in apoptotic cortical lymphocytes and tangible macrophages (which had ingested apoptotic cells) was noted.
In two studies by Rockwell et al. (2013, 2017), male and female 8‐week‐old C57Bl mice (n = 5) were administered a single dose of 0.1 mmol/kg PFNA by i.p. injection (0.46 mg/kg bw). In a substudy, mice were given 1 mg/kg LPS i.p., 28 days after treatment with PFNA. Twenty‐eight days after i.p injection of PFNA, spleen and thymus atrophy persisted. A decreased spleen weight was still noted; percentages of CD4 and CD8 cells were still up, at the cost of percentages of CD19 and CD14 cells. The TNFα response in serum to LPS treatment was markedly increased. The study supports the immunotoxicity of PFNA after i.p. injection, as well as its persistence over time. Although the mice were administered PFNA intraperitoneally, the study supports the immunotoxicity of PFNA after oral administration as observed by Fang et al. (2008, 2009).
Frawley et al. (2018) reported on the effects of PFDA on the immune system (see also Section 3.3.3.2.1 on effects following repeated exposure), when applied to female rats and mice for a period of 28 days. Female Harlan Sprague‐Dawley rats were exposed to 0, 0.125, 0.25, 0.5, 1 or 2 mg PFDA/kg bw per day by oral gavage for 28 days. Rats treated with the two highest doses showed decreased body weight gain and these dose groups were excluded from further evaluation. Treatment‐related hepatocyte necrosis and hepatomegaly were observed in rats treated with 0.5 mg PFDA/kg per day, while effects on liver weights were found at 0.125 mg/kg body weight. No effects on spleen or thymus weight were noted, except on one group treated with 0.125 mg/kg bw, which was sensitised to SRBC. Female B6C3F1/N mice were exposed once per week to 0, 0.3125, 0.625, 1.25, 2.5 or 5 mg PFDA/kg bw by gavage for 4 weeks. Body weight was not affected. Increased liver weight and hepatomegaly was observed following exposure to 0.625 mg PFDA/kg bw per week and higher, while splenic atrophy was observed at 5 mg PFDA/kg bw per week. At higher doses in mice, total numbers of spleen cells were decreased, while also subpopulations of spleen cells were altered at lower doses, i.e. from 1.25 mg/kg bw, whereas in PFDA‐exposed rats, no changes were observed in leucocyte subpopulations. The phagocytosis of sheep red blood cells (SRBC) by fixed tissue macrophages in liver was measured in rats after i.v. administration of 51Cr‐labelled SRBCs. The vascular half‐life for 51Cr‐SRBC was not significantly altered, but the specific activity of fixed tissue macrophages in the liver was decreased by 24–39% at 0.25–0.5 mg PFDA/kg per day. The PFDA‐induced effects on the functionality of the immune system (i.e. humoral‐ and cell‐mediated immunity, host resistance) were limited.
In summary, the literature database, including the publications that appeared after the publication of the EFSA CONTAM Opinion (2018), if any, support the notion that PFOS exposure, possibly more than PFOA, causes immunosuppression, as evidenced by decreased antibody responses to sensitisation to an antigen, and that suppressed immune functionality may lead to reduced resistance to infection. Pachkowski et al. (2019) derived a reference dose of 1.8 × 10–6 mg/kg per day for PFOS, based on the study by Dong et al. (2009). They preferred to derive a PoD from the Dong et al. (2009) study rather than the Peden‐Adams et al. (2008) study, because of the longer exposure duration in the former. They did not, however, take into consideration that in the Dong et al. (2009) study a shorter, and perhaps suboptimal sensitisation schedule was used, rendering the parameter tested less sensitive. The CONTAM Panel concluded that the study by Peden‐Adams et al. (2008), showing effects at the lower serum levels, is the critical study for immune effects in animals.
PFOA effects in mice are similar to those of PFOS in mice, with both structural and functional parameters influenced, but effects were observed at higher doses compared to PFOS; the NOAEL for immunotoxicity of PFOA was 1 mg/kg bw per day based on suppression of anti‐SRBC IgM titres in mice.
The information on other PFASs is rather limited.
Immune effects of PFNA indicate an LOAEL of 1 mg/kg bw per day, but this was not based on similar outcome parameters as measured for PFOS and PFOA (i.e. the functional parameter antibody production specific for sheep red blood cells was not measured).
PFDA did not influence antibody responses at the concentrations tested, but did cause other immune effects, i.e. effects on fixed liver macrophages, at a dose of 0.25–0.5 mg PFDA/kg per day.
3.3.3.6. Genotoxicity
3.3.3.6.1. PFOS and PFOA
In 2018, the EFSA CONTAM Panel concluded that for PFOS and PFOA, the available genotoxicity data are inconclusive. No evidence for a direct genotoxic mode of action for PFOS and PFOA was identified. There has been some evidence for oxidative stress induced by both PFOS and PFOA (EFSA CONTAM Panel, 2018). Three new studies and two recent NTP reports have been identified since the literature deadline for this EFSA CONTAM Panel 2018 Opinion on PFOS and PFOA.
PFOS
Chen et al. (2016) used a transgenic fish model, the λ transgenic medaka, to evaluate the potential mutagenicity of PFOS in liver tissue following a subchronic exposure (0, 6.7, 27.6, 87.6 μg/L in water) for 30 days. The dose‐dependent increase in mutation frequency at the cII target gene (twofold at the maximal dose) was due to +1 frameshift mutations.
Treatment of rats with PFOS (0, 0.6, 1.25 and 2.5 mg/kg bw per day) by gavage for 30 days at 48 h intervals significantly increased both micronucleus frequency and DNA single strand breaks (SSBs) as assessed by the comet assay as well as the number of apoptotic cells in the liver (Eke et al., 2017).
In the 2019 NTP study, no mutagenic activity was seen for PFOS (NTP, 2019b) in E. coli (WP2 uvrA/pKM101, 100–‐10,000 μg/plate) or S. Typhimirium (TA100 or TA 98, 50–5,000 μg/plate). In a 28‐day study in female rats, PFOS (5 mg/kg bw per day) significantly increased the frequency of micronucleated polychromatic erythrocytes. Since this increase was within the historical control range, NTP judged the result to be equivocal. No increase in micronucleated erythrocyte frequency was seen in males. A significant dose‐dependent decreases in the percentage of polychromatic erythrocytes in the peripheral blood of both sexes was observed.
PFOA
In the 2019 NTP study, no mutagenic activity was seen for PFOA (NTP, 2019a) in E. coli (WP2 uvrA/pKM101, 100–10,000 μg/plate) or S. Typhimirium (TA100 or TA 98, 50–5,000 μg/plate).
In mice after a 5‐week administration of PFOA (0, 0.2, 1, 5 mg/kg bw per day in drinking water), no evidence of treatment‐related genotoxicity was observed. This was assessed by measurements of SSBs by comet assay in liver and testis and by micronuclei in reticulocytes and spleenocytes (Crebelli et al., 2019). No evidence of lipid peroxidation and oxidative stress (decreased antioxidant capacity) was observed at the highest PFOA dose associated with marked liver hypertrophy and signs of cell injury (elevated ALT and AST).
No increase in micronucleated reticulocytes were observed in female rats administered PFOA (6.25–100 mg/kg bw per day) for 28 days. In male rats, a significant increase in micronucleated reticulocytes was seen over the administered doses (0.625–10 mg/kg bw per day). Since this increase was within the historical control range NTP questioned the biological significance. No changes were observed in the percentage of immature erythrocytes in peripheral blood of both sexes.
In summary, the new studies do not change the conclusion made in 2018 (EFSA CONTAM Panel, 2018).
3.3.3.6.2. PFASs other than PFOS and PFOA
Appendix I, Table I.1, lists available in vitro genotoxicity studies identified in peer reviewed papers for relevant PFASs other than PFOS and PFOA. In addition, a report from industry about PFBS was available.
Table I.1.
In vitro genotoxicity studies
| Test system | Cells/animals | Concentration/Treatment for genotoxicity endpoints | Result | Comment | Reference |
|---|---|---|---|---|---|
| In vitro | |||||
| Bacterial reverse mutation assay (Ames test) | S. Typhimurium TA98,TA100, TA1535, TA1537, TA1538 |
PFBA: 20 μmol/plate (+/−S9) PFHxA: 20 μmol/plate (+/−S9) PFHpA: 10 μmol/plate (+/−S9) PFNA: 5 μmol/plate (+/−S9) PFDA: 5 μmol/plate (+/−S9) PFDoDA: 1 μmol/plate (+/−S9) |
Negative Negative Negative Negative Negative Negative |
Only highest applied non‐cytotoxic dose shown; two independent experiments Only highest applied non‐cytotoxic dose shown; independent experiments Only highest applied non‐cytotoxic dose shown; independent experiments Only highest applied non‐cytotoxic dose shown; independent experiments Only highest applied non‐cytotoxic dose shown; independent experiments Only highest applied non‐cytotoxic dose shown; independent experiments |
Buhrke et al. (2013) |
| Bacterial reverse mutation assay (Ames test) | S. Typhimurium TA98,TA100, TA1535, TA1537, WP2uvrA | PFHxA: 0, 333–5,000 μg/plate (+/−S9) | Negative | Loveless et al. (2009) | |
| Bacterial reverse mutation assay (Ames test) | S. Typhimurium TA98,TA100, E. Coli WP2 uvrA pKM 101 |
PFHxA: 10–750 μg/plate (TA98, TA100, +/−S9) PFHxA: 100–2,000 μg/plate E. Coli WP2 uvrA pKM 101, +/−S9) PFNA: 50–500 μg/plate (TA98, TA100, +/−S9) PFNA: 500–5,000 μg/plate E. Coli WP2 uvrA pKM 101, +/−S9) PFDA: 100–1,000 μg/plate (TA98, TA100, +/−S9) PFDA: 500–5,000 μg/plate E. Coli WP2 uvrA pKM 101, +/−S9) PFBS: 50–5,000 μg/plate (TA98, +/−S9) PFBS: 50–5,000 μg/plate (TA100, +/−S9) PFBS: 50–1,000 μg/plate E. Coli WP2 uvrA pKM 101, +/−S9) |
Negative Negative Negative Negative Negative Negative Equivocal Negative Negative |
NTP (2019a) NTP (2019b) |
|
| Chromosomal aberration assay | Human peripheral blood lymphocytes |
PFHxA: 0, 2000–3,860 μg/mL (−S9), 4 h PFHxA: 0, 250–1,000, (+S9), 4 h PFHxA: 0, 250–1,000, (−S9), 22 h |
Negative Negative Negative |
No significant increase of structural or numerical chromosome aberrations activated test systems | Loveless et al. (2009) |
| DNA strand breaks and FPG sensitive sites (Comet assay) | HepG2 cells |
PFHxA: 0, 100–400 μM, 24 h PFNA: 0, 100–400 μM, 24 h PFBS: 0, 100–400 μM, 24 h |
Negative Positive ≥ 200 μM Negative |
No significant increase in ROS production (DCFH‐DA), 0.4–2,000 μM, 3h; 400 μM for 24 h resulted in a LDH release of ≤ 5% No significant increase in ROS production (DCFH‐DA), 0.4‐2,000 μM, 3 h; 400 μM for 24 h resulted in a LDH release of 66%. No significant increase in ROS production (DCFH‐DA), 0.4‐2,000 μM, 3h; 400 μM for 24 h resulted in a LDH release of ≤ 5% |
Eriksen et al. (2010) |
| DNA strand breaks (Comet assay | HepG2 cells |
PFHxS: 0, 0.2–20 μM, 24 h PFNA: 0, 0.2–20 μM, 24 h PFDA: 0, 0.2–20 μM, 24 h PFUnDA: 0, 0.2–20 μM, 24 h PFDoDA: 0, 0.2–20 μM, 24 h |
Positive 1, ≥ 10 μM Positive 2, 20 μM Negative Negative Negative |
Cytotoxicity > 200 μM, 24 h; no clear dose dependency, positive for ROS (DCFDA fluorescence) ≥ 0.2 μM Cytotoxicity > 200 μM, 24 h; no clear dose dependency, positive for ROS (DCFDA fluorescence) 0.2, ≥ 20 μM Cytotoxicity > 200 μM, 24 h; positive for ROS (DCFDA fluorescence) 0.2, ≥ 20 μM Cytotoxicity > 200 μM, 24 h; positive for ROS (DCFDA fluorescence) ≥ 2 μM Cytotoxicity > 20 μM, 24 h; positive for ROS (DCFDA fluorescence) ≥ 2 μM |
Wielsøe et al. (2015) |
| DNA strand breaks (Comet assay) | Human lymphoblastoid (TK6) cells | PFNA: 0, 125, 250 ppm, 2 h |
Positive 125, 250 μg/mL |
The authors state that cells are viable (as measured by trypan blue). However, data are not shown. | Yahia et al. (2014) |
|
8‐OHdG (LC‐MS/MS) |
Human lyphoblastoid (TK6) cells | PFNA: 0, 125, 250 ppm, 2 h |
Positive 125, 250 μg/mL |
The authors state that cells are viable (as measured by trypan blue). However, data are not shown. 8‐OHdG induction was greater than that produced by PFOA. | Yahia et al. (2014) |
|
Micronuclei (OECD 487) |
V79 cells |
PFBA: 100 μM (–/+S9), 3 h (+21 h) PFHxA: 100 μM (–/+S9), 3 h (+21 h) PFHpA: 100 μM (–/+S9), 3 h (+21 h) PFNA: 10 μM (–/+S9), 3 h (+21 h) PFDA: 10 μM (–/+S9), 3 h (+21 h) PFDoDA: 1 μM (–/+S9), 3 h (+21 h) |
Negative Negative Negative Negative Negative Negative |
IC50 (neutral red) > 1,000 μM, 72 h IC50 (neutral red): 344 μM, 72 h IC50 (neutral red): 128 μM, 72 h IC50 (neutral red): 28 μM, 72 h IC50 (neutral red): 15 μM, 72 h IC50 (neutral red): 7 μM, 72 h |
Buhrke et al. (2013) |
DCFDA: dichlorodihydrofluorescein diacetate; IC50: half maximal inhibitory concentration; ROS: reactive oxygen species.
PFBS did not induce mutations in a bacterial reverse mutation assay or chromosome aberrations in the Chinese Hamster Ovary‐W‐B1 cell line (NICNAS, 2005). PFBS mutagenicity was judged to be equivocal in S. Typhimirium TA 98, but negative in TA 100 and E. coli WP2 uvrA/pKM101 (NTP, 2019b). No induction of SSBs as measured by comet assay was observed in HepG2 cells (Eriksen et al., 2010). No increase of micronucleated erythrocytes was seen in peripheral blood samples from male and female rats administered PFBS (62.5–500 mg/kg bw per day) for 28 days. A significant decrease in polychromatic erythrocytes was seen in the peripheral blood of both sexes (NTP, 2019b).
PFBA and PFHpA did not induce mutations in bacteria and was negative in the micronuclei test in V79 cells (Buhrke et al., 2013).
PFDoDA did not induce mutations in bacteria and was negative in the micronuclei test in V79 cells (Buhrke et al., 2013). No induction of SSBs as measured by comet assay was observed in HepG2 cells (Wielsøe et al., 2015). In Japanese medaka fish exposed to water levels of 100, 500 and 2,500 μg/L PFDoDA (for 1, 4, 7, 14, 21 and 28 days) a significant time‐ and concentration‐dependent increase of SSBs in liver DNA and erythrocyte abnormalities including micronuclei was observed (Ayanda et al., 2018).
PFUnDA did not induce SSBs as measured by comet assay in HepG2 cells (Wielsøe et al., 2015).
PFNA did not induce mutations in bacteria and was negative in the micronuclei test in V79 cells (Buhrke et al., 2013; NTP, 2019a). Three studies reported increased SSBs, including Fpg sensitive sites and 8‐OHdG in HepG2 cells (Eriksen et al., 2010; Wielsøe et al., 2015) and TK6 cells (Yahia et al., 2014). In the green mussel (Perna viridis) PFNA (water levels 0, 0.01–1,000 μg/L, 7 days) increased SSBs as measured by comet assay (Liu et al., 2014b). A 28‐day PFNA administration to female (1.56–6.25 mg/kg bw per day) and male (0.626–2.5 mg/kg bw per day) rats did not increase micronucleated reticulocytes. Severe toxicity to the bone marrow was observed in male rats (at 2.5 mg/kg bw per day), while none was evident in the females (NTP, 2019a).
PFDA did not induce mutations in bacteria and was negative in the micronuclei test in V79 cells (Buhrke et al., 2013; NTP, 2019a). In HepG2 cells PFDA was negative in the Comet assay (Eriksen et al., 2010). In the green mussel (Perna viridis) PFDA (water levels 0, 0.01–1,000 μg/L, 7 days) exerted genotoxicity as assessed by the alkaline comet assay (Liu et al., 2014b). A 28‐day PFDA administration did not affect the frequency of micronucleated reticulocytes in female rats (0.156–1.25 mg/kg bw per day). However, an increase in micronuclei was seen in male rats at the highest administered PFDA dose (2.5 mg/kg bw per day) which induced severe toxicity to the bone marrow (NTP, 2019a) see Section 3.3.3.2.1 and Appendix E Table E.1 for further details on this study.
PFHxA did not induce mutations in bacteria and was negative in the micronuclei test in V79 cells (Buhrke et al., 2013; Loveless et al., 2009; NTP, 2019a). Two studies reported no genotoxic effects (SSBs including Fpg sensitive sites and chromosomal aberations) after PFHxA treatment of HepG2 cells (Eriksen et al., 2010) or human peripheral blood lymphocytes (Loveless et al., 2009). In a 28‐day study no increase in micronucleated reticulocytes was observed in peripheral blood of male and female rats administered PFHxA (31.5–500 mg/kg bw twice daily), whereas the percentage of circulating immature erythrocytes was markedly increased (NTP, 2019a).
PFHxS induced SSBs as measured by comet assay in HepG2 cells (Wielsøe et al., 2015). No increase of micronucleated erythrocytes was seen in peripheral blood samples from male and female rats administered perfluorohexane sulfonate potassium salt (PFHxSK) (males: 0.625–10 mg/kg bw per day, females: 3.12–60 mg/kg bw per day) for 28 days. PFHxSK exerted significant decreases in polychromatic erythrocytes in the peripheral blood of males (NTP, 2019b).
For PFASs relevant to this Opinion, no further genotoxicity studies in experimental animals and published in peer‐reviewed papers were identified.
The CONTAM Panel concluded that for PFASs other than PFOS and PFOA the number of studies and data are limited. Structural similarity for PFHxS and PFOS as well as for PFNA and PFOA and some evidence for oxidative stress induced by PFHxS and PFNA (See Appendix I, Table I.1) might indicate that, as reported for PFOS and PFOA before, (EFSA CONTAM Panel, 2018), for PFHxS and PFNA a direct genotoxic mode of action is unlikely.
3.3.3.7. Long‐term toxicity and carcinogenicity
Previous long‐term/carcinogenicity studies showed that PFOS and PFOA are tumour promoters in rodent liver, that PFOA also may induce Leydig cell tumours in rats, and that data on tumour formation in mammary gland and pancreas of PFOA‐treated animals are equivocal (EFSA, 2008; EFSA CONTAM Panel, 2018). The CONTAM Panel has not identified relevant new carcinogenicity studies for PFOS and PFOA in experimental animals.
Klaunig et al. (2015) treated SD rats with 0, 2.5, 15 or 100 mg of PFHxA/kg bw per day (males) and 0, 5, 30 or 200 mg of PFHxA/kg bw per day (females) by gavage for 104 weeks. There were no effects on body weights, food consumption, haematology, hormone parameters, several functional observational endpoints, or motor activity. In females a dose‐dependent decrease in survival and histological changes in the kidney (papillary necrosis) (at 200 mg/kg bw per day) could be observed. Other changes included decreases in triglyceride serum levels for males at 2.5 mg/kg bw day and for females decreases in serum LDL/VLDL and increases in urinary volume were noted at 200 mg/kg bw per day. There was no indication for carcinogenicity of PFHxA at any of the dosages tested.
Benninghoff et al. (2012) used a two‐stage chemical carcinogenesis model in trout to study whether PFNA, PFOS, PFOA, PFDA or 8:2FTOH are complete carcinogens or tumour promoters. Hepatocarcinogenesis was initiated with aflatoxin B1 (AFB1). Both, PFNA and PFDA, were applied via the diet 5 days per week for 6 months starting with a concentration of 2,000 mg/kg diet (approximately 50 mg/kg bw per day), which was reduced to 200 mg/kg diet (approximately 5 mg/kg bw per day). 8:2 FTOH was applied at 2,000 mg/kg diet throughout the experiment. PFNA and PFDA enhanced incidence and size of liver tumours when compared with AFB1‐initiated animals fed a control diet. In the groups, receiving PFNA or PFDA only, there were no tumours or a few liver adenomas, respectively. This indicates a tumour promoting capacity of both compounds in this animal species. However, incidence and size of liver tumours in AFB1‐treated trouts fed diets containing 8:2 FTOH were unaltered when compared with AFB1‐initiated animals fed control diet. Thus 8:2 FTOH exerted no liver tumour promoting activity in trout. Also, no tumours were found in animals treated with 8:2 FTOH only.
No long‐term toxicity or carcinogenicity studies were identified for the other PFASs under consideration in this assessment.
In summary, there is a long‐term study for PFHxA only, providing no evidence for any carcinogenicity of this PFAS. PFNA and PFDA showed a liver tumour promoting capacity in a trout two‐stage model of hepatocarcinogenesis, while 8:2FTOH failed to do so. For the remaining PFASs in this Opinion information on their carcinogenic potency is missing.
3.3.4. Observations in humans
In this section, the body of evidence on epidemiologic studies in humans is presented and assessed. Under the various subheadings (per outcome), the basic order is chronological, but if a specific cohort is re‐examined later, then this breaks the chronological order, so that several studies of a specific outcome in the same cohort are placed together. For clarity the type of study design is also emphasised in the review below.
Regarding the four outcomes (increased serum cholesterol, impaired antibody response after vaccination, increased serum ALT, and decreased birth weight) that were considered potential critical effects in the Opinion on PFOS and PFOA (EFSA CONTAM Panel, 2018), key studies published after the deadline of the literature review for the PFOS and PFOA Opinion (EFSA CONTAM Panel, 2018), were also considered. These will be commented in Section 3.4.1 on Critical effects, where the evidence regarding PFOS and PFOA is discussed again.
3.3.4.1. Fertility and pregnancy outcomes
3.3.4.1.1. Birth weight
In the Opinion on PFOS and PFOA, (EFSA CONTAM Panel, 2018), 13 prospective studies (maternal pregnancy or pre‐pregnancy serum) and four cross‐sectional studies (cord blood) were reviewed that had examined associations between PFOS and/or PFOA and birth weight. Only studies with more than 100 participants were included in that review. In addition, two large studies from the C8 cohort were reviewed that examined associations between retrospectively modelled exposure to PFOA and birth weight. Among the reviewed studies, relatively modest but consistent inverse associations with birth weight were observed for both compounds. No clear associations with low birth weight (LBW < 2,500 g) or small for gestational age (SGA) were observed in six studies addressing those outcomes. Also, no association was observed between PFOA and birth weight in the two studies from the C8 cohort relying on modelled exposures. While acknowledging that the association between PFOS and PFOA and birth weight might be partly confounded by physiological changes in pregnancy (Savitz, 2007; Verner et al., 2015), and the lack of association with LBW or SGA, the CONTAM Panel still concluded that there may well be an association between exposure to PFOS and PFOA and birth weight’.
Since the publication of the previous Opinion on PFOS and PFOA in 2018 (EFSA CONTAM Panel, 2018), several papers on PFASs and birth weight have been published. These include both original research papers and meta‐analyses. Below, these publications are reviewed first, for all compounds. Then results on PFASs other than PFOS and PFOA are summarised for those papers previously included in the Opinion (EFSA CONTAM Panel, 2018). Finally, a review of published findings from meta‐analyses is given prior to a short summary.
New publications not reviewed in the EFSA CONTAM Panel 2018 Opinion
In a study from the Danish National birth cohort, Meng et al. (2018) examined associations between maternal concentrations (median) of PFOS (30.1 ng/mL), PFOA (4.6 ng/mL), PFHxS (1.0 ng/mL), PFNA (0.5 ng/mL), PFHpS (0.4 ng/mL) and PFDA (0.2 ng/mL) and birth weight, gestational age, LBW and preterm delivery (< 37 weeks of gestation). First trimester serum samples from 3,535 women were analysed for PFOS and PFOA, and from 2,120 women for the other PFASs. This included 1,400 women that had previously been described in another publication (Fei et al., 2007). In this updated analysis, PFOS, PFOA, PFNA and PFHpS (but not PFHxS and PFDA) were significantly and inversely associated with birth weight and gestational age as continuous outcomes. For birth weight, the mean decrease in birth weight ranged from 50 to 100 g across quartiles of exposure. For all compounds no significant associations with LBW were observed.
In a cohort study of 424 mother–infant pairs from China, Wang et al. (2019a) examined associations between cord blood concentrations (median) of PFOS (0.7 ng/mL) and PFOA (2.0 ng/mL) with birth weight and length. No associations were observed for any of the assessed outcomes.
In the UK, Marks et al. (2019) examined associations between maternal serum concentrations (median) of PFOS (14 ng/mL), PFOA (3 ng/mL) PFHxS (2 ng/mL) and PFNA (0.4 ng/mL) with birth size in 457 male infants whose mothers had been recruited into the ALSPAC birth cohort. Serum samples were drawn between week 12 and 33 of gestation. Maternal PFOS concentrations were significantly and inversely associated with birth weight, birth length and head circumference. Non‐significant inverse associations were observed for the other compounds.
In a study of 2,071 singleton and 1,040 twin infants born to mothers in New York State, Bell et al. (2018) examined associations between concentrations (median) of PFOS (1.7 ng/mL) and PFOA (1.1 ng/mL) quantified in newborn bloodspots and measures of birth size. No consistent associations were observed.
Manzano‐Salgado et al. (2017a) examined in 1,202 Spanish pregnant women, associations between first trimester serum (mean) PFHxS (0.6 ng/mL), PFOS (6.1 ng/mL), PFOA (2.4 ng/mL) and PFNA (0.7 ng/mL) with birth weight, LBW, and SGA. No consistent associations were observed for any of the outcomes examined.
In a cohort of 638 pregnant women from Denmark, providing blood samples between gestation weeks 8 to 16, Lind et al. (2017a) examined associations between maternal serum concentrations (median) of PFOS (8.1 ng/mL), PFOA (1.7 ng/mL), PFHxS (0.3 ng/mL), PFNA (0.7 ng/mL) and PFDA (0.3 ng/mL) with birth weight. No consistent associations with birth weight as continuous outcome were observed for individual PFASs. Maternal concentrations of PFOA were, however, significantly higher among those few cases (n = 13) who gave birth to LBW infants (< 2,500 g).
Buck Louis et al. (2018) examined associations between several PFASs and birth weight in a cohort of 2,106 healthy pregnant women recruited across 12 US cities. The median concentrations of individual PFAS (all in ng/mL) were 0.06 for N‐methylperfluoro‐1‐octane‐sulfonamidoacetic acid (N‐MeFOSAA), 0.25 for PFDA, 0.03 for PFDoDA, 0.01 for PFDS, 0.02 for PFHpA, 0.71 for PFHxS, 0.76 for PFNA, 1.99 for PFOA, 5.13 for PFOS and 0.18 for PFUNDA. In this study PFOA was inversely associated with birth weight while for other PFASs no association were observed with birth weight or length. Several PFAS were inversely associated with upper thigh‐length. Although of interest the interpretation of such inverse association is unclear in the absence of consistent inverse association with birth weight and length.
In a study of 693 mother–child pairs from four European birth cohorts, Govarts et al. (2018) examined the association between PFOS (median: 2 ng/mL) and PFOA (median: 0.5 ng/mL) in cord blood and small for gestational age (SGA). PFOS was not associated with SGA [OR: 0.82 (95%CI: 0.74, 0.91) per interquartile range increase in exposure] while the corresponding estimate for PFOA showed a borderline significant increased risk of SGA [OR: 1.64 (95%CI: 0.97, 2.76)].
Summary of existing meta‐analyses not reviewed in the 2018 EFSA CONTAM Panel Opinion
Steenland et al. (2018) published a meta‐analysis based on 24 studies (n = 5,772) that had measured serum concentrations of PFOA. In addition to these studies, they also included 4,142 women from the C8 cohort where exposure to PFOA had been retrospectively modelled (not included in the other meta‐analyses). Based on the 24 studies with measured concentrations, a –11 g decrease (–17, –4) in birth weight was observed for every 1 ng/mL increase in PFOA. Adding the modelled exposures, the corresponding estimate was non‐significant [–1.0 g decrease (95% CI –2.4, –0.4)]. The authors also concluded that studies based on serum samples drawn early compared to late in pregnancy showed weaker associations. This difference was interpreted as evidence of bias due to confounding by physiological changes during pregnancy. The conclusions by Steenland et al. (2018) are largely in line with the conclusions on a consistent association between PFOA and birth weight in the previous Opinion (EFSA CONTAM Panel, 2018) on PFOS and PFOA, where the conclusion was based on studies with measured concentrations only, as the modelled concentrations in the C8 studies did not take the inter‐pregnancy interval in mothers into consideration when modelling their exposure, which is a strong determinant of pregnancy concentrations for parous women (Bach et al., 2018). The updated study from the Danish National birth cohort by Meng et al. (2018) with serum samples drawn early in pregnancy and reporting around 50–100 g reduction in birth weight is, however, not in line with the conclusion by Steenland et al. (2018) that samples drawn early in pregnancy show much weaker associations.
In contrast to the meta‐analyses by Steenland et al. (2018), another previous review on PFOA only included studies using quantified levels of PFOA in serum or plasma. Johnson et al. (2014) used the Navigation Guide methodology to evaluate the evidence for an association between developmental exposure to PFOA and fetal growth. Nine studies met the inclusion criteria. Based on these studies each 1 ng/mL increase in serum PFOA concentration was associated with a 19 g decrease in birth weight (95% CI: –30, –8). Three years later, Negri et al. (2017) published another meta‐analysis of 12 studies with maternal plasma/serum concentrations of PFOA and eight studies with plasma/serum concentrations for PFOS. The regression coefficient for decrease in birth weight per ~ 3‐fold (1‐Ln) increase in PFOS and PFOA was –46 g (95% CI –80, –12) and –27 g (95% CI –51, –4), respectively.
Associations with PFASs other than PFOS and PFOA in papers reviewed for only PFOS and PFOA the EFSA CONTAM Panel 2018 Opinion
Kwon et al. (2016) examined associations between cord blood concentrations (median) of PFHxS (0.4 ng/mL), PFNA (0.2 ng/mL), PFDA (0.1 ng/mL), PFUnDA (0.3 ng/mL), PFDoDA (0.1 ng/mL) and PFTrDA (0.4 ng/mL) and birth weight in 268 Korean infants. Serum levels of PFHxS, PFNA, PFDA and PFUnDA were all significantly and inversely associated with birth weight.
Wang et al. (2016) examined associations between maternal serum concentrations (medians) of PFNA (1.6 ng/mL), PFDA (0.4 ng/mL), PFUnDA (3.4 ng/mL), and PFDoDA (0.4 ng/mL) and birth weight among 223 Taiwanese mothers and their term infants. Serum samples were drawn in the third trimester. Among female infants (n = 106), all four PFASs were significantly and inversely associated with birth weight, while no associations were observed for male infants (n = 117).
Maisonet et al. (2012) examined associations between maternal serum concentrations (median) of PFHxS (2 ng/mL) with birth size in 447 female infants whose mothers had been recruited into the ALSPAC birth cohort. Serum samples were drawn mainly between weeks 10 and 28 (IQR) of gestation. Maternal PFHxS concentrations were significantly and inversely associated with birth weight and birth length.
Using maternal serum sampled in gestation week 9–20, Bach et al. (2016) studied associations between serum concentrations (medians) of PFHxS (0.5 ng/mL), PFHpS (0.2 ng/mL), PFNA (0.8 ng/mL), PFDA (0.3 ng/mL) and PFUnDA (0.3 ng/mL) in 1,507 primiparous women from Aarhus, Denmark. No significant associations were observed.
Robledo et al. (2015) examined the association between pre‐pregnancy maternal serum concentrations of (means) 2‐(n‐ethyl‐perfluorooctane sulfonamide) acetate (Et‐PFOSA‐AcOH) (0.1 ng/mL), 2‐(n‐methyl‐perfluorooctane sulfonamide) acetate (Me‐PFOSA‐AcOH) (0.3 ng/mL), PFDeA (0.5 ng/mL), PFNA (1.6 ng/mL) and FOSA (0.1 ng/mL) and birth weight and ponderal index in 243 US mothers. Analyses were performed for male and female infants separately. Only FOSA was significantly and inversely associated with birth weight in male infants (n = 113).
Chen et al. (2012) examined the associations between cord blood concentrations of PFNA (mean 2.4 ng/mL) and PFUnDA (10.6 ng/mL) among 429 mother–infant pairs from Taiwan. Non‐significant associations were observed for both compounds.
Hamm et al. (2010) studied associations between maternal concentrations during pregnancy of serum PFHxS (mean 2.1 ng/mL) and birth weight in 252 Canadian mother–child pairs. No association was observed.
Lenters et al. (2016) studied associations between PFHxS, PFNA, PFDA, PFUnDA and PFDoDA and birth weight in a combined cohort of 1,250 mothers from Greenland (n = 513), Poland (n = 180) and Ukraine (n = 557) who gave birth at term (≥ week 37 of gestation). With the exception of PFHxS (~ 3.5 ng/mL), median concentrations were below 1 ng/mL. No significant associations were observed.
Shi et al. (2017) examined associations between cord blood concentrations (medians) of PFHxS (0.2 ng/mL), PFNA (0.2 ng/mL), PFDA (0.01 ng/mL) and PFUnDA (0.06 ng/mL) and birth weight and height in 170 Chinese infants. No significant associations were observed.
Summary
Eight new studies on PFOS and PFOA have been published after the 2018 Opinion (Bell et al., 2018; Buck Louis et al., 2018; Govarts et al., 2018; Lind et al., 2017a; Manzano‐Salgado et al., 2017a; Marks et al., 2019; Meng et al., 2018; Wang et al., 2019a). Several but not all studies observed no association. For example, the large (n = 3,535) study by Meng et al. (2018) observed consistent inverse associations between PFOS and PFOA and birth weight. In that study, samples were drawn in early pregnancy (first trimester), which should minimise the risk of confounding by physiological changes in pregnancy (Steenland et al., 2018). The difference between the study by Meng et al. (2018) and some other studies are the two‐ to fourfold higher serum levels, as women were recruited around the year 2000 when environmental contamination was at peak levels. Taking into consideration these differences in serum levels, the additional studies reviewed on PFOS and PFOA do not contradict the previous conclusion from the 2018 Opinion (EFSA CONTAM Panel, 2018) that ‘there may well be a causal association between PFOS and PFOA and birth weight’. That conclusion is also in agreement with most meta‐analyses that have been conducted to date.
Concerning PFASs other than PFOS and PFOA, concentrations were generally much lower compared to PFOS and PFOA and inconsistent associations with birth weight were observed.
3.3.4.1.2. Preterm delivery
In the Opinion on PFOS and PFOA (EFSA CONTAM Panel, 2018) many of the studies reviewed above in relation to birth weight also examined associations with preterm delivery (gestational age less than 259 days). Overall, no consistent associations were observed for PFOS and PFOA in these studies. This was also the case for other PFASs (see Appendix J, Section J.1 for details on these studies).
Since the publication of the previous Opinion (EFSA CONTAM Panel, 2018), only one large (n = 3,535) study by Meng et al. (2018) has reported results on preterm delivery. In that study maternal serum PFAS concentrations in early pregnancy showed relatively consistent associations with preterm delivery. Despite this report, and in line with the conclusions from the previous Opinion, the evidence for an association between PFASs and preterm delivery is still limited.
3.3.4.1.3. Time to pregnancy
In the Opinion on PFOS and PFOA (EFSA CONTAM Panel, 2018), six studies reporting results on time to pregnancy were reviewed. Based on that review the Panel's conclusion was that ‘there is insufficient evidence to suggest that exposure to PFOS/PFOA may adversely affect fecundity’. The same conclusion was reached when reviewing these same studies for PFASs other than PFOS and PFOA (see Appendix J, Section J.1).
3.3.4.1.4. Miscarriage
In the Opinion on PFOS and PFOA, five studies reporting results on miscarriage were reviewed. Based on that review the Panel's conclusion was that there is ‘limited evidence to suggest that PFOS or PFOA are associated with increased risk of pregnancy loss’. The same conclusion was reached when reviewing these same studies for PFASs other than PFOS and PFOA (see Appendix J, Section J.1).
3.3.4.1.5. Hypertension in pregnancy – Preeclampsia
In the Opinion on PFOS and PFOA (EFSA CONTAM Panel, 2018), six studies reporting results on pregnancy hypertension were reviewed. Five of those studies were from the same study population, the C8 cohort. The CONTAM Panel's conclusion was that ‘there is insufficient evidence to suggest that PFOS or PFOA are associated with pregnancy induced hypertension or preeclampsia’. No new studies have since been identified for PFOS, PFOA or other PFASs.
3.3.4.2. Developmental effects
PFOS and PFOA
In the Opinion on PFOS and PFOA, 30 studies on 18 cohorts were identified with PFOS/PFOA measurements in serum of pregnant mothers, in cord blood or in breast milk and various follow‐up outcomes in children between 6 months and 22 years (EFSA CONTAM Panel, 2018). Most studies examined associations between prenatal or perinatal exposure to PFOS/PFOA and later neurobehavioural developmental effects. The CONTAM Panel concluded that support for associations between prenatal exposure to PFOS or PFOA and early life neurobehavioural development or overweight was considered insufficient (EFSA CONTAM Panel, 2018).
PFASs other than PFOS and PFOA
For PFASs other than PFOS and PFOA, 17 publications on 14 longitudinal studies were identified with PFHxS/PFNA measurements in serum/plasma of pregnant mothers or in cord blood and follow‐up of various outcomes in children between 6 months and 22 years. Most studies have examined associations between prenatal or perinatal exposure to PFHxS/PFNA and later neurobehavioural development and overall, they do not provide support for any association. Also, for overweight/obesity and timing of puberty, no consistent associations were found.
Fewer studies examined associations between these outcomes and prenatal exposure to PFASs other than PFOS, PFOA, PFHxS and PFNA, and no significant associations were found.
A description of the individual studies can be found in Appendix J, Section J.2.
The CONTAM Panel concludes that there is insufficient evidence to suggest that exposure to PFAS may adversely affect neurobehavioural development or overweight.
3.3.4.3. Neurotoxic outcomes
PFOS and PFOA
In the Opinion on PFOS and PFOA, eight cross‐sectional studies, five in children and three in adults, assessing various neurobehavioural, neuropsychiatric and cognitive outcomes were reviewed (EFSA CONTAM Panel, 2018). Consistent adverse associations were not found with serum levels of PFOS or PFOA. Several studies found inverse associations (‘protective effect’).
PFASs other than PFOS and PFOA
Three cross‐sectional studies assessed different endpoints relevant to neurotoxicity in children. One of them was a large cross‐sectional study from the C8 cohort (Stein and Savitz, 2011) which showed a statistically significant association between serum PFHxS and diagnosis of ADHD (based on interview, including medications) in children and adolescents. Same‐direction non‐statistically significant associations between were also reported for PFNA (and PFOS). There was no association between PFHxS and learning problems which would indicate that, should the association be causal, it would be based on aspects of ADHD that do not affect learning. In contrast to PFOA where contaminated drinking water was the main source of exposure in this cohort, the serum PFHxS levels were not higher than in other populations. Another study showed an association with behavioural changes at age 5 but not at age 7. The third study was very small (N = 83).
Three cross‐sectional studies were performed in adults. They reported possible inverse (‘protective direction’) associations for PFNA and PFHxS.
A description of the individual studies can be found in Appendix J, Section J.3.
The CONTAM Panel concludes that there is insufficient evidence to suggest that exposure to PFASs may adversely affect neurobehavioural, neuropsychiatric and cognitive outcomes.
3.3.4.4. Immune outcomes
3.3.4.4.1. Asthma and allergies in children and adults
PFOS and PFOA
In the Opinion on PFOS and PFOA, four prospective studies and six cross‐sectional studies were reviewed that had examined associations between PFOS and PFOA with asthma and allergies (EFSA CONTAM Panel, 2018). The CONTAM Panel concluded that ‘there is not much evidence to suggest that PFOS or PFOA are associated with asthma and allergies in children and adults’. Since then, five new prospective studies on asthma and allergies have been published. The associations reported in these new studies were reviewed for all PFASs and the studies previously reviewed for PFOS and PFOA were reviewed for other PFASs as well (See Appendix J, Section J.4).
In summary, new studies on PFOS and PFOA showed no or inconsistent associations with asthma and allergies for both prenatal and postnatal exposures. These studies do not change the conclusion of the previous Opinion on PFOS and PFOA.
PFASs other than PFOS and PFOA
Concerning PFASs other than PFOS and PFOA, prospective studies in children with prenatal exposure assessment have produced inconsistent results (Wang et al., 2011b; Timmermann et al., 2017b; Averina et al., 2019). Results of studies focusing on prenatal exposures have also been inconclusive (Goudarzi et al., 2016; Impinen et al., 2018, 2019; Manzano‐Salgado et al., 2019; Okada et al., 2014; Timmermann et al., 2017b). Two well‐conducted cross‐sectional studies by Dong et al. (2013) and Qin et al. (2017) from the same study population in Taiwan, suggest that several PFASs may be positively associated with asthma in children. Three other cross‐sectional studies in adolescents and adults (Buser and Scinicariello, 2016; Stein et al., 2016a; Tao et al., 2008) found no association. One possible explanation for a positive association with asthma being observed in a cross‐sectional study is that asthmatic cases might be differently exposed through altered behaviour because of their underlying disease condition.
In conclusion, the available evidence is insufficient to suggest that exposure to PFASs are associated with allergy and asthma in children and adults.
3.3.4.4.2. Vaccination response
In the previously published Opinion on PFOS and PFOA (EFSA CONTAM Panel, 2018), a total of six studies were reviewed. Since then, three additional publications on two cohorts have been published (Grandjean et al., 2017a,b; Zeng et al., 2019). All these studies are reviewed below for all PFASs, including also PFOS and PFOA.
Grandjean et al. (2012) examined associations between both pre‐ (gestation week 32) and postnatal (5 years) serum concentrations of PFASs and offspring antibody concentrations against tetanus and diphtheria following booster vaccination at age 5 years (cohort 3, n = 456–587, 1997–2000). Postnatally, serum PFASs and pre‐booster antibody concentrations were measured at a mean age (SD) of 5.0 (0.1) years. Serum antibody response was then measured ~4 weeks later after booster vaccination and at offspring age 7.5 (0.1) years. The median concentrations for antibody titres to tetanus were 0.22 IU/mL at 5 years pre‐booster, 35 IU/mL at 5 years post‐booster and 1.6 IU/mL at 7.5 years. For diphtheria the corresponding numbers were 0.12, 13.0 and 0.68 IU/mL, respectively. Associations between offspring PFAS concentrations at age 5 pre‐booster with antibody titres at age 5‐ and 7.5‐year post‐booster can be interpreted as a short‐ and long‐term influence on the efficacy of the booster vaccination, respectively. The strength of this study is that it is not purely observational and has an interventional component. The large increase in antibody concentration is initiated through vaccination and this increase is examined in relation to baseline PFASs concentrations. The interpretation of associations reported between maternal PFAS concentrations and offspring antibody concentrations during childhood are, however, more challenging, as several vaccinations are administered from birth at various time points. Furthermore, among breastfed infants, maternal PFAS concentrations are, due to exposure through breastfeeding, strong determinants of offspring concentrations during the first few years of life. Several associations were explored in this study and the results are summarised below:
Association between maternal PFAS concentrations and antibody concentrations at ages 5 (pre‐ and post‐booster) and 7.5:
PFOS: Mean concentration in maternal serum was 27.3 ng/mL. Each twofold increase in maternal PFOS concentrations was associated with –39% (95%CI: –55, –17) and –21% (95%CI: –38, 1) decrease in diphtheria antibody concentrations at 5 years pre‐ and post‐booster, respectively. Non‐significant but inverse direction associations were observed for tetanus antibody concentrations.
PFHxS: Maternal concentrations of PFHxS (mean: 4.4 ng/mL) were not associated with antibody concentrations to tetanus or diphtheria at age 5 years pre‐ and post‐booster.
PFOA: Maternal concentrations of PFOA (mean: 3.2 ng/mL) showed a non‐significant inverse association with antibody concentrations to diphtheria at age 5 years pre‐ and post‐booster while the associations for tetanus were in opposite directions at pre‐ and post‐booster, neither of them being significant.
PFNA: Similar to PFOA, maternal concentrations of PFNA (mean: 0.6 ng/mL) showed a non‐significant inverse association with antibody concentrations to diphtheria at age 5 years pre‐ and post‐booster, while the associations for tetanus were centred around the NULL.
PFDA: Maternal concentrations of PFDA (mean: 0.3 ng/mL) were significantly and inversely associated with antibody concentrations to diphtheria (around 20% decrease per twofold increase) at age 5 years pre‐ and post‐booster. No association was observed for tetanus.
Combined exposures: Structural equations were used to evaluate the associations for combined exposure to PFOS, PFHxS and PFOA during pregnancy and in relation to offspring antibody response to diphtheria and tetanus at age 5.0 years pre‐booster and at age 7.5 years post‐booster. A twofold increase in maternal concentrations during pregnancy was significantly associated with ‐48% (95%CI: –68, –16) and –42% (95%CI: –66, –1) decrease in serum antibody response to diphtheria at age 5 pre‐booster and age 7.5 post‐booster, respectively. No associations were observed for tetanus.
Offspring PFAS concentrations at age 5 and offspring antibody concentrations at ages 5 and 7.5 years:
PFOS: Each twofold increase in offspring PFOS concentrations at 5‐year pre‐booster (mean 16.7 ng/mL) was associated with −29% (95%CI: −46, −6) and −24% (95%CI: −44, 4) change in post‐booster antibody response to tetanus at age 5 and 7.5 years, respectively. The corresponding estimates for diphtheria were −16% (95%CI: −32, 4) and −28% (−46, −3), respectively.
PFHxS: At age 5 years pre‐booster, two‐fold offspring concentrations of PFHxS (0.6 ng/mL) were significantly associated with −19% (95%CI: −30, −7) lower tetanus antibody concentration at 5 years post‐booster and −20% (95%CI: −32, −6) lower concentration at age 7.5 years. A non‐significant −10% lower concentration was observed for diphtheria for these two time points.
PFOA: At 5 years of age, pre‐booster offspring concentrations of PFOA (4.1 ng/mL) showed a weak but inverse association with antibody response to tetanus and diphtheria post‐booster at age 5 years (6–13% decrease). At age 7.5 years the association for both antibody titres to diphtheria and tetanus was, however, strongly significant, corresponding to around ~ 25% decrease per twofold increase in PFOA.
PFNA: At 5 years pre‐booster, each twofold increase in offspring PFNA concentrations (mean: 1.0 ng/mL) was associated with around 15–20% decrease in antibody response to diphtheria and tetanus at age 5 and 7.5 years, although statistical significance was not always reached.
PFDA: At 5‐year pre‐booster, each twofold increase in PFDA (mean: 1.0 ng/mL) concentrations was associated with around 10–20% decrease in antibody response to diphtheria and tetanus at 5‐ and 7.5‐year post‐booster, although statistical significance was reached only for tetanus.
Combined exposures: Structural equations were used to evaluate the associations for combined exposures to PFOS, PFHxS and PFOA at offspring age 5 years (pre‐booster) in relation to offspring antibody response to diphtheria and tetanus at age 5 years pre‐booster and at age 7.5 years post‐booster. A twofold increase in offspring serum levels at age 5 years pre‐booster showed a non‐significant inverse association with antibody concentrations age 5 years pre‐booster. A twofold increase in combined exposure at age 5.0 years pre‐booster was, however, significantly associated with a −44% (95%CI: −66, −11) and −55% (95%CI: −73, −25) decrease in serum antibody response to diphtheria and tetanus at age 7.5, respectively.
Low antibody levels At age 5 years pre‐booster, a twofold increase in PFOS concentrations was associated with 1.6 (95%CI: 1.1, 2.3) higher odds of being below a protective level (0.1 IU/mL) against diphtheria. The corresponding estimates for PFOA was OR 1.2, 95%CI: 0.8–1.7. Slightly elevated but non‐significant OR were observed for tetanus. At age 7.5 years concentrations of PFOS and PFOA at 5 years were associated with 2.4 (95%CI: 0.9, 6.4) and 3.3 (95%CI: 1.4, 7.5) higher odds of being below protective levels against diphtheria. Similar elevated odds were reported for tetanus at age 7.5.
Co‐exposures: Concerning possible confounding by other co‐exposures, PCBs in maternal samples and offspring samples at age 5.0 years showed a weak correlation with individual PFASs. Adjustment for these co‐exposures had no impact on the effect estimates. With respect to individual PFASs, the correlation between the five substances at offspring age 5 years ranged between 0.2 and 0.8. The strongest correlation was observed between PFNA and PFDA, while for PFOS and PFOA the correlation was ~ 0.5. Other pair‐wise correlations were weaker. The authors performed benchmark dose (BMD) analyses for each of the five PFASs in serum of the 5‐year‐old children in relation to antibody response at 5 and 7.5 years. The results were reported with and without mutual adjustment for PFOS and PFOA (Budtz‐Jorgensen and Grandjean, 2018). In short, the modelling showed that both PFOS and PFOA, in statistical terms, were associated with antibody concentrations independent of each other (not confounded).
In summary, combined serum levels of PFOS, PFHxS and PFOA during pregnancy and at age 5 years pre‐booster vaccination showed a strong inverse association with serum antibody titres to diphtheria and/or tetanus at age 7.5 years. Use of combined exposure is supported by evidence suggesting that the associations observed for each PFAS are at least partly independent (Budtz‐Jorgensen and Grandjean, 2018). For individual PFASs at age 5 years, the associations with antibody concentrations to tetanus and diphtheria at 5 and 7.5 years post‐booster were consistently inverse. Associations for tetanus were generally weaker and less consistent compared to diphtheria. A lack of statistical significance for some of the associations reported is not unexpected, given the different concentrations of individual PFASs. Higher odds of being below protective levels for diphtheria at age 5.0 and 7.5 years suggest that the associations observed are of clinical relevance.
Looker et al. (2014) examined, among 411 US adults, associations between serum concentrations of PFOA and PFOS and antibody response following influenza vaccination. Participants had been exposed to PFOA contaminated drinking water and were enrolled into the C8 project in 2005–2006. They were again followed up and recruited for this study in 2010. Median serum concentrations of PFOA and PFOS in 2010, at baseline prior to vaccination, were 31.5 and 9.2 ng/mL, respectively. After vaccination (after 21 ± 3 days), seroconversion for influenza type B, A/H1N1 and A/H2N3 was 62%, 84% and 65%, respectively. Mean antibody concentrations for influenza type B post vaccination were significantly lower among those in the highest compared to the lowest quartile of serum PFOA levels. Non‐significant decreases in antibody titres were also observed across quartiles for A/H1N1 and A/H2N3. After adjustment for age and sex, the association between PFOA and antibody titres to influenza Type B was weakened and non‐significant, while the inverse association for A/H2N3 became more pronounced, showing a significant risk of not attaining the antibody threshold considered to offer long‐term protection. No association was observed for PFOS.
Kielsen et al. (2016) recruited 12 healthy adult volunteers from Denmark, aged 23–67, and examined associations between serum concentrations of several PFASs with changes in antibody concentrations to diphtheria and tetanus following booster vaccination in an exploratory study. Serum samples were drawn at baseline prior to booster vaccination. Baseline concentrations (medians) were 0.4 ng/mL for PFHxS, 9.5 ng/mL for PFOS, 0.12 ng/mL for PFHpA, 1.7 ng/mL for PFOA, 0.7 ng/mL for PFNA, 0.3 ng/mL for PFDA, 0.21 ng/mL for PFUnDA and 0.04 ng/mL for PFDoDA. Changes in antibody concentration were quantified at days 2, 4, 7, 10, 14 and 30 after booster vaccination. Concentrations of both diphtheria and tetanus antibodies increased by more than 10‐fold and reached a plateau by day 10. The outcome measure was, as a result, defined as the individual change in serum antibody titres between days 4 and 10 post‐booster. Baseline concentrations of PFHxS, PFOS, PFNA, PFDA, PFUnDA and PFDoDA were significantly and inversely associated with the rate of increase in diphtheria antibody concentrations between 4 and 10 days. A non‐significant inverse association was observed for PFOA (p = 0.25) and PFHpA (p = 0.75). The percent reduction in diphtheria antibody concentrations ranged between ~8 to ~18% for a twofold increase in PFAS concentrations. However, for tetanus, only PFUnDA and PFDoDA were significantly inversely associated with the rate of increase in antibody response, corresponding to ~10% reduction in antibody concentrations per twofold increase in exposure.
Stein et al. (2016b) examined the association between serum concentrations (means) of PFOS (5.2 ng/mL), PFHxS (1.1 ng/mL), PFOA (2.3 ng/mL) and PFNA (0.8 ng/mL) with immune response following vaccination against influenza (FluMist). A total of 78 subjects from the US, aged 18–49, provided a baseline blood sample and were then immunised. Blood samples were then drawn after 48–72 h and again, at least 30 days after vaccination. A total of 13 immune outcomes were explored including cytokine, chemokine and immunoglobulin concentrations in serum. Overall, no significant associations were observed between baseline concentrations of these four compounds with the outcomes explored. When interpreting these results, it is worth noting that the FluMist generated a limited systemic response to the vaccination. More precisely, seroconversion was only observed in 7 and 19 out of 78 participants as measured by haemagglutinin inhibition and immunohistochemistry, respectively. Such limited response to the vaccination is a clear limitation, making any association with antibody response difficult to detract.
In a cohort of 101 infants from Germany, Abraham et al. (2020) examined the association between plasma concentrations of PFHxS, PFOS, PFOA and PFNA and antibodies to diphtheria, tetanus and haemophilus influence type b (Hib). Mothers and their children were recruited in 1997–1999 when the infants were between 341 and 369 days old. Of these, 21 were formula fed (≤ 2 weeks of breastfeeding) and 80 were breastfed for > 4 months. When combining exclusive and partial breastfeeding into ‘equivalent to exclusive breastfeeding’ the median duration was 7.4 months. Mean levels of PFASs in plasma from, respectively, non‐breastfed and breastfed infants were for PFOA 3.8 and 16.8 ng/mL, for PFOS 6.8 and 15.2 ng/mL, for PFHxS 1.7 and 2.1 ng/mL and for PFNA 0.2 and 0.6 ng/mL. For the mothers, the mean concentrations in plasma among those who did not breastfeed (n = 21) and those who breastfed (n = 80) were for PFOA 4.9 and 3.2 ng/mL, for PFOS 17.2 and 14.1 ng/mL, for PFHxS 1.8 and 1.0 ng/mL and for PFNA 0.4 and 0.3 ng/mL. Higher concentrations in plasma among breastfed infants and lower concentrations among mothers who breastfed is explained by lactational transfer of PFASs from the mother to the baby. This transfer into breast milk is more effective for PFOA compared to PFOS, which also explains the differences in PFOS/PFOA ratio between mothers and infants (see human TK section).
Concentrations of PFOA in infant plasma were significantly and inversely correlated with antibody concentrations to diphtheria (r = –0.23, p = 0.02), tetanus (r = –0.25, p = 0.01) and Hib (r = –0.32, p = 0.001). Analyses were adjusted for time since last vaccination and for tetanus also the number of vaccinations. Adjustment for other co‐contaminants quantified in infant blood, including PCBs, dioxins (I‐TEQ), organochlorine pesticides, mercury, cadmium and lead did not influence these associations. Adjustment for duration of exclusive breastfeeding had no relevant influence. The NOAECs for PFOA, estimated by dividing exposure into quintiles, ranged between 18.9 and 19.4 ng/mL, depending on the type of antibody titres. In terms of effect size the mean reduction in antibody response when comparing the highest to lowest quintile of PFOA exposure was –57%, –53% and –78% for diphtheria, tetanus and Hib, respectively. Associations for PFOS, PFHxS and PFNA were not significant. Upon request from EFSA, the authors provided analyses of the associations with the sum of PFOA, PFNA, PFHxS and PFOS (see Appendix K). Similar as for PFOA, the sum of the four PFASs was significantly and inversely correlated with tetanus and Hib, while the correlation for diphtheria was borderline significant.
Since the first study from the Faroe Islands (Grandjean et al., 2012), reviewed above, two additional studies using partly the same data have been published. One study was based on later follow‐up of the children, while the other examined associations with PFAS concentrations at earlier age. Both these studies, and others reviewed in this section, are of more observational character as the timing of exposure assessment is not standardised and assessed in relation to administration of the booster vaccination.
Using the same cohort as in the first study (Grandjean et al., 2012; cohort 3, n = 587, 1997–2000), additional follow‐up was conducted when the children were 13 years of age (Grandjean et al., 2017b). In that study, associations between offspring PFAS concentrations at ages 7.5 and 13 years were examined in relation to serum antibody titres to tetanus and diphtheria at age 13 years. At age 7.5 years, serum concentrations (means) were 15.3 ng/mL for PFOS, 4.4 ng/mL for PFOA, 0.5 ng/mL for PFHxS, 1.1 ng/mL for PFNA and 0.4 ng/mL for PFDA. These median concentrations were quite similar to those observed at age 5 (Grandjean et al., 2012). Serum PFOS concentrations at age 7.5 were inversely associated with antibody concentrations to diphtheria at age 13 corresponding with around –24% (95%CI: –43, –2) decrease per twofold increase in serum levels. The corresponding estimate for PFDA was –22% (95%CI: –34, –6). For PFOA, PFHxS and PFNA a modest inverse association was observed, being far from significant (p > 0.20). After exclusion of subjects having received booster vaccinations between age 7.5–13, slightly stronger associations were observed. At age 13 years, serum PFAS concentrations were approximately halved compared to those at that age 7.5. At age 13 years, the cross‐sectional analyses showed a modest and non‐significant inverse association for all five compounds. After exclusion of those having received booster vaccinations, the association for PFOA became significant, showing a –25% (95%CI: –43, –3) decrease per twofold increase in exposure, while the associations for the other four compounds were still inverse but non‐significant. For tetanus antibodies, the associations were, however, in the opposite direction for some of the compounds, suggesting an increase in antibody concentration at 13 years with higher exposure at ages 7.5 and 13. One methodological limitation of this study, as explained by the authors, is that children and adolescents that visit the emergency room for cuts or other injuries, frequently receive a tetanus booster for protection, which adds considerable variability to the outcome measures. Around 46% of the subjects in that study were suspected of having received a booster vaccination against tetanus between ages 7.5 and 13 years, which makes it difficult to evaluate the association for tetanus with the same precision as for diphtheria.
Another study aimed at examining the effect of PFASs on antibody response in young children (Grandjean et al., 2017a), using partly the same data (cohort 3, n = 587–532, 1997–2000) along with another comparable cohort recruited 10 years later (cohort 5, n = 275–239, 2007–2009). The aim of this study was to explore associations with serum PFAS concentrations at birth, age 18 months and 5 years in relation to serum antibody concentrations at age 5 years prior to booster vaccination at that age. In Cohort 5 maternal blood samples were drawn around birth, while in Cohort 3 serum PFAS concentrations at birth were estimated based on maternal samples drawn in gestation week 32.
For tetanus, both cohorts individually and combined showed a consistent inverse association between serum PFAS concentrations at birth, age 18 months and 5 years and serum antibody concentrations at 5 years (pre‐booster). Statistical significance was, however, only consistently reached for PFOA.
For diphtheria, serum PFAS concentrations at birth showed an inverse association with serum antibody concentrations at 5 years (pre‐booster) in both cohorts. In combined analyses of both cohorts, significant associations were observed for PFOS, PFOA and PFNA. For PFAS levels at 18 months and 5 years, the associations with antibody concentrations at 5 years (pre‐booster) were inconsistent, with cohort 3 showing in general inverse associations, while cohort 5 frequently showing positive associations. In cohort 3, serum PFOS and PFOA concentrations were ~17 and 4 ng/mL. Due to phase‐out of long‐chain PFASs, environmental concentrations had decreased continuously and for cohort 5 the concentrations were two‐ to threefold lower (~5 ng/mL for PFOS and 2 ng/mL for PFOA). Decreasing and lower exposures in cohort 5 may well explain these inconsistent findings.
Modelled exposures: The authors also estimated offspring serum concentrations of PFOS and PFOA at months 3, 6 and 12 based on maternal serum concentrations at birth and information on duration of breastfeeding. Associations with serum antibody concentrations to tetanus and diphtheria at age 5 years pre‐booster were then explored. The strongest associations were generally observed for predicted concentrations at 3 months of age, while the associations became increasingly weaker at ages 6 and 12 months.
Granum et al. (2013) examined in a subset (n = 50) of children from the Norwegian Mother and Child Cohort study the associations between maternal serum concentrations (medians) of PFOS (5.5 ng/mL), PFHxS (0.3 ng/mL), PFOA (1.1 ng/mL) and PFNA (0.3 ng/mL), measured at delivery with serum antibody concentrations in offspring that had all followed a routine vaccination program, where vaccines against tetanus and Hib were given at ages 3, 5, and 12 months, and vaccination against measles and rubella at 15 months. Maternal serum concentrations were for all compounds significantly and inversely associated with offspring antibody concentrations to rubella at age 3 years (n = 50). Similar conclusions were reached when each compound was mutually adjusted for the other. Non‐significant inverse associations were observed for Hib, tetanus and measles antibodies.
Stein et al. (2016a) examined cross‐sectionally associations between concentrations (means) of PFHxS (2.5 ng/mL), PFOS (20.8 ng/mL), PFOA (4.1 ng/mL) and PFNA (0.8 ng/mL) and serum antibody concentrations to measles, mumps and rubella among 1,188 12‐ to 19‐year‐old US children from the US National Health and Nutrition Examination Survey (NHANES). Among seropositive individuals (96%) a 1‐ng/mL increase in concentrations of PFOS, PFHxS and PFOA was associated with, respectively, −13% (95%CI: −20, −6), −6% (95%CI: −11, −2) and −9% (95%CI: −16, −3) lower serum antibody concentrations to rubella. PFOS and PFOA showed slightly weaker but significant inverse associations with antibody concentrations to Mumps (~ 6–7% decrease per 1‐ng/mL increase). Non‐significant, but inverse associations were observed for PFHxS and non‐significant associations were observed for measles for all four compounds.
Finally, Zeng et al. (2019) examined in 201 Chinese mother–child pairs associations between several PFASs measured in cord blood and antibodies to hand, foot and mouth disease (CA16 and EV71) both in cord blood and at 3 months of age. PFAS cord‐blood concentrations were (medians) PFOA (1.2 ng/mL), PFOS (3.2 ng/mL), PFDA (0.1 ng/mL), PFDoDA (0.05 ng/mL), PFHxS (4.0 ng/mL), PFNA (0.2 ng/mL), PFUnDA (0.1 ng/mL) and sum of all PFASs (10.6 ng/mL). Except for PFHxS and PFDoDA, all PFASs quantified (including the sum) were inversely associated with antibodies to hand, foot and mouth disease in cord blood. Similar inverse associations were observed at 3 months, but statistical significance was not reached for several compounds. Cord blood concentrations of individual PFASs (including the sum) were, however, associated with increased odds of having serum antibodies below protective levels in both cord blood and in offspring serum drawn at 3 months. The only exception from this pattern was PFHxS.
Summary
Results from six vaccination response studies in children and adults have been reported to date (Grandjean et al., 2012; Granum et al., 2013; Looker et al., 2014; Kielsen et al., 2016; Stein et al., 2016b; Abraham et al., 2020). Three of these studies show, for several PFASs, relatively strong inverse associations with antibody response following booster vaccination to tetanus and diphtheria in both children (Abraham et al., 2020; Grandjean et al., 2012) and adults (Kielsen et al., 2016). One study showed an inverse association between maternal PFAS levels and antibodies to rubella in children (Granum et al., 2013) and one study showed some, but more modest inverse associations with antibody titres to influenza in adults (Looker et al., 2014). The null findings by Stein et al. (2016b) on influenza vaccination do not contradict these results, as most subjects did not respond to the vaccination. Associations with antibody titres falling below protective levels were also reported (Grandjean et al., 2012; Looker et al., 2014). Concerns have been raised previously that findings on vaccination response among the Faroese children (Grandjean et al., 2012) could be confounded by other seafood contaminants. However, stability of those findings after adjustment for PCBs (Grandjean et al., 2012) and replication of findings in infants (Abraham et al., 2020) and a small group (12) of Danish adults (Kielsen et al., 2016), where exposure to seafood‐borne contaminants differs, make such confounding less likely. Consistent findings from observational studies in other study populations provide further support for an adverse association between several PFASs and vaccination response in children and adolescents (Stein et al., 2016a; Zeng et al., 2019). The two recent studies from the Faroe Islands are in line with other studies showing associations in early infancy (Grandjean et al., 2017a) and at adolescent age (Grandjean et al., 2017b). It is, however, difficult to conclude from these studies if one age period is more sensitive than another.
Overall, different compounds appear to show significant findings across different studies. Such inconsistencies are not unexpected given the differences in concentrations and mixture composition. As a result, it is difficult to make strong conclusions if one compound is much more potent than the other. Based on more detailed analyses from the Grandjean et al. (2012) study, it seems likely that both PFOS and PFOA may affect antibody response independently (Budtz‐Jorgensen and Grandjean, 2018).
In line with the conclusion from the Opinion on PFOS and PFOA (EFSA CONTAM Panel, 2018), additional studies published since then strengthen the conclusion that both PFOS and PFOA are associated with reduced antibody response to vaccination. Given the lower concentration of other PFASs, the evidence for association is weaker.
3.3.4.4.3. Clinical infections
In the previously published Opinion on PFOS and PFOA (EFSA CONTAM Panel, 2018), a total of five studies were reviewed. Since then three other studies have been published. Given the relevance of this outcome for the Opinion, all these studies are reviewed below for all PFASs (including PFOS and PFOA).
In the previously mentioned study by Granum et al. (2013), maternal concentrations of PFHxS, PFOS, PFOA and PFNA were also examined in relation to common cold in the offspring, that was assessed by parental report at ages 1, 2 and 3 years (n = 99). The authors observed a significant positive association between the total number of reported episodes of common cold in the offspring up to 3 years of age (n = 99) and maternal PFHxS, PFOA and PFNA, but not PFOS. For PFOA and PFHxS a positive association with a number of reported episodes of gastroenteritis was also observed.
In the previously mentioned study on asthma and allergies by Impinen et al. (2019), associations between maternal PFHxS, PFHpS, PFOS, PFOA and PFUnDA concentrations and offspring risk of infections at ages 0–3 years and 6–7 years, assessed by parental report, were also examined. Although positive associations with certain types of infections were observed for individual PFASs (pseudocroup, bronchitis/pneumonia at age 0–3, and Diarrhoea/gastric flu at age 7), inverse associations were also observed (such as for episodes of common cold at age 0–3 years). Overall, the results of this study are inconclusive.
Dalsager et al. (2016) examined associations between maternal serum concentrations (median) for PFHxS (0.3 ng/mL), PFOS (8.1 ng/mL), PFOA (1.7 ng/mL), PFNA (0.7 ng/mL) and PFDA (0.3 ng/mL) and infections in children among 649 mother–child pairs from Odense, Denmark. Maternal serum samples were drawn prior to week 16 of gestation and episodes of infection were recorded every 2 weeks over 12 months from birth, using mobile phone text messages. Both the number of days and information on symptoms (11 different categories) were recorded. Among the 649 participants, 354 reported symptoms ranging from rare events such as blood in stools (n = 4) to temperature over 38.5 degrees (n = 283) and runny nose (n = 337). Maternal PFOS concentrations in the upper tertile (> 10.2 ng/mL) compared with the lowest (< 6.9 ng/mL) were significantly associated with increased proportion of days with fever (incidence rate‐ratio: 1.7 (95% CI: 1.2, 2.2), p‐trend = 0.001). No association was observed for the other compounds. An increased odds ratio of experiencing days with fever above the median was found for PFOS (OR: 2.4 (95% CI: 1.3, 4.1)) and for PFOA (OR: 2.0 (95% CI: 1.1, 3.6), but no such association was observed for PFHxS, PFNA and PFDA. For other symptoms than fever, different PFASs showed no associations with other symptoms including coughing, nasal discharge, diarrhoea or vomiting.
In a cohort of 1,558 mother–offspring pairs, Goudarzi et al. (2017) examined the association between maternal concentrations (means) of PFHxS (0.3 ng/mL), PFOS (5.5 ng/mL) PFHxA (< 0.1 ng/mL), PFHpA (< 0.1 ng/mL), PFOA (2.7 ng/mL), PFNA (1.1 ng/mL), PFDA (0.5 ng/mL), PFUnDA (1.4 ng/mL), PFDoDA (0.2 ng/mL), PFTrDA (0.3 ng/mL) and PFTeDA (< 0.1 ng/mL) and prevalence of offspring infectious diseases up to 4 years of age. Samples were drawn in gestation week 28 to 32. At the 4‐year follow‐up, mothers filled out a questionnaire that included questions on common offspring infectious diseases that had occurred up to 4 years of age, including otitis media, pneumonia, varicella and respiratory syncytial virus. Associations between maternal PFASs and total infection diseases, defined as cases with at least one of these four common infectious diseases up to 4 years of age, were then explored. Maternal PFOS was associated with increased propensity for total infections up to 4 years of age [OR of 1.6 (95% CI 1.2, 2.2), when comparing those with > 6.7 ng/mL vs. < 2.7 ng/mL]. No associations were observed for the other compounds.
In a cohort of 641 mother–child pairs from Norway, Impinen et al. (2018) examined the association between cord blood concentrations of (medians): PFHxS (0.2 ng/mL), PFOS (5.2 ng/mL), FOSA (0.4 ng/mL), PFOA (1.6 ng/mL), PFNA (0.2 ng/mL) and PFUnDA (0.1 ng/mL) and the number of episodes of common cold infections in the offspring from 0 to 2 years, and number of episodes of respiratory tract infection in the offspring from 0 to 10 years. The number of episodes was based on parental report at follow‐up at offspring age 2 and 10 years, respectively. PFUnDA was positively associated with the number of episodes of infections in the offspring until age 2 years. No associations were observed for other PFASs. PFOS, PFOA, FOSA, PFNA and PFUnDA (but not PFHxS) were, however, all strongly and significantly associated with the number of episodes of respiratory tract infection until 10 years of age.
In the previously reviewed study by Looker et al. (2014) on vaccination associations, self‐reported occurrence of recent (< 12 months) respiratory episodes of colds or influenza in adults (n = 411) were also examined. No associations were observed for PFOS and PFOA (median levels 31.5 and 9.2 ng/mL).
Fei et al. (2010a) examined in a subset of participants from the Danish National Birth Cohort (n = 1,400, 1996–2002), associations between maternal concentrations of PFOS and PFOA and risk of hospitalisation due to infectious diseases, up to 2008. Information on hospital admission was extracted from nationwide registries. Mean concentrations for PFOS and PFOA in maternal serum, drawn in gestation week 4–14, were 35 and 5.6 ng/mL, respectively. At the end of follow‐up, 26% of the offspring had been hospitalised at least once. Offspring age at the end of follow‐up ranged from 6 to 11 years. In this study, no associations were observed between maternal concentrations of PFOS and PFOA and offspring hospitalisation. The authors noted that they were only able to examine associations with resistance to infections in general, and the study was not designed to examine if prenatal exposure could predict occurrence of specific infections.
Okada et al. (2012) also found no association between second trimester maternal serum concentrations (median) of PFOS (5.2 ng/mL) and PFOA (1.3 ng/mL) and offspring otitis media (parental report) up to 18 months of age (n = 343).
Abraham et al. (2020) also examined associations between PFOS and PFOA and number of infections in the children (n = 101) occurring from birth, until examination based on maternal report. No association was observed. The limitations of this study are small sample size for studying infections and the fact that most of the infants would have been partly protected by maternal antibodies from breastfeeding.
Summary
Based on the results from the vaccination studies reviewed above, findings on increased propensity of infections would seem plausible. Overall four studies on infection in children found some positive associations between maternal concentrations of PFASs with offspring's later risk of infections (Granum et al., 2013; Dalsager et al., 2016; Goudarzi et al., 2017; Impinen et al., 2018). Two studies in children reported no such association (Fei et al., 2010a; Abraham et al., 2020) and one study reported both positive and negative associations (Impinen et al., 2019). In general, associations were most frequently reported for PFOS, but as for the vaccination studies, differences in concentration and mixture composition makes it difficult to evaluate the evidence for each compound independently. One study in adults also reported no association with infections (Looker et al., 2014). All but two of these studies (Fei et al., 2010a; Dalsager et al., 2016) were based on self‐reported episodes of infection, which are prone to bias. Since those reporting such infections should have been blinded to the exposure, such bias is likely to be non‐differential. Overall, there is some evidence to suggest that exposures to PFASs are associated with increased propensity of infections but more studies with objective measures of infections (not self‐reports) are needed.
3.3.4.5. Endocrine effects
3.3.4.5.1. Thyroid function and disease
PFOS and PFOA
In the Opinion on PFOS and PFOA, six studies on associations with thyroid disease and 20 studies on associations with thyroid hormones were reviewed (EFSA CONTAM Panel, 2018). Some were performed in children and some in adults. Most of them were cross‐sectional. It was concluded that there is insufficient support for associations between PFOS or PFOA and thyroid disease or changes in thyroid hormones.
PFASs other than PFOS and PFOA
Most studies examined associations with PFHxS and PFNA, and the 19 studies are tabulated in Appendix J, Section J.5, where the individual studies are reviewed.
Regarding thyroid disease, one study found an association between PFHxS and thyroid disease (as defined by TSH levels outside a certain range), but only in women and not consistent with results when TSH was used as a continuous variable. No associations were reported between PFNA and thyroid disease.
Associations between serum levels of PFHxS and/or PFNA and thyroid hormones were investigated in seventeen cross‐sectional studies
In summary, the available evidence is insufficient to suggest that exposure to PFASs are associated with thyroid disease or changes in thyroid hormones.
3.3.4.5.2. Male fertility and puberty
In the Opinion on PFOS and PFOA (EFSA CONTAM Panel, 2018), a number of cross‐sectional studies on semen quality and sex hormones in men were reviewed. In summary, those studies did not provide support for serum PFOS/PFOA as predictors of adverse effects on semen quality or reproductive hormones. Some of these studies also examined other PFASs than PFOS and PFOA, as described in Appendix J, Section J.5. In addition, one additional study reporting results on PFOS and PFOA have been published after the PFOS/PFOA Opinion.
In summary, the available evidence is insufficient to suggest that pre‐ or postnatal exposures to PFASs are associated with effects on pubertal development or male fertility.
3.3.4.5.3. Female fertility, menstrual cycle and puberty
In the Opinion on PFOS and PFOA (EFSA CONTAM Panel, 2018), a number of studies on semen quality and sex hormones in men were reviewed. In summary, those studies provided insufficient evidence that exposure to PFOS or PFOA is related to menstrual cycle length, menopause or puberty development. Likewise, the studies reviewed in this Opinion (Appendix J, Section J.5) do not provide consistent associations between pre‐ or postnatal exposures to PFASs and female reproduction outcomes or puberty.
3.3.4.6. Metabolic outcomes
3.3.4.6.1. Blood lipids
PFOS and PFOA
In the Opinion of PFOS and PFOA 26 publications in 16 study cohorts on associations between PFOS and/or PFOA and serum lipids were reviewed (EFSA CONTAM Panel, 2018). Some were performed in occupational cohorts, but most of them in the general population. Most were cross‐sectional but there were also some longitudinal studies. It was concluded that it is likely that associations between serum PFOS and PFOA levels and serum cholesterol are causal and that an increase in cholesterol was considered adverse.
After the Opinion on PFOS and PFOA, some new studies examined associations with blood lipids. Convertino et al. (2018) in a before–after study with no external control group examined 49 patients with advanced solid tumours, who failed standard therapy. The patients received oral doses of between 50 and 1,200 mg weekly for 6 weeks of ammonium perfluorooctanoate (APFO) as a possible chemotherapeutic agent. The serum PFOA concentrations were about 10,000–150,000 ng/mL after the first dose and about 60,000–500,000 ng/mL after 6 weeks. Overall there was a significant, but slight, decrease of total and LDL cholesterol with increasing dose and serum levels of PFOA. The authors concluded that at these serum PFOA levels the decrease in total cholesterol (and LDL), was consistent with results in animal models, and the effects of PPARα‐agonists such as clofibrate in humans. Additionally, the authors noted that these changes of total cholesterol (TC) and LDL at very high serum PFOA levels contradict the epidemiological findings of increasing cholesterol with increasing serum PFOA at much lower serum PFOA concentrations.
However, the median TC in the study with cancer patients seemed to increase at the lowest serum PFOA categories, although there were only few patients in each category. Moreover, the small sample size, the singularity of the exposure conditions (extremely high level, non‐dietary medicinal administration etc.), the unique characteristics of the population under study and the lack of a control group hinders both the internal and the external validity of this study. Thus, the CONTAM Panel noted that this report cannot shed additional light on effects in humans at the much lower PFOA intake levels occurring from normal diet or contaminated drinking water.
Graber et al. (2019) examined the association between PFAS and self‐reported physician‐diagnosed ‘high’ cholesterol in 105 adults exposed to contaminated drinking water in New Jersey, MA, USA. The geometric means of serum PFOS and PFOA were 5.4 and 3.0 ng/mL. After adjustment for potential confounders, there were no significant associations between PFOS/PFOA and self‐reported high cholesterol.
Dong et al. (2019) studied cross‐sectional associations between serum levels of PFAS and cholesterol in six US NHANES surveys 2003–2014, including about 9,000 adults and 3,000 adolescents. The median PFOS (10.9 ng/mL) and PFOA (3.0 ng/mL) levels in adults were only slightly higher than in adolescents. After adjustment for a number of potential confounders, there were significant linear associations between serum PFOS/PFOA and total cholesterol. BMD was estimated using a ‘hybrid approach’ where the outcome was ‘high’ cholesterol and the BMR was 10%. BMDLs for PFOS and PFOA were estimated to be 24 and 5.6 ng/mL.
Jain and Ducatman (2019) studied associations between (log‐transformed) PFAS and (log‐transformed) serum lipids cross‐sectionally in 3,629 adults from NHANES surveys 2005–2014. The aim was to examine if associations differed by gender and obesity (BMI > 30). After adjustment for a number of potential confounders, serum PFOA (but not PFOS) was positively associated with total and LDL cholesterol in obese males, but not in the other three groups (women and non‐obese males). Serum PFOS (but not PFOA) was positively associated with LDL cholesterol in obese females, but not in the other three groups. The authors propose that PFAS exposure also contributes to liver steatosis and/or that increased serum cholesterol from PFAS is more likely to occur in individuals with steatosis.
Donat‐Vargas et al. (2019) studied associations between serum PFAS and blood lipids in a longitudinal study of 187 individuals from a population‐based cohort followed up for 10 years. The median PFOS and PFOA levels were 20 and 2.9 ng/mL at baseline and 15 and 2.7 ng/mL at follow‐up. Total cholesterol decreased from 5.5 to 5.4 mmol/L, but there was no significant association between changes of PFOS/PFOA and cholesterol within individuals at the prospective follow‐up of 172 individuals, not taking cholesterol‐lowering medications. There was, however, a significant inverse association between the change of PFOS and the change of triglycerides.
Lin et al. (2019) studied associations between baseline serum PFAS and blood lipids cross‐sectionally, and in a longitudinal follow‐up of 888 prediabetic adults followed up for 15 years, half of which had an intervention aiming at lifestyle changes. The median serum PFOS and PFOA levels were 27 and 4.9 ng/mL at baseline in the late 1990s. At baseline, there were significant positive associations between log‐transformed serum PFOA and serum cholesterol (total, LDL, VLDL, non‐HDL) as well as the risk of hypercholesterolaemia, while the associations with PFOS were statistically significant only for VLDL and non‐HDL cholesterol. The prospective risk of hypercholesterolaemia was associated with PFOA in the subgroup without lifestyle intervention (n = 361), with a hazard ratio of 1.4 (95% CI 1.1–1.9), when comparing individuals with PFOA > 4.9 ng/mL (median) and PFOA ≤ 4.9 ng/mL, while the risk was not increased in the intervention group. For PFOS, the corresponding risk was not significantly increased (HR 1.2, 95% CI 0.9–1.6). There were also some positive associations between PFOS/PFOA and serum triglycerides.
These new cross‐sectional studies in general population samples are consistent with the large number of previous studies finding positive associations between serum levels of PFOS and/or PFOA and serum cholesterol. More interesting are the two new longitudinal studies, one of which did not find any association between the change of PFOS/PFOA and change of cholesterol (Donat‐Vargas et al., 2019) in 172 individuals, and the other one finding an association between baseline PFOA and prospective risk of hypercholesterolemia in 361 prediabetics (Lin et al., 2019).
Associations between PFOS/PFOA and cholesterol have been reviewed again after external comments to the previous Opinion. Further details are presented in Section 3.4. As illustrated there, the CONTAM panel now considers the uncertainty regarding causality is larger than what was stated in the previous Opinion.
PFASs other than PFOS and PFOA
Nelson et al. (2010) performed a cross‐sectional study of the association between PFOS, PFOA, PFNA and PFHxS and blood lipids, body weight and insulin resistance in 860 adults from US NHANES 2003–2004, 20–80 years of age, not taking cholesterol‐lowering medication. Median serum levels were PFOS: 20 ng/mL, PFOA: 3.8 ng/mL, PFNA: 1.0 ng/mL and PFHxS: 1.8 ng/mL (all four > LOD in > 98% of samples). Total cholesterol (TC) and non‐HDL cholesterol (total minus HDL) in serum were determined in all participants and LDL cholesterol in half of them. A number of potential confounders were adjusted for. TC was about 7% higher (14 mg/dL higher) in the fourth quartile (Q4) of PFNA than in the first quartile (Q1); the difference in PFNA medians between Q4 and Q1 was 1.6 ng/mL. The result for non‐HDL cholesterol and LDL showed similar results, but for LDL, the association was not statistically significant. PNFA was moderately correlated with PFOS and PFOA (Spearman r = 0.5), which were also associated with cholesterol (almost as strong as for PFNA). PFHxS was inversely associated with cholesterol (statistically significant with PFHxS as a continuous variable, and almost so when analysed by quartiles).
Lin et al. (2011) studied the association between PFNA (median 1.7 ng/mL) and PFUnDA (median 7.1 ng/mL) and metabolic outcomes including blood lipids (HDL, LDL, total cholesterol, triglycerides) in a cross‐sectional study of 287 young people from Taiwan. No significant associations were found with blood lipids in adjusted models.
Fisher et al. (2013) examined cross‐sectional associations between PFHxS and plasma lipids, glucose homoeostasis, and metabolic syndrome in 2,700 fasting participants aged 18–74 years in the national Canadian Health Measures Survey (CHMS). The GM PFHxS level was 1.9–2.8 ng/mL in the different age groups. In analyses adjusted for age, sex and a number of other potential confounders (and excluding participants taking cholesterol‐lowering drugs), there was a positive association between PFHxS and total cholesterol, LDL and TG, and a negative association with HDL. The OR for high cholesterol increased by PFHxS quartiles.
Fu et al. (2014) performed a small (N = 133) cross‐sectional study of the association between PFASs and blood lipids in a Chinese sample aged 0–80 (mean 30) years, coming for a health check‐up. Analyses were adjusted for age, gender and BMI, but there was no information on use of lipid‐lowering medications. Median serum levels were PFOS: 1.5 ng/mL, PFOA: 1.4 ng/mL, PFNA: 0.4 ng/mL. Other PFASs were < 0.3 ng/mL or below the LOD in > 25% of samples. There was a statistically significant association between total cholesterol and LDL and PFNA. TC was about 6% higher (0.25 mmol/L higher) in the fourth quartile (Q4) of PFNA than in the first quartile (Q1); the difference in PFNA medians between Q4 and Q1 was 0.9 ng/mL. There were also positive associations between PFDA (median 0.2 ng/mL) and cholesterol and HDL. There were no associations between total cholesterol and PFHxA, PFHpA, PFUndA.
In a cross‐sectional study of 891 pregnant women from Norway, part of which had been selected for a study of subfecundity, Starling et al. (2014) studied the association between PFASs and blood lipids. About 40% of invited women participated. A number of potential confounders were adjusted for. Mean levels for the four most abundant PFASs were PFOS: 13, PFOA: 2.3, PFHxS: 0.6 and PFNA: 0.4 ng/mL. Total cholesterol was significantly associated with PFOS, about 4 mg/dL (2%) higher per interquartile range (about 6 ng/mL). There was no significant association with the other PFASs and specifically for PFNA (difference between means of Q4 and Q1 about 0.5 ng/mL) there was no tendency towards a positive association. The Spearman correlation coefficient between PFOS and PFNA was 0.66.
Zeng et al. (2015) performed a cross‐sectional study in 225 Taiwanese children 12–15 year old, serving as controls in a study of asthma (participation rate 72% of those approached). PFNA (median 0.8 ng/mL), PFBS and PFTeDA were positively associated with total cholesterol in some adjusted models.
Seo et al. (2018) published a cross‐sectional study on associations between several PFASs and blood lipids in 786 adults from Seoul, Korea, participating in a medical check‐up. A significant positive association was found between PFNA (median 1.7 ng/mL) and total cholesterol, and a significant inverse association between PFHxS (median 7.1 ng/mL) and cholesterol. The authors reported that there were also positive rank correlations between PFDA (median 1.1 ng/mL) and PFUnDA (median 1.5 mg/mL), but no details were presented. All associations reported were unadjusted for age, sex, and other PFASs.
The study by Graber et al. (2019), mentioned above, examined the cross‐sectional associations between PFHxS (GM 2.0 ng/mL) and PFNA (GM 3.5 ng/mL) and self‐reported physician‐diagnosed ‘high’ cholesterol in 105 adults exposed to contaminated drinking water. After adjustment for potential confounders, there was a significant association (OR 1.16, 95% CI 1.03–1.29) between PFNA (but not PFHxS) and self‐reported high cholesterol.
The study by Dong et al. (2019), mentioned above, found a significant linear association between serum levels of PFNA (median 1.0 ng/mL) and total and LDL cholesterol. No associations were found for PFHxS (median 1.6 ng/mL) or PFDA (0.2 ng/mL). BMD for PFNA was estimated using a ‘hybrid approach’ where the outcome was ‘high’ cholesterol (> 95 percentile) and the BMR was 10%. The BMDL for PFNA and total cholesterol was estimated to be 2.0 ng/mL, i.e. lower than for PFOS/PFOA.
The study by Donat‐Vargas et al. (2019), mentioned above, found no significant associations between changes of PFHxS, PFNA, PFDA, or PFUnDA and cholesterol within 172 individuals at the prospective follow‐up for 10 years. There were, however, significant inverse associations between the changes of PFNA, PFDA, PFUnDA and the change of triglycerides.
The study by Lin et al. (2019) mentioned above had median serum levels of PFHxS = 2.3 ng/mL, PFNA = 0.6 ng/mL, EtFOSAA = 1.1 ng/mL, and MeFOSAA = 1.0 ng/mL at baseline. At baseline there were significant positive associations between log‐transformed serum PFNA and serum cholesterol (total, LDL, and non‐HDL) as well as the risk of hypercholesterolemia, while this was not the case for PFHxS, EtFOSAA, or MeFOSAA. The prospective risk of hypercholesterolaemia was significantly associated with PFNA in the subgroup without lifestyle intervention (N = 361), with a hazard ratio of 1.2 (95% CI 1.0–1.3) per doubling of PFNA at baseline, while the risk was not increased in the intervention group. There were also some positive associations between PFAS and serum triglycerides.
The study by Jain and Ducatman (2019) mentioned above found that serum PFNA (GM 1.0 ng/mL) was positively associated with total and LDL cholesterol in obese males and females, but not in non‐obese individuals. Serum PFDA (GM 0.25 ng/mL) was positively associated with total and LDL cholesterol in obese females, but not in the other three groups. No such associations were found for PFHxS (GM 1.6 ng/mL), PFUnDA (GM 0.12 ng/mL), or MePFOSAA (GM 0.18 ng/mL). PFHxS, PFDA, and PFUnDA were also positively associated with HDL cholesterol. The authors propose that PFAS exposure also contributes to liver steatosis and/or that increased serum cholesterol from PFAS is more likely to occur in individuals with steatosis.
Thus, stratified by each examined PFAS, the findings regarding associations between various PFASs and total cholesterol were as follows:
-
–
For PFHxA, Fu et al. (2014) and Zeng et al. (2015) found no association with TC.
-
–
For PFHpA, and PFBA, Fu et al. (2014) found no association with TC.
-
–
For PFNA, Nelson et al. (2010), Fu et al. (2014), Zeng et al. (2015), Seo et al. (2018), Graber et al. (2019), Dong et al. (2019), Lin et al. (2019), and Jain and Ducatman (2019) found a positive association with TC, while Starling et al. (2014) found no association.
-
–
For PFDA, Fu et al. (2014) and Jain and Ducatman (2019) found a positive association with TC, while Starling et al. (2014), Dong et al. (2019), and Donat‐Vargas et al. (2019) found no such association.
-
–
For PFUnDA, Fu et al. (2014), Starling et al. (2014), Donat‐Vargas et al. (2019), and Jain and Ducatman (2019) found no association with TC.
-
–
For PFDoDA, and PFTeDA, Zeng et al. (2015) found no association with TC.
-
–
For PFHxS, Nelson et al. (2010) and Seo et al. (2018) found an inverse association with TC, while Fisher et al. (2013) found a positive association, and Starling et al. (2014), Zeng et al. (2015), Graber et al. (2019), Dong et al. (2019), Donat‐Vargas et al. (2019), Lin et al. (2019), and Jain and Ducatman (2019) found no association.
-
–
For PFHpS, Starling et al. (2014) found no association with TC.
-
–
For PFBS, Zeng et al. (2015) found a positive association with TC.
Summary
There were 12 cross‐sectional studies on associations between cholesterol and PFAS other than PFOS and PFOA, and mostly results were not consistent. The only exception is PFNA, where almost all studies (Fu et al., 2014; Graber et al., 2019; Jain and Ducatman, 2019; Lin et al., 2019; Nelson et al., 2010; Seo et al., 2018, and Zeng et al., 2015) found significant associations with TC, while Starling et al. (2014) did not. In most of these studies the median or GM PFNA level was < 1 ng/mL. Several of the studies were large and adjusted for potential confounders. In most of the studies PFNA was moderately correlated with PFOS/PFOA (Pearson r 0.4–0.6). In the smallest studies, it is difficult to disentangle the possible impact of PFNA exposure from that of PFOS and PFOA, overall the data suggest that PFNA has an association with serum cholesterol which is independent from PFOS/PFOA.
3.3.4.6.2. Diabetes, obesity and metabolic syndrome
PFOS and PFOA
In the Opinion on PFOS and PFOA (EFSA CONTAM Panel, 2018), 15 studies were reviewed on the associations between PFOS and or PFOA and glucose homoeostasis or diagnosis of diabetes. Overall, the results did not indicate adverse effects on glucose homoeostasis or increased risk of diabetes. Several studies also examined adiposity and overall, the results did not indicate an increased risk of overweight or obesity due to exposure to PFOS or PFOA. Thus, it was concluded that there is no evidence that PFOS or PFOA increases the risk of metabolic syndrome.
PFASs other than PFOS and PFOA
In summary, eight studies were reviewed on associations between PFASs other than PFOS and PFOA, and diabetes or metabolic syndrome, and the results are not consistent. Details on the individual studies are shown in Appendix J, Section J.6.
3.3.4.6.3. Liver
PFOS and PFOA
In the Opinion on PFOS and PFOA (EFSA CONTAM Panel, 2018), 11 studies on associations between PFOS/PFOA and liver enzymes, other liver biomarkers, or liver disease were summarised. Most studies on associations between serum PFOA and serum levels of the liver enzyme ALT at occupational exposure showed no significant associations. The association between PFOA and ALT was considered likely to be causal, but the adversity of the increase in the normal range was considered uncertain since the increase in ALT per unit of PFOS/PFOA was small and no association with liver disease had been shown. For PFOS, data were inconsistent. In the large C8 cohort an association was also found between PFOA and ALT above the reference range, and this was considered adverse. Since the increase in ALT was a potential critical effect (EFSA CONTAM Panel, 2018), the association has now been reviewed again.
After the publication of the Opinion on PFOS and PFOA, several studies have been published on associations between PFOS/PFOA and markers of effects on liver cells and are summarised below.
Bassler et al. (2019) studied associations between PFOS (mean 27 ng/mL) and PFOA (mean 95 ng/mL) and two biochemical markers of hepatocyte apoptosis: Cytokeratin 18 M30 (CK18 M30) and CK18 M65 in a cross‐sectional study of 200 participants in the C8 study, randomly selected among those 40–70 years of age, and without high alcohol consumption or history of hepatitis. The rationale for examining these biomarkers was the findings of hepatocyte steatosis and apoptosis in experimental studies, as well as associations with ALT in human epidemiological studies. In addition, several biomarkers of inflammation (cytokines and C3a) were examined. The rationale for this was that PFAS are immunosuppressors and may have anti‐inflammatory properties. If so, the observed association with ALT would not be caused by inflammation, which is a common cause of liver damage. The results were consistent with the hypotheses. Positive associations were found between serum levels of PFOA and CK18 M65 and, especially, CK18 M30. For PFOS, the positive trends were not statistically significant. For markers associated with inflammation, associations suggested anti‐inflammatory effects. The authors’ interpretation is that PFOA causes hepatocellular apoptosis, and maybe non‐alcoholic fatty liver disease (NAFLD).
Salihovic et al. (2018) examined such associations longitudinally in a population‐based study of 1,016 old (70 years) individuals. They were followed up at 75 and 80 years of age. The initial mean levels of PFOS and PFOA were 13.2 and 3.3 ng/mL. For ALT, there was a statistically significant association between the change of PFOS/PFOA and the change of serum ALT.
Nian et al. (2019) reported cross‐sectional findings regarding associations between PFOS/PFOA and liver biomarkers in 1605 adult government employees or residents in Shenyang, China (see also Bao et al., 2017). The median PFOS and PFOA concentrations in serum were 24 and 6.2 ng/mL. After adjustment for potential confounders, serum ALT was positively associated with PFOA and PFOS, although for PFOS not when individuals taking lipid‐lowering medications were excluded. There was also a positive association between serum PFOS and ALT outside the reference range, but only for branched PFOS.
Jain (2019) examined associations between PFOS (GM 6.0 ng/mL), and PFOA (GM 2.1 ng/mL) and liver enzymes (ALT, AST, GGT) in 2883 individuals from NHANES 2011–2014 with the specific aim to study if associations were more pronounced among obese (BMI ≥ 30) participants. Statistically significant (but modestly sized) associations were found between log‐transformed serum levels of PFOA (but not PFOS) and log‐transformed ALT in the 1082 obese individuals but not in the others. The authors’ interpretation is that obese individuals already have a tendency towards fatty liver (NAFLD) and therefore are more susceptible regarding an impact of PFASs.
In summary, the conclusion in the Opinion on PFOS/PFOA (EFSA CONTAM Panel, 2018) regarding associations between serum ALT and exposure to PFOA is in agreement with these newer studies. Of special interest is the study by Salihovic et al. (2018) because the longitudinal design of examining ‘change of ALT versus change of PFAS’ is superior to cross‐sectional studies and minimises the potential confounding by individual factors. While previous studies were not consistent regarding associations between PFOS and ALT (EFSA CONTAM Panel, 2018), the study by Salihovic et al. (2018) finds a significant association between PFOS and ALT, and suggests that also PFOS exposure might increase serum ALT. The study by Bassler et al. (2019) provides more insight in the mechanisms behind the associations.
PFASs other than PFOS and PFOA
Lin et al. (2010) examined associations between PFNA and PFHxS and liver function biomarkers (ALT, GGT and total bilirubin) in 2200 adults from US NHANES surveys 1999–2004. The mean serum PFNA was 0.8 ng/mL and for PFHxS it was 2.0 ng/mL. In adjusted models, there were no significant positive associations between log‐transformed PFNA or PFHxS and liver enzymes, but there was a tendency towards a positive association with total bilirubin for both PFNA and PFHxS.
Gleason et al. (2015) studied associations between serum PFNA and PFHxS and liver function biomarkers (ALT, AST, GGT, ALP and bilirubin) in about 4300 individuals from US NHANES surveys 2007–2010. The median levels of PFNA and PFHxS were 1.4 ng/mL and 1.8 ng/mL. A number of potential confounders were adjusted for. Significant positive associations were found between ln‐transformed PFNA and ln‐transformed levels of ALT, GGT and bilirubin, and between ln‐transformed PFHxS and ln‐transformed ALT. There was also a positive trend for PFNA when calculating odds ratios of having levels of ALT higher than the 75th percentile of levels in the NHANES surveys.
Rantakokko et al. (2015) studied serum levels of PFHxA, PFNA, PFDA, PFUnDA and PFHxS in extremely obese subjects undergoing bariatric surgery. There were some inverse associations (less inflammation) between PFNA, PFDA and PFHxS levels and grade of histologic inflammation in the liver. There was, however, also a positive association between PFHxA and ALT at 12 months after surgery.
Salihovic et al. (2018) examined associations between PFHpA, PFNA, PFDA, PFUnDA and PFHxS (apart from PFOS and PFOA) and markers of effects on liver cells, including ALT, ALP, bilirubin and GGT. The associations were examined longitudinally in a population‐based study of 1,016 70‐year‐old individuals. They were followed up at 75 and 80 years of age. The initial median levels were PFHpA: 0.05, PFNA: 0.7, PFDA: 0.3, PFUnDA: 0.3 and PFHxS: 2.1 ng/mL. For ALT, there was a statistically significant association (trend) over the three examinations between the changes of these PFASs and the change of serum ALT. Many of the p‐values were very low; the highest one (for PFHxS) was 0.01, which was not statistically significant if applying a Bonferroni adjustment for multiple comparisons. For most PFASs, there were also significant positive associations between PFASs and ALP, and significant inverse associations with bilirubin.
The above‐mentioned study by Jain (2019) examined associations between PFHxS (GM 1.3 ng/mL), and PFNA (GM 0.8 ng/mL) and liver enzymes (ALT, AST, GGT) in 2,883 individuals from NHANES 2011–2014 with the specific aim to study if associations were more pronounced among obese (BMI ≥ 30) participants. Statistically significant (but modestly sized) associations were found between log‐transformed serum levels of PFHxS/PFNA and log‐transformed ALT in the 1,082 obese individuals but not in the others. The authors’ interpretation is that obese individuals already have a tendency towards fatty liver (NAFLD) and therefore are more susceptible regarding an impact of PFASs.
The study by Nian et al. (2019), mentioned above, reported findings regarding associations between PFNA and PFDA, and liver biomarkers in 1,605 adult government employees or residents, in Shenyang, China. The median serum concentrations were 2.0 (PFNA) and 0.9 (PFDA) ng/mL. After adjustment for potential confounders, serum ALT was positively associated with PFNA and PFDA, but the magnitude was limited; 6.2% and 3.1% increase for a 2.7‐fold (1 ln) increase in PFNA and PFDA, respectively. For PFNA, there was also a significant association with prevalence of abnormal (outside the reference range) ALT (OR 1.5 per 2.7‐fold increase of PFNA, 95% CI 1.2–1.9). Also, PFBA, PFHxA, PFUnDA, PFDoDA, PFTrDA and PFHxS could be detected in > 50% of individuals, but levels were relatively low, and had no significant association with serum ALT.
The study by Bassler et al. (2019) mentioned above, found significant positive associations between PFHxS and PFNA and CK18 M30, and for PFHxS also with CK18 M65, the two biochemical markers of hepatocyte apoptosis in 200 participants in the C8 study. This finding was similar to what was found for PFOA, and as for PFOA results for other biomarkers suggested anti‐inflammatory effects. The authors’ interpretation is that the class of PFAS causes hepatocellular apoptosis, and maybe non‐alcoholic fatty liver disease (NAFLD).
In summary, there were several studies on associations between liver biomarkers and PFASs other than PFOS and PFOA, and the results indicate positive associations between PFHxS/PFNA and serum ALT. In particular the longitudinal study by Salihovic et al. (2018) provides support for this. The magnitude of the association was, however, modest in most of the studies and only one of them reported an association with ALT outside the normal range (as found for PFOA, see previous Opinion (EFSA CONTAM Panel, 2018)).
In summary, the available evidence on associations between ALT and PFASs is insufficient to use as a basis for a health‐based guidance value.
3.3.4.7. Kidney function and uric acid
PFOS and PFOA
In the EFSA Opinion on PFOS and PFOA, three studies on associations between PFOS/PFOA and estimated (based on serum creatinine) glomerular filtration rate (EFSA CONTAM Panel, 2018) were reviewed. In addition, seven studies examined associations with serum uric acid, a compound which usually increases when glomerular filtration rate (GFR) decreases. In summary, there were relatively strong associations between serum PFOS/PFOA and estimated GFR as well as serum uric acid. Taking into account that some reverse causality is plausible, that there may also be confounding by variables such as age, sex, height and weight, which are used in the eGFR equations, and that no significant association were shown between PFOS/PFOA and chronic kidney disease, the CONTAM Panel considered the evidence that PFOS/PFOA exposure causes reduced GFR insufficient. The possibility of reverse causality is very relevant also for other outcomes based on serum markers, which are eliminated by renal excretion, e.g. uric acid
PFASs other than PFOS and PFOA
In summary, five studies examined associations between PFASs other than PFOS/PFOA and estimated glomerular filtration rate and/or serum uric acid. Some associations were found, but not consistent between studies. As mentioned above for PFOS/PFOA, associations with eGFR may be affected by reverse causation and the variables used to calculate eGFR.
A description of the individual studies can be found in Appendix J, Section J.7.
In summary, there is insufficient evidence to conclude that exposure to PFASs decrease GFR or increase uric acid in serum.
3.3.4.8. Carcinogenicity outcomes
In the Opinion on PFOS and PFOA (EFSA CONTAM Panel, 2018), a number of studies on cancer incidence or cancer mortality at occupational or environmental exposure were reviewed. In summary, those studies provided insufficient support for carcinogenicity of PFOS and PFOA in humans. It was noted that this is in line with the conclusion from the recent IARC report on PFOA (IARC, 2017), which concluded that there was limited evidence for carcinogenicity. After the PFOS/PFOA Opinion, some additional studies have been published on other PFASs (see Appendix J, Section J.8). In addition, one study has been published reporting only results on PFOS and PFOA. These studies have not changed the previous conclusion for PFOS and PFOA. For other PFASs, limited information was identified.
3.3.4.9. Cardiovascular disease and mortality
PFOS and PFOA
In the Opinion on PFOS and PFOA (EFSA CONTAM Panel, 2018), five cross‐sectional and four longitudinal studies examining associations with cardiovascular outcomes (mortality, coronary heart disease, stroke, hypertension and atherosclerosis) were reviewed. Altogether, these studies did not show any clear association between PFOS/PFOA and cardiovascular disease. It was noted, however, that the longitudinal studies could not demonstrate a very small increase of the relative risk.
PFASs other than PFOS and PFOA
In summary, six studies report findings on cardiovascular disease in relation to exposure to other PFASs than PFOS and PFOA. The individual studies are reviewed in Appendix J, Section J.9. The CONTAM Panel notes that some recent studies (Bao et al., 2017; Huang et al., 2018, Mastrantonio et al., 2018) suggest an association between exposure to PFAS and cardiovascular disease, but considers the evidence yet insufficient to use as a basis for a health‐based guidance value.
3.3.4.10. Bone mineral density
PFOS and PFOA
In the Opinion on PFOS and PFOA (EFSA CONTAM Panel, 2018), two cross‐sectional US NHANES studies examining associations with bone mineral density were reviewed. There were some inverse associations between PFOS/PFOA and bone mineral density, but only in subgroups and for some sites, with limited consistency between the two studies. The magnitudes of the associations were small and may be due to residual confounding and/or reverse causation. The latter would be mediated by higher PFOS/PFOA in women with early menopause, which in turn decreases bone mineral density.
PFASs other than PFOS and PFOA
One of the US NHANES studies reviewed in the Opinion on PFOS and PFOA (EFSA CONTAM Panel, 2018), namely a study by Khalil et al. (2016) examined associations also with PFHxS (mean 2.5 ng/mL) and PFNA (mean 1.9 ng/mL). The study was performed in 1,914 individuals aged 12–80 years, and bone mineral density was measured by DXA in spine and femur. In addition, self‐reported physician‐diagnosed osteoporosis was examined. In post‐menopausal women, significant inverse associations were found between serum PFNA and BMD in femur and spine. The odds ratios for self‐reported osteoporosis in women were significantly increased in the upper two quartiles of PFHxS and in the highest quartile of PFNA. These analyses were based on only 77 cases of self‐reported physician‐diagnosed osteoporosis. With the findings from this single study, the evidence on an impact of PFNA or PFHxS on bone mineral density is insufficient.
3.3.5. Mode of action
In animals, the most commonly reported effects are those on the liver (increased weight, hypertrophy, increased fat content) and the levels of thyroid hormones, cholesterol and triglycerides, and liver transaminases in serum. In addition, some PFASs were shown to cause liver tumours. Furthermore, effects on the immune system, as well as on the development of the mammary gland, were observed in various studies, often at lower levels than those causing effects on the liver and thyroid hormones (see Sections 3.3.3.3 and 3.3.3.5). Certain effects seem associated with binding of PFASs to the PPARα receptor, but the question is to what extent this mechanism can explain the adverse effects, as will be discussed below. In a separate section, the effects of PFASs on thyroid hormone levels will be discussed, but it is unclear if such effects are underlying the effects on immune response, mammary gland development and liver effects. In subsequent paragraphs, possible mechanisms behind the effects on liver (including lipid metabolism), mammary gland development and the immune system will be discussed, including data from knockout (KO) animals and in vitro models. In particular PPARα KO mice but also mice expressing human PPARα have been used to study the role of PPARα, and potential differences between rodents and humans. It should be realised that both such models and in vitro models do not necessarily represent the in vivo situation, also due to kinetic differences. The MoA section focuses on liver effects and important endpoints for the risk assessment and is not intended to discuss all effects observed for PFASs.
3.3.5.1. Receptor mediated effects
3.3.5.1.1. PFASs transactivate PPARα
In vivo studies
Mechanistic studies showed that many PFASs are ligands of the nuclear receptor PPARα, as demonstrated in vivo and in vitro (see below). When activated by ligand binding, PPARα heterodimerises with another nuclear receptor family member, the retinoid X receptor (RXR), the receptor for 9‐cis‐retinoic acid. The heterodimer complex attaches to peroxisome proliferator response elements, being present in the promoter or enhancer regions of genes regulated by PPARα, such as enzymes of the peroxisomal ß‐oxidation pathway but also mitochondrial enzymes, as well as transporters. PPARα plays an important role not only in lipid metabolism but also various other metabolic processes like lipoprotein metabolism, gluconeogenesis and bile acid metabolism. The activation of this nuclear receptor also appears to be the critical key‐event in the induction of liver hyperplasia in rodents, as is given in detail in Section 3.3.5.1.2. A previous prevailing view has been that PPARα is less important in human than in rodent liver, but it needs to be kept in mind that PPARα plays a prominent role in regulation of lipid metabolism also in human liver (reviewed by Kersten and Stienstra, 2017).
Analyses of rodent liver tissue samples revealed that PFBA, PFHxA, PFHpA, PFOA, PFNA, PFDA, PFDoDA, PFBS, PFHxS, PFOS and 8:2 FTOH induce peroxisomal ß‐oxidation or at least the expression of essential enzymes of this biochemical pathway. There are few in vivo studies comparing the relative potencies of PFASs to induce peroxisomal ß‐oxidation and/or hepatomegaly. Daily i.p. administrations of four PFCAs to male rats for 5 days induced hepatic peroxisomal ß‐oxidation in the order of PFDA > PFNA > PFOA >>> PFHpA (Kudo et al., 2000). In female rats, PFDA was also slightly more potent than PFNA, but PFOA and PFHpA were inactive. This is likely to be related to differences in toxicokinetics between male and female rats (see Section 3.3.1.1). In a subsequent study, Kudo et al. (2006) compared daily i.p. treatments with PFHxA, PFHpA, PFOA and PFNA for 5 days in male and female mice. All compounds induced hepatomegaly and peroxisomal beta‐oxidation with PFNA and PFOA being most effective, followed by PFHpA and PFHxA.
NTP (2019a,b) treated male and female rats for 28 days by gavage with different daily doses of PFBS, PFHxS, PFOS, PFHxA, PFOA, PFNA, PFDA, as well as the PPARα agonist Wyeth (WY)‐14,643. Absolute and relative liver weight were increased by all compounds in both males and females, although with clear differences in the potencies. Based on the applied dose, PFOS, PFOA, PFNA and PFDA were the most potent in males followed by PFHxA and WY‐14,643, and PFBS and PFHxA. In females, PFDA was the most potent, followed by PFOS, PFNA and WY‐14,643, PFOA, PFHxS, PFBS and PFHxA. The order changed when basing it on measured serum or liver levels. In females, e.g. PFOA treatment resulted in relatively low serum levels. Most compounds also induced the liver mRNA levels of Acox1, as well as the enzyme activity (the latter only measured in males), and mRNA levels of CYP4a1, CYP2b1 and CYP2b2. An exception was PFHxS, which did not affect mRNA levels of Acox1 in females, and only had a weak effect on CYP4a1. Similar was true for PFHxA in males and females. In general, however, when studied, most endpoints are somehow affected by all PFASs, and differences seem merely due to kinetics and relative potencies.
In vitro studies
In vitro models may be suitable tools for studying effects mediated by PPARα, although the PPARα‐expression and related processes in cell lines and isolated hepatocytes may differ from the in vivo situation (Kersten and Stienstra, 2017). A further severe limitation is the frequent lack of information on the actual uptake of PFASs by the cultured cells, which may be very low (Rosenmai et al., 2018).
A number of studies investigated the relationship between the structure of PFASs and induction of genes involved in peroxisomal ß‐oxidation or other steps of lipid metabolism in in vitro assays. Naile et al. (2012) reported on the impact of PFBA, PFPeA, PFHxA, PFOA, PFNA, PFUnDA, PFDoDA, PFBS, PFHxS and PFOS on transcript levels of seven genes, five of them involved in fatty acid and cholesterol synthesis, in rat H4IIE hepatoma cells. The PPARα‐regulated peroxisomal 3‐ketoacyl‐CoA thiolase was induced most strongly by PFHxS followed by PFOA, PFPeA, PFHxA and PFDoDA, but was downregulated by PFBA, PFBS, PFOS, PFNA and PFUnDA. However, overall the full picture of effects on gene expression did not show consistent patterns related to e.g. chain length or PFCAs vs. PFSAs, and lacked clear dose response relationships across the wide range of concentrations applied. In addition, the results seem not consistent with the NTP‐studies with rats, showing upregulation of Acox1 by all PFASs tested and also increased activity of acyl‐CoA oxidase (although with clear differences in potency).
Bjork and Wallace (2009) showed that in primary rat hepatocytes, peroxisomal Acox and Cte/Acot1 genes were induced, in the order of PFOA > PFHxA > PFOS > PFHxS > PFBA = PFBS = PFPeA, when compounds were applied at 25 μM for 24 h. In human primary hepatocytes or human HepG2 cells, no effect on expression of these genes could be observed when compounds were applied at concentrations up to 200 μM. Cyp4A1/11, however, was induced by some PFASs in human primary hepatocytes. The authors speculated that – unlike to rat – no peroxisome proliferator responsive element (PPRE) has been identified within the 5′‐flanking region of the human Cyp4A11 gene (Kawashima et al., 2000), indicating that the regulation of this gene differs between the two species. Bjork and Wallace (2009) concluded that there is 1) a chain length‐dependent increase in gene expression, 2) higher transcriptional activation by PFCAs than the corresponding PFSAs, 3) the absence of induction of expression of peroxisome‐related fatty acid oxidation genes by the compounds in human liver cells.
Wolf et al. (2012) transfected COS‐1 cells (monkey fibroblast‐like cell line) with mouse or human PPARα‐luciferase reporter plasmids. In the case of mouse PPARα, all tested compounds showed a response, but C20max concentrations (the concentration showing 20% of the maximal activity) varied between 5 and 317 μM. The transcriptional activation (based on C20max values) was in the order of: PFNA (5 μM) > PFOA (6 μM) > PFHpA (11 μM) > PFDA (20 μM) > PFDoDA (33 μM) > PFHxA (38 μM) > PFPeA (45 μM) > PFBA (51 μM) > PFHxS (76 μM) > PFOS (94 μM)> PFBS (317 μM). In the case of human PPARα, C20max values varied between 11 and 471 μM: PFNA (11 μM) > PFHpA (15 μM) > PFOA (16 μM) > PFHxA (47 μM) > PFPeA (52 μM) > PFBA (75 μM) > PFHxS (81 μM) > PFBS (206 μM) > PFOS (262 μM) > PFHxA (471 μM) > PFDA and PFDoDA (no response). Overall, these studies show that PFASs are able to activate PPARα with little difference between the mouse and human receptor, with similar order. In addition, the chain length determines the transcriptional activation of PPARα, and the PFSAs seem less potent than the PFCAs.
Activation of PPARα was also assessed in NIH‐3T3 cells (murine fibroblast cell line) transiently transfected with mouse PPARα or PPARγ (Rosenmai et al., 2016). The assay investigated the dependency of PPAR activation, assessed by a reporter gene, on the chain length for PFBA, PFPA, PFHxA, PFHpA, PFOA, PFNA, PFDA, PFUnDA and PFDoDA. All PFCAs activated PPARα around twofold at 100 μM. In another study, Rosenmai et al. (2018) studied the PPARα transactivating potency by transiently transfecting human HepG2 liver cells with a human‐PPARα reporter gene plasmid. They also studied intracellular concentrations of the PFASs by LC–MS/MS. The uptake of the PFASs by HepG2 cells was generally very low (0.04–4.1%). There was a trend that the intracellular concentrations of PFCAs increased with the chain length, but this was the reverse for the three PFSAs, PFOS showing the lowest cellular concentrations of all PFASs tested. The PPARα transactivating potencies of PFCAs were in a similar range (~ 1.5‐ to ~ 3.5‐fold), increased with chain length up to C8 (PFOA), and then decreased. PFOS and FOSA were inactive.
To conclude: in in vitro studies, a tendency towards elevated transcriptional activation of rodent and human PPARα was found with increasing chain length of PFASs up to the length of C9 and a trend to lower activity for PFCAs with chain lengths of > C9. PFSAs show lower potencies, and decreasing with chain length. In addition, compound‐specific effects on the regulation of peroxisomal but also other genes and enzyme activities were reported but not always consistent with in vivo studies. This could be explained by the variable uptake of PFASs by cells which may be relatively low and differ between individual PFASs, as well as between the cell models applied.
3.3.5.1.2. Interaction of PFASs with nuclear receptors other than PPARα
In vivo and in vitro studies also addressed the question whether further nuclear receptors may be involved in the effects observed with PFASs in the liver. Rosen et al. (2008) compared the transcript profiles of the livers of WT and PPARα KO mice treated with PFOA (1 or 3 mg/kg bw per day) or the PPARα agonist WY 14,643 (50 mg/kg bw per day) for 7 days. It was concluded that approximately 85% of the genes altered by PFOA were dependent on PPARα; the PPARα‐independent genes were often involved in lipid homeostasis and xenobiotic metabolism and it was speculated that this may be due to PFOA‐induced activation of PPARγ, CAR (constitutive activated/androstane receptor) or the transcription factor Nrf2 (nuclear factor erythroid 2‐related factor 2). In a follow‐up study, Rosen et al. (2017) treated male mice for 7 days by oral gavage with PFNA (1 or 3 mg/kg bw per day) or PFHxS (3 or 10 mg/kg bw per day), and compared the hepatic gene expression patterns with historical data obtained from PFOS, PFOA or WY‐14,643. They found that roughly 11–24% of up‐ or down‐regulated genes in PFAS‐treated animals were independent of PPARα, and argued that this might be due to suppression of STAT5B or transactivation of the constitutive activated receptor (CAR), oestrogen receptor alpha (ERα) and/or PPARγ. In the NTP studies (NTP, 2019a,b), all PFASs tested induced mRNA levels of Cyp2b1 and Cyp2b2 in livers of male and female rats, supporting the activation of CAR. There were clear differences in potencies. Also, WY‐14,643 induced these genes but it is unclear if this implies that this compound is less specific as assumed, or whether there is an indirect mechanism.
In HEK239 cells, Li et al. (2019c) determined the potency of PFASs to bind to human PPARβ/δ and to activate the receptor, applying a reporter‐gene assay. The binding capacity was elevated with increasing chain length until PFDoDA (C12) and then decreased again. The three PFSAs tested (PFBS, PFHxS, PFOS) showed stronger binding than their carboxylic acid (PFCA) counterparts, while 6:2 FTOH and 8:2 FTOH did not interact with PPARβ/δ. The transactivating potency of PFASs showed a similar inverted U‐shaped curve peaking at PFTrDA. Bjork et al. (2011) deduced from the analysis of transcriptome patterns, induced by PFOS or PFOA in rat and human primary hepatocytes, that multiple receptor systems are activated by these compounds, such as PPARα, CAR, PXR and LXRa. The activation of PXR by PFOS and PFDA was shown by Bijland et al. (2011) and Ma et al. (2005), respectively.
3.3.5.1.3. Thyroid hormone levels and function
There is considerable evidence from animal studies that PFASs interfere with the thyroid hormone homoeostasis. Observations in human epidemiological studies do not provide sufficient support for associations between the PFASs examined and thyroid disease or changes in thyroid hormones at serum levels observed in people exposed occupationally or via food. For thyroid hormone function, the levels of the free hormones (free thyroxine (fT4) and free triiodothyronine (fT3)) are particularly important. In the NTP studies (NTP, 2019a,b), most PFASs reduced serum levels of fT4 and total T4, in some cases by more than 80%. Also, total T3 levels were often decreased but only in some cases there was also a decrease in serum TSH levels. Disturbance of thyroid hormone function may not only occur at the level of the two thyroid hormone receptors (TRα and TRβ), but also at the level of biogenesis, distribution and metabolism of thyroid hormones. This potentially includes disturbances at the level of the sodium iodide symporter, haemoprotein thyroperoxidase, iodinases and thyroid hormone conjugating enzymes, the T4 distributor proteins thyroxine‐binding globulin (TBG), transthyretin (TTR) and deiodinases (deiodinisation of T4 in target organs to the more active T3) (Köhrle, 2008). Ultimately, disturbances on either of the listed levels of biosynthesis, distribution and function may result in variation of fT4 and fT3 levels and consequently in compromised functions of organs including adverse health outcomes. It should be pointed out that the measurement of fT4 levels may be influenced by the competition between PFASs and T4 for binding to proteins, which may be resolved by applying an additional dialysis step (Chang et al., 2007). This could explain why the strong decrease in fT4 is not accompanied by increased TSH levels and clear effects on the thyroid. This does not apply to the decreased levels of T3 and T4, which are also of potential concern.
Competition between the binding of T4 and PFASs to the human variant of TTR was assessed with a radioligand‐binding assay (Weiss et al., 2009). The binding potency (IC50) was reported to decrease in the order: PFHxS (0.7 μM) > PFOS/PFOA (0.9 μM) > PFHpA (1.6 μM) > PFOSi (1.7 μM) > PFNA (2.7 μM) > PFHxA (8.2 μM) > PFDA (9.0 μM) > PFBS (19 μM) > PFUnDA (22 μM) > PFTeDA (29 μM) > PFDoDA (47 μM). Overall, TTR binding potencies of the most potent PFASs were 12.5–50 times lower than those of T4. Some shorter (PFBS) but also longer chain PFASs (PFDA and larger) were even less potent (> 100 times less potent) than T4. A comparative binding study to human TTR and TBG was performed by Ren et al. (2016). The compounds tested comprised PFBA, PFHxA, PFHpA, PFOA, PFNA, PFDA, PFUnDA, PFDoDA, PFTrDA, PFTeDA, PFBS, PFHxS, PFOS and FTOH (8:2). All compounds tested, except for the telomer alcohol, were identified as TTR binders, again showing highest potencies for PFHxS, PFOS, PFOA (IC50s of 0.6, 0.1 and 0.4 μM). Only two compounds bound to TBG and at a very high concentration (PFTrDA and PFTeDA, IC50s of 170 and 190 μM).
In summary, the findings of the two studies above on binding of PFASs to the thyroid hormone distributor proteins TBG and particularly TTR may contribute to understanding of potentially altered thyroid hormone levels in relation to exposure to PFASs. However, there is some debate on the importance of TTR for humans since thyroid hormones also bind to TBG.
Ren et al. (2015) also investigated the binding affinities of 15 PFASs to the ligand binding domain of TRα. All PFASs tested, except PFBA and FTOH (8:2), could be identified as binders. However, most PFASs were more than 100‐fold less potent than T3 (IC50 = 0.3 μM), with the exception of six compounds, with the following rank order of potency (IC50): PFDA (6 μM) > PFOS (16 μM) > PFODA ~ PFTeDA ~ PFUnDA (18–20 μM) > PFHxDA (24 μM). In conclusion, these results show that PFASs are able to interfere with thyroid hormone function on the level of thyroid hormone receptors, with PFDA showing the most potent activity.
A study by Long et al. (2013) investigated the effect of PFASs on proliferation control of rat pituitary GH3 cells in the T‐screen assay. T3 was used as a positive control. At 10‐8 M, T3 induced a 12‐fold increase in proliferation in comparison to solvent control. All PFASs significantly decreased GH3 cell proliferation, being dose‐dependent for PFOS, PFHxS, PFNA, PFDA and PFUnDA. PFASs also decreased T3 induced proliferation of GH3 cells. The other PFASs reduced growth only at higher doses. No inhibitory effect could be detected for PFOA. These results provide further evidence that PFASs are capable to interfere with thyroid hormone function at the level of the control of proliferation of pituitary cells.
The studies by NTP (NTP, 2019a,b) showed that all seven tested PFASs induced transcription of Acox1, Cyp4a1, Cyp2b1 and 2b2, showing that they are activators of PPARα and CAR. This may also result in an increase of glucuronosyltransferases involved in conjugation of thyroid hormones. Yu et al. (2009) showed increased transcription of hepatic UGT1A1 in SD rats treated with PFOS.
At this stage, it appears that the effects on thyroid hormone levels occur at relatively high doses, i.e. higher than dose resulting in effects on antibody response or mammary gland development.
3.3.5.2. Liver toxicity
3.3.5.2.1. Liver hypertrophy and hyperplasia
Hepatotoxic effects of PFASs are indicated by steatosis, lipid peroxidation, occurrence of necrotic hepatocytes and elevated liver transaminases in serum. The elevated relative liver weight in PFAS‐treated rodents appears to be a combination of net gain of liver cells (hyperplasia) and increased volume of hepatocytes (hypertrophy) due to proliferation of peroxisomes, endoplasmic reticulum and steatosis. Most mechanistic studies on the induction of hepatic hypertrophy and hyperplasia used rodents, which were treated with clofibrate, ciprofibrate, fenofibrate, WY‐14,643 or diethylhexylphtalate (DEHP) (Corton et al., 2017). These well‐studied PPARα‐ligands are often referred to as peroxisome proliferators (PP). Such PPs induce an initial and transient wave of DNA replication in rodent liver, followed by a plateau of an elevated number of cells (hyperplasia), as long as the treatment with the ligand is continued. This hyperplasia is frequently associated with increases in cell size (hypertrophy) due to proliferation of peroxisomes and of the smooth endoplasmic reticulum, which occurs preferentially in hepatocytes close to the central vein in the liver lobule. However, hyperplasia due to steatosis does not occur in livers of rodents treated with PPs. Upon withdrawal of the PPARα ligand, the liver quickly returns to its original mass and eliminates the excess of cells via apoptosis. Retreatment with PPARα ligands rapidly stops the apoptotic activity in the liver (Bursch et al., 1984; James et al., 1998). Thus, the PPARα‐induced hyperplasia of rodent liver results from both an increased transient wave of cell replication and a concomitant suppression of cell elimination by apoptosis.
Considering the PFAS‐induced hepatomegaly in more detail, Wolf et al. (2008) reported a dose‐dependent increase in hepatocellular proliferation in PFOA‐treated WT mice, which was associated with increased liver weight and hepatocellular hypertrophy. Signs of a mild hepatocellular steatosis were documented in livers from PFOA treated animals by light microscopy. Das et al. (2017) studied WT mice treated with 10 mg/kg bw per day of PFNA, PFOA or PFHxS. PFOA induced the largest increase in relative liver weight, followed by PFNA and PFHxS. The DNA content, given as μg/mg liver tissue was decreased by all compounds, indicating considerable hepatocellular hypertrophy. However, considering the two‐ to threefold elevated absolute liver weight in the PFAS‐treated animals, there was an overall net gain in hepatic DNA content, indicating a net gain in liver cells, and thus liver hypertrophy ‐ similar to observations with the PP WY‐14,643. However, contrary to the latter PP, steatosis was evident in the PFAS‐treated WT mice. Das et al. (2017) documented an about two‐ to threefold elevated content of hepatic triglyceride content in the PFAS‐treated WT mice, which may contribute considerably to the hepatocellular hypertrophy observed.
To understand the relevance of PP‐induced hepatomegaly for humans, primates have been investigated. Cynomolgus monkeys were treated with the PP di‐ethyl‐hexylphthalate (DEHP) or clofibrate for 2 weeks, which did not elicit any effect on liver weight or replicative DNA synthesis in the organ (Pugh et al., 2000). A 15‐month treatment with DEHP elevated liver weight in mice and rats but not in marmosets (Ito et al., 2007). When marmosets received DEHP or clofibrate over a period of 13 weeks, again no increase in liver mass and hypertrophy of hepatocytes was found (Kurata et al., 1998). Hoivik et al. (2004) applied fenofibrate or ciprofibrate to cynomolgus monkeys for up to 15 days. Both compounds caused slight hepatocellular hypertrophy but no cell proliferation as determined by the number of mitoses and Ki‐67 positive nuclei. To conclude, these data show convincingly that PPARα agonists fail to induce cell replication and hyperplasia in liver of monkeys. Occasionally, there are reports on hypertrophy of hepatocytes in the monkey liver.
Primary cultures of hepatocytes from rats and humans were used to study species differences in the response to PPs. Mono‐ethyl‐hexylphthalate (MEHP), the active metabolite of the DEHP, caused dose‐dependent induction of DNA synthesis in rat but not in human hepatocytes (Hasmall et al., 1999, 2000). Similar reports exist for monoisononyl phthalate (MINP), the primary and active metabolite of the PP diisononyl phthalate (DINP) (Shaw et al., 2002). Goll et al. (1999) compared effects of various PPs, including DEHP and some fibrates, in primary cultures of rat and human hepatocytes. An increase in DNA synthesis was reported for ciprofibrate, nafenopin, bezafibrate, clofibrate and DEHP in rat hepatocytes only. Ciprofibrate, nafenopin, fomesafen, 2‐ethylhexanoic acid and methylclofenapate caused induction of DNA synthesis in primary rat hepatocytes, but none did so in human hepatocytes (Doull et al., 1999). To conclude, these in vivo and in vitro studies provide strong evidence that monkey and human liver cells are insensitive to PP‐induced growth effects.
Several mouse models served to study the molecular mechanisms underlying the different response of human and rodent hepatocytes towards activated PPARα. Lee et al. (1995) knocked out the ligand binding domain of murine PPARα and introduced a truncated gene, being expressed in all cells of mice. When treated with typical PPs (clofibrate, WY‐14,643), homozygous KO mice did not show the typical PP‐mediated pleiotropic response in the liver, such as increases in peroxisomes, liver cell proliferation and hepatomegaly. Wolf et al. (2008) addressed the question whether PPARα also mediates the induction of liver growth by PFOA. WT (CD‐1 and Sv/129) and KO mice (Sv/129 background) were treated by daily gavage with the ammonium salt of PFOA (1, 3, or 10 mg/kg bw per day) for 1 week. In WT mice, PFOA induced a dose‐dependent increase in hepatocyte hypertrophy, vacuoles, peroxisome proliferation and a dose‐dependent increase in PCNA‐positive nuclei, the latter reaching significance only at the highest dose. KO mice also showed a dose‐dependent increase in hepatocyte vacuolation, but these were much larger than in WT mice. There was also an increased number of PCNA‐positive nuclei, as in WT being significant at the highest dose only, and at that dose higher than the increase seen in WT animals treated with the highest PFOA dose or WY‐14,643 (positive control). The authors stated that the elevated cell replication in the PFOA‐treated KO mice was probably due to the physical accumulation of material, which may be interpreted as a regenerative and not as hyperplastic growth reaction of the liver. This assumption is based on the fact that hepatocytes were full of large electron‐lucent, non‐membrane‐bound spaces throughout the hepatocellular cytoplasm. These ‘deposits’ may have contributed also to the increased relative liver weight in the KO mice. Since Das et al. (2017) reported elevated hepatic lipid content in PFOA‐treated KO mice, the CONTAM Panel assumed these deposits to be lipid droplets.
Das et al. (2017) used PPARα KO mice exposed to 10 mg/kg bw per day of PFNA, PFOA or PFHxS for 7 days (see also above). In the KO animals, PFOA induced the largest increase in relative liver weight, followed by PFNA and PFHxS. The hepatic triglyceride content was elevated considerably in the untreated KO mice and was elevated even further in the PFAS‐treated KO mice. Similar to the WT mice, the DNA content per mg liver tissue was decreased being indicative for hypertrophy. Considering the increased liver weight, there was also a net gain in liver cells in the KO mice, based on the increase of total DNA per liver, especially for PFOA and PFNA; this increase in total DNA content per liver was in the range of the DNA gain seen in the WT animals. The authors did not provide an explanation for this finding. However, in the study of Minata et al. (2010) PFOA was applied to KO mice for a longer period (4 weeks). At this time point, not only steatosis but also severe cholangiopathy, enhanced blood levels of biliary acids, hepatocellular damage and apoptotic cells (especially in bile ducts) became evident. An increase in serum bile acid levels was also observed in the NTP studies with rats (NTP, 2019a,b) exposed to PFBS, PFOS, PFHxA, PFOA, PFNA and PFDA, but also WY‐14,643, pointing at cholestasis. The concomitant development of hepatic fibrosis by proliferating fibroblasts and the infiltration of immune cells might have started to develop already after one week of PFOA treatment leading to the elevated total hepatic DNA content, observed by Das et al. (2017). The study of Minata et al. (2010) substantiates also the assumption that the elevated DNA replication, reported by Wolf et al. (2008) after a 7 days′ PFOA treatment of KO animals, may be interpreted as regenerative DNA replication following severe hepatocellular and bile epithelial damage, especially in the highest dose group.
Das et al. (2017) also investigated the mechanism of PFAS‐induced steatosis by microarray analyses of hepatic gene expression patterns of WT and KO mice treated with PFOA, PFOS, PFNA, PFHxS or WY‐14,643. In WT mice, the PFASs and WY‐14,643 induced genes involved in fatty acid uptake into hepatocytes as well as the synthesis of fatty acids, triglycerides and cholesterol. In KO animals almost none of the gene expression changes induced by WY in WT were evident, while the expression levels of some fatty acid and TG synthesis genes were retained or even elevated by PFAS treatment. This indicates that some genes involved in triglyceride and cholesterol synthesis may be regulated independent of PPARα, explaining partly the pronounced steatosis seen in KO animals. These findings show that PFASs induce hepatocellular steatosis in rodents in a PPARα‐independent way, which may increase the volume of the hepatocytes (hypertrophy) and contribute to the elevated liver weight.
In order to create a more physiological model, Cheung et al. (2004) used KO mice and introduced the human PPARα under the control of a liver‐specific promoter for stable hepatic expression. Both the WT and humanised mice responded to treatment with the PP Wy‐14,643 as proven by upregulated genes of peroxisomal ß‐oxidation. However, WT mice but not the humanised animals, showed hepatocellular proliferation. The relative liver weight was only slightly increased in the humanised mice probably due to induction of peroxisomal ß‐oxidation and the concomitant peroxisomal proliferation, contributing to the development of a hepatocellular hypertrophy. Yang et al. (2008) replaced the mouse PPARα gene by a construct designated hPPARαPAC containing only the human PPARα. The hPPARαPAC mice expressed the construct in the same tissues as WT animals. By a 2‐weeks treatment with WY‐14,643, hepatomegaly was induced in the transgenics but to a considerably lower extent than in WT mice. Likewise, cell replication was not induced in the humanised mice when exposed to WY‐14,643, indicating that the hepatomegaly may be due to hypertrophic hepatocytes.
With regard to PFASs, Nakagawa et al. (2012) used the mouse model, developed by Cheung et al. (2004), and observed an increased absolute and relative liver weight in WT, KO and humanised mice, when treated with PFOA doses at 1 or 5 mg/kg bw per day for 6 weeks. The hepatomegaly appeared to be mainly due to enhanced intrahepatic storage of triglycerides, which was highest in the KO mice, followed by humanised mice, but in WT mice much less and only at the intermediate dose. At lower doses and shorter treatment (0.1 or 0.3 mg/kg bw per day, 2 weeks), Nakamura et al. (2009) observed increased liver weight and hepatic triglyceride levels only in WT mice.
In humanised PPARα mouse models, hPPARα is expressed in cells made up exclusively of mouse proteins, factors and (co‐)regulators. It was argued that this might suppress the human phenotype. This objection was refuted by a study of Tateno et al. (2015). Liver of SCID mice was repopulated, thereby creating chimeric organs consisting of > 70% human hepatocytes. Treatment with the PP fenofibrate induced hepatocellular hypertrophy, cell proliferation and peroxisome proliferation exclusively in the murine but not in the human hepatocytes. It should be stressed that fenofibrate is a weaker PP than WY‐14,643, which induced some proliferation in KO mice (Yang et al., 2008).
It should be noted that in the chimeric livers, used by Tateno et al. (2015), human hepatocytes were surrounded by murine mesenchymal liver cells, which might have disturbed epithelial‐mesenchymal interactions in this organ. To address this question, and to gain a profound understanding of the MoA of PFASs, the following studies should be considered. Some groups proposed that non‐parenchymal Kupffer cells, macrophages located in the liver sinusoids, are critical for growth induction by PPARα ligands. When being activated, Kupffer cells start to produce and release cytokines, like tumour necrosis factor‐alpha (TNFα) (Bojes et al., 1997; Rolfe et al., 1997). TNFα is able to induce replication and to suppress apoptosis in cultured rodent hepatocytes (Holden et al., 2000; Rolfe et al., 1997). Also in vivo, hepatocyte proliferation was antagonised by pretreating animals with antibodies blocking either TNFα (Bojes et al., 1997; Rolfe et al., 1997) or the TNFα receptor (West et al., 1999). TNFα is an important activator of NF‐kB. NF‐kB coordinates not only adaptive and innate immune responses, but is also involved in the regulation of apoptosis. In dependence of the context, NF‐kB triggers either pro‐ or anti‐apoptotic pathways and is involved in the decision whether a cell may survive or die. Several PPARα activators were found to enhance the secretion of TNFα and to activate NF‐kB in liver cells (Rose et al., 2000; Calfee‐Mason et al., 2004). On the other hand, in purified hepatocyte cultures without Kupffer cells, PPARα ligands failed to induce DNA replication (Hasmall et al., 2000; Parzefall et al., 2001).
Several groups reported on elevated expression of cyclins and/or the proto‐oncogene c‐myc in rat or murine liver when treated with PPARα ligands (Ma et al., 1997; Jolly et al., 2005; Urbanek‐Olejnik et al., 2016). Shah et al. (2007) described molecular mechanisms underlying the differences in c‐myc regulation by human and murine PPARα. The microRNA let‐7c destabilises the mRNA of c‐myc. In WT mice, induction of hepatocellular proliferation by activated PPARα caused downregulation of the let‐7c, which elevated the expression of c‐myc and enhanced hepatocellular proliferation. In contrast, PPARα‐humanised mice did not enhance c‐myc expression via downregulation of let‐7c.
A further interesting aspect is that human livers express the protein of a truncated form of PPARα at relatively high levels (Thomas et al., 2015). The truncated PPARα is unable to bind to peroxisome proliferator‐responsive elements (PPRE) in the DNA, and acts as dominant negative regulator of the full‐length PPARα and functions as an endogenous inhibitor of proliferative genes. The absence of the truncated form in mice may contribute to species‐specific differences in PP induced liver growth.
These findings provide evidence that PPARα‐mediated pathways controlling peroxisomal ß‐oxidation are independent from those controlling the hepatocellular proliferation pathways. Structural differences between human and mouse PPARα exist, leading to the differential susceptibility towards the PP‐induced hyperplastic liver growth. PFASs appear to induce liver hyperplasia via PPARα, similar to PPs, assuming that the observed increase in PCNA content and total DNA in KO mice is due to other processes following severe liver damage by PFOA treatment.
3.3.5.2.2. Hepatocellular cytotoxicity, steatosis and increased liver transaminases in serum
Several in vitro studies investigated the impact of chain length of PFASs on the cytotoxicity of the compounds in cells of hepatocellular origin. Buhrke et al. (2013) reported a positive correlation between chain length and cytotoxicity in human HepG2 cells, i.e. the toxicity (IC50) was as follows: PFDoDA (7 μM) > PFDA (15 μM) > PFNA (23 μM) > PFOA (47 μM) > PFHpA (128 μM) > PFHxA (344 μM) > PFBA (1,000 μM). There was no evidence for the induction of apoptosis by any of the compounds tested. Rand et al. (2014) studied the relative cytotoxicity of PFCAs in human liver epithelial (THLE‐2) cells. The EC50 values ranged from 65 ± 41 μM (PFDA) to 1,361 ± 146 μM (PFBA). Toxicity decreased with shorter chain length, as follows: PFDA > PFNA> PFOA >PFHpA > PFHxA > PFPeA > PFBA.
To conclude, there is some association between the chain length of PFASs and cytotoxicity, which may be partly due to the higher cell membrane permeability of long‐chain PFASs.
For many PFASs, hepatotoxic effects were documented in in vivo experiments. In the studies performed by NTP (NTP, 2019a,b), increased ALT levels were observed in male rats at dose levels of 500 mg/kg bw per day for PFBS, 0.625 mg/kg bw per day for PFOS, 500 mg/kg bw per day for PFHxA, 0.625 mg/kg bw per day for PFOA, 2.5 mg/kg bw per day for PFNA and 0.312 mg/kg bw per day for PFDA. In female rats, this occurred at dose levels of 250 mg/kg bw per day for PFBS, 2.5 mg/kg bw per day for PFOS, 500 mg/kg bw per day for PFHxA, 50 mg/kg bw per day for PFOA, 3.12 mg/kg bw per day for PFNA and 1.25 mg/kg bw per day for PFDA. Also treatment with WY‐14,643 resulted in increased ALT levels, but PFHxS showed no effects. PFBS and PFHxA showed the effects at much higher doses. Increases in serum levels of ALT and/or AST were also documented in previous studies in which rodents were treated with PFOS (1 mg/kg bw per day Han et al., 2018a,b), PFHxA (20 mg/kg bw per day, Loveless et al., 2009), PFNA (1 mg/kg bw per day, Fang et al., 2015), PFUnDA (1 mg/kg bw per day, Takahashi et al., 2014) or PFODA (1,000 mg/kg bw per day, Hirata‐Koizumi et al., 2012). Hepatocellular necrosis was induced by PFBA (175 mg/kg bw per day, Foreman et al., 2009), PFDoDA (2.5 mg/kg bw per day, Kato et al., 2015b), PFHxS (3 mg/kg bw per day, Chang et al., 2018) or 8:2 FTOH (100 mg/kg bw per day, Iwase et al., 2006).
The reasons for the increased serum transaminases, which indicate membrane damage of hepatocytes, and the occurrence of necrotic hepatocytes in the histopathological evaluations, are not completely understood. It has been assumed repeatedly that this damage may be related to the profound alterations in the hepatocellular lipid metabolism of PFAS‐treated rodents. Via activation of PPARα in rodents, PFASs induce the ß‐oxidation cycle of fatty acids in the hepatic peroxisomes, which degrades the long‐chain fatty acids to acyl‐CoA‐moieties for use in other metabolic pathways. Despite an increased peroxisomal degradation of long‐chain fatty acids, the hepatic lipid content appears to be elevated by PFASs (EFSA CONTAM Panel, 2018). This is due to accumulation of triglycerides, as described for PFOA (5 mg/kg bw per day, Hui et al., 2017; Wu et al., 2018), PFOS (0.09 mg/kg bw per day, Huck et al., 2018), PFNA (0.2 mg/kg bw per day, Wang et al., 2015a), PFDA (1.5 mg/g bw per day, Kawashima et al., 1995) and PFDoDA (0.2 mg/kg bw per day, Zhang et al., 2013e). Also enhanced storage of cholesterol may account for this effect, as observed after application of PFNA (0.2 mg/kg bw per day, Wang et al., 2015a) or PFDoDA (5 mg/kg bw per day, Zhang et al., 2013e). Steatosis does not occur in livers of rodents treated with typical PPs and appears to be very specific for PFASs. Mechanistic studies indicate a disbalance between (i) the reduced export of lipids from hepatocytes and expression/activity of lipid catabolism genes and (ii) increased expression/activity of genes involved in lipid uptake by hepatocytes and in fatty acid, triglyceride and cholesterol biosynthesis. The development of steatosis is independent of a PPARα‐mediated mechanism and is even very pronounced in KO mice, as described in the subchapter above.
Intrahepatic lipid overload is a symptom for severe metabolic disturbances and predisposes the hepatocytes to undergo necrosis. Necrotic cells alarm the immune system and may elicit a pro‐inflammatory response in the liver. Indeed, elevated intrahepatic levels of pro‐inflammatory cytokines, like IL1ß or TNFa, were observed after treatment with PFNA (Fang et al., 2012a). It has also been hypothesised that the increased peroxisomal ß‐oxidation of long‐chain fatty acids leads to enhanced production of hydrogen peroxide and subsequent lipid peroxidation. Eriksen et al. (2010) did not find evidence that PFNA (0–2,000 μM) induces ROS production or oxidative stress in HepG2 cells. However, elevated hepatic levels of malondialdehyde (MDA)/thiobarbituric acid reactive substances (TBARS) were seen after in vivo treatment of rodents with PFOS (1 mg/kg bw per day; Han et al., 2018a), PFOA (5 mg/kg bw per day; Wang et al., 2017b) or PFNA (5 mg/kg bw per day; Fang et al., 2012b). Also, PFDoDA was found to cause intrahepatic lipid peroxidation when applied at 0.5 mg/kg bw per day (Liu et al., 2016) and to induce SOD activity at 1 mg/kg bw per day (Zhang et al., 2008).
To conclude, like PPs, PFASs seem to cause liver hyperplasia via murine PPARα, considering no clear hyperplastic liver growth in the KO and humanised mouse models. PFASs induce steatosis in rodent hepatocytes in a PPARα‐independent way. The accumulated lipids increase the hepatocellular volume and contribute to the elevated weight of the liver due to hypertrophy. The hepatocellular steatosis seems to be causally related to the occurrence of necrotic hepatocytes and the increased serum transaminase levels. These various effects in the rodent liver appear to be common for most of the PFASs, investigated so far. Thorough knowledge of the MoA underlying the development of hepatocellular steatosis in PFAS‐treated rodents is missing, as outlined also in EFSA CONTAM Panel et al. (2018) Opinion.
3.3.5.3. Immunotoxicity
A clear mode of action of immunotoxicity by PFOS and PFOA has not been established. Just anecdotal data are available that may offer some leads for further investigation, but do as yet not offer a clear mechanism along which PFASs influence the immune system. Information on immunotoxicity by PFASs other than PFOS and PFOA and whether such effects on the immune system might be brought about by similar mechanisms as PFOS and PFOA is even more limited.
In some studies on PFOS and PFOA, effects on the immune system were shown at doses where also effects on body weight were observed and it might be argued that effects on the immune system may have been secondary to general toxicity. However, other studies showed effects at concentrations where no effects on body weight were observed (see Section 3.3.3.5). The pivotal studies showing effects on the antibody response to immunisation with sheep red blood cells (Dong et al., 2009; Peden‐Adams et al., 2008), showed effects at dose levels below those that induced effects on body weights. As PFASs have been shown to affect thyroid hormone levels (see Section 3.3.3.4.16), Fair et al. (2011) investigated whether this could explain the effects observed by Peden‐Adams et al. (2008) and found effects on the immune system at doses lower than where effects on thyroid hormone levels occurred. Dong et al. (2009) also measured corticosterone levels in serum and did not find changes that might have been responsible for the suppressed antibody response.
As reviewed in the previous Opinion on PFOS and PFOA (EFSA CONTAM Panel, 2018), one component of a mechanism along which immunotoxic effects by these chemicals could be brought about, is by impacting NF‐κB regulated gene transactivation and affecting gene expression of apoptotic regulators, resulting in apoptosis of lymphoid cells, and consequently suppressed proliferation and activation of T‐cell receptor signalling and depressed immune functionality.
An association between immune parameters, PPARβ/δ mRNA expression and PFASs has been observed in cord blood, in addition to mRNA expression of several immunomodulatory genes (Pennings et al., 2016). Qazi et al. (2009b), comparing WT and PPARα KO mice, reported effects of PFOS on the thymus and the spleen that were partly dependent on PPARα. These findings would be in line with the notion that different PPARs have been described to interact with both the innate, as well as the acquired immune system (Daynes and Jones, 2002), and that PPAR agonists in fact induce anti‐inflammatory activity (Le Menn and Neels, 2018; Wang et al., 2018b). This is in keeping with suppressed LPS‐dependent release of TNFα in human peripheral blood leukocytes by PFOS (Brieger et al. (2011) and IL‐18 by PFOA in THO‐1 cells (Corsini et al., 2011). In addition, also immune effects independent of PPARα have been shown, in vitro by PPAR silencing (Corsini et al., 2011) and in vivo by using PPARα KO animals (DeWitt et al., 2009, 2016). This is also shown by unpublished data obtained from Peden‐Adams (see Figure H.3), showing a similar decrease in PFCs in wild‐type C57Bl/6 mice and a mice containing a PPARα targeted mutation. Hence a clear picture on the involvement of PPARs is lacking, and further research on this topic is necessary.
There may be a common MoA behind the immunotoxic effects of PFASs. However, there may also be differences in the mechanisms behind some of the immunotoxic effects observed with different PFASs. For instance, cytokine profiles in lymphoid cells have shown to be differentially affected by PFOS and PFOA (Corsini et al., 2011; Midgett et al., 2015). It should be noted, however, that individual interleukin values, influenced by PFASs, cannot easily be interpreted in terms of how they play a role in the suppressive effect on antibody production by PFOS and PFOA.
At least for PFOS, other mechanisms, that may or may not be related to those mentioned above, have been put forward as well:
Zarei et al. (2018) investigated the cytotoxic activity of PFOS on human lymphocytes after in vitro incubation. The authors noted increased ROS production, lipid peroxidation, glutathione depletion, activation of caspase‐3, as well as damage to cell organelles such as mitochondria and lysosomes.
Suo et al. (2017) showed that PFOS increased IL‐22 excretion by lamina proprial lymphocytes isolated from the large intestines of wild‐type mice. This effect was not observed using F1 mice from an AhRf/f and a RORc‐cre cross to specifically delete the Ah receptor and it was concluded that this response must be dependent on the Ah receptor.
Lee et al. (2018), using rat basophilic leukaemia cells in vitro, showed that PFOS significantly increased histamine release by IgE stimulated cells, which coincided with enhanced gene expression of proinflammatory cytokines and activation of nuclear NF‐κB. However, even if IgE dependent allergic responses are immune‐related, it is not clear if there is a relation to immune suppression such as evidenced by depressed antibody responses.
As indicated above, information on immunotoxicity by other PFASs than PFOS and PFOA is even more limited.
Fang et al. (2008, 2009) studied effects of oral exposure to PFNA in both mice and rats, and RT‐PCR of thymus cells revealed increased gene expression of PPARα and PPARγ. However, PFNA did not seem to influence genes encoding for the NF‐κB signalling pathway.
In an in vitro study performed by Zhou et al. (2017), the human gastric cell line AGS was exposed to PFDA, and increased IL‐13 and IL‐18 expression, both at the protein level and the mRNA level, were observed. Also NLRP3 and NF‐κB were increased, indicating enhanced inflammasome activity. Increased IL‐13 and IL‐18 production were also observed in the stomach of mice, exposed to PFDA in drinking water, accompanied by disorganised alignment of cells and increased inflammatory cell infiltration.
Both these studies by Fang et al. (2008, 2009) and by Zhou et al. (2017) do therefore indicate that components of the immune system are affected by PFNA and PFDA. Whether such effects of PDFA would have an impact on the functionality of the immune system, including antibody production, has not been studied.
In a study on PFHpA, PFNA, PFDA and PFUnDA, Lee and Kim (2018) compared effects of the chain length of PFASs in an in vivo system of anaphylaxis and an in vitro assay using IgE stimulated mast cells. The longer chain compounds PFDA and PFUnDA increased the release of histamine and proinflammatory cytokines and intracellular calcium concentrations. RBL cells, transfected with a NF‐κB–luciferase reporter construct, showed enhanced luciferase activity when treated with PFDA and PFUnDA, but not the other compounds. In vivo, PFNA, PFDA and PFUnDA aggravated allergic reactions in an ovalbumin model, such as an aggravation in the decrease of rectum temperature after injection of ovalbumin. These results indicate that the chain length is a determinant in effects on allergic processes. Again, these data support the notion that components of the immune system are influenced by these compounds. However, even if allergic responses are immune‐related, it is not clear whether these effects may lead to suppressed antibody responses to antigens other than the allergen ovalbumin, and if so would follow similar mechanisms as such potential immunosuppressive effects, if they would be present.
In summary, the studies that were performed to shed light on possible mechanisms, support the notion that PFASs influence the immune system. Immune effects of PFOS and PFOA have been mostly studied, but their mode of action has not been fully identified. NF‐κB regulated gene transactivation seems involved. There are interactions of PPARs with the immune system, but it is not clear in how far these receptors may be involved in immune effects by PFASs.
3.3.5.4. Impaired mammary gland development
The mammary gland develops through three major stages into a fully lactating mammary gland. In the embryonic stage, distinct placodes are formed from the ectoderm‐derived mammary line that descend into the mesenchyme and form the rudimentary ductal structure present at birth. This occurs under the control of epithelial/mesenchymal interactions. During later major developmental stages during puberty (ductal elongation), and in reproduction (alveolar growth in pregnancy and lactation, and tissue remodelling during involution at weaning), the development is regulated by both hormones and epithelial/mesenchymal interactions (Macias and Hinck, 2012). The transition stages of the mammary gland development are regarded as highly sensitive windows of susceptibility in both humans and rodents, with the terminal end buds and ductal growth as the most fragile structures in the rodent (Martinson et al., 2013; Russo and Russo, 1996).
The in vivo studies on mammary gland development in mice (described in Section 3.3.3.3.2) indicate that all the developmental stages can be affected by PFOA. However, the cross‐fostering study with PFOA implies that postnatal exposure is sufficient to decrease mammary gland development lasting beyond sexual maturity in mice (White et al., 2009). Of note, the MoA is not necessarily similar at the different developmental stages. There are no studies on other PFASs than PFOA for this effect.
After PFOA dosing of C57Bl/6 mice, starting in peri‐puberty, both inhibitory but also stimulatory effects on mammary gland development have been reported (see 3.3.3.3.2), whereas in other mouse strains only inhibitory effects have been reported. Prenatal and/or early postnatal exposure affected mammary gland development at lower levels than exposure later in life.
Only few studies on effects of PFASs on mammary cells in vitro were identified. When grown on reconstituted extracellular matrix, the cell line MCF‐10A can be used as a model for studying ER‐independent breast tissue development, as the cells form acini, which are highly‐organised epithelial structures that share some aspects of glandular breast tissue architecture. Acini development was dose‐relatedly disturbed, starting at 0.6 μM (lowest concentration tested) of PFOS, PFNA (both from day 5) and PFDA (from day 3), and completely absent at 60 μM on day 10 (Halsne et al., 2016). Acini development was disturbed to a much lesser extent with PFOA and PFUnDA, and started to occur at least at a 10‐fold higher concentration and at later time points. When grown in monolayer, only at much higher concentrations (100 to 500 μM for 72 h) than those causing effects on acini, some effects on cell cycle were observed with PFOS, PFNA, or PFDA, whereas PFOA and PFUnDA had no effect. It is however doubtful that this model, which according to Qu et al. (2015) ‘exhibit a unique differentiated phenotype in 3D culture which may not exist or be rare in normal human breast tissue’ can mimic early‐life breast gland differentiation. Similar doubt applies to results from studies on PFOS or PFOA in MCF‐10A cells in monolayer (Pierozan and Karlsson, 2018, Pierozan et al., 2018, 2018).
The involvement of PPARα in mammary gland development is of interest since PFOA can activate this receptor. PPARα mRNA is expressed in luminal epithelial cells and stromal adipocytes in the mouse mammary gland, and the level of PPARα mRNA decreases during pregnancy and lactation (Gimble et al., 1998). The mammary gland develops normally in PPARα KO mice. However, PPARα activation influences mammary lobuloalveolar development in pregnancy. Transgenic mice expressing constitutively active PPARα in basal epithelial cells (and not, as is normal, in luminal epithelial cells) in the mammary gland during pregnancy had defects in lactation, resulting in mortality of new‐borns (Yang et al., 2006a). This was due to impaired development of lobuloalveoli, and reduced proliferation together with increased apoptosis in mammary epithelia, which were accompanied by reduction in β‐catenin expression and the target gene Cyclin D1. Treatment of pregnant WT mice with synthetic PPARα ligand (WY‐14,643) also led to impaired development of lobuloalveoli, an effect not observed in similarly treated PPARα KO mice (Yang et al., 2006b).
The stimulating effect (increased growth) in the mammary gland, observed in C57BL/6 mice after 5 mg/kg bw PFOA exposure five days per week during puberty, as reported by Yang et al. (2009), occurred also in PPARα KO mice on a C57BL/6 background, and was thus independent of PPARα (Zhao et al., 2010). In contrast, the decrease in mammary gland development at higher PFOA dose (7.5 mg/kg bw per day, 5 days per week) during puberty was not seen in PPARα KO mice (Zhao et al., 2012). However, since the serum PFOA concentration in PPARα C57BL/6 KO mice was approximately only 50% of that in WT at both 5 and 7.5 mg/kg bw per day, the dependency on PPARα expression for the observed effects could not be fully elucidated in the study.
An important mechanistic issue to understand is whether effects induced by PFOA on mammary gland development occur without general effects on pubertal transition. In CD‐1 mice treated during gestation, it appears that puberty is not affected, since Tucker et al. (2015) reported no impact of PFOA treatment on vaginal opening. However, when mice were treated during puberty at higher levels that resulted in decreased mammary gland development, Zhao et al. (2012) reported later vaginal opening in Balb/c and absent vaginal opening in C57Bl/6 mice, and there was absence of oestrus cycle in both strains.
Zhao et al. (2012) observed in two mice strains treated with PFOA during puberty (Balb/c with 2.5 mg/kg bw per day and C57Bl/6 with 7.5 mg/kg bw per day, 5 days per week), a decrease of the number of terminal end buds, a decrease in number of stimulated terminal ducts and a reduced ductal length (see 3.3.3.3.2). In the ovaries, reduced levels of proteins important for steroidogenesis (StAR, CYP11A1, HSD3β1 and HSD17β1) were reported, whereas levels of aromatase were not affected. In the mammary gland, the important growth factors HGF and AREG were down‐regulated, whereas IGF‐1 levels were unaffected. In line with this, levels of the proliferation marker PCNA were significantly reduced in mammary glands. The effects of PFOA could partially, but not entirely, be prevented by co‐treatment with estradiol or progesterone, or combinations thereof. The effects of PFOA on mammary gland development in animals dosed during puberty thus seem to involve interference with ovarian steroid production, and expression of growth factors in the mammary gland.
In summary, the MoA behind the most sensitive PFOA effect, which is a decrease in mammary gland development in animals dosed during gestation and neonatally, is unknown. Normal mammary gland development does not require PPARα expression, but PPARα activation in pregnancy can reduce mammary gland development in the dam.
3.4. Critical effects, dose‐response assessment and derivation of a health‐based guidance value
3.4.1. Critical effects
The potential effects of PFASs have been studied extensively, both in laboratory animals and in humans. In animals the effects that were most often reported were increased liver weight and effects on thyroid hormones. In addition, effects on the immune system and mammary gland development were observed in animals at lower levels than those showing effects on liver and thyroid hormone levels.
For several PFASs, exposure of particularly mice resulted in a decrease in the T‐cell dependent antibody response. In the two most informative animal studies on PFOS (Dong et al., 2009; Peden‐Adams et al., 2008), applying multiple dose levels, the lowest NOAEC serum level of 17.8 ng/mL was noted for Peden‐Adams et al. (2008) (see Section 3.3.3.5). Effects were also observed with other PFASs and also in rats, but the studies currently available do not allow a formal comparison of potential differences in potencies (see Section 3.3.3.5, Table 20).
In addition, various effects have been observed in developmental studies with rodents. The effect shown at the lowest exposure is that of PFOA on the development of the mammary glands in mice, observed in several studies with different strains. These effects concerned an impaired development of the mammary glands of animals exposed in utero and/or during lactation, and effect on the further development when exposed during peri‐puberty (Macon et al., 2011; Tucker et al., 2015; White et al., 2011), but also a decreased involution of the glands in the dams after the lactation period (White et al., 2007). In particular the impaired development was associated with relatively low serum levels in three different studies (LOAECs around 20 ng/mL on PND 22 corresponding to a maternal LOAEC of around 66 ng/mL, no NOAECs). Effects on mammary gland development have thus far not been studied for other PFASs.
In human studies, various associations between serum levels and a number of outcomes have been reported. In the previous Opinion (EFSA CONTAM Panel, 2018), four endpoints were selected as potential critical effects for PFOS and/or PFOA. These were (i) increased serum total and LDL cholesterol (risk factor for cardiovascular disease), (ii) increased ALT levels (indicating effects on liver cells), (iii) reduced birth weight, and (iv) effects on the immune system as shown by decreased antibody response to vaccines.
In the previous Opinion (EFSA CONTAM Panel, 2018), the CONTAM Panel used the effects on serum cholesterol levels to derive TWIs for both PFOS and PFOA. Those TWIs were also protecting towards the other potential critical endpoints. Although the association with increased cholesterol was observed in a large number of studies, the CONTAM Panel now considers the uncertainty regarding causality larger. This is primarily due to a postulated biological process around the enterohepatic cycling of both PFASs and bile acids, the latter affecting serum cholesterol levels.
The issue of possible confounding of associations between PFASs and serum cholesterol due to shared intestinal reabsorption of PFASs and bile acids was noted by the German risk assessment institute BfR in an expert meeting about the previous EFSA Opinion on PFOS and PFOA (described in the meeting minutes31 (EFSA/CONTAM/3503)). PFASs are eliminated not only by renal clearance but also via the bile. Both bile acids and PFASs are extensively reabsorbed in the intestine and subject to enterohepatic circulation. Common intestinal reabsorption of bile salts and PFASs, and uptake into the liver via shared membrane transport pathways is a possible mechanism of confounding since inter‐individual variability in reabsorption could lead to a positive association between serum levels of PFASs and cholesterol, the latter being affected by reabsorption of bile acids. In a recent small ‘proof‐of‐concept’ study many associations between PFASs and bile acids were found, both in a positive and negative direction (Salihovic et al., 2020). Variability in intestinal reabsorption might therefore explain the observed association between serum cholesterol and serum PFAS levels. This is a reasonable potential mechanism for confounding, but it has not been convincingly demonstrated. There are also studies arguing against such confounding. A recent Swedish study (Li et al., 2020) showed significantly higher levels of serum cholesterol in an area with high exposure to PFASs from contaminated drinking water than in a reference area with low PFAS exposure. So in this case, the association with increased serum cholesterol is based on higher intake rather than higher serum PFAS levels. Nevertheless, because of this potential source of confounding there is uncertainty regarding causality, making it less appropriate to use increased serum cholesterol as the basis for a health‐based guidance value.
As already explained in the previous Opinion on PFOA and PFOS (EFSA CONTAM Panel, 2018), the association with reduced birth weight might at least partly be explained by changes in the physiology during pregnancy, although a recent study seemed to strengthen the causality of the effect (Meng et al., 2018; see also Section 3.3.4.1.1). The remaining decrease in birth weight after adjusting for confounders was not large and the potential longer term consequences of this decrease are unclear. Thus far, there is little evidence for an increase in the proportion of children with low birth weight (< 2,500 g).
The consistent increase in ALT levels in general population studies seems supported by observed effects in animal studies, but was not observed in most of the occupational studies with much higher exposure (EFSA CONTAM Panel, 2018). In the critical study (Gallo et al., 2012), the increase in subjects with high ALT levelled off at relatively low serum concentrations (about 30 ng/mL of PFOS and PFOA) and above that, it did not increase further. This is in contrast to rodent studies showing increase in ALT only at the high end of the dose‐response curve. This inconsistency creates some uncertainty that should be addressed in future studies. For these reasons, this endpoint was not considered as the critical effect.
Reduction in thyroid hormone levels is often observed in animal studies. Epidemiological studies provide insufficient support for associations between exposure to PFASs and changes in thyroid hormone levels or thyroid function.
Considering the selection of the most critical effect, those on the immune system were observed at the lowest serum levels in both animals and humans. The findings were considered robust since they were consistently observed for several PFASs and for several species. This is not the case for effects on mammary gland development, which are observed at similar low serum levels in mice but have not been studied in other animal models or humans. Therefore, the CONTAM Panel decided to base the present assessment on PFASs on effects on the immune system.
A decrease in vaccination response is seen as adverse by the scientific community, as summarised by WHO/IPCS (2012) in the Guidance for immunotoxicity risk assessment for chemicals. This may in particular apply to vulnerable population groups, i.e. infants and the elderly, considering their higher infection risk.
For compounds that accumulate in the body, the preference of the CONTAM Panel is to identify serum or tissue levels associated with adverse effects. The CONTAM Panel decided to base its assessment on the serum levels for the sum of four PFASs, i.e. PFOA, PFNA, PFHxS and PFOS. At present, these are the PFASs that contribute most to the levels observed in human serum (see Section 3.3.2 on biomonitoring). Based on structural similarities, other PFASs may cause similar effects, but the critical studies in humans did not report these in the blood of the participants. In particular PFASs with long half‐lives may be relevant. Although some other PFASs like PFBA and PFHxA also contribute significantly to the exposure (see Section 3.2.1 on current exposure), these compounds show much shorter half‐lives in humans and thus much higher exposure is required to obtain similar serum levels as the 4 PFASs selected. As will be discussed below, the available data are insufficient to derive potency factors for the different PFASs, although such differences in potency are likely to exist.
A study on children in the Faroe Islands (Grandjean et al., 2012) showed several inverse associations between serum levels of PFOA, PFNA, PFHxS and PFOS, as well as the sum of PFOA, PFHxS and PFOS at five years of age, before booster vaccination, and antibody titres against diphtheria and tetanus at both the age of 5, shortly after booster vaccination, and at 7.5 years. In the previous Opinion (EFSA CONTAM Panel, 2018), BMD analysis was performed on the PFOS data in 5‐year‐old children from the Faroe Islands, resulting in a BMD05 and BMDL05 of, respectively, 11.6 and 10.5 ng/mL. However, the modelling approach was criticised during the expert meeting (EFSA/CONTAM/3503), including the use of the antibody titre in the lowest decile as the reference value rather than extrapolate and evaluate the BMR for a serum PFOS concentration of zero. Data for PFOA were not modelled, since the levels were much lower than those for PFOS, and there were no indications that PFOA was more potent than PFOS. For this study, additional data on the sum of PFOA, PFNA, PFHxS and PFOS were obtained (see Appendix L). Modelling of the data by EFSA with the recommended BMD modelling software (PROAST and BMDS) resulted in wide BMDL‐BMDU intervals, as a consequence of extrapolating to zero exposure, well below the lowest observed serum levels. Therefore, the CONTAM Panel identified an NOAEC serum level at the age of 5 years for the sum of PFOA, PFNA, PFHxS and PFOS of 27.0 ng/mL, based on decreased antibody titres for diphtheria at the age of 7 years.
This study was supported by a more recent study from Germany. In this latter study, an inverse association was observed between serum levels of PFOA (Abraham et al., 2020), but also the sum of PFOA, PFNA, PFOS and PFHxS (Appendix K), and antibody titres against haemophilus influenzae type b (Hib), diphtheria and tetanus in serum sampled from 1‐year‐old children predominantly breastfed for a median duration of 7.4 months (see Section 3.3.4.4.2). A lowest BMDL10 of 17.5 ng/mL at the age of 1 year was derived for the sum of PFOA, PFNA, PFOS, and PFHxS based on an association with reduction in antibody titres against diphtheria (See Appendix K). For PFOS, PFHxS and PFNA alone, no significant associations were observed in this study.
The possible confounding by a number of contaminants like PCBs, dioxins, organochlorine pesticides, lead and mercury was examined in these studies but these had no effect on the observed associations.
A substantial decrease in antibody response after vaccination was reported, with inverse association for several antigens in several independent cohorts, but not all (see Section 3.3.4.4.2). In the German study, at the highest quintile the mean antibody titres for Hib, diphtheria and tetanus were 63%, 42% and 49% lower, respectively, than those in the first quintile (see Appendix K). In the study from the Faroe Islands, at the LOAEC, the antibody titres for diphtheria were lower by around 50%. In that study, the proportion of children having vaccination titres below the protective limit after vaccination was also increased at higher PFAS levels. Such decreases in antibody responses are clearly adverse on a population level, not only in terms of protection against the pathogen to which the vaccine is directed, but also in terms of general immunologic defence against other pathogens. Indeed, there are some data suggesting that PFAS exposure is associated with increased infection risk, and also with decreased specific antibody formation after virus exposure in infants.
The study by Abraham et al. (2020) indicates that children are sensitive in their first year, and this was also suggested by Grandjean et al. (2017a), comparing measured and predicted serum levels of PFASs during the first 18 months with measured antibody titres against diphtheria and tetanus at 5 years of age before applying a booster. However, the observed associations between serum levels at 5 years of age and antibody titres at 7 years (Grandjean et al., 2012) imply that the decreased antibody response also occurs in older children. Although performed with a small group and thus not suitable for deriving a critical serum level, the study by Kielsen et al. (2016a) with 12 adults shows that the decrease in vaccination response may also occur at much older age and low serum levels of PFASs (median levels for PFOA, PFHxS and PFOS of, respectively, 1.7, 0.7, 0.4 and 9.5 ng/mL). This is supported by the results from mice studies that were performed with adult animals (see Section 3.3.3.5). One study performed with treatment of pregnant dams did not show higher sensitivity of the offspring
3.4.2. Mixture approach
In the previous Opinion (EFSA CONTAM Panel, 2018) it was decided to derive separate TWIs for PFOS and PFOA, ‘since both toxicity as well as underlying modes of toxic action for PFOS and PFOA are not sufficiently understood and might differ, but also overlap.’ Since that Opinion, EFSA launched a new Guidance document on how to evaluate the effects of mixtures (EFSA Scientific Committee, 2019) and it was considered that similarities in chemical properties and effects warrant a mixture approach for a number of PFASs. The CONTAM Panel decided to focus on four PFASs (PFOA, PFNA, PFHxS and PFOS) but acknowledges that more data are needed on other PFASs in the future. In humans, these four PFASs share toxicokinetic properties and show similar accumulation and long half‐lives. Furthermore, these four PFASs show the highest concentrations in blood plasma and serum (See Section 3.3.2). Also in terms of effects, these compounds in general show the same effects when studied in animals. This is exemplified by the NTP studies (NTP, 2019a,b), which studied a number of effects on organs and tissues, but e.g. also serum levels of hormones, bile acids, cholesterol, triglycerides and the expression of a several genes in the liver, like Cyp4a1, Cyp2b1, Cyp2b2, and Acox1. Overall, most of the seven PFASs showed an effect on these parameters, confirming that in general these compounds cause similar effects (see Table 21). Differences in potencies were also observed and these could partly be explained by differences in kinetics. There are also sex‐related differences in kinetics, in particular in rats, but these are not observed in humans.
Table 21.
| PFAS | Liver weight | Serum total T4 | Serum free T4 | Serum T3 | Serum triglycerides | Serum cholesterol | Serum bile salts/acids | Gene expression liver | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (g) | (μg/dL) | (ng/dL) | (ng/dL) | (mg/dL) | (mg/dL) | (μmol/mL) | Acox1 | Cyp4a1 | Cyp2b1 | |||||||||||
| M | F | M | F | M | F | M | F | M | F | M | F | M | F | M | F | M | F | M | F | |
| PFBS | 1.5 | 1.3 | 0.03 | 0.3 | 0.1 | 0.2 | 0.4 | 0.6 | 0.3 | −−− | 0.2 | 0.8 | 1.9 | 5.3 | 4.8 | 3.1 | 24 | 4.5 | 63 | 510 |
| PFHxS | 1.5 | 1.2 | 0.3 | 0.7 | 0.2 | 0.6 | 0.6 | −−− | 0.6 | −−− | 0.7 | −−− | −−− | −−− | 2.8 | −−− | 16 | 1.3 | 27 | 38 |
| PFOS | 1.6 | 1.5 | 0.1 | 0.1 | 0.1 | 0.3 | 0.6 | 0.6 | 0.2 | 0.7 | 0.2 | 0.7 | 3.4 | 3.7 | 5.3 | 3.0 | 30 | 2.4 | 349 | 802 |
| PFHxA | 1.4 | 1.4 | 0.4 | −−− | 0.3 | – | 0.7 | −−− | −−− | −−− | 0.8 | −−− | 2.2 | 2.6 | 2.0 | 1.6 | 12 | 2.5 | 6 | 68 |
| PFOA | 1.3 | 1.6 | 0.03 | 0.5 | 0.1 | 0.7 | 0.6 | −−− | 0.6 | 1.6 | 0.6 | 1.2 | 4.5 | −−− | 5.7 | 3.9 | 30 | 7.6 | 18 | 71 |
| PFNA | 1.3 | 1.3 | 0.1 | 0.6 | 0.1 | 0.5 | −−− | −−− | 0.5 | −−− | 0.7 | −−− | 16.9 | 2.8 | 5.9 | 5.2 | 22 | 13 | 6.8 | 14 |
| PFDA | 1.2 | 1.3 | 0.7 | −−− | 0.1 | 0.3 | −−− | 2.1 | 0.5 | −−− | 0.6 | 0.7 | 13.1 | 7.6 | 9.1 | 5.4 | 60 | 17 | 10 | 89 |
| Wyeth | 1.4 | 1.6 | 0.8 | −−− | 0.6 | 1.5 | −−− | 1.3 | −−− | −−− | 0.8 | 0.9 | 7.1 | −−− | 6.5 | 5.6 | 43 | 7.1 | 3.7 | 33 |
PFAS: perfluoroalkyl substance; F: female; M: male; MoA: mode of action; T4: thyroxine; TSH: thyroid stimulating hormone.
Dose levels (mg/kg bw per day; males/females): PFBS (62.5–1000/62.6–1000), PFHxS (0.625–10/3.12–50), PFOS (0.312–5/0.312–5), PFHxA (62.6–1,000/62.6–1,000), PFOA (0.625–10/6.25–100), PFNA (0.625–10/1.56–25), PFDA (0.156–2.5/0.156–2.5), Wyeth 14,346 (6.25–25/6.25–25). Blue colour indicates significantly higher than controls, purple colour significantly lower.
Serum TSH levels decreased in males treated with PFOA, PFNA, and Wyeth 14,643, but increased in females treated with PFOA and Wyeth 14,643. For free T4 there may be an issue with the measurement (see Section on MoA).
Figures express the lowest/highest ratio compared to the controls observed for either one of the dose levels. In some cases most animals died and these dose groups were not included.
An important question is whether these four PFASs show similar potencies for the critical effects. As shown in Section 3.3.3.5, effects of PFOS and PFOA on vaccination response have been studied in animals as well as more general effects of the immune system for some other PFASs (in addition to PFOS and PFOA, also PFNA and PFDA). However, there are no comparative studies that provide reliable insight in the relative potencies.
The study by Abraham et al. (2020) showed that only for PFOA there was a significant association with antibody titres against three different vaccines. For PFOS, PFHxS and PFNA this was not observed. The association was, however, also significant for the sum of the four PFASs, based on additional information from the authors (see Appendix K). Grandjean et al. (2012) showed significant associations for PFOS, PFOA and the sum of PFOS, PFOA and PFHxS. Furthermore, the decrease in titres was also significant for the sum of four PFASs (PFOA, PFNA, PFHxS and PFOS), based on additional data provided by the authors (see Appendix L). Although in a later study by Grandjean et al. (2017a), focussing on serum levels at young age and antibody titres at 5 years before the booster in two cohorts, PFOA showed stronger associations than PFOS, these were also observed for PFOS. Granum et al. (2013) reported an inverse association between maternal PFASs and rubella titres at age 3 years for all four PFASs, and similar conclusions were reached when each compound was mutually adjusted for the other (Granum et al., 2013 and see Appendix K). In summary all three studies showed positive associations for PFOA. Since PFOA and PFOS serum concentrations are higher as compared to serum concentrations of PFNA and PFHxS, and PFOA highly correlates with the serum levels of the other PFASs, it is uncertain whether PFOA has a higher potency for this critical endpoint as compared to the other PFASs and thus drives the association. Furthermore, the CONTAM Panel noted that a potential higher potency of PFOA seems to contradict the outcome of various studies with animals, which thus far showed effects at much lower serum levels for PFOS than for PFOA, keeping in mind that a thorough comparison was never made. The latter also applies to effects on mammary glands in mice, which were only studied for PFOA.
Another issue is whether to base relative potencies on applied dose or e.g. serum levels (internal dose). The NTP studies (NTP, 2019a,b) reported effects on absolute liver weight, and also serum levels at the last day of treatment. This allows comparison of dose‐response curves based on external dose but also on internal dose, i.e. the serum level. This comparison reveals large differences between the two approaches, due to kinetic differences. This adds additional uncertainty to deriving potency factors for different PFASs.
Figure 11.
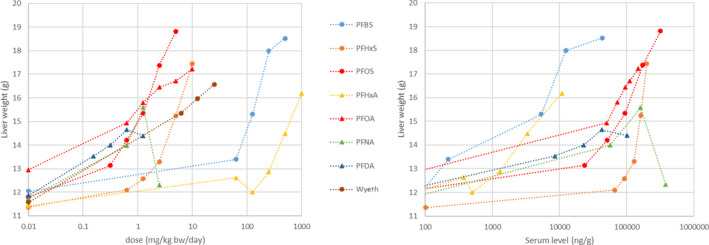
Absolute liver weights in male rats exposed to various PFASs, based on applied dose (left) or serum levels (right)
- PFAS: perfluoroalkyl substance; PFBS: perfluorobutane sulfonic acid; PFOS: perfluoroheptane sulfonate. For WY‐14,643 serum levels were not determined. There is a clear shift in the order of the different PFASs; compare e.g. PFBS (blue dots) and PFOS (red dots).
Despite many studies, the mode of action behind various adverse effects is still unclear. This applies to effects on the liver but certainly on the immune system and mammary gland development. It has been shown that some PFASs act on the PPARα receptor and it was hypothesised that activation of this receptor is an important event behind some of the observed adverse effects. However, PFASs show partly different effects than classical agonists, also in PPARα KO mice. Therefore, the MoA is still unclear, even for effects on the liver, like steatosis. Due to the lack of a clear MoA, results of in vitro studies can help to improve our understanding but cannot be used to derive potency factors at this stage. Also the differences in kinetics between species, sex and PFASs makes this rather complicated. The absence of a clear MoA is not a reason for not applying a mixture approach.
As a pragmatic approach, the CONTAM Panel decided to restrict the mixture approach to the four most abundant PFASs in human serum and in the absence of more specific information, to assume equal potencies by default for these four PFASs on immune outcomes. Although scientifically less correct, this was done on a weight base rather than a molar base, to allow easier comparison with the exposure assessment.
3.4.3. Dose‐response assessment
3.4.3.1. Effects on the immune system
Since for the study from the Faroe Islands (Grandjean et al., 2012), BMD modelling did not provide a BMDL that was considered suitable for the risk assessment (see Section 3.4.1), an NOAEC was derived for the sum of the four PFASs (PFOA, PFNA, PFHxS and PFOS) of 27.0 ng/mL in serum of 5‐year‐old children (serum level in 4th quintile, see Appendix L).
BMD modelling on the data from a study from Germany (Abraham et al., 2020; Appendix K) showed for the association between serum levels of the 4 PFASs and the titres of antibodies against diphtheria and tetanus BMDL‐BMDU intervals of, respectively, 17.5–46.6 and 18.8–56.3 ng/mL, based on four individual models (Appendix K). These models provided very similar results. For Hib the modelling of the dose response did not reach statistical significance for the sum of the 4 PFASs. It was decided to use a critical effect size of 10%, instead of the default 5%, considering the large variation in the response. The model averaging provided BMDL‐BMDU intervals of, respectively, 2.1–45.4 and 3.9–49.5 ng/mL for diphtheria and tetanus. The Panel noted that these BMDLs of 2.1 and 3.9 ng/mL are eight‐ and fivefold lower than the lowest BMDLs from the individual models. This seems to be caused by the fact that several of the 500 bootstrap runs performed for model averaging provided curve fits that deviated from the individual models. Furthermore, these BMDLs obtained with model averaging were below the observed range of the serum levels of the 4 PFASs in this study. Therefore, it was decided to use the lowest BMDL from the individual models, being 17.5 ng/mL as the Reference Point.
The lowest BMDL10 of 17.5 ng/ml was used to estimate the daily intake by mothers of the sum of PFOA, PFNA, PFHxS and PFOS that would result in this critical serum concentration at 1 year of age in breastfed children. This daily intake was subsequently used to derive the health‐based guidance value. In the previous Opinion (EFSA CONTAM Panel, 2018), a PBPK model was used to translate the critical serum levels into a daily intake. This model was originally developed for adults, but was adjusted to estimate the serum levels also in growing children and to include exposure via breastfeeding. Data from human biomonitoring studies on milk versus plasma levels, were used to estimate the levels in human milk corresponding to a certain serum level in the mother. In addition, the prenatal exposure and resulting body burden of the new‐born was estimated based on levels in cord blood and mother's blood. This modelling was previously performed for PFOS only, but in the current Opinion this model was extended to PFOA, based on available data (see Appendix M for a description of the PBPK modelling). It has been shown that during breastfeeding, a substantial part of the PFASs in the mother is transferred to the infant, and as a result, serum levels in the mother but also levels in the breast milk decrease over the lactation period. This decline was also included in the model. Data available for PFNA and PFHxS were insufficient, but considering similarities in the toxicokinetics and structural similarities it was assumed that these compounds behave like PFOA and PFOS, respectively.
In the case of the German study, the serum level of 17.5 ng/mL was the sum of levels of PFOA, PFNA, PFHxS and PFOS. Based on the mean levels of these PFASs in 1‐year‐old breastfed infants (Abraham et al., 2020), relative contributions of, respectively, 48.4, 1.7, 6.1 and 43.8% were calculated, and used to estimate the contribution of each PFAS to a serum level of 17.5 ng/mL. These contributions were for PFOA, PFNA, PFHxS and PFOS, respectively, 8.47, 0.30, 1.06 and 7.67 ng/mL, or 8.78 ng/mL for the PFCAs and 8.72 ng/mL for the PFSAs. The PBPK models were then used to calculate the critical milk and corresponding serum levels in the mother at 35 years that would result in these levels for PFOA/PFNA and PFHxS/PFOS in the one‐year‐old infant, followed by an estimation of the daily intakes by the mothers that lead to this critical serum level at 35 years. Assuming 12 months of breastfeeding, it was estimated that for PFOA/PFNA this corresponds to an intake by the mother of 0.187 ng/kg bw per day and for PFHxS/PFOS of 0.444 ng/kg bw per day, or combined 0.631 ng/kg bw per day for the sum of the four PFASs. Such intakes would result in serum levels in the mother at 35 years of age of 1.998 ng/mL for PFOA/PFNA and 4.888 ng/mL PFHxS/PFOS, or combined 6.886 ng/mL. These serum levels would result in initial milk levels of 0.060 and 0.073 ng/mL for PFOA/PFNA and PFHxS/PFOS, respectively, based on the applied milk to serum ratios of 0.03 and 0.015. For the sum of 4 PFASs this is 0.133 ng/mL. The CONTAM Panel decided to apply a breastfeeding duration of 12 months, based on the WHO recommendations to breastfeed exclusively for 6 months with continued breastfeeding along with appropriate complementary foods up to two years of age or beyond.
Figures 12 and 13 show the estimated serum levels in women exposed in utero, via breastfeeding for 12 months and subsequently regular food consumption, the latter resulting in an intake of 0.19 ng/kg bw per day of PFOA or 0.44 ng/kg bw per day of PFOS. The model is developed for PFOA and PFOS, but these are assumed to be representing also PFNA and PFHxS, respectively. The major purpose is to show the serum level around child‐bearing age. The graphs do not include the effect of having a child and of subsequent breastfeeding, which results in a (temporary) decline in the serum levels of the mothers.
Figure 12.
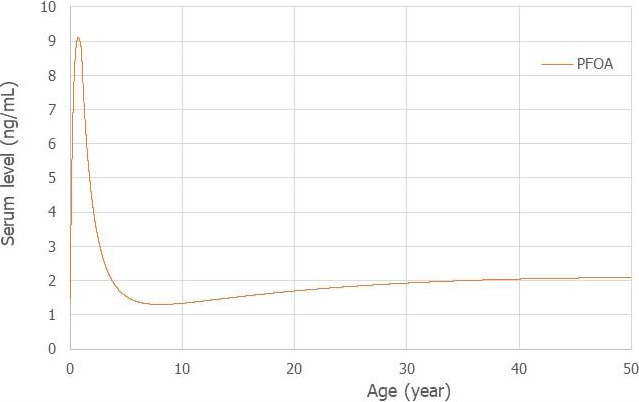
Estimated serum levels of perfluorooctanoic acid (PFOA) in a woman exposed in utero, via breastfeeding for 12 months and subsequently via food intake for 49 years to 0.187 ng/kg bw per day
- The level peaks at 9 months of breastfeeding around 9.1 ng/mL, decreases and then increases to 2.0 ng/mL at 35 years and a steady state level at 50 years around 2.1 ng/mL.
Figure 13.
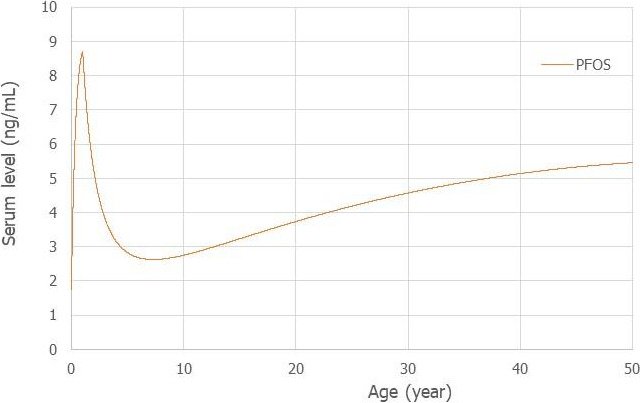
Estimated serum levels of perfluoroheptane sulfonate (PFOS) in a woman exposed in utero, via breastfeeding for 12 months and subsequently via food intake for 49 years to 0.444 ng/kg bw per day
- The level peaks at the end of breastfeeding around 8.7 ng/mL, decreases and then increases to 4.9 ng/mL at 35 years and a steady state level at 50 years around 5.5 ng/mL.
Figures 14 and 15 show the estimated serum levels in a child during the first 10 years of age, born to the mothers described above, and being exposed in utero, then breastfed for 12 months, followed by 9 years exposure via food, for either PFOA or PFOS. Exposure during the age 1 to 10 years was assumed to be double that of the mothers (for PFOA and PFOS 0.374 and 0.888 ng/kg bw per day, respectively), to compensate for the higher food per kg bw intake and resulting higher exposure (see Section 3.2.1). This resulted in serum levels at the age of 5 years of 2.3 and 4.2 ng/mL for PFOA and PFOS, respectively, or 6.5 ng/mL for the sum. This is much lower than the NOAEC of 27.0 ng/mL observed in the study from the Faroe Islands, showing that the BMDL10 from the German study corresponds to a lower intake by the mothers.
Figure 14.
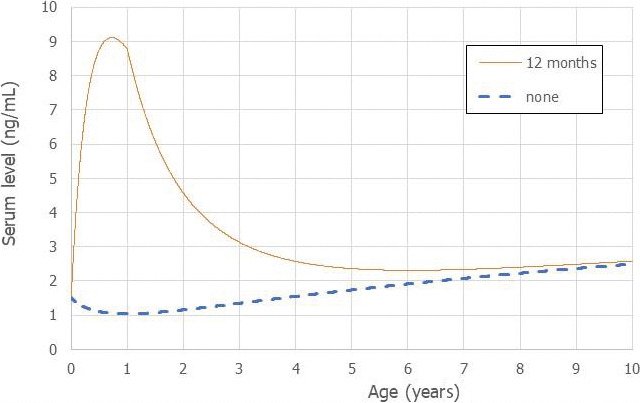
Estimated serum levels of perfluorooctanoic acid (PFOA) in a child exposed in utero, via breastfeeding for 12 months and subsequently via food intake for 9 years to 0.374 ng/kg bw per day (exposure from food twofold that of the mother)
- The level peaks at 9 months before the end of breastfeeding around 9.1 ng/mL, decreases and then reaches levels of 8.8, 2.4 and 2.6 ng/mL at, respectively, 1, 5 and 10 years of age. Estimated levels of PFOA in children who are not breastfed are also shown (none).
Figure 15.
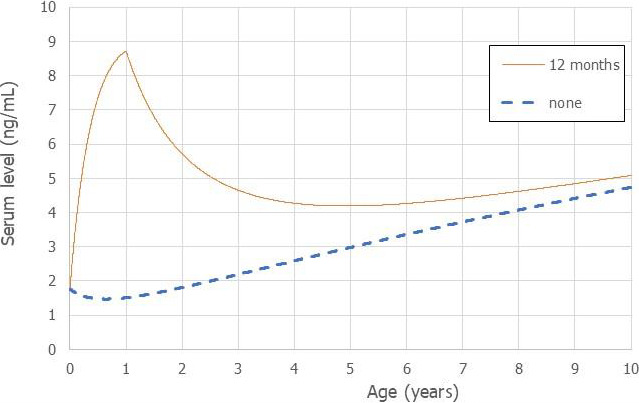
Estimated serum levels of perfluoroheptane sulfonate (PFOS) in a child exposed in utero, via breastfeeding for 12 months and subsequently via food intake for 9 years to 0.888 ng/kg bw per day (exposure from food twofold that of the mother)
- The level peaks at the end of breastfeeding around 8.7 ng/mL, decreases and then reaches levels of 4.2 and 5.1 ng/mL at, respectively, 5 and 10 years of age. Estimated levels of PFOS in children who are not breastfed are also shown (none).
Figures 14 and 15 also show the estimated serum levels for children that are not breastfed. After birth there is an initial decline in the levels followed by a gradual increase over time. This shows the impact of breastfeeding during the first years but also that at later age the serum levels are primarily determined by the intake from food.
The CONTAM Panel decided to use the daily intake of 0.63 ng/kg bw per day as the starting point for the derivation of a HBGV for the sum of PFOA, PFNA, PFHxS and PFOS.
Two animal studies (Dong et al., 2009; Peden‐Adams et al., 2008) showed effects for PFOS at rather low doses and serum levels, but also detectable serum levels of PFOS in the control groups. Due to the lack of animals with zero serum levels and the high variability in response between animals, the BMD‐modelling with PROAST did not provide a useful BMDL. In these studies, the lowest NOAEC observed for immune effects was 18 ng/mL for mice treated with PFOS (see Section 3.3.3.5.1). Using this critical serum level means that the UF of 4 for interspecies toxicokinetic differences should not be applied and only a total UF of 25 is required to consider possible inter‐ and intraspecies differences. That would result in a critical serum level of 0.72 ng/mL (18/25). This level is 24‐fold lower than the BMDL10 of 17.5 ng/mL observed in the German study. This critical serum level would correspond to a much lower daily intake than the one estimated from the human studies. It should be noted that the age of the mice in these studies corresponds more to an adult situation in humans and at this age serum levels will be 2.5‐fold lower at the TWI intake (see Figures 12 and 13). This comparison of the results from animal and human studies suggests that the application of the various uncertainty factors is too conservative and supports the use of the human data to derive a HBGV.
3.4.3.2. Mammary gland effects
The CONTAM Panel considered also the mammary gland effects as potentially adverse for humans. Three different studies imply that such effects are associated with serum levels in pups around 20 ng/mL, as measured at PND 21. It should be stressed that the critical window for this effect is unclear and that serum levels in pups vary during lactation. Therefore, it may be more appropriate to use the critical serum level in the dam, which in the three‐generation study was around 66 ng/mL (see Section 3.3.3.3.2 on mammary gland development). This is a LOAEC and a UF of 3 was applied to determine an NOAEC of 20 ng/mL in the dam. Using the critical serum level means that the UF of 4 for interspecies differences in toxicokinetic differences should not be applied and only a UF of 25 is required to consider possible inter‐ and intraspecies differences. This would result in a critical serum level in the mother of 20/25 = 0.8 ng/mL. This level is ninefold lower than the serum level of 6.9 ng/mL in mothers around the age of 35 years, corresponding to the BMDL10 of 17.5 ng/mL in 1‐year‐old children (see above). This shows that basing the assessment on the effects on mammary gland development would result in a much lower HBGV.
Based on the uncertainties on whether these effects on mammary gland development may occur in humans and the high uncertainties in extrapolation between species, the CONTAM Panel decided to use the effects on vaccination response in humans as the critical endpoint. Nevertheless, this developmental effect is of potential concern.
3.4.4. Derivation of a health based guidance value
The effects on the immune system were consistently observed in both animals and humans and support that the immune effects from PFAS exposure in humans are causally related. The critical serum level, if it had been derived from animal studies, would have corresponded to a lower HBGV than the HBGV based on human studies, when taking standard UFs into consideration. The CONTAM Panel decided to derive a Health Based Guidance Value (HBGV) based on immune effects in humans. Three studies showed a dose‐response, of which two measured serum levels in children and from the most sensitive of these the BMDL10 of 17.5 ng/mL was used to calculate the exposure corresponding with this Reference Point, being 0.63 ng/kg bw per day for the sum of the 4 PFASs (PFHxS, PFOS, PFOA and PFNA).
Since a decreased vaccination response is regarded as a risk factor for disease rather than a disease, and since the study was based on infants, which appear to be a vulnerable population group, no additional UFs for potential intraindividual differences in toxicokinetics and toxicodynamics were deemed necessary.
The CONTAM Panel established a group tolerable weekly intake (TWI) of 7 × 0.63 = 4.4 ng/kg bw per week, in order to take into account the long half‐lives of these PFASs.
This TWI should prevent that mothers reach a body burden that results in levels in milk that would lead to serum levels in the infant that are associated with effects on the immune system. In doing this, the higher exposure of breastfed infants is already taken into account in the derivation of the TWI, and the intake by infants should therefore not be compared with this TWI. Since serum levels in infants normally reach the highest levels in the population at background exposure, this TWI is thought to protect also other age groups against effects on the immune system. However, the CONTAM Panel noted that the information on older age groups is limited.
The CONTAM Panel noticed that this TWI is protective for the other potential critical endpoints (increase in serum cholesterol, reduced birth weight and high serum levels of ALT) considered in the previous Opinion on PFOS and PFOA (EFSA CONTAM Panel et al., 2018). This is based on the fact that the serum levels in adults exposed at the TWI, stay below the combined BMDLs that were derived in the previous Opinion on PFOS and PFOA.
3.5. Risk characterisation
Based on the occurrence data obtained from the Member States, the exposure to individual PFASs was estimated for different age groups across different food consumption surveys. Since it was decided to limit the risk assessment to PFOA, PFNA, PFHxS and PFOS, also the combined exposure to these four PFASs was estimated. Table 22 shows the exposure, but on a weekly basis to allow a better comparison with the TWI of 4.4 ng/kg bw per week.
Table 22.
Summary statistics of the mean and 95th percentile for the combined LB and UB chronic dietary exposure to the sum of PFOA, PFOS, PFHxS and PFNA extended to 1 week for age groups across surveys
| Age group | Range of mean dietary exposure (LB‐UB) (ng/kg bw per week) | |||||
|---|---|---|---|---|---|---|
| Mean LB dietary exposure | Mean UB dietary exposure | |||||
| Minimum | Median | Maximum | Minimum | Median | Maximum | |
| Toddlers | 10 | 21 | 46 | 428 | 519 | 785 |
| Other children | 6 | 11 | 21 | 270 | 373 | 572 |
| Adolescents | 3 | 6 | 11 | 144 | 185 | 290 |
| Adults | 4 | 6 | 9 | 95 | 112 | 154 |
| Elderly | 5 | 6 | 15 | 81 | 105 | 131 |
| Very elderly | 3 | 6 | 22 | 88 | 108 | 139 |
| Age group | Range of 95th percentile dietary exposure (LB‐UB) (ng/kg bw per week) | |||||
| 95th percentile LB dietary exposure | 95th percentile UB dietary exposure | |||||
| Minimum | Median | Maximum | Minimum | Median | Maximum | |
| Toddlers | 23 | 53 | 96 | 705 | 938 | 1,603 |
| Children | 19 | 29 | 68 | 553 | 763 | 1,157 |
| Adolescents | 9 | 15 | 37 | 309 | 399 | 626 |
| Adults | 9 | 16 | 35 | 184 | 229 | 439 |
| Elderly | 12 | 17 | 39 | 161 | 201 | 327 |
| Very elderly | 9 | 16 | 70 | 153 | 198 | 294 |
LB: lower bound; UB: upper bound; TWI: tolerable weekly intake.
Note: Infants are not shown, since a higher intake was taken into account when deriving the TWI.
For the group of adolescents, adults, elderly and very elderly, mean LB exposure varied between 3 and 22 ng/kg bw per week, as compared to P95 exposure between 9 and 70 ng/kg bw per week. UB exposure for these age groups was much higher, for the mean varying between 81 and 290 ng/kg bw per week, with P95 exposure between 153 and 294 ng/kg bw per week. The highest mean LB exposure for adolescents and adults, exceeds the TWI by a factor of 5. At the highest LB P95 exposure this is a factor 16. At the UB exposure the exceedance is much larger.
Toddlers and other children, showed in general, higher exposure, due to the higher intake of food per kg bw. The mean LB exposure varied between 6 and 46 ng/kg bw per week, as compared to P95 exposure between 19 and 96 ng/kg bw per week. UB exposure was again much higher, for the mean varying between 270 and 785 ng/kg bw per week, with P95 UB exposure between 553 and 1,603 ng/kg bw per week. In addition, the CONTAM Panel noted that the high exposure from food by toddlers is partly due to a high intake of PFNA, associated with high uncertainty, since, based on only one measured level in an important food category (see Section 3.2.1). It is difficult to evaluate the impact of the exceedance of the TWI by toddlers and other children. As shown in Section 3.4.3, a twofold higher intake than the TWI by children did not result in serum levels higher than the NOAEC derived from the Faroe Islands. It seems possible that some of the children have an exposure that could result in adverse effects on the immune response.
For infants, mean LB exposure varied between 17 and 85 ng/kg bw per week, as compared to P95 exposure between 32 and 195 ng/kg bw per week. UB exposure for these age groups was much higher, for the mean varying between 299 and 802 ng/kg bw per week, with P95 exposure between 649 and 1,574 ng/kg bw per week. The issue of the high PFNA contribution based on only one measurement also applied for infants. As mentioned above, the exposure of infants from breastfeeding was taken into account in the derivation of the TWI. For this reason, the risk for the breastfed infant should be evaluated by assessing the long‐term exceedance of the TWI by the mothers rather than comparing the exposure of infants with the TWI. To exemplify this, Figure 16 shows the daily intake of children during the first two years of life, based on breastfeeding for 12 months followed by other food. The initial intake for the sum of 4 PFASs is estimated to be 29 ng/kg bw per day, decreasing to 7.6 ng/kg bw per day at the end of the breastfeeding period. This decrease is due to the decline in the levels in milk but also the growth of the child during this period (from 3.7 to 8 kg bw). After 12 months the daily intake from food becomes 1.26 ng/kg bw per day. When expressed on a weekly base these figures are, respectively, 203, 53 and 8.8 ng/kg bw per week. So even if the mother is exposed at the TWI, in order to prevent too high serum levels in the infant, the intake by infants during the first year is much higher than the TWI. Therefore, comparing the intake of infants via breastfeeding with the TWI is not appropriate. This also applies to the relatively high exposure of infants from food, as shown in Table 6 (but omitted from Table 22 to stress this issue).
Figure 16.
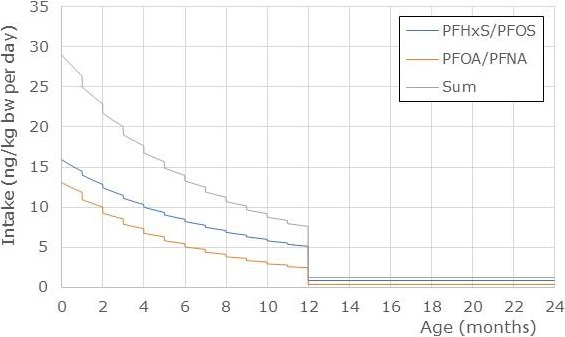
Estimated daily intake of PFOA/PFNA, PFHxS/PFOS or the sum of 4 PFASs during the first 2 years considering exposure breastfeeding for the first 12 months of life and thereafter from food
- Exposure from food during the second year was 0.374 and 0.888 ng/kg bw per day for PFOA/PFNA and PFHxS/PFOS, respectively, being twice that of the mothers. Serum levels of the mother at 35 years were 2.00 and 4.89 ng/mL for PFOA/PFNA and PFHxS/PFOS, respectively. Based on milk/serum ratios of 0.015 and 0.03, this resulted in initial milk levels of 0.073 and 0.060 ng/mL for PFOA/PFNA and PFHxS/PFOS, respectively. These levels were modelled to decline with 7.7 and 3.1% each month for PFOA/PFNA and PFHxS/PFOS, respectively.
The modelled serum level in the mothers at 35 years, corresponding to the TWI is 6.9 ng/mL (see dose‐response modelling). As shown in Tables 16 and 17 (see Section 3.3.2), biomonitoring data showed median serum levels of PFOA, PFNA, PFHxS and PFOS of, respectively, 1.9, 0.6, 0.7 and 7.7 ng/mL or combined 10.9 ng/mL. This level is higher than the level corresponding to the TWI and suggests that a major part of the adult population exceeds this level. The same conclusion could be derived from the estimated dietary intake. On the other hand, it also indicates that the LB exposure is a more accurate prediction of the exposure than the UB estimates which would lead to a much higher exceedance of the critical serum levels.
The CONTAM Panel decided to focus on 4 PFASs (PFOA, PFNA, PFHxS and PFOS). Based on the exposure assessment, these four PFASs contribute about half of the sum of the 17 PFASs that were included. This seems to contradict the biomonitoring data that show a much higher contribution of these four. However, a major part of the additional exposure came from PFBA and PFHxA, which have a shorter half‐life and accumulate much less in humans.
3.6. Uncertainty analysis
3.6.1. Uncertainty in exposure estimates
The number of occurrence data submitted to EFSA on PFASs differed in terms of both food products and compounds, with most of the samples relating to fish meat (33% of the results), and to PFOS and PFOA. Most of the food samples (~ 84%) were submitted by only three European countries (Germany, Norway and France), thus levels of PFASs in food commodities might not be representative for all of Europe. This may introduce uncertainty concerning the occurrence values used in the exposure assessment and lead to both an under‐ and overestimation of exposure.
While PFASs released from materials in contact with food may not be a significant contributor to exposure on a population basis, this may have a disproportionately large impact on some individuals. It is not known to what extent such release is covered by the occurrence database. This might lead to an underestimation of the exposure.
The LODs/LOQs of the PFAS data reported to EFSA varied between laboratories, food matrices and substances. Overall, 95% of the results were reported below LOD/LOQ, increasing the disparity between LB and UB exposure values and thus contributing to the uncertainty associated with the dietary exposure estimations (an underestimation linked to the LB value and an overestimation linked to the UB value). This varied between substances.
High LODs/LOQs did also result in a very limited set of detected data for specific compounds in some food groups, especially in cases where the number of samples available were low. This has for example resulted in ‘Food for infants and small children’ being a major contributor to the total exposure to the sum of PFOA, PFNA, PFHxS and PFOS for infants and children, based on one sample with concentrations above the LOQ for PFNA.
There are also food groups where the measured levels were below the LOQs/LODs of most other reported datasets, meaning that the mean levels used in the assessment were underestimated. This is especially relevant for widely consumed products that still contribute to the exposure despite the low levels (e.g. fruits and vegetables). As a result the exposure is underestimated.
For several food groups, it was assumed that measured levels in a subgroup are representative for the whole food group, despite the absence of data for other products (e.g. data in apples used for all fruit). This could lead to both an overestimation (other subgroups not liable to contamination) or underestimation (other subgroups more contaminated) of the exposure.
During the last fifty years different time trends have been observed for various PFASs in the environment and thus in food. This is among others due to restrictions on the use of PFOS and PFOA and at the same time increased use of short chain PFASs. This means that estimates of exposure that are based on data collected over a period of time will not necessarily reflect the current situation.
Uncertainties and limitations related to the use of the EFSA Comprehensive Food Consumption Database, as described by EFSA (EFSA, 2011a), are applicable in the present assessment and are not further described in this Scientific Opinion.
For the most prevalent PFASs in human serum, i.e. PFOA, PFNA, PFHxS and PFOS, diet has been recognised as the main source of exposure in the general population. For other PFASs, almost nothing is known on the relative contribution of various exposure routes. Also, human metabolism of precursors may contribute to internal exposure. In the current exposure assessment, neither non‐dietary exposure nor exposure to precursors have been considered, but both of these are included when biomonitoring data are used as a measure of exposure. This contributes to an underestimation of the exposure.
3.6.2. Uncertainty in biomonitoring data
Blood serum and blood plasma have been the most commonly used matrices for biomonitoring of PFASs, even though for some specific PFASs (not PFHxS, PFOS, PFOA and PFNA, but PFHxA and FOSA), whole blood may be more appropriate.
The representativeness of the biomonitoring data is affected by several factors, such as a limited amount of data for many PFASs and a non‐equal distribution of studies between the European countries. Also, the studies included samples collected over ten years. As significant time trends have been observed in human samples from several studies, the collection time points may have had an influence on the aggregated data such as mean and median concentrations.
3.6.3. Uncertainties in hazard identification and characterisation
3.6.3.1. Experimental animal data
For PFASs other than PFOS and PFOA, no or only a limited number of chronic exposure studies are available.
There is a lack of data on effects on the immune system and in particular the T‐cell directed antibody response for PFASs other than PFOS and PFOA. Moreover, the underlying immunotoxic mode of action of PFASs is unknown.
Concerning mammary gland development, there are no studies on other PFASs than PFOA. Information on the possible effects on milk production in animals affected by impaired mammary gland development after perinatal exposure is limited. This adds to the uncertainty related to this developmental endpoint.
3.6.3.2. Epidemiological studies in humans
The previous Opinion (EFSA CONTAM Panel, 2018) described in detail the uncertainties related to epidemiological studies. In general, studies performed adjustment for potential confounding factors (factors associated with both exposure to PFASs and the outcome considered) in the statistical models. Examples are age, sex, occupation and diet. There is, however, always a possibility of residual confounding not taken into account, for example certain life‐style factors associated with socioeconomic status. The possibility of confounding is discussed in the respective section on the various outcomes, especially for the potential critical effects considered, i.e. effects on the immune response.
For rare outcomes, the number of cases in the study base is usually small and therefore the statistical power to demonstrate or refute an association with PFAS exposure is usually limited. In most cases this will result in risk estimates that are non‐informative, or at least not statistically significant.
For the selected critical effect in this risk assessment, the conclusion on adversity is based on reduced antibody response, which is an effect marker of impaired immune function, while the evidence for increased risk of infections is more limited.
3.6.4. Uncertainties in dose‐response assessment and HBGV derivation
3.6.4.1. Estimation of the reference point
For the data from Abraham et al. (2020), NOAECs could be derived for the antibody titres against Hib and tetanus but not diphtheria, following grouping of the data into quintiles (see Appendix K). The NOAECs depend on the exposure level in the lowest quantile and is therefore less protective compared to the BMD approach which estimates the Reference Point relative to zero dose. In addition, EFSA guidance prescribes the use of model averaging instead of using the lowest BMDL from the individual models. BMD modelling of the data provided BMDL10 values for diphtheria and tetanus, which were very similar for the four different models. Model averaging provided BMDLs that were several‐fold lower than the lowest BMDLs from individual models and below the range of serum levels in the study population. An evaluation of the 500 bootstraps revealed that part of the runs resulted in curves that did not level off at lower serum concentrations, thereby reducing the BMDL. This is likely to be caused by the lack of data close to zero. Extrapolation well beyond the lowest observed exposure level (no zero dose) and high variability between humans are sources of uncertainty that have not been reflected on or addressed in existing EFSA guidance or experimental work. In the case of PFASs, the serum concentrations were well above zero in both critical studies, the means for the sum of the 4 PFASs in the lowest quintile being 10 and 16 ng/mL for, respectively, Abraham et al. (2020) and Grandjean et al. (2012). Furthermore, booster vaccination in children results in an increase in antibody titres that has high between‐person variability. This combined with a rather steep decrease (reduction in antibody titres > 50% for levels of the sum of 4 PFASs at the LOAECs) was considered a logical explanation for the wide BMDL/BMDU confidence intervals observed when attempting to apply existing guidance (model averaging) on these studies. Instead, the lowest BMDL of the individual models was used to provide an estimate for the Reference Point.
Overall, both the few number of data points in the critical dataset (n = 101), particularly at higher serum concentrations of PFASs, and the relatively large variability between individuals results in the (mean) dose‐response curve not being clear. This introduces uncertainty in the shape of the dose response curve as well as the location of the established Reference Point.
Similar issues as with the modelling of human data were observed with animal data on effects on the immune response. In two independent studies on effects of PFOS on the antibody response after immunisation of mice with sheep red blood cells, the BMD modelling resulted in wide BMDL/BMDU confidence intervals and extrapolation outside the range of observed PFOS serum levels.
Also in these experiments there were background PFOS levels measured in the untreated controls, i.e. there were no animals with zero serum levels in these experiments.
3.6.4.2. Mixture effects and potency factors
Most PFASs cause similar effects in experimental animals, although there are differences in the effective doses, partly due to kinetic differences. For potential critical effects identified in this Opinion including vaccination response and mammary gland development, no studies are available that compare the dose‐response curves for different PFASs and thus would allow to derive potencies for these PFASs. Furthermore, there are clear interspecies and even sex‐related differences that further complicate the derivation of potency factors. Therefore, it was decided to assume equal potencies for the four selected PFASs, which in humans share similar long half‐lives. To allow easier comparison with estimated exposure, this was based on a weight basis rather than molar basis, although the latter is likely to be scientifically more correct. Given the quite similar molecular weight of the four compounds (PFHxS = 400, PFOS = 538, PFOA = 414 and PFNA = 464) this would contribute little to the overall uncertainty in the assessment. The fact that the NOAECs are based on the sum of 4 PFASs adds to the uncertainty, since the observed associations may be more related to one of the PFASs in the mixture.
3.6.4.3. PBPK modelling
Human PBPK models for PFOA and PFOS were used to estimate exposure to PFOA/PFNA and PFOS/PFHxS for women of 35 years (child‐ bearing age) leading to a defined serum level in mothers, and to simulate PFAS concentrations in the infant at 1 year of age after 12‐months breastfeeding. This was done to calculate the intake by the mothers that would eventually result in serum levels in the 1‐year‐old infant equal to the NOAEC. Due to the lack of PBPK models for PFHxS and PFNA, the PBPK modelling was based on summing PFOA and PFNA, as well as PFHxS and PFOS and applying the models for PFOA and PFOS, respectively. Since in practice PFNA and PFHxS contribute little to the overall exposure, this will have a minor effect on the estimations.
The variation in reported half‐lives for PFOS and PFOA is a source of uncertainty, since in the respective PBPK models defined values of 5.4 and 2.3 years for PFOS and PFOA, respectively, were taken into account for developing the models. Tissue/plasma partition coefficients for PFASs for all tissues in the model were calculated from tissue concentration data derived from experimental animals rather than humans. Although validated against human studies, uncertainty remains for extrapolation from animal to human.
The models do not take into account possible physiological changes during pregnancy (bodyweight, volume of distribution), which may alter the toxicokinetics of PFASs during this period and could influence the transfer into the fetus. This also applies for the decrease in BW from the delivery and during the lactation period. Body weight is a sensitive parameter and all tissue volumes and flows are scaled to this parameter.
There is uncertainty in the modelling of plasma levels of infants, in terms of the contribution from breast milk. Data on PFOS/PFOA transfer to breast milk are available, and the average intake in mL per day is known. The milk consumption was kept constant over 1 year, i.e. the known decline in the milk volumes after 6 months was not taken into account, which could result in an overestimation of the exposure. If taken into account, it would have slowed down the decline in milk PFAS levels, so the PFAS exposure from a lower milk volume might not have been that different. Furthermore, additional exposure from other food in the breastfeeding period was not taken into account. The parameters used for the transfer to milk, based on breast milk to serum ratio in women, and decline in the milk levels are based on limited information. It was decided to apply a breastfeeding duration of 12 months, because of the current breastfeeding practice in Europe (Theurich et al., 2019) and based on the WHO recommendations to breastfeed exclusively for 6 months with continued breastfeeding along with appropriate complementary foods up to two years of age or beyond. This is supported by information on breastfeeding 6 to 12 months and beyond in a number of European countries. Using a duration of breastfeeding of less than 12 months would imply that the TWI would not be protective for women breastfeeding for periods in line with the WHO recommendations. However, applying a longer period than 12 months might be too conservative and not in line with common practice to breastfeed up to 12 months. When assuming a 6‐month breastfeeding period, corresponding daily intakes by the mothers for PFOA/PFNA, PFHxS/PFOS or the sum would be, respectively, 0.30, 0.72 and 1.02 ng/kg bw per day. This would result in serum levels in the mothers at 35 years of 3.2, 7.9 and 11.1 ng/mL, and initial milk levels of 0.10 and 0.12 ng/mL for PFOA/PFNA and PFHxS/PFOS, respectively, based on ratios of 0.03 and 0.015. These intakes and critical serum levels in mothers are higher than those estimated for 12 months of breastfeeding. With 6 months of breastfeeding the peak serum levels in the children are achieved at 6 months of age, being 13.9 and 11.8 ng/mL for PFOA/PFNA and PFHxS/PFOS, respectively, and then decrease during the next 6 months to the serum levels of 8.8 and 8.7 ng/mL corresponding to the BMDL10.
Further, there is neither information on possible differences between infants and adults regarding absorption and/or excretion of PFOS/PFOA, nor on specific kinetic parameters, such as plasma protein binding and renal clearance in infants.
In a second comparison, the PBPK models were also used to estimate the serum levels in a 35‐year‐old consumer resulting from the mean LB exposure of adults to PFOA and PFOS, as presented in table 10. For PFOA, the exposure of 0.13, 0.18 and 0.28 ng/kg bw per day (min, median, max across surveys), would result in serum levels of 1.4, 1.9 and 3.0 ng/mL. These levels are in the range observed in biomonitoring data with a median of 1.9 (min–max 0.76–4.9) ng/mL (Table 17). For PFOS, the mean exposure of 0.29, 0.58 and 0.93 ng/kg bw per day (min, median, max), would result in serum levels of 3.2, 6.4 and 10.2 ng/mL, as compared to the median of biomonitoring data being 7.7 (min–max 1.7–27.4) ng/mL (Table 16). This implies that both for PFOA and PFOS, the PBPK models correspond quite well with the serum levels in the adults.
Table 10.
Summary statistics of the mean and 95th percentile LB and UB chronic dietary exposure to 17 PFASs (ng/kg bw per day) for adults in 19 surveys across European countries
| PFAS | Range of mean dietary exposure in adults (LB–UB) (ng/kg bw per day) | |||||
|---|---|---|---|---|---|---|
| Mean LB dietary exposure | Mean UB dietary exposure | |||||
| Minimum | Median | Maximum | Minimum | Median | Maximum | |
| PFBA | 0.12 | 0.32 | 0.51 | 2.19 | 2.68 | 3.11 |
| PFPeA | 0.04 | 0.07 | 0.15 | 3.97 | 4.80 | 5.80 |
| PFHxA | 0.22 | 0.29 | 0.37 | 3.47 | 4.05 | 5.58 |
| PFHpA | 0.06 | 0.12 | 0.17 | 2.45 | 2.84 | 4.59 |
| PFOA | 0.13 | 0.18 | 0.28 | 3.60 | 4.18 | 5.71 |
| PFNA | 0.02 | 0.04 | 0.07 | 3.08 | 3.68 | 5.25 |
| PFDA | 0.02 | 0.05 | 0.11 | 2.98 | 3.52 | 5.12 |
| PFUnDA | 0.01 | 0.02 | 0.06 | 3.32 | 3.76 | 5.46 |
| PFDoDA | 0.01 | 0.02 | 0.04 | 2.72 | 3.25 | 4.97 |
| PFTrDA | < 0.01 | 0.01 | 0.04 | 0.48 | 0.66 | 0.85 |
| PFTeDA | < 0.01 | < 0.01 | 0.01 | 0.44 | 0.53 | 0.70 |
| PFBS | 0.03 | 0.03 | 0.04 | 3.46 | 4.04 | 5.68 |
| PFHxS | 0.06 | 0.08 | 0.11 | 2.86 | 3.45 | 5.06 |
| PFHpS | < 0.01 | < 0.01 | < 0.01 | 0.62 | 0.76 | 0.94 |
| PFOS | 0.29 | 0.58 | 0.93 | 3.82 | 4.47 | 5.94 |
| PFDS | < 0.01 | < 0.01 | < 0.01 | 0.51 | 0.67 | 0.91 |
| FOSA | 0.01 | 0.04 | 0.33 | 7.86 | 9.26 | 11.64 |
| PFAS | Range of 95th percentile dietary exposure in adults (LB–UB) (ng/kg bw per day) | |||||
| 95th percentile LB dietary exposure | 95th percentile UB dietary exposure | |||||
| Minimum | Median | Maximum | Minimum | Median | Maximum | |
| PFBA | 0.48 | 0.86 | 1.29 | 3.52 | 4.46 | 5.30 |
| PFPeA | 0.10 | 0.21 | 0.88 | 7.51 | 9.32 | 12.09 |
| PFHxA | 0.43 | 0.61 | 0.77 | 6.31 | 8.02 | 15.78 |
| PFHpA | 0.14 | 0.25 | 0.30 | 4.16 | 6.12 | 14.70 |
| PFOA | 0.32 | 0.40 | 0.59 | 6.76 | 8.37 | 15.92 |
| PFNA | 0.06 | 0.10 | 0.18 | 5.80 | 7.46 | 15.42 |
| PFDA | 0.06 | 0.13 | 0.32 | 5.64 | 7.19 | 15.27 |
| PFUnDA | 0.04 | 0.08 | 0.30 | 5.62 | 7.26 | 15.76 |
| PFDoDA | 0.03 | 0.05 | 0.17 | 4.95 | 6.72 | 15.10 |
| PFTrDA | 0.02 | 0.05 | 0.18 | 0.93 | 1.25 | 1.81 |
| PFTeDA | < 0.01 | 0.01 | 0.06 | 0.75 | 0.93 | 1.50 |
| PFBS | 0.05 | 0.07 | 0.09 | 6.19 | 8.10 | 15.88 |
| PFHxS | 0.15 | 0.18 | 0.24 | 5.36 | 7.13 | 15.22 |
| PFHpS | < 0.01 | < 0.01 | < 0.01 | 1.11 | 1.33 | 1.78 |
| PFOS | 0.84 | 1.71 | 4.79 | 7.53 | 9.31 | 16.31 |
| PFDS | < 0.01 | < 0.01 | < 0.01 | 1.09 | 1.37 | 2.00 |
| FOSA | 0.02 | 0.13 | 2.30 | 13.12 | 16.03 | 22.98 |
3.6.5. Summary of Uncertainties
In Table 23, a summary of the uncertainty evaluation is presented, highlighting the main sources of uncertainty and indicating an estimate of whether the respective source of uncertainty might have led to an over‐ or underestimation of the exposure or the resulting risk.
Table 23.
Summary of qualitative evaluation of the impact of uncertainties on the risk assessment of PFASs in food
| Sources of uncertainty | Direction |
|---|---|
| Extrapolation of occurrence data few Member States to whole of Europe | +/–a |
| Limited occurrence data for some PFASs and several food groups | +/– |
| Large proportion of left‐censored data in the final dataset | +/– |
| Using the substitution method at the lower bound (LB) and upper bound (UB) scenario | +/– |
| Including data over the last 10 years while levels in food and humans are declining | + |
| Non‐dietary exposure and contribution from precursors | – |
| Residual confounding, e.g. from life‐style factors in human epi studies | +/– |
| Limited data on PFASs other than PFOS and PFOA | +/– |
| Evidence for an association between PFASs exposure and increased risk of infections is limited | + |
| The derivation of Reference Point | +/– |
| PBPK modelling | +/– |
| Lack of information on the relative potencies of PFASs/ mixture approach | +/– |
| Variability in vaccination response | +/– |
PFAS: perfluoroalkyl substance; PFOA: perfluorooctanoic acid; PFOS: perfluoroheptane sulfonate.
+ = uncertainty with potential to cause over‐estimation of exposure/risk; – = uncertainty with potential to cause under‐estimation of exposure/risk.
The CONTAM Panel has used LB exposures for the risk characterisation as it considers that the UB exposure assessment results in a large overestimation, also based on comparison of the critical serum level in mothers with the data from biomonitoring (see Section 3.5 risk characterisation). Therefore, the risk characterisation is better based on the LB exposure, although there are also issues with levels based on too few quantified results, so that not all contributing food groups may me covered in the exposure assessment.
Overall, the CONTAM Panel considered that the impact of the uncertainties on the risk assessment for the sum of PFOA, PFNA, PFHxS and PFOS is high.
4. Conclusions
4.1. Occurrence/Exposure
An initial number of 97,434 results for food samples analysed for PFASs from 16 European countries were available for the assessment. The final cleaned dataset contained 69,433 analytical results for 26 PFASs with 92% of left‐censored (LC) data (results below LOD/LOQ).
The exposure assessment was limited to 17 PFASs (PFBA, PFPeA, PFHxA, PFHpA, PFOA, PFNA, PFDA, PFUnDA, PFDoDA, PFTrDA, PFTeDA, PFBS, PFHxS, PFHpS, PFOS, PFDS and FOSA). For this 67,839 analytical data were available. Most data were on PFOS (n = 8,498), PFOA (n = 8,197), PFDA (n = 5,770), PFNA (n = 5,594), PFHxA (n = 5,448) and PFHxS (n = 4,745).
For most food categories and PFASs, calculated mean UB levels were much higher than LB levels. Due to high proportions of results below LOD/LOQ and/or only limited availability of data for all PFASs, the exposure calculations should be considered only as a rough indication of the range of chronic dietary exposure, and should thus be interpreted with caution. The CONTAM Panel concluded that the calculated LB exposure is likely to be more realistic than UB exposure.
The CONTAM Panel decided to limit the risk assessment to the sum of PFOA, PFNA, PFHxS and PFOS. For this sum, the mean exposure in adolescents, adults, elderly and very elderly ranged from 0.42 to 3.1 ng/kg bw per day at the LB. Toddlers and other children had approximately two‐fold higher mean intake than older age groups (adolescents, adults, elderly, very elderly), ranging from 0.84 to 6.5 ng/kg bw per day at the LB. In infants the mean exposure ranges were at the LB 2.4 to 12.2 ng/kg bw per day. The 95th percentile exposures ranged across surveys and age groups at the LB from 1.3 (adults) to 27.9 (infants) ng/kg bw per day.
The four PFASs contributed in adults approximately 46% to the sum of all PFASs for which the exposure was calculated. Other PFASs that contributed more than 5% to this sum were PFBA (16%) and PFHxA (15%), which have short half‐lives in humans.
For the combined exposure to PFOA, PFNA, PFHxS and PFOS, the main contributing categories were ‘Fish meat’ and ‘Fruit and fruit products’ and ‘Eggs and egg products’ for all population groups.
Diet is the major source of PFAS exposure for most of the population, but other routes such as dust ingestion and indoor air inhalation may also contribute substantially.
4.2. Hazard identification and characterisation
4.2.1. Toxicokinetics
Many of the 27 PFASs considered in this Opinion are shown to be readily absorbed through the gastrointestinal tract in mammals including humans; then they distribute to the plasma and other parts of the body and depending on the specific PFAS, tend to accumulate in the liver. They are excreted in both urine and faeces.
Neither PFCAs nor PFSAs are metabolised by animals or humans, whereas precursors such as fluorotelomer alcohols (FTOHs) and polyfluoroalkyl phosphate esters (PAPs) are biotransformed to several metabolites, including PFCAs.
Routes and rates of elimination of PFASs vary according to the chemical end‐group, the chain length and the species. In rats, but not in humans, sex differences were observed in toxicokinetics.
In rodents, half‐lives vary from a few hours to several weeks and are in general much shorter than in humans.
In humans, the estimated half‐lives for short‐chain PFASs (such as PFBA, PFBS and PFHxA) were found to range from a few days to approximately one month whereas for compounds having a long perfluorinated carbon chain length (such as PFOA, PFNA, PFDA, PFHxS or PFOS), it can be several years.
The long elimination half‐lives of PFASs mainly originate from their interactions with various transporters involved in the reabsorption processes occurring at the hepatic, intestinal and renal level.
The maternal transfer of PFASs to offspring occurs both prenatally (in utero) and postnatally (via breastfeeding).
4.2.2. Transfer to plants and animals
PFASs are transferred from soil to plants. In general, transfer rates are higher for the short chain PFASs and decrease from roots to leaves to fruits.
In food producing animals, PFASs transfer from feed to animal derived food like milk, eggs and meat, with clear differences between species and the type of PFAS. This is also relevant for soil ingestion by foraging farm animals.
4.2.3. Biomonitoring
Serum and plasma are suitable matrices for monitoring the exposure to PFASs.
Generally, after the year 2000, the concentrations in serum/plasma of PFOS, PFOA and in some studies PFHxS have decreased, while the concentrations of PFNA, PFDA and PFUnDA have increased. No clear trends have been reported for the remaining PFASs.
Summary statistics was performed on median levels from the various studies published from 2007 to 2018. Median values for the study medians were determined, referred to as median concentrations.
The most prominent PFAS for adults was PFOS (64%), followed by PFOA (16%), PFHxS (5.6%) and PFNA (5.1%). For children, PFOS and PFOA contributed almost the same with 35.0% and 36.6% of the total, followed by PFNA (8.8%) and PFHxS (6.7%).
For adults the median concentrations in serum or plasma were 7.7, 1.9, 0.67, 0.61, 0.30 and 0.28 ng/mL for PFOS, PFOA, PFHxS, PFNA, PFDA and PFUnDA, respectively, while the concentrations of the remaining PFASs were below 0.25 ng/mL.
For children the median concentrations in plasma were 3.2, 3.3, 0.79, 0.60 and 0.30 ng/mL for PFOS, PFOA, PFNA, PFHxS and PFDA, respectively, while the concentrations of the remaining PFASs were below 0.25 ng/mL.
Considerably higher concentrations have been observed for some individuals, including both occupationally exposed adults, and children and adults, which have experienced elevated exposure from, e.g. contaminated drinking water. In these cases, the relative abundance of the various PFASs may deviate considerably from what is observed in general populations.
4.2.4. Toxicity in experimental animals
Repeated dose exposure
For most PFASs, repeated dose toxicity studies were identified, the most consistent endpoint was increased liver weight. This was seen for all PFASs studied, but with clear differences in relative potencies. Disturbances in lipid metabolism, including hepatocellular steatosis as well as hepatotoxic effects were evident mostly at higher dose levels.
Many PFASs decreased the levels of thyroid hormones (both T4 and T3).
For some PFASs, increased relative kidney weight, alterations of the mucosa in the nasal cavity and olfactory epithelium were observed.
Developmental toxicity
The most sensitive developmental effect observed was impaired development of mammary gland in mice, after exposure late in gestation or via lactation. This effect persisted beyond sexual maturity. Only PFOA has been investigated in relation to this outcome, with a maternal LOAEC around 66 ng/mL serum.
Developmental toxicity studies in rodents were identified for most of the PFASs. The effects most often observed were increased fetal and/or neonatal mortality and reduction in fetal weight and/or postnatal growth. In general, developmental toxicity occurred at similar or slightly lower doses than those inducing maternal toxicity. LOAELs and NOAELs were orders of magnitude higher than those for developmental effects on mammary glands seen with PFOA.
Reproductive toxicity
Effects on male reproductive parameters, including atrophy of the testicular interstitium, accompanied by reduced serum testosterone levels and epidydimal and testicular weights have been reported for PFNA and PFDA. Degenerative changes and spermatid retention in seminiferous tubules were also observed.
Neurotoxicity
PFOS and PFOA exert developmental neurotoxic effects in rodents at doses of 0.1–0.3 mg/kg bw per day or higher. Studies with PFDA, PFHxS and PFDoDA indicate developmental neurotoxic effects.
Immunotoxicity
PFOS and PFOA have been shown to cause a reduced response to vaccination (T‐cell dependent antibody response) and PFOS also caused a reduced resistance to infection. Effects were noted at doses where no overt toxicity was evident.
Whereas effects on the immune system have also been observed for other PFASs, i.e. PFNA and PFDA, the available data base for these compounds is more limited and does not include vaccination response.
The CONTAM Panel concluded that the immune system is a prime target of PFASs.
Genotoxicity
For PFOS and PFOA no evidence for a direct genotoxic mode of action was identified.
For PFASs other than PFOS and PFOA, the number of studies and data are limited. However, structural similarity for PFHxS and PFOS, as well as for PFNA and PFOA, indicates that also for these PFASs a direct genotoxic mode of action is unlikely.
Long‐term toxicity and carcinogenicity
Available studies indicate that PFOS and PFOA are tumour promoters in rodent liver and that PFOA also induces Leydig cell tumours in rats.
The only long‐term study available for PFHxA provides no evidence for carcinogenicity. PFNA and PFDA promoted liver tumour formation in a trout two‐stage model of hepatocarcinogenesis, while 8:2 FTOH failed to do so.
4.2.5. Human observations
Immune outcomes
Epidemiological studies published since the publication of the previous EFSA CONTAM Panel Opinion on PFOS and PFOA, provide further support for the conclusion that PFOS and PFOA are associated with reduced antibody response to vaccination, observed in several studies.
The evidence for other PFASs is weaker, related to the mixture of PFASs in the blood, the lower concentrations compared to PFOS and PFOA, and the small number of studies.
Studies with children on the Faroe Islands showed various associations between the serum levels of individual PFASs but also the sum of PFOA, PFNA, PFHxS and PFOS and antibody titres against diphtheria and tetanus. An NOAEC of 27.0 ng/mL was identified for the association between the sum of these four PFASs at 5 years of age and the antibody titres against diphtheria at 7 years.
The CONTAM Panel identified a new study with children from Germany showing an inverse association between serum levels of PFOA, but also the sum of PFOA, PFNA, PFHxS and PFOS, and antibody titres against haemophilus influenzae type b (HiB), diphtheria and tetanus in serum sampled from 1‐year-old children predominantly breastfed. A BMDL10 of 17.5 ng/mL at the age of 1 year was derived for the sum of PFOA, PFNA, PFHxS and PFOS. This study supports the results from the study on the Faroe Islands.
Some of the studies suggest that serum levels of PFOS and PFOA are associated with increased propensity for infection.
Epidemiological studies provide insufficient evidence to conclude on associations between exposure to PFASs and asthma and allergies.
Metabolic outcomes
Epidemiological studies provide clear evidence for an association between exposure to PFOS, PFOA and PFNA and increased serum levels of cholesterol.
Epidemiological studies provide evidence for an association between exposure to PFASs and increased serum levels of the liver enzyme alanine transferase (ALT). The magnitude of the associations was small, however, and few studies found associations with ALT outside the reference range. There were no associations with liver disease.
There is insufficient evidence for associations with diabetes, obesity and metabolic syndrome.
Fertility and Pregnancy outcomes
Studies on PFOS and PFOA published since 2018 confirm previous conclusions from the PFOS and PFOA Opinion (EFSA CONTAM Panel, 2018) that ‘there may well be a causal association between PFOS and PFOA and birth weight’.
Maternal serum levels in studies reporting results on other PFASs were generally much lower and those studies provide no evidence for an adverse association for other PFASs and birth weight.
Epidemiological studies show no associations between other PFASs and fertility and reproductive outcomes in both males and females.
Other potential effects in humans
As for PFOS and PFOA, epidemiological studies provide no evidence for associations between other PFASs and fertility and reproductive outcomes in both males and females.
Epidemiological studies provide no evidence for associations between exposure to PFASs and neurodevelopmental outcomes, growth in infancy or childhood, neurobehavioural, neuropsychiatric, cognitive outcomes or thyroid function.
Epidemiological studies provide insufficient support for carcinogenicity of PFOS and PFOA in humans. This conclusion applies to studies conducted both in occupationally exposed individuals and in the general population. Limited information was identified for other PFASs.
Epidemiological studies provide insufficient evidence to conclude on associations between exposure to PFASs and increased risk of cardiovascular disease.
Epidemiological studies provide insufficient evidence for associations between exposure to PFASs and changes in kidney function or serum levels of uric acid, as well as low bone mineral density or osteoporosis.
4.2.6. Mode of action
Like peroxisome proliferators, PFASs seem to cause liver hyperplasia in rodents via activation of PPARα.
PFASs induce steatosis in rodent hepatocytes in a PPARα‐independent way. Hepatocellular steatosis appears to be causally related to the occurrence of necrotic hepatocytes and the increased serum transaminase levels. Thorough knowledge of the mode of action causing steatosis is missing.
There is no clear mode of action behind the strong decrease observed in thyroid hormone levels (T4, T3) in rodents exposed to PFASs, which is normally not accompanied by increased TSH levels. PFASs compete with T4 for binding to transthyretin and can induce glucuronosyltransferase activity in the liver, but it is unclear if this is behind the decrease in hormone levels observed in experimental animals.
A mode of action of immunotoxicity by PFASs has not been established. Data from in vivo and in vitro studies on PFOS and PFOA suggests that immunotoxic effects may originate from modulation of PPARs, NF‐κB regulated gene transactivation and/or regulation of apoptosis.
The MOA behind the impaired mammary gland development in mice dosed with PFOA is unknown.
4.3. Critical effects, dose response assessment, derivation of HBGV
In human studies, various associations between serum levels and a number of outcomes have been reported. In the previous Opinion (EFSA CONTAM Panel, 2018), four endpoints were selected as potential critical effects for PFOS and/or PFOA. These were (i) increased serum total and LDL cholesterol (risk factor for cardiovascular disease), (ii) increased ALT levels (indicating effects on liver cells), (iii) reduced birth weight, and (iv) effects on the immune system, as shown by decreased antibody response to vaccines.
The CONTAM Panel concluded that effects on the immune system, which were observed at the lowest serum PFAS levels in both animals and humans, is the critical effect. The findings were considered robust since in animals they were consistently observed for the two studied PFASs (PFOA, PFOS) and also in humans.
The CONTAM Panel noted that this is not the case for effects on mammary gland development, which are observed at similar low serum levels in mice but have not been studied in other animal models or humans.
Therefore, the CONTAM Panel decided to base the present assessment on PFASs on effects on the immune system.
Based on observations on animals and humans, the CONTAM Panel decided to combine its assessment on the sum of four PFASs, i.e. PFOA, PFNA, PFHxS and PFOS. At present, these are the PFASs that contribute most to the levels observed in human serum.
In humans, these four PFASs share toxicokinetic properties and show similar accumulation and long half‐lives. Also in terms of effects, these compounds in general show the same effects when studied in animals.
Current data do not allow the derivation of potency factors. As a pragmatic approach, the CONTAM Panel assumed by default equal potencies for effects of these four PFASs on immune outcomes.
The CONTAM Panel identified an NOAEC serum level at the age of 5 years for the sum of PFOA, PFNA, PFHxS and PFOS of 27.0 ng/mL, based on decreased diphtheria antibody titres at the age of 7 years, from the Faroe Islands study.
From the study from Germany, a lowest BMDL10 of 17.5 ng/mL at the age of 1 year was derived for the sum of PFOA, PFNA, PFHxS, PFOS, based on the inverse association between serum levels of the sum of these 4 PFASs and antibody titres against diphtheria.
The BMDL10 of 17.5 ng/mL corresponds to a lower intake by the child and thus the mother in her life up to pregnancy, since PFAS serum levels in breastfed children are in general higher at 1 year of age than at 5 years. Therefore, this BMDL10 was used to estimate the daily intake by mothers that would result in this critical serum concentration at 1 year of age in breastfed children. This daily intake was subsequently used to derive an HBGV for the sum of PFOA, PFNA, PFHxS and PFOS.
Using a PBPK model, and assuming 12 months of breastfeeding, it was estimated that the BMDL10 in infants corresponds to an intake by the mother of 0.63 ng/kg bw per day for the sum of the four PFASs. Such intake would result in a serum level in the mother at 35 years of age of 6.9 ng/mL.
The CONTAM Panel decided to use the daily intake of 0.63 ng/kg bw per day as the starting point and established a group tolerable weekly intake (TWI) of 7 × 0.63 = 4.4 ng/kg bw per week for the sum of PFOA, PFNA, PFHxS and PFOS.
Since a decreased vaccination response is regarded as a risk factor for disease rather than a disease, and since the study was based on infants, which appear to be a vulnerable population group, no additional UFs for potential intraindividual differences in toxicokinetics and toxicodynamics were deemed necessary.
This TWI should prevent that mothers reach a body burden that results in levels in milk that would lead to serum levels in the infant, associated with decreases in vaccination response. As a result, the higher exposure of breastfed infants is taken into account in the derivation of the TWI and the intake by infants should therefore not be compared with this TWI.
The CONTAM Panel noted that this TWI is protective for the other potential critical endpoints (increase in serum cholesterol, reduced birth weight and high serum levels of ALT) considered in the previous Opinion on PFOS and PFOA (EFSA CONTAM Panel, 2018).
4.4. Risk Characterisation
Based on the occurrence data obtained from the Member States, the exposure to individual PFASs was estimated for different age groups across different food consumption surveys. Since it was decided to limit the risk assessment to PFOA, PFNA, PFHxS and PFOS, also the combined exposure to these four PFASs was estimated.
The CONTAM Panel noted that several factors contribute to the high uncertainty of the present exposure assessment. Large differences between LB and UB concentrations were observed in foods, as a result of analytical methods being used that are not sufficiently sensitive. This results in a large difference between maximum UB and minimum LB chronic dietary exposure estimates for PFASs.
The CONTAM Panel considers that the exposure levels for PFOA, PFNA, PFHxS and PFOS are closer to the LB than the UB values.
Regarding the combined exposure of the four PFASs, toddlers and other children showed in general, higher exposure, due to the higher intake of food per kg bw. The mean LB exposure varied between 6 and 46 ng/kg bw per week. The high (P95) LB exposure ranged from 19 and 96 ng/kg bw per week. For toddlers, the high exposure from food is influenced by a high contribution from PFNA and associated with high uncertainty.
For the group of adolescents, adults, elderly and very elderly, mean LB exposure varied between 3 and 22 ng/kg bw per week, as compared to high (P95) LB exposure between 9 and 70 ng/kg bw per week. The highest mean LB exposure for adolescents and adults exceeds the TWI by a factor of 5. At the highest LB P95 exposure this is a factor of 16.
These exceedances of the TWI at LB exposure estimates indicate a health concern.
5. Recommendations
For individual PFASs more sensitive analytical methods with high levels of quality control (to avoid matrix effects or impact of background contamination) are needed in order to reduce uncertainty in the dietary exposure assessment.
Occurrence data are needed for all PFASs found in the environment and in a broad range of widely consumed food products.
For the determination of the total amount of PFASs, sensitive and accurate methods, which facilitate determination in samples of food and drinks are needed.
Exposure assessment should be frequently updated especially when analytical data obtained from more sensitive methods become available.
Additional studies on the relative contribution of sources other than food are needed, especially for PFASs which are present in the highest concentrations in indoor air and house dust, such as n:2 FTOHs and PAPs.
More studies on the effect of cooking and food processing, in particular in relation to transfer to food from food contact materials that contain PFASs, are needed.
More information is needed on the transfer of PFASs along the food chain.
Additional studies on paired human samples are needed to identify the relevant matrices for biomonitoring of various PFASs.
Studies on effects of other PFASs, and in particular PFOS, PFNA and PFHxS, on mammary gland development should be conducted.
Studies on effects of other PFASs, and in particular PFNA and PFHxS on the immune system should be conducted.
Studies for the critical effects that allow for a derivation of potency factors for PFASs should be conducted.
Studies to characterise the mode of action of immunotoxicity and mammary gland development of PFASs should be performed.
The effects of PFASs on thyroid hormone levels and potential consequences for neurodevelopment should be further investigated.
More longitudinal epidemiological studies are needed on human endpoints, in particular prospective vaccination studies covering more varied types of vaccines, different populations, as well as more studies on other immune outcomes in humans, including risk of infections.
Epidemiological studies should include the sum of several PFASs.
Experimental evidence is needed to understand and quantify the association between PFASs and blood lipids, for example the importance of enterohepatic circulation.
PBPK models for PFASs should be further optimised.
Abbreviations
- ACOX
acyl‐CoA oxidase
- AD
Alzheimer's disease
- ADHD
attention deficit hyperactivity disorder
- ADME
absorption, distribution, metabolism and excretion
- AFB1
aflatoxin B1
- AFFF
Aqueous Film Forming Foam
- ALP
Alkaline phosphatase
- ALT
Alanine aminotransferase
- APFO
ammonium perfluorooctanoate
- AST
aspartate aminotransferase
- AT
Austria
- ATSDR
Agency for Toxic Substances and Disease Registry
- BAF
Bioaccumulation factor
- BE
Belgium
- BfR
Federal Institute for Risk Assessment in Germany
- BMD
Benchmark dose
- BMDL10
Benchmark dose for a 10% increase
- BMDU
Benchmark dose upper confidence limit
- BMR
Benchmark response
- br‐ PFOA/PFOS
Branched PFOA/PFOS
- BSA
bovine serum albumin
- BUN
blood urea nitrogen
- bw
Body weight
- CA
Canada
- CAR
constitutive activated/androstane receptor
- CHMS
Canadian Health Measures Survey
- CHO
Chinese hamster ovary
- CI
Confidence interval
- CIMT
carotid artery intima media thickness
- CKD
chronic kidney disease
- CS
Cross‐sectional study
- CSF
cerebrospinal fluid
- CY
Cyprus
- CZ
The Czech Republic
- DATA Unit
EFSA former EFSA Dietary and Chemical Monitoring Unit
- DCFDA
dichlorofluorescin diacetate
- DCFH‐DA
dichlorodihydrofluorescein diacetate
- DE
Germany
- DK
Denmark
- dw
Dry weight
- DXA
dual‐energy x‐ray absorptiometry
- eGFR
estimated glomerular filtration rate
- EPA
Environmental Protection Agency
- ES
Spain
- ESI
electrospray ionisation
- EtFASAs
N‐ethyl perfluoroalkane sulfonamides
- EtFASEs
N‐ethylperfluoroalkane sulfonamidoethanols
- EtFOSE
N‐ethyl perfluorooctane sulfonamido ethanol
- f
female
- FASAs
perfluoroalkane sulfonamides
- FAT
fatty acid translocase
- FSH
follicle stimulating hormone
- FSANZ
Food Standards Australia New Zealand
- FTOHs
fluoroteleomer alcohols
- GC
gas chromatography
- GD
Gestation day
- GDM
gestational diabetes
- GFR
glomerular filtration rate
- GGT
gamma‐glutamyl transferase
- GL
Greenland
- GM
Geometric mean
- GPx
glutathione peroxidase
- GSH
glutathione
- GSSG
oxidized glutathione
- HA
Health Advisory
- HBM
Human Biomonitoring
- HC
High consumer
- HDL
high‐density lipoproteins
- HOMA‐IR
Homeostasis Model Assessment – Insulin resistance
- IARC
International Agency for Research on Cancer
- IC50
half maximal inhibitory concentration
- IgG
Immunoglobulin G
- IgM
Immunoglobulin M
- IGT
impaired glucose tolerance
- IL
interleukin
- i.p.
intraperitoneal
- IPE
ion‐pair extraction
- IQR
Interquartile range
- i.v.
intravenous
- Ka
association constant
- KLH
keyhole limpet haemocyanin
- KR
Korea (South)
- L
Longitudinal study
- LB
Lower bound
- LC
Left‐censored
- LC‐MS/MS
LC coupled to quadrupole tandem mass spectrometry
- LDH
Lactate dehydrogenase
- L‐FABP
liver fatty acids binding protein
- LH
luteinizing hormone
- Li
labelling index
- LOAEC
Lowest‐observed‐adverse‐effect‐concentration
- LOAEL
Lowest‐observed‐adverse‐effect‐level
- LOD
limit of detection
- LOQ
limit of quantification
- LPS
Lipopolysaccharide
- m
male
- MAC
maximum acceptable concentration
- MDA
malondialdehyde
- MoA
Mode of action
- MRI
magnetic resonance imaging
- MS
mass spectrometry
- MT
Malta
- N/A
Not applicable
- NAFLD
non‐alcoholic fatty liver disease
- NASH
non‐alcoholic steatohepatitis
- NC
Non consumer
- NK
natural killer (cell)
- NO
Norway
- NOAEC
no‐observed‐adverse‐effect‐concentration
- NOAEL
no‐observed‐adverse‐effect‐level
- n‐PFOA/PFOS
Linear PFOA/PFOS
- NR
Not reported
- Nrf2
nuclear factor erythroid 2‐related factor 2
- NTP
National Toxicology Program
- OAT
organic anion transport protein
- Oatp
organic anion‐transporting polypeptide
- OR
Odds ratio
- PAD
peripheral artery disease
- PAPs
polyfluoroalkyl phosphoric acid esters
- PBPK
Physiologically‐based pharmacokinetic (model)
- PCBs
polychlorinated biphenyls
- PCNA
proliferating cell nuclear antigen
- PD
postnatal day
- PFASs
perfluoroalkyl substances
- PFBS
perfluorobutane sulfonic acid
- PFCAs
perfluoroalkyl carboxylic acids
- PFDA
perfluorodecanoic acid
- PFDoDA
Perfluorododecanoic acid
- PFHpA
perfluoroheptanoic acid
- PFHxA
perfluorohexanoic acid
- PFHxS
perfluorohexane sulfonic acid
- PFNA
perfluorononanoic acid
- PFOA
Perfluorooctanoic acid
- PFOS
perfluorooctane sulfonic acid
- PFOSF
perfluorooctane sulfonyl fluoride
- PFSAs
perfluoroalkyl sulfonic acids
- PFUnDA
perfluoroundecanoic acid
- PK
pharmacokinetic
- PO
Poland
- POD
point of departure
- POP
persistent organic pollutant
- POSF
perfluorooctane sulfonyl fluoride
- PPAR
peroxisome proliferator‐activated receptors
- PTFE
Polytetrafluoroetane
- Q
quartile
- RCR
risk characterisation ratio
- REACH
Registration, Evaluation, Authorisation and Restriction of Chemicals
- ROS
Reactive oxygen species
- SD
Standard deviation
- SE
Sweden
- SGA
small for gestational age
- SHE
Syrian hamster emrbyo
- SI
Slovenia
- SOD
superoxide dismutase
- SOP
standard operational procedure
- SRBC
sheep red blood cells
- S‐UA
serum uric acid
- SVHC
Substances of Very High Concern
- T
tertile
- T3
triiodothyronine
- T4
thyroxine
- TAD
total administered dose
- TBG
thyroxin binding globulin
- TC
Total cholesterol
- TDI
tolerable daily intake
- TDS
Total Diet Study
- TG
triglycerides
- TH
tyrosine hydroxylase
- TL
telomerisation
- TNF
Tumour necrosis factor
- TNP
tri‐nitrophenyl
- TPOab
thyroid peroxidase antibodies
- TSH
thyroid stimulating hormone
- TTR
transthyretin
- TW
Taiwan
- UA
Ukraine
- UB
Upper bound
- UF
uncertainty factor
- UK
The United Kingdom
- URAT
urate transporter
- US
United States
- VLDL
very low density lipoprotein
- WG
working group
- WHO
World Health Organization
- ww
Wet weight
Appendix A – Levels of PFOS, PFOA, PFNA and PFHxS in different species of fish, arranged according to the LB level for PFOS
1.
| Fish species | PFOS | PFOA | PFNA | PFHxS | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | %LC | LB | UB | N | %LC | LB | UB | N | %LC | LB | UB | N | %LC | LB | UB | |
| Carp (Cyprinus) | 145 | 14% | 14.12 | 14.21 | 149 | 32% | 4.10 | 4.33 | 125 | 65% | 0.84 | 1.47 | 126 | 97% | 0.07 | 1.01 |
| Eels (Apodes) | 164 | 35% | 9.23 | 9.44 | 177 | 96% | 0.07 | 0.68 | 54 | 91% | 0.98 | 1.66 | 58 | 98% | 0.02 | 0.73 |
| Roach (Rutilus) | 8 | 13% | 8.05 | 8.18 | 10 | 100% | 0.00 | 1.00 | 10 | 100% | 0.00 | 1.00 | 10 | 100% | 0.00 | 1.00 |
| Perch (Perca) | 47 | 31% | 6.08 | 6.31 | 49 | 99% | 0.04 | 0.45 | 17 | 100% | 0.00 | 0.95 | 15 | 100% | 0.00 | 0.88 |
| Bream (Charax) | 41 | 49% | 6.03 | 6.29 | 45 | 100% | 0.00 | 0.55 | 16 | 100% | 0.00 | 0.94 | 16 | 100% | 0.00 | 0.94 |
| Barbel (Barbus) | 13 | 8% | 5.16 | 5.24 | 14 | 100% | 0.00 | 0.56 | 5 | 100% | 0.01 | 0.60 | 5 | 100% | 0.00 | 0.51 |
| Sardine and pilchard (Sardina) | 14 | 0% | 4.73 | 4.73 | 28 | 64% | 0.10 | 0.37 | 14 | 57% | 0.08 | 0.53 | 14 | 64% | 0.01 | 0.45 |
| Sea catfish and wolf‐fish (Anarhichas) | 20 | 70% | 3.04 | 3.46 | 16 | 94% | 0.11 | 0.80 | 13 | 100% | 0.00 | 0.79 | 13 | 100% | 0.00 | 0.73 |
| Plaice (Pleuronectes) | 39 | 46% | 2.95 | 3.29 | 39 | 97% | 0.08 | 0.72 | 28 | 100% | 0.00 | 0.85 | 5 | 100% | 0.00 | 0.51 |
| Whitefish (Coregonus) | 18 | 23% | 1.52 | 1.62 | 18 | 100% | 0.00 | 0.29 | 1 | 100% | 0.01 | 0.60 | – | – | – | – |
| ‐Bass (Marone) | 6 | 67% | 1.40 | 1.74 | 6 | 100% | 0.00 | 0.44 | 3 | 100% | 0.01 | 0.60 | 3 | 100% | 0.00 | 0.51 |
| Grey mullet (Mugil) | 13 | 8% | 0.93 | 0.97 | 18 | 11% | 0.17 | 0.20 | 8 | 25% | 0.15 | 0.15 | 8 | 38% | 0.02 | 0.04 |
| Sprat (Sprattus sprattus) | 51 | 12% | 0.86 | 0.92 | 56 | 84% | 0.05 | 0.35 | 58 | 84% | 0.04 | 0.33 | 58 | 84% | 0.01 | 0.30 |
| Shrimps (Crangon crangon) | 39 | 25% | 0.74 | 0.76 | 38 | 76% | 0.02 | 0.09 | 34 | 93% | 0.02 | 0.12 | 19 | 100% | 0.00 | 0.09 |
| Norway lobster (Nephrophs norvegicus) | 2 | 50% | 0.74 | 0.76 | 2 | 100% | 0.02 | 0.10 | 2 | 100% | 0.03 | 0.13 | 2 | 100% | 0.00 | 0.10 |
| Crayfish (Astacus spp.) | 2 | 100% | 0.74 | 0.76 | 2 | 100% | 0.02 | 0.10 | 1 | 100% | 0.03 | 0.13 | 1 | 100% | 0.00 | 0.10 |
| Flounder (Platichthys flesus) | 16 | 50% | 0.72 | 1.04 | 17 | 100% | 0.00 | 0.52 | 7 | 100% | 0.00 | 0.74 | 1 | 100% | 0.00 | 0.51 |
| Sole (Limanda; Solea) | 15 | 67% | 0.70 | 1.08 | 16 | 88% | 0.13 | 0.55 | 11 | 82% | 0.02 | 0.63 | 3 | 100% | 0.00 | 0.51 |
| Crab (Cancer spp.) | 16 | 44% | 0.69 | 0.93 | 13 | 46% | 0.38 | 0.54 | 16 | 50% | 0.35 | 0.50 | 20 | 85% | 0.30 | 0.78 |
| Char (Salvelinus) | 3 | 100% | 0.58 | 0.98 | 1 | 100% | 0.12 | 0.53 | 3 | 100% | 0.01 | 0.60 | 2 | 100% | 0.00 | 0.51 |
| Lophiiformes (Pediculati) | 4 | 50% | 0.58 | 0.98 | 7 | 100% | 0.00 | 0.31 | 7 | 100% | 0.00 | 0.36 | 3 | 100% | 0.00 | 0.51 |
| Rays (Hypotremata) | 2 | 50% | 0.58 | 0.98 | 2 | 100% | 0.12 | 0.53 | 2 | 100% | 0.01 | 0.60 | 1 | 100% | 0.00 | 0.51 |
| Bonito (Sarda Sarda) | 1 | 0% | 0.58 | 0.98 | 1 | 100% | 0.12 | 0.53 | – | – | – | – | – | – | – | – |
| ‐Cod and whiting (Gadus spp.) | 174 | 67% | 0.47 | 1.05 | 145 | 93% | 0.01 | 0.74 | 130 | 92% | 0.02 | 0.78 | 27 | 100% | 0.00 | 0.53 |
| Mackeral (Scomber) | 125 | 79% | 0.36 | 0.93 | 136 | 81% | 0.31 | 0.88 | 129 | 96% | 0.00 | 0.74 | 122 | 99% | 0.00 | 0.74 |
| Prawns (Palaemon serratus) | 8 | 38% | 0.33 | 0.50 | 9 | 33% | 0.20 | 0.42 | 2 | 100% | 0.03 | 0.13 | 2 | 100% | 0.00 | 0.10 |
| Herring (Clupea) | 288 | 74% | 0.32 | 0.62 | 290 | 96% | 0.02 | 0.38 | 243 | 90% | 0.02 | 0.38 | 237 | 99% | 0.00 | 0.38 |
| Salmon and trout (Salmo spp.) | 574 | 88% | 0.31 | 0.83 | 521 | 95% | 0.13 | 0.63 | 522 | 100% | 0.00 | 0.70 | 365 | 100% | 0.00 | 0.63 |
| Hake (Merluccius) | 32 | 11% | 0.27 | 0.31 | 35 | 93% | 0.06 | 0.12 | 19 | 97% | 0.00 | 0.07 | 15 | 100% | 0.00 | 0.03 |
| Halibut (Hippoglossus spp.) | 487 | 71% | 0.26 | 0.81 | 106 | 99% | 0.00 | 0.30 | 487 | 100% | 0.00 | 0.77 | 487 | 100% | 0.00 | 0.69 |
| Tuna (Thunsnus) | 21 | 39% | 0.16 | 0.26 | 34 | 100% | 0.00 | 0.12 | 17 | 100% | 0.00 | 0.13 | 17 | 100% | 0.00 | 0.11 |
| Mussel (Mytilus edulis) | 55 | 21% | 0.08 | 0.17 | 58 | 100% | 0.00 | 0.14 | 53 | 100% | 0.00 | 0.15 | 33 | 100% | 0.00 | 0.08 |
| Water molluscs | 10 | 60% | 0.06 | 0.35 | 10 | 40% | 0.01 | 0.33 | 9 | 44% | 0.00 | 0.34 | 9 | 100% | 0.00 | 0.31 |
| Squid (Loligo vulgaris) | 4 | 100% | 0.06 | 0.35 | 4 | 100% | 0.01 | 0.33 | – | – | – | – | – | – | – | – |
| ‐Cuttlefish (Sepia officinalis) | 2 | 0% | 0.06 | 0.35 | 2 | 50% | 0.01 | 0.33 | 1 | 100% | 0.00 | 0.34 | 1 | 100% | 0.00 | 0.31 |
| Cockle (Cardium edule) | 1 | 100% | 0.06 | 0.35 | 1 | 100% | 0.01 | 0.33 | 1 | 100% | 0.00 | 0.34 | – | – | – | – |
| ‐Scallop (Pecten spp.) | 19 | 83% | 0.01 | 0.18 | 19 | 66% | 0.01 | 0.18 | 19 | 100% | 0.00 | 0.18 | 19 | 100% | 0.00 | 0.16 |
| Oyster (Ostrea edulis) | 36 | 93% | 0.00 | 0.77 | 37 | 100% | 0.00 | 0.78 | 37 | 100% | 0.00 | 0.78 | 33 | 100% | 0.00 | 0.78 |
| Queen scallop (Chlamys opercularis) | 9 | 100% | 0.00 | 0.05 | 9 | 100% | 0.00 | 0.05 | 9 | 100% | 0.00 | 0.06 | 9 | 100% | 0.00 | 0.05 |
–: No data provided to EFSA; LB: lower bound: LC: left‐censored; N: number of samples, UB: upper bound; PFOS: perfluoroheptane sulfonate; PFOA: perfluorooctanoic acid; PFNA: perfluorononanoic acid; PFHxS: perfluorohexane sulfonic acid.
Appendix B – Biomonitoring
1.
Appendix C– Toxicokinetics in Experimental Animals
1.
During the previous decades, most of the information on the fate of PFASs was based on PFOS and PFOA (EFSA, 2008; EFSA CONTAM Panel, 2018). These compounds have been shown to be readily absorbed in the gastrointestinal tract in mammals, including humans, and to distribute to the plasma and other parts of the body and depending on the specific PFAS, tend to accumulate in the liver. PFOS and PFOA are not metabolised and are excreted in both urine and faeces. They may be subject to extensive enterohepatic recirculation. For PFOS, the serum elimination half‐lives in rats and mice were slightly higher than one month, whereas in rabbits and monkeys, the serum elimination half‐life was 3–4 months. Significant sex differences in the elimination of PFOA are observed in some species such as rats, for which half‐lives may vary from few hours (in females) to several days (in males). Differences in biological half‐lives between species for both PFOS and PFOA and between sexes for PFOA are mainly due to differences in renal clearance. For both PFOS and PFOA, maternal transfer occurs prenatally to the fetus through placental transfer and postnatally through the consumption of maternal milk.
C.1. PFCAs
In the past 10 years, a significant amount of data were published on the toxicokinetics of shorter chain PFCAs such as PFBA, PFHxA and PFHpA, as well as on longer chain perfluorinated compounds, including PFNA, PFDA, PFUnDA, PFDoDA, PFTrDA and PFTeDA. Oral exposure of experimental animals to PFCAs having a perfluorinated carbon chain length of 3–11 was shown to result in an estimated absorption fraction greater than 95% of the administered dose (ATSDR, 2018). None of the experimental studies observed the formation of metabolites, suggesting, as previously reported for PFOA (EFSA CONTAM Panel, 2018), that the biotransformation of PFCAs is unlikely in mammals, irrespective of their chain length. As it is known that transport proteins play a key role in PFCA elimination, in particular in renal tubular reabsorption, information on this process is given at the end of Section C.1.
No data were identified regarding the toxicokinetics of PFPeA, PFPeDA, PFHxDA and PFODA.
Regarding PFOA, some papers and reports published recently (Gomis et al., 2018; NTP, 2019a; Pizzuro et al., 2019) confirm that this PFAS is well absorbed following oral exposure (> 90%) is not biotransformed by xenobiotic metabolising enzymes and is preferentially distributed to the liver, in addition to serum and other tissues in most species. The NTP study showed that in male Sprague‐Dawley rats administered once daily by gavage, for 28 days to PFOA doses from 0.625 to 10 mg/kg bw per day, the liver/plasma ratios ranged from 0.87 to 1.07 (NTP, 2019a). Although the ratio was not provided for females, the plasma concentrations found in males were 2 or 3 levels of magnitude higher than in females exposed to similar doses. The reasons for such sex differences in tissue distribution are unclear, but could be related to longer half‐lives of PFOA in male vs. female rats (Pizzurro et al., 2019). The half‐life of PFOA in male and female Sprague‐Dawley rats was found to range from 3 to 14 h in females and from 8 to 12 days in males (Dzierlenga et al., 2019, cited by NTP, 2019a). Table C.2 summarises selected TK parameters for PFOA.
The toxicokinetics of PFBA was investigated in male and female Sprague‐Dawley rats given either single oral doses by gavage (3, 10, 30, 100, 300 mg/kg bw) or a single i.v. dose (30 mg/kg bw) of ammonium PFBA (Chang et al., 2008). Gastrointestinal absorption was estimated to be rapid (mean Tmax ranging from 0.63 h in females to 1.25 h in males for the 30 mg/kg bw dose) and almost complete in both sexes (similar Cmax values obtained after oral or i.v. dose). For male rats, 24 h after oral dosing, mean liver concentrations ranged from 22% to 27% of the mean serum levels, depending on the dose. Values in females were not reported and concentrations in other tissues were not measured. Clearance for the 30 mg/kg bw oral dose (corresponding to 140 μmol/kg bw) varied from 444 mL/kg per day in males to 1,718 mL/kg per day in females and mean serum elimination half‐life was 9.2 h in males and 1.8 h in females (Table C.3). PFBA was mainly excreted in urine ranging from 51% to 64% of the administered dose (for doses ranging from 3 to 100 mg/kg bw) in males at 24 h and representing 100% of the administered dose in females. Within the first 24 h, faecal excretion was between 0.1% and 3% in males. There was no evidence indicating that PFBA was metabolised in rats. A similar study was carried out in CD1 mice by the same authors (Chang et al., 2008). Male and female animals were given by gavage single oral doses of ammonium PFBA (10, 30, 100, 300 mg/kg bw). At the 30 mg/kg bw dose (corresponding to 140 μmol/kg bw), clearance varied from 254 mL/kg per day in males to 835 mL/kg per day in females and the serum elimination half‐life value was 16.3 and 3.1 h in males and females, respectively (Table C.3). The percent of dose recovered in urine of female mice after 24 h (ranging from 65% to 68%) was approximately twice that recovered in urine of male mice. A small proportion of the ingested dose of PFBA (4–11%) was found in faeces at 24 h, suggesting incomplete absorption and/or biliary elimination. PFBA toxicokinetics was also investigated in male and female cynomolgus monkeys given a single i.v. dose (10 mg/kg bw) (Chang et al., 2008). In both males and females, the clearance was approximately 1,700 mL/kg per day, whereas elimination in urine at 24 h was 42% and 36% of the administered dose, respectively. The serum elimination half‐lives were similar in males and females (approximately 40 h, Table C.2).
Butenhoff et al. (2012) carried out 28‐day and 90‐day studies in male and female Sprague‐Dawley rats exposed by gavage to ammonium PFBA at doses up to 150 and 30 mg/kg bw per day, respectively. Each study also included recovery groups that were sacrificed 3 weeks after the end of the dosing period. Serum and liver concentrations of PFBA were measured at the end of the treatment and recovery periods. At the end of both 28‐day and 90‐day dosing periods, males and females had mean serum PFBA concentrations that increased with dose. For the 30 mg/kg bw per day dose, concentrations measured in the serum of males were approximately 38 and 52 μg/mL at the end of the 28‐day and 90‐day dosing periods, respectively. These values decreased to approximately 0.2 and 0.5 μg/mL at the end of the 3 weeks recovery periods, respectively. At the same dose, in females, mean serum PFBA concentrations at the end of the exposure period ranged from 1.7 μg/mL (28 day) to 5.2 μg/mL (90 day), and concentrations at the end of recovery were approximately 2–4% of those at the end of the dosing period. PFBA levels were also measured in the liver. In males exposed during 28 and 90 days at a dose of 30 mg/kg bw, hepatic concentrations were 17.4 and 16.1 μg/g, respectively, whereas females had mean concentrations of 0.4 and 0.9 μg/g, respectively. At the end of the recovery period, concentrations of PFBA in the liver were close to or below the LOQ (0.050 μg/g).
Chengelis et al. (2009) investigated the toxicokinetic parameters of PFHxA in rats and cynomolgus monkeys. In a first experiment, male and female Sprague‐Dawley rats were given a single iv dose of 10 mg PFHxA/kg bw (corresponding to 31.8 μmol/kg bw). The mean half‐life of PFHxA in serum was shorter for female than for male rats (0.4 h compared to 1.0 h, Table C.3). In a second experiment, male and female Sprague‐Dawley rats were given daily by gavage during 26 days 50, 150, or 300 mg PFHxA/kg bw (corresponding to 159.2, 477.7, 955.4 μmol/kg bw). No significant differences were observed between the mean serum concentrations of PFHxA measured 24 h after the first oral dose compared to the concentrations measured 24 h after the last dose. The half‐life of PFHxA in serum was about 2–3 h regardless of dosage level, sex or number of doses (Table C.3. Approximately 90% of the administered daily dose of PFHxA was recovered in the urine of male rats during 24 h post‐dosing; in the same conditions, urinary elimination in female rats was about 80% of the administered dose. There were no significant sex differences in the toxicokinetics of PFHxA in monkeys following a single iv dose of 10 mg/kg bw (Table C.3). The average half‐life in serum ranged from 2.4 to 5.3 h.
The toxicokinetics of sodium 14C‐PFHxA was investigated in rats and mice following a single dose (2 or 100 mg/kg bw by gavage) or after 14 consecutive doses (2 mg/kg unlabelled PFHxA by gavage) followed by a single oral dose of 14C‐PFHxA (Gannon et al., 2011). Absorption was almost complete and rapid (T max between 15 and 30 min after dosing in both species) and bioavailability was nearly 100% at both levels in both species. In rats, the plasma elimination half‐life was slightly longer in males (1.5–1.7 h) than in females (0.5–0.7 h). Half‐lives were not calculated in mice. In all tissues except skin, PFHxA was not quantifiable 24 h after dosing in both sexes of the two species. The primary route of elimination was via urine (approximately 99% of the dose) in male and female rats and mice, irrespective of the administered dose. The route and extent of elimination was unchanged after 14 days of daily dosing. No metabolites were observed in plasma, urine or faeces samples. The absence of biotransformation was confirmed in vitro (incubation of 14C‐PFHxA with rat and mouse hepatocytes) in the same study (Gannon et al., 2011).
The rapid and extensive absorption of PFHxA in rodents as well as its efficient elimination via urine were confirmed by Iwai (2011) in a study carried out in rats and mice in which animals of both sexes were orally administered single or daily doses (50 mg/kg bw) of ammonium 14C‐PFHxA.
Based on the data from Gannon et al. (2011), Russell et al. (2013) estimated the plasma clearance of PFHxA at 1957 and 6,654 mL/kg per day for male and female rats, respectively.
In a paper dealing with the toxicokinetics of PFHxS in different species, Sundström et al. (2012) reported, as unpublished data, half‐life values of PFHxA in monkeys (1.5 and 0.8 days for males and females, respectively).
Fujii et al. (2015) investigated the toxicokinetics of eight PFCAs with six (C6) to 14 (C14) carbon atoms, in male and female FVB/NJcl mice, administered by iv injection (0.31 μmol/kg bw, single dose, corresponding to 0.1 mg/kg bw) or by gavage (3.13 μmol/kg bw, single dose, corresponding to 1 mg/kg bw), each PFAS separately. After gavage administration or iv injection, PFHxA was not detected in the serum at any sampling time (LOD = 0.2 nmol/g).
Iwabuchi et al. (2017) administered PFHxA to male rats either as a single dose (gavage, 100 μg/kg bw) or in drinking water (1, 5 or 25 μg/L) for 1 or 3 months. PFHxA was very rapidly and completely absorbed, distributed and eliminated from the body with a half‐life ranging from 2 to 4 h (Table C.3). No tissue accumulation was observed in the chronic study and the steady state was reached within a day.
More recently, PFHxA was evaluated in male and female Sprague‐Dawley rats administered by gavage doses of 62.6, 125, 250, 500 and 1,000 mg/kg bw per day for 28 days (NTP, 2019a). At the end of the experiment, males generally had a higher (1.6‐ to 3‐fold) plasma concentration compared to females. The liver/plasma ratios, calculated in males only, were less than 1 (0.5 or lower) for animals treated with the three highest doses (hepatic concentrations > LOQ).
Kudo et al. (2001) investigated the elimination of different PFCAs in rats. In a first experiment, male and female Wistar rats were administered a single i.p. dose (20 mg/kg bw) of PFHpA and urine and faeces were collected up to 5 days. In a second experiment, rats were bile duct cannulated prior to administration of a single i.v. dose (25 mg/kg bw) of PFHpA. Bile samples were collected up to 5 h after the injection. PFHpA was rapidly eliminated in urine, > 90% of the dose within 120 min in both males and females. In contrast, faecal elimination was limited (< 2% for both sexes). Part of faecal elimination was due to biliary excretion of PFHpA, with a significantly faster biliary elimination rate in females compared to males.
The toxicokinetics of several PFCAs, including among others PFHpA, were studied by Ohmori et al. (2003) in male and female Wistar rats administered with a single i.v. dose (48.63 μmol/kg bw, corresponding to approximately 25 mg/kg bw) of the perfluorinated substance. Half‐lives in male and female rats were calculated to be 0.10 and 0.05 days, respectively; total clearances were 1,604 and 3,070 mL/kg per day, respectively, and distribution volumes were approximately 200 mL/kg in both sexes (Table C.3).
The toxicokinetics of PFHpA was investigated in male and female mice by Fujii et al. (2015, see above). Estimated gastrointestinal absorption was > 94% of the orally administered dose (3.13 μmol/kg, corresponding to approximately 1.14 mg/kg bw) in both sexes. In males, 24 h post‐dosing via gavage, PFHpA was not quantifiable in the sampled tissues (serum, liver, kidney, brain, adipose tissue), whereas in females, 1.8% and 0.2% of the administered dose were found in liver and kidney, respectively. The major route of elimination was via urine (approximately 46% of the dose at 24 h) whereas faecal excretion represented less than 8% of the dose during the same period for both sexes. In animals exposed to PFHpA via gavage, total clearances were found to be 293 and 190 mL/kg per day in males and females, respectively (Table C.3).
After intraperitoneal injection of PFNA (20 mg/kg bw) in Wistar rats (Kudo et al., 2001, see above), elimination in urine within 5 days post‐dosing amounted to 2.0% and 52% of the dose in males and females, respectively; during the same period, faecal elimination was < 5% of the dose in males and < 2% of the dose in females. Biliary excretion was investigated in male and female rats injected intravenously with PFNA at a dose of 25 mg/kg bw and was found to occur at a higher extent in females compared to males (approximately 0.4% of the dose eliminated within 5 h after injection in females compared to less than 0.1% in males), suggesting a more efficient re‐absorption mechanism of biliary‐excreted PFNA in females. At the end of the experiment, the concentrations of PFNA for males were approximately 45 μg/mL and 90 μg/g in serum and liver, respectively; in females, these levels were approximately 18 and 8 times lower, respectively.
Toxicokinetic parameters of PFNA injected intravenously to Wistar rats at a dose of 48 μmol/kg bw (corresponding to 22.3 mg/kg bw) were reported by Ohmori et al. (2003). Half‐lives in male and female rats were calculated to be 29.5 and 2.44 days, respectively (Table C.3). A significant sex‐related difference was observed for total clearance (6.9 and 105.7 mL/kg per day, in males and females, respectively) and this disparity was mainly due to a significantly lower renal clearance in males compared to females. Protein binding, estimated in vitro using plasma protein from male and female rats, was over 98%.
The toxicokinetics of PFNA was investigated in Sprague‐Dawley rats and CD‐1 mice by Tatum‐Gibbs et al. (2011). Male and female rats were given a single dose of PFNA by oral gavage at 1, 3 or 10 mg/kg bw, and blood was collected for analysis at 1, 2, 3, 4, 7, 16, 21, 28, 35, 42 and 50 days after treatment. In addition, PFNA concentrations in liver and kidney were measured at the end of the experiment. For the 10 mg/kg dose, the Cmax was 89.8 and 68.4 μg/mL in males and females, respectively. For the 3 mg/kg bw dose, corresponding to 6.47 μmol/kg bw, an average estimated half‐life of 23.6 and 32.0 days for males and females, respectively, was reported (Table C.3). PFNA was found to be stored preferentially in the liver. Male and female CD‐1 mice were given a single oral dose (1 or 10 mg/kg, corresponding to 2.16 or 21.6 μmol/kg bw) of PFNA, and animals were killed at time intervals similar to the rat experiment; blood, liver and kidney were collected. In the mouse, the rates of PFNA serum elimination were slightly faster in females than males, with estimated serum half‐life of 25.7–68.8 days and 34.4–228 days, respectively, depending on the dose (Table C.3). PFNA was found to be stored preferentially in the mouse liver. A significantly higher hepatic retention of PFNA was observed in male mice than in females. The liver/serum concentration ratios ranged from 5 to 15, while kidney/serum ratios typically range between 0.2 and 0.4.
The toxicokinetics of PFNA was also investigated by Fujii et al. (2015) using male and female FVB/NJcl mice as models (see PFHxA subsection for experimental details). Gastrointestinal absorption was found to be complete in both sexes. After iv injection, total clearance was 3.9 mL/kg per day in males and 5.1 mL/kg per day in females, whereas after gavage administration, the values were 4.0 and 2.4 mL/kg per day, respectively (Table C.3). The distribution volumes reported for mice injected with PFNA were 220 and 150 mL/kg in males and females, respectively. Twenty‐four hours after iv injection, a limited portion of the administered dose was eliminated in urine (1.3% for males and 2.2% for females) and even less was excreted in faeces (< 1% for both sexes). The majority of the dose was retained in serum (27% of the dose for males and 32% for females) and liver (69% for males and 46% for females). For males and females treated by gavage, urinary elimination and faecal excretion were close to, or below 1% of the administered dose and the distribution pattern was similar to that found in animals exposed by iv injection.
Iwabuchi et al. (2017) administered PFNA to male rats either as a single dose (gavage, 50 μg/kg bw) or in drinking water (1, 5 or 25 μg/L) for 1 or 3 months. After a 3‐month exposure period, PFNA was found to accumulate mainly in the liver: at the highest dose tested (25 μg/L), the concentration in this organ was 2.4 mg/kg. The estimated plasmatic half‐life ranged from 18 to 64 days (Table C.3), but for the liver, the average estimated half‐life was 160 days.
In a 28‐day study, Sprague‐Dawley rats were given repeated oral gavage doses of PFNA ranging from 0.625 to 10 mg/kg bw per day in males and from 1.56 to 25 mg/kg bw per day in females. At similar doses, PFNA plasma concentrations were generally five‐ to ninefold higher in males compared to females. The liver/plasma ratios (calculated in males only) ranged from 0.9 to 2.6 (NTP, 2019a).
Kim et al. (2019) investigated the tissue distribution and excretion of PFNA in rats i.v. administered PFNA at a dose of 3 mg/kg bw. PFNA distributed preferentially in the liver and the kidney. The liver distribution of PFNA in male rats was about 2.5 times higher than that in female rats. In males, the cumulative excretion of PFNA in urine and faeces was 14.33 ± 9.30% and 1.28 ± 0.45% of the dose, respectively, whereas corresponding values in females were 34.56 ± 2.21% and 3.13 ± 2.18%, respectively, showing that urine is the major excretion route of PFNA in rats and that elimination occurs at a higher extent in females than in males. Based on the i.v. study and using a one‐compartment model, the serum elimination half‐lives in male and female rats were estimated to be 40.2 and 4.4 days, respectively, and the clearances were 7.4 and 16.6 mL/kg per day, respectively (Table C.3).
Disposition of PFAS was investigated in rats by Kudo et al. (2001, see above). In males and females, the concentrations of PFDA in the serum and the liver at the end of the experiment were approximately 37 μg/mL and 130 μg/g, respectively.
Ohmori et al. (2003) investigated the toxicokinetics of PFDA in rats (see above experimental conditions for PFHpA). Half‐lives in male and female rats were found to be 40 and 59 days, respectively; distribution volumes were 348 and 441 mL/kg, respectively, and total clearances were approximately 5 mL/kg per day in both sexes (Table C.3).
Toxicokinetic parameters of PFDA in mice were also reported by Fujii et al. (2015, see PFHxA subsection for experimental details). Gastrointestinal absorption was found to be almost 100% of the administered dose for both sexes. After iv injection, total clearance was 2.2 mL/kg per day in males and 2.8 mL/kg per day in females, whereas after gavage administration, the values were 3.9 and 2.2 mL/kg per day, respectively (Table C.3). The distribution volumes reported for mice injected with PFNA were 250 and 200 mL/kg in males and females, respectively. Irrespective of the route of administration and of the sex, 0–24 h urinary and faecal excretions were close to, or below 1% of the dose and most of the administered PFDA was retained in the liver.
Male and female Sprague‐Dawley rats were given for 28 days oral gavage doses of PFDA ranging from 0.156 to 2.5 mg/kg bw per day. PFDA plasma concentrations were slightly higher (30% or less) in females compared to males. The liver/plasma ratios (calculated in males only) decreased with dose from 5.3 to 1.6 (NTP, 2019a). The toxicokinetics of PFDA were recently examined in male and female rats by Kim et al. (2019). After i.v. administration of PFDA to rats at a dose of 1 mg/kg bw, PFDA was mainly distributed to the liver, followed by the kidney, with slightly higher values in males compared to females. The cumulative excretion of PFDA in urine and faeces was 11.22 ± 2.96% and 18.25 ± 2.72% in male rats, respectively, and 22.17 ± 5.28% and 16.44 ± 0.70% in female rats, respectively. Based on the i.v. study and using a one‐compartment model, the serum elimination half‐lives in male and female rats was estimated to be 109 and 50 days, respectively, and the clearances were 0.76 and 0.81 mL/kg per day, respectively (Table C.3).
The toxicokinetics of PFUnDA, PFDoDA, PFTrDA and PFTeDA were studied by Fujii et al. (2015) in mice (see experimental details in PFHxA subsection). The gastrointestinal absorption of these perfluorinated compounds having between 11 and 14 perfluorinated carbon atoms was at or near 100% for both males and females. The analyses of the tissues and organs 24 h after i.v. injection or gavage administration showed that most of the perfluorinated compounds were distributed to the liver and, to a lesser extent to the serum in both males and females. For C11–C14 compounds, urinary elimination during the 0–24 h period was ≤ 0.1% of the dose, irrespective of the route of administration and of the sex. During the same period, faecal excretion was approximately 1% of the dose for C11 to C14 injected intravenously. When administration occurred by gavage, faecal excretion was slightly higher than via i.v. for PFTrDA (1.7–3.1% of the dose, depending on the sex) and PFTeDA (3.0–6.1% of the dose, depending on the sex). Most of the C11–C14 compounds were retained in the liver (64–78% for males, 47–53% for females). After i.v. injection, total clearance varied from 2.8 mL/kg per day for PFUnDA to 10.4 mL/kg per day for PFTeDA, whereas after gavage administration, the values were from 3.1 mL/kg per day for PFUnDA to 106.3 mL/kg per day for PFTeDA (Table C.3). When the total clearances of gavage‐ and i.v.‐administered mice are compared, disparities exist in the long‐chain perfluroalkyl carboxylic acids (C13 and C14), suggesting for these compounds that bile is an important elimination route.
There were no marked differences between sexes. The distribution volumes reported for mice injected with PFUnDA, PFDoDA, PFTrDA and PFTeDA, varied from 280 to 430 mL/kg in males and from 330 to 580 mL/kg in females.
Interactions with binding proteins, including carriers and transporters
Interactions with proteins, namely serum albumin, liver fatty acid binding proteins (L‐FABP), and organic anion transporters influence the toxicokinetics of PFASs.
Although several authors investigated binding of PFASs (mainly PFCAs) to albumin, few studies tested a series of perfluorinated compounds using the same methodology. Using in vitro approaches, PFNA and PFDA were found to bind to bovine serum albumin (BSA) at levels greater than 99% and 80%, respectively (Bischel et al., 2010; Vanden Heuvel et al., 1992). The association constants are on the order of 105M−1 for PFNA, PFDA and PFUnDA (Bischel et al., 2010; MacManus‐Spencer et al., 2010). Bischel et al. (2011) investigated the associations, at low ligand concentrations, of PFBA, PFPA, PFHxA, PFHpA, PFOA, PFNA, PFDA, PFUnDA and PFDoDA with BSA. All the PFCAs tested were highly bound (> 95%) to BSA, except PFDoDA (80%). Affinity of PFCAs for BSA, estimated on the basis of the protein‐water distribution coefficient (K PW) increases with PFCAs hydrophobicity, but decreases from 8 to 11 perfluorinated carbons, probably due to steric hindrances associated with longer and more rigid perfluoroalkyl chains. Log K PW ranges from 3.3 to 4.3. Additional observations at pH‐induced changes in binding affinity support evidence that short‐ and long‐chain PFCAs bind at different locations on BSA.
Liver fatty acid binding protein (L‐FABP) is a lipid‐binding protein highly expressed in the liver as well as in the intestine and the kidney. L‐FABP appears to have a higher affinity for PFCAs than albumin. Using purified recombinant rat liver L‐FABP, Woodcroft et al. (2010) tested its interaction with PFCAs having perfluorinated carbons chain length of 4–8. They found an increasing affinity from PFPA to PFNA. Additional data on interaction of PFCAs with L‐FABP are presented in Section 3.3.1.22.
In rat as in human, PFCA renal active excretion and reabsorption are mediated through an organic anion transport system (Table C.1). The roles of the five rat renal organic anion transporters (OAT1, OAT2, OAT3, URAT1 and OATP1A1) in transporting PFCAs with different chain lengths (C2–C18) were investigated by Weaver et al. (2010). OAT1 and OAT3 reside in the basolateral membrane of the proximal tubular cells, and would facilitate PFCA renal tubular secretion. In contrast, due to their expression in the apical membrane of the proximal tubular cells, OATP1A1, OAT2, OAT4 and URAT1 would be the transporters involved in PFCA renal tubular reabsorption (Han et al., 2012). Inhibition of uptake of model substrates was measured for the different anion transporters. PFHxA, PFHpA and PFOA inhibited OAT1‐mediated p‐aminohippurate transport, with PFHpA being the strongest inhibitor. PFOA and PFNA were the strongest inhibitors for OAT3‐mediated estrone‐3‐sulfate transport, while OATP1A1‐mediated estradiol‐17β‐glucuronide uptake was inhibited by PFNA, PFDA and PFUnDA, with PFDA giving the strongest inhibition. No strong inhibitors were found for OAT2 or URAT1. Kinetic analysis was performed for the strongest inhibitors. OAT1 transported PFHpA with a Km value of 50.5 μM, OAT3 transported PFNA with a Km value 174.5 μM. OATP1A1‐mediated transport yielded Km values of 20.5 (PFNA) and 28.5 μM (PFDA). These data suggest that OAT1 and OAT3 are involved in renal secretion of PFHpA and PFNA, whereas OATP1A1 can contribute to the renal reabsorption of PFNA and PFDA.
C.2. PFSAs
PFOS, the most investigated PFSA, is known to be well absorbed in the gastrointestinal tract, to be resistant to biotransformation and to be mainly retained in liver and plasma (EFSA, 2008; EFSA CONTAM Panel, 2018). These properties are confirmed in articles and reports published recently (Chou and Lin, 2019, Gomis et al., 2018; Huang et al., 2019a; NTP, 2019b; Pizzuro et al., 2019). The NTP study showed that in male and female Sprague‐Dawley rats administered once daily by gavage, for 28 days, to PFOS doses from 0.312 to 5 mg/kg bw per day, plasma concentrations were similar in males and females and the liver/plasma ratios (measured in males only) ranged from 2.7 to 3.8 (NTP, 2019b). After a single gavage administration of 2 mg/kg PFOS to male and female Sprague‐Dawley rats, Huang and co‐workers found a liver/plasma ratio of 3–4 in females, whereas in males, the ratio increased from ca 5 (1 day post‐dose) to 30 (140 days post‐dose); a half‐life of about 38 days was calculated in both sexes (Huang et al., 2019a). Table C.2 summarises selected TK parameters for PFOS.
To date, only limited data were published on the toxicokinetics of PFSAs other than PFOS. PFBS and PFHxS were investigated in rodents and cynomolgus monkeys, but no data were identified regarding the toxicokinetics of PFHpS and PFDS. Based on animal experiments, the gastrointestinal absorption of investigated PFSAs is estimated between 50% of the dose for PFBS to almost 100% for PFHxS (ATSDR, 2018). As previously reported for PFOS, there are no indications that PFSAs are metabolised.
A series of studies was undertaken by Olsen et al. (2009) to evaluate the toxicokinetics of PFBS in rats and monkeys. Male and female Sprague‐Dawley rats were given a single dose of 30 mg potassium PFBS/kg bw by either i.v. injection or oral gavage. In male and female rats dosed orally, mean Tmax values were 0.4 and 0.3 h, respectively, suggesting a rapid gastrointestinal absorption of PFBS. The mean serum PFBS concentrations at 24 h were significantly higher in males (0.38 ± 0.07 μg/mL) than in females (0.02 ± 0.01 μg/mL). At 96 h, the amount of PFBS found in the liver of males and females corresponded to approximately 0.03% and 0.05% of the administered dose, respectively. The comparison of the Area Under the Curve (AUC) values obtained from the i.v. and oral studies resulted in a bioavailability of 100% in females, and 55% in males. In orally treated animals, approximately 69% and 74% of the administered dose were found in urine after 24 h in males and females, respectively, whereas faecal excretion was ≤ 0.5% in both sexes. The mean serum half‐life values were significantly shorter in males (4.7 h) than females (7.4 h) for animals orally exposed, but no difference was observed for animals intravenously injected (Table C.3).
In cynomolgus monkeys given a single i.v. dose of 10 mg/kg potassium PFBS, there were no statistically significant differences between males and females for any of the toxicokinetic parameters (Olsen et al., 2009). Mean serum concentration at 24 h was approximately 8 μg/mL. The percentage of administered dose recovered in urine from 0 to 24 h ranged from 33.8% to 86.8%. Mean serum elimination half‐lives in male and female monkeys were 95 h and 83 h, respectively (Table C.3).
Chengelis et al. (2009) investigated the toxicokinetic parameters of PFBS in rats and monkeys, using the same experimental design as for PFHxA (see Section C.1). In Sprague‐Dawley rats given a single i.v. dose of 10 mg PFBS/kg bw, the half‐life of PFBS in serum was shorter for female than male rats (0.64 h compared to 2.1 h) and apparent clearance from the serum was approximately seven‐ to eightfold higher for female rats than for male rats (Table C.3). Approximately 70% of the administered dose of PFBS was recovered in the urine of male and female rats during the 0–24 h period. In monkeys given a single i.v. dose of 10 mg/kg bw, the half‐life of PFBS in serum ranged from 8.1 to 15 h.
Adult male C57/BL6 mice were dietary exposed for 1–5 days to 16 mg/kg bw per day of 35S‐PFBS (Bogdanska et al., 2014). PFBS was found to distribute to most of the 20 tissues examined. The tissue levels increased from 1 to 3 days of exposure but appeared thereafter to level‐off in most cases. After 5 days of treatment, the highest PFBS levels were detected in liver, gastrointestinal tract, blood, kidney, cartilage, whole bone, lungs and thyroid gland.
Recently, PFBS was evaluated in male and female Sprague‐Dawley rats administered by repeated gavage doses of 62.6, 125, 250, 500 and 1,000 mg/kg bw per day for 28 days (NTP, 2019b). Males generally had higher (5‐ to 18‐fold) plasma concentrations compared to females across all dose groups. The liver/plasma ratios (calculated in males only) ranged from 0.4 to 0.6 across the doses.
Huang et al. (2019a) investigated the toxicokinetics of PFBS in male and female Sprague‐Dawley rats. After a single i.v. or gavage administration, concentrations were measured in the plasma, liver, kidney and brain. In all tissues, concentrations decreased slightly over time, with females having a faster decrease than males, and were as follows: liver > kidney > brain. In males, the liver/plasma ratio was generally above 1, dropping below 1 at 12 h, whereas in females, the ratio was 1.5–2 times lower than in males. After i.v. administration, plasma half‐lives were 0.4 and 2.3 h in females and males, respectively, whereas after gavage, these values averaged 1.3 and 3.3 h (Table C.3).
Butenhoff et al. (2009) exposed by oral gavage male and female Sprague‐Dawley rats to potassium PFHxS at dose levels of 0.3, 1, 3 and 10 mg for 2 weeks prior to mating and during mating, gestation and lactation (postnatal day 22) for parental females as well as during 6 weeks for males. The mean serum PFHxS concentrations in parent males at the end of the exposure period ranged from 44 μg/mL at 0.3 mg/kg to 201 μg/mL at 10 mg/kg. In pooled pup serum from postnatal day 22, serum PFHxS concentrations ranged from 9 μg/mL at 0.3 mg/kg to 94 μg/mL at 10 mg/kg. At the end of gestation, maternal serum PFHxS ranged from 3 μg/mL at 0.3 mg/kg to 60 μg/mL at 10 mg/kg. At doses of 1.0 mg/kg per day or higher, PFHxS‐treated rats appeared to have reached serum steady state by after 2 weeks as their serum PFHxS concentrations were not statistically different between 2 and 6 weeks. Mean liver to serum PFHxS concentration ratio determined after 6 weeks of exposure ranged from approximately 1–3 for parent males, depending on dose, whereas in females and pups, this ratio never exceeded 0.4.
Comparative toxicokinetics of PFHxS in Sprague‐Dawley rats, CD‐1 mice and cynomolgus monkeys were reported by Sundström et al. (2012). After a single oral dose of potassium PFHxS (10 mg/kg bw per day), given to male and female rats either by gavage or i.v., the toxicokinetics parameters were estimated using a two‐compartment model. Based on the i.v. study, the serum elimination half‐lives in male and female rats were 6.83 and 1.83 days, respectively, and the clearances were 40.3 and 119 mL/kg per day, respectively (Table C.3). At 24 h, the PFHxS mean serum concentrations after oral administration were 61 and 30 μg/mL, for males and females, respectively. For females, the comparison of the AUC value obtained from the i.v. and oral studies resulted in a bioavailability of 50%. The female Tmax value after oral dosing was estimated to be at approximately 30 min, suggesting a rapid gastro‐intestinal absorption process. Due to the short duration of the observation period (24 h) and hence to the lack of significant serum elimination over this period, the estimation of most of the parameters was considered as poorly reliable, especially for males. A second experiment was conducted in male and female rats given a single i.v. dose of potassium PFHxS (10 mg/kg bw per day) followed up for 10 weeks. The toxicokinetics parameters were estimated using a two‐compartment model for males and one‐compartment model for females. At the end of the experiment, the mean serum PFHxS concentration in males was approximately 6 μg/mL, whereas it was below the LOQ (0.01 μg/mL) in females. The half‐life was estimated to be approximately 29 days in male rats, but only 1.6 days in females (Table C.3), indicating strong sex‐related differences. The clearances were 6.71 and 53.35 mL/kg per day in males and females, respectively. A third experiment was designed to investigate the distribution and elimination routes of PFHxS in male and female Sprague‐Dawley rats. The percentage of the PFHxS administered dose recovered in serum, liver, urine and faeces 96 h after a single oral dose of either 1, 10 or 100 mg potassium PFHxS/kg bw was determined. Regardless of sex, mean serum PFHxS concentrations were non‐linearly related to dose. Female serum and liver concentrations were considerably lower than those of males given an equivalent dose. For instance, for males, at the lowest dose tested (1 mg/kg bw), approximately 18% and 31% of the dose was found in serum and liver, respectively, whereas for females, these values were 7% and 2%, respectively. Urine was the major route of excretion in male and female rats. Within 96 h following a single oral dose at 1, 10 and 100 mg PFHxS/kg bw, females excreted 35%, 28% and 41% of the dose in urine, respectively, whereas urinary excretion for males was only about 6–7% of the dose at the 1 and 10 mg/kg dose level, but 30% at the 100 mg/kg dose level. Faecal excretion was limited (< 1% of administered dose), irrespective of the dose or the sex.
Additional studies were carried out in male and female mice given a single oral dose of potassium PFHxS (1 or 20 mg/kg bw) and followed for 23 weeks (Sundström et al., 2012). Regardless of sex, dose or sampling time, mean PFHxS concentrations were highest in serum followed by liver and then kidney. As indicated in Table C.3, mean serum half‐life values were quite similar between male and female mice (approximately 30 vs. 25 days for 1 mg/kg bw and 28 vs. 27 days for 20 mg/kg bw for males and females, respectively). Clearances were similar between sexes and were approximately 3 and 4 mL/kg bw per day for the doses of 1 and 20 mg/kg bw, respectively. Urinary elimination predominated, but was slow and no indication of a clear sex‐related difference was observed. Based on the data provided by the authors, a urinary elimination of about 30% of the administered dose within 2 months may be estimated. During the same period, total faecal excretion was likely close to or below 1% of the administered dose.
In male and female cynomolgus monkeys given a single i.v. dose of 10 mg potassium PFHxS/kg bw and followed during 24 weeks, mean serum elimination half‐lives were less for females (87 ± 27 days) than males (141 ± 30 days); however, this difference was not statistically significant (Sundström et al., 2012).
The toxicokinetics of PFHxS in female and male Sprague‐Dawley rats after a single i.v. or oral administration of 10 mg/kg bw were investigated by Kim et al. (2016a). They found that PFHxS was almost completely absorbed in both sexes and was more rapidly absorbed in the female rats (Tmax of 1.37 h) than in the male rats (3.11 days). The measurement of PFHxS concentrations in different tissues at the end of the experiment (72 days in males, 14 days in females) showed that the highest values were found in the liver and the kidney. Based on the i.v. study and using a two‐compartment model, the serum elimination half‐lives in male and female rats were 20.7 and 0.88 days, respectively, and the clearances were 9.0 and 227.9 mL/kg per day, respectively (Table C.3). The calculated excreted percentage of the i.v. dose of PFHxS in female and male rats was 28.02 and 8.26% in urine, respectively, showing a significant sex difference, probably due to differences in the transport and renal re‐absorption process of PFHxS between male and female rats.
Male and female Sprague‐Dawley rats were given for 28 days oral gavage doses of PFHxS ranging from 0.625 to 2.5 mg/kg bw per day (males) or from 3.12 to 50 mg/kg bw per day (females). Although females were administered doses five times higher than those administered to males, the female plasma concentrations were about half of male concentrations. The liver/plasma ratios (calculated in males only) ranged from 0.6 to 1.2 (NTP, 2019b). The toxicokinetic parameters of PFHxS after a single i.v. or gavage administration in male and female Sprague‐Dawley rats were reported recently by Huang et al. (2019a). In both sexes, concentrations of PFHxS were highest in the liver, around one‐ to threefold less in the kidney and 40‐fold less in the brain. Liver/plasma ratios ranged from 0.5 to 0.82 and from 0.29 to 0.55 in males and females, respectively. Based on the i.v. study and using a two‐compartment model, the serum elimination half‐lives in female and male rats were 0.7 and 13 days, respectively. After gavage administration with the lower dose tested (4 mg/kg bw), the half‐life based on a one compartment model was 17.6 days in males whereas it was 7.5‐fold shorter in females (Table C.3).
Interactions with binding proteins including carrier and transporters
Bischel et al. (2011) investigated the associations, at low ligand concentrations, of PFBS, PFHxS and PFOS with BSA. All the PFSAs tested were highly bound (> 99%) to BSA. PFBS exhibits higher affinity for BSA than the equivalent chain‐length PFCA (PFPA).
Zhao et al. (2015) demonstrated that the uptake of PFBS, PFHxS and PFOS into freshly isolated rat and human hepatocytes is mediated by sodium‐dependent mechanisms through Na+/taurocholate co‐transporting polypeptide (Ntcp), a bile salt transporter expressed at the sinusoidal membrane of hepatocytes (See Table C.1). More recently, the same team found that rat organic anion‐transporting polypeptides OATP1A1, OATP1A5, OATP1B2 and OATP2B1 which are expressed in hepatocytes and enterocytes can transport PFBS, PFHxS and PFOS (Zhao et al., 2017). Thus, both Na+/taurocholate co‐transporting polypeptide and the transporters of the OATP family could contribute to the enterohepatic circulation of PFSAs in rodents. It is also plausible that these transporters play a role in the accumulation of PFSAs in the liver.
C.3. Mixtures
Benskin et al. (2009) conducted an isomer‐specific disposition study following a single oral dose of a PFAS mixture administered by gavage to male Sprague‐Dawley rats. The dose consisted of 400 μg/kg bw PFOS, 500 μg/kg bw PFOA, 390 μg/kg bw PFNA (200 μg/kg bw n‐PFNA and 190 μg/kg bw iso‐PFNA), and 30 μg/kg bw PFHxS isomers which were present as impurities. The PFNA isomer profiles in the dose and in blood suggested both preferential uptake and elimination of iso‐PFNA. The half‐lives for iso‐PFNA and n‐PFNA, were 20.7 days and 40.6 days respectively. On day 3 post‐dosing, maximum concentrations were found in liver (2.7 and 2.3 μg/g for iso‐PFNA and n‐PFNA, respectively). Approximately 33% of administered PFNA was excreted in urine throughout the experiment (38 days), with the remainder in faeces. The portion of iso‐PFNA in urine and faeces was 63% and 57% of the dose, respectively. The same team repeated the experiment selecting a subchronic exposure instead of a single dose study (De Silva et al., 2009). Male and female Sprague‐Dawley rats were dietary exposed to the same mixture of isomers for 12 weeks, followed by a 12‐week depuration period. On day 38 of the exposure period (steady state), the greatest site of accumulation was the liver. Levels of n‐PFNA and iso‐PFNA in female liver tissue corresponded to 1.3 and 0.37 μg/g, respectively, whereas in males the corresponding levels were 9.9 and 6.3 μg/g, respectively. Half‐lives of 47 and 31 days for n‐PFNA and iso‐PFNA in male rats and 2.1 and 0.8 days in females were reported. PFHxS data suggest preferential absorption of the linear isomer. Branched isomers were eliminated quickly from all tissues, such that only n‐PFHxS was detectable on day 38. In blood, the half‐life of linear PFHxS was 15.9 days whereas for branched isomers, it varied from 3.5 days to 6.9 days. Liver half‐life of n‐PFHxS was over three times longer than that estimated for blood. Elimination of branched isomers occurred primarily via urine.
In a study carried out by Numata et al. (2014), the fate of seven PFCAs and PFSAs, was investigated in male and female pigs fed during 3 weeks a diet contaminated with a mixture of these PFASs. Absorption, tissue distribution and excretion were measured and the half‐lives were estimated. The concentration of PFBS, PFHxS, PFHxA, PFHpS, PFHpA, PFOS and PFOA in feed was 132 ± 11, 91.3 ± 8.0, 47.8 ± 4.4, 3.99 ± 0.50, 10.2 ± 1.7, 137 ± 13, 22.4 ± 2.6 μg/kg. Only the data for PFHxA suggest a steady state after 3 weeks of exposure. For PFBS, the estimation of the time to 95% completion of the steady state was 217 days, whereas it was between 1 and 10 years for the rest of the compounds. At the end of the experiment, more than 80% of the total mass of ingested PFBS, PFHxS, PFHpS, PFOS, PFOA was found in blood and other tissues. For PFHpA and PFHxA, this percentage was approximately 60% and 20%, respectively. For all investigated substances except PFOS, blood plasma was the largest reservoir of unexcreted PFAS and less than 7% of unexcreted PFAS was present in the liver (for PFOS approximately 35% of the ingested dose was found in the liver). Faecal excretion occurred at a limited extent for all investigated PFASs (less than 8% of the ingested dose). Elimination in urine was < 5% of the dose for PFHxS, PFHpS, PFOS and PFOA, ranging from 10 to 20% for PFHpA and PFBS and higher than 60% for PFHxA. No significant differences were observed between males and females. The half‐life was estimated to be 4, 43 and 74 days for PFHxA, PFBS and PFHpA, respectively. It was approximately 0.6, 1.1, 1.8 and 2 years for PFOA, PFHpS, PFOS and PFHxS, respectively.
Guruge et al. (2016) investigated the toxicokinetics of a mixture of 10 PFASs (PFOS, PFBA, PFPeA, PFHxA, PFHpA, PFOA, PFNA, PFDA, PFUnDA and PFDoDA) in adult female micro‐minipigs weighing 9–14 kg. After a single gelatin capsule filled with a mixture, corresponding to a dose of 3 mg/kg bw of each of the 10 tested substances, was given, they found that absorption of PFBA, PFPeA and PFHxA was rapid, with concentrations in the blood reaching the maximum in less than 12 h. The maximum levels of the rest of the PFASs in the blood were reached at 24–48 h after the exposure. PFPeA had the shortest blood half‐life followed by PFHxA, being 1.6 and 2.7 days, respectively. In contrast, PFOS elimination from the blood was the slowest, with a half‐life of 86.6 days. The half‐lives of PFBA, PFHpA, PFOA, PFNA, PFDA, PFUnDA and PFDoDA were 13.9, 34.7, 63.0, 49.5, 40.8, 38.5 and 31.5 days, respectively. Three weeks post‐dosing, PFDoDA, PFUnDA, PFOS, PFDA and PFNA were mainly present in the liver, whereas for PFBA, PFHpA and PFOA, the concentration was higher in the blood than in the liver. At the end of the experiment, PFPeA and PFHxA were undetected in blood and liver whereas the PFNA body burden was the highest among the tested PFASs.
C.4. Other PFASs
Some other PFASs can be precursors and degrade or biotransform to PFSAs and PFCAs. The environmental degradation is reported in the environmental fate section, whereas the biotransformation in experimental animals or in biological in vitro models is described hereinafter. Most of the studies have been performed on 8:2 fluorotelomer alcohol (8:2 FTOH), whereas there have been limited investigations on non‐FTOH metabolism.
The toxicokinetics of 8:2 fluorotelomer alcohol was reviewed by ECHA (ECHA, 2013) and is summarised in Table C.4. In rats 8:2 FTOH is rapidly absorbed (absorption rate estimated between 27 and 57%, depending on the dose), and the parent compound and metabolites are quickly distributed to blood and tissues (Hagen et al., 1981; Martin et al., 2005; Fasano et al., 2006). At 7 days following the administration of radiolabelled 8:2 FTOH, 4–7% of the orally administered dose was present in the tissues as parent compound and related metabolites and the levels in the majority of these tissues were greater than in whole blood, with the highest levels observed in fat, liver, thyroid and adrenals (Fasano et al., 2006). Elimination was mainly via faeces (> 70%) and biliary excretion was between 20 and 45% of the administered dose, depending on the dose. Less than 4% of the administered dose was excreted in urine, and females eliminated more than males (Fasano et al., 2006). Metabolites identified in plasma, urine and faeces were in principle glucuronide and glutathione conjugates of the parent compound, oxidised and reduced intermediates and PFOA, PFNA, PFHpA and PFHxA (Table C.4). 8:2 FTOH and most metabolites were rapidly or completely cleared from the tissues (8:2 FTOH t1/2 = approximately 5 h), with the exception of PFOA (particularly in males). The half‐life based on total radioactivity (Fasano et al., 2006) was approximately 9 and 7 days in males and females, respectively. Whereas single‐dose studies showed no differences between sexes in metabolic profiles, the repeated dose study carried out by Fasano et al. (2009) resulted in levels of PFCAs (PFNA, PFOA and PFHpA) consistently higher in male livers than in female livers (Table C.4).
Dagnino et al. (2016) administered by oral gavage a single dose of 8:2 FTOH at 5 or 50 mg/kg bw to male Sprague‐Dawley rats and blood, urine and faeces samples were collected at 8, 24, 48, 72, 96, and 120 h after dosing. In the 50 mg/kg bw group, the highest concentration of metabolites measured in serum was for PFOA (1,995 ng/mL), whereas PFNA was found at a concentration of 25.66 ng/mL. In urine, PFOA (303.6 ng/mL) was the main transformation product and PFNA concentration was 0.84 ng/mL. Other PFCAs were not examined (Table C.4).
Recently, Huang et al. (2019b) investigated the toxicokinetics of 8:2 FTOH in male and female Sprague‐Dawley rats given a single dose via gavage or i.v. of 8:2 FTOH. The parent substance and its two metabolites (PFOA and 7:3‐fluorotelomer acid [7:3‐FTA]) were determined in plasma, liver, kidney and brain. There was rapid absorption and distribution of 8:2‐FTOH after gavage administration and the plasma elimination half‐life ranged from 1.1 to 1.7 h. Bioavailability of 8:2‐FTOH ranged from 22 to 41% for both sexes with no dose‐dependent trends. 8:2‐FTOH metabolites, PFOA and 7:3‐FTA were detected in plasma following administration of the parent FTOH. The plasma half‐life of PFOA was longer in males than in females (198–353 h and 4.47–6.9 h, respectively). The plasma half‐life of 7:3‐FTA was around 2–3 days in both sexes. 8:2‐FTOH and 7:3‐FTA were detected in all tissues; PFOA was found in the liver and kidney (Table C.4), but not in the brain. Detectable concentrations of metabolites persisted longer than the parent FTOH. Sex differences were observed in the tissue distribution and elimination of PFOA, but not 8:2‐FTOH and 7:3‐FTA.
Henderson and Smith (2007) examined the metabolism and disposition of 8:2 FTOH in timed‐pregnant mice exposed to a single gavage dose (30 mg /kg bw) (Table C.4). During gestation (GD9 to GD18), maternal serum and liver concentrations of PFOA decreased from 789 ± 41 to 668 ± 23 ng/mL and from 673 ± 23 to 587 ± 55 ng/g, respectively. PFOA was transferred to the developing fetuses as early as 24‐h post‐treatment with concentrations increasing from 45 ± 9 ng/g (GD10) to 140 ± 32 ng/g (GD18), while PFNA was quantifiable only at GD18 (31 ± 4 ng/g). Post‐partum, maternal serum PFOA concentrations decreased from 451 ± 21 ng/mL on postnatal day (PND) 1 to 52 ± 19 ng/mL on PND15 and PFNA concentrations, although fivefold less, exhibited a similar trend. Immediately after birth, pups were cross‐fostered with dams that had been treated during gestation with 8:2 FTOH or vehicle in order to investigate exposure through lactation. At both PND3 and PND15, PFOA and PNDA were detected in serum and liver from neonates exposed pre‐ and/or postnatally, indicating that maternal exposure to 8:2 FTOH results in both in utero and lactational exposure to PFOA and PNDA.
In vitro data (Table C.4) suggest that hepatocytes from rats, mice and humans have the ability to biotransform 8:2 FTOH into several PFCAs (Nabb et al., 2007). Human hepatocytes produced about 20‐ and 12‐fold less PFOA than mouse and rat hepatocytes, respectively.
PAPs biotransformation proceeds via the hydrolysis of the phosphate linkage, yielding the corresponding FTOH, which is then available for oxidation. Both mono‐AP and di‐PAP congener uptake, biotransformation and elimination were investigated in rats (see Table C.4).
Male Sprague‐Dawley rats were administered a single dose of 200 mg/kg by oral gavage of mono‐phosphate (8:2 monoPAPS), or the corresponding di‐phosphate (8:2 diPAPS), with blood taken over 15 days post‐dosing (D'Eon and Mabury, 2007). Both compounds were synthesised by the authors and were 97% pure and contained < 0.01% PFOA. Upon completion of the time‐course study, the animals were redosed using an identical dosing procedure, with sacrifice and necropsy 24 h after the second dosing. Increased levels of PFOA, along with both 8:2 PAPs congeners, were observed in the blood of the dosed animals. In the 8:2 monoPAPS‐dosed animals, 8:2 monoPAPs and PFOA blood concentrations peaked at 7,900 ± 1,200 ng/g and 34 ± 4 ng/g, respectively. In the 8:2 diPAPS‐dosed animals, 8:2 diPAPS concentration peaked at 32 ± 6 ng/g, and 8:2 monoPAPs and PFOA peaked at 900 ± 200 ng/g and 3.8 ± 0.3 ng/g, respectively. As indicated in Table C.4, in addition to PFOA, PFHpA was also observed in the animals dosed with monoPAPs. Consistent with other fluorinated contaminants, the tissue distributions showed increased levels of both PFOA and the 8:2 PAPs congeners in the liver relative to the other tissues measured.
A subsequent study by the same authors carried out in rats administered by gavage or by iv injection a single dose of various congeners of mono‐PAPs or di‐PAPs (see Table C.4), resulted in biotransformation yields of 1% for 6:2 diPAP (to PFHxA), 9% for 8:2 diPAP (to PFOA) and 8% for 10:2 diPAP (to PFDA). Half‐lives of the investigated mono‐ and di‐PAP congeners were estimated to be in the 1.6–4.8 day range. (D'Eon and Mabury, 2011).
Dagnino et al. (2016) investigated the metabolism of 8:2 diPAP in male Sprague‐Dawley rats (see above experimental details). PFOA and PFNA highest concentrations in serum of animals dosed at 50 mg/kg dosed animals were 36.1 ng/mL and 1.5 ng/mL, respectively. In urine, PFOA and PFNA concentrations were approximately 6 and 0.6 ng/mL, respectively. Other PFCAs were not examined (Table C.4).
Ross et al. (2012) investigated the isomer‐specific fate of perfluorooctane sulfonamide (FOSA) in male Sprague‐Dawley rats exposed to commercial FOSA (purity not indicated) via food for 77 days (83.0 ng/kg bw per day), followed by 27 days of depuration. Elimination half‐lives of the two major branched FOSA isomers (2.5 ± 1.0 days and 3.7 ± 1.2 days) were quicker than for linear FOSA (5.9 ± 4.6 days), resulting in a depletion of branched FOSA isomers in blood and tissues relative to the total dosed FOSA. A significant enrichment of 5m‐PFOS and a significant depletion of 1m‐PFOS were observed in serum, relative to authentic electrochemical PFOS. The results confirm that in vivo exposure to commercially relevant PFOS‐precursors can result in a distinct PFOS isomer profile.
Female Sprague‐Dawley rats were administered EtFOSE (purity > 99%) by gavage for 3 weeks, at 5 mg/kg bw per day and several putative metabolites were analysed in liver and serum (Xie et al., 2009). Levels of EtFOSE and FOSE in serum and liver were in the low μg/kg range, whereas EtFOSA was not detected in either matrix. In contrast, levels of EtFOSAA were in the low mg/kg range in the liver and serum. FOSA hepatic levels were also in the low mg/kg range, but were approximately one order of magnitude lower in serum. The major metabolite detected in both liver and serum was PFOS (Table C.4), with levels that were at least 10 times higher compared to the five perfluorooctanesulfonamides. The liver‐to‐serum ratios ranged from 1.3 for EtFOSAA to 10.1 for FOSA and decreased in the order FOSA > FOSE > PFOS > EtFOSAA. The formation of EtFOSAA, FOSA and PFOS was also observed in rat liver slices incubated with EtFOSE (Xu et al., 2004; data not presented in Table C.4).
Luebker et al. (2002) determined the relative effectiveness of EtFOSA and EtFOSE to inhibit 11‐(5‐dimethylaminonapthalenesulphonyl)‐undecanoic acid (DAUDA) binding to L‐FABP, compared to PFOS and PFOA. They found that PFOS exhibited the highest level of inhibition of DAUDA‐L‐FABP binding in the competitive binding assays, followed by EtFOSA, and, with equal IC50s, N‐EtFOSE and PFOA.
Appendix D – Effects following acute exposure
1.
Due to the limited number of published data, studies were considered even if PFASs were not applied via water or food or by gavage (oral exposure). Reports on acute exposure effects were identified for PFHxA, PFDA, EtFOSE (as listed in Table D.1) and 8:2 FTOH. Studies on effects following acute exposure to PFOS and PFOA, which have been published between 2008 and 2016, are documented in the previous Opinion (EFSA CONTAM Panel, 2018). An additional study on PFOS has been published.
Table D.1.
Acute Studies
| Substance (purity) | Species/dose route/doses | Observed effects | Highest dose with no effect (mg/kg bw) | Lowest dose with effect (mg/kg bw) | LD50 (mg/kg bw) | Reference |
|---|---|---|---|---|---|---|
| PFHxA | ||||||
| PFHxA (sodium salt; 100% purity) |
SD rats (f) No/sex/group: not given single application: 0, 175, 550, 1,750, 5,000 mg/kg bw; route not specified |
> 1,750 < 5,000 |
Loveless et al. (2009) | |||
| PFDA | ||||||
| PFDA (96% purity) |
C57BL/6N mice (f) No/sex/group: 10 Single gavage: 0, 20, 40, 80, 160 or 320 mg/kg bw |
120 | Harris et al. (1989b) | |||
|
PFDA (96% purity) |
C57BL/6J mice (f) No/sex/group: 19–20 Single gavage: 0, 20, 40, 80, or 160 mg/kg bw |
129 | Harris et al. (1989b) | |||
|
PFDA (> 96% purity) |
C57BL/6J mice (f) No/sex/group: 3–4 Single gavage: 0, 40, 80, 100 or 160 mg/kg bw Sacrifice after 2, 7, 14 or 30 days |
Decr. body weight Incr. abs liver weight Incr fatty‐acyl‐CoA oxidase activity |
40 (day 30) 40 (day 2–30) 40 (day 2–30) |
Brewster and Birnbaum (1989) | ||
|
PFDA (purity not specified) |
F344 rats (m) No/sex/group: ≤ 6 single i.p. administration: 0, 5, 15, 25, 50 mg/kg bw |
Fatty‐acyl‐CoA oxidase activity | 5 | N/A | Adinehzadeh et al. (1999) | |
|
PFDA (free acid, 98% purity) |
C57BL/6 mice (m) No/sex/group: 4–5 single i.p. administration: 0, 0.5, 1.0, 10, 20, 40 or 80 mg/kg bw |
Decr mRNA of Oatp1a1, Oatp1a4, Oatp1b2 |
10 20 |
40 0.5 40 |
N/A | Cheng and Klaassen (2008a) |
|
PFDA (98% purity) |
C57BL/6 mice (m) No/sex/group: 5 single i.p. administration: 0, 0.5, 1.0, 10, 20, 40 or 80 mg/kg bw |
Incr mRNA of: Cyp4A14 Cyp2B10 |
1 40 |
10 80 |
N/A | Cheng and Klaassen (2008b) |
| PFDA (> 99% purity) |
C57BL/6 mice (m) No/sex/group: 5 single i.p. administration: 0, 0.25, 0.5, 1, 10, 20, 40 or 80 mg/kg bw |
Incr rel liver weight Incr mRNA of Cyp4a14 Mrp3 Mrp4 |
1 10 |
0.25 0.25 10 20 |
N/A | Maher et al. (2008) |
| PFDA (98% purity) |
129/Sv wt mice (m) No/sex/group: 5 single i.p. administration: 0 or 80 mg/kg bw |
Incr ALP, AST, ALT Incr total bile acids in serum Incr indirect and direct serum bilirubin Hepatic inflammation |
80 80 80 80 |
N/A | Luo et al. (2017) | |
| EtFOSE | ||||||
|
EtFOSE (purity not specified) |
SD rats (m) No/sex/group: 3 single i.p. administration: 0 or 100 mg/kg bw |
Peroxisomal ß‐oxidation | 100 | N/A | Berthiaume and Wallace (2002) | |
| PFOS | ||||||
| PFOS (Potassium salt from 3M, approx. 88.9% purity, containing PFHxS at 3.2%, PFHpS at 1.2%, PFPeS at 1.1%, PFBS at 0.97%, PFPS at 0.74%) |
Cynomolgus monkey (m/f) No/sex/group: 6 Single gavage of 9 mg/kg bw Observation period for 316 days post gavage |
Non‐significant reduction of serum cholesterol post‐treatment (m) | Chang et al. (2017) | |||
| PFOA, > 98% pure | Sprague‐Dawley rats, 10–11 weeks old at start. 0, 0.625, 1.25, 2.5, 5, 10 mg/kg bw per day, N=10 per sex per group 28 days |
Reduced epidydimal weight Reduced epidydimal sperm count |
5 5 |
10 10 |
NTP (2019a) | |
ALP: alanine phosphatase; ALT: alanine aminotransferase; AST: aspartate aminotransferase; bw: body weight; f: female; m: male; i.p.: intraperitoneal; PFAS: perfluoroalkyl substance; PFOS: perfluoroheptane sulfonate; PFOA: perfluorooctanoic acid; EtFOSE: N‐ethyl perfluorooctane sulfonamido ethanol; PFHxA: perfluorohexanoic acid; PFHxS: perfluorohexane sulfonic acid; PFDA: perfluorodecanoic acid; PFPeS: perfluoropropanesulfonate; PFPS: perfluoropropanesulfonate.
For PFHxA, Loveless et al. (2009) reported that the LD50 ranged between 1,750 and 5,000 mg/kg bw in female rats.
For PFDA, Harris et al. (1989b) determined an oral LD50 of 120 mg/kg bw in female C57BL/6N mice and of 129 mg/kg bw for female C57BL/6J mice.
Adinehzadeh et al. (1999) studied the impact of PFDA (Sigma‐Aldrich, commercial source,) at single i.p. doses of 2–50 mg/kg bw in male F344 rats (6 rats/group). At doses of < 20 mg/kg bw, PFDA was less toxic than above, as indicated by measurements of daily food intake. At 50 mg/kg bw, serum concentrations of tumour necrosis factor (TNF)‐alpha were elevated about eightfold. Hepatic fatty acyl‐CoA oxidase activity showed a dose‐dependent increase from 5 to 25 mg/kg bw. At 15 or 50 mg/kg bw, there were increases in hepatic phosphatidylcholine and phosphatidylethanolamine concentrations. Both doses, however, did not alter liver ATP content.
Cheng and Klaassen (2008a) investigated the effects of PFDA on Na(+)‐taurocholate cotransporting polypeptide (Ntcp) and organic anion‐transporting polypeptides (Oatp) 1a1, 1a4 and 1b2, major transporters for the uptake of bile acids and other organic compounds into the liver. Male C57BL/6 mice received a single i.p. administration of PFDA at 0.5, 1, 10, 20, 40 or 80 mg/kg bw. The highest dose of PFDA elevated serum bile acid concentrations about threefold. mRNA and protein expression of all four transporters were lowered after PFDA exposure. The subsequent use of PPARα‐/‐ mice indicated that the downregulation of the transporters appears to involve PPARα.
Cheng and Klaassen (2008b) treated male C57BL/6 mice with a single i.p. administration of PFDA at 0.5, 1, 10, 20, 40 or 80 mg/kg bw. Two days after treatment, the expression of Cyp2B10, Cyp3A11 and Cyp4A14 was increased significantly. By using CAR‐, PXR‐, PPARα‐ or FXR‐knockout mice, it was determined that PPARα and CAR contribute to the induction of Cyps by PFDA.
Maher et al. (2008) treated male C57BL/6 mice with single i.p administrations of PFDA at 0, 0.25, 0.50, 1, 10, 20, 40 or 80 mg/kg bw. PFDA elevated the liver/body weight ratios and mRNA of the PPARα‐target gene Cyp4a14 at all doses tested. The transporter proteins Mrp3 and Mrp4 were induced by 10 and 20 mg/kg bw, respectively. Single application of 80 mg/kg bw of PFDA elevated transcript levels of hepatic Mrp3 (fourfold) and Mrp4 (31‐fold) and also serum levels of conjugated bilirubin and bile acids, indicating that PFDA interferes with the transporters for hepatic efflux of bilirubin and bile acids to serum.
Luo et al. (2017) treated wild‐type and PPARα‐null 129/Sv mice with a single i.p. dose of PFDA at 80 mg/kg bw. Five days after treatment, metabolomic analyses of blood and liver tissue samples revealed elevated direct and indirect bilirubin levels, increases in liver enzymes ALP, ALT and AST in the serum, and weak hepatocellular injury and inflammation in wild‐type mice, associated with adaptive regulations of bile acid synthesis and transport. While both wild‐type and PPARα‐null mice exhibited elevated liver/body weight ratios, there was no disruption of bile acid homoeostasis, hepatocellular injury or inflammation in the knockout animals.
Berthiaume and Wallace (2002) treated male Sprague‐Dawley rats with a single i.p. injection of 100 mg/kg bw of EtFOSE. PFOA and PFOS at 100 mg/kg bw served as positive control. Animals were sacrificed on the third day post‐treatment. Peroxisome proliferation was determined by lauroyl CoA oxidase activity, reduction of serum cholesterol concentration and relative liver weights were recorded as well. The degree of mitochondrial biogenesis was estimated by measurements of cytochrome oxidase activity, cytochrome content and mitochondrial DNA copy number. In contrast to PFOS and PFOA, EtFOSE exhibited no potency as peroxisome proliferator and exerted no effects.
Chang et al (2017) treated cynomolgus monkeys with a single dose of 9 mg PFOS/kg bw by gavage and reported on an insignificant reduction of serum cholesterol in the post‐treatment phase. No further alterations could be observed.
Finlay (2008, cited from ECHA, 2012) treated male and female SD rats with 8:2 FTOH by single gavage with 2,000 and 500 mg/kg bw, respectively. The authors reported on no mortality, no signs of toxicity, no body weight loss and no gross lesions at necropsy.
Appendix E – Effects following repeated exposure tables
1.
Appendix F – Developmental and Reproductive toxicity tables
1.
Appendix G – Developmental Neurotoxicity tables
1.
Appendix H – Immunotoxicity
1.
Table H.1.
Immunotoxicity studies perfluorooctanesulfonic acid (PFOS) from 2018 Opinion (EFSA CONTAM Panel, 2018)
| Substance/ (Purity) | Species/Experimental design and doses | Most sensitive endpoints | Highest dose with no effect (mg/kg bw per day) | Significant effect level (mg/kg bw per day) | Serum/tissue levels of compound | Reference |
|---|---|---|---|---|---|---|
| PFOS, 98% | C57BL/6 mice (4–8 males per group), administered via diet for 10 days, at 0.001 or 0.02% (2 or 40 mg/kg bw per day) (0.02% equals a total of 6 mg/animal over 10 days) | Several immune parameters up or down, but also reduced body weight gain | N/A | 0.02% (40 mg/kg bw per day) | 340 μg/mL | Qazi et al. (2009a,b) |
| PFOS, 98% | C57BL/6 mice (4 males), restricted food diet, 10 days, 0.001, 0.002, 0.02 (1.6, 3.1 or 23.5 mg/kg bw per day) | Reduced B‐cell numbers |
0.001% (1.6 mg/kg bw per day) |
0.02% (23.5 mg/kg bw per day) |
50.8 μg/mL at 0.001% 340 μg/mL at 0.02% |
Qazi et al. (2012) |
| PFOS, 98% | Balb/c mice (8 per group, males and females), gavage, 5, 20 mg/kg bw per day, 14 days | Thymus and spleen histopathology, but in addition to liver effects and PPARα changes | 5 | 20 |
4.89 μg/mL at 5 mg/kg bw per day 25.2 μg/mL at 20 mg/kg bw per day |
Wang et al. (2011a) |
| PFOS, 85% | Balb/c mice (5 females per group), gavage, 20 mg/kg bw per day, 7 days | Values various immune parameters reduced, but in addition to body weights | N/A | 20 | NR | Vetvicka and Vetvickova (2013) |
| PFOS, 98% |
C57BL/6 mice (12 males per group), gavage, 1, 5, 10 mg/kg bw per day, 7 days |
Inconsistent; some ex vivo apoptotic parameters in splenocytes and thymocytes up and others go down | 1 | 5 |
24.37 μg/mL at 1 mg/kg bw per day 87.56 μg/mL at 5 mg/kg bw per day |
Zhang et al. (2013d) |
| PFOS, 98% |
C57BL/6 mice (12 males per group), gavage, 5, 20, 40 mg/kg bw per day, for 7 days |
Reduced NK activity, antibody response, lymphocyte proliferation | N/A | 5 | 97.25 μg/mL | Zheng et al. (2009, 2011) |
| PFOS, 85% | BALB/c mice (5 females per group), gavage, 20 mg/kg bw per day, 21 days | Reduced antibody response and NK activity, but accompanied by body weight effects | N/A | 30 | NR | Vetvicka and Vetvickova (2013) |
| PFOS | B6C3F1 mice (5 males per group), food, 0.25 mg/kg bw per day for 28 days |
Effects on body weight and liver No immune effects |
0.25 | N/A | 11.6 μg/mL | Qazi et al. (2010) |
| PFOS, 98% | B6C3F1 mice (5 females per group), gavage, 0.0331, 0.0993, 9.3 mg/kg bw per day, 28 days | TNFα and IL‐6 up and down, but no dose‐response | N/A | 0.0331 | NR | Mollenhauer et al. (2011) |
| PFOS, 98% | B6C3F1 mice (5–10 females), gavage, 3.31, 16.6, 33.1, 166 μg/kg bw per day, 28 days | Increased ex vivo IL‐6 | N/A |
3.31 μg/kg bw per day (0.00331 mg/kg bw per day) |
< 1 ng/mL (LOQ) | Fair et al. (2011) |
| PFOS, 98% | Sprague‐Dawley rats (15 males or female per group), diet, 0.14, 1.33, 3.21, 6.31 mg/kg bw per day, 28 days | Reduced total IgG1 levels in serum | 0.14 | 1.33 |
0.95 μg/mL at 0.14 mg/kg bw per day 13.45 μg/mL at 1.33 mg/kg bw per day |
Lefebvre et al. (2008) |
| PFOS, 98% | B6C3F1 mice (5 males or females per group), gavage, 0.166, 1.66, 3.31, 16.6, 33.1, 166 μg/kg per day, 28 days |
Reduced specific antibody Response (PFCs) |
0.166 μg/kg per day (0.000166 mg/kg bw per day) |
1.66 μg/kg bw per day (0.00166 mg/kg bw per day) |
17.8n/ ng/mL at 0.000166 mg/kg bw per day 91.5 ng/mL at 0.00166 mg/kg bw per day |
Peden‐Adams et al. (2008) |
| PFOS | B6C3F1 mice (30 females per group), gavage, 5, 25 μg/kg per day, 21 days | Reduced survival after challenge with influenza virus |
5 μg/kg per day (0.005 mg/kg bw per day) |
25 μg/kg per day (0.025 mg/kg bw per day) |
189 ng/mL at 0.005 mg/kg bw per day 670 ng/mL at 0.025 mg/kg bw per day |
Guruge et al. (2009) |
| PFOS, 98% | C57BL/6 mice (10–12 males per group), gavage, 8.3, 16.7, 83.33, 416.7, 833.3 μg/kg bw per day for 60 days | Antibody responses down. |
8.3 μg/kg per day (0.0083 mg/kg bw per day) |
83.33 μg/kg bw per day (0.0833 mg/kg bw per day) |
0.84 μg/mL at 0.0083 mg/kg bw per day 8.21 μg/mL at 0.0833 mg/kg bw per day |
Dong et al. (2009) |
| PFOS, 98% | C57BL/6 mice (6 males per group), gavage, 8.3, 16.7, 83.33, 416.7, 833.3 μg/kg bw per day for 60 days |
IL‐4 and IL‐10, TNF‐a, IL‐1b, values up and down. IgM decrease IgG, IgE increase |
16.7 μg/kg per day (0.0167 mg/kg per day) |
83.33 μg/kg per day (0.0833 mg/kg per day) |
10.75 μg/mL at 0.0833 mg/kg per day 2.36 μg/mL at 0.0167 mg/kg per day |
Dong et al. (2011) |
| PFOS, 98% | C57BL/6 mice (12 males per group), gavage, 8.3, 16.7, 83.33, 416.7, 833.3 μg/kg bw per day for 60 days | Apoptotic lymphocytes, | 0.0167 mg/kg per day |
83.33 μg/kg per day (0.0833 mg/kg per day) |
0.84 μg/mL at 0.0833 mg/kg per day NR at 0.0167 mg/kg per day |
Dong et al. (2012) |
| PFOS, 98% | C57BL/6 mice (4 per group), 6‐8 weeks old, exposed to 2 mg PFOS/kg bw per day for a period of 7 days | IL 12 production after Citrobacter rodentium infection | N/A | 2 mg/kg bw per day | NR | Suo et al. (2017) |
| PFOS (purity NR) | ICR mice (number, sex NR), sensitised to ovalbumin on day 0, and orally exposed to 50‐150 mg PFOS/kg bw on days 9, 11, and 13. | Aggrevated allergic inflammation in an ovalbumin model. | N/A | 50 mg/kg, three times | NR | Lee et al. (2018) |
bw: body weight; IgG: immunoglobulin G; IL: interleukin; LOQ: limit of quantification; NK: natural killer (cell); N/A: not applicable; NR: not reported; PFC: perfluorinated compound; PFOS: perfluorooctane sulfonic acid; PPAR: peroxisome proliferator activated receptors; TNF: tumour necrosis factor.
Table H.2.
Immunotoxicity studies perfluorooctanoic acid (PFOA) from 2018 Opinion (EFSA CONTAM Panel, 2018)
| Substance/(Purity) | Species/Experimental design and doses | Most sensitive endpoints | Highest dose with no effect (mg/kg bw per day) | Significant effect level (mg/kg bw per day) | Serum/tissue levels of compound | Reference |
|---|---|---|---|---|---|---|
| PFOA, 97% | BALB/c mice (5 females per group), 7 days, gavage, 20 mg/kg bw per day |
Several immune parameters reduced, but also reduced body weight gain and increased liver weight |
N/A | 20 | NR | Vetvicka and Vetvickova (2013) |
| PFOA, 98% | C57BL/6J mice (16 females), 10 days, drinking water, 0, 30 mg/kg bw per day. Followed by nil or exposure for 5 days |
Reduced IgM antibody responses but enhanced IgG response. Also effects on body weight gain |
N/A | 30 | NR | DeWitt et al. (2008) |
| PFOA, 98% |
C57BL/6J mice (6 males), Drinking water for 15 days 3.75, 7.5, 15, 30 mg/kg bw per day |
Reduced IgM antibody response | 3.75 | 7.5 | NR | Dewitt et al. (2009) |
| PFOA, 98% | C57BlL6N (6 females) drinking water for 15 days 0.94, 1.88, 3.75, 7.5, 30 mg/kg bw per day | Reduced T‐cell dependent and T‐cell independent IgM antibody responses | 1.88 | 3.75 | NR | DeWitt et al. (2016) |
| PFOA, 98% | C57BL/6 mice (4–8 males per group), food for 10 days, 0.001 or 0.02% (2 or 40 mg/kg bw per day) (0.02% equals a total of 5.2 mg/animal over 10 days) |
Values several immune parameters up or down, but also body weight gain |
N/A | 0.02% (40 mg/kg bw per day) | 152 μg/mL | Qazi et al. (2009a,b) |
| PFOA, 98% |
C57BL/6 mice (4 males per group), restricted food diet, 10 days, 0.001, 0.002 or 0.02% (1.6, 3.1 or 23.5 mg/kg bw per day) |
Reduced B‐cell numbers |
0.002% (3.1 mg/kg bw per day) |
0.02% (23.5 mg/kg bw per day) |
87.6 μg/mL at 0.002% (3.1 mg/kg bw per day) 152 μg/mL At 0.02% (23.5 mg/kg bw per day) |
Qazi et al. (2012) |
| PFOA, 96% | BALB/c mice (5 females per group), gavage, 20 mg/kg bw per day, 21 days |
Various immune parameters reduced |
N/A | 20 | NR | Vetvicka and Vetvickova (2013) |
| PFOA, 100% |
ICR mice (5–6 males per group), drinking water, 21 days, 0.49, 2.64, 17.63, 47.21 mg/kg bw per day |
Reduced CD8 levels | N/A | 0.49 | NR | Son et al. (2009) |
| PFOA, 100% | CD‐1 mice (10–20 males), gavage, 0.3, 1, 10, 30 mg/kg bw per day, 29 days |
Various immune parameters reduced, but also body weight gain, increased liver weight |
1 | 10 |
27 μg/mL at 1 mg/kg bw per day 190 μg/mL at 10 mg/kg bw per day |
Loveless et al. (2008) |
| PFOA, 100% | CD(SD) rats, gavage (10–20 males, 0.3, 1, 10, 30 mg/kg bw per day, 29 days |
Body weight, haematopoiesis, but no immune effects |
No immune effects | N/A | NR | Loveless et al. (2008) |
| PFOA | BALB/c mice (8–10 females per group, diet, 4 mg/kg diet at day 2 of gestation though to 12 weeks of age |
Increased airway hyperresponsiveness |
N/A | 4 mg/kg diet | 4.8 μg/mL | Ryu et al. (2014) |
bw: body weight; IgM: immunoglobulin M; N/A: not applicable; NR: not reported; PFOA: perfluorooctanoic acid.
Figure H.2.
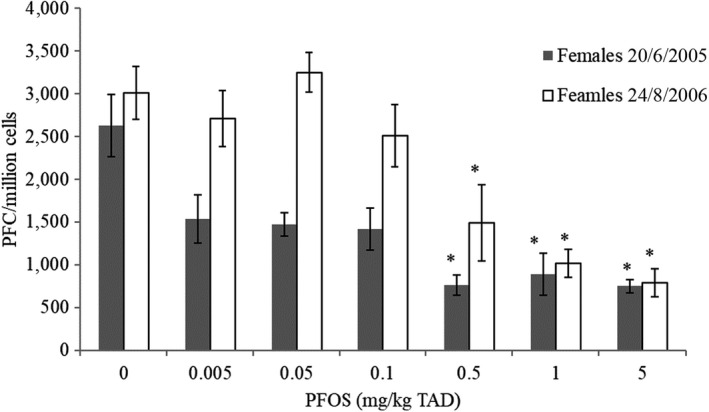
PFC response in female B6C3F1 mice treated with 0, 0.005, 0.05, 0.1, 0.5, 1 or 5 mg PFOS/kg bw (TAD) for 28 days by oral gavage (n = 5)
-
* Significantly different from control (p < 0.05). (Mean and SEM). Two independent experiments.PFC: perfluorinated compound; PFOS: Perfluorooctanesulfonic acid; SEM: standard error of mean; TAD: total applied dose.
Appendix I – In vitro genotoxicity of PFASs tables
1.
Appendix J – Human observations
J.1. Fertility and pregnancy outcomes
Preterm delivery
Papers previously reviewed only for PFOS and PFOA (EFSA CONTAM Panel, 2018)
Maisonet et al. (2012) examined the association between maternal serum concentrations of PFHxS (median: 1.6 ng/mL) with gestational age or preterm delivery in 447 singleton female infants from the ALSPAC cohort. No differences in PFHxS concentrations were observed among preterm cases and non‐cases. In a cohort of 638 pregnant women from Denmark, providing blood samples between gestation weeks 8–16, Lind et al. (2017a) examined differences in maternal serum concentrations (medians) of PFOS (8.1 ng/mL), PFOA (1.7 ng/mL), PFHxS (0.3 ng/mL), PFNA (0.7 ng/mL) and PFDA (0.3 ng/mL) among preterm cases and non‐cases. Non‐significant differences were observed. Similarly, Hamm et al. (2010) found no association with preterm delivery for PFHxS (mean 2.1 ng/mL) among 252 pregnant women in Canada. Chen et al. (2012) examined, among 429 pregnant women from Taiwan, the association between cord blood concentrations (means) of PFNA (2.4 ng/mL) and PFUnDA (10.3 ng/mL) with preterm delivery. No associations were observed. Bach et al. (2016) examined associations between serum concentrations (medians) of PFHxS (0.5 ng/mL), PFHpS (0.2 ng/mL), PFNA (0.8 ng/mL), PFDA (0.3 ng/mL) and PFUnDA (0.3 ng/mL) in 1,507 primiparous women from Aarhus Denmark with preterm delivery. No associations were observed.
Time to pregnancy
Bach et al. (2015) observed no association between maternal serum concentrations (medians) of PFHxS (0.5 ng/mL), PFHpS (0.2 ng/mL), PFNA (0.8 ng/mL), PFDA (0.3 ng/mL) and PFUnDA (0.3 ng/mL) with time to pregnancy, among 1,372 women from the Aarhus Birth Cohort (2008–2013). Serum samples were drawn during pregnancy prior to week 20 of gestation and time to the current pregnancy was recorded through questionnaires in early pregnancy.
In another study, 222 Danish women, planning their first time pregnancy (1992–1995), were followed for six menstrual cycles or until conception. No associations between pre‐pregnancy serum concentrations (medians) of PFHxS (~ 1.2 ng/mL) and PFNA (~ 0.5 ng/mL), PFDA (~ 0.1 ng/mL), and time to pregnancy (Vestergaard et al., 2012) were observed.
Jørgensen et al. (2014) examined the relationship between serum concentrations (medians) of PFHxS (1.9 ng/mL) and PFNA (0.6 ng/mL) with time to pregnancy in 938 pregnant women from Greenland (48%), Poland (22%) and Ukraine (30%). Time to pregnancy in the current pregnancy was based on self‐report and serum samples were drawn in the 2nd or 3rd trimester. PFNA was significantly associated with time to pregnancy of more than 13 months. No association was observed for PFHxS.
Vélez et al. (2015) examined associations between maternal serum concentrations of PFHxS (median 1.0 ng/mL) and subfecundity and infertility (defined as time to pregnancy > 12 months or infertility treatment for the current pregnancy) among 2,001 women recruited before week 10 of gestation in 10 cities across Canada in 2008–2011. Blood samples were drawn during the first trimester. Maternal PFHxS concentrations were associated with longer time to pregnancy, that was also reflected in increased odds of infertility (OR for infertility per 1‐SD increase in PFHxS: 1.3 (95%: 1.1, 1.5)). Results stratified by parity were not reported in this study, and no adjustment was made for parity.
A cohort of 501 US couples who were followed over a 12‐month period (Buck Louis et al., 2013) showed a significant association between maternal pre‐pregnancy concentrations of FOSA (mean: 0.1 ng/mL) and longer time to pregnancy.
As explained in the Opinion on PFOS and PFOA (EFSA CONTAM Panel, 2018), studies relying on samples drawn in the current pregnancy as proxy for pre‐pregnancy exposure are prone to reverse causation. The one study relying on pre‐pregnancy samples (Vestergaard et al., 2012) did not observe any association with time to pregnancy for PFHxS and PFNA. Longer time to pregnancy for FOSA in the study by Buck Louis et al. (2013) needs to be confirmed in an independent setting before any reliable conclusions can be drawn. Based on the small number of studies reviewed above there is little to suggest that PFNA, PFHXs or FOSA are associated with reduced fertility in terms of time to pregnancy.
Miscarriage
Using a case–control design nested within a prospective cohort of 2,874 women from Odense Denmark, Jensen et al. (2015) examined the association between serum concentrations (medians) of PFHxS (~ 0.3 ng/mL), PFNA (~ 0.7 ng/mL) and PFDA (~ 0.3 ng/mL) and the risk of miscarriage. Of the women recruited into the cohort, 88 suffered miscarriage and 56 of them had stored serum samples drawn prior to week 12 of gestation. These 56 cases were compared with 336 randomly selected controls from the cohort who also had serum drawn prior to week 12 of gestation. In addition, 51 miscarriage cases were compared to 204 controls that were matched on parity and gestational day of serum sampling. In serum samples drawn before week 12 of gestation (prior to miscarriage), higher serum concentrations were observed for miscarriage cases compared to non‐cases for PFNA (1.16 vs. 0.68. ng/mL) and PFDA (0.33 vs. 0.26 ng/mL). In terms of risk, both compounds showed a dose‐related increase in risk with an odds ratio of around 38 (95% CI: 10, 145) and 3.7 (95% CI: 1.6, 8.6) when comparing the highest to the lowest tertile of exposure to PFNA and PFDA, respectively.
Buck Louis et al. (2016) examined, in a cohort of 501 US couples, the association between pre‐pregnancy serum concentrations of several PFASs and pregnancy loss. Concentrations of (mean or % < LOD) of Et‐PFOSA‐AcOH (97% < LOD), Me‐PFOSA‐AcOH (0.3 ng/mL), FOSA (92% < LOD), PFDA (0.4 ng/mL) and PFNA (1.2 ng/mL) were quantified. Of these 501 women, 344 became pregnant and 98 of them suffered pregnancy loss. However, information on 24 out of the 344 pregnancies was missing due to loss to follow‐up. Concentrations of Me‐PFOSA‐AcOH showed, by linear trend test, an inverse (protective) association with pregnancy loss. For other compounds, no association with pregnancy loss was observed.
J.2. Developmental effects
PFHxS and PFNA
Neurodevelopment
Liew et al. (2014) studied the association between plasma PFAS levels in pregnant women and the risk of cerebral palsy (CP) in their children in a case–control study (156 CP cases and 550 controls) from the Danish National Birth Cohort (N = 1,400) recruited between 1996 and 2002. Cases were collected from a National CP register with validated diagnoses. A number of potential confounders were taken into account. Median PFHxS and PFNA levels were 0.9 and 0.4 ng/mL and not associated with risk of CP.
Liew et al. (2015) also performed a case–control study of attention deficit hyperactivity disorder (ADHD) and autism in the same cohort. Cases of ADHD (N = 220) and autism (N = 220) were randomly selected among cases identified in the cohort by linking with national disease registries, and 515 random controls were selected from the cohort, frequency‐matched for sex. A number of potential confounders were adjusted for. Median PFHxS and PFNA levels in maternal plasma were 0.9 ng/mL and 0.4 ng/mL. Relative risks in the fourth quartile of PFHxS and PFNA were 0.6 (95% CI 0.4–0.7) and 1.6 (95% CI 1.2–2.1) for ADHD, respectively, and 1.3 (95% CI 0.8–1.1) and 1.0 (95% CI 0.6–1.6) for autism, respectively.
Braun et al. (2014) examined associations between PFHxS and PFNA in pregnant mothers (N = 175) in Ohio (the HOME study), and autistic behaviour (a Social Responsiveness Scale, SRS, based on questionnaires to mothers) in their children at 4–5 years of age. The median levels were 1.6 ng/mL for PFHxS and 0.9 ng/mL for PFNA in the women (mostly at 16 weeks), recruited in 2003–2006. A number of potential confounders were adjusted for. There were no statistically significant associations between PFHxS or PFNA and results from the SRS scale.
The HOME cohort was also used to study associations between maternal serum PFHxS/PFNA (levels as above) and executive function (flexibility, goal planning and information processing), as estimated from a parent‐reported questionnaire (named BRIEF) in about 200 children at 5 and 8 years of age (Vuong et al., 2016). There were no consistent significant associations between maternal PFHxS or PFNA and executive functions in children.
In a Taiwanese cohort of 430 pregnant women, serum PFHxS and PFNA levels were measured in the third trimester (year 2000–2001) and then 120 children were followed up at 5 and 8 years of age for assessing IQ (Wang et al., 2015b). The median maternal serum PFHxS was 0.7 ng/mL, and for PFNA, the median was 1.4 ng/mL. After adjustment for a number of potential confounders, no statistically significant associations were found between maternal PFHxS/PFNA and children's IQ at 5 or 8 years of age.
Two Taiwanese birth cohorts were used to examine the association between PFNA in cord blood and ADHD‐related symptoms (parental questionnaires) in 282 children at 7 years of age (Lien et al., 2016). The response rate for follow‐up was low. The mean PFNA levels were 4.4 ng/mL. A number of potential confounders were adjusted for. Several scores for hyperactivity and inattention were positively associated with PFNA.
Oulhote et al. (2016) studied associations between maternal serum PFHxS and PFNA at the end of pregnancy and child behaviour (parent‐reported scales) in 539 Faroese children (born 1997–2000) at 7 years of age. In addition, associations between children's behaviour and their PFHxS/PFNA levels (at age 5 and 7) were examined. No associations between behavioural problems and maternal (medians PFHxS 4.5 ng/mL, PFNA 0.6 ng/mL) or children's levels at age 7 (PFHxS 0.5, PFNA 1.1 ng/mL) were found. There was, however, a significant association between serum PFNA levels (but not PFHxS) in the children at age 5 and unfavourable behavioural changes (see Section 3.3.4.4.2).
Dutch children were examined by Quaak et al. (2016) for associations between the sum of PFASs (including PFHxS and PFNA) in cord blood and parental‐assessed scales for ADHD and ‘externalising behaviour’ (attention problems and aggressive behaviour) in 59 children. A number of potential confounders were taken into account. No adverse associations were found.
Growth in infancy and childhood, overweight, metabolic risk factors and timing of puberty
Halldorsson et al. (2012) performed a longitudinal study of the association between PFNA in serum in 665 pregnant women, recruited in 1988–1989 from the Aarhus cohort, Denmark and risk of overweight (BMI, waist circumference, insulin, leptin and adiponectin) in their offspring at 20 years of age. The median PFNA level was 0.3 ng/mL. After adjustment for PFOA, no significant associations with these outcomes were reported.
Maisonet et al. (2012) studied the association between prenatal PFHxS (serum levels in pregnant mothers; median 1.6 ng/mL) and birth weight and weight at 20 months in 448 girls from Avon, UK (ALSPAC study). The girls were selected for another study (of menarche, see Christensen et al. (2011) below). A number of potential confounders were adjusted for. Findings on birth weight are reported in Section 3.3.4.1.11. No association was found with maternal PFHxS.
A Faroese mother–child cohort (recruited 2007–2009) was used by Karlsen et al. (2016) to study the associations between maternal PFHxS and PFNA levels and overweight at 18 months and 5 years of age. Median PFHxS and PFNA levels 2 weeks post‐partum were 0.2 and 0.7 ng/mL. A number of potential confounders were adjusted for. There were no significant associations between these maternal PFAS levels and risk of overweight.
Associations between maternal plasma PFHxS/PFNA in early pregnancy and adiposity in children were examined by Mora et al. (2017) in a US birth cohort. BMI, waist circumference and skinfold thickness were measured at about 3 and 8 years of age (1006, and 876 children, participation rate 61 and 53% of children with prenatal PFHxS/PFNA levels). At 8 years, also fat mass was estimated (using DXA). A number of potential confounders were adjusted for. Serum albumin and estimated pre‐pregnancy eGFR were also taken into account. Median maternal PFHxS and PFNA levels were 2.4 and 0.6 ng/mL. No consistent associations were found between maternal PFHxS/PFNA and adiposity at about 3 years of age or adiposity in boys at about 8 years. In girls, there were significant associations between PFHxS/PFNA and measures of adiposity at 8 years. Positive associations with PFHxS/PFNA remained after further adjustment for eGFR and albumin, but were attenuated when levels of several PFASs (PFOS, PFOA, PFHxS, PFNA) were all included in the models.
Fleisch et al. (2017) studied associations between prenatal (maternal plasma at about 10 weeks of gestation in 1999–2002) PFHxS and PFNA and glucose homoeostasis (glucose, insulin, HOMA‐IR), leptin and adiponectin in about 500 children from Boston at the median age of 8 years. A number of potential confounders were adjusted for. GM maternal PFHxS and PFNA were 2.5 and 0.7 ng/mL. Associations between prenatal PFHxS and PFNA and insulin resistance, leptin and adiponectin were null. As mentioned in Section 3.3.4.2, associations examined cross‐sectionally using PFASs in children's plasma showed an inverse association between PFNA and HOMA‐IR.
Associations between prenatal PFHxS and PFNA exposure (serum levels in mothers at week 16) and adiposity at the age of 8 in 204 US children from the HOME study were examined by Braun et al. (2016). GM for PFHxS and PFNA were 1.5 and 1.0 ng/mL. A number of potential confounders were adjusted for. There were no associations between PFHxS/PFNA and adiposity.
Using a nested case–control design, Christensen et al. (2011) examined the association between maternal concentrations of PFHxS (median 1.6 ng/mL), PFNA (median 0.6 ng/mL) and FOSA (median 0.2 ng/mL) with age of menarche in the British ALSPAC cohort. They selected 218 early menarche cases, which reported age at menarche before 11.5 years (median 11.1 years) and 230 random controls (mean age at menarche 12.6 years). Age at menarche was assessed through self‐reported questionnaires administered with 2‐year intervals between the age of 8–13. Serum PFAS concentrations were quantified in archived serum samples taken during pregnancy (1991–1992). No significant association was observed with early age of menarche with odds ratios centred around 1.
In the same cohort, Hartman et al. (2017) studied the association between prenatal PFHxS and PFNA (serum levels in pregnant mothers; median 1.6 ng/mL and 0.5 ng/mL) and body fat (measured by DXA) at age 9 years in 359 girls from Avon, UK (ALSPAC study). The girls were selected for a study of early menarche. A number of potential confounders were adjusted for. No associations were found between body fat and maternal PFHxS or PFNA.
In a Spanish mother–child cohort (INMA), Manzano‐Salgado et al. (2017b) examined associations between maternal PFHxS and PFNA and various outcomes at age 6 months, and/or 4 and 7 years (BMI, waist circumference, blood pressure, blood lipids and a cardiovascular risk score based on these factors) in their children (N = 1,086–1,230). Maternal GM levels of PFHxS and PFNA were 0.6 and 0.7 ng/mL. No significant associations were found with BMI, waist circumference or blood pressure, or total cholesterol. The metabolic score at age 4 years was positively associated with maternal PFNA.
Other PFASs
Some of the above‐mentioned studies also examined associations between the same outcomes as mentioned above and prenatal exposure to other PFASs than PFHxS and PFNA. This was the case for PFDA (Liew et al., 2014, 2015; Vuong et al., 2016; Oulhote et al., 2016; Wang et al., 2015b; Karlsen et al., 2016; Fleisch et al., 2017; Berg et al., 2017), PFUnDA (Wang et al., 2015b; Lien et al., 2016; Berg et al., 2017), PFDoDA (Wang et al., 2015b) and PFHpS (Berg et al., 2017). None of these studies showed significant associations between prenatal exposure to PFDA, PFUnDA, PFDoDA or PFHpS and adverse outcomes.
J.3. Neurotoxic Outcomes
Associations between PFASs and Attention Deficit Hyperactivity Disorder (ADHD) and learning problems in children were investigated by Stein and Savitz (2011). This was a cross‐sectional study in the C8 cohort exposed to PFOA from contaminated drinking water. Apart from levels of PFOS and PFOA, also PFNA and PFHxS were measured in serum in 2005–2006 in 10,000–11,000 children aged 5–18 years. The median PFNA and PFHxS levels were 1.5 ng/mL and 5.2 ng/mL. Classification of ADHD was based on interviews (diagnosis made in the health sector, or medication against ADHD). A number of potential confounders were adjusted for. There was no association between PFNA and ADHD. The OR was significantly increased in the three upper quartiles of PFHxS (upper limit of Q1 = 2.9 ng/mL) with ORs of 1.3–1.5, based on about 1,300 cases. The ORs were also increased in Q2–Q4 (ORs 1.4–1.6) for ADHD with medication (about 500 cases). ‘Learning problems’ were not more prevalent in the upper quartiles of PFNA or PFHxS.
In the C8 cohort, cross‐sectional associations between PFNA and PFHxS and self‐reported memory impairment were examined in 21,000 adults ≥ 50 years of age (Gallo et al., 2013). The GM PFNA and PFHxS levels in 2005–2006 were 1.4 and 3.2 ng/mL. A number of potential confounders were adjusted for. About 20% reported some memory impairment. Statistically significant inverse (‘protective’) associations were found between PFNA as well as PFHxS and memory impairment. The OR for memory impairment was 0.89 (95% CI 0.80–0.99) for Q5 vs. Q1 of S‐PFNA. For S‐PFHxS, the OR was 0.89 (95% CI 0.79–0.99). The authors discuss anti‐inflammatory effects of PFNA/PFHxS (via PPARγ) or residual confounding as possible explanations.
Gump et al. (2011) studied the association between PFNA, PFDA, PFHxS and FOSA, and impulsivity in a cross‐sectional study of 83 children aged 9–11 years from north‐west USA. Impulsivity was assessed by a procedure reinforcing delayed response in a computer task, which could be learned during the task. Median PFNA and PFDA levels were 0.7 and 0.3 ng/mL. Median PFHxS and FOSA levels were 3.7 and 0.6 ng/mL. A number of potential confounders were taken into account. Higher levels of these four compounds were inversely associated with the (reinforced) response, which was assumed to indicate less ability to inhibit the optimal delay in response, and thus increased impulsivity.
Power et al. (2013) performed a cross‐sectional study on the associations between serum levels of PFNA and PFHxS and self‐reported memory problems or confusion periods in about 1,800 individuals, aged 60–85 years, from the U.S. NHANES 1999–2008. A number of potential confounders were taken into account. The GM for PFNA was 1.0 ng/mL and for PFHxS 2.1 ng/mL. The authors found no significant associations between PFNA or PFHxS and these cognitive symptoms. The OR for memory problems or confusion was 0.91 (0.79–1.04) for a doubling of serum PFNA and 0.93 (95% CI 0.82–1.06) for a doubling of serum PFOA. The OR for memory problems was significantly below 1.0 in some subanalyses of diabetics both for PFNA and PFHxS.
Berk et al. (2014) studied the cross‐sectional association between self‐reported depressive symptoms and serum levels of FOSA, Me‐PFOSA‐AcOH, Et‐PFOSAAcOH, PFBS, PFHxS, PFOS, PFHpA, PFNA, PFDA, PFUnDA and PFDoDA in 5,400 individuals > 18 years in NHANES surveys 2005–2010. Levels are not presented. There were relatively strong inverse (‘protective’) associations between levels of PFNA, PFDA and PFHxS and depressive symptoms after adjustment for some potential confounders: OR 0.62 (95% CI 0.42–0.92) for Q4 vs. Q1 of PFNA, and OR 0.62 (95% CI 0.45–0.85) for Q4 vs. Q1 of PFDA, and 0.66 (95% CI 0.47–0.93) for PFHxS.
As mentioned above, Oulhote et al. (2016) studied associations between PFHxS and PFNA at age 5 and 7 and child behaviour (parent‐reported scales) in 539 Faroese children (born 1997–2000) at 7 years of age. No associations between behavioural problems and children's levels at age 7 (PFHxS 0.5, PFNA 1.1 ng/mL) were found. There was, however, a significant association between serum PFNA levels (but not PFHxS) in the children at age 5 and unfavourable behavioural changes.
In a nested case–control from the Danish National Birth Cohort Long et al. (2019) examined associations between concentrations of PFHpS, PFBS, PFHxS, PFDS, FOSA, PFHxA, PFHpA, PFNA, PFDA, PFUnDA, PFDoDA, PFPeA in amniotic fluid in relation to autism spectrum disorders (75 cases and 135 matched controls). Concentrations in amniotic fluid were much lower (< LOQ up to ~ 1 ng/mL for the median) compared to levels observed in maternal serum from the same cohort (Ernst et al. 2019). No increase in risk of autism spectrum disorders was observed. If anything, the associations suggested a modest reduction in risk.
J.4. Immune outcomes
Asthma and allergies in children and adults
Prospective studies – New publications not reviewed in the 2018 Opinion (EFSA CONTAM Panel, 2018)
Manzano‐Salgado et al. (2019) examined associations between maternal concentrations (means) of PFOS (6.1 ng/mL), PFHxS (0.6 ng/mL), PFOA (2.4 ng/mL) and PFNA (0.7 ng/mL) and certain outcomes. Blood samples were drawn during the first trimester. Information on the occurrence of lower respiratory tract infections, wheezing, asthma, and eczema was obtained by parental report at ages 1, 1.5, 4 and 7 years (n = 1,071 to 1,188). Lung function was also assessed at age 4 years (n = 992). Overall, no associations between PFASs and any of the outcomes reported were observed. PFOS was significantly inversely associated with eczema.
Based on the 641 mother–child pairs from the Environment and Childhood Asthma Study from Oslo, Impinen et al. (2018) examined the association between cord blood concentrations (medians) of PFOA (1.6 ng/mL), PFNA (0.2 ng/mL), PFUnDA (0.1 ng/mL), PFOS (5.2 ng/mL), PFHxS (0.2 ng/mL) and FOSA (0.4 ng/mL) and offspring lung function at birth, and asthma and allergy at 2 and 10 years. Information on offspring asthma, allergy and lung function (spirometry test) at ages 2 and 10 years were based on parental report and clinical examination. Of the 100 different comparisons made for asthma and allergies (17 different outcomes with 6 substances), only four comparisons reached statistical significance, which is not more than what would be expected by chance.
In a study from the Norwegian Mother and Child Cohort, Impinen et al. (2019) examined associations between maternal concentrations (median) of PFOS (12.3 ng/mL), PFOA (2.5 ng/mL), PFHxS (0.7 ng/mL), PFUnDA (0.2 ng/mL) and PFHpS (0.2 ng/mL) and offspring risk of allergies and asthma (doctor diagnosed and parental reports) at 3 years (n = 1,270) and 7 years (n = 972). Overall, no association of an association with asthma or allergies was observed.
In a Faroese birth cohort (1997–2000) of 559 mother–child pairs with prospective follow‐up, Timmermann et al. (2017b) examined the association between maternal concentrations of (medians) PFNA (0.6 ng/mL), PFDA (0.3 ng/mL) and PFHxS (4.2 ng/mL) and asthma and allergies in the offspring assessed by parental report at ages 5 and 13 years. Associations with immunoglobulin E and A (IgE, IgA) levels measured at age 7 years were also explored. In addition, the offspring's own concentrations at age 5 of (medians) PFNA (1.0 ng/mL), PFDA (0.3 ng/mL) and PFHxS (0.6 ng/mL) and age 13 years (similar median concentrations as at age 5) were examined in relation to asthma and allergies at ages 5 and 13 years. In short, neither maternal concentrations of PFNA, PFDA and PFHxS nor the offspring's own concentrations at age 5 were associated with asthma, allergy or immunoglobulin levels at age 7 and no association was observed cross‐sectionally at age 13. The authors also performed secondary analyses among those 22 subjects that had not been vaccinated to measles, mumps and rubella prior to age 5 and observed elevated risk of asthma and allergy with higher PFAS concentrations at age 5 and 13 years. Although interesting, those analyses were based on far too few subjects to allow for any meaningful conclusions.
In a cohort of 675 Norwegian adolescents, aged 13–19 years, Averina et al. (2019) examined both cross‐sectionally and prospectively the association between concentrations (medians) of the sum of 18 PFASs (11 ng/mL), PFOA (2 ng/mL), PFOS (6.5 ng/mL) and PFHxS (1 ng/mL) and asthma and allergies. Outcome assessments were based on both self‐report and clinical examinations. Cross‐sectionally, both the sum of 18 PFASs and PFOS were positively associated (linear trend test) with self‐reported doctor‐diagnosed asthma. A positive but non‐significant association was observed for PFOA and PFHxS. No consistent associations were observed for other PFASs. Prospectively, similar elevated odds ratios for asthma were observed for the sum of 18 PFASs and PFOS in a follow‐up 3 years later, although a test for linear trend did not reach statistical significance. In that follow‐up, the sum of 18 PFASs and PFHxS were positively associated with eosinophilic airway inflammation (defined as FeNO > 25 ppb). For all compounds, no consistent associations were observed for self‐reported pollen allergy, food allergy and atopic eczema (cross‐sectional analyses).
Prospective studies – Papers previously reviewed only for PFOS and PFOA (EFSA CONTAM Panel, 2018)
Wang et al. (2011b) examined the association between serum cord blood concentrations (median) of PFNA (2.3 ng/mL)) with both cord blood levels (cross‐sectional) and offspring levels (prospective) of IgE and atopic dermatitis at age 2 years (n = 244). No associations were observed in cross‐sectional or prospective analyses.
Okada et al. (2014) examined 2,063 mother–child pairs from the Hokkaido Study on Environment and Children's Health (2003–2009) for associations between maternal concentrations (medians) of PFHxS (0.3 ng/mL), PFHxA (< 0.1 ng/mL), PFHpA (< 0.1 ng/mL), PFNA (0.9 ng/mL), PFDA (0.4 ng/mL), PFUnDA (1.0 ng/mL), PFDoDA (0.1 ng/mL), PFTrDA (0.3 ng/mL) and PFTeDA (< 0.1 ng/mL) and allergic diseases in the offspring at 12 and 24 months postpartum. Allergic diseases were defined using a modified part of the Japanese version of the International Study of Asthma and Allergies in Childhood (ISAAC) and associations with allergic diseases and eczema were reported. No positive associations between any of the PFAS quantified and allergic disease or eczema were observed. If anything, the observed associations were in some cases inverse (protective).
In a cohort of 1,558 mother–child pairs form Japan with offspring follow‐up at 4 years of age, Goudarzi et al. (2016) examined associations between pregnancy concentrations (medians) of PFHxS (0.3 ng/mL), PFNA (0.9 ng/mL), PFDA (0.4 ng/mL), PFUnDA (1.0 ng/mL), PFDoDA (0.1 ng/mL) and PFTrDA (0.2 ng/mL) and prevalence of allergic diseases in the offspring (wheezing, eczema and rhinoconjunctivitis symptoms). The prevalence of offspring allergy was assessed based on maternal report using a modified section of the Japanese version of the International Study of Asthma and Allergies in Childhood (ISAAC). No increase in risk for total allergic diseases or wheeze was observed with higher concentrations of these compounds. If anything, the associations were in the direction of being protective.
Cross sectional studies – Papers previously reviewed only for PFOS and PFOA (EFSA CONTAM Panel, 2018)
Dong et al. (2013) examined associations between serum concentrations (medians) of several PFASs, i.e. PFOS (~ 31 ng/mL), PFHxS (~ 2 ng/mL), PFBS (~ 0.5 ng/mL), PFOA (median ~ 1 ng/mL), PFDA (~ 1 ng/mL), PFDoDA (~ 3 ng/mL), PFHpA (~ 0.2 ng/mL), PFHxA (~ 0.2 ng/mL), PFNA (~ 1 ng/mL) and PFTeDA (~ 5 ng/mL), and asthma in 10‐ to 15‐year‐old Taiwanese children. The study recruited 231 children who had received doctor diagnosis of asthma in the previous year and 225 non‐asthmatic controls. Significant and positive dose‐response associations with asthma (p for trend < 0.05) were observed for PFOA, PFDA, PFDoDA and PFNA. For these acids the odds ratios when comparing the highest to the lowest quartile ranged from 1.6 to 4.1. Significant associations were also observed for the three sulfonates, PFOS, PFHxS and PFBS (corresponding odds ratios ranging between 1.9 and 3.8). Among asthmatic cases, mean serum concentrations of immunoglobulin E, absolute eosinophil counts and eosinophilic cationic protein concentrations also tended to increase across quartiles of PFAS concentrations. Severity of asthma also appeared to increase with higher concentrations. The Spearman correlation coefficient between individual PFASs ranged from 0.79 (PFDA and PFNA) to 0.02 (PFHpA and PFTeDA).
Using a subset (300 out of 456) of the children from this study, Qin et al. (2017) measured lung function by spirometry in 168 controls and 132 asthma cases. In line with previous findings from this cohort, positive associations between PFOS, PFHxS, PFOA, PFDA and PFNA and asthma were observed. In contrast to findings by Dong et al. (2013), no association was observed for PFBS and a positive association was observed for PFTeDA. Among asthmatic cases, PFOS, PFNA and PFHxS were associated with reduced pulmonary function in terms of forced vital capacity (FVC) and forced expiratory volume in the first second (FEV1), while PFOA was only associated with FEV1. Compared to their previous study, these inconsistencies may, at least partly, relate to lower participation rate.
Buser and Scinicariello (2016) examined in adolescents aged 12–19 years from the NHANES (2005–2006) study, the cross‐sectional association between serum concentrations (medians) of PFHxS (2.1 ng/mL) and PFNA (0.9 ng/mL) with food sensitisation (defined as having at least one food‐specific IgE level ≥ 0.35 kU/L) and food allergies (self‐reported yes to the question ‘What foods are you allergic to’) in 637–701 participants. Non‐significant associations were observed between PFNA and PFHxS with food sensitisation and self‐reported food allergies.
A US study examined associations between serum concentrations of (medians) PFNA (~ 1.0 ng/mL) and PFHxS (~ 4 ng/mL) with respiratory conditions among 458 New York State (NYS) employees and National Guard personnel working near the World Trade Centre after its collapse (Tao et al., 2008). Non‐significant differences in concentrations for PFNA and PFHxS were observed among subjects classified as having symptomatic and asymptomatic respiratory conditions.
In a study of 12‐ to 19‐year‐old children and young adults (n = 640) from the US NHANES, Stein et al. (2016a) examined the associations between (means) PFNA (0.9 ng/mL) and PFHxS (2.1 ng/mL) with self‐reported asthma (n = 70), wheeze (n = 70), allergy (n = 102) and rhinitis (n = 164). Sensitisation to 19 different allergens was also measured (plants, dust mite, pets, cockroach, shrimp, rodents, mould and food). No consistent associations were observed between the different PFAS quantified and outcomes for asthma and allergies.
In a pregnancy cohort of 1,258 women, Ashley‐Martin et al. (2015) examined the cross‐sectional association between cord blood concentrations of PFHxS (mean:1.0 ng/mL) and Immunoglobulin E, thymic stromal lymphopoietin and interleukin‐33. No significant associations were observed.
J.5. Endocrine Effects
Thyroid Function and disease
PFHxS and PFNA
PFHxS and PFNA are the most studied compounds (apart from PFOS and PFOA). Therefore, they are summarised first (listed in Table J.1).
Adults
Bloom et al. (2010) compared serum PFHxS/PFNA levels, cross‐sectionally, with levels of thyroid hormones in 31 New York anglers. No significant associations were found.
Chan et al. (2011) performed a cross‐sectional study of pregnant (15–20 weeks) Canadian women subject to prenatal screening. Out of 974 maternal sera, the authors selected all women (N = 96 ‘cases’) with normal TSH and low (< 10th percentile) free T4. Age‐matched controls (N = 175) were selected among women with normal TSH and free T4 levels between the 50th and 90th percentiles. PFHxS was measured in these sera. The a priori hypothesis was that PFHxS would cause hypothyroxinaemia (still with normal TSH), but no such association was found.
Ji et al. (2012) examined the association between serum concentrations of PFHxS/PFNA and total T4 and TSH among 633 Koreans aged < 12 years. Median serum levels were 1.5 ng/mL for PFHxS and 2.1 ng/mL for PFNA. A number of potential confounders were adjusted for. No associations were found with TSH or T4.
Jain (2013) examined associations between PFHxS/PFNA (levels not reported) and thyroid hormones in 1,540 individuals ≥ 12 years from the NHANES survey 2007–2008. Those with thyroid problems or medications, current pregnancy or missing variables had been removed. A number of potential confounders were taken into account. No associations were found with free T3, free T4 or TSH.
Wen et al. (2013) studied associations between PFHxS/PFNA levels and thyroid hormones in adults in US NHANES 2007–2010. Subclinical hyper‐ and hypothyroidism was defined from low and high serum TSH, respectively. There was a positive association between PFHxS and both hyper‐ and hypothyroidism, but this was not supported by any association between PFHxS and TSH, with the latter as a continuous variable.
Wang et al. (2013b) examined the association between PFHxS/PFNA and TSH in 903 pregnant women (about 18th week of gestation) in the Norwegian Mother and Child cohort, MoBa. About half of the women had been selected due to subfecundicity. Those who reported previous thyroid disease were excluded. The GM of serum PFHxS and PFNA were 0.6 and 0.4 ng/mL, respectively. Several potential confounders were adjusted for. PFHxS was positively associated with free T4, but not with TSH. No associations were found between PFNA and thyroid hormones.
Lin et al. (2013a) examined associations between PFNA (GM 1.5 ng/mL) and thyroid hormones (TSH, free T4) in 567 Taiwanese individuals aged 12–30 years. A number of potential confounders were adjusted for. PFNA was positively associated with free T4, but not with TSH.
Wang et al. (2014c) performed a cross‐sectional study of PFHxS/PFNA and thyroid hormones (TSH, T3, T4 and free T4) in 285 pregnant women (third trimester) in Taiwan with no known thyroid disease, and thyroid hormones were also measured in cord blood in 116 of their neonates. The median maternal PFHxS and PFNA concentrations were 0.8 and 1.5 ng/mL, respectively. Several potential confounders were adjusted for. In women, PFHxS was positively associated with TSH, but not with free T4, total T4 or total T3 and not with thyroid hormones in the newborns. PFNA was inversely associated with free T4 and total T4 but not with TSH. Maternal PFNA was inversely associated with total T4 and total T3, but not with free T4 or TSH.
Webster et al. (2014) examined associations between PFHxS/PFNA and thyroid hormones (free T4 and TSH) in 152 pregnant (measured twice in early 2nd trimester) Canadian nonsmoking women. The median serum PFHxS and PFNA levels were 1.0 and 0.6 ng/mL. Analyses were adjusted for week of gestation and presence of TPO antibodies (positive in 14 women, indicating possible autoimmune thyroid disease). There was a positive association between PFNA and TSH, but no association with free T4, and no associations between PFHxS and thyroid hormones.
Lewis et al. (2015) studied associations between PFHxS/PFNA and thyroid hormones (TSH, T4, free T4, T3, free T3) in 1,682 individuals 12–80 years enrolled in NHANES 2011–2012. Median serum PFHxS and PFNA levels were, respectively, 0.8–1.8 ng/mL and 0.7–1.1 ng/mL (in various age groups in men and women). There were no overall associations between PFHxS/PFNA and thyroid hormones. In results stratified by sex and four age groups, there were a few significant findings for the most important hormones (TSH, free T4 and free T3): a positive association between PFNA and TSH in male adolescents, and a positive association between PFNA and free T4 in women 20–39 years. But many (24) such associations were tested.
Berg et al. (2015) examined associations between PFHxS/PFNA and thyroid hormones in 375 pregnant women in Norway. PFHxS/PFNA were measured around gestation week 18, and thyroid hormones (TSH, free T4, T4, free T3, T3), as well as thyroid hormone binding proteins were measured in the same samples, and also 3 days and 6 weeks postpartum. Median PFHxS and PFNA levels were 0.4 and 0.6 ng/mL. Some potential confounders were adjusted for and also binding proteins in repeated measures analyses (mixed effects models). No significant associations were found with hormone levels.
Berg et al. (2017) also reported on associations between PFHxS/PFNA and thyroid hormones in these pregnant women in Norway and TSH in their infants at birth. Heel prick TSH in infants was measured 3 days after birth. Some potential confounders were adjusted for. No significant associations were found with maternal thyroid hormones or infant TSH.
Webster et al. (2016) studied associations between PFHxS/PFNA and thyroid hormones (TSH, T4, free T4, T3, free T3) in 1,525 adults from NHANES 2007–2008, excluding those with a history of thyroid disease. GM PFHxS and PFNA levels were 1.9 and 1.5 ng/mL. Several confounders were adjusted for, and results were also stratified for the presence of thyroid peroxidase antibodies (TPOab, present in 9%, suggesting possible autoimmune thyroid disease) and urinary iodine classified as low (< 100 μg/L) in 26%. In individuals with normal urinary iodine and no TPOab there were no associations between PFHxS/PFNA and thyroid hormones. However, in 26 individuals with TPOab and low urinary iodine, there were positive associations between PFHxS/PFNA and TSH, and free T3, and an inverse association with free T4 (PFHxS only). The authors suggest that the results could support a ‘multiple hit’ hypothesis with those who have TPO antibodies and low urinary iodine (about 1% of the US population) being a sensitive group.
In a study of 157 mother–infant pairs, Yang et al. (2016) examined cross‐sectional associations between maternal concentrations of PFHxS/PFNA and maternal and infant thyroid hormones (TSH, free T4, T4, free T3, T3). Median maternal serum PFHxS and PFNA were 0.5 ng/mL for both compounds, while cord serum levels were 0.2 ng/mL for PFHxS and 1.2 ng/mL for PFNA. In analyses adjusted for potential confounders, there was an inverse association between maternal PFNA and maternal TSH. There were no associations between maternal or infant PFHxS/PFNA and infant thyroid hormones.
Crawford et al. (2017) studied associations between PFHxS/PFNA (GM 1.6 and 0.8 ng/mL) and thyroid hormones (in 99 US women attempting to conceive). There was a positive association between PFNA and free T4, but no association with TSH, and no association between PFHxS and thyroid hormones. Only age was adjusted for.
Li et al. (2017) examined associations between PFHxS (median 0.2 ng/mL) and thyroid hormones in 202 persons (mainly adults, but two infants and 10 adolescents, which had been selected in order to include both participants with normal (N = 62) and ‘abnormal’ levels (N = 140) of thyroid hormones (TSH, free T4 or free T3). In analyses adjusted for age and sex but not for region, there were no significant associations between PFHxS and thyroid hormones.
In summary, for PFHxS, only one of 13 studies in adults showed an overall positive association with TSH (Wang et al., 2014c) and no studies showed any overall association with free T3 or free T4. In addition, in a small study subgroup with positive TPO antibodies and low iodine (Webster et al., 2016; N = 26), there was a positive association with TSH, and free T3, and an inverse association with free T4. There were few studies in newborns/infants and no consistent findings. For PFNA, out of 12 studies in adults, there was one study in pregnant Chinese women (Yang et al., 2016) sampled just before delivery that showed an inverse association with TSH, while a study in pregnant Canadian women (Webster et al., 2014) showed a positive association with TSH. In addition, the small study subgroup with positive TPO antibodies and low iodine by Webster et al. (2016) showed a positive association with both TSH and free T3. Two small studies (Lin et al., 2013a, Crawford et al., 2017) showed positive associations with free T4, while another (Wang et al., 2014c) showed an inverse association. Most studies showed no significant associations. There were few studies in newborns/infants and no consistent findings.
Newborns and children
Lopez‐Espinosa et al. (2012) examined cross‐sectional associations between PFNA (median 1.5 ng/mL) with total T4 and TSH among about 12,000 children in the C8 cohort aged 1–17 years. A number of potential confounders were adjusted for. There was no significant association with parent‐reported thyroid disease with serum PFNA, but the number of cases was small (N = 39).
As mentioned above, Wang et al. (2014c) found no significant associations between PFHxS/PFNA in maternal serum and cord blood free T4 or TSH.
Shah‐Kulkarni et al. (2016) examined associations between PFHxS/PFNA and thyroid hormones (TSH, T4, T3) in cord blood of 279 newborns from Seoul, Korea. GM levels were 0.4 (PFHxS) and 0.2 (PFNA) ng/mL. Several potential confounders were adjusted for. In girls, but not in boys, there was an inverse association between PFNA and TSH. There were no associations between PFHxS and TSH.
Kim et al. (2016b) compared serum concentrations of PFHxS/PFNA between 27 cases of infants with congenital hypothyroidism and 13 control infants. Concentrations of PFNA (median: 1.9 vs. 0.6 ng/mL) were significantly higher in the cases compared to controls, while PFHxS levels were similar (1.2 ng/mL for both groups). Maternal PFOS/PFOA levels were not available.
As mentioned above, Yang et al. (2016) found no associations between maternal or infant levels of PFHxS/PFNA and infant thyroid hormones.
The above‐mentioned studies are summarised in Table J.1.
Other PFASs
There are 11 studies on associations between PFASs other than PFOS, PFOA, PFHxS, and PFNA and thyroid disease or function, namely Bloom et al. (2010), Ji et al. (2012), Jain (2013), Wang et al. (2013b), Lin et al. (2013a), Wang et al. (2014c), Berg et al. (2015), Shah‐Kulkarni et al. (2016), Kim et al. (2016b), Yang et al. (2016), and Li et al. (2017). For PFASs examined, see Table J.1. There were no consistent associations (reported in more than one study) between these other PFASs and thyroid disease or function.
Male fertility and puberty
Di Nisio et al. (2019) examined the association between exposure PFOA and fertility males from the Veneto region in Italy. The study was conducted within routine reproductive health survey and included 383 subjects (median age 18 years, range 18–24 years), which were clinically examined and provided semen samples. A subset of these participants (n = 212) were living in areas with known environmental PFOA contamination. To verify differences in exposure serum samples from 50 subjects from non‐contaminated area (controls) and 50 subjects form the contaminated areas (exposed) were quantified. The median concentrations for PFOA among exposed vs. controls was 7.4 and 4.7 ng/mL, respectively. For the full sample anogenital distance, testicular volume and penis length were smaller among subjects in the exposed vs. controlled area (p < 0.001). Sperm concentrations and subjects with % normal morphology were also lower among subjects form the exposed area. When restricting the analyses to subjects with quantified PFOA levels (n = 50/50), similar differences were observed for genital development while significant differences were not observed for semen quality. Serum PFOA concentrations were also positively associated with testosterone. Even though these results suggest that residential exposure may affect semen quality, this simple ecological comparison between subjects presumed to be exposed vs. controls, is prone to confounding. The exposure gradients between controls and exposed as reported from a subsample of study participants was also modest, and no associations based on actual individual measured concentrations were reported for comparison. Differences in penis length, testicular volume and anogenital distance reflect different genital development during earlier years and are difficult to interpret in relation to current adult exposure to PFOA.
In a sample of 105 Danish men from the general population, Joensen et al. (2009) examined associations between several PFAS and semen quality and reproductive hormones. Serum PFAS concentrations were (medians in ng/mL) 6.6 for PFHxS, 0.2 for PFHpA, 0.8 for PFNA, 0.9 for PFDA, 0.1 for PFUnDA, 0.08 for PFDoDA, < 0.01 for PFTrDA and 0.06 for FOSA. As reviewed previously (EFSA 2008), the sum of PFOS and PFOA was associated with reduced normal spermatozoa. No results were, however, reported for other PFAS. Concerning reproductive hormones, FOSA was associated with higher testosterone concentrations while no associations were observed for other PFASs.
Toft et al. (2012) examined associations between PFHxS (1.1 ng/mL) and PFNA (1.2 ng/mL) and semen quality in a cohort of 588 men from Greenland, Poland and Ukraine. Overall no consistent associations with semen quality were observed.
In cross‐sectional analyses among 103 Chinese males living in an area with high industrial activity, Song et al (2018b) examined correlations between several PFAS measured in blood and semen quality. Blood concentrations (medians in ng/mL) were 3.0 for PFBA, 0.6 for PFPrA, 3.0 for PFPeA, 29 for PFHxA, 0.2 for PFHBS, 0.4 for PFHpA and 3.9 for PFHxS. PFAS concentrations in blood and semen were not correlated with sperm concentrations. Blood concentrations for individual PFAS showed varying concentrations (positive and inverse) with progressive sperm motility. Overall no consistent associations were observed between PFBA, PFPrA, PFPeA, PFHxA, PFHBS, PFHpA and PFHxS and semen quality.
In a subcohort within the Danish National birth cohort, Ernst et al. (2019) examined the associations between maternal concentrations of PFAS and pubertal development in 455 mother–child pairs. Median (in ng/mL) serum concentrations (1st or 2nd trimester samples) were ~ 1 for PFHxS, ~ 0.5 for PFNA and ~ 0.2 for PFDA. Outcomes assessment was based on self‐report and covered questions on Tanner stages 2–5 for pubic hair, breast and genital development, as well as axillary hair growth and acne. In addition, information on age at first ejaculation and voice break (for boys) and age at menarche (for girls) was reported. Overall maternal concentrations of different PFAS appeared to suggest slightly slower pubertal development in both boys and girls. However, few associations reached formal statistical significance, consistency with different outcomes measures was in many cases absent and clear dose response was not observed.
Female fertility, menstrual cycle and puberty
In a cohort of 495 women aged 18–44 scheduled for laparoscopy in Salt Lake City US, Buck Louis et al. (2012) examined associations between PFNA, PFDA and PFHxS and endometriosis (mean or median concentrations not reported). The same associations were also examined among 131 women from the same area who were not seeking clinical care. After adjustment for covariates, a non‐significant increased risk was observed for PFNA and PFDA among women seeking clinical care (n = 495). In the smaller sub‐sample of women not seeking clinical care (n = 131), similar non‐significant associations were also observed but with much more imprecise/wide confidence intervals.
With a case–control design, Zhang et al. (2018) examined associations between (medians in ng/mL) PFNA (~ 2), PFDA (1.8), PFUnDA (1.3), PFDoDA (0.2), PFHpA (0.2), PFHxS (0.3) and PFBS (0.05) and primary ovarian insufficiency among 120 diagnosed cases and 120 healthy controls. A significant and positive association was observed between PFHxS and primary ovarian insufficiency. No association was observed for other PFASs. Associations between individual PFASs and reproductive hormones (FSH, luteinising hormone, estradiol and prolactin) were also explored among cases only. In those analyses, an inverse association was observed between PFHxS and estradiol.
In a cohort of 2,731 women form the NHANES study, Taylor et al. (2014) examined associations between PFNA (median: ~ 1.1 ng/mL) and PFHxS (median: 1.3 ng/mL) with age at natural menopause among women aged 20–65 years. Positive association with PFHxS and PFNA for age at natural menopause was observed. Much stronger association was also observed between these two substances and rate of hysterectomy. In addition, the authors noted that the concentrations of these two substances increased with time since natural menopause. Those findings strongly suggest that the association between PFNA and PFHxS and natural age at menopause are driven by reverse causation.
Singer et al. (2018) examined in a cohort of 1977 Norwegian pregnant women, the associations between several PFAS and menstrual cycle characteristics. Median (in ng/mL) levels of PFAS in maternal serum drawn around gestation week 17 were 0.33 for PFNA, 0.09, 0.13 for PFDA, 0.21 for PFUnDA, 0.65 for PFHxS and 0.15 for PFHpS. Overall no consistent associations were observed between individual PFASs and irregular menstrual cycles.
Using a case–control design, Heffernan et al. (2018) examined the associations between several PFAS and polycystic ovarian syndrome (PCOS) among 29 cases and 30 controls matched for age and BMI. Mean serum concentrations (in ng/mL) were 1.0 for PFHxS, 0.6 for PFNA and 0.41 for PFDA. No associations were observed for these substances. In this study associations with biomarkers of glucose homoeostasis (fasting glucose, insulin HbA1 and HOMA‐IR) and reproductive hormones (testosterone, SHBG, FAI, androstenedione and oestradiol) were also explored for cases and controls separately. Some inconsistent associations were observed among cases and controls. Given the modest statistical power (n ~ 30), limited conclusions can be drawn.
Using a case–control design, Wang et al. (2019b) examined the associations between PFASs and polycystic ovarian syndrome (PCOS) in Chinese women. A total of 180 infertile PCOS cases and 187 healthy controls were recruited into the study. Medium (ng/mL) plasma PFAS concentrations were 0.11 for PFBS, 0.08 for PFHpA, 0.24 for PFHxS, 0.52 for PFNA, 0.45 for PFDA, 0.40 for PFUnDA, 0.24 for PFDoDA and 12.16 for the sum of all PFASs, including PFOS and PFOA. PFDoDA was significantly associated with increased odds of PCOS‐related infertility, while PFUnDA showed a significant inverse association.
With a nested design, the UK ALSPAC cohort was used by Christensen et al. (2011) to examine the association between different PFAS and age at menarche. Maternal pregnancy samples among 218 female cases who had age at menarche before the age of 11.5 y and 230 randomly selected controls were quantified. Median concentrations (in ng/mL) in pregnancy serum were 0.2 for FOSA, 0.6 for Et‐PFOSA‐AcOH, 0.4 for Me‐PFOSA‐AcOH, 19.8 for PFOS, 1.6 for PFHxS, 3.7 for PFOA, 0.6 for PFNA. Overall no consistent associations with age at menarche were observed.
Regarding puberty in girls, see also the study by Ernst et al. (2019) in Section 3.3.4.5.2.
Duration of Breast Feeding
In the Opinion on PFOS and PFOA (EFSA CONTAM Panel, 2018), three human studies on duration of breastfeeding were reviewed and it was concluded that shorter duration reported for higher concentrations of PFOS and PFOA was likely to be caused by reverse causation. This conclusion was based on inconsistent findings from nulliparous and parous women (Fei et al., 2010b).
Concerning other PFASs than PFOS and PFOA, Timmermann et al. (2017a) examined associations between serum PFOS and PFOA concentrations and duration of breastfeeding by combining two Faroese birth cohorts formed in 1997–2000 and 2007–2009 with 640 and 490 pregnant women, respectively (n = 1,130 in total). Both cohorts relied on serum samples drawn in week 34–36 of gestation. Duration of breastfeeding was based on maternal report at 18 months in the 1997–2000 cohort. However, in the 2007–2009 cohort, duration of breastfeeding was assessed by maternal report, 5 years post‐partum. In these combined data, the median was 1.5 ng/mL for PFHxS, 0.6 ng/mL for PFNA and 0.3 ng/mL for PFDA. PFNA and PFDA were significantly and inversely associated with duration of breastfeeding.
For the inverse association with duration of breastfeeding reported by Timmermann et al. (2017a), the same mechanism for reverse causation exists as explained for PFOS and PFOA (EFSA CONTAM Panel, 2018) Overall, the existing evidence is too weak to conclude that PFASs may adversely affect duration of breastfeeding.
Other endocrine effects
In a cohort of 349 Chinese pregnant women, Yao et al. (2019) examined cross‐sectional associations between several PFAS in cord blood and reproductive hormones (estradiol and testosterone) and steroidogenic enzymes (P450arom, 3β‐HSD1, 17β‐HSD1). Median cord blood concentrations (ng/mL) were 0.2 for PFBS, 0.2 for PFDA, 0.1 for PFDoDA, 0.1 for PFHpA, 0.2 for PFHxS, 0.3 for PFNA, 0.1 for FOSA and 0.1 for PFUnDA. PFHxS was positively associated with estradiol while PFUnDA and PFNA were positively associated with testosterone. PFHxS was positively associated with P450arom, 3β‐HSD1 and 17β‐HSD1. Sum of all PFASs (incl. PFOS ~ 1 ng/mL and PFOA ~ 36 ng/mL), PFUnDA, PFNA and PFDA also showed a positive association with P450arom. On their own, the biological relevance of these cross‐sectional association in cord‐blood is far from clear. Replication in an independent data would be needed to draw any meaningful conclusions on causality.
In a study of 80 mother–child pairs form the Faroese Islands, Shelly et al. (2019) examined the association between maternal and childhood (age 5, 7 and 9 y) exposures to PFHxS, PFDA and PFNA (median or mean concentrations not reported) and adipokine hormone levels at these same ages. For these substances, no consistent associations were observed.
Limited conclusions can be taken on the basis of these two studies.
J.6. Metabolic Outcomes
Diabetes
Lin et al. (2009) examined cross‐sectional associations between PFOS, PFOA, PFNA (GM 0.7 ng/mL) and PFHxS (GM 2.6 ng/mL) and glucose homoeostasis and metabolic syndrome in 1,443 adults and adolescents (12–19 years) from US NHANES samples 1999–2004. Data were available on fasting plasma glucose and insulin, and beta cell function and insulin resistance were assessed. Plasma glucose, blood lipids, waist, blood pressure and medications were used to define metabolic syndrome. There was an inverse association between PFNA and metabolic syndrome in adolescents, but null findings in adults. Also for PFHxS, the point estimates for the odds ratio of metabolic syndrome were below 1.
The study by Nelson et al. (2010) (see also Section 3.3.4.6.1 above) found no consistent associations between PFNA (median 1.0 ng/mL) or PFHxS (median 1.8 ng/mL) and body weight, BMI or insulin resistance (HOMA‐IR).
The study by Lin et al. (2011) (see Section 3.3.4.6.1) found no consistent associations between PFNA or PFUnDA and indicators of metabolic syndrome, but few details were given.
The study by Fisher et al. (2013), see Section 3.3.4.6.1, found no consistent associations between PFHxS and indicators of metabolic syndrome.
Lind et al. (2014) studied the association between diabetes, insulin secretion and insulin resistance, and serum levels of PFOS, PFOA, PFHpA, PFNA, PFUnDA, PFHxS, FOSA (all of these with detection rates > 90%; seven other PFASs were determined but had lower detection rates and were not considered). For PFNA, but not for the other PFASs, there was a significant (nonlinear) association with prevalent diabetes. There were no associations between PFASs and insulin resistance.
A cross‐sectional study of associations between PFOS, PFOA, PFNA (mean 3.8 ng/mL) and PFUnDA (mean 6.4 ng/mL) and glucose homoeostasis among 571 Taiwanese adults from outpatient cardiology clinics (but free of self‐reported coronary heart disease, stroke or diabetes) was reported by Su et al. (2016). The odds ratio for diabetes was decreased with increasing PFNA and PFUnDA (i.e. ‘protective’ association).
The association between PFOS, PFOA and PFHxS (GM 1.0 ng/mL) levels in early pregnancy and development of GDM and impaired glucose tolerance (IGT) at the end of pregnancy was examined in a prospective study of 1,259 women in Canada (Shapiro et al., 2016). The odds ratio for IGT and GDM was increased in quartile 2 of PFHxS, but not significantly increased in Q3 or Q4.
Cardenas et al. (2017) examined associations between PFNA (GM 0.5 ng/mL), PFHxS (GM 2.4 ng/mL) N‐methyl‐perfluorooctane sulfonamido acetic acid (Et‐FOSA‐AcOH) (GM 1.1 ng/mL), N‐methyl‐perfluorooctane sulfonamido acetic acid (Me‐FOSA‐AcOH) (GM 0.9 ng/mL) and glycaemic indicators at baseline and during 5 years of follow‐up in 957 individuals with high risk of diabetes. PFHxS, Et‐PFOSA‐AcOH and Me‐PFOSA‐AcOH were positively associated with insulin resistance (HOMA‐IR) and insulin response (HOMA‐beta), and HbA1c at baseline, but there were no associations between baseline levels of any of the afore‐mentioned PFASs and incident diabetes changes or in changes in glycaemic indicators during follow‐up.
J.7. Kidney and Uric acid
The association between PFHxS and PFNA and eGFR (Schwarz formula) was examined in 9,660 children and adolescents aged 1–18 years (mean 12 years) from the C8 cohort by Watkins et al. (2013). The median serum levels of PFHxS and PFNA were 5.2 and 1.5 ng/mL in 2005–2006. There was a significant inverse association with eGFR both for PFHxS and PFNA. For PFNA, most of this difference was found already between Q1 and Q2. The change in eGFR per interquartile range of PFHxS and PFNA was about 1 mL/min/1.73 m2, which was similar to what was found (per IQR) for PFOS and PFOA. When the analysis for PFOA was repeated but using estimated S‐PFOA (from drinking water), there was no association between estimated S‐PFOA and eGFR. The authors conclude that reverse causation is a likely cause of the association between (measured) S‐PFOA and eGFR, i.e. that lower GFR causes higher S‐PFOA, rather than vice versa, and this argument is valid also for PFHxS and PFNA.
Kataria et al. (2015) studied, cross‐sectionally, the association between PFHxS/PFNA and kidney function (and uric acid) among 1,960 adolescents in the US NHANES 2003–2010. Median PFHxS and PFNA levels were 2.0 and 1 ng/mL. A number of potential confounders were adjusted for. No significant associations were found with eGFR (Schwartz formula) or uric acid.
Lin et al. (2013b) examined associations between PFNA and PFUnDA and serum uric acid in a cross‐sectional study of 644 young people from Taiwan, who had been subject to a mass urine screening. The focus of the study was carotid intima–media thickness. Most of them were in the age 20–30 years and the GM PFNA and PFUnDA levels were about 1.1 and 5.9 ng/mL. No significant associations were found between PFNA/PFUnDA and uric acid in adjusted models including a number of potential confounders.
In the cross‐sectional study by Gleason et al. (2015), mentioned above in the Liver section (Section 3.3.4.6.3), associations between serum PFHxS/PFNA and serum uric acid were examined in about 4,300 individuals from US NHANES surveys 2007–2010. The median levels of PFHxS and PFNA were 1.8 ng/mL and 1.2 ng/mL. A number of potential confounders were adjusted for. A significant positive association was found between ln serum PFNA (but not ln PFHxS) and uric acid.
Associations between a number of PFAS (PFBS, PFHxS, PFHxA, PFNA, PFDA, PFDoDA, PFTeDA) and serum uric acid were examined in a cross‐sectional study of 225 Taiwanese children (12–15 years) by Qin et al. (2016). There was a significant positive association between ln serum PFHxS (but not the other PFASs) and uric acid in a model adjusted for potential confounders.
J.8. Carcinogenicity outcomes
In a nested case–control study from France, Mancini et al. (2019) examined the association between serum PFOS (median ng/mL) and PFOA (median ng/mL) with breast cancer risk. Blood samples were drawn between 1994 and 1999 and cancer cases were prospectively identified until 2013. PFOS and PFOA were not associated with overall breast cancer risk (all cases). PFOS was, however, positively associated with oestrogen receptor‐positive and progesterone receptor‐positive tumours. However, the effect estimates did not indicate a clear dose response as all estimates were similarly elevated above the referent quartile. No consistent associations were observed for PFOA.
In another nested case–control design, Cohn et al. (2019) examined the association between pregnancy concentration of PFAS with breast cancer risk in the female offspring after 54 years of follow‐up. Out of 9,300 daughters born in 1959 to 167 archived maternal samples drawn during pregnancy whose daughters had been diagnosed (cases: n = 102) and matched controls (n = 310) were selected. Maternal concentrations were (median in ng/mL) 0.3 for EtFOSAA, and 2.0 for PFHxS. No association with breast cancer was found for these two substances. In a subset of women with high maternal cholesterol, EtFOSAA was positively associated with later offspring risk of breast cancer. Without further replication, this study provides limited evidence for a link between in utero exposure to EtFOSAA and PFHxS and later breast cancer risk.
In a nested case–control (902 breast cancer cases and matched 858 controls) design of US women, Hurley et al (2018) examined association between baseline concentrations (median in ng/mL) of PFNA (0.9), PFUnDA (0.1), PFHxS (1.6) and MeFOSAA (0.2) and later breast cancer risk over 10–19 years of follow‐up time. No increased risks of breast cancer were observed. If anything, the observed associations tended to show a modest reduction in risk.
J.9. Cardiovascular disease and mortality
Lin et al. (2013b) studied, cross‐sectionally, the association between intima‐media thickness (as a measure of atherosclerosis) in the carotid artery (CIMT) and serum levels of PFNA and PFUnDA (in addition to PFOS and PFOA) in 644 individuals from Taiwan, aged 12–30 years, recruited at a mass screening of urine for glucose and protein. S‐PFNA and S‐PFUnDA were about 1.1 and 5.9 ng/mL. No positive associations were found between PFNA or PFUnDA and CIMT after adjustment for potential confounders. Similar results were found be the same authors (Lin et al., 2016) when analysing some additional subjects and performing some subanalyses.
Mattsson et al. (2015) performed a case–control study (nested in a Swedish cohort) of the association between several PFAS (apart from PFOS/PFOA): PFHpDA (median at recruitment 1990–1991, 0.05 ng/mL), PFNA (median 0.5 ng/mL), PFDA (median 0.2 ng/mL), PFUnDA (median 0.2 ng/mL), PFDoDA (median 0.02 ng/mL) and PFHxS (median 1.6 ng/mL) and incident coronary heart disease (CHD, fatal or non‐fatal). Cases and age‐matched controls (231 pairs) were obtained from a cohort of 1,782 men living in rural Sweden. For part of the age‐control pairs still alive, blood samples were collected also in 2002–2003, and then levels had decreased about 10%. An association with risk of CHD was found only for PFHpA (OR for Q3 of S‐PFHpA 2.6 (95% CI 1.4–4.8) and for Q4 OR = 1.7, 95% CI 0.9–3.2).
Lind et al. (2017b) studied cross‐sectional associations between several PFASs (apart from PFOS and PFOA): PFHxS (mean 2.1 ng/mL), PFHpA (mean 0.05 ng/mL), PFNA (mean 0.7 ng/mL), PFDA (mean 0.3 ng/mL), PFUnDA (mean 0.3 ng/mL) and FOSA (mean 0.1 ng/mL) in serum in about 1,000 elderly Swedish individuals (see Lind et al. 2014, Section 3.3.4.6.2 on diabetes, obesity and metabolic syndrome). The authors found a positive association between ln PFNA and echogenicity (a marker of plaque vulnerability) of the intima–media complex, but only in women, and a positive association between PFUnDA and prevalence of carotid atherosclerotic plaques, but again only in women.
Bao et al. (2017) examined associations between PFASs in serum and blood pressure among 1,612 adult government employees or residents in Shenyang, China. Apart from PFOS and PFOA, also PFBA (0.2 ng/mL), PFHxDA (0.03 ng/mL), PFNA (median 1.2 ng/mL), PFDA (median 0.8 ng/mL), PFUnDA (median 0.5 ng/mL), PFDoDA (median 0.1 ng/mL), PFTrDA (median 0.4 ng/mL), PFTeDA (median 0.1 ng/mL) and PFHxS (median 0.7 ng/mL) could be quantified in > 50% of the participants. After adjustment for relevant potential confounders, statistically significant positive associations were found between PFNA and PFBA and prevalence of hypertension as well as blood pressure. For a threefold increase in PFNA, the adjusted OR was 1.2 (95% CI 1.04–1.36). For PFBA, the corresponding adjusted OR was 1.1 (95% CI 1.04–1.17). Adjusted models indicated increase in systolic and diastolic blood pressure with 3 mm Hg for a threefold increase in PFNA and 1 mm Hg for PFBA. Associations with hypertension and blood pressure were stronger (and statistically significant) for PFOS and PFOA. Huang et al. (2018) studied associations between a number of PFASs in serum and self‐reported physician‐diagnosed occurrence of cardiovascular disease (about 1,200 cases) in seven rounds of the US NHANES (1999–2014) including about 11 000 participants. Apart from PFOS and PFOA, PFHpA (median 0.2 ng/mL), PFNA (median 1.0 ng/mL), PFDA (0.2 ng/mL), PFUnDA (0.2 ng/mL), PFDoDA 0.1 ng/mL), PFHxS (1.6 ng/mL) could be quantified in > 50% of participants. A large number of potential confounders and cardiovascular risk factors (including serum cholesterol) were adjusted for. There were significant associations between PFNA, PFUnDA and PFDoDA and the odds ratios for any cardiovascular disease. This was also the case when all PFASs (including PFOS and PFOA) were summed up. Regarding specific cardiovascular diseases, positive associations were found for coronary heart disease and associations were also found between PFASs and cardiovascular risk factors, such as serum cholesterol.
Mastrantonio et al. (2018) studied mortality in an ecological study of 24 municipalities with drinking water contaminated by PFASs (from a manufacturing company operating since 1964), and 56 municipalities with non‐contaminated drinking water, all of them in the Veneto region of Italy. Selection of municipalities with contaminated drinking water was based on water levels of PFOS > 30 ng/L, PFOA > 500 ng/L or other PFAS > 500 ng/L, while municipalities with uncontaminated water had PFAS levels < LOQ = 10 ng/L. Other PFASs than PFOS and PFOA included PFBA, PFPeDA, PFHxA, PFHpDA, PFNA, PFDA, PFUnDA, PFDoDA, PFBS and PFHxS. The contaminated municipalities had 144 000 inhabitants and the uncontaminated had 588,000 inhabitants. Age‐ and sex‐standardised mortality (SMR) rates for 1980–2013 were based on mortality for Italy. The specific causes of death examined were selected a priori, based on previous literature. Apart from sex and age, no individual potential confounders could be assessed, but a deprivation index and municipality‐based smoking habits were adjusted for. Increased relative risks (RR) in contaminated municipalities were found for total mortality (RR = 1.11, 95% CI 1.10–1.12), myocardial infarction (RR 1.13, 95% CI 1.09–1.18), cerebrovascular disease (RR = 1.21, 95% CI 1.17–1.25), diabetes (RR 1.27, 95% CI 1.17–1.37) and Alzheimers disease (RR 1.24, 95% CI 1.08–1.43). The RR for liver cancer was significantly decreased (RR 0.84, 95% CI 0.74–0.94).
In summary, six studies report findings on cardiovascular disease in relation to exposure to other PFASs than PFOS and PFOA. Regarding the study by Lin et al. (2013b), it should be noted that CIMT is not a good marker of atherosclerosis at this young age. The findings by Lind et al. (2017b) on atherosclerosis (PFNA and PFUnDA) were restricted to women and have not yet been replicated. For associations with blood pressure/hypertension (Bao et al., 2017), replication in other studies is lacking. The same is true for the longitudinal study by Mattsson et al. (2015) – PFHpA has rarely been studied in relation to cardiovascular disease. The study by Huang et al. (2018) is large and has good confounder‐control. Self‐reported disease is a limitation, but on the other hand information bias is not likely since the participants were unaware of their PFASs levels. Therefore, it supports the hypothesis that the aforementioned PFASs (PFNA, PFUnDA and PFDoDA) contribute to the risk of, especially coronary heart disease, the major outcome reported. The findings have, however, not been replicated.
The longitudinal ecological study by Mastrantonio et al. (2018) is relevant for all PFASs since it used drinking water contamination as exposure instead of serum levels. Without individual data, it is, however, difficult to know if established cardiovascular risk factors differ between municipalities (apart from smoking and socioeconomy, which could be taken into account on area level). The large size and the fact that results were averaged over many (contaminated and uncontaminated) municipalities are strengths of this study and it does provide some support for associations between PFASs and cardiovascular disease. This is true also for PFOS and PFOA (this paper was published after the literature deadline for the PFOS and PFOA Opinion (EFSA CONTAM Panel, 2018). For Alzheimers disease and diabetes, data on morbidity are more appropriate than data from death certificates, and there is no corresponding support from morbidity studies (see Sections 3.3.4.6.2 (Diabetes) and 3.3.4.3 (Neurotoxic effects)).
Appendix K – Additional information on the study from Abraham et al. (2020) and Benchmark dose modelling
K.1. Additional information
Figure K.1–K.3.
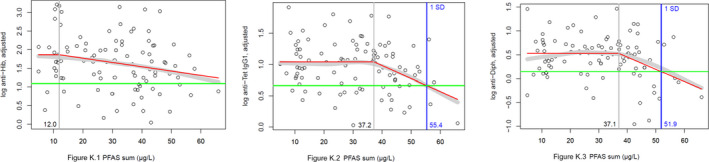
Scatter plot of levels of vaccine antibodies (K.1 Hib, K.2 Tetanus, K.3 Diphtheria) adjusted for the number of vaccinations (in the case of tetanus only) and for the time since the last vaccination for Hib (K.1, n = 98), tetanus IgG1 (K.2, n = 100) and diphtheria (K.3, n = 100), in relation to the PFAS sum (PFOA, PFNA, PFHxS and PFOS) levels
- Titres are presented as log 10 transformed values. Broad grey band: moving average; red line: Fitted ‘knee’ function; horizontal green line: mean minus one standard deviation of the antibody levels below the ‘knee’; vertical grey line: PFAS sum level of the ‘knee’; vertical blue line: PFAS sum level of the ‘knee’ function with antibody levels averagely diminished by one standard deviation.
Figure K.4.
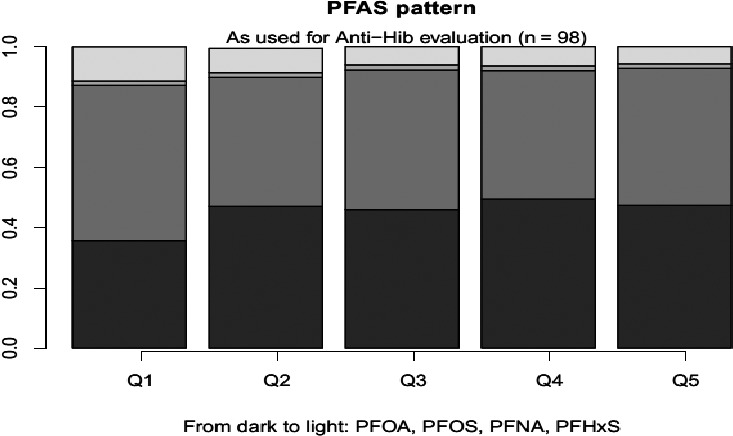
Relative contribution of PFOA, PFOS, PFNA and PFHxS to the PFAS sum in the quintiles
Figure K.5.
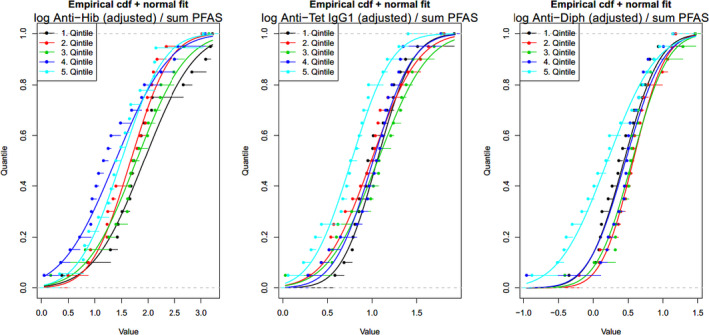
Empirical cumulative distribution functions of PFAS sum (PFOA, PFNA, PFHxS and PFOS) quintiles together with the respective fitted normal distribution curves
- Titres on the X‐axis presented as log 10 transformed values.
K.2. Benchmark dose modelling: Vaccination response
Data used
The BMD modelling of the individual data on antibody titres against tetanus and diphtheria, from the study by Abraham et al. (2020) was performed by the authors since these could not be provided to EFSA. However, the CONTAM Panel was able to reproduce the results using data extracted from the graphs provided by the authors.
Data on rubella are from the study by Granum et al. (2013) and obtained from the authors. Analysis performed by the CONTAM Panel.
Selection of the BMR
The BMR (benchmark response) used is a 10% change in mean response compared to the controls. The BMD (benchmark dose) is the dose corresponding with the BMR of interest.
It was decided to use a critical effect size of 10% instead of the default 5% considering a large variation in the response.
A 90% confidence interval around the BMD will be estimated, the lower bound is reported by BMDL and the upper bound by BMDU.
Software Used
Results are obtained using the EFSA web‐tool for BMD analysis, which uses the R‐package PROAST, version 69.0, for the underlying calculations.
Specification of Deviations from Default Assumptions
Dose‐response models
Default set of fitted models:
| Model | Number of parameters | Formula | |
|---|---|---|---|
| Null | 1 | y = a | |
| Full | no. of groups | y = group mean | |
| Exp model 3 | 3 | y = a · exp(bxd) | |
| Exp model 4 | 4 |
|
|
| Hill model 3 | 3 |
|
|
| Hill model 4 | 4 |
|
|
| Inverse Exponential | 4 |
|
|
| Log‐Normal Family | 4 |
|
Procedure for selection of BMDL
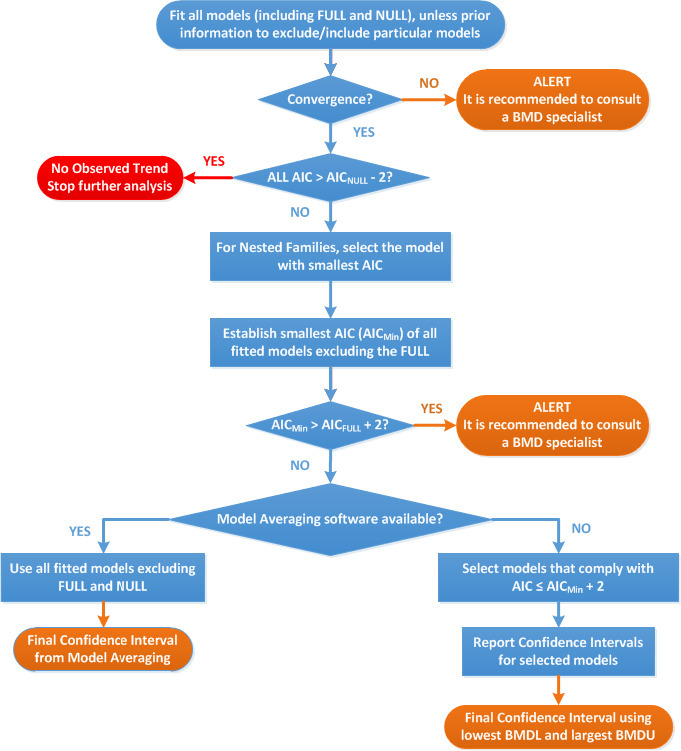
Flowchart for selection of BMDL (EFSA Scientific Committee, 2017)
K.2.1. Tetanus (Abraham et al., 2020)
Results
Response variable: saktet10. 32
Fitted Models
| Model | Converged | loglik | npar | AIC |
|---|---|---|---|---|
| full | NA | NA | NA | NA |
| null model | Yes | –129.12 | 2 | 262.24 |
| Expon. m3‐ | Yes | –124.70 | 4 | 257.40 |
| Expon. m5‐ | Yes | –124.73 | 5 | 259.46 |
| Hill m3‐ | Yes | –124.72 | 4 | 257.44 |
| Hill m5‐ | Yes | –124.78 | 5 | 259.56 |
| Inv.Expon. m3‐ | Yes | –124.51 | 4 | 257.02 |
| Inv.Expon. m5‐ | Yes | –124.55 | 5 | 259.10 |
| LN m3‐ | Yes | –124.49 | 4 | 256.98 |
| LN m5‐ | Yes | –124.51 | 5 | 259.02 |
Estimated Model Parameters
EXP
estimate for var‐ : 0.709
estimate for a‐ : 11.38
estimate for CED‐ : 33.13
estimate for d‐ : 4
HILL
estimate for var‐ : 0.7092
estimate for a‐ : 11.38
estimate for CED‐ : 33.06
estimate for d‐ : 4
INVEXP
estimate for var‐ : 0.7064
estimate for a‐ : 10.78
estimate for CED‐ : 40.01
estimate for d‐ : 1.284
LOGN
estimate for var‐ : 0.706
estimate for a‐ : 10.79
estimate for CED‐ : 39.72
estimate for d‐ : 2.184
BMDL‐BMDU from single models
| BMDL(a) | BMD(a) | BMDU(a) | P value | |
|---|---|---|---|---|
| EXP (m3‐) | 18.9 | 33.1 | 40.4 | 0.01 |
| HILL (m3‐) | 18.9 | 33.1 | 40.4 | 0.01 |
| INVEXP (m3‐) | 25.5 | 40.0 | 56.3 | 0.01 |
| LOGN (m3‐) | 24.0 | 39.7 | 56.0 | 0.01 |
(a): ng/mL; p value calculated with likelihood ratio test.
Weights for Model Averaging
| EXP | HILL | INVEXP | LOGN |
|---|---|---|---|
| 0.23 | 0.22 | 0.27 | 0.28 |
Final BMD Values
ng/mL
Confidence intervals for the BMD are based on 500 bootstrap datasets.
Visualisation
Conclusion
The CONTAM Panel decided to deviate from the Guidance by using the lowest BMDL from individual models instead of the BMDL obtained with model averaging (see Dose‐response assessment section 3.4.3.1, for further details).
K.2.2. Diphtheria (Abraham et al., 2020)
Results
Response variable: sakdiph0 33
Fitted Models
| Model | Converged | loglik | npar | AIC |
|---|---|---|---|---|
| full | NA | NA | NA | NA |
| null model | Yes | –143.43 | 2 | 290.86 |
| Expon. m3‐ | Yes | –138.62 | 4 | 285.24 |
| Expon. m5‐ | Yes | –138.63 | 5 | 287.26 |
| Hill m3‐ | Yes | –138.62 | 4 | 285.24 |
| Hill m5‐ | Yes | –138.64 | 5 | 287.28 |
| Inv.Expon. m3‐ | Yes | –138.40 | 4 | 284.80 |
| Inv.Expon. m5‐ | Yes | –137.46 | 5 | 284.92 |
| LN m3‐ | Yes | –138.48 | 4 | 284.96 |
| LN m5‐ | Yes | –137.56 | 5 | 285.12 |
Estimated Model Parameters
EXP
estimate for var‐ : 0.9366
estimate for a‐ : 3.485
estimate for CED‐ : 31.61
estimate for d‐ : 4
HILL
estimate for var‐ : 0.9367
estimate for a‐ : 3.49
estimate for CED‐ : 31.5
estimate for d‐ : 4
INVEXP
estimate for var‐ : 0.9325
estimate for a‐ : 3.37
estimate for CED‐ : 36.19
estimate for d‐ : 1.121
LOGN
estimate for var‐ : 0.934
estimate for a‐ : 3.387
estimate for CED‐ : 35.19
estimate for d‐ : 1.818
BMDL‐BMDU from single models
| BMDLa | BMDa | BMDUa | p value | |
|---|---|---|---|---|
| EXP (m3‐) | 17.5 | 31.6 | 38.1 | 0.01 |
| HILL (m3‐) | 17.6 | 31.5 | 38.0 | 0.01 |
| INVEXP (m3‐) | 23.1 | 36.2 | 46.5 | 0.01 |
| LOGN (m3‐) | 21.1 | 35.2 | 46.6 | 0.01 |
ng/mL; P value calculated with likelihood ratio test.
Weights for Model Averaging
| EXP | HILL | INVEXP | LOGN |
|---|---|---|---|
| 0.23 | 0.23 | 0.28 | 0.26 |
Final BMD Values
ng/mL
Confidence intervals for the BMD are based on 500 bootstrap datasets.
Visualisation
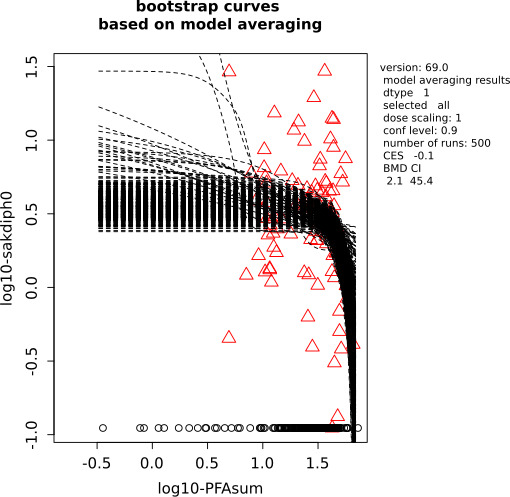
Conclusion
The CONTAM Panel decided to deviate from the Guidance by using the lowest BMDL from individual models instead of the BMDL obtained with model averaging (see Dose‐response assessment section 3.4.3.1, for further details).
K.2.3. Rubella (Granum et al., 2013)
Data Description
The endpoint to be analysed is: Rubella_OD.
Data used for analysis are shown in the Appendix K.2.3.1.
Results
Response variable: Rubella_OD
Fitted Models
| Model | Converged | loglik | npar | AIC |
|---|---|---|---|---|
| full | NA | NA | NA | NA |
| null model | Yes | –6.95 | 2 | 17.90 |
| Expon. m3‐ | Yes | –2.10 | 4 | 12.20 |
| Expon. m5‐ | Yes | –2.05 | 5 | 14.10 |
| Hill m3‐ | Yes | –2.10 | 4 | 12.20 |
| Hill m5‐ | Yes | –1.98 | 5 | 13.96 |
| Inv.Expon. m3‐ | Yes | –2.09 | 4 | 12.18 |
| Inv.Expon. m5‐ | Yes | –1.92 | 5 | 13.84 |
| LN m3‐ | Yes | –2.10 | 4 | 12.20 |
| LN m5‐ | Yes | –1.98 | 5 | 13.96 |
Estimated Model Parameters
EXP
estimate for var‐ : 0.06328
estimate for a‐ : 3.177
estimate for CED‐ : 0.1836
estimate for d‐ : 0.4876
HILL
estimate for var‐ : 0.06328
estimate for a‐ : 3.164
estimate for CED‐ : 0.1937
estimate for d‐ : 0.4947
INVEXP
estimate for var‐ : 0.06327
estimate for a‐ : 2.785
estimate for CED‐ : 0.8405
estimate for d‐ : 0.1394
LOGN
estimate for var‐ : 0.06327
estimate for a‐ : 2.939
estimate for CED‐ : 0.4863
estimate for d‐ : 0.216
Weights for Model Averaging
| EXP | HILL | INVEXP | LOGN |
|---|---|---|---|
| 0.25 | 0.25 | 0.25 | 0.25 |
Final BMD Values
| endpoint | subgroup | BMDL | BMDU |
|---|---|---|---|
| Rubella_OD | all | 0 | 9.05 |
Confidence intervals for the BMD are based on 500 bootstrap datasets.
Visualisation
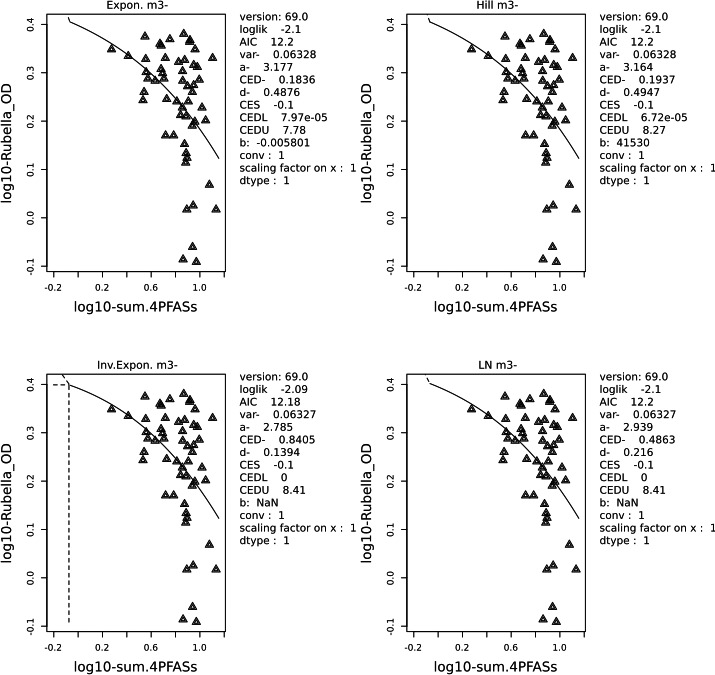
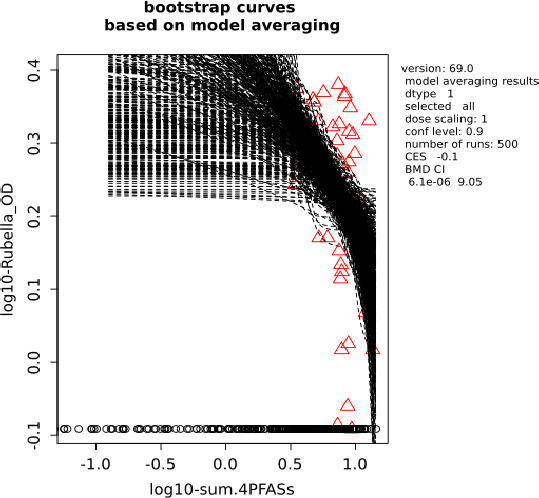
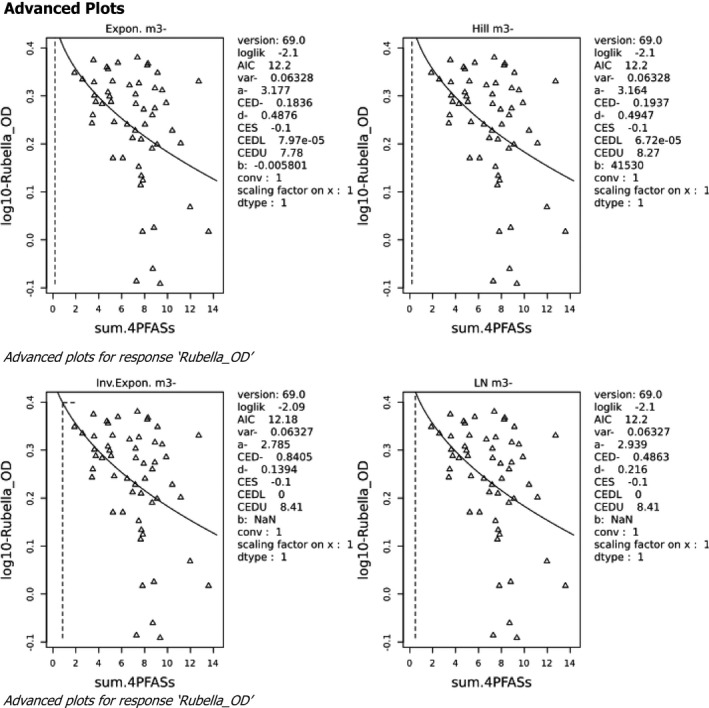
K.2.3.1. Data used for analysis (Granum et al., 2013)
| sum.4PFASs | Rubella_OD |
|---|---|
| 1.88 | 2.23 |
| 2.58 | 2.16 |
| 3.41 | 1.75 |
| 3.48 | 1.82 |
| 3.54 | 2.37 |
| 3.59 | 2.13 |
| 3.62 | 2.00 |
| 3.74 | 1.94 |
| 4.29 | 1.92 |
| 4.70 | 2.29 |
| 4.80 | 2.03 |
| 4.81 | 2.27 |
| 4.94 | 1.99 |
| 5.07 | 1.94 |
| 5.20 | 2.14 |
| 5.23 | 1.48 |
| 5.34 | 1.76 |
| 5.67 | 2.34 |
| 6.09 | 1.48 |
| 6.47 | 1.74 |
| 6.68 | 2.10 |
| 6.94 | 1.63 |
| 7.18 | 1.69 |
| 7.21 | 2.01 |
| 7.23 | 1.92 |
| 7.28 | 0.82 |
| 7.37 | 2.40 |
| 7.48 | 1.42 |
| 7.50 | 2.12 |
| 7.65 | 1.30 |
| 7.69 | 1.62 |
| 7.71 | 1.36 |
| 7.81 | 1.04 |
| 7.84 | 1.33 |
| 7.92 | 1.87 |
| 8.03 | 1.74 |
| 8.23 | 2.31 |
| 8.29 | 2.33 |
| 8.65 | 1.55 |
| 8.67 | 1.82 |
| 8.71 | 0.87 |
| 8.81 | 1.06 |
| 8.89 | 1.88 |
| 8.90 | 2.07 |
| 9.09 | 1.58 |
| 9.13 | 2.23 |
| 9.35 | 0.81 |
| 9.53 | 2.05 |
| 9.88 | 1.93 |
| 10.41 | 1.69 |
| 11.15 | 1.59 |
| 11.97 | 1.17 |
| 12.72 | 2.14 |
| 13.58 | 1.04 |
EFSA obtained individual data on PFOA, PFNA, PFHxS and PFOS, but it was decided to only analyse the data for the sum of the 4 PFASs. Summary data on the levels of the individual PFASs are in the table below, for those mothers for which rubella data in the infants were available.
PFAS levels (ng/mL) in serum of mothers, related to antibodies to rubella in 54 infants based on individual data submitted to EFSA by B. Granum (Granum et al., 2013 )
| PFAS | Mean | SD | Median | Min | Max |
|---|---|---|---|---|---|
| PFOA | 1.11 | 0.46 | 1.04 | 0.27 | 2.12 |
| PFNA | 0.28 | 0.11 | 0.26 | 0.10 | 0.66 |
| PFHxS | 0.33 | 0.43 | 0.24 | 0.04 | 2.79 |
| PFOS | 5.29 | 1.99 | 5.61 | 1.40 | 10.71 |
| Sum 4 PFASs | 7.11 | 2.55 | 7.43 | 1.88 | 13.58 |
Appendix L – Additional information from the study of Grandjean et al. (2012)
1.
Table L.1.
PFOS serum levels at age 5 vs. diphtheria antibody titres (log 2) at age 7
| Deciles | PFOS (ng/mL) | N | Mean | SD | t‐test | %change |
|---|---|---|---|---|---|---|
| p‐value | ||||||
| 1 | 10.3 | 43 | 0.17 | 1.7 | Referent | |
| 2 | 12.7 | 43 | 0.33 | 1.9 | 0.69 | 11 |
| 3 | 14.2 | 43 | –0.41 | 2.1 | 0.17 | –33 |
| 4 | 15.6 | 43 | 0.27 | 1.7 | 0.79 | 7 |
| 5 | 16.6 | 43 | –0.42 | 1.9 | 0.12 | –34 |
| 6 | 18.2 | 43 | –0.54 | 1.9 | 0.07 | –39 |
| 7 | 19.8 | 43 | 0.09 | 1.8 | 0.22 | –6 |
| 8 | 21.3 | 43 | –0.41 | 2.0 | 0.16 | –33 |
| 9 | 23.6 | 43 | –0.19 | 2.1 | 0.37 | –22 |
| 10 | 28.4 | 43 | –0.59 | 1.8 | 0.048 | –41 |
| Quintiles | PFOS (ng/mL) | N | Mean | SD | t‐test | %change |
|---|---|---|---|---|---|---|
| p‐value | ||||||
| 1 | 11.5 | 86 | 0.25 | 1.81 | Referent | |
| 2 | 14.9 | 86 | –0.07 | 1.91 | 0.32 | –20 |
| 3 | 17.4 | 86 | –0.48 | 1.89 | 0.01 | –40 |
| 4 | 20.6 | 86 | –0.16 | 1.90 | 0.15 | –25 |
| 5 | 26.0 | 86 | –0.39 | 1.94 | 0.03 | –36 |
Yellow highlighted text = NOAEC; Blue highlighted text = LOAEC.
Total number of individuals in these analyses = 431. Divided into deciles: ~ 43 and quintiles ~ 86. Mean and standard error provided on log‐2 scale SD derived. p‐value: t‐test (assuming log‐normal distribution). % change estimated based on mean difference relative to the lowest (referent) decile corresponds to % shift on median concentration on the original (back‐transformed scale). Mean and SD pooled (from deciles) to estimate NOAEC in quintiles.
Table L.3.
Sum PFAS serum levels at age 5 vs. diphtheria antibody titres (log 2) at age 7
| Decile | Sum_PFAS (ng/mL) | N | Mean | SE | SD | t‐test | %change |
|---|---|---|---|---|---|---|---|
| p | |||||||
| 1 | 14.7 | 43 | 0.14 | 0.25 | 1.63 | referent | |
| 2 | 17.5 | 43 | 0.49 | 0.25 | 1.67 | 0.33 | 27 |
| 3 | 19.5 | 43 | –0.31 | 0.29 | 1.88 | 0.24 | –27 |
| 4 | 20.8 | 43 | –0.44 | 0.28 | 1.80 | 0.12 | –33 |
| 5 | 22.5 | 43 | –0.38 | 0.36 | 2.35 | 0.23 | –30 |
| 6 | 24.2 | 43 | –0.06 | 0.29 | 1.90 | 0.60 | –13 |
| 7 | 25.8 | 43 | –0.17 | 0.31 | 2.02 | 0.43 | –19 |
| 8 | 28.2 | 43 | –0.01 | 0.27 | 1.76 | 0.68 | –10 |
| 9 | 31.2 | 43 | –0.74 | 0.27 | 1.80 | 0.02 | –46 |
| 10 | 38.5 | 43 | –0.66 | 0.30 | 1.98 | 0.04 | –43 |
| Quintiles | Sum_PFAS | N | Mean | SD | t‐test | %change | |
|---|---|---|---|---|---|---|---|
| p | |||||||
| 1 | 16.1 | 86 | 0.31 | 1.65 | Referent | ||
| 2 | 20.2 | 86 | –0.37 | 1.84 | 0.02 | –38 | |
| 3 | 23.4 | 86 | –0.22 | 2.14 | 0.08 | –31 | |
| 4 | 27.0 | 86 | –0.09 | 1.90 | 0.16 | –24 | |
| 5 | 34.8 | 86 | –0.70 | 1.89 | 0.0005 | –50 | |
Yellow highlighted text = NOAEC; Blue highlighted text = LOAEC.
Total number of individuals in these analyses = 431. Divided into deciles: ~ 43 and quintiles ~ 86. Mean and standard error provided on log‐2 scale SD derived. p‐value: t‐test (assuming log‐normal distribution). % change estimated based on mean difference relative to the lowest (referent) decile corresponds to % shift on median concentration on the original (back‐transformed scale). Mean and SD pooled (from deciles) to estimate NOAEC in quintiles.
Table L.4.
Sum PFASs serum levels at age 5 vs. tetanus antibody titres (log 2) at age 7
| Decile | Sum_PFAS (ng/mL) | N | Mean | SE | SD | t‐test | %change | |
|---|---|---|---|---|---|---|---|---|
| p | ||||||||
| 1 | 14.7 | 43 | 1.225 | 0.282 | 1.85 | Referent | No NOAEC for deciles | |
| 2 | 17.5 | 43 | 1.343 | 0.335 | 2.20 | 0.79 | 9 | |
| 3 | 19.5 | 43 | 1.205 | 0.287 | 1.88 | 0.96 | –1 | |
| 4 | 20.8 | 43 | 0.534 | 0.293 | 1.92 | 0.09 | –38 | NOAEC identified in quintile below |
| 5 | 22.5 | 43 | 1.232 | 0.263 | 1.72 | 0.99 | 0 | |
| 6 | 24.2 | 43 | 1.163 | 0.32 | 2.10 | 0.88 | –4 | |
| 7 | 25.8 | 43 | 0.852 | 0.321 | 2.10 | 0.47 | –23 | |
| 8 | 28.2 | 43 | 0.608 | 0.317 | 2.08 | 0.19 | –35 | |
| 9 | 31.2 | 43 | 0.51 | 0.321 | 2.10 | 0.10 | –39 | |
| 10 | 38.5 | 43 | 0.57 | 0.372 | 2.44 | 0.16 | –36 | |
| Quintiles | Sum_PFAS | N | Mean | SD | t‐test | %change | ||
| p | ||||||||
| 1 | 16.1 | 86 | 1.28 | 2.03 | Referent | |||
| 2 | 20.2 | 86 | 0.87 | 1.90 | 0.17 | –25 | ||
| 3 | 23.4 | 86 | 1.20 | 1.92 | 0.79 | –6 | ||
| 4 | 27.0 | 86 | 0.73 | 2.09 | 0.08 | –32 | ||
| 5 | 34.8 | 86 | 0.54 | 2.28 | 0.03 | –40 |
Yellow highlighted text = NOAEC; Blue highlighted text = LOAEC.
Total number of individuals in these analyses = 431. Divided into deciles: ~ 43 and quintiles ~ 86. Mean and standard error provided on log‐2 scale SD derived. p‐value: t‐test (assuming log‐normal distribution). % change estimated based on mean difference relative to the lowest (referent) decile corresponds to % shift on median concentration on the original (back‐transformed scale). Mean and SD pooled (from deciles) to estimate NOAEC in quintiles.
Appendix M – PBPK Modelling
M.1. Model description
The model codes were based on the supplementary material in Loccisano et al. (2011), with some corrections provided by a co‐author of the original paper (see EFSA CONTAM Panel, 2018). The original Loccisano model was slightly modified by integrating a growth equation based on a French survey. This study (EAT for French total Diet Study) includes 4,078 subjects aged between 3 and 60 years, and 703 subjects of less than 3 years. The reported data (weight, age) from this study allowed building an equation describing the increase in weight according to age.
Briefly, PFOA and PFOS are taken up either directly into the plasma (i.v.), via the skin (both routes not used in the assessment) or into the gut (oral). From the gut, PFOA or PFOS is transported to the liver by the portal blood. Only the free fractions of PFOA and/or PFOS in plasma are assumed to be available for partitioning into tissues. PFOA or PFOS are eliminated through the filtrate compartment in the kidneys to storage into urine, but before that PFOA or PFOS in the filtrate compartment can be reabsorbed into the plasma through a saturable process with a transporter maximum constant (Tmc) and affinity constant (Kt). The Q parameters indicate blood flows into and out of tissues, the P parameters the partitioning between tissues and plasma, the V parameters the volume of the various tissues. Qfil is the flow from the plasma to the filtrate compartment and is analogous to glomerular filtration rate (GFR).
M.1.1. Modification of the original model
Slight modifications were made by the EFSA CONTAM Panel in 2018. Explanation of the modifications from the original model was given in notes in the respective model codes (see EFSA CONTAM Panel 2018, opinion). In the present opinion, the PFOA and PFOS human PBPK models were used to estimate maternal exposure (daily intake) to PFOA/PFNA and PFOS/PFHxS for women corresponding to a critical serum level in one‐year old infants and corresponding milk level at 35 years. Therefore, they were also used to simulate PFAS concentrations in the infant at 1 year after 12 months of breastfeeding. These models were run for 35 years in order to calculate the serum and milk concentrations at 35 years which were used as starting concentrations at the end of pregnancy (at delivery); then, these concentrations were used for estimating the starting serum concentrations for the new born infant and during the breastfeeding. During this period, human milk replaces the intake via food. Milk levels are based on published data on milk to serum ratios and also include the observed decline over time in serum levels during breastfeeding. To perform these simulations, constants for placental transfer, transfer from blood to milk and decline of PFASs in milk (per month) have been added to the model codes to allow estimation of the serum level of PFASs at birth and during breastfeeding based on the serum level of the mother at birth. This means that the development of the serum levels in the mother during pregnancy and lactation are not part of the models. Only the mother's serum level at birth is used as a starting parameter for the child and the milk levels.
The formula for body weight describes the body weight over the whole life time. For the uncertainty analysis also other growth curves were evaluated based on data from the WHO for boys and girls. These are described in the model codes and can be activated. However, they were not used for deriving the daily intake that is the basis for the TWI.
General constant
The following line codes represent the placental transfer, ratio for milk concentration/maternal serum PFAS concentration, the decline of PFASs in milk due to transfer to the infant. These allow the calculation of initial amount at birth and intake via breastfeeding:
BWbirth = 3.68; Body weight at birth in kg, new add opinion 2020
PT; placental transfer, new add opinion 2020
Ratio; Milk concentration/maternal serum concentration, new add opinion 2020; for PFOA, a ratio of 0.03 was used, for PFOS of 0.015, based on previous studies (Table M.1a )
Table M.1a.
Ratios of concentrations in breast milk and maternal serum of PFOA, PFNA, PFHxS and PFOS, calculated based on mean or median values reported by various authors
| Reference | Milk/serum ratio | |||||||
|---|---|---|---|---|---|---|---|---|
| Based on mean values | Based on median values | |||||||
| PFHxS | PFOS | PFOA | PFNA | PFHxS | PFOS | PFOA | PFNA | |
| Kärrman et al. (2007) | 0.018 | 0.0097 | NA | NA | 0.018 | 0.0089 | NA | NA |
| Haug et al. (2011a,b) | NA | 0.014 | 0.038 | NA | NA | 0.013 | 0.018 | NA |
| Kim et al. (2011a) | 0.006 | 0.009 | 0.018 | NA | 0.018 | 0.011 | 0.025 | NA |
| Liu et al. (2011) | NA | 0.018 | 0.11 | 0.047 | NA | 0.014 | 0.096 | 0.039 |
| Median | 0.012 | 0.012 | 0.038 | 0.047 | 0.018 | 0.012 | 0.025 | 0.039 |
NA: not applicable.
Note: Median ratios were the basis for the values used in the PBPK modelling, being 0.015 for PFHxS/PFOS and 0.03 for PFOA/PFNA.
DECLINE; decline of PFAS concentration in milk per month, new add opinion 2020; for PFOA, a rate of 7.7% per month was used, for PFOS 3.1% per month, based on previous studies (Table M.1b )
Table M.1b.
Monthly decrease in breast milk concentration (%), values from Thomsen et al. (2010) were the basis for the values used in the PBPK modelling, being 3.1% for PFHxS/PFOS and 7.7% for PFOA/PFNA
| PFOS | PFOA | Comments | Reference | |
|---|---|---|---|---|
| Repeated measurements in breast milk | 3.1 | 7.7 | Collected samples monthly up to 12 months | Thomsen et al. (2010) |
| Repeated measurements in serum | 1.6 | 3.5 | Serum at birth and at 6 months | Fromme et al. (2010) |
| Measurement in serum and questionnaire data | 3 | 3 | Serum after birth, child < 3.5 years | Mondal et al. (2014) |
| 1.6 | 1.3 | Serum in pregnancy | Lauritzen et al. (2016) | |
| 1.2 | 2.4 | Serum in pregnancy | Brantsæter et al. (2013) | |
| 2.7 | 3.8 | Serum | Bjermo et al. (2013) |
Line code for the initial amount at birth (example for PFOA)
These line codes allow the calculation of the initial amount of PFOA in several compartments at birth according to the maternal level at delivery and the placental transfer. The number (e.g. AGbirth= APlasbirth*0.99) represents the ratio between the initial amount at birth in a specific compartment (here the gut)/initial amount in plasma compartment. They have been calculated by running a simple PFOA PBPK model with different exposure, and are constant. The initial amount at birth in the plasma is calculated with the following formula:
Aplas = CA × free × Vplas = CA × Free × Vplac × BW
APlasbirth = CONCbirth *Free*VPlasC*BWbirth;initial amount in plasma at birth (μg)
AGbirth = APlasbirth* 0.99929853;initial amount in gut at birth (μg)
ALbirth = APlasbirth*66.8360764;initial amount in liver at birth (μg)
AFbirth = APlasbirth* 10.000151;initial amount at in fat birth (μg)
AKbirth = APlasbirth* 5.88575746;initial amount in kidney at birth (μg)
ARbirth = APlasbirth* 74.9108792;initial amount in rest of the body at birth (μg)
Afilbirth = APlasbirth* 2.09606E‐05;initial amount in filtrate at birth (μg)
Adelaybirth = APlasbirth* 42.27910714;initial amount in storage at birth (μg)
Aurinebirth = APlasbirth* 769.1347494;initial amount in urine at birth (μg)
Line code for Breastfeeding 12 months (example for PFOA)
These line codes allow the calculation of the amount in milk per month according to the milk concentration/maternal serum PFOA concentration ratio and the decline of PFOA in milk per month. Based on studies values of 0.03 for the ratio and 7.7% per month for the decline were used for PFOA.
Decline in PFOA milk concentration over the lactation period (new add in opinion 2020)
Intakemilka=PFOaMilkconcentration*Milkconsumption; initial intake via breastfeeding first month (μg/day)
Intakemilkb=Intakemilka*(1‐DECLINE);intake via breastfeeding second month (μg/day)
Intakemilkc=Intakemilkb*(1‐DECLINE);intake via breastfeeding 3th month (μg/day)
Intakemilkd=Intakemilkc*(1‐DECLINE);intake via breastfeeding 4th month (μg/day)
Intakemilke=Intakemilkd*(1‐DECLINE);intake via breastfeeding 5th month (μg/day)
Intakemilkf=Intakemilke*(1‐DECLINE);intake via breastfeeding 6th. Month (μg/day)
Intakemilkg=Intakemilkf*(1‐DECLINE);intake via breastfeeding 7th month (μg/day)
Intakemilkh=Intakemilkg*(1‐DECLINE);intake via breastfeeding 8th month (μg/day)
Intakemilki=Intakemilkh*(1‐DECLINE);intake via breastfeeding 9th month (μg/day)
Intakemilkj=Intakemilki*(1‐DECLINE);intake via breastfeeding 10th month (μg/day)
Intakemilkk=Intakemilkj*(1‐DECLINE);intake via breastfeeding 11th month (μg/day)
Intakemilkl=Intakemilkk*(1‐DECLINE);;intake via breastfeeding 12th month (μg/day)
Oralconc=IF year < 0.083 THEN Intakemilka/BW ELSE IF year >= 0.083 AND year < 0.167 THEN Intakemilkb/BW ELSE IF year >= 0.167 and year < 0.250 THEN Intakemilkc/BW ELSE IF year >= 0.250 AND year < 0.333 THEN Intakemilkd /BW ELSE IF year >= 0.333 AND year < 0.417 THEN Intakemilke/BW ELSE IF year >= 0.417 AND year < 0.500 THEN Intakemilkf/BW ELSE IF year >= 0.500 AND year < 0.583 THEN Intakemilkg/BW ELSE IF year >= 0.583 AND year < 0.667 THEN Intakemilkh/BW ELSE IF year >= 0.667 AND year < 0.750 THEN Intakemilki/BW ELSE IF year >= 0.750 AND year < 0.833 THEN Intakemilkj/BW ELSE IF year >= 0.833 AND year < 0.917 THEN Intakemilkk/BW ELSE IF year >= 0.917 AND year < 1 THEN Intakemilkl/BW ELSE IF year >= 1 THEN oralexpo ELSE 0.0
M.1.1.1. Model code for PFOA
METHOD Stiff
STARTTIME = 0
STOPTIME=438,000;end of simulation (h); 50 years
DT = 0.01
TOLERANCE = 0.01;default tolerance
DTMAX = 10.0
DTMIN = 0.000001
year = TIME/(24*365)
;Physiological parameters (from Brown et al., 1997)
;fractional blood flows
QCC = 12.5; Cardiac blood output (L/h/kg^0.75)
QFC = 0.052; Fraction cardiac output going to fat
QLC = 0.069; Fraction cardiac output going to liver, through hepatic artery
QKC = 0.175; Fraction cardiac output going to kidney
QSkC = 0.058; Fraction cardiac output going to skin
QGC = 0.181; Fraction of cardiac output going to gut and in the liver via portal artery
; Not used; QfilC = 0.035; Fraction cardiac output to the filtrate compartment (20% of;kidney blood flow)
;body weight curve Turn on, To turn off between French curve used in the opinion and WHO curves (only available from birth to 5 years, do not use after 5 years except for the French curve), use parameter window to switch
;Bwc=0 if BW French curve is used
; Bwc=1 if P50 WHO boys curve is used
; Bwc=2 if P05 boys WHO curve is used
;Bwc=3 if P95 boys WHO curve is used
;Bwc=4 if P50 WHO girls curve is used
;Bwc=5 if P05 WHO girls curve is used
;Bwc=6 if P95 WHO girls curve is used
Bwc=0
BW = IF Bwc=0 then 3.68+4.47*year‐0.093*year^2+0.00061*year^3 ELSE IF Bwc=1 then 3.3+ 8.0155*year − 2.1478*year^2 + 0.2342*year^3 ELSE IF Bwc=2 then 2.6+ 0.2063*year^3− 1.9117*year^2 + 6.9393*year ELSE IF Bwc=3 then 4.2+0.2595*year^3 − 2.3486*year^2 + 9.1534*year ELSE IF Bwc=4 then 3.2 +0.1891*year^3 − 1.7351*year^2+ 7.0589*year ELSE IF Bwc=5 then 2.5 +0.1609*year^3 − 1.5129*year^2 + 6.0135*year ELSE IF Bwc=6 then 4+ 0.2359*year^3 − 2.0807*year^2 + 8.5496*year ELSE 0.0
;body weight equation for Monte Carlo analysis, see ‘uncertainty analysis chapter’ in the appendix for explanation
;BW=(3.68+4.47*year‐0.093*year^2+0.00061*year^3)*(MCc)
MCc= 1.58; calculated for P95 percentile BW=3.68+4.47*year−0.093*year^2+0.00061*year^3 + 2*log(1.96)*(3.68+4.47*year‐0.093*year^2+0.00061*year^3)
;MCc= 0.42; calculated for P05 percentile corresponding to BW=3.68+4.47*year−0.093*year^2+0.00061*year^3 − 2*log(1.96)*(3.68+4.47*year‐0.093*year^2+0.00061*year^3)
;body weight equation (based on French equation) for Sensitivity analysis, see ‘sensitivity analysis chapter’ in the appendix for explanation; BW=3.68+4.47*year‐0.093*year^2+0.00061*year^3+0.1*(3.68+4.47*year‐0.093*year^2+0.00061*year^3)
;fractional tissue volumes
VLC = 0.026;Fraction liver volume
VFC = 0.214;Fraction fat volume
VKC = 0.004;Fraction kidney volume
VfilC = 0.0004;Fraction filtrate compartment volume (10% of kidney volume)
VGC = 0.0171;Fraction gut volume
VPlasC = 0.0428;Fraction plasma volume (58% of blood)
Htc = 0.44;haematocrit
BWbirth=3.68;Body weight at birth in Kg, new add opinion 2020
PT= 0.74;placenta transfer, new add opinion 2020
Ratio= 0.03;maternal/Milk concentration during breastfeeding, new add opinion 2020
DECLINE = 0.077;decline of PFOA in milk was 7.7% per month, new add opinion 2020
;dermal uptake
SkinTarea = 9.1*((BW*1,000)**0.666);Total area of skin (cm^2)
Skinthickness = 0.1;Skin thickness (cm)
; Chemical‐specific parameters (PFOA)
Tmc = 6,000;Maximum resorption rate
Kt = 55;Resorption affinity; same as monkey
Free = 0.02;Free fraction of PFOA in plasma; same as monkey
PL = 2.2;Liver/plasma partition coefficient
PF = 0.04;Fat/plasma partition coefficient
PK = 1.05;Kidney/plasma partition coefficient
PSk = 0.1;Skin/plasma partition coefficient
PR = 0.12;Rest of the body/plasma partition coefficient
PG = 0.05;Gut/blood plasma coefficient
kurinec = 0.0003;urinary elimination rate constant (/h/kg^‐0.25); estimated from Harada et al. (2005)
kurine = kurinec*BW**(‐0.25); urinary elimination rate constant (1/h)
; Free fraction of chemical in tissues
FreeL = Free/PL;liver
FreeF = Free/PF;fat
FreeK = Free/PK;kidney
FreeSk = Free/PSk;skin
FreeR = Free/PR;rest of tissues
FreeG = Free/PG;gut
; Exposure parameters
tchng = 438,000 ;Duration of exposure (h); 50 years
;turn dose on/off
DoseOn = IF time<tchng THEN 1.0 ELSE 0.0
;direct input to plasma (IV dose)
;IVconc = 0;iv uptake (μg/kg/day)
;IVdose = IVconc*BW;(μg/day)
; Dermal exposure
Dermconc = 0.0;Dermal concentration (mg/mL)
Dermvol = 0.001;Dermal exposure volume (mL)
Dermdose = Dermconc*Dermvol*1,000;(μg)
Skinarea = 972;Exposed area on skin (cm^2)
;Drinking water exposure
Drinkconc = 0;Drinking water concentration (μg/L or ppb)
Drinkrate = 13;Drinking water rate (mL/kg/day)
Drinkdose = (Drinkconc*Drinkrate/1,000)*BW;(μg/day)
; Oral exposure
oralexpo= 0.187e‐3;μg/kg/day new add opinion 2020
;oralconc =0.0;Oral uptake (μg/kg/day) not used in opinion 2020
Oraldose = Oralconc*BW;(μg/day)
PFOamaternal=2.0; PFOA maternal serum concentration μg/L at delivery (new add opinion 2020)
PFOaMilkconcentration=PFOamaternal*ratio; initial Milk concentration at birth(μg/L)
Milkconsumption=0.8; milk consumption in (L/day)
;Decline in PFOA milk concentration over the lactation period (new add in opinion 2020)
Intakemilka=PFOaMilkconcentration*Milkconsumption; initial intake via breastfeeding first month (μg/day)
Intakemilkb=Intakemilka*(1‐DECLINE);intake via breastfeeding second month (μg/day)
Intakemilkc=Intakemilkb*(1‐DECLINE);intake via breastfeeding 3th month (μg/day)
Intakemilkd=Intakemilkc*(1‐DECLINE);intake via breastfeeding 4th month (μg/day)
Intakemilke=Intakemilkd*(1‐DECLINE);intake via breastfeeding 5th month (μg/day)
Intakemilkf=Intakemilke*(1‐DECLINE);intake via breastfeeding 6th. Month (μg/day)
Intakemilkg=Intakemilkf*(1‐DECLINE);intake via breastfeeding 7th month (μg/day)
Intakemilkh=Intakemilkg*(1‐DECLINE);intake via breastfeeding 8th month (μg/day)
Intakemilki=Intakemilkh*(1‐DECLINE);intake via breastfeeding 9th month (μg/day)
Intakemilkj=Intakemilki*(1‐DECLINE);intake via breastfeeding 10th month (μg/day)
Intakemilkk=Intakemilkj*(1‐DECLINE);intake via breastfeeding 11th month (μg/day)
Intakemilkl=Intakemilkk*(1‐DECLINE);intake via breastfeeding 12th month (μg/day)
Oralconc=IF year < 0.083 THEN Intakemilka/BW ELSE IF year>= 0.083 AND year <0.167 THEN Intakemilkb/BW ELSE IF year>=0.167 and year <0.250 THEN Intakemilkc/BW ELSE IF year >=0.250 AND year <0.333 THEN Intakemilkd /BW ELSE IF year>=0.333 AND year <0.417 THEN Intakemilke/BW ELSE IF year>=0.417 AND year <0.500 THEN Intakemilkf/BW ELSE IF year>=0.500 AND year <0.583 THEN Intakemilkg/BW ELSE IF year>=0.583 AND year <0.667 THEN Intakemilkh/BW ELSE IF year>=0.667 AND year <0.750 THEN Intakemilki/BW ELSE IF year>=0.750 AND year <0.833 THEN Intakemilkj/BW ELSE IF year>=0.833 AND year <0.917 THEN Intakemilkk/BW ELSE IF year>=0.917 AND year <1 THEN Intakemilkl/BW ELSE IF year>=1 THEN oralexpo ELSE 0.0
CONCbirth= PFOamaternal*PT;PFOA concentration at birth (μg/L), new add in 2020
APlasbirth= CONCbirth *Free*VPlasC*BWbirth;initial amount in plasma at birth (μg)
AGbirth= APlasbirth* 0.99929853;initial amount in gut at birth (μg)
ALbirth= APlasbirth*66.8360764;initial amount in liver at birth (μg)
AFbirth= APlasbirth* 10.000151;initial amount at in fat birth (μg)
AKbirth= APlasbirth* 5.88575746;initial amount in kidney at birth (μg)
ARbirth= APlasbirth* 74.9108792;initial amount in rest of the body at birth (μg)
Afilbirth= APlasbirth* 2.09606E‐05;initial amount in filtrate at birth (μg)
Adelaybirth= APlasbirth* 42.27910714;initial amount in storage at birth (μg)
Aurinebirth= APlasbirth* 769.1347494;initial amount in urine at birth (μg)
Tinput = 24; duration of dose (h) the CONTAM Panel increased the Tinput to 24h (instead;of 0.6) considering continuous exposure from food.
;oral dose
Input1 = IF MOD(time,24) <=Tinput THEN Oraldose/Tinput ELSE 0.0
;drinking water
Input2 = IF MOD(time,24) <= Tinput THEN Drinkdose/Tinput ELSE 0.0
; Scaling parameters
QC = QCC*BW**0.75;Cardiac output (L/h)
QCP = QC*(1‐Htc);adjust for plasma flow (L/h)
QL = QLC*QCP;Plasma flow to liver (L/h)
QF = QFC*QCP;Plasma flow to fat (L/h)
QK = QKC*QCP;Plasma flow to kidney (L/h)
Qfil = 0.2*QK;Plasma flow to filtrate compartment (L/h); 20% of QK
QG = QGC*QCP;Plasma flow to gut (L/h)
QSk = IF Dermconc > 0.0 THEN QSkC*QCP*(Skinarea/SkinTarea) else 0.0;plasma flow to skin (L/h)
QR = QCP ‐ QL ‐ QF ‐ QK ‐ QG ‐QSk; Plasma flow to rest of the body (L/h)
Qbal = QCP ‐ (QR+QL+QF+QK+QG+QSk); balance check
VL = VLC*BW;Liver volume (L)
VF = VFC*BW;Fat volume (L)
VK = VKC*BW;Kidney volume (L)
Vfil = VfilC*BW;Filtrate compartment volume (L)
VG = VGC*BW;Gut volume (L)
VPlas = VPlasC*BW;Plasma volume (L)
VSk = (Skinarea*Skinthickness)/1,000;Skin volume (L)
VR = 0.84*BW ‐ VL ‐ VF ‐ VK ‐ Vfil ‐ VG ‐ VPlas ‐ VSk;Rest of the body volume (L)
Vbal = (0.84*BW)‐(VL+VF+VK+VFil+VG+VPlas+VSk+VR);Balance check
Tm = Tmc*BW**0.75;transporter maximum
;>>>>>>>>>>>>>>Model equations<<<<<<<<<<<<<<<<<<
;Plasma compartment
APlas’ = QF*CF*FreeF+(QL+QG)*CL*FreeL+QR*CR*FreeR+QSk*CSk*FreeSk+QK*CK*FreeK ‐
QCP*CA*Free ‐ Qfil*CA*Free; rate change of free PFOA in plasma compartment (μg/h)
init APlas = 0 + APlasbirth;initial amount in plasma compartment (μg)
CAFree = APlas/VPlas;free concentration of chemical in plasma in μg/L (ng/mL)
CA = CAFree/Free;total concentration of chemical in plasma in μg/L (ng/mL)
; Gut compartment
AG’ = QG*(CA*Free‐CG*FreeG) + Input1*DoseOn + Input2*DoseOn ;rate change in gut compartment (μg/h)
init AG = 0.0 + AGbirth;initial amount in plasma compartment (μg)
CG = AG/VG;Concentration in gut (μg/L or ng/mL)
CVG = CG/PG;Concentration leaving gut (μg/L or ng/mL)
; Liver compartment
AL’ = (QL*(CA*Free))+(QG*CG*FreeG) ‐ ((QL+QG)*CL*FreeL);Rate of change in liver (μg/h)
init AL = 0.0 + ALbirth;initial amount in liver compartment (μg)
CL = AL/VL;Concentration in liver (μg/L or ng/mL)
CVL = CL/PL;Concentration leaving liver (μg/L or ng/mL)
; Fat compartment
AF’ = QF*(CA*Free‐CF*FreeF);Rate of change in fat (μg/h)
init AF = 0.0 + AFbirth;initial amount in liver compartment (μg)
CF = AF/VF;Concentration in fat (μg/L or ng/mL)
CVF = CF/PF;Concentration leaving fat (μg/L or ng/mL)
; Kidney compartment
AK’ = QK*(CA*Free‐CK*FreeK) + (Tm*Cfil)/(Kt+Cfil);Rate of change in kidneys (μg/h)
init AK = 0.0 + AKbirth;initial amount in kidney compartment (μg)
CK = AK/VK;Concentration in kidneys (μg/L or ng/mL)
CVK = CK/PK;Concentration leaving kidneys (μg/L or ng/mL)
; Filtrate compartment
Afil’ = Qfil*(CA*Free‐Cfil) ‐ (Tm*Cfil)/(Kt+Cfil);Rate of change in filtrate compartment (μg/h)
init Afil = 0.0 + Afilbirth;initial amount in filtrate compartment (μg)
Cfil = Afil/Vfil;Concentration in filtrate compartment (μg/L or ng/mL)
; Storage compartment for urine
Adelay’ = Qfil*Cfil‐kurine*Adelay;Rate of change in storage compartment (μg/h)
init Adelay = 0.0 + Adelaybirth;initial amount in storage compartment (μg)
; Urine
Aurine’ = kurine*Adelay;Rate of change in urine (μg/h)
init Aurine = 0.0 + Aurinebirth;initial amount in urine (μg)
; Skin compartment
ASk’ = QSk*(CA*Free‐CSk*FreeSk);Rate of change in skin(μg/h)
init ASk = 0.0;initial amount in skin (μg)
CSk = ASk/VSk;Concentration in skin compartment (μg/L or ng/mL)
CVSk = CSk/PSk;Concentration leaving skin compartment (μg/L or ng/mL)
; Rest of the body
AR’ = QR*(CA*Free‐CR*FreeR);Rate of change in rest of the body (μg/h)
init AR = 0.0 + ARbirth;initial amount in rest of the body (μg)
CR = AR/VR;Concentration in rest of the body (μg/L or ng/mL)
CVR = CR/PR;Concentration leaving rest of the body (μg/L or ng/mL)
;mass balance checking (not present in Loccisano et al., 2011, equations were taken from Loccisano et al., 2013)
Atissu = Aplas +AG+AL+AF+AK+Afil +Ask+AR +Adelay ;amount in tissues (μg)
Aloss=Aurine ‐ Aurinebirth ;amount loss (μg)
Atissubirth= Aplasbirth +AGbirth+ALbirth+AFbirth+AKbirth +ARbirth + Adelaybirth + Afilbirth ;amount in body at birth (μg)
Atissutotal= Atissu‐Atissubirth ;total amount in tissues (μg)
Atotalbody= Atissutotal+ Aloss ;total amount in body (μg)
d/dt(cumdose) = Input1 rate from oral exposure during simulation (μg/h)
INIT cumdose = 0.0 ;initial dose (μg)
Totalexpo = cumdose ;total amount from oral exposure during simulation (μg)
MB = Totalexpo ‐ Atotalbody ;mass balance
Display Bwc, MCc, CA,ASk,Atotalbody,Atissutotal,Atissubirth,Atissu,Aloss,Totalexpo, MB, Oralconc, TmC, Kt, Free, PL,PK,PF,PR,PSK,PG, tchng,BW,QCC,QFC,QLC,QKC,QGC,QSkC,VFC,VLC,VKC,VGC,VFilC,VPlasC,Dermvol,Drinkrate, Qfil, APlas, AG, AL, AF, AK, AR,PFOaMilkconcentration, Oralconc, Oraldose, oralexpo, PFOamaternal, PT, ratio, DECLINE; for parameters window
Display ASk,Atotalbody,Atissutotal,Atissubirth,Atissu,Aloss,MB,CA, year, BW,CVK, APlas, AG, AL, AF, AK, AR, PFOaMilkconcentration, Oralconc, Oraldose, oralexpo, PFOamaternal, Qbal;for plotting
M.1.1.2. Model code for PFOS
METHOD Stiff
STARTTIME = 0
STOPTIME=438,000;end of simulation (h); 50 years
DT = 0.01
TOLERANCE = 0.01 ;default tolerance
DTMAX = 10.0
DTMIN = 0.000001
year= TIME/(24*365)
; Physiological parameters (from Brown et al., 1997)
;fractional blood flows
QCC = 12.5;Cardiac blood output (L/h/kg^0.75)
QFC = 0.052;Fraction cardiac output going to fat
QLC = 0.069 ;Fraction cardiac output going to liver, through hepatic artery
QKC = 0.175 ;Fraction cardiac output going to kidney
QSkC = 0.058 ;Fraction cardiac output going to skin
QGC = 0.181 ;Fraction of cardiac output going to gut and in the liver via portal artery
; Not used;QfilC = 0.035; Fraction cardiac output to the filtrate compartment (20% of kidney blood flow)
;body weight curve Turn on, To turn off between French curve used in the opinion and WHO curves (only available from birth to 5 years, do not use after 5 years except for the French curve), use parameter window to switch
;Bwc=0 if BW French curve is used
; Bwc=1 if P50 WHO boys curve is used
; Bwc=2 if P05 boys WHO curve is used
;Bwc=3 if P95 boys WHO curve is used
;Bwc=4 if P50 WHO girls curve is used
;Bwc=5 if P05 WHO girls curve is used
;Bwc=6 if P95 WHO girls curve is used
Bwc=0
BW = IF Bwc=0 then 3.68+4.47*year‐0.093*year^2+0.00061*year^3 ELSE IF Bwc=1 then 3.3+ 8.0155*year ‐ 2.1478*year^2 + 0.2342*year^3 ELSE IF Bwc=2 then 2.6+ 0.2063*year^3 ‐ 1.9117*year^2 + 6.9393*year ELSE IF Bwc=3 then 4.2+0.2595*year^3 ‐ 2.3486*year^2 + 9.1534*year ELSE IF Bwc=4 then 3.2 +0.1891*year^3 ‐ 1.7351*year^2+ 7.0589*year ELSE IF Bwc=5 then 2.5 +0.1609*year^3 ‐ 1.5129*year^2 + 6.0135*year ELSE IF Bwc=6 then 4+ 0.2359*year^3 ‐ 2.0807*year^2 + 8.5496*year ELSE 0.0
;body weight equation for Monte Carlo analysis see ‘uncertainty analysis chapter’ in the appendix for explanation
;BW=(3.68+4.47*year‐0.093*year^2+0.00061*year^3)*(MCc)
MCc= 1.58; calculated for P95 percentile BW=3.68+4.47*year‐0.093*year^2+0.00061*year^3 + 2*log(1.96)*(3.68+4.47*year‐0.093*year^2+0.00061*year^3)
;MCc=0.42 ;calculated for P05 percentile corresponding to BW=3.68+4.47*year‐0.093*year^2+0.00061*year^3 ‐ 2*log(1.96)*(3.68+4.47*year‐0.093*year^2+0.00061*year^3)
;body weight equation (based on French equation) see ‘sensitivity analysis chapter’ in the appendix for explanation
;BW=3.68+4.47*year‐0.093*year^2+0.00061*year^3+0.1*(3.68+4.47*year‐0.093*year^2+0.00061*year^3)
;fractional tissue volumes
VLC = 0.026; Fraction liver volume
VFC = 0.214; Fraction fat volume
VKC = 0.004; Fraction kidney volume
VfilC = 0.0004; Fraction filtrate compartment volume (10% of kidney volume)
VGC = 0.0171; Fraction gut volume
VPlasC = 0.0428 ;Fraction plasma volume (58% of blood)
Htc = 0.44; haematocrit
BWbirth=3.68; Body weight at birth in kg new add opinion 2020
PT= 0.36; placenta transfer, new add opinion 2020
Ratio= 0.015; maternal milk/serum ratio during breastfeeding, new add opinion 2020
DECLINE = 0.031 ;decline of PFOS in milk was 3.1% per month, new add opinion 2020
;for dermal exposure
SkinTarea = 9.1*((BW*1,000)**0.666); Total area of skin (cm^2)
Skinthickness = 0.1; Skin thickness (cm)
; Chemical‐specific parameters (PFOS)
Tmc =3,500. ;Maximum resorption rate, Changed from 3.5 in the original Loccisano 2011 model and expressed in μg, to be consistent with other parameters
Kt = 23.0 ;Resorption affinity, Changed from 0.023 in the original Loccisano 2011 model and expressed in μg, to be consistent with other parameters
Free = 0.025 ;Free fraction of PFOS in plasma
PL = 3.72 ;Liver/plasma partition coefficient
PF = 0.14 ;Fat/ plasma partition coefficient
PK = 0.8 ;Kidney/ plasma partition coefficient
PSk = 0.29 ;Skin/ plasma partition coefficient
PR = 0.2 ;Rest of the body/ plasma partition coefficient
PG = 0.57 ;Gut/ plasma partition coefficient
kurinec = 0.001 ;urinary elimination rate constant (/h/kg^‐0.25); estimated from ;Harada et al. (2005)
kurine = kurinec*BW**(‐0.25); urinary elimination rate constant (1/h)
; Free fraction of chemical in tissues
FreeL = Free/PL ;liver
FreeF = Free/PF ;fat
FreeK = Free/PK ;kidney
FreeSk = Free/PSk ;skin
FreeR = Free/PR ;rest of tissues
FreeG = Free/PG ;gut
; Exposure parameters
tchng =438,000 ;Duration of exposure (h); 50 years
;turn dose on/off
DoseOn = IF time<tchng THEN 1.0 else 0.0
; Dermal exposure
Dermconc = 0.0 ;Dermal concentration (μg/mL)
Dermvol = 0.0 ;Dermal exposure volume (mL)
Dermdose = Dermconc*Dermvol*1,000; (μg)
Skinarea = 5 ;Exposed area on skin (cm^2)
; Oral exposure
oralexpo= 0.444e‐3 ;μg/kg/day new add opinion 2020
Oraldose = Oralconc*BW ;(μg/day)
;Drinking water exposure
Drinkconc = 0.0 ;Drinking water concentration (μg/L or ppb)
Drinkrate = 13 ;Drinking water rate (mL/kg/day)
Drinkdose = (Drinkconc*Drinkrate/1,000)*BW; (μg/day)
; Inhalation exposure
Inhalation = 0.0 ;Inhalation dose (ppm)
Tinput = 24 ;duration of dose (h) the CONTAM Panel increased the Tinput to 24h (instead of 0.6) considering continuous exposure from food.
PFOSmaternal=4.89 ;maternal serum concentration μg/l at delivery (new add opinion 2020)
PFOSMilkconcentration=PFOSmaternal*ratio ;initial Milk concentration at birth μg/L
Milkconsumption=0.8 ;milk consumption in (L/day)
;Decline in PFOS milk concentration over the lactation period (new add in opinion 2020)
Intakemilka=PFOSMilkconcentration*Milkconsumption;initial intake via breastfeeding first month (μg/day)
Intakemilkb=Intakemilka*(1‐DECLINE);intake via breastfeeding second month (μg/day)
Intakemilkc=Intakemilkb*(1‐DECLINE);intake via breastfeeding 3th month (μg/day)
Intakemilkd=Intakemilkc*(1‐DECLINE);intake via breastfeeding 4th month (μg/day)
Intakemilke=Intakemilkd*(1‐DECLINE);intake via breastfeeding 5th month (μg/day)
Intakemilkf=Intakemilke*(1‐DECLINE);intake via breastfeeding 6th. Month (μg/day)
Intakemilkg=Intakemilkf*(1‐DECLINE);intake via breastfeeding 7th month (μg/day)
Intakemilkh=Intakemilkg*(1‐DECLINE);intake via breastfeeding 8th month (μg/day)
Intakemilki=Intakemilkh*(1‐DECLINE);intake via breastfeeding 9th month (μg/day)
Intakemilkj=Intakemilki*(1‐DECLINE);intake via breastfeeding 10th month (μg/day)
Intakemilkk=Intakemilkj*(1‐DECLINE);intake via breastfeeding 11th month (μg/day)
Intakemilkl=Intakemilkk*(1‐DECLINE);;intake via breastfeeding 12th month (μg/day)
Oralconc=IF year <0.083 THEN Intakemilka/BW ELSE IF year>= 0.083 AND year <0.167 THEN Intakemilkb/BW ELSE IF year>=0.167 and year <0.250 THEN Intakemilkc/BW ELSE IF year >=0.250 AND year <0.333 THEN Intakemilkd /BW ELSE IF year>=0.333 AND year <0.417 THEN Intakemilke/BW ELSE IF year>=0.417 AND year <0.500 THEN Intakemilkf/BW ELSE IF year>=0.500 AND year <0.583 THEN Intakemilkg/BW ELSE IF year>=0.583 AND year <0.667 THEN Intakemilkh/BW ELSE IF year>=0.667 AND year <0.750 THEN Intakemilki/BW ELSE IF year>=0.750 AND year <0.833 THEN Intakemilkj/BW ELSE IF year>=0.833 AND year <0.917 THEN Intakemilkk/BW ELSE IF year>=0.917 AND year <1 THEN Intakemilkl/BW ELSE IF year>=1 THEN oralexpo ELSE 0.0
CONCbirth= PFOSmaternal *PT; PFOA concentration at birth, new add in 2020
APlasbirth= CONCbirth *Free*VPlasC*BWbirth;initial amount in plasma at birth (μg)
AGbirth= APlasbirth* 9.112506818;initial amount in gut at birth (μg)
ALbirth= APlasbirth* 90.41427413;initial amount in liver at birth (μg)
AFbirth= APlasbirth* 27.99955778;initial amount at in fat birth (μg)
AKbirth= APlasbirth* 3.587821811;initial amount in kidney at birth (μg)
ARbirth= APlasbirth* 100.1289877;initial amount in rest of the body at birth (μg)
Afilbirth= APlasbirth* 1.50221E‐05;initial amount in filtrate at birth (μg)
Adelaybirth= APlasbirth* 9.156014881;initial amount in storage at birth (μg)
Aurinebirth= APlasbirth* 565.1438095;initial amount in urine at birth (μg)
;oral dose
Input1 = IF MOD(time,24) <=Tinput THEN Oraldose/Tinput ELSE 0.0
;drinking water
Input2 = IF MOD(time,24) <= Tinput THEN Drinkdose/Tinput ELSE 0.0
; Scaling parameters
QC = QCC*BW**0.75;Cardiac output (L/h)
QCP = QC*(1‐Htc);adjust for plasma flow (L/h)
QL = QLC*QCP;Plasma flow to liver (L/h)
QF = QFC*QCP;Plasma flow to fat (L/h)
QK = QKC*QCP;Plasma flow to kidney (L/h)
Qfil = 0.2*QK;Plasma flow to filtrate compartment (L/h); 20% of QK
QG = QGC*QCP;Plasma flow to gut (L/h)
QSk = IF Dermconc > 0.0 THEN QSkC*QCP*(Skinarea/SkinTarea) else 0.0;plasma flow to skin
QR = QCP ‐ QL ‐ QF ‐ QK ‐ QG ‐QSk;Plasma flow to rest of the body (L/h)
Qbal = QCP ‐ (QR+QL+QF+QK+QG+QSk);balance check
VL = VLC*BW;Liver volume (L)
VF = VFC*BW;Fat volume (L)
VK = VKC*BW;Kidney volume (L)
Vfil = VfilC*BW;Filtrate compartment volume (L)
VG = VGC*BW;Gut volume (L)
VPlas = VPlasC*BW;Plasma volume (L)
VSk = (Skinarea*Skinthickness)/1,000; Skin volume (L)
VR = 0.84*BW ‐ VL ‐ VF ‐ VK ‐ Vfil ‐ VG ‐ VPlas ‐ VSk;Rest of the body volume (L)
Vbal = (0.84*BW)‐(VL+VF+VK+VFil+VG+VPlas+VSk+VR);Balance check
Tm = Tmc*BW**0.75;transporter maximum
;>>>>>>>>>>>>>>>>>>>> Model equations <<<<<<<<<<<<<<<<<<<<<<<<<<<<
; Plasma compartment
APlas’ = QF*CF*FreeF+(QL+QG)*CL*FreeL+QR*CR*FreeR+QSk*CSk*FreeSk+QK*CK*FreeK‐ QCP*CA*Free ‐ Qfil*CA*Free;rate change of free PFOS in plasma compartment (μg/h)
init APlas = 0 + APlasbirth;initial amount in plasma compartment (μg)
CAFree = APlas/VPlas;free concentration of PFOS in plasma in μg/L (ng/mL)
CA = CAfree/Free;total concentration in plasma in μg/L (ng/mL)
; Gut compartment
AG’ = QG*(CA*Free‐CG*FreeG) + Input1*DoseOn + Input2*DoseOn; rate change in gut compartment (μg/h)
init AG = 0.0 + AGbirth;initial amount in plasma compartment (μg)
CG = AG/VG;Concentration in gut (μg/L or ng/mL)
CVG = CG;Concentration leaving gut (μg/L or ng/mL)
; Liver compartment
AL’ = (QL*(CA*Free))+(QG*CG*Freeg) ‐ ((QL+QG)*CL*FreeL);Rate of change in liver (μg/h)
init AL = 0.0 + ALbirth;initial amount in liver compartment (μg)
CL = AL/VL;Concentration in liver (μg/L or ng/mL)
CVL = CL/PL;Concentration leaving liver (μg/L or ng/mL)
; Fat compartment
AF’ = QF*(CA*Free‐CF*FreeF);Rate of change in fat (μg/h)
init AF = 0.0 + AFbirth;initial amount in liver compartment (μg)
CF = AF/VF;Concentration in fat (μg/L or ng/mL)
CVF = CF/PF;Concentration leaving fat (μg/L or ng/mL)
; Kidney compartment
AK’ = QK*(CA*Free‐CK*FreeK) + Tm*Cfil/(Kt+Cfil); Rate of change in kidneys (μg/h)
init AK = 0.0 + AKbirth; initial amount in kidney compartment (μg)
CK = AK/VK; Concentration in kidneys (μg/L or ng/mL)
CVK = CK/PK; Concentration leaving kidneys (μg/L or ng/mL)
; Filtrate compartment
Afil’ = Qfil*(CA*Free‐Cfil) ‐ (Tm*Cfil)/(Kt+Cfil); Rate of change in filtrate compartment (μg/h)
init Afil = 0.0 + Afilbirth; initial amount in filtrate compartment (μg)
Cfil = Afil/Vfil; Concentration in filtrate compartment (μg/L or ng/mL)
; Storage compartment for urine
Adelay’ = Qfil*Cfil‐kurine*Adelay;Rate of change in storage compartment (μg/h)
init Adelay = 0.0 + Adelaybirth;initial amount in storage compartment (μg)
; Urine
Aurine’ = kurine*Adelay; Rate of change in urine (μg/h)
;Aurine’ = Qfil*Cfil ‐ kurine*Aurine
init Aurine = 0.0 +Aurinebirth; initial amount in urine (μg)
; Skin compartment
ASk’ = QSk*(CA*Free‐CSk*FreeSk);Rate of change in skin (μg/h)
init ASk = 0.0;initial amount in skin (μg)
CSk = ASk/VSk;Concentration in skin compartment (μg/L or ng/mL)
CVSk = CSk/PSk;Concentration leaving skin compartment (μg/L or ng/mL)
; Rest of the body
AR’ = QR*(CA*Free‐CR*FreeR);Rate of change in rest of the body (μg/h)
init AR = 0.0 + ARbirth;initial amount in rest of the body (μg)
CR = AR/VR;Concentration in rest of the body (μg/L or ng/mL)
CVR = CR/PR;Concentration leaving rest of the body (μg/L or ng/mL)
; mass balance checking (not present in Loccisano et al 2011, equations were taken from Loccisano et al 2013)
Atissu = Aplas +AG+AL+AF+AK+Afil +Ask+AR +Adelay; amount in tissues (μg)
Aloss=Aurine ‐ Aurinebirth; amount loss (μg)
Atissubirth= Aplasbirth +AGbirth+ALbirth+AFbirth+AKbirth +ARbirth + Adelaybirth + Afilbirth; amount in body at birth (μg)
Atissutotal= Atissu‐Atissubirth;total amount in tissues (μg)
Atotalbody= Atissutotal+ Aloss;total amount in body (μg)
d/dt(cumdose) = Input1;rate from oral exposure during simulation (μg/h)
INIT cumdose = 0.0;initial dose (μg)
Totalexpo = cumdose;total amount from oral exposure during simulation (μg)
MB = Totalexpo ‐ Atotalbody;mass balance
Display MCc,Bwc,Adelay,Afil,Aurine,Atotalbody,Atissutotal,Atissubirth,Atissu,Aloss,Totalexpo, MB, Oralconc, TmC, Kt, Free, PL,PK,PF,PR,PSK,PG, tchng,BW,QCC,QFC,QLC,QKC,QGC,QSkC,VFC,VLC,VKC,VGC,VFilC,VPlasC,Dermvol,Drinkrate, Qfil, APlas, AG, AL, AF, AK, AR,PFOSMilkconcentration, Oralconc, Oraldose, oralexpo, PFOSmaternal, PT, ratio, DECLINE;for parameters window
Display MCc,Adelay, Afil,Aurine, Atotalbody,Atissutotal,Atissubirth,Atissu,Aloss,MB,CA, year, BW,CVK, APlas, AG, AL, AF, AK, AR, PFOSMilkconcentration, Oralconc, Oraldose, oralexpo, PFOSmaternal, Qbal, Vbal;for plotting
M.2. Model evaluation
M.2.1. General approach
M.2.1.1. Sensitivity analysis
Sensitivity analysis (SA) provides a quantitative evaluation of how parameters influence the dose metrics (PFAS serum concentration).
In the previous opinion (EFSA CONTAM Panel, 2018), it was described that the highest values of sensitivity analysis results (value > 0.5) were obtained for the following parameters according to Loccisano et al. (2011): Cardiac output, elimination parameters (glomerular filtration rate and resorption maximum parameters (Tm)), the free fraction and the haematocrit. Loccisano et al. (2011) performed the sensitivity analysis at daily doses of 3 and 10 mg/kg for PFOA, and 0.3 and 0.75 mg/kg for PFOS.
A new sensitivity analysis was performed in order to determine the impact of newly added parameters in the model (BW growth curve, placental transfer, ratio milk concentration/maternal serum concentration (milk/serum ratio), decline in PFAS concentration in breastmilk over time, breastfeeding duration and daily milk consumption) on the predicted serum PFOA and PFOS concentrations in the child.
Sensitivity coefficients were determined based on the predicted serum concentrations resulting from a 10% change increase in the value of each parameter using the forward difference method (according to the WHO/IPCS, 2010). Sensitivity coefficients were normalised using the following equation:
Sensitivity Coefficient = ((A – B)/B)/(C – D)/D))
Where A is the serum concentration resulting from e.g. a 10% increase in the parameter value, B is the serum concentration resulting from the initial parameter value, C is the value of parameter increased by 10% and D is the initial parameter value.
For the BW growth curve (parameter that changes during simulation time), the following equation was used in the assessment of the sensitivity, based on French data on body weight (including a 10% increase).
BW=3.68+4.47*year‐0.093*year^2+0.00061*year^3+0.1*(3.68+4.47*year‐0.093*year^2+0.00061*year^3) (based on a forward difference method)
This equation has been added in the model codes for PFOA and PFOS.
M.2.1.2. Uncertainty analysis
The notion of uncertainty encompasses both true uncertainty (i.e. in model parameter value) and variability (i.e. from population variability).
In order to evaluate the impact of parameter variability on the predicted serum PFOA and PFOS concentration, parameters were randomly varied based on a predefined distribution (with 100 iterations), with the mean values set to those used during model calibration. The uncertainty coefficients were calculated using the following equation:
Uncertainty coefficients = Ratio ‘P95/P50’ = (Mean + 2 x standard deviation)/mean
For the French BW growth curve (parameter that changes during simulation time), it was not possible to obtain the 95th nor the 5th percentile of the distribution, so the CONTAM Panel assumed that the distribution was lognormal (accepted distribution for a biological parameter), and applied a factor of 2 to the mean to roughly estimate the 95th and the 5th percentile) according to JECFA guidance (CF JECFA Principles and Methods for the Risk Assessment of Chemicals in Food, chapter 6).
The following equation was used:
BW=3.68+4.47*year‐0.093*year^2+0.00061*year^3 ‐ 2*log(1.96)*(3.68+4.47*year‐0.093*year^2+0.00061*year^3)
(Or BW=(3.68+4.47*year‐0.093*year^2+0.00061*year^3)*(1–0.29*2))
This equation has been added in the model codes for PFOA and PFOS.
Note: The CONTAM panel is aware that this approach is very conservative.
M.2.1.3. Simulations data used for sensitivity and uncertainty analysis
The following intakes were used for sensitivity and uncertainty analysis:
For PFOA, simulations were run for 35 years with exposure from breast milk during the first year of life followed by a daily oral exposure of 0.187 in ng/kg bw per day for 34 years.
For PFOS, simulations were run for 35 years with exposure from breast milk the first year of life followed by a daily oral exposure of 0.445 in ng/kg bw per day for 34 years.
M.2.1.4. Conclusions of the sensitivity and uncertainty analysis
The outcome of the sensitivity and uncertainty analyses might inform the reliability of a model to provide dose metric predictions of use in the risk assessment, as illustrated in Table M.2 (WHO/IPCS, 2010).
Table J.1.
Reports on associations between serum levels of PFHxS and/or PFNA and thyroid disease or thyroid hormones. In addition, the last column shows if also other PFASs were assessed (apart from PFOS and PFOA, see previous Opinion (EFSA CONTAM Panel, 2018))
| Author | Population, country and number of subjects | Type | Serum PFNA/PFHxS levels (ng/mL) | Findings | Comments | Other compounds |
|---|---|---|---|---|---|---|
| Thyroid disease | ||||||
| Lopez‐Espinosa et al. (2012) | C8, 12,000 children 1–17 years | CS, L | Median PFNA 1.5 | Null for hypo‐or hyperthyroidism. PFNA vs. TT4 +, but null for TSH | Few (39) cases. Adjusted for potential confounders | – |
| Wen et al. (2013) | NHANES, USA, 1181 | CS |
PFHxS: median 2.0 PFNA: median 1.5 |
PFHxS vs. hypothyroidism + hyperthyroidism + in women only PFNA null PFHxS vs. TT4, TT3, and FT3 + in women, but null vs. TSH |
Adjusted for potential confounders. Few (24) cases. Diagnoses based on cut‐offs for TSH. Not consistent with results on continuous scale | – |
| Thyroid hormones | ||||||
| Bloom et al. (2010) | Anglers, USA, 31 | CS |
PFHxS GM 0.75 PFNA GM 0.79 |
Null | Too small study to be informative | PFDA, PFUnDA |
| Chan et al. (2011) | Pregnant women, Canada, 96+175 | CS, analysed as case–control | PFHxS GM 1.1 |
Cases: high FT4, Controls: normal FT4, Both: normal TSH No association btw PFHxS and case/ctrl status |
Matched + further adjustment for potential confounders | – |
| Ji et al. (2012) | Adults, Korea, 633 | CS |
PFHxS median 1.5 PFNA median 2.1 |
Null for TSH and TT4 for both compounds | Adjusted for potential confounders | PFDA, PFUnDA, PFDoDA, PFTrDA, PFHpS |
| Jain (2013) | NHANES, USA, ≥ 12 years, 1540 | CS | PFHxS and PFNA measured but mean or median levels not reported | PFHxS vs. TT4 +, but null for FT3, FT4 and TSH for both compounds | Adjusted for potential confounders | Several, but data not presented |
| Wang et al. (2013b) | Pregnant women, Norway, 903 | CS |
PFHxS GM 0.6 PFNA GM 0.4 |
Null for TSH for both compounds | Adjusted for potential confounders | PFDA, PFUnDA, PFHpDA |
| Lin et al. (2013a) | Young individuals, Taiwan, 567 | CS | GM PFNA 1.0 |
PFNA vs. FT4 +, but null for PFHxS Null for TSH vs. both compounds |
Adjusted for potential confounders | PFUnDA |
| Wang et al. (2014c) | Pregnant women, 285 newborns, Taiwan, 116 | CS | PFHxS median 0.8, PFNA median 1.5 |
PFHxS: In women TSH +, but null for FT4, TT4 and TT3. In newborns null PFNA: In women FT4 ‐ and TT4 ‐, but null for TSH. In newborns – for TT3 and TT4, but null for FT4 and TSH |
Adjusted for potential confounders | PFDA, PFUnDA, PFDoDA |
| Webster et al. (2014) | Pregnant women, Canada, 152 | CS |
PFHxS median 1.0 PFNA median 0.6 |
PFNA vs. TSH + in all women and in 14 women with TPO antibodies. Null for FT4 PFHxS vs. TSH and FT4 null |
Adjusted for gestational week and TPO antibodies | – |
| Lewis et al. (2015) | NHANES, USA, 1682, results by sex and age groups | CS |
PFHxS: medians 0.8–1.8 PFNA: medians 0.7–1.1 |
Overall null for both compounds Some significant findings in subgroups by age and sex) |
Adjusted for potential confounders | – |
| Berg et al. (2015) | Pregnant women, Norway, 375 | CS, but repeated sampling |
PFHxS: median 0.4 PFNA: median 0.6 |
Null for both compounds vs. TSH, FT4, T4, FT3, T3 | Hormones measured three times. Adjusted for some potential confounders | PFDA, PFUnDA, PFHpS |
| Webster et al. (2016) | Adults NHANES, USA, 1525 | CS |
PFHxS GM 1.9 PFNA GM 1.5 |
No TPOab and normal U‐I: Null for both compounds vs. FT4, TSH, T3, T4, FT4. TPOab and low U‐I: Several sign. assoc for PFHxS and PFNA |
Adjusted for potential confounders 26 out of 1,525 had TPO antibodies and low U‐iodine |
– |
| Shah‐Kulkarni et al. (2016) | Newborns, Korea, 279 | CS | PFHxS GM 0.4 and PFNA 0.2 in cord blood | PFHxS and PFNA vs. TSH, T4, T3: overall and in boys null. In girls PFHxS vs. TT3 +, and PFNA vs. TSH − | Adjusted for potential confounders | PFPeA, PFDA, PFUnDA, PFDoDA, PFTrDA, PFTeDA |
| Kim et al. (2016b) | 27 infants with congenital hypothyroidism, 13 controls | CS |
PFHxS: Medians 1.2 and 1.2 PFNA: Medians 1.9 and 0.6 |
PFNA higher in cases. No difference for PFHxS | No confounding adjustment Maternal levels not available | PFBA, PFPeDA, PFHpDA, PFDA, PFUnDA, PFDoDA, PFTrDA, PFTeDA, PFBS, PFHpS, PFDS |
| Yang et al. (2016) | Mother–infant pairs, Beijing, 157 | CS |
PFHxS: median maternal 0.5, infant 0.2 PFNA: median maternal 0.5, infant 1 |
Maternal PFNA vs. maternal TSH: – Infant PFHxS and PFNA vs.: infant FT3, FT4, TSH: null |
Adjusted for potential confounders | PFDA, PFUnDA, PFDoDA |
| Crawford et al. (2017) | Healthy women in a time to pregnancy study, US, 99 | CS | GM PFHxS 1.6 and PFNA 0.8 |
PFNA vs. FT4 +, null for TT3, TT4, TSH PFHxS vs. FT4, TT3, TT4 and TSH: null |
Adjusted only for age | – |
| Li et al. (2017) | 140 persons with ‘abnormal thyroid hormones’ and 62 with normal levels, China | CS | Median PFHxS 0.2 | PFHxS vs. FT4, FT3, TSH, antibodies: null | Unadjusted | PFPrPA, PFBA, PFPeDA, PFPrA, PFBS |
| Berg et al. (2017) | 370 mother‐infant pairs from Norway | CS | Median PFHxS 0.4 and PFNA 0.6 | Maternal PFHxS and PFNA vs. infant TSH: null | Adjusted for some confounders and for other persistent OPs | PFDA, PFUnDA, PFHpS |
CS: cross‐sectional (study); FT4: free T4; FT3: free T3; GM: geometric mean; L: longitudinal (study); N: number of subjects; PFHxS: perfluorohexane sulfonic acid; PFNA: perflurononanoic acid; T3: triiodothyronine; TT3: total T3, T4: thyroxine; TT4: total T4; TPO: thyroid peroxidase; TSH: thyroid‐stimulating hormone. A ‘+’ sign denotes a positive association and a ‘–’ denotes an inverse association.
The reliability of the model predictions regarding dose metrics that can be used for risk assessment is based on the level of sensitivity of the predictions to the model parameters and the level of uncertainty of the parameter values. If the highly sensitive parameters are also the ones that are highly uncertain, then the reliability of the model for risk assessment applications would be questionable (WHO/IPCS, 2010).
Table M.2.
Sensitivity and uncertainty analyses in determining the reliability of PBPK model predictions of dose metrics for risk assessment (see WHO/IPCS 2010)
| Uncertainty | ||||
|---|---|---|---|---|
| High | medium | low | ||
| Sensitivity | high | |||
| medium | ||||
| low | ||||
Low reliability: black box; medium reliability: grey boxes; high reliability: white boxes (see WHO/IPCS, 2010).
M.2.2. Results for PFOA
M.2.2.1. Sensitivity analysis for PFOA
Table M.3.
Results of sensitivity analysis for PFOA
| Parameter | Value | Sensitivity coefficient | ||||
|---|---|---|---|---|---|---|
| At birth | At 6 months | At 1 year | At 5 years | At 35 years | ||
| Placental transfer | 0.74 | 1 | 0.08 | 0.049 | 0.022 | 0 |
| Milk/serum ratioa | 0.03 | 0 | 0.92 | 0.95 | 0.44 | 0 |
| Body weight curve (10% increase) | – | –0.91 | –0.89 | –0.86 | –0.20 | –0.23 |
| Decline | 0.077 | 0 | –0.18 | –0.38 | –0.18 | 0 |
| Milk volume | 0.8 | 0 | 0.92 | 0.95 | 0.44 | 0 |
Note: If Normalised Coefficient > 1, parameter error is amplified.
Milk/serum ratio corresponds to milk concentration/maternal serum concentration ratio.
According to the WHO/IPCS (2010), the sensitivity of the parameter (see Table M.3) is considered as high (absolute value greater than or equal to 0.5), medium (absolute value greater than or equal to 0.2 but less than 0.5) or low (absolute value greater than or equal to 0.1 but less than 0.2).
Table K.1.
Results of the statistical evaluation of the influence of PFAS sum (PFOA, PFNA, PFHxS and PFOS) on vaccine antibodies against Hib, tetanus and diphtheria (presented as log 10 values) in children vaccinated at least two times
| Groups | Hib | Tetanus IgG1 | Diphtheria | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Quantile | Grp. mean | N | Mean | SD | p‐value | % Diff. | Quantile | Grp. mean | N | Mean | SD | p value | % Diff. | Quantile | Grp. mean | N | Mean | SD | p‐value | % Diff. | |
| 5 | Q1 | 10 | 20 | 1.92 | 0.75 | 0.127 | – | Q1 | 10 | 20 | 1.06 | 0.32 | 0.0914 | – | Q1 | 10 | 20 | 0.44 | 0.4 | 0.059 | – |
| Q2 | 19.8 | 20 | 1.67 | 0.59 | 0.247 | –44 | Q2 | 19.8 | 20 | 0.99 | 0.41 | 0.573 | –15 | Q2 | 19.8 | 20 | 0.57 | 0.36 | 0.286 | 35 | |
| Q3 | 31.9 | 20 | 1.78 | 0.74 | 0.547 | –28 | Q3 | 30.9 | 20 | 1.06 | 0.44 | 0.955 | 0 | Q3 | 30.9 | 20 | 0.56 | 0.4 | 0.365 | 32 | |
| Q4 | 40.5 | 20 | 1.38 | 0.79 | 0.034 | –71 | Q4 | 39.8 | 20 | 1.01 | 0.35 | 0.677 | –11 | Q4 | 39.8 | 20 | 0.46 | 0.42 | 0.869 | 5 | |
| Q5 | 50.4 | 18 | 1.49 | 0.63 | 0.065 | –63 | Q5 | 49.7 | 20 | 0.77 | 0.35 | 0.010 | –49 | Q5 | 49.7 | 20 | 0.2 | 0.55 | 0.126 | –42 | |
| Quantile | grp. mean | N | Mean | SD | p.value | % Diff. | Quantile | grp. mean | N | Mean | SD | p.value | % Diff. | Quantile | Grp. mean | N | Mean | SD | p.value | % Diff. | |
| 10 | Q1 | 8.4 | 10 | 1.8 | 0.82 | 0.233 | – | Q1 | 8.4 | 10 | 1.1 | 0.4 | 0.387 | – | Q1 | 8.4 | 10 | 0.54 | 0.52 | 0.210 | – |
| Q2 | 11.5 | 10 | 2.04 | 0.71 | 0.482 | 74 | Q2 | 11.5 | 10 | 1.01 | 0.22 | 0.575 | –19 | Q2 | 11.5 | 10 | 0.35 | 0.21 | 0.303 | –35 | |
| Q3 | 15.7 | 10 | 1.7 | 0.75 | 0.790 | –21 | Q3 | 15.7 | 10 | 1.01 | 0.43 | 0.643 | –19 | Q3 | 15.7 | 10 | 0.63 | 0.31 | 0.650 | 23 | |
| Q4 | 23.9 | 10 | 1.63 | 0.4 | 0.580 | –32 | Q4 | 23.9 | 10 | 0.97 | 0.42 | 0.493 | –26 | Q4 | 23.9 | 10 | 0.52 | 0.41 | 0.922 | –5 | |
| Q5 | 29.6 | 10 | 1.6 | 0.69 | 0.574 | –37 | Q5 | 28.7 | 10 | 0.97 | 0.45 | 0.507 | –26 | Q5 | 28.7 | 10 | 0.47 | 0.42 | 0.7354 | –15 | |
| Q6 | 34.2 | 10 | 1.95 | 0.78 | 0.675 | 41 | Q6 | 33.1 | 10 | 1.16 | 0.42 | 0.748 | 15 | Q6 | 33.1 | 10 | 0.65 | 0.37 | 0.588 | 29 | |
| Q7 | 38.7 | 10 | 1.14 | 0.66 | 0.063 | –78 | Q7 | 38 | 10 | 1.05 | 0.25 | 0.742 | –11 | Q7 | 38 | 10 | 0.51 | 0.19 | 0.883 | –7 | |
| Q8 | 42.3 | 10 | 1.63 | 0.86 | 0.658 | –32 | Q8 | 41.6 | 10 | 0.98 | 0.44 | 0.525 | –24 | Q8 | 41.6 | 10 | 0.42 | 0.58 | 0.624 | –24 | |
| Q9 | 46.3 | 10 | 1.58 | 0.75 | 0.543 | –40 | Q9 | 45.1 | 10 | 0.78 | 0.29 | 0.062 | –52 | Q9 | 45.1 | 10 | 0.28 | 0.62 | 0.3354 | –45 | |
| Q10 | 55.5 | 8 | 1.38 | 0.47 | 0.196 | –62 | Q10 | 54.3 | 10 | 0.75 | 0.42 | 0.077 | –55 | Q10 | 54.3 | 10 | 0.12 | 0.49 | 0.084 | –62 | |
Hib: Haemophilus influenza type b; NOAEC: No‐Observed‐Adverse‐Effect Concentration; PFOA: perfluorooctanoic acid; PFNA: perfluorononanoic acid; PFHxS: perfluorohexane sulfonate; PFOS: perfluorooctanesulfonic acid; SD: standard deviation.
Note: Yellow colour marks the quantile corresponding to the NOAECs for the PFAS sum derived as the quantile above the quantile significantly different from the first quantile. ANOVA results (p values) of group evaluations are given in green.
M.2.2.2. Uncertainty analysis for PFOA
Table M.4.
Results of uncertainty analysis for PFOA
| Parameter | Value | Distribution used | Uncertainty coefficient (P95/P50) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| initial | min | max | 100 iterations | At birth | At 6 months | At 1 year | At 5 years | At 35 years | |
| Placental transfer | 0.74 | 0.37 | 1.48 | Uniform | 0.70 | 0.07 | 0.042 | 0.02 | 0 |
| Milk/serum ratiob | 0.03 | 0.015 | 0.045 | Uniform | 0 | 0.53 | 0.56 | 0.25 | 0 |
| Body weight curve* | – | – | – | lognormal | 2.4 | 2.4 | 2.3 | 1.2 | 0.8 |
| Body weight curvea | – | – | – | lognormal | 1.6 | 1.6 | 1.5 | 1.1 | 0.9 |
| Decline | 0.077 | 0.0385 | 0.1155 | Uniform | 0 | 0.11 | 0.23 | 0.11 | 0 |
| Milk volume | 0.8 | 0.4 | 1.2 | Uniform | 0 | 0.54 | 0.56 | 0.26 | 0 |
The following equation was used: BW=3.68+4.47*year‐0.093*year^2+0.00061*year^3 ‐ 2*log(1.96)*(3.68+4.47*year‐0.093*year^2+0.00061*year^3) corresponding to the P05 of the curve. Note that the calculated uncertainty coefficient is P50/P05.
The following equation was used:
BW=3.68+4.47*year‐0.093*year^2+0.00061*year^3 + 2*log(1.96)*(3.68+4.47*year‐0.093*year^2+0.00061*year^3) corresponding to the P95 of the curve. Note that the calculated uncertainty coefficient is P95/P50; this equation has been added in the model codes for PFOA and PFOS.
Milk/serum ratio corresponds to milk concentration/maternal serum concentration ratio.
M.2.2.3. Coupling the results of sensitivity and uncertainty analysis for PFOA
Table M.5.
Results of sensitivity and uncertainty analysis for PFOA
| Uncertainty | ||||
|---|---|---|---|---|
| High | medium | Low | ||
| Sensitivity | High | BW |
‐ Milk/serum ratio ‐ Milk consumption |
|
| Medium | Decline | |||
| Low | Placental transfer | |||
According to the WHO/IPCS (2010) guidance, the BW appears to have a low reliability, the milk/serum ratio appears to have a medium reliability (see Table M.5). Placental transfer and decline show a high reliability.
Table L.2.
PFOA serum levels at age 5 vs. diphtheria antibody titres (log 2) at age 7
| Deciles | PFOA (ng/mL) | N | Mean | SE | SD | t‐test | %change |
|---|---|---|---|---|---|---|---|
| p | |||||||
| 1 | 2.5 | 43 | 0.23 | 0.24 | 1.58 | Referent | |
| 2 | 3 | 43 | 0.06 | 0.25 | 1.61 | 0.61 | –11 |
| 3 | 3.3 | 43 | –0.11 | 0.32 | 2.12 | 0.4 | –21 |
| 4 | 3.6 | 43 | –0.35 | 0.28 | 1.82 | 0.11 | –33 |
| 5 | 3.9 | 43 | –0.51 | 0.31 | 2.04 | 0.06 | –40 |
| 6 | 4.2 | 43 | –0.05 | 0.27 | 1.74 | 0.48 | –18 |
| 7 | 4.6 | 43 | 0.26 | 0.31 | 2.05 | 0.95 | 2 |
| 8 | 4.9 | 43 | 0.00 | 0.33 | 2.14 | 0.71 | –15 |
| 9 | 5.5 | 43 | –0.52 | 0.26 | 1.73 | 0.04 | –41 |
| 10 | 6.7 | 43 | –0.69 | 0.31 | 2.04 | 0.02 | –47 |
| Quintiles | PFOA (ng/mL) | N | Mean | SE | SD | t‐test | %change |
|---|---|---|---|---|---|---|---|
| p | |||||||
| 1 | 2.75 | 86 | 0.15 | 0.17 | 1.60 | 0.17 | |
| 2 | 3.45 | 86 | –0.23 | 0.21 | 1.97 | 0.22 | –23 |
| 3 | 4.05 | 86 | –0.28 | 0.20 | 1.89 | 0.11 | –26 |
| 4 | 4.75 | 86 | 0.13 | 0.23 | 2.09 | 0.94 | –1 |
| 5 | 6.1 | 86 | –0.60 | 0.20 | 1.9 | 0.007 | –41 |
Yellow highlighted text = NOAEC; Blue highlighted text = LOAEC.
Total number of individuals in these analyses = 431. Divided into deciles: ~ 43 and quintiles ~ 86. Mean and standard error provided on log‐2 scale SD derived. p‐value: t‐test (assuming log‐normal distribution). % change estimated based on mean difference relative to the lowest (referent) decile corresponds to % shift on median concentration on the original (back‐transformed scale). Mean and SD pooled (from deciles) to estimate NOAEC in quintiles.
M.2.2.4. Additional analysis on Body weight influence
For body weight, the CONTAM Panel explored the body weight influence with different growth curves from the WHO until the age of 5 years:
1‐BW=3.68+4.47*year‐0.093*year^2+0.00061*year^3
2‐BW = 3.3+ 8.0155*year ‐ 2.1478*year^2 + 0.2342*year^3; P50 boys until 5 years old
3‐BW =3.2 +0.1891*year^3 ‐ 1.7351*year^2+ 7.0589*year; P50 girls until 5 years old
4‐BW = 2.5 +0.1609*year^3 ‐ 1.5129*year^2 + 6.0135*year; P05 girls until 5 years old
5‐BW=2.6+ 0.2063*year^3 ‐ 1.9117*year^2 + 6.9393*year; P05 boys until 5 years old
6‐ BW =4+ 0.2359*year^3 ‐ 2.0807*year^2 + 8.5496*year; P95 girls until 5 years old
7‐ BW=4.2+0.2595*year^3 2.3486*year^2 + 9.1534*year; P95 boys until 5 years old
Results are presented in the Table M.6 below:
Table M.6.
Impact of different growth curves on the calculated serum levels at birth, 6 months, 1 and 5 years of age, following 12 months of breastfeeding
| Body weight growth curve | At birth | 6 months | 1 years | 5 years |
|---|---|---|---|---|
| present opinion | 1.48 | 8.77 | 8.78 | 1.53 |
| P05 girls from WHO | 2.18 | 9.99 | 9.80 | 1.83 |
| P05 boys from WHO | 2.09 | 9.17 | 9.00 | 1.83 |
| P50 girls from WHO | 1.70 | 8.18 | 8.15 | 1.71 |
| P50 boys from WHO | 1.65 | 7.61 | 7.58 | 1.73 |
| P95 girls from WHO | 1.36 | 6.67 | 6.71 | 1.58 |
| P95 boys from WHO | 1.30 | 6.32 | 6.40 | 1.63 |
Note: Mothers and children were exposed to 0.187 ng/kg bw per day. The target concentration at 1 year was 8.78 ng/mL.
As shown in Figure M.1, when using different body weight equations (P50 in boys and girls), this resulted in a PFOA serum concentration (CA) at 1 year between 7.61 (CA2) and 8.18 ng/mL (CA3). By comparison, CA1 is the PFOA serum concentration (8.77 ng/mL) using the French growth equation.
Figure M.1.
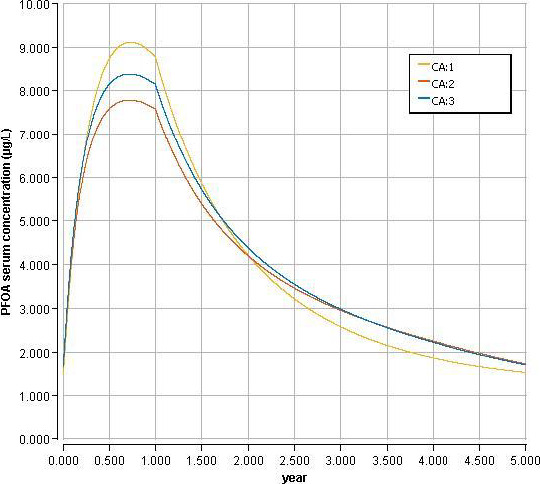
Body weight curves influence on PFOA serum concentration (ng/mL)
- CA1 corresponds to equation used in the current opinion (French survey), CA2 to the WHO curve (P50 in boys), CA3 to the WHO curve (P50 in girls).
Figure M.2 Below shows the body weight curves according to age with the different equations.
Figure M.2.
- BW1 represents the body weight curve from the French survey (used in the current opinion), BW2 the body weight curve from P50 WHO curve for boys and BW3 the body weight curve from P50 WHO curve for girls.
M.2.2.5. Breastfeeding duration: 6 months vs. 12 months
Table M.7 below resumes the influence of breastfeeding for 6 vs. 12 months on the PFOA serum concentration in children, with the same exposure after breastfeeding. The target serum concentration is 8.78 ng/mL, the exposure of the mother and child after 6 months breastfeeding was 0.187 ng/kg bw per day (so not double as used in the assessment).
Table M.7.
Influence of breastfeeding for 6 vs. 12 months on the PFOA serum concentration in children
| Breastfeeding duration | 6 months | 1 year |
|---|---|---|
| 6 months | 8.76 | 5.32 |
| 12 months | 8.77 | 8.78 |
Conclusion:
When breastfeeding duration is varied between 6 and 12 months, this results in a PFOA serum concentration at 1 year between 5.32 and 8.78 ng/mL (following an oral exposure (daily intake) in ng/kg per day of 0.187, after 6‐ or 12‐month breastfeeding period).
M.2.2.6. Conclusions for PFOA
According to the analysis of sensitivity and uncertainty, only the BW appears to have a low reliability, i.e. a high impact on the PFOA serum concentration.
Nevertheless, according to additional analyses, e.g. comparison of serum prediction with different growth curves (WHO vs. French total Diet Study), the predictions remain acceptable, whatever the BW equation used. The calculated PFOA serum concentration in ng/mL with the PBPK model at 1 year, depending of the growth curve (WHO P50 boys and girls) used is less than a factor 2 (e.g. 1.16 for boys, 1.08 for girls) different from the predicted PFOA serum concentration used as RP in the current opinion.
In conclusion, the CONTAM Panel decided to use the growth curve based on the French study (EAT for French total Diet Study) in the modelling which represents the complete lifetime.
M.2.3. Results for PFOS
M.2.3.1. Sensitivity analysis for PFOS
Table M.8.
Results of sensitivity analysis for PFOS
| Parameter | Value | Sensitivity coefficient | ||||
|---|---|---|---|---|---|---|
| At birth | At 6 months | At 1 year | At 5 years | At 35 years | ||
| Placental transfer | 0.36 | 1 | 0.13 | 0.07 | 0.04 | 0.0 |
| Milk/serum ratio a | 0.015 | 0.0 | 0.87 | 0.93 | 0.48 | 0.0 |
| Body weight curve (10% increase) | – | –0.91 | –0.89 | –0.88 | –0.35 | 0.19 |
| Decline | 0.031 | 0.0 | –0.07 | –0.16 | –0.08 | 0.0 |
| Milk volume | 0.8 | 0.0 | 0.87 | 0.92 | 0.48 | 0.0 |
Note: If Normalised Coefficient > 1, parameter error is amplified.
Milk/serum ratio corresponds to milk concentration/maternal serum concentration ratio.
According to the WHO/IPCS (2010), the sensitivity of the parameter is considered as high (absolute value greater than or equal to 0.5), medium (absolute value greater than or equal to 0.2 but less than 0.5) or low (absolute value greater than or equal to 0.1 but less than 0.2).
M.2.3.2. Uncertainty analysis for PFOS
Table M.9.
Results of uncertainty analysis for PFOS
| Parameter | Value | Distribution | Uncertainty coefficient | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Initial | Min | Max | 100 iterations | At birth | At 6 months | At 1 year | At 5 years | At 35 years | |
| Placenta transfer | 0.36 | 0.18 | 0.72 | Uniform | 0.70 | 0.11 | 0.06 | 0.03 | 0.0 |
| Milk/serum ratio | 0.015 | 0.0075 | 0.0225 | Uniform | 0.0 | 0.51 | 0.54 | 0.28 | 0.0 |
| Body weight curve* | – | – | – | Lognormal | 2.38 | 2.34 | 2.31 | 1.46 | 0.83 |
| Body weight curvea | – | – | – | Lognormal | 1.58 | 1.57 | 1.56 | 1.17 | 0.91 |
| Decline | 0.031 | 0.0155 | 0.0465 | Uniform | 0.0 | 0.04 | 0.09 | 0.05 | 0.0 |
| Milk volume | 0.8 | 0.4 | 1.2 | Uniform | 0.0 | 0.51 | 0.54 | 0.28 | 0.0 |
*: The following equation was used: BW=3.68+4.47*year‐0.093*year^2+0.00061*year^3 ‐ 2*log(1.96)*(3.68+4.47*year‐0.093*year^2+0.00061*year^3) corresponding to the P05 of the curve. Note that the calculated uncertainty coefficient is P50/P05
The following equation was used:
BW=3.68+4.47*year‐0.093*year^2+0.00061*year^3 + 2*log(1.96)*(3.68+4.47*year‐0.093*year^2+0.00061*year^3) corresponding to the P95 of the curve. Note that the calculated uncertainty coefficient is P95/P50; This equation has been added in the model codes for PFOA and PFOS.
M.2.3.3. Coupling the results of sensitivity and uncertainty analysis for PFOS
According the WHO/IPCS (2010) guidance, the BW appears to have a low reliability, the maternal milk/serum ratio appears to have a medium reliability (see Table M.10). Placental transfer and decline show a high reliability.
Table M.10.
results of sensitivity and uncertainty analysis for PFOS
| Uncertainty | ||||
|---|---|---|---|---|
| High | medium | Low | ||
| Sensitivity | High | BW |
‐Milk/serum ratio ‐Milk consumption |
|
| Medium | ||||
| Low | ‐Decline | ‐Placental transfer | ||
M.2.3.4. Additional analysis on Body weight influence
For body weight, the CONTAM Panel explored the body weight influence with different growth curves from the WHO until the age of 5 years:
1‐BW=3.68+4.47*year‐0.093*year^2+0.00061*year^3
2‐BW = 3.3+ 8.0155*year ‐ 2.1478*year^2 + 0.2342*year^3; P50 boys until 5 years old
3‐BW =3.2 +0.1891*year^3 ‐ 1.7351*year^2+ 7.0589*year; P50 girls until 5 years old
4‐BW = 2.5 +0.1609*year^3 ‐ 1.5129*year^2 + 6.0135*year; P05 girls until 5 years old
5‐BW=2.6+ 0.2063*year^3 ‐ 1.9117*year^2 + 6.9393*year; P05 boys until 5 years old
6‐BW =4+ 0.2359*year^3 ‐ 2.0807*year^2 + 8.5496*year; P95 girls until 5 years old
7‐BW=4.2+0.2595*year^3 ‐ 2.3486*year^2 + 9.1534*year; P95 boys until 5 years old
Results are presented in Table M.11 below:
Table M.11.
Impact of different growth curves on the calculated serum levels at birth, 6 months, 1 and 5 years of age, following 12 months of breastfeeding
| Body weight growth curve | At birth | 6 months | 1 year | 5 years |
|---|---|---|---|---|
| Present opinion | 1.76 | 7.38 | 8.73 | 2.83 |
| P05 girls from WHO | 2.60 | 8.42 | 9.79 | 3.64 |
| P05 boys from WHO | 2.50 | 7.72 | 8.97 | 3.63 |
| P50 girls from WHO | 2.03 | 6.88 | 8.09 | 3.26 |
| P50 boys from WHO | 1.97 | 6.40 | 7.52 | 3.28 |
| P95 girls from WHO | 1.62 | 5.61 | 6.63 | 2.90 |
| P95 boys from WHO | 1.55 | 5.31 | 6.31 | 2.98 |
Note: Mothers and children were exposed to 0.445ng/kg bw per day. The target concentration at 1 year was 8.73 ng/mL.
As shown in Figure M.3 , when using different body weight equations (P50 in boys and girls), this is resulting in a PFOS serum concentration (CA) at 1 year between 7.52 (CA2) and 8.09 ng/mL (CA3). By comparison, CA1 is the PFOS serum concentration (8.73 ng/mL) using the French growth equation. Body weight curves are shown in Figure M.2 .
Figure M.3.
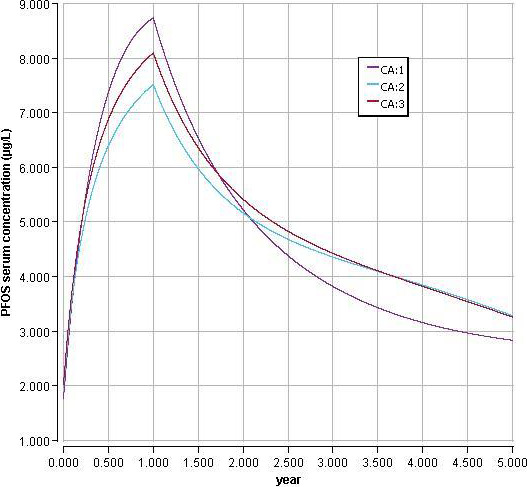
- CA1 corresponds to equation used in the current opinion (French survey), CA2 to the WHO curve (P50 in boys), CA3 to the WHO curve (P50 in girls).
M.2.3.5. Breastfeeding influence: PFOS 6 months vs. 12 months
Table M.12 below resumes the breastfeeding influence on PFOS serum concentration at 6 and 12 months, with the same exposure after breastfeeding.
Table M.12.
influence of breastfeeding for 6 vs. 12 months on the PFOS serum concentration in children
| Breastfeeding duration | 6 months | 1 year |
|---|---|---|
| 6 months | 7.37 | 5.11 |
| 12 months | 7.38 | 8.73 |
Conclusion: When breastfeeding duration is varied between 6 and 12 months, this results in a PFOS serum concentration at 1 year between 5.11 and 8.73 ng/mL (following an oral exposure (daily intake) in ng/kg bw per day of 0.445, after a 6 or 12 months breastfeeding period).
M.2.3.6. Conclusions for PFOS
According to the analysis of sensitivity and uncertainty, only the BW appears to have a low reliability, i.e. a high impact on the PFOS serum concentration.
Nevertheless, according to additional analyses, e.g. comparison of serum prediction with different growth curves (WHO vs. growth curve from French total Diet Study), it appears that the predictions remain acceptable, whatever the BW equation used.
The calculated PFOS serum concentration in ng/mL with PBPK at 1 year, depending of the growth curve used is less than a factor 2 (e.g. 1.16 for boys, 1.07 for girls) different from the predicted PFOS serum concentration used as RP in the current opinion.
In conclusion, the CONTAM Panel decided to use the growth curve based on the French study (EAT for French total Diet Study) in the modelling which represents the complete lifetime.
M.2.4. Model prediction
M.2.4.1. Comparison of model prediction with experimental data in infant
The study from Fromme et al. (2010) was used to evaluate the model with different dose metric (mother's serum level during pregnancy, at delivery, infant serum level at birth and at 6 months), and the ratio between observed and predicted value (serum concentration at birth and at 6 months) are less than a factor 2, which is acceptable according to the WHO/IPCS (2010) guidance:
‐ For PFOS: observed value = 3.3 ng/mL, predicted value = 5.1 ng/mL (serum concentration at 6 months, with breastfeeding),
‐ For PFOA: observed value = 8 ng/mL, predicted value = 10 ng/mL (serum concentration at 6 months, with breastfeeding)
M.2.4.2. Comparison of model prediction with experimental data in adult
In Loccisano et al. (2011, 2013), the data used for evaluating the human model consisted of serum measurements in residents from Little Hocking (Ohio, USA) who were exposed to PFOA‐contaminated drinking water. It was assumed that drinking water was the only source of exposure to PFOA (Figure M.4 ). The CONTAM Panel decided to use the same data to re‐evaluate the model used the current opinion.
Figure M.4.
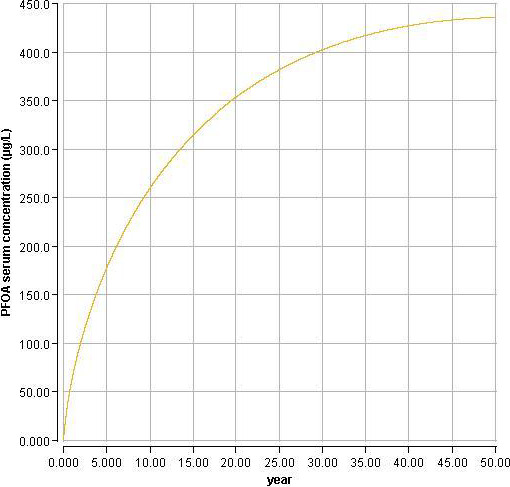
Comparison of model PFOA simulation with experimental data from Emmett et al. (2006) used by Loccisano et al. (2011) for validation
A drinking water concentration of 3.55 ppb PFOA was assumed, leading to a PFOA serum concentration at 30 years old of 400 ng/mL. The reported mean measured by Emmett et al. (2006) was 448 ng/mL. The prediction of the model (with additional input, i.e. BW curve) remains acceptable, with the ratios of predicted to observed concentrations within a factor of 2.
Conclusion: The models are able to reproduce the experimental data reported in adults and in children.
M.2.5. Model comparison with MDH model
Table M.13 below resumes the differences and similarities between the MDH toxicokinetic (Goeden et al., 2019) and the applied PBPK models for PFOA.
Table M.13.
Models comparison between the MDH toxicokinetics and applied PBPK models for PFOA
| Parameters | MDH (1 compartment model) | PBPK |
|---|---|---|
| Language | Excel | Berkeley Madonna |
| Body weight | According to US EPA | According French survey |
| Volume of distribution in children | Adjusted between 0 and 10 years | none |
| Volume of distribution in adult | 0.17 L/kg | – |
| Milk volume consumption | < 1 month (510 mL/day), 1 to < 3 month (690 mL/day), 3 to < 6 month (770 mL/day), 6 to < 12 month (620 mL/day) | 800 mL/day |
| Decline in PFOA concentration | None | 0.077 |
| Placenta transfer | 0.87 | 0.77 |
| Milk/serum ratio | 0.052 | 0.03 |
| Intake | Water consumption and breastfeeding | Oral intake and breastfeeding (water consumption, dermal, inhalation but not used in modelling; water part of oral intake) |
| Body weight change during pregnancy | none | none |
| Maternal age when breastfeeding begins (year) | 30 | 35 |
| Model evaluation | Serum level at delivery, infant serum level at birth and at 6 months from Fromme et al. (2010): observed/predicted value within a factor 2 | Serum level at delivery, infant serum level at birth and at 6 months from Fromme et al. (2010): observed/predicted value within a factor 2 |
M.2.5.1. Simulation comparison between MDH toxicokinetic model and PBPK model for PFOA
MDH simulation was performed according to the MDH selected parameters (body weight, volume of distribution (infant, adult), placental transfer and maternal milk/serum ratio). For comparison purpose, modifications in intake (water consumption and concentration in water was converted to oral intake) was the only change made in the MDH model (Table M.14 ).
Table M.14.
Comparison of model outcomes of the MDH and the applied PBPK model for PFOA, using a maternal intake of 0.187 ng/kg bw per day, a serum level in mothers at birth of 2.0 ng/mL and 12 months of breastfeeding
| Model used | PFOA target concentration at 1 year (BMDL ng/mL) | Estimated PFOA serum at birth | Estimated PFOA serum at 6 months | Estimated PFOA serum concentration at 1 year |
|---|---|---|---|---|
| MDH model | 8.78 | 1.74 | 8.80 | 10.77 |
| PBPK | 1.48 | 8.77 | 8.78 |
Conclusion
Despite parameter differences between the MDH model and PBPK model (e.g. body weight, volume of distribution (infant, adult), placenta transfer and maternal milk/serum ratio), both models provided similar prediction of the PFOA serum concentration estimated at 6 months and 1 year, using the following scenario: 12 months breastfeeding, maternal oral exposure of 0.187 ng/kg bw per day, with maternal PFOA serum concentration of 2.0 ng/mL at delivery. Moreover, both models can reproduce with accuracy the observed value reported by Fromme et al. 2010 (Goeden et al., 2019).
Appendix N – EFSA Literature search
1.
Appendix O – EFSA guidance documents applied for the risk assessment
1.
EFSA (European Food Safety Authority), 2010a. Standard sample description for food and feed. EFSA Journal 2010;8(1):1457, 54 pp. https://doi.org/10.2903/j.efsa.2010.1457
EFSA (European Food Safety Authority), 2010b. Management of left‐censored data in dietary exposure assessment of chemical substances. EFSA Journal 2010;8(3):1557, 96 pp. https://doi.org/10.2903/j.efsa.2010.1557
EFSA (European Food Safety Authority), 2011a. Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment. EFSA Journal 2011;9(3):2097, 34 pp. https://doi.org/10.2903/j.efsa.2011.2097
EFSA (European Food Safety Authority), 2011b. Evaluation of the FoodEx, the food classification system applied to the development of the EFSA Comprehensive European Food Consumption Database. EFSA Journal 2011;9(3):1970, 27 pp. https://doi.org/10.2903/j.efsa.2011.1970
EFSA (European Food Safety Authority), 2011c. Report on the development of a Food Classification and Description System for exposure assessment and guidance on its implementation and use. EFSA Journal 2011;9(12):2489, 84 pp. https://doi.org/10.2903/j.efsa.2011.2489
EFSA Scientific Committee, 2012. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579, 32 pp. https://doi.org/10.2903/j.efsa.2012.2579
EFSA (European Food Safety Authority), 2015. The food classification and description system FoodEx2 (revision 2). EFSA supporting publication 2015;EN‐804, 90 pp. https://doi.org/10.2903/sp.efsa.2015.en-804
EFSA Scientific Committee, Hardy A, Benford D, Halldorsson T, Jeger MJ, Knutsen KH, More S, Mortensen A, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Silano V, Solecki R, Turck D, Aerts M, Bodin L, Davis A, Edler L, Gundert‐Remy U, Sand S, Slob W, Bottex B, Abrahantes JC, Marques DC, Kass G and Schlatter JR, 2017. Update: Guidance on the use of the benchmark dose approach in risk assessment. EFSA Journal 2017;15(1):4658, 41 pp. https://doi.org/10.2903/j.efsa. 2017.4658
EFSA Scientific Committee, More SJ, Bampidis V, Benford D, Bennekou SH,Bragard C, Halldorsson TI, Hernandez‐Jerez AF, Koutsoumanis K, Naegeli H, Schlatter JR, Silano V,Nielsen SS, Schrenk D, Turck D, Younes M, Benfenati E, Castle L, Cedergreen N, Hardy A, Laskowski R,Leblanc JC, Kortenkamp A, Ragas A, Posthuma L, Svendsen C, Solecki R, Testai E, Dujardin B, Kass GEN, Manini P, Jeddi MZ, Dorne J‐LCM and Hogstrand C, 2019. Guidance on harmonised methodologies for human health, animal health and ecological risk assessment of combined exposure to multiple chemicals. EFSA Journal 2019;17(3):5634, 77 pp. https://doi.org/10.2903/j.efsa.2019.5634
Annex A – Occurrence and exposure data
1.
Available as Excel file on EFSA Knowledge Junction on zenodo at: https://doi.org/10.5281/zenodo.3974423
Annex B – Distribution of analytical results
1.
Available as Excel file on EFSA Knowledge Junction on zenodo at: https://doi.org/10.5281/zenodo.3974423
Annex C – Comparison of PFOA and PFOS occurrence and exposure data with previous assessment (EFSA CONTAM Panel, 2018)
1.
Available as Excel file on EFSA Knowledge Junction on zenodo at: https://doi.org/10.5281/zenodo.3974423
Suggested citation: EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) , Schrenk D, Bignami M, Bodin L, Chipman JK, del Mazo J, Grasl‐Kraupp B, Hogstrand C, Hoogenboom LR, Leblanc J‐C, Nebbia CS, Nielsen E, Ntzani E, Petersen A, Sand S, Vleminckx C, Wallace H, Barregård L, Ceccatelli S, Cravedi J‐P, Halldorsson TI, Haug LS, Johansson N, Knutsen HK, Rose M, Roudot A‐C, Van Loveren H, Vollmer G, Mackay K, Riolo F and Schwerdtle T, 2020. Scientific Opinion on the risk to human health related to the presence of perfluoroalkyl substances in food. EFSA Journal 2020;18(9):6223, 391 pp. 10.2903/j.efsa.2020.6223
Requestor: European Commission
Question number: EFSA‐Q‐2017‐00549
Panel members: Margherita Bignami, Laurent Bodin, James Kevin Chipman, Jesús del Mazo, Bettina Grasl‐Kraupp, Christer Hogstrand, Laurentius (Ron) Hoogenboom, Jean‐Charles Leblanc, Carlo Stefano Nebbia, Elsa Nielsen, Evangelia Ntzani, Annette Petersen, Salomon Sand, Dieter Schrenk, Tanja Schwerdtle, Christiane Vleminckx and Heather Wallace.
Acknowledgements: The Panel wishes to thank the following for their support provided to this scientific output as Hearing experts: Klaus Abraham, Esben Budtz‐Jørgensen, Tony Fletcher, Philippe Grandjean, Hans Mielke and Hans Rumke and EFSA staff members: Davide Arcella, Marco Binaglia, Petra Gergelova, Elena Rovesti and Marijke Schutte. The Panel wishes to acknowledge all European competent institutions, Member State bodies and other organisations that provided data for this scientific output. The Panel would also like to thank the following authors and co‐authors for providing additional information in relation to their respective studies: Berit Granum, Margie M Peden‐Adams, Thomas Webster.
Adopted: 9 July 2020
This publication is linked to the following EFSA Supporting Publications article: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2020.EN-1931/full
Notes
Opinion of the Scientific Panel on Contaminants in the Food chain on Perfluooctane sulfonate (PFOS) perfluorooctanoic acid (PFOA) and their salts. EFSA Journal 2008; Journal number, 653, pp. 1–131.
Commission Recommendation 2010/161/EU on the monitoring of perfluoroalkylated substances in food. OJ L 68, 18.3.2010, p. 22.
European Food Safety Authority, perfluoroalkylated substances in food: occurrence and dietary exposure. EFSA Journal 2012;10(6):2743.
PERFOOD is a consortium of several European research institutions which collaborate in an FP7 EU project with the aim to improve the analytical tools for the determination of PFASs in food items, to contribute to the understanding of PFASs transfer from the environment into dietary items and to quantify the possible contribution of food/beverage contact materials and food and water processing to the overall PFASs levels in the diet ( www.perfood.eu).
UNEP/POPS/POPRC.12/INF/15/Rev.1.
H360 may damage fertility or the unborn child.
H372 causes damage to organs through prolonged or repeated exposure.
From 1 January 2014 onwards, Evidence Management Unit (DATA).
Web of Science (WoS), formerly ISI Web of Knowledge, Thomson Reuters. Available online: http://thomsonreuters.com/thomson-reuters-web-of-science/
PubMed, Entrez Global Query Cross‐Database Search System, National Center for Biotechnology Information (NCBI), National Library of Medicine (NLM), Department of the National Institutes of Health (NIH), United States Department of Health and Human Services. Available online: http://www.ncbi.nlm.nih.gov/pubmed/
EndNote X5, Thomson Reuters. Available online: http://endnote.com/
Targeted samples are based on certain sampling criteria e.g. monitor the background contamination in matrices with high accumulation potential, controlling compliance with the maximum levels laid down in the relevant legislation.
Commission Recommendation of 17 March 2010 on the monitoring of perfluoroalkylated substances in food (2010/161/EU).
Based on standard conversion factors in EFSA Scientific Committee (2012), Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579. 32 pp. https://doi.org/10.2903/j.efsa.2012.2579.
Results from NTP (NTP, 2019b) were not included in the table, since the test substance was administered to adult non‐pregnant rats.
Saktet = serum antibody titres of tetanus.
sakdiph = serum antibody titres of diphtheria.
References
- Abbott BD, Wolf CJ, Schmid JE, Das KP, Zehr RD, Helfant L, Nakayama S, Lindstrom AB, Strynar MJ and Lau C, 2007. Perfluorooctanoic acid induced developmental toxicity in the mouse is dependent on expression of peroxisome proliferator activated receptor‐alpha. Toxicological Sciences, 98, 571–581. [DOI] [PubMed] [Google Scholar]
- Abbott BD, Wolf CJ, Das KP, Zehr RD, Schmid JE, Lindstrom AB, Strynar MJ and Lau C, 2009. Developmental toxicity of perfluorooctane sulfonate (PFOS) is not dependent on expression of peroxisome proliferator activated receptor‐alpha (PPARα) in the mouse. Reproductive Toxicology, 27, 258–265. [DOI] [PubMed] [Google Scholar]
- Abbott BD, Wood CR, Watkins AM, Tatum‐Gibbs K, Das KP and Lau C, 2012. Effects of perfluorooctanoic acid (PFOA) on expression of peroxisome proliferator‐activated receptors (PPAR) and nuclear receptor‐regulated genes in fetal and postnatal CD‐1 mouse tissues. Reproductive Toxicology, 33, 491–505. [DOI] [PubMed] [Google Scholar]
- Abe T, Takahashi M, Kano M, Amaike Y, Ishii C, Maeda K, Kudoh Y, Morishita T, Hosaka T, Sasaki T, Kodama S, Matsuzawa A, Kojima H and Yoshinari K, 2017. Activation of nuclear receptor CAR by an environmental pollutant perfluorooctanoic acid. Archives of Toxicology, 91, 2365–2374. 10.1007/s00204-016-1888-3 [DOI] [PubMed] [Google Scholar]
- Abraham K, Mielke H, Fromme H, Volkel W, Menzel J, Peiser M, Zepp F, Willich SN and Weikert C, 2020. Internal exposure to perfluoroalkyl substances (PFASs) and biological marker in 101 healthy 1‐year‐old children: associations between levels of perfluorooctanoic acid (PFOA) and vaccine response. Archives of Toxicology, 94, 2131–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adinehzadeh M, Reo NV, Jarnot BM, Taylor CA and Mattie DR, 1999. Dose‐response hepatotoxicity of the peroxisome proliferator, perfluorodecanoic acid and the relationship to phospholipid metabolism in rats. Toxicology, 134, 179–195. [DOI] [PubMed] [Google Scholar]
- Ahrens L and Bundschuh M, 2014. Fate and effects of poly‐ and perfluoroalkyl substances in the aquatic environment: a review. Environmental Toxicology and Chemistry, 33, 1921–1929. [DOI] [PubMed] [Google Scholar]
- Ahrens L, Siebert U and Ebinghaus R, 2009. Temporal trends of polyfluoroalkyl compounds in harbor seals (Phoca vitulina) from the German Bight, 1999–2008. Chemosphere, 76, 151–158. 10.1016/j.chemosphere.2009.03.053 [DOI] [PubMed] [Google Scholar]
- Ahrens L, Yeung LWY, Taniyasu S, Lam PKS and Yamashita N, 2011a. Partitioning of perfluorooctanoate (PFOA), perfluorooctane sulfonate (PFOS) and perfluorooctane sulfonamide (PFOSA) between water and sediment. Chemosphere, 85, 731–737. [DOI] [PubMed] [Google Scholar]
- Ahrens L, Herzke D, Huber S, Bustnes JO, Bangjord G and Ebinghaus R, 2011b. Temporal trends and pattern of polyfluoroalkyl compounds in Tawny Owl (Strix aluco) eggs from Norway, 1986–2009. Environmental Science and Technology, 45, 8090–8097. 10.1021/es103473v [DOI] [PubMed] [Google Scholar]
- Ahrens L, Shoeib M, Harner T, Lee SC, Guo R and Reiner EJ, 2011c. Wastewater treatment plant and landfills as sources of polyfluoroalkyl compounds to the atmosphere. Environmental Science and Technology, 45, 8098–8105. 10.1021/es1036173 [DOI] [PubMed] [Google Scholar]
- Albrecht PP, Torsell NE, Krishnan P, Ehresman DJ, Frame SR, Chang SC, Butenhoff JL, Kennedy GL, Gonzalez FJ and Peters JM, 2013. A species difference in the peroxisome proliferator‐activated receptor a‐dependent response to the developmental effects of perfluorooctanoic acid. Toxicological Sciences, 131, 568–582. 10.1093/toxsci/kfs318 [DOI] [PubMed] [Google Scholar]
- Alves A, Kucharska A, Erratico C, Xu F, Den Hond E, Koppen G, Vanermen G, Covaci A and Voorspoels S, 2014. Human biomonitoring of emerging pollutants through non‐invasive matrices: state of the art and future potential. Analytical and Bioanalytical Chemistry, 406, 4063–4088. 10.1007/s00216-014-7748-1 [DOI] [PubMed] [Google Scholar]
- Alves A, Jacobs G, Vanermen G, Covaci A and Voorspoels S, 2015. New approach for assessing human perfluoroalkyl exposure via hair. Talanta, 144, 574–583. 10.1016/j.talanta.2015.07.009 [DOI] [PubMed] [Google Scholar]
- Alves RN, Maulvault AL, Barbosa VL, Cunha S, Kwadijk CJAF, Álvarez‐Muñoz D, Rodríguez‐Mozaz S, Aznar‐Alemany Ò, Eljarrat E, Barceló D, Fernandez‐Tejedor M, Tediosi A and Marques A, 2017. Preliminary assessment on the bioaccessibility of contaminants of emerging concern in raw and cooked seafood. Food and Chemical Toxicology, 104, 69–78. 10.1016/j.fct.2017.01.029 [DOI] [PubMed] [Google Scholar]
- ANSES (French Agency for Food, Environmental and Occupational Health & Safety), 2017. Opinion on the development of chronic reference values by the oral route for four perfluorinated compounds: perfluorohexanoic acid (PFHxA), perfluorohexane sulfonic acid (PFHxS), perfluorobutanoic acid (PFBA), and perfluorobutane sulfonic acid (PFBS). Available online: https://www.anses.fr/fr/system/files/SUBSTANCES2015SA0129EN.pdf
- Apelberg BJ, Witter FR, Herbstman JB, Calafat AM, Halden RU, Needham LL and Goldman LR, 2007a. Cord serum concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in relation to weight and size at birth. Environmental Health Perspectives, 115, 1670–1676. 10.1289/ehp.10334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apelberg BJ, Goldman LR, Calafat AM, Herbstman JB, Kuklenyik Z, Heidler J, Needham LL, Halden RU and Witter FR, 2007b. Determinants of fetal exposure to polyfluoroalkyl compounds in Baltimore, Maryland. Environmental Science and Technology, 41, 3891–3897. 10.1021/es0700911 [DOI] [PubMed] [Google Scholar]
- Armstrong DL, Lozano N, Rice CP, Ramirez M and Torrents A, 2016. Temporal trends of perfluoroalkyl substances in limed biosolids from a large municipal water resource recovery facility. Journal of Environmental Management, 165, 88–95. 10.1016/j.jenvman.2015.09.023 [DOI] [PubMed] [Google Scholar]
- Arrebola JP, Castaño A, Esteban M, Bartolomé M, Pérez‐Gómez B, Ramos JJ and Bioambient ES, 2018. Differential contribution of animal and vegetable food items on persistent organic pollutant serum concentrations in Spanish adults. Data from BIOAMBIENT.ES project. Science of the Total Environment, 634, 235–242. 10.1016/j.scitotenv.2018.03.283 [DOI] [PubMed] [Google Scholar]
- Arvaniti OS and Stasinakis AS, 2015. Review on the occurrence, fate and removal of perfluorinated compounds during wastewater treatment. Science of the Total Environment, 524–525, 81–92. 10.1016/j.scitotenv.2015.04.023 [DOI] [PubMed] [Google Scholar]
- Ashley‐Martin J, Dodds L, Levy AR, Platt RW, Marshall JS and Arbuckle TE, 2015. Prenatal exposure to phthalates, bisphenol A and perfluoroalkyl substances and cord blood levels of IgE, TSLP and IL‐33. Environmental Research, 140, 360–368. 10.1016/j.envres.2015.04.010 [DOI] [PubMed] [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry), 2013. Health consultation. Exposure investigation report. Perfluorochemical serum sampling in the vicinity of Decatur, Alabama, Morgan, Lawrence, and Limestone counties. U.S. Department of Health and Human Services, Atlanta. Available online: http://www.atsdr.cdc.gov/HAC/pha/Decatur/Perfluorochemical_Serum%20Sampling.pdf
- ATSDR (Agency for Toxic Substances and Disease Registry), 2018. Draft Toxicological Profile for Perfluoroalkyls. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp200.pdf [PubMed]
- Averina M, Brox J, Huber S and Furberg AS, 2018. Perfluoroalkyl substances in adolescents in northern Norway: Lifestyle and dietary predictors. The Tromsø study, Fit Futures 1. Environment International, 114, 123–130. [DOI] [PubMed] [Google Scholar]
- Averina M, Brox J, Huber S, Furberg AS and Sørensen M, 2019. Serum perfluoroalkyl substances (PFAS) and risk of asthma and various allergies in adolescents. The Tromsø study Fit Futures in Northern Norway. Environmental Research, 169, 114–121. [DOI] [PubMed] [Google Scholar]
- Axmon A, Axelsson J, Jakobsson K, Lindh CH and Jönsson BA, 2014. Time trends between 1987 and 2007 for perfluoroalkyl acids in plasma from Swedish women. Chemosphere, 102, 61–67. 10.1016/j.chemosphere.2013.12.021 [DOI] [PubMed] [Google Scholar]
- Ayanda I, Min Y, Yu Z and Zha J, 2018. Cytotoxic and genotoxic effects of perfluorododecanoic acid (PFDoA) in Japanese medaka. Knowledge and Management of Aquatic Ecosystems, 419, 7 pp 10.1051/kmae/2017058 [DOI] [Google Scholar]
- Bach CC, Bech BH, Nohr EA, Olsen J, Matthiesen NB, Bossi R, Uldbjerg N, Bonefeld‐Jørgensen EC and Henriksen TB, 2015. Serum perfluoroalkyl acids and time to pregnancy in nulliparous women. Environmental Research, 142, 535–541. 10.1016/j.envres.2015.08.007 [DOI] [PubMed] [Google Scholar]
- Bach CC, Bech BH, Nohr EA, Olsen J, Matthiesen NB, Bonefeld‐Jørgensen EC, Bossi R and Henriksen TB, 2016. Perfluoroalkyl acids in maternal serum and indices of fetal growth: the Aarhus birth cohort. Environmental Health Perspectives, 124, 848–854. 10.1289/ehp.1510046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach CC, Matthiesen NB, Olsen J and Henriksen TB, 2018. Conditioning on parity in studies of perfluoroalkyl acids and time to pregnancy: an example from the Danish National Birth Cohort. Environmental Health Perspectives, 126, 117003 10.1289/EHP1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baduel C, Lai FY, Townsend K and Mueller JF, 2014. Size and age‐concentration relationships for perfluoroalkyl substances in stingray livers from eastern Australia. Science of the Total Environment, 496, 523–530. 10.1016/j.scitotenv.2014.07.010 [DOI] [PubMed] [Google Scholar]
- Bao J, Kärrman A, Bavel B and Jin Y, 2014. Perfluoroalkyl substances in the blood samples from a male population of Sweden. Chinese Science Bulletin, 59, 388–395. [Google Scholar]
- Bao WW, Qian ZM, Geiger SD, Liu E, Liu Y, Wang SQ, Lawrence WR, Yang BY, Hu LW, Zeng XW and Dong GH, 2017. Gender‐specific associations between serum isomers of perfluoroalkyl substances and blood pressure among Chinese: isomers of C8 Health Project in China. Science of the Total Environment, 607–608, 1304–1312. 10.1016/j.scitotenv.2017.07.124 [DOI] [PubMed] [Google Scholar]
- Barbosa V, Maulvault AL, Alves RN, Kwadijk C, Kotterman M, Tediosi A, Fernández‐Tejedor M, Sloth JJ, Granby K, Rasmussen RR, Robbens J, De Witte B, Trabalón L, Fernandes JO, Cunha SC and Marques A, 2018. Effects of steaming on contaminants of emerging concern levels in seafood. Food and Chemical Toxicology, 118, 490–504. 10.1016/j.fct.2018.05.047 [DOI] [PubMed] [Google Scholar]
- Bartell SM, Calafat AM, Lyu C, Kato K, Ryan PB and Steenland K, 2010. Rate of decline in serum PFOA concentrations after granular activated carbon filtration at two public water systems in Ohio and West Virginia. Environmental Health Perspectives, 118, 222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolome M, Gallego‐Pico A, Cutanda F, Huetos O, Esteban M, Perez‐Gomez B and Castano A, 2017. Perfluorinated alkyl substances in Spanish adults: geographical distribution and determinants of exposure. Science of the Total Environment, 603–604, 352–360. 10.1016/j.scitotenv.2017.06.031 [DOI] [PubMed] [Google Scholar]
- Bassler J, Ducatman A, Elliott M, Wen S, Wahlang B, Barnett J and Cave MC, 2019. Environmental perfluoroalkyl acid exposures are associated with liver disease characterized by apoptosis and altered serum adipocytokines. Environmental Pollution, 247, 1055–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley TH, White K, Honigfort P, Twaroski ML, Neches R and Walker RA, 2005. Perfluorochemicals: potential sources of and migration from food packaging. Food Additives and Contaminants, 22, 1023–1031. [DOI] [PubMed] [Google Scholar]
- Bell EM, Yeung E, Ma W, Kannan K, Sundaram R, Smarr MM and Buck Louis GM, 2018. Concentrations of endocrine disrupting chemicals in newborn blood spots and infant outcomes in the upstate KIDS study. Environment International, 121, 232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benninghoff AD, Orner GA, Buchner CH, Hendricks JD, Duffy AM and Williams DE, 2012. Promotion of hepatocarcinogenesis by perfluoroalkyl acids in rainbow trout. Toxicological Sciences, 125, 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benskin JP, De Silva AO, Martin LJ, Arsenault G, McCrindle R, Riddell N, Mabury SA and Martin JW, 2009. Disposition of perfluorinated acid isomers in Sprague‐Dawley rats; part 1: single dose. Environmental Toxicology and Chemistry, 28, 542–554. 10.1897/08-239.1 [DOI] [PubMed] [Google Scholar]
- Benskin JP, Phillips V, St Louis VL and Martin JW, 2011. Source elucidation of perfluorinated carboxylic acids in remote alpine lake sediment cores. Environmental Science and Technology, 45, 7188–7194. [DOI] [PubMed] [Google Scholar]
- Berg V, Nøst TH, Huber S, Rylander C, Hansen S, Veyhe AS, Fuskevåg OM, Odland JØ and Sandanger TM, 2014. Maternal serum concentrations of per‐ and polyfluoroalkyl substances and their predictors in years with reduced production and use. Environment International, 69, 58–66. [DOI] [PubMed] [Google Scholar]
- Berg V, Nost TH, Hansen S, Elverland A, Veyhe AS, Jorde R, Odland JO and Sandanger TM, 2015. Assessing the relationship between perfluoroalkyl substances, thyroid hormones and binding proteins in pregnant women; a longitudinal mixed effects approach. Environment International, 77, 63–69. 10.1016/j.envint.2015.01.007 [DOI] [PubMed] [Google Scholar]
- Berg V, Nost TH, Pettersen RD, Hansen S, Veyhe AS, Jorde R, Odland JO and Sandanger TM, 2017. Persistent organic pollutants and the association with maternal and infant thyroid homeostasis: a multipollutant assessment. Environmental Health Perspectives, 125, 127–133. 10.1289/ehp152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk M, Williams LJ, Andreazza AC, Pasco JA, Dodd S, Jacka FN, Moylan S, Reiner EJ and Magalhaes PV, 2014. Pop, heavy metal and the blues: secondary analysis of persistent organic pollutants (POP), heavy metals and depressive symptoms in the NHANES National Epidemiological Survey. British Medical Journal Open, 4, e005142 10.1136/bmjopen-2014-005142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntsen HF, Bjorklund CG, Audinot JN, Hofer T, Verhaegen S, Lentzen E, Gutleb AC and Ropstad E, 2017. Time‐dependent effects of perfluorinated compounds on viability in cerebellar granule neurons: dependence on carbon chain length and functional group attached. Neurotoxicology, 63, 70–83. 10.1016/j.neuro.2017.09.005 [DOI] [PubMed] [Google Scholar]
- Berntsen HF, Bjorklund CG, Strandabo R, Haug TM, Moldes‐Anaya A, Fuentes‐Lazaro J, Verhaegen S, Paulsen RE, Tasker RA and Ropstad E, 2018. PFOS‐induced excitotoxicity is dependent on Ca2+ influx via NMDA receptors in rat cerebellar granule neurons. Toxicology and Applied Pharmacology, 15, 19–32. 10.1016/j.taap.2018.08.015 [DOI] [PubMed] [Google Scholar]
- Berthiaume J and Wallace KB, 2002. Perfluorooctanoate, perflourooctanesulfonate, and N‐ethyl perfluorooctanesulfonamido ethanol; peroxisome proliferation and mitochondrial biogenesis. Toxicological Letters, 129, 23–32. [DOI] [PubMed] [Google Scholar]
- Bijland S, Rensen PC, Pieterman EJ, Maas AC, van der Hoorn JW, van Erk MJ, Havekes LM, Willems van Dijk K, Chang SC, Ehresman DJ, Butenhoff JL and Princen HM, 2011. Perfluoroalkyl sulfonates cause alkyl chain length‐dependent hepatic steatosis and hypolipidemia mainly by impairing lipoprotein production in APOE*3‐Leiden CETP mice. Toxicological Sciences, 123, 290–303. [DOI] [PubMed] [Google Scholar]
- Binnington MJ, Lei YD, Pokiak L, Pokiak J, Ostertag SK, Loseto LL, Chan HM, Yeung LWY, Huang H and Wania F, 2017. Effects of preparation on nutrient and environmental contaminant levels in Arctic beluga whale (Delphinapterus leucas) traditional foods. Environmental Science: Processes and Impacts, 19, 1000–1015. 10.1039/c7em00167c [DOI] [PubMed] [Google Scholar]
- Bischel HN, MacManus‐Spencer LA and Luthy R, 2010. Noncovalent interactions of long‐chain perfluoroalkyl acids with serum albumin. Environmental Science and Technology, 32_44. [DOI] [PubMed] [Google Scholar]
- Bischel HN, MacManus‐Spencer LA, Zhang C and Luthy RG, 2011. Strong associations of short‐chain perfluoroalkyl acids with serum albumin and investigation of binding mechanisms. Environmental Toxicology and Chemistry, 30, 2423–2430. 10.1002/etc.647 [DOI] [PubMed] [Google Scholar]
- Bjermo H, Darnerud PO, Pearson M, Barbieri HE, Lindroos AK, Nälsén C, Lindh CH, Jönsson BA and Glynn A, 2013. Serum concentrations of perfluorinated alkyl acids and their associations with diet and personal characteristics among Swedish adults. Molecular Nutrition Food Research, 57, 2206–2215. [DOI] [PubMed] [Google Scholar]
- Bjerregaard‐Olesen C, Bach CC, Long M, Ghisari M, Bossi R, Bech BH, Nohr EA, Henriksen TB, Olsen J and Bonefeld‐Jørgensen EC, 2016. Time trends of perfluorinated alkyl acids in serum from Danish pregnant women 2008–2013. Environment International, 1, 14–21. [DOI] [PubMed] [Google Scholar]
- Bjork JA and Wallace KB, 2009. Structure‐activity relationships and human relevance for perfluoroalkyl acid‐induced transcriptional activation of peroxisome proliferation in liver cell cultures. Toxicological Sciences, 111, 89–99. [DOI] [PubMed] [Google Scholar]
- Bjork JA, Butenhoff JL and Wallace KB, 2011. Multiplicity of nuclear receptor activation by PFOA and PFOS in primary human and rodent hepatocytes. Toxicology, 288, 8–17. [DOI] [PubMed] [Google Scholar]
- Blaine AC, Rich CD, Sedlacko EM, Hundal LS, Kuma K, Lau C, Mills MA, Harris KM and Higgins CP, 2014. Perfluoroalkyl acid distribution in various plant compartments of edible crops grown in biosolids‐amended soils. Environmental Science and Technology, 48, 7858–7865. [DOI] [PubMed] [Google Scholar]
- Bloom MS, Kannan K, Spliethoff HM, Tao L, Aldous KM and Vena JE, 2010. Exploratory assessment of perfluorinated compounds and human thyroid function. Physiology and Behavior, 99, 240–245. 10.1016/j.physbeh.2009.02.005 [DOI] [PubMed] [Google Scholar]
- Blum A, Balan SA, Scheringer M, Trier X, Goldenman G, Cousins IT, Diamond M, Fletcher T, Higgins C, Lindeman AE, Peaslee G, Voogt Pd, Wang Z and Weber R, 2015. The madrid statement on poly‐ and perfluoroalkyl substances (PFASs). Environmental Health Perspectives, 123, A107–A111. 10.1289/ehp.1509934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanska J, Sundstrom M, Bergstrom U, Borg D, Abedi‐Valugerdi M, Bergman A, DePierre J and Nobel S, 2014. Tissue distribution of 35S‐labelled perfluorobutanesulfonic acid in adult mice following dietary exposure for 1‐5 days. Chemosphere, 98, 28–36. 10.1016/j.chemosphere.2013.09.062 [DOI] [PubMed] [Google Scholar]
- Bojes HK, Germolec DR, Simeonova P, Bruccolrei A, Schoonhoven R, Luster MI and Thurman RG, 1997. Antibodies to tumor necrosis factor alpha prevent increases in cell replication in liver due to the potent peroxisome proliferator WY14,643. Carcinogenesis, 18, 669–674. [DOI] [PubMed] [Google Scholar]
- Brantsæter AL, Whitworth KW, Ydersbond TA, Haug LS, Haugen M, Knutsen HK, Thomsen C, Meltzer HM, Becher G, Sabaredzovic A, Hoppin JA, Eggesbø M and Longnecker MP, 2013. Determinants of plasma concentrations of perfluoroalkyl substances in pregnant Norwegian women. Environment International, 54, 74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalkbrenner AE, Just AC, Yolton K, Calafat AM, Sjödin A, Hauser R, Webster GM, Chen A and Lanphear BP, 2014. Gestational exposure to endocrine‐disrupting chemicals and reciprocal social, repetitive, and stereotypic behaviors in 4‐ and 5‐year‐old children: the HOME study. Environmental Health Perspectives, 122, 513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Chen A, Romano ME, Calafat AM, Webster GM, Yolton K and Lanphear BP, 2016. Prenatal perfluoroalkyl substance exposure and child adiposity at 8 years of age: the HOME study. Obesity, 24, 231–237. 10.1002/oby.21258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster DW and Birnbaum LS, 1989. The biochemical toxicity of perfluorodecanoic acid in the mouse is different from that of 2,3,7,8‐tetrachlorodibenzo‐p‐dioxin. Toxicology and Applied Pharmacology, 99, 444–554. [DOI] [PubMed] [Google Scholar]
- Brieger A, Bienefeld N, Hasan R, Goerlich R and Haase H, 2011. Impact of perfluorooctane sulfonate and perfluorooctanoic acid on human peripheral leukocytes. Toxicology In Vitro, 25, 960–968. [DOI] [PubMed] [Google Scholar]
- Brown RP, Delp MD, Lindstedt SL, Rhomberg LR and Beliles RP, 1997. Physiological parameter values for physiologically based pharmacokinetic models. Toxicology and Industrial Health, 13, 407–484. [DOI] [PubMed] [Google Scholar]
- Buck Louis GM, Peterson CM, Chen Z, Hediger ML, Croughan MS, Sundaram R, Stanford JB, Fujimoto VY, Varner MW, Giudice LC, Kennedy A, Sun L, Wu Q and Kannan K, 2012. Perfluorochemicals and endometriosis the ENDO study. Epidemiology, 23, 799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck Louis GM, Sundaram R, Schisterman EF, Sweeney AM, Lynch CD, Gore‐Langton RE, Maisog J, Kim S, Chen Z and Barr DB, 2013. Persistent environmental pollutants and couple fecundity: the LIFE study. Environmental Health Perspectives, 121, 231–236. 10.1289/ehp.1205301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck Louis GM, Zhai S, Smarr MM, Grewal J, Zhang C, Grantz KL, Hinkle SN, Sundaram R, Lee S, Honda M, Oh J and Kannan K, 2018. Endocrine disruptors and neonatal anthropometry, NICHD fetal growth studies – singletons. Environmental International, 119, 515–526. 10.1016/j.envint.2018.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, Jensen AA, Kannan K, Mabury SA and van Leeuwen SPJ, 2011. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integrated Environmental Assessment and Management, 7, 513–541. 10.1002/ieam.258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck Louis GM, Sapra KJ, Barr DB, Lu Z and Sundaram R, 2016. Preconception perfluoroalkyl and polyfluoroalkyl substances and incident pregnancy loss, LIFE Study. Reproductive Toxicology, 65, 11–17. 10.1016/j.reprotox.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budtz‐Jorgensen E and Grandjean P, 2018. Application of benchmark analysis for mixed contaminant exposures: mutual adjustment of perfluoroalkylate substances associated with immunotoxicity. PLoS ONE, 13, e0205388 10.1371/journal.pone.0205388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhrke T, Kibellus A and Lampen A, 2013. In vitro toxicological characterization of perfluorinated carboxylic acids with different carbon chain lengths. Toxicology Letters, 218, 97–104. [DOI] [PubMed] [Google Scholar]
- Bull S, Burnett K, Vassaux K, Ashdown L, Brown T and Rushton L, 2014. Extensive literature search and provision of summaries of studies related to the oral toxicity of perfluoroalkylated substances (PFASs), their precursors and potential replacements in experimental animals and humans. Area 1: data on toxicokinetics (absorption, distribution, metabolism, excretion) in in vitro studies, experimental animals and humans. Area 2: Data on toxicity in experimental animals. Area 3: data on observations in humans. EFSA Supporting Publication 2014;11(4):EN‐572, 345 pp. 10.2903/sp.efsa.2014.EN-572 [DOI] [Google Scholar]
- Bursch W, Lauer B, Timmermann‐Trosiener I, Barthel G, Schuppler J and Schulte‐Hermann R, 1984. Controlled death (apoptosis) of normal and putative preneoplastic cells in rat liver following withdrawal of tumor promoters. Carcinogenesis, 5, 453–458. [DOI] [PubMed] [Google Scholar]
- Buser MC and Scinicariello F, 2016. Perfluoroalkyl substances and food allergies in adolescents. Environment International, 88, 74–79. 10.1016/j.envint.2015.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butenhoff JL, Kennedy GL Jr, Hinderliter PM, Lieder PH, Jung R, Hansen KJ, Gorman GS, Noker PE and Thomford PJ, 2004. Pharmacokinetics of perfluorooctanoate in cynomolgus monkeys. Toxicological Sciences, 82, 394–406. 10.1093/toxsci/kfh [DOI] [PubMed] [Google Scholar]
- Butenhoff JL, Olsen GW and Pfahles‐Hutchens A, 2006. The applicability of biomonitoring data for perfluorooctanesulfonate to the environmental public health continuum. Environmental Health Perspectives, 114, 1776–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butenhoff JL, Chang SC, Ehresman DJ and York RG, 2009. Evaluation of potential reproductive and developmental toxicity of potassium perfluorohexanesulfonate in Sprague Dawley rats. Reproductive Toxicology, 27, 331–341. [DOI] [PubMed] [Google Scholar]
- Butenhoff JL, Bjork JA, Chang SC, Ehresman DJ, Parker GA, Das K, Lau C, Lieder PH, van Otterdijk FM and Wallace KB, 2012. Toxicological evaluation of ammonium perfluorobutyrate in rats: twenty‐eight‐day and ninety‐day oral gavage studies. Reproductive Toxicology, 33, 513–530. [DOI] [PubMed] [Google Scholar]
- Butt CM, Mabury SA, Kwan M, Wang X and Muir DC, 2008. Spatial trends of perfluoroalkyl compounds in ringed seals (Phoca hispida) from the Canadian Arctic. Environmental Technology and Chemistry, 27, 542–553. [DOI] [PubMed] [Google Scholar]
- Bytingsvik J, Simon E, Leonards PE, Lamoree M, Lie E, Aars J, Derocher AE, Wiig O, Jenssen BM and Hamers T, 2013. Transthyretin‐binding activity of contaminants in blood from polar bear (Ursus maritimus) cubs. Environmental Science and Technology, 47, 4778–4786. 10.1021/es305160v [DOI] [PubMed] [Google Scholar]
- Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Tully JS and Needham LL, 2007a. Serum concentrations of 11 polyfluoroalkyl compounds in the U.S. population: data from the national health and nutrition examination survey (NHANES). Environmental Science and Technology, 41, 2237–2242. [DOI] [PubMed] [Google Scholar]
- Calfee‐Mason KG, Spear BT and Glauert HP, 2004. Effects of vitamin E on the NF‐kappaB pathway in rats treated with the peroxisome proliferator, ciprofibrate. Toxicology and Applied Pharmacology, 199, 1–9. [DOI] [PubMed] [Google Scholar]
- Campo J, Masiá A, Picó Y, Farré M and Barceló D, 2014. Distribution and fate of perfluoroalkyl substances in mediterranean Spanish sewage treatment plants. Science of The Total Environment, 472, 912–922. [DOI] [PubMed] [Google Scholar]
- Campo J, Pérez F, Masiá A, Picó Y, Farré M and Barceló D, 2015. Perfluoroalkyl substance contamination of the llobregat river ecosystem (Mediterranean area, NE Spain). Science of the Total Environment, 503–504, 48–57. [DOI] [PubMed] [Google Scholar]
- Cardenas A, Gold DR, Hauser R, Kleinman KP, Hivert MF, Calafat AM, Ye X, Webster TF, Horton ES and Oken E, 2017. Plasma concentrations of per‐ and polyfluoroalkyl substances at baseline and associations with glycemic indicators and diabetes incidence among high‐risk adults in the diabetes prevention program trial. Environmental Health Perspectives, 125, 107001 10.1289/ehp1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cariou R, Veyrand B, Yamada A, Berrebi A, Zalko D, Durand S, Pollono C, Marchand P, Leblanc JC, Antignac JP and Le Bizec B, 2015. Perfluoroalkyl acid (PFAA) levels and profiles in breast milk, maternal and cord serum of French women and their newborns. Environment International, 84, 71–81. [DOI] [PubMed] [Google Scholar]
- Case MT, York RG and Christian MS, 2001. Rat and rabbit oral developmental toxicology studies with two perfluorinated compounds. International Journal of Toxicology, 20, 101–109. [DOI] [PubMed] [Google Scholar]
- Cerveny D, Grabic R, Fedorova G, Grabicova K, Turek J, Kodes V, Golovko O, Zlábek V and Randak T, 2016. Perfluoroalkyl substances in aquatic environment‐comparison of fish and passive sampling approaches. Environmental Research, 144, 92–98. [DOI] [PubMed] [Google Scholar]
- Chan E, Burstyn I, Cherry N, Bamforth F and Martin JW, 2011. Perfluorinated acids and hypothyroxinemia in pregnant women. Environmental Research, 111, 559–564. 10.1016/j.envres.2011.01.011 [DOI] [PubMed] [Google Scholar]
- Chang SC, Thibodeaux JR, Eastvold ML, Ehresman DJ, Bjork JA, Froehlich JW, Laub CS, Singh RJ, Wallace KB and Butenhoff JL, 2007. Negative bias from analog methods used in the analysis of free thyroxine in rat serum containing perfluorooctanesulfonate (PFOS). Toxicology, 234, 21–33. [DOI] [PubMed] [Google Scholar]
- Chang SC, Thibodeaux JR, Eastvold ML, Ehresman DJ, Bjork JA, Froehlich JW, Lau C, Singh RJ, Wallace KB and Butenhoff JL, 2008. Thyroid hormone status and pituitary function in adult rats given oral doses of perfluorooctanesulfonate (PFOS). Toxicology, 20, 330–339. [DOI] [PubMed] [Google Scholar]
- Chang S, Noker PE, Gorman GS, Gibson SJ, Hart JA, Ehresman DJ and Butenhoff JL, 2012. Comparative pharmacokinetics of perfluorooctanesulfonate (PFOS) in rats, mice, and monkeys. Reproductive Toxicology, 33, 428–440. [DOI] [PubMed] [Google Scholar]
- Chang S, Allen BC, Andres KL, Ehresman DJ, Falvo R, Provencher A, Olsen GW and Butenhoff JL, 2017. Evaluation of serum lipid, thyroid, and hepatic clinical chemistries in association with serum perfluorooctanesulfonate (PFOS) in cynomolgus monkeys after oral dosing with potassium PFOS. Toxicological Sciences, 156, 387–401. 10.1093/toxsci/kfw267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Butenhoff JL, Parker GA, Coder PS, Zitzow JD, Krisko RM, Bjork JA, Wallace KB and Seed JG, 2018. Reproductive and developmental toxicity of potassium perfluorohexanesulfonate in CD‐1 mice. Reproductive Toxicology, 78, 150–168. 10.1016/j.reprotox.2018.04.007 [DOI] [PubMed] [Google Scholar]
- Chen MH, Ha EH, Wen TW, Su YN, Lien GW, Chen CY, Chen PC and Hsieh WS, 2012. Perfluorinated compounds in umbilical cord blood and adverse birth outcomes. PLoS ONE, 7, e42474 10.1371/journal.pone.0042474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Hu W, Huang C, Hua S, Wei Q, Bai C, Chen J, Norris MB, Winn R, Yang D and Dong Q, 2016. Subchronic perfluorooctanesulfonate (PFOS) exposure induces elevated mutant frequency in an in vivo λ transgenic medaka mutation assay. Science Reports, 6, 38466 10.1038/srep38466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W‐L, Bai F‐Y, Chang Y‐C, Chen P‐C and Chen C‐Y, 2018. Concentrations of perfluoroalkyl substances in foods and the dietary exposure among Taiwan general population and pregnant women. Journal of Food and Drug Analysis, 26, 994–1004. 10.1016/j.jfda.2017.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Li H, Mo J, Chen X, Wu K, Ge F, Ma L, Li X, Guo X, Zhao J and Ge RS, 2019. Perfluorododecanoic acid blocks rat leydig cell development during prepuberty. Chemical Research in Toxicology, 32, 146–155. [DOI] [PubMed] [Google Scholar]
- Cheng X and Klaassen CD, 2008a. Critical role of PPAR‐alpha in perfluorooctanoic acid‐ and perfluorodecanoic acid‐induced downregulation of Oatp uptake transporters in mouse livers. Toxicological Sciences, 106, 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X and Klaassen CD, 2008b. Perfluorocarboxylic acids induce cytochrome P450 enzymes in mouse liver through activation of PPAR‐alpha and CAR transcription factors. Toxicological Sciences, 106, 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chengelis CP, Kirkpatrick JB, Radovsky A and Shinohara M, 2009. A 90‐day repeated dose oral (gavage) toxicity study of perfluorohexanoic acid (PFHxA) in rats (with functional observational battery and motor activity determinations). Reproductive Toxicology, 27, 342–351. [DOI] [PubMed] [Google Scholar]
- Cheung C, Akiyama TE, Ward JM, Nicol CJ, Feigenbaum L, Vinson C and Gonzalez FJ, 2004. Diminished hepatocellular proliferation in mice humanized for the nuclear receptor peroxisome proliferator‐activated receptor alpha. Cancer Research, 64, 3849–3854. [DOI] [PubMed] [Google Scholar]
- Choi H, Bae IA, Choi JC, Park SJ and Kim M, 2018. Perfluorinated compounds in food simulants after migration from fluorocarbon resin‐coated frying pans, baking utensils, and non‐stick baking papers on the Korean market. Food Additives and Contaminants B, 11, 264–272. 10.1080/19393210.2018.1499677 [DOI] [PubMed] [Google Scholar]
- Chou WC and Lin Z, 2019. Bayesian evaluation of a physiologically based pharmacokinetic (PBPK) model for perfluorooctane sulfonate (PFOS) to characterize the interspecies uncertainty between mice, rats, monkeys, and humans: development and performance verification. Environment International, 129, 408–422. 10.1016/j.envint.2019.03.058 [DOI] [PubMed] [Google Scholar]
- Christensen KY, Maisonet M, Rubin C, Holmes A, Calafat AM, Kato K, Flanders WD, Heron J, McGeehin MA and Marcus M, 2011. Exposure to polyfluoroalkyl chemicals during pregnancy is not associated with offspring age at menarche in a contemporary British cohort. Environment International, 37, 129–135. 10.1016/j.envint.2010.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu S, Wang J, Leong G, Woodward LA, Letcher RJ and Li QX, 2015. Perfluoroalkyl sulfonates and carboxylic acids in liver, muscle and adipose tissues of black‐footed albatross (Phoebastria nigripes) from Midway Island, North Pacific Ocean. Chemosphere, 138, 60–66. 10.1016/j.chemosphere.2015.05.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn BA, La Merrill MA, Krigbaum NY, Wang M, Park JS, Petreas M, Yeh G, Hovey RC, Zimmermann L and Cirillo PM, 2019. In Utero exposure to poly and perfluoroalkyl substances (PFASs) and subsequent breast cancer. Reproductive Toxicology, pii: S0890‐6238(18)30486‐6. 10.1016/j.reprotox.2019.06.012 [DOI] [PubMed] [Google Scholar]
- Convertino M, Church TR, Olsen GW, Liu Y, Doyle E, Elcombe CR, Barnett AL, Samuel LM, MacPherson IR and Evans TRJ, 2018. Modeling for assessing the systemic health risk of perfluorooctanoate (PFOA). Toxicological Sciences, 163, 293–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsini E, Avogadro A, Galbiati V, dell'Agli M, Marinovich M, Galli CL and Germolec DR, 2011. In vitro evaluation of the immunotoxic potential of perfluorinated compounds (PFCs). Toxicology and Applied Pharmacology, 250, 108–116. [DOI] [PubMed] [Google Scholar]
- Corton JC, Peters JM and Klaunig JE, 2017. The PPARα‐dependent rodent liver tumor response is not relevant to humans: addressing misconceptions. Archives of Toxicology, 92, 83–119. 10.1007/s00204-017-2094-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford NM, Fenton SE, Strynar M, Hines EP, Pritchard DA and Steiner AZ, 2017. Effects of perfluorinated chemicals on thyroid function, markers of ovarian reserve, and natural fertility. Reproductive Toxicology, 69, 53–59. 10.1016/j.reprotox.2017.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crebelli R, Caiola S, Conti L, Cordelli E, De Luca G, Dellatte E, Eleuteri P, Iacovella N, Leopardi P, Marcon F, Sanchez M, Sestili P, Siniscalchi E and Villani P, 2019. Can sustained exposure to PFAS trigger a genotoxic response? A comprehensive genotoxicity assessment in mice after subacute oral administration of PFOA and PFBA. Regulatory Toxicology and Pharmacology, 106, 169–177. 10.1016/j.yrtph.2019.05.005 [DOI] [PubMed] [Google Scholar]
- Cui Q, Pan Y, Zhang H, Sheng N and Dai J, 2018. Elevated concentrations of perfluorohexanesulfonate and other per and polyfluoroalkyl substances in Baiyangdian Lake (China): source characterization and exposure assessment. Environmental Pollution, 241, 684–691. 10.1016/j.envpol.2018.05.099 [DOI] [PubMed] [Google Scholar]
- Dagnino S, Strynar MJ, McMahen RL, Lau CS, Ball C, Garantziotis S, Webster TF, McClean MD and Lindstrom AB, 2016. Identification of biomarkers of exposure to FTOHs and PAPs in humans using a targeted and nontargeted analysis approach. Environmental Science and Technology, 50, 10216–10225. 10.1021/acs.est.6b01170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalsager L, Christensen N, Husby S, Kyhl H, Nielsen F, Host A, Grandjean P and Jensen TK, 2016. Association between prenatal exposure to perfluorinated compounds and symptoms of infections at age 1‐4 years among 359 children in the Odense Child Cohort. Environment International, 96, 58–64. 10.1016/j.envint.2016.08.026 [DOI] [PubMed] [Google Scholar]
- Daly ER, Chan BP, Talbot EA, Nassif J, Bean C, Cavallo SJ, Metcalf E, Simone K and Woolf AD, 2018. Per‐ and polyfluoroalkyl substance (PFAS) exposure assessment in a community exposed to contaminated drinking water, New Hampshire. International Journal of Hygiene and Environmental Health, 221, 569–577. [DOI] [PubMed] [Google Scholar]
- Danish EPA (Danish Environmental Protection Agency), 2015. Perfluoroalkylated substances: PFOA, PFOS and PFOSA. Evaluation of health hazards and proposal of a health based quality criterion for drinking water, soil and ground water. Environmental project No. 1665, 2015. 90 pp.
- Das KP, Grey BE, Zehr RD, Wood CR, Butenhoff JL, Chang SC, Ehresman DJ, Tan YM and Lau C, 2008. Effects of perfluorobutyrate exposure during pregnancy in the mouse. Toxicological Sciences, 105, 173–181. 10.1093/toxsci/kfn099 [DOI] [PubMed] [Google Scholar]
- Das KP, Grey BE, Rosen MB, Wood CR, Tatum‐Gibbs KR, Zehr RD, Strynar MJ, Lindstrom AB and Lau C, 2015. Developmental toxicity of perfluorononanoic acid in mice. Reproductive Toxicology, 51, 133–144. 10.1016/j.reprotox.2014.12.012 [DOI] [PubMed] [Google Scholar]
- Das KP, Wood CR, Lin MT, Starkov AA, Lau C, Wallace KB, Corton JC and Abbott BD, 2017. Perfluoroalkyl acids‐induced liver steatosis: effects on genes controlling lipid homeostasis. Toxicology, 378, 37–52. 10.1016/j.tox.2016.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassuncao C, Hu XC, Nielsen F, Weihe P, Grandjean P and Sunderland EM, 2018. Shifting global exposures to poly‐ and perfluoroalkyl substances (PFASs) evident in longitudinal birth cohorts from a seafood‐consuming population. Environmental Science and Technology, 52, 3738–3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daynes RA and Jones DC, 2002. Emerging roles of PPARS in inflammation and immunity. Nature Reviews Immunology, 2, 748–759. [DOI] [PubMed] [Google Scholar]
- De Silva AO and Mabury SA, 2006. Isomer distribution of perfluorocarboxylates in human blood: potential correlation to source. Environmental Science and Technology, 40, 2903–2909. [DOI] [PubMed] [Google Scholar]
- De Silva AO, Benskin JP, Martin LJ, Arsenault G, McCrindle R, Riddell N, Martin JW and Mabury SA, 2009. Disposition of perfluorinated acid isomers in Sprague‐Dawley rats; part 2: subchronic dose. Environmental Toxicology and Chemistry, 28, 555–567. 10.1897/08-254.1 [DOI] [PubMed] [Google Scholar]
- Del Vento S, Halsall C, Gioia R, Jones K and Dachs J, 2012. Volatile per‐ and polyfluoroalkyl compounds in the remote atmosphere of the western Antarctic Peninsula: an indirect source of perfluoroalkyl acids to Antarctic waters? Atmospheric Pollution Research, 3, 450–455. 10.5094/APR.2012.051 [DOI] [Google Scholar]
- Denys S, Fraize‐Frontier S, Moussa O, Le Bizec B, Veyrand B and Volatier JL, 2014. Is the fresh water fish consumption a significant determinant of the internal exposure to perfluoroalkylated substances (PFAS)? Toxicology Letters, 231, 233–238. [DOI] [PubMed] [Google Scholar]
- D'Eon JC and Mabury SA, 2007. Production of perfluorinated carboxylic acids (PFCAs) from the biotransformation of polyfluoroalkyl phosphate surfactants (PAPS): exploring routes of human contamination. Environmental Science and Technology, 41, 4799–4805. [DOI] [PubMed] [Google Scholar]
- D'Eon JC and Mabury SA, 2011. Exploring indirect sources of human exposure to perfluoroalkyl carboxylates (PFCAs): evaluating uptake, elimination, and biotransformation of polyfluoroalkyl phosphate esters (PAPs) in the rat. Environmental Health Perspectives, 119, 344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt JC, Copeland CB, Strynar MJ and Luebke RW, 2008. Perfluorooctanoic acid‐induced immunomodulation in adult C57BL/6J or C57BL/6N female mice. Environmental Health Perspectives, 116, 644–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewitt JC, Copeland CB and Luebke RW, 2009. Suppression of humoral immunity by perfluorooctanoic acid is independent of elevated serum corticosterone concentration in mice. Toxicological Sciences, 109, 106–112. [DOI] [PubMed] [Google Scholar]
- DeWitt JC, Williams WC, Creech NJ and Luebke RW, 2016. Suppression of antigen‐specific antibody responses in mice exposed to perfluorooctanoic acid: role of PPARα and T‐ and B‐cell targeting. Journal of Immunotoxicology, 13, 38–45. 10.3109/1547691X.2014.996682 [DOI] [PubMed] [Google Scholar]
- Di Nisio A, Sabovic I, Valente U, Tescari S, Rocca MS, Guidolin D, Dall'Acqua S, Acquasaliente L, Pozzi N, Plebani M, Garolla A and Foresta C, 2019. Endocrine Disruption of Androgenic Activity by Perfluoroalkyl Substances: Clinical and Experimental Evidence. Clin Endocrinol Metab, 104, 1259–1271. 10.1210/jc.2018-01855 [DOI] [PubMed] [Google Scholar]
- Ding L, Hao F, Shi Z, Wang Y, Zhang H, Tang H and Dai J, 2009. Systems biological responses to chronic perfluorododecanoic acid exposure by integrated metabonomic and transcriptomic studies. Journal of Proteome Research, 8, 2882–2891. 10.1021/pr9000256 [DOI] [PubMed] [Google Scholar]
- Dixon D1, Reed CE, Moore AB, Gibbs‐Flournoy EA, Hines EP, Wallace EA, Stanko JP, Lu Y, Jefferson WN, Newbold RR and Fenton SE, 2012. Histopathologic changes in the uterus, cervix and vagina of immature CD‐1 mice exposed to low doses of perfluorooctanoic acid (PFOA) in a uterotrophic assay. Reproductive Toxicology, 33, 506–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobraca D, Israel L, McNeel S, Voss R, Wang M, Gajek R, Park JS, Harwani S, Barley F, She J and Das R, 2015. Biomonitoring in California firefighters: metals and perfluorinated chemicals. Occupational and Environmental Medicine, 57, 88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo JL, 2011. Influence of cooking processes on the concentrations of environmental pollutants in food: a review of the published literature. Critical Reviews in Food Science and Nutrition, 51, 29–37. [DOI] [PubMed] [Google Scholar]
- Domingo JL and Nadal M, 2017. Per‐ and Polyfluoroalkyl Substances (PFASs) in food and human dietary intake: a review of the recent scientific literature. Journal of Agricultural and Food Chemistry, 65, 533–543. 10.1021/acs.jafc.6b04683 [DOI] [PubMed] [Google Scholar]
- Domingo JL, Jogsten IE, Eriksson U, Martorella I, Perello G, Nadala M and van Bavel B, 2012. Human dietary exposure to perfluoroalkyl substances in Catalonia, Spain. Temporal Trend. Food Chemistry, 135, 1575–1582. [DOI] [PubMed] [Google Scholar]
- Donat‐Vargas C, Bergdahl IA, Tornevi A, Wennberg M, Sommar J, Koponen J, Kiviranta H and Akesson A, 2019. Associations between repeated measure of plasma perfluoroalkyl substances and cardiometabolic risk factors. Environment International, 124, 58–65. [DOI] [PubMed] [Google Scholar]
- Dong GH, Zhang YH, Zheng L, Liu W, Jin YH and He QC, 2009. Chronic effects of perfluorooctanesulfonate exposure on immunotoxicity in adult male C57BL/6 mice. Archives of Toxicology, 83, 805–815. [DOI] [PubMed] [Google Scholar]
- Dong GH, Liu MM, Wang D, Zheng L, Liang ZF and Jin YH, 2011. Sub‐chronic effect of perfluorooctanesulfonate (PFOS) on the balance of type 1 and type 2 cytokine in adult C57BL6 mice. Archives of Toxicology, 85, 1235–1244. [DOI] [PubMed] [Google Scholar]
- Dong GH, Wang J, Zhang YH, Liu MM, Wang D, Zheng L and Jin YH, 2012. Induction of p53‐mediated apoptosis in splenocytes and thymocytes of C57BL/6 mice exposed to perfluorooctane sulfonate (PFOS). Toxicology and Applied Pharmacology, 264, 292–299. [DOI] [PubMed] [Google Scholar]
- Dong GH, Tung KY, Tsai CH, Liu MM, Wang D, Liu W, Jin YH, Hsieh WS, Lee YL and Chen PC, 2013. Serum polyfluoroalkyl concentrations, asthma outcomes, and immunological markers in a case‐control study of Taiwanese children. Environmental Health Perspectives, 121, 507–513. 10.1289/ehp.1205351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z, Wang H, Yu YYB, Naidu R and Liu Y, 2019. Using 2003‐2014 U.S. NHANES data to determine the associations between per‐ and polyfluoroalkyl substances and cholesterol: trend and implications. Ecotoxicology and Environmental Safety, 173, 461–468. [DOI] [PubMed] [Google Scholar]
- Doull J, Cattley R, Elcombe C, Lake BG, Swenberg J, Wilkinson C, Williams G and van Gemert MA, 1999. Cancer risk assessment of di(2‐ethylhexyl)phthalate: application of the new U.S. EPA risk assessment guidelines. Regulatory Toxicology and Pharmacology, 29, 327–357. [DOI] [PubMed] [Google Scholar]
- Dreyer A, Weinberg I, Temme C and Ebinghaus R, 2009. Polyfluorinated compounds in the atmosphere of the atlantic and southern oceans: evidence for a global distribution. Environmental Science and Technology, 43, 6507–6514. 10.1021/es9010465 [DOI] [PubMed] [Google Scholar]
- Dzierlenga AL, Robinson VG, Waidyanatha S, DeVito MJ, Eifrid MA, Gibbs ST, Granville CA and Blystone CR, 2019. Toxicokinetics of perfluorohexanoic acid (PFHxA), perfluorooctanoic acid (PFOA) and perfluorodecanoic acid (PFDA) in male and female Hsd: Sprague dawley SD rats following intravenous or gavage administration. Xenobiotica. 10.1080/00498254.2019.1683776 [DOI] [PubMed] [Google Scholar]
- ECHA (European Chemicals Agency), 2012. CLH report (Proposal for Harmonised Classification and Labelling). 8:2 Fluorotelomer alcohol (8:2 FTOH). Version number: 1 Date: 20 March 2012. Available online: https://echa.europa.eu/documents/10162/570253b4-9e95-4e29-a394-89ece2050905
- ECHA (European Chemicals Agency), 2013. Committee for Risk Assessment RAC Annex 1 Background document to the Opinion proposing harmonised classification and labelling at EU level of 8:2 Fluorotelomer alcohol (8:2 FTOH). Available online: https://echa.europa.eu/documents/10162/983ac315-260c-12a8-661a-3f5302e4941b
- EFSA (European Food Safety Authority), 2008. Opinion of the Scientific Panel on Contaminants in the Food chain on Perfluorooctane sulfonate (PFOS), perfluorooctanoic acid (PFOA) and their salts. EFSA Journal 2008;6(7):653, 131 pp. 10.2903/j.efsa.2008.653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2010a. Standard sample description for food and feed. EFSA Journal 2010;8(1):1457, 54 pp. 10.2903/j.efsa.2010.1457 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2010b. Management of left‐censored data in dietary exposure assessment of chemical substances. EFSA Journal 2010;8(3):1557, 96 pp. 10.2903/j.efsa.2010.1557 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011a. Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment. EFSA Journal 2011;9(3):2097, 34 pp. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011b. Evaluation of the FoodEx, the food classification system applied to the development of the EFSA Comprehensive European Food Consumption Database. EFSA Journal 2011;9(3):1970, 27 pp. 10.2903/j.efsa.2011.1970 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011c. Report on the development of a Food Classification and Description System for exposure assessment and guidance on its implementation and use. EFSA Journal 2011;9(12):2489, 84 pp. 10.2903/j.efsa.2011.2489 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2012. Perfluoroalkylated substances in food: occurrence and dietary exposure. EFSA Journal 2012;10(6):2743, 55 pp. 10.2903/j.efsa.2012.2743 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2015. The food classification and description system FoodEx2 (revision 2). EFSA supporting publication 2015;EN‐804, 90 pp. 10.2903/sp.efsa.2015.en-804 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2020. Outcome of a public consultation on the draft risk assessment of perfluoroalkyl substances in food. EFSA supporting publication 2020:EN‐1931, 10.2903/sp.efsa.2020.1931 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), Knutsen HK, Alexander J, Barregård L, Bignami M, Brüschweiler B, Ceccatelli S, Cottrill B, Dinovi M, Edler L, Grasl‐Kraupp B, Hogstrand C, Hoogenboom LR, Nebbia CS, Oswald IP, Petersen A, Rose M, Roudot A‐C, Vleminckx C, Vollmer G, Wallace H, Bodin L, Cravedi J‐P, Halldorsson TI, Haug LS, Johansson N, van Loveren H, Gergelova P, Mackay K, Levorato S, van Manen M and Schwerdtle T, 2018. Scientific Opinion on the risk to human health related to the presence of perfluorooctane sulfonic acid and perfluorooctanoic acid in food. EFSA Journal 2018;16(12):5194, 284 pp. 10.2903/j.efsa.2018.5194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2013. Scientific Opinion on nutrient requirements and dietary intakes of infants and young children in the European Union. EFSA Journal 2013;11(10):3408, 103 pp. 10.2903/j.efsa.2013.3408 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579, 32 pp. 10.2903/j.efsa.2012.2579 [DOI] [Google Scholar]
- EFSA Scientific Committee , Hardy A, Benford D, Halldorsson T, Jeger MJ, Knutsen KH, More S, Mortensen A, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Silano V, Solecki R, Turck D, Aerts M, Bodin L, Davis A, Edler L, Gundert‐Remy U, Sand S, Slob W, Bottex B, Abrahantes JC, Marques DC, Kass G and Schlatter JR, 2017. Update: Guidance on the use of the benchmark dose approach in risk assessment. EFSA Journal 2017;15(1):4658, 41 pp. 10.2903/j.efsa. 2017.4658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , More SJ, Bampidis V, Benford D, Bennekou SH,Bragard C, Halldorsson TI, Hernandez‐Jerez AF, Koutsoumanis K, Naegeli H, Schlatter JR, Silano V,Nielsen SS, Schrenk D, Turck D, Younes M, Benfenati E, Castle L, Cedergreen N, Hardy A, Laskowski R,Leblanc JC, Kortenkamp A, Ragas A, Posthuma L, Svendsen C, Solecki R, Testai E, Dujardin B, Kass GEN,Manini P, Jeddi MZ, Dorne J‐LCM and Hogstrand C, 2019. Guidance on harmonised methodologies for human health, animal health and ecological risk assessment of combined exposure to multiple chemicals. EFSA Journal 2019;17(3):5634, 77 pp. 10.2903/j.efsa.2019.5634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeghy PP and Lorber M, 2011. An assessment of the exposure of Americans to perfluorooctane sulfonate: a comparison of estimated intake with values inferred from NHANES data. Journal of Exposure Science and Environmental Epidemiology, 21, 150 10.1038/jes.2009.73 [DOI] [PubMed] [Google Scholar]
- Ehresman DJ, Froehlich JW, Olsen GW, Chang SC and Butenhoff JL, 2007. Comparison of human whole blood, plasma, and serum matrices for the determination of perfluorooctanesulfonate (PFOS), perfluorooctanoate (PFOA), and other fluorochemicals. Environmental Research, 103, 176–184. [DOI] [PubMed] [Google Scholar]
- Eke D, Celik A, Yilmaz MB, Aras N, Kocaturk Sel S and Alptekin D, 2017. Apoptotic gene expression profiles and DNA damage levels in rat liver treated with perfluorooctane sulfonate and protective role of curcumin. International Journal of Biological Macromolecules, 104, 515–520. 10.1016/j.ijbiomac.2017.06.075 [DOI] [PubMed] [Google Scholar]
- Ellis DA, Martin JW, De Silva AO, Mabury SA, Hurley MD, Sulbaek Andersen MP and Wallington TJ, 2004. Degradation of fluorotelomer alcohols: a likely atmospheric source of perfluorinated carboxylic acids. Environmental Science & Technology, 38, 3316–3321. [DOI] [PubMed] [Google Scholar]
- Emmett EA, Shofer FS, Zhang H, Freeman D, Desai C and Shaw LM, 2006. Community exposure to perfluorooctanoate: relationships between serum concentrations and exposure sources. Journal of Occupational and Environmental Medicine, 48, 759–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Era S, Harada KH, Toyoshima M, Inoue K, Minata M, Saito N, Takigawa T, Shiota K and Koizumi A, 2009. Cleft palate caused by perfluorooctane sulfonate is caused mainly by extrinsic factors. Toxicology, 256, 42–47. 10.1016/j.tox.2008.11.003 [DOI] [PubMed] [Google Scholar]
- Ericson Jogsten I, Nadal M, van Bavel B, Lindström G and Domingo JL, 2012. Per‐ and polyfluorinated compounds (PFCs) in house dust and indoor air in Catalonia, Spain: implications for human exposure. Environment International, 39, 172–180. [DOI] [PubMed] [Google Scholar]
- Eriksen KT, Raaschou‐Nielsen O, Sørensen M, Roursgaard M, Loft S and Møller P, 2010. Genotoxic potential of the perfluorinated chemicals PFOA, PFOS, PFBS, PFNA and PFHxA in human HepG2 cells. Mutation Research‐Genetic Toxicology and Environmental Mutagenesis, 700, 39–43. [DOI] [PubMed] [Google Scholar]
- Eriksen KT, Sørensen M, McLaughlin JK, Tjonneland A, Overvad K and Raaschou‐Nielsen O, 2011. Determinants of plasma PFOA and PFOS levels among 652 Danish men. Environmental Science and Technology, 45, 8137–8143. 10.1021/es100626h [DOI] [PubMed] [Google Scholar]
- Eriksson U and Kärrman A, 2015. World‐wide indoor exposure to polyfluoroalkyl phosphate esters (PAPs) and other PFASs in household dust. Environmental Science and Technology, 49, 14503–14511. [DOI] [PubMed] [Google Scholar]
- Eriksson U, Roos A, Lind Y, Hope K, Ekblad A and Kärrman A, 2016. Comparison of PFASs contamination in the freshwater and terrestrial environments by analysis of eggs from osprey (Pandion haliaetus), tawny owl (Strix aluco), and common kestrel (Falco tinnunculus). Environmental Research, 149, 40–47. 10.1016/j.envres.2016.04.03 [DOI] [PubMed] [Google Scholar]
- Eriksson U, Mueller JF, Toms LL, Hobson P and Kärrman A, 2017. Temporal trends of PFSAs, PFCAs and selected precursors in Australian serum from 2002 to 2013. Environmental Pollution, 220, 168–177. 10.1016/j.envpol.2016.09.036 [DOI] [PubMed] [Google Scholar]
- Ernst A, Brix N, Lauridsen LLB, Olsen J, Parner ET, Liew Z, Olsen LH and Ramlau‐Hansen CH, 2019. Exposure to perfluoroalkyl substances during fetal life and pubertal development in boys and girls from the danish national birth cohort. Environmental Health Perspectives, 127, 17004 10.1289/EHP3567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eryasa B, Grandjean P, Nielsen F, Valvi D, Zmirou‐Navier D, Sunderland E, Weihe P and Oulhote Y, 2019. Physico‐chemical properties and gestational diabetes predict transplacental transfer and partitioning of perfluroralkyl substances. Environment International, 130, 104874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschauzier C, Raat KJ, Stuyfzand PJ and De Voogt P, 2013. Perfluorinated alkylated acids in groundwater and drinking water: identification, origin and mobility. Science of Total Environment, 458–460, 477–485. 10.1016/j.scitotenv.2013.04.066 [DOI] [PubMed] [Google Scholar]
- Fair PA, Driscoll E, Mollenhauer MAM, Bradshaw SG, Yun SH, Kannan K, Bossart GD, Keil DE and Peden‐Adams MM, 2011. Effects of environmentally‐relevant levels of perfluorooctane sulfonate on clinical parameters and immunological functions in B 6C 3F 1 mice. Journal of Immunotoxicology, 8, 17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Zhang L, Feng Y, Zhao Y and Dai J, 2008. Immunotoxic effects of perfluorononanoic acid on BALB/c mice. Toxicological Sciences, 105, 312–321. 10.1093/toxsci/kfn127 [DOI] [PubMed] [Google Scholar]
- Fang X, Feng Y, Shi Z and Dai J, 2009. Alterations of cytokines and MAPK signaling pathways are related to the immunotoxic effect of perfluorononanoic acid. Toxicological Sciences, 108, 367–376. 10.1093/toxsci/kfp019 [DOI] [PubMed] [Google Scholar]
- Fang X, Gao G, Xue H, Zhang X and Wang H, 2012a. In vitro and in vivo studies of the toxic effects of perfluorononanoic acid on rat hepatocytes and Kupffer cells. Environmental Toxicology and Pharmacology, 34, 484–494. [DOI] [PubMed] [Google Scholar]
- Fang X, Gao G, Xue H, Zhang X and Wang H, 2012b. Exposure of perfluorononanoic acid suppresses the hepatic insulin signal pathway and increases serum glucose in rats. Toxicology, 294, 109–115. [DOI] [PubMed] [Google Scholar]
- Fang S, Zhao S, Zhang Y, Zhong W and Zhu L, 2014. Distribution of perfluoroalkyl substances (PFASs) with isomer analysis among the tissues of aquatic organisms in Taihu Lake, China. Environmental Pollution, 193, 224–232. 10.1016/j.envpol.2014.07.006 [DOI] [PubMed] [Google Scholar]
- Fang X, Gao G, Zhang X and Wang H, 2015. Perfluorononanoic acid disturbed the metabolism of lipid in the liver of streptozotocin‐induced diabetic rats. Toxicology Mechanisms and Methods, 25, 622–627. [DOI] [PubMed] [Google Scholar]
- Fang X, Wu C, Li H, Yuan W and Wang X, 2018. Elevation of intracellular calcium and oxidative stress is involved in perfluorononanoic acid‐induced neurotoxicity. Toxicology and Industrial Health, 34, 139–145. 10.1177/0748233717742262 [DOI] [PubMed] [Google Scholar]
- Fasano WJ, Kennedy GL, Szostek B, Farrar DG, Ward RJ, Haroun L and Hinderliter PM, 2005. Penetration of ammonium perfluorooctanoate through rat and human skin in vitro . Drug and Chemical Toxicology, 28, 79–90. [DOI] [PubMed] [Google Scholar]
- Fasano WJ, Carpenter SC, Gannon SA, Snow TA, Stadler JC, Kennedy GL, Buck RC, Korzeniowski SH, Hinderliter PM and Kemper RA, 2006. Absorption, distribution, metabolism, and elimination of 8‐2 fluorotelomer alcohol in the rat. Toxicological Sciences, 91, 341–355. 10.1093/toxsci/kfj160 [DOI] [PubMed] [Google Scholar]
- Fasano WJ, Sweeney LM, Mawn MP, Nabb DL, Szostek B, Buck RC and Gargas ML, 2009. Kinetics of 8‐2 fluorotelomer alcohol and its metabolites, and liver glutathione status following daily oral dosing for 45 days in male and female rats. Chemico‐Biological Interactions, 180, 281–295. [DOI] [PubMed] [Google Scholar]
- Fei C, McLaughlin JK, Tarone RE and Olsen J, 2007. Perfluorinated chemicals and fetal growth: a study within the Danish National Birth Cohort. Environmental Health Perspectives, 115, 1677–1682. 10.1289/ehp.10506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei C, McLaughlin JK, Lipworth L and Olsen J, 2010a. Prenatal exposure to PFOA and PFOS and risk of hospitalization for infectious diseases in early childhood. Environmental Research, 110, 773–777. 10.1016/j.envres.2010.08.004 [DOI] [PubMed] [Google Scholar]
- Fei C, McLaughlin JK, Lipworth L and Olsen J, 2010b. Maternal concentrations of perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) and duration of breastfeeding. Scandinavian Journal of Work, Environment & Health, 36, 413–421. [DOI] [PubMed] [Google Scholar]
- Felizeter S, McLachlan MS and de Voogt P, 2012. Uptake of perfluorinated alkyl acids by hydroponically grown lettuce (Lactuca sativa). Environmental Science and Technology, 46, 11735–11743. 10.1021/es302398u [DOI] [PubMed] [Google Scholar]
- Feng X, Cao X, Zhao S, Wang X, Hua X, Chen L and Chen L, 2017. Exposure of pregnant mice to perfluorobutanesulfonate causes hypothyroxinemia and developmental abnormalities in female offspring. Toxicological Sciences, 155, 409–419. 10.1093/toxsci/kfw219 [DOI] [PubMed] [Google Scholar]
- Filgo AJ, Quist EM, Hoenerhoff MJ, Brix AE, Kissling GE and Fenton SE, 2015. Perfluorooctanoic acid (PFOA)‐induced liver lesions in two strains of mice following developmental exposures: PPARα is not required. Toxicologic Pathology, 43, 558–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay C, 2008. 8‐2 Telomer B Alcohol: acute oral toxicity‐fixed dose method. DuPont‐6711. (as cited in ECHA, 2012, CLH report).
- Fisher M, Arbuckle TE, Wade M and Haines DA, 2013. Do perfluoroalkyl substances affect metabolic function and plasma lipids? ‐ Analysis of the 2007–2009, Canadian Health Measures Survey (CHMS) Cycle 1. Environmental Research, 121, 95–103. 10.1016/j.envres.2012.11.006 [DOI] [PubMed] [Google Scholar]
- Fisher M, Arbuckle TE, Liang CL, LeBlanc A, Gaudreau E, Foster WG, Haines D, Davis K and Fraser WD, 2016. Concentrations of persistent organic pollutants in maternal and cord blood from the maternal‐infant research on environmental chemicals (MIREC) cohort study. Environmental Health, 15, 59 10.1186/s12940-016-0143-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisch AF, Rifas‐Shiman SL, Mora AM, Calafat AM, Ye X, Luttmann‐Gibson H, Gillman MW, Oken E and Sagiv SK, 2017. Early‐life exposure to perfluoroalkyl substances and childhood metabolic function. Environmental Health Perspectives, 125, 481–487. 10.1289/ehp303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman JE, Chang SC, Ehresman DJ, Butenhoff JL, Anderson CR, Palkar PS, Kang BH, Gonzalez FJ and Peters JM, 2009. Differential hepatic effects of perfluorobutyrate mediated by mouse and human PPAR‐alpha. Toxicological Sciences, 110, 204–211. [DOI] [PubMed] [Google Scholar]
- Framtiden i våre hender , online. Om Framtiden i våre henders kartlegging av PFAS i kosmetikk, mars 2018. Available online: https://www.framtiden.no/bilder/dokumenter/bakgrunn_kosmetikk_mars2018.pdf
- Franko J, Meade BJ, Frasch HF, Barbero AM and Anderson SE, 2012. Dermal penetration potential of perfluorooctanoic acid (PFOA) in human and mouse skin. Journal of Toxicology and Environmental Health A, 75, 50–62. [DOI] [PubMed] [Google Scholar]
- Frawley RP, Smith M, Cesta MF, Hayes‐Bouknight S, Blystone C, Kissling GE, Harris S and Germolec D, 2018. Immunotoxic and hepatotoxic effects of perfluoro‐n‐decanoic acid (PFDA) on female Harlan Sprague‐Dawley rats and B6C3F1/N mice when administered by oral gavage for 28 days. Journal of Immunotoxicology, 15, 41–52. 10.1080/1547691x.2018.1445145 [DOI] [PubMed] [Google Scholar]
- Freberg BI, Haug LS, Olsen R, Daae HL, Hersson M, Thomsen C, Thorud S, Becher G, Molander P and Ellingsen DG, 2010. Occupational exposure to airborne perfluorinated compounds during professional ski waxing. Environmental Science & Technology, 44, 7723–7728. 10.1021/es102033k [DOI] [PubMed] [Google Scholar]
- Frisbee SJ, Brooks AP Jr, Maher A, Flensborg P, Arnold S, Fletcher T, Steenland K, Shankar A, Knox SS, Pollard C, Halverson JA, Vieira VM, Jin C, Leyden KM and Ducatman AM, 2009. The C8 health project: design, methods, and participants. Environmental Health Perspectives, 117, 1873–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromme H, Tittlemier SA, Völkel W, Wilhelm M and Twardella D, 2009. Perfluorinated compounds ‐ Exposure assessment for the general population in western countries. International Journal of Hygiene and Environmental Health, 212, 239–270. [DOI] [PubMed] [Google Scholar]
- Fromme H, Mosch C, Morovitz M, Alba‐Alejandre I, Boehmer S, Kiranoglu M, Faber F, Hannibal I, Genzel‐Boroviczény O, Koletzko B and Völkel W, 2010. Pre‐ and postnatal exposure to perfluorinated compounds (PFCs). Environmental Science & Technology, 44, 7123–7129. [DOI] [PubMed] [Google Scholar]
- Fromme H, Dreyer A, Dietrich S, Fembacher L, Lahrz T and Völkel W, 2015. Neutral polyfluorinated compounds in indoor air in Germany‐the LUPE 4 study. Chemosphere, 139, 572–578. [DOI] [PubMed] [Google Scholar]
- Fromme H, Wöckner M, Roscher E and Völkel W, 2017. ADONA and perfluoroalkylated substances in plasma samples of German blood donors living in South Germany. International Journal of Hygiene and Environmental Health, 220 (2 Pt B), 455–460. https://www.ncbi.nlm.nih.gov/pubmed/28073630 [DOI] [PubMed] [Google Scholar]
- FSANZ (Food Standards Australia New Zealand), 2017. Hazard assessment report – Perfluorooctane sulfonate (PFOS), Perfluorooctanoic acid (PFOA), Perfluorohexane sulfonate (PFHxS). Available online: https://www.ehealth.gov.au/internet/main/publishing.nsf/Content/2200FE086D480353CA2580C900817CDC/File/2.Summary-Hazard-Assessment-Report.pdf
- Fu Y, Wang T, Fu Q, Wang P and Lu Y, 2014. Associations between serum concentrations of perfluoroalkyl acids and serum lipid levels in a Chinese population. Ecotoxicology and Environmental Safety, 106, 246–252. [DOI] [PubMed] [Google Scholar]
- Fu J, Gao Y, Wang T, Liang Y, Zhang A, Wang Y and Jiang G, 2015. Elevated levels of perfluoroalkyl acids in family members of occupationally exposed workers: the importance of dust transfer. Scientific Reports, 5, 9313 10.1038/srep09313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Gao Y, Cui L, Wang T, Liang Y, Qu G, Yuan B, Wang Y, Zhang A and Jiang G, 2016. Occurrence, temporal trends, and half‐lives of perfluoroalkyl acids (PFAAs) in occupational workers in China. Scientific Reports, 6, 38039 10.1038/srep38039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii Y, Harada KH and Koizumi A, 2013. Occurrence of perfluorinated carboxylic acids (PFCAs) in personal care products and compounding agents. Chemosphere, 93, 538–544. 10.1016/j.chemosphere.2013.06.049 [DOI] [PubMed] [Google Scholar]
- Fujii Y, Niisoe T, Harada KH, Uemoto S, Ogura Y, Takenaka K and Koizumi A, 2015. Toxicokinetics of perfluoroalkyl carboxylic acids with different carbon chain lengths in mice and humans. Journal of Occupational Health, 57, 1–12. 10.1539/joh.14-0136-OA [DOI] [PubMed] [Google Scholar]
- Furdui VI, Stock NL, Ellis DA, Butt CM, Whittle DM, Crozier PW, Reiner EJ, Muir DC and Mabury SA, 2007. Spatial distribution of perfluoroalkyl contaminants in lake trout from the Great Lakes. Environmental Science & Technology, 41, 1554–1559. [DOI] [PubMed] [Google Scholar]
- Gaillard J, Veyrand B, Thomas M, Dauchy X, Boiteux V, Marchand P, Le Bizec B, Banas D and Feidt C, 2017. Tissue uptake, distribution, and elimination of perfluoroalkyl substances in juvenile perch through perfluorooctane sulfonamidoethanol based phosphate diester dietary exposure. Environmental Science & Technology, 51, 7658–7666. 10.1021/acs.est.6b05598 [DOI] [PubMed] [Google Scholar]
- Gallo V, Leonardi G, Genser B, Lopez‐Espinosa MJ, Frisbee SJ, Karlsson L, Ducatman AM and Fletcher T, 2012. Serum perfluorooctanoate (PFOA) and perfluorooctane sulfonate (PFOS) concentrations and liver function biomarkers in a population with elevated PFOA exposure. Environmental Health Perspectives, 120, 655–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V, Leonardi G, Brayne C, Armstrong B and Fletcher T, 2013. Serum perfluoroalkyl acids concentrations and memory impairment in a large cross‐sectional study. British Medical Journal Open, 3, e002414 10.1136/bmjopen-2012-002414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon SA, Johnson T, Nabb DL, Serex TL, Buck RC and Loveless SE, 2011. Absorption, distribution, metabolism, and excretion of [1‐14C]‐perfluorohexanoate ([14C]‐PFHx) in rats and mice. Toxicology, 283, 55–62. 10.1016/j.tox.2011.02.004 [DOI] [PubMed] [Google Scholar]
- Gao Y, Fu J, Cao H, Wang Y, Zhang A, Liang Y, Wang T, Zhao C and Jiang G, 2015. Differential accumulation and elimination behavior of perfluoroalkyl Acid isomers in occupational workers in a manufactory in China. Environmental Science & Technology, 49, 6953–6962. 10.1021/acs.est.5b00778 [DOI] [PubMed] [Google Scholar]
- Gao K, Gao Y, Li Y, Fu J and Zhang A, 2016. A rapid and fully automatic method for the accurate determination of a wide carbon‐chain range of per‐ and polyfluoroalkyl substances (C4‐C18) in human serum. Journal of Chromatography A, pii: S0021‐9673(16)31266‐3. [DOI] [PubMed] [Google Scholar]
- García‐Valcárcel AI, Molero E, Escorial MC, Chueca MC and Tadeo JL, 2014. Uptake of perfluorinated compounds by plants grown in nutrient solution. Science of the Total Environment, 472, 20–26. [DOI] [PubMed] [Google Scholar]
- Gebbink WA, Glynn A and Berger U, 2015. Temporal changes (1997–2012) of perfluoroalkyl acids and selected precursors (including isomers) in Swedish human serum. Environmental Pollution, 199, 166–173. [DOI] [PubMed] [Google Scholar]
- Gebbink WA, Bignert A and Berger U, 2016. Perfluoroalkyl acids (PFAAs) and selected precursors in the Baltic seaenvironment: do precursors play a role in food web accumulation of PFAAs? Environmental Science Technology, 50, 6354–6362. [DOI] [PubMed] [Google Scholar]
- Gewurtz SB, Backus SM, De Silva AO, Ahrens L, Armellin A, Evans M, Fraser S, Gledhill M, Guerra P, Harner T, Helm PA, Hung H, Khera N, Kim MG, King M, Lee SC, Letcher RJ, Martin P, Marvin C, McGoldrick DJ, Myers AL, Pelletier M, Pomeroy J, Reiner EJ, Rondeau M, Sauve MC, Sekela M, Shoeib M, Smith DW, Smyth SA, Struger J, Spry D, Syrgiannis J and Waltho J, 2013. Perfluoroalkyl acids in the Canadian environment: multi‐media assessment of current status and trends. Environment International, 59, 183–200. 10.1016/j.envint.2013.05.008 [DOI] [PubMed] [Google Scholar]
- Ghisi R, Vamerali T and Manzetti S, 2019. Accumulation of perfluorinated alkyl substances (PFAS) in agricultural plants: a review. Environmental Research, 169, 326–341. 10.1016/j.envres.2018.10.023 [DOI] [PubMed] [Google Scholar]
- Giesy JP and Kannan K, 2001. Global distribution of perfluorooctane sulfonate in wildlife. Environmental Science & Technology, 35, 1339–1342. 10.1021/es001834k [DOI] [PubMed] [Google Scholar]
- Gimble JM, Pighetti GM, Lerner MR, Wu X, Lightfoot SA, Brackett DJ, Darcy K and Hollingsworth AB, 1998. Expression of peroxisome proliferator activated receptor mRNA in normal and tumorigenic rodent mammary glands. Biochemical and Biophysical Research Communications, 253, 813–817. 10.1006/bbrc.1998.9858 [DOI] [PubMed] [Google Scholar]
- Gleason JA, Post GB and Fagliano JA, 2015. Associations of perfluorinated chemical serum concentrations and biomarkers of liver function and uric acid in the US population (NHANES), 2007‐2010. Environmental Research, 136, 8–14. 10.1016/j.envres.2014.10.004 [DOI] [PubMed] [Google Scholar]
- Glynn A, Berger U, Bignert A, Ullah S, Aune M, Lignell S and Darnerud PO, 2012. Perfluorinated alkyl acids in blood serum from primiparous women in Sweden: serial sampling during pregnancy and nursing, and temporal trends 1996‐2010. Environmental Science & Technology, 46, 9071–9079. [DOI] [PubMed] [Google Scholar]
- Gobelius L, Lewis J and Ahrens L, 2017. Plant uptake of per‐ and polyfluoroalkyl substances at a contaminated fire training facility to evaluate the phytoremediation potential of various plant species. Environmental Science & Technology, 51, 12602–12610. [DOI] [PubMed] [Google Scholar]
- Goeden HM, Greene CW and Jacobus JA, 2019. A transgenerational toxicokinetic model and its use in derivation of Minnesota PFOA water guidance. Journal of Exposure Science & Environmental Epidemiology, 29, 183–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeritz I, Falk S, Stahl T, Schafers C and Schlechtriem C, 2013. Biomagnification and tissue distribution of perfluoroalkyl substances (PFASs) in market‐size rainbow trout (Oncorhynchus mykiss). Environmental Toxicology and Chemistry, 32, 2078–2088. 10.1002/etc.2279 [DOI] [PubMed] [Google Scholar]
- Goll V, Alexandre E, Viollon‐Abadie C, Nicod L, Jaeck D and Richert L, 1999. Comparison of the effects of various peroxisome proliferators on peroxisomal enzyme activities, DNA synthesis, and apoptosis in rat and human hepatocyte cultures. Toxicology and Applied Pharmacology, 160, 21–32. [DOI] [PubMed] [Google Scholar]
- Gómez‐Canela C, Fernandez‐Sanjuan M, Farres M and Lacorte S, 2015. Factors affecting the accumulation of perfluoroalkyl substances in human blood. Environmental Science and Pollution Research International, 22, 1480–1486. 10.1007/s11356-014-3439-x [DOI] [PubMed] [Google Scholar]
- Gomis MI, Vestergren R, Borg D and Cousins IT, 2018. Comparing the toxic potency in vivo of long‐chain perfluoroalkyl acids and fluorinated alternatives. Environment International, 113, 1–9. 10.1016/j.envint.2018.01.011 [DOI] [PubMed] [Google Scholar]
- Goudarzi H, Miyashita C, Okada E, Kashino I, Kobayashi S, Chen CJ, Ito S, Araki A, Matsuura H, Ito YM and Kishi R, 2016. Effects of prenatal exposure to perfluoroalkyl acids on prevalence of allergic diseases among 4‐year‐old children. Environment International, 94, 124–132. 10.1016/j.envint.2016.05.020 [DOI] [PubMed] [Google Scholar]
- Goudarzi H, Miyashita C, Okada E, Kashino I, Chen CJ, Ito S, Araki A, Kobayashi S, Matsuura H and Kishi R, 2017. Prenatal exposure to perfluoroalkyl acids and prevalence of infectious diseases up to 4 years of age. Environment International, 104, 132–138. 10.1016/j.envint.2017.01.024 [DOI] [PubMed] [Google Scholar]
- Govarts E, Iszatt N, Trnovec T, de Cock M, Eggesbo M, Murinova LP, van de Bor M, Guxens M, Chevrier C, Koppen G, Lamoree M, Hertz‐Picciotto I, Lopez‐Espinosa M‐J, Lertxundi A, Grimalt JO, Torrent M, Goni‐Irigoyen F, Vermeulen R, Legler J and Schoeters G, 2018. Prenatal exposure to endocrine disrupting chemicals and risk of being born small for gestational age: pooled analysis of seven European birth cohorts. Environmental International, 115, 267–278. 10.1016/j.envint.2018.03.017 [DOI] [PubMed] [Google Scholar]
- Graber JM, Alexander C, Laumbach RJ, Black K, Strickland PO, Georgopoulos PG, Marshall EG, Shendell DG, Alderson D, Mi Z, Mascari M and Weisel CP, 2019. Per and polyfluoroalkyl substances (PFAS) blood levels after contamination of a community water supply and comparison with 2013‐2014 NHANES. Journal of Exposure Science and Environmental Epidemiology, 29, 172–182. 10.1038/s41370-018-0096-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Andersen EW, Budtz‐Jorgensen E, Nielsen F, Molbak K, Weihe P and Heilmann C, 2012. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA, 307, 391–397. 10.1001/jama.2011.2034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Heilmann C, Weihe P, Nielsen F, Mogensen UB, Timmermann A and Budtz‐Jorgensen E, 2017a. Estimated exposures to perfluorinated compounds in infancy predict attenuated vaccine antibody concentrations at age 5‐years. Journal of Immunotoxicology, 14, 188–195. 10.1080/1547691x.2017.1360968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Heilmann C, Weihe P, Nielsen F, Mogensen UB and Budtz‐Jorgensen E, 2017b. Serum vaccine antibody concentrations in adolescents exposed to perfluorinated compounds. Environmental Health Perspectives, 125, 077018 10.1289/ehp275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granum B, Haug LS, Namork E, Stølevik SB, Thomsen C, Aaberge IS, van Loveren H, Løvik M and Nygaard UC, 2013. Pre‐natal exposure to perfluoroalkyl substances may be associated with altered vaccine antibody levels and immune‐related health outcomes in early childhood. Journal of Immunotoxicology, 10, 373–379. [DOI] [PubMed] [Google Scholar]
- Gremmel C, Fromel T and Knepper TP, 2016. Systematic determination of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in outdoor jackets. Chemosphere, 160, 173–180. 10.1016/j.chemosphere.2016.06.043 [DOI] [PubMed] [Google Scholar]
- Gribble MO, Bartell SM, Kannan K, Wu Q, Fair PA and Kamen DL, 2015. Longitudinal measures of perfluoroalkyl substances (PFAS) in serum of Gullah African Americans in South Carolina: 2003–2013. Environmental Research, 143(Pt B), 82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gump BB, Wu Q, Dumas AK and Kannan K, 2011. Perfluorochemical (PFC) exposure in children: associations with impaired response inhibition. Environmental Science & Technology., 45, 8151–8159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guruge KS, Hikono H, Shimada N, Murakami K, Hasegawa J, Yeung LW, Yamanaka N and Yamashita N, 2009. Effect of perfluorooctane sulfonate (PFOS) on influenza A virus‐induced mortality in female B6C3F1 mice. Journal of Toxicological Sciences, 34, 687–691. [DOI] [PubMed] [Google Scholar]
- Guruge KS, Noguchi M, Yoshioka K, Yamazaki E, Taniyasu S, Yoshioka M, Yamanaka N, Ikezawa M, Tanimura N, Sato M, Yamashita N and Kawaguchi H, 2016. Microminipigs as a new experimental animal model for toxicological studies: comparative pharmacokinetics of perfluoroalkyl acids. Journal of Applied Toxicology, 36, 68–75. 10.1002/jat.3145 [DOI] [PubMed] [Google Scholar]
- Gützkow KB, Haug LS, Thomsen C, Sabaredzovic A, Becher G and Brunborg G, 2012. Placental transfer of perfluorinated compounds is selective‐a Norwegian Mother and Child sub‐cohort study. International Journal of Hygiene and Environmental Health, 215, 216–219. [DOI] [PubMed] [Google Scholar]
- Gyllenhammar I, Berger U, Sundström M, Lignell S, Aune M, Darnerud PO, Kotova N and Glynn A, 2013. Perfluoroalkyl Acids in Serum from Nursing Women Living in an Area in Sweden with Drinking Water Contamination. Nationell miljöövervakning på uppdrag av naturvårdsverket. Report NV‐02409‐12. [Google Scholar]
- Gyllenhammar I, Berger U, Sundström M, McCleaf P, Eurén K, Eriksson S, Ahlgren S, Lignell S, Aune M, Kotova N and Glynn A, 2015. Influence of contaminated drinking water on perfluoroalkyl acid levels in human serum‐A case study from Uppsala, Sweden. Environmental Research, 140, 673–683. [DOI] [PubMed] [Google Scholar]
- Hadrup N, Pedersen Mikael, Skov Kasper, Hansen Niels Lund, Berthelsen LO, Kongsbak K, Boberg J, Dybdahl M, Hass U, Frandsen H and Vinggaard AM, 2016. Perfluorononanoic acid in combination with 14 chemicals exerts low‐dose mixture effects in rats. Archives of Toxicology, 90, 661–675. [DOI] [PubMed] [Google Scholar]
- Hagen DF, Belisle J, Johnson JD and Venkateswarlu P, 1981. Characterization of fluorinated metabolites by a gas chromatographic‐helium microwave plasma detector. The biotransformation of 1H,1H,2H,2H‐perfluorodecanolto perfluorooctanoate. Analytical Biochemistry, 118, 336–343. [DOI] [PubMed] [Google Scholar]
- Haines DA and Murray J, 2012. Human biomonitoring of environmental chemicals–early results of the 2007‐2009 Canadian Health Measures Survey for males and females. Hygiene and Environmental Health, 215, 133–137. [DOI] [PubMed] [Google Scholar]
- Halldorsson TI, Fei C, Olsen J, Lipworth L, McLaughlin JK and Olsen SF, 2008. Dietary predictors of perfluorinated chemicals: a study from the Danish National Birth Cohort. Environmental Science and Technology, 42, 8971–8977. [DOI] [PubMed] [Google Scholar]
- Halldorsson TI, Rytter D, Haug LS, Bech BH, Danielsen I, Becher G, Henriksen TB and Olsen SF, 2012. Prenatal exposure to perfluorooctanoate and risk of overweight at 20 years of age: a prospective cohort study. Environmental Health Perspectives, 120, 668–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halsne R, Tandberg JI, Lobert VH, Østby GC, Thoen E, Ropstad E and Verhaegen S, 2016. Effects of perfluorinated alkyl acids on cellular responses of MCF‐10A mammary epithelial cells in monolayers and on acini formation in vitro. Toxicology Letters, 259, 95–107. 10.1016/j.toxlet.2016.08.004 [DOI] [PubMed] [Google Scholar]
- Hamm MP, Cherry NM, Chan E, Martin JW and Burstyn I, 2010. Maternal exposure to perfluorinated acids and fetal growth. Journal of Exposure Science and Environmental Epidemiology, 20, 589–597. 10.1038/jes.2009.57 [DOI] [PubMed] [Google Scholar]
- Han X, Nabb DL, Russell MH, Kennedy GL and Rickard RW, 2012. Renal elimination of perfluorocarboxylates (PFCAs). Chemical Research in Toxicology, 25, 35–46. 10.1021/tx200363w [DOI] [PubMed] [Google Scholar]
- Han R, Hu M, Zhong Q, Wan C, Liu L, Li F, Zhang F and Ding W, 2018a. Perfluorooctane sulphonate induces oxidative hepatic damage via mitochondria‐dependent and NF‐kappaB/TNF‐alpha‐mediated pathway. Chemosphere, 191, 1056–1064. 10.1016/j.chemosphere.2017.08.070 [DOI] [PubMed] [Google Scholar]
- Han R, Zhang F, Wan C, Liu L, Zhong Q and Ding W, 2018b. Effect of perfluorooctane sulphonate‐induced Kupffer cell activation on hepatocyte proliferation through the NF‐kappaB/TNF‐alpha/IL‐6‐dependent pathway. Chemosphere, 200, 283–294. 10.1016/j.chemosphere.2018.02.137 [DOI] [PubMed] [Google Scholar]
- Hansen S, Vestergren R, Herzke D, Melhus M, Evenset A, Hanssen L, Brustad M and Sandanger TM, 2016. Exposure to per‐ and polyfluoroalkyl substances through the consumption of fish from lakes affected by aqueous film‐forming foam emissions ‐ A combined epidemiological and exposure modeling approach. The SAMINOR 2 Clinical Study. Environment International, 94, 272–282. [DOI] [PubMed] [Google Scholar]
- Hanssen L, Dudarev AA, Huber S, Odland JO, Nieboer E and Sandanger TM, 2013. Partition of perfluoroalkyl substances (PFASs) in whole blood and plasma, assessed in maternal and umbilical cord samples from inhabitants of arctic Russia and Uzbekistan. Science of the Total Environment, 447, 430–437. 10.1016/j.scitotenv.2013.01.029 [DOI] [PubMed] [Google Scholar]
- Harada K, Inoue K, Morikawa A, Yoshinaga T, Saito N and Koizumi A, 2005. Renal clearance of perfluorooctane sulfonate and perfluorooctanoate in humans and their species‐specific excretion. Environmental Research, 99, 253–261. 10.1016/j.envres.2004.12.003 [DOI] [PubMed] [Google Scholar]
- Harada KH, Hashida S, Kaneko T, Takenaka K, Minata M, Inoue K, Saito N and Koizumi A, 2007. Biliary excretion and cerebrospinal fluid partition of perfluorooctanoate and perfluorooctane sulfonate in humans. Environmental Toxicology and Pharmacology, 24, 134–139. 10.1016/j.etap.2007.04.003 [DOI] [PubMed] [Google Scholar]
- Hardell E, Kärrman A, van Bavel B, Bao J, Carlberg M and Hardell L, 2014. Case‐control study on perfluorinated alkyl acids (PFAAs) and the risk of prostate cancer. Environment International, 63, 35–39. [DOI] [PubMed] [Google Scholar]
- Harrad S, de Wit CA, Abdallah MA, Bergh C, Björklund JA, Covaci A, Darnerud PO, de Boer J, Diamond M, Huber S, Leonards P, Mandalakis M, Ostman C, Haug LS, Thomsen C and Webster TF, 2010. Indoor contamination with hexabromocyclododecanes, polybrominated diphenyl ethers, and perfluoroalkyl compounds: an important exposure pathway for people? Environmental Science & Technology, 44, 3221–3231. [DOI] [PubMed] [Google Scholar]
- Harris MW and Birnbaum LS, 1989a. Developmental toxicity of perfluorodecanoic acid in C57BL/6N mice. Fundamental and Applied Toxicology, 12, 442–448. [DOI] [PubMed] [Google Scholar]
- Harris MW, Uraih LC and Birnbaum LS, 1989b. Acute toxicity of perfluorodecanoic acid in C57BL/6 mice differs from 2,3,7,8‐tetrachlorodibenzo‐p‐dioxin. Fundamental and Applied Toxicology, 13, 723–736. 10.1016/0272-0590(89)90330-8 [DOI] [PubMed] [Google Scholar]
- Hartman TJ, Calafat AM, Holmes AK, Marcus M, Northstone K, Flanders WD, Kato K and Taylor EV, 2017. Prenatal exposure to perfluoroalkyl substances and body fatness in girls. Child Obesity, 13, 222–230. 10.1089/chi.2016.0126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasmall SC, James NH, Macdonald N, West D, Chevalier S, Cosulich SC and Roberts RA, 1999. Suppression of apoptosis and induction of DNA synthesis in vitro by the phthalate plasticizers monoethylhexylphthalate (MEHP) and diisononylphthalate (DINP): a comparison of rat and human hepatocytes in vitro . Archives of Toxicology, 73, 451–456. [DOI] [PubMed] [Google Scholar]
- Hasmall SC, James NH, Macdonald N, Gonzalez FJ, Peters JM and Roberts RA, 2000. Suppression of mouse hepatocyte apoptosis by peroxisome proliferators: role of PPARα and TNFαlpha. Mutation Research, 448, 193–200. [DOI] [PubMed] [Google Scholar]
- Haug LS, Thomsen C and Becher G, 2009. Time trends and the influence of age and gender on serum concentrations of perfluorinated compounds in archived human samples. Environment and Science Technology, 43, 2131–2136. [DOI] [PubMed] [Google Scholar]
- Haug LS, Thomsen C, Brantsæter AL, Kvalem HE, Haugen M, Becher G, Alexander J, Meltzer HM and Knutsen HK, 2010a. Diet and particularly seafood are major sources of perfluorinated compounds in humans. Environment International, 36, 772–778. [DOI] [PubMed] [Google Scholar]
- Haug LS, Salihovic S, Ericson Jogsten I, Thomsen C, van Bavel B, Linström G and Becher G, 2010b. Levels in food and beverages and daily intake of perfluorinated compounds in Norway. Chemosphere, 80, 1137–1143. [DOI] [PubMed] [Google Scholar]
- Haug LS, Huber S, Schlabach M, Becher G and Thomsen C, 2011a. Investigation on per‐ and polyfluorinated compounds in paired samples of house dust and indoor air from Norwegian homes. Environmental Science & Technology, 45, 7991–7998. [DOI] [PubMed] [Google Scholar]
- Haug LS, Huber S, Becher G and Thomsen C, 2011b. Characterisation of human exposure pathways to perfluorinated compounds‐comparing exposure estimates with biomarkers of exposure. Environment International, 37, 687–693. [DOI] [PubMed] [Google Scholar]
- HBM , 2018. (Statement of the German Human Biomonitoring Commission). [HBM‐I values for Perfluorooctanoic acid (PFOA) and Perfluorooctanesulfonic acid (PFOS) in blood plasma. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz, 61, 474–487. 10.1007/s00103-018-2709-z [DOI] [PubMed] [Google Scholar]
- HBM , 2020. (Statement by the Human Biomonitoring Commission of the German Environment Agency). HBM‐II values for perfluorooctanoic acid (PFOA) and perfluorooctane‐ sulfonic acid (PFOS) in blood plasma. Translation of the German version in Bundesgesundheitsbl, 63, 356–360 Available on line: https://www.umweltbundesamt.de/sites/default/files/medien/4031/dokumente/hbm-ii_values_for_pfoa_and_pfos_0.pdf [Google Scholar]
- Hebert PC and MacManus‐Spencer LA, 2010. Development of a fluorescence model for the binding of medium‐ to long‐chain perfluoroalkyl acids to human serum albumin through a mechanistic evaluation of spectroscopic evidence. Analytical Chemistry, 82, 6463–6471. [DOI] [PubMed] [Google Scholar]
- Heffernan AL, Cunningham TK, Drage DS, Alyward LL, Thompson K, Vijayasarathy S, Mueller JF, Atkin SL and Sathypalan T, 2018. Perfluorinated alkyl acids in the serum and follicular fluid of UK women with and without polycystic ovarian syndrome undergoing fertility treatment and associations with hormonal and metabolic parameters. International Journal of Hygiene and Environmental Health, 221, 1068–1075. [DOI] [PubMed] [Google Scholar]
- Henderson WM and Smith MA, 2007. Perfluorooctanoic acid and perfluorononanoic acid in fetal and neonatal mice following in utero exposure to 8‐2 fluorotelomer alcohol. Toxicological Science, 95, 452–461. 10.1093/toxsci/kfl162 [DOI] [PubMed] [Google Scholar]
- Higgins CP and Luthy RG, 2006. Sorption of Perfluorinated Surfactants on Sediments. Environmental Science & Technology, 40, 7251–7256. [DOI] [PubMed] [Google Scholar]
- Hines EP, White SS, Stanko JP, Gibbs‐Flournoy EA, Lau C and Fenton SE, 2009. Phenotypic dichotomy following developmental exposure to perfluorooctanoic acid (PFOA) in female CD‐1 mice: low doses induce elevated serum leptin and insulin, and overweight in mid‐life. Molecular and Cellular Endocrinology, 304, 97–105. 10.1016/j.mce.2009.02.021 [DOI] [PubMed] [Google Scholar]
- Hirata‐Koizumi M, Fujii S, Furukawa M, Ono A and Hirose A, 2012. Repeated dose and reproductive/developmental toxicity of perfluorooctadecanoic acid in rats. Journal of Toxicological Sciences, 37, 63–79. [DOI] [PubMed] [Google Scholar]
- Hirata‐Koizumi M, Fujii S, Hina K, Matsumoto M, Takahashi M, Ono A and Hirose A, 2015. Repeated dose and reproductive/developmental toxicity of long‐chain perfluoroalkyl carboxylic acids in rats: perfluorohexadecanoic acid and perfluorotetradecanoic acid. Fundamental Toxicological Sciences, 2, 177–190. [Google Scholar]
- Hoivik DJ, Qualls CW Jr, Mirabile RC, Cariello NF, Kimbrough CL, Colton HM, Anderson SP, Santostefano MJ, Morgan RJ, Dahl RR, Brown AR, Zhao Z, Mudd PN Jr, Oliver WB Jr, Brown HR and Miller RT, 2004. Fibrates induce hepatic peroxisome and mitochondrial proliferation without overt evidence of cellular proliferation and oxidative stress in cynomolgus monkeys. Carcinogenesis, 25, 1757–1769. [DOI] [PubMed] [Google Scholar]
- Holden P, Hasmall S, James N, West D, Brindle R, Gonzalez F, Peters J and Roberts R, 2000. Tumour necrosis factor a (TNFα): role in suppression of apoptosis by peroxisome proliferators. Cellular and Molecular Biology, 46, 29–39. [PubMed] [Google Scholar]
- Holmström KE, Johansson A‐K, Bignert A, Lindberg P and Berger U, 2010. Temporal trends of perfluorinated surfactants in Swedish peregrine falcon eggs (Falco peregrinus), 1974–2007. Environmental Science & Technology, 44, 4083–4088. [DOI] [PubMed] [Google Scholar]
- Hölzer J, Midasch O, Rauchfuss K, Kraft M, Reupert R, Angerer J, Kleeschulte P, Marschall N and Wilhelm M, 2008. Biomonitoring of perfluorinated compounds in children and adults exposed to perfluorooctanoate‐contaminated drinking water. Environmental Health Perspectives, 116, 651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houde M, Martin JW, Letcher RJ, Solomon KR and Muir DCG, 2006. Biological monitoring of polyfluoroalkyl substance: a review. Environmental Science & Technology, 40, 3463–3473. [DOI] [PubMed] [Google Scholar]
- Houde M, De Silva AO, Muir DCG and Letcher RJ, 2011. Monitoring of perfluorinated compounds in aquatic biota: an updated review. Environmental Science & Technology, 45, 7962–7973. [DOI] [PubMed] [Google Scholar]
- Hu XC, Dassuncao C, Zhang X, Grandjean P, Weihe P, Webster GM, Nielsen F and Sunderland EM, 2018. Can profiles of poly‐ and Perfluoroalkyl substances (PFASs) in human serum provide information on major exposure sources? Environmental Health, 17, 11 10.1186/s12940-018-0355-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M, Jiao J, Zhuang P, Chen X, Wang J and Zhang Y, 2018. Serum polyfluoroalkyl chemicals are associated with risk of cardiovascular diseases in national US population. Environment International, 119, 37–46. 10.1016/j.envint.2018.05.051 [DOI] [PubMed] [Google Scholar]
- Huang MC, Dzierlenga AL, Robinson VG, Waidyanatha S, DeVito MJ, Edifrid MA, Granville CA, Gibbs ST and Blystone CR, 2019a. Toxicokinetics of perfluorobutane sulfonate (PFBS), perfluorohexane‐1‐sulphonic acid (PFHxS), and perfluorooctane sulfonic acid (PFOS) in male and female Hsd: Sprague Dawley SD rats after intravenous and gavage administration. Toxicology Reports, 6, 645–655. 10.1016/j.toxrep.2019.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang MC, Robinson VG, Waidyanatha S, Dzierlenga AL, DeVito MJ, Edifrid MA, Gibbs ST and Blystone CR, 2019b. Toxicokinetics of 8:2 fluorotelomer alcohol (8:2‐FTOH) in male and female Hsd: Sprague Dawley SD rats after intravenous and gavage administration. Toxicology Reports, 6, 924–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huck I, Beggs K and Apte U, 2018. Paradoxical protective effect of perfluorooctanesulfonic acid against high‐fat diet‐induced hepatic steatosis in mice. International Journal of Toxicology, 37, 383–392. 10.1177/1091581818790934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui Z, Li R and Chen L, 2017. The impact of exposure to environmental contaminant on hepatocellular lipid metabolism. Gene, 622, 67–71. 10.1016/j.gene.2017.04.024 [DOI] [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer), 2017. Some chemicals used as solvents and in polymer manufacture IARC monographs on the evaluation of carcinogenic risks to humans volume 110. Available online: http://monographs.iarc.fr/ENG/Monographs/vol110/mono110-01.pdf [PubMed]
- Impinen A, Nygaard UC, Lodrup Carlsen KC, Mowinckel P, Carlsen KH, Haug LS and Granum B, 2018. Prenatal exposure to perfluoralkyl substances (PFASs) associated with respiratory tract infections but not allergy‐ and asthma‐related health outcomes in childhood. Environmental Research, 160, 518–523. 10.1016/j.envres.2017.10.012 [DOI] [PubMed] [Google Scholar]
- Impinen A, Longnecker MP, Nygaard UC, London SJ, Ferguson KK, Haug LS and Granum B, 2019. Maternal levels of perfluoroalkyl substances (PFASs) during pregnancy and childhood allergy and asthma related outcomes and infections in the Norwegian Mother and Child (MoBa) cohort. Environment International, 124, 462–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingelido AM, Marra V, Abballe A, Valentini S, Iacovella N, Barbieri P, Porpora MG, Domenico Ad and De Felip E, 2010. Perfluorooctanesulfonate and perfluorooctanoic acid exposures of the Italian general population. Chemosphere, 80, 1125–1130. [DOI] [PubMed] [Google Scholar]
- Ingelido AM, Abballe A, Gemma S, Dellatte E, Iacovella N, De Angelis G, Zampaglioni F, Marra V, Miniero R, Valentini S, Russo F, Vazzoler M, Testai E and De Felip E, 2018. Biomonitoring of perfluorinated compounds in adults exposed to contaminated drinking water in the Veneto Region, Italy. Environment International, 110, 149–159. 10.1016/j.envint.2017.10.026 [DOI] [PubMed] [Google Scholar]
- Inoue K, Okada F, Ito R, Kato S, Sasaki S, Nakajima S, Uno A, Saijo Y, Sata F, Yoshimura Y, Kishi R and Nakazawa H, 2004. Perfluorooctane sulfonate (PFOS) and related perfluorinated compounds in human maternal and cord blood samples: assessment of PFOS exposure in a susceptible population during pregnancy. Environmental Health Perspectives, 112, 1204–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y, Yamanoshita O, Kurata Y, Kamijima M, Aoyama T and Nakajima T, 2007. Induction of peroxisome proliferator‐activated receptor alpha (PPARa)‐related enzymes by di(2‐ethylhexyl) phthalate (DEHP) treatment in mice and rats, but not marmosets. Archives of Toxicology, 81, 219–226. [DOI] [PubMed] [Google Scholar]
- Iwabuchi K, Senzaki N, Mazawa D, Sato I, Hara M, Ueda F, Liu W and Tsuda S, 2017. Tissue toxicokinetics of perfluoro compounds with single and chronic low doses in male rats. Journal of Toxicological Sciences, 42, 301–317. 10.2131/jts.42.301 [DOI] [PubMed] [Google Scholar]
- Iwai H, 2011. Toxicokinetics of ammonium perfluorohexanoate. Drug and Chemical Toxicology, 34, 341–346. 10.3109/01480545.2011.585162 [DOI] [PubMed] [Google Scholar]
- Iwai H and Hoberman AM, 2014. Oral (Gavage) combined developmental and perinatal/postnatal reproduction toxicity study of ammonium salt of perfluorinated hexanoic acid in mice. International Journal of Toxicology, 33, 219–237. 10.1177/1091581814529449 [DOI] [PubMed] [Google Scholar]
- Iwai H, Hoberman AM, Goodrum PE, Mendelsohn E and Anderson JK, 2019. Addendum to Iwai and Hoberman (2014)—reassessment of developmental toxicity of PFHxA in mice. International Journal of Toxicology, 38, 183–191. [DOI] [PubMed] [Google Scholar]
- Iwase Y, Kudo N, Toyama T, Tamura M, Mitsumoto A and Kawashima Y, 2006. Effects of 8‐2 fluorotelomer alcohol on oleic acid formation in the liver of rats. Biological and Pharmaceutical Bulletin, 29, 1740–1746. [DOI] [PubMed] [Google Scholar]
- Jahnke A and Berger U, 2009. Trace analysis of per‐ and polyfluorinated alkyl substances in various matrices—How do current methods perform? Journal of Chromatography A, 1216, 410–421. 10.1016/j.chroma.2008.08.098 [DOI] [PubMed] [Google Scholar]
- Jain RB, 2013. Association between thyroid profile and perfluoroalkyl acids: data from NHANES 2007–2008. Environmental Research, 126, 51–59. 10.1016/j.envres.2013.08.006 [DOI] [PubMed] [Google Scholar]
- Jain RB, 2014. Contribution of diet and other factors to the levels of selected polyfluorinated compounds: data from NHANES 2003‐2008. International Journal of Hygiene and Environmental Health, 217, 52–61. [DOI] [PubMed] [Google Scholar]
- Jain RB, 2019. Concentration of selected liver enzymes across the stages of glomerular function: the associations with PFOA and PFOS. Heliyon, 5, e02168 10.1016/j.heliyon.2019.e02168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RB and Ducatman A, 2019. Roles of gender and obesity in defining correlations between perfluoroalkyl substances and lipid/lipoproteins. Science of the Total Environment, 653, 74–81. [DOI] [PubMed] [Google Scholar]
- Jakobsson K, Kronholm Diab K, Lindh C, Persson B and Jönsson B, 2014. Exponering för perfluorerade ämnen (PFAS) i dricksvatten i Ronneby kommun. Rapport 8/2014. Available online: https://sodrasjukvardsregionen.se/download/exponering-for-perfluorerade-amnen-pfas-i-dricksvatten-i-ronneby-kommun/
- James NH, Soames AR and Roberts RA, 1998. Suppression of hepatocyte apoptosis and induction of DNA synthesis by the rat and mouse hepatocarcinogen diethylhexylphthalate (DEHP) and the mouse hepatocarcinogen 1,4‐dichlorobenzene (DCB). Archives of Toxicology, 72, 784–790. [DOI] [PubMed] [Google Scholar]
- Jensen TK, Andersen LB, Kyhl HB, Nielsen F, Christesen HT and Grandjean P, 2015. Association between perfluorinated compound exposure and miscarriage in Danish pregnant women. PLoS ONE, 10, e0123496 10.1371/journal.pone.0123496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji K, Kim S, Kho Y, Paek D, Sakong J, Ha J, Kim S and Choi K, 2012. Serum concentrations of major perfluorinated compounds among the general population in Korea: dietary sources and potential impact on thyroid hormones. Environment International, 45, 78–85. 10.1016/j.envint.2012.03.007 [DOI] [PubMed] [Google Scholar]
- Jin H, Zhang Y, Jiang W, Zhu L and Martin JW, 2016. Isomer‐specific distribution of perfluoroalkyl substances in blood. Environmental Science & Technology, 50, 7808–7815. 10.1021/acs.est.6b01698 [DOI] [PubMed] [Google Scholar]
- Joensen UN, Bossi R, Leffers H, Jensen AA, Skakkebaek NE and Jorgensen N, 2009. Do perfluoroalkyl compounds impair human semen quality? Environmental Health Perspectives, 117, 923–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joensen UN, Veyrand B, Antignac JP, Blomberg Jensen M, Petersen JH, Marchand P, Skakkebæk NE, Andersson AM, Le Bizec B and Jørgensen N, 2013. PFOS (perfluorooctanesulfonate) in serum is negatively associated with testosterone levels, but not with semen quality, in healthy men. Human Reproduction, 28, 599–608. [DOI] [PubMed] [Google Scholar]
- Jogsten IE, Perello G, Llebaria X, Bigas E, Marti‐Cid R, Kärrman A and Domingo JL, 2009. Exposure to perfluorinated compounds in Catalonia, Spain, through consumption of various raw and cooked foodstuffs, including packaged food. Food and Chemical Toxicology, 47, 1577–1583. 10.1016/j.fct.2009.04.004 [DOI] [PubMed] [Google Scholar]
- Johansson N, Fredriksson A and Eriksson P, 2008. Neonatal exposure to perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) causes neurobehavioural defects in adult mice. Neurotoxicology, 29, 160–169. 10.1016/j.neuro.2007.10.008 [DOI] [PubMed] [Google Scholar]
- Johansson JH, Berger U, Vestergren R, Cousin IT, Bignert A, Glynn A and Darnerud PO, 2014. Temporal trends (1999‐2010) of perfluoroalkyl acids in commonly consumed food items. Environmental Pollution, 188, 102–108. 10.1016/j.envpol.2014.01.026 [DOI] [PubMed] [Google Scholar]
- Johnson PI, Sutton P, Atchley DS, Koustas E, Lam J, Sen S, Robinson KA, Axelrad DA and Woodruff TJ, 2014. The navigation guide‐evidence‐based medicine meets environmental health: systematic review of human evidence for PFOA effects on fetal growth. Environmental Health Perspectives, 122, 1028–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly RA, Goldstein KM, Wei T, Gao H, Chen P, Huang S, Colet JM, Ryan TP, Thomas CE and Estrem ST, 2005. Pooling samples within microarray studies: a comparative analysis of rat liver transcription response to prototypical toxicants. Physiological Genomics, 22, 346–355. [DOI] [PubMed] [Google Scholar]
- Jørgensen KT, Specht IO, Lenters V, Bach CC, Rylander L, Jönsson BAG, Lindh CH, Giwercman A, Heederik D, Toft G and Bonde JP, 2014. Perfluoroalkyl substances and time to pregnancy in couples from Greenland, Poland and Ukraine. Environmental Health, 13, 116 10.1186/1476-069X-13-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan K, Corsolini S, Falandysz J, Fillmann G, Kumar KS, Loganathan BG, Mohd MA, Olivero J, Van Wouwe N, Yang KH and Aldous KM, 2004. Perfluorooctanesulfonate and related fluorochemicals in human blood from several countries. Environmental Science & Technology, 38, 4489–4495. [DOI] [PubMed] [Google Scholar]
- Karlsen M, Grandjean P, Weihe P, Steuerwald U, Oulhote Y and Valvi D, 2016. Early‐life exposures to persistent organic pollutants in relation to overweight in preschool children. Reproductive Toxicology, 68, 145–153. 10.1016/j.reprotox.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kärrman A, van Bavel B, Jarnberg U, Hardell L and Lindstrom G, 2006. Perfluorinated chemicals in relation to other persistent organic pollutants in human blood. Chemosphere, 64, 1582–1591. 10.1016/j.chemosphere.2005.11.040 [DOI] [PubMed] [Google Scholar]
- Kärrman A, Ericson I, van Bavel B, Darnerud PO, Aune M, Glynn A, Lignell S and Lindström G, 2007. Exposure of perfluorinated chemicals through lactation: levels of matched human milk and serum and a temporal trend, 1996‐2004, in Sweden. Environmental Health Perspectives, 115, 226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataria A, Trachtman H, Malaga‐Dieguez L and Trasande L, 2015. Association between perfluoroalkyl acids and kidney function in a cross‐sectional study of adolescents. Environmental Health, 14, 89 10.1186/s12940-015-0077-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Wong LY, Jia LT, Kuklenyik Z and Calafat AM, 2011. Trends in exposure to polyfluoroalkyl chemicals in the U.S. Population: 1999‐2008. Environment Science and Technology, 45, 8037–8345. [DOI] [PubMed] [Google Scholar]
- Kato K, Wong LY, Chen A, Dunbar C, Webster GM, Lanphear BP and Calafat AM, 2014. Changes in serum concentrations of maternal poly‐ and perfluoroalkyl substances over the course of pregnancy and predictors of exposure in a multiethnic cohort of Cincinnati, Ohio pregnant women during 2003−2006. Environmental Science & Technology, 48, 9600–9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Ye X and Calafat AM, 2015a. PFASs in the general population In: De Witt JC, ed. Toxicological Effects of Perfluoroalkyl and Polyfluoralkyl Substances. Series: Molecular and Integrative Toxicology. Springer International Publishing, Switzerland, 51–76. 10.1007/978-3-319-15518-0_3 [DOI] [Google Scholar]
- Kato H, Fujii S, Takahashi M, Matsumoto M, Hirata‐Koizumi M, Ono A and Hirose A, 2015b. Repeated dose and reproductive/developmental toxicity of perfluorododecanoic acid in rats. Environmental Toxicology, 30, 1244–1263. 10.1002/tox.21996 [DOI] [PubMed] [Google Scholar]
- Kato K, Kalathil AA, Patel AM, Ye X and Calafat AM, 2018. Per‐ and polyfluoroalkyl substances and fluorinated alternatives in urine and serum by on‐line solid phase extraction‐liquid chromatography‐tandem mass spectrometry. Chemosphere, 209, 338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata K, Matsuzaki H, Nukui S, Okazaki M, Sakai A, Kawashima Y and Kudo N, 2017. Perfluorododecanoic acid induces cognitive deficit in adult rats. Toxicological Sciences, 157, 421–428. 10.1093/toxsci/kfx058 [DOI] [PubMed] [Google Scholar]
- Kawashima Y, Kobayashi H, Miura H and Kozuka H, 1995. Characterization of hepatic responses of rat to administration of perfluorooctanoic and perfluorodecanoic acids at low levels. Toxicology, 99, 169–178. [DOI] [PubMed] [Google Scholar]
- Kawashima H, Naganuma T, Kusunose E, Kono T, Yasumoto R, Sugimura K and Kishimoto T, 2000. Human fatty acid omegahydroxylase, CYP4A11: determination of complete genomic sequence and characterization of purified recombinant protein. Archives of Biochemistry and Biophysics., 378, 333–339. [DOI] [PubMed] [Google Scholar]
- Keil DE, Mehlmann T, Butterworth L and Peden‐Adams MM, 2008. Gestational exposure to perfluorooctane sulfonate suppresses immune function in B6C3F1 mice. Toxicological Sciences, 103, 77–85. [DOI] [PubMed] [Google Scholar]
- Kelly BC, Ikonomou MG, Blair JD, Surridge B, Hoover D, Grace R and Gobas FAPC, 2009. Perfluoroalkyl contaminants in an arctic marine food web: trophic magnification and wildlife exposure. Environmental Science & Technology, 43, 4037–4043. [DOI] [PubMed] [Google Scholar]
- Kersten S and Stienstra R, 2017. The role and regulation of the peroxisome proliferator activated receptor alpha in human liver. Biochimie, 136, 75–84. 10.1016/j.biochi.2016.12.019 [DOI] [PubMed] [Google Scholar]
- Khalil N, Chen A, Lee M, Czerwinski SA, Ebert JR, DeWitt JC and Kannan K, 2016. Association of perfluoroalkyl substances, bone mineral density, and osteoporosis in the U.S. population in NHANES 2009‐2010. Environmental Health Perspectives, 124, 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielsen K, Shamim Z, Ryder LP, Nielsen F, Grandjean P, Budtz‐Jorgensen E and Heilmann C, 2016a. Antibody response to booster vaccination with tetanus and diphtheria in adults exposed to perfluorinated alkylates. Journal of Immunotoxicology, 13, 270–273. 10.3109/1547691x.2015.1067259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Choi K, Ji K, Seo J, Kho Y, Park J, Kim S, Park S, Hwang I, Jeon J, Yang H and Giesy JP, 2011a. Trans‐placental transfer of thirteen perfluorinated compounds and relations with fetal thyroid hormones. Environmental Science & Technology, 45, 7465–7472. [DOI] [PubMed] [Google Scholar]
- Kim SK, Lee KT, Kang CS, Tao L, Kannan K, Kim KR, Kim CK, Lee JS, Park PS, Yoo YW, Ha JY, Shin YS and Lee JH, 2011b. Distribution of perfluorochemicals between sera and milk from the same mothers and implications for prenatal and postnatal exposures. Environmental Pollution, 159, 169–174. [DOI] [PubMed] [Google Scholar]
- Kim DH, Lee MY and Oh JE, 2014. Perfluorinated compounds in serum and urine samples from children aged 5‐13 years in South Korea. Environmental Pollution, 192, 171–178. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Heo SH, Lee DS, Hwang IG, Lee YB and Cho HY, 2016a. Gender differences in pharmacokinetics and tissue distribution of 3 perfluoroalkyl and polyfluoroalkyl substances in rats. Food and Chemical Toxicology, 97, 243–255. [DOI] [PubMed] [Google Scholar]
- Kim DH, Kim UJ, Kim HY, Choi SD and Oh JE, 2016b. Perfluoroalkyl substances in serum from South Korean infants with congenital hypothyroidism and healthy infants–Its relationship with thyroid hormones. Environmental Research, 147, 399–404. 10.1016/j.envres.2016.02.037 [DOI] [PubMed] [Google Scholar]
- Kim SJ, Shin H, Lee YB and Cho HY, 2018. Sex‐specific risk assessment of PFHxS using a physiologically based pharmacokinetic model. Archives of Toxicology, 92, 1113–1131. 10.1007/s00204-017-2116-5 [DOI] [PubMed] [Google Scholar]
- Kim SJ, Choi EJ, Choi GW, Lee YB and Cho HY, 2019. Exploring sex differences in human health risk assessment for PFNA and PFDA using a PBPK model. Archives of Toxicology, 93, 311–330. [DOI] [PubMed] [Google Scholar]
- Kissa E, 2001. Fluorinated surfactants and repellents, 2nd Edition. Surfactant Science Series, Volume 97. Marcel Dekker Inc., New York, 623 pp. ISBN: 0‐8247-0472‐X [Google Scholar]
- Klaunig JE, Shinohara M, Iwai H, Chengelis CP, Kirkpatrick JB, Wang Z and Bruner RH, 2015. Evaluation of the chronic toxicity and carcinogenicity of perfluorohexanoic acid (PFHxA) in Sprague‐Dawley rats. Toxicologic Pathology, 43, 209–220. [DOI] [PubMed] [Google Scholar]
- Köhrle J, 2008. Environment and endocrinology: the case of thyroidology. Annals of Endocrinology, 69, 116–122. [DOI] [PubMed] [Google Scholar]
- Koskela A, Finnilä MA, Korkalainen M, Spulber S, Koponen J, Hakansson H, Tuukkanen J and Viluksela M, 2016. Effects of developmental exposure to perfluorooctanoic acid (PFOA) on long bone morphology and bone cell differentiation. Toxicology and Applied Pharmacology, 301, 14–21. 10.1016/j.taap.2016.04.002 [DOI] [PubMed] [Google Scholar]
- Kotthoff M, Müller J, Jürling H, Schlummer M and Fiedler D, 2015. Perfluoroalkyl and polyfluoroalkyl substances in consumer products. Environmental Science and Pollution Research, 22, 14546–14549. 10.1007/s11356-015-4202-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk J, Ehlers S, Oberhausen A, Tischer M, Furst P, Schafft H and Lahrssen‐Wiederholt M, 2013. Absorption, distribution, and milk secretion of the perfluoroalkyl acids PFBS, PFHxS, PFOS, and PFOA by dairy cows fed naturally contaminated feed. Journal of Agricultural and Food Chemistry, 61, 2903–2912. 10.1021/jf304680j [DOI] [PubMed] [Google Scholar]
- Kratzer J, Ahrens L, Roos A, Bäcklin BM and Ebinghaus R, 2011. Temporal trends of polyfluoroalkyl compounds (PFCs) in liver tissue of grey seals (Halichoerus grypus) from the Baltic Sea, 1974–2008. Chemosphere, 85, 253–261. [DOI] [PubMed] [Google Scholar]
- Kudo N, Bandai N, Suzuki E, Katakura M and Kawashima Y, 2000. Induction by perfluorinated fatty acids with different carbon chain length of peroxisomal beta‐oxidation in the liver of rats. Chemico‐Biological Interactions, 124, 119–132. [DOI] [PubMed] [Google Scholar]
- Kudo N, Suzuki E, Katakura M, Ohmori K, Noshiro R and Kawashima Y, 2001. Comparison of the elimination between perfluorinated fatty acids with different carbon chain length in rats. Chemico‐Biological Interactions, 134, 203–216. [DOI] [PubMed] [Google Scholar]
- Kudo N, Katakura M, Sato Y and Kawashima Y, 2002. Sex hormone‐regulated renal transport of perfluorooctanoic acid. Chemico‐Biological Interactions, 139, 301–316. [DOI] [PubMed] [Google Scholar]
- Kudo N, Iwase Y, Okayachi H, Yamakawa Y and Kawashima Y, 2005. Induction of hepatic peroxisome proliferation by 8‐2 telomer alcohol feeding in mice: formation of perfluorooctanoic acid in the liver. Toxicological Sciences, 86, 231–238. [DOI] [PubMed] [Google Scholar]
- Kudo N, Suzuki‐Nakajima E, Mitsumoto A and Kawashima Y, 2006. Responses of the liver to perfluorinated fatty acids with different carbon chain length in male and female mice. In relation to induction of hepatomegaly, peroxisomal beta‐oxidation and microsomal 1‐acylglycerophosphocholine acyltransferase. Biological and Pharmaceutical Bulletin, 29, 1952–1957. 10.1248/bpb.29.1952 [DOI] [PubMed] [Google Scholar]
- Kurata Y, Kidachi F, Yokoyama M, Toyota N, Tsuchitani M and Katoh M, 1998. Subchronic toxicity of di(2‐ethylhexyl)phthalate in common marmosets: lack of hepatic peroxisome proliferation, testicular atrophy, or pancreatic acinar cell hyperplasia. Toxicological Sciences, 42, 49–56. [DOI] [PubMed] [Google Scholar]
- Kwon EJ, Shin JS, Kim BM, Shah‐Kulkarni S, Park H, Kho YL, Park EA, Kim YJ and Ha EH, 2016. Prenatal exposure to perfluorinated compounds affects birth weight through GSTM1 polymorphism. Journal of Occupational and Environmental Medicine, 58, e198–e205. 10.1097/JOM.0000000000000739 [DOI] [PubMed] [Google Scholar]
- Lacina O, Hradkova P, Pulkrabova J and Hajslova J, 2011. Simple, high throughput ultra‐high performance liquid chromatography/tandem mass spectrometry trace analysis of perfluorinated alkylated substances in food of animal origin: milk and fish. Journal of Chromatography A, 1218, 4312–4321. [DOI] [PubMed] [Google Scholar]
- Ladics GS, Kennedy GL, O'Connor J, Everds N, Malley LA, Frame SR, Gannon S, Jung R, Roth T, Iwai H and Shin‐ya S, 2008. 90‐day oral gavage toxicity study of 8‐2 fluorotelomer alcohol in rats. Drug and Chemical Toxicology, 31, 189–216. 10.1080/01480540701873103 [DOI] [PubMed] [Google Scholar]
- Lai KP, Ng AHM, Wan HL, Wong AYM, Leung CCT, Li R and Wong CKC, 2018. Dietary exposure to the environmental chemical, PFOS on the diversity of gut microbiota, associated with the development of metabolic syndrome. Frontiers in Microbiology, 9, 2552 10.3389/fmicb.2018.02552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laitinen JA, Koponen J, Koikkalainen J and Kiviranta H, 2014. Firefighters’ exposure to perfluoroalkyl acids and 2‐butoxyethanol present in firefighting foams. Toxicology Letters, 231, 227–232. [DOI] [PubMed] [Google Scholar]
- Land M, de Wit CA, Bignert A, Cousins IT, Herzke D, Johansson JH and Martin JW, 2018. What is the effect of phasing out long‐chain per‐ and polyfluoroalkyl substances on the concentrations of perfluoroalkyl acids and their precursors in the environment? A systematic review Environmental Evidence, 7, 4 10.1186/s13750-017-0114-y [DOI] [Google Scholar]
- Lange FT, Wenz M, Schmidt CK and Brauch HJ, 2007. Occurrence of perfluoroalkyl sulfonates and carboxylates in German drinking water sources compared to other countries. Water Science and Technology, 56, 151–158. [DOI] [PubMed] [Google Scholar]
- Lau C, Thibodeaux JR, Hanson RG, Rogers JM, Grey BE, Stanton ME, Butenhoff JL and Stevenson LA, 2003. Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse: II Postnatal evaluation. Toxicological Sciences, 74, 382–392. [DOI] [PubMed] [Google Scholar]
- Lau C, Thibodeaux JR, Hanson RG, Narotsky MG, Rogers JM, Lindstrom AB and Strynar MJ, 2006. Effects of perfluorooctanoic acid exposure during pregnancy in the mouse. Toxicological Sciences, 90, 510–518. [DOI] [PubMed] [Google Scholar]
- Lauritzen HB, Larose TL, Øien T, Odland JØ, van de Bor M, Jacobsen GW and Sandanger TM, 2016. Factors associated with maternal serum levels of perfluoroalkyl substances and organochlorines: a descriptive study of parous women in Norway and Sweden. PLoS ONE, 11, e0166127 10.1371/journal.pone.0166127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Menn G and Neels JG, 2018. Regulation of immune cell function by PPARs and the connection with metabolic and neurodegenerative diseases, 2018. International Journal of Molecular Sciences, 19, 1575. 10.3390/ijms19061575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK and Kim SH, 2018. Correlation between mast cell‐mediated allergic inflammation and length of perfluorinated compounds. Journal of Toxicology and Environmental Health, Part A, 81, 302–313. 10.1080/15287394.2018.1440188 [DOI] [PubMed] [Google Scholar]
- Lee I and Viberg H, 2013. A single neonatal exposure to perfluorohexane sulfonate (PFHxS) affects the levels of important neuroproteins in the developing mouse brain. Neurotoxicology, 37, 190–196. 10.1016/j.neuro.2013.05.007 [DOI] [PubMed] [Google Scholar]
- Lee SS, Pineau T, Drago J, Lee EJ, Owens JW, Kroetz DL, Fernandez‐Salguero PM, Westphal H and Gonzalez FJ, 1995. Targeted disruption of the alpha isoform of the peroxisome proliferator‐activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Molecular & Cellular Toxicology, 15, 3012–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, D'Eon J and Mabury SA, 2010. Biodegradation of polyfluoroalkyl phosphates as a source of perfluorinated acids to the environment. Environmental Science & Technology, 44, 3305–3310. 10.1021/es9028183 [DOI] [PubMed] [Google Scholar]
- Lee YJ, Kim M‐K, Bae J and Yang JH, 2013. Concentrations of perfluoroalkyl compounds in maternal and umbilical cord sera and birth outcomes in Korea. Chemosphere, 90, 1603–1609. [DOI] [PubMed] [Google Scholar]
- Lee CK, Kang SG, Lee JT, Lee SW, Kim JH, Kim DH, Son BC, Kim KH, Suh CH, Kim SY and Park YB, 2015. Effects of perfluorooctane sulfuric acid on placental PRL‐family hormone production and fetal growth retardation in mice. Molecular and Cellular Endocrinology, 401, 165–172. 10.1016/j.mce.2014.10.026 [DOI] [PubMed] [Google Scholar]
- Lee JK, Lee S, Choi YA, Jin ML, Kim YY, Kang BC, Kim MJ, Dhakal H, Lee SR, Kim SU, Khang S and Kim SH, 2018. Perfluorooctane sulfonate exacerbates mast cell‐mediated allergic inflammation by the release of histamine. Molecular & Cellular Toxicology, 14, 173–181. 10.1007/s13273-018-0019-z [DOI] [Google Scholar]
- Lefebvre DE, Curran I, Armstrong C, Coady L, Parenteau M, Liston V, Barker M, Aziz S, Rutherford K, Bellon‐ Gagnon P, Shenton J, Mehta R and Bondy G, 2008. Immunomodulatory effects of dietary potassium perfluorooctane sulfonate (PFOS) exposure in adult sprague‐dawley rats. Journal of Toxicology and Environmental Health‐Part A‐Current Issues, 71, 1516–1525. [DOI] [PubMed] [Google Scholar]
- Lenters V, Portengen L, Rignell‐Hydbom A, Jonsson BA, Lindh CH, Piersma AH, Toft G, Bonde JP, Heederik D, Rylander L and Vermeulen R, 2016. Prenatal phthalate, perfluoroalkyl acid, and organochlorine exposures and term birth weight in three birth cohorts: multi‐pollutant models based on elastic net regression. Environmental Health Perspectives, 124, 365–372. 10.1289/ehp.1408933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letcher RJ, Su G, Moore JN, Williams LL, Martin PA, de Solla SR and Bowerman WW, 2015. Perfluorinated sulfonate and carboxylate compounds and precursors in herring gull eggs from across the Laurentian Great Lakes of North America: temporal and recent spatial comparisons and exposure implications. Science of the Total Environment, 538, 468–477. 10.1016/j.scitotenv.2015.08.083 [DOI] [PubMed] [Google Scholar]
- Lewis RC, Johns LE and Meeker JD, 2015. Serum biomarkers of exposure to perfluoroalkyl substances in relation to serum testosterone and measures of thyroid function among adults and adolescents from NHANES 2011‐2012. International Journal of Environmental Research and Public Health, 12, 6098–6114. 10.3390/ijerph120606098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Ramdhan DH, Naito H, Yamagishi N, Ito Y, Hayashi Y, Yanagiba Y, Okamura A, Tamada H, Gonzalez FJ and Nakajima T, 2011. Ammonium perfluorooctanoate may cause testosterone reduction by adversely affecting testis in relation to PPARα. Toxicology Letters, 205, 265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Guo F, Wang Y, Zhang J, Zhong Y, Zhao Y and Wu Y, 2013. Can nail, hair and urine be used for biomonitoring of human exposure to perfluorooctane sulfonate and perfluorooctanoic acid? Environmental International, 53, 47–52. [DOI] [PubMed] [Google Scholar]
- Li Y, Cheng Y, Xie Z and Zeng F, 2017. Perfluorinated alkyl substances in serum of the southern Chinese general population and potential impact on thyroid hormones. Scientific Reports, 7, 43380 10.1038/srep43380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Fletcher T, Mucs D, Scott K, Lindh CH, Tallving P and Jakobsson K, 2018a. Half‐lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occupational and Environmental Medicine, 75, 46–51. 10.1136/oemed-2017-104651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Song P, Liu L and Wang X, 2018b. Perfluorooctanoic acid exposure during pregnancy alters the apoptosis of uterine cells in pregnant mice. I International Journal of Clinical and Experimental Pathology, 11, 5602–5611. [PMC free article] [PubMed] [Google Scholar]
- Li X, Wang Z and Klaunig JE, 2019a. The effects of perfluorooctanoate on high fat diet induced non‐alcoholic fatty liver disease in mice. Toxicology, 416, 1–14. 10.1016/j.tox.2019.01.017 [DOI] [PubMed] [Google Scholar]
- Li D, Zhang L, Zhang Y, Guan S, Gong X and Wang X, 2019b. Maternal exposure to perfluorooctanoic acid (PFOA) causes liver toxicity through PPAR‐alpha pathway and lowered histone acetylation in female offspring mice. Environmental Science and Pollution Research, 26, 18866–18875. [DOI] [PubMed] [Google Scholar]
- Li CH, Ren X‐M, Cao L‐Y, Qina W‐P and Guo L‐H, 2019c. Investigation of binding and activity of perfluoroalkyl substances to the human peroxisome proliferator‐activated receptor β/δ. Environmental Science: Processes & Impacts, 21, 1908–1914. [DOI] [PubMed] [Google Scholar]
- Li Y, Barregard L, Xu Y, Scott K, Pineda D, Lindh CH, Jakobsson K and Fletcher T, 2020. Associations between perfluoroalkyl substances and serum lipids in a Swedish adult population with contaminated drinking water. Environmental Health, 19, 33 10.1186/s12940-020-00588-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Xie G, Wu X, Su M and Yang B, 2019. Effect of prenatal PFOS exposure on liver cell function in neonatal mice. Environmental Science and Pollution, 26, 18240–18246. 10.1007/s11356-019-05245-4 [DOI] [PubMed] [Google Scholar]
- Lieder PH, Chang SC, York RG and Butenhoff JL, 2009a. Toxicological evaluation of potassium perfluorobutanesulfonate in a 90‐day oral gavage study with Sprague‐Dawley rats. Toxicology, 255, 45–52. 10.1016/j.tox.2008.10.002 [DOI] [PubMed] [Google Scholar]
- Lieder PH, York RG, Hakes DC, Chang SC and Butenhoff JL, 2009b. A two‐generation oral gavage reproduction study with potassium perfluorobutanesulfonate (K+PFBS) in Sprague Dawley rats. Toxicology, 259, 33–45. [DOI] [PubMed] [Google Scholar]
- Lien GW, Huang CC, Shiu JS, Chen MH, Hsieh WS, Guo YL and Chen PC, 2016. Perfluoroalkyl substances in cord blood and attention deficit/hyperactivity disorder symptoms in seven‐year‐old children. Chemosphere, 156, 118–127. 10.1016/j.chemosphere.2016.04.102 [DOI] [PubMed] [Google Scholar]
- Liew Z, Ritz B, Bonefeld‐Jorgensen EC, Henriksen TB, Nohr EA, Bech BH, Fei C, Bossi R, von Ehrenstein OS, Streja E, Uldall P and Olsen J, 2014. Prenatal exposure to perfluoroalkyl substances and the risk of congenital cerebral palsy in children. American Journal of Epidemiology, 180, 574–581. 10.1093/aje/kwu179 [DOI] [PubMed] [Google Scholar]
- Liew Z, Ritz B, von Ehrenstein OS, Bech BH, Nohr EA, Fei C, Bossi R, Henriksen TB, Bonefeld‐Jørgensen EC and Olsen J, 2015. Attention deficit/hyperactivity disorder and childhood autism in association with prenatal exposure to perfluoroalkyl substances: a nested case‐control study in the Danish National Birth Cohort. Environmental Health Perspectives, 123, 367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilja K, Norström K, Remberger M, Kaj L, Egelrud L, Junedahl E, Brorström‐Lundén E, Ghebremeskel M and Schlabach M, 2009. Screening of Selected Hazardous Substances in the Eastern Baltic Marine Environment. IVL Swedish Environmental Research Institute Ltd. B1874, Project report NCM/ HELCOM “Screening of hazardous substances in the eastern Baltic Sea”, 57 p. Available online: https://www.ivl.se/download/18.343dc99d14e8bb0f58b7598/1445517412208/B1874.pdf
- Lin CY, Chen PC, Lin YC and Lin LY, 2009. Association among serum perfluoroalkyl chemicals, glucose homeostasis, and metabolic syndrome in adolescents and adults. Diabetes Care, 32, 702–707. 10.2337/dc08-1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CY, Lin LY, Chiang CK, Wang WJ, Su YN, Hung KY and Chen PC, 2010. Investigation of the associations between low‐dose serum perfluorinated chemicals and liver enzymes in US adults. Am Journal of Gastroenterology, 105, 1354–1363. 10.1038/ajg.2009.707 [DOI] [PubMed] [Google Scholar]
- Lin CY, Wen LL, Lin LY, Wen TW, Lien GW, Chen CY, Hsu SH, Chien KL, Sung FC, Chen PC and Su TC, 2011. Associations between levels of serum perfluorinated chemicals and adiponectin in a young hypertension cohort in Taiwan. Environmental Science & Technology, 45, 10691–10698. 10.1021/es201964x [DOI] [PubMed] [Google Scholar]
- Lin CY, Wen LL, Lin LY, Wen TW, Lien GW, Hsu SH, Chien KL, Liao CC, Sung FC, Chen PC and Su TC, 2013a. The associations between serum perfluorinated chemicals and thyroid function in adolescents and young adults. Journal of Hazardous Materials, 244–245, 637–644. 10.1016/j.jhazmat.2012.10.049 [DOI] [PubMed] [Google Scholar]
- Lin C‐Y, Lin L‐Y, Wen T‐W, Lien G‐W, Chien K‐L, Hsu SHJ, Liao C‐C, Sung F‐C, Chen P‐C and Su T‐C, 2013b. Association between levels of serum perfluorooctane sulfate and carotid artery intima–media thickness in adolescents and young adults. International Journal of Cardiology, 168, 3309–3316. 10.1016/j.ijcard.2013.04.042 [DOI] [PubMed] [Google Scholar]
- Lin CY, Chen PC, Lo SC, Torng PL, Sung FC and Su TC, 2016. The association of carotid intima‐media thickness with serum Level of perfluorinated chemicals and endothelium‐platelet microparticles in adolescents and young adults. Environment International, 94, 292–299. 10.1016/j.envint.2016.06.004 [DOI] [PubMed] [Google Scholar]
- Lin PD, Cardenas A, Hauser R, Gold DR, Kleinman KP, Hivert MF, Fleisch AF, Calafat AM, Webster TF, Horton ES and Oken E, 2019. Per‐ and polyfluoroalkyl substances and blood lipid levels in pre‐diabetic adults‐longitudinal analysis of the diabetes prevention program outcomes study. Environment International, 129, 343–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind L, Zethelius B, Salihovic S, van Bavel B and Lind PM, 2014. Circulating levels of perfluoroalkyl substances and prevalent diabetes in the elderly. Diabetologia, 57, 473–479. 10.1007/s00125-013-3126-3 [DOI] [PubMed] [Google Scholar]
- Lind DV, Priskorn L, Harmer Lassen T, Nielsen F, Boye Kyhl H, Møbjerg Kristensen D, Christesen HT, Jørgensen JS, Grandjean P and Kold Jensen T, 2017a. Prenatal exposure to perfluoroalkyl substances and anogenital distance at 3 months of age in a Danish mother‐child cohort. Reproductive Toxicology, 68, 200–206. 10.1016/j.reprotox.2016.08.019 [DOI] [PubMed] [Google Scholar]
- Lind PM, Salihovic S, van Bavel B and Lind L, 2017b. Circulating levels of perfluoroalkyl substances (PFASs) and carotid artery Atherosclerosis. Environmental Research, 152, 157–164. 10.1016/j.envres.2016.10.002 [DOI] [PubMed] [Google Scholar]
- Lindstrom AB, Strynar MJ and Laurence EL, 2011. Polyfluorinated compounds: past, present, and future. Environmental Science & Technology, 45, 7954–7961. [DOI] [PubMed] [Google Scholar]
- Liu J, Li J, Liu Y, Chan HM, Zhao Y, Cai Z and Wu Y, 2011. Comparison on gestation and lactation exposure of perfluorinated compounds for newborns. Environment International, 37, 1206–1212. [DOI] [PubMed] [Google Scholar]
- Liu X, Guo Z, Krebs KA, Pope RH and Roache NF, 2014a. Concentrations and trends of perfluorinated chemicals in potential indoor sources from 2007 through 2011 in the US. Chemosphere, 98, 51–57. [DOI] [PubMed] [Google Scholar]
- Liu C, Chang VW, Gin KY and Nguyen VT, 2014b. Genotoxicity of perfluorinated chemicals (PFCs) to the green mussel (Perna viridis). Science of the Total Environment, 487, 117–122. 10.1016/j.scitotenv.2014.04.017 [DOI] [PubMed] [Google Scholar]
- Liu Y, Pereira AS, Beesoon S, Vestergren R, Berger U, Olsen GW, Glynn A and Martin JW, 2015. Temporal trends of perfluorooctanesulfonate isomer and enantiomer patterns in archived Swedish and American serum samples. Environment International, 75, 215–222. [DOI] [PubMed] [Google Scholar]
- Liu H, Zhang H, Cui R, Guo X, Wang D and Dai J, 2016. Activation of peroxisome proliferator‐activated receptor α ameliorates perfluorododecanoic acid‐induced production of reactive oxygen species in rat liver. Archives of Toxicology, 90, 1383–1397. [DOI] [PubMed] [Google Scholar]
- Liu X, Fang M, Xu F and Chen D, 2019. Characterization of the binding of per‐ and poly‐fluorinated. Trends in Analytical Chemistry, 116, 177–185. 10.1016/j.trac.2019.05.017 [DOI] [Google Scholar]
- Loccisano AE, Campbell JL Jr, Andersen ME and Clewell HJ, 2011. Evaluation and prediction of pharmacokinetics of PFOA and PFOS in the monkey and human using a PBPK model. Regulatory Toxicology and Pharmacology, 59, 157–175. 10.1016/j.yrtph.2010.12.004 [DOI] [PubMed] [Google Scholar]
- Loccisano AE, Longnecker MP, Campbell JL Jr, Andersen ME and Clewell HJ, 2013. Development of PBPK models for PFOA and PFOS for human pregnancy and lactation life stages. Journal of Toxicology and Environmental Health. Part A, 76, 25–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löfstedt Gilliam J, Leonel J, Cousins IT and Benskin JP, 2016. Is Ongoing Sulfluramid Use in South America a Significant Source of Perfluorooctanesulfonate (PFOS)? Production Inventories, Environmental Fate, and Local Occurrence. Environmental Science & Technology, 50, 653–659. [DOI] [PubMed] [Google Scholar]
- Löfstrand K, Jorundsdottir H, Tomy G, Svavarsson J, Weihe P, Nygard T and Bergman K, 2008. Spatial trends of polyfluorinated compounds in guillemot (Uria aalge) eggs from North‐Western Europe. Chemosphere, 72, 1475–1480. 10.1016/j.chemosphere.2008.05.011 [DOI] [PubMed] [Google Scholar]
- Long M, Ghisari M and Bonefeld‐Jørgensen EC, 2013. Effects of perfluoroalkyl acids on the function of the thyroid hormone and the aryl hydrocarbon receptor. Environmental Science and Pollution Research, 20, 8045–8056. 10.1007/s11356-013-1628-7 [DOI] [PubMed] [Google Scholar]
- Long M, Knudsen AK, Pedersen HS and Bonefeld‐Jørgensen EC, 2015. Food intake and serum persistent organic pollutants in the Greenlandic pregnant women: the ACCEPT sub‐study. Science of the Total Environment, 529, 198–212. [DOI] [PubMed] [Google Scholar]
- Long M, Ghisari M, Kjeldsen L, Wielsøe M, Nørgaard‐Pedersen B, Lykke Mortensen E, Abdallah MW and Bonefeld‐Jørgensen EC, 2019. Autism spectrum disorders, endocrine disrupting compounds, and heavy metals in amniotic fluid: a case‐control study. Molecular Autism, 10, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looker C, Luster MI, Calafat AM, Johnson VJ, Burleson GR, Burleson FG and Fletcher T, 2014. Influenza vaccine response in adults exposed to perfluorooctanoate and perfluorooctanesulfonate. Toxicological Sciences, 138, 76–88. 10.1093/toxsci/kft269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez‐Espinosa MJ, Mondal D, Armstrong B, Bloom MS and Fletcher T, 2012. Thyroid function and perfluoroalkyl acids in children living near a chemical plant. Environmental Health Perspectives, 120, 1036–1041. 10.1289/ehp.1104370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo M, Campo J, Farré M, Pérez F, Picó Y and Barceló D, 2016. Perfluoroalkyl substances in the Ebro and Guadalquivir river basins (Spain). Science of the Total Environment, 540, 191–199. 10.1016/j.scitotenv.2015.07.045 [DOI] [PubMed] [Google Scholar]
- Loveless SE, Hoban D, Sykes G, Frame SR and Everds NE, 2008. Evaluation of the immune system in rats and mice administered linear ammonium perfluorooctanoate. Toxicological Sciences, 105, 86–96. [DOI] [PubMed] [Google Scholar]
- Loveless SE, Slezak B, Serex T, Lewis J, Mukerji P, O'Connor JC, Donner EM, Frame SR, Korzeniowski SH and Buck RC, 2009. Toxicological evaluation of sodium perfluorohexanoate. Toxicology, 264, 32–44. [DOI] [PubMed] [Google Scholar]
- Lu Z, Lu R, Zheng H, Yan J, Song L, Wang J, Yang H and Cai M, 2018. Risk exposure assessment of per‐ and polyfluoroalkyl substances (PFASs) in drinking water and atmosphere in central eastern China. Environmental Science and Pollution Research, 25, 9311–9320. 10.1007/s11356-017-0950-x [DOI] [PubMed] [Google Scholar]
- Luebker DJ, Hansen KJ, Bass NM, Butenhoff JL and Seacat AM, 2002. Interactions of fluorochemicals with rat liver fatty acid‐binding protein. Toxicology, 176, 175–185. [DOI] [PubMed] [Google Scholar]
- Luebker DJ, Case MT, York RG, Moore JA, Hansen KJ and Butenhoff JL, 2005a. Two‐generation reproduction and cross‐foster studies of perfluorooctanesulfonate (PFOS) in rats. Toxicology, 215, 126–148. [DOI] [PubMed] [Google Scholar]
- Luebker DJ, York RG, Hansen KJ, Moore JA and Butenhoff JL, 2005b. Neonatal mortality from in utero exposure to perfluorooctanesulfonate (PFOS) in Sprague‐Dawley rats: dose‐response, and biochemical and pharamacokinetic parameters. Toxicology, 215, 149–169. [DOI] [PubMed] [Google Scholar]
- Luo M, Tan Z, Dai M, Song D, Lin J, Xie M, Yang J, Sun L, Wei D, Zhao J, Gonzalez FJ and Liu A, 2017. Dual action of peroxisome proliferator‐activated receptor alpha in perfluorodecanoic acid‐induced hepatotoxicity. Archives of Toxicology, 91, 897–907. 10.1007/s00204-016-1779-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luster MI, Portier C, Pait DG, Rosenthal GJ, Germolec DR, Corsini E, Blaylock BL, Pollock P, Craig Y and Kouchi W, 1993. Risk assessment in immunotoxicology. II. Relationships between immune and host resistance tests. Fundamental and Applied Toxicology, 21, 71–82. [DOI] [PubMed] [Google Scholar]
- Lv N, Zhao M, Han Y, Cui L, Zhong W, Wang C and Jiang Q, 2018. The roles of bone morphogenetic protein 2 in perfluorooctanoic acid induced developmental cardiotoxicity and l‐carnitine mediated protection. Toxicology and Applied Pharmacology, 352, 68–76. 10.1016/j.taap.2018.05.028 [DOI] [PubMed] [Google Scholar]
- Ma X, Stoffregen DA, Wheelock GD, Rininger JA and Babish JG, 1997a. Discordant hepatic expression of the cell division control enzyme p34cdc2 kinase, proliferating cell nuclear antigen, p53 tumor suppressor protein, and p21Waf1 cyclin‐dependent kinase inhibitory protein after WY14,643 ([4‐chloro‐6‐(2,3‐xylidino)‐2‐pyrimidinylthio]acetic acid) dosing to rats. Molecular Pharmacology, 51, 69–78. [DOI] [PubMed] [Google Scholar]
- Ma Y, Sachdeva K, Liu J, Song X, Li Y, Yang D, Deng R, Chichester CO and Yan B, 2005. Clofibrate and perfluorodecanoate both upregulate the expression of the pregnane X receptor but oppositely affect its ligand‐dependent induction on cytochrome P450 3A23. Biochemical Pharmacology, 69, 1363–1371. [DOI] [PubMed] [Google Scholar]
- Macias H and Hinck L, 2012. Mammary gland development. Wiley Interdisciplinary Reviews‐Developmental Biology, 1, 533–557. 10.1002/wdev.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacManus‐Spencer LA, Tse ML, Hebert PC, Bischel HN and Luthy RG, 2010. Binding of perfluorocarboxylates to serum albumin: a comparison of analytical methods. Toxicology and Chemistry, 30, 2423–2430. 10.1002/etc.647 [DOI] [PubMed] [Google Scholar]
- Macon MB, Villanueva LR, Tatum‐Gibbs K, Zehr RD, Strynar MJ, Stanko JP, White SS, Helfant L and Fenton SE, 2011. Prenatal perfluorooctanoic acid exposure in CD‐1 mice: low‐dose developmental effects and internal dosimetry. Toxicological Sciences, 122, 134–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher JM, Aleksunes LM, Dieter MZ, Tanaka Y, Peters JM, Manautou JE and Klaassen CD, 2008. Nrf2‐ and PPAR alpha‐mediated regulation of hepatic Mrp transporters after exposure to perfluorooctanoic acid and perfluorodecanoic acid. Toxicological Sciences, 106, 319–328. 10.1093/toxsci/kfn177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonet M, Terrell ML, McGeehin MA, Christensen KY, Holmes A, Calafat AM and Marcus M, 2012. Maternal concentrations of polyfluoroalkyl compounds during pregnancy and fetal and postnatal growth in British girls. Environmental Health Perspectives, 120, 1432–1437. 10.1289/ehp.1003096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini FR, Cano‐Sancho G, Gambaretti J, Marchand P, Boutron‐Ruault MC, Severi G, Arveux P, Antignac JP and Kvaskoff M, 2019. Perfluorinated alkylated substances serum concentration and breast cancer risk: Evidence from a nested case‐control study in the French E3N cohort. International Journal of Cancer, 146, 917–928. 10.1002/ijc.32357 [DOI] [PubMed] [Google Scholar]
- Manzano‐Salgado CB, Casas M, Lopez‐Espinosa MJ, Ballester F, Iniguez C, Martinez D, Costa O, Santa‐Marina L, Pereda‐Pereda E, Schettgen T, Sunyer J and Vrijheid M, 2017a. Prenatal exposure to perfluoroalkyl substances and birth outcomes in a Spanish birth cohort. Environment International, 108, 278–284. 10.1016/j.envint.2017.09.006 [DOI] [PubMed] [Google Scholar]
- Manzano‐Salgado CB, Casas M, Lopez‐Espinosa MJ, Ballester F, Iniguez C, Martinez D, Romaguera D, Fernández‐Barrés S, Santa‐Marina L, Basterretxea M, Schettgen T, Valvi D, Vioque J, Sunyer J and Vrijheid M, 2017b. Prenatal exposure to perfluoroalkyl substances and cardiometabolic risk in children from the Spanish INMA Birth Cohort Study. Environmental Health Perspectives, 125, 097018 10.1289/EHP1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzano‐Salgado CB, Granum B, Lopez‐Espinosa MJ, Ballester F, Iñiguez C, Gascón M, Martínez D, Guxens M, Basterretxea M, Zabaleta C, Schettgen T, Sunyer J, Vrijheid M and Casas M, 2019. Prenatal exposure to perfluoroalkyl substances, immune‐related outcomes, and lung function in children from a Spanish birth cohort study. International Journal of Hygiene and Environmental Health, 222, 945–954. [DOI] [PubMed] [Google Scholar]
- Marks KJ, Cutler AJ, Jeddy Z, Northstone K, Kato K and Hartman TJ, 2019. Maternal serum concentrations of perfluoroalkyl substances and birth size in British boys. International Journal of Hygiene and Environmental Health, 222, 889–895. 10.1016/j.ijheh.2019.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JW, Mabury SA, Solomon KR and Muir DCG, 2003a. Dietary accumulation of perfluorinated acids in juvenile rainbow trout (Oncorhynchus Mykiss). Environmental Toxicology and Chemistry, 22, 189–195. [PubMed] [Google Scholar]
- Martin JW, Mabury SA, Solomon KR and Muir DCG, 2003b. Bioconcentration and tissue distribution of perfluorinated acids in rainbow trout (Oncorhynchus Mykiss). Environmental Toxicology and Chemistry, 22, 196–204. [PubMed] [Google Scholar]
- Martin JW, Mabury SA and O'Brien PJ, 2005. Metabolic products and pathways of fluorotelomer alcohols in isolated rat hepatocytes. Chemical‐Biological Interactions, 155, 165–180. 10.1016/j.cbi.2005.06.007 [DOI] [PubMed] [Google Scholar]
- Martin JW, Ellis DA, Mabury SA, Hurley MD and Wallington TJ, 2006. Atmospheric chemistry of perfluoroalkanesulfonamides: kinetic and product studies of the OH radical and Cl atom initiated oxidation of N‐ethyl perfluorobutanesulfonamide. Environmental Science & Technology, 40, 864–872. [DOI] [PubMed] [Google Scholar]
- Martin JW, Asher BJ, Beesoon S, Benskin JP and Ross MS, 2010. PFOS or PreFOS? Are perfluorooctane sulfonate precursors (PreFOS) important determinants of human and environmental perfluorooctane sulfonate (PFOS) exposure? Journal of Environmental Monitoring, 12, 1979–2004. [DOI] [PubMed] [Google Scholar]
- Martinson HA, Lyons TR, Giles ED, Borges VF and Schedin P, 2013. Developmental windows of breast cancer risk provide opportunities for targeted chemoprevention. Experimental Cell Research, 319, 1671–1678. 10.1016/j.yexcr.2013.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrantonio M, Bai E, Uccelli R, Cordiano V, Screpanti A and Crosignani P, 2018. Drinking water contamination from perfluoroalkyl substances (PFAS): an ecological mortality study in the Veneto Region, Italy. European Journal of Public Health, 28, 180–185. 10.1093/eurpub/ckx066 [DOI] [PubMed] [Google Scholar]
- Mattsson K, Rignell‐Hydbom A, Holmberg S, Thelin A, Jonsson BA, Lindh CH, Sehlstedt A and Rylander L, 2015. Levels of perfluoroalkyl substances and risk of coronary heart disease: findings from a population‐based longitudinal study. Environmental Research, 142, 148–154. 10.1016/j.envres.2015.06.033 [DOI] [PubMed] [Google Scholar]
- MDH (Minnesota Department of Health), 2012. Perfluorochemical contamination in southern Washington county, northern Dakota county, and southeastern Ramsey county. Minnesota, US DHHS ATSDR, Atlanta: Available online: http://www.health.state.mn.us/divs/eh/hazardous/topics/pfcs/pha/woodbury/pha3m0112.pdf [Google Scholar]
- Meng Q, Inour K, Ritz B, Olsen J and Liew Z, 2018. Prenatal exposure to perfluroralkyl substances and birth outcomes: an updated analysis from the Danish National Birth Cohort. International Journal of Environmental Research and Public Health, 15, 1832 10.3390/ijerph15091832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michigan Science Advisory Workgroup 71 , 2019. Health‐based drinking water value recommendations for PFAS in Michigan. Available online: https://www.michigan.gov/documents/pfasresponse/Health-Based_Drinking_Water_Value_Recommendations_for_PFAS_in_Michigan_Report_659258_7.pdf
- Midgett K, Peden‐Adams MM, Gilkeson GS and Kamen DL, 2015. In vitro evaluation of the effects of perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) on IL‐2 production in human T‐cells. Journal of Applied Toxicology, 35, 459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minata M, Harada KH, Kärrman A, Hitomi T, Hirosawa M, Murata M, Gonzalez FJ and Koizumi A, 2010. Role of peroxisome proliferator‐activated receptor‐alpha in hepatobiliary injury induced by ammonium perfluorooctanoate in mouse liver. Industrial Health, 48, 96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miralles‐Marco A and Harrad S, 2015. Perfluorooctane sulfonate: a review of human exposure, biomonitoring and the environmental forensics utility of its chirality and isomer distribution. Environment International, 77, 148–159. [DOI] [PubMed] [Google Scholar]
- Mollenhauer MAM, Bradshaw SG, Fair PA, McGuinn WD and Peden‐Adams MM, 2011. Effects of perfluorooctane sulfonate (PFOS) exposure on markers of inflammation in female B6C3F1 mice. Journal of Environmental Science and Health Part A‐Toxic/Hazardous Substances & Environmental Engineering, 46, 97–108. [DOI] [PubMed] [Google Scholar]
- Möller A, Ahrens L, Surm R, Westerveld J, van der Wielen F, Ebinghaus R and de Voogt P, 2010. Distribution and sources of polyfluoroalkyl substances (PFAS) in the River Rhine watershed. Environmental Pollution, 158, 3243–3250. 10.1016/j.envpol.2010.07.019 [DOI] [PubMed] [Google Scholar]
- Mondal D, Weldon RH, Armstrong BG, Gibson LJ, Lopez‐Espinosa MJ, Shin HM and Fletcher T, 2014. Breastfeeding: a potential excretion route for mothers and implications for infant exposure to perfluoroalkyl acids. Environmental Health Perspectives, 122, 187–192. 10.1289/ehp.1306613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora AM, Oken E, Rifas‐Shiman SL, Webster TF, Gillman MW, Calafat AM, Ye X and Sagiv SK, 2017. Prenatal exposure to perfluoroalkyl substances and adiposity in early and mid‐childhood. Environmental Health Perspectives, 125, 467–473. 10.1289/ehp246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mørck TA, Nielsen F, Nielsen JKS, Siersma VD, Grandjean P and Knudsen LE, 2015. PFAS concentrations in plasma samples from Danish school children and their mothers. Chemosphere, 129, 203–209. 10.1016/j.chemosphere.2014.07.018 [DOI] [PubMed] [Google Scholar]
- Müeller CE, De Silva AO, Small J, Williamson M, Wang X, Morris A, Katz S, Gamberg M and Muir DCG, 2011. Biomagnification of perfluorinated compounds in a remote terrestrial food chain: Lichen‐Caribou‐Wolf. Environmental Science & Technology, 45, 8665–8673. [DOI] [PubMed] [Google Scholar]
- Munoz G, Giraudel JL, Botta F, Lestremau F, Devier MH, Budzinski H and Labadie P, 2015. Spatial distribution and partitioning behavior of selected poly‐ and perfluoroalkyl substances in freshwater ecosystems: a French nationwide survey. Science of the Total Environment, 517, 48–56. 10.1016/j.scitotenv.2015.02.043 [DOI] [PubMed] [Google Scholar]
- Mylchreest E, Munley SM and Kennedy GL Jr, 2005. Evaluation of the developmental toxicity of 8‐2 telomer B alcohol. Drug and Chemical Toxicology, 28, 315–328. 10.1081/dct-200064491 [DOI] [PubMed] [Google Scholar]
- Nabb DL, Szostek B, Himmelstein MW, Mawn MP, Gargas ML, Sweeney LM, Stadler GC, Buck RC and Fasano WJ, 2007. In vitro metabolism of 8‐2 fluorotelomer alcohol: interspecies comparisons and metabolic pathway refinement. Toxicological Sciences, 100, 333–344. [DOI] [PubMed] [Google Scholar]
- Naile JE, Wiseman S, Bachtold K, Jones PD and Giesy JP, 2012. Transcriptional effects of perfluorinated compounds in rat hepatoma cells. Chemosphere, 86, 270–277. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Ramdhan DH, Tanaka N, Naito H, Tamada H, Ito Y, Li Y, Hayashi Y, Yamagishi N, Yanagiba Y, Aoyama T, Gonzalez FJ and Nakajima T, 2012. Modulation of ammonium perfluorooctanoate‐induced hepatic damage by genetically different PPARa in mice. Archives of Toxicology, 86, 63–74. 10.1007/s00204-011-0704-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Ito Y, Yanagiba Y, Ramdhan DH, Kono Y, Naito H, Hayashi Y, Li Y, Aoyama T, Gonzalez FJ and Nakajima T, 2009. Microgram‐order ammonium perfluorooctanoate may activate mouse peroxisome proliferator‐activated receptor alpha, but not human PPARalpha. Toxicology, 265, 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negri E, Metruccio F, Guercio V, Tosti L, Benfenati E, Bonzi R, La Vecchia C and Moretto A, 2017. Exposure to PFOA and PFOS and fetal growth: a critical merging of toxicological and epidemiological data. Critical Reviews in Toxicology, 47, 489–515. [DOI] [PubMed] [Google Scholar]
- Nelson JW, Hatch EE and Webster TF, 2010. Exposure to polyfluoroalkyl chemicals and cholesterol, body weight, and insulin resistance in the general U.S. population. Environmental Health Perspectives, 118, 197–202. 10.1289/ehp.0901165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JW, Scammell MK, Hatch EE and Webster TF, 2012. Social disparities in exposures to bisphenol A and polyfluoroalkyl chemicals: a cross‐sectional study within NHANES 2003‐2006. Environmental Health, 11, 10 10.1186/1476-069X-11-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng CA and Hungerbühler K, 2014. Bioaccumulation of perfluorinated alkyl acids: observations and models. Environmental Science & Technology, 48, 4637–4648. 10.1021/es404008g [DOI] [PubMed] [Google Scholar]
- Ngo HT, Hetland RB, Sabaredzovic A, Haug LS and Steffensen IL, 2014. In utero exposure to perfluorooctanoate (PFOA) or perfluorooctane sulfonate (PFOS) did not increase body weight or intestinal tumorigenesis in multiple intestinal neoplasia (Min/+) mice. Environmental Research, 132, 251–263. [DOI] [PubMed] [Google Scholar]
- Nian M, Li Q‐Q, Bloom M, Qian Z, Syberg KM, Vaughn MG, Wang S‐Q, Wei Q, Zeeshan M, Gurram N, Chu C, Wang J, Tian Y‐P, Hu L‐W, Liu K‐K, Yang B‐Y, Liu R‐Q, Feng D, Zeng X‐W and Dong G‐H, 2019. Liver function biomarkers disorder is associated with exposure to perfluoroalkyl acids in adults: isomers of C8 Health Project in China. Environmental Research, 172, 81–88. 10.1016/j.envres.2019.02.013 [DOI] [PubMed] [Google Scholar]
- NICNAS (National Industrial Chemicals Notification and Assessment Scheme), 2005. Potassium perfluorobutane sulfonate: Hazard assessment. Australia. Available online: http://www.nicnas.gov.au/Publications/CAR/Other/PotassiumPerfluorobutaneSulfonatePDF.pdf
- Nilsson H, Kärrman A, Rotander A, van Bavel B, Lindström G and Westberg H, 2010. Inhalation exposure to fluorotelomer alcohols yield perfluorocarboxylates in human blood? Environmental Science & Technology, 44, 7717–7722. 10.1021/es101951t [DOI] [PubMed] [Google Scholar]
- Nilsson H, Kärrman A, Rotander A, van Bavel B, Lindstrom G and Westberg H, 2013. Professional ski waxers’ exposure to PFAS and aerosol concentrations in gas phase and different particle size fractions. Environmental Science: Processes & Impacts, 15, 814–822. 10.1039/c3em30739e [DOI] [PubMed] [Google Scholar]
- Noorlander CW, van Leeuwen SPJ, te Biesebeek JD, Mengelers MJB and Zeilmaker MJ, 2011. Levels of perfluorinated compounds in food and dietary intake of PFOS and PFOA in The Netherlands. Journal of Agriculture and Food Chemistry, 59, 7496–7505. [DOI] [PubMed] [Google Scholar]
- Nordén M, Berger U and Engwall M, 2013. High levels of perfluoroalkyl acids in eggs and embryo livers of great cormorant (Phalacrocorax carbo sinensis) and herring gull (Larus argentatus) from Lake Vanern, Sweden. Environmental Science and Pollution Research, 20, 8021–8030. 10.1007/s11356-013-1567-3 [DOI] [PubMed] [Google Scholar]
- Nøst TH, Vestergren R, Berg V, Nieboer E, Odland JØ and Sandanger TM, 2014. Repeated measurements of per‐ and polyfluoroalkyl substances (PFASs) from 1979 to 2007 in males from Northern Norway: assessing time trends, compound correlations and relations to age/birth cohort. Environment International, 67, 43–53. [DOI] [PubMed] [Google Scholar]
- NTP (National Toxicology Program), 2019a. NTP technical report on the toxicity studies of perfluoroalkyl carboxylates (perfluorohexanoic acid, perfluorooctanoic acid, perfluorononanoic acid, and perfluorodecanoic acid) administered by gavage to sprague dawley (Hsd:Sprague Dawley SD) rats. NTP TOX 97. Research Triangle Park, North Carolina, USA. Available online: http://ntp.niehs.nih.gov
- NTP (National Toxicology Program), 2019b. NTP technical report on the toxicity studies of perfluoroalkyl sulfonates (perfluorobutane sulfonic acid, perfluorohexane sulfonate potassium salt, and perfluorooctane sulfonic acid) administered by gavage to sprague dawley (Hsd:Sprague Dawley SD) rats. NTP TOX 96. Research Triangle Park, North Carolina, USA. Available online: http://ntp.niehs.nih.gov
- Numata J, Kowalczyk J, Adolphs J, Ehlers S, Schafft H, Fuerst P, Müller‐Graf C, Lahrssen‐Wiederholt M and Greiner M, 2014. Toxicokinetics of seven perfluoroalkyl sulfonic and carboxylic acids in pigs fed a contaminated diet. Journal of Agricultural and Food Chemistry, 62, 6861–6870. 10.1021/jf405827u [DOI] [PubMed] [Google Scholar]
- Nyberg E, Awad R, Bignert A, Ek C, Sallsten G and Benskin JP, 2018. Inter‐individual, inter‐city, and temporal trends of per‐ and polyfluoroalkyl substances in human milk from Swedish mothers between 1972 and 2016. Environmental Science: Processes & Impacts, 20, 1136–1147. [DOI] [PubMed] [Google Scholar]
- Ode A, Rylander L, Lindh CH, Källén K, Jönsson BA, Gustafsson P, Olofsson P, Ivarsson SA and Rignell‐Hydbom A, 2013. Determinants of maternal and fetal exposure and temporal trends of perfluorinated compounds. Environmental Science and Pollution Research, 20, 7970–7978. [DOI] [PubMed] [Google Scholar]
- OECD (The Organisation for Economic Co‐operation and Development), 2018. Toward a New Comprehensive Global Database of Per‐ and Polyfluoroalkyl Substances (PFASs): Summary Report on Updating the OECD 2007 List of Per‐ and Polyfluoroalkyl Substances (PFASs). Series on Risk Management No. 39. Available online: http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ENV-JM-MONO(2018)7&doclanguage=en
- Ohmori K, Kudo N, Katayama K and Kawashima Y, 2003. Comparison of the toxicokinetics between perfluorocarboxylic acids with different carbon chain length. Toxicology, 184, 135–140. [DOI] [PubMed] [Google Scholar]
- Okada E, Sasaki S, Saijo Y, Washino N, Miyashita C, Kobayashi S, Konishi K, Ito YM, Ito R, Nakata A, Iwasaki Y, Saito K, Nakazawa H and Kishi R, 2012. Prenatal exposure to perfluorinated chemicals and relationship with allergies and infectious diseases in infants. Environmental Research, 112, 118–125. 10.1016/j.envres.2011.10.003 [DOI] [PubMed] [Google Scholar]
- Okada E, Kashino I, Matsuura H, Sasaki S, Miyashita C, Yamamoto J, Ikeno T, Ito YM, Matsumura T, Tamakoshi A and Kishi R, 2013. Temporal trends of perfluoroalkyl acids in plasma samples of pregnant women in Hokkaido, Japan, 2003‐2011. Environment International, 60, 89–96. [DOI] [PubMed] [Google Scholar]
- Okada E, Sasaki S, Kashino I, Matsuura H, Miyashita C, Kobayashi S, Itoh K, Ikeno T, Tamakoshi A and Kishi R, 2014. Prenatal exposure to perfluoroalkyl acids and allergic diseases in early childhood. Environment International, 65, 127–134. 10.1016/j.envint.2014.01.007 [DOI] [PubMed] [Google Scholar]
- Olsen GW, 2015. PFAS biomonitoring in Higher Exposed Populations. Springer International Publishing, Switzerland, Toxicological Effects of Perfluoroalkyl and Polyfluoralkyl Substances, Molecular and Integrative Toxicology. 10.1007/978-3-319-15518-0_4 [DOI] [Google Scholar]
- Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL and Zobel LR, 2007. Half‐life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environmental Health Perspectives, 115, 1298–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW, Butenhoff JL and Zobel LR, 2009. Perfluoroalkyl chemicals and human fetal development: an epidemiologic review with clinical and toxicological perspectives. Reproductive Toxicology, 27, 212–230. 10.1016/j.reprotox.2009.02.001 [DOI] [PubMed] [Google Scholar]
- Oulhote Y, Steuerwald U, Debes F, Weihe P and Grandjean P, 2016. Behavioral difficulties in 7‐year old children in relation to developmental exposure to perfluorinated alkyl substances. Environment International, 97, 237–245. 10.1016/j.envint.2016.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachkowski B, Post GB and Stern AH, 2019. The derivation of a Reference Dose (RfD) for perfluorooctane sulfonate (PFOS) based on immune suppression. Environmental Research, 171, 452–469. 10.1016/j.envres.2018.08.004 [DOI] [PubMed] [Google Scholar]
- Papadopoulou E, Sabaredzovic A, Namork E, Nygaard UC, Granum B and Haug LS, 2016. Exposure of Norwegian toddlers to perfluoroalkyl substances (PFAS): the association with breastfeeding and maternal PFAS concentrations. Environment International, 94, 687–694. [DOI] [PubMed] [Google Scholar]
- Papadopoulou E, Poothong S, Koekkoek J, Lacattini L, Padilla‐Sanchez JA, Haugen M, Herzke D, Valdersnes S, Maage A, Cousins IT, Leonards PEG and Haug LS, 2017. Estimating human exposure to perfluoroalkyl acids via solid food and drinks: implementation and comparison of different dietary assessment methods. Environmental Research, 158, 269–276. 10.1016/j.envres.2017.06.011 [DOI] [PubMed] [Google Scholar]
- Parzefall W, Berger W, Kainzbauer E, Teufelhofer O, Schulte‐Hermann R and Thurman RG, 2001. Peroxisome proliferators do not increase DNA synthesis in purified rat hepatocytes. Carcinogenesis, 22, 519–523. [DOI] [PubMed] [Google Scholar]
- Peden‐Adams MM, Keller JM, Eudaly JG, Berger J, Gilkeson GS and Keil DE, 2008. Suppression of humoral immunity in mice following exposure to perfluorooctane sulfonate. Toxicological Sciences, 104, 144–154. [DOI] [PubMed] [Google Scholar]
- Peng H, Zhang S, Sun J, Zhang Z, Giesy JP and Hu J, 2014. Isomer‐Specific Accumulation of Perfluorooctanesulfonate from (N‐Ethyl perfluorooctanesulfonamido) ethanol‐based Phosphate Diester in Japanese Medaka (Oryzias latipes). Environmental Science & Technology, 48, 1058–1066. [DOI] [PubMed] [Google Scholar]
- Pennings JL, Jennen DG, Nygaard UC, Namork E, Haug LS, vanLoveren H and Granum B, 2016. Cord blood gene expression supports that prenatal exposure to perfluoroalkyl substances causes depressed immune functionality in early childhood. Journal of Immunotoxicology, 13, 173–180. [DOI] [PubMed] [Google Scholar]
- Pérez F, Nadal M, Navarro‐Ortega A, Fabrega F, Domingo JL, Barcelo D and Farre M, 2013. Accumulation of perfluoroalkyl substances in human tissues. Environment International, 59, 354–362. 10.1016/j.envint.2013.06.004 [DOI] [PubMed] [Google Scholar]
- Persson S, Rotander A, Kärrman A, van Bavel B and Magnusson U, 2013. Perfluoroalkyl acids in subarctic wild male mink (Neovison vison) in relation to age, season and geographical area. Environment International, 59, 425–430. 10.1016/j.envint.2013.06.025 [DOI] [PubMed] [Google Scholar]
- Pierozan P and Karlsson O, 2018. PFOS induces proliferation, cell‐cycle progression, and malignant phenotype in human breast epithelial cells. Archives of Toxicology, 92, 705–716. 10.1007/s00204-017-2077-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierozan P, Jerneren F and Karlsson O, 2018. Perfluorooctanoic acid (PFOA) exposure promotes proliferation, migration and invasion potential in human breast epithelial cells. Archives of Toxicology, 92, 1729–1739. 10.1007/s00204-018-2181-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzurro DM, Seeley M, Kerper LE and Beck BD, 2019. Interspecies differences in perfluoroalkyl substances (PFAS) toxicokinetics and application to health‐based criteria. Regulatory Toxicology and Pharmacology, 106, 239–250. [DOI] [PubMed] [Google Scholar]
- Poothong S, Thomsen C, Padilla‐Sanchez JA, Papadopoulou E and Haug LS, 2017. Distribution of Novel and Well‐Known Poly‐ and Perfluoroalkyl Substances (PFASs) in Human Serum, Plasma, and Whole Blood. Environmental Science & Technology, 51, 13388–13396. 10.1021/acs.est.7b03299 [DOI] [PubMed] [Google Scholar]
- Poothong S, Papadopoulou E, Padilla‐Sánchez JA, Thomsen C and Haug LS, 2020. Multiple pathways of human exposure to poly‐ and perfluoroalkyl substances (PFASs): From external exposure to human blood. Environment International, 134, 105244 10.1016/j.envint.2019.105244 [DOI] [PubMed] [Google Scholar]
- Porpora MG, Lucchini R, Abballe A, Ingelido AM, Valentini S, Fuggetta E, Cardi V, Ticino A, Marra V, Fulgenzi AR and De Felip E, 2013. Placental transfer of persistent organic pollutants: a preliminary study on mother‐newborn pairs. International Journal of Environmental Research and Public Health, 10, 699–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post GB, Cohn PD and Cooper KR, 2012. Perfluorooctanoic acid (PFOA), an emerging drinking water contaminant: a critical review of recent literature. Environmental Research, 116, 93–117. [DOI] [PubMed] [Google Scholar]
- Power MC, Webster TF, Baccarelli AA and Weisskopf MG, 2013. Cross‐sectional association between polyfluoroalkyl chemicals and cognitive limitation in the National Health and Nutrition Examination Survey. Neuroepidemiology, 40, 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevedouros K, Cousins IT, Buck RC and Korzeniowski SH, 2006. Sources, fate and transport of perfluorocarboxylates. Environmental Science & Technology, 40, 32–44. [DOI] [PubMed] [Google Scholar]
- Pugh G Jr, Isenberg JS, Kamendulis LM, Ackley DC, Clare LJ, Brown R, Lington AW, Smith JH and Klaunig JE, 2000. Effects of di‐isononyl phthalate, di‐2‐ethylhexyl phthalate, and clofibrate in cynomolgus monkeys. Toxicological Sciences, 56, 181–188. [DOI] [PubMed] [Google Scholar]
- Qazi MR, Bogdanska J, Butenhoff JL, Nelson BD, DePierre JW and Abedi‐Valugerdi M, 2009a. High‐dose, short‐term exposure of mice to perfluorooctanesulfonate (PFOS) or perfluorooctanoate (PFOA) affects the number of circulating neutrophils differently, but enhances the inflammatory responses of macrophages to lipopolysaccharide (LPS) in a similar fashion. Toxicology, 262, 207–214. [DOI] [PubMed] [Google Scholar]
- Qazi MR, Xia Z, Bogdanska J, Chang SC, Ehresman DJ, Butenhoff JL, Nelson BD, DePierre JW and Abedi‐Valugerdi M, 2009b. The atrophy and changes in the cellular compositions of the thymus and spleen observed in mice subjected to short‐term exposure to perfluorooctanesulfonate are high dose phenomena mediated in part by peroxisome proliferator‐activated receptor‐alpha (PPARa). Toxicology, 260, 68–76. [DOI] [PubMed] [Google Scholar]
- Qazi MR, Nelson BD, DePierre JW and Abedi‐Valugerdi M, 2010. 28‐Day dietary exposure of mice to a low total dose (7 mg/kg) of perfluorooctanesulfonate (PFOS) alters neither the cellular compositions of the thymus and spleen nor humoral immune responses: does the route of administration play a pivotal role in PFOS‐induced immunotoxicity? Toxicology, 267, 132–139. [DOI] [PubMed] [Google Scholar]
- Qazi MR, Dean Nelson B, DePierre JW and Abedi‐Valugerdi M, 2012. High‐dose dietary exposure of mice to perfluorooctanoate or perfluorooctane sulfonate exerts toxic effects on myeloid and B‐lymphoid cells in the bone marrow and these effects are partially dependent on reduced food consumption. Food and Chemical Toxicology, 50, 2955–2963. [DOI] [PubMed] [Google Scholar]
- Qin XD, Qian Z, Vaughn MG, Huang J, Ward P, Zeng XW, Zhou Y, Zhu Y, Yuan P, Li M, Bai Z, Paul G, Hao YT, Chen W, Chen PC, Dong GH and Lee YL, 2016. Positive associations of serum perfluoroalkyl substances with uric acid and hyperuricemia in children from Taiwan. Environmental Pollution, 212, 519–524. 10.1016/j.envpol.2016.02.050 [DOI] [PubMed] [Google Scholar]
- Qin XD, Qian ZM, Dharmage SC, Perret J, Geiger SD, Rigdon SE, Howard S, Zeng XW, Hu LW, Yang BY, Zhou Y, Li M, Xu SL, Bao WW, Zhang YZ, Yuan P, Wang J, Zhang C, Tian YP, Nian M, Xiao X, Chen W, Lee YL and Dong GH, 2017. Association of perfluoroalkyl substances exposure with impaired lung function in children. Environmental Research, 155, 15–21. 10.1016/j.envres.2017.01.025 [DOI] [PubMed] [Google Scholar]
- Qu Y, Han B, Yu Y, Yao W, Bose S, Karlan BY, Giuliano AE and Cui X, 2015. Evaluation of MCF10A as a reliable model for normal human mammary epithelial cells. PLoS ONE, 10, e0131285 10.1371/journal.pone.0131285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaak I, de Cock M, de Boer M, Lamoree M, Leonards P and van de Bor M, 2016. Prenatal exposure to perfluoroalkyl substances and behavioral development in children. International Journal of Environmental Research and Public Health, 13, 10.3390/ijerph13050511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiñones O and Snyder SA, 2009. Occurrence of perfluoroalkyl carboxylates and sulfonates in drinking water utilities and related waters from the United States. Environmental Science & Technology, 43, 9089–9095. 10.1021/es9024707 [DOI] [PubMed] [Google Scholar]
- Ramhøj L, Hass U, Boberg J, Scholze M, Christiansen S, Nielsen F and Axelstad M, 2018. Perfluorohexane sulfonate (PFHxS) and a mixture of endocrine disrupters reduce thyroxine levels and cause antiandrogenic effects in rats. Toxicological Sciences, 163, 579–591. 10.1093/toxsci/kfy055 [DOI] [PubMed] [Google Scholar]
- Rand AA, Rooney JP, Butt CM, Meyer JN and Mabury SA, 2014. Cellular toxicity associated with exposure to perfluorinated carboxylates (PFCAs) and their metabolic precursors. Chemical Research in Toxicology, 27, 42–50. [DOI] [PubMed] [Google Scholar]
- Rantakokko P, Mannisto V, Airaksinen R, Koponen J, Viluksela M, Kiviranta H and Pihlajamaki J, 2015. Persistent organic pollutants and non‐alcoholic fatty liver disease in morbidly obese patients: a cohort study. Environmental Health, 14, 79 10.1186/s12940-015-0066-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayne S and Forest K, 2009. Congener‐specific organic carbon‐normalized soil and sediment‐water partitioning coefficients for the C1 through C8 perfluoroalkyl carboxylic and sulfonic acids. Journal of Environmental Science and Health Part A Toxic/Hazardous Substances & Environmental Engineering, 44, 1374–1387. 10.1080/10934520903217229 [DOI] [PubMed] [Google Scholar]
- Ren XM, Zhang YF, Guo LH, Qin ZF, Lv QY and Zhang LY, 2015. Structure‐activity relations in binding of perfluoroalkyl compounds to human thyroid hormone T3 receptor. Archives of Toxicology, 89, 233–242. [DOI] [PubMed] [Google Scholar]
- Ren XM, Qin WP, Cao LY, Zhang J, Yang Y, Wan B and Guo LH, 2016. Binding interactions of perfluoroalkyl substances with thyroid hormone transport proteins and potential toxicological implications. Toxicology, 366–367, 32–42. 10.1016/j.tox.2016.08.011 [DOI] [PubMed] [Google Scholar]
- Riddell N, Arsenault G, Benskin JP, Chittim B, Martin JW, McAlees A and McCrindle R, 2009. Branched perfluorooctane sulfonate isomer quantification and characterization in blood serum samples by HPLC/ESI‐MS(/MS). Environmental Science & Technology, 43, 7902–7908. 10.1021/es901261v [DOI] [PubMed] [Google Scholar]
- Robledo CA, Yeung E, Mendola P, Sundaram R, Maisog J, Sweeney AM, Barr DB and Louis GM, 2015. Preconception maternal and paternal exposure to persistent organic pollutants and birth size: the LIFE study. Environmental Health Perspectives, 123, 88–94. 10.1289/ehp.1308016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwell CE, Turley AE, Cheng X, Fields PE and Klaassen CD, 2013. Acute immunotoxic effects of perfluorononanoic acid (PFNA) in C57BL/6 mice. Clinical and Experimental Pharmacology, Suppl, 4 10.4172/2161-1459.s4-002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwell CE, Turley AE, Cheng X, Fields PE and Klaassen CD, 2017. Persistent alterations in immune cell populations and function from a single dose of perfluorononanoic acid (PFNA) in C57Bl/6 mice. Food and Chemical Toxicology, 100, 24–33. 10.1016/j.fct.2016.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JM, Ellis‐Hutchings RG, Grey BE, Zucker RM, Norwood J Jr, Grace CE, Gordon CJ and Lau C, 2014. Elevated blood pressure in offspring of rats exposed to diverse chemicals during pregnancy. Toxicological Sciences, 137, 436–446. 10.1093/toxsci/kft248 [DOI] [PubMed] [Google Scholar]
- Rolfe M, James NH and Roberts RA, 1997. Tumour necrosis factor alpha (TNF‐alpha) suppresses apoptosis and induces S‐phase in rodent hepatocytes: a mediator of the hepatocarcinogenicity of peroxisome proliferators? Carcinogenesis, 18, 2277–2280. [DOI] [PubMed] [Google Scholar]
- Roos A, Berger U, Jarnberg U, van Dijk J and Bignert A, 2013. Increasing concentrations of perfluoroalkyl acids in Scandinavian otters (Lutra lutra) between 1972 and 2011: a new threat to the otter population? Environmental Science & Technology, 47, 11757–11765. 10.1021/es401485t [DOI] [PubMed] [Google Scholar]
- Rose ML, Rusyn I, Bojes HK, Belyea J, Cattley RC and Thurman RG, 2000. Role of Kupffer cells and oxidants in signaling peroxisome proliferator‐induced hepatocyte proliferation. Mutation Research, 448, 179–192. [DOI] [PubMed] [Google Scholar]
- Rosen MB, Lee JS, Ren H, Vallanat B, Liu J, Waalkes MP, Abbott BD, Lau C and Corton JC, 2008. Toxicogenomic dissection of the perfluorooctanoic acid transcript profile in mouse liver: evidence for the involvement of nuclear receptors PPAR alpha and CAR. Toxicological Sciences, 103, 46–56. [DOI] [PubMed] [Google Scholar]
- Rosen MB, Das KP, Rooney J, Abbott B, Lau C and Corton JC, 2017. PPARalpha‐independent transcriptional targets of perfluoroalkyl acids revealed by transcript profiling. Toxicology, 387, 95–107. 10.1016/j.tox.2017.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmai AK, Taxvig C, Svingen T, Trier X, van Vugt‐Lussenburg BM, Pedersen M, Lesné L, Jégou B and Vinggaard AM, 2016. Fluorinated alkyl substances and technical mixtures used in food paper‐packaging exhibit endocrine‐related activity in vitro. Andrology, 4, 662–672. [DOI] [PubMed] [Google Scholar]
- Rosenmai AK, Ahrens L, le Godec T, Lundqvist J and Oskarsson A, 2018. Relationship between peroxisome proliferator‐activated receptor alpha activity and cellular concentration of 14 perfluoroalkyl substances in HepG2 cells. Journal of Applied Toxicology, 38, 219–226. 10.1002/jat.3515 [DOI] [PubMed] [Google Scholar]
- Rose MI, Rusyn I, Bojes HK, Belyea J, Cattley RC and Thurman RG, 2000. Role of Kupffer cells and oxidants in signaling peroxisome proliferator‐induced hepatocyte proliferation. Mutation Research, 448, 179–192. [DOI] [PubMed] [Google Scholar]
- Ross MS, Wong CS and Martin JW, 2012. Isomer‐specific biotransformation of perfluorooctane sulfonamide in Sprague‐Dawley rats. Environmental Science & Technology, 46, 3196–3203. 10.1021/es204028v [DOI] [PubMed] [Google Scholar]
- Rotander A, Toms LM, Aylward L, Kay M and Mueller JF, 2015. Elevated levels of PFOS and PFHxS in firefighters exposed to aqueous film forming foam (AFFF). Environment International, 82, 28–34. [DOI] [PubMed] [Google Scholar]
- Route WT, Russell RE, Lindstrom AB, Strynar MJ and Key RL, 2014. Spatial and temporal patterns in concentrations of perfluorinated compounds in bald eagle nestlings in the upper Midwestern United States. Environmental Science & Technology, 48, 6653–6660. 10.1021/es501055d [DOI] [PubMed] [Google Scholar]
- Rubarth J, Dreyer A, Guse N, Einax JW and Ebinghaus R, 2011. Perfluorinated compounds in red‐throated divers from the German Baltic Sea: new findings from their distribution in 10 different tissues. Environmental Chemistry, 8, 419–428. 10.1071/EN10142 [DOI] [Google Scholar]
- Rush EL, Singer AB, Longnecker MP, Haug LS, Sabaredzovic A, Symanski E and Whitworth KW, 2018. Oral contraceptive use as a determinant of plasma concentrations of perfluoroalkyl substances among women in the Norwegian Mother and Child Cohort (MoBa) study. Environment International, 112, 156–164. 10.1016/j.envint.2017.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell MH, Nilsson H and Buck RC, 2013. Elimination kinetics of perfluorohexanoic acid in humans and comparison with mouse, rat and monkey. Chemosphere, 93, 2419–2425. 10.1016/j.chemosphere.2013.08.060 [DOI] [PubMed] [Google Scholar]
- Russo J and Russo IH, 1996. Experimentally induced mammary tumors in rats. Breast Cancer Research and Treatment, 39, 7–20. [DOI] [PubMed] [Google Scholar]
- Rylander C, Phi DT, Odland JØ and Sandanger TM, 2009. Perfluorinated compounds in delivering women from south central Vietnam. Journal of Environmental Monitoring, 11, 2002–2008. 10.1039/b908551c [DOI] [PubMed] [Google Scholar]
- Ryu MH, Jha A, Ojo OO, Mahood TH, Basu S, Detillieux KA, Nikoobakht N, Wong CS, Loewen M, Becker AB and Halayko AJ, 2014. Chronic exposure to perfluorinated compounds: impact on airway hyperresponsiveness and inflammation. American Journal of Physiology‐Lung Cellular and Molecular Physiology, 307, L765–L774. 10.1152/ajplung.00100.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv SK, Rifas‐Shiman SL, Webster TF, Mora AM, Harris MH, Calafat AM, Ye X, Gillman MW and Oken E, 2015. Sociodemographic and perinatal predictors of early pregnancy per‐ and polyfluoroalkyl substance (PFAS) concentrations. Environmental Science Technology, 49, 11849–11858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito N, Harada K, Inoue K, Sasaki K, Yoshinaga T and Koizumi A, 2004. Perfluorooctanoate and perfluorooctanesulfonate concentrations in surface water in Japan. Journal of Occupational Health, 46, 49–59. [DOI] [PubMed] [Google Scholar]
- Salihovic S, Nilsson H, Hagberg J and Lindström G, 2013. Trends in the analysis of persistent organic pollutants (POPs) in human blood. TrAC Trends in Analytical Chemistry, 46, 129–138. 10.1016/j.trac.2012.06.009 [DOI] [Google Scholar]
- Salihovic S, Stubleski J, Kärrman A, Larsson A, Fall T, Lind L and Lind PM, 2018. Changes in markers of liver function in relation to changes in perfluoroalkyl substances ‐ a longitudinal study. Environment International, 117, 196–203. 10.1016/j.envint.2018.04.052 [DOI] [PubMed] [Google Scholar]
- Salihovic S, Dickens AM, Schoultz I, Fart F, Sinisalu L, Lindeman T, Halfvarson J, Orešič M and Hyötyläinen T, 2020. Simultaneous determination of perfluoroalkyl substances and bile acids in human serum using ultra‐high‐performance liquid chromatography–tandem mass spectrometry. Analytical and Bioanalytical, 412, 2251–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz DA, 2007. Guest editorial: biomarkers of perfluorinated chemicals and birth weight. Environmental Health Perspectives, 115, A528–A529. 10.1289/ehp.10923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaider LA, Balan SA, Blum A, Andrews DQ, Strynar MJ, Dickinson ME, Lunderberg DM, Lang JR and Peaslee GF, 2017. Fluorinated Compounds in U.S. Fast Food Packaging. Environmental Science & Technology Letters, 4, 105–111. 10.1021/acs.estlett.6b00435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher DP, Kelly JE, Huset CA, Barry KM, Hoffbeck RW, Yingling VL and Messing RB, 2018. Occurrence of perfluoroalkyl substances (PFAS) in garden produce at homes with a history of PFAS‐contaminated drinking water. Chemosphere, 196, 548–555. 10.1016/j.chemosphere.2017.12.179 [DOI] [PubMed] [Google Scholar]
- Schröter‐Kermani C, Müller J, Jürling H, Conrad A and Schulte C, 2013. Retrospective monitoring of perfluorocarboxylates and perfluorosulfonates in human plasma archived by the German Environmental Specimen Bank. International Journal of Hygiene and Environmental Health, 216, 633–640. [DOI] [PubMed] [Google Scholar]
- Seals R, Bartell SM and Steenland K, 2011. Accumulation and clearance of perfluorooctanoic acid (PFOA) in current and former residents of an exposed community. Environmental Health Perspectives, 119, 119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo SH, Son MH, Choi SD, Lee DH and Chang YS, 2018. Influence of exposure to perfluoroalkyl substances (PFASs) on the Korean general population: 10‐year trend and health effects. Environment International, 113, 149–161. 10.1016/j.envint.2018.01.025 [DOI] [PubMed] [Google Scholar]
- Shah YM, Morimura K, Yang Q, Tanabe T, Takagi M and Gonzalez FJ, 2007. Peroxisome proliferator‐activated receptor alpha regulates a microRNA‐mediated signaling cascade responsible for hepatocellular proliferation. Molecular and Cellular Biology, 27, 4238–4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah‐Kulkarni S, Kim BM, Hong YC, Kim HS, Kwon EJ, Park H, Kim YJ and Ha EH, 2016. Prenatal exposure to perfluorinated compounds affects thyroid hormone levels in newborn girls. Environment International, 94, 607–613. 10.1016/j.envint.2016.06.024 [DOI] [PubMed] [Google Scholar]
- Shapiro GD, Dodds L, Arbuckle TE, Ashley‐Martin J, Ettinger AS, Fisher M, Taback S, Bouchard MF, Monnsier P, Dallaire R, Morisset AS and Fraser W, 2016. Exposure to organophosphorus and organochlorine pesticides, perfluoroalkyl substances, and polychlorinated biphenyls in pregnancy and the association with impaired glucose tolerance and gestational diabetes mellitus: the MIREC Study. Environmental Research, 147, 71–81. [DOI] [PubMed] [Google Scholar]
- Shaw D, Lee R and Roberts RA, 2002. Species differences in response to the phthalate plasticizer monoisononylphthalate (MINP) in vitro: a comparison of rat and human hepatocytes. Archives of Toxicology, 76, 344–350. [DOI] [PubMed] [Google Scholar]
- Shaw SD, Berger ML, Harris JH, Yun SH, Wu Q, Liao C, Blum A, Stefani A and Kannan K, 2013. Persistent organic pollutants including polychlorinated and polybrominated dibenzo‐p‐dioxins and dibenzofurans in firefighters from Northern California. Chemosphere, 91, 1386–1394. [DOI] [PubMed] [Google Scholar]
- Shelly C, Grandjean P, Oulhote Y, Plomgaard P, Frikke‐Schmidt R, Nielsen F, Zmirou‐Navier D, Weihe P and Valvi D, 2019. Early life exposures to perfluoroalkyl substances in relation to adipokine hormone levels at birth and during childhood. Journal of Clinical Enocrinology Metabolism, 104, 5338–5348. 10.1210/jc.2019-00385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z, Zhang H, Ding L, Feng Y, Xu M and Dai J, 2009a. The effect of perfluorododecanonic acid on endocrine status, sex hormones and expression of steroidogenic genes in pubertal female rats. Reproductive Toxicology, 27, 352–359. [DOI] [PubMed] [Google Scholar]
- Shi Z, Ding L, Zhang H, Feng Y, Xu M and Dai J, 2009b. Chronic exposure to perfluorododecanoic acid disrupts testicular steroidogenesis and the expression of related genes in male rats. Toxicology Letters, 188, 192–200. [DOI] [PubMed] [Google Scholar]
- Shi Y, Yang L, Li J, Lai J, Wang Y, Zhao Y and Wu Y, 2017. Occurrence of perfluoroalkyl substances in cord serum and association with growth indicators in newborns from Beijing. Chemosphere, 169, 396–402. 10.1016/j.chemosphere.2016.11.050 [DOI] [PubMed] [Google Scholar]
- Shoeib M, Harner T and Vlahos P, 2006. Perfluorinated chemicals in the arctic atmosphere. Environmental Science & Technology, 40, 7577–7583. 10.1021/es0618999 [DOI] [PubMed] [Google Scholar]
- Shoeib M, Harner T G MW and Lee SC, 2011. Indoor sources of poly‐ and perfluorinated compounds (PFCS) in Vancouver, Canada: implications for human exposure. Environmental Science & Technology, 45, 7999–8005. 10.1021/es103562v [DOI] [PubMed] [Google Scholar]
- Sinclair E, Kim SK, Akinleye HB and Kannan K, 2007. Quantitation of gas‐phase perfluoroalkyl surfactants and fluorotelomer alcohols released from nonstick cookware and microwave popcorn bags. Environmental Science & Technology, 41, 1180–1185. [DOI] [PubMed] [Google Scholar]
- Singer AB, Whitworth KW, Haug LS, Sabaredzovic A, Impinen A, Papadopoulou E and Longnecker MP, 2018. Menstrual cycle characteristics as determinants of plasma concentrations of perfluoroalkyl substances (PFASs) in the Norwegian Mother and Child Cohort (MoBa study). Environmental Research, 166, 78–85. 10.1016/j.envres.2018.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S and Singh SK, 2019a. Effect of gestational exposure to perfluorononanoic acid on neonatal mice testes. Journal of Applied Toxicology, 39, 1663–1671. 10.1002/jat.3883 [DOI] [PubMed] [Google Scholar]
- Singh S and Singh SK, 2019b. Chronic exposure to perfluorononanoic acid impairs spermatogenesis, steroidogenesis and fertility in male mice. Journal of Applied Toxicology, 39, 420–431. 10.1002/jat.3733 [DOI] [PubMed] [Google Scholar]
- Singh S and Singh SK, 2019c. Acute exposure to perfluorononanoic acid in prepubertal mice: effect on germ cell dynamics and an insight into the possible mechanisms of its inhibitory action on testicular functions. Ecotoxicology and Environmental Safety, 183, 109499 10.1016/j.ecoenv.2019.109499 [DOI] [PubMed] [Google Scholar]
- Singh S and Singh SK, 2019d. Prepubertal exposure to perfluorononanoic acid interferes with spermatogenesis and steroidogenesis in male mice. Ecotoxicology and Environmental Safety, 170, 590–599. 10.1016/j.ecoenv.2018.12.034 [DOI] [PubMed] [Google Scholar]
- Sochorová L, Hanzlíková L, Černá M, Drgáčová A, Fialová A, Svarcova A, Gramblička T and Pulkrabová J, 2017. Perfluorinated alkylated substances and brominated flame retardants in serum of the Czech adult population. International Journal of Hygiene and Environmental Health, 220, 235–243. 10.1016/j.ijheh.2016.09.003 [DOI] [PubMed] [Google Scholar]
- Son HY, Lee S, Tak EN, Cho HS, Shin HI, Kim SH and Yang JH, 2009. Perfluorooctanoic acid alters T lymphocyte phenotypes and cytokine expression in mice. Environmental Toxicology, 24, 580–588. [DOI] [PubMed] [Google Scholar]
- Song P, Li D, Wang X and Zhong X, 2018a. Effects of perfluorooctanoic acid exposure during pregnancy on the reproduction and development of male offspring mice. Andrologia, 50(e13059), PMID: 29862542. 10.1111/and.13059 [DOI] [PubMed] [Google Scholar]
- Song X, Tang S, Zhu H, Chen Z, Zang Z, Zhang Y, Niu X, Wang X, Yin H, Zeng F and He C, 2018b. Biomonitoring PFAAs in blood and semen samples: investigation of a potential link between PFAAs exposure and semen mobility in China. Environment International, 113, 50–54. 10.1016/j.envint.2018.01.010 [DOI] [PubMed] [Google Scholar]
- Stahl T, Heyn J, Thiele H, Hϋther J, Failing K, Georgii S and Brunn H, 2009. Carryover of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) from Soil to Plants. Archives of Environmental Contamination and Toxicology, 57, 289–298. [DOI] [PubMed] [Google Scholar]
- Starling AP, Engel SM, Whitworth KW, Richardson DB, Stuebe AM, Daniels JL, Haug LS, Eggesbo M, Becher G, Sabaredzovic A, Thomsen C, Wilson RE, Travlos GS, Hoppin JA, Baird DD and Longnecker MP, 2014. Perfluoroalkyl substances and lipid concentrations in plasma during pregnancy among women in the Norwegian Mother and Child Cohort Study. Environment International, 62, 104–112. 10.1016/j.envint.2013.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenland K, Barry V and Savitz D, 2018. Serum perfluorooctanoic acid (PFOA) and birthweight: an updated meta‐analysis with bias analysis. Epidemiology, 29, 765–776. 10.1097/EDE.0000000000000903 [DOI] [PubMed] [Google Scholar]
- Stein CR and Savitz DA, 2011. Serum perfluorinated compound concentration and attention deficit/hyperactivity disorder in children 5‐18 years of age. Environment Health Perspectives, 119, 1466–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein CR, McGovern KJ, Pajak AM, Maglione PJ and Wolff MS, 2016a. Perfluoroalkyl and polyfluoroalkyl substances and indicators of immune function in children aged 12‐19 y: National Health and Nutrition Examination Survey. Pediatric Research, 79, 348–357. 10.1038/pr.2015.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein CR, Ge Y, Wolff MS, Ye X, Calafat AM, Kraus T and Moran TM, 2016b. Perfluoroalkyl substance serum concentrations and immune response to FluMist vaccination among healthy adults. Environmental Research, 149, 171–178. 10.1016/j.envres.2016.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock NL, Muir DCG and Mabury SA, 2010. Perfluoroalkyl compounds In: Harrad S, ed. Persistent organic pollutants. Wiley, Chichester, UK: pp. 25–70. [Google Scholar]
- Stubleski J, Salihovic S, Lind L, Lind PM, van Bavel B and Kärrman A, 2016. Changes in serum levels of perfluoroalkyl substances during a 10‐year follow‐up period in a large population‐based cohort. Environment International, 95, 86–92. [DOI] [PubMed] [Google Scholar]
- Su TC, Kuo CC, Hwang JJ, Lien GW, Chen MF and Chen PC, 2016. Serum perfluorinated chemicals, glucose homeostasis and the risk of diabetes in working‐aged Taiwanese adults. Environment International, 88, 15–22. 10.1016/j.envint.2015.11.016 [DOI] [PubMed] [Google Scholar]
- Su M, Liang X, Xu X, Wu X and Yang B, 2019. Hepatoprotective benefits of vitamin C against perfluorooctane sulfonate induced liver damage in mice through suppressing inflammatory reaction and ER stress. Environmental Toxicology and Pharmacology, 65, 60–65. 10.1016/j.etap.2018.12.004 [DOI] [PubMed] [Google Scholar]
- Suh CH, Cho NK, Lee CK, Lee CH, Kim DH, Kim JH, Son BC and Lee JT, 2011. Perfluorooctanoic acid‐induced inhibition of placental prolactin‐family hormone and fetal growth retardation in mice. Molecular and Cellular Endocrinology, 337, 7–15. 10.1016/j.mce.2011.01.009 [DOI] [PubMed] [Google Scholar]
- Sundström M, Ehresman DJ, Bignert A, Butenhoff JL, Olsen GW, Chang SC and Bergman A, 2011. A temporal trend study (1972‐2008) of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in pooled human milk samples from Stockholm, Sweden. Environment International, 37, 178–183. [DOI] [PubMed] [Google Scholar]
- Sundström M, Chang SC, Noker PE, Gorman GS, Hart JA, Ehresman DJ, Bergman A and Butenhoff JL, 2012. Comparative pharmacokinetics of perfluorohexanesulfonate (PFHxS) in rats, mice, and monkeys. Reproductive Toxicology, 33, 441–451. 10.1016/j.reprotox.2011.07.004 [DOI] [PubMed] [Google Scholar]
- Suo C, Fan Z and Qiu J, 2017. Perfluorooctane sulfonate affects intestinal immunity against bacterial infection. Scientific Reports, 7, 5166 10.1038/s41598-017-04091-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susmann H, Schaider LA, Rodgers KM and Rudel RA, 2019. Dietary habits related to food packaging and population exposure to PFASs. Environmental Health Perspectives, 127, 107003–107010. 10.1289/EHP4092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swedish Chemical Agency , 2015. Occurrence and use of highly fluorinated substances and alternatives. Report from a government assignment. Report 7/15. Available online: https://www.kemi.se/en/global/rapporter/2015/report-7-15-occurrence-and-use-of-highly-fluorinated-substances-and-alternatives.pdf
- Swedish EPA (Swedish Environmental Protection Agency), 2012. Environmental and Health Risk Assessment of Perfluoroalkylated and Polyfluoroalkylated Substances (PFASs) in Sweden. Report 6513, September 2012, 139 pp. Available online: http://www.naturvardsverket.se/Documents/publikationer6400/978-91-620-6513-3.pdf?pid=3822
- Sznajder‐Katarzyńska K, Surma M, Cieślik E and Wiczkowski W, 2018. The perfluoroalkyl substances (PFASs) contamination of fruits and vegetables. Food Additives & Contaminants: Part A, 35, 1776–1786. 10.1080/19440049.2018.1502477 [DOI] [PubMed] [Google Scholar]
- Takahashi M, Ishida S, Hirata‐Koizumi M, Ono A and Hirose A, 2014. Repeated dose and reproductive/developmental toxicity of perfluoroundecanoic acid in rats. Journal of Toxicological Sciences Kato, 39, 97–108. [DOI] [PubMed] [Google Scholar]
- Taniyasu S, Senthilkumar K, Yamazaki E, Yeung LW, Guruge KS, Kannan K and Yamashita N, 2013. Perfluoroalkyl substances in the blood of wild rats and mice from 47 prefectures in Japan: use of samples from nationwide specimen bank. Archives of Environmental Contamination and Toxicology, 65, 149–170. 10.1007/s00244-013-9878-4 [DOI] [PubMed] [Google Scholar]
- Tao L, Kannan K, Aldous KM, Mauer MP and Eadon GA, 2008. Biomonitoring of perfluorochemicals in plasma of New York State personnel responding to the World Trade Center disaster. Environmental Science & Technology, 42, 3472–3478. [DOI] [PubMed] [Google Scholar]
- Tateno C, Yamamoto T, Utoh R, Yamasaki C, Ishida Y, Myoken Y, Oofusa K, Okada M, Tsutsui N and Yoshizato K, 2015. Chimeric mice with hepatocyte‐humanized liver as an appropriate model to study human peroxisome proliferator‐activated receptor‐α. Toxicologic Pathology, 43, 233–248. [DOI] [PubMed] [Google Scholar]
- Tatum‐Gibbs K, Wambaugh JF, Das KP, Zehr RD, Strynar MJ, Lindstrom AB, Delinsky A and Lau C, 2011. Comparative pharmacokinetics of perfluorononanoic acid in rat and mouse. Toxicology, 281, 48–55. 10.1016/j.tox.2011.01.003 [DOI] [PubMed] [Google Scholar]
- Taylor KW, Hoffman K, Thayer KA and Daniels JL, 2014. Polyfluoroalkyl chemicals and menopause among women 20‐65 years of age (NHANES). Environmental Health Perspectives, 122, 145–150. 10.1289/ehp.1306707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theurich MA, Davanzo R, Busck‐Rasmussen M, Diaz‐Gomez NM, Brennan C, Kylberg E, Boerug A, McHugh L, Weikart C, Abraham K and Koletzko B, 2019. Breastfeeding rates and programs in europe: a survey of 11 national breastfeeding committees and representatives. JPGN, 68, 400–407. [DOI] [PubMed] [Google Scholar]
- Thomas M, Bayha C, Klein K, Müller S, Weiss TS, Schwab M and Zanger U, 2015. The truncated splice variant of peroxisome proliferator‐activated receptor alpha, PPARα‐tr, autonomously regulates proliferative and pro‐inflammatory genes. BMC Cancer, 15, 488 10.1186/s12885-015-1500-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J, Lorber M, Toms LL, Kato K, Calafat AM and Mueller JF, 2010. Use of simple pharmacokinetic modeling to characterize exposure of Australians to perfluorooctanoic acid and perfluorooctane sulfonic acid. Environment International, 36, 390–397. 10.1016/j.envint.2010.02.008 [DOI] [PubMed] [Google Scholar]
- Thomsen C, Haug LS, Stigum H, Frøshaug M, Broadwell SL and Becher G, 2010. Changes in concentrations of perfluorinated compounds, polybrominated diphenyl ethers, and polychlorinated biphenyls in Norwegian breast‐milk during twelve months of lactation. Environmental Science & Technology, 44, 9550–9556. [DOI] [PubMed] [Google Scholar]
- Timmermann CAG, Budtz‐Jørgensen E, Petersen MS, Weihe P, Steuerwald U, Nielsen F, Jensen TK and Grandjean P, 2017a. Shorter duration of breastfeeding at elevated exposures to perfluoroalkyl substances. Reproductive Toxicology, 68, 164–170. 10.1016/j.reprotox.2016.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermann CAG, Budtz‐Jørgensen E, Jensen TK, Osuna CE, Petersen MS, Steuerwald U, Nielsen F, Poulsen LK, Weihe P and Grandjean P, 2017b. Association between perfluoroalkyl substance exposure and asthma and allergic disease in children as modified by MMR vaccination. Journal of Immunotoxicology, 14, 39–49. 10.1080/1547691X.2016.1254306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toft G, Jonsson BA, Lindh CH, Giwercman A, Spano M, Heederik D, Lenters V, Vermeulen R, Rylander L, Pedersen HS, Ludwicki JK, Zviezdai V and Bonde JP, 2012. Exposure to perfluorinated compounds and human semen quality in Arctic and European populations. Human Reproduction, 27, 2532–2540. 10.1093/humrep/des185 [DOI] [PubMed] [Google Scholar]
- Toms LM, Thompson J, Rotander A, Hobson P, Calafat AM, Kato K, Ye X, Broomhall S, Harden F and Mueller JF, 2014. Decline in perfluorooctane sulfonate and perfluorooctanoate serum concentrations in an Australian population from 2002 to 2011. Environment International, 71, 74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudel D, Horowitz L, Wormuth M, Scheringer M, Cousins IT and Hungerbühler K, 2008. Estimating consumer exposure to PFOS and PFOA. Risk Analysis, 28, 251–269. [DOI] [PubMed] [Google Scholar]
- Tucker DK, Macon MB, Strynar MJ, Dagnino S, Andersen E and Fenton SE, 2015. The mammary gland is a sensitive pubertal target in CD‐1 and C57Bl/6 mice following perinatal perfluorooctanoic acid (PFOA) exposure. Reproductive Toxicology, 54, 26–36. 10.1016/j.reprotox.2014.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl M, 2016. Unpublished report Um‐MuKi Bratislava‐Vienna study. Personal communication from Maria Uhl (Umweltbundesamt, Austria) to Line S. Haug. Email 30 August 2016.
- Ullah S, Huber S, Bignert A and Berger U, 2014. Temporal trends of perfluoroalkane sulfonic acids and their sulfonamide‐based precursors in herring from the Swedish west coast 1991‐2011 including isomer‐specific considerations. Environment International, 65, 63–72. 10.1016/j.envint.2014.01.005 [DOI] [PubMed] [Google Scholar]
- Urbanek‐Olejnik K, Liszewska M, Winczura A and Kostka G, 2016. Changes of c‐Myc and DNMT1 mRNA and protein levels in the rat livers induced by dibutyl phthalate treatment. Toxicology & Industrial Health, 32, 801–808. [DOI] [PubMed] [Google Scholar]
- US EPA (US Environmental Protection Agency), 2002. Telomer Research Program, 2002. Telomer Research Program Update, Presented to the USEPA OPPT, November 25, 2002; U.S. Environmental Protection Agency public docket AR226–1141, Washington, DC.
- Van Esterik JC, Bastos Sales L, Dolle ME, Hakansson H, Herlin M, Legler J and van der Ven LT, 2016. Programming of metabolic effects in C57BL/6JxFVB mice by in utero and lactational exposure to perfluorooctanoic acid. Archives of Toxicology, 90, 701–715. 10.1007/s00204-015-1488-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Loveren H, Van Amsterdam JGC, Vandebriel RJ, Kimman TG, Rümke HC, Steerenberg PS and Vos JG, 2001. Vaccine‐induced antibody responses as parameters of the influence of endogenous and environmental factors. Environmental Health Perspectives, 109, 757–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Heuvel JP, Kuslikis BI and Peterson RE, 1992. Covalent binding of perfluorinated fatty acids to proteins in the plasma, liver and testes of rats. Chemico‐Biological Interactions, 82, 317–328. [DOI] [PubMed] [Google Scholar]
- Vélez MP, Arbuckle TE and Fraser WD, 2015. Maternal exposure to perfluorinated chemicals and reduced fecundity: the MIREC study. Human Reproduction, 30, 701–709. 10.1093/humrep/deu350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verner MA, Loccisano AE, Morken NH, Yoon M, Wu H, McDougall R, Maisonet M, Marcus M, Kishi R, Miyashita C, Chen MH, Hsieh WS, Andersen ME, Clewell HJ 3rd and Longnecker MP, 2015. Associations of Perfluoroalkyl Substances (PFAS) with lower birth weight: an evaluation of potential confounding by glomerular filtration rate using a physiologically based pharmacokinetic model (PBPK). Environmental Health Perspectives, 123, 1317–1324. 10.1289/ehp.1408837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergaard S, Nielsen F, Andersson AM, Hjøllund NH, Grandjean P, Andersen HR and Jensen TK, 2012. Association between perfluorinated compounds and time to pregnancy in a prospective cohort of Danish couples attempting to conceive. Human Reproduction, 27, 873–880. 10.1093/humrep/der450 [DOI] [PubMed] [Google Scholar]
- Vestergren R, Ullah S, Cousins IT and Berger U, 2012. A matrix effect‐free method for reliable quantification of perfluoroalkyl carboxylic acids and perfluoroalkane sulfonic acids at low parts per trillion levels in dietary samples. Journal of Chromatography A, 1237, 64–71. [DOI] [PubMed] [Google Scholar]
- Vetvicka V and Vetvickova J, 2013. Reversal of perfluorooctanesulfonate‐induced immunotoxicity by a glucan‐resveratrol‐vitamin C combination. Oriental Pharmacy and Experimental Medicine, 13, 77–84. [Google Scholar]
- Viberg H, Lee I and Eriksson P, 2013. Adult dose‐dependent behavioral and cognitive disturbances after a single neonatal PFHxS dose. Toxicology, 304, 185–191. 10.1016/j.tox.2012.12.013 [DOI] [PubMed] [Google Scholar]
- Vorkamp K, Nielsen F, Kyhl HB, Husby S, Nielsen LB, Barington T, Andersson AM and Jensen TK, 2014. Polybrominated diphenyl ethers and perfluoroalkyl substances in serum of pregnant women: levels, correlations, and potential health implications. Archives of Environmental Contamination and Toxicology, 67, 9–20. [DOI] [PubMed] [Google Scholar]
- Vuong AM, Yolton K, Webster GM, Sjodin A, Calafat AM, Braun JM, Dietrich KN, Lanphear BP and Chen A, 2016. Prenatal polybrominated diphenyl ether and perfluoroalkyl substance exposures and executive function in school‐age children. Environmental Research, 147, 556–564. 10.1016/j.envres.2016.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan HT, Leung PY, Zhao YG, Wei X, Wong MH and Wong CK, 2013. Blood plasma concentrations of endocrine disrupting chemicals in Hong Kong populations. Journal of Hazardous Materials, 261, 763–769. [DOI] [PubMed] [Google Scholar]
- Wan HT, Zhao YG, Leung PY and Wong CKC, 2014. Perinatal exposure to perfluorooctane sulfonate affects glucose metabolism in adult offspring. PLoS ONE, 9, e87137 10.1371/journal.pone.0087137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Szostek B, Buck RC, Folsom PW, Sulecki LM and Gannon JT, 2009. 8‐2 Fluorotelomer alcohol aerobic soil biodegradation: Pathways, metabolites, and metabolite yields. Chemosphere, 75, 1089–1096. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang L, Liang Y, Qiu W, Zhang J, Zhou Q and Jiang G, 2011a. Modulation of dietary fat on the toxicological effects in thymus and spleen in BALB/c mice exposed to perfluorooctane sulfonate. Toxicology Letters, 204, 174–182. [DOI] [PubMed] [Google Scholar]
- Wang IJ, Hsieh WS, Chen CY, Fletcher T, Lien GW, Chiang HL, Chiang CF, Wu TN and Chen PC, 2011b. The effect of prenatal perfluorinated chemicals exposures on pediatric atopy. Environmental Research, 111, 785–791. [DOI] [PubMed] [Google Scholar]
- Wang Z, Cousins IT, Scheringer M and Hungerbühler K, 2013a. Fluorinated alternatives to long‐chain perfluoroalkyl carboxylic acids (PFCAs), perfluoroalkane sulfonic acids (PFSAs) and their potential precursors. Environment International, 60, 242–248. [DOI] [PubMed] [Google Scholar]
- Wang Y, Starling AP, Haug LS, Eggesbo M, Becher G, Thomsen C, Travlos G, King D, Hoppin JA, Rogan WJ and Longnecker MP, 2013b. Association between perfluoroalkyl substances and thyroid stimulating hormone among pregnant women: a cross‐sectional study. Environmental Health, 12, 76 10.1186/1476-069x-12-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Cousins IT, Scheringer M, Buck RC and Hungerbühler K, 2014a. Global emission inventories for C14–C14 perfluoroalkyl carboxylic acid (PFCA) homologues from 1951 to 2030, Part I: production and emissions from quantifiable sources. Environment International, 70, 62–75. [DOI] [PubMed] [Google Scholar]
- Wang Z, Cousins IT, Scheringer M, Buck RC and Hungerbühler K, 2014b. Global emission inventories for C14–C14 perfluoroalkyl carboxylic acid (PFCA) homologues from 1951 to 2030, Part II: The remaining pieces of the puzzle. Environment International, 69, 166–176. [DOI] [PubMed] [Google Scholar]
- Wang Y, Rogan WJ, Chen PC, Lien GW, Chen HY, Tseng YC, Longnecker MP and Wang SL, 2014c. Association between maternal serum perfluoroalkyl substances during pregnancy and maternal and cord thyroid hormones: Taiwan maternal and infant cohort study. Environmental Health Perspectives, 122, 529–534. 10.1289/ehp.1306925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Yan Shengmin, Zhang Wei, Zhang Hongxia and Dai Jiayin, 2015a. Integrated proteomic and miRNA transcriptional analysis reveals the hepatotoxicity mechanism of PFNA exposure in mice. Journal of Proteome Research, 14, 330–341. [DOI] [PubMed] [Google Scholar]
- Wang Y, Rogan WJ, Chen HY, Chen PC, Su PH, Chen HY and Wang SL, 2015b. Prenatal exposure to perfluroalkyl substances and children's IQ: the Taiwan maternal and infant cohort study. International Journal of Hygiene and Environmental Health, 218, 639–644. 10.1016/j.ijheh.2015.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Adgent M, Su PH, Chen HY, Chen PC, Hsiung CA and Wang SL, 2016. Prenatal exposure to perfluorocarboxylic acids (PFCAs) and fetal and postnatal growth in the Taiwan Maternal and Infant Cohort Study. Environmental Health Perspectives, 124, 1794–1800. 10.1289/ehp.1509998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Boucher JM, Scheringer M, Cousins IT and Hungerbühler K, 2017a. Toward a Comprehensive Global Emission Inventory of C4–C10 Perfluoroalkanesulfonic Acids (PFSAs) and related precursors: focus on the life Cycle of C8‐Based Products and Ongoing Industrial Transition. Environmental Science & Technology, 51, 4482–4493. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhao F, Kan M, Wen Z, Zhou Y, Yu L and Liu H, 2017b. Effects of perfluorooctanoic acid on oxidative stress and PPARα and its related CYP4A1 gene expression in rat liver. Wei Sheng Yan Jiu, 46, 802–806. [PubMed] [Google Scholar]
- Wang Z, DeWitt JC, Higgins CP and Cousins IT, 2017c. A never‐ending story of per‐ and polyfluoroalkyl substances (PFASs)? Environmental Science & Technology, 51, 2508–2518. [DOI] [PubMed] [Google Scholar]
- Wang Y, Shi Y, Vestergren R, Zhou Z, Liang Y and Cai Y, 2018a. Using hair, nail and urine samples for human exposure assessment of legacy and emerging per‐ and polyfluoroalkyl substances. Science of the Total Environment, 636, 383–391. [DOI] [PubMed] [Google Scholar]
- Wang X, Kong B, He B, Wei L, Zhu J, Jin Y, Shan Y, Wang W, Pan C and Fu Z, 2018b. 8:2 Fluorotelomer alcohol causes immunotoxicity and liver injury in adult male C57BL/6 mice. Environmental Toxicology, 34, 141–149. [DOI] [PubMed] [Google Scholar]
- Wang H, Du H, Yang J, Jiang H, O K, Xu L, Liu S, Yi J, Qian X, Chen Y, Jiang Q and He G, 2019a. PFOS, PFOA, estrogen homeostasis, and birth size in Chinese infants. Chemosphere, 221, 349–355. 10.1016/j.chemosphere.2019.01.061 [DOI] [PubMed] [Google Scholar]
- Wang W, Zhou W, Wu S, Liang F, Li Y, Zhang J, Cui L, Feng Y and Wang Y, 2019b. Perfluoroalkyl substances exposure and risk of polycystic ovarian syndrome related infertility in Chinese women. Environmental Pollution, 247, 824e831. 10.1016/j.envpol.2019.01.039 [DOI] [PubMed] [Google Scholar]
- Washburn ST, Bingman TS, Braithwaite SK, Buck RC, Buxton LW, Clewell HJ, Haroun LA, Kester JE, Rickard RW and Shipp AM, 2005. Exposure assessment and risk characterization for perfluorooctanoate in selected consumer articles. Environmental Science & Technology, 39, 3904–3910. [DOI] [PubMed] [Google Scholar]
- Watkins DJ, Josson J, Elston B, Bartell SM, Shin HM, Vieira VM, Savitz DA, Fletcher T and Wellenius GA, 2013. Exposure to perfluoroalkyl acids and markers of kidney function among children and adolescents living near a chemical plant. Environmental Health Perspectives, 121, 625–630. 10.1289/ehp.1205838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver YM, Ehresman DJ, Butenhoff JL and Hagenbuch B, 2010. Roles of rat renal organic anion transporters in transporting perfluorinated carboxylates with different chain lengths. Toxicological Sciences, 113, 305–314. 10.1093/toxsci/kfp275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster GM, Venners SA, Mattman A and Martin JW, 2014. Associations between perfluoroalkyl acids (PFASs) and maternal thyroid hormones in early pregnancy: a population‐based cohort study. Environmental Research, 133, 338–347. 10.1016/j.envres.2014.06.012 [DOI] [PubMed] [Google Scholar]
- Webster GM, Rauch SA, Marie NS, Mattman A, Lanphear BP and Venners SA, 2016. Cross‐Sectional Associations of Serum Perfluoroalkyl Acids and Thyroid Hormones in U.S. Adults: Variation According to TPOAb and Iodine Status (NHANES 2007‐2008). Environmental Health Perspectives, 124, 935–942. 10.1289/ehp.1409589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JM, Andersson PL, Lamoree MH, Leonards PE, van Leeuwen SP and Hamers T, 2009. Competitive binding of poly‐ and perfluorinated compounds to the thyroid hormone transport protein transthyretin. Toxicological Sciences, 109, 206–216. 10.1093/toxsci/kfp05 [DOI] [PubMed] [Google Scholar]
- Wen LL, Lin LY, Su TC, Chen PC and Lin CY, 2013. Association between serum perfluorinated chemicals and thyroid function in U.S. adults: the National Health and Nutrition Examination Survey 2007‐2010. Journal of Clinical Endocrinoliocy and Metabolism, 98, E1456–E1464. [DOI] [PubMed] [Google Scholar]
- West D, James N, Holden P, Brindle R, Rolfe M and Roberts R, 1999. Role for tumour necrosis factor a (TNFα) receptor 1 (TNFR1) and interleukin 1 receptor (IL1R) in the suppression of apoptosis by peroxisome proliferators. Hepatology, 30, 1417–1424. [DOI] [PubMed] [Google Scholar]
- White SS, Calafat AM, Kuklenyik Z, Villanueva L, Zehr RD, Helfant L, Strynar MJ, Lindstrom AB, Thibodeaux JR, Wood C and Fenton SE, 2007. Gestational PFOA exposure of mice is associated with altered mammary gland development in dams and female offspring. Toxicological Sciences, 96, 133–144. [DOI] [PubMed] [Google Scholar]
- White SS, Kato K, Jia LT, Basden BJ, Calafat AM, Hines EP, Stanko JP, Wolf CJ, Abbott BD and Fenton SE, 2009. Effects of perfluorooctanoic acid on mouse mammary gland development and differentiation resulting from cross‐foster and restricted gestational exposures. Reproductive Toxicology, 27, 289–298. 10.1016/j.reprotox.2008.11.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SS, Stanko JP, Kato K, Calafat AM, Hines EP and Fenton SE, 2011. Gestational and chronic low‐dose PFOA exposures and mammary gland growth and differentiation in three generations of CD‐1 mice. Environmental Health Perspectives, 119, 1070–1076. 10.1289/ehp.1002741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO/IPCS (World Health Organization/International Programme on Chemical Safety), 2009. Environmental Health Criteria 240. Principles and methods for the assessment of chemicals in food.
- WHO/IPCS , 2010. Characterization and application of physiologically based pharmacokinetic models in risk assessment IPCS Harmonization Project Document No. 9, WHO/IPCS, Geneva, Switzerland.
- WHO/IPCS (World Health Organization/International Programme on Chemical Safety), 2012. Guidance for immunotoxicity risk assessment for chemicals. Available online: http://www.inchem.org/documents/harmproj/harmproj/harmproj10.pdf
- Wielsøe M, Long M, Ghisari M and Bonefeld‐Jørgensen EC, 2015. Perfluoroalkylated substances (PFAS) affect oxidative stress biomarkers in vitro . Chemosphere, 129, 239–245. 10.1016/j.chemosphere.2014.10.014 [DOI] [PubMed] [Google Scholar]
- Wielsøe M, Kern P and Bonefeld‐Jorgensen EC, 2017. Serum levels of environmental pollutants is a risk factor for breast cancer in Inuit: a case control study. Environmental Health, 16, 56 10.1186/s12940-017-0269-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIL Research Laboratories , 2005. A combined 28‐day repeated dose oral toxicity study with the reproduction/developmental toxicity screening test of perfluorohexanoic acid and 1H, 1H, 2H, 2H‐tridecafluoro‐1-octanol in rats, with recovery. Study number WIL‐534001. Sponsor: AGC Chemical. Available online: http://mail.agc-chemicals.com/file.jsp?id=file/6-2FTOH-5.pdf
- Wilhelm M, Hölzer J, Dobler L, Rauchfuss K, Midasch O, Kraft M, Angerer J and Wiesmüller G, 2009. Preliminary observations on perfluorinated compounds in plasma samples (1977‐2004) of young German adults from an area with perfluorooctanoate‐contaminated drinking water. International Journal of Hygiene and Environmental Health, 212, 142–145. [DOI] [PubMed] [Google Scholar]
- Wilhelm M, Wittsiepe J, Völkel W, Fromme H and Kasper‐Sonnenberg M, 2015. Perfluoroalkyl acids in children and their mothers: Association with drinking water and time trends of inner exposures—Results of the Duisburg birth cohort and Bochum cohort studies. International Journal of Hygiene and Environmental Health, 218, 645–655. 10.1016/j.ijheh.2015.07.001 [DOI] [PubMed] [Google Scholar]
- Wolf DC, Moore T, Abbott BD, Rosen MB, Das KP, Zehr RD, Lindstrom AB, Strynar MJ and Lau C, 2008. Comparative hepatic effects of perfluorooctanoic acid and WY 14,643 in PPAR‐alpha knockout and wild‐type mice. Toxicologic Pathology, 36, 632–639. [DOI] [PubMed] [Google Scholar]
- Wolf CJ, Schmid JE, Lau C and Abbott BD, 2012. Activation of mouse and human peroxisome proliferator‐activated receptor‐alpha (PPARα) by perfluoroalkyl acids (PFAAs): further investigation of C4‐C12 compounds. Reproductive Toxicology, 33, 546–551. 10.1016/j.reprotox.2011.09.009 [DOI] [PubMed] [Google Scholar]
- Wong F, MacLeod M, Mueller JF and Cousins IT, 2014. Enhanced elimination of perfluorooctane sulfonic acid by menstruating women: evidence from population‐based pharmacokinetic modeling. Environmental Science & Technology, 48, 8807–8814. [DOI] [PubMed] [Google Scholar]
- Wong F, MacLeod M, Mueller JF and Cousins IT, 2015. Response to Comment on “Enhanced Elimination of Perfluorooctane Sulfonic Acid by Menstruating Women: evidence from population‐based pharmacokinetic modeling. Environmental Science and Technology, 49, 5838–5839. [DOI] [PubMed] [Google Scholar]
- Woodcroft MW, Ellis DA, Rafferty SP, Burns DC, March RE, Stock NL, Trumpour KS, Yee J and Munro K, 2010. Experimental characterization of the mechanism of perfluorocarboxylic acids’ liver protein bioaccumulation: the key role of the neutral species. Environmental Toxicology and Chemistry, 29, 1669–1677. 10.1002/etc.199 [DOI] [PubMed] [Google Scholar]
- Worley RR, Moore SM, Tierney BC, Ye X, Calafat AM, Campbell S, Woudneh MB and Fisher J, 2017. Per‐ and polyfluoroalkyl substances in human serum and urine samples from a residentially exposed community. Environment International, 106, 135–143. 10.1016/j.envint.2017.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Xie G, Xu X, Wu W and Yang B, 2018. Adverse bioeffect of perfluorooctanoic acid on liver metabolic function in mice. Environmental Science and Pollution Research International, 25, 4787–4793. 10.1007/s11356-017-0872-7 [DOI] [PubMed] [Google Scholar]
- Xia W, Wan Y, Li YY, Zeng H, Lv Z, Li G, Wei Z and Xu SQ, 2011. PFOS prenatal exposure induce mitochondrial injury and gene expression change in hearts of weaned SD rats. Toxicology, 282, 23–29. [DOI] [PubMed] [Google Scholar]
- Xie W, Wu Q, Kania‐Korwel I, Tharappel JC, Telu S, Coleman MC, Glauert HP, Kannan K, Mariappan SV, Spitz DR, Weydert J and Lehmler HJ, 2009. Subacute exposure to N‐ethyl perfluorooctanesulfonamidoethanol results in the formation of perfluorooctanesulfonate and alters superoxide dismutase activity in female rats. Archives of Toxicology, 83, 909–924. 10.1007/s00204-009-0450-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Krenitsky DM, Seacat AM, Butenhoff JL and Anders MW, 2004. Biotransformation of N‐Ethyl‐N‐(2‐hydroxyethyl)perfluorooctane sulfonamide by Rat Liver Microsomes, Cytosol, and Slices and by Expressed Rat and Human Cytochromes P450. Chemical Research in Toxicology, 17, 767–775. 10.1021/tx034222x [DOI] [PubMed] [Google Scholar]
- Yahia D, Tsukuba C, Yoshida M, Sato I and Tsuda S, 2008. Neonatal death of mice treated with perfluorooctane sulfonate. Journal of Toxicological Sciences, 33, 219–226. [DOI] [PubMed] [Google Scholar]
- Yahia D, El‐Nasser MA, Abedel‐Latif M, Tsukuba C, Yoshida M, Sato I and Tsuda S, 2010. Effects of perfluorooctanoic acid (PFOA) exposure to pregnant mice on reproduction. Journal of Toxicological Sciences, 35, 527–533. [DOI] [PubMed] [Google Scholar]
- Yahia D, Haruka I, Kagashi Y and Tsuda S, 2014. 8‐Hydroxy‐2’‐deoxyguanosine as a biomarker of oxidative DNA damage induced by perfluorinated compounds in TK6 cells. Environmental Toxicology, 31, 192–200. 10.1002/tox.22034 [DOI] [PubMed] [Google Scholar]
- Yang Q, Yamada A, Peters S, Kimura JM and Gonzalez F, 2006a. Alterations in Skin and Stratified Epithelia by Constitutively Activated PPARα. Journal of Investigative Dermatology, 126, 374–385. [DOI] [PubMed] [Google Scholar]
- Yang Q, Kurotani R, Yamada A, Kimura S and Gonzalez F, 2006b. PPARα activation during pregnancy severely impairs mammary lobuloalveolar development in mice. Endocrinology, 147, 4772–4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Nagano T, Shah Y, Cheung C, Ito S and Gonzalez FJ, 2008. The PPAR alpha‐humanized mouse: a model to investigate species differences in liver toxicity mediated by PPAR alpha. Toxicological Sciences, 101, 132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Tan YS, Harkema JR and Haslam SZ, 2009. Differential effects of peripubertal exposure to perfluorooctanoic acid on mammary gland development in C57Bl/6 and Balb/c mouse strains. Reproductive Toxicology, 27, 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CH, Glover KP and Han X, 2010. Characterization of cellular uptake of perfluorooctanoate via organic anion transporting polypeptide 1A2, organic anion transporter 4, and urate transporter 1 for their potential roles in mediating human renal reabsorption of perfluorocarboxylates. Toxicological Sciences, 117, 294–302. [DOI] [PubMed] [Google Scholar]
- Yang L, Li J, Lai J, Luan H, Cai Z, Wang Y, Zhao Y and Wu Y, 2016. Placental transfer of perfluoroalkyl substances and associations with thyroid hormones: Beijing prenatal exposure study. Scientific Reports, 6, 21699 10.1038/srep21699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Q, Shi R, Wang CF, Han WC, Gao Y, Zhang Y, Zhou YJ, Ding GD and Tian Y, 2019. Cord blood Per‐ and polyfluoroalkyl substances, placental steroidogenic enzyme, and cord blood reproductive hormone. Environment International, 129, 573–582. 10.1016/j.envint.2019.03.047 [DOI] [PubMed] [Google Scholar]
- Yeung LW, Robinson SJ, Koschorreck J and Mabury SA, 2013a. Part I. A temporal study of PFCAs and their precursors in human plasma from two German cities 1982‐2009. Environmental Science & Technology, 47, 3865–3874. [DOI] [PubMed] [Google Scholar]
- Yeung LW, Robinson SJ, Koschorreck J and Mabury SA, 2013b. Part II. A temporal study of PFOS and its precursors in human plasma from two German cities in 1982‐2009. Environmental Science & Technology, 47, 3875–3882. [DOI] [PubMed] [Google Scholar]
- Young CJ and Mabury SA, 2010. Atmospheric perfluorinated acid precursors: chemistry, occurrence, and impacts. Reviews of Environmental Contamination and Toxicology, 208, 1–109. [DOI] [PubMed] [Google Scholar]
- Yu WG, Liu W and Jin YH, 2009. Effects of perfluorooctane sulfonate on rat thyroid hormone biosynthesis and metabolism. Environmental Toxicology and Chemistry, 28, 990–996. [DOI] [PubMed] [Google Scholar]
- Zabaleta I, Negreira N, Bizkarguenaga E, Prieto A, Covaci A and Zuloaga O, 2017. Screening and identification of per‐ and polyfluoroalkyl substances in microwave popcorn bags. Food Chemistry, 230, 497–506. 10.1016/j.foodchem.2017.03.074 [DOI] [PubMed] [Google Scholar]
- Zarei MH, Hosseini Shirazi SF, Aghvami M and Pourahmad J, 2018. Perfluorooctanesulfonate (PFOS) Induces Apoptosis Signaling and Proteolysis in Human Lymphocytes through ROS Mediated Mitochondrial Dysfunction and Lysosomal Membrane Labialization. Iranian Journal of Pharmaceutical Research, 17, 995–1007. [PMC free article] [PubMed] [Google Scholar]
- Zeilmaker M, Fragki S, Verbruggen E, Bokkers B and Lijzen J, 2018Mixture exposure to PFAS: A Relative Potency Factor approach. RIVM Report 2018‐0070. 10.21945/RIVM-2018-0070. Available online: https://www.rivm.nl/bibliotheek/rapporten/2018-0070.pdf [DOI]
- Zeng XW, Qian Z, Emo B, Vaughn M, Bao J, Qin XD, Zhu Y, Li J, Lee YL and Dong GH, 2015. Association of polyfluoroalkyl chemical exposure with serum lipids in children. Science of Total Environment, 512–513, 364–370. [DOI] [PubMed] [Google Scholar]
- Zeng XW, Bloom MS, Dharmage SC, Lodge CJ, Chen D, Li S, Guo Y, Roponen M, Jalava P, Hirvonen MR, Ma H, Hao YT, Chen W, Yang M, Chu C, Li QQ, Hu LW, Liu KK, Yang BY, Liu S, Fu C and Dong GH, 2019. Prenatal exposure to perfluoroalkyl substances is associated with lower hand, foot and mouth disease viruses antibody response in infancy: Findings from the Guangzhou Birth Cohort Study. Science of the Total Environment, 663, 60–67. 10.1016/j.scitotenv.2019.01.325 [DOI] [PubMed] [Google Scholar]
- Zhang T and Qin X, 2014. Assessment of fetal exposure and maternal elimination of perfluoroalkyl substances. Environmental Sciences: Processes & Impacts, 16, 1878–1881. 10.1039/C4EM00129J [DOI] [PubMed] [Google Scholar]
- Zhang H, Shi Z, Liu Y, Wei Y and Dai J, 2008. Lipid homeostasis and oxidative stress in the liver of male rats exposed to perfluorododecanoic acid. Toxicology and Applied Pharmacology, 227, 16–25. [DOI] [PubMed] [Google Scholar]
- Zhang H, Ding L, Fang X, Shi Z, Zhang Y, Chen H, Yan X and Dai J, 2011. Biological responses to perfluorododecanoic acid exposure in rat kidneys as determined by integrated proteomic and metabonomic studies. PLoS ONE, 6, e20862 10.1371/journal.pone.0020862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Beesoon S, Zhu L and Martin JW, 2013a. Biomonitoring of perfluoroalkyl acids in human urine and estimates of biological half‐life. Environmental Science & Technology, 47, 10619–10627. [DOI] [PubMed] [Google Scholar]
- Zhang LY, Ren XM and Guo LH, 2013b. Structure‐based investigation on the interaction of perfluorinated compounds with human liver fatty acid binding protein. Environmental Science & Technology, 47, 11293–11301. 10.1021/es4026722 [DOI] [PubMed] [Google Scholar]
- Zhang T, Sun H, Lin Y, Qin X, Zhang Y, Geng X and Kannan K, 2013c. Distribution of poly‐ and perfluoroalkyl substances in matched samples from pregnant women and carbon chain length related maternal transfer. Environmental Science & Technology, 47, 7974–7981. [DOI] [PubMed] [Google Scholar]
- Zhang Y‐H, Wang J, Dong G‐H, Liu M‐M, Wang D, Zheng L and Jin Y‐H, 2013d. Mechanism of perfluorooctanesulfonate (PFOS)‐induced apoptosis in the immunocyte. Journal of Immunotoxicology, 10, 49–58. [DOI] [PubMed] [Google Scholar]
- Zhang H, Hou J, Cui R, Guo X, Shi Z, Yang F and Dai J, 2013e. Phosphoproteome analysis reveals an important role for glycogen synthase kinase‐3 in perfluorododecanoic acid‐induced rat liver toxicity. Toxicology Letters, 218, 61–69. 10.1016/j.toxlet.2013.01.012 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Jiang W, Fang S, Zhu L and Deng J, 2014a. Perfluoroalkyl acids and the isomers of perfluorooctanesulfonate and perfluorooctanoate in the sera of 50 new couples in Tianjin, China. Environment International, 68, 185–191. [DOI] [PubMed] [Google Scholar]
- Zhang H, Lu Y, Luo B, Yan S, Guo X and Dai J, 2014b. Proteomic analysis of mouse testis reveals perfluorooctanoic acid‐induced reproductive dysfunction via direct disturbance of testicular steroidogenic machinery. Journal of Proteome Research, 13, 3370–3385. 10.1021/pr500228d [DOI] [PubMed] [Google Scholar]
- Zhang H, Vestergren R, Wang T, Yu J, Jiang G and Herzke D, 2017. Geographical Differences in Dietary Exposure to Perfluoroalkyl Acids between Manufacturing and Application Regions in China. Environmental Science & Technology, 51, 5747–5755. 10.1021/acs.est.7b00246 [DOI] [PubMed] [Google Scholar]
- Zhang S, Tan R, Pan R, Xiong J, Tian Y, Wu J and Chen L, 2018. Association of perfluoroalkyl and polyfluoroalkyl substances with premature ovarian insufficiency in chinese women. Clinical Endocrinology and Metabolism, 103, 2543–2551. 10.1210/jc.2017-02783 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Tan YS, Haslam SZ and Yang C, 2010. Perfluorooctanoic acid effects on steroid hormone and growth factor levels mediate stimulation of peripubertal mammary gland development in C57BL/6 mice. Toxicological Sciences, 115, 214–224. 10.1093/toxsci/kfq030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Tan YS, Strynar MJ, Perez G, Haslam SZ and Yang C, 2012. Perfluorooctanoic acid effects on ovaries mediate its inhibition of peripubertal mammary gland development in Balb/c and C57Bl/6 mice. Reproductive Toxicology, 33, 563–576. 10.1016/j.reprotox.2012.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Li L, Liu J, Li H, Zhang C, Han P, Zhang Y, Yuan X, Ge RS and Chu Y, 2014. Exposure to perfluorooctane sulfonate in utero reduces testosterone production in rat fetal Leydig cells. PLoS ONE, 9, e78888 10.1371/journal.pone.007888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Zitzow JD, Ehresman DJ, Chang SC, Butenhoff JL, Forster J and Hagenbuch B, 2015. Na+/Taurocholate Cotransporting Polypeptide and Apical Sodium‐Dependent Bile Acid Transporter Are Involved in the Disposition of Perfluoroalkyl Sulfonates in Humans and Rats. Toxicological Sciences, 146, 363–373. 10.1093/toxsci/kfv102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Zitzow JD, Weaver Y, Ehresman DJ, Chang SC, Butenhoff JL and Hagenbuch B, 2017. Organic Anion Transporting Polypeptides Contribute to the Disposition of Perfluoroalkyl Acids in Humans and Rats. Toxicological Sciences, 156, 84–95. 10.1093/toxsci/kfw236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Dong GH, Jin YH and He QC, 2009. Immunotoxic changes associated with a 7‐day oral exposure to perfluorooctanesulfonate (PFOS) in adult male C57BL/6 mice. Archives of Toxicology, 83, 679–689. [DOI] [PubMed] [Google Scholar]
- Zheng L, Dong GH, Zhang YH, Liang ZF, Jin YH and He QC, 2011. Type 1 and Type 2 cytokines imbalance in adult male C57BL/6 mice following a 7‐day oral exposure to perfluorooctanesulfonate (PFOS). Journal of Immunotoxicology, 8, 30–38. [DOI] [PubMed] [Google Scholar]
- Zheng F, Sheng N, Zhang H, Yan S, Zhang J and Wang J, 2017. Perfluorooctanoic acid exposure disturbs glucose metabolism in mouse liver. Toxicology and Applied Pharmacology, 335, 41–48. 10.1016/j.taap.2017.09.019 [DOI] [PubMed] [Google Scholar]
- Zhou X, Dong T, Fan Z, Peng Y, Zhou R, Wang X, Song N, Han M, Fan B, Jia J and Liu S, 2017. Perfluorodecanoic acid stimulates NLRP3 inflammasome assembly in gastric cells. Scientific Reports, 7, 45468 10.1038/srep45468 [DOI] [PMC free article] [PubMed] [Google Scholar]


