Abstract
The DNA methyltransferase inhibitors (DNMTi) 5-azacytidine (AZA) and 5-aza-2-deoxycytidine have been approved for the treatment of different types of hematological malignancies. However, only about 50% of patients respond to treatment. Therefore, a more comprehensive understanding of the molecular changes in patients treated with DNMTi is needed. Here we examined gene expression profiles in a total of 150 RNA samples from two adult cohorts and one pediatric cohort with hematological cancers taken before, during, and after treatment with AZA (40 patients; 15 non-responders, 25 responders). Using each patient as their own control, malignant cells showed preferential activation of a subset of evolutionarily young transposable elements (TE), including endogenous retroviral long terminal repeats (LTR), short and long interspersed nuclear elements (SINE and LINE), and the type I interferon (IFN) pathway in responders, all independent of disease classification. Transfection of eight upregulated LTR into recipient human cells in culture showed robust and heterogenous activation of six genes in the type I IFN pathway. These results, obtained in diverse hematological disease entities, show that common targets (TE) activated by the same drug (AZA) elicit an immune response which may be important for patient’s responses to DNMTi.
Keywords: hematological cancer, 5-azayitidine, transposable elements, endogenous retrovirus, innate immune system
Introduction
The DNA methyltransferase inhibitor (DNMTi) 5-azacytidine (AZA) has been approved by the U.S. Food and Drug Administration and the European Medicines Agency to treat higher-risk myelodysplastic syndrome (MDS), chronic myelomonocytic leukemia (CMML), and acute myeloid leukemia (AML). Except for allogeneic bone marrow transplantation, AZA is the only drug that has been shown to extend the survival of patients with MDS. Yet only about half of the patients respond to the treatment, and most patients become resistant over time (1, 2). Despite intense investigation, the molecular factors associated with the variable clinical responses have not yet been identified (3, 4).
Numerous studies have reported that aberrant DNA methylation is one of the most common molecular changes in cancer cells (5). Hypomethylation of abnormally silenced tumor suppressor genes has been suggested to be the most likely mechanism for response to DNMTis (5–7), but upregulation of cancer testis antigens (CTAs) has also been suggested to play important roles in the activation of the immune system and in killing cancer cells (8–10). Recently, we and other groups have described “viral mimicry”, in which the destruction of cancer cells is mediated by the innate immune system in response to single-and double-stranded transcripts of transposable elements (TEs) that are induced by DNMTi treatment (11, 12).
Activation of the viral mimicry pathway has also been demonstrated in cancer cell lines treated with other types of drugs that target the epigenome, such as inhibitors of G9a (histone lysine 9 methyltransferase) or histone lysine-specific demethylase I (13, 14). Therefore, epigenetic mechanisms including DNA methylation and histone modifications keep TEs silenced (13–15). Despite these extensive in vitro data, the expression profiles of genes involved in the viral mimicry pathway have not yet been extensively investigated or validated in cancer patients treated with DNMTis.
Here we first confirmed that the three major classes of TEs, which are present in all human cells, were indeed upregulated in patients in response to the same drug (AZA) in different hematological malignancies. We then show that eight upregulated LTRs are capable of activating genes in the type I interferon (IFN) pathway providing direct mechanistic evidence for a role for viral mimicry in patients.
Materials and Methods
Patients and drug treatment
The Nordic cohort included 12 patients (MDS, n = 6; CMML, n = 5; AML, n = 1) all treated with AZA from whom consecutive peripheral blood or bone marrow samples were available. All patients had provided written informed consent in accordance with the Declaration of Helsinki, and the study was approved by the local ethics committees and IRB. AZA treatment was performed according to the Nordic MDS guidelines, in which AZA was administered subcutaneously (100 mg/m2) for five consecutive days on a 28-d schedule. Patient characteristics at treatment initiation are shown in Supplementary Table S1. All patients had been diagnosed with higher-risk disease except for one, who had lower-risk MDS but elevated blast counts before the onset of treatment. Clinical response to AZA was evaluated using IWG criteria (16) and defined as the best response during the whole treatment course. Patients with complete remission (CR), partial remission (PR), marrow complete remission (mCR), and hematologic improvement (HI) were considered as responders. The patient characteristics of the Australian (MDS, n = 7; CMML, n = 7) and the pediatric (AML, n = 11; ALL: acute lymphoblastic leukemia, n = 3) cohorts were described in our and others’ previous reports, respectively (17,18).
Isolation of cell populations and RNA extraction
Peripheral blood (PB) and bone marrow (BM) mononuclear cells were isolated immediately upon sample collection using Ficoll-Paque PLUS (GE Healthcare) or Lymphoprep (StemCell Technologies) density gradient centrifugation and cryopreserved at –196 °C for later use. The cells were thawed and immediately isolated into either CD14+ monocytes, CD3+ T cells, or CD34+ stem and progenitor cells using magnetic bead-based separation on a RoboSep magnetic cell separator device (StemCell Technologies) or using MACS columns (Miltenyi Biotec) according to the manufacturer’s instructions. For all five CMML patients, CD14+ cells were used. CD34+ cells were used for two MDS patients with BM samples available. For three of the MDS and AML patients, the CD3 and CD19 double-negative population was used for further analysis of mainly myeloid cells. These were isolated by stepwise positive selection of CD3+ and CD19+ cells. The isolated cells were subjected to total RNA extraction using the AllPrep DNA/RNA Kit (Qiagen) according to the manufactureŕs instructions, including stringent DNase I cleanup of any contaminating genomic DNA, and the concentration was measured using the Qubit fluorometer with RNA HS assay (Thermo Fischer Scientific). For two MDS patients, total RNA was extracted directly from PB mononuclear cells without further isolation. For each individual patient, the same malignant cell populations were isolated and used for RNA-seq at each time point before and during treatment (Supplementary Table S1).
RNA-seq
Total RNA-seq was performed on the 63 RNA samples from the 12 Nordic patients. cDNA libraries were prepared using RNA HyperPrep kits with RiboErase (KAPA Biosystems) according to the manufacturer’s instructions and were sequenced as single-end 75 bases on a NextSeq 500 instrument (Illumina). Sequencing reads were aligned against the human GRCh37 reference genome in order to report results consistent with our previous study (19, 20) by using Bowtie2 and TopHat2 v.2.1.0 (21) with the following parameters: “bowtie2–2.2.5/bowtie2/bowtie2-build –f ~/genomes/hg19.fa Index-hg19” and “tophat-2.1.0.OSX_x86_64/tophat2 -g 1 -I 5000 -o tophat_out bowtie2/hg19 sequence_file.fastq”. The number of overlapping mapped reads with coding genes and TEs was obtained using Samtools-1.2 and BEDTool2–2.24.0 (22) with the following parameters: “samtools-1.2/samtools index accepted_hits.bam” and “bedtools2/bin/coverageBed -a reference_file.bed -b sequence_file_hits.bed -split -sorted > hits.count.txt”. RPKM values (reads per kilobase per million mapped reads) were calculated using uniquely mapped reads. The raw and processed data sets from this study have been submitted to the NCBI Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/) under accession number GSE118558.
Gene ontology and gene set enrichment analysis
Gene ontology analysis of upregulated genes was performed using biological processes in the Gene Ontology database (http://www.geneontology.org) (23, 24). Upregulated genes were defined as those increased by twofold or more posttreatment relative to pretreatment using median RPKM values of the non-responders and responders (the highest values from the multiple time points were used). Gene set enrichment analysis (GSEA) was performed based on P-values obtained by a two-tailed Wilcoxon Signed-Rank Test comparing pretreatment and posttreatment RPKM values (the highest values from the multiple time points were used) in the non-responders and responders. A standard Kolmogorov-Smirnov test (25) was used to test whether a specific gene set showed differential expression between the pretreatment and posttreatment sample. The significance of GSEA was evaluated by permutating the target gene set. Tumor suppressor genes were identified in the NCG 5.0 database (n = 38) (26). Ninety-nine out of 208 CTAs identified by CTdatabase (27) were used, because the other 109 CTAs were not expressed. Immune response-related genes (n = 2,312) were identified following Gene Ontology terms (GO:0002376 and GO:0045321).
Transposable elements analysis
The number of reads from TE transcripts in the Nordic and Australian cohorts was obtained using BEDTool after unique mapping by TopHat2 v.2.1.0. A majority of TEs can be detected by the mapping, as reported in our previous study (20). The definitions of TEs were based on RepeatMasker (http://www.repeatmasker.org). The TEs overlapping with coding genes or with long noncoding RNAs were excluded from the analysis in order to determine the transcripts emanating from the TE elements, as described in our previous report (20). The TEs expressed at low level (less than 20 read counts) were also filtered out from this study. For the analysis of endogenous retroviruses (ERVs), we only included reads mapped to their LTR regions, because LTRs are putative promoters of ERVs capable of initiating transcription.
RT-qPCR
cDNA synthesis using RNA samples from the pediatric cohort was performed with an iScript cDNA synthesis kit (BIO-RAD) after digestion of genomic DNA by TURBO DNase (Thermo Fisher) according to the manufacturer’s instructions. qPCR reactions were carried out in CFX96 Real-Time PCR detection system (Bio-Rad) with a KAPA SYBR FASTqPCR kit (KAPA Biosystems). The conditions of qPCR reaction were as follows: 3 min at 95 °C, followed by 40 cycles of 10 s at 95 °C, 20 s at 60 °C, and 20 s at 72 °C. Primer sequences are listed in Supplementary Table S2.
Synthesis of transcribed LTRs and transfection in vitro
Eight individual LTRs were transcribed from a T7 promoter in PCR products using the mMESSAGE mMACHINE™ T7 Transcription Kit (Thermo Fisher Scientific). Transfection was performed using Lipofectamine 2000 Transfection Reagent (Thermo Fisher Scientific) using 500 ng of in vitro transcribed LTRs for 5×104 HCT116 cells. Total RNA from HCT116 was purified 48 h after treatment using a Direct-zol RNA MiniPrep Kit (Zymo Research). Primer sequences are listed in Supplementary Table S2.
Results
RNA-seq data from patients with hematological cancer
We first performed RNA-seq on sorted malignant myeloid cells and T cells from the Nordic cohort of MDS, CMML, and AML patients (malignant cells: four non-responders and eight responders; T cells: five responders; see Supplementary Table S1 and Supplementary Fig. S1). In addition, we downloaded RNA-seq data on CD34+-sorted MDS and CMML hematopoietic stem and progenitor cells (HSPCs) from an Australian cohort in a publicly available database (17) (five non-responders and nine responders; see Supplementary Table S1 and Supplementary Fig. S1).
Genes involved in immune response and leukocyte activation are activated in responders following AZA treatment
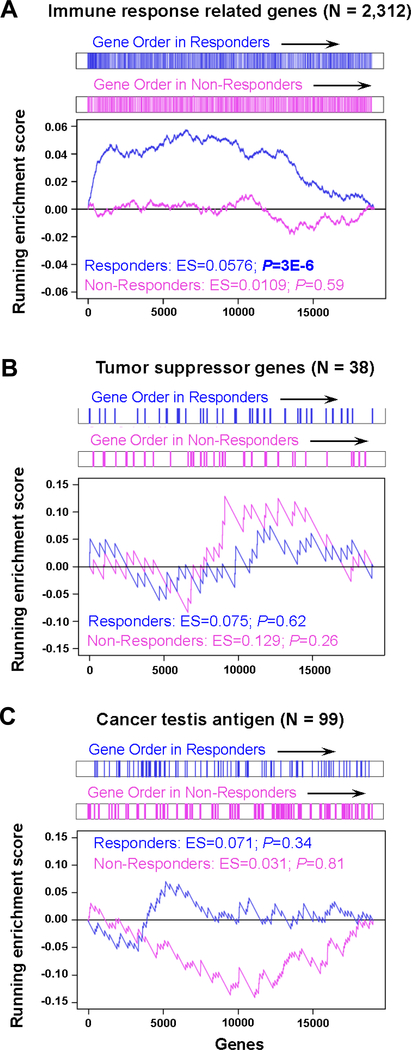
We analyzed RNA-seq data by supervised cluster analysis to identify differential expression patterns between non-responders and responders. We concentrated on upregulated genes for the following analyses, because previous reports from us and others using cultured cells showed that upregulation of tumor suppressor genes, CTAs, and innate immune genes might be involved in anti-tumor effects of the drug in vitro (5, 28). In the non-responders, 385 coding genes were identified as being highly upregulated in the malignant cells when comparing posttreatment to pretreatment values (fold change [FC] ≥ 2; Supplementary Fig. S2A and Supplementary Table S3). Among responders, 1,117 coding genes were identified as being highly upregulated (FC ≥ 2; Supplementary Fig. S2A and Supplementary Table S3). Gene ontology analysis of the upregulated genes showed that immune response gene sets, including those involved in defense response and innate immune response genes, were upregulated in samples from malignant cells of patients responding to AZA treatment (Supplementary Fig. S2B and Supplementary Table S4), but not malignant cells of non-responders or T cells from responders consistent with recent findings in cancer cell lines after DNMTi treatment (11–13, 19, 20). Earlier studies reported that treatment with DNMTis alone or together with histone deacetylase inhibitors enhance T cell rejuvenation accompanied by tumor T cell infiltration facilitated by cytokine production by the cancer cells (29–30). We confirmed the upregulation of leukocyte activation gene sets in the malignant cells from responders (Supplementary Fig. S2B and Supplementary Table S4); however, these gene sets were not upregulated in the gene ontology analysis of the non-responders and T cells from responders (Supplementary Fig. S2B and Supplementary Table S4). Gene set enrichment analysis (GSEA) confirmed that immune response–related genes were significantly upregulated following AZA treatment in responders (P=3×10−6) but not in non-responders (Fig. 1A). The activation of the innate immune system in normal cells may lead to off-target effects for patients (5); however, we found no upregulation of these gene sets among the 3,164 genes in the presumably normal T cells from responders (FC ≥ 2; Supplementary Fig. S2A and S2B and Supplementary Table S4). Thus, the AZA-inducible innate immune response might be cancer cell–specific. This specificity may be due to the fact that AZA has an absolute requirement for incorporation into DNA to inhibit DNA methylation (31). Since normal cells often have lower proliferation rates and many are quiescent, this may help explain the preferential effects on cancer cells (5). Activation of the innate immune system may therefore play a key role in the clinical response to AZA in patients with hematological cancer.
Figure 1. Genes related to immune response are significantly upregulated in responders, but not in non-responders, following AZA treatment.
Gene set enrichment analysis (GSEA) plots show the enrichment in responders (n = 17) and non-responders (n = 9) A, Immune response–related genes; B, Tumor suppressor genes; C, Cancer testis antigens.
Abnormal silencing of tumor suppressor genes by DNA methylation has long been suggested to be the most likely target for epigenetic therapy, because reactivation of such genes by epigenetic drugs might inhibit the growth of cancer cells (6, 7, 32). However, most tumor suppressor genes identified in the NCG 5.0 database (26) (n = 38) did not show significant upregulation in GSEA (Fig. 1B and Supplementary Fig. S3A). Interestingly, both CDKN2A and CDKN2B (which encode the cyclin dependent kinase inhibitors p16 and p15, respectively) were upregulated at low levels in responders (Supplementary Fig. S3A and S3B), but not in non-responders (Supplementary Fig. S3B). Earlier studies have reported abnormal silencing of CDKN2B (but not CDKN2A) by promoter hypermethylation in patients with myeloid cancer (32). The mechanisms of CDKN2A reactivation in this study are unclear, but CDKN2B protein expression can be upregulated by promoter demethylation during DNMTi treatment (33). Whereas the reactivation of CDKN2A and CDKN2B might also influence the differential clinical responses to AZA treatment, the absolute expression levels of the genes were relatively low following AZA treatment in responders (median of RPKM; CDKN2A = 0.37, CDKN2B = 0.30). Therefore, activation of the innate immune system is more likely associated with the clinical responses. This is supported by recent findings in cancer cell lines (11, 12) showing that knocking down genes in the type I IFN signaling pathway can abolish the anti-tumor effect of DNMTis, suggesting that the activation of the innate immune system may play a key role in DNMTi effectiveness as therapeutic drugs.
The derepression of CTAs by DNMTis has also been associated with activation of an immune response (8, 9). However, GSEA showed that the CTAs were not significantly upregulated following AZA treatment in responders (Fig. 1C). Our data therefore suggest that CTAs are unlikely to trigger immune response genes in this group of patients.
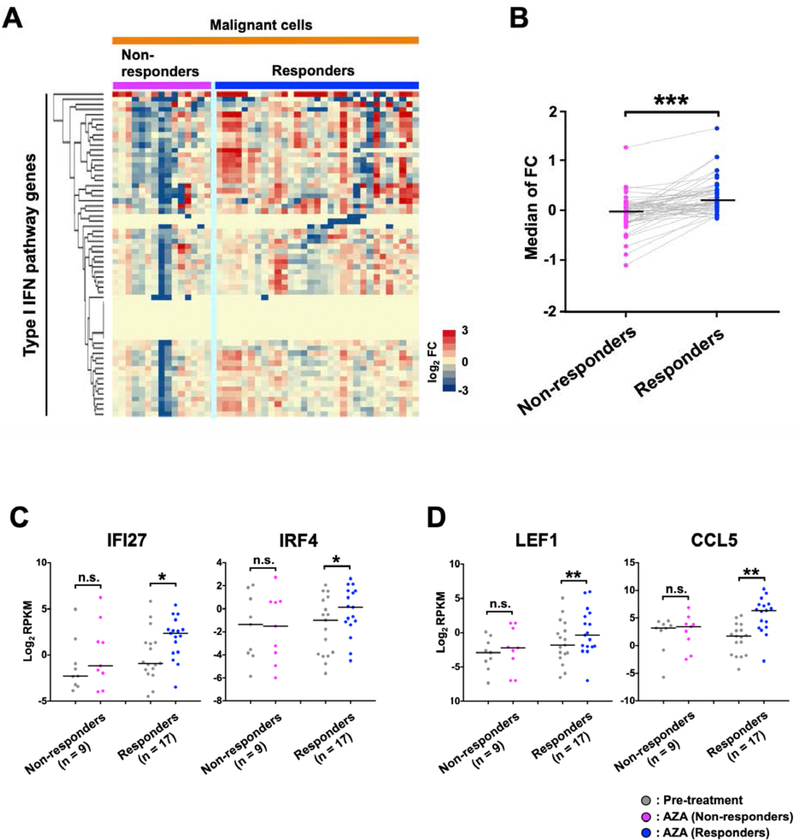
We also applied unsupervised analyses for type I IFN signaling pathway genes (GO:0060337), which are a subset of innate immune response genes (GO:0045087). These genes were preferentially upregulated in all types of malignant cells from responders in both the Nordic and Australian patient cohorts (Supplementary Fig. S4A-S4G). When the RNA-seq data were assembled into one data set, a differential upregulation of the genes in responders was also apparent (Fig. 2A and 2B; P<0.001). The RNA-seq data included the genes for IFI27, a type I IFN inducible protein, and IRF4, an IFN regulatory transcription factor (Fig. 2C; P<0.05). Responder-specific upregulation was also found in the leukocyte activation genes LEF1, encoding a T cell receptor-alpha enhancer-binding transcription factor, and CCL5, encoding a chemokine also known as RANTES (Fig. 2D; P<0.05). However, the upregulation of type I IFN signaling pathway genes and leukocyte activation genes was not linked to clinical responses in all patients, and other molecular changes may also be important for the responses to AZA. Further, some of the type I IFN signaling pathway genes showed downregulation following AZA treatment by an unknown mechanism (Fig. 2A and Supplementary Fig. S4B and S4G). The average mapping rate in the samples from the Nordic patients (average; 91%, highest; 94%, lowest; 77%) was comparable to that of Australian patients (average; 83%, highest; 87%, lowest; 78%). Because, the variation in mapping rates might have effects on the results obtained for expression changes of type I IFN signaling pathway genes, we reanalyzed the data using only those samples with high mapping rates (> 90%). The preferential upregulation of type I IFN signaling pathway genes in responders was confirmed (Supplementary Fig. S4H). Thus the variations in RNA quality as seen in the mapping rates did not change the conclusion.
Figure 2. Responder-specific upregulation of type I IFN signaling pathway genes following AZA treatment.
A, The expression changes of type I IFN signaling pathway genes (GO:0060337: n = 64) based on RNA-seq data. B, This graph represents the median fold change (FC) of the type I IFN signaling pathway genes in non-responders and responders shown in (A). Gray lines connect the same genes in non-responders and responders. P-values were calculated using the two-tailed Wilcoxon Signed-Rank Test for comparisons between the two groups. (***); P < 0.001. C, Expression of selected immune response–related genes by RNA-seq. IFI27 and IRF4 are involved in the type I IFN signaling pathway (GO:0060337). D, Expression of LEF1 and CCL5, which are involved in leukocyte activation (GO:0045321). The highest values obtained at any of the multiple time points are shown as the posttreatment value. P-values were calculated using two-tailed Wilcoxon Signed-Rank Test. (*); P < 0.05, (**); P < 0.01, (n.s.); not significant.
The innate immune system may be activated in response to specific classes of evolutionarily young transposable elements
Recent studies in cell lines and mouse models have reported that the innate immune system is activated by the presence of transcripts from LTRs, an effect we have called “viral mimicry” (11, 12). However, non-LTR retrotransposons such as long and short interspersed nuclear elements (LINEs and SINEs) may also affect the activation of the antiviral innate immune response (34, 35). Chiappinelli et al. (11) showed a correlation between the expression of LTRs and of type I IFN signaling pathway genes in patients with epithelial ovarian cancer without any history of anti-cancer treatment in a study based on The Cancer Genome Atlas (TCGA) consortium data. Unnikrishnan et al. (17) showed that immune and inflammatory response genes were induced by AZA treatment in CMML and MDS responders. Furthermore, we previously reported the upregulation of LTRs by AZA treatment for separated CD34+ stem and progenitor cells cultured in vitro from bone marrow cells of MDS patients (36). However, there are no reports of the upregulation of TEs (including LTRs, LINEs, and SINEs) in cancer patients after DNMTi treatment. Because TEs are widely distributed throughout the human genome, we simplified the analyses by focusing on TEs in intergenic regions, as we have done in a previous comprehensive in vitro analysis (20) (Supplementary Fig. S5, S6, and S7).
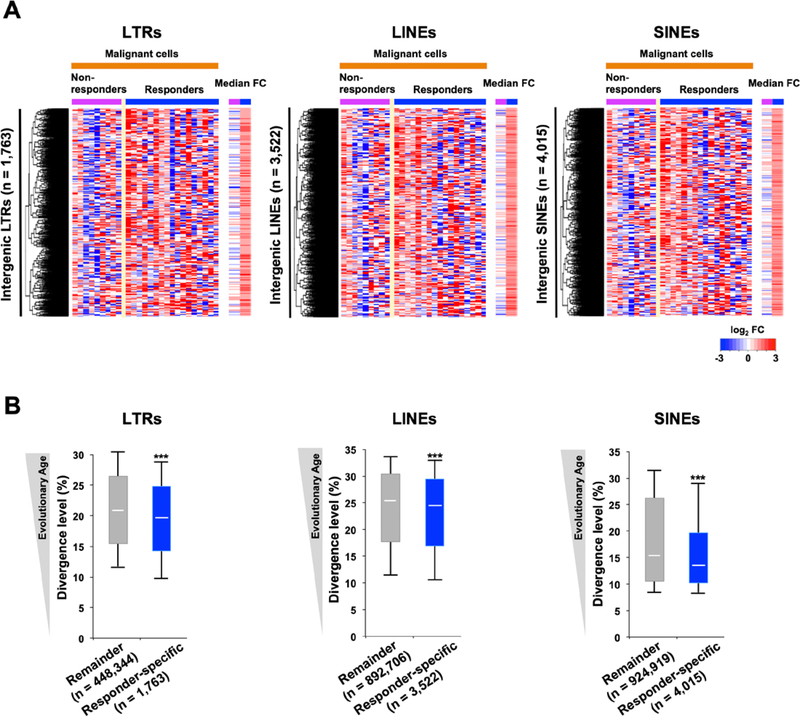
Although numerous TEs were upregulated in the patient samples following AZA treatment, as reported for cancer cell lines (19, 20, 37), we found no significant differences between non-responders and responders in terms of the global upregulation of TEs, i.e., the total number of TE transcripts was not correlated with activation of the innate immune system (Supplementary Fig. S5B-S5C, S6B-S6C, and S7B-S7C). However, as we previously found that evolutionarily young LTRs might be responsible for activation of the innate immune system in vitro (20), we examined the evolutionary ages of responder-specific upregulated TEs (Fig. 3A) based on levels of genetic divergence (Fig. 3B), which can be considered indices of evolutionary age (38, 39). The responder-specific upregulated LTRs had significantly lower divergence levels than LTRs that were not upregulated (P<0.001). These results are remarkably in line with our previous in vitro data, which identified evolutionarily young LTRs as potential triggers for the activation of the innate immune system (20). Moreover, evolutionarily young LINEs and SINEs were also found in the responder-specific upregulated elements (Fig. 3B). We also observed specific classes of evolutionarily young TEs in responder-specific upregulated TEs in both CD14+ cells from Nordic CMML patients at C1D5 (cycle 1, day 5) and mononuclear cells from Nordic MDS patients at C3D1 (Supplementary Fig. S8; P<0.001).
Figure 3. Specific evolutionarily young transposable elements show responder-specific induction by AZA treatment.
A, Intergenic transposable elements (TEs) that were upregulated in responders (median FC ≥ 2) but not in non-responders (median FC < 2). The median FC is calculated by using posttreatment comparing to pretreatment median RPKM values in the non-responders or responders (highest value from the multiple time points is used). B, The distribution of divergence levels in individual TEs shown in A. “Remainder” refers to LTRs that were not responder-specific upregulated by treatment. Each box represents the data between the 25th and 75th quartiles. The whiskers are drawn down to the 10th percentile and up to the 90th percentile. The difference between the two groups is significant for all three TE groups. P-values were calculated using two-tailed Mann-Whitney U test: (∗∗∗); P < 0.001.
We next categorized the responder-specific upregulated TEs into different TE families (Supplementary Fig. S5D, S6D, and S7D). Some specific families were upregulated (FC ≥ 2) in AZA responders, such as LTR12C and LTR7 (Supplementary Fig. S5D and S5E). We previously showed that LTR12 superfamilies such as LTR12C and LTR12 might play key roles in activation of the type I IFN signaling pathway, because these evolutionarily young super-families were among the most abundantly expressed LTRs in cancer cell lines treated with DNMTis (19, 20, 37). Ahmad et al. (34) reported that cytoplasmic Alu:Alu hybrids, overexpressed as a result of adenosine deaminase ADAR1 deficiency, can act as endogenous ligands for the viral RNA sensor MDA5. These earlier reports and the present study suggest that not only AZA-induced evolutionarily young LTRs, but also LINEs and SINEs, may potentially trigger activation of the innate immune system in responders to AZA treatment.
Validation of responder-specific upregulation of immune response, leukocyte activation genes, and evolutionarily young TEs in a pediatric cohort
As shown in the Nordic and Australian cohorts of adult patients with aggressive myeloid cancers, activation of the innate immune system is responder-specific. To extend the generality of this finding, we analyzed samples from pediatric leukemia patients (AML, n = 11; ALL, n = 3; Supplementary Table S1, Fig. 4A,B, Supplementary Fig. S9, and Supplementary Fig. S10A-S10C) from The Therapeutic Advances in Childhood Leukemia & Lymphoma (TACL) consortium (18). We were not able to perform total RNA-seq on these samples; therefore we used RT-qPCR for selected immune response genes, leukocyte activation genes, and LTRs.
Figure 4. The innate immune genes and LTRs show responder-specific upregulation in pediatric patients.
A, Relative expression of immune response– and leukocyte activation–related gene in pediatric AML and ALL patients (n = 14). B, Relative expression of two LTRs in pediatric AML and ALL patients (n = 14). Black lines represent median values. The highest values obtained at any of the multiple time points are shown as the posttreatment value. P-values were calculated using the two-tailed Wilcoxon Signed-Rank Test for comparisons between the two groups. (*); P < 0.05, (**); P < 0.01, (n.s.); not significant.
Consistent with the results from the adult myeloid cancers, both the innate immune system genes (IFI27 and LEF1) and evolutionarily young LTRs (LTR12C and LTR7) were significantly upregulated in the eight responders (P<0.05), but not in the six non-responders, of the pediatric cohort (Fig. 4A,B). Upregulation of CDKN2A and CDKN2B were also observed in some responders (P<0.01), but the absolute expression of these two induced genes following AZA treatment in responders was low (Supplementary Fig. S9). The low expression, as observed in some ALL patients, may be due to genomic deletions of the genes as previously reported (40). Further, CDKN2B was also increased in some non-responders by AZA treatment, although this was not significant (P = 0.125). The pediatric patients also received chemotherapy (fludarabine and cytarabine) along with AZA. It is possible that the combination treatment may have an effect on enhanced innate immune activity, but the activation of the innate immune system could still distinguish a variable clinical response to anticancer therapies. These results support the hypothesis that the activation of the innate immune system is associated with clinical responses to AZA across different types of hematological malignancies independently of exact treatment conditions suggesting a common mechanism of action.
Type I IFN related genes are upregulated by transfection of in vitro transcribed LTRs
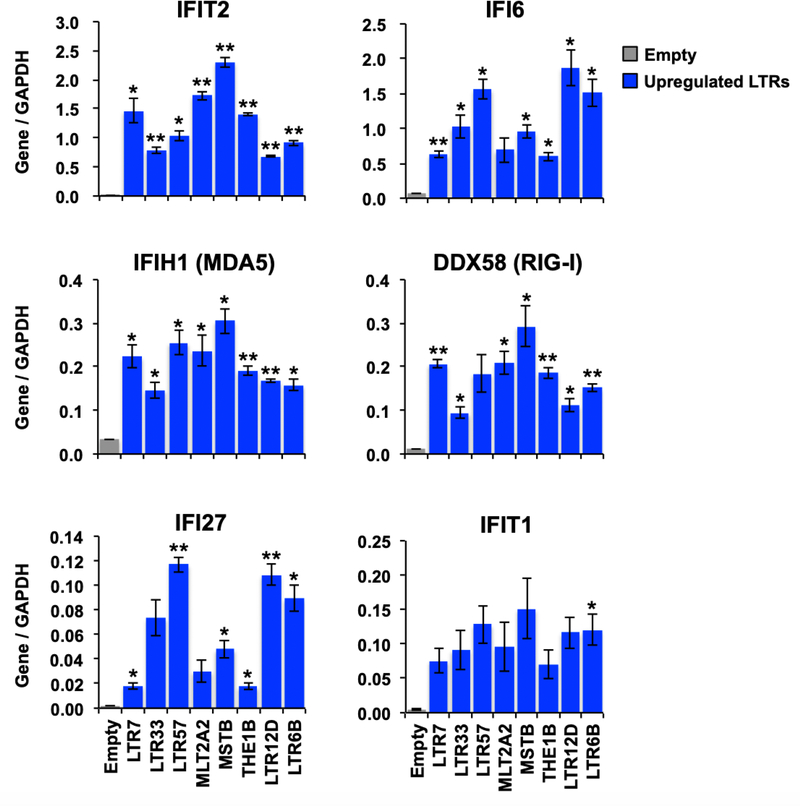
Our analyses in treated patients suggested that upregulated TEs might be responsible for the activation of the host innate immune system. To confirm that specific examples of these LTRs could in fact trigger viral mimicry, in vitro transcribed RNAs for eight selected LTRs strongly induced in responders, were transfected into the HCT116 colon cancer cell line which we have previously shown is competent to respond to induced TE expression (12) (Fig. 5). The expression of type I IFN genes was increased by transfection of all of the LTRs. However, the abilities of individual LTRs to induce genes in the type I IFN pathway were quite variable. For example, MSTB, MTL2A2, and LTR7 induced the highest levels of expression of IFIT2, whereas LTR12D, LTR57, and LTR6B induced the highest levels of IFI6. The abilities of individual LTRs to induce specific viral mimicry responses are therefore highly dependent on the LTR. The tested in vitro transcribed LTRs are single-stranded RNAs, therefore the variable responses for LTR-derived RNA molecules may be due to differences in secondary structures such as hairpin loops or RNA modifications such as tri-phosphorylation (41). Further studies are needed to elucidate the potential function of the responder-specific LTRs. Nevertheless, the data provide mechanistic support for the concept that activation of LTRs in patients is capable of triggering an innate immune response.
Figure 5. The type I IFN related genes are activated by transfection of in vitro transcribed LTRs.
Relative expression of type I IFN related genes based on RT-qPCR after transfection of in vitro transcribed LTRs in the HCT116 cell line. Error bars represent SEM from three independent biological replicates. P-values were calculated using the two-tailed Student’s t-test for comparisons with Empty samples: (*); P < 0.05, (**); P < 0.01.
Discussion
Recent studies in vitro have shown that the innate immune system is activated by the presence of transcripts from LTRs and other types of TEs (20, 34, 35). Here, our analysis of gene expression profiles in patients before and after AZA treatment shows responder-specific activation of the innate immune system in three heterogenous cohorts of patients with different types of hematological malignancies, one of which was solely pediatric patients. The activation of the innate immune system is likely due to the induced expression of evolutionarily young TEs. This is possibly because evolutionarily young TEs are more dependent on DNA methylation for silencing and hence show a more prominent induction by DNMTis, as described in our previous in vitro study (20). We and others have recently shown that disruption of genes in the type I IFN signaling pathway abolishes the anti-tumor effect of DNMTis (11, 12). Moreover, the potential functions of evolutionarily young LTRs, LINEs, and other noncoding RNAs as tumor-intrinsic triggers of innate immune signaling pathways have been reported in earlier studies (20, 34, 35). Chiappinelli et al. (11) showed the upregulation of type l IFN signaling pathway genes following overexpression of plasmid vectors containing LTRs. Further, Guler et al. (35) revealed that silencing of evolutionarily young LINEs using siRNAs targeting conserved regions of the LINEs contributed to the establishment of lethal cancer cells that were tolerant of anti-tumor drugs. We therefore suggest that the viral mimicry pathway plays a role in the patients’ response to treatment with AZA. Although our data in vitro also suggested that evolutionarily young LTRs are responsible for the activation of the host innate immune system, further evidence will be needed to confirm this finding.
Additional molecular mechanisms may clearly also be important for the clinical response to AZA. Numerous studies have reported that reactivation of silenced tumor suppressor, DNA repair, or cell differentiation genes could normalize growth behavior in DNMTi-treated cells (42, 43). Moreover, upregulation of CTAs following DNA demethylation might increase the immunogenicity of, and consequently induce apoptosis of, cancer cells (5). In the current study, the activation of these gene sets was not associated with the clinical response to AZA. However, the gene ontology analysis and GSEA were performed on the fold change of median expression values in all 26 patients. Therefore, the reactivation of silenced tumor suppressor, DNA repair, and differentiation genes might also contribute to an improved clinical response in some patients.
Our study was limited to 40 patients who had hematological cancers and were grouped according to their clinical response rather than classical immuno-morphological classification subtypes. We were able to confirm a statistically robust responder-specific activation of the innate immune system in two adult cohorts (Nordic and Australian) and one pediatric cohort of leukemia patients and in different cell types. The fact that AZA may target the many thousands of TEs, which make up a large proportion of the human genome, may indicate a generalized mechanism of action. Therefore, the statistically significant correlation between viral mimicry pathway activation and drug response also suggests the possibility of a common mechanism in different cell types.
Our results support the concept that the activation of the innate immune system following AZA treatment is important in patients. Further large-scale prospective clinical and translational studies are indeed required to clearly validate this association, and we have recently opened such a clinical trial (# NCT03999723). Our data also show for the first time that although all three major classes of TEs (LTRs, LINEs, and SINEs) are upregulated following AZA treatment, only specific classes of evolutionarily young TEs seem to be associated with induction of the innate immune system and clinical responses to AZA. Therefore, we suggest that the activation of evolutionarily young TEs and the innate immune system may be common factors that are important for the clinical responses to AZA.
Supplementary Material
Significance.
Activation of specific classes of evolutionarily young transposable elements can lead to activation of the innate immune system.
Acknowledgments
We thank the Van Andel Research Institute (VARI) Genomics and Bioinformatics Core; David Nadziejka for technical editing of the article; and all members in Grønbæk and Jones group for discussion. This work was supported by the National Cancer Institute (grant R35CA209859), the Folz Fund of the Grand Rapids Community Foundation, and by Van Andel Institute through the Van Andel Institute – Stand Up to Cancer Epigenetics Dream Team. Stand Up to Cancer is a division of the Entertainment Industry Foundation. Research grants are administered by the American Association for Cancer Research, the Scientific Partner of SU2C. The study was also funded by the Lundbeck Foundation (grant R180–2014-3675), the Danish Cancer Society (grant R124-A7695), and the Novo Nordisk Foundation (grant NNF13OC0003435). The Grønbæk Lab is funded by center grants from The Danish Cancer Society (Danish Research Center for Precision Medicine in Blood Cancer; grant 223-A13071–18-S68), the Novo Nordisk Foundation (Novo Nordisk Foundation Center for Stem Cell Biology, DanStem; grant NNF17CC0027852), and from Greater Copenhagen Health Science Partners (Clinical Academic Group in Translational Hematology).
Footnotes
Conflict of interest: K.G. serves on advisory boards for Celgene and Otsuka Pharma. P.A.J. is consultant for Zymo Inc.
References
- 1.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10:223–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ørskov AD, Grønbæk K. DNA Methyltransferase Inhibitors in Myeloid Cancer: Clonal Eradication or Clonal Differentiation? Cancer J Sudbury Mass. 2017;23:277–85. [DOI] [PubMed] [Google Scholar]
- 3.Treppendahl MB, Kristensen LS, Grønbæk K. Predicting response to epigenetic therapy. J Clin Invest. 2014;124:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuendgen A, Müller-Thomas C, Lauseker M, Haferlach T, Urbaniak P, Schroeder T, et al. Efficacy of azacitidine is independent of molecular and clinical characteristics-an analysis of 128 patients with myelodysplastic syndromes or acute myeloid leukemia and a review of the literature. Oncotarget. 2018;9:27882–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones PA, Issa J-PJ, Baylin S. Targeting the cancer epigenome for therapy. Nat Rev Genet. 2016;17:630–41. [DOI] [PubMed] [Google Scholar]
- 6.Bender CM, Pao MM, Jones PA. Inhibition of DNA methylation by 5-aza-2’-deoxycytidine suppresses the growth of human tumor cell lines. Cancer Res. 1998;58:95–101. [PubMed] [Google Scholar]
- 7.McGarvey KM, Fahrner JA, Greene E, Martens J, Jenuwein T, Baylin SB. Silenced tumor suppressor genes reactivated by DNA demethylation do not return to a fully euchromatic chromatin state. Cancer Res. 2006;66:3541–9. [DOI] [PubMed] [Google Scholar]
- 8.Weber J, Salgaller M, Samid D, Johnson B, Herlyn M, Lassam N, et al. Expression of the MAGE-1 tumor antigen is up-regulated by the demethylating agent 5-aza-2’-deoxycytidine. Cancer Res. 1994;54:1766–71. [PubMed] [Google Scholar]
- 9.De Smet C, De Backer O, Faraoni I, Lurquin C, Brasseur F, Boon T. The activation of human gene MAGE-1 in tumor cells is correlated with genome-wide demethylation. Proc Natl Acad Sci U S A. 1996;93:7149–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gang AO, Frøsig TM, Brimnes MK, Lyngaa R, Treppendahl MB, Grønbæk K, et al. 5-Azacytidine treatment sensitizes tumor cells to T-cell mediated cytotoxicity and modulates NK cells in patients with myeloid malignancies. Blood Cancer J. 2014;4:e197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiappinelli KB, Strissel PL, Desrichard A, Li H, Henke C, Akman B, et al. Inhibiting DNA Methylation Causes an Interferon Response in Cancer via dsRNA Including Endogenous Retroviruses. Cell. 2015;162:974–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roulois D, Loo Yau H, Singhania R, Wang Y, Danesh A, Shen SY, et al. DNA-Demethylating Agents Target Colorectal Cancer Cells by Inducing Viral Mimicry by Endogenous Transcripts. Cell. 2015;162:961–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu M, Thomas SL, DeWitt AK, Zhou W, Madaj ZB, Ohtani H, et al. Dual Inhibition of DNA and Histone Methyltransferases Increases Viral Mimicry in Ovarian Cancer Cells. Cancer Res. 2018;78:5754–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheng W, LaFleur MW, Nguyen TH, Chen S, Chakravarthy A, Conway JR, et al. LSD1 Ablation Stimulates Anti-tumor Immunity and Enables Checkpoint Blockade. Cell. 2018;174:549–563.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karimi MM, Goyal P, Maksakova IA, Bilenky M, Leung D, Tang JX, et al. DNA methylation and SETDB1/H3K9me3 regulate predominantly distinct sets of genes, retroelements, and chimeric transcripts in mESCs. Cell Stem Cell. 2011;8:676–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheson BD, Greenberg PL, Bennett JM, Lowenberg B, Wijermans PW, Nimer SD, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108:419–25. [DOI] [PubMed] [Google Scholar]
- 17.Unnikrishnan A, Papaemmanuil E, Beck D, Deshpande NP, Verma A, Kumari A, et al. Integrative Genomics Identifies the Molecular Basis of Resistance to Azacitidine Therapy in Myelodysplastic Syndromes. Cell Rep. 2017;20:572–85. [DOI] [PubMed] [Google Scholar]
- 18.Sun W, Triche T, Malvar J, Gaynon P, Sposto R, Yang X, et al. A phase 1 study of azacitidine combined with chemotherapy in childhood leukemia: a report from the TACL consortium. Blood. 2018;131:1145–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu M, Ohtani H, Zhou W, Ørskov AD, Charlet J, Zhang YW, et al. Vitamin C increases viral mimicry induced by 5-aza-2’-deoxycytidine. Proc Natl Acad Sci U S A. 2016;113:10238–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohtani H, Liu M, Zhou W, Liang G, Jones PA. Switching roles for DNA and histone methylation depend on evolutionary ages of human endogenous retroviruses. Genome Res. 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14:R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinforma Oxf Engl. 2010;26:841–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The Gene Ontology Consortium. Expansion of the Gene Ontology knowledgebase and resources. Nucleic Acids Res. 2017;45:D331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.An O, Dall’Olio GM, Mourikis TP, Ciccarelli FD. NCG 5.0: updates of a manually curated repository of cancer genes and associated properties from cancer mutational screenings. Nucleic Acids Res. 2016;44:D992–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Almeida LG, Sakabe NJ, deOliveira AR, Silva MCC, Mundstein AS, Cohen T, et al. CTdatabase: a knowledge-base of high-throughput and curated data on cancer-testis antigens. Nucleic Acids Res. 2009;37:D816–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones PA, Ohtani H, Chakravarthy A, De Carvalho DD. Epigenetic therapy in immune-oncology. Nat Rev Cancer. 2019;19:151–61. [DOI] [PubMed] [Google Scholar]
- 29.Topper MJ, Vaz M, Chiappinelli KB, DeStefano Shields CE, Niknafs N, Yen R-WC, et al. Epigenetic Therapy Ties MYC Depletion to Reversing Immune Evasion and Treating Lung Cancer. Cell. 2017;171:1284–1300.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghoneim HE, Fan Y, Moustaki A, Abdelsamed HA, Dash P, Dogra P, et al. De Novo Epigenetic Programs Inhibit PD-1 Blockade-Mediated T Cell Rejuvenation. Cell. 2017;170:142–157.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones PA, Laird PW. Cancer epigenetics comes of age. Nat Genet. 1999;21:163–7. [DOI] [PubMed] [Google Scholar]
- 32.Daskalakis M, Nguyen TT, Nguyen C, Guldberg P, Köhler G, Wijermans P, et al. Demethylation of a hypermethylated P15/INK4B gene in patients with myelodysplastic syndrome by 5-Aza-2’-deoxycytidine (decitabine) treatment. Blood. 2002;100:2957–64. [DOI] [PubMed] [Google Scholar]
- 33.Cechova H, Lassuthova P, Novakova L, Belickova M, Stemberkova R, Jencik J, et al. Monitoring of methylation changes in 9p21 region in patients with myelodysplastic syndromes and acute myeloid leukemia. Neoplasma. 2012;59:168–74. [DOI] [PubMed] [Google Scholar]
- 34.Ahmad S, Mu X, Yang F, Greenwald E, Park JW, Jacob E, et al. Breaching Self-Tolerance to Alu Duplex RNA Underlies MDA5-Mediated Inflammation. Cell. 2018;172:797–810.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guler GD, Tindell CA, Pitti R, Wilson C, Nichols K, KaiWai Cheung T, et al. Repression of Stress-Induced LINE-1 Expression Protects Cancer Cell Subpopulations from Lethal Drug Exposure. Cancer Cell. 2017;32:221–237.e13. [DOI] [PubMed] [Google Scholar]
- 36.Tobiasson M, Abdulkadir H, Lennartsson A, Katayama S, Marabita F, De Paepe A, et al. Comprehensive mapping of the effects of azacitidine on DNA methylation, repressive/permissive histone marks and gene expression in primary cells from patients with MDS and MDS-related disease. Oncotarget. 2017;8:28812–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brocks D, Schmidt CR, Daskalakis M, Jang HS, Shah NM, Li D, et al. DNMT and HDAC inhibitors induce cryptic transcription start sites encoded in long terminal repeats. Nat Genet. 2017;49:1052–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang AS, Gonzalgo ML, Zingg JM, Millar RP, Buckley JD, Jones PA. The rate of CpG mutation in Alu repetitive elements within the p53 tumor suppressor gene in the primate germline. J Mol Biol. 1996;258:240–50. [DOI] [PubMed] [Google Scholar]
- 39.Moorjani P, Amorim CEG, Arndt PF, Przeworski M. Variation in the molecular clock of primates. Proc Natl Acad Sci U S A. 2016;113:10607–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mirebeau D, Acquaviva C, Suciu S, Bertin R, Dastugue N, Robert A, et al. The prognostic significance of CDKN2A, CDKN2B and MTAP inactivation in B-lineage acute lymphoblastic leukemia of childhood. Results of the EORTC studies 58881 and 58951. Haematologica. 2006;91:881–5. [PubMed] [Google Scholar]
- 41.Pichlmair A, Schulz O, Tan CP, Näslund TI, Liljeström P, Weber F, et al. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5’-phosphates. Science. 2006;314:997–1001. [DOI] [PubMed] [Google Scholar]
- 42.Tsai H-C, Li H, Van Neste L, Cai Y, Robert C, Rassool FV, et al. Transient low doses of DNA-demethylating agents exert durable antitumor effects on hematological and epithelial tumor cells. Cancer Cell. 2012;21:430–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin JC, Jeong S, Liang G, Takai D, Fatemi M, Tsai YC, et al. Role of nucleosomal occupancy in the epigenetic silencing of the MLH1 CpG island. Cancer Cell. 2007;12:432–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.