Abstract
In Nepal, the prevalence of anaemia decreased by 1% from 2006 to 2011 but increased by 6% from 2011 to 2016. In this study, we examined the changes in prevalence by possible factors from 2006 to 2016 along with the factors associated with anaemia among women of reproductive age (15–49 years) using the Nepal Demographic and Health Survey (NDHS) data from years 2006, 2011 and 2016. We used rate of change analysis to explore average annual rate of change (AARC) in anaemia prevalence and concentration curves and indices to assess unequal distribution of anaemia prevalence among socio‐economic quintiles. Multilevel regression was performed to examine the association of multilevel factors with anaemia. Our results showed higher AARC increase in anaemia prevalence from 2006 to 2016, among women aged 30–39 years, with secondary or higher education, who had two or fewer children, not working women, from higher wealth quintiles and who were overweight or obese. Shifting of concentration curve from ‘above the line of equality’ in 2006 and 2011 to ‘under the line of equality’ in 2016 was observed. Women aged 20–29 years, with more than four children, who underwent female sterilization, had experienced violence and from Provinces 1, 2 and 5 were at higher risk of anaemia. Overweight and obese women using hormonal contraception and from lowest wealth quintiles were at lower risk. The change in trends and the associated multilevel factors identified should be considered in designing multilevel interventions that particularly target women at risk for sustainable anaemia reduction.
Keywords: anaemia, concentration curve, concentration index, demographic and health survey, hierarchical data, multilevel analysis, women of reproductive age
Key messages.
Women aged 30–39 years, having secondary or higher education, not working, who had one or two children, who were overweight and belonging to Bagmati (Province 3) had the highest annual increase in anaemia prevalence from 2006 to 2016.
From 2006 to 2016, a shift in anaemia distribution from poorer women towards women belonging to higher wealth quintiles was noted.
Women aged 20–29 years, with more than four children, who underwent female sterilization, , had experienced violence and from Provinces 1, 2, and 5 were at higher risk of anaemia.
1. INTRODUCTION
The estimated global anaemia prevalence was 29%, translating to 496 million nonpregnant anaemic women of reproductive age (WRA) (15–49 years) in 2011 (Stevens et al., 2013). In South Asia, anaemia affects nearly half (47%; confidence interval [CI] 33%–59%) of the total WRA (Stevens et al., 2013) and is associated with adverse health consequences such as maternal death (Brabin, Hakimi, & Pelletier, 2001; Daru et al., 2018), preterm birth, low birth weight (Bondevik, Lie, Ulstein, & Kvale, 2001; M. M. Rahman et al., 2016), stillbirths and neonatal death (Black et al., 2008). In the last decade, many countries in South Asia implemented policies to meet the Millennium Development Goals, which led to significant increases in health care service utilization and reduction in undernutrition and anaemia (United Nations, 2015).
In South Asia, anaemia reduced from 53% in 1995 to 47% in 2011 (Stevens et al., 2013). Nepal was also able to reduce the prevalence of anaemia among WRA from 36% in 2006 to 35% in 2011 (Ministry of Health, New ERA, & ICF International Inc, 2017). After the development of the national strategy to control anaemia in 2002 (Ministry of Health, 2002), Nepal continued to prioritize anaemia as a significant public health problem. Consequently, public health programmes were implemented to increase the coverage of several anaemia prevention programmes such as iron‐folic acid (IFA) supplementation and deworming programme targeted at pregnant and postpartum women and children (Pokharel, Maharjan, Mathema, & Harvey, 2011) along with nutrition awareness programmes targeted at the general population. Despite the continued programme efforts, the prevalence of anaemia increased from 2011 to 2016 (41% in 2016), and the reasons behind this is not clearly known.
The occurrence of anaemia is associated with different etiological factors, such as nutritional deficiencies (e.g., iron deficiency, vitamin B12 and folate deficiency), infectious diseases (malaria and hook worm infestations) or blood disorders (World Health Organization, 2017). Also significant to this risk are different social and environmental factors at multiple levels (Y. Balarajan, Ramakrishnan, Ozaltin, Shankar, & Subramanian, 2011). Further investigation of factors at multiple levels, such as individual, household and community levels, is needed to better understand the problem and guide the design of interventions.
Previous studies conducted to analyse the factors associated with anaemia among WRA in Nepal have only explored a few specific individual and household factors (Chandyo et al., 2007; Gautam, Min, Kim, & Jeong, 2019; Harding, Aguayo, Namirembe, & Webb, 2018), and some are based on the analysis of a specific sample or subnational data (Baral & Onta, 2009; Mishra, Marasini, Gupta, Agrawal, & Gautam, 2018; Sinha, Majumdar, & Yadav, 2011). For instance, a study conducted by Gautam et al. in 2019 (using 2016 Nepal Demographic and Health Survey [NDHS]) identified that source of drinking water, women's use of hormonal contraception, overweight/obesity and smoking status were significantly associated with anaemia (Gautam et al., 2019). Harding et al. in 2018 (using 2011 NDHS) analysed individual, household and environmental determinants of anaemia and reported that month of interview, ecological zones, source of water, age, body mass index (BMI) and breastfeeding status were associated with anaemia among WRA in Nepal (Harding et al., 2018). Both studies used single‐level analysis techniques for analysing the NDHS datasets, which were hierarchical and therefore overestimated or underestimated the actual effects.
The previous studies analysing anaemia risk in Nepal have not considered multilevel modelling approach, which is generally recommended for the analysis of hierarchical structure of the demographic and health survey data (Hox, Moerbeek, & Van de Schoot, 2017). Moreover, there is a gap in existing analyses in assessment of changes in possible factors of anaemia and whether socio‐economic inequalities in prevalence of anaemia have changed from the year 2006 to 2016. In the current study, we applied a statistically robust method to analyse NDHS 2006, 2011 and 2016 datasets with consideration of an extensive range of confounders to overcome the limitations of previous studies and present the factors of anaemia among women in Nepal at individual, household and community levels. We used the United Nations Children's Fund's (UNICEF) conceptual framework for undernutrition (UNICEF, 1990) and performed broad literature search to identify possible factors of anaemia among WRA (Figure 1). Moreover, recent studies from South Asia and low‐ and middle‐income countries were reviewed and discussed in order to enable comparability of the findings (Y. Balarajan et al., 2011; Y. S. Balarajan, Fawzi, & Subramanian, 2013; Christian, Khatry, & West, 2004; Dreyfuss et al., 2000; Gautam et al., 2019; Harding et al., 2018; Kamruzzaman, Rabbani, Saw, Sayem, & Hossain, 2015; Makhoul et al., 2012; Nguyen, Scott, Avula, Tran, & Menon, 2018; Pasricha et al., 2008; Shah & Baig, 2005).
FIGURE 1.
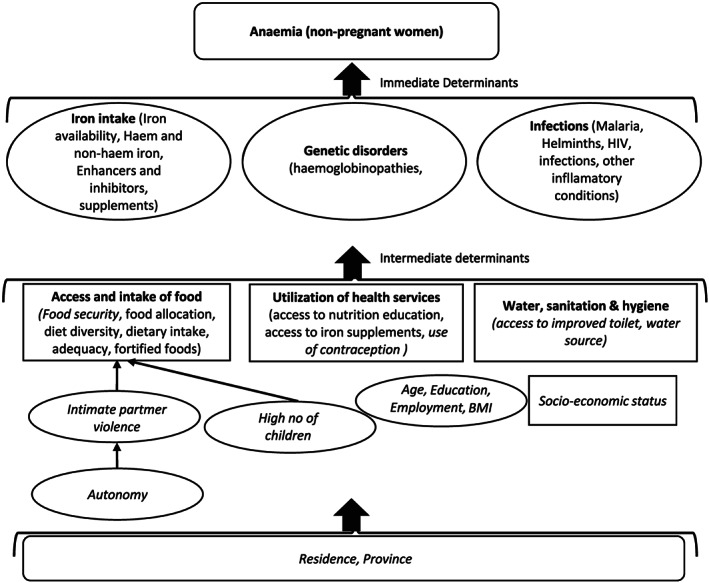
Conceptual framework for determinants of anaemia
2. METHODS
2.1. Study design and setting
In this study, three rounds of NDHS data collected in 2006 (February to August), 2011 (January to June) and 2016 (June 2016 to January 2017) were used for rate of change analysis. The number of households, response rates and sample sizes used in this study are presented in Table 1. In the 2016 NDHS, a two‐stage stratified sampling procedure was performed. The primary sampling units (clusters) were selected using probability proportional to size, followed by listing of households in selected clusters, which served as the sampling frame in the second stage. Then, using equal probability systematic selection, 30 households per sampling unit (cluster) were selected from sampling frame (Ministry of Health, New ERA, & ICF International Inc, 2017).
TABLE 1.
Characteristics of NDHS data and of WRA (15–49 years) in 2006, 2011 and 2016 NDHS
| Characteristics of NDHS | NDHS year | |||||
|---|---|---|---|---|---|---|
| 2006 | 2011 | 2016 | ||||
| Total households (N) | 8,707 | 10,826 | 11,490 | |||
| Response rate (%) | 99.6 | 99.4 | 98.5 | |||
| Total women sampled (N) | 10,793 | 12,674 | 12,862 | |||
| Pregnant during survey (N (%)) | 626 (5.8) | 614 (4.8) | 536 (4.2) | |||
| Nonpregnant women with no haemoglobin measurements (N (%)) | 126 (1.2) | 6,266 (52.0) | 6,192 (50.0) | |||
| Nonpregnant women with haemoglobin measurements available (N) a | 10,041 | 5,794 | 6,134 | |||
| Weighted N b | 10,043 | 5,795 | 6,124 | |||
| Characteristics of WRA | N c | Mean (±SD) | N c | Mean (±SD) | N c | Mean (±SD) |
| Age of women (years) | 10,043 | 29.0 (10.0) | 5,795 | 29.1 (9.8) | 6,124 | 29.5 (9.8) |
| Education (years of schooling) | 10,043 | 3.2 (4.0) | 5,795 | 4.4 (4.2) | 6,124 | 5.1 (4.4) |
| Total number of children | 10,043 | 2.2 (0.8) | 5,795 | 2.1 (0.9) | 6,124 | 2.0 (0.9) |
| % [95% CI] | % [95% CI] | % [95% CI] | ||||
| Education | ||||||
| No education | 5,310 | 52.9 [51.9, 53.8] | 2,284 | 39.4 [38.2, 40.7] | 2,073 | 33.9 [32.7, 35.0] |
| Primary | 1,762 | 17.5 [16.8, 18.3] | 1,026 | 17.7 [16.7, 18.7] | 1,007 | 16.4 [15.5, 17.4] |
| Secondary | 2,571 | 25.6 [24.8, 26.5] | 2,052 | 35.4 [34.2, 36.7] | 2,167 | 35.4 [34.2, 36.6] |
| Higher secondary and above | 399 | 4.0 [3.6, 4.4] | 433 | 7.5 [6.8, 8.2] | 877 | 14.3 [13.5, 15.2] |
| Currently working | 7,208 | 71.8 [70.9, 72.6] | 3,593 | 62.0 [60.7, 63.2] | 3,563 | 58.2 [56.9, 59.4] |
| Prevalence of underweight | 2,429 | 24.2 [23.3, 25.1] | 1,052 | 18.2 [17.2, 19.2] | 1,051 | 17.2 [16.2, 18.1] |
| Prevalence of overweight/obesity | 856 | 8.5 [8.0, 9.1] | 770 | 13.3 [12.4, 14.2] | 1,354 | 22.1 [21.1, 23.2] |
| Prevalence of anaemia | ||||||
| Not anaemic | 6,480 | 64.5 [63.6, 65.5] | 3,804 | 65.6 [64.4, 66.9] | 3,643 | 59.5 [58.3, 60.7] |
| Anaemic | 3,563 | 35.5 [34.5, 36.4] | 1,991 | 34.4 [33.1, 35.6] | 2,481 | 40.5 [39.3, 41.7] |
Abbreviations: CI, confidence interval; NDHS, Nepal Demographic and Health Survey; WRA, women of reproductive age.
Data used for average annual rate of change analysis.
Weighted data used for multilevel analysis.
Weighted N.
In the NDHS, haemoglobin (capillary blood samples) measurements of women were conducted in every household (2006) and every second household (2011) eligible for household questionnaire. In 2016, women of every second households selected for men's questionnaire were measured for haemoglobin levels. All WRA in the selected households were interviewed through a face‐to‐face interview using a structured questionnaire. The detailed methodology of the NDHS has been published elsewhere (Ministry of Health, New ERA, & ICF International Inc, 2017; Ministry of Health and Population, New ERA, & ICF International Inc, 2012; Ministry of Health and Population, New ERA, & Macro International Inc, 2007).
2.2. Target population
Women who were aged 15–49 years, were not pregnant during the survey and had haemoglobin measurements available were included in this study.
2.3. Study variables
2.3.1. Dependent variable
Our dependent variable was diagnosis of anaemia, measured in the NDHS by testing the level of serum haemoglobin using the HemoCue (Hb 201 Photometer) system in all three rounds of NDHS. This involved taking a drop of blood from a finger prick collected in a microcuvette and analysing onsite using a portable HemoCue analyser. Adjusted measurements of haemoglobin levels for altitude and smoking status were used in this study, which were then categorized into the following: not anaemic (≥120 g/L); severe anaemia (<80 g/L); moderate anaemia (80–109 g/L) and mild anaemia (110–119 g/L). We used the World Health Organization's cut‐off points for nonpregnant women 15 years or older (World Health Organization, 2011). For our analyses, we dichotomized these categories as anaemic (severe, moderate and mild anaemia) and not anaemic (no anaemia).
2.3.2. Independent variables
We categorized our independent variables into individual, household and community levels.
Individual level variables included women's age, education, current work status (professional/technical/managerial, clerical, sales/services, agriculture, and skilled and unskilled manual, and others were categorized as ‘currently working’ if they were working during the survey period) and total number of children born. Physical and/or sexual violence was categorized as intimate partner violence (IPV) and defined as ‘yes’ if they had ever experienced IPV, and use of contraception was categorized as none, hormonal, female sterilization, male contraception and traditional methods. Autonomy was calculated using multiple correspondence analysis for decision on women's health care, large household purchases and decision to visit family and relatives. We categorized BMI as underweight (<18.5 kg/m2), normal weight (18.5 to 24.9 kg/m2), overweight (25.0 to 29.9 kg/m2) and obese (≥30.0 kg/m2) (World Health Organization, 1995).
Household level variables included the following: Household wealth quintile available from NDHS and NDHS standard categories was used to classify improved toilet and improved water source (Ministry of Health, New ERA, & ICF International Inc, 2017). Food security was calculated and categorized as food secure, mildly food insecure, moderately food insecure and severely food insecure (Coates, Swindale, & Bilinsky, 2007).
Place of residence (urban or rural) and province were included as community level variables.
2.4. Statistical analyses
Descriptive statistics were used to define the datasets and present the demographic profile of women included in this study. Rate of change analysis was used to explore average annual rate of change (AARC) in anaemia prevalence from 2006 to 2011, 2011 to 2016 and 2006 to 2016 across selected factors using the following formula (Khan, Islam, Shariff, Alam, & Rahman, 2017):
where Y t = prevalence of anaemia of any given year, r = annual rate of change, n = number of years between two surveys and Y t + n = prevalence of anaemia of the (t + n)th year.
We used concentration curves and indices to assess whether the prevalence of anaemia was unequally distributed among socio‐economic quintiles in years 2006, 2011 and 2016. Concentration curve plots the cumulative percentage of anaemia in y axis against the cumulative percentage of wealth in x axis (lowest to highest). The concentration index quantifies the socio‐economic inequality in anaemia and is derived from the concentration curve. It estimates the area between the concentration curve and line of equality (45° angle), which is calculated as
where yi is the health status of the ith individual and Ri is the fractional rank of the ith individual in terms of the wealth index; μ is the weighted mean of y, and COV ω is the weighted covariance. The concentration index takes values between −1 and +1. The curve lies above the line of equality when the values are negative, indicating that anaemia is disproportionately concentrated among the poorer socio‐economic quintiles. The curve lies below the line of equality when the values are positive, indicating that anaemia is disproportionately concentrated among women from higher socio‐economic quintiles. If there is no socio‐economic status‐related inequality, the value is zero and the curve lies along the 45° line of equality (O'Donnell, Van Doorslaer, Wagstaff, & Lindelow, 2007). We graphed concentration curves and calculated concentration indices using Stata command ‘conindex’ (O'Donnell, O'Neill, Van Ourti, & Walsh, 2016).
We performed multilevel logistic regression analysis using the data from NDHS 2006, 2011 and 2016 to identify factors associated with anaemia. The dataset we analysed was sampled using multistage sampling method, which often introduces multilevel dependency among observations. These dependencies often come from several levels of hierarchy. In this study, the lower level of hierarchy is WRA (Level 1), who are nested within clusters (Level 2) and the clusters within the next higher level (residence, province: Level 3). The odds of women experiencing anaemia are not independent because of the nested structure of the dataset as the women from the same cluster may share similar community characteristics. Using single‐level logistic regression without considering clustering effect would overestimate or underestimate the actual effect. We therefore used multilevel logistic regression model considered appropriate approach to account for multiple hierarchy and dependency in data (Hox et al., 2017).
We used intraclass correlation (ICC) coefficient to assess the cluster level differences in anaemia. ICC equals 0 if all the observations are independent of one another, and if all observations are the same, the ICC equals 1. A nonzero ICC implies that the observations are not independent (Hox et al., 2017). Four different multilevel logistic regression models were run separately. The first model was without any independent variable (null model—Model 1) followed by addition of individual and household level variables in Model 2; Model 3 included only the community level variables; and finally, Model 4 included individual‐, household‐ and community‐level variables. Median odds ratio (MOR), Akaike information criterion (AIC), Bayesian information criterion (BIC) and variance of the random intercept at cluster level are reported for each model. Results are reported as adjusted odds ratios (AOR) and 95% CIs. All analyses were accounted for sampling design by using weights. We used Stata/SE 13.0 for the analyses.
2.5. Ethical considerations
Ethical approval of NDHS (including the approval for haemoglobin testing) was obtained from the Nepal Health Research Council. Therefore, we did not require any ethical approval to conduct this study.
2.6. Data availability and statement
The NDHS data used in the study are a third‐party dataset. We requested for access to these datasets through the Demographic and Health Survey programme. These are open‐source dataset and available to anyone on request at https://dhsprogram.com/data/dataset/Nepal_Standard-DHS_2016.cfm?flag=0.
3. RESULTS
Sociodemographic characteristics of the women from 2006, 2011 and 2016 are presented in Table 1. The prevalence of anaemia was 35.5% in 2006, 34.4% in 2011 and 40.5% in 2016. The mean age of women was around 29 years in all NDHS rounds. The mean years of education increased in 2016 (5.1 years) from 3.2 years in 2006 and 4.4 years in 2011. Prevalence of overweight/obesity increased from 8.5% to 13.3% and 22.1% in 2006, 2011 and 2016, respectively, whereas underweight decreased from 24.2% in 2006, 18.2% in 2011 to 17.2% in 2016.
The AARC in anaemia prevalence from years 2006 to 2011, years 2011 to 2016 and from 2006 to 2016 by different sociodemographic factors are presented in Table 2. From 2006 to 2011, there was a decline in AARC, on an average of 0.20, whereas there was an increase of 2.39 from 2011 to 2016. Overall, from 2006 to 2016, the increase was 0.65 per year. Prevalence of anaemia among women aged 30–39 and 20–29 years increased per year with an average of 0.36% and 0.17%, respectively, from 2006 to 2016. Women who had one to two children born, overweight women and women from Bagmati (Province 3) had highest AARC increase, whereas women with more than four children and underweight women had AARC decrease in anaemia prevalence from 2006 to 2016.
TABLE 2.
AARC in prevalence of anaemia from 2006 to 2016
| Percent anaemic in 2006 (N = 10,043) | Percent anaemic in 2011 (N = 5,795) | Percent AARC (2006–2011) | Percent anaemic in 2016 (N = 6,124) | Percent AARC (2011–2016) | Percent AARC (2006–2016) | |
|---|---|---|---|---|---|---|
| Women's age | ||||||
| 15–19 | 8.8 | 8.3 | −0.10 | 8.8 | 0.11 | 0.00 |
| 20–29 | 12.1 | 11.7 | −0.08 | 13.7 | 0.49 | 0.17 |
| 30–39 | 7.8 | 8.1 | 0.06 | 10.9 | 0.75 | 0.36 |
| 40–49 | 6.8 | 6.3 | −0.10 | 7.1 | 0.17 | 0.03 |
| Education | ||||||
| No education | 20.4 | 14.4 | −0.70 | 14 | −0.08 | −0.47 |
| Primary | 5.6 | 5.5 | −0.02 | 6.2 | 0.15 | 0.06 |
| Secondary | 8.4 | 12.1 | 1.10 | 15 | 0.79 | 0.93 |
| Higher secondary and above | 1.1 | 2.3 | 0.27 | 5.3 | 0.82 | 0.52 |
| Currently working | ||||||
| No | 11 | 14.1 | 0.86 | 18 | 1.18 | 1.01 |
| Yes | 24.5 | 20.2 | −0.58 | 22.5 | 0.58 | −0.18 |
| Children ever born | ||||||
| 0 | 9.9 | 10.5 | 0.13 | 11.7 | 0.27 | 0.20 |
| 1–2 | 10.0 | 11.0 | 0.22 | 14.6 | 1.05 | 0.58 |
| 3–4 | 8.8 | 8.4 | −0.08 | 9.9 | 0.35 | 0.12 |
| >4 | 6.6 | 4.5 | −0.36 | 4.3 | −0.04 | −0.21 |
| Women's BMI | ||||||
| Normal weight | 23.1 | 23.8 | 0.15 | 25.6 | 0.43 | 0.28 |
| Underweight | 10.6 | 7.6 | −0.45 | 8.3 | 0.15 | −0.21 |
| Overweight | 1.6 | 2.4 | 0.17 | 5.2 | 0.75 | 0.43 |
| Obese | 0.3 | 0.5 | 0.04 | 1.4 | 0.20 | 0.12 |
| Household wealth | ||||||
| Lowest | 5.4 | 5.6 | 0.04 | 5.5 | −0.02 | 0.01 |
| Second | 7.8 | 6.6 | −0.21 | 7.9 | 0.30 | 0.01 |
| Middle | 8.6 | 7.7 | −0.16 | 9.9 | 0.55 | 0.14 |
| Higher | 7.1 | 7.4 | 0.06 | 9.6 | 0.55 | 0.28 |
| Highest | 6.5 | 7.1 | 0.13 | 7.6 | 0.11 | 0.12 |
| Province | ||||||
| 1 (Eastern region) | 4.9 | 7.3 | 0.62 | 7.3 | 0.00 | 0.27 |
| 2 (Central/Eastern plains) | 9.3 | 7.7 | −0.27 | 11.1 | 0.97 | 0.20 |
| 3 (Bagmati) | 3.9 | 4.1 | 0.04 | 6.4 | 0.58 | 0.28 |
| 4 (Gandaki) | 1.8 | 3.1 | 0.30 | 2.2 | −0.16 | 0.04 |
| 5 (Western/mid‐western region) | 6.9 | 7.3 | 0.08 | 8 | 0.15 | 0.12 |
| 6 (Karnali) | 1.3 | 1.1 | −0.04 | 2 | 0.20 | 0.07 |
| 7 (Sudurpaschim) | 7.4 | 3.7 | −0.52 | 3.5 | −0.04 | −0.32 |
| Total a | 35.5 | 34.4 | −0.20 | 40.5 | 2.39 | 0.65 |
Abbreviations: AARC, average annual rate of change; BMI, body mass index.
Total AARC in anaemia prevalence across all women included in this study.
Figure 2 shows that the concentration curves lie slightly above the 45° line of equality for year 2006 and 2016. Even though the concentration index was very small for 2006 (CI −0.035, SE: 0.012, p = 0.004) and 2011 (CI −0.034, SE: 0.016, p = 0.033), the negative values indicate that anaemia was unequally distributed among poor women. From 2006 to 2016, we found very small AARC increases in anaemia among women from lower socio‐economic quintiles and larger per year increase among women from higher socio‐economic quintiles (Table 2). This is also reflected in the concentration curve for 2016 with small shift of the curve under the line of equality (Figure 2). However, we did not observe any significant socio‐economic inequality when using concentration index (Figure 2), even though the curve is under the 45° line of equality and concentration index is positive (CI: 0.013, SE:0.015, p = 0.382). Because p > 0.05, the results indicate no inequality in distribution of anaemia ranked by wealth scores in 2016 (Figure 2).
FIGURE 2.
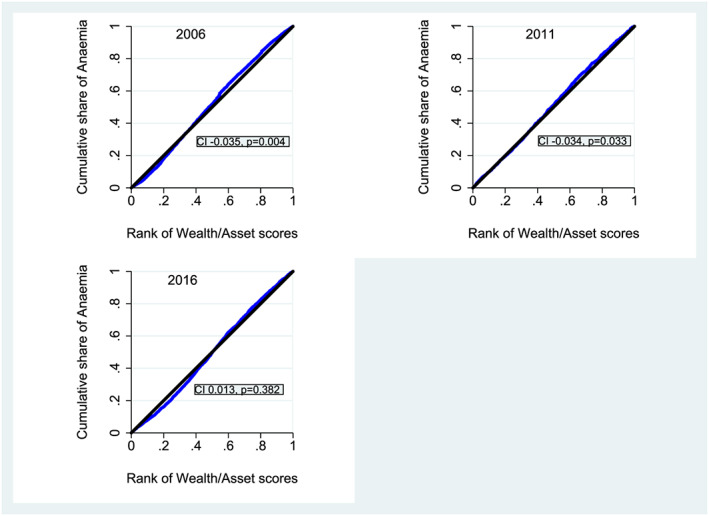
Concentration curves and indices for anaemia for years 2006, 2011 and 2016. Note: Blue line is the concentration curve and the black is the 45° line of equality
The results of the multilevel logistic regression models for 2016 dataset are presented in Table 3. The null model (Model 1) found 16% variation in anaemia prevalence across clusters (ICC: 0.16). However, this difference was reduced to 12% (ICC: 0.12) when individual‐, household‐ and community‐level factors were adjusted in the final model (Model 4).
TABLE 3.
Multilevel logistic regression analysis for determinants of anaemia among women of reproductive age (15–49) using the 2016 NDHS
| Null model (N = 6,134) (Model 1) | Individual–household‐level model (N = 4,448) (Model 2) | Community‐level model (N = 6,134) (Model 3) | Individual–household–community‐level model (N = 4,448) (Model 4) | |
|---|---|---|---|---|
| AOR [95% CI] | AOR [95% CI] | AOR [95% CI] | ||
| Women's age | ||||
| 15–19 | 1.31 [0.86, 1.99] | 1.22 [0.80, 1.85] | ||
| 20–29 | 1.43 [1.11, 1.85] ** | 1.33 [1.04, 1.72] * | ||
| 30–39 | 1.18 [0.97, 1.45] | 1.13 [0.93, 1.39] | ||
| 40–49 | Ref. | Ref. | ||
| Education | ||||
| No education | 0.93 [0.69, 1.26] | 0.86 [0.64, 1.16] | ||
| Primary | 0.90 [0.67, 1.22] | 0.86 [0.63, 1.16] | ||
| Secondary | 1.02 [0.78, 1.33] | 1.00 [0.76, 1.30] | ||
| Higher secondary and above | Ref. | Ref. | ||
| Currently working | ||||
| No | Ref. | Ref. | ||
| Yes | 0.86 [0.73, 1.00] | 0.89 [0.76, 1.05] | ||
| Total no of children | ||||
| 0 | Ref. | Ref. | ||
| 1–2 | 1.21 [0.90, 1.63] | 1.22 [0.91, 1.65] | ||
| 3–4 | 1.37 [0.97, 1.93] | 1.33 [0.94, 1.87] | ||
| >4 | 1.66 [1.11, 2.47] * | 1.54 [1.03, 2.28] * | ||
| Use of contraception | ||||
| Not using | Ref. | Ref. | ||
| Hormonal | 0.60 [0.48, 0.74] *** | 0.61 [0.50, 0.76] *** | ||
| Female sterilization | 1.59 [1.26, 2.02] *** | 1.50 [1.19, 1.90] ** | ||
| Male contraception | 0.79 [0.61, 1.02] | 0.82 [0.64, 1.06] | ||
| Traditional methods | 1.00 [0.78, 1.29] | 1.01 [0.79, 1.30] | ||
| Women's BMI | ||||
| Normal weight | Ref. | Ref. | ||
| Underweight | 1.21 [0.98, 1.50] | 1.16 [0.94, 1.42] | ||
| Overweight | 0.56 [0.46, 0.69] *** | 0.58 [0.47, 0.72] *** | ||
| Obese | 0.59 [0.41, 0.84] ** | 0.62 [0.43, 0.89] *** | ||
| Autonomy | 1.00 [0.90, 1.10] | 1.01 [0.91, 1.11] | ||
| Intimate partner violence | ||||
| No | Ref. | Ref. | ||
| Yes | 1.25 [1.04, 1.52] * | 1.23 [1.02, 1.49] * | ||
| Improved source of water | ||||
| No | Ref. | Ref. | ||
| Yes | 1.03 [0.75, 1.40] | 0.93 [0.68,1.26] | ||
| Improved toilet | ||||
| No | Ref. | Ref. | ||
| Yes | 1.01 [0.85, 1.19] | 1.03 [0.87, 1.22] | ||
| Household wealth | ||||
| Lowest | 0.53 [0.38, 0.75] *** | 0.59 [0.41, 0.85] ** | ||
| Second | 0.85 [0.63, 1.15] | 0.89 [0.65, 1.20] | ||
| Middle | 1.00 [0.75, 1.34] | 0.99 [0.74, 1.32] | ||
| Higher | 1.00 [0.77, 1.31] | 0.99 [0.76, 1.29] | ||
| Highest | Ref. | Ref. | ||
| Food security | ||||
| Food insecure | Ref. | Ref. | ||
| Mildly food insecure | 0.96 [0.79, 1.17] | 0.93 [0.76, 1,13] | ||
| Moderately food insecure | 0.97 [0.79, 1.20] | 0.98 [0.80, 1.20] | ||
| Severely food insecure | 1.17 [0.87, 1.57] | 1.16 [0.86, 1.56] | ||
| Type of residence | ||||
| Urban | Ref. | Ref. | ||
| Rural | 0.94 [0.78, 1.12] | 1.04 [0.83, 1.29] | ||
| Province | ||||
| 1 (Eastern region) | 1.81 [1.31, 2,51] | 1.78 [1.20, 2.63] ** | ||
| 2 (Central/Eastern plains) | 3.42 [2.48, 4.72] | 2.37 [1.57, 3.57] *** | ||
| 3 (Bagmati) | Ref. | Ref. | ||
| 4 (Gandaki) | 0.82 [0.58, 1.17] | 0.82 [0.53, 1.24] | ||
| 5 (Western/mid‐western region) | 1.88 [1.37, 2.57] | 1.70 [1.17, 2.48] ** | ||
| 6 (Karnali) | 1.22 [0.88, 1.70] | 1.24 [0.83, 1.86] | ||
| 7 (Sudurpaschim) | 1.69 [1.21, 2.35] | 1.49 [1.00, 2.23] | ||
| Model summary | ||||
| AIC | 7,940.75 | 5,661.06 | 7,875.71 | 5,639.53 |
| BIC | 7,960.92 | 5,859.47 | 7,936.20 | 5,882.74 |
| ICC | 0.16 | 0.13 | 0.12 | 0.12 |
| MOR | 2.24 | 2.03 | 1.88 | 1.94 |
| Variance of the random effect intercept at cluster level | 0.84 | 0.74 | 0.66 | 0.69 |
Abbreviations: AIC, Akaike information criterion; BIC, Bayesian information criterion; BMI, body mass index; ICC, intraclass correlation; MOR, median odds ratio; NDHS, Nepal Demographic and Health Survey.
p < 0.05.
p < 0.01.
p < 0.001.
In Model 4, women aged 20–29 years compared with those aged 40–49 years were 1.33 times (95% CI [1.04, 1.72]), women with four or more children compared with no children were 1.54 times (95% CI [1.03, 2.28]) and women who had undergone female sterilization compared with those not using contraception were 1.53 times (AOR 1.50, 95% CI [1.19, 1.90]) more likely to be anaemic. Similarly, women who had ever experienced IPV had higher odds of being anaemic (AOR 1.23, 95% CI [1.02, 1.49]) than those who had not experienced IPV.
Women using hormonal contraception were 39% (AOR 0.61, 95% CI [0.50, 0.76]), overweight women were 42% (AOR 0.58, 95% CI [0.47, 0.72]), obese women were 38% (AOR 0.62, 95% CI [0.43, 0.89]) and women from lowest socio‐economic quintiles were 41% (AOR 0.59, 95% CI [0.41, 0.85]) less likely to be anaemic. Compared with women who resided in Bagmati (Province 3), women in Province 1 (AOR 1.78, 95% CI [1.20, 2.63]), Province 2 (AOR 2.37, 95% CI [1.57, 3.57]) and Province 5 (AOR 1.70, 95% CI [1.17, 2.48]) had higher odds of being anaemic (Model 4). We did not observe any association of education, current work status, improved source of water, improved toilet, household food security and urban/rural residence with anaemia in Model 4.
Multilevel modelling results for 2006 and 2011 are summarized in supplementary tables, but no comparison of the findings was performed. In 2006, the ICC for null model was 0.22, which reduced to 0.14 in Model 4. We found that women aged 15–19 and 20–29 years of age, women with one to two children and women from Provinces 1, 2, 5 and Sudurpaschim (Province 7) were at increased odds of being anaemic, whereas women using hormonal contraception and overweight women were at decreased odds of being anaemic (Table S1).
In 2011, the ICC for null model was 0.13, which reduced to 0.08 in Model 4. No association between age of women and children ever born was observed. However, use of hormonal contraception and being overweight were still protective factors of anaemia. In addition, obesity was found to be a protective factor of anaemia. Women from Provinces 1, 2, Gandaki (Province 4), 5 and Sudurpaschim (Province 7) were also at increased risk of being anaemic (Table S2).
4. DISCUSSION
In this study, we found AARC increases in anaemia prevalence from 2006 to 2016 among women aged 30–39 years, with secondary or higher education, who had two or fewer children, not working, belonging to higher wealth quintiles and who were overweight or obese.
The prevalence of anaemia for 2016 was 40.5% which is double the prevalence of 20.4% reported in the Nepal National Micronutrient Status Survey (NNMSS) conducted in same year. This difference could be explained by the fact that NNMSS used venous blood samples to measure haemoglobin (Ministry of Health and Population, New ERA, UNICEF, EU, USAID, & CDC, 2018), whereas NDHS used capillary blood samples. Both surveys collected data in different months, and thus, seasonality could also be a reason for the variation in anaemia prevalence between the surveys (Bondevik, Lie, Ulstein, & Kvale, 2000; Jiang, Christian, Khatry, Wu, & West, 2005). A recent systematic review has reported significant differences in haemoglobin concentration between capillary and venous blood and also highlighted that instrument error, measurement error and true biological variability between capillary and venous haemoglobin may have led to the variation (Neufeld et al., 2019). Future lab‐based studies measuring both venous and capillary blood samples from same participants and using a uniform blood‐analysis method (i.e., laboratory or HemoCue) could further shed light on this variability.
We observed socio‐economic inequalities in the distribution of anaemia in 2006 and 2011 but not in 2016. Most importantly, the shifting of concentration curve from ‘above the line of equality’ in 2006 and 2011 to ‘under the line of equality’ in 2016 confirms a shift in the distribution of anaemia from poorer women towards women belonging to higher wealth quintiles. The inequality in 2006 and 2011 might imply that anaemia is result of the inability of poor women to access adequate food, IFA supplements (Makhoul et al., 2012) and treatment of infectious diseases such as malaria and helminths (Dreyfuss et al., 2000; Morey, Sharma, & Mills, 2003). Moreover, lack of hygiene and sanitation and the higher risk of infectious diseases along with open defecation being more prevalent during 2006 and 2011 would explain the relationship between poor socio‐economic status and anaemia. For instance, only 22.7% households in 2006 and 39.5% in 2011 were reported to have toilet facilities in Nepal (Ministry of Health and Population, New ERA, & Macro International Inc, 2007, 2012).
One of the interesting findings of our study is that anaemia in Nepal is not the problem of poorer women only (concentration index of 2016). We found women from the lowest wealth quintile were at lower risk of anaemia. Contrary to our findings, Gautam et al., 2019, on the basis of the analysis of the 2016 NDHS dataset, did not find any association between wealth and anaemia among women in Nepal (Gautam et al., 2019). The difference in findings might be because their study included ethnicity in the model, which could have influenced the association of wealth and anaemia as wealth considerably differs by ethnicity in Nepal (Bennett, Dahal, & Govindasamy, 2008). Because studies on ethnicity and wealth using recent data is lacking in Nepal, and this being a general curiosity, we performed a simple logistic regression to investigate whether ethnicity influences the association of wealth on anaemia. The results confirmed our assumption. In Gautam et al., 2019, wealth was found to be significantly associated with anaemia in their unadjusted model, but the association disappeared when ethnicity was coadjusted with other variables in their adjusted model (Gautam et al., 2019).
The first justification for women from the lowest wealth quintile being at lower risk of anaemia is the nationwide open defection free (ODF) campaign initiated after 2011, which was targeted at poor households. This may have contributed to the reduction of anaemia through the reduction in prevalence of helminthiasis. ODF campaign in Nepal has been identified as a priority programme since 2011 and has two major activities: (a) awareness programme on hygiene and sanitation and (b) toilet construction. The campaign was successful in improving use of improved toilets (95% in 2016) (Ministry of Health, New ERA, & ICF International Inc, 2017). Second, the reduced risk of anaemia among poorer women could be due to the effectiveness of malaria prevention and treatment programme, which were also targeted at poorer households in malaria‐endemic areas. However, no evidence supporting this fact is documented in Nepal.
Third, households with lower wealth quintile in Nepal usually depend on vegetables available from their kitchen garden (Osei et al., 2017) such as iron‐rich green leafy vegetables (fresh and dried), which might provide up to 60% of their daily iron needs (Chandyo et al., 2007). Even though bio‐available iron from vegetables is relatively low, there were several ongoing interventions that were targeted at poorer households (e.g., consumption of animal source foods and dietary iron consumption). Interventions such as Suaahara‐I (from 2011 to 2016) and Sunaula Hazar Din (from 2014 to 2017), which provided financial and technical support to poor households for poultry farming, found increased consumption of meat and eggs among WRA (Cunningham et al., 2017; The World Bank, 2018). These interventions also included aspects of water, sanitation and hygiene interventions, deworming and open defecation eradication, which could have influenced the association between wealth and anaemia.
Younger women were found to be at risk in our study. It may be because young girls and women are often underrepresented in the public health programmes that aim to prevent anaemia. Even though the national strategy to control anaemia among women and children developed in 2002 mentions extending IFA supplementation to nonpregnant WRA, they are still being excluded from the IFA programme (Ministry of Health, 2002). Given that adolescent girls (up to 78%) and young women (16%–65%) in Nepal share a huge burden of anaemia (Baral & Onta, 2009; Konishi et al., 2011), targeting adolescent girls and reproductive aged women before pregnancy could have produced some gains in anaemia reduction during the 2011–2016 period (Cavalli‐Sforza, 2005; Gunaratna et al., 2015). The Government of Nepal initiated weekly IFA supplementation to adolescent girls 10–19 years only after 2016 (Ministry of Health and Population, New ERA, UNICEF, EU, USAID, & CDC, 2018); however, no evidence on the programme implementation and uptake is available, which may be because proper implementation is lacking. Therefore, implementation studies are needed to understand whether and how these interventions that focus on nonpregnant, young girls and women have been effective. Contrary to our finding, few studies did not find any association of age with anaemia (Chandyo et al., 2007; Gautam et al., 2019; Harding et al., 2018), which could be due to the use of multilevel model and control for other additional variables in our study.
Interestingly, we did find some consistency in findings between Gautam et al. (2019) and other studies from Nepal (Bellizzi & Ali, 2018; Konishi et al., 2011) on the following two factors: (a) use of hormonal contraception and (b) women's overweight and obesity status, associated with lower odds of being anaemic. A review on noncontraceptive benefits of hormonal contraception suggests reduction in menstrual blood loss and risk of anaemia (Bahamondes, Valeria Bahamondes, & Shulman, 2015). Surprisingly, our study found that female sterilization method put women at risk of anaemia. Whereas some studies have reported that female sterilization leads to menstrual disorders (Sadatmahalleh, Ziaei, Kazemnejad, & Mohamadi, 2016) and cause anaemia, others did not find any significant difference in menstrual disorders between the cases of female sterilization and the controls(Sadatmahalleh et al., 2016; Shobeiri & Atashkhoii, 2005). There may be other pathways through which female sterilization may influence anaemia (such as number of children/sons and gender difference), which should be unpacked by future studies through path analysis.
In our findings, BMI was inversely associated with anaemia, which is consistent with other studies from Nepal (Gautam et al., 2019; Harding et al., 2018), India (Y. S. Balarajan et al., 2013; Bentley & Griffiths, 2003) and Bangladesh (Kamruzzaman et al., 2015). Majority of the women's diet in Nepal is monotonous, with starchy foods constituting most of the diet (Harris‐Fry et al., 2018), and even though it does not meet the daily recommended allowances (Harris‐Fry et al., 2018; Henjum et al., 2015) it is still the main source of energy and iron (Chandyo et al., 2007). This might suggest that having a high‐energy diet providing sufficient energy to maintain bodyweight of overweight and obese women might suffice in providing iron. Because of the limitations in the current dataset, we are unable to assess how diet influences the association between overweight, obesity and anaemia.
Besides, women who had experienced violence were more likely to be anaemic in the present study, and this is supported by previous studies conducted in India (Ackerson & Subramanian, 2008) and Bangladesh (M. Rahman, Nakamura, Seino, & Kizuki, 2013), but not in Nepal (Gautam et al., 2019). Existing evidence on the possible pathways between violence and anaemia highlight the act of withholding of food by partner and inadequate amount of food to eat, leading to adverse nutritional outcomes (Lentz, 2018). IPV prevention interventions should include women and their partners and other household members and address social and gender norms (Shai et al., 2019).
Although we cannot identify the mechanism between provinces and anaemia, it is unlikely that food security/insecurity in Provinces 1, 2 and 5 would have led to anaemia because we found no association between food insecurity and anaemia. Other evidence from Nepal also report that women in Province 2 have poor nutritional status (Rai, Gurung, Thapa, & Saville, 2019; Saville et al., 2018). We speculate that burden of malaria and hookworm, which is high in low plains of Nepal where Provinces 1, 2 and 5 lie (Singh, Sah, & Pokharel, 2015), might be contributing to the increased risk of anaemia. In addition, household dynamics, household food allocation and social norms around food intake and inadequate consumption of iron‐rich foods in their diet could have consequently led to anaemia (Gittelsohn, Thapa, & Landman, 1997; Harris‐Fry et al., 2018).
In general, agricultural approaches that prioritize dietary improvement, such as promotion of kitchen garden to produce green leafy vegetables, promotion of animal sourced foods (poultry raising) and food fortification, have been shown to improve intake of iron (English et al., 1997) and reduce anaemia among women and children (Osei et al., 2017). The Government of Nepal is also considering fortification of rice with micronutrients including iron, folic acid and vitamins (World Food Programme & Government of Nepal, 2018). The fortified rice was found to be acceptable among remote Nepalese population (Rai et al., 2019). These interventions may not show immediate effect but are sustainable and cost‐effective in the long term (Baltussen, Knai, & Sharan, 2004). In Nepal, whereas vegetables and cereals are consumed almost daily and are sources of nonhaem iron, intake of haem iron from animal sources is deficient (Makhoul et al., 2012). Vegetables and cereals are high in phytate and polyphenols, and other dietary behaviours such as tea and coffee consumption inhibit iron absorption (Gillooly et al., 1983). Anaemia prevention programme regardless of their focus on nutrition‐sensitive or nutrition‐specific approaches should prioritize nutrition knowledge on inhibitors (such as phytate, polyphenols, tea and coffee) and enhancers (such as ascorbic acids and animal tissue) of iron absorption (Zijp, Korver, & Tijburg, 2000). Although promoting animal source food could be one of the approaches to improve iron absorption, different approach for vegetarian communities such as blanching of vegetables (Yadav & Sehgal, 2003) and soaking and germination of pulses to reduce phytate and polyphenols levels are required (Afify Ael, El‐Beltagi, El‐Salam, & Omran, 2011).
4.1. Limitations of the study
Our study has some limitations: (a) We cannot infer causality of independent variables upon anaemia status because this study utilizes survey data; (b) we were unable to include other factors of anaemia such as helminth, malaria infection, haemoglobinopathies and inflammatory conditions because these are not collected in NDHS; (c) information on dietary diversity, IFA supplementation and deworming was limited to women age 15–49 with a live birth in the 5 years preceding the survey and hence not included in the analyses; and (d) NDHS uses capillary haemoglobin measurements using HemoCue, and therefore, caution should be taken when comparing our findings with studies using venous blood haemoglobin measurements.
5. CONCLUSIONS
Given that multilevel factors are associated with anaemia risk in women, changes at policy level and programme level should consider addressing associated factors, the changes in prevalence and trends of socio‐economic inequality associated with anaemia. An immediate and interim solution would be the expansion of current IFA interventions along with infection prevention approaches; however, a life‐course‐based programme for nutrition behaviour change, ensuring access to iron rich foods and improving nutrition knowledge on infectious disease‐anaemia interactions and how food choices can enhance or inhibit iron absorption, might produce a longer‐term impact. In addition, inclusion of information on IPV in teaching curriculum might contribute to both changes in social norms and anaemia reduction. Critically, the design and implementation of such interventions should take into account the provincial context, and we recommend future studies to evaluate intervention pathways for anaemia reduction and evaluate interventions particularly in Provinces 1, 2 and 5 where women were at higher risk of being anaemic.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONTRIBUTIONS
AR and NK designed the study. AR conducted the data analysis with support of NK; AR wrote the manuscript. All authors contributed to data interpretation and manuscript revision. AR had primary responsibility for the final content. All authors read and approved the manuscript.
Supporting information
Table S1: Multilevel logistic regression analysis for predictors of anaemia among women of reproductive age (15–49) using the 2006 NDHS
Table S2: Multilevel logistic regression analysis for predictors of anaemia among women of reproductive age (15–49) using the 2011 NDHS
Rai A, Khan MN, Thapa S. Trends and determinants of anaemia in women of Nepal: a multilevel analysis. Matern Child Nutr. 2020;16:e13044 10.1111/mcn.13044
REFERENCES
- Ackerson, L. K. , & Subramanian, S. V. (2008). Domestic violence and chronic malnutrition among women and children in India. American Journal of Epidemiology, 167(10), 1188–1196. 10.1093/aje/kwn049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afify Ael, M. , El‐Beltagi, H. S. , El‐Salam, S. M. , & Omran, A. A. (2011). Bioavailability of iron, zinc, phytate and phytase activity during soaking and germination of white sorghum varieties. PLoS ONE, 6(10), e25512 10.1371/journal.pone.0025512, http://www.ncbi.nlm.nih.gov/pubmed/22003395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahamondes, L. , Valeria Bahamondes, M. , & Shulman, L. P. (2015). Non‐contraceptive benefits of hormonal and intrauterine reversible contraceptive methods. Human Reproduction Update, 21(5), 640–651. 10.1093/humupd/dmv023, http://www.ncbi.nlm.nih.gov/pubmed/26037216 [DOI] [PubMed] [Google Scholar]
- Balarajan, Y. , Ramakrishnan, U. , Ozaltin, E. , Shankar, A. H. , & Subramanian, S. V. (2011). Anaemia in low‐income and middle‐income countries. Lancet, 378(9809), 2123–2135. 10.1016/S0140-6736(10)62304-5, http://www.ncbi.nlm.nih.gov/pubmed/21813172 [DOI] [PubMed] [Google Scholar]
- Balarajan, Y. S. , Fawzi, W. W. , & Subramanian, S. V. (2013). Changing patterns of social inequalities in anaemia among women in India: Cross‐sectional study using nationally representative data. BMJ Open, 3(3), e002233 10.1136/bmjopen-2012002233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltussen, R. , Knai, C. , & Sharan, M. (2004). Iron fortification and iron supplementation are cost‐effective interventions to reduce iron deficiency in four subregions of the world. The Journal of Nutrition, 134(10), 2678–2684. 10.1093/jn/134.10.2678, http://www.ncbi.nlm.nih.gov/pubmed/15465766 [DOI] [PubMed] [Google Scholar]
- Baral, K. , & Onta, S. (2009). Prevalence of anemia amongst adolescents in Nepal: A community based study in rural and urban areas of Morang District. Nepal Medical College Journal, 11(3), 179–182. https://www.ncbi.nlm.nih.gov/pubmed/20334065 [PubMed] [Google Scholar]
- Bellizzi, S. , & Ali, M. M. (2018). Effect of oral contraception on anemia in 12 low‐ and middle‐income countries. Contraception, 97(3), 236–242. 10.1016/j.contraception.2017.11.001, http://www.ncbi.nlm.nih.gov/pubmed/29133111 [DOI] [PubMed] [Google Scholar]
- Bennett, L. , Dahal, D. R. , & Govindasamy, P. (2008). Caste, ethnic and regional identity in Nepal: Further analysis of the 2006 Nepal Demographic and Health Survey. Maryland, USA: Calverton; https://dhsprogram.com/pubs/pdf/FA58/FA58.pdf [Google Scholar]
- Bentley, M. E. , & Griffiths, P. L. (2003). The burden of anemia among women in India. European Journal of Clinical Nutrition, 57(1), 52–60. 10.1038/sj.ejcn.1601504, http://www.ncbi.nlm.nih.gov/pubmed/12548297 [DOI] [PubMed] [Google Scholar]
- Black, R. E. , Allen, L. H. , Bhutta, Z. A. , Caulfield, L. E. , de Onis, M. , Ezzati, M. , … Child Undernutrition Study, G . (2008). Maternal and child undernutrition: Global and regional exposures and health consequences. Lancet, 371(9608), 243–260. 10.1016/S0140-6736(07)61690-0, http://www.ncbi.nlm.nih.gov/pubmed/18207566 [DOI] [PubMed] [Google Scholar]
- Bondevik, G. T. , Lie, R. T. , Ulstein, M. , & Kvale, G. (2000). Seasonal variation in risk of anemia among pregnant Nepali women. International Journal of Gynaecology and Obstetrics, 69(3), 215–222. 10.1016/s0020-7292(00)00206-x, http://www.ncbi.nlm.nih.gov/pubmed/10854862 [DOI] [PubMed] [Google Scholar]
- Bondevik, G. T. , Lie, R. T. , Ulstein, M. , & Kvale, G. (2001). Maternal hematological status and risk of low birth weight and preterm delivery in Nepal. Acta Obstetricia et Gynecologica Scandinavica, 80(5), 402–408. 10.1034/j.1600-0412.2001.d01-5.x [DOI] [PubMed] [Google Scholar]
- Brabin, B. J. , Hakimi, M. , & Pelletier, D. (2001). An analysis of anemia and pregnancy‐related maternal mortality. J Nutr, 131(2S‐2), 604S–614S; discussion 614S‐615S. 10.1093/jn/131.2.604S [DOI] [PubMed] [Google Scholar]
- Chandyo, R. K. , Strand, T. A. , Ulvik, R. J. , Adhikari, R. K. , Ulak, M. , Dixit, H. , & Sommerfelt, H. (2007). Prevalence of iron deficiency and anemia among healthy women of reproductive age in Bhaktapur, Nepal. European Journal of Clinical Nutrition, 61(2), 262–269. 10.1038/sj.ejcn.1602508 [DOI] [PubMed] [Google Scholar]
- Christian, P. , Khatry, S. K. , & West, K. P. Jr. (2004). Antenatal anthelmintic treatment, birthweight, and infant survival in rural Nepal. Lancet, 364(9438), 981–983. 10.1016/S0140-6736(04)17023-2 [DOI] [PubMed] [Google Scholar]
- Coates, J. , Swindale, A. , & Bilinsky, P. (2007). Household Food Insecurity Access Scale (HFIAS) for measurement of household food access: Indicator guide (v. 3). Washington, DC. https://www.fantaproject.org/monitoring-and-evaluation/household-food-insecurity-access-scale-hfias
- Cunningham, K. , Singh, A. , Pandey Rana, P. , Brye, L. , Alayon, S. , Lapping, K. , … Klemm, R. D. W. (2017). Suaahara in Nepal: An at‐scale, multi‐sectoral nutrition program influences knowledge and practices while enhancing equity. Maternal & Child Nutrition, 13(4). 10.1111/mcn.12415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daru, J. , Zamora, J. , Fernández‐Félix, B. M. , Vogel, J. , Oladapo, O. T. , Morisaki, N. , … Jayaratne, K. (2018). Risk of maternal mortality in women with severe anaemia during pregnancy and post partum: A multilevel analysis. The Lancet Global Health, 6(5), e548–e554. 10.1016/S2214-109X(18)30078-0 [DOI] [PubMed] [Google Scholar]
- Dreyfuss, M. L. , Stoltzfus, R. J. , Shrestha, J. B. , Pradhan, E. K. , LeClerq, S. C. , Khatry, S. K. , … West, K. P. Jr. (2000). Hookworms, malaria and vitamin A deficiency contribute to anemia and iron deficiency among pregnant women in the plains of Nepal. The Journal of Nutrition, 130(10), 2527–2536. 10.1093/jn/130.10.2527, http://www.ncbi.nlm.nih.gov/pubmed/11015485 [DOI] [PubMed] [Google Scholar]
- English, R. , Badcock, J. , Giay, T. , Ngu, T. , Waters, A. , & Bennett, S. (1997). Effect of nutrition improvement project on morbidity from infectious diseases in preschool children in Vietnam: Comparison with control commune. BMJ, 315(7116), 1122–1125. 10.1136/bmj.315.7116.1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam, S. , Min, H. , Kim, H. , & Jeong, H. S. (2019). Determining factors for the prevalence of anemia in women of reproductive age in Nepal: Evidence from recent national survey data. PLoS ONE, 14(6), e0218288 10.1371/journal.pone.0218288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillooly, M. , Bothwell, T. H. , Torrance, J. D. , MacPhail, A. P. , Derman, D. P. , Bezwoda, W. R. , … Mayet, F. (1983). The effects of organic acids, phytates and polyphenols on the absorption of iron from vegetables. The British Journal of Nutrition, 49(3), 331–342. 10.1079/bjn19830042 [DOI] [PubMed] [Google Scholar]
- Gittelsohn, J. , Thapa, M. , & Landman, L. T. (1997). Cultural factors, caloric intake and micronutrient sufficiency in rural Nepali households. Social Science & Medicine, 44(11), 1739–1749. 10.1016/s0277-9536(96)00375-9 [DOI] [PubMed] [Google Scholar]
- Harding, K. L. , Aguayo, V. M. , Namirembe, G. , & Webb, P. (2018). Determinants of anemia among women and children in Nepal and Pakistan: An analysis of recent national survey data. Maternal & Child Nutrition, 14, e12478 10.1111/mcn.12478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris‐Fry, H. A. , Paudel, P. , Shrestha, N. , Harrisson, T. , Beard, B. J. , Jha, S. , … Saville, N. M. (2018). Status and determinants of intra‐household food allocation in rural Nepal. European Journal of Clinical Nutrition, 72, 1524–1536. 10.1038/s41430-017-0063-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henjum, S. , Torheim, L. E. , Thorne‐Lyman, A. L. , Chandyo, R. , Fawzi, W. W. , Shrestha, P. S. , & Strand, T. A. (2015). Low dietary diversity and micronutrient adequacy among lactating women in a peri‐urban area of Nepal. Public Health Nutrition, 18(17), 3201–3210. 10.1017/S1368980015000671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hox, J. J. , Moerbeek, M. , & Van de Schoot, R. (2017). Multilevel analysis: Techniques and applications (Second ed.). Routledge. [Google Scholar]
- Jiang, T. , Christian, P. , Khatry, S. K. , Wu, L. , & West, K. P. Jr. (2005). Micronutrient deficiencies in early pregnancy are common, concurrent, and vary by season among rural Nepali pregnant women. The Journal of Nutrition, 135(5), 1106–1112. 10.1093/jn/135.5.1106 [DOI] [PubMed] [Google Scholar]
- Kamruzzaman, M. , Rabbani, M. G. , Saw, A. , Sayem, M. A. , & Hossain, M. G. (2015). Differentials in the prevalence of anemia among non‐pregnant, ever‐married women in Bangladesh: Multilevel logistic regression analysis of data from the 2011 Bangladesh Demographic and Health Survey. BMC Women's Health, 15(1), 54 10.1186/s12905-015-0211-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, M. N. , Islam, M. M. , Shariff, A. A. , Alam, M. M. , & Rahman, M. M. (2017). Socio‐demographic predictors and average annual rates of caesarean section in Bangladesh between 2004 and 2014. PLoS ONE, 12(5), e0177579 10.1371/journal.pone.0177579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi, S. , Parajuli, R. P. , Takane, E. , Maharjan, M. , Sharma, S. , Tachibana, K. , … Watanabe, C. (2011). Health status of married women residing five communities in Nepal: Unexpectedly high prevalence of anemia in a well‐off community of Kathmandu. Nepal Medical College Journal, 13(1), 1–6. http://www.ncbi.nlm.nih.gov/pubmed/21991691 [PubMed] [Google Scholar]
- Lentz, E. C. (2018). Complicating narratives of women's food and nutrition insecurity: Domestic violence in rural Bangladesh. World Development, 104, 271–280. 10.1016/j.worlddev.2017.11.019 [DOI] [Google Scholar]
- Makhoul, Z. , Taren, D. , Duncan, B. , Pandey, P. , Thomson, C. , Winzerling, J. , … Shrestha, R. (2012). Risk factors associated with anemia, iron deficiency and iron deficiency anemia in rural Nepali pregnant women. The Southeast Asian Journal of Tropical Medicine and Public Health, 43(3), 735–746. http://www.ncbi.nlm.nih.gov/pubmed/23077854 [PubMed] [Google Scholar]
- Ministry of Health . (2002). National strategy for control of anaemia among women and children in Nepal. Kathmandu, Nepal. http://www.nnfsp.gov.np/PortalContent.aspx?Doctype=Resources&ID=66
- Ministry of Health and Population, New ERA, & ICF International Inc . (2012). Nepal Demographic and Health Survey 2011. https://dhsprogram.com/pubs/pdf/FR257/FR257%5B13April2012%5D.pdf
- Ministry of Health and Population, New ERA, & Macro International Inc . (2007). Nepal Demographic and Health Survey 2006 . https://dhsprogram.com/pubs/pdf/FR191/FR191.pdf
- Ministry of Health and Population, New ERA, UNICEF, EU, USAID, & CDC . (2018). Nepal National Micronutrient Status Survey, 2016. Nepal. https://www.unicef.org/nepal/reports/nepal-national-micronutrient-status-survey-report-2016
- Ministry of Health, New ERA, & ICF International Inc . (2017). Nepal Demographic and Health Survey 2016. https://www.dhsprogram.com/pubs/pdf/fr336/fr336.pdf
- Mishra, S. K. , Marasini, S. , Gupta, B. K. , Agrawal, K. K. , & Gautam, N. (2018). Prevalence of iron deficiency anemia in anemic patients: A hospital based study. Journal of Universal College of Medical Sciences, 6(2), 41–45. 10.3126/jucms.v6i2.22494 [DOI] [Google Scholar]
- Morey, E. R. , Sharma, V. R. , & Mills, A. (2003). Willingness to pay and determinants of choice for improved malaria treatment in rural Nepal. Social Science & Medicine, 57(1), 155–165. 10.1016/s0277-9536(02)00338-6 [DOI] [PubMed] [Google Scholar]
- Neufeld, L. M. , Larson, L. M. , Kurpad, A. , Mburu, S. , Martorell, R. , & Brown, K. H. (2019). Hemoglobin concentration and anemia diagnosis in venous and capillary blood: Biological basis and policy implications. Annals of the new York Academy of Sciences, 1450(1), 172–189. 10.1111/nyas.14139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, P. H. , Scott, S. , Avula, R. , Tran, L. M. , & Menon, P. (2018). Trends and drivers of change in the prevalence of anaemia among 1 million women and children in India, 2006 to 2016. BMJ Global Health, 3(5), e001010 10.1136/bmjgh-2018-001010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell, O. , O'Neill, S. , Van Ourti, T. , & Walsh, B. (2016). Conindex: Estimation of concentration indices. The Stata Journal, 16(1), 112–138. http://www.ncbi.nlm.nih.gov/pubmed/27053927, 10.1177/1536867X1601600112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell, O. , Van Doorslaer, E. , Wagstaff, A. , & Lindelow, M. (2007). Analyzing health equity using household survey data: A guide to techniques and their implementation. Washington, DC: The World Bank; https://openknowledge.worldbank.org/handle/10986/6896 [Google Scholar]
- Osei, A. , Pandey, P. , Nielsen, J. , Pries, A. , Spiro, D. , Davis, D. , … Haselow, N. (2017). Combining home garden, poultry, and nutrition education program targeted to families with young children improved anemia among children and anemia and underweight among nonpregnant women in Nepal. Food and Nutrition Bulletin, 38(1), 49–64. 10.1177/0379572116676427 [DOI] [PubMed] [Google Scholar]
- Pasricha, S. R. , Caruana, S. R. , Phuc, T. Q. , Casey, G. J. , Jolley, D. , Kingsland, S. , … Biggs, B. A. (2008). Anemia, iron deficiency, meat consumption, and hookworm infection in women of reproductive age in northwest Vietnam. The American Journal of Tropical Medicine and Hygiene, 78(3), 375–381. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5619638/, 10.4269/ajtmh.2008.78.375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokharel, R. K. , Maharjan, M. R. , Mathema, P. , & Harvey, P. W. J. (2011). Success in delivering interventions to reduce maternal anemia in Nepal: A case study of the intensification of maternal and neonatal micronutrient program. Washington, DC. https://www.spring-nutrition.org/publications/projects/a2z/success-delivering-interventions-reduce-maternal-anemia-nepal
- Rahman, M. , Nakamura, K. , Seino, K. , & Kizuki, M. (2013). Intimate partner violence and chronic undernutrition among married Bangladeshi women of reproductive age: Are the poor uniquely disadvantaged? European Journal of Clinical Nutrition, 67(3), 301–307. 10.1038/ejcn.2012.202 [DOI] [PubMed] [Google Scholar]
- Rahman, M. M. , Abe, S. K. , Rahman, M. S. , Kanda, M. , Narita, S. , Bilano, V. , … Shibuya, K. (2016). Maternal anemia and risk of adverse birth and health outcomes in low‐ and middle‐income countries: Systematic review and meta‐analysis. The American Journal of Clinical Nutrition, 103(2), 495–504. 10.3945/ajcn.115.107896 [DOI] [PubMed] [Google Scholar]
- Rai, A. , Gurung, S. , Thapa, S. , & Saville, N. M. (2019). Correlates and inequality of underweight and overweight among women of reproductive age: Evidence from the 2016 Nepal Demographic Health Survey. PLoS ONE, 14(5), e0216644 10.1371/journal.pone.0216644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai, A. , Maharjan, M. R. , Harris Fry, H. A. , Chhetri, P. K. , Wasti, P. C. , & Saville, N. M. (2019). Consumption of rice, acceptability and sensory qualities of fortified rice amongst consumers of social safety net rice in Nepal. PLoS ONE, 14(10), e0222903 10.1371/journal.pone.0222903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadatmahalleh, S. J. , Ziaei, S. , Kazemnejad, A. , & Mohamadi, E. (2016). Menstrual pattern following tubal ligation: A historical cohort study. Int J Fertil Steril , 9(4), 477‐482. doi: 10.22074/ijfs.2015.4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saville, N. M. , Shrestha, B. P. , Style, S. , Harris‐Fry, H. , Beard, B. J. , Sen, A. , … Costello, A. (2018). Impact on birth weight and child growth of participatory learning and action women's groups with and without transfers of food or cash during pregnancy: Findings of the low birth weight South Asia cluster‐randomised controlled trial (LBWSAT) in Nepal. PLoS ONE, 13(5), e0194064 10.1371/journal.pone.0194064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah, B. K. , & Baig, L. A. (2005). Association of anemia with parasitic infestation in pregnant Nepalese women: Results from a hospital‐based study done in eastern Nepal. Journal of Ayub Medical College Abbottabad, 17(1). https://www.ncbi.nlm.nih.gov/pubmed/15929517 [PubMed] [Google Scholar]
- Shai, N. , Pradhan, G. D. , Chirwa, E. , Shrestha, R. , Adhikari, A. , & Kerr‐Wilson, A. (2019). Factors associated with IPV victimisation of women and perpetration by men in migrant communities of Nepal. PLoS ONE, 14(7), e0210258 10.1371/journal.pone.0210258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shobeiri, M. J. , & Atashkhoii, S. (2005). The risk of menstrual abnormalities after tubal sterilization: A case control study. BMC Womens Health, 5(1), 5 10.1186/1472-6874-5-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, K. R. N. , Sah, R. B. , & Pokharel, P. K. (2015). A study on malaria cases in hilly areas and Terai belt of Nepal. Health Renaissance, 13(1), 4–12. 10.3126/hren.v13i1.17942 [DOI] [Google Scholar]
- Sinha, A. K. , Majumdar, B. , & Yadav, S. K. (2011). Prevalence and significance of iron deficiency anaemia among people of Morang District of Nepal. Journal of Nobel Medical College, 1(1), 40–44. https://www.ncbi.nlm.nih.gov/pubmed/20334065 [Google Scholar]
- Stevens, G. A. , Finucane, M. M. , De‐Regil, L. M. , Paciorek, C. J. , Flaxman, S. R. , Branca, F. , … Nutrition Impact Model Study Group . (2013). Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non‐pregnant women for 1995–2011: A systematic analysis of population‐representative data. The Lancet Global Health, 1(1), e16–e25. 10.1016/S2214-109X(13)70001-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The World Bank . (2018). Nepal Sunaula Hazar Din Community Action for Nutrition Project: Endline Report. Kathmandu, Nepal. http://documents.worldbank.org/curated/en/276351468062668345/Nepal-Community-Action-for-Nutrition-Project-Sunaula-Hazar-Din
- United Nations . (2015). The Millenium Development Goals Report 2015. Newyork. https://www.un.org/millenniumgoals/2015_MDG_Report/pdf/MDG2015rev(July1).pdf
- United Nations Children's Fund . (1990). Strategy for improved nutrition of children and women in developing countries. A UNICEF Policy Review. New York., 58, 13–24. 10.1007/BF02810402 [DOI] [PubMed] [Google Scholar]
- World Food Programme, & Government of Nepal . (2018). Landscape analysis for rice fortification in Nepal. Kathmandu, Nepal
- World Health Organization . (1995). Physical status: The use and interpretation of anthropometry. Geneva. https://www.who.int/childgrowth/publications/physical_status/en/ [PubMed]
- World Health Organization . (2011). Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System. Geneva. https://www.who.int/vmnis/indicators/haemoglobin/en/
- World Health Organization . (2017). Nutritional anaemias: Tools for effective prevention and control (9241513063). Geneva. https://www.who.int/nutrition/publications/micronutrients/anaemias-tools-prevention-control/en/
- Yadav, S. K. , & Sehgal, S. (2003). Effect of domestic processing and cooking methods on total, HCl extractable iron and in vitro availability of iron in bathua and fenugreek leaves. Nutrition and Health, 17(1), 61–63. 10.1177/026010600301700107 [DOI] [PubMed] [Google Scholar]
- Zijp, I. M. , Korver, O. , & Tijburg, L. B. (2000). Effect of tea and other dietary factors on iron absorption. Critical Reviews in Food Science and Nutrition, 40(5), 371–398. 10.1080/10408690091189194 [DOI] [PubMed] [Google Scholar]
- Cavalli‐Sforza, T (2005, Dec). Effectiveness of weekly iron‐folic acid supplementation to prevent and control anemia among women of reproductive age in three Asian countries: development of the master protocol and implementation plan. Nutr Rev, 63(12 Pt 2), S77–80. 10.1301/nr.2005.dec.s77-s80. [DOI] [PubMed] [Google Scholar]
- Gunaratna, N. S , Masanja, H , Mrema, S , Levira, F , Spiegelman, D , Hertzmark, E …, & Fawzi, W (2015). Multivitamin and iron supplementation to prevent periconceptional anemia in rural tanzanian women: a randomized, controlled trial.. PLoS One, 10(4), e0121552 10.1371/journal.pone.0121552–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health and Population , New ERA , UNICEF , EU , USAID , & CDC (2018). Nepal National Micronutrient Status Survey, 2016. Nepal. https://www.unicef.org/nepal/reports/nepal-national-micronutrient-status-survey-report-2016
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Multilevel logistic regression analysis for predictors of anaemia among women of reproductive age (15–49) using the 2006 NDHS
Table S2: Multilevel logistic regression analysis for predictors of anaemia among women of reproductive age (15–49) using the 2011 NDHS
Data Availability Statement
The NDHS data used in the study are a third‐party dataset. We requested for access to these datasets through the Demographic and Health Survey programme. These are open‐source dataset and available to anyone on request at https://dhsprogram.com/data/dataset/Nepal_Standard-DHS_2016.cfm?flag=0.


